Abstract
CARD14 (caspase activation and recruitment domain) mutations have been associated with psoriasis vulgaris, psoriatic arthritis, generalized and palmoplantar pustular psoriasis, pityriasis rubra pilaris, and atopic dermatitis. We present a pediatric patient with a novel CARD14: c.394A > T/− (Ile123Phe) mutation, diagnosed with CARD14-associated papulosquamous eruption (CAPE), who was successfully treated with biological treatment.
1 Introduction
CARD14 (caspase activation and recruitment domain) gene activates a group of interacting proteins known as nuclear factor-kappa-B (NF-κB), which regulate the activity of multiple genes, including those that control the immune responses and inflammatory reactions of the body (1, 2). Until now, CARD14 mutations have been associated with psoriasis vulgaris (PsV), psoriatic arthritis (PsA), generalized and palmoplantar pustular psoriasis (GPP and PPP), pityriasis rubra pilaris (PRP), and atopic dermatitis (AD) (3–8).
In 2018, a new dermatological condition, CARD14-associated papulosquamous eruption (CAPE) was described for a group of patients with clinical features of psoriasis and PRP that also bear some resemblance to atopic dermatitis or even ichthyosis (6). Due to the limited data, there are no treatment guidelines for CAPE.
2 Case report
We present an 11-year-old patient who developed skin problems by the age of 2. According to the patient’s parents, there was no significant history of skin diseases in the family. His diagnoses included psoriasis vulgaris and pityriasis rubra pilaris; however, no definitive diagnosis was made. The patient presented with well-demarcated pink-red patches and thin plaques involving bilateral cheeks and chin with sparing of the infralabial area and substantial involvement of the trunk and extremities in the form of erythema and significant scaling (Figure 1A). Histopathological examinations of several skin biopsies revealed features of PsV (parakeratosis mounded with neutrophils, hypogranulosis, and regular acanthosis) and PRP (alternating parakeratosis and orthokeratosis in a vertical and horizontal pattern, irregular acanthosis, and follicular plugging). He was treated with topical medications, including 0.5% betamethasone cream, 1% hydrocortisone cream, 0.1% mometasone furoate cream, systemic acitretin 0.8 mg/kg/day from 3 to 6 years old, cyclosporine 5 mg/kg/day for 4 months, methotrexate 0.4 mg/kg/week, and dimethyl fumarate 20 mg/kg/day for 11 months, all with poor response. Next-generation sequencing (NGS) panel targeted for mutations associated with ichthyosis, psoriasis, PRP, and EB revealed novel CARD14: c.394A > T/− (Ile123Phe) mutation. The gene variant has not been reported in the Human Gene Mutation Database, ClinVar, GnomAD, and ExAc databases. Bioinformatics analysis using the Alamut program software indicated that nucleotide A at position 394 and amino acid Ile at position 132 are highly evolutionarily conserved. The PolyPhen-2 algorithm and SIFT software analysis indicated the potentially pathogenic nature of the mutation. The patient was eventually diagnosed with CARD14-associated papulosquamous eruption (CAPE). Before initiating treatment with biologics. Children’s Dermatology Life Quality Index (CDLQI) and Family Dermatology Life Quality Index (FDLQI) questionnaires were filled out by the patient and his parents and assessed. Investigator Global Assessment (IGA) was also evaluated (see Table 1). Initial scores for CDLQI, FDLQI, and IGA were 17 (very large impact of the disease on the patient’s life), 28 (extremely large impact of the disease on the patient’s family life), and 4 (severe skin symptoms), respectively. He started biological therapy with tumor necrosis factor-α (TNF-α) inhibitor, adalimumab, with a dose of 40 mg SC every 14 days showing clinical improvement of his skin lesions for a period of 18 months without a total remission (Figure 1B). During the next 3 months, deterioration was observed and the frequency of administering the drug was modified to every 7 days (Figure 1C). Due to a lack of improvement, it was decided to change the biological treatment for ustekinumab, which is a monoclonal IgG1k antibody that targets both IL-12 and IL-23 cytokines by binding to their shared p40 subunit. Initially, he was treated with a dose of 45 mg (1.14 mg per kg), then in the fourth week and then every 12 weeks thereafter, which is standard dosing for PsV and PsA (9). The patient additionally applied topical steroids, tacrolimus, and cholesterol ointment. Two months after the therapy initiation, the patient presented lower therapy effectiveness, and deterioration of skin lesions was observed. A possible cause could be the psychological trauma after a car accident that the patient was involved in. He suffered no physical injuries, and the treatment was uninterrupted. Therefore, based on available data in the literature and previously reported cases of patients with CAPE (Table 2), it was decided to increase the frequency of ustekinumab injections to every other 8 weeks with significant clinical improvement (Figure 1D). The patient did not report any side effects while undergoing therapy, and no side effects were observed by physicians. The patient’s parents also reported substantial improvement in his schoolwork and contact with peers, which is also noticeable in the FDLQI (3 points—small effect on the family’s life quality) and CDLQI (0 points—no effect on patient’s life quality) scores (Table 1).
Figure 1

Skin symptoms of CAPE and case timeline. Photographs of the patient before treatment with adalimumab: well-demarcated pink-red patches and thin plaques, bilateral cheeks and chin with sparing of the infralabial area, involvement of the trunk, and extremities—erythema and significant scaling (A), after 2 months on adalimumab therapy (B), before ustekinumab therapy (C), and after 13 months on ustekinumab therapy (D).
Table 1
| Date of assessment | IGA1 | FDLQI2 | CDLQI3 | Height (cm) | Height percentile (%) | Weight (kg) | Weight percentile (%) | Biologics | Dose (mg) | Dose per body mass (dose mg/body mass kg) | Frequency (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| August 12, 2019 | 4 | 28 | 17 | 138 | 89.2 | 27 | 56.6 | QUALIFICATION | |||
| September 02, 2019 | 4 | 139 | 98.6 | 28 | 63.8 | Adalimumab | 40 | 1.43 | 14 | ||
| October 7, 2019 | 3 | 140 | 97.1 | 30 | 75 | Adalimumab | 40 | 1.33 | 14 | ||
| December 16, 2019 | 3 | 13 | 0 | 141 | 94.5 | 30 | 71.1 | Adalimumab | 40 | 1.33 | 14 |
| March 30, 2020 | 2 | 142 | 93.1 | 33 | 81 | Adalimumab | 40 | 1.21 | 14 | ||
| May 05, 2020 | 2 | 143 | 93.9 | 34 | 83.1 | Adalimumab | 40 | 1.18 | 14 | ||
| July 13, 2020 | 2 | 144 | 93.6 | 33 | 76 | Adalimumab | 40 | 1.21 | 14 | ||
| August 18, 2020 | 2 | 145 | 94.4 | 35 | 82.4 | Adalimumab | 40 | 1.14 | 14 | ||
| October 14, 2020 | 2 | 145 | 92.7 | 35 | 80 | Adalimumab | 40 | 1.14 | 14 | ||
| January 20, 2021 | 3 | 146 | 91.4 | 34 | 70.6 | Adalimumab | 40 | 1.18 | 14 | ||
| February 16, 2021 | 4 | 147 | 92.7 | 32 | 56.8 | Adalimumab | 40 | 1.25 | 14 | ||
| May 11, 2021 | 4 | 149 | 94.1 | 33 | 57.8 | Adalimumab | 40 | 1.21 | 14 | ||
| August 03, 2021 | 3 | 150 | 93.6 | 34 | 58.3 | Adalimumab | 40 | 1.18 | 7 | ||
| September 13, 2021 | 4 | ND | ND | ND | ND | Adalimumab | 40 | ND | 7 | ||
| December 7, 2021 | 4 | 24 | 14 | ND | ND | ND | ND | Adalimumab | 40 | ND | 7 |
| March 02, 2022 | 4 | 19 | 15 | ND | ND | ND | ND | QUALIFICATION | |||
| May 24, 2022 | 3 | 157 | 96.9 | 39.5 | 62.8 | Ustekinumab | 45 | 1.14 | 28 | ||
| June 21, 2022 | 2 | 158 | 97.4 | 38 | 59.6 | Ustekinumab | 45 | 1.18 | 84 | ||
| September 22, 2022 | 4 | 159 | 96.9 | 39.5 | 61 | Ustekinumab | 45 | 1.14 | 84 | ||
| December 13, 2022 | 2 | 160 | 96.5 | 41 | 62.7 | Ustekinumab | 45 | 1.10 | 56 | ||
| February 14, 2023 | 1 | 162 | 97.3 | 42 | 63.3 | Ustekinumab | 45 | 1.07 | 56 | ||
| April 11, 2023 | 1 | 4 | 2 | 163 | 97.3 | 43 | 64.1 | Ustekinumab | 45 | 1.05 | 56 |
| June 09, 2023 | 1 | 164 | 97.2 | 43 | 64.5 | Ustekinumab | 45 | 1.03 | 56 | ||
| August 21, 2023 | 1 | 3 | 0 | 165 | 96.7 | 44 | 60.3 | Ustekinumab | 45 | 102 | 56 |
Presentation of the therapeutic course of the presented patient, taking into account the IGA, FDLQI, CDLQI scales, height, weight, biological agent, dose and frequency of the drug istration.
1Investigator’s Global Assessment (IGA) interpretation: 0 = clear skin, 1 = almost clear skin, 2 = mild severity of lesions, 3 = moderate severity of lesions, 4 = severe skin lesions. 2Family and 3Children’s Dermatology Life Quality scores: 0–1 = no effect on life; 2–6 = small effect; 7–12 = moderate effect; 13–18 = very large effect; 19–30 = extremely large effect. The bold values and color shading inside is intended to facilitate the reception of the table.
Table 2
| Patients with CAPE treated with ustekinumab | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication | Described mutation | Age of onset | Facial involvement | Trunk involvement | Palmoplantar keratoderma | Follicular papules | Island of sparing | Family history for PsV, PsA, m/pGF, CAPE | Conventional treatment | Outcome | Biologic treatment | Outcome |
| Craiglow et al. (6) | c.349G > A, p.G117S (homozygous) | 8 months | Yes | Yes | Yes | Yes | No | Positive | Isotretinoin | Partial | Ustekinumab 0.7 mg/kg q12w + methotrexate | Near complete |
| c.34915G > C | 2 years | Yes | Yes | Yes | No | No | Positive | Isotretinoin | Partial | Ustekinumab 1.1 mg/kg q12w | Near complete | |
| c.412G > A, p.E138K (de novo) | 3 weeks | Yes | Yes | Yes | No | No | Negative | Acitretin | Minimal | Ustekinumab 0.87 mg/kg q12w | Partial | |
| c.467 T > C, p.L156P | 6 months | Yes | Yes | Yes | No | Yes | Positive | Ustekinumab 1.2 mg/kg q8w | Near complete | |||
| c.349G > A, p.G117S | 1 year | Yes | Yes | No | No | No | Positive | Methotrexate | Partial | Ustekinumab 0.87 mg/kg q12w | Near complete | |
| Isotretinoin | Partial | |||||||||||
| c.371 T > C, p.L124P (de novo) | 3 months | Yes | Yes | Yes | Yes | Yes | Negative | Methotrexate | Minimal | Ustekinumab 0.9 mg/kg q12w | Near complete | |
| Acitretin | Partial | |||||||||||
| Cyclosporine | Partial | |||||||||||
| Psoralen ultraviolet A | Worsening | |||||||||||
| Signa et al. (21) | c.446 T > G, p.L149R (dizygotic twins) | 9 months | Yes | Yes | No data | No data | No | Positive | Cyclosporine | Partial | Ustekinumab (2 mg/kg) q12w | Total remission |
| Nieto-Benito et al. (18) | c.277A > C, p.Lys(93Glu) | 2 months | Yes | Yes | Yes | No data | Yes | Positive | Oral retinoids | No response | Ustekinumab 90 mg q12w | Near complete |
| Frare et al. (17) | c.1604A > G, p.Gln535Arg | 10 months | Yes | Yes | No | No | Yes | Positive | Topical steroids | Minimal | Ustekinumab 45 mg q12w + methotrexate | Partial |
| c.365 T > C, p.Met119Thr | 3 months | Yes | Yes | Yes | Yes | Yes | Positive | Isotretinoin + mometasone | Partial | |||
| Methotrexate | Worsening | Ustekinumab 45 mg q12w | Worsening | |||||||||
| Ustekinumab 45 mg q8w | Near complete | |||||||||||
| Kiszewski et al. (25) | c.349 + 2 T > C | 5 months | Yes | Yes | Yes | Yes | Yes | No data | Cyclosporine | No response | Ustekinumab 10.8 mg q2w | Near complete |
| Methotrexate | Minimal | |||||||||||
| Noguiera et al. (24) | c.349 + 5G > C | 8 months | Yes | Yes | No data | Yes | Yes | Positive | Topical agents | Minimal | Ustekinumab 0.75–1.0 mg q8w | Near complete |
| Niedźwiedź et al. (26) | c.394A > T/− (Ile123Phe) | 2 years of age | Yes | Yes | Yes | Yes | Yes | Negative | Systemic acitretin, cyclosporine, methotrexate, dimethyl fumarate | No or poor response | Adalimumab 40 mg q1-2w | Partial with decreased response to the drug |
| Ustekinumab 1.0–1.18 mg/kg q8-12w | Near complete | |||||||||||
Summary of the patients diagnosed with CAPE and treated with ustekinumab.
The color shading is intended to facilitate the reception of the table.
3 Discussion
The CARD14 gene provides instructions for making a protein that activates a group of interacting proteins known as nuclear factor-kappa-B (NF-κB). The NF-κB protein complex is responsible for the activation and regulation of multiple genes, including those that are responsible for inflammatory reactions. The NF-κB protein complex also protects cells from certain signals that would otherwise cause them to undergo apoptosis (1, 10, 11). NF-κB signaling plays a vital role in regulating inflammatory reactions in the skin and in promoting the survival of the skin (1, 10–12). CARD14 gain-of-function (GOF) mutations are linked with clinical features of PsV and PRP, while loss-of-function mutations are associated with atopic dermatitis. GOF mutation in CARD14 results in heightened nuclear factor κB (NF-κB) signaling (6, 11, 12). Elevated NF-κB activity leads to increased levels of chemokines such as IL-8 and CCL20, which, in turn, lead to the recruitment and differentiation of inflammatory cells, including the production of IL-23 by dendritic cells and IL-17 and IL-22 by T cells.
The role of CARD14 in the pathogenesis of several inflammatory skin conditions was initially described through publications of familial cases of PsV and PRP (13–15). Gál et al. (16) identified several CARD14 variants in almost half of their cases of PRP, but no correlation was found between the therapeutic response and the genetic background, which could have been due to a limited number of patients. To date, there are several reports that indicate that various CARD14 mutations may lead to autoinflammatory skin diseases such as plaque, pustular and/or erythrodermic types of psoriasis, pityriasis rubra pilaris, ichthyosis, and psoriatic arthritis (Figure 2). Dominant loss of function mutations in CARD14 resulted in an unusually severe form of atopic dermatitis (11).
Figure 2
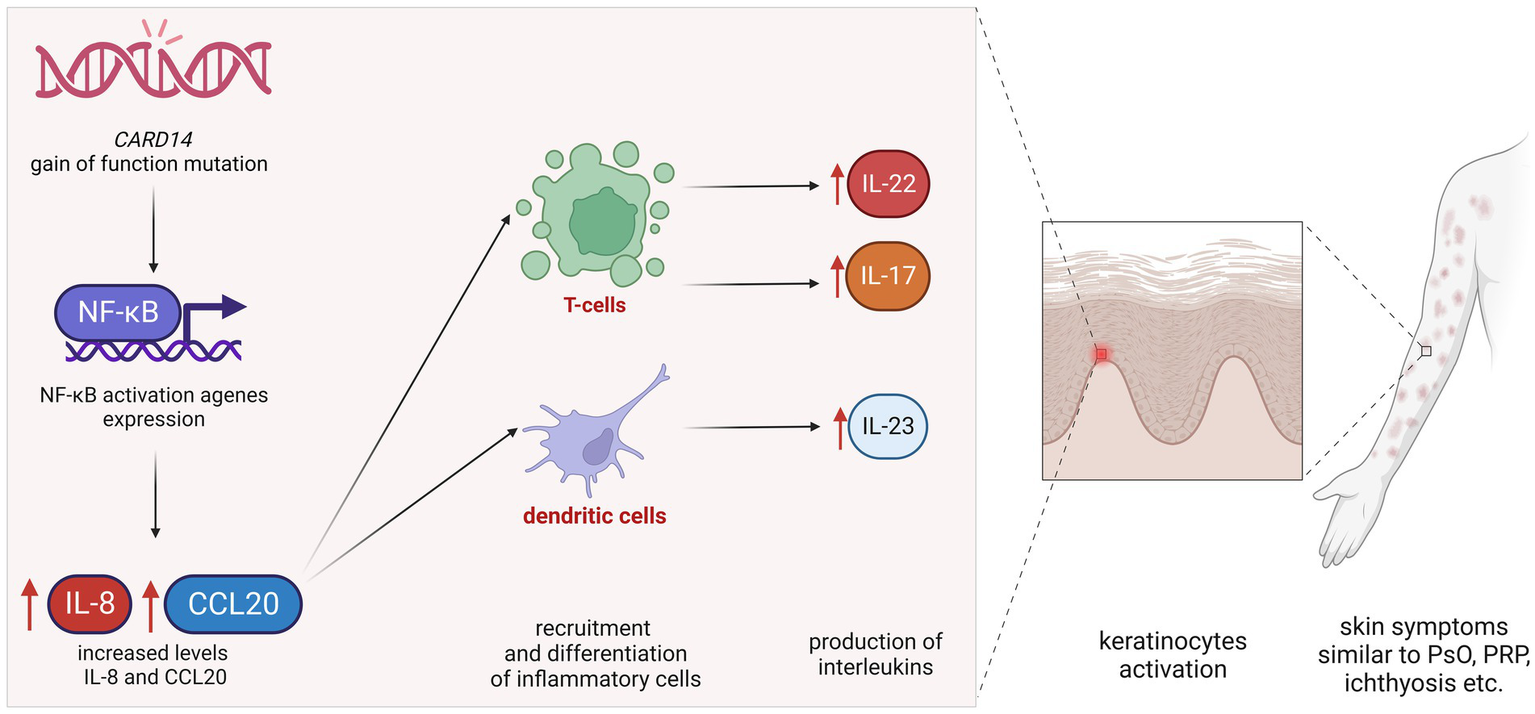
Simplified pathomechanism of CARD14 gain-of-function mutations. GOF mutation in CARD14 results in heightened nuclear factor κB signaling. Elevated NF-κB activity leads to increased levels of chemokines, such as IL-8 and CCL20, which, in turn, lead to the recruitment and differentiation of inflammatory cells, including the production of IL-23 by dendritic cells and IL-17 and IL-22 by T cells. Increased levels of these interleukins cause parakeratosis leading to skin symptoms similar to psoriasis, pityriasis rubra pilaris, and/or ichthyosis.
In 2018, a new term, CARD-14-associated papulosquamous eruption (CAPE) was introduced by Craiglow et al. (6) to describe a group of patients with clinical features of psoriasis and pityriasis rubra pilaris (PRP) bearing some resemblance to atopic dermatitis or even ichthyosis. There was no definite diagnosis in those patients, and topical or systemic treatment, including cyclosporin or methotrexate, was unsuccessful. All patients with CAPE had CARD14 mutations. Clinical characteristics of patients with CAPE are as follows: (1) young onset of skin symptoms, (2) facial involvement with well-demarcated pink-red plaques involving the cheeks, chin, and ears with sparing of the infralabial region, (3) palmoplantar keratoderma, (4) trunk involvement, (5) follicular papules, and (6) island of sparing (6, 17). Patients diagnosed with CAPE are reported to present a low quality of life and tend to present psychological symptoms such as depression (18).
Histopathological examinations are reported to be not diagnostic enough because biopsies showed conflicting microscopic pictures, which also occurred in our patient (19). Ring et al. evaluated biopsies of skin lesions from patients diagnosed with CAPE and compared them with biopsies of PsV and PRP patients (18). In the studied skin samples, CAPE shared more histopathologic features with PRP than with psoriasis, including checkerboard parakeratosis and orthokeratosis, acanthosis, follicular plugging, and similar thickness of the epidermis below the stratum corneum and a lack of relative suprapapillary plate thinning. CAPE samples demonstrated regular psoriasiform acanthosis with elongated rete ridges in contrast to PRP specimens. Similar to PsV, CAPE also lacked acantholysis, while approximately half of the PRP specimens presented with acantholysis.
Patients with CAPE are reported to present poor responses to conventional topical and systemic therapy such as acitretin, cyclosporine, or methotrexate (6, 17). The overlapping stimulation of the IL-23/Th17 axis caused by CARD14 mutations indicates that blocking this pathway may be the best treatment option for patients with CAPE. Biologics, such as ustekinumab, guselkumab, secukinumab, and ixekizumab, are reported to present beneficial treatment responses in CARD14-related diseases (6, 20, 21).
Based on the known effects of GOF mutations in the CARD14 gene and its effects on NF-κB, ustekinumab appears to be a pathogenesis-based treatment for CAPE, as shown by the several clinical responses in published case reports (Table 2). In available literature data including our patient, 11 of 12 described patients treated with high doses of ustekinumab responded to biological treatment with at least a good response. Patients with CAPE may require a more frequent or higher dose of biologics to achieve remission than patients with psoriasis. Nieto-Benito et al. (18) describe a 36-year-old man with CAPE who was treated for 14 years for ichthyosis and progressive symmetric erythrokeratoderma with acitretin with poor response. After a genetic investigation, it was decided to start therapy with ustekinumab with a good response.
Despite promising data, long-term follow-up of patients treated with these biological molecules is still lacking. Our presented patient with CAPE is currently undergoing treatment with high doses of ustekinumab for 21 months and is showing clinical improvement; however, the dosing and the frequency of medicine administration should be assessed individually (22–24). The remaining question is whether CARD14 mutations can be associated with severe inflammatory skin condition resistance to treatment.
A limitation of our study is a lack of histopathological images of performed biopsies. These were evaluated by a non-university, external company and provided only the descriptions of the images. We also did not perform molecular assessment during the course of the treatment for levels of inflammatory markers, such as IL-17, IL-22 and IL-23, or TNF-α.
4 Conclusion
Patients with CAPE share clinical findings, mostly similar to psoriasis and/or pityriasis rubra pilaris. Patients with gain-of-function CARD14 mutations and diagnosed with CARD-14-associated papulosquamous eruption present similar phenotypes such as young onset of the skin symptoms, facial involvement with well-demarcated pink-red plaques involving the cheeks, chin, and ears with sparing of the infralabial region, palmoplantar keratoderma, trunk involvement, follicular papules, and the island of sparing. Patients with severe inflammatory skin conditions and presenting phenotypical features, who do not respond to standard treatment, should be considered for genetic investigations for CARD14 mutations. Patients diagnosed with CAPE had poor responses to conventional psoriasis treatment, acitretin, cyclosporine, or methotrexate. Unfortunately, there are still not enough data to establish generally accepted therapeutic guidelines for CARD14-related dermatological conditions; however, treatment with high doses of biologics targeting psoriasis pathways, IL-23 and IL-17, such as ustekinumab, shows promising results.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minor’s legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
MN: Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Conceptualization. JN: Funding acquisition, Supervision, Writing – review & editing. AS: Data curation, Writing – original draft. MS: Supervision, Writing – review & editing. BK: Writing – review & editing, Data curation. DS-S: Data curation, Writing – original draft. MC: Supervision, Writing – review & editing. KP-K: Data curation, Writing – review & editing. AG: Supervision, Writing – review & editing. AL: Formal analysis, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by statutory activities funds of the Medical University of Lodz no. 503/5-064-04/503-01.
Acknowledgments
The authors would like to thank our patient and his family for their consent to the publication of this manuscript. Figures were created with BioRender software.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Harden JL Lewis SM Pierson KC Suárez-Fariñas M Lentini T Ortenzio FS et al . CARD14 expression in dermal endothelial cells in psoriasis. PLoS One. (2014) 9:e111255. doi: 10.1371/JOURNAL.PONE.0111255
2.
Scudiero I Zotti T Ferravante A Vessichelli M Vito P Stilo R . Alternative splicing of CARMA2/CARD14 transcripts generates protein variants with differential effect on NF-κB activation and endoplasmic reticulum stress-induced cell death. J Cell Physiol. (2011) 226:3121–31. doi: 10.1002/JCP.22667
3.
Msafiri Makene A Liu JL . Association between CARD14 gene polymorphisms and psoriasis vulgaris in Hainan Han population based on exon sequencing: a case-control study. Medicine. (2022) 101:E30890. doi: 10.1097/MD.0000000000030890
4.
Yang SF Lin MH Chou PC Hu SK Shih SY Yu HS et al . Genetics of generalized pustular psoriasis: current understanding and implications for future therapeutics. Genes. (2023) 14:1297. doi: 10.3390/GENES14061297
5.
Queiro R Coto P González-Lara L Coto E . Genetic variants of the NF-κB pathway: unraveling the genetic architecture of psoriatic disease. Int J Mol Sci. (2021) 22:13004. doi: 10.3390/IJMS222313004
6.
Craiglow BG Boyden LM Hu R Virtanen M Su J Rodriguez G et al . CARD14 – associated Papulosquamous eruption (CAPE): a Spectrum including features of psoriasis and Pityriasis Rubra pilaris. J Am Acad Dermatol. (2018) 79:487–94. doi: 10.1016/J.JAAD.2018.02.034
7.
Takeichi T Terawaki S Kubota Y Ito Y Tanahashi K Muro Y et al . A patient with CARD14-associated papulosquamous eruptions showing atopic dermatitis-like features. J Eur Acad Dermatol Venereol. (2021) 35:e58–9. doi: 10.1111/JDV.16799
8.
Genovese G Moltrasio C Cassano N Maronese CA Vena GA Marzano AV . Pustular psoriasis: from pathophysiology to treatment. Biomedicines. (2021) 9:1746. doi: 10.3390/BIOMEDICINES9121746
9.
Stelara | European Medicines Agency . Available at:https://www.ema.europa.eu/en/medicines/human/EPAR/stelara (Accessed October 22, 2023)
10.
Sundberg JP Pratt CH Silva KA Kennedy VE Qin W Stearns TM et al . Gain of function p.E138A alteration in Card14 leads to psoriasiform skin inflammation and implicates genetic modifiers in disease severity. Exp Mol Pathol. (2019) 110:104286. doi: 10.1016/J.YEXMP.2019.104286
11.
Howes A O’Sullivan PA Breyer F Ghose A Cao L Krappmann D et al . Psoriasis mutations disrupt CARD14 autoinhibition promoting BCL10-MALT1-dependent NF-κB activation. Biochem J. (2016) 473:1759–68. doi: 10.1042/BCJ20160270
12.
Peled A Sarig O Sun G Samuelov L Ma CA Zhang Y et al . Loss-of-function mutations in caspase recruitment domain-containing protein 14 (CARD14) are associated with a severe variant of atopic dermatitis. J Allergy Clin Immunol. (2019) 143:173–181.e10. doi: 10.1016/J.JACI.2018.09.002
13.
Fuchs-Telem D Sarig O Van Steensel MAM Isakov O Israeli S Nousbeck J et al . Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am J Hum Genet. (2012) 91:163–70. doi: 10.1016/J.AJHG.2012.05.010
14.
Berki DM Liu L Choon SE Burden AD Griffiths CEM Navarini AA et al . Activating CARD14 mutations are associated with generalized pustular psoriasis but rarely account for familial recurrence in psoriasis vulgaris. J Invest Dermatol. (2015) 135:2964–70. doi: 10.1038/JID.2015.288
15.
Ammar M Jordan CT Cao L Lim E Bouchlaka Souissi C Jrad A et al . CARD14 alterations in Tunisian patients with psoriasis and further characterization in European cohorts. Br J Dermatol. (2016) 174:330–7. doi: 10.1111/BJD.14158
16.
Gál B Göblös A Danis J Farkas K Sulák A Varga E et al . The management and genetic background of pityriasis rubra pilaris: a single-centre experience. J Eur Acad Dermatol Venereol. (2019) 33:944–9. doi: 10.1111/JDV.15455
17.
Frare CP Blumstein AJ Paller AS Pieretti L Choate KA Bowcock AM et al . CARD14-associated papulosquamous eruption (CAPE) in pediatric patients: three additional cases and review of the literature. Pediatr Dermatol. (2021) 38:1237–42. doi: 10.1111/PDE.14779
18.
Nieto-Benito LM Baniandrés-Rodríguez O Moreno-Torres A Hernández-Martín A Torrelo-Fernández A Campos-Domínguez M . Clinical response to ustekinumab in CARD14-associated papulosquamous eruption (CAPE) with a new missense mutation in CARD14: a case report and systematic review. J Eur Acad Dermatol Venereol. (2020) 34:e728–30. doi: 10.1111/JDV.16548
19.
Ring NG Craiglow BG Panse G Antaya RJ Ashack K Ashack R et al . Histopathologic findings characteristic of CARD14-associated papulosquamous eruption. J Cutan Pathol. (2020) 47:425–30. doi: 10.1111/CUP.13633
20.
Lwin SM Hsu CK Liu L Huang HY Levell NJ McGrath JA . Beneficial effect of ustekinumab in familial pityriasis rubra pilaris with a new missense mutation in CARD14. Br J Dermatol. (2018) 178:969–72. doi: 10.1111/BJD.15462
21.
Signa S Campione E Rusmini M Chiesa S Grossi A Omenetti A et al . Whole exome sequencing approach to childhood onset familial erythrodermic psoriasis unravels a novel mutation of CARD14 requiring unusual high doses of ustekinumab. Pediatr Rheumatol Online J. (2019) 17:38. doi: 10.1186/S12969-019-0336-3
22.
Camela E Potestio L Fabbrocini G Pallotta S Megna M . The holistic approach to psoriasis patients with comorbidities: the role of investigational drugs. Expert Opin Investig Drugs. (2023) 32:537–52. doi: 10.1080/13543784.2023.2219387
23.
Megna M Camela E Battista T Genco L Martora F Noto M et al . Efficacy and safety of biologics and small molecules for psoriasis in pediatric and geriatric populations. Part I: focus on pediatric patients. Expert Opin Drug Saf. (2023) 22:25–41. doi: 10.1080/14740338.2023.2173170
24.
Napolitano M Fabbrocini G Neri I Stingeni L Boccaletti V Piccolo V et al . Dupilumab treatment in children aged 6-11 years with atopic dermatitis: a multicentre, real-life study. Paediatr Drugs. (2022) 24:671–8. doi: 10.1007/S40272-022-00531-0
25.
Kiszewski AE De Almeida HL . Successful treatment with ustekinumab in CARD14-associated papulosquamous eruption in a Brazilian child. Dermatol Ther. (2022) 35:e15939. doi: 10.1111/DTH.15939
26.
Niedźwiedź M Narbutt J Siekierko A Skibińska M Kwiek B Sobolewska-Sztychny D et al . Case report: Successful treatment with biologics in a pediatric patient with a severe inflammatory skin disease and novel CARD14 mutation. Front. Med. (2024) 11:1360248. doi: 10.3389/fmed.2024.1360248
Summary
Keywords
CARD14 , CAPE, biologics, adalimumab, ustekinumab, case report, CARD14: c.394A > T/− (Ile123Phe), CARD14-associated papulosquamous eruption
Citation
Niedźwiedź M, Narbutt J, Siekierko A, Skibińska M, Kwiek B, Sobolewska-Sztychny D, Ciążyńska M, Poznańska-Kurowska K, Gostyński A and Lesiak A (2024) Case report: Successful treatment with biologics in a pediatric patient with a severe inflammatory skin disease and novel CARD14 mutation. Front. Med. 11:1360248. doi: 10.3389/fmed.2024.1360248
Received
22 December 2023
Accepted
18 January 2024
Published
05 February 2024
Volume
11 - 2024
Edited by
Simone Ribero, University of Turin, Italy
Reviewed by
Luca Potestio, University of Naples Federico II, Italy
Chiara Moltrasio, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, Italy
Updates
Copyright
© 2024 Niedźwiedź, Narbutt, Siekierko, Skibińska, Kwiek, Sobolewska-Sztychny, Ciążyńska, Poznańska-Kurowska, Gostyński and Lesiak.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michał Niedźwiedź, michal.niedzwiedz@umed.lodz.pl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.