Abstract
Introduction:
There is a growing body of evidence suggesting a causal relationship between interstitial lung disease (ILD) and air pollution, both for the development of the disease, and driving disease progression. We aim to provide a comprehensive literature review of the association between air pollution, and ILD, including idiopathic pulmonary fibrosis (IPF).
Methods:
We systematically searched from six online database. Two independent authors (DL and CF) selected studies and critically appraised the risk of bias using the Newcastle-Ottawa Scale (NOS). Findings are presented through a narrative synthesis and meta-analysis. Meta-analyses were performed exclusively when there was a minimum of three studies examining identical pollutant-health outcome pairs, all evaluating equivalent increments in pollutant concentration, using a random effects model.
Results:
24 observational studies conducted in 13 countries or regions were identified. Pollutants under investigation encompassed ozone (O3), nitrogen dioxide (NO2), Particulate matter with diameters of 10 micrometers or less (PM10) and 2.5 micrometers or less (PM2.5), sulfur dioxide (SO2), carbon monoxide (CO), nitric oxide (NO) and nitrogen oxides (NOx). We conducted meta-analyses to assess the estimated Risk Ratios (RRs) for acute exacerbations (AE)-IPF in relation to exposure to every 10 μg/m3 increment in air pollutant concentrations, including O3, NO2, PM10, and PM2.5. The meta-analysis revealed a significant association between the increased risk of AE-IPF in PM2.5, yielding RR 1.94 (95% CI 1.30–2.90; p = 0.001). Findings across all the included studies suggest that increased exposure to air pollutants may be linked to a range of health issues in individuals with ILDs.
Conclusion:
A scarcity of available studies on the air pollutants and ILD relationship underscores the imperative for further comprehensive research in this domain. The available data suggest that reducing levels of PM2.5 in the atmosphere could potentially reduce AE frequency and severity in ILD patients.
1 Introduction
Interstitial lung disease (ILD) refers to a group of chronic lung disorders (1) characterized by inflammation and fibrosis of the lung interstitium (2, 3) including idiopathic pulmonary fibrosis (IPF), sarcoidosis, hypersensitivity pneumonitis, and connective tissue disease associated ILD (CTD-ILD) (4). Symptoms vary, but commonly include breathlessness, cough, and fatigue (5–8), and these symptoms can significantly decrease quality of life (QoL) for individuals with ILD. Moreover, prevalent comorbid conditions including gastro-oesophageal reflux disease (GORD), obstructive sleep apnoea (OSA), and depression can further contribute to the negative impact on QoL (5).
Prognosis is highly variable amongst ILD patients, with some patients having very rapid progression, and others a more indolent disease course. IPF, in particular, is associated with a high symptom burden, significant comorbidities, and a shortened life expectancy (9–12), with a 5-year mortality rate of 69% when not receiving antifibrotic treatment (13).
Recognized risk factors for the development of ILD include advanced age, male sex, genetic factors, smoking, medications, systemic autoimmune diseases, and occupational or environmental exposures (14–22). In the past decade, the impact of geographic factors, specifically air pollution, on lung health has received growing attention. Air pollutants have been identified as triggers for exacerbations of COPD and are associated with worsened asthma symptoms (23, 24). These pollutants also play a role in increasing the likelihood of lung cancer in individuals at risk (23, 24). Furthermore, there is evidence indicating an elevated risk of respiratory infections (24, 25), including tuberculosis (25) associated with exposure to air pollutants.
Air pollution is a complex combination of solid particles, liquid droplets, and various gases (26). It originates from a wide array of sources, including household fuel combustion, industrial smokestacks, vehicle emissions, power generation, the open burning of waste, agricultural activities, desert dust, and numerous other origins (26). Common air pollutants measured include particulate matters, where particles with an aerodynamic diameter of equal or less than 2.5 micrometre (PM2.5) and particles with an aerodynamic diameter of 10 micrometre (PM10), ozone (O3), nitrogen dioxide (NO2), carbon monoxide (CO) and sulfur dioxide (SO2) (26).
There has been increasing interest in the relationship between air quality and ILD with most studies and review papers focusing on IPF. In this systematic review with meta-analysis, we evaluate the relationship between air pollution and the broader ILD population. By incorporating all ILD subgroups, we aim to provide a comprehensive overview of the association between various pollutants and health outcomes, and to inform the understanding of underlying mechanisms and the potential public health implications.
2 Methods
2.1 Review protocol
The review followed the PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline (27). Given the specific focus on potential associations between exposure and clinical outcomes, instead of applying the conventional PICO (Population, Interventions, Comparators, and Outcomes) framework, the PEO (Patient, Exposure of interest, and Outcome) approach was employed (28). In this framework, the population of interest focused on two primary groups: those with a diagnosis of ILD and those who subsequently develop ILD. The exposure of interest is air pollution. The outcome examined is the impact of air pollution on ILD patients, including but not restricted to, acute exacerbation of ILD, disease progression, hospitalisation, and mortality rates associated with ILD and the risk of ILD development in the general population exposed to air pollution (Table 1). Our primary objective is to comprehensively explore the diverse impacts of air pollution on ILD. We anticipate encountering a spectrum of outcomes and surrogate markers beyond those explicitly specified, allowing for a thorough examination of this complex relationship.
Table 1
| Population | Individuals with ILD or general population |
|---|---|
| Exposure of interest (independent variable) | Air pollution |
| Outcome (dependent variable) | Impact of air pollution on individuals with ILD, or risk of developing ILD in general population |
PEO worksheet (impact of air pollution on interstitial lung disease).
2.2 Eligibility criteria
All authors engaged in thorough deliberation and reached a consensus regarding the inclusion and exclusion criteria. Inclusion criteria for this review were: (1) original research studies; (2) human studies; (3) studies examining the pollutants of interest in individuals diagnosed with ILD or in the general population who develop ILD. Exclusion criteria were: (1) studies exploring the impact of air pollution on general lung health and respiratory disease other than ILD; (2) studies focusing on other risk factors such as occupational factors in ILD; (3) studies measuring indoor exposure; and (4) qualitative studies.
2.3 Search strategy and information sources
Articles were searched for using the following six electronic databases: PubMed, Medline, Cochrane Library, CINAHL, Embase, and Scopus with latest search carried out on 20 July 2023. The key terms used were: (Air quality or air pollution or environment*) and (impact or outcome or relationship or effect or association) and (Pulmonary Fibrosis or interstitial lung disease or ILD). Key terms were searched as key words rather than subject headings to explore the topic as widely as possible. The search was then limited to the years 2000 to the present and “English Language”.
Apart from the electronic databases search, a snowball search technique (29) was used to find similar papers to the identified key papers. A secondary manual search of the reference lists was also carried out if the title suggested relevance to the searching terms. In addition, articles that are not able to be obtained from on-line library were requested through the Royal Prince Alfred Hospital library.
2.4 Study selection
Two independent authors (DL and CF) selected studies for inclusion. During the selection process, disagreements regarding the inclusion of specific articles meeting the predefined criteria were successfully resolved through discussion and consensus between the selecting authors. Studies lacking individual-level data, such as ecological studies, were considered for inclusion in the review but were ultimately excluded from the meta-analysis.
2.5 Data collection and data items
In this systematic review, data from each of the included studies were extracted, encompassing the first author’s name, publication year, study design, study population, sample size, country, mean age, sex, ILD diagnosis criteria, air pollution metrics, and the key findings. We collected risk estimates from the preferred model of choice by each study author (some of which were presented in abstracts). These estimates included hazard ratios (HR), risk ratios (RR), or odds ratios (OR), along with their corresponding 95% confidence intervals (CIs). We then entered the extracted data from the articles into an Excel spreadsheet for analysis. To ensure reliability, the primary author (DL) extracted the complete data, while co-author (CF) independently verified the accuracy and completeness of the extracted information. Any disagreements were adjudicated and resolved by senior investigator (TC).
2.6 Study risk of bias assessment
The risk of bias (30) of included studies was performed using the Newcastle-Ottawa Scale (NOS), a validated tool for assessing the quality of nonrandomized studies (31). It follows a “star system” approach, evaluating three perspectives: selection of study groups, comparability of groups, and ascertainment of exposure or outcome of interest in case–control or cohort studies. Each item is assigned one point, except for comparability, which can score up to two points.
To ensure precision and clarity in evaluating the comparability section of this review, a study receives one star if it controls or adjusts the age and sex for analysis. Additionally, if the study incorporates additional factors, such as smoking status, forced vital capacity (FVC), antifibrotic treatment, average temperature, and humidity, into the analysis to adjust for potential confounding effects, it qualifies for an additional star.
The adequacy of follow-up time of the cohorts was determined based on the assessed outcomes within each study, including long-term and short-term outcomes according to the definition provided in the 2021 World Health Organization’s (WHO) Air Quality Guidelines (AQG). Long-term exposure is characterized by an average measurement taken over one or several years, while short-term exposure is defined as a measurement taken over a period ranging from minutes to days (26).
The maximum possible score is nine. Quality is categorised as good, fair, or poor based on stars awarded in each domain. The majority of the included studies in this review were cohort and case–control studies. The evaluation of bias risk in these types of studies using the NOS was conducted independently by two investigators (DL and CF). It is worth noting that for ecologic studies, there is no established and validated tool for evaluation of quality (32). As a result, the assessment of the five ecologic studies was primarily descriptive. The remaining one study is a panel study. Its limitations are also described.
2.7 Data analysis
Due to the scarcity of studies focusing on the same pollutant and health outcome, the findings are presented through a narrative synthesis method. Meta-analyses were performed exclusively when there was a minimum of three studies examining identical pollutant-health outcome pairs, all evaluating equivalent increments in pollutant concentration. Due to variations in study design, methodology, and study populations, a random effects model was implemented.
For meta-analysis, given the variations in methodology across the studies, an inverse-variance approach was applied for the assessment of their relative significance (33). Within each study, we computed the standard error (SE) and the natural logarithm (log) of both Hazard Ratios (HR) and Odds Ratios (OR). These log-transformed HR and OR values were treated as equivalent to log Risk Ratio (logRRs). Alongside the SE, we calculated the pooled Risk Ratio (RR) for the meta-analysis. This approach mirrors the methodology previously employed (34). The 0.05 level (two-sided) was used to indicate significance of p-values.
For studies included in the meta-analysis, the RRs were used for the measure of association between air pollutants and health outcomes for comparing equivalent increases in pollutant concentration. In cases where RRs or ORs for gaseous pollutants were in parts per billion (ppb), they were converted to μg/m3 at standard conditions (25°C) first. The heterogeneity in effect sizes was assessed using the I2 statistic (35). The consideration of publication bias was deemed unnecessary due to the limited number of studies addressing the same pollutant and health outcome. All statistical analyses and meta-analyses were conducted with RevMan 5.4 software (Cochrane Collaboration).
3 Results
3.1 Study selection
The study selection process is summarised in Figure 1. Initially, a total of 6,291 records were identified through database searching. All search results from the database were imported into Endnote 20 to remove duplicates. After eliminating 1,200 duplicates, 5,091 records remained and underwent the initial screening based on their titles and abstracts. Subsequently, 5,035 records were excluded based on specific inclusion and exclusion criteria. The remaining 56 studies underwent a comprehensive review. During this review, 34 records were deemed ineligible and were subsequently removed, leaving a total of 22 papers sourced from the electronic database for inclusion in this literature review. Among the 34 ineligible papers, six contained duplicate content that was already present in other included papers, despite having different titles. Therefore, these six duplicate papers were removed from the selection to avoid redundancy in the literature review (36–41).
Figure 1

PRISMA 2020 flow diagram for study selection.
Five additional records were identified through citation and manual searching. One study emerged as a preliminary report (42). An attempt to communicate with the authors to gain a deeper comprehension of methods and analysis was unsuccessful, resulting in the exclusion of that report. Of the remaining four studies, two meeting the eligibility criteria were included. In summary, our systematic literature review comprised a total 24 papers (Figure 1).
3.2 Study characteristics
Table 2
| First author & year (reference) | Study population | Sample size | Country | Age years (mean ± SD) | Males/females | ILD diagnosis criteria | Air pollution metrics | Key findings |
|---|---|---|---|---|---|---|---|---|
| Johannson K. A., 2014 (43) | Patients with IPF | 436 | South Korea | 62.8 ± 7.9 | 345/91 | IPF diagnosis re-confirmed as per 2011 ATS/ERS/JRS/LARA Guideline | Mean and maximum Levels of O3, NO2, PM 10, SO2 and CO over the 6 weeks preceding the date of AE. Cumulative exposures were calculated over the entire period, from IPF diagnosis to date of censoring. |
|
| Sack C., 2017 (44) | Participants of the Multi-Ethnic Study on Atherosclerosis (MESA) | 2,265 | United States | 59.8 (9.4) | 1059/1206 | ILD defined based on HRCT scans | Annual average exposure levels of PM 2.5, NOX, NO2, and O3 |
|
| Conti S., 2018 (45) | Inhabitants of Lombardy, Italy | Almost 10 million inhabitants | Italy | IPF diagnosis based on International Classification of Diseases (ICD-9-CM) code 516.3 | Average concentrations over 2005–2010 for NO2 and O3, and over 2005–2009 for PM10 |
|
||
| Johannson K. A., 2018 (46) | Patients with IPF | 25 | United States | 73.6 ± 7.5 | 21/4 | IPF diagnosis re-confirmed as per 2011 ATS/ERS/JRS/LARA Guideline | Mean levels of O3, NO2, PM2.5, and PM10 over 2 to 5 weeks preceding clinical measurements; mean and maximum level over 40 weeks period |
|
| Sesé L., 2018 (47) | Patients with IPF | 192 | France | 67.9 ± 11.2 | 148/44 | IPF diagnosis based on 2000 ATS/ERS diagnosis criteria | Short-term effect: mean level of O3, NO2, PM2.5, and PM10 over the 6 weeks preceding the date of AE. Long-term effect: cumulative exposure levels from IPF diagnosis to the date of the event or censoring |
|
| Winterbottom C. J., 2018 (48) | Patients with IPF | 135 | United States | 68 (46–92) | 101/34 | IPF diagnosis re-confirmed as per 2011 ATS/ERS/JRS/LARA Guideline | Daily mean levels of PM 2.5 and PM10 between 2007 and 2013 |
|
| Pirozzi C., 2018 (49) | Patients with fibrotic sarcoidosis | 16 | United States | 59 (53.25, 62.5) | 4/12 | Sarcoidosis diagnosis based on American Thoracic Society (ATS) criteria (1999) | Average Levels of PM2.5 and O3 for 7, 10, and 14 days preceding each study visit |
|
| Rice M. B., 2019 (50) | Participants from Framingham study | 2,618 | United States | 59.5 ± 11.9 | 1299/1319 | ILAs diagnosis Based on CT scans | 5-year (2004–2008) average levels of PM2.5, Elemental carbon (EC) and O3 |
|
| Dales R., 2020 (51) | Patients with a primary diagnosis of IPF | 3,989 | Chile | 1665/2324 | IPF diagnosis based on International Classification of Diseases, 10th Revision (ICD-10) J84.1 | Annual average of 24 h means of CO, NO2, SO2, PM2.5 and PM 10, and 8-h daily maximum level of O3 over 0 to 6 days before admission and over the 30 days before admission |
|
|
| Chen H. H., 2020 (52) | Patients with connective tissue diseases (CTDs)-ILD | 505 CTD-ILD and 2020 non-ILD CTD | Taiwan, China | CTD-ILD 60.1 ± 14.7/ non-ILD CTD 59.4 ± 14.0 | CTD-ILD 125/380 non-ILD CTD 500/1,520 | CTD-ILD diagnosis based on ICD-9 code 515 and 516.36 | Mean levels of PM2.5, PM10, NO2, CO, SO2 and O3 1 year prior to the index date. |
|
| Mariscal-Aguilar P., 2021 (53) | Patients with IPF | 52 | Spain | 66 ± 10 | 41/11 | IPF diagnosis based on ATS/ERS criteria (unsure which version) | Mean levels of PM2.5, PM10, NO2, CO, SO2, and O3 (from the previous 12 weeks prior to death for mortality, mean level between 2013 and 2019 for disease progression.) |
|
| Shull J. G., 2021 (54) | Patients with IPF | 379 | Spain | IPF diagnosis based on central expert ILD multidisciplinary discussion (MDD) | Annual mean concentration of PM2.5 over 1 year in 2015 |
|
||
| Tahara M., 2021 (55) | Patients with idiopathic interstitial pneumonias (IIPs) (152 IPF and 200 other subtypes of IIPs) | 352 | Japan | IIPs diagnosis based on HRCT, surgical lung biopsy (SLB) samples and MDD | Daily and monthly mean concentrations of SO2, NO, NO 2, NOx, CO, O 3, PM 2.5 and PM 10 |
|
||
| Tomos I., 2021 (56) | Patients with IPF | 118 | Greece | 72 ± 8.3 | 88/30 | IPF diagnosis based on 2011 ATS/ERS/JRS/LARA Guideline | Annual mean levels of O3, NO2, PM10 and PM2.5 |
|
| Yoon H-Y, 2021 (57) | Patients with IPF | 1,114 | South Korea | 65.7 ± 8.2 | 897/217 | IPF diagnosis based on 2011 ATS/ERS/JRS/LARA Guideline and MDD | Annual mean levels of PM10 and NO2 |
|
| Goobie G. C., 2022 (58) | 3 cohort patients with fibrotic interstitial lung disease (fILD) | 6,683 | United States and Canada | 67 (57–73) | 3653/3030 | fILD diagnosis were made by specialist ILD physicians: IPF diagnosis as per ATS/ERS/JRS/ALAT 2018; Hypersensitivity pneumonitis diagnosis as per ATS/JRS/ALAT 2020 | Monthly mean exposures 5 years pre-enrolment or precensoring for total PM 2.5 and its constituents (SO42−, NO3−, NH4+, black carbon, organic matter, sea salt, and soil) |
|
| Liang L., 2022 (59) | Patients with IPF | 11,974 IPF admissions | China | Primary discharge diagnosis of IPF based on ICD-10: J84.1 | Daily mean concentrations of PM10, PM2.5, NO2, O3, SO2 |
|
||
| Liu B., 2022 (60) | Patients hospitalized for rheumatoid arthritis associated with interstitial lung disease (RA ILD) | 221 RA-ILD admissions | China | Diagnosis of RA-ILD as per 1987 ACR criteria for RA, ILD by HRCT reviewed by rheumatologist and radiologist. | Monthly mean concentrations of PM2.5, PM10, SO2, O3 and NO2 |
|
||
| Cui F., 2023 (61) | Participants from the UK Biobank | 433,738 | United Kingdom | 56.4 ± 8.1 | 199,363/ 234,375 | IPF diagnosis based on ICD-10: J84.1 | Annual average concentrations of NO2, NOx, PM2.5 and PM 10 |
|
| Goobie G. C., 2023 (62) | Two cohort of IPF patients | 1,059 | United States | 69 (61–74) | 772/287 | IPF diagnosis made by a specialist ILD clinician, as per clinical practice guidelines | Monthly mean of PM2.5 (from 2000 to 2018) and its constituents (SO42−, NO3−, NH4+, black carbon, organic matter, sea salt, and soil) (from 2000 to 2017) |
|
| Mariscal-Aguilar P., 2023 (63) | Patients with IPF | 69 | Spain | 73.70 ± 7.72 | 53/16 | IPF diagnosis based on patients’ medical record at the time of each admission | Mean concentration of CO, NO2, O3, and NOx over 1, 3,6, 12 and 36 months before an event |
|
| Yoon H. Y., 2023 (64) | Patients with IPF | 946 | South Korea | 65.4 ± 8.1 | 765/181 | IPF diagnosis based on 2011 ATS/ERS/JRS/LARA Guideline and MDD | Annual average concentrations of NO2 and PM10 |
|
| Roeser A., 2023 (65) | Patients with Systemic sclerosis (SSc)-associated ILD | 181 | France | 53 (42.5–64) | 37/144 | SSc diagnosis based on 2013 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria and ILD diagnosis based on 2018 ATS/ERS/JRS/ALAT Guidelines | Annual mean concentrations of PM2.5, PM10, NO2, and O3 |
|
| Zheng Q., 2023 (66) | Patients with IPF | 570 | Australia | 70.9 (8.2) | 401/169 | IPF patients from Australian IPF Registry – IPF diagnosis made by referring respiratory physician | 5-year annual mean concentrations of PM2.5 and NO2 |
|
Summary of studies examined the impact of air pollution on interstitial lung disease (ILDs).
O3, ozone; NO2, nitrogen dioxide; PM, particulate matter; PM2.5, particulate matter with a diameter of 2.5 micrometres or less; PM10, particulate matter with a diameter of 10 micrometres or less; SO2, sulfur dioxide; CO, carbon monoxide; NO, Nitric Oxide; IPF, idiopathic pulmonary fibrosis; ILAs, interstitial lung abnormalities; FVC, Forced vital capacity; AE, Acute exacerbation; NOx, Nitrogen Oxides; SGRQ, Saint George Respiratory Questionnaire (SGRQ) measures the impact of respiratory symptoms on overall health, daily life, and perceived well-being, and queries symptoms over the past three months and “these days”; LCQ, Leicester cough questionnaire (LCQ) is a 19-item quality of life (QoL) measure of cough over the prior two weeks; KSQ, King’s Sarcoidosis Questionnaire (KSQ) is a validated questionnaire for assessing general health status and lung specific health status; Elemental carbon (EC), a traffic related PM2.5 constituent; DLco, diffusing capacity of the lungs for carbon monoxide; SO42−, sulfate; NH4+, ammonium; NO3−, nitrate; PFF, Pulmonary Fibrosis Foundation; Simmons, Simmons Center for Interstitial Lung Disease Registry; aOR, adjusted odds ratio; aHR, adjusted hazard ratio.
Studies were conducted across various countries, representing diversity of geography, culture, and population ethnicity (Table 2). Six studies were conducted in the United States (44, 46, 48–50, 62), representing a quarter of all the included studies. Three studies were conducted each in Spain (53, 54, 63) and South Korea (43, 57, 64). Two studies each came from France (47, 65) and mainland China (59, 60). The remaining studies were conducted in Italy (45), Chile (51), Taiwan (52), Japan (55), Greece (56), the United Kingdom (61), and Australia (66). A collaborative effort between investigators from the USA and Canada resulted in a study evaluating North American ILD populations and air pollution (58).
All 24 selected studies were observational in nature. Among these, 17 studies were published in the last 5 years (2019–2023), while the remaining seven studies were published between 2014 and 2018 (43–49). Population sizes varied widely, ranging from 16 participants to 10 million inhabitants. The mean population ages ranged from 53 to 73.7 years old. Of the studies that reported sex distribution, 207,108 (46.5%) participants were male, and 238,166 (53.5%) participants were female.
The majority of studies (20 studies) enrolled participants from individuals diagnosed with ILD groups, with the exception of four studies that recruited individuals from the general community (44, 45, 50, 61). Among the patient groups with ILD participants, 14 studies included participants with IPF only in their study populations. Five studies focused on non-IPF ILD patients including fibrotic sarcoidosis (49), a connective tissue disease (CTD) population with and without associated ILD (52), fibrotic ILD (fILD) (58), rheumatoid arthritis associated ILD (RA-ILD) (60), and systemic sclerosis (SSc)-associated ILD (SSc-ILD) (65). The last study is a case–control study that involved individuals who had received diagnoses of idiopathic interstitial pneumonias (IIPs) (55) including those with IPF.
Among the 24 studies incorporated in the analysis, seven studies scrutinized the link between short-term exposure to air pollution and its impact on health outcomes (43, 46, 49, 51, 55, 59, 60). Fourteen studies delved into the connection between long-term exposure to air pollution and health outcomes (44, 45, 48, 50, 52, 54, 56–58, 62, 64–66). The remaining three studies examined the effects of both short-term and long-term exposure (47, 53, 63).
3.3 Risk of bias in studies
Eighteen of the 24 analysed studies were cohort or case control studies, and their assessment was conducted using the NOS tool as described above. NOS scores were assigned to each study by consensus. Among the appraised 18 studies, 12 received a full score of 9/9 (43, 44, 47, 48, 50, 56–58, 61, 62, 65, 66), indicating high quality research. Four studies received a score of 8/9 (49, 52, 53, 64), one study received a score of 7/9 (55), and one study was scored as 6/9 (46). All 18 studies were considered to be of good to high quality.
The limitations identified in the remaining six studies involved data sources, accuracy of diagnoses, measurement of exposure, and potential confounding factors. Most studies had limitations of potential underestimation or misclassification of cases due to data sources or diagnostic coding (45, 51, 54, 59). Additionally, there was a lack of adjustment for individual-level factors (45, 51, 59, 60), and limited data on certain pollutants (54, 59) or occupational history (45, 59). Some studies had limited statistical power due to small sample size and limiting generalisability to broader populations or regions (60, 63) (Table 3).
Table 3
| Newcastle-Ottawa scale appraisal | ||||||||
|---|---|---|---|---|---|---|---|---|
| First author & year (reference) | Study design | Sample size | Limitations | Selection | Comparability | Outcome | Score | Quality |
| Johannson, 2014 (43) | Cohort | 436 | **** | ** | *** | 9 | Good | |
| Sack C., 2017 (44) | Cohort | 2,265 | **** | ** | *** | 9 | Good | |
| Johannson, 2018 (46) | Cohort | 25 | *** | ** | * | 6 | Good | |
| Sesé, 2018 (47) | Cohort | 192 | **** | ** | *** | 9 | Good | |
| Winterbottom, 2018 (48) | Cohort | 135 | **** | ** | *** | 9 | Good | |
| Pirozzi, 2018 (49) | Cohort | 16 | *** | ** | *** | 8 | Good | |
| Rice, 2019 (50) | Cohort | 2,618 | **** | ** | *** | 9 | Good | |
| Mariscal-Aguilar, 2021 (53) | Cohort | 52 | *** | ** | *** | 8 | Good | |
| Tomos, 2021 (56) | Cohort | 118 | **** | ** | *** | 9 | Good | |
| Yoon, 2021 (57) | Cohort | 1,114 | **** | ** | *** | 9 | Good | |
| Goobie, 2022 (58) | Cohort | 6,683 | **** | ** | *** | 9 | Good | |
| Cui, 2023 (61) | Cohort | 433,738 | **** | ** | *** | 9 | Good | |
| Goobie, 2023 (62) | Cohort | 1,059 | **** | ** | *** | 9 | Good | |
| Yoon, 2023 (64) | Cohort | 946 | **** | ** | ** | 8 | Good | |
| Roeser, 2023 (65) | Cohort | 181 | **** | ** | *** | 9 | Good | |
| Zheng, 2023 (66) | Cohort | 570 | **** | ** | *** | 9 | Good | |
| Chen, 2020 (52) | Case–Control | 2,525 | **** | ** | ** | 8 | Good | |
| Tahara, 2021 (55) | Case–Control | 352 | *** | ** | ** | 7 | Good | |
| Conti, 2018 (45) | Ecologic | Almost 10 million inhabitants | 1. Potential underestimation of incident cases of IPF due to reliance on administrative databases. 2. Uncertainty regarding the accuracy of diagnosis codes. 3. Proxy use of incident diagnosis date as disease onset. 4. Limited data on individuals who moved before 2005. 5. Potential residual confounding from unmeasured risk factors such as occupation and smoking. |
|||||
| Shull, 2021 (54) | Ecologic | 379 | 1. Inability to quantify precise air pollution exposure prior to diagnosis. 2. Potential underestimation of prevalence in rural areas and small towns. 3. Other pollutants such as NO2 and O3 were not examined. |
|||||
| Liang, 2022 (59) | Ecologic | 11,974 | 1. Ecological study design limits individual-level predictions. 2. Individual-level factors like smoking and socioeconomic status were not adjusted for. 3. Lack of data on time-varying factorsand specific residential addresses. 4. Outcome measurement limitations due to the primary diagnosis of IPF was coded by clinicians at discharge, which may have led to misclassification. 5. Constraints in analysing multiple pollutants and lack of occupational history. |
|||||
| Liu, 2022 (60) | Ecologic | 221 | 1. Exclusion of hospital outpatients may underestimate the impact of exposure. 2. Use of average daily data from fixed-site monitoring stations may not reflect individual exposure levels accurately. 3. Low statistical power due to limited incidences of RA-ILD. 4. Study conducted in a single district, limiting generalizability of results |
|||||
| Dales, 2020 (51) | Ecologic | 3,989 | 1. Lack of detailed personal information relevant to interstitial disease like smoking. 2. Lack of a more detailed description of the diagnosis. Inaccuracies in disease definition due to errors in both clinical diagnosis and diagnostic coding. |
|||||
| Mariscal-Aguilar, 2023 (63) | Panel | 69 | 1. Two-centre and limited sample size. 2. Lack of consideration for workplace air pollution. 3. Missing data on temperature and humidity. 4. Inability to control for all confounding factors. 5. Lack of diversity in exposure levels since the majority of patients reside in the same region. |
|||||
Critical appraisal of selected studies.
Thresholds for converting the Newcastle-Ottawa scales to AHRQ standards (good, fair, and poor): good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain. Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain. Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome/exposure domain.
3.4 Findings: impact of air pollutants on ILDs
Air pollution have been found to have multiple impacts on ILDs (Supplementary Table S1).
3.4.1 Air pollution and mortality in IPF or fibrotic interstitial lung disease (fILD)
Six studies involving 8,546 participants examined the association between air pollution and mortality in individuals with IPF (43, 47, 53, 57, 63) or fILD (58). Among these studies, five studies investigated NO2 (43, 47, 53, 57, 63), four studies involved O3 (43, 47, 53, 63), and three studies each examined PM10 (47, 53, 57), and PM2.5 (47, 53, 62).
The findings regarding the association between NO2 and O3 were inconclusive. Specifically, three studies did not detect any association between the average levels of NO2 and O3 in the 12 weeks preceding an individual’s death (53), the cumulative long-term NO2 and O3 exposure from the time of IPF diagnosis to the event date (43, 47), and IPF mortality. However, Mariscal-Aguilar et al. (63) reported a link between elevated mean levels of NO2 and O3 and higher probability of IPF mortality in individuals exposed to these pollutants for 1–3 months for O3, and 2–36 months for NO2. Additionally, Yoon et al. (57) found a positive association between NO2 and IPF mortality (95% CI 1.030–1.344, p = 0.016).
Variable impact of PM10 exposure has been described. Although two studies (53, 57) failed to detect any connection between short and long-term mean levels of PM10 and IPF mortality, a separate study (47) revealed a positive link. This study demonstrated that long-term cumulative exposure to elevated PM10 levels was associated with increased IPF mortality (HR 2.01; 95% CI 1.07–3.77; p = 0.03).
Regarding PM2.5 exposure, Mariscal-Aguilar et al. (53) reported no discernible link between the mean level of PM2.5 over the 12 weeks preceding death and IPF mortality, whereas Sesé et al. (47) revealed a significant correlation between long term cumulative exposure and IPF mortality (HR 7.93; 95% CI 2.93–21.33; p < 0.001). Another study exploring the effects of PM2.5 and its constituents on mortality in patients with fILD (58) showed that PM2.5 exposure of 8 μg/m3 or higher over a 5-year period was associated with increased mortality across all fILD patient groups (HR, 1.18; 95% CI, 1.02–1.37; p = 0.03).
In Mariscal-Aguilar et al. study (53), the mean CO level assessed 12 weeks before death was associated with increased IPF mortality (OR 2.45; 95% CI 1.39–4.56; p = 0.005). Conversely, Mariscal-Aguilar et al. (63), did not find any association between CO exposure and IPF mortality. However, this same study did reveal a positive association between increased mean concentrations of nitrogen oxides (NOx) and IPF mortality over a 12-36-month period (63).
3.4.2 Air pollution and disease progression (IPF and systemic sclerosis-associated ILD, SSc-ILD)
Three studies (1708 participants) explored the link between air pollution and the progression of IPF (47, 64, 66). Among the four pollutants, namely PM10, PM2.5, O3, and NO2, the results showed that PM10 (47, 64), PM2.5 (47, 66), and O3 (47) had no relationship with the disease progression in IPF patients. However, the results regarding NO2 were inconclusive.
In the study conducted by Sesé et al. (47), researchers examined cumulative concentrations of these pollutants from the date of IPF diagnosis to the event, while Zheng et al. (66) assessed 5-year annual mean concentrations. Neither study found a link between NO2 levels and progression of IPF. However, Yoon et al. study (64) reported that a 10-ppb increase in NO2 concentration was associated with a 10.5% increase in the risk of IPF disease progression (HR 1.105; 95% CI 1.000–1.219, p = 0.048) after adjusting for covariates.
Additionally, a study of 181 participants with SSc-ILD, examined the relationship between air pollution and the severity at diagnosis and with progression of ILD at 24 months (65). Increased O3 mean (SD) exposure levels during the 5 years preceding ILD diagnosis were associated with worse disease severity at diagnosis (adjusted OR: 1.12, 95% CI 1.05–1.21; p = 0.002) and disease progression at 24 months (OR: 1.10, 95% CI 1.02–1.19; p = 0.02), while PM2.5, PM10, and NO2 showed no association with the severity or progression of ILD.
Furthermore, one study involving 181 participants diagnosed with SSc-ILD investigated the association between air pollution and the severity of the disease at diagnosis and its progression over a 24-month period (65). Elevated levels of O3 exposure were significantly linked to more severe SSc-ILD both at the time of diagnosis (aOR: 1.12, 95% CI 1.05–1.21; p = 0.002) and progression at 24 months (aOR: 1.10, 95% CI 1.02–1.19; p = 0.02). However, no significant associations were identified between exposure to particulate matters (PM10 and PM2.5) or NO2 and the disease severity or its progression.
3.4.3 Air pollution and the risk of acute exacerbation of ILD (AE-IPF and AE-IIP)
Four studies (1,098 participants) investigated the potential association between air pollution and acute exacerbation of IPF (AE-IPF) (43, 47, 55, 56) including O3, NO2, PM10, PM2.5, SO2, CO, NO and NOx. Among these, SO2 and CO were examined in two of the studies, with no association found between short-term exposure to higher levels of these pollutants and incidence of AE-IPF (43, 55). A study conducted by Tahara et al. (55) also examined NO and NOx, with significant and near-significant association between increase in NO (OR 1.46; 95% CI 1.11–1.93; p = 0.008) and NOx (OR 1.24, 95% CI 0.99–1.53; p = 0.052) concentrations and AE-IPF.
We conducted meta-analyses to assess the RRs for AE-IPF in relation to exposure to every 10 μg/m3 increment in air pollutant concentrations, including O3, NO2, PM10, and PM2.5 (as illustrated in Figure 2). The study by Johannson et al. (43) expressed effect sizes per standard deviation increase in pollutant. However, it was excluded because we could not locate, in the main or supplementary text, a description of the SD on a continuous scale (43).
Figure 2
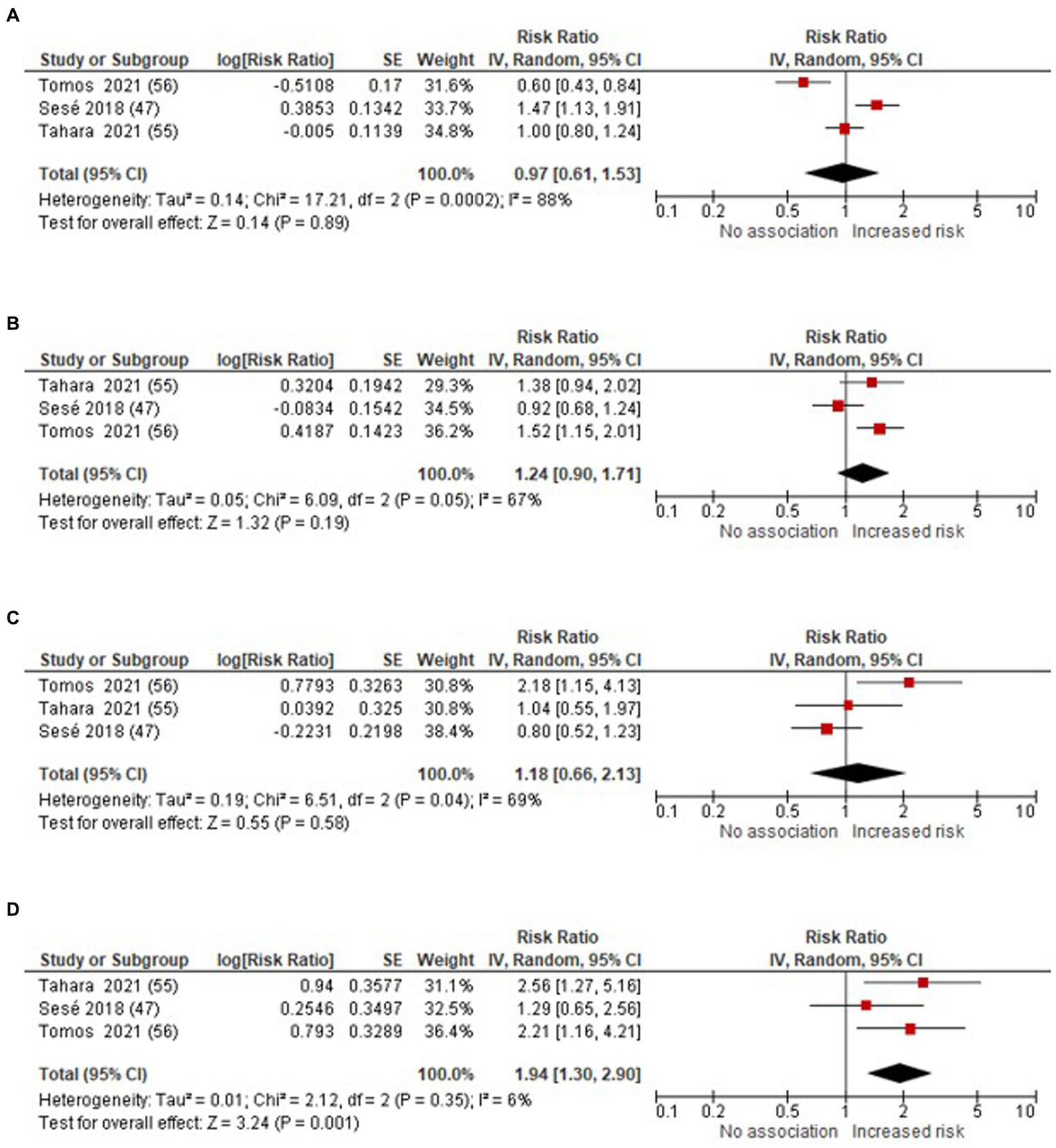
Forest plot: air pollutants (per 10 μg/m3 increase in air pollutants concentrations) and the risk of AE-IPF (A) O3(B) NO2(C) PM10(D) PM2.5.
The meta-analysis revealed a significant association between the increased risk of AE-IPF in PM2.5, yielding RR 1.94 (95% CI 1.30–2.90; p = 0.001). However, the link between O3, NO2 and PM10 and the risk of AE-IPF remained uncertain, with RR 0.97 (95% CI: 0.61–1.53; p = 0.89) for O3, RR 1.24 (95% CI 0.90–1.71; p = 0.19) for NO2, and RR 1.18 (95% CI 0.66–2.13; p = 0.58) for PM10, respectively.
Tahara et al. (55) (352 participants) also investigated the link between air pollution and the risk of acute exacerbations of idiopathic interstitial pneumonias (AE-IIPs). Their study revealed positive associations between the average monthly exposure to NO, NO2, NOX, and PM2.5, and AE-IIPs. Specifically, for every 10 ppb increase, the aOR was 1.50 (95% CI 1.19–1.88; p = 0.001) for NO, 1.99 (95% CI 1.22–3.27; p = 0.006) for NO2, and 1.29 (95% CI 1.08–1.53; p = 0.004) for NOX. Additionally, for every 10 ug/m3 increase, the aOR was 2.88 (95% CI 1.69–4.91; p ≤ 0.001) for PM2.5. However, there was no significant association found between the average monthly levels of SO2, O3, CO, PM10, and AE-IIPs.
3.4.4 Air pollution and lung function (baseline and decline in IPF, decline in fibrotic sarcoidosis patients)
Five studies (46, 48, 49, 58, 66) involving a total of 5,907 participants explored the relationship between air pollution and the decline in lung function among individuals with IPF or other fibrotic ILD. The pollutants investigated included PM2.5, PM10, O3, and NO2. Four studies found no significant association between PM2.5 levels and decline in FVC (46, 48, 49, 66). The sole study indicating an association was conducted by Goobie et al. (58) in patients with fILD. They observed that a 1 μg/m3 increase in the 5-year pre-censoring PM2.5 exposure, as assessed through adjusted meta-analysis across three cohorts, was linked to an additional 0.15% decrease in FVC percentage estimated per year (HR0.15, 95% CI -0.42 to 0.12, p = 0.29). Investigation of the constituents of PM2.5 revealed that higher exposures to sulfate, nitrate, and ammonium were correlated with a more accelerated annual decrease in the FVC percent predicted (SO42: β −2.53, 95% CI -4.45 to −0.62, p = 0.01); (NO3-: β-1.72, 95% CI -2.86 to −0.58, p = 0.003); (NH4+: β-5.93, 95% CI -10.18to −1.69, p = 0.006).
The same study (58) also identified associations between PM2.5 and its constituents, particularly SO42 and NH4+, and diffusing capacity of the lungs for carbon monoxide (DLco) decline. The adjusted meta-analysis showed non-significant association between total PM 2.5 (HR: −0.05; 95% CI −0.31 to 0.21; p = 0.70), but significant association with its constituents SO42 (β: −2.12; 95% CI −3.93 to −0.30; p = 0.02), and NH4+ (β: −4.66; 95% CI −8.77 to −0.54; p = 0.03). In the study by Zheng et al. (66), they also found a positive link between increased PM2.5 levels and DLco decline in IPF patients. Specifically, for each interquartile range (IQR) increase of 2.2 μg/m3 in PM2.5, there was an additional 0.9% predicted/year (95% CI −1.6 to −0.3) accelerated decline in DLco.
For PM10, Johannson et al. (46) did not observe any association between higher cumulative mean exposures or maximum exposures to air pollution and a more rapid decline in FVC or FEV1 over a duration of up to 40 weeks. In contrast to this, Winterbottom et al. (48) demonstrated that for every 5 μg/m3 increase in PM10 concentration, there was an additional decline of 46 mL per year in FVC (95% CI, 12–81 mL/y; p = 0.008). Neither NO2 (46, 66) nor O3 (46, 49), were found to be associated with a more rapid decline in lung function.
Among the five studies, two studies (6,708 participants) examined the association between air pollutants and baseline lung function (46, 58). Both studies identified a correlation between elevated air pollution levels and lower baseline lung functions. Johannson et al. (46) observed that increased mean levels of NO2(p = 0.03), PM2.5(p = 0.02), and PM10(p = 0.003) over the study period were significantly linked to reduced FVC % predicted. Goobie et al. (46) found that an increase in PM2.5 and its constituent mixture by one quantile was associated with decreased baseline FVC, specifically, higher MC is associated with a significant 3.38% decrease in the estimated baseline percentage of FVC (β-3.38; 95% CI −4.88 −1.87; p < 0.001) and a 3.64% decrease in the estimated baseline percentage of DLCO (β −3.64; 95% CI −4.61 to −2.66; p < 0.001).
3.4.5 Air pollution and incidence of ILD
Three studies, which collectively involved nearly 10 million inhabitants from Italy (45), 379 IPF participants from Spain (54), and 433,738 participants from a biobank in the UK (61), investigated the connection between air pollution exposure and the incidence of IPF. They showed that NO2, PM2.5, and NOx were linked to an increased incidence of IPF. In the Italian study (45), the authors demonstrated that an increase of 10 μg/m3 NO2, was associated with an increase in the IPF incidence rate from 6.39% (95% CI −3.62–17.45) to 7.55% (95% CI −0.76–16.56%), depending on the season. In the UK study (61), the adjusted hazard ratios for IPF were aHR 1.11 (95% CI 1.03–1.19; p = 0.006) for NO2, aHR 1.07 (95% CI 1.01–1.13; p = 0.02) for NOx, and aHR 1.09 (95% CI 1.02–1.17, p = 0.01) for PM2.5, for each interquartile rise in these pollutants. Shull et al. (54) underscored the positive link between increased incidence of ILD and PM2.5, revealing that the highest PM2.5 concentrations, ranging from 20 to 24.6 μg/m3, were in areas of traffic congestion, industrial zones, the airport, and the ports of Barcelona. These areas, rather than the most densely populated ones, exhibited a higher aggregation of IPF cases. No significant association was discovered between the incidence of IPF and the concentrations of PM10 (45, 61) or O3 (45).
Two studies (4,883 participants) investigated the association between ambient air pollution and the likelihood of subclinical ILD or ILA (44, 50). Both studies observed a positive connection between NOx and elemental carbon (EC) exposure and the occurrence of ILD or ILA. Sack et al. (44) showed that for each 40-ppb increase in NOx concentration, the OR for ILD was 1.77 (95% CI 1.06–2.95, p = 0.03). In a separate study (50), an IQR difference in 5-year EC exposure of 0.14 μg/m3 was associated with an OR of 1.27 (95% CI 1.04–1.55, p = 0.02) for ILA. The same study also noted an OR of 1.33 (CI 1.00 to 1.76, p = 0.05) for the progression of ILA. Neither study showed an association between exposure to PM2.5 and ozone (O3) and the presence of ILA and Sack et al. study (44) showed no association between ILA incidence and NO2 exposure.
One case–control study (52) involving 2,525 participants examined the link between air pollution exposure and the development of ILD in patients with a variety of CTD diagnoses. The study revealed that individuals with CTD-ILD had somewhat lower average exposure to PM2.5, PM10, and SO2 when compared to patients with non-ILD CTD. However, these differences were not statistically significant. For every 10-ppb increase in O3 exposure, there was a decrease in the risk of developing ILD (aOR 0.51; 95% CI 0.33–0.79; p = 0.002).
3.4.6 Air pollution and hospitalisation (IPF and RA-ILD)
Three studies (51, 59, 63) involving a total of 16,032 participants explored the impact of various air pollutants, namely NO2, O3, PM2.5, CO, SO2, NOX, on hospitalisation incidence in patients with IPF. In all three studies, the investigation focused on the exposure to NO2 and O3, and consistently revealed a positive correlation between the mean concentration of NO2, whether it was from short-term or long-term exposure, and the incidence of hospitalization. Nevertheless, the findings regarding O3 were inconclusive. Mariscal-Aguilar et al. (63) found an association between elevated mean O3 levels during the month preceding admission and a heightened rate of hospital admissions. In contrast, Dale et al. (51) did not observe a statistically significant association in their two-pollutant model. Liang et al. (59) showed a positive association with short-term increased level of O3 exposure in men and hospital admissions (RR: 1.045; 95% CI 1.000–1.092; p = 0.05) per IQR increase of 85 μg/m3, but not with average O3 exposure over the 30 days period before admission.
Two of these studies also investigated PM2.5, Dale et al. (51) found a positive association (RR 1.29, 95% CI 1.09–1.54, p = 0.003) between short-term elevated average level of PM2.5 (for an IQR Increase) and an increased incidence of hospitalisation. Liang et al. (59) also observed a positive association with short-term PM2.5 exposure (RR: 1.049, 95% CI 1.024–1.074, p = 0.0001) for lag0 and RR 1.031 (95% CI 1.007–1.056; p = 0.01) for a moving average 0-1 days, both per IQR of 72 μg/m3. However, this association was not evident for average PM2.5 exposure over 0–30 days. PM10 level was found to be one of the strongest independent pollutants associated with increased hospitalisation (51) (RR 1.31; 95% CI 1.12–1.53; p < 0.001), but less statistically significant in Liang et al.’s study (per 86 μg/m3: RR 1.021, 95% CI 0.994–1.049, p = 0.13) (59).
CO exposure was initially found to have a positive association with hospitalisation (51), but this association was lost when using two-pollutant models. In contrast, Mariscal-Aguilar et al. (63) did not find any association between incidence of hospitalisation and CO levels. Regarding SO2 exposure, Dale et al. (51) initially demonstrated a positive correlation between elevated SO2 concentration and hospitalization during univariable analysis, with a relative risk (RR) of 1.20 (95% CI 1.07–1.34, p < 0.05), but this relationship lost significance when two-pollutant models were applied. Liang et al. (59) identified an association between short-term SO2 exposure and hospitalization in men. Elevated exposure to NOX concentrations, as examined by Mariscal-Aguilar et al. (63), was significantly linked to the incidence of hospitalization in IPF patients for respiratory causes, particularly with exposures spanning 6, 12, and 36 months.
A single study focusing on RA-ILD patients (221 participants) (60), found that per 10 μg/m3 increase in PM2.5, PM10, SO2, and NO2 were each significantly associated with increased monthly hospitalisation admissions. The study showed both higher mean and cumulative concentrations of O3, for every 10 mg/m3 increase in the 0–2-month lag, displayed an inverse relationship (Excess risk −0.304 to −0.393; and − 3.383 to −0.406) implying that higher levels of O3 were linked to lower rates of hospitalisations for RA-ILD.
3.4.7 Air pollution and supplemental oxygen requirement
One study (135 patients with IPF) (48) examined the link between air pollution and supplemental oxygen requirement during the 6-min walk test (6MWT) in IPF patients. The findings revealed an association between higher mean level of PM2.5 and an increase in oxygen requirement to maintain saturation > =88% during the 6MWT, with each 5 μg/m3 increase in PM2.5 exposure corresponding to a 1.15 (95% CI, 0.03–2.26; p = 0.044) increase in O2 requirement.
3.4.8 Air pollution and development of chronic respiratory failure in IPF
In a study involving 69 individuals with IPF (63), researchers examined the relationship between air pollution and the occurrence of chronic respiratory failure, as defined by baseline arterial blood gases (ABG), measured while they were breathing room air, with a partial pressure of oxygen (PO2) below 60 mmHg (63). Elevated exposure to CO, NO2, and NOx were found to be significantly associated with a higher risk of developing chronic respiratory failure. Specifically, for every 0.1 mg/m3 increase in CO, the OR was 1.62 (1.11–2.36) (p = 0.01) over a 3-month period and 1.84 (1.1–3.06) during the 6 months leading up to the event. For each 10 μg/m3 increase in NO2 and NOx: the OR for NO2 was 1.65 (1.01–2.66) (p = 0.04) at 6 month prior to the event. For NOx, the OR was 1.12 (1.01–1.23) (p = 0.03) at 3 months and 1.20 (1.03–1.38) (p = 0.01) at 6 months of exposure before the event.
3.4.9 Air pollution and questionnaire outcomes in fibrotic sarcoidosis patients
In a study involving 16 participants with fibrotic sarcoidosis (49), correlations between short-term air pollution exposure and health outcomes were evaluated through validated health related QoL questionnaires. With each IQR increase in the 14-day average of PM2.5 levels, a decrease in general and lung-specific health status was observed as measured by the King’s Sarcoidosis Questionnaire (KSQ) (General: −6.60, 95% CI −12.51 to −0.68,; Lung-specific: −6.91, 95% CI −12.73 to −1.09) The study did not find any association between short-term O3 exposure and respiratory symptoms or QoL metrics, using the Saint George Respiratory Questionnaire (SGRQ), Leicester cough questionnaire (LCQ), and KSQ.
3.4.10 Air pollution and surrogate markers
DNA methylation (DNAm) can be inherited and influenced by air pollution in adults (67, 68). Aberrant DNAm patterns lead to reduced DNA stability and changes in gene expression and mutations, which can contribute to health problems (67, 68). Goobie et al. examined 1,059 participants from two groups of patients with IPF: 313 from the Simmons cohort and 746 from the PFF cohort (62). They investigated the association between PM2.5 (and its constituent components) with global DNAm (%5 mC) in patients with IPF. This study found an association between monthly mean levels of PM2.5 and its constituents’ exposure and increased level in %5 mC (β = 0.02, 95%CI 0.0003–0.05, p = 0.047) over the 3-month period before sampling. The results in the 1-year period before sampling were consistent but did not reach statistical significance in both cohorts (Simmons cohort: β = 0.03, 95%CI −0.004 to 0.06, p = 0.09; PFF cohort: β = 0.03, 95%CI −0.007 to 0.06, p = 0.12). Increased levels of sulfate, nitrate, ammonium, and black carbon components were also linked to elevated %5 mC across multiple models.
One study of 118 participants with IPF (56) examined the relationship between prolonged exposure to air pollution and the presence of serum inflammatory markers. The study found that an increase of 10 μg/m3 in the mean O3 level from the previous year was positively correlated with the percentage change in IL-4 (an inflammatory mediator) among AE-IPF patients (p = 0.014). Conversely, PM2.5, PM10, and NO2 levels showed an inverse association with the percentage changes in IL-4 (p = 0.003, p = 0.003, p = 0.032) and osteopontin (p = 0.013, p = 0.013, p = 0.085) among the same group of patients.
4 Discussion
In this comprehensive systematic review, of the link between air pollution and ILD, including IPF we evaluated 24 studies which met the inclusion criteria. The pollutants under investigation include PM2.5, PM10, O3, NO2, CO, SO2, NOx, and NO. All of these pollutants were considered in the assessment of AE-IPF risk. The meta-analyses performed showing a significant association between AE-IPF and PM2.5, while the association between O3, NO2, PM10, and AE-IPF risk remained uncertain.
4.1 Particulate matter (PM2.5 and PM10)
Particulate matter, a blend of solid particles and liquid droplets present in the atmosphere, includes PM2.5 and PM10 (69). PM2.5 is characterized as fine inhalable particles, typically measuring 2.5 micrometres or less in diameter, while PM10 is described as inhalable particles, generally having diameters of 10 micrometres or less (69). PM2.5 arises as a result of sophisticated atmospheric interactions involving combustion and the conversion of gases into particles (70, 71). It originates predominantly from a range of sources, encompassing the combustion of coal, oil, gasoline, diesel, and wood, as well as high-temperature industrial operations including those conducted in smelters and steel mills (71). Additionally, vehicle exhaust emissions and the burning of biomass contribute significantly to the production of PM2.5 (71, 72). PM10 is produced through the fragmentation of larger solid particles, the presence of biological materials like pollen grains, and the dispersion of wind-blown dust from agricultural areas, soil surfaces, unpaved roads, and dust generated by traffic (70). Additionally, it can originate from non-combustible substances such as fly ash (70).
Particulate matter has the potential to lead to severe health issues. PM2.5 has the tendency to settle deep within the respiratory airways, potentially penetrating into the alveoli, in some cases, being absorbed into the bloodstream (69, 70). This makes it particularly challenging to eliminate from the body. PM10 comprises particles that are larger in size, yet they are still small enough to penetrate into the lungs (70). Elevated levels of both PM2.5 and PM10 have been associated with higher rates of all-cause mortality, cardiovascular mortality, respiratory mortality, and mortality due to lung cancer (73).
Most of the included studies in this analysis evaluated PM2.5and PM10. It is interesting that both PM2.5 and PM10 were associated with increased hospital admissions without apparent influence on the progression of IPF. One possible explanation for this finding lies in the temporal dynamics of PM2.5 and PM10 exposure. PM2.5 infiltrates alveoli (69, 70), while PM10 deposits in the upper respiratory tract (25), both capable of precipitating acute respiratory exacerbations necessitating hospitalization. However, it appears that these exposures may not have a lasting impact on the lung function decline (FVC%), which serves as an indicator of disease progression in the studies under consideration (47, 64, 66). This is consistent with the findings that there was no significant association between PM2.5 (46, 48, 49) or PM10 (46, 48, 66) with lung function decline.
4.2 Ozone (O3)
O3 can be categorized into two types: beneficial stratospheric ozone and detrimental ground-level O3 (74). Stratospheric ozone serves as a shield against the harmful ultraviolet radiation from the sun, protecting living organisms (74). Conversely, ground-level O3 has been linked to elevated all-cause mortality rates, respiratory mortality, hospital admissions for asthma-related issues, and visits to emergency rooms (73).
Ground-level O3 is not directly released into the atmosphere; rather, it forms through a complex sequence of reactions involving oxides of nitrogen (NOx) and volatile organic compounds (VOC) when exposed to heat and sunlight (70, 74). VOCs are compounds that characterized by their high vapor pressure, and their primary sources predominantly include emissions from industrial and agricultural activities (25). Additionally, VOCs can stem from sources such as wildfires, vegetation, animals, and vehicles (25). Due to the reaction mechanism with heat and sunlight, O3 tends to attain elevated, unhealthy levels primarily during hot, sunny days in urban settings (25, 74).
Our findings regarding O3 are varied. Interestingly, an inverse correlation was found between O3 concentration and hospitalization incidence in RA-ILD and the development of ILD in patients with CTDs (52). This interesting outcome warrants careful interpretation because the protective influence of O3 against ILD risk appears to be consistently observed solely in patients with systemic lupus erythematosus (SLE), rather than in those with other CTDs (52). Increased O3 levels necessitate a reaction mechanism involving heat and sunlight (25, 74). Given that photosensitivity is a well-established exacerbating factor for SLE (75), it implies that individuals with SLE may opt to stay indoors to avoid direct sunlight. This behaviour could potentially explain why heightened levels of O3 appear to correlate with a reduced risk of ILD among SLE patients, as they may, in fact, experience lower O3 exposure.
4.3 Nitrogen oxides (NOx), nitric oxide (No) and nitrogen dioxide (NO2)
NOx encompasses a collection of exceedingly reactive gases, consisting of varying proportions of nitrogen and oxygen, with the most common components being NO2, NO, and nitrous oxide (N2O) (76). The primary sources of NOx emissions encompass motor vehicles, electric utilities, and a diverse array of industrial, commercial, and residential activities involving fuel combustion (76).
This review encompassed an exploration of NOx in four distinct studies. Positive correlations were identified for all health outcomes under investigation. Remarkably, the findings indicated significant links between NOx and the occurrence of subclinical ILD (44), heightened mortality rates in individuals with IPF (63), an increased risk of experiencing AE-IPF and AE-IIPs (55), elevated occurrences of IPF (61), higher hospitalization rates among those with IPF (63), and a hastened progression towards chronic respiratory failure in this population (63). Although each health outcome was examined in separate studies, it’s noteworthy that NOx consistently showed a positive association with these risks. This recurring pattern strongly suggests that NOx may play a significant role in exacerbating these health issues in ILDs. This finding underscores the importance of further research and attention to the potential health impacts of NOx exposure and calls for comprehensive efforts to mitigate NOx emissions and protect public health.
Within industrial combustion processes, NO predominates, often surpassing NO2 in both quantity and concentration (76, 77). However, the majority of NO molecules eventually undergo oxidation, transforming into the more toxic NO2 gas (77). NO is harmful to human health and can result in eye and throat irritation, chest tightness, nausea, headaches, and a gradual decline in physical strength (76). NO was assessed in a single study, which revealed that an increased concentration of NO was significantly linked to an elevated risk of AE-IPF and AE-IIPs (55). Elevated levels of nitric oxide are recognized for their inflammatory characteristics (78), potentially fostering an inflammatory setting that could trigger exacerbation events in interstitial lung diseases. However, it is crucial to note that this finding originates from a solitary study, emphasizing the necessity for further investigations to validate and substantiate these results.
NO2 is a gas and strong oxidant (70). NO2, in conjunction with other NOx components, engages in chemical reactions with the previously mentioned VOCs in the air, leading to the formation of O3 (70, 79). Additionally, NO2 serves as a crucial precursor to other secondary pollutants, including organic, nitrate, and sulfate particles, which are measured as part of PM10 or PM2.5 particulate matter (70). NO2 predominantly enters the atmosphere through the combustion of fuel (79). It arises from the emissions produced by vehicles such as cars, trucks, and buses, as well as from power plants and off-road machinery (79). Elevated levels of NO2 are associated with increased rates of all-cause mortality, respiratory mortality, hospital admissions related to asthma, and visits to the emergency room (73). It’s interesting to note that NO2, which is a component of NOx, appears to have distinct effects on various health outcomes. Our findings found that NO2 exhibited a significant positive correlation with an increased incidence of hospitalization in IPF (51, 59, 63) and RA-ILD (60), a heightened risk of AE-IIPs (55), lower lung function (46), and the development of chronic respiratory failure (63). However, no associations were detected between NO2 and the severity at diagnosis and progression at 24 months of SSc-ILD (65), lung function decline in IPF (46), or the incidence of subclinical ILD/ILAs (44).
4.4 Carbon monoxide (CO)
CO is a toxic gas that is colourless, has no odour or taste (26). It is generated when carbonaceous fuels such as wood, gasoline, coal, natural gas, and kerosene undergo incomplete combustion (26). CO exhibits a greater affinity for binding with haemoglobin, potentially resulting in diminished oxygen transport to vital organs (70). Elevated levels of CO are linked to a rise in hospital admissions and visits to the emergency room attributable to ischemic heart disease (73).
Our review found that CO was positively associated with the development of chronic respiratory failure (63) and had mixed results in terms of its relationship with mortality in IPF (53, 63). The presence of a positive association with the onset of chronic respiratory failure suggests a potential mechanism wherein increased levels of CO in the ambient air may bind to haemoglobin and lead to a decrease in the PO2 (63).
4.5 Sulfur dioxide (SO2)
SO2 is an invisible gas that readily dissolves in water (70). Its primary source arises from the combustion of fossil fuels conducted by power plants and various industrial activities (70, 80). Smaller contributions to SO2 emissions come from natural occurrences such as volcanic activity, as well as vehicles, locomotives, ships, and heavy machinery utilizing high-sulfur content fuels (70, 80). Increased SO2 concentrations have been reported as contributing to heightened rates of all-cause mortality, hospital admissions for asthma-related issues, visits to emergency rooms, and respiratory mortality (73).
This review found a positive link between SO2 and hospitalization in RA-ILD (60). However, for hospitalization incidence in IPF patients, the findings were mixed (51, 59). No significant associations were identified between SO2 and mortality in IPF (53), the risk of AE-IPF (43, 55), or the risk of AE-IIPs (55). The diversity in these findings may stem from varying geographical locations and levels of exposure. Two studies showing a positive association both reported a higher mean SO2 concentration than the other four studies, with a mean SO2 concentration of 28.15 ± 19.08 μg/m3 (60) and 23.6 μg/m3 (51).
4.6 Limitations
In this review we included a substantial number of participants. Nevertheless, it’s imperative to acknowledge certain limitations. Firstly, the studies encompassed in this review only spanned 13 countries or regions, which represents a relatively small portion of the global population. Secondly, a limited number of studies examined each specific health outcome, potentially not providing a comprehensive reflection of the true outcomes. It’s crucial to be caution when interpreting the meta-analysis results concerning the association between air pollutants and the risk of AE-IPF, primarily due to the significant heterogeneity observed, ranging from 67 to 88% across the studies, except for PM2.5(6%). Additionally, we acknowledge that potential confounding factors such as temperature, humidity, seasons, and occupational environments can impact the results. Socioeconomic status can also play a significant role in ILD incidence and outcomes. IPF patients with lower economic status experienced poorer survival outcomes, despite adjusting for occupational exposure and geographical origin (81).
Another significant constraint is that the various studies opted for different outcome measures, making it impossible to conduct a meta-analysis encompassing all the desired outcomes. Moreover, a notable limitation stems from the substantial variation in population sizes observed across studies, spanning from 16 participants to 10 million. Lastly, our study was not pre-registered with PROSPERO.
5 Conclusion
This comprehensive review highlights the need for further research efforts to gain a deeper understanding of the complex relationship between air pollution and ILDs. Future investigations should endeavour to incorporate real-time data collection methodologies, encompassing factors including the duration spent indoors and in traffic, which could provide valuable insights into individual exposure patterns. Additionally, there is a need for the standardization of the parameters being measured across various studies, ensuring consistency and comparability of findings. Moreover, employing multi-pollutant models in future research would be beneficial in resolving the complex interaction of various pollutants in affecting ILD outcomes.
The available data hints at the likely benefit for public health intervention. Specifically, there is compelling evidence to suggest that reducing levels of PM2.5 in the atmosphere could potentially lead to a reduction in the frequency and severity of acute exacerbations (AE) in individuals with ILDs. This finding bares significance, as AE in ILD patients has been consistently linked to high mortality rates, frequent hospitalizations, and elevated healthcare costs (82). The potential benefits from the interventions could not only improve individual health outcomes but also alleviate the burden on healthcare systems and resources.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft. CF: Supervision, Validation, Writing – review & editing. LT: Supervision, Writing – review & editing. LK: Conceptualization, Supervision, Writing – review & editing. TC: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Centre of Research Excellence in Pulmonary Fibrosis, which is funded by the NHMRC (GNT1116371 and GNT2015613), Lung Foundation Australia, anonymous philanthropy and Foundation partner Boehringer Ingelheim.
Acknowledgments
The authors acknowledge the technical assistance provided by the Sydney Informatics Hub, a Core Research Facility of the University of Sydney.
Conflict of interest
TC reports fees for work on scientific advisory boards for Boehringer Ingelheim, Roche, Bristol Myers Squibb, Vicore, and DevPro, grants from Boehringer Ingelheim, Roche, and Bristol Myers Squibb, Galapagos, Biogen, outside the submitted work. LT reports speaker’s fees for Boehringer Ingelheim, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1321038/full#supplementary-material
References
1.
Demedts M Wells AU Antó JM Costabel U Hubbard R Cullinan P et al . Interstitial lung diseases: an epidemiological overview. Eur Respir J. (2001) 18:2S–16S. doi: 10.1183/09031936.01.18s320002
2.
Ryerson CJ Collard HR . Update on the diagnosis and classification of ILD. Curr Opin Pulm Med. (2013) 19:453–9. doi: 10.1097/MCP.0b013e328363f48d
3.
Mari PV GJ M Richeldi L . Contemporary concise review 2018: interstitial lung disease. Respirology. (2019) 24:809–16. doi: 10.1111/resp.13572
4.
Wells AU Hirani N . Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and new Zealand and the Irish thoracic society. Thorax. (2008) 63:v1–v58. doi: 10.1136/thx.2008.101691
5.
Carvajalino S Reigada C Johnson MJ Dzingina M Bajwah S . Symptom prevalence of patients with fibrotic interstitial lung disease: a systematic literature review. BMC Pulm Med. (2018) 18:78. doi: 10.1186/s12890-018-0651-3
6.
Senanayake S Harrison K Lewis M McNarry M Hudson J . Patients’ experiences of coping with idiopathic pulmonary fibrosis and their recommendations for its clinical management. PLoS One. (2018) 13:e0197660. doi: 10.1371/journal.pone.0197660
7.
Kreuter M Swigris J Pittrow D Geier S Klotsche J Prasse A et al . Health related quality of life in patients with idiopathic pulmonary fibrosis in clinical practice: insights-IPF registry. Respir Res. (2017) 18:139. doi: 10.1186/s12931-017-0621-y
8.
Akhtar AA Ali MA Smith RP . Depression in patients with idiopathic pulmonary fibrosis. Chron Respir Dis. (2013) 10:127–33. doi: 10.1177/1479972313493098
9.
Behr J Kreuter M Hoeper MM Wirtz H Klotsche J Koschel D et al . Management of patients with idiopathic pulmonary fibrosis in clinical practice: the INSIGHTS-IPF registry. Eur Respir J. (2015) 46:186–96. doi: 10.1183/09031936.00217614
10.
Kreuter M Ehlers-Tenenbaum S Palmowski K Bruhwyler J Oltmanns U Muley T et al . Impact of comorbidities on mortality in patients with idiopathic pulmonary fibrosis. PLoS One. (2016) 11:e0151425. doi: 10.1371/journal.pone.0151425
11.
Choi WI Park SH Dauti S Park BJ Lee CW . Interstitial lung disease and risk of mortality: 11-year nationwide population-based study. Int J Tuberc Lung Dis. (2018) 22:100–5. doi: 10.5588/ijtld.17.0167
12.
Choi WI Park SH Park BJ Lee CW . Interstitial lung disease and lung Cancer development: a 5-year Nationwide population-based study. Cancer Res Treat. (2018) 50:374–81. doi: 10.4143/crt.2017.119
13.
Khor YH Ng Y Barnes H Goh NSL McDonald CF Holland AE . Prognosis of idiopathic pulmonary fibrosis without anti-fibrotic therapy: a systematic review. Eur Respir Rev. (2020) 29:190158. doi: 10.1183/16000617.0158-2019
14.
López-Ramírez C Suarez Valdivia L Rodríguez Portal JA . Causes of pulmonary fibrosis in the elderly. Med Sci (Basel). (2018) 6:58. doi: 10.3390/medsci6030058
15.
Choi W-I Dauti S Kim HJ Park SH Park JS Lee CW . Risk factors for interstitial lung disease: a 9-year Nationwide population-based study. BMC Pulm Med. (2018) 18:96. doi: 10.1186/s12890-018-0660-2
16.
Lederer DJ Enright PL Kawut SM Hoffman EA Hunninghake G van Beek EJ et al . Cigarette smoking is associated with subclinical parenchymal lung disease: the multi-ethnic study of atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. (2009) 180:407–14. doi: 10.1164/rccm.200812-1966OC
17.
Alarcon-Calderon A Vassallo R Yi ES Ryu JH . Smoking-related interstitial lung diseases. Immunol Allergy Clin N Am. (2023) 43:273–87. doi: 10.1016/j.iac.2023.01.007
18.
Trethewey SP Walters GI . The role of occupational and environmental exposures in the pathogenesis of idiopathic pulmonary fibrosis: a narrative literature review. Medicina (Kaunas). (2018) 54:108. doi: 10.3390/medicina54060108
19.
Furukawa H Oka S Shimada K Tsuchiya N Tohma S . Genetics of interstitial lung disease: Vol de Nuit (night flight). Clin Med Insights Circ Respir Pulm Med. (2015) 9s1:CCRPM.S23283. doi: 10.4137/CCRPM.S23283
20.
Camus P Kudoh S Ebina M . Interstitial lung disease associated with drug therapy. Br J Cancer. (2004) 91:S18–23. doi: 10.1038/sj.bjc.6602063
21.
Azadeh N Limper AH Carmona EM Ryu JH . The role of infection in interstitial lung diseases: a review. Chest. (2017) 152:842–52. doi: 10.1016/j.chest.2017.03.033
22.
Khalil N Churg A Muller N O'Connor R . Environmental, inhaled and ingested causes of pulmonary fibrosis. Toxicol Pathol. (2007) 35:86–96. doi: 10.1080/01926230601064787
23.
Marino E Caruso M Campagna D Polosa R . Impact of air quality on lung health: myth or reality?Ther Adv Chronic Dis. (2015) 6:286–98. doi: 10.1177/2040622315587256
24.
Kurt OK Zhang J Pinkerton KE . Pulmonary health effects of air pollution. Curr Opin Pulm Med. (2016) 22:138–43. doi: 10.1097/MCP.0000000000000248
25.
Bălă G-P Râjnoveanu R-M Tudorache E Motișan R Oancea C . Air pollution exposure-the (in)visible risk factor for respiratory diseases. Environ Sci Pollut Res Int. (2021) 28:19615–28. doi: 10.1007/s11356-021-13208-x
26.
WHO global air quality guidelines . Particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. Geneva: World Health Organization (2021) Licence: CC BY-NC-SA 3.0 IGO.
27.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
28.
Moola S Munn Z Sears K Sfetcu R Currie M Lisy K et al . Conducting systematic reviews of association (etiology): the Joanna Briggs Institute's approach. Int J Evid Based Healthc. (2015) 13:163–9. doi: 10.1097/XEB.0000000000000064
29.
Wohlin C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering; London, England, United Kingdom: Association for Computing Machinery; (2014). p. Article 38.
30.
Boutron I PM Higgins JPT Altman DG Lundh A Hróbjartsson A . Chapter 7: considering bias and conflicts of interest among the included studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022) Cochrane, (2022). Available at: www.training.cochrane.org/handbook
31.
Wells GS B O’connell D Peterson J Welch V Losos M Tugwell P . The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2021) [updated 2021; cited 2023 Jun 22]. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
32.
Stanhope J Weinstein P . Critical appraisal in ecology: what tools are available, and what is being used in systematic reviews?Res Synth Methods. (2023) 14:342–56. doi: 10.1002/jrsm.1609
33.
Deeks JJ Higgins JPT Altman DG . Analysing data and undertaking meta-analyses. Chichester, UK: John Wiley & Sons, Ltd; (2019). p. 241–284.
34.
Schwingshackl L Hoffmann G . Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med (Malden, MA). (2015) 4:1933–47. doi: 10.1002/cam4.539
35.
Higgins JPT Thompson SG Deeks JJ Altman DG . Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
36.
Roeser A Sese L Chassagnon G Chaigne B Tran Ba S Jebri S et al . Impact of air pollution on systemic sclerosis associated interstitial lung disease severity: a retrospective two-center cohort study. Eur Respir J. (2022) 60:1513. doi: 10.1183/13993003.congress-2022.1513
37.
Aguilar PM Carrera LG Segura CC De Lucas EZ Sanchez MH Hernan GB et al . Association between mortality of idiopathic pulmonary fibrosis and air pollution levels in Madrid. Eur Respir J. (2021) 58:PA3761. doi: 10.1183/13993003.congress-2021.PA3761
38.
Aguilar PM Carrera LG De Lucas EZ Segura CC Sanchez MH Hernan GB et al . Association between severity of idiopathic pulmonary fibrosis and air pollution levels in Madrid. Eur Respir J. (2021) 58:PA3760. doi: 10.1183/13993003.congress-2021.PA3760
39.
Abramson MJ Walters EH . Mapping air pollution and idiopathic pulmonary fibrosis. Respirology. (2021) 26:292–3. doi: 10.1111/resp.14004
40.
Sesé L Annesi-Maesano I Nunes H . Impact of particulate matter on the natural history of IPF: a matter of concentrations?Chest. (2018) 154:726–7. doi: 10.1016/j.chest.2018.05.043
41.
Chen HH Chao WC Chen YH Chen DY Lin CH . Air pollutants and development of interstitial lung disease in patients with autoimmune disease. Ann Rheum Dis. (2020) 79:865.1–865.86865. doi: 10.1136/annrheumdis-2020-eular.204
42.
Singh S Collins BF Bairwa M Joshi JM Talwar D Singh N et al . Hypersensitivity pneumonitis and its correlation with ambient air pollution in urban India. Eur Respir J. (2019) 53:1801563. doi: 10.1183/13993003.01563-2018
43.
Johannson KA Vittinghoff E Lee K Balmes JR Ji W Kaplan GG et al . Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J. (2014) 43:1124–31. doi: 10.1183/09031936.00122213
44.
Sack C Vedal S Sheppard L Raghu G Barr RG Podolanczuk A et al . Air pollution and subclinical interstitial lung disease: the multi-ethnic study of atherosclerosis (MESA) air-lung study. Eur Respir J. (2017) 50:1700559. doi: 10.1183/13993003.00559-2017
45.
Conti S Harari S Caminati A Zanobetti A Schwartz JD Bertazzi PA et al . The association between air pollution and the incidence of idiopathic pulmonary fibrosis in northern Italy. Eur Respir J. (2018) 51:1700397. doi: 10.1183/13993003.00397-2017
46.
Johannson KA Vittinghoff E Morisset J Wolters PJ Noth EM Balmes JR et al . Air pollution exposure is associated with lower lung function, but not changes in lung function, in Patients With Idiopathic Pulmonary Fibrosis. CHEST. (2018) 154:119–25. doi: 10.1016/j.chest.2018.01.015
47.
Sesé L Nunes H Cottin V Sanyal S Didier M Carton Z et al . Role of atmospheric pollution on the natural history of idiopathic pulmonary fibrosis. Thorax. (2018) 73:145–50. doi: 10.1136/thoraxjnl-2017-209967
48.
Winterbottom CJ Shah RJ Patterson KC Kreider ME Jr Panettieri RA Rivera-Lebron B et al . Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest. (2018) 153:1221–8. doi: 10.1016/j.chest.2017.07.034
49.
Pirozzi CS Mendoza DL Xu Y Zhang Y Scholand MB Baughman RP . Short-term particulate air pollution exposure is associated with increased severity of respiratory and quality of life symptoms in patients with fibrotic sarcoidosis. Int J Environ Res Public Health. (2018) 15:1077. doi: 10.3390/ijerph15061077
50.
Rice MB Li W Schwartz J Di Q Kloog I Koutrakis P et al . Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: the Framingham heart study. Thorax. (2019) 74:1063–9. doi: 10.1136/thoraxjnl-2018-212877
51.
Dales R Blanco-Vidal C Cakmak S . The association between air pollution and hospitalization of patients with idiopathic pulmonary fibrosis in Chile: a daily time series analysis. Chest. (2020) 158:630–6. doi: 10.1016/j.chest.2020.02.017
52.
Chen H-H Yong Y-M Lin C-H Chen Y-H Chen D-Y Ying J-C et al . Air pollutants and development of interstitial lung disease in patients with connective tissue disease: a population-based case–control study in Taiwan. BMJ Open. (2020) 10:e041405. doi: 10.1136/bmjopen-2020-041405
53.
Mariscal-Aguilar P Carrera LG Segura CC Sánchez MIT Peña MF-V Hernán GB et al . Relationship between air pollution levels in Madrid and the natural history of idiopathic pulmonary fibrosis: severity and mortality. J Int Med Res. (2021) 49:3000605211029058. doi: 10.1177/03000605211029058
54.
Shull JG Pay MT Lara Compte C Olid M Bermudo G Portillo K et al . Mapping IPF helps identify geographic regions at higher risk for disease development and potential triggers. Respirology (Carlton, Vic). (2021) 26:352–9. doi: 10.1111/resp.13973
55.
Tahara M Fujino Y Yamasaki K Oda K Kido T Sakamoto N et al . Exposure to PM2.5 is a risk factor for acute exacerbation of surgically diagnosed idiopathic pulmonary fibrosis: a case-control study. Respir Res. (2021) 22:80. doi: 10.1186/s12931-021-01671-6
56.
Tomos I Dimakopoulou K Manali ED Papiris SA Karakatsani A . Long-term personal air pollution exposure and risk for acute exacerbation of idiopathic pulmonary fibrosis. Environ Health. (2021) 20:99. doi: 10.1186/s12940-021-00786-z
57.
Yoon H-Y Kim S-Y Kim O-J Song JW . Nitrogen dioxide increases the risk of mortality in idiopathic pulmonary fibrosis. Eur Respir J. (2021) 57:2001877. doi: 10.1183/13993003.01877-2020
58.
Goobie GC Carlsten C Johannson KA Khalil N Marcoux V Assayag D et al . Association of Particulate Matter Exposure with Lung Function and Mortality among Patients with Fibrotic Interstitial Lung Disease. JAMA Intern Med. (2022) 182:1248–59. doi: 10.1001/jamainternmed.2022.4696
59.
Liang L Cai Y Lyu B Zhang D Chu S Jing H et al . Air pollution and hospitalization of patients with idiopathic pulmonary fibrosis in Beijing: a time-series study. Respir Res. (2022) 23:81. doi: 10.1186/s12931-022-01998-8
60.
Liu B Sun G Liu Y Hou Y Tao J . Observational studies: ambient air pollution and hospitalization for RA-ILD in a heavily polluted city in China. Medicine. (2022) 101:e29309. doi: 10.1097/MD.0000000000029309
61.
Cui F Sun Y Xie J Li D Wu M Song L et al . Air pollutants, genetic susceptibility and risk of incident idiopathic pulmonary fibrosis. Eur Respir J. (2023) 61:2200777. doi: 10.1183/13993003.00777-2022
62.
Goobie GC Li X Ryerson CJ Carlsten C Johannson KA Fabisiak JP et al . PM2.5 and constituent component impacts on global DNA methylation in patients with idiopathic pulmonary fibrosis. Environ Pollut. (2023) 318:120942. doi: 10.1016/j.envpol.2022.120942
63.
Mariscal-Aguilar P Gómez-Carrera L Carpio C Zamarrón E Bonilla G Fernández-Velilla M et al . Relationship between air pollution exposure and the progression of idiopathic pulmonary fibrosis in Madrid: chronic respiratory failure, hospitalizations, and mortality. A retrospective study. Front Public Health. (2023) 11:1135162. doi: 10.3389/fpubh.2023.1135162
64.
Yoon HY Kim SY Kim OJ Song JW . Nitrogen dioxide increases the risk of disease progression in idiopathic pulmonary fibrosis. Respirology. (2023) 28:254–61. doi: 10.1111/resp.14373
65.
Roeser A Sese L Chassagnon G Chaigne B Dunogue B Tran Ba S et al . The association between air pollution and the severity at diagnosis and progression of systemic sclerosis-associated interstitial lung disease: results from the retrospective ScleroPol study. Respir Res. (2023) 24:151. doi: 10.1186/s12931-023-02463-w
66.
Zheng Q Cox IA Leigh L de Graaff B Johnston FH Corte TJ et al . Long-term exposure to low concentrations of air pollution and decline in lung function in people with idiopathic pulmonary fibrosis: evidence from Australia. Respirology (Carlton, VIC). (2023) 28:916–24. doi: 10.1111/resp.14552
67.
Wu Y Qie R Cheng M Zeng Y Huang S Guo C et al . Air pollution and DNA methylation in adults: a systematic review and meta-analysis of observational studies. Environ Pollut. (2021) 284:117152. doi: 10.1016/j.envpol.2021.117152
68.
Jiang C-L He S-W Zhang Y-D Duan H-X Huang T Huang Y-C et al . Air pollution and DNA methylation alterations in lung cancer: a systematic and comparative study. Oncotarget. (2016) 8:1369–91. doi: 10.18632/oncotarget.13622
69.
US EPA . Particulate matter (PM) pollution. Washington (DC): United States Environmental Protection Agency; (2023) [updated 2023 Jul 11; cited 2023 Sep 17]. Available at: https://www.epa.gov/pm-pollution/particulate-matter-pm-basics#PM.
70.
WHO . Air quality guidelines global update 2005: particulate matter, ozone, nitrogen dioxide, and sulfur dioxide. Copenhagen, Denmark: World Health Organization (2006).
71.
Pui DYH Chen S-C Zuo Z . PM2.5 in China: measurements, sources, visibility and health effects, and mitigation. Particuology. (2014) 13:1–26. doi: 10.1016/j.partic.2013.11.001
72.
de Kok TMCM Driece HAL Hogervorst JGF Briedé JJ . Toxicological assessment of ambient and traffic-related particulate matter: a review of recent studies. Mutat Res. (2006) 613:103–22. doi: 10.1016/j.mrrev.2006.07.001
73.
Goshua A Akdis CA Nadeau KC . World Health Organization global air quality guideline recommendations: executive summary. Allergy (Copenhagen). (2022) 77:1955–60. doi: 10.1111/all.15224
74.
US EPA . Ground-level ozone pollution. Washington (DC): United States Environmental Protection Agency; (2023) [updated 2023 Jue 02; cited 2023 Sep 17]. Available at: https://www.epa.gov/ground-level-ozone-pollution/ground-level-ozone-basics#effects.
75.
Scheinfeld N Deleo VA . Photosensitivity in lupus erythematosus. Photodermatol Photoimmunol Photomed. (2004) 20:272–9. doi: 10.1111/j.1600-0781.2004.00094.x
76.
Baukal C . Everything you need to know about NOx. Met Finish. (2005) 103:18–24. doi: 10.1016/S0026-0576(05)80816-5
77.
Panigrahi TH Sahoo SR Murmu G Maity D Saha S . Current challenges and developments of inorganic/organic materials for the abatement of toxic nitrogen oxides (NOx) – a critical review. Prog Solid State Chem. (2022) 68:100380. doi: 10.1016/j.progsolidstchem.2022.100380
78.
Guzik TJ Korbut R Adamek-Guzik T . Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. (2003) 54:469–87. PMID:
79.
US EPA . Nitrogen Dioxide (NO2) Pollution. Washington (DC): United States Environmental Protection Agency; (2023). Available at: https://www.epa.gov/no2-pollution/basic-information-about-no2#What%20is%20NO2.
80.
US EPA . Sulfur Dioxide (SO2) Pollution. Washington (DC): United States Environmental Protection Agency; (2023). Available at: https://www.epa.gov/so2-pollution/sulfur-dioxide-basics#what%20is%20so2.
81.
Sesé L Caliez J Annesi-Maesano I Cottin V Pesce G Didier M et al . Low income and outcome in idiopathic pulmonary fibrosis: an association to uncover. Respir Med. (2021) 183:106415. doi: 10.1016/j.rmed.2021.106415
82.
Kolb M Bondue B Pesci A Miyazaki Y Song JW Bhatt NY et al . Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. (2018) 27:180071. doi: 10.1183/16000617.0071-2018
Summary
Keywords
air pollution, interstitial lung disease (ILD), idiopathic pulmonary fibrosis (IPF), ozone (O3), nitrogen dioxide (NO2), particulate matter (PM2.5, PM10), sulfur dioxide (SO2)
Citation
Lan D, Fermoyle CC, Troy LK, Knibbs LD and Corte TJ (2024) The impact of air pollution on interstitial lung disease: a systematic review and meta-analysis. Front. Med. 10:1321038. doi: 10.3389/fmed.2023.1321038
Received
13 October 2023
Accepted
27 December 2023
Published
17 January 2024
Volume
10 - 2023
Edited by
Dawei Yang, Fudan University, China
Reviewed by
Cathryn Lee, The University of Chicago, United States; Nicol Bernardinello, University of Padua, Italy
Updates
Copyright
© 2024 Lan, Fermoyle, Troy, Knibbs and Corte.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doris Lan, greenlan5@hotmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.