Abstract
Purpose:
The aim of this study was to prospectively quantify the level of agreement among the deep learning system, non-physician graders, and general ophthalmologists with different levels of clinical experience in detecting referable diabetic retinopathy, age-related macular degeneration, and glaucomatous optic neuropathy.
Methods:
Deep learning systems for diabetic retinopathy, age-related macular degeneration, and glaucomatous optic neuropathy classification, with accuracy proven through internal and external validation, were established using 210,473 fundus photographs. Five trained non-physician graders and 47 general ophthalmologists from China were chosen randomly and included in the analysis. A test set of 300 fundus photographs were randomly identified from an independent dataset of 42,388 gradable images. The grading outcomes of five retinal and five glaucoma specialists were used as the reference standard that was considered achieved when ≥50% of gradings were consistent among the included specialists. The area under receiver operator characteristic curve of different groups in relation to the reference standard was used to compare agreement for referable diabetic retinopathy, age-related macular degeneration, and glaucomatous optic neuropathy.
Results:
The test set included 45 images (15.0%) with referable diabetic retinopathy, 46 (15.3%) with age-related macular degeneration, 46 (15.3%) with glaucomatous optic neuropathy, and 163 (55.4%) without these diseases. The area under receiver operator characteristic curve for non-physician graders, ophthalmologists with 3–5 years of clinical practice, ophthalmologists with 5–10 years of clinical practice, ophthalmologists with >10 years of clinical practice, and the deep learning system for referable diabetic retinopathy were 0.984, 0.964, 0.965, 0.954, and 0.990 (p = 0.415), respectively. The results for referable age-related macular degeneration were 0.912, 0.933, 0.946, 0.958, and 0.945, respectively, (p = 0.145), and 0.675, 0.862, 0.894, 0.976, and 0.994 for referable glaucomatous optic neuropathy, respectively (p < 0.001).
Conclusion:
The findings of this study suggest that the accuracy of this deep learning system is comparable to that of trained non-physician graders and general ophthalmologists for referable diabetic retinopathy and age-related macular degeneration, but the deep learning system performance is better than that of trained non-physician graders for the detection of referable glaucomatous optic neuropathy.
Introduction
Diabetic retinopathy (DR), glaucomatous optic neuropathy (GON), and age-related macular degeneration (AMD) are responsible for more than 18% of visual impairment and blindness cases globally (1–6). While it is estimated that 80% of vision loss is avoidable through early detection and intervention (7–9), approximately 50% of cases remain undiagnosed (10, 11). High rates of undiagnosed disease can be attributed to these conditions being asymptomatic in their early stages, coupled with a disproportionately low availability of eye care services, particularly within developing countries and under-served populations (12).
Previous research has demonstrated that color fundus photography is an effective tool for the diagnosis of AMD, GON, and DR (13–15). Despite this, accurate interpretation of the optic nerve and retina is highly dependent on clinical experts, limiting the utility in low recourse settings. Deep learning represents an advancement of artificial neural networks that permits improved predictions from raw image data (16). Recently, several studies have investigated the application of deep learning algorithms for the automated classification of common ophthalmic disorders (17–21), with promising results for disease classification (sensitivity and specificity range = 80–95%). Thereby, these systems offer great promise to improve the accessibility and cost-effectiveness of ocular disease screening in developing countries.
Despite this, most previous systems could only detect a single ocular disorder, thus would omit severe blinding eye diseases. In addition, previous studies have evaluated on retrospective datasets, and there is a paucity of data directly comparing the performance of deep learning system (DLS) capable to detect common blindness diseases to that of general ophthalmologists or non-physician graders. Given the fact that in real world screening programs, human graders or general ophthalmologists may also make mistakes, a robust study to directly compare DLS and general ophthalmologists or non-physician graders is of paramount importance for healthcare decision makers and patients to make informed decisions relating to the deployment of these systems.
Therefore, in the present study, we investigated the diagnostic agreement between ophthalmologists with varying levels of experience, non-physician graders, and validated deep learning models (22) for DR, GON, and AMD on an independent dataset in China.
Methods
This study was approved by the Institutional Review Board of the Zhongshan Ophthalmic Center, China (2017KYPJ049) and conducted in accordance with the Declaration of Helsinki. All graders and ophthalmologists have been informed that their data will be compared with the DLS. Informed consent for the use of fundus photographs was not required as images were acquired retrospectively and were fully anonymized.
Test set development, reference standard, and definitions
A total of 300 fundus photographs were randomly selected from a subset of 42,388 independent gradable images from the online LabelMe dataset (http://www.labelme.org, Guangzhou, China) (22, 23). The LabelMe dataset includes images from 36 hospital ophthalmology departments, optometry clinics, and screening settings in China that include various kinds of eye diseases, such as DR, glaucoma, and AMD. The data will be available upon request. Retinal photographs were captured using a variety of common conventional desktop retinal cameras, including Topcon, Canon, Heidelberg, and Digital Retinography System. The LabelMe dataset was graded for DR, GON, and AMD by 21 ophthalmologists who previously achieved an unweighted kappa of ≥0.70 (substantial) on a test set of images. Images were randomly assigned to a single ophthalmologist for grading and were returned to the pooled dataset until three consistent grading outcomes were achieved. Once an image was given a reference standard label it was removed from the grading dataset. This process has been described in detail elsewhere (22, 23).
Stratified random sampling was used to select 50 images of each disease category and an additional 150 images classified as normal or a disease other than DR, AMD, and GON. Poor quality images (defined as ≥50% of the fundus photograph area obscured) were excluded. Images that were included in the training and internal validation datasets of the deep learning models were not eligible for inclusion. Following the selection of images, experienced retinal (n = 5) specialists independently labeled all 300 images to establish a reference standard for DR and AMD. Similarly, glaucoma specialists (n = 5) independently graded all images to determine the GON reference standard. Specialists were blinded to any previous medical history or retinal diagnosis for the included images. Once all images were graded, they were converted to a two-level classification for each disease: non-referable and referable. Each image was only assigned a conclusive label if more than 50% of the specialists reported a consistent grading outcome.
A website1 was developed to allow human graders to log in and interpret images. Diabetic retinopathy severity was classified as none, mild non-proliferative DR (NPDR), moderate NPDR, severe NPDR, and proliferative DR using the International Clinical Diabetic Retinopathy scale (24). Diabetic macular edema (DME) was defined as any hard exudates within one-disk diameter of the fovea or an area of hard exudates in the macular area at least 50% of the disk area (25). Referable DR was defined as moderate NPDR or worse with or without the presence of DME. The severity of AMD was graded according to the clinical classification of AMD, which has been described elsewhere (26). For the purpose of this study, referable AMD was defined as late wet AMD as it was the only subtype of AMD that could be managed with effective therapy currently. Glaucomatous optic neuropathy was classified as absent or referable GON according to definitions utilized by previous population-based studies (27–29). The definition of referable GON included the presence of any of the following: vertical cup to disk ratio (VCDR) ≥0.7; rim width ≤0.1 disk diameter; localized notches; and presence of retinal nerve fiber layer (RNFL) defect and/or disk hemorrhage.
Development of the deep learning system
The development and validation of the DR, GON, and AMD models have been described in detail elsewhere (22, 30–32). In brief, referable GON, DR, and AMD deep learning algorithms were developed using a total of 210,473 fundus photographs (referable DR, 106,244; referable GON, 48,116; referable AMD 56,113). Several pre-processing steps were performed for normalization to control for variations in image size and resolution. This included augmentation to enlarge heterogeneity, applying local space average color for color constancy and downsizing image resolution to 299 × 299 pixels (33). Finally, eight convolutional neural networks were contained within the DLS (Version 20,171,024), all adopting Inception-v3 architecture (34). The development of the networks was described in our previous studies (22, 23, 32). Briefly, the networks were downsized to 299 × 299, and local space average color and data augmentation were adopted. These networks were trained from scratch and included (1) classification for referable DR, (2) classification of DME, (3) classification of AMD, (4) classification of GON, and (5) assessment of the availability of the macular region and rejection of non-retinal photographs.
Graders and ophthalmologists identification and recruitment
Five trained non-physician graders, who also previously received training for DR, AMD, and GON classification, usually graded images from 50 to 100 participants for common blindness diseases every workday and underwent tests per quarter, from Zhongshan Ophthalmic Center Image Grading Center with National Health Screening (NHS) DR grader certification were recruited to grade all these images.
We also invited general ophthalmologists from four provincial hospitals and five county hospitals in seven provinces in China (Guangdong, Guangxi, Fujian, Jiang Su, Yunnan, Xinjiang, and Inner Mongolia province). General ophthalmologists who had at least 3 years clinical practice including residency were eligible to participate.
Selected ophthalmologists were sent an invitation to participate via email or mobile phone text message. Those who did not respond were followed up with a telephone call. The clinical practice characteristics of invited ophthalmologists were obtained from publicly available resources or personally via telephone.
Of the 330 ophthalmologists who were eligible to participate, 66 (20%) were randomly selected and subsequently invited to participate in the study. Nineteen ophthalmologists (28.8%) declined or did not respond and 47 ophthalmologists (71.2%) agreed to participate. A flow chart outlining the recruitment of ophthalmologists is shown in Figure 1.
Figure 1
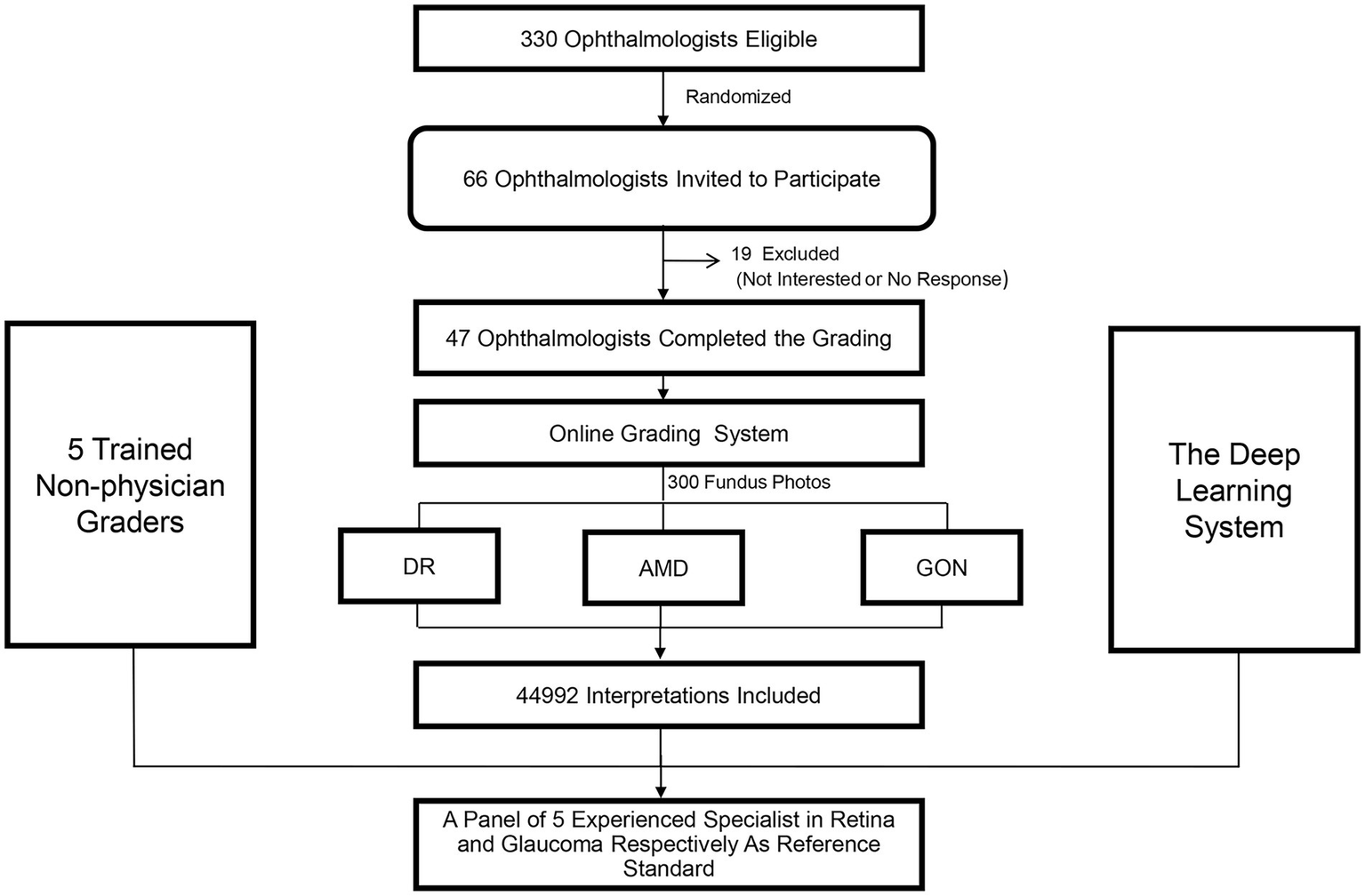
Recruitment, workflow, and grading of ophthalmologists and non-physician graders.
Test set implementation
Participants independently reviewed all 300 images in a random order. They were blinded to the reference standard and the grades assigned by other participants. Due to the variability in existing classification criteria for GON, a standardized grading criteria was provided to all participants. Participants were not provided with details of the comprehensive grading criterion utilized for the grading of DR and AMD, as it was assumed that the participants’ experience would be sufficient to enable them to classify these disorders into the specific categories (DR: mild, moderate, severe NPDR and proliferative DR; AMD: early or moderate AMD, late dry AMD, and late wet AMD). There was no time limit for the interpretation of each image. All grading results were converted to a two-level classification for each disease (referable and non-referable disorders) and then compared against the reference standard. The eight deep learning models were also tested using the same images.
In order to characterize the features of misclassified images by DLS and human graders, an experienced ophthalmologist (Z.X.L.) reviewed misclassified fundus photographs and classified them into categories arbitrarily developed by a consensus meeting by investigators.
Statistical analysis
The area under the receiver operating characteristic curve (AUC), rate of agreement and unweighted kappa were calculated. Agreement was defined as the proportion of images that were correctly classified by participants or the DLS models using the gold standard label as a reference standard. Firstly, data from all participants were used and in this situation, the CIs accounting for within and between subject variability by estimating the variance using the form; {var.(parameterp) + [avg(parameterp) × (1−avg(parameterp))]/nc}/np, where avg.(parameterp) denotes the average corresponding parameter (AUC, agreement rate or kappa) among participants, var.(parameterp) denotes the sample variance of parameter among participants, nc denotes the number of images interpreted by each participant, and np denotes the number of participants.
Then, a representative grading result for graders and ophthalmologists was made when more than 50% of group members achieved consistent grading outcomes. As the DLS can generate a continuous probability between 0 and 1 for referable disorders, AUC for DLS was calculated using these continuous probabilities to compared with reference standard, whereas the agreement rate and unweighted kappa were dichotomized by assigning a certain probability when reaching the highest accuracy. The AUCs of graders, ophthalmologists, and DLS were calculated by comparing with reference standard for two-level classification (referable and non-referable).
We investigated the extent to which the clinical experience of ophthalmologists was associated with agreement. Logistic regression models of ophthalmologist agreement that simultaneously incorporated several ophthalmologist characteristics (hospital level, academic affiliation, clinical practice years, and clinical expertise) were modeled. Non-physician graders were not included in this analysis due to the relatively small sample size (n = 5).
Sensitivity analyses was used to explore whether the grading results would change by using an alternate reference standard instead of the specialist-derived standard. Firstly, cases where the reference standard was different from the most frequent (≥80.0%) grading result of the participants were identified (8 of 300 images). Then, the results were reanalyzed by substituting the most frequent grading outcome of participants as the reference standard for the eight images, or just excluding the eight images. A p value of less than 0.05 was regarded as statistically significant. Stata statistical software (version 14; College Station, Texas, United States) was used.
Results
Reference dataset
Of the 300 images included in the dataset, the total number of images labeled as referable DR, AMD, and GON according to the final specialist grading were 45 (15.0%), 46 (15.3%), and 46 (15.3%), respectively. The remaining 163 (54.4%) images were classified as normal or a disease other than DR, AMD, and GON.
Graders and ophthalmologists characteristics
The five trained non-physician graders were all females with a mean age of 30.4 ± 2.2 years (range, 27–34 years) and an average of 3.6 ± 0.6 years (range, 2–5 years) of grading experience in DR screening support and research image grading. There were 6, 23, 12, and 6 general ophthalmologists aged <30, 30–40, 40–50, and ≥50 years, respectively. Among these ophthalmologists, there were 22 males and 25 females. Twenty-seven were from affiliated hospitals and the other were from nonaffiliated hospitals. Their lengths of clinical practice were 5 years (n = 13), 5–10 years (n = 16), and ≥10 years (n = 18).
Diagnostic agreement among deep learning models, trained non-physician graders, and ophthalmologists
Table 1 displays the agreement distribution by individual grading outcomes of specialists performing initial reference standard grading compared to the final reference standard. The overall agreement rate of the initial independent specialist diagnoses was 96.5% for referable DR, 98.1% for referable AMD, and 92.8% for referable GON.
Table 1
| Specialist ophthalmologists independent gradings | ||||
|---|---|---|---|---|
| Final reference standard | Absent | Present | Missing | Total |
| Referable DRb | ||||
| Absent | 1,269 | 6 | 0 | 1,275 |
| Present | 45 | 178 | 2 | 225 |
| Total | 1,314 | 184 | 2 | 1,500 |
| Late wet AMDc | ||||
| Absent | 1,258 | 12 | 0 | 1,270 |
| Present | 16 | 214 | 0 | 230 |
| Total | 1,274 | 226 | 0 | 1,500 |
| Referable GONd | ||||
| Absent | 1,176 | 94 | 0 | 1,270 |
| Present | 14 | 216 | 0 | 230 |
| Total | 1,190 | 310 | 0 | 1,500 |
Comparison of the five specialist ophthalmologist’s independent gradings vs. final expert consensus reference standard for 300 fundus photographs.a
aThe overall all agreement rate for referable DR, late wet AMD, and GON were 96.5, 98.1, and 92.8%, respectively. b,cThe members to make reference standard were consisted of five retina specialists, and each disorder was graded for multiple categories and then converted to two levels for analysis.dThe members were consisted of five glaucoma specialists. DR, diabetic retinopathy; AMD, age-related macular degeneration; GON, glaucomatous optic neuropathy.
Table 2 provides a comparison between the DLS and general ophthalmologists. The sensitivity and specificity of the DLS for referable DR were 97.8% (44/45) and 92.5% (236/255), respectively. The results for general ophthalmologists for referable DR were 91.1% (41/45) and 99.6% (254/255), respectively.
Table 2
| Reference standard | Deep learning system | Ophthalmologists | |||||
|---|---|---|---|---|---|---|---|
| Agreement (%) | Misclassification (%) | Total | Agreement (%) | Misclassification (%) | Total | ||
| Diabetic | Referable | 44 (97.8) | 1 (2.2) | 45 | 41 (91.1) | 4 (8.9) | 45 |
| Retinopathy | Non-referable | 236 (92.5) | 19 (7.5) | 255 | 254 (99.6) | 1 (0.4) | 255 |
| Age related macular degeneration | Referable | 39 (83.0) | 8 (7.0) | 47 | 43 (91.5) | 4 (8.5) | 47 |
| Non-referable | 245 (96.8) | 8 (3.2) | 253 | 248 (98.0) | 5 (2.0) | 253 | |
| Glaucomatous optic neuropathy | Referable | 45 (97.8) | 1 (2.2) | 46 | 42 (91.3) | 4 (8.7) | 46 |
| Non-referable | 252 (99.2) | 2 (0.8) | 254 | 249 (98.0) | 5 (2.0) | 254 | |
Comparison of deep learning system and general ophthalmologists to the expert consensus reference standard.
A representative grading result for graders and ophthalmologists were made when more than 50% of group members achieved a consistent grading.
Table 3 compares the grading agreement of trained non-physician graders, ophthalmologists, and the DLS versus the reference standard. There were no significant differences in the AUC of non-physician graders, general ophthalmologists with different levels of clinical experience, and the DLS for the interpretation of referable DR (p = 0.415, compared with expert consensus reference diagnosis) and referable AMD (p = 0.145, compared with expert consensus reference diagnosis). For the classification of GON, the DLS achieved a superior AUC result compared to non-physician graders (p < 0.001).
Table 3
| Trained non-physician graders (95% CI) | Ophthalmologists (95% CI) | Deep learning systema (95% CI) | p value | ||||
|---|---|---|---|---|---|---|---|
| Clinical experience 3–5 years | Clinical experience 5–10 years | Clinical experience >10 years | Total | ||||
| Referable DR | |||||||
| Model 1 | |||||||
| AUC | 0.984 (0.960–1.000) | 0.964 (0.926–1.000) | 0.965 (0.927–1.000) | 0.954 (0.911–0.996) | 0.954 (0.911–0.995) | 0.990 (0.982–0.999) | 0.415 |
| Kappa | 0.959 (0.845–1.000) | 0.946 (0.832–1.000) | 0.947 (0.834–1.000) | 0.933 (0.820–1.000) | 0.933 (0.820–1.000) | 0.775 (0.665–0.886) | |
| Agreement rate | 0.989 (0.971–0.998) | 0.983 (0.961–0.996) | 0.987 (0.966–0.996) | 0.983 (0.961–0.995) | 0.983 (0.962–0.995) | 0.933 (0.899–0.959) | |
| Referable AMD | |||||||
| Model 1 | |||||||
| AUC | 0.912 (0.859–0.964) | 0.933 (0.887–0.979) | 0.946 (0.904–0.987) | 0.958 (0.922–0.995) | 0.948 (0.906–0.989) | 0.945 (0.903–0.986) | 0.145 |
| Kappa | 0.823 (0.710–0.936) | 0.851 (0.738–0.964) | 0.876 (0.762–0.989) | 0.901 (0.788–1.000) | 0.887 (0.774–1.000) | 0.798 (0.685–0.911) | |
| Agreement rate | 0.953 (0.923–0.974) | 0.960 (0.931–0.979) | 0.967 (0.940–0.983) | 0.973 (0.948–0.988) | 0.970 (0.944–0.986) | 0.947 (0.915–0.969) | |
| Referable GON | |||||||
| Model 1 | |||||||
| AUC | 0.675 (0.604–0.746) | 0.862 (0.797–0.926) | 0.894 (0.836–0.953) | 0.976 (0.946–1.000) | 0.953 (0.911–0.994) | 0.994 (0.988–0.999) | <0.001 |
| Kappa | 0.445 (0.341–0.549) | 0.779 (0.666–0.891) | 0.825 (0.712–0.938) | 0.961 (0.848–1.000) | 0.922 (0.809–1.00) | 0.926 (0.813–1.00) | |
| Agreement rate | 0.887 (0.845–0.920) | 0.947 (0.914–0.969) | 0.957 (0.927–0.977) | 0.990 (0.971–0.998) | 0.980 (0.957–0.993) | 0.980 (0.956–0.993) | |
Agreement of image interpretation by trained non-physician graders, general ophthalmologists, and deep learning system versus the expert consensus reference standard.a
DR, diabetic retinopathy; AMD, age-related macular degeneration; GON, glaucomatous optic neuropathy; AUC, area under receiver operator characteristic curve; CI, confidence interval. aThe AUC, kappa, and agreement rate of graders and ophthalmologists were calculated using a representative grading result for each group when there was at least 50% of group members reached consistent grading.
Ophthalmologist characteristics related with image interpretation agreement
The agreement between general ophthalmologists’ image grading and the reference standard is shown in Table 4. Table 4 shows that the overall agreement was higher for referable DR in ophthalmologists with greater clinical experience (p = 0.009) and those who were specialists (p = 0.040). Agreement was significantly higher for referable AMD in ophthalmologists from provincial level hospitals (p = 0.017), adjunct academic affiliations (p = 0.002), ophthalmologists with more years of clinical practice (p = 0.009), and those who were glaucoma or retinal specialist ophthalmologists (p = 0.006). Similarly, the level of agreement for referable GON was greater among ophthalmologists from provincial level hospitals (p < 0.001), those from adjunct academic affiliations (p < 0.001), those with more years of clinical experience (p < 0.001) and those who were glaucoma or retinal specialist ophthalmologists (p < 0.001).
Table 4
| Characteristics | Referable diabetic retinopathy | Referable age-related macular degeneration | Referable glaucomatous optic neuropathy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | AUC (95% CI) | Agreement rate (95% CI) | p | n | AUC (95% CI) | Agreement rate (95% CI) | p | n | AUC (95% CI) | Agreement rate (95% CI) | p | |
| Hospital | ||||||||||||
| County level (n = 20) | 5,794 | 0.929 (0.929–0.930) | 0.955 (0.955–0.956) | 5,878 | 0.871 (0.871–0.872) | 0.929 (0.929 0.930) | 5,868 | 0.818 (0.818–0.820) | 0.903 (0.902–0.903) | |||
| Provincial level (n = 27) | 7,894 | 0.932 (0.931–0.932) | 0.956 (0.956–0.957) | 7,971 | 0.872 (0.871–0.872) | 0.929 (0.929–0.930) | 8,030 | 0.875 (0.875–0.876) | 0.933 (0.932–0.933) | |||
| 0.808a | 0.017a | <0.001a | ||||||||||
| Academic affiliation | ||||||||||||
| None (n = 18) | 5,196 | 0.926 (0.925–0.926) | 0.954 (0.954–0.955) | 5,281 | 0.867 (0.867–0.868) | 0.926 (0.926–0.927) | 5,269 | 0.810 (0.810–0.811) | 0.897 (0.897–0.898) | |||
| Adjunct affiliation (n = 29) | 8,492 | 0.934 (0.934–0.935) | 0.957 (0.957–0.958) | 8,568 | 0.891 (0.891–0.892) | 0.941 (0.941–0.942) | 8,629 | 0.877 (0.876–0.878) | 0.934 (0.934–0.935) | |||
| 0.343b | 0.002b | <0.001b | ||||||||||
| Clinical practice (yrs) | ||||||||||||
| ≤5 (n = 13) | 3,718 | 0.925 (0.924–0.925) | 0.951 (0.950–0.951) | 3,780 | 0.875 (0.875–0.876) | 0.928 (0.927–0.928) | 3,782 | 0.806 (0.805–0.807) | 0.569 (0.892–0.893) | |||
| 5–10 (n = 16) | 4,637 | 0.929 (0.928–0.929) | 0.953 (0.953–0.954) | 4,703 | 0.876 (0.876–0.877) | 0.934 (0.934–0.935) | 4,743 | 0.848 (0.847–0.849) | 0.919 (0.919–0.920) | |||
| >10 (n = 18) | 5,333 | 0.937 (0.937–0.938) | 0.839 (0.838–0.840) | 5,366 | 0.892 (0.891–0.892) | 0.942 (0.942–0.943) | 5,373 | 0.887 (0.886–0.888) | 0.941 (0.940–0.041) | |||
| 0.009c | 0.009c | <0.001c | ||||||||||
| Expertise in ophthalmology | ||||||||||||
| Nonexpert (n = 27) | 7,797 | 0.929 (0.929–0.930) | 0.953 (0.953–0.954) | 7,919 | 0.873 (0.873–0.874) | 0.930 (0.930–0.931) | 7,934 | 0.817 (0.816–0.817) | 0.902 (0.901–0.902) | |||
| Expert (n = 20) | 5,891 | 0.933 (0.933–0.934) | 0.960 (0.960–0.961) | 5,930 | 0.894 (0.894–0.895) | 0.942 (0.942–0.943) | 5,964 | 0.898 (0.898–0.899) | 0.944 (0.944–0.945) | |||
| 0.040d | 0.006d | <0.001d | ||||||||||
Ophthalmologist characteristics for image interpretation versus expert consensus reference standard.
A test for trend based on logistic regression model which diagnostic agreement for corresponding disorder was considered as the outcome variable and a two-category variable for hospital level was regarded as independent variable.
A test for trend based on logistic regression model which diagnostic agreement for corresponding disorder was considered as the outcome variable and a two-category variable for whether to be an adjunct affiliation was regarded as independent variable.
A test for trend based on logistic regression model which diagnostic agreement for corresponding disorder was considered as the outcome variable and a three-category variable for clinical practice years was regarded as independent variable.
A test for trend based on logistic regression model which diagnostic agreement for corresponding disorder was considered as the outcome variable and a two-category variable for expertise in ophthalmology was regarded as independent variable.
CI, confidence interval.
Image disagreement characteristics
The interpretations of non-physician graders, ophthalmologists, and the DLS compared with the reference standard for each of the 300 fundus photographs for diabetic retinopathy are shown in Figure 2. This figure also demonstrates that several images caused mistakes common to nonphysician graders, ophthalmologists, and the DLS; for example, images #1 and #87 triggered consistent false positives. In the same way, images #71, #97, #140, #181, #232, and #239 displayed consistent false negatives. These images are shown in Figure 3. The general features of images that were misclassified by human participants (trained non-physician graders and ophthalmologists) are summarized in Table 5.The primary reason for false negative of referable DR was the presence of DME (n = 10, 58.9%), while two cases (100.0%) with microaneurysm/s and artifacts resulted in false positive by human participants. For referable AMD, false negative cases were mostly related to the presence of subtle subretinal hemorrhage (n = 6, 50.0%). False positives resulted from misclassification of earlier forms of AMD (n = 9, 75.1%). Among human participants, the most common reason for false negative of referable GON were those images with borderline VCDR (n = 8, 27.7%), while false positives occurred in those images which displayed physiological cupping (n = 14, 93.3%).
Figure 2
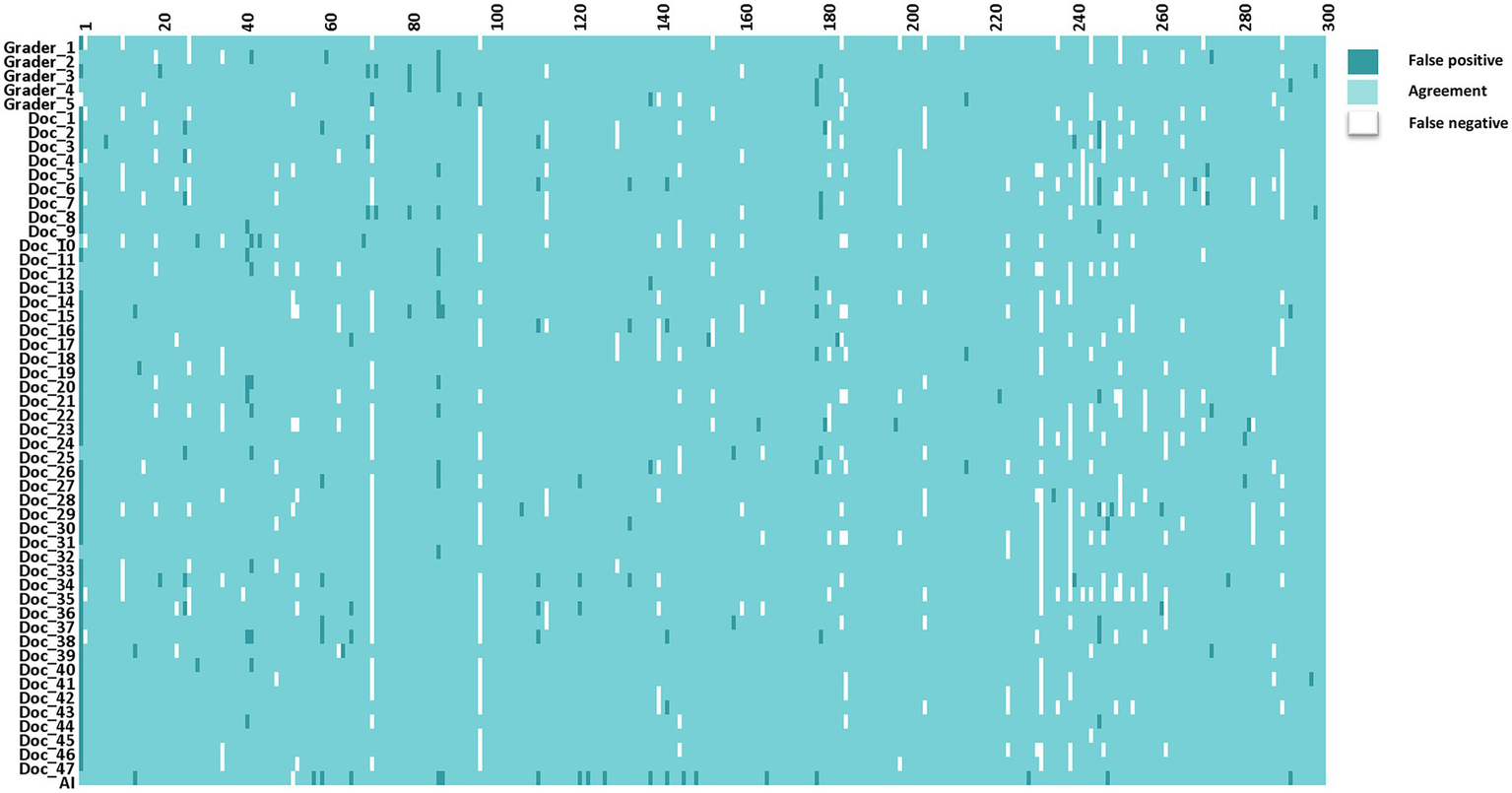
The interpretations of graders, ophthalmologists, and artificial intelligence compared with the reference standards for each of the 300 fundus photographs for diabetic retinopathy.
Figure 3
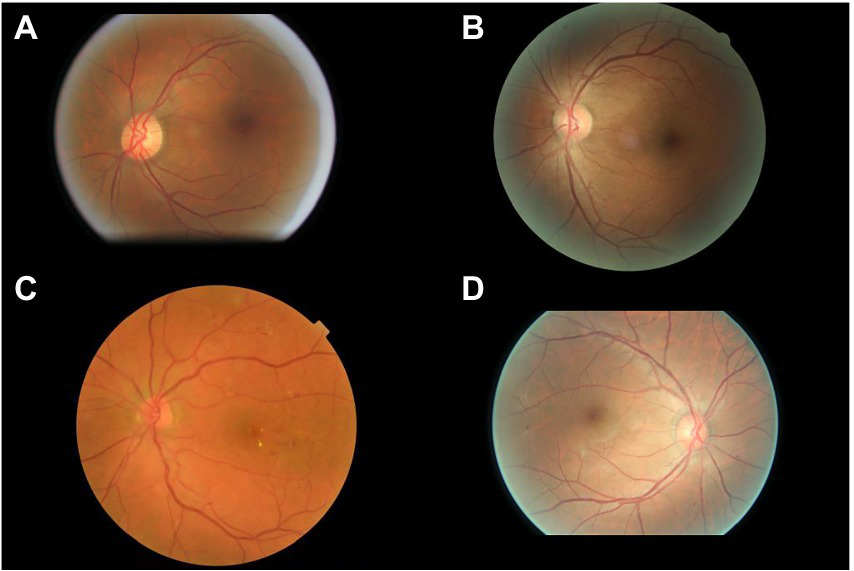
Sample images consistently misclassified by human participants. (A,B) Images with only microaneurysm misclassified as referable diabetic retinopathy. (C) Images of diabetic macular edema misclassified as non-referable diabetic retinopathy. (D) Microaneurysm and dot hemorrhage misclassified as non-referable diabetic retinopathy.
Table 5
| Reason | No. | Proportion (%) |
|---|---|---|
| Referable DR | ||
| False negative | ||
| MA, hemorrhage, DME | 10 | 58.9 |
| Dot hemorrhage, MA | 4 | 23.5 |
| MA, hemorrhage, HEs, CWS | 3 | 17.6 |
| Subtotal | 17 | 100.0 |
| False positive | ||
| Microaneurysm/s, Artifacts | 1 | 100.0 |
| Subtotal | 1 | 100.0 |
| Referable AMD | ||
| False negative | ||
| Subretinal Hemorrhage | 6 | 50.0 |
| Sub-retinal/Sub-RPE fibrovascular proliferation | 3 | 25.0 |
| Serous detachment of the sensory retina or RPE | 3 | 25.0 |
| Sub-total | 12 | 100.0 |
| False positive | ||
| Other macular degeneration | 9 | 75.1 |
| Myopic maculopathy | 1 | 8.3 |
| Choroidal osteoma | 1 | 8.3 |
| Other diseases (Pre-macular hemorrhage) | 1 | 8.3 |
| Sub-total | 12 | 100.0 |
| Referable GON | ||
| False negative | ||
| Borderline VCDR | 8 | 27.7 |
| Borderline VCDR with RNFL defect | 6 | 20.7 |
| Optic disk with tilt or rotation | 5 | 17.2 |
| With other diseases | 3 | 10.3 |
| Rim < 0.1 | 3 | 10.3 |
| Notch | 2 | 6.9 |
| Linear hemorrhage around optic disk | 2 | 6.9 |
| Sub-total | 29 | 100.0 |
| False positive | ||
| Physiological large cupping (0.5 ≤ VCDR < 0.7) | 14 | 93.3 |
| Juxtapapillary capillary hemangioma | 1 | 7.7 |
| Sub-total | 15 | 100.0 |
Characteristics of the disagreement images by human participants.a
The cases included in this analysis were those with more than 20% of the individual human participants (graders and ophthalmologists) inconsistent with the reference standard. DR, diabetic retinopathy; MA, microaneurysm; HEs, hard exudates; CWS, cotton-wool spot; DME, diabetic macular edema; AMD, age-related macular degeneration; RPE, retina pigment epithelium; and GON, glaucomatous optic neuropathy; VCDR, vertical cup to disc ratio; RNFL, retinal nerve fiber layer.
One fundus image demonstrated coexisting intraretinal microvascular abnormality and DME that were not identified by the DLS. The most common reason for false positives by the DLS was the presence of microaneurysm/s only (n = 10, 55.5%; Table 6). For referable AMD, the presence of subretinal hemorrhage (n = 5, 71.4%) was the primary reason for false negative and other diseases (n = 7, 87.5%) including DR or GON. For referable GON, the DLS under-interpreted one image with VCDR less than 0.7, while two images with physiological large cupping (n = 2, 40%) and three images with other diseases (n = 3, 60%) were incorrectly classified as positive.
Table 6
| Reason | No. | Proportion (%) |
|---|---|---|
| Referable DR | ||
| False negative | ||
| MA, IRMA, DME | 1 | 100.0 |
| Sub-total | 1 | 100.0 |
| False positive | ||
| MA only | 10 | 55.5 |
| Other diseases | ||
| Late wet AMD | 4 | 22.2 |
| Retinal degeneration | 3 | 16.7 |
| RVO | 1 | 5.6 |
| Subtotal | 18 | 100.0 |
| Referable AMD | ||
| False negative | ||
| Subretinal hemorrhage | 5 | 71.4 |
| Serous detachment of the sensory retina or RPE | 2 | 28.6 |
| Subtotal | 7 | 100.0 |
| False positive | ||
| Other diseases | ||
| DR | 7 | 87.5 |
| GON | 1 | 12.5 |
| Subtotal | 8 | 100.0 |
| Referable GON | ||
| False negative | ||
| VCDR < 0.7 with notch | 1 | 100.0 |
| Sub-total | 1 | 100.0 |
| False positive | ||
| Physiologic large cupping (0.5 ≤ VCDR < 0.7) | 2 | 40.0 |
| Other diseases | ||
| AMD | 2 | 40.0 |
| Juxtapapillary capillary hemangioma | 1 | 20.0 |
| Subtotal | 5 | 100.0 |
Characteristics of the disagreement images by deep learning system.
DR, diabetic retinopathy; MA, microaneurysm; IRMA, intra-retinal microvascular abnormality; DME, diabetic macular edema; AMD, age-related macular degeneration; VRO, retinal vein occlusion; RPE, retina pigment epithelium; and GON, glaucomatous optic neuropathy.
Discussion
In this study, we prospectively compared the diagnostic agreement of trained non-physician graders and ophthalmologists using three validated deep learning models for the detection of referable DR, late wet AMD, and GON from color fundus photographs. Our results suggest that the performance of the deep learning models for referable DR and AMD are comparable to non-physician graders and ophthalmologists. As for referable GON, the DLS outperformed non-physician graders.
There was no difference among the non-physician graders, ophthalmologists with different years of clinical practice, and the DLS for the diagnostic accuracy of referable DR. The non-physician graders included in this study all had grader certification from the NHS DR screening program, underwent regular assessments every month, and routinely interpreted fundus photographs of diabetic patients from nationwide screening programs, which may explain their relatively high agreement compared to the gold standard. While the DLS also exhibited comparably good performance when compared with non-physician graders and general ophthalmologists.
Comparison of the DLS with general ophthalmologists found that the DLS had higher sensitivity (97.8 vs. 91.1%) and lower specificity (92.5 vs. 99.6%) for the classification of referable DR. However, nearly half of the false positive cases identified by the DLS included (n = 8, 44.5%) other disorders, for example, late wet AMD and retinal degeneration. The remaining false positive images (n = 10, 55.5%) had mild NPDR. Those images identified as false positive by the DLS would receive a referral and be identified during confirmatory examination conducted by a specialist.
Previous studies have shown that the majority of referral cases for DR (73%) are as a result of DME (35). There are 100 million patients with DR worldwide which corresponds to 7.6 million DME patients (36). However, our results showed that images that were characterized as DME (n = 10, 58.9%) were under interpreted by human graders more often than other DR lesions. DR changes related to DME displayed considerable variation among graders and ophthalmologists, with an overall agreement rate of 71% when compared with the reference standard. Therefore, the importance of not overlooking the diagnosis of DME among graders and ophthalmologists should be emphasized.
The DLS outperformed non-physician graders in the classification of referable GON in this study. The variability in inter-assessor agreement among non-physician graders and ophthalmologists for the classification of ocular disorders is well known, especially glaucoma (37, 38). The Glaucomatous optic neuropathy evaluation (GONE) project previously reported that ophthalmology trainees underestimated glaucoma likelihood in 22.1% of optic disks and overestimated 13.0% of included optic disks. This has been similar in our study where general ophthalmologists underestimated 23.8% and underestimated 8.9% of included optic disks (37). Furthermore, Breusegem et al. (38) reported that non-expert ophthalmologists had significantly lower accuracy compared with experts in the diagnosis of glaucoma. Our results are in agreement with previous studies and showed that ophthalmologists with more clinical experience and specialist training in ophthalmology achieve higher inter-assessor agreement. The experience and knowledge obtained through years of clinical practice is likely to play a significant role in interpretation and performance accuracy. In contrast, the DLS is easily able to adopt labels from experienced ophthalmologists to learn the most representative characteristics of GON. Fundus photography is an important method to evaluate GON, however, the diagnosis of glaucoma requires the results of visual field analysis, optical coherence tomography, and intra ocular pressure measurements to make an accurate diagnosis. Thus, further studies to compare DLS with ophthalmologists using multi-modality clinical data is warranted.
The main strength of our study was to prospectively compare the performance of a DLS for the detection of three common blinding eye diseases to non-physician graders and ophthalmologists of varying levels of experience and with different specialties. Our study is also distinctly different from previous reports (19, 39–42). First, we evaluated three ocular diseases at the same time. Second, no prospective comparison of ophthalmologists with varying levels of clinical experience and trained non-physician graders with a DLS for common ocular disorders has been reported. Previous authors have compared the performance of the DLS with that of graders or specialists; this is often considered the gold standard for the development of the DLS (39, 41, 43). Non-physician graders and ophthalmologists are susceptible to making diagnostic mistakes. Our study included independent graders and ophthalmologists to evaluate the performance of the DLS. Therefore, the current study will provide information on the accuracy of the DLS, as well as a more comprehensive understanding and acceptance of how AI systems might work or contribute.
There are several limitations of this study which warrant further consideration. On one hand, human participants included in this study were recruited from China. This has the potential to affect the generalizability of these results to other human graders, especially those in developed countries. In the future, similar studies should be attempted in other countries with different physician or specialist training system. On the other hand, the use of single-field, non-stereoscopic fundus photographs without the inclusion of optical coherence tomography may lead to a reduced sensitivity for DR and particularly DME detection for human participants and the DLS.
In conclusion, our DLS demonstrated sufficient agreement with non-physician graders and general ophthalmologists when compared to the reference standard diagnosis agreement for referable DR and AMD. The DLS performance was better than non-physician graders and ophthalmologists with ≤10 years of clinical experience for referable GON. Further investigation is required to validate the performance in real-world, clinical settings which display the full spectrum and distribution of lesions and manifestations encountered in clinical practice.
Funding
This work was supported by National Key R&D Program of China (2018YFC0116500), the Fundamental Research Funds of the State Key Laboratory in Ophthalmology, National Natural Science Foundation of China (81420108008), and Science and Technology Planning Project of Guangdong Province (2013B20400003).
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Zhongshan Ophthalmic Center, China. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ZL and MH were involved in the concept, design, and development of the deep learning algorithm. ZL, XG, JZ, XL, RC, and MH contributed to the acquisition, analysis, and interpretation of data. ZL wrote the manuscript. All authors revised and edited the manuscript. MH is the guarantor of this work and as such has full access to all the data in the study and takes responsibility for data integrity and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors wish to thank the staffs from the Image Grading Center, Zhongshan Ophthalmic Center, Sun Yat-Sen University for their grading assistance during this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- DLS
Deep learning systems
- DR
Diabetic retinopathy
- AMD
Age related macular degeneration
- GON
Glaucomatous optic neuropathy
- AUC
Area under receiver operator characteristic curve
- GONE
Glaucomatous optic neuropathy evaluation
- VCDR
Vertical cup to disc ratio
- RNFL
Retinal nerve fiber layer
- NHS
English national health screening
- DESP
Diabetic eye screening program
- DME
Diabetic macular edema
Abbreviations
Footnotes
References
1.
Cheung N Mitchell P Wong TY . Diabetic retinopathy. Lancet. (2010) 376:124–36. doi: 10.1016/S0140-6736(09)62124-3
2.
Bressler NM . Age-related macular degeneration is the leading cause of blindness. JAMA. (2004) 291:1900–1. doi: 10.1001/jama.291.15.1900
3.
Pascolini D Mariotti SP Pokharel GP Pararajasegaram R Etya’ale D Négrel AD et al . 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. (2004) 11:67–115. doi: 10.1076/opep.11.2.67.28158
4.
Stevens GA White RA Flaxman SR Price H Jonas JB Keeffe J et al . Global prevalence of vision impairment and blindness: magnitude and temporal trends, 1990–2010. Ophthalmology. (2013) 120:2377–84. doi: 10.1016/j.ophtha.2013.05.025
5.
Bourne RR Stevens GA White RA Smith JL Flaxman SR Price H et al . Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. (2013) 1:e339–49. doi: 10.1016/S2214-109X(13)70113-X
6.
Tham YC Li X Wong TY Quigley HA Aung T Cheng CY . Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. (2014) 121:2081–90. doi: 10.1016/j.ophtha.2014.05.013
7.
Frick KD Foster A . The magnitude and cost of global blindness: an increasing problem that can be alleviated. Am J Ophthalmol. (2003) 135:471–6. doi: 10.1016/S0002-9394(02)02110-4
8.
Armstrong KL Jovic M Vo-Phuoc JL Thorpe JG Doolan BL . The global cost of eliminating avoidable blindness. Indian J Ophthalmol. (2012) 60:475–80. doi: 10.4103/0301-4738.100554
9.
Pizzarello L Abiose A Ffytche T Duerksen R Thulasiraj R Taylor H et al . VISION 2020: the right to sight: a global initiative to eliminate avoidable blindness. Arch Ophthalmol. (Chicago, Ill: 1960). (2004) 122:615–20. doi: 10.1001/archopht.122.4.615
10.
Tapp RJ Shaw JE Harper CA de Courten MP Balkau B McCarty DJ et al . The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care. (2003) 26:1731–7. doi: 10.2337/diacare.26.6.1731
11.
Weih LM Nanjan M McCarty CA Taylor HR . Prevalence and predictors of open-angle glaucoma: results from the visual impairment project. Ophthalmology. (2001) 108:1966–72. doi: 10.1016/S0161-6420(01)00799-0
12.
Subburaman GB Hariharan L Ravilla TD Ravilla RD Kempen JH . Demand for tertiary eye Care Services in Developing Countries. Am J Ophthalmol. (2015) 160:619–627.e1. doi: 10.1016/j.ajo.2015.06.005
13.
Scanlon PH . The english national screening programme for diabetic retinopathy 2003–2016. Acta Diabetol. (2017) 54:515–25. doi: 10.1007/s00592-017-0974-1
14.
Klein R Klein BE Neider MW Hubbard LD Meuer SM Brothers RJ . Diabetic retinopathy as detected using ophthalmoscopy, a nonmydriatic camera and a standard fundus camera. Ophthalmology. (1985) 92:485–91. doi: 10.1016/S0161-6420(85)34003-4
15.
Chan HH Ong DN Kong YX O'Neill EC Pandav SS Coote MA et al . Glaucomatous optic neuropathy evaluation (GONE) project: the effect of monoscopic versus stereoscopic viewing conditions on optic nerve evaluation. Am J Ophthalmol. (2014) 157:936–944.e1. doi: 10.1016/j.ajo.2014.01.024
16.
LeCun Y Bengio Y Hinton G . Deep learning. Nature. (2015) 521:436–44. doi: 10.1038/nature14539
17.
Hassan SS Bong DB Premsenthil M . Detection of neovascularization in diabetic retinopathy. J Digit Imaging. (2012) 25:437–44. doi: 10.1007/s10278-011-9418-6
18.
Abramoff MD Lou Y Erginay A Clarida W Amelon R Folk JC et al . Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci. (2016) 57:5200–6. doi: 10.1167/iovs.16-19964
19.
Chakrabarty L Joshi GD Chakravarty A Raman GV Krishnadas SR Sivaswamy J . Automated detection of glaucoma from topographic features of the optic nerve head in color fundus photographs. J Glaucoma. (2016) 25:590–7. doi: 10.1097/IJG.0000000000000354
20.
Issac A Partha Sarathi M Dutta MK . An adaptive threshold based image processing technique for improved glaucoma detection and classification. Comput Methods Prog Biomed. (2015) 122:229–44. doi: 10.1016/j.cmpb.2015.08.002
21.
Zheng Y Hijazi MH Coenen F . Automated "disease/no disease" grading of age-related macular degeneration by an image mining approach. Invest Ophthalmol Vis Sci. (2012) 53:8310–8. doi: 10.1167/iovs.12-9576
22.
Li Z He Y Keel S Meng W Chang RT He M . Efficacy of a deep learning system for detecting glaucomatous optic neuropathy based on color fundus photographs. Ophthalmology. (2018) 125:1199–206. doi: 10.1016/j.ophtha.2018.01.023
23.
Li Z Keel S Liu C He Y Meng W Scheetz J et al . An automated grading system for detection of vision-threatening referable diabetic retinopathy on the basis of color fundus photographs. Diabetes Care. (2018) 41:2509–16. doi: 10.2337/dc18-0147
24.
Ophthalmology AAo. International clinical diabetic retinopathy disease severity scale detailed table. (2002)
25.
Programme D. Revised grading definitions for the NHS diabetic eye screening Programme. (2012)
26.
Ferris FL 3rd Wilkinson CP Bird A Chakravarthy U Chew E Csaky K et al . Clinical classification of age-related macular degeneration. Ophthalmology. (2013) 120:844–51. doi: 10.1016/j.ophtha.2012.10.036
27.
Iwase A Suzuki Y Araie M Yamamoto T Abe H Shirato S et al . The prevalence of primary open-angle glaucoma in Japanese: the Tajimi study. Ophthalmology. (2004) 111:1641–8. doi: 10.1016/S0161-6420(04)00665-7
28.
He M Foster PJ Ge J Huang W Zheng Y Friedman DS et al . Prevalence and clinical characteristics of glaucoma in adult Chinese: a population-based study in Liwan District. Guangzhou Invest Ophthalmol Vis Sci. (2006) 47:2782–8. doi: 10.1167/iovs.06-0051
29.
Topouzis F Wilson MR Harris A Anastasopoulos E Yu F Mavroudis L et al . Prevalence of open-angle glaucoma in Greece: the Thessaloniki eye study. Am J Ophthalmol. (2007) 144:511–519.e1. doi: 10.1016/j.ajo.2007.06.029
30.
Zhixi Li SK Liu C He Y Meng W Scheetz J Lee PY et al . An automated grading system for vision-threatening referable diabetic retinopathy detection based on color fundus photographs. Diabetes Care. (2018) 41:2509–16. doi: 10.2337/dc18-0147
31.
Keel S Lee PY Scheetz J Li Z Kotowicz MA MacIsaac RJ et al . Feasibility and patient acceptability of a novel artificial intelligence-based screening model for diabetic retinopathy at endocrinology outpatient services: a pilot study. Sci Rep. (2018) 8:4330. doi: 10.1038/s41598-018-22612-2
32.
Keel S Li Z Scheetz J Robman L Phung J Makeyeva G et al . Development and validation of a deep-learning algorithm for the detection of neovascular age-related macular degeneration from colour fundus photographs. Clin Exp Ophthalmol. (2019) 47:1009–18. doi: 10.1111/ceo.13575
33.
Ebner M . Color constancy based on local space average color. Mach Vis Appl. (2009) 20:283–301. doi: 10.1007/s00138-008-0126-2
34.
Christian Szegedy VV . Rethinking the inception architecture for computer vision. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. (2016).
35.
Looker HC Nyangoma SO Cromie DT Olson JA Leese GP Black MW et al . Rates of referable eye disease in the Scottish National Diabetic Retinopathy Screening Programme. Br J Ophthalmol. (2014) 98:790–5. doi: 10.1136/bjophthalmol-2013-303948
36.
Yau JW Rogers SL Kawasaki R Lamoureux EL Kowalski JW Bek T et al . Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. (2012) 35:556–64. doi: 10.2337/dc11-1909
37.
O'Neill EC Gurria LU Pandav SS Kong YX Brennan JF Xie J et al . Glaucomatous optic neuropathy evaluation project: factors associated with underestimation of glaucoma likelihood. JAMA Ophthalmol. (2014) 132:560–6. doi: 10.1001/jamaophthalmol.2014.96
38.
Breusegem C Fieuws S Stalmans I Zeyen T . Agreement and accuracy of non-expert ophthalmologists in assessing glaucomatous changes in serial stereo optic disc photographs. Ophthalmology. (2011) 118:742–6. doi: 10.1016/j.ophtha.2010.08.019
39.
Gulshan V Peng L Coram M Stumpe MC Wu D Narayanaswamy A et al . Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. (2016) 316:2402–10. doi: 10.1001/jama.2016.17216
40.
Silva PS Horton MB Clary D Lewis DG Sun JK Cavallerano JD et al . Identification of diabetic retinopathy and ungradable image rate with ultrawide field imaging in a national teleophthalmology program. Ophthalmology. (2016) 123:1360–7. doi: 10.1016/j.ophtha.2016.01.043
41.
Gargeya R Leng T . Automated identification of diabetic retinopathy using deep learning. Ophthalmology. (2017) 124:962–9. doi: 10.1016/j.ophtha.2017.02.008
42.
Burlina PM Joshi N Pekala M Pacheco KD Freund DE Bressler NM . Automated grading of age-related macular degeneration from color fundus images using deep convolutional neural networks. JAMA Ophthalmol. (2017) 135:1170–6. doi: 10.1001/jamaophthalmol.2017.3782
43.
Ting DSW Cheung CY Lim G Tan GSW Quang ND Gan A et al . Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. (2017) 318:2211–23. doi: 10.1001/jama.2017.18152
Summary
Keywords
deep learning, diabetic retinopathy, age-related macular degeneration, glaucomatous optic neuropathy, fundus photograph
Citation
Li Z, Guo X, Zhang J, Liu X, Chang R and He M (2023) Using deep leaning models to detect ophthalmic diseases: A comparative study. Front. Med. 10:1115032. doi: 10.3389/fmed.2023.1115032
Received
03 December 2022
Accepted
03 February 2023
Published
01 March 2023
Volume
10 - 2023
Edited by
Tyler Hyungtaek Rim, Mediwhale Inc., Republic of Korea
Reviewed by
Tae Keun Yoo, B&VIIT Eye Center/Refractive Surgery & AI Center, Republic of Korea; Wen Fan, Nanjing Medical University, China
Updates
Copyright
© 2023 Li, Guo, Zhang, Liu, Chang and He.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingguang He, mingguang_he@yahoo.com
This article was submitted to Ophthalmology, a section of the journal Frontiers in Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.