Abstract
Purpose:
To further evaluate the efficacy and safety of anti-vascular endothelial growth factor (VEGF) agents in management of primary pterygium.
Methods:
Randomized controlled trials (RCTs) in databases of PubMed, Web of Science, Embase, and the Cochrane Central Register of Controlled Trials were searched from inception to September 2022. Recurrences and complications were evaluated as the pooled risk ratio (RR) and 95% confidence interval (CI) using random-effects model.
Results:
In total of 1,096 eyes in 19 RCTs were included. Anti-VEGF agents statistically decreased recurrence rate of pterygium following surgery (RR 0.47, 95% CI 0.31–0.74, P < 0.001). Subgroup analysis showed that anti-VEGF as an adjunct to bare sclera (RR 0.34, 95% CI 0.13–0.90, P = 0.03) and conjunctival autograft (RR 0.50, 95% CI 0.26–0.96, P = 0.04) statistically reduced recurrence rate, while the effect was not favorable for conjunctivo-limbo autograft (RR 0.99, 95% CI 0.36–2.68, P = 0.98). Anti-VEGF agents statistically decreased recurrence in White patients (RR 0.48, 95% CI 0.28–0.83, P = 0.008), while didn't in Yellow patients (RR 0.43, 95% CI 0.12–1.47, P = 0.18). Both topical (RR 0.19, 95% CI 0.08–0.45, P < 0.001) and subconjunctival anti-VEGF agents (RR 0.64, 95% CI 0.45–0.91, P = 0.01) had a positive influence on recurrence. There was no statistically significant difference in complications between the groups (RR 0.80, 95% CI 0.52–1.22, P = 0.29).
Conclusions:
As adjuvant treatment, anti-VEGF agents statistically reduced the recurrence following pterygium surgery, especially among White patients. Anti-VEGF agents were well tolerated without increased complications.
1. Introduction
As a frequent ocular disease, pterygium is the growth of the fibrovascular conjunctiva tissue into the cornea (1). Surgery is often required when pterygium causes blur of vision, ocular mobility restriction, or even cosmetic dissatisfaction (2). However, the main concern about the surgery is the high level of recurrence, with about 1.9–8% in conjunctival autograft (3), 38–88% in bare sclera, (4) and 0–17% in limbal conjunctival autograft (5). Therefore, many adjuvant treatments, including 5-FU (6), mitomycin C (7), and ciclosporin A (8, 9), have been developed trying to diminish recurrence.
Among the risk factors responsible for pterygium growth, vascular endothelial growth factor (VEGF) plays an important role (10). Compared to normal conjunctiva, a higher level of VEGF was presented in pterygium (11). Consequently, several anti-VEGF agents, mainly bevacizumab, were afterward administered in treating pterygium. Dozens of randomized controlled trials (RCTs) on the safety and efficacy of bevacizumab in pterygium showed inconsistent conclusions (12–30). Although our previous meta-analysis (2) and a recently published paper by Zhang (31) revealed that bevacizumab reduced recurrence after pterygium surgery, the finding wasn't conclusively supported by researches thereafter and the data just focused on bevacizumab. Some other anti-VEGF agents, including ranibizumab (32–35), conbercept (36), and aflibercept (37) also showed different efficacy in management of pterygium. Overall, the current evidence does not convincingly support the use of anti-VEGF in pterygium surgery (10). Whether anti-VEGF drugs can reduce recurrence following pterygium surgery remains unanswered.
The current study is therefore designed to further evaluate the influence of all anti-VEGF agents on primary pterygium in terms of recurrence and complication.
2. Methods
2.1. Search strategy
The study was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. Databases of PubMed, Web of Science, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched from inception to September 2022. Relevant keywords and medical subject heading (MeSH) terms were used, which included: (1) “anti-vascular endothelial growth factor” OR “anti-VEGF” OR “Bevacizumab” OR “Ranibizumab” OR “Conbercept” OR “aflibercept”; (2) “pterygium” OR “pterygia”. Details of the literature searching were demonstrated in the supplemental Search Strategy file. Endnote software was used. Titles and/or abstracts were screened to subtract evidently irrelevant literatures. Full texts were estimated for qualification. To discern studies not found by the electronic searches, we performed a manual search by checking the reference lists of all preliminarily enrolled studies. Language limitation was not utilized.
2.2. Inclusion and exclusion criteria
The qualified articles should fulfill the inclusion criteria: (1) Participants: patients with primary pterygium; (2) Intervention: topical or subconjunctival anti-VEGF agents, despite operation or not. The dose of anti-VEGF agents or follow-up duration were not restricted; (3) Comparison: anti-VEGF agents and control; (4) Outcomes: recurrence rate and/or complication; (5) Study type: RCT. RCTs were excluded if the raw data was unavailable for extraction.
2.3. Outcome measurements
The recurrence rates and complications were the primary outcome measurements. Fibrovascular growth developing cross the cornea was diagnosed as recurrence. The number of recurrences was calculated at the endpoint of the follow-up. The number of complications such as subconjunctival hemorrhage, corneal dellen, and systemic complications during the follow-up in each study was counted.
2.4. Data extraction
Two authors (BWZ and XMD) independently performed the data extraction. The information collected from each study included the first author's last name, year of publication, location, sample size, type of anti-VEGF, route of administration, age, follow-up duration, and treatment method. Discrepancies between the authors were resolved by discussion to obtain a consensus.
2.5. Risk of bias assessment
According to the methods represented in the Cochrane Handbook for Systematic Reviews of Interventions 5.3, two authors (BWZ and XMD) independently assessed the risk of bias in each study. The authors reviewed each study and rated “low”, “high”, or “unclear” to the following items: (1) selection bias (Was there sufficient generation of the allocation concealment and randomization sequence?); (2) performance and detection bias (Was there blinding of personnel, participants, and outcome assessors?); (3) attrition bias (Were there incomplete outcome data and how to deal with this?); (4) reporting bias (Was there evidence of reporting outcome selectively?); and (5) other sources of bias (Were there any other potential threats to validity?). Any conflict was discussed until agreement was reached.
2.6. Statistical analysis
Statistical analyses were administered using RevMan 5.3 (The Cochrane Collaboration, Copenhagen, Denmark). The recurrence rates and complications were considered as dichotomous variables, which were measured as risk ratio (RR) with a 95% confidence interval (CI). It was assumed that heterogeneity still existed because of the diversity in clinical characteristics and differences in sample size among the studies, even when no statistical significance was observed. Thus, random-effects model was used to pool the data. Statistical heterogeneity was evaluated by calculating an I2 statistic and a Cochran Q statistic. Subgroup analysis were conducted to further assess the influence of the following factors on the recurrence: (a) topical or subconjunctival injection of anti-VEGF agents; (b) types of surgery; (c) races of patients. Sensitivity analysis was performed by leaving studies one-by-one to evaluate the stability of the results. Publication biases were detected according to symmetry in funnel plots. A P value <0.05 was considered statistically significant.
3. Results
3.1. Literature search
Process of literature search was summarized in Figure 1. Initially, 149 articles were enrolled. The abstracts of the left studies were screened following the removed duplications. A 37 articles with probably related topic were further reviewed in full texts. In total of 19 RCTs were finally included in the study.
Figure 1

Flow diagram for the literature search and selection process.
3.2. Characteristics and quality assessment of the eligible studies
Characteristics of the enrolled studies were shown in Table 1. A total of 19 RCTs were included (12–26, 30, 32, 36, 38). There were 18 English articles and 1 Chinese articles. In total of 1,096 eyes were included: 570 in the anti-VEGF group whereas 526 in the control group. Based on Cochrane Handbook for Systematic Reviews of Interventions 5.3, quality assessment was performed. The risks of bias for the studies were listed in Supplementary Figures S1A, B.
Table 1
| References | Location | No. of eyes (Anti-VEGF/Control) | Type of anti-VEGF | Route of administration | Mean age (Anti-VEGF/Control, years) | Follow-up (months) | Treatment method |
|---|---|---|---|---|---|---|---|
| Razeghinejad et al. (21) | Iran | 15/15 | Bevacizumab | Subconjunctival | 45.8/41.6 | 8 vs. 7.4 | Conjunctival autograft |
| Banifatemi et al. (16) | Iran | 22/22 | Bevacizumab | Subconjunctival | 41.95/44.13 | 1 | Conjunctival autograft |
| Enkvetchakul et al. (15) | Thailand | 34/40 | Bevacizumab | Subconjunctival | 51.5/49 | 6 | Non-surgery |
| Shenasi et al. (26) | Iran | 33/33 | Bevacizumab | Subconjunctival | 58.67/55.94 | 9 | Bare sclera |
| Shahin et al. (20) | Egypt | 20/21 | Bevacizumab | Subconjunctival | 58.40/57.58 | 8 | Conjunctivo-limbal autograft |
| Xu et al. (38) | China | 40/40 | Bevacizumab | Subconjunctival | 44/41 | 12 | Conjunctivo-limbal autograft |
| Nava-Castaneda et al. (23) | Mexico | 33/16 | Bevacizumab | Subconjunctival | 48.75/47.8 | 12 | Conjunctival autograft |
| Karalezli et al. (17) | Turkey | 42/46 | Bevacizumab | Topical | 58.82/53.04 | 29.3 vs. 28.5 | Conjunctival autograft |
| Razeghinejad et al. (25) | Iran | 20/21 | Bevacizumab | Subconjunctival | 41.95/44.13 | 6 | Conjunctival autograft |
| Ozsutcu et al. (24) | Turkey | 30/30 | Bevacizumab | Subconjunctival | 43.25/41.68 | 9 | Conjunctival autograft |
| Kasetsuwan et al. (22) | Thailand | 12/10 | Bevacizumab | Topical | 50.7/59.3 | 3 | Bare sclera |
| Hwang et al. (30) | Korea | 36/33 | Bevacizumab | Topical | 71.3/73.4 | 6 | Bare sclera |
| Singh et al. (14) | India | 30/30 | Bevacizumab | Subconjunctival | 37.33 | 3 | Conjunctival autograft |
| Bekibele et al. (12) | Nigeria | 26/27 | Bevacizumab | Subconjunctival | 49.2/52.0 | 18.35 | Conjunctival autograft |
| Motarjemizadeh et al. (13) | Iran | 60/30 | Bevacizumab | Topical | 39.47/40.97 | 12 | Bare sclera |
| Nuzzi et al. (19) | Italy | 42/41 | Bevacizumab | Subconjunctival | 52.39/54.02 | 6 | Bare sclera |
| Mohamed et al. (18) | Egypt | 22/18 | Bevacizumab | Subconjunctival | 31–58 | 1 | Conjunctival autograft |
| Zhang et al. (36) | China | 48/48 | Conbercept | Subconjunctival | 60.13/61.02 | 6 | Bare sclera/Conjunctival autograft |
| Mandalos et al. (32) | Greece | 5/5 | Ranibizumab | Subconjunctival | 66.6 | / | Bare sclera |
Characteristics of the enrolled randomized clinical trials.
VEGF, vascular endothelial growth factor.
3.3. Meta-analysis
Recurrence was reported in 15 studies. The definitions of recurrence in the included RCTs were summarized in Table 2. Overall recurrence in the current study was presented in Figure 2. The results showed that anti-VEGF agents significantly decreased recurrence (RR 0.47, 95% CI 0.31–0.74, P < 0.001; Pheterogeneity = 0.18, I2 = 26%). The sensitivity analysis for the recurrence was stable. Subgroup analysis stratified by races indicated that anti-VEGF agents statistically decreased recurrence in White patients (RR 0.48, 95% CI 0.28–0.83, P = 0.008; Pheterogeneity = 0.10, I2 = 42%), while didn't in Yellow patients (RR 0.43, 95% CI 0.12–1.47, P = 0.18; Pheterogeneity = 0.25, I2 = 28%) (Figure 3). Subgroup analysis in terms of the surgery options showed that bare sclera (RR 0.34, 95% CI 0.13–0.90, P = 0.03; Pheterogeneity = 0.02, I2 = 71%) and conjunctival autograft (RR 0.50, 95% CI 0.26–0.96, P = 0.04; Pheterogeneity = 0.82, I2 = 0) statistically reduced recurrence, while conjunctivo-limbo autograft did not (RR 0.99, 95% CI 0.36–2.68, P = 0.98; Pheterogeneity = 0.48, I2 = 0) (Supplementary Figure S2A). Subgroup analysis based on the administration of anti-VEGF agents demonstrated that both topical (RR 0.19, 95% CI 0.08–0.45, P < 0.001; Pheterogeneity = 0.55, I2 = 0) and subconjunctival application (RR 0.64, 95% CI 0.45–0.91, P = 0.01; Pheterogeneity = 0.49, I2 = 0) could significantly reduce recurrence (Supplementary Figure S2B).
Table 2
| References | Definition of recurrence |
|---|---|
| Razeghinejad et al. (21) | Fibrovascular tissue extending more than 1.5 mm across limbus |
| Shenasi et al. (26) | Fibrovascular growth crossing limbus and extending over the cornea to any distance |
| Shahin et al. (20) | 4 grades classified |
| Xu et al. (38) | Fibrovascular tissue invading cornea |
| Nava-Castaneda, A et al. (23) | 4 grades classified |
| Karalezli et al. (17) | Fibrovascular growth passing the corneal limbus by more than 1 mm |
| Razeghinejad et al. (25) | More than 1.5 mm of fibrovascular tissue overgrowth on cornea and any fibrovascular tissue crossing limbus |
| Ozsutcu et al. (24) | Any fibrovascular growth of conjunctival tissue extending more than 1.5 mm across limbus |
| Kasetsuwan et al. (22) | 4 grades classified |
| Singh et al. (14) | 4 grades classified |
| Bekibele et al. (12) | Growth of fibrovascular tissue 1 mm or more into cornea |
| Motarjemizadeh et al. (13) | New vessels or fibrovascular connective tissues crossing corneal limbus |
| Nuzzi et al. (19) | Growth of fibrovascular tissue extending more than 1 mm across the limbus |
| Mohamed et al. (18) | No definition |
| Zhang et al. (36) | 4 grades classified |
Definition of recurrence of pterygium in the enrolled studies.
Figure 2

Forest plot for the overall recurrence of anti-VEGF drugs for primary pterygium.
Figure 3

Forest plot for the recurrence of anti-VEGF drugs for primary pterygium which was stratified by races.
Complications were reported in 19 RCTs. There was no statistically significant difference in complications between anti-VEGF group and control group (RR 0.80, 95% CI 0.52–1.22, P = 0.29; Pheterogeneity = 0.04, I2 = 45%) (Figure 4). Especially, rate of subconjunctival hemorrhage between the both groups was not statistically different (RR 1.44, 95% CI 0.76–2.71, P = 0.27; Pheterogeneity = 0.41, I2 = 3%) (Supplementary Figure S3). The sensitivity analysis for the complications was stable.
Figure 4
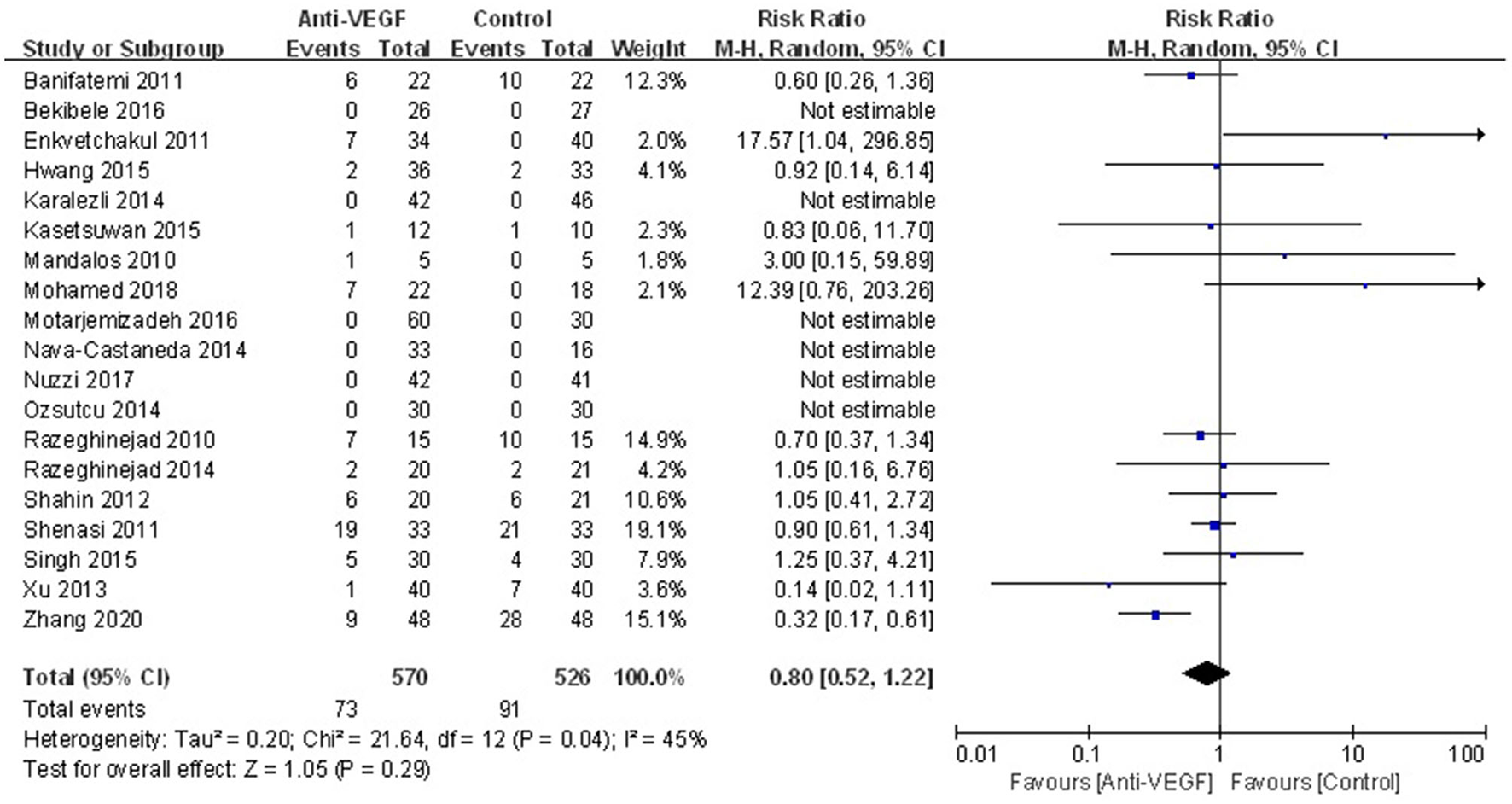
Forest plot for the overall complications associated with anti-VEGF drugs for primary pterygium.
Funnel plots displayed insignificant publication biases for recurrence and complication (Supplementary Figures S4A, B).
4. Discussion
The present study is a comprehensive analysis on the efficacy and safety of anti-VEGF adjuvant treatments for primary pterygium, which includes not only the commonly used bevacizumab, but also ranibizumab and conbercept. Results from the study found out that, anti-VEGF agents, regardless of topical or subconjunctival administration, were statistically effective for reducing recurrence following pterygium excision by bare sclera or conjunctival autograft, while the complications were not increased.
Although there are several meta-analyses about the effect of anti-VEGF drugs on pterygium, all of them focused on bevacizumab (2, 3, 31, 39–42), probably due to its lower cost. The efficacy of other newer anti-VEGF adjuncts, for instance, conbercept and ranibizumab, was not involved in any meta-analysis. This is an important reason why the current study was carried out. The most recent meta-analysis regarding the efficacy of bevacizumab on pterygium was conducted by Zhang et al. (31), the study type of which was RCTs. But among the included studies, two are retrospective analysis instead of RCTs. Thus, its conclusion is in question. Compared to our previous meta-analysis on the relevant topic in 2018 (2), 4 RCTs are added thereafter to the current study. So, it is more likely for us to supply newer and definite evidence for the unresolved issue.
Subgroup analysis showed that compared to control, anti-VEGF in combination with conjunctivo-limbal autograft didn't statistically reduce recurrence rate. The reason probably lies in the limited power due to only 2 involved studies and the trivial efficacy of anti-VEGF agents compared to corneal limbus stem cells. Only 1 RCT studied the efficacy of anti-VEGF agent on recurrent pterygia, making it difficult to pool the data. Therefore, we didn't reanalyze the effect of anti-VEGF agents in recurrent pterygia. It is believed that anti-VEGF agents were not as much effective as in primary pteryium than in recurrent pteriugium because these drugs affect neovascularization rather than old and organized vessels (43).
An interesting finding is that anti-VEGF agents were effective in reducing recurrence among White patients. The reasons for that remain unknown. Future researches focusing on the variation of VEGF among pterygium patients with different races may partly reveal the underlying cause.
Except bevacizumab, there were few studies on the effect of other anti-VEGF agents in pterygium, probably due to the higher costs. Therefore, the sample size was also usually small, which might draw inconclusive results. For example, regarding ranibizumab, subconjunctival ranibizumab had no effect on the extent of vascularization of primary pterygium (32), but it appeared to arrest growth in early recurrent pterygium (33). In another study (34), the recurrence was 3/10 among primary pterygium patients underwent surgery with subconjunctival ranibizumab. Regarding conbercept, there was only one study showing that multiple subconjunctival conbercept injections were effective and safe (36). As for aflibercept, several non-RCT studies indicated that aflibercept was a safe method of reducing inflammation, fibrovascular proliferation, and recurrence (37, 44). Therefore, more well-designed RCTs with large sample size are needed to further determine whether ranibizumab, conbercept, and aflibercept actually decrease pterygium recurrence, and which one is superior.
Ocular complications including subconjunctival hemorrhage, conjunctival cyst, graft edema, corneal epithelial defect, aseptic scleritis and infections, as well as the systematic complications were mainly evaluated. The pooled results showed that anti-VEGF drugs were not associated with more complications compared to controls, indicating their safety. It is consistent with many studies on bevacizumab (2, 31, 42). Subconjunctival administration especially requiring more frequent injection probably cause more side effects than topical administration. Subconjunctival hemorrhage was particularly concerned. We believe it is not appropriate to consider subconjunctival hemorrhage as a complication in the study by Hwang et al. (30) because bevacizumab was only used topically.
Several limitations must be mentioned in the study. First, the definition of recurrence varied among studies. According to Tseng's criteria (45), Grade IV, which is defined as fibrovascular tissue extending past the limbus, is the true recurrence. But different criteria were used in the included primary studies. Second, studies showed that half of recurrences probably occur within 4 months, and 97% probably occur within 12 months (46). Therefore, follow-up of 1 year or over is necessary. Most of the included studies reported recurrence with follow-up of <1 year, which might underestimate the true recurrence. Third, the optimal route and dosage of anti-VEGF drugs, remains unanswered. Therefore, caution is required in the interpretation and further well-designed studies are still needed.
In conclusion, the study showed that topical or subconjunctival anti-VEGF agents could significantly decrease recurrence following pterygium excision by bare sclera or conjunctival autograft, while the complications were not increased. Anti-VEGF agents were especially effective in reducing recurrence among White patients. Anti-VEGF is an effective and safe method in the management of primary pterygium.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YS: conception, design, and data interpretation. BZ and XD: collection and assembly of data and data analysis. All authors: manuscript writing and final approval of manuscript.
Funding
The study was supported by the Science and Technology Program of Meizhou (2021C0301095). The funding organization played no role in the design or conduct of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1166957/full#supplementary-material
References
1.
Chu WK Choi HL Bhat AK Jhanji V . Pterygium: new insights. Eye. (2020) 34:1047–50. 10.1038/s41433-020-0786-3
2.
Sun Y Zhang B Jia X Ling S Deng J . Efficacy and safety of bevacizumab in the treatment of pterygium: an updated meta-analysis of randomized controlled trials. J Ophthalmol. (2018) 2018:4598173. 10.1155/2018/4598173
3.
Fonseca EC Rocha EM Arruda GV . Comparison among adjuvant treatments for primary pterygium: A network meta-analysis. Br J Ophthalmol. (2018) 102:748–56. 10.1136/bjophthalmol-2017-310288
4.
Janson BJ Sikder S . Surgical management of pterygium. Ocul Surf. (2014) 12:112–9. 10.1016/j.jtos.2014.01.001
5.
Zheng K Cai J Jhanji V Chen H . Comparison of pterygium recurrence rates after limbal conjunctival autograft transplantation and other techniques: meta-analysis. Cornea. (2012) 31:1422–7. 10.1097/ICO.0b013e31823cbecb
6.
Lee BWH Sidhu AS Francis IC Coroneo MT . 5-Fluorouracil in primary, impending recurrent and recurrent pterygium: Systematic review of the efficacy and safety of a surgical adjuvant and intralesional antimetabolite. Ocul Surf. (2022) 26:128–41. 10.1016/j.jtos.2022.08.002
7.
Guo Q Li X Cui MN Liang Y Li XP Zhao J et al . Low-Dose Mitomycin C Decreases the Postoperative Recurrence Rate of Pterygium by Perturbing NLRP3 Inflammatory Signalling Pathway and Suppressing the Expression of Inflammatory Factors. J Ophthalmol. (2019) 2019:9472782. 10.1155/2019/9472782
8.
Zhang Q Bao N Liang K Tao L . Adjuvant Use of Cyclosporine A in the Treatment of Primary Pterygium: A Systematic Review and Meta-Analysis. Cornea. (2018) 37:1000–7. 10.1097/ICO.0000000000001542
9.
Patel ED Rhee MK . Surgical Techniques and Adjuvants for the Management of Pterygium. Eye Contact Lens. (2022) 48:3–13. 10.1097/ICL.0000000000000849
10.
Mak RK Chan TC Marcet MM Choy BN Shum JW Shih KC et al . Use of anti-vascular endothelial growth factor in the management of pterygium. Acta Ophthalmol. (2017) 95:20–7. 10.1111/aos.13178
11.
Gumus K Karakucuk S Mirza GE Akgun H Arda H Oner AO . Overexpression of vascular endothelial growth factor receptor 2 in pterygia may have a predictive value for a higher postoperative recurrence rate. Br J Ophthalmol. (2014) 98:796–800. 10.1136/bjophthalmol-2012-301944
12.
Bekibele CO Sarimiye TF Ogundipe A Olaniyan S . 5-Fluorouracil vs avastin as adjunct to conjunctival autograft in the surgical treatment of pterygium. Eye. (2016) 30:515–21. 10.1038/eye.2016.29
13.
Motarjemizadeh Q Aidenloo NS Sepehri S A . comparative study of different concentrations of topical bevacizumab on the recurrence rate of excised primary pterygium: a short-term follow-up study. Int Ophthalmol. (2016) 36:63–71. 10.1007/s10792-015-0076-4
14.
Singh P Sarkar L Sethi HS Gupta VS A . randomized controlled prospective study to assess the role of subconjunctival bevacizumab in primary pterygium surgery in Indian patients. Indian J Ophthalmol. (2015) 63:779–84. 10.4103/0301-4738.171508
15.
Enkvetchakul O Thanathanee O Rangsin R Lekhanont K Suwan-Apichon O . A randomized controlled trial of intralesional bevacizumab injection on primary pterygium: preliminary results. Cornea. (2011) 30:1213–1218. 10.1097/ICO.0b013e31821c9b44
16.
Banifatemi M Razeghinejad MR Hosseini H Gholampour A . Bevacizumab and ocular wound healing after primary pterygium excision. J Ocular Pharmacol Therapeutics. (2011) 27:17–21. 10.1089/jop.2010.0094
17.
Karalezli A Kucukerdonmez C Akova YA Koktekir BE . Does topical bevacizumab prevent postoperative recurrence after pterygium surgery with conjunctival autografting?Int J Ophthalmol. (2014) 7:512–6. 10.3980/j.issn.2222-3959.2014.03.23
18.
Mohamed TA Soliman W Fathalla AM El Refaie A . Effect of single subconjunctival injection of bevacizumab on primary pterygium: clinical, histopathological and immunohistochemical study. Int J Ophthalmol. (2018) 11:797–801. 10.18240/ijo.2018.05.13
19.
Nuzzi R Tridico F . Efficacy of Subconjunctival Bevacizumab Injections before and after Surgical Excision in Preventing Pterygium Recurrence. J Ophthalmol. (2017) 2017:6824670. 10.1186/ISRCTN11424742
20.
Shahin MM Elbendary AM Elwan MM . Intraoperative subconjunctival bevacizumab as an adjunctive treatment in primary pterygium: A preliminary report. Ophthal Surg Lasers Imag. (2012) 43:459–66. 10.3928/15428877-20120802-02
21.
Razeghinejad MR Hosseini H Ahmadi F Rahat F Eghbal H . Preliminary results of subconjunctival bevacizumab in primary pterygium excision. Ophthalmic Res. (2010) 43:134–8. 10.1159/000252980
22.
Kasetsuwan N Reinprayoon U Satitpitakul V . Prevention of recurrent pterygium with topical bevacizumab 0.05% eye drops: a randomized controlled trial. Clin Therap. (2015) 37:2347–2351. 10.1016/j.clinthera.2015.08.023
23.
Nava-Castañeda A Olvera-Morales O Ramos-Castellon C Garnica-Hayashi L Garfias Y . Randomized, controlled trial of conjunctival autografting combined with subconjunctival bevacizumab for primary pterygium treatment: 1-year follow-up. Clin Experiment Ophthalmol. (2014) 42:235–41. 10.1111/ceo.12140
24.
Ozsutcu M Ayintap E Akkan JC Koytak A Aras C . Repeated bevacizumab injections versus mitomycin C in rotational conjunctival flap for prevention of pterygium recurrence. Indian J Ophthalmol. (2014) 62:407–11. 10.4103/0301-4738.120220
25.
Razeghinejad MR Banifatemi M . Subconjunctival bevacizumab for primary pterygium excision; a randomized clinical trial. J Ophthalmic Vis Res. (2014) 9:22–30.
26.
Shenasi A Mousavi F Shoa-Ahari S Rahimi-Ardabili B Fouladi RF . Subconjunctival bevacizumab immediately after excision of primary pterygium: the first clinical trial. Cornea. (2011) 30:1219–22. 10.1097/ICO.0b013e31820ca63f
27.
Fallah MR Khosravi K Hashemian MN Beheshtnezhad AH Rajabi MT Gohari M . Efficacy of topical bevacizumab for inhibiting growth of impending recurrent pterygium. Curr Eye Res. (2010) 35:17–22. 10.3109/02713680903395273
28.
Lekhanont K Patarakittam T Thongphiew P Suwan-apichon O Hanutsaha P . Randomized controlled trial of subconjunctival bevacizumab injection in impending recurrent pterygium: a pilot study. Cornea. (2012) 31:155–161. 10.1097/ICO.0b013e3182151e0e
29.
Ozgurhan EB Agca A Kara N Yuksel K Demircan A Demirok A . Topical application of bevacizumab as an adjunct to recurrent pterygium surgery. Cornea. (2013) 32:835–838. 10.1097/ICO.0b013e3182772d4e
30.
Hwang S Choi S A . Comparative Study of Topical Mitomycin C, Cyclosporine, and Bevacizumab after Primary Pterygium Surgery. Korean J Ophthalmol. (2015) 29:375–81. 10.3341/kjo.2015.29.6.375
31.
Zhang X Jiang Y Fu Q Zhang X Chen Y . Efficacy of bevacizumab in the treatment of pterygium: An updated meta-analysis of randomized controlled trials. Int Immunopharmacol. (2021) 98:107921. 10.1016/j.intimp.2021.107921
32.
Mandalos A Tsakpinis D Karayannopoulou G Tsinopoulos I Karkavelas G Chalvatzis N et al . The effect of subconjunctival ranibizumab on primary pterygium: a pilot study. Cornea. (2010) 29:1373–9. 10.1097/ICO.0b013e3181d927b9
33.
Rose L Byrd JM Qaseem Y . Subtenon Injections of Ranibizumab Arrest Growth in Early Recurrent Pterygium. Eye Contact Lens. (2017) 43:399–405. 10.1097/ICL.0000000000000292
34.
Galor A Yoo SH Piccoli FV Schmitt AJ Chang V Perez VL . Phase I study of subconjunctival ranibizumab in patients with primary pterygium undergoing pterygium surgery. Am J Ophthalmol. (2010) 149:926–931.e922. 10.1016/j.ajo.2010.01.015
35.
Hurmeric V Vaddavalli P Galor A Perez VL Roman JS Yoo SH . Single and multiple injections of subconjunctival ranibizumab for early, recurrent pterygium. Clin Ophthalmol. (2013) 7:467–73. 10.2147/OPTH.S40400
36.
Zhang J Tian Q Zheng T Chen D Wang Q Ke M . Effect of multiple subconjunctival conbercept injections as an adjuvant to the surgical treatment of pterygium: a prospective randomised comparative 6-month follow-up study. Eye. (2020) 34:408–14. 10.1038/s41433-019-0596-7
37.
Mansour AM . Regression of Inflamed Pterygia by Frequent High-Dose Intralesional Ziv-Aflibercept. Cornea. (2017) 36:1002–5. 10.1097/ICO.0000000000001251
38.
Xu QB Zhu LW Xu GZ . Comparative study of pterygium surgery combined with bevacizumab or mitomycin C. Int Eye Sci. (2013) 13:2532–4. 10.3980/j.issn.1672-5123.2013.12.52
39.
Zeng W Liu Z Dai H Yan M Luo H Ke M et al . Anti-fibrotic, anti-VEGF or radiotherapy treatments as adjuvants for pterygium excision: a systematic review and network meta-analysis. BMC Ophthalmol. (2017) 17:211. 10.1186/s12886-017-0601-5
40.
Huang ST Tian BS Xiao O Yang YJ Zhou SY . Safety of antivascular endothelial growth factor administration in the ocular anterior segment in pterygium and neovascular glaucoma treatment: Systematic review and meta-analysis. Medicine. (2018) 97:e11960. 10.1097/MD.0000000000011960
41.
Liu J Xu JH Xu W Liang GL Lou JX Wang Y et al . Bevacizumab as adjuvant therapy in the management of pterygium: a systematic review and Meta-analysis. Int J Ophthalmol. (2017) 10:1126–33. 10.18240/ijo.2017.07.17
42.
Hu Q Qiao Y Nie X Cheng X Ma Y . Bevacizumab in the treatment of pterygium: A meta-analysis. Cornea. (2014) 33:154–60. 10.1097/ICO.0000000000000037
43.
Ghiasian L Samavat B Hadi Y Arbab M Abolfathzadeh N . Recurrent Pterygium: A Review. J Curr Ophthalmol. (2021) 33:367–78. 10.4103/joco.joco_153_20
44.
Malozhen SA Trufanov SV Krakhmaleva DA . Antiangiogenic therapy in the surgical treatment of pterygium. Vestn Oftalmol. (2020) 136:177–83. 10.17116/oftalma2020136052177
45.
Prabhasawat P Barton K Burkett G Tseng SC . Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. (1997) 104:974–85. 10.1016/S0161-6420(97)30197-3
46.
Varssano D Shalev H Lazar M Fischer N . Pterygium excision with conjunctival autograft: true survival rate statistics. Cornea. (2013) 32:1243–50. 10.1097/ICO.0b013e31828ce09c
Summary
Keywords
anti-vascular endothelial growth factor, primary pterygium, efficacy, safety, meta-analysis
Citation
Zhang B, Dong X and Sun Y (2023) Efficacy and safety of anti-vascular endothelial growth factor agents in the treatment of primary pterygium. Front. Med. 10:1166957. doi: 10.3389/fmed.2023.1166957
Received
23 February 2023
Accepted
05 May 2023
Published
23 May 2023
Volume
10 - 2023
Edited by
Alejandro Navas, Instituto de Oftalmología Fundación de Asistencia Privada Conde de Valenciana, I.A.P, Mexico
Reviewed by
Du Liping, Chongqing Eye Institute, China; Mohd Radzi Hilmi, International Islamic University Malaysia, Malaysia; Pir Salim Mahar, Aga Khan University, Pakistan
Updates
Copyright
© 2023 Zhang, Dong and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Sun sunyidoctor@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.