Abstract
Background:
Cadmium (Cd) is a heavy metal associated with several human disorders. Preeclampsia is a major cause of maternal mortality worldwide. The association between maternal Cd exposure and preeclampsia remains elusive.
Methods:
To better understand this relationship, we conducted a systematic review and meta-analysis of eligible studies from five databases (PubMed, Embase, Web of Science, Scopus, and CNKI) from their inception to September 10, 2022. The quality of these studies was evaluated using the Newcastle-Ottawa quality assessment scale (NOS). We use random-effects models to calculate overall standardized mean differences (SMDs) and 95% confidence intervals (CIs). Sensitivity analyses were performed to assess the robustness of our results. We also evaluated publication bias using Egger’s and Begg’s tests. Additionally, we conducted meta-regression and sub-group analyses to identify potential sources of heterogeneity between studies.
Results:
Our analysis included a total of 17 studies with 10,373 participants. We found a significant association between maternal cadmium exposure and the risk of preeclampsia (SMD 0.27, 95% CI 0.09–0.44, p < 0.01). No significant publication bias was detected in Begg’s or Egger’s tests. Meta-regression suggested that geographical location, year of publication, cadmium samples, sample size, and measurement methods did not contribute to heterogeneity between studies.
Conclusion:
Our findings suggest that maternal blood cadmium levels are associated with an increased risk of preeclampsia. In contrast, the pregnant women’s urine or placental levels of cadmium may not suggest preeclamptic risk during pregnancy. Further high-quality clinical studies and animal experiments are needed to understand this association better.
Systematic review registration:
PROSPERO, https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=361291, identifier: CRD42022361291.
1 Introduction
Cadmium (Cd) is a natural element usually exists in soil, rocks, coal, and mineral fertilizer (1). As a heavy metal, it can co-exist with zinc, copper, and lead minerals. Cadmium is widely used in the mining and smelting of non-ferrous metals, the manufacture of fertilizers, and the burning of fossil fuels and wastes. Human exposure to Cd is primarily via taking contaminated food, especially leafy green vegetables. Spinach (0.124 mg/kg) and lettuce (0.051 mg/kg) contain the highest levels of Cd. Regular consumption of shellfish and animal organs (e.g., liver and kidney) can increase the risk of cadmium exposure (2). According to the World Health Organization and the International Agency for Research on Cancer, Cd is the group I human carcinogen (3). Epidemiological studies have shown that cadmium exposure via respiration or drinking contaminated water could lead to cancer of the lung (3, 4), prostate (3), kidney (3), and bladder (5).
Preeclampsia (PE) is a major feto-maternal threat that affects around 5% of pregnancies worldwide (6–9). It is defined as either a systolic blood pressure (BP) of 140 mmHg or more or a diastolic blood pressure of 90 mmHg or more, or both, on two occasions at least 4 h apart after 20 weeks of gestation in a previously normotensive woman (10). Apart from hypertension, preeclampsia also involves multiple systemic presentations, including reduced maternal platelets, headache, and fetal growth restriction, etc. This has caused global concerns, imposing a heavy burden on public health. Despite multiple genetic, angiogenic, and immune predispositions identified in recent decades, its etiology remains unclear (11).
The relationship between cadmium and preeclampsia has not been fully explored before, but we may extrapolate it from existing studies focusing on the association between Cd exposure and varieties in BP. Gallagher et al. were the first to investigate the association between cadmium exposure and hypertension systematically (12). They identified a positive correlation between blood Cd and BP and a negative correlation between urine Cd and BP. These were more prominent among females. More recent studies have explored the intrinsic role of Cd in the development of vasculopathy, eventually leading to hypertension (12–15). Possible mechanisms of cadmium-induced hypertension can be renal failure, calcium signaling disruption, oxidative stress disorder, obstruction of the renin-angiotensin system, and vascular endothelial disorder (16, 17).
Reproductive age women have notably higher blood levels of Cd than men (median 0.41 vs. 0.17 ug/L, p < 0.01) (18, 19). Whether increased levels of Cd predispose them to developing preeclampsia has not been investigated. We only know that the placenta is an effective barrier against Cd, with only 0.01% of maternal Cd passed on to fetuses (20). Cd is hence partially concentrated in the placenta, causing potential placental and feto-maternal damage, such as fetal growth restriction (FGR), and maternal gestational diabetes mellitus (GDM) (21).
To our knowledge, we are the first to conduct an updated systematic review to focus specifically on the relationship between Cd exposure and PE.
2 Methods
The study protocol was registered with PROSPERO (No. CRD42022361291). The article was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (22).1
2.1 Search strategy
Two investigators (ZX.Z. and C.L.) independently searched four international electronic databases (PubMed, Scopus, Embase and Web of Science) as well as the China National Knowledge Infrastructure (CNKI) from database inception to September 10, 2022. The search syntax applied in the databases was (Cadmium OR “heavy metal” OR “trace element”) AND (preeclampsia OR eclampsia OR pre-eclampsia OR gestosis OR “pregnancy hypertension” OR “pregnancy-induced hypertension” OR “pregnancy-associated hypertension” OR “hypertensive disorders of pregnancy” OR “pregnancy toxemia” OR “gestational hypertension” OR “HELLP syndrome*”). The detailed search strategies can be accessed in Supplementary Search Strategy. Two independent reviewers (C.L. and ZX.Z.) initially screened all articles to assess for eligibility by the title and abstract. In addition, we searched for a list of the references and related review articles for additional articles. These include studies written in English or Chinese.
2.2 Eligibility criteria and study selection
The criteria for inclusion and exclusion of studies were established prior to the literature search. Articles were included if the following criteria were met: (1) observational studies involving maternal levels of cadmium and preeclampsia; (2) the exposure of interest should include cadmium while the outcome of interest should include preeclampsia; (3) the control should be pregnant women instead of non-pregnant women; (4) investigated the relationship between exposure to cadmium and any results mentioned in the search statement. These studies were included regardless of the age range, gravidity, parity and singleton/ multiple pregnancies of participants.
Articles were excluded if: (1) Studies not based on Cd level in maternal serum or placenta or urine, for instance, Cd exposure level in soil, or Cd level in maternal hair. (2) Duplicates, irrelevant studies, letters, reviews, commentaries, and conference abstracts were excluded.
Selected articles were retrieved thoroughly and further assessed for eligibility. ZX.Z. and C.L. screened the studies, and a third reviewer, JM.L. was to resolve any discrepancies between the two.
2.3 Data extraction and quality assessment
The following information was extracted by two researchers (ZX.Z. and C.L.) from the studies included: the first author and year of publication, location of the study, study types, number of participants, types of specimen, sample size, Cd level, average maternal age with standard deviation, sample collect time, methods of measurement and diagnostic criteria.
The quality of case–control and cohort studies was evaluated by the Newcastle-Ottawa Scale (NOS). A nine-star rating system evaluated by three dimensions, such as selection, comparability, outcome ascertainment. A score between 7 and 9 indicates good quality, while 4 to 6 was considered moderate quality. Poor quality was defined if the score was ≤3. Cross-sectional studies were assessed by a modified form of NOS, scoring zero to ten. Seven or more suggests a good quality, four to six indicates fair quality, while poor quality is considered for studies being score three or less (23).
2.4 Sub-group analysis and meta-regression analysis
Sub-group analysis and meta-regression were performed to assess whether sample types (blood, urine, placenta), geographic locations, year of publication, or type of measurement or sample size influenced the relationship. We divided all studies into five groups based on the original location of the study population: Asian studies were all from China. Studies from the USA were allocated to the American group. African studies consisted of reports from the Democratic Republic of Congo and South Africa. Middle-East studies covered reports from Iran and Turkey. European studies contained reports from France and the Republic of Serbia.
2.5 Statistical analysis
We calculated the results and performed data analysis via Review Manager 5.1 (The Nordic Cochrane Center, Copenhagen, Denmark) and Stata version 16.0 (StataCorp, College Station, TX, United States). The standardized mean differences (SMDs) with 95% confidence intervals (CIs) were used to summarize maternal cadmium exposure levels. Heterogeneity was tested by I2 (I2 ≥ 50% indicates high heterogeneity). The forest plot was used to visualize the overall results, with the random-effect model (REM) being adopted for calculation as the heterogeneity was considered significant. A sensitivity analysis was performed, removing each study once to assess whether any study influenced the overall results. Publication bias was visualized via funnel plot and verified by Begg’s and Egger’s tests.
3 Results
3.1 Study selection
Six hundred and ninety-two articles were identified via searching five databases (PubMed, Scopus, Embase, Web of Science, and CNKI). After examining all the references from full-text articles, eight additional studies were identified. Of the total 700 studies, 246 were deleted due to duplication. Four hundred and fifty-four literatures were further screened, and 422 were excluded after preliminarily browsing the title and abstract. Thirty-two records were being evaluated in the full-text assessment. Fifteen of them were removed for reasons: Six studies were excluded for not reporting cadmium samples (24–29). Four that reported amniotic fluid or nail samples were not included in our study (30–33). One article was excluded for overlapping the study population with another article, which was included (34). One was a conference paper (35). Two studies reported incomplete or absent data, such as no integral data for normal pregnancy and PE (36, 37). One article failed to make it into the finalists as it mainly focused on the relationship between Cd exposure and obstetric outcomes during pregnancy without preeclampsia (38). The remaining 17 reports were included in a systematic review and meta-analysis (39–55). Details of the study selection process can be illustrated in Figure 1.
Figure 1

PRISMA flow diagram for study selection process on correlation between maternal cadmium exposure and preeclampsia.
3.2 Characteristics of included studies
The 17 articles from 9 countries spanning over two decades. The size of the population between different studies was contrastingly different, ranging from 46 (minimum) to 5,429 (maximum). Differences were also observed in varied types of samples (blood, urine, placenta) and methods of measurement, such as inductively coupled plasma optical emission spectrometry (ICP-OES), inductively coupled plasma mass spectrometry (ICP-MS), and atomic absorption spectrometry (AAS). Despite these differences, most studies adopted ACOG’s diagnostic criteria for preeclampsia. More detailed information can be seen in Table 1.
Table 1
| Study | Nation | Design | Age of PE | Age of control | Cd of PE | Cd of control | Sample type (unit) | Methods of measurement | diagnostic criteria of PE | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | N | Mean ± SD | N | Mean ± SD | Mean ± SD | ||||||
| Laine et al. (44) | US | NCC | 24.0 ± 6.0 | 86 | 25.0 ± 6.3 | 86 | 3.7 ± 3.4 | 3.5 ± 2.1 | Placental (ng/g) | ICP-MS | ACOG |
| Wang et al. (49) | China | CC | 26.3 ± 4.0 | 51 | 27.0 ± 2.9 | 51 | 1.2 ± 0.8 4.3 ± 2.0 0.3 ± 0.2 |
1.1 ± 0.4 3.6 ± 3.6 0.4 ± 0.2 |
Blood (μg/L) Placental (μg/kg) UCB (μg/L) |
ICP-MS | ACOG |
| Bommarito et al. (51) | US | CC | 33.0 ± 4.5 | 28 | 32.7 ± 4.9 | 355 | 0.1 ± 0.1 | 0.1 ± 0.1 | Urine (μg/L) | ICP-MS | ACOG |
| Li et al. (55) | China | CC | 31.2 ± 8.1 | 23 | 32.4 ± 3.3 | 23 | 0.6 ± 0.6 15.1 ± 1.7 |
0.4 ± 0.4 11.2 ± 1.1 |
Blood (μg/dL) Placental (ng/g) |
ICP-MS | ACOG |
| Ovayolu et al. (54) | Turkey | CC | 30.6 ± 7.7 | 46 | 28.0 ± 6.6 | 46 | 0.6 ± 0.8 | 0.5 ± 0.6 | Blood (μg/L) | ICP-MS | ACOG |
| Wang et al. (53) | China | CC | NS | 427 | NS | 427 | 1.1 ± 2.4 | 1.2 ± 5.3 | Blood (μg/L) | ICP-MS | (56) |
| Liu et al. (52) | US | CS | 29.1 ± 6.2 | 115 | 28.0 ± 6.3 | 1,159 | 0.8 ± 0.4 | 0.7 ± 0.4 | Blood (μg/L) | ICP-MS | ACOG |
| Liu et al. (47) | China | CS | 29.4 ± 4.1 | 199 | 28.5 ± 3.7 | 5,230 | 0.6 ± 2.7 | 0.4 ± 2.0 | Urine (μg/L) | ICP-MS | (57) |
| Maduray et al. (46) | South Africa | CC | 25.0 ± 5.0 | 43 | 24.0 ± 5.0 | 23 | 0.1 ± 0.0 4.0 ± 0.9 |
0.1 ± 0.3 3.8 ± 0.6 |
Blood (μg/L) Hair (ng/g) |
ICP-OES | (58) |
| Elongi Moyene et al. (45) | DR Congo | CC | 27.1 ± 6.1 | 88 | 26.7 ± 5.9 | 88 | 2.1 ± 2.4 | 0.5 ± 0.3 | Urine (μg/L) | ICP-MS | (59) |
| Wang et al. (42) | China | CC | NS | 10 | NS | 88 | 5.7 ± 4.2 1.0 ± 6.1 |
4.8 ± 4.2 1.0 ± 6.4 |
Blood Urine (μg/L) |
AAS | NS |
| Kolusari et al. (41) | Turkey | CC | 27.9 ± 5.2 | 47 | 27.9 ± 4.3 | 48 | 0.0 ± 0.0 | 0.0 ± 0.0 | Blood (μg/dL) | ICP-OES | ACOG |
| Vigeh et al. (40) | Iran | CC | 26.0 ± 4.0 | 31 | 26.9 ± 5.7 | 365 | 0.5 ± 0.3 0.3 ± 0.4 |
0.5 ± 0.3 0.4 ± 0.4 |
Blood UCB (μg/L) |
ICP-MS | ACOG |
| Zhang et al. (50) | China | CC | 28.6 ± 2.0 | 40 | 27.7 ± 2.2 | 40 | 38.3 ± 11.4 | 18.5 ± 6.2 | Blood (μg/L) | ICP-MS | ACOG |
| Yazbeck et al. (43) | French | Cohort | NS | 106 | NS | 865 | 0.9 ± 0.5 | 0.9 ± 0.6 | Blood (μg/L) | AAS | (60) |
| Kosanovic et al. (39) | Serbia | CC | NS | 23 | NS | 37 | 1.5 ± 0.5 0.4 ± 0.1 |
1.3 ± 0.9 0.3 ± 0.1 |
Blood UCB (μg/L) |
AAS | NS |
| Musa Obadia et al. (48) | DR Congo | CC | 30.6 ± 6.4 | 40 | 31.4 ± 4.7 | 40 | 0.7 ± 0.4 3.5 ± 5.1 |
0.7 ± 0.2 0.7 ± 0.5 |
Blood Urine (μg/L) |
ICP-MS | NS |
Characteristics of included studies.
NS, not stated; NCC, Nested case-control; CC, Case-control study; CS, Cross sectional; PC, Prospective cohort.
ICP-MS, Inductively coupled plasma mass spectrometry; ICP-OES, Inductively coupled plasma optical emission spectrometer; AAS, Atomic absorption spectrophotometry; UCB, Umbilical cord blood.
3.3 Results of the systematic review
The 17 studies were further divided into 14 case–control or nested case–control, two cross-sectional and 1 cohort studies, and assessed by NOS. Since their inclusion and exclusion criteria are similar, we combine them to increase the sample size. One article was assessed as moderate quality and none of the studies was evaluated as poor quality. The specific score is accessible in Supplementary Tables S1–S3.
3.4 Results of meta-analysis
The meta-analysis of the 17 reports included a total of 10,373 participants. The number of healthy pregnant controls was much higher than the case group. There were 1,403 preeclamptic women, and a six-fold group of non-preeclamptic pregnant women (1,403 vs. 8,970). The overall results showed that maternal cadmium exposure in preeclamptic women was significantly higher than that of healthy pregnant control (SMD 0.27, 95% CI 0.09–0.44); (I2 = 82.6%; p < 0.01), see Figure 2. After systematic assessment, we extracted the variables associated with maternal Cd exposure to pool the overall results. The funnel plot showed insignificant publication bias, as shown in Figure 3. Publication bias was evaluated quantitatively by Begg’s test and Egger’s test (z = 2.06, p = 0.039; t = 1.86, p = 0.082; see Supplementary Figure S1). The leave-one-out sensitivity analysis showed that Moyene et al. and Zhang et al. reported inverse contributions to the combined results (Supplementary Figure S2) (45, 50).
Figure 2
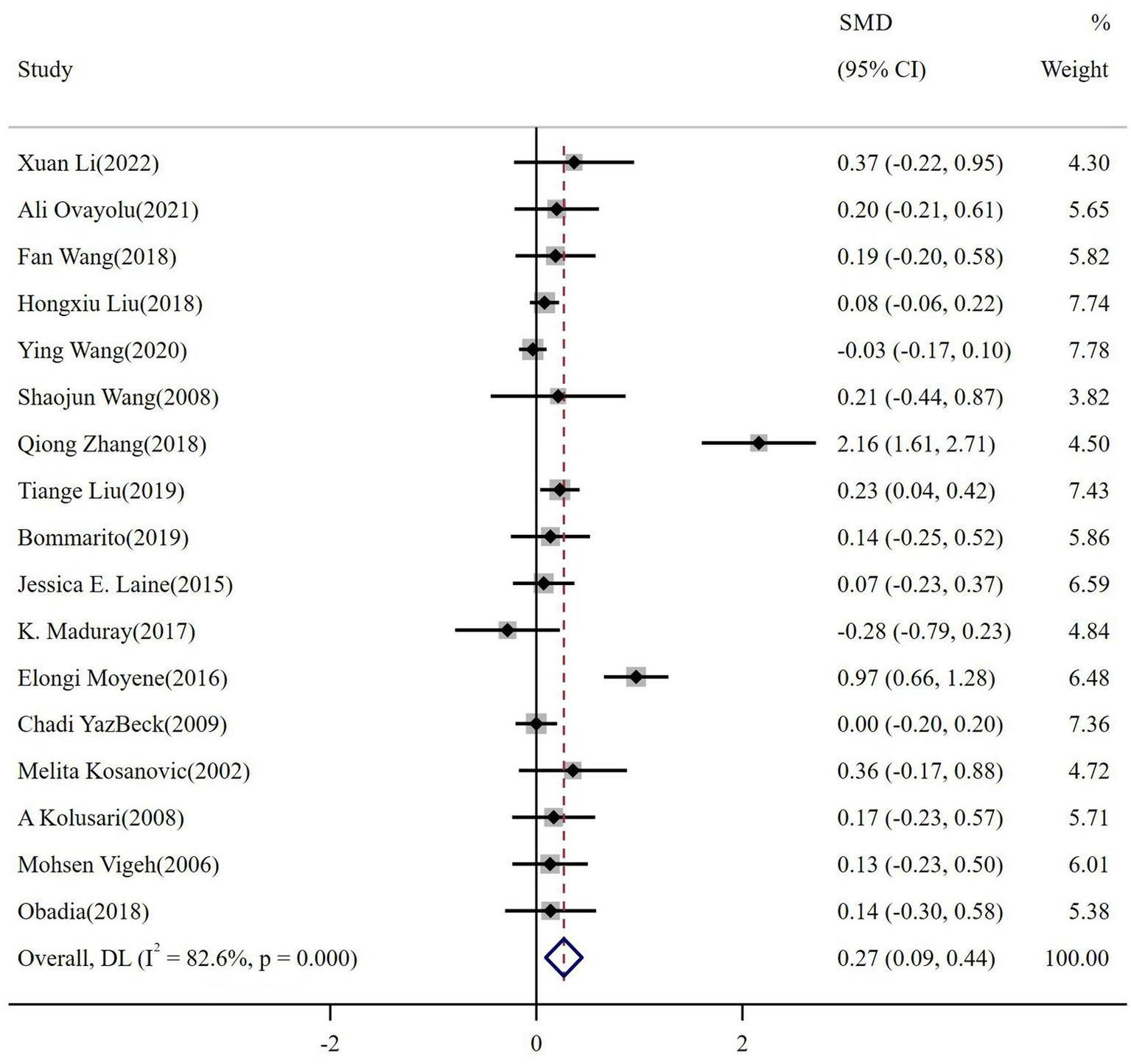
The forest plot of correlation between maternal cadmium exposure levels in preeclamptic and healthy pregnant women.
Figure 3
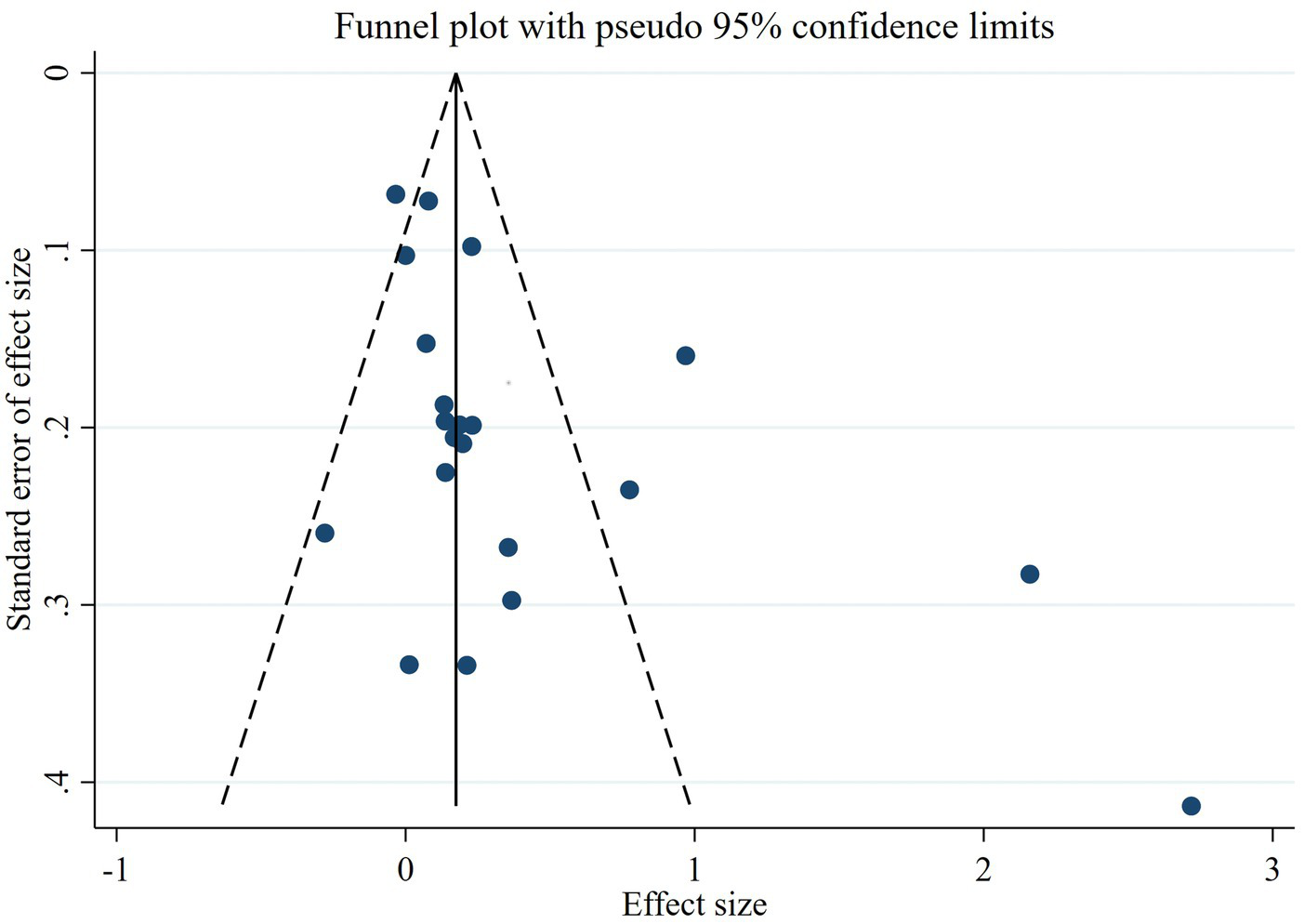
The funnel plot to assess publication bias.
3.5 Results of sub-group meta-analysis
We performed subgroup analysis to further identify any contributing factors that lead to the heterogeneity between studies. Thirteen studies involved maternal blood Cd levels between preeclamptic and normotensive were allocated in the same sub-group. Five records provided maternal urine cadmium levels, while three showed the placental levels of Cd in the two populations. The pooled results demonstrated that maternal blood cadmium levels were associated with an increased risk of preeclampsia (SWDBCd = 0.26; 95% CI: 0.04–0.47, Figure 4). By contrast, no significant association was found in maternal urine or placental Cd levels (SWDUCd = 0.40; 95% CI: −0.02 to 0.83; SWDPCd = 0.93; 95% CI: −0.17 to 2.02; respectively, Figure 4).
Figure 4
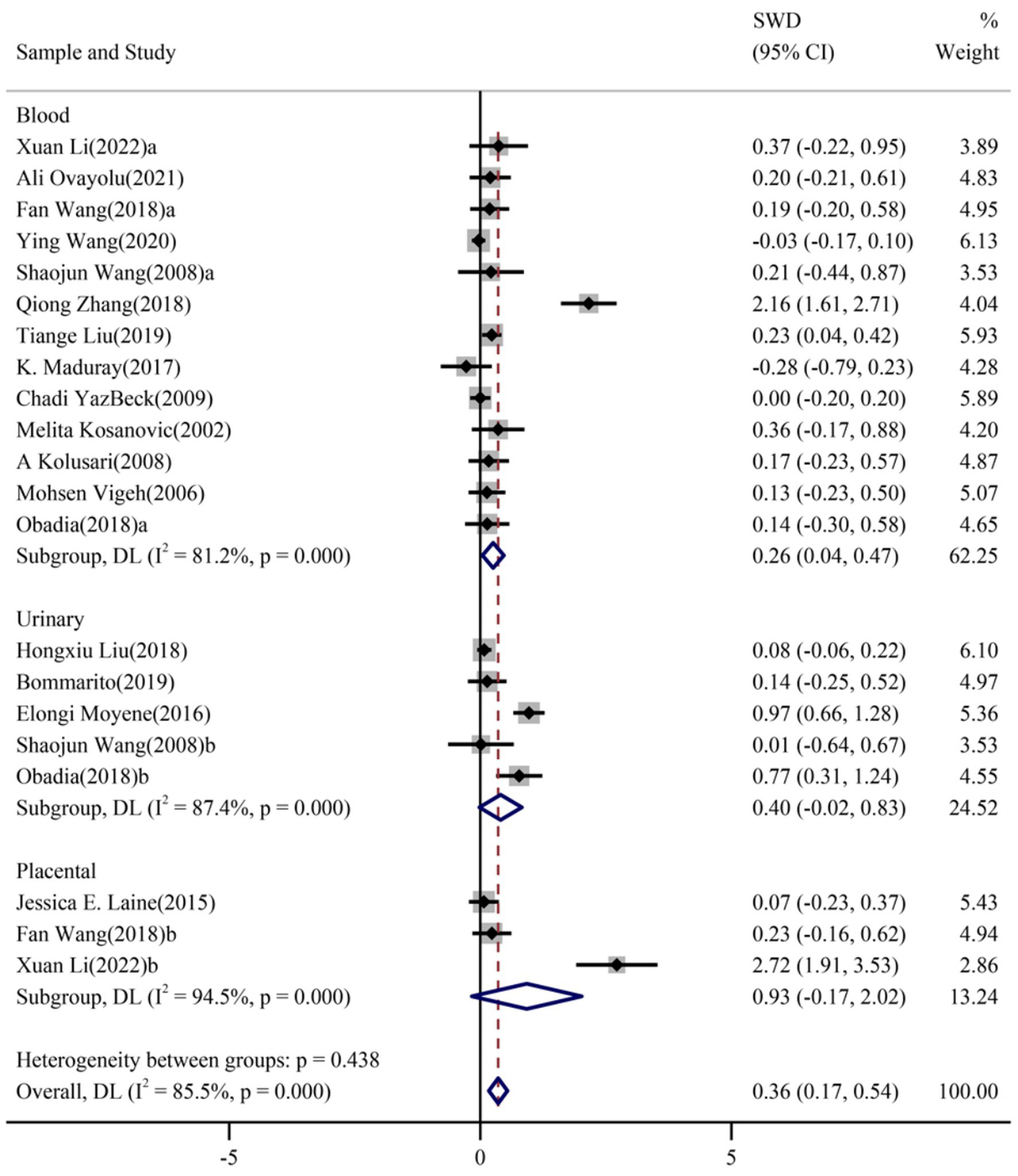
Forest plots of the combined associations of cadmium with PE stratified by types of samples of cadmium.
3.6 Results of meta-regression
Meta-regression was performed because of significant heterogeneity between studies. Different year of publication, geographical locations, different types of samples of cadmium, sample size, and measurement methods were further tested to look for potential causes of heterogeneity. However, it turned out that none of them was the major contributor (p-value of sample type: 0.139; p-value of location: 0.465; p-value of measurements: 0.685; p-value of year: 0.609; p-value of sample size: 0.145). Detailed information can be seen in Figure 5.
Figure 5
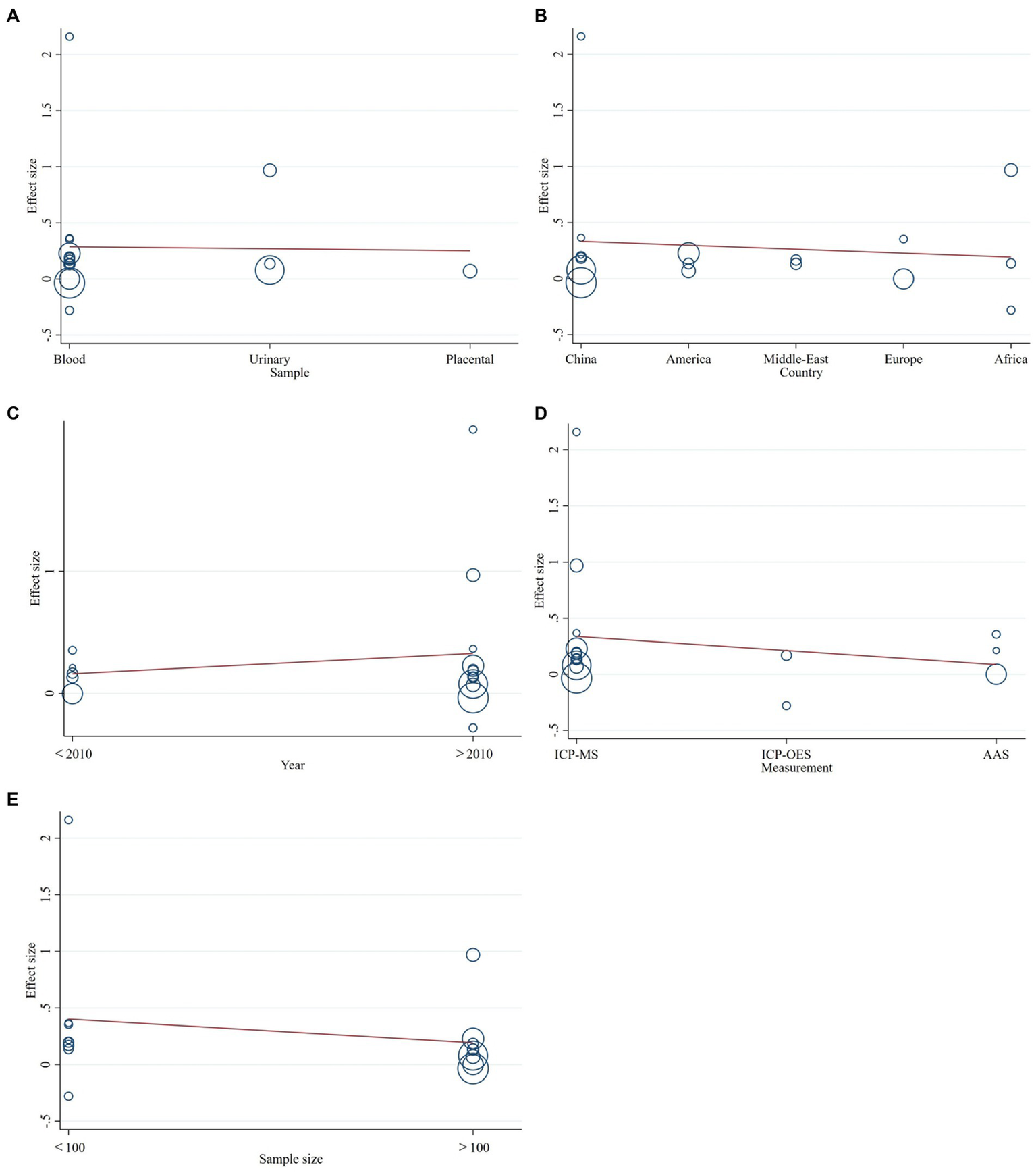
The meta-regression to assess publication bias. (A) types of samples of cadmium, (B) country of the study, (C) year of publication, (D) measurement methods, or (E) sample size.
4 Discussion
In the systematic review and meta-analysis, we recruited 17 studies involving 10,373 pregnant women to confirm the positive association between Cd exposure and preeclampsia. We also observed that sample selection bias, measurements, study designs may not contribute to the heterogeneity between studies. The meta-analysis found that maternal cadmium exposure in preeclamptic women was significantly higher than that of healthy pregnant controls. Generally, blood Cd usually reflects recent Cd exposure, while urine Cd levels generally reflect long-term Cd exposure (2, 61). The evidence linking maternal Cd exposure with preeclampsia implies that reproductive-age women should stop smoking and choose their diet carefully, avoiding potentially contaminated foods, particularly leafy vegetables, potatoes and grains, peanuts, and soybeans. Most of which are popular food choices in daily life. Due to the long process of Cd metabolism, the preparation should be as early as a couple’s family planning (62, 63).
The correlation between Cd and hypertensive disorders was first proposed by Henry. In his animal study in 1965, hypertensive animal models were successfully replicated through injection into pregnant rats with a water solution of Cd (64). The pregnant rats developed some forms of typical clinical manifestations, such as hypertension, albuminuria and FGR during pregnancy. Autopsy histopathological report revealed endothelial cells swelling, thickening of the media of the renal vessel walls, and protein tube formation in the renal tubules. The impaired placental angiogenesis was the typical change in human with PE (34, 65). Cd can change the placental structure, thereby affecting the function of placental transfer, predisposing pregnant women to PE (66).
Cadmium can also express a potent estrogen-like activity in vivo, stimulating the growth of myometrium (67, 68). In animal models, exposure to Cd can increase the weight of uterus and induce hormone-regulating gene expression even after oophorectomy (69). Cadmium enhances Lipopolysaccharide (LPS) and interleukin 4 (IL-4) mediated activation. The activation-induced cytosine deaminase (AID) is expressed in B cells via the estrogen receptor (34, 70). It can up-regulate the angiotensin II type 1-receptor-agonistic autoantibodies (AT1-AAs), inducing hypertension (21, 34). Cadmium-mediated complement C5a receptor activates C5, which further activates AT1R and leads to increased blood pressure (34). In vitro studies have shown that cadmium up-regulates gene expression in the transforming growth factor-β (TGF-β) pathway and IL-8 (71–73), thereby inhibiting the migration of placental trophoblast cells (73). Reduced migration of trophoblast cells is arguably one of the mechanisms of the development of preeclampsia (74, 75). Cd induces IL-6 production in trophoblast cells through a reactive oxygen species (ROS) dependent activation of the extracellular signal-regulated kinases (ERK)1/2 to and increased ERK1/2, c-Jun N-terminal kinases (JNK), and c-Jun phosphorylation (71), it then stimulates B cell production of AT1R (66, 76), contributing to the endothelial dysfunction and eventually hypertension in pregnancy. Furthermore, normal pregnant rats with long-term injection of IL-6 showed a significant increase in arterial pressure (77). Previous studies have identified that cadmium exposure decreased the expression of vascular endothelial growth factor (VEGF) and placental growth factor (PLGF) (51, 55), which activate systemic maternal endothelia, leading to vascular injury and hypertension (74, 78). In vivo studies have suggested that cadmium may induce preeclampsia by impairing the immune function (34), increasing oxidative DNA damage in the placenta (79), and damaging the kidneys (80). However, Sutoo and Akiyama found contradictory facts in rats (81). They observed that exposure to Cd increases dopamine through calmodulin, which in turn lowers blood pressure (81). Whether cadmium raises blood pressure remains controversial.
There was a systematic review that reported the correlation between Cd exposure and preeclampsia. Pollack et al. study in 2014 included only three studies. Two of the investigations were cross-sectional. One did not adjust for possible confounder factors, while the other only reported term preeclampsia in pregnancy outcomes (82). The limited number of articles may give a biased perspective.
By comparison, our report has several strengths. First, this is the first meta-analysis to comprehensively pool blood, urine, and placental Cd to explore the association between Cd and preeclampsia. Second, our meta-analysis included all the recent available studies to avoid selection bias maximally. We also included articles written in Chinese, as many articles focus on this topic. This is because the environmental contamination of heavy metals is a hot research topic in China currently. This helps complete evidence for this research. Third, we conducted meta-regression to further explore the association and determine the source of heterogeneity between studies.
However, we also noted some limitations in our study. The studies that met the inclusion criteria were still limited compared to other heavy metal studies (83), which confined us from exploring deeper. Furthermore, some studies reported the correlation between Cd and preeclampsia but did not report their data completely, and hence, we could not pool them together for further analysis (36, 38). Thirdly, not all studies followed ACOG’s diagnostic criteria. For those which followed ACOG’s, the version of the guidelines may be different. This may impact the study group, causing selection bias in the original study. Lastly, we failed to identify the causes for significant heterogeneity. This might be attributed to the abovementioned reasons, i.e., different study designs, different diagnostic criteria and various measurement methods. Future reviews with more well-designed original studies may alleviate the in-between study heterogeneity.
5 Conclusion
In summary, our meta-analysis provides quantitative evidence that Cd exposure is positively associated with preeclampsia in pregnancy. Large cohort studies and animal studies are needed to further clarify cadmium’s role in PE’s pathogenesis.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CL: Conceptualization, Investigation, Data curation, Methodology, Software, Writing – original draft. JL: Conceptualization, Data curation, Investigation, Software, Writing – original draft, Formal Analysis. YY: Data curation, Investigation, Software, Methodology, Writing – original draft. QW: Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. YZ: Formal Analysis, Investigation, Supervision, Writing – review & editing, Data curation, Project administration, Software, Validation. ZZ: Investigation, Conceptualization, Formal Analysis, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was co-supported by the Zhejiang Provincial Project for Medical and Health Science [2022503241], and Zhejiang Provincial Project for Education [Y202249319].
Acknowledgments
We have published a preprint in the Research Square (84).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1259680/full#supplementary-material
References
1.
Satarug S . Dietary Cadmium intake and its effects on kidneys. Toxics. (2018) 6:15. doi: 10.3390/toxics6010015
2.
Faroon O Ashizawa A Wright S Tucker P Jenkins K Ingerman L et al . Toxicological Profile for Cadmium. Agency for Toxic Substances and Disease Registry, Dept. of Health and Human Services (2012). 1–439.
3.
Straif K Benbrahim-Tallaa L Baan R Grosse Y Secretan B El Ghissassi F et al . A review of human carcinogens--part C: metals, arsenic, dusts, and fibres. Lancet Oncol. (2009) 10:453–4. doi: 10.1016/s1470-2045(09)70134-2
4.
Nawrot T Plusquin M Hogervorst J Roels HA Celis H Thijs L et al . Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol. (2006) 7:119–26. doi: 10.1016/S1470-2045(06)70545-9
5.
Kellen E Zeegers MP Hond ED Buntinx F . Blood cadmium may be associated with bladder carcinogenesis: the Belgian case-control study on bladder cancer. Cancer Detect Prev. (2007) 31:77–82. doi: 10.1016/j.cdp.2006.12.001
6.
Wallis AB Saftlas AF Hsia J Atrash HK . Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens. (2008) 21:521–6. doi: 10.1038/ajh.2008.20
7.
Duley L . The global impact of pre-eclampsia and eclampsia. Semin Perinatol. (2009) 33:130–7. doi: 10.1053/j.semperi.2009.02.010
8.
Abalos E Cuesta C Grosso AL Chou D Say L . Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. (2013) 170:1–7. doi: 10.1016/j.ejogrb.2013.05.005
9.
Ananth CV Keyes KM Wapner RJ . Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. (2013) 347:f6564. doi: 10.1136/bmj.f6564
10.
Gestational Hypertension and Preeclampsia . ACOG practice bulletin, number 222. Obstet Gynecol. (2020) 135:e237–60. doi: 10.1097/AOG.0000000000003891
11.
Phipps EA Thadhani R Benzing T Karumanchi SA . Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. (2019) 15:275–89. doi: 10.1038/s41581-019-0119-6
12.
Chowdhury R Ramond A O'Keeffe LM Shahzad S Kunutsor SK Muka T et al . Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. (2018) 362:k3310. doi: 10.1136/bmj.k3310
13.
Jarup L Akesson A . Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. (2009) 238:201–8. doi: 10.1016/j.taap.2009.04.020
14.
Martins AC Almeida Lopes ACB Urbano MR Carvalho MFH Silva AMR Tinkov AA et al . An updated systematic review on the association between cd exposure, blood pressure and hypertension. Ecotoxicol Environ Saf. (2021) 208:111636. doi: 10.1016/j.ecoenv.2020.111636
15.
Aramjoo H Arab-Zozani M Feyzi A Naghizadeh A Aschner M Naimabadi A et al . The association between environmental cadmium exposure, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Sci Pollut Res Int. (2022) 29:35682–706. doi: 10.1007/s11356-021-17777-9
16.
Prozialeck WC Edwards JR Nebert DW Woods JM Barchowsky A Atchison WD . The vascular system as a target of metal toxicity. Toxicol Sci. (2008) 102:207–18. doi: 10.1093/toxsci/kfm263
17.
Almenara CCP Oliveira TF Padilha AS . The role of antioxidants in the prevention of Cadmium-induced endothelial dysfunction. Curr Pharm Des. (2020) 26:3667–75. doi: 10.2174/1381612826666200415172338
18.
Vahter M Akesson A Liden C Ceccatelli S Berglund M . Gender differences in the disposition and toxicity of metals. Environ Res. (2007) 104:85–95. doi: 10.1016/j.envres.2006.08.003
19.
Fransson MN Barregard L Sallsten G Akerstrom M Johanson G . Physiologically-based toxicokinetic model for cadmium using Markov-chain Monte Carlo analysis of concentrations in blood, urine, and kidney cortex from living kidney donors. Toxicol Sci. (2014) 141:365–76. doi: 10.1093/toxsci/kfu129
20.
Whelton BD Toomey JM Bhattacharyya MH . Cadmium-109 metabolism in mice. IV. Diet versus maternal stores as a source of cadmium transfer to mouse fetuses and pups during gestation and lactation. J Toxicol Environ Health. (1993) 40:531–46. doi: 10.1080/15287399309531817
21.
Vidal AC Semenova V Darrah T Vengosh A Huang Z King K et al . Maternal cadmium, iron and zinc levels, DNA methylation and birth weight. BMC Pharmacol Toxicol. (2015) 16:20. doi: 10.1186/s40360-015-0020-2
22.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. (2009) 6:e123–30. doi: 10.1371/journal.pmed.1000097
23.
Modesti PA Reboldi G Cappuccio FP Agyemang C Remuzzi G Rapi S et al . Panethnic differences in blood pressure in Europe: a systematic review and Meta-analysis. PLoS One. (2016) 11:e0147601. doi: 10.1371/journal.pone.0147601
24.
Akinloye O Oyewale OJ Oguntibeju OO . Evaluation of trace elements in pregnant women with pre-eclampsia. Afr J Biotechnol. (2010) 9:5196–202. doi: 10.5897/AJB10.343
25.
Hamdan HZ Hassan AA Adam I . Minerals in pregnancy and newborns In: Molecular nutrition: Mother and infant (2020). 155–77.
26.
Kahn LG Trasande L . Environmental toxicant exposure and hypertensive disorders of pregnancy: recent findings. Curr Hypertens Rep. (2018) 20:87. doi: 10.1007/s11906-018-0888-5
27.
Rduch T Tsolaki E El Baz Y Leschka S Born D Kinkel J et al . The role of inorganics in preeclampsia assessed by multiscale multimodal characterization of placentae. Front Med (Lausanne). (2022) 9:857529. doi: 10.3389/fmed.2022.857529
28.
Zhang M Wu H Yu L Luo T Wen C Chai Y . Serum microelements amino acids and acyl carnitines levels in pregnancies complicated with preeclampsia: a prospective study. Clin Exp Obstet Gynecol. (2022) 49:121. doi: 10.31083/j.ceog4905121
29.
Harrington JM Young DJ Fry RC Weber FX Sumner SS Levine KE . Validation of a Metallomics analysis of placenta tissue by inductively-coupled plasma mass spectrometry. Biol Trace Elem Res. (2016) 169:164–73. doi: 10.1007/s12011-015-0431-3
30.
Dawson EB Evans DR Nosovitch J . Third-trimester amniotic fluid metal levels associated with preeclampsia. Arch Environ Health. (1999) 54:412–5. doi: 10.1080/00039899909603372
31.
Ebrahim K Ashtarinezhad A . The association of amniotic fluid cadmium levels with the risk of preeclampsia, prematurity and low birth weight. Iran J Neonatol. (2015) 6:1–6. doi: 10.22038/IJN.2015.4482
32.
Soobramoney C Maduray K Moodley J Moodley R Naicker T . The screening of nails for selected essential and toxic elements in normotensive and pre-Eclamptic women. Biol Trace Elem Res. (2019) 189:28–33. doi: 10.1007/s12011-018-1465-0
33.
Suliburska J Kocylowski R Komorowicz I Grzesiak M Bogdanski P Baralkiewicz D . Concentrations of mineral in amniotic fluid and their relations to selected maternal and fetal parameters. Biol Trace Elem Res. (2016) 172:37–45. doi: 10.1007/s12011-015-0557-3
34.
Zhang Q Huang Y Zhang K Huang Y Yan Y Wang F et al . Cadmium-induced immune abnormality is a key pathogenic event in human and rat models of preeclampsia. Environ Pollut. 218:770–82. doi: 10.1016/j.envpol.2016.07.073
35.
Liu T Zhang M Guallar E Wang G Hong X Wang X et al . Metal exposures and preeclampsia in the Boston birth cohort. Circulation. (2019) 139:139. doi: 10.1161/circ.139.suppl_1.P264
36.
Osorio-Yañez C Gelaye B Miller RS Enquobahrie DA Baccarelli AA Qiu C et al . Associations of maternal urinary Cadmium with trimester-specific blood pressure in pregnancy: role of dietary intake of micronutrients. Biol Trace Elem Res. (2016) 174:71–81. doi: 10.1007/s12011-016-0705-4
37.
Padula AM Ma C Huang H Morello-Frosch R Woodruff TJ Carmichael SL . Drinking water contaminants in California and hypertensive disorders in pregnancy. Environ Epidemiol (Philadelphia, PA). (2021) 5:e149. doi: 10.1097/ee9.0000000000000149
38.
Kovalchuk L Tarkhanova A Tarkhanov A . The essential and toxic effects of trace elements in the biological tissues of pregnant women and newborn babies (an ecologically unfavourable region). Int J Safety Secur Eng. (2013) 3:265–77. doi: 10.2495/SAFE-V3-N4-265-277
39.
Kosanovic M Jokanovic M Jevremovic M Dobric S Bokonjic D . Maternal and fetal cadmium and selenium status in normotensive and hypertensive pregnancy. Biol Trace Elem Res. (2002) 89:97–104. doi: 10.1385/BTER:89:2:97
40.
Vigeh M Yokoyama K Ramezanzadeh F Dahaghin M Sakai T Morita Y et al . Lead and other trace metals in preeclampsia: a case-control study in Tehran, Iran. Environ Res. (2006) 100:268–75. doi: 10.1016/j.envres.2005.05.005
41.
Kolusari A Kurdoglu M Yildizhan R Adali E Edirne T Cebi A et al . Catalase activity, serum trace element and heavy metal concentrations, and vitamin a, D and E levels in pre-eclampsia. J Int Med Res. (2008) 36:1335–41. doi: 10.1177/147323000803600622
42.
Wang S Wang K Wang H Yang L . The association between maternal zinc status and cadmium levels and changes in blood pressure in pregnancy. Acta Acad Med Mil Tert. (2008) 30:1678. doi: 10.3321/j.issn:1000-5404.2008.17.030
43.
Yazbeck C Thiebaugeorges O Moreau T Goua V Debotte G Sahuquillo J et al . Maternal blood lead levels and the risk of pregnancy-induced hypertension: the EDEN cohort study. Environ Health Perspect. (2009) 117:1526–30. doi: 10.1289/ehp.0800488
44.
Laine JE Ray P Bodnar W Cable PH Boggess K Offenbacher S et al . Placental cadmium levels are associated with increased preeclampsia risk. PLoS One. (2015) 10:e0139341. doi: 10.1371/journal.pone.0139341
45.
Elongi Moyene JP Scheers H Tandu-Umba B Haufroid V Buassa-Bu-Tsumbu B Verdonck F et al . Preeclampsia and toxic metals: a case-control study in Kinshasa, DR Congo. Environ Health. (2016) 15:48. doi: 10.1186/s12940-016-0132-1
46.
Maduray K Moodley J Soobramoney C Moodley R Naicker T . Elemental analysis of serum and hair from pre-eclamptic south African women. J Trace Elem Med Biol. (2017) 43:180–6. doi: 10.1016/j.jtemb.2017.03.004
47.
Liu H Xia W Xu S Zhang B Lu B Huang Z et al . Cadmium body burden and pregnancy-induced hypertension. Int J Hyg Environ Health. (2018) 221:246–51. doi: 10.1016/j.ijheh.2017.11.001
48.
Musa Obadia P Kayembe-Kitenge T Haufroid V Banza Lubaba Nkulu C Nemery B . Preeclampsia and blood lead (and other metals) in Lubumbashi, DR Congo. Environ Res. (2018) 167:468–71. doi: 10.1016/j.envres.2018.07.032
49.
Wang F Fan F Wang L Ye W Zhang Q Xie S . Maternal Cadmium levels during pregnancy and the relationship with preeclampsia and fetal biometric parameters. Biol Trace Elem Res. (2018) 186:322–9. doi: 10.1007/s12011-018-1312-3
50.
Zhang Q Huang Y Zhang K Yan Y Wu J Wang F et al . Progesterone attenuates hypertension and autoantibody levels to the angiotensin II type 1 receptor in response to elevated cadmium during pregnancy. Placenta. (2018) 62:16–24. doi: 10.1016/j.placenta.2017.12.004
51.
Bommarito PA Kim SS Meeker JD Fry RC Cantonwine DE McElrath TF et al . Urinary trace metals, maternal circulating angiogenic biomarkers, and preeclampsia: a single-contaminant and mixture-based approach. Environm Health Glob Access Sci Sour. (2019) 18:63. doi: 10.1186/s12940-019-0503-5
52.
Liu T Zhang M Guallar E Wang G Hong X Wang X et al . Trace minerals, heavy metals, and preeclampsia: findings from the Boston birth cohort. J Am Heart Assoc. (2019) 8:e012436. doi: 10.1161/JAHA.119.012436
53.
Wang Y Wang K Han T Zhang P Chen X Wu W et al . Exposure to multiple metals and prevalence for preeclampsia in Taiyuan, China. Environ Int. (2020) 145:106098. doi: 10.1016/j.envint.2020.106098
54.
Ovayolu A Turksoy VA Gun I Karaman E Dogan I Turgut A . Analyses of maternal plasma cadmium, lead, and vanadium levels in the diagnosis and severity of late-onset preeclampsia: a prospective and comparative study. J Matern Fetal Neonatal Med. (2021) 35:4803–9. doi: 10.1080/14767058.2020.1864318
55.
Li X Yu T Zhai M Wu Y Zhao B Duan C et al . Maternal cadmium exposure impairs placental angiogenesis in preeclampsia through disturbing thyroid hormone receptor signaling. Ecotoxicol Environ Saf. (2022) 244:114055. doi: 10.1016/j.ecoenv.2022.114055
56.
Wang Y Zhao N Qiu J He X Zhou M Cui H et al . Folic acid supplementation and dietary folate intake, and risk of preeclampsia. Eur J Clin Nutr. (2015) 69:1145–50. doi: 10.1038/ejcn.2014.295
57.
Lin LT Tsui KH Cheng JT Cheng JS Huang WC Liou WS et al . Increased risk of intracranial hemorrhage in patients with pregnancy-induced hypertension: a Nationwide population-based retrospective cohort study. Medicine (Baltimore). (2016) 95:e3732. doi: 10.1097/MD.0000000000003732
58.
Lambert G Brichant JF Hartstein G Bonhomme V Dewandre PY . Preeclampsia: an update. Acta Anaesthesiol Belg. (2014) 65:137–49.
59.
Lindheimer MD Roberts JM Cunningham FG Introduction LC . History, controversies, and definitions In: LindheimerMDRobertsJMCunninghamFG, editors. Chesley’s hypertensive disorders in pregnancy. San Diego, CA; Burlington, MA, London: Academic Press-Elsevier (2009). 1–23.
60.
National High Blood Pressure Education Program . Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. (2000) 183:S1–S22. doi: 10.1067/mob.2000.107928
61.
Adams SV Newcomb PA . Cadmium blood and urine concentrations as measures of exposure: NHANES 1999-2010. J Expo Sci Environ Epidemiol. (2014) 24:163–70. doi: 10.1038/jes.2013.55
62.
Rosen EM Munoz MI McElrath T Cantonwine DE Ferguson KK . Environmental contaminants and preeclampsia: a systematic literature review. J Toxicol Environ Health B Crit Rev. (2018) 21:291–319. doi: 10.1080/10937404.2018.1554515
63.
Akerstrom M Barregard L Lundh T Sallsten G . The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol Appl Pharmacol. (2013) 268:286–93. doi: 10.1016/j.taap.2013.02.009
64.
Schroeder HA Buckman J . Cadmium hypertension. Its reversal in rats by a zinc chelate. Arch Environ Health. (1967) 14:693–7. doi: 10.1080/00039896.1967.10664821
65.
Wang F Zhang Q Zhang X Luo S Ye D Guo Y et al . Preeclampsia induced by cadmium in rats is related to abnormal local glucocorticoid synthesis in placenta. Reprod Biol Endocrinol. (2014) 12:77. doi: 10.1186/1477-7827-12-77
66.
Zhang K Hu M Zhang L Zhang Q Huang Y . The effect of BML-111 in preeclampsia rat model induced by the low dose of Cadmium chloride. AJP Rep. (2019) 9:e201–8. doi: 10.1055/s-0039-1693016
67.
Byrne C Divekar SD Storchan GB Parodi DA Martin MB . Cadmium--a metallohormone?Toxicol Appl Pharmacol. (2009) 238:266–71. doi: 10.1016/j.taap.2009.03.025
68.
Knazicka Z Forgacs Z Lukacova J Roychoudhury S Massanyi P Lukac N . Endocrine disruptive effects of cadmium on steroidogenesis: human adrenocortical carcinoma cell line NCI-H295R as a cellular model for reproductive toxicity testing. J Environ Sci Health A Tox Hazard Subst Environ Eng. (2015) 50:348–56. doi: 10.1080/10934529.2015.987520
69.
Johnson MD Kenney N Stoica A Hilakivi-Clarke L Singh B Chepko G et al . Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med. (2003) 9:1081–4. doi: 10.1038/nm902
70.
Pauklin S . Erratum to: regulation of activation induced deaminase (AID) by estrogen. Methods Mol Biol. (2016) 1366:E1. doi: 10.1007/978-1-4939-3127-9_44
71.
Hu J Wang H Hu YF Xu XF Chen YH Xia MZ et al . Cadmium induces inflammatory cytokines through activating Akt signaling in mouse placenta and human trophoblast cells. Placenta. (2018) 65:7–14. doi: 10.1016/j.placenta.2018.03.008
72.
Adebambo OA Shea D Fry RC . Cadmium disrupts signaling of the hypoxia-inducible (HIF) and transforming growth factor (TGF-beta) pathways in placental JEG-3 trophoblast cells via reactive oxygen species. Toxicol Appl Pharmacol. (2018) 342:108–15. doi: 10.1016/j.taap.2018.01.010
73.
Brooks SA Fry RC . Cadmium inhibits placental trophoblast cell migration via miRNA regulation of the transforming growth factor beta (TGF-beta) pathway. Food Chem Toxicol. (2017) 109:721–6. doi: 10.1016/j.fct.2017.07.059
74.
Burton GJ Redman CW Roberts JM Moffett A . Pre-eclampsia: pathophysiology and clinical implications. BMJ. (2019) 366:l2381. doi: 10.1136/bmj.l2381
75.
Kadyrov M Kingdom JC Huppertz B . Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am J Obstet Gynecol. (2006) 194:557–63. doi: 10.1016/j.ajog.2005.07.035
76.
Paniagua L Diaz-Cueto L Huerta-Reyes M Arechavaleta-Velasco F . Cadmium exposure induces interleukin-6 production via ROS-dependent activation of the ERK1/2 but independent of JNK signaling pathway in human placental JEG-3 trophoblast cells. Reprod Toxicol (Elmsford, NY). (2019) 89:28–34. doi: 10.1016/j.reprotox.2019.06.008
77.
Gadonski G LaMarca BB Sullivan E Bennett W Chandler D Granger JP . Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension. (2006) 48:711–6. doi: 10.1161/01.HYP.0000238442.33463.94
78.
Chappell LC Cluver CA Kingdom J Tong S . Pre-eclampsia. Lancet. (2021) 398:341–54. doi: 10.1016/S0140-6736(20)32335-7
79.
Zhang X Xu Z Lin F Wang F Ye D Huang Y . Increased oxidative DNA damage in placenta contributes to Cadmium-induced Preeclamptic conditions in rat. Biol Trace Elem Res. (2016) 170:119–27. doi: 10.1007/s12011-015-0438-9
80.
Jacobo-Estrada T Santoyo-Sanchez M Thevenod F Barbier O . Cadmium handling, toxicity and molecular targets involved during pregnancy: lessons from experimental models. Int J Mol Sci. (2017) 18:1590. doi: 10.3390/ijms18071590
81.
Sutoo D Akiyama K . Effect of cadmium or magnesium on calcium-dependent central function that reduces blood pressure. Arch Toxicol. (2000) 74:1–4. doi: 10.1007/s002040050644
82.
Pollack AZ Ranasinghe S Sjaarda LA Mumford SL . Cadmium and reproductive health in women: a systematic review of the epidemiologic evidence. Curr Environ Health Rep. (2014) 1:172–84. doi: 10.1007/s40572-014-0013-0
83.
Zhong Z Yang Q Li C Chen X Zhou F . A global perspective of correlation between maternal blood lead levels and risks of preeclampsia: an updated systematic review and meta-analysis. Front Public Health. (2022) 10:1072052. doi: 10.3389/fpubh.2022.1072052
84.
Li C Yang Y Wang Q Zheng Y Zhong Z . The relationship between cadmium exposure and preeclampsia: a systematic review and meta-analysis. Research Square [Preprint]. (2023). doi: 10.21203/rs.3.rs-2966265/v1
Summary
Keywords
Cadmium, heavy metal, preeclampsia, blood pressure, systematic review, meta-analysis
Citation
Li C, Luo J, Yang Y, Wang Q, Zheng Y and Zhong Z (2023) The relationship between cadmium exposure and preeclampsia: a systematic review and meta-analysis. Front. Med. 10:1259680. doi: 10.3389/fmed.2023.1259680
Received
18 July 2023
Accepted
15 November 2023
Published
01 December 2023
Volume
10 - 2023
Edited by
Abraham A. Pouliakis, National and Kapodistrian University of Athens, Greece
Reviewed by
Timothy Abiola Olusesan Oluwasola, University of Ibadan, Nigeria; Sonia Minooee, James Cook University, Australia
Updates
Copyright
© 2023 Li, Luo, Yang, Wang, Zheng and Zhong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zixing Zhong, zhongzixing@hmc.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.