Abstract
Introduction:
Coronavirus disease 2019 (COVID-19) is a highly contagious viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has had a dramatic effect on the world, resulting in millions of deaths worldwide and causing drastic changes in daily life. A study reported that septic complications were associated with high mortality in COVID-19 patients. This study aimed to evaluate how the COVID-19 pandemic changed the pre-pandemic and post-pandemic prevalence of sepsis in ICUs and to evaluate the different risk factors associated with mortality and the different diffusion of microorganisms and their resistance.
Materials and methods:
We conducted a single-center retrospective observational clinical study, observing all patients in the ICU of the SS Annunziata Hospital in Chieti (Italy) who were diagnosed with sepsis and had a bacterial isolate from their blood culture. Sepsis was diagnosed by SEPSIIS III criteria. We enrolled all in-patients in the ICU from January 2018 to December 2021. We divided the patients into three groups: (1) non-pandemic period (Np) hospitalized in 2018–2019, (2) pandemic period (Pp)-COVID hospitalized in 2020–2021 with a diagnosis of COVID-19, and (3) Pp-non-COVID patients hospitalized in 2020–2021 without a diagnosis of COVID-19.
Results:
From January 2018 to December 2021, 1,559 patients were admitted to the ICU, of which 211 patients [36 (17.1%) in 2018, 52 (24.6%) in 2019, 73 (34.6%) in 2020, and 50 (23.7%) in 2021, respectively] met the selection criteria: 88 patients in period Np, 67 patients in Pp without COVID-19, and 56 patients Pp with COVID-19. The overall mortality of these patients was high (65.9% at 30 days in Np), but decreased during the Pp (60.9%): Pp-non-COVID was 56.7% vs. Pp-COVID 66.1%, with a statistically significant association with APACHE III score (OR 1.08, 95%CI 1.04–1.12, p < 0.001), SOFA score (OR 1.12, 95%CI 1.03–1.22, p = 0.004), and age (OR 1.04, 95%CI 1.02–1.07, p < 0.0001). Between the Np vs. Pp periods, we observed an increase in a few Gram-positive bacteria such as S. capitis (1 pt. −0.9% vs. 14 pt. −7.65%- p = 0.008), S. epidermidis, Streptococcus spp., and E. faecalis, as well as a decrease in a case of blood culture positive for S. aureus, S. hominis, and E. faecium. In Gram-negative bacteria, we observed an increase in cases of Acinetobacter spp. (Np 6 pt. −5.1%- vs. Pp 20 pt. −10.9%, p = 0.082), and Serratia spp., while cases of sepsis decreased from E. faecium (Np 11 pt. −9.4%- vs. Pp 7 pt. −3.8%, p = 0.047), and Enterobacter spp., S. haemolyticus, S. maltophilia, Proteus spp., and P. aeruginosa have not changed. Finally, we found that resistance to OXA-48 (p = 0.040), ESBL (p = 0.002), carbapenems (p = 0.050), and colistin (p = 0.003) decreased with time from Np to Pp, particularly in Pp-COVID.
Conclusion:
This study demonstrated how the COVID-19 pandemic changed the prevalence of sepsis in the ICU. It emerged that the risk factors associated with mortality were APACHE and SOFA scores, age, and, above all, the presence of ESBL-producing bacteria. Despite this, during the pandemic phase, we have observed a significant reduction in the emergence of resistant germs compared to the pre-pandemic phase.
Introduction
Coronavirus disease 2019 (COVID-19) is a contagious viral disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has had a dramatic effect on the world, causing almost 7 million deaths worldwide and changing daily life. COVID-19 has many reports concerning different clinical manifestations and different risk factors and biomarkers associated with the worsening (1–3). According to a report from the end of May 2020, 1.3 million cases were reported to the United States Centers for Disease Control and Prevention (CDC), with 14% requiring hospitalization, 2% admitted to the intensive care unit (ICU), and 5% dying. The individual risk of severe illness varies by age, underlying comorbidities, and vaccination status. Sepsis is one of the leading causes of death associated with SARS-CoV-2 infection, accounting for 65% (4).
A 2016 SCCM/ESICM task force has defined sepsis as life-threatening organ dysfunction caused by a dysregulated host response to infection (Sepsis-3) as evidenced by organ dysfunction and infection (5).
The Global Burden of Disease Study in 2017 reported an estimated 48.9 million incident cases of sepsis (6). Approximately 11 million deaths were reported, representing 19.7% of all global deaths. Overall mortality decreased by almost 53% between 1990 and 2017.
The importance of identifying risk factors for sepsis was highlighted in one epidemiologic study, which found that septic shock was the fifth leading cause of years of lost productive life due to premature mortality (6). Sepsis risk factors include ICU admission (approximately 50% of ICU patients have a nosocomial infection), advanced age (≥65 years), bacteremia, immunosuppression, diabetes and obesity, cancer, previous hospitalization, genetic factors, community-acquired pneumonia, and severe acute respiratory illness from SARS-CoV-2. COVID-19 can predispose people to sepsis from secondary infections (7–10).
A study reported that sepsis, occurring as a complication of COVID-19, was associated with high mortality in COVID-19 patients (11).
Ventilated COVID-19 patients often receive multiple antibiotic courses. At the height of the pandemic, antibiotic stewardship policies were overridden (12), and ICU capacity was increased. A Spanish hospital reported increased antibiotic use (13). Such data raise concerns that resistance in hospitals may increase as a result of COVID-19 pressures, notwithstanding a lack of evidence that this has occurred.
This study aimed to evaluate how the COVID-19 pandemic changed the pre-pandemic and post-pandemic prevalence of sepsis in ICUs and to evaluate the different risk factors associated with mortality and different diffusions of microorganisms and their resistance.
Methods
Study design, setting, and population
We carried out a single-center retrospective observational clinical study that observed all the patients in the ICU of the SS Annunziata Hospital in Chieti (Italy) who were diagnosed with sepsis and who presented a bacterial isolate from blood culture.
We enrolled all in-patients in the ICU from January 2018 to December 2021. We divided the patients into three groups: (1) non-pandemic period (Np) hospitalized in 2018–2019, (2) pandemic period (Pp)-COVID hospitalized in 2020–2021 with a diagnosis of COVID-19, and (3) Pp-non-COVID patients hospitalized in 2020–2021 without a diagnosis of COVID-19.
Inclusion criteria:
-
all patients admitted to intensive care for more than 48 h;
-
age over 18 years old; and
-
presence of two or more positive blood cultures in patients with clinical signs of active infection and sepsis.
Exclusion criteria:
-
admission to the ICU for ongoing sepsis;
-
the presence of only one positive blood culture kit; and
-
pregnant women.
The study protocol was approved by the Ethics Internal Committee at the University “G. d’Annunzio” Chieti-Pescara (Ethics Committee Project No. 02 02/02/2022) and was performed according to the ethical standards established in the 1964 Declaration of Helsinki.
Variables, data sources, and measurement
Demographic data such as age and gender, as well as the presence of comorbidities such as diabetes, active malignancies, chronic kidney disease (CKD), drug addiction, and immunodeficiency were analyzed. Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) score and Sequential Organ Failure Assessment (SOFA) score were calculated for all patients (14).
The days of hospitalization and the presence and type of the isolated germ were evaluated, with the characteristics of resistance and antibiotic therapy carried out empirically and after susceptibility testing.
Sepsis was diagnosed by SEPSIIS III criteria (15) and EUCAST guidelines.
Antimicrobial susceptibility testing
Every blood culture was placed in a BacT/ALERT® BPA (bioMérieux), which provided both a microbial detection system and a culture media with suitable nutritional and environmental conditions for organisms that might be present in the test sample. Inoculated bottles were incubated in the instrument and continuously monitored for the presence of microorganisms that would grow in the BacT/ALERT BPA bottles. The antimicrobial agents tested included ampicillin, amoxicillin/clavulanic acid, piperacillin/tazobactam, cefuroxime, cefotaxime, ceftriaxone, ceftazidime, cefepime, imipenem, ertapenem, gentamicin, amikacin, ciprofloxacin, levofloxacin, and trimethoprim/sulfamethoxazole. The results were interpreted by the EUCAST guidelines. Extended-spectrum beta-lactamase (ESBL) production was confirmed phenotypically using a combination disk test according to the EUCAST guidelines. Phenotypic screening for carbapenemase production in Enterobacteriaceae was performed using the Carba NP test. An antimicrobial sensitivity test was performed by Vitek 2 (bioMérieux).
Statistical analysis
Descriptive analysis was carried out using mean and standard deviation (±SD) or median and interquartile range (IQR) for the quantitative variables and percentage values for the qualitative ones. Normality distribution for quantitative variables was assessed by the Shapiro–Wilk test. The association between groups and explicative variables was investigated by Pearson χ2 test and Analysis of Variance (ANOVA) or analog test non-parametric Kruskal Wallis’s test followed by the appropriate post-hoc test if significant. Bonferroni’s correction for multiple comparison tests was applied. The prevalence of patients admitted for infection per year with 95% confidence intervals (CIs) was calculated. In addition, the occurrence of mortality per year was also estimated. Crude odds ratios (ORs) and corresponding 95% CI were calculated to quantify the risk associated with mortality as an explicative variable using the Wald test. Statistical significance was set at a level of ≤0.05, unless adjustments for multiple comparisons were required. All analyses were performed using Stata software v17.1 (StataCorp, College Station, Texas, United States).
Results
From January 2018 to December 2021, 1,559 patients were admitted to the ICU, and 211 patients satisfied the selection criteria [36 (17.1%) in 2018, 52 (24.6%) in 2019, 73 (34.6%) in 2020, and 50 (23.7%) in 2021, respectively]: 88 patients in period Np, 67 patients in Pp without COVID-19, and 56 patients in Pp with COVID-19 (Figure 1). The demographic characteristics of the overall study population and their comorbidities are reported in Table 1. Briefly, the patient’s median age was 70 (IQR 62–78) years, of which 65.6% were male. We found significant differences in the mean score of the SOFA score between the three groups (p = 0.028). Specifically, the SOFA score was higher in Pp-COVID 12.2 (±3.46) vs. Np 10.3 (±3.8) with p = 0.008.
Figure 1

Study flow chart.
Table 1
| Total | Np | Pp-non-COVID | Pp-COVID | ||
|---|---|---|---|---|---|
| N = 211 | N = 88 | N = 67 | N = 56 | p-value | |
| Sex, n(%) | |||||
| Female | 72 (34.1%) | 32 (36.4%) | 23 (34.3%) | 17 (30.4%) | 0.759 |
| Male | 139 (65.9%) | 56 (63.6%) | 44 (65.7%) | 39 (69.6%) | |
| Age, years | 70 (62–78) | 71 (60.5–80.5) | 70 (62–77) | 69 (64–75) | 0.845 |
| Comorbidity, n(%) | |||||
| No | 15 (7.1%) | 3 (3.4%) | 4 (6.0%) | 8 (14.3%) | 0.056 |
| Yes | 196 (92.9%) | 85 (96.6%) | 63 (94.0%) | 48 (85.7%) | |
| Diabetes mellitus, n(%) | |||||
| No | 163 (77.3%) | 64 (72.7%) | 53 (79.1%) | 46 (82.1%) | 0.563 |
| Yes | 48 (22.7%) | 24 (26.3%) | 14 (20.9%) | 10 (17.9%) | |
| Cancer | |||||
| No | 183 (86.7%) | 75 (85.2%) | 56 (83.6%) | 52 (92.9%) | 0.276 |
| Yes | 28 (13.3%) | 13 (14.8%) | 11 (16.4%) | 4 (7.1%) | |
| Acute renal failure, n(%) | |||||
| No | 172 (81.5%) | 69 (78.4%) | 54 (80.6%) | 49 (87.5%) | 0.381 |
| Yes | 39 (18.5%) | 19 (21.6%) | 13 (19.4%) | 7 (12.5%) | |
| Drug addiction, n(%) | |||||
| No | 203 (96.2%) | 82 (93.2%) | 65 (97.0%) | 56 (100.0%) | 0.103 |
| Yes | 8 (3.8%) | 6 (6.8%) | 2 (3.0%) | 0 (0.0%) | |
| HIV, n(%) | |||||
| No | 202 (95.7%) | 83 (94.3%) | 63 (94.0%) | 56 (100.0%) | 0.184 |
| Yes | 9 (4.3%) | 5 (5.7%) | 4 (6.0%) | 0 (0.0%) | |
| SOFA Score | 11.2 (3.7) | 10.6 (3.8)* | 11.2 (3.6) | 12.2 (3.4)* | 0.028 |
| APACHE Score | 25.9 (8.2) | 27.3 (8.9) | 25.1 (7.5) | 24.9 (7.6) | 0.122 |
| ICU, days | 18 (7–35) | 14 (3–37) | 19 (9–38) | 18 (10–31) | 0.293 |
Characteristics of sample.
N (%) mean and (sd) or median and interquartile range (IQR) are shown when appropriate. *p-value < α/3 for Bonferroni multiple testing correction vs. Np.
The APACHE score remained unchanged significantly throughout the observation period. In the Pp group, patients remained longer in the ICU for 19 (9–38) days compared to 14 (3–37) days in the Np group (p = 0. 293).
In the two study periods, with our strict criteria, Np and Pp sepsis showed an overall prevalence of 13.53%, with data of 9.33% in 2018, 13.40% in 2019, 18.02% in 2020, and 13.16% in 2021, respectively (Figure 2).
Figure 2
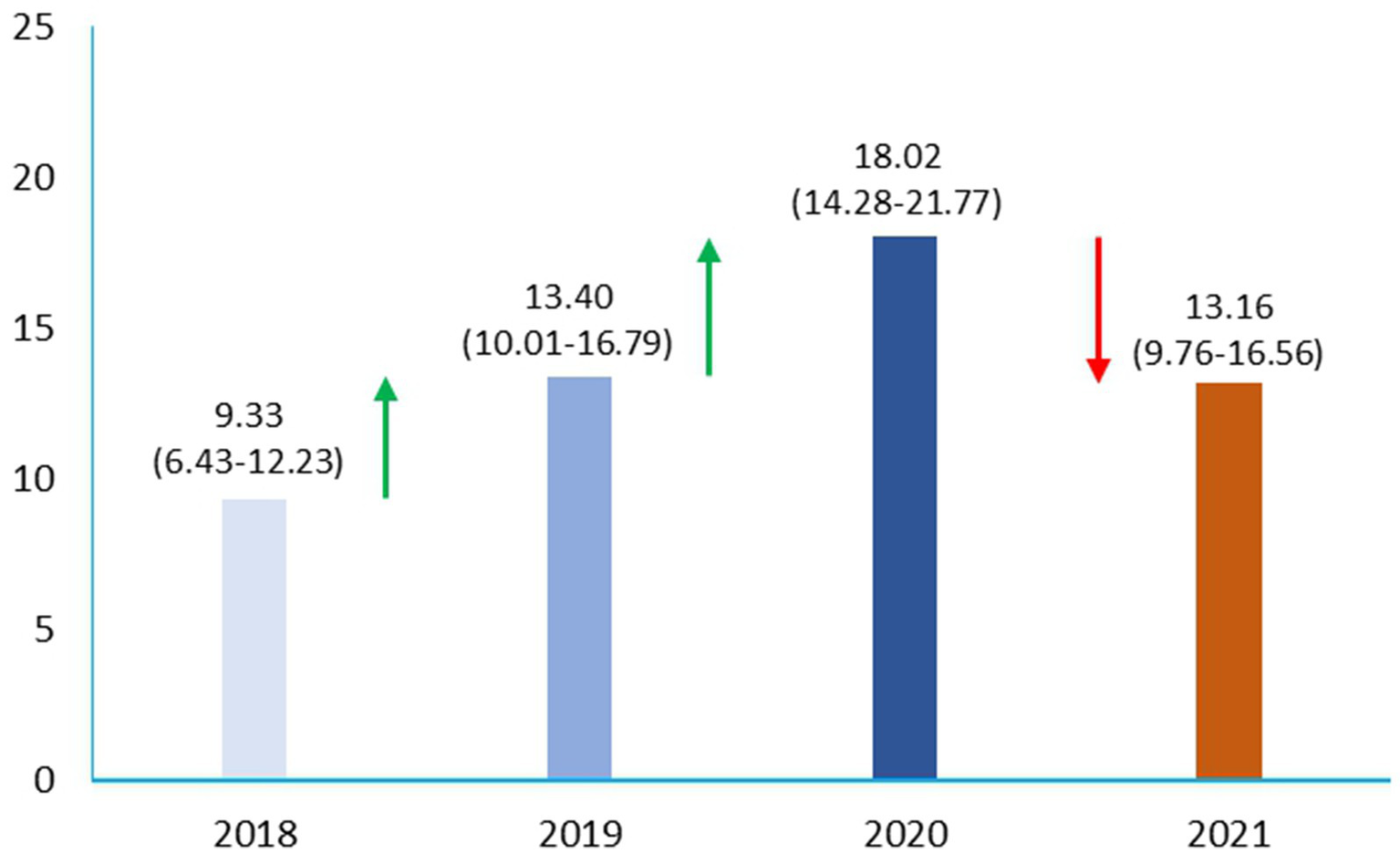
Prevalence of sepsis shock in pre-pandemic and pandemic era in ICU.
We found that among patients enrolled in the study, 135 (64%) had sepsis of medical origin and 76 (36%) had sepsis of surgical origin. However, the overall mortality rate for these patients was high, 65.9% in 30 days, but mortality decreased during the Pp to 60.9%: Pp-non-COVID was 56.7%, compared to Pp-COVID of 66.1%. Furthermore, in the overall population, ESBL microorganisms were associated with increased mortality (68% vs. 55%, with p = 0.028).
Crude OR indicates that the occurrence of mortality increased with the SOFA score (OR 1.12, 95%CI 1.03–1.22, p = 0.004), APACHE score (OR 1.08, 95%CI 1.04–1.12, p < 0.0001), and age (OR 0.98, 95%CI 0.97–0.99, p = 0.023; Table 2).
Table 2
| *ORc (95% CI) | P-value | |
|---|---|---|
| Sex | ||
| Female | 1 | |
| Male | 0.86 (0.47–1.57) | 0.631 |
| Groups | ||
| Np | 1 | |
| Pp-non-COVID | 0.49 (0.25–0.96) | 0.039 |
| Pp-COVID | 0.73 (0.35–1.50) | 0.396 |
| Age, years | 1.04 (1.02–1.07) | <0.0001 |
| Comorbidity | ||
| No | 1 | |
| Yes | 1.31 (0.44–3.84) | 0.619 |
| Diabetes mellitus | ||
| No | 1 | |
| Yes | 0.89 (0.45–1.77) | 0.757 |
| Cancer | ||
| No | 1 | |
| Yes | 0.65 (0.28–1.46) | 0.298 |
| Acute renal failure | ||
| No | 1 | |
| Yes | 1.92 (0.85–4.31) | 0.111 |
| Drug addiction | ||
| No | 1 | |
| Yes | 0.50 (0.12–2.07) | 0.343 |
| HIV | ||
| No | 1 | |
| Yes | 1.03 (0.25–4.27) | 0.959 |
| SOFA Score | 1.12 (1.03–1.22) | 0.004 |
| APACHE Score | 1.08 (1.04–1.12) | <0.0001 |
| ICU, days | 0.98 (0.97–0.99) | 0.023 |
Crude OR and 95% CI for identifying risk associated with mortality.
*ORc, crude odds ratio; 95% CI, 95% confidence interval.
Characteristics of the overall germs in the ICU
The blood cultures from CVC were positive on 23.1% in Np and an increase in the Pp-noCOVID compared with Pp-COVID 67.3% vs. 72.4% (p < 0.0001). In addition, we found that resistance to OXA-48 (p = 0.040), ESBL (p = 0.002), carbapenems (p = 0.050), and colistin (p = 0.003) decreased with time from Np to Pp, particularly in Pp-COVID (Table 3).
Table 3
| Total | Np | Pp-non-COVID | Pp-COVID | ||
|---|---|---|---|---|---|
| N = 300 | N = 117 | N = 107 | N = 76 | P-value | |
| S. aureus | |||||
| No | 274 (91.3%) | 105 (89.7%) | 95 (88.8%) | 74 (97.4%) | 0.093 |
| Yes | 26 (8.7%) | 12 (10.3%) | 12 (11.2%) | 2 (2.6%) | |
| S. hominis | |||||
| No | 290 (96.7%) | 112 (95.7%) | 104 (97.2%) | 74 (97.4%) | 0.848 |
| Yes | 10 (3.3%) | 5 (4.3%) | 3 (2.8%) | 2 (2.6%) | |
| S. capitis | |||||
| No | 285 (95.0%) | 116 (99.1%) | 102 (95.3%) | 67 (88.2%) | 0.003 |
| Yes | 15 (5.0%) | 1 (0.9%)* | 5 (4.7%) | 9 (11.8%) | |
| S. epidermidis | |||||
| No | 217 (72.3%) | 87 (74.4%) | 76 (71.0%) | 54 (71.1%) | 0.821 |
| Yes | 83 (27.7%) | 30 (25.6%) | 31 (29.0%) | 22 (28.9%) | |
| Streptococcus | |||||
| No | 294 (98.0%) | 117 (100.0%) | 104 (97.2%) | 73 (96.1%) | 0.075 |
| Yes | 6 (2.0%) | 0 (0.0%) | 3 (2.8%) | 3 (3.9%) | |
| E. faecalis | |||||
| No | 286 (95.3%) | 113 (96.6%) | 103 (96.3%) | 70 (92.1%) | 0.325 |
| Yes | 14 (4.7%) | 4 (3.4%) | 4 (3.7%) | 6 (7.9%) | |
| E. faecium | |||||
| No | 282 (94.0%) | 106 (90.6%) | 101 (94.4%) | 75 (98.7%) | 0.064 |
| Yes | 18 (6.0%) | 11 (9.4%) | 6 (5.6%) | 1 (1.3%) | |
| E. coli | |||||
| No | 274 (91.3%) | 102 (87.2%) | 101 (94.4%) | 71 (93.4%) | 0.130 |
| Yes | 26 (8.7%) | 15 (12.8%) | 6 (5.6%) | 5 (6.6%) | |
| K. pneumoniae | |||||
| No | 269 (89.7%) | 105 (89.7%) | 96 (89.7%) | 68 (89.5%) | 0.998 |
| Yes | 31 (10.3%) | 12 (10.3%) | 11 (10.3%) | 8 (10.5%) | |
| Proteus | |||||
| No | 298 (99.3%) | 116 (99.1%) | 106 (99.1%) | 76 (100.0%) | 0.709 |
| Yes | 2 (0.7%) | 1 (0.9%) | 1 (0.9%) | 0 (0.0%) | |
| Acinetobacter | |||||
| No | 274 (91.3%) | 111 (94.9%) | 97 (90.7%) | 66 (86.8%) | 0.146 |
| Yes | 26 (8.7%) | 6 (5.1%) | 10 (9.3%) | 10 (13.2%) | |
| S. maltophilia | |||||
| No | 296 (98.7%) | 115 (98.3%) | 106 (99.1%) | 75 (98.7%) | 0.880 |
| Yes | 4 (1.3%) | 2 (1.7%) | 1 (0.9%) | 1 (1.3%) | |
| P. aeruginosa | |||||
| No | 284 (94.7%) | 111 (94.9%) | 98 (91.6%) | 75 (98.7%) | 0.108 |
| Yes | 16 (5.3%) | 6 (5.1%) | 9 (8.4%) | 1 (1.3%) | |
| Enterobacter | |||||
| No | 290 (96.7%) | 112 (95.7%) | 105 (98.1%) | 73 (96.1%) | 0.656 |
| Yes | 10 (3.3%) | 5 (4.3%) | 2 (1.9%) | 3 (3.9%) | |
| S. haemolyticus | |||||
| No | 293 (97.7%) | 112 (95.7%) | 107 (100.0%) | 74 (97.4%) | 0.080 |
| Yes | 7 (2.3%) | 5 (4.3%) | 0 (0.0%) | 2 (2.6%) | |
| Serratia | |||||
| No | 294 (98.0%) | 115 (98.3%) | 104 (97.2%) | 75 (98.7%) | 0.770 |
| Yes | 6 (2.0%) | 2 (1.7%) | 3 (2.8%) | 1 (1.3%) | |
| Peripheral blood cultures | |||||
| No | 41 (13.7%) | 6 (5.1%) | 17 (15.9%) | 18 (23.7%) | 0.001 |
| Yes | 259 (86.3%) | 111 (94.9%) | 90 (84.1%)* | 58 (76.3%)* | |
| CVC blood cultures | |||||
| No | 146 (48.7%) | 90 (76.9%) | 35 (32.7%) | 21 (27.6%) | <0.0001 |
| Yes | 154 (51.3%) | 27 (23.1%) | 72 (67.3%)* | 55 (72.4%)* | |
| Oxa R | |||||
| No | 82 (27.3%) | 23 (19.7%) | 32 (29.9%) | 27 (35.5%) | 0.041 |
| Yes | 218 (72.7%) | 94 (80.3%) | 75 (70.1%)* | 49 (64.5%)* | |
| ESBL | |||||
| No | 111 (37.0%) | 30 (25.6%) | 43 (40.2%) | 38 (50.0%) | 0.002 |
| Yes | 189 (63.0%) | 87 (74.4%) | 64 (59.8%) | 38 (50.0%)* | |
| R Carbapenemi | |||||
| No | 133 (44.3%) | 42 (35.9%) | 51 (47.7%) | 40 (52.6%) | 0.050 |
| Yes | 167 (55.7%) | 75 (64.1%) | 56 (52.3%) | 36 (47.4%)* | |
| R Aminoglicosidi | |||||
| No | 162 (54.0%) | 61 (52.1%) | 58 (54.2%) | 43 (56.6%) | 0.832 |
| Yes | 138 (46.0%) | 56 (47.9%) | 49 (45.8%) | 33 (43.4%) | |
| R Glycopentide | |||||
| No | 149 (49.7%) | 50 (42.7%) | 59 (55.1%) | 40 (52.6%) | 0.150 |
| Yes | 151 (50.3%) | 67 (57.3%) | 48 (44.9%) | 36 (47.4%) | |
| R Daptomicina | |||||
| No | 170 (56.7%) | 61 (52.1%) | 64 (59.8%) | 45 (59.2%) | 0.447 |
| Yes | 130 (43.3%) | 56 (47.9%) | 43 (40.2%) | 31 (40.8%) | |
| R Fluorochinoloni | |||||
| No | 87 (29.0%) | 33 (28.2%) | 30 (28.0%) | 24 (31.6%) | 0.848 |
| Yes | 213 (71.0%) | 84 (71.8%) | 77 (72.0%) | 52 (68.4%) | |
| R Fosfomicina | |||||
| No | 236 (78.7%) | 93 (79.5%) | 80 (74.8%) | 63 (82.9%) | 0.401 |
| Yes | 64 (21.3%) | 24 (20.5%) | 27 (25.2%) | 13 (17.1%) | |
| R Colistina | |||||
| No | 132 (44.0%) | 42 (35.9%) | 44 (41.1%) | 46 (60.5%) | 0.003 |
| Yes | 168 (56.0%) | 75 (64.1%)* | 63 (58.9%)* | 30 (39.5%) | |
| R Linezolid | |||||
| No | 175 (58.3%) | 60 (51.3%) | 65 (60.7%) | 50 (65.8%) | 0.111 |
| Yes | 125 (41.7%) | 57 (48.7%) | 42 (39.3%) | 26 (34.2%) | |
Characteristics of the overall bacteria in ICU.
N (%) are shown when appropriate. p-value < α for Pearson’s chi-square test between couples: S. capitis (Np vs. Pp-COVID); peripheral blood cultures (Pp-non-COVID vs. Np and Pp-COVID vs. Np); CVC blood cultures (Pp-non-COVID vs. Np and Pp-COVID vs. Np); Oxa R (Pp-non-COVID vs. Np and Pp-COVID vs. Np); ESBL (Pp-COVID vs. Np); R Carbapenemi (Pp-COVID vs. Np); R Colistina (Np vs. Pp-COVID and Pp-non-COVID vs. Pp-COVID).
About germs, there was no significant difference in germ circulation between Gram-positive vs. Gram-negative among groups (Np 58.1%, Pp-noCOVID 59.8%, Pp-COVID 61.8% vs. Np 41.9%, Pp-noCOVID 40.2%, Pp-COVID 38.2%, p = 0.875, respectively). Between the Np vs. Pp period, we observed an increase in a few Gram-positive bacteria, such as S. capitis (1 patient 0.9% vs. 14 patients 7.65%, p = 0.008), S. epidermidis, Streptococcus spp., and E. faecalis and a decrease in cases of blood culture positive for S. aureus, S. hominis, and E. faecium.
Finally, in Gram-negative bacteria, we observed an increase in cases of Acinetobacter spp. (Np 6 pt. −5.1%- vs. Pp 20 pt. −10.9%, p = 0.082) and Serratia spp., while the cases of sepsis decreased from E. faecium (Np 11 pt. −9.4%- vs. Pp 7 pt. −3.8%, p = 0.047), and Enterobacter spp., S. haemolyticus, S. maltophilia, Proteus spp., and P. aeruginosa have not changed over the time of the study.
Discussion
We found a substantial increase in the prevalence of cases of sepsis and septic shock in patients admitted to the ICU from 2018 to 2020 and a reduction in 2021.
Sepsis is characterized by a high mortality rate. The rate estimates range from 10 to 52%, depending on how the data are collected (16–20). Mortality rates increase linearly according to the severity of the disease (19). In one study, the mortality rates of septic shock were 46% (21). In another study, the mortality associated with sepsis was ≥10%, while in the case of septic shock, it was ≥40% (22). We did not detect differences in the medical or surgical origin of sepsis in the study periods, probably due to the sample size. In addition, we know that patients with sepsis and positive blood cultures have a higher severity of illness and higher mortality (23) and this represents a particular group risk population.
There are different studies on patients in ICU with a mortality rate of 59% in COVID-19 sepsis vs. 29% in the same period without COVID-19, or 58.7% vs. 40%, respectively (24, 25). Our data show that in ICU patients, there was an increase in the 30-day mortality rate from 2018 to 2020, with a reduction in 2021 and a return to mortality values in the pre-COVID-19 era. This trend can probably be traced back to the different phases of the COVID-19 disease that impacted patient survival. Indeed, in 2020, there was a pandemic that caught healthcare systems unprepared with the absence of knowledge related to COVID-19 physiopathology and its treatment. Initially, the physiopathology of the SARS-CoV-2 infection was unknown. COVID-19 is classically divided into two phases: the first is characterized by a high viral load, while the second is associated with the activation of an inflammatory response, including the appearance of a cytokine storm, which is then responsible for the evolution of ARDS and MOF, and eventually death (3, 26, 27).
Numerous therapies were attempted in the first half of 2020 that proved largely ineffective. Only in the second half of 2020 did pathophysiological knowledge increase, and the discovery of effective therapeutic strategies made it possible to approach patients better, allowing for better survival even in patients with septic complications, as can be seen from the 2021 data (27–29).
The subsequent diffusion of the massive vaccination strategy resulted in the modification of the severity of COVID-19, allowing for the development of vaccine immunity, which changed the natural history of the disease due to a more ready immune system response to infection. Finally, the greater availability and increasingly correct use of DPI have probably contributed to the reduction of the incidence of sepsis and mortality in patients observed in 2021.
There are no clear data on mortality rates in the COVID-19 era, but in particular, there are no data on the mortality rates of patients in the ICU who died from sepsis and SARS-CoV-2 infection.
In the general population, COVID-19-related mortality appears to be lower in younger patients (<44 years) without comorbidities (<10%) (30). Risk factors for mortality are known in COVID-19 and sepsis, such as advancing age, immunosuppression, and hospitalization (31, 32). This concordance of factors could help explain why we have seen an increase in sepsis in the first phase of COVID-19.
In this ICU population, age is a significant risk factor for mortality; data about this are widely available in the literature (33, 34). Another risk factor highlighted in our study is the correlation between mortality and days of stay in the ICU. Furthermore, as expected, other risk factors associated with mortality included comorbidities, which affected fewer COVID-19 patients than non-COVID-19 patients in this study. Patients with sepsis who were diagnosed with COVID-19 had fewer comorbidities. These data are attributable to the fact that patients with SARS-CoV-2 infection were mainly hospitalized for severe respiratory insufficiency, while, as is known, patients admitted to intensive care without a diagnosis of COVID-19 were hospitalized for the appearance of septic shock, which we know is linked to the presence of comorbidities. In this study with septic patients, the SOFA and APACHE scores were correlated with mortality, but the SOFA score is higher in COVID-19 patients vs. non-COVID-19 patients. These data can also be explained by the clinical conditions that were secondary to the cytokine storm that evolves in ARDS or MOF.
Neutrophils are the first immune cells recruited to the site of inflammation following stimulation by chemotactic factors released from damaged pulmonary tissues. Both exogenous and endogenous inflammatory stimuli can be recognized by specific receptors in human neutrophils. This further promotes the recruitment and activation of circulating neutrophils. These activated neutrophils produce several cytotoxic products and various proinflammatory cytokines. The overwhelming activation of neutrophils contributes to surrounding tissue damage and even lung dysfunction (35). Therefore, in COVID-19 ARDS patients, higher counts of neutrophils are observed and represent a predictor of poor outcome (36, 37).
A previous study on COVID-19 patients showed that neutrophils are an early marker in high-risk COVID-19 patients for acute respiratory failure and organ damage. Based on these results, we believe that classic inflammation markers such as CRP are not sufficient for stratification on COVID-19 patients. Instead, the dosing of factors among the relationship between neutrophils and lymphocytes (NRLs), IL-6, LDH, and ferritin could be useful for the early identification of patients at high risk of ARDS and death (3, 36).
Furthermore, in our study, it emerged that in the pre-pandemic era, the cases of sepsis associated with blood culture from a peripheral vein were statistically higher, while in the COVID-19 period, the cases of sepsis isolated from CVC increased. These data have never been found in the literature, and the reasons for this increased incidence of CVC-related infections could be associated with increased use of CVC and immunosuppression secondary to SARS-CoV-2 infection and to the cytokine storm phase that makes the immune system particularly dysregulated (27).
In the literature, we know that the types of sepsis-related microorganisms have changed over time. Gram-positive bacteria are mostly responsible for sepsis in the United States, although the number of cases of Gram-negative sepsis remains remarkable (32, 38). The incidence of fungal sepsis has increased over the past decade but remains lower than bacterial sepsis (16). In approximately one-half of cases of sepsis, the microorganism is not identified, so we have culture-negative sepsis (39). In our study, we have highlighted in the pandemic era a significant increase in cases of sepsis from the CNS and Acinetobacter spp. The epidemiological report of the European Centre for Disease Prevention and Control (ECDC) on hospital-acquired infections in the ICU, computed from 2017 data, showed a predominance of Gram-positive pathogens in bloodstream infections (40). Gram-negative bacilli cause approximately a quarter to a half of all bloodstream infections, and this depends on geographic region, whether the onset of the infection is in the hospital or the community, and other patient risk factors (41). This study showed an increase in S. capitis and S. epidermidis, but also in Gram-negative bacteria such as Acinetobacter spp. and Serratia spp. These data agree with the data relating to germs usually circulating in the ICU, but they do not seem to agree with a recent study in Iraq that instead shows a high incidence of Gram-positive sepsis mainly caused by Streptococcus, Haemophilus, and Moraxella (42). Probably, the different circulation of Gram-positive bacteria is related to the different characteristics of the patients and the countries.
We observed a significant reduction in the number of resistances of isolated germs, which may also be linked to a reduced selective pressure of antibiotic therapy for a better and more appropriate use of antibiotic therapy especially in the COVID-19 period. Patients in the ICU frequently are on or have recently been on antibiotics, which increases the risk of infections with P. aeruginosa and other non-fermenting Gram-negative bacilli, such as Acinetobacter species, that have intrinsic or acquired resistance to commonly used agents.
In our study, we particularly observed over time during the pandemic era a decreased resistance related to OXA-48, ESBL, carbapenems, and colistin. Our data contrast with unique but recently published data on non-ICU patients showing a higher incidence of ESBL-producing E. coli in COVID-19 patients than in non-COVID-19 patients. ESBL infections are associated with longer hospital stays and higher mortality rates in different population situations (43); these data are probably linked to a decrease in the number of Gram-negative bacteria that also led to the reduction of ESBLs.
In agreement with the literature data, we found a significant correlation between mortality from sepsis or septic shock and the presence of an ESBL germ. Neither was associated with the APACHE score and therefore with the patient’s clinical severity or with the diagnosis of COVID-19 (44, 45).
We know that the limitations of this study are the lack of information on colonization rates and molecular analysis for clusters of bacteria isolates. Additionally, our study is a single-center study, and therefore, our results cannot be generalized to all conditions. Perhaps during the pandemic phase, there was a more appropriate use of antibiotic therapies, but above all, PPE was used more correctly in ICUs.
Conclusion
This study demonstrated how the COVID-19 pandemic changed the prevalence of sepsis in the ICU. It emerged that the risk factors associated with mortality were SOFA and APACHE scores, age, days in the ICU, and, above all, the presence of ESBL-producing bacteria. Despite this, during the pandemic phase, we have observed a significant reduction in the emergence of resistant germs compared to the pre-pandemic phase. The COVID-19 pandemic revealed the critical need for effective infection-control policies and the correct use of antibiotic stewardship, along with a number of interventions to reduce sepsis and other co-infections in COVID-19 units. Compliance with guidelines for infection control and standards of antibiotic care is imperative.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was approved by the Ethics Internal Committee at the University “G. d’Annunzio” ChietiPescara (Ethics Committee Project No. 02 the 02/02/2022) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Written informed consent from the patients was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
KF: Conceptualization, Writing – original draft. LV: Data curation, Validation, Writing – original draft. PB: Data curation, Formal analysis, Writing – review & editing. MN: Data curation, Formal analysis, Writing – review & editing. CU: Conceptualization, Funding acquisition, Writing – review & editing. AG: Funding acquisition, Methodology, Writing – review & editing. MB: Investigation, Methodology, Writing – review & editing. GT: Data curation, Methodology, Resources, Writing – review & editing. JV: Conceptualization, Supervision, Writing – original draft. SM: Conceptualization, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Dufrusine B Valentinuzzi S Bibbo S Damiani V Lanuti P Pieragostino D et al . Iron Dyshomeostasis in COVID-19: biomarkers reveal a functional link to 5-lipoxygenase activation. Int J Mol Sci. (2022) 24:15. doi: 10.3390/ijms24010015
2.
Ucciferri C Di Gasbarro A Borrelli P Di Nicola M Vecchiet J Falasca K . New therapeutic options in mild moderate COVID-19 outpatients. Microorganisms. (2022) 10:131. doi: 10.3390/microorganisms10112131
3.
Ucciferri C Caiazzo L Di Nicola M Borrelli P Pontolillo M Auricchio A et al . Parameters associated with a diagnosis of COVID-19 in emergency department. Immun Inflamm Dis. (2021) 9:851–61. doi: 10.1002/iid3.440
4.
Santos TA Oliveira JE Fonseca CDD Barbosa DA Belasco A Miura CRM . Sepsis and COVID-19: outcomes in young adults in intensive care. Rev Bras Enferm. (2023) 76:e20230037. doi: 10.1590/0034-7167-2023-0037
5.
Seymour CW Liu VX Iwashyna TJ Brunkhorst FM Rea TD Scherag A et al . Assessment of clinical criteria for Sepsis: for the third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:762–74. doi: 10.1001/jama.2016.0288
6.
Rudd KE Johnson SC Agesa KM Shackelford KA Tsoi D Kievlan DR et al . Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
7.
Dremsizov T Clermont G Kellum JA Kalassian KG Fine MJ Angus DC . Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course?Chest. (2006) 129:968–78. doi: 10.1378/chest.129.4.968
8.
Netea MG van der Meer JW . Immunodeficiency and genetic defects of pattern-recognition receptors. N Engl J Med. (2011) 364:60–70. doi: 10.1056/NEJMra1001976
9.
Prescott HC Dickson RP Rogers MA Langa KM Iwashyna TJ . Hospitalization type and subsequent severe Sepsis. Am J Respir Crit Care Med. (2015) 192:581–8. doi: 10.1164/rccm.201503-0483OC
10.
Vincent JL Bihari DJ Suter PM Bruining HA White J Nicolas-Chanoin MH et al . The prevalence of nosocomial infection in intensive care units in Europe. Results of the European prevalence of infection in intensive care (EPIC) study. EPIC international advisory committee. JAMA. (1995) 274:639–44.
11.
Zhou F Yu T Du R Fan G Liu Y Liu Z et al . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
12.
Rawson TM Moore LSP Zhu N Ranganathan N Skolimowska K Gilchrist M et al . Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. (2020) 71:2459–68. doi: 10.1093/cid/ciaa530
13.
Abelenda-Alonso G Padulles A Rombauts A Gudiol C Pujol M Alvarez-Pouso C et al . Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Control Hosp Epidemiol. (2020) 41:1371–2. doi: 10.1017/ice.2020.381
14.
Vincent JL Moreno R Takala J Willatts S De Mendonca A Bruining H et al . The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10.
15.
Singer M Deutschman CS Seymour CW Shankar-Hari M Annane D Bauer M et al . The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
16.
Martin GS Mannino DM Eaton S Moss M . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. (2003) 348:1546–54. doi: 10.1056/NEJMoa022139
17.
Kaukonen KM Bailey M Suzuki S Pilcher D Bellomo R . Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. (2014) 311:1308–16. doi: 10.1001/jama.2014.2637
18.
Dombrovskiy Y Martin AA Sunderram J Paz HL . Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. (2007) 35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9
19.
Kaukonen KM Bailey M Pilcher D Cooper DJ Bellomo R . Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. (2015) 372:1629–38. doi: 10.1056/NEJMoa1415236
20.
Epstein L Dantes R Magill S Fiore A . Varying estimates of Sepsis mortality using death certificates and administrative codes--United States, 1999-2014. MMWR Morb Mortal Wkly Rep. (2016) 65:342–5. doi: 10.15585/mmwr.mm6513a2
21.
Rangel-Frausto MS Pittet D Costigan M Hwang T Davis CS Wenzel RP . The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. (1995) 273:117–23. PMID:
22.
Annane D Bellissant E Cavaillon JM . Septic shock. Lancet. (2005) 365:63–78. doi: 10.1016/S0140-6736(04)17667-8
23.
Lal A Rayes H O'Horo JC Singh TD Gajic O Kashyap R . Septic shock definitions and associated outcomes in blood culture positive critically ill patients. Ann Transl Med. (2023) 11:192. doi: 10.21037/atm-22-5147
24.
Heubner L Hattenhauer S Guldner A Petrick PL Rossler M Schmitt J et al . Characteristics and outcomes of sepsis patients with and without COVID-19. J Infect Public Health. (2022) 15:670–6. doi: 10.1016/j.jiph.2022.05.008
25.
Buetti N Tabah A Loiodice A Ruckly S Aslan AT Montrucchio G et al . Eurobact 2 study, different epidemiology of bloodstream infections in COVID-19 compared to non-COVID-19 critically ill patients: a descriptive analysis of the Eurobact II study. Crit Care. (2022) 26:319. doi: 10.1186/s13054-022-04166-y
26.
Di Biagio A Ricci E Calza L Squillace N Menzaghi B Rusconi S et al . Factors associated with hospital admission for COVID-19 in HIV patients. AIDS. (2020) 34:1983–5. doi: 10.1097/QAD.0000000000002663
27.
Ucciferri C Vecchiet J Falasca K . Role of monoclonal antibody drugs in the treatment of COVID-19. World J Clin Cases. (2020) 8:4280–5. doi: 10.12998/wjcc.v8.i19.4280
28.
Katia F Myriam DP Ucciferri C Auricchio A Di Nicola M Marchioni M et al . Efficacy of canakinumab in mild or severe COVID-19 pneumonia. Immun Inflamm Dis. (2021) 9:399–405. doi: 10.1002/iid3.400
29.
Ucciferri C Barone M Vecchiet J Falasca K . Pidotimod in Paucisymptomatic SARS-CoV2 infected patients. Mediterr J Hematol Infect Dis. (2020) 12:e2020048. doi: 10.4084/mjhid.2020.048
30.
Ioannidis JPA Axfors C Contopoulos-Ioannidis DG . Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ Res. (2020) 188:109890. doi: 10.1016/j.envres.2020.109890
31.
Harrison DA Welch CA Eddleston JM . The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC case mix Programme database. Crit Care. (2006) 10:R42. doi: 10.1186/cc4854
32.
Uslan DZ Crane SJ Steckelberg JM Cockerill FR St Sauver JL Wilson WR et al . Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. (2007) 167:834–9. doi: 10.1001/archinte.167.8.834
33.
Purdy FR Tweeddale MG Merrick PM . Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. (1997) 44:1256–61. doi: 10.1007/BF03012772
34.
Andrew MK Godin J LeBlanc J Boivin G Valiquette L McGeer A et al . Older age and frailty are associated with higher mortality but lower ICU admission with COVID-19. Can Geriatr J. (2022) 25:183–96. doi: 10.5770/cgj.25.546
35.
Aulakh GK . Neutrophils in the lung: "the first responders". Cell Tissue Res. (2018) 371:577–88. doi: 10.1007/s00441-017-2748-z
36.
Liu Y Du X Chen J Jin Y Peng L Wang HHX et al . Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. (2020) 81:e6–e12. doi: 10.1016/j.jinf.2020.04.002
37.
Yang SC Tsai YF Pan YL Hwang TL . Understanding the role of neutrophils in acute respiratory distress syndrome. Biom J. (2021) 44:439–46. doi: 10.1016/j.bj.2020.09.001
38.
Klotz SA Chasin BS Powell B Gaur NK Lipke PN . Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn Microbiol Infect Dis. (2007) 59:401–6. doi: 10.1016/j.diagmicrobio.2007.07.001
39.
Gupta S Sakhuja A Kumar G McGrath E Nanchal RS Kashani KB . Culture-negative severe Sepsis: Nationwide trends and outcomes. Chest. (2016) 150:1251–9. doi: 10.1016/j.chest.2016.08.1460
40.
Bijkerk P Monnier AA Fanoy EB Kardamanidis K Friesema IH Knol MJ . ECDC round Table report and ProMed-mail most useful international information sources for the Netherlands early warning committee. Euro Surveill. (2017) 22:502. doi: 10.2807/1560-7917.ES.2017.22.14.30502
41.
Sievert DM Ricks P Edwards JR Schneider A Patel J Srinivasan A et al . National Healthcare Safety Network, and N.F. Participating, antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. (2013) 34:1–14. doi: 10.1086/668770
42.
Sh R . Study of microbial infections and some immunological parameters among Covid-19 in ICU patients in Najaf governorate. Iraq Arch Razi Inst. (2022) 77:1569–74. doi: 10.22092/ARI.2022.358102.2151
43.
Ling W Paterson DL Harris PNA Furuya-Kanamori L Edwards F Laupland KB . Population-based incidence and characteristics of adult Escherichia coli bloodstream infection in Queensland, Australia, from 2000 to 2019. Open forum. Infect Dis. (2023) 10:ofad071. doi: 10.1093/ofid/ofad071
44.
Giannitsioti E Louka C Mamali V Kousouli E Velentza L Papadouli V et al . Bloodstream infections in a COVID-19 non-ICU department: microbial epidemiology, resistance profiles and comparative analysis of risk factors and Patients' outcome. Microorganisms. (2022) 10:314. doi: 10.3390/microorganisms10071314
45.
Deana C Rovida S Orso D Bove T Bassi F De Monte A et al . Learning from the Italian experience during COVID-19 pandemic waves: be prepared and mind some crucial aspects. Acta Biomed. (2021) 92:e2021097. doi: 10.23750/abm.v92i2.11159
Summary
Keywords
COVID-19, sepsis, ICU, mortality, antimicrobial therapy
Citation
Falasca K, Vetrugno L, Borrelli P, Di Nicola M, Ucciferri C, Gambi A, Bazydlo M, Taraschi G, Vecchiet J and Maggiore SM (2024) Antimicrobial resistance in intensive care patients hospitalized with SEPSIS: a comparison between the COVID-19 pandemic and pre-pandemic era. Front. Med. 11:1355144. doi: 10.3389/fmed.2024.1355144
Received
13 December 2023
Accepted
25 April 2024
Published
15 May 2024
Volume
11 - 2024
Edited by
Shisan Bao, The University of Sydney, Australia
Reviewed by
Pier Maria Fornasari, REGENHEALTHSOLUTIONS, Italy
Antonio Lalueza, University Hospital October 12, Spain
Updates
Copyright
© 2024 Falasca, Vetrugno, Borrelli, Di Nicola, Ucciferri, Gambi, Bazydlo, Taraschi, Vecchiet and Maggiore.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katia Falasca, k.falasca@unich.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.