Abstract
Introduction:
This study explored the feasibility and preliminary efficacy of a music-based, multicomponent exercise intervention among community-dwelling older adults with mild-to-moderate cognitive impairment.
Methods:
16 older adults aged 85±9 years with mild-to-moderate cognitive impairment received music-based multicomponent exercise training for 20 weeks at an independent living facility. Participants received aerobic, resistance, and balance training paired with beat-accentuated music stimulation. Participants’ adherence to the training was tracked down and their cognitive and physical functioning and health-related quality of life were assessed at pre- and post-test.
Results:
3 participants withdrew due to unexpected issues unrelated to the intervention and thus 13 participants (7 females) attended an average of 4.6 days/week over 20 weeks and reported high satisfaction with the intervention (90.6%). Participants showed significant improvement in global cognition, cognitive processing speed, and walking endurance/aerobic fitness at post-test.
Discussion:
These findings support the feasibility of music-based, multicomponent exercise training for older adults in an independent living facility and set the stage for future studies to test the efficacy of music on physical activity and ensuing health outcomes. We conclude that music-based, multicomponent exercise training can be beneficial for community-dwelling older adults with mild-to-moderate cognitive decline. As a form of rhythmic auditory stimulation, beat-accentuated music can be combined with exercise training to manipulate exercise tempo and may provide a source of motivation to help older adults adhere to exercise.
1. Introduction
It is estimated that 13.8 million United States adults will be living with Alzheimer’s disease (AD) by 2050, which will result in $1.1 trillion in health care costs (1). With no curative treatment currently available, lifestyle interventions reducing risk factors are imperative to prevent or delay the onset of AD (2). This is an important direction because delaying the onset of AD by 5 years could lower the prevalence of the disease by 42% and reduce the health care costs by $367 billion (1). Notably, about one-third of AD cases worldwide are related to modifiable risk factors; the largest proportion of cases in the US is attributable to the lack of physical activity (PA) (3). Hence, it is important to promote PA in the aging population to protect against the progression of cognitive decline and dementia.
The global and national PA guidelines (PAG) prescribe older adults to regularly engage in moderate-intensity aerobic exercise for 150–300 min/week as well as resistance and balance training for 2–3 times/week (4, 5). However, current population data show that only 9–18% of US older adults adhere to the PAG (6, 7). This trend is untoward especially given the meta-analytic evidence that PA interventions with both aerobic and resistance training have shown greater benefits for older adults’ cognitive health compared with aerobic training only (8). Despite the cumulative evidence supporting the benefits of exercise training for cognitive and brain health, the rate of citizens maintaining regular exercise is lower than desired (9). Thus, a new approach is needed to promote adherence to the PAG in the growing number of older adults.
Cumulative evidence indicates that music listening can have motivational effects on PA (10–13). A meta-analysis revealed that listening to music prior to or during acute exercise bouts increases positive affective valence (feeling good versus bad; g = 0.48, CI [0.39, 0.56]), reduces ratings of perceived exertion (RPE; g = 0.22, CI [0.14, 0.30]), enhances physical performance (g = 0.31, CI [0.25, 0.36]), and improves oxygen utilization efficiency (VO2max; g = 0.15, CI [0.02, 0.27]) among healthy adults (14). These findings support the notion that music helps exercise bouts to be perceived as more joyous, less arduous, and more energizing. These findings are congruous with the view that humans are predisposed to take pleasure in moving with music, as evidenced in preverbal infants’ positive affective responses and rhythmic motor reactions when hearing musical or rhythmic stimuli (e.g., Mozart, Saint-Saëns, children’s song, or drumbeats) to a greater extent than non-musical stimuli (e.g., adult speech) (15). In-depth interviews of older adults indicate that a negative affective response to exercise is a key barrier to adherence to PA whereas enjoyment is an important motivator for being physically active (16, 17). Therefore, exercising with music may help older adults enjoy exercise more as a motivational stimulant.
Our lab conceptualized a theoretical model to account for how music influences acute and long-term PA based on the theory of hedonic motivation (18). In an acute context, music can help people like a PA session more (or dislike it less) and thus increase wanting (or decrease dread) to exercise more or harder (18). Over the long-term phase, hedonic (affective) responses to PA serve as the inputs into the process of generating hedonic motivation for another bout of PA. In other words, when people experience a PA session as pleasant, this positive hedonic response is linked to enhanced motivation for upcoming PA, which increases the chance of maintaining long-term PA. In our conceptualization, we particularly stressed the importance of beat accentuation and tempo synchronization to facilitate auditory-motor synchronization in music listeners (18). This argument was made based on (1) the neurobiological evidence that affect- and reward-related brain regions are activated when moving in sync with pleasurable music (19–21) and (2) the behavioral evidence that infants’ rhythmic motor responses to music coincided with smiles and the duration of smiles showed meaningful correlations with the degree of music-motor synchrony accuracy (r = 0.42 and 0.26 in two experiments) (15). Therefore, facilitating auditory-motor synchronization may maximize the effect of music on positive hedonic response to PA and thereby promote adherence to a PA program (18).
We developed an exercise program paired with Beat-accentuated Music Stimulation (BMS) and found its affective and ergogenic effects on an acute bout of exercise compared with exercising without music among community-dwelling older adults (22). We here define BMS as the application of pulsed, tempo-synchronous music stimuli embedded with sonically enhanced beats to facilitate rhythmic body movement. BMS has been employed in cardiac rehabilitation (23) or gait rehabilitation in people with Parkinson’s disease (24–26) or stroke (27, 28). A randomized controlled trial (RCT) (23) found that self-directed walking-for-exercise in sync with BMS led to nearly twofold increases in accelerometer-measured weekly volume of PA at all intensities and in caloric expenditure over 3 months among midlife-to-older adults in a home-based cardiac rehab program relative to the same exercise program with beat-unaccented, tempo-synchronous music or without music. These findings suggest that the distinctive combination of music with accented beats can dramatically increase PA above and beyond beat-unaccented music. However, to our knowledge, no study to date has applied BMS to multicomponent exercise training among older adults with cognitive decline.
We conducted a single-arm intervention trial to test the feasibility, acceptability, and preliminary efficacy of a 20-week BMS-based multicomponent exercise training program for community-dwelling older adults with mild-to-moderate cognitive impairment. Outcomes of interest were adherence to and overall satisfaction with the intervention as well as changes in cognitive and physical functioning and health-related quality of life (QoL). Our approach to prevent or delay the onset of AD or other dementias through multicomponent exercise training could be particularly urgent among older adults in early stages of cognitive decline. Given the low rate of PA among older adults in the US and the strong association between low PA and the prevalence of AD, developing and implementing a novel PA intervention for older adults will have implications for dementia prevention. This preliminary study will set the stage for an RCT to fully test the efficacy of BMS-based exercise training in the growing aging population at risk of dementia.
2. Methods
2.1. Participants
Sixteen community-dwelling older adults (9 females) who were 86.2 ± 8.6 years old (M ± SD), previously low-active (< 60 min/week of exercise, determined by self-report and confirmed by facility staff), and with mild-to-moderate cognitive impairment [determined by Montreal Cognitive Assessment (MoCA)] were recruited from an independent senior living facility. Prior to recruitment, approval was obtained from the institutional review board of the university. Eligibility screening was conducted in person by appointment in a designated room at the senior living facility. Individuals were ineligible if they were incapable of walking, unable to hear verbal instructions and music, physically active (> 90 min/week of exercise), had severe cognitive impairment [determined by MoCA total score < 11; 29], or had anxiety or depression (determined by a single-item on the EuroQol Health Questionnaire [EQ-5D-5L] ≥ 4) (29). Participants who could walk with or without an assistive device (walker, cane, etc.) or who could hear using hearing aids were eligible for the study. Participants also completed the Physical Activity Readiness Questionnaire for Everyone (30) to determine if they had an ongoing medical condition that might put them at risk by engaging in moderate-intensity exercise training. Through this screening procedure, we excluded 1 individual with advanced Parkinson’s disease, 1 individual with post-stroke hemiparesis, and 2 individuals with MoCA scores < 11.
We were able to include participants with mild Parkinson’s disease, arthritis, or osteoporosis, taking medications to manage heart conditions and/or blood pressure, or using a walker for ambulation. These individuals were instructed to exercise at a light intensity and/or in a seated position within the training protocol. Eligible participants were provided with the study procedure and completed a Brief Informed Consent Test (31) through which we confirmed their ability to understand the study information. All participants provided written informed consent on their own. Among 16 enrollees, 3 withdrew participation due to unexpected lower body injuries or visual impairment (unrelated to the intervention) and thus 13 participants (7 females) with MoCA score ranging from 16 to 25 (M = 20.38, SD = 2.98) completed the intervention and were included in data analysis.
2.2. Music-based multicomponent exercise intervention
All participants were provided with music-based multicomponent group exercise training at 9:30 AM for 30–35 min/day, 6 days/week over 20 weeks in a designated room at the senior living facility. The exercise goal for all participants was to attend the group exercise training > 3 days per week. The exercise program was open to all members in the residential facility and thus a few additional individuals often attended without participating in the study, but adherence was only tracked for study participants. To be consistent with the PAG, the group exercise program consisted of a dynamic warm-up (5 min), aerobic training (15 min), resistance and balance training (10 min), and cool-down stretches (5 min). See Table 1 for an overview of the exercise program. Most exercises were chair-assisted and thus adaptable across fitness levels and were safely implemented for participants with fall risks. Participants were instructed to exercise in a standing position with at least one hand holding a chair or in a seated position. The exercise program was developed and consistently delivered by two researchers along with three staff members in the facility, who were all CPR-certified and experienced exercise instructors. Participants were instructed to exercise at light-to-moderate intensity based on the Borg RPE Category-Ratio scale (32) which was posted in the exercise room.
Table 1
| Training goal | Dynamic warm-up | Aerobic step training | Muscle strengthening and balance training | Cool-down | ||
|---|---|---|---|---|---|---|
| Upper body | Lower body | Core and balance | ||||
| Frequency | 6 days/week | 6 days/week | 2 days/week (M, TH) | 2 days/week (T, F) | 2 days/week (W, S) | 6 days/week |
| Duration | 5 min | 15 min | 10 min | 10 min | 10 min | 5 min |
| Exercises | • Marching in place • Elbow to knee taps • Forward taps with punches • Side taps with arm reaching | • Forward taps • Backward taps • Side taps • Forward backward steps • Side steps • Triangular steps • Crossover steps | • Punches• Bicep curls• Bent over rows• Shoulder presses• Front arm raises• Lateral arm raises | • Chair-assisted squats• Lifting buttocks from the chair• Straight leg raises—Front, Lateral, Rear• Calf raises | • Chair-assisted deadlift • Alternate knee raises • Seated abdominal crunches• Russian twists | • Trunk rotations • Neck stretches • Arm crosses • Calf stretches • Quadriceps stretches • Hamstring stretches • Gluteus maximus stretches |
Multicomponent exercise intervention with beat-accentuated music stimulation.
All exercises were performed in a chair-seated position or chair-assisted standing position. Participants were encouraged to exercise within light-to-moderate intensity by staying in 2–3 on the Ratings of Perceived Exertion (RPE) CR10 scale. M = Monday, T = Tuesday, W=Wednesday, TH = Thursday, F=Friday, S=Saturday.
Participants were trained to exercise in sync with the tempo of a BMS playlist that was made of 35 music excerpts, which were played in a randomized order. Exercise pace and the music tempo were incrementally increased by five beats per min (BPM) every 5 weeks during the intervention, from 85 BPM to 100 BPM. Participants were not asked to use BMS for their self-directed walking outdoors for safety reasons.
2.3. Beat-accentuated music stimuli
All music excerpts in the BMS playlist were slow-to-medium tempo country and pop songs that were rigorously chosen by a certified music production specialist, in consideration of participants’ music preference initially surveyed as well as their unchanging tempos and a clearly discernible rhythm in 2/4 or 4/4 meter. The emotions and lyrics of all songs were scrutinized and therefore any songs with provocative lyrics (e.g., cursing, drugs, alcohol, sexual references, racism, violence) or negative emotions (e.g., sad, anger, fear, regret.) were excluded. More than half of the songs we chose were instrumental music. For the songs selected, we sonically enhanced each quarter note beat by adding lower- and/or higher-frequency drum sounds (kick-drum, snares, hihats, and rides) to correspond with one paced step or muscle contraction when exercising. The beats were added as a secondary track and recorded concurrently with the original music, using musical instrument digital interface (MIDI) keyboard-drum instruments (Pro Tools 2021, Avid Technology Inc., Burlington, MA, United States), in a similar manner with the prior study (23). Our intention was to implement beat accentuation at frequencies and volumes just beyond minimal detection levels without detracting from the authenticity of the original music. The tempo of music excerpts was adjusted without damaging the harmony or pitch via open-source sound-editing software (Audacity 3.0.4, The Audacity Team, available at: audacityteam.org).
2.4. Procedure
Individuals who expressed interest were scheduled for the screening by appointment in a designated area at the senior living facility. After screening, eligible participants completed pre-test to assess cognitive and physical functioning and health-related QoL. The total testing procedure was completed within an hour. Testing was conducted by research staff with the aid of facility staff. After a month of pre-testing all participants, the 5-month exercise intervention started and their adherence to the intervention was tracked down. After the intervention, participants were scheduled for post-test to assess the same outcome measures with the pre-test. At post-test, participants’ satisfaction with the intervention was also assessed.
2.5. Outcome measures
Adherence to the intervention was tracked during the intervention through a sign-up sheet that was self-reported by participants and confirmed by the exercise instructor after every session. At the post-test, participants’ satisfaction with the intervention was assessed using the Client Satisfaction Questionnaire (CSQ-8), an 8-item 4-point Likert scale with the total possible score ranging from 4 to 32 and a higher score indicating greater satisfaction (33).
General cognitive functioning was assessed using the MoCA, a widely used test to access memory, executive function, and other symptoms of cognitive decline (34). To prevent potential practice effects, version 8.1 and 8.2 of the MoCA were used at the pre- and post-test, respectively. Test scores were calculated based on pre-established algorithms to obtain the MoCA total score and memory index score (MoCA-MIS). The MoCA-MIS is calculated by summing the number of words correctly remembered after a delay in free recall, category-cued recall, and multiple-choice recall multiplied by 3, 2 and 1, respectively (35). There are five words to be recalled and thus a participant can obtain a score ranging from 0 to 15. This new scoring method was developed to reflect encoding memory performance (35).
Inhibitory control and cognitive processing speed was assessed using the Flanker Inhibitory Control and Attention Test and the Pattern Comparison Processing Speed Test in the NIH Toolbox Cognition Battery (2022 Toolbox Assessments, Inc., available at nihtoolbox.org), The Flanker Test requires participants to indicate the left–right orientation of an arrow stimulus presented centrally while inhibiting attention to the potentially incongruent arrow stimuli surrounding the central stimulus. Accuracy and reaction time on the incongruent versus congruent items serve as measures of inhibitory control (36). The Pattern Comparison Processing Speed Test asks participants quickly identify whether two images are the same or not and the number of correct items completed in 90 s is counted (37). Both NIH Toolbox tests were validated in young-to-older adults aged 18–65 years (38) and oldest older adults aged 85–99 years (39).
Health-related QoL was self-reported through the 5-item EQ-5D-5L (29). Validated in older adults with multimorbidity (40), the EQ-5D-5L is based on descriptions of self-perceived health based on 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has 5 response options corresponding to no problems, slight problems, moderate problems, severe problems, and incapability. Participants also reported their overall health on the day on a hash-marked, vertical visual analogue scale (EQ-VAS) marked from 0 (the worst health you can imagine) to 100 (the best health you can imagine).
Physical functioning was assessed through the Timed Up and Go (TUG), 4-Stage Balance Test (4SBT), 6-Minute Walk Test (6MWT), and 30-Second Chair Stand Test (30SCST) performed in that order. The TUG, 4SBT, and 30SCST are part of the CDC’s STEADI toolkit for the assessment of fall risks in older adults (41). The 6MWT is found to be a reliable and valid measure of physical endurance and aerobic fitness in older adults (42, 43). We measured the duration to complete a TUG trial (after a practice trial), the sum of durations to maintain the posture required in the first, second, and third stage of the 4SBT, the total distance walked for 6 min (6MWT), and the reps of sit-to-stand maneuvers completed in 30 s (30SCST). Some participants completed the tests using a walker as needed, consistently across the pre- and post-test.
2.6. Data analysis
All statistical analyses were conducted with R 4.2.2 (44). Normality of the data was first checked with descriptive statistics and the Shapiro–Wilk test. For the outcome variables showing normal distribution, we conducted student’s paired-sample t-tests to examine the pre- and post-test differences with alpha (α) at < 0.05 for two-sided tests of statistical significance. Cohen’s d effect sizes were computed and interpreted based on the criterion of 0.2 (small effect), 0.5 (moderate effect), and 0.8 (large effect). For the outcome variables with non-normal distributions, we conducted the Wilcoxon signed-rank test, the non-parametric counterpart of a paired-sample t-test, using the ‘wilcox_test’ R package with alpha (α) at < 0.05 for two-sided tests. For non-parametric tests, effect sizes were calculated using the ‘wilcox_effsize’ R package, where r value is interpreted to be a small effect (0.10–< 0.30), moderate effect (0.30–< 0.50), and large effect (≥ 0.50).
3. Results
3.1. Feasibility and acceptability of the intervention
Thirteen participants attended 4.6 days/week over 20 weeks in average (min = 3.6 days/week, max = 5.3 days/week; SD = 0.5 days/week). The CSQ-8 total score indicated that participants were highly satisfied with the intervention (M = 29.1, SD = 3.1). These results support the feasibility and acceptability of BMS-based multicomponent exercise training for 20 weeks in community-dwelling older adults with mild-to-moderate cognitive impairment.
3.2. Cognitive functioning
Statistically significant changes were found in the MoCA total score (t = 4.71, df = 11, p < 0.001) with a large effect size (d = 1.36); the post-test score (M = 22.3, SD = 3.31) was higher than the pre-test score (M = 20.6, SD = 3.15). Ten out of 13 participants made improvements in the MoCA total score at post-test, whereas 2 participants scored the same with the pre-test, and 1 participant refused the test at post-test. However, no significant differences were found in the MoCA-MIS (t = −0.899, df = 11, p = 0.388). The results of the NIH Toolbox test indicated statistically significant changes in the Pattern Comparison Processing Speed test score (t = 2.83, df = 11, p < 0.05) with a large effect size (d = 0.816); the post-test score (M = 67.8, SD = 15.4) was higher than the pre-test score (M = 60.1, SD = 9.69). Ten out of 13 participants made improvements in the Pattern Comparison Processing Speed test score at post-test, whereas two participants scored lower than the pre-test, and 1 participant refused completing the test at post-test. Differences in the Flanker score did not reach significance (t = 1.81, df = 12, p = 0.098) although a moderate effect size (d = 0.522) was obtained; the post-test score (M = 77.5, SD = 12.7) was marginally higher than the pre-test score (M = 74.2, SD = 13). See Figures 1A–D for a summary of these results.
Figure 1
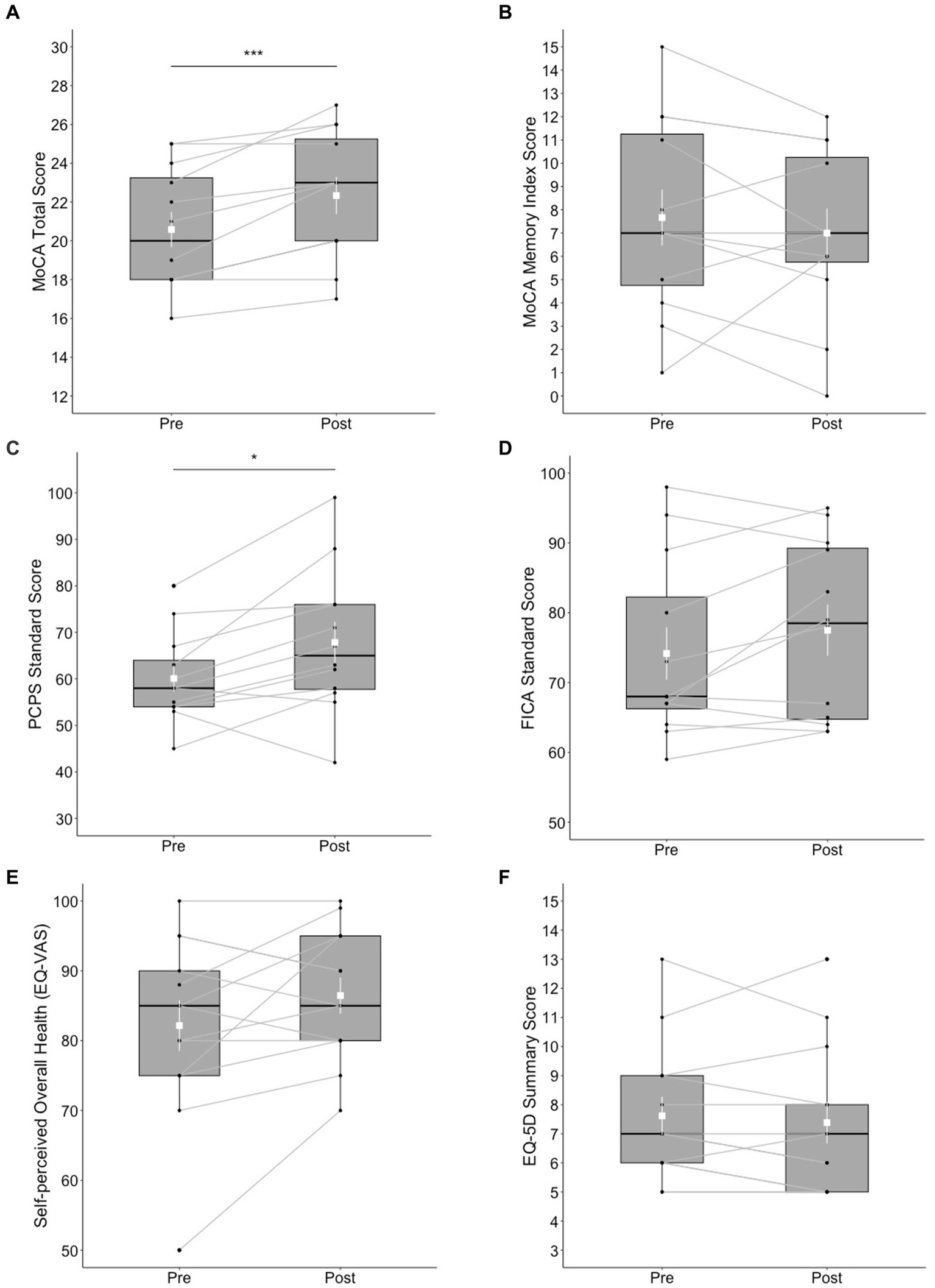
Box plots of cognitive and mental health outcomes at pre- and post-test; (A) MoCA total score, (B) MoCA Memory Index Score, (C) NIH Toolbox Pattern Comparison Processing Speed (PCPS) test, (D) NIH Toolbox Flanker Inhibitory Control and Attention (FICA) test, (E) self-perceived overall health reported on the EQ visual analogue scale (VAS), and (F) EQ-5D Summary Score. The shapes of the distribution are shown on the boxes and whiskers. The box bounds the IQR divided by the median (solid horizontal line) and whiskers extend to a maximum of 1.5 × IQR beyond the box. Mean and standard errors are indicated by small, white squares and appended lines. Significant differences between pre- and post-test are indicated by *p < 0.05, ***p < 0.001.
3.3. Health-related quality of life
No significant differences between pre- and post-test were found in the EQ-5D-5L summary score (V = 29, p = 0.273). The differences between pre- and post-test in EQ-VAS was not significant (t = 1.74, df = 12, p = 0.107); post-test score (M = 86.5, SD = 9.32) versus pre-test score (M = 82.2, SD = 13.1). See Figures 1E,F for a summary of these results.
3.4. Physical functioning
The ranks of 6MWT total distance were significantly higher (V = 66, p < 0.05) at post-test (Mdn = 282 m) than at pre-test (Mdn = 249 m) with a large effect size (r = 0.601). Eleven out of 13 participants made an improvement in the 6MWT total distance. No significant differences between pre- and post-test were found in the ranks of TUG duration (V = 29, p = 0.273), 4SBT duration (V = 43, p = 0.398), and 30CST reps (V = 24.5, p = 0.798). See Figure 2 for a summary of these results.
Figure 2
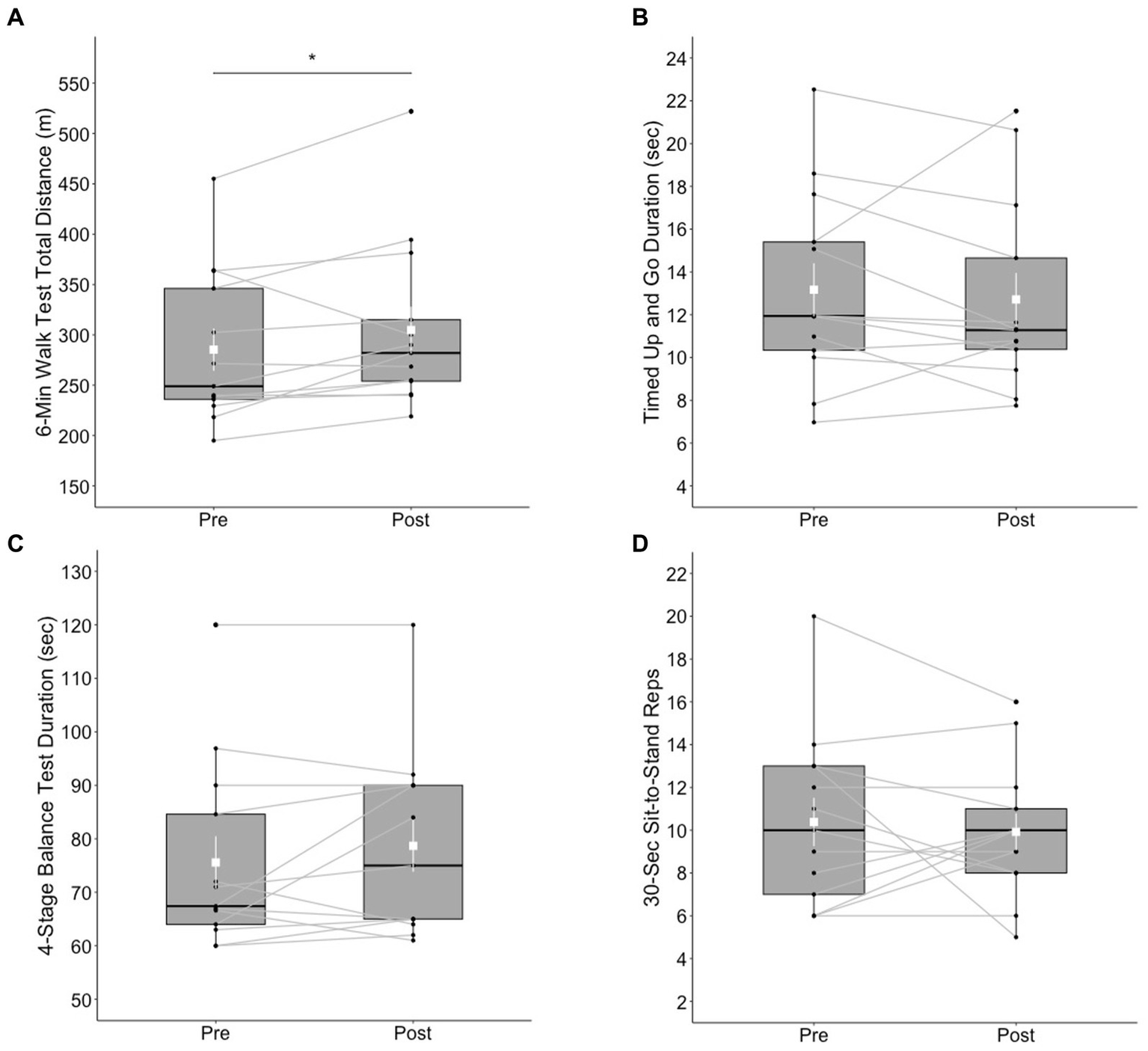
Box plots of physical functioning outcomes at pre- and post-test; (A) 6-Minute Walk Test total distance (m), (B) Timed Up and Go test, (C) 4-Stage Balance Test, and (D) 30-Second Chair Stand test. The shapes of distribution are shown on the boxes and whiskers. The box bounds the IQR divided by median (solid horizontal line) and whiskers extend to a maximum of 1.5 × IQR beyond the box. Mean and standard errors are indicated by small, white squares and appended lines. Significant differences between pre- and post-test are indicated by *p < 0.05.
4. Discussion
Our participants demonstrated high adherence and satisfaction with the BMS-based multicomponent exercise intervention for 20 weeks. This finding supports the feasibility and acceptability of the intervention among older adults with mild-to-moderate cognitive impairment in an independent living facility. Our data also provide preliminary evidence in support of the efficacy of BMS-based multicomponent exercise training for cognitive and physical functioning in cognitively impaired older adults. Most participants who completed the intervention showed improvements in general cognitive functioning assessed by the MoCA, visual information processing assessed by the Pattern Comparison Processing Speed Test, and walking endurance and aerobic fitness assessed by 6MWT. These findings are meaningful because we, for the first time, combined multicomponent exercise training with BMS and demonstrated its feasibility and preliminary efficacy for cognitive and physical health among cognitively impaired older adults.
It is possible that music stimulation played a positive role in participants’ high adherence and satisfaction with the exercise training. Scientists have demonstrated that listening to music prior to or during acute bouts of aerobic and resistance training have beneficial effects on affective valence, RPE, physical performance, and oxygen utilization, and thus becomes a motivational stimulant to PA bouts [for a review, see (14)]. Despite this body of literature, there is an inadequate level of empirical evidence to substantiate the effects of music on long-term adherence to PA (18, 45, 46). This gap in the literature was partly addressed by a theoretical model accounting for the putative mechanisms through which music acts to promote long-term adherence to PA (18). From the view of the theory of hedonic motivation (47, 48), music can help people like an exercise session more (or dislike it less) and thus increase wanting (or decrease dread) to exercise more or harder (18). When people experience pleasure during an exercise session, this positive affective response is linked to enhanced motivation for another bout of exercise, which increases the chance of long-term adherence to PA (18). Therefore, it is possible that participants in this study could benefit from positive affective responses to exercising with music. This is a tentative assertion at the moment because we did not measure affective response to PA nor did we include a control condition. Social interactions and the environmental factors in the independent living facility could have also made positive impacts on adherence. Future researchers may conduct an RCT to investigate the unique effects of BMS on exercise adherence among older adults by including a non-music exercise control group and to identify psychological mechanisms underlying such effects.
Recent systematic reviews identified a few RCTs that demonstrated small but beneficial effects of music on long-term adherence to PA among older adults in a cardiac and pulmonary rehabilitation setting (45, 46). The methodologies employed in this study, beat accentuation and tempo synchronization, may have played an important role in the observed high adherence rates given that music had little effect on long-term PA behaviors in RCTs without such methodologies. Specifically, older adults in a cardiac rehabilitation who were prescribed to walk-for-exercise with beat-unaccented, tempo-asynchronous music stimuli demonstrated trivial differences in the rate of meeting the PAG and accelerometer-measured PA over 26 weeks compared with controls who received the same exercise prescription without music (49). In another RCT, people with COPD who received an 8-week walking intervention with beat-unaccented, tempo-asynchronous music stimuli showed little difference in pedometer-measured and self-reported PA compared with controls in the same intervention without music (50). However, in the RCT (23), walking-for-exercise with BMS led to nearly twofold increases in accelerometer-measured PA and in caloric expenditure over 3 months among midlife-to-older adults relative to the same exercise program with beat-unaccented, tempo-synchronous music and without music. This view would be supported by prior evidence that beat accentuation facilitates beat perception and auditory-motor synchronization when moving with music (51, 52). Acknowledging the limitation of a single-arm intervention study, future studies may be conducted using an RCT designed to decompose the unique effects of beat accentuation on exercise behaviors by including a beat-unaccented music control group.
BMS is a form of rhythmic auditory stimulation (RAS) that refers to an application of pulsed rhythmic auditory stimuli (e.g., metronome and/or music) for the facilitation of body movements that are intrinsically rhythmic (53). RAS have been frequently implemented to foster motor behaviors—especially walking—mostly in people with movement disorders [for reviews, see (54–59)] and occasionally for cardiovascular rehabilitation [for reviews, see (45, 46)] and also for people with Alzheimer’s disease (60). RAS interventions have used varying forms of tempo-synchronous stimuli such as metronome pulse [for reviews, see (59, 61, 62)], contemporary music (63–65), or contemporary music with sonically-enhanced (accentuated) beats (24–26)—referred to as BMS herein—mostly for older adults with Parkinson’s disease. The evidence that RAS facilitates motor behaviors implies its beneficial application for exercise training, yet no study to date has made such approach. Therefore, the novelty of this study comes from the combination of RAS with exercise training following the PAG for cognitively impaired older adults.
It is promising that BMS-based multicomponent exercise training for 20 weeks led to improvements in general cognition, cognitive processing speed, and walking endurance/aerobic fitness in cognitively impaired older adults. These findings are consistent with previous findings that exercise training has beneficial effects on cognitive functioning among older adults with normal cognition (66, 67), self-reported memory complaints (68–70), mild cognitive impairment (71–73), and dementia (72, 74). Although two different, validated versions of the MoCA were used in pre- and post-test to prevent potential practice effect, it is possible that improved general cognition is due to repeated testing. This suggest that future studies should include a control group to determine if the observed cognitive benefit is attributable to exercise training and/or BMS. Moreover, the multicomponent nature of exercise training may have played a role for the positive outcomes in this study and thus has implications for future interventions to help older adults comply with the PAG. Our approach to prevent or delay the onset of AD or other dementias through multicomponent exercise training would be particularly urgent among older adults in early stages of cognitive decline. Given the low rate of PA among older adults and the strong association between low PA and the prevalence of AD, developing and implementing a novel PA intervention for older adults will have implications for dementia prevention. This preliminary study will set the stage for an RCT to fully test the efficacy of BMS-based exercise training in the growing aging population at risk of dementia.
It should be noted that our intervention led to no changes in verbal memory (MoCA-MIS) and only nearly significant improvements in inhibitory control of attention (Flanker test). These findings are partly coherent with the meta-analytic findings that, in older adults with mild cognitive impairment, multicomponent exercise training has resulted in improvements in global cognition, attention, and executive function but not in memory (72) and aerobic training strongly improved global cognition but weakly improved memory (73). We also attribute the lack of changes in the Flanker test to the lack of validity in some of our oldest-old participants at + 90 years of age who had difficulties in understanding the test instructions and practice trials as well as the small sample size. This interpretation may be supported by the recent study which validated the NIH Toolbox Cognition Battery among healthy oldest older adults at 85–99 years of age who had MoCA total scores of 22–30 (39), which is higher than the participants in this study. We also note that our intervention led to no changes in balance (TUG, 4SBT) and lower-body strength (30CST). We attribute these results to the limited capacity for balance training and lower body strength in the intervention. Due to the risks for falls and limited mobility, some oldest-older participants performed all exercises in a seated position, which minimized the training benefits for balance and lower body strength.
Limitations of this study are acknowledged. The small sample size and mild-to-moderate cognitive impairment of our participants and the convenient selection of an independent living facility might limit the ability to generalize these findings to the broader older adult population with and without cognitive decline. Furthermore, given the small sample size, we included all participants in the single-arm exercise intervention and were not able to include a control group. Future studies may conduct an RCT to rigorously test the efficacy of BMS for exercise adherence by having a no-music exercise control group or to test the efficacy of BMS-based exercise training for physical, cognitive, and mental health versus a no-exercise control group among older adults with varying clinical conditions. Despite these limitations, the findings of this study are of value because they demonstrate the feasibility and acceptability of this intervention for cognitive impaired older adults and providing promising effect sizes that can be used to design future research. We conclude that multicomponent exercise training can be beneficial for general cognition, cognitive processing speed, walking endurance, and aerobic fitness of older adults with mild-to-moderate cognitive decline in an independent living facility and that beat-accented music can be paired with exercise training to manipulate exercise tempo, which may be associated with good adherence to the training regimen by older adults.
Funding
This study was funded by the Undergraduate Research, Scholarship and Creativity Office (URSCO) and the Office of Leadership and Civic Engagement (OLCE) at the University of North Carolina at Greensboro.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by UNC Greensboro Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
KP designed the study with input from LB, JH, and JE. LB contributed to the development and implementation of the intervention and testing sessions. JH contributed to the arrangement of music stimuli and its implication for exercise training. KP, LB, and JH drafted an early version of the manuscript. JE revised and completed the writing of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors sincerely thank to Gina Rice, Matthew Ward, and Csilla Roper, the wellness team members at the Heritage Greens Senior Living Community, for their support and cooperation for the implementation of intervention and testing in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Alzheimer’s Association . 2020 Alzheimer's disease facts and figures. Alzheimers Dement. (2020) 16:391–460. doi: 10.1002/alz.12068
2.
Livingston G Sommerlad A Orgeta V Costafreda SG Huntley J Ames D et al . Dementia prevention, intervention, and care. Lancet. (2017) 390:2673–734. doi: 10.1016/S0140-6736(17)31363-6
3.
Norton S Matthews FE Barnes DE Yaffe K Brayne C . Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. (2014) 13:788–94. doi: 10.1016/S1474-4422(14)70136-X
4.
Piercy KL Troiano RP Ballard RM Carlson SA Fulton JE Galuska DA et al . The physical activity guidelines for Americans. JAMA. (2018) 320:2020–8. doi: 10.1001/jama.2018.14854
5.
Bull FC Al-Ansari SS Biddle S Borodulin K Buman MP Cardon G et al . World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955
6.
Bennie JA De Cocker K Teychenne MJ Brown WJ Biddle SJH . The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among 383,928 U.S. adults. Int J Behav Nutr Phys Act. (2019) 16:34. doi: 10.1186/s12966-019-0797-2
7.
Clarke TC Norris T Schiller JS . Early release of selected estimates based on data from the 2016 National Health Interview Survey. National Center for Health Statistics [Internet] (2017). Available at: https://www.cdc.gov/nchs/data/nhis/earlyrelease/earlyrelease201705.pdf
8.
Colcombe S Kramer AF . Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. (2003) 14:125–30. doi: 10.1111/1467-9280.t01-1-01430
9.
Kramer AF Colcombe S . Fitness effects on the cognitive function of older adults: a meta-analytic study—revisited. Perspect Psychol Sci. (2018) 13:213–7. doi: 10.1177/1745691617707316
10.
Karageorghis CI Terry PC Lane AM . Development and initial validation of an instrument to assess the motivational qualities of music in exercise and sport: the Brunel music rating inventory. J Sports Sci. (1999) 17:713–24. doi: 10.1080/026404199365579
11.
Bigliassi M Silva VB Karageorghis CI Bird JM Santos PC Altimari LR . Brain mechanisms that underlie the effects of motivational audiovisual stimuli on psychophysiological responses during exercise. Physiol Behav. (2016) 158:128–36. doi: 10.1016/j.physbeh.2016.03.001
12.
Karageorghis CI Terry PC . The psychophysical effects of music in sport and exercise: a review. J Sport Behav. (1997) 20:54–68.
13.
Karageorghis CI Priest D-L . Music in the exercise domain: a review and synthesis (part I). Int Rev Sport Exerc Psychol. (2012) 5:44–66. doi: 10.1080/1750984x.2011.631026
14.
Terry PC Karageorghis CI Curran ML Martin OV Parsons-Smith RL . Effects of music in exercise and sport: a meta-analytic review. Psychol Bull. (2020) 146:91–117. doi: 10.1037/bul0000216
15.
Zentner M Eerola T . Rhythmic engagement with music in infancy. Proc Natl Acad Sci U S A. (2010) 107:5768–73. doi: 10.1073/pnas.1000121107
16.
Buman MP Daphna Yasova L Giacobbi PR . Descriptive and narrative reports of barriers and motivators to physical activity in sedentary older adults. Psychol Sport Exerc. (2010) 11:223–30. doi: 10.1016/j.psychsport.2010.02.002
17.
Gray PM Murphy MH Gallagher AM Simpson EEA . Motives and barriers to physical activity among older adults of different socioeconomic status. J Aging Phys Act. (2016) 24:419–29. doi: 10.1123/japa.2015-0045
18.
Park KS Williams DM Etnier JL . Exploring the use of music to promote physical activity: from the viewpoint of psychological hedonism. Front Psychol. (2023) 14:1021825. doi: 10.3389/fpsyg.2023.1021825
19.
Koelsch S . Brain correlates of music-evoked emotions. Nat Rev Neurosci. (2014) 15:170–80. doi: 10.1038/nrn3666
20.
Ferreri L Mas-Herrero E Zatorre RJ Ripollés P Gomez-Andres A Alicart H et al . Dopamine modulates the reward experiences elicited by music. Proc Natl Acad Sci. (2019) 116:3793–8. doi: 10.1073/pnas.1811878116
21.
Matthews TE Witek MAG Lund T Vuust P Penhune VB . The sensation of groove engages motor and reward networks. NeuroImage. (2020) 214:116768. doi: 10.1016/j.neuroimage.2020.116768
22.
Park KS Hong J Etnier JL . Affective and ergogenic effects of beat-accented synchronous music on acute exercise training in older adults with mild cognitive impairment or mild dementia. Alzheimers Dement. (2022) 18:S8. doi: 10.1002/alz.069303
23.
Alter DA O’Sullivan M Oh PI Redelmeier DA Marzolini S Liu R et al . Synchronized personalized music audio-playlists to improve adherence to physical activity among patients participating in a structured exercise program: a proof-of-principle feasibility study. Sports Med Open. (2015) 1:23. doi: 10.1186/s40798-015-0017-9
24.
Thaut MH McIntosh GC Rice RR Miller RA Rathbun J Brault JM . Rhythmic auditory stimulation in gait training for Parkinson's disease patients. Mov Disord. (1996) 11:193–200. doi: 10.1002/mds.870110213
25.
Benoit C-E Dalla Bella S Farrugia N Obrig H Mainka S Kotz SA . Musically cued gait-training improves both perceptual and motor timing in Parkinson's disease. Front Hum Neurosci. (2014) 8:494. doi: 10.3389/fnhum.2014.00494
26.
McIntosh GC Brown SH Rice RR Thaut MH . Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. (1997) 62:22–6. doi: 10.1136/jnnp.62.1.22
27.
Prassas S Thaut M McIntosh G Rice R . Effect of auditory rhythmic cuing on gait kinematic parameters of stroke patients. Gait Posture. (1997) 6:218–23. doi: 10.1016/s0966-6362(97)00010-6
28.
Thaut MH McIntosh GC Rice RR . Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. J Neurol Sci. (1997) 151:207–12. doi: 10.1016/S0022-510X(97)00146-9
29.
Herdman M Gudex C Lloyd A Janssen M Kind P Parkin D et al . Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. (2011) 20:1727–36. doi: 10.1007/s11136-011-9903-x
30.
Warburton DER Jamnik VK Bredin SSD Gledhill N . The physical activity readiness questionnaire for everyone (PAR-Q+) and electronic physical activity readiness medical examination (ePARmed-X+). Health Fitness J Canada. (2011) 4:3–17. doi: 10.14288/hfjc.v4i2.103
31.
Buckles VD Powlishta KK Palmer JL Coats M Hosto T Buckley A et al . Understanding of informed consent by demented individuals. Neurology. (2003) 61:1662–6. doi: 10.1212/01.WNL.0000098933.34804.FC
32.
Borg GA . Psychophysical bases of perceived exertion. Med Sci Sports Exerc. (1982) 14:377–81. doi: 10.1249/00005768-198205000-00012
33.
Larsen DL Atkisson CC Hargreaves WA Nguyen T . Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann. (1979) 2:197–207. doi: 10.1016/0149-7189(79)90094-6
34.
Nasreddine ZS Phillips NA Bédirian V Charbonneau S Whitehead V Collin I et al . The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
35.
Julayanont P Brousseau M Chertkow H Phillips N Nasreddine ZS . Montreal cognitive assessment memory index score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer's disease. J Am Geriatr Soc. (2014) 62:679–84. doi: 10.1111/jgs.12742
36.
Zelazo PD Anderson JE Richler J Wallner-Allen K Beaumont JL Weintraub S . NIH toolbox cognition battery (CB): measuring executive function and attention. Monogr Soc Res Child Dev. (2013) 78:16–33. doi: 10.1111/mono.12032
37.
Carlozzi NE Tulsky DS Kail RV Beaumont JL . NIH toolbox cognition battery (CB): measuring processing speed. Monogr Soc Res Child Dev. (2013) 78:88–102. doi: 10.1111/mono.12036
38.
Weintraub S Dikmen SS Heaton RK Tulsky DS Zelazo PD Slotkin J et al . The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: validation in an adult sample. J Int Neuropsychol Soc. (2014) 20:567–78. doi: 10.1017/s1355617714000320
39.
Nolin SA Cowart H Merritt S McInerney K Bharadwaj PK Franchetti MK et al . Validity of the NIH toolbox cognitive battery in a healthy oldest-old 85+ sample. J Int Neuropsychol Soc. (2022) 29:605–14. doi: 10.1017/s1355617722000443
40.
Bhadhuri A Kind P Salari P Jungo KT Boland B Byrne S et al . Measurement properties of EQ-5D-3L and EQ-5D-5L in recording self-reported health status in older patients with substantial multimorbidity and polypharmacy. Health Qual Life Outcomes. (2020) 18:317. doi: 10.1186/s12955-020-01564-0
41.
Nithman RW Vincenzo JL . How steady is the STEADI? Inferential analysis of the CDC fall risk toolkit. Arch Gerontol Geriatr. (2019) 83:185–94. doi: 10.1016/j.archger.2019.02.018
42.
Rikli RE Jones CJ . The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. J Aging Phys Act. (1998) 6:363–75. doi: 10.1123/japa.6.4.363
43.
Mänttäri A Suni J Sievänen H Husu P Vähä-Ypyä H Valkeinen H et al . Six-minute walk test: a tool for predicting maximal aerobic power (VO2 max) in healthy adults. Clin Physiol Funct Imaging. (2018) 38:1038–45. doi: 10.1111/cpf.12525
44.
R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing (2022).
45.
Clark IN Taylor NF Peiris CL . Music listening interventions for physical activity: a systematic review and meta-analysis of randomised controlled trials. Disabil Rehabil. (2022):1–8. doi: 10.1080/09638288.2022.2155715
46.
Chair SY Zou H Cao X . A systematic review of effects of recorded music listening during exercise on physical activity adherence and health outcomes in patients with coronary heart disease. Ann Phys Rehabil Med. (2021) 64:101447. doi: 10.1016/j.rehab.2020.09.011
47.
Williams DM . Psychological hedonism, hedonic motivation, and health behavior In: WilliamsDMRhodesREConnerMT, editors. Affective Determinants of Health Behavior. New York, NY: Oxford University Press (2018). 204–34.
48.
Williams DM . The theory of hedonic motivation In: WilliamsDM, editor. Darwinian Hedonism and the Epidemic of Unhealthy Behavior. Cambridge: Cambridge University Press (2019). 139–46.
49.
Clark IN Baker FA Peiris CL Shoebridge G Taylor NF . Participant-selected music and physical activity in older adults following cardiac rehabilitation: a randomized controlled trial. Clin Rehabil. (2017) 31:329–39. doi: 10.1177/0269215516640864
50.
Bauldoff GS Hoffman LA Zullo TG Sciurba FC . Exercise maintenance following pulmonary rehabilitation: effect of distractive stimuli. Chest. (2002) 122:948–54. doi: 10.1378/chest.122.3.948
51.
Burger B Thompson MR Luck G Saarikallio S Toiviainen P . Influences of rhythm- and timbre-related musical features on characteristics of music-induced movement. Front Psychol. (2013) 4:183. doi: 10.3389/fpsyg.2013.00183
52.
Chen JL Zatorre RJ Penhune VB . Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. NeuroImage. (2006) 32:1771–81. doi: 10.1016/j.neuroimage.2006.04.207
53.
Thaut MH Hoemberg V von Wild K . Handbook of Neurologic Music Therapy. Oxford: Oxford University Press (2016).
54.
Thaut MH . The future of music in therapy and medicine. Ann N Y Acad Sci. (2005) 1060:303–8. doi: 10.1196/annals.1360.023
55.
Schaefer RS . Auditory rhythmic cueing in movement rehabilitation: findings and possible mechanisms. Phil Trans R Soc Lond Ser B Biol Sci. (2014) 369:20130402. doi: 10.1098/rstb.2013.0402
56.
Ghai S . Effects of real-time (sonification) and rhythmic auditory stimuli on recovering arm function post stroke: a systematic review and Meta-analysis. Front Neurol. (2018) 9:488. doi: 10.3389/fneur.2018.00488
57.
Ghai S Ghai I . Effects of (music-based) rhythmic auditory cueing training on gait and posture post-stroke: a systematic review & dose-response meta-analysis. Sci Rep. (2019) 9:2183. doi: 10.1038/s41598-019-38723-3
58.
Ghai S Ghai I Effenberg AO . Effect of rhythmic auditory cueing on gait in cerebral palsy: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. (2017) 14:43–59. doi: 10.2147/NDT.S148053
59.
Ghai S Ghai I Schmitz G Effenberg AO . Effect of rhythmic auditory cueing on parkinsonian gait: a systematic review and meta-analysis. Sci Rep. (2018) 8:506. doi: 10.1038/s41598-017-16232-5
60.
Wittwer JE Winbolt M Morris ME . Home-based gait training using rhythmic auditory cues in Alzheimer's disease: feasibility and outcomes. Front Med. (2020) 6:335. doi: 10.3389/fmed.2019.00335
61.
Lim I van Wegen E de Goede C Deutekom M Nieuwboer A Willems A et al . Effects of external rhythmical cueing on gait in patients with Parkinson's disease: a systematic review. Clin Rehabil. (2005) 19:695–713. doi: 10.1191/0269215505cr906oa
62.
Nombela C Hughes LE Owen AM Grahn JA . Into the groove: can rhythm influence Parkinson's disease?Neurosci Biobehav Rev. (2013) 37:2564–70. doi: 10.1016/j.neubiorev.2013.08.003
63.
Park KS Hass CJ Patel B Janelle CM . Musical pleasure beneficially alters stride and arm swing amplitude during rhythmically-cued walking in people with Parkinson's disease. Hum Mov Sci. (2020) 74:102718. doi: 10.1016/j.humov.2020.102718
64.
Park KS Hass CJ Janelle CM . Familiarity with music influences stride amplitude and variability during rhythmically-cued walking in individuals with Parkinson’s disease. Gait Posture. (2021) 87:101–9. doi: 10.1016/j.gaitpost.2021.04.028
65.
de Bruin N Doan JB Turnbull G Suchowersky O Bonfield S Hu B et al . Walking with music is a safe and viable tool for gait training in Parkinson's disease: the effect of a 13-week feasibility study on single and dual task walking. Parkinsons Dis. (2010) 2010:1–9. doi: 10.1016/j.apmr.2008.09.559
66.
Northey JM Cherbuin N Pumpa KL Smee DJ Rattray B . Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. (2018) 52:154–60. doi: 10.1136/bjsports-2016-096587
67.
Kelly ME Loughrey D Lawlor BA Robertson IH Walsh C Brennan S . The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. (2014) 16:12–31. doi: 10.1016/j.arr.2014.05.002
68.
Lautenschlager NT Cox KL Flicker L Foster JK van Bockxmeer FM Xiao J et al . Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. (2008) 300:1027–37. doi: 10.1001/jama.300.9.1027
69.
Boa Sorte Silva NC Nagamatsu LS Gill DP Owen AM Petrella RJ . Memory function and brain functional connectivity adaptations following multiple-modality exercise and mind-motor training in older adults at risk of dementia: an exploratory sub-study. Front Aging Neurosci. (2020) 12:22. doi: 10.3389/fnagi.2020.00022
70.
Barnes DE Santos-Modesitt W Poelke G Kramer AF Castro C Middleton LE et al . The mental activity and eXercise (MAX) trial. JAMA Intern Med. (2013) 173:797–804. doi: 10.1001/jamainternmed.2013.189
71.
Baker LD Frank LL Foster-Schubert K Green PS Wilkinson CW McTiernan A et al . Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. (2010) 67:71–9. doi: 10.1001/archneurol.2009.307
72.
Wang X Wang H Ye Z Ding G Li F Ma J et al . The neurocognitive and BDNF changes of multicomponent exercise for community-dwelling older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Aging. (2020) 12:4907–17. doi: 10.18632/aging.102918
73.
Zheng G Xia R Zhou W Tao J Chen L . Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. (2016) 50:1443–50. doi: 10.1136/bjsports-2015-095699
74.
Groot C Hooghiemstra AM Raijmakers PGHM van Berckel BNM Scheltens P Scherder EJA et al . The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev. (2016) 25:13–23. doi: 10.1016/j.arr.2015.11.005
Summary
Keywords
adherence, aerobic training, cognitive impairment, dementia, music therapy, physical activity, rhythmic auditory stimulation, resistance training
Citation
Park KS, Buseth L, Hong J and Etnier JL (2023) Music-based multicomponent exercise training for community-dwelling older adults with mild-to-moderate cognitive decline: a feasibility study. Front. Med. 10:1224728. doi: 10.3389/fmed.2023.1224728
Received
18 May 2023
Accepted
24 July 2023
Published
21 August 2023
Volume
10 - 2023
Edited by
Amy Clements-Cortes, University of Toronto, Canada
Reviewed by
Thomas Lowder, University of Central Arkansas, United States; Fahad Naveed Ahmad, Wilfrid Laurier University, Canada; Amanda Ferland, Tongji Hospital Affiliated to Tongji University, China
Updates
Copyright
© 2023 Park, Buseth, Hong and Etnier.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyoung Shin Park, k_park4@uncg.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.