Abstract
Introduction:
Leprosy is a chronic infectious disease caused by the agent Mycobacterium leprae, characterized by its high disabling power. Data points to Brazil being the second country with the highest number of cases in the world, behind only India, representing a major challenge for public health. This work aims to determine the prevalence of paucibacillary (PB) cases in relation to leprosy cases in Brazil, using data published in the literature.
Methods:
This is a systematic review and meta-analysis carried out with studies from the last 20 years, being developed based on the Preferred Reporting Items for Systematic Review and Meta-analyzes (PRISMA).The search was carried out in the databases: PUBMED, SciELO, LILACS (via VHL)and Science Direct in October 2023, using the following descriptors (((“Brazil” [Mesh]) AND (“Leprosy, paucibacillary” [Mesh])) AND “Epidemiology” [Mesh]), in English, Portuguese and Spanish. Original studies of the analytical case–control, cohort, cross-sectional, epidemiological types were selected, as well as articles with satisfactory information for numerical extraction with separate data on the paucibacillary and multibacillary clinical forms. The methodological quality assessment followed the JBI critical appraisal checklist. Statistical analysis was performed using the Comprehensive Meta-Analyses-CMA software, version 3.0 (Biostat, Engewood, NJ, United States).
Results:
The meta-analysis of the 48 studies obtained a paucibacillary prevalence rate in Brazil of 50.5% or 0.505 (95% CI = 0.502–0.509).The differences in the analyzes were statistically significant (Q-value 4302.681;df 47; I 98.905), with a high heterogeneity value evidenced by I2 (98.905). This analysis demonstrated that the frequency in the Midwest region was the highest and the South region was the lowest (21.4%). Begg’s (Kendall Tau p = 0.35) and Egger’s tests (p = 0.20) were performed, in which no high publication bias was noted. Subgroup analysis indicated that paucibacillary cases varied from region to region, with the Midwest region having the highest prevalence and the South region having the lowest.
Conclusion:
The results stand out significantly for the research gaps that investigate PB cases, requiring more research aimed at investigating the paucibacillary clinical form that can contribute to the early diagnosis of leprosy.
Systematic review registration:
PROSPERO code: CRD42024514106.
1 Introduction
Leprosy is a chronic infectious disease caused by the agents Mycobacterium leprae and recently discovered Mycobacterium lepromatosis, characterized by its high disabling power that can present itself in different clinical forms with dermatological and neurological signs and symptoms, with affinity for phagocytic cells, such as macrophages and Schwann cells. It has exogenous transmission through the upper airways, occurring through close and prolonged contact with the untreated bacilliferous patient (1–3).
According to the World Health Organization (WHO), leprosy remains a persistent challenge to public health. Despite significant global efforts and established strategies, indicators continue to point to the persistence of morbidity. The current strategy adopted by the WHO aims to interrupt transmission and achieve the elimination of indigenous cases, aiming to achieve a zero-incidence rate for leprosy [3]. Brazil in recent years occupies the second position in terms of leprosy cases in the world, behind only India. According to the 2022 epidemiological bulletin from the Ministry of Health (MS), between 2016 and 2020, 155,359 new cases were registered, with 2020 being the year with the lowest global detection rate, which is associated with a reduction in diagnoses due to overload in health services during the COVID-19 pandemic in which health services were overloaded (4).
The persistence of the spread of the disease is associated with several issues, with late diagnosis highlighted as one of the main factors, as it favors the spread between contacts and contributes to the advancement of the condition, resulting in the development of disabilities that compromise nervous function (5, 6). Inadequate diagnosis, especially when differentiating leprosy cases, reveals a hidden presence of the disease, compromising the accuracy of officially reported epidemiological data (7). Furthermore, delay in diagnosis becomes an alarming factor that contributes to the emergence of disabilities, which consequently cause numerous biopsychosocial and economic impacts for both the individual and society (8). Territorial expansion and population increase are correlated with the advancement of leprosy, being determinant for the high detection rates of the disease (9). In this context, early identification combined with timely administration of treatment can reduce the chances of progression and worsening of leprosy (10).
The manifestation of clinical forms of leprosy is linked to the host’s immune response. The WHO classifies patients based on the number of lesions and/or smear results, with additional categorization as multibacillary in cases of uncertainty. Paucibacillary (PB) individuals have up to five skin lesions on sputum smear microscopy, while Multibacillary (MB) individuals have six or more lesions and/or a positive result on sputum smear microscopy. The Madrid classification considers clinical, microscopic, bacteriological, immunological and histological aspects, dividing leprosy into indeterminate, tuberculoid, dimorphic or borderline and lepromatous forms. The WHO defines as paucibacillary those with indeterminate, tuberculoid or borderline-tuberculoid forms, and a bacilloscopic index of less than five lesions in all sites surveyed (11).
The paucibacillary classification of leprosy refers to the initial phase of the disease, characterizing individuals with less symptomatic or asymptomatic forms. This category is marked by the limited presence of skin and neurological lesions, with a low bacterial load, making these individuals less likely to be significant sources of transmission. Diagnosis faces challenges due to the similarity of clinical manifestations with several other dermatological conditions, such as pityriasis alba, granulomatous rosacea, granuloma nulla, among others (12). The detection of leprosy in the PB clinical form faces challenges due to several factors, including the lack of confirmatory laboratory methods. Although the effectiveness of real-time PCR (qPCR) has been demonstrated with respect to specificity and sensitivity in several studies, more comprehensive investigations are needed to evaluate its applicability in clinical practice (13).
Considering the challenging detection of the paucibacillary form in individuals with leprosy, the clinical evolution of the disease tends to worsen and progress to the multibacillary form. Due to this scenario, confirmed cases of paucibacillary leprosy are less frequent in Brazil compared to multibacillary cases (14). However, the prevalence of cases, especially in endemic regions, is predominantly associated with intense local transmission. Given this scenario, in endemic regions, targeting surveillance initiatives to prioritize early diagnosis, treatment and assessment of contacts become essential to interrupt the transmission chain (15).
Systematic analyzes provide a high degree of evidence in clinical epidemiology, playing a crucial role in guiding decisions in both public and private spheres. Meta-analysis, in turn, is a statistical approach that synthesizes the results of two or more independent studies that address the same research question, consolidating the summarized data into a comprehensive statistical measure (16). In this sense, we intend to carry out a systematic review and meta-analysis study to understand the epidemiological situation of paucibacillary (PB) cases of leprosy in Brazil, providing a quantitative assessment of these factors.
This research can help identify patterns of geographic variation and distribution, highlighting the regions with the highest incidences, contributing to the development of specific strategies in the most affected regions, and may be fundamental for directing public health policies, guiding prevention strategies and early diagnosis. In addition to contributing significantly to the scientific literature, providing a synthesis of the available evidence from the last 20 years regarding the prevalence of PB cases, providing important insights to identify areas in which more research is needed related to the topic, as well as providing information for researchers, healthcare professionals, health and policy development. In this sense, the research question guiding this study is: what is the prevalence of paucibacillary cases in the last 20 years in Brazil?
2 Materials and methods
2.1 Type of study, protocol, and registration
This is a systematic review and meta-analysis with the objective of determining the prevalence of paucibacillary (PB) cases in relation to leprosy cases in Brazil, using data published in the literature. Following the recommendations of the Preferred Report Items of a Systematic Review and Meta-Analyses (PRISMA) 2020 Statement (17). This ratio meta-analysis was carried out in this study for two correlated investigations: (I) To identify the prevalence of paucibacillary cases in Brazil and (II) to determine the prevalence of cases by region. The article was registered on the PROSPERO platform with the code CRD42024514106.
2.2 Research strategy
Regarding the purpose of this review, the formulation of the research question was based on the acronym POT (Population, Outcome and Type of study), being derived from the acronym PICO (Population, Intervention, Comparator and Outcome). Which consisted of the following question: “What is the prevalence of paucibacillary cases in the last 20 years in Brazil?” The anagram for its formation was composed of “P” for problem (paucibacillary cases), “O” for outcome (prevalence of cases in Brazil) and T for type of study (original and epidemiological studies).
The databases investigated for the search were PubMed (accessed October 17, 2023),1 SciELO (accessed on October 17, 2023),2 Science Direct (accessed October 17, 2023),3 and LILACS (via VHL) (accessed on October 17, 2023),4 as these databases in Latin America have weight and contain the majority of Brazilian publications.
The health descriptors available in Medical Subject Headings (MeSH) were used. The search strategy that was developed for the databases had the following descriptors: (((“Brazil” [Mesh]) AND (“Leprosy, paucibacillary” [Mesh])) AND “Epidemiology” [Mesh]). And after the search, filters related to eligibility criteria such as full texts, languages and publication period were applied.
2.3 Eligibility criteria
Original studies published in Portuguese, English or Spanish, from January 2003 to October 2023, focused on inclusion criteria of particular types of research designs (descriptive and analytical observational studies) of the types case–control, cohort, cross-sectional, epidemiological, and with sufficient data for numerical extraction with separate data from clinical forms (paucibacillary and multibacillary) were eligible. For the research provided to this meta-analysis, only observational studies were selected as the selection technique since bias can arise in original studies due to defects in the included study’s design, which tend to distort the size or direction of relationships in the data (18).
Brief/short communications, letters to the editor, clinical trials, reviews, editorials, articles unavailable in their complete form, articles outside the stipulated time frame, review articles and studies outside the inclusion criteria mentioned above were excluded.
2.4 Study selection and data extraction
Data was collected from all databases on October 17, 2023. The selection process was carried out in two phases. In the first phase, two researchers (BEBG and MJAS) independently screened the titles and abstracts to identify eligible studies using online software (Rayyan, Qatar Computing Research Institute). Then, in the second phase, three researchers (BEBG, AMPR and AVCB) read the eligible studies in full. Any disagreements between the three reviewers regarding the inclusion, exclusion and/or extraction of research data were resolved by re-evaluation and agreement between their two supervisors (DMS and MJAS) to make the final decision. For the qualitative analysis, only studies that met the minimum eligibility criteria were included.
For data extraction and synthesis, all extracted data were stored in Microsoft Excel file format and the following information was extracted from each study: Authors and year of publication; Title; Kind of study; Data base; Purpose of the article; Location, State and Brazilian Region analyzed in the appropriate added study and period; Total population analyzed; Operational classification with the occurrence of multibacillary cases (n, %) and paucibacillary cases (n; %); and JBI score.
2.5 Assessment of methodological quality
Quality assessment was performed by two researchers (DMS and BEBG) using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Analytical Cross-Sectional Studies (0–8), the JBI Checklist for Case Studies- control (0–10), and the JBI Checklist for Cohort Studies (0–11). Only when the conditioned answer was “Yes” were the scores for completing the checklist questions taken into account. A third researcher (MJAS) provided an opinion in case of disagreement (19).
2.6 Statistical analysis
The Comprehensive Meta-Analyses—CMA software, version 4.0 (Biostat, Engewood, NJ, United States), was used to perform the statistical analyzes of the meta-analysis. The analysis was conducted using a randomized effect approach, considering a p < 0.05 to indicate statistical significance. Heterogeneity was assessed using the I-square test (I2). Furthermore, the Egger and Begg classification correlation tests were applied, together with the use of a funnel plot, to investigate possible publication bias, being considered statistically significant when p < 0.05. Subgroup analysis was carried out according to Brazilian regions and meta-regression was used to explore the origin of the identified heterogeneity.
3 Results
3.1 Selection of the studies
After searching health databases, 313 publications were tracked: 104 PubMed, 1 SciELO, 51 LILACS, 157 Science Direct. Of these results, 249 remained after applying the filters (languages, period of studies and complete texts), screening of relevant titles was carried out (studies with data from paucibacillary cases), 148 studies were excluded, these being because they were not related to the study objective, as they did not provide data to compose the sample and 26 duplicate studies were also excluded. In this sense, we evaluated 75 complete articles in full, for data extraction and composing the sample (Supplementary material S1). After reading and analysis, 27 studies were excluded and 48 were eligible to compose the sample and included in this systematic review (Figure 1).
Figure 1

PRISMA flow diagram (2020) and selection of included studies.
3.2 Characterization of included studies
A total of 48 studies were included in the final analysis. The publication period of the studies was a 20-year segment covering the period from 2003 to 2023. The most frequent language was English, followed by Portuguese. The Northeast (n = 17; 35.42%), North (n = 12; 25%) and Southeast (n = 12; 25%) regions were the most represented in relation to the studies developed (Table 1; Figure 2).
Table 1
| No. | Authors (year) | Title | Kind of study | Data base | Purpose of the article | Location, state, and Brazilian region/period | Total analyzed | Classification | JBI score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MB | n(%) | PB | n(%) | |||||||||
| 1 | Paz et al. (2021) | Association of the polymorphism of the vitamin D receptor gene (VDR) with the risk of leprosy in the Brazilian Amazon (62). | Case–control | PUBMED | To evaluate the association of SNPs FokI (rs2228570), TaqI (rs731236), ApaI (rs7975232) of the VDR gene with leprosy. | Rondom do Pará, Cúrialândia, Goiânia and cities of Redenção, state of Pará, Region | 157 | 100 | 24.60 | 57 | 14.10 | 8/10 |
| 2 | Teixeira et al. (2010) | Epidemiological and clinical characteristics of leprosy reactions in paucibacillary and multibacillary individuals treated at two leprosy reference centers in the city of Recife, state of Pernambuco (63) | Cross-sectional | SciELO, LILACS | Describe the epidemiological and clinical characteristics of leprosy reactions in paucibacillary and multibacillary individuals. | Amazon, Brazil, North | 201 | 101 | 50.20 | 100 | 49.80 | 8/8 |
| 3 | Da Silva et al. (2021) | Epidemiological scenario of leprosy and differences by sex (64) | Ecological time series study | LILACS | Describe the epidemiological scenario of leprosy according to clinical and demographic characteristics among female and male individuals. | Municipality of Paulo Afonso, in Bahia, Northeast (2014–2017) | 130 (new cases) | 58 | 44.60 | 70 | 53.90 | 9/9 |
| 4 | De Souza and Silva Matos (2017) | Characterization of leprosy in children under 15 years in an important municipality of north-eastern Brazil (53) | Observational, cross-sectional and descriptive | LILACS | To identify the family, clinical and epidemiological characteristics of individuals under 15 years of age who were diagnosed with leprosy in the city of Juazeiro-BA. | Juazeiro-BA/Brazil, (2012–2014) | 42 (NEW CASES) | 4 | 9.50 | 38 | 90.50 | 6/8 |
| 5 | Patrocínio et al. (2005) | Detection of Mycobacterium leprae in nasal mucosa biopsies by the polymerase chain reaction (65) | Cross-sectional | ScienceDirect, PUBMED | To evaluate the clinical application of PCR for detecting M. leprae in lower nasal turbinate biopsies in untreated leprosy patients and their contacts. | Uberlândia, MG, Brazil, Southeast | 52 | 37 | 71.20 | 15 | 28.80 | 6/8 |
| 6 | Marciano et al. (2018) | Epidemiological and geographical characterization of leprosy in a Brazilian hyperendemic municipality (8) | Retrospective, observational and descriptive ecological design | SciELO, PUBMED | To identify the distribution pattern of leprosy in a hyperendemic municipality in Brazil and determine its relationship with the clinical-epidemiological situation over 11 years. | Rondonópolis, state of Mato Grosso/Brazil, mid-west (2000–2010) | 1832 | 921 | 50.4 | 906 | 49.6 | 9/9 |
| 7 | Da Cruz Silva et al. (2015) | Epidemiological aspects of leprosy in Juazeiro-BA, from 2002 to 2012 (66) | Descriptive, cross-sectional | SciELO, PUBMED | To analyze the epidemiology of leprosy in Juazeiro-BA, from 2002 to 2012. | Juazeiro-BA/Brazil, Northeast (2002–2012) | 1916 | 810 | 42.30 | 1,106 | 57.70 | 9/9 |
| 8 | Moraes et al. (2023) | Epidemiological characteristics of leprosy from 2000 to 2019 in a state with low endemicity in southern Brazil (28) | Retrospective observational | ScienceDirect, SciELO, PUBMED | Characterize the epidemiological profile of leprosy in the state of Rio Grande do Sul from 2000 to 2019. | Rio Grande do Sul/Brazil, South (2000–2019) | 4,233 | 3,318 | 79 | 882 | 21 | 9/9 |
| 9 | De Sousa et al. (2012) | Epidemiological Profile of Leprosy in the Brazilian state of Piauí between 2003 and 2008 (67) | Descriptive | SciELO | To evaluate the clinical and epidemiological profile of patients with leprosy between 2003 and 2008 in the Brazilian state of Piauí and analyze the detection and prevalence rates in the general population and in the child population under 15 years of age. | Piauí/Brazil, northeast (2003–2008) | 12,238 | 5,632 | 46.02 | 6,551 | 53.53 | 6/8 |
| 10 | Moreira et al. (2014) | Epidemiological situation of leprosy in Salvador from 2001 to 2009 (68) | Retrospective cross-sectional | SciELO | To analyze the epidemiological situation, the detection rate and evaluate the clinical and epidemiological profile of leprosy in Salvador, in the period 2001–2009. | Salvador-BA/Brazil, Northeast (2001–2009) | 3,226 | 1,535 | 47.6 | 1,667 | 51.7 | 6/8 |
| 11 | Da Corrêa et al. (2012) | Epidemiological, clinical, and operational aspects of leprosy patients assisted at a referral service in the state of Maranhão, Brazil (23) | Cross-sectional | SciELO, LILACS, PUBMED | Describe the epidemiological, clinical and operational aspects of leprosy patients. | São Luis - MA, northeast (2008–2009) | 85 | 62 | 72.90 | 23 | 27.10 | 9/9 |
| 12 | Matos et al. (2021) | Epidemiological, neurofunctional profile and prevalence of factors associated with the occurrence of physical disabilities due to leprosy in a reference center in Northeast Brazil: a sectional study (69) | Cross-sectional | SciELO, PUBMED | To describe the epidemiological and neurofunctional profile, as well as the prevalence of factors associated with the occurrence of physical disabilities due to leprosy in a reference center in Northeast Brazil. | Juazeiro-BA/Brazil, Northeast (JAN-JUN, 2018) | 50 | 26 | 52 | 24 | 48 | 6/8 |
| 13 | Albuquerque et al. (2020) | Epidemiological, temporal and spatial dynamics of leprosy in a municipality in northeastern Brazil (2008–2017): an ecological study (70) | Ecological | SciELO, LILACS, PUBMED | To analyze the epidemiological, temporal and spatial dynamics of leprosy in a municipality in the Northeast of Brazil. | Arapiraca-AL/Brazil, Northeast (2008–2017) | 292 | 136 | 46.60 | 156 | 53.40 | 8/9 |
| 14 | Francisco et al. (2019) | Estimation of the hidden prevalence of leprosy in a municipality in the interior of the State of São Paulo (9) | Descriptive, epidemiological | LILACS | Analyze the leprosy situation in a municipality in the interior of the State of São Paulo in relation to the global situation, and calculate the hidden prevalence | Municipality in the interior of the State of São Paulo, (2006–2016) | 295 cases reported | 221 | 74.10 | 74 | 25.90 | 9/9 |
| 15 | Durães et al. (2010) | Epidemiological study of 107 familial leprosy outbreaks in the city of Duque de Caxias - Rio de Janeiro, Brazil (71) | Descriptive, epidemiological | SciELO, LILACS | Analyze epidemiological data on the variables: sex, age, years of study, degree of kinship with the IC and type of residential contact (intra-domestic or per-domestic) with the IC in 107 leprosy families. | Duque de Caxias-RJ, (1998–2002) | 1,040 | 176 | 17 | 149 | 14 | 8/9 |
| 16 | Silva et al. (2018) | Factors associated with leprosy in a municipality of the Pre-Amazon region, state of Maranhão, Brazil (72) | Descriptive, analytics | SciELO, LILACS, PUBMED | Evaluate the clinical and epidemiological characteristics of the disease to reduce the detection rate of new cases by 2015. | Buriticupu, Maranhão/Brazil, Northeast (2003–2015) | 879 | 488 | 55.50 | 391 | 44.50 | 6/8 |
| 17 | Monteiro et al. (2019) | Hansen’s disease in children under 15 years old in the state of Tocantins, Brazil, 2001–2012: epidemiological patterns and temporal trends (73) | Descriptive, Epidemiological | PUBMED, LILACS, SciELO | To describe the epidemiological characteristics and temporal trends of leprosy indicators in children under 15 years of age in Tocantins between 2001–2012. | State of Tocantins/Brazil, (2001–2012) | 1,225 | 296 | 24.20 | 929 | 75.80 | 9/9 |
| 18 | Imbiriba et al. (2009) | Leprosy in indigenous populations of Amazonas, Brazil: an epidemiological study in the municipalities of Autazes, Eirunepé and São Gabriel da Cachoeira (2000 to 2005) (74) | Descriptive, epidemiological | SciELO, | Description and analysis of the epidemiological characteristics of leprosy notifications in the municipalities of Autazes, Eirunepé and São Gabriel da Cachoeira, comparing findings between indigenous and non-indigenous people, according to variables of interest. Cases reported in SINAN from 2000 to 2005 were analyzed. | Municipalities of Autazes, Eirunepé and São Gabriel da Cachoeira, Amazonas, Northern Region (2000 to 2005) | 386 | 157 | 40.70 | 228 | 59.30 | 9/9 |
| 19 | Vieira et al. (2014) | Leprosy in Rondônia: incidence and characteristics of reported cases, 2001 to 2012 (75) | Descriptive | SciELO, LILACS | Describe and analyze the incidence of leprosy and the characteristics of cases reported in the State of Rondônia, Brazil, from 2001 to 2012 | Rondônia, Northern Region (2001–2012) | 15,648 | 8,353 | 54.4 | 7,282 | 46.6 | 9/9 |
| 20 | Araújo et al. (2021) | Hanseniasis in the municipality of Western Amazon (Acre, Brazil): are we far from the goal of the World Health Organization?: Hansen and Western Amazon (76) | Ecological, temporal | ScienceDirect, SciELO, PUBMED | Analyze the epidemiological profile and trends of leprosy from 2005 to 2018, in addition to the clinical characteristics of the patients. | Hospital of Sanitary Dermatology, Cruzeiro do Sul, Acre, North (2005 to 2018) | 422 | 307 | 73.9 | 109 | 25.8 | 9/9 |
| 21 | Barreto et al. (2012) | High rates of undiagnosed leprosy and subclinical infection among school children in the Amazon Region (59) | Cross-sectional | PUBMED, SciELO | Determine the prevalence of subclinical infection (defined in this study as seropositivity for IgM anti-PGL-I without clinical signs or symptoms of leprosy) and the prevalence of undiagnosed leprosy among CS in selected municipalities in the state. SHOVEL. | Altamira - PA; Briefs -PA; Castanhal - PA; Marituba - PA; Oriximiná - PA; Paragominas - PA; Parauapebas - PA; Redemption - PA. North (2009–2011) | 87 | 33 | 37.9 | 54 | 62.1 | 9/9 |
| 22 | Figueiredo and Da Silva (2003) | [Increase in leprosy detection rates in São Luís, Maranhão, Brazil, from 1993 to 1998: is the endemic expanding?] (77) | Epidemiological, descriptive | PUBMED, SciELO | Write and analyze aspects of the detection of new cases of leprosy in São Luís from 1993 to 1998 | São Luis - MA, northeast (1993–1998) | 2,796 | 1,263 | 45.17 | 1,533 | 54.83 | 9/9 |
| 23 | Pedrosa et al. (2018) | Leprosy among schoolchildren in the Amazon region: A cross-sectional study of active search and possible source of infection by contact tracing (54) | Cross-sectional | PUBMED | Determine the detection rate of previously undiagnosed leprosy cases among schoolchildren under 15 years of age living in Manaus, Amazonas, Brazil, and their possible source of infection through contact tracing | Manaus, Amazonas, North (2014–2016) | 40 | 8 | 20 | 32 | 80 | 6/8 |
| 24 | De Lima et al. (2015) | Leprosy in a University Hospital in Southern Brazil (55) | Descriptive, retrospective, cross-sectional | SciELO, PUBMED | Evaluate the epidemiological profile of patients monitored at a University Hospital | Hospital de Clinicas UFPR, Paraná, south (2005–2010) | 81 | 57 | 70.32 | 22 | 26.56 | 5/8 |
| 25 | Lima Neto et al. (2020) | Leprosy in children under 15 years of age in a municipality in northeastern Brazil: evolutionary aspects from 2003 to 2015 (56) | Descriptive | SciELO, PUBMED | Evaluate the epidemiological characteristics of the disease in children under 15 years of age | Buriticupu, Maranhão, northeast (2003–2015) | 61 | 20 | 32.8 | 41 | 67.2 | 8/10 |
| 26 | Diniz and Maciel (2018) | Leprosy: clinical and epidemiological study in patients over 60 years in Espírito Santo State – Brazil (78) | Observational, descriptive and retrospective | SciELO, LILACS | Describe the epidemiology and clinical aspects of leprosy cases in individuals over 60 years of age | Espirito Santo, (2001–2011), Southeast Region | 2,510 | 1,030 | 41 | 1,480 | 59 | 9/9 |
| 27 | Monteiro et al. (2014) | Limited activity and social participation after hospital discharge from leprosy treatment in a hyperendemic area in northern Brazil (79) | Cross-sectional | SciELO, PUBMED | Characterize the limitation of activity and social participation and its correlation with disabilities and/or disabilities in individuals after discharge from multidrug therapy for leprosy. | Araguaína, Tocantins, North (2004–2009) | 282 | 112 | 39.7 | 170 | 60.3 | 8/8 |
| 28 | Reis et al. (2014) | Mycobacterium leprae DNA in peripheral blood may indicate a bacilli migration route and high-risk for leprosy onset (80) | Cross-sectional | ScienceDirect, PUBMED | To evaluate the presence of M. leprae DNA in peripheral blood samples from leprosy patients and their household contacts | CREDESH, Minas Gerais, southeast | 1,026 | 775 | 75.5 | 251 | 24.5 | 6/8 |
| 29 | De Abreu et al. (2014) | Mycobacterium leprae is identified in the oral mucosa from paucibacillary and multibacillary leprosy patients (81) | Cross-sectional, retrospective | ScienceDirect, PUBMED | Investigate the presence of M. leprae, through IHC and PCR, in the oral mucosa of leprosy patients and compare the results of PB and MB patients | Institute of Tropical Medicine, Faculty of Medicine, University of São Paulo, Southeast Region | 50 | 29 | 58.00 | 21 | 42 | 6/8 |
| 30 | Dennison et al. (2021) | Mycobacterium leprae–helminth co-infections and vitamin D deficiency as potential risk factors for leprosy: A case–control study in south-eastern Brazil (82) | Case–control | ScienceDirect, PUBMED | Investigate possible associations between leprosy, helminth infections and micronutrients | Governador Valadares and Mantena, Minas Gerais, southeast (2016–2018) | 79 | 53 | 67 | 23 | 29.1 | 8/10 |
| 31 | Da Martins et al. (2015) | Nasal mucosa study of leprosy contacts with positive serology for the phenolic glycolipid 1 antigen (83) | Cross-sectional, prospective | ScienceDirect, SciELO, PUBMED | Identify specific and early leprosy lesions through endoscopic, bacilloscopy, histopathological examinations and real-time polymerase chain reaction of the nasal cavity mucosa in household and household contacts with positive serology for the phenolic glycolipid antigen 1. | Duque de Caxias, Rio de Janeiro, Southeast Region (2003–2006) | 31 | 24 | 77.4 | 7 | 22.6 | 6/8 |
| 32 | Guzman (2011) | Clinical-epidemiological profile of leprosy cases reported in the municipality of Assis Brasil, Acre, from 2003 to 2010 (84) | Epidemiological, descriptive | LILACS | To describe the epidemiological characteristics of leprosy in the municipality of Assis Brasil, in Acre, from 2003 to 2010. | Assis Brasi, Acre (2003–2010) | 25 | 17 | 68 | 8 | 32 | 9/9 |
| 33 | Franco et al. (2014) | Profile of cases and risk factors for leprosy, in children under 15 years of age, in a hyperendemic municipality in the northern region of Brazil (85) | Ecological, longitudinal | LILACS | Demonstrate the temporal pattern of leprosy, clinical aspects and contact relationships, in children under 15 years of age, in an area of a former leprosy colony in the North of Brazil, | Igarapé Açu, Pará, north (2003–2013) | 29 | 10 | 34.5 | 19 | 65.5 | 9/9 |
| 34 | Gomes et al. (2017) | Epidemiological profile of leprosy in a hyperendemic municipality in northeastern Brazil (86) | Epidemiological, cross-sectional and descriptive | SciELO, LILACS | Analyze the epidemiological profile of leprosy in Floriano, Piauí. | Floriano, Piauí. Northeast (2009–2013) | 388 | 170 | 43.81 | 217 | 55.93 | 9/9 |
| 35 | Pereira et al. (2011) | Epidemiological profile of leprosy in the municipality of Teresina, in the period 2001–2008 (87) | Descriptive | SciELO, LILACS | Describe the epidemiological profile of the municipality of Teresina from 2001–2008. | municipality of Teresina, northeast (2001–2008) | 5,921 | 2,231 | 38 | 3,685 | 62 | 9/9 |
| 36 | Melão et al. (2011) | Epidemiological profile of patients with leprosy in the extreme south of Santa Catarina, from 2001 to 2007 (88) | Cross-sectional and retrospective study | SciELO, LILACS | To know the profile of patients with leprosy in the municipalities of the Association of Municipalities of the Carboniferous Region (AMREC), from 01/01/2001 to 12/31/2007 | Association of Municipalities of the Carboniferous Region, Santa Catarina, South (2001–2007) | 54 | 27 | 50 | 26 | 48.15 | 6/8 |
| 37 | Azevedo et al. (2021) | Epidemiological profile and spatial distribution of leprosy in Paulo Afonso, Bahia (89) | Descriptive epidemiological | SciELO, LILACS | analyze the epidemiological characteristics and distribution of new leprosy cases in the population of Paulo Afonso, Bahia, between 2000 and 2015. | Paulo Afonso, Bahia, Northeast (2000 and 2015). | 1,069 | 403 | 37.7 | 666 | 62.30 | 8/9 |
| 38 | Santos et al. (2020) | Epidemiological profile and leprosy trend in children under 15 years of age (27) | Quantitative, cross-sectional and descriptive | SciELO, LILACS | To evaluate the epidemiological characteristics and trend of new cases of leprosy in children under 15 years of age in the state of Bahia, Brazil, between 2007 and 2017 | In the state of Bahia, northeast Brazil, between 2007 and 2017 | 2,298 | 844 | 37 | 1,454 | 63.27 | 9/9 |
| 39 | Freitas et al. (2017) | Sociodemographic, clinical and epidemiological profile of leprosy in children under 15 years of age, Mato Grosso, Brazil (90) | Cross-sectional epidemiological | LILACS | Characterize the sociodemographic, clinical and epidemiological profile of leprosy in children under 15 years of age registered in Mato Grosso, from 2001 to 2013 | Mato Grosso, Midwest (2001–2013) | 2,455 | 722 | 29.40 | 1,652 | 67.30 | 9/9 |
| 40 | De Camargo et al. (2018) | Polymorphisms in the TGFB1 and IL2RA genes are associated with clinical forms of leprosy in Brazilian population (91) | Cross-sectional and descriptive | SciELO, PUBMED | We performed an association study for IL2RA and TGFB! genes with clinical forms of leprosy based on two case–control samples. These scenes encode molecules important for the immunosuppressive activity of Trea cells and present differential expressions according to the clinical forms of leprosy. Furthermore. IL2RA is a positional candidate gene because it is located close to the region of chromosome 10o13. presenting a link to PB leprosy | Rondonópolis/Mato Grosso/Center-West, São Paulo/southeast | 885 | 690 | 77.97 | 195 | 22.03 | 9/10 |
| 41 | Pacheco et al. (2014) | Prevalence and control of leprosy: research in an urban settlement in São Luís, Maranhão, Brazil (92) | Descriptive retrospective | LILACS | To investigate the prevalence of disabilities in patients with Leprosy in three Basic Health Units (UBS) in São Luís in Maranhão and preliminarily discuss possible ways to control the disease. | Urban occupation of São Luís, Maranhão, Brazil 2008–2009 | 24 | 21 | 87.5 | 3 | 12.5 | 6/8 |
| 42 | De Sousa et al. (2021) | Reviewing the therapeutic management of leprosy in primary care: demand case series referred to a University Hospital in the Midwest region of Brazil (93) | Analytical retrospective | SciELO, PUBMED | To analyze the therapeutic management of leprosy patients referred from primary health services to a specialized service. | University Hospital of the Midwest region of Brazil (2016–2017) | 225 | 210 | 93.3 | 14 | 6.22 | 9/9 |
| 43 | Goulart et al. (2008) | Risk and protective factors for leprosy development determined by epidemiological surveillance of household contacts (57) | Descriptive and cross-sectional | Pubmed | Establish the relative risks or preventive effects of the presence of BCG vaccination, the Mitsuda test and the ML-Flow assay. | Hospital das Clínicas of the Federal University of Uberlândia, Uberlândia, Minas Gerais, Southeast (2002–2007) | 28 | 8 | 28.6 | 20 | 71.40 | 6/8 |
| 44 | Oliveira et al. (2020) | Serological evidence of Toxoplasma gondii infection as potential risk for the development of lepromatous leprosy in an endemic area for both neglected tropical diseases in Brazil (94) | Descriptive analytical | BMC | Investigate the possible influence of M. leprae/T. gondii in the manifestation of leprosy and its clinical forms. | City of Campos dos Goytacazes, state of Rio de Janeiro, Southeast (2015–2019) | 291 | 136 | 46.7 | 63 | 21.6 | 10/10 |
| 45 | Ramos et al. (2021) | Social inequalities and their association with the leprosy burden in a Brazilian city of low endemicity: An ecological study (95) | Ecological | Pubmed | To analyze the association between social inequalities and leprosy burden in a low endemicity scenario in the state of São Paulo, Brazil. | Municipality of Ribeirão Preto, state of São Paulo, southeast, Brazil, 2006–2026 | 608 | 482 | 79.28 | 126 | 20.72 | 7/9 |
| 46 | Barbosa et al. (2020) | Spatial analysis of epidemiological and quality indicators of health services for leprosy in hyperendemic areas in Northeastern Brazil (96) | Ecological | Pubmed, SciELO | Analyze spatial patterns through epidemiological indicators | Pernambuco, Northeast, Brazil 2005–2014 | 28,895 | 14,109 | 49 | 14,740 | 51 | 9/9 |
| 47 | Nicchio et al. (2016) | Spatial and temporal epidemiology of Mycobacterium leprae infection among leprosy patients and household contacts of an endemic region in Southeast Brazil (60) | Epidemiological | Science DIRECT, SciELO | Objective of evaluating the distribution pattern of the disease and the areas at greatest risk of illness, in a highly endemic municipality (Ituiutaba, MG) in the southeast region of Brazil | Municipality of Ituiutaba, in the state of Minas Gerais located in southeastern Brazil, 2004–2014 | 371 | 213 | 57.40 | 158 | 42.60 | 8/9 |
| 48 | Dos Santos et al. (2020) | Study of TNF- α, IFN- γ, TGF- β, IL-6, and IL-10 gene polymorphism in individuals from the leprosy endemic area in the Brazilian Amazon (97) | Cross-sectional | ScienceDirect | Verify the relationship between TNF-ÿ rs1800629 cytokine polymorphisms; IFN-ÿ rs2430561; TGF-ÿ rs1982073 and rs1800471; IL-6 rs180079 and IL-10 rs1800896, rs1800871 and rs1800872 leprosy | Curionopolis, Goianésia do Pará, Canaã dos Carajás, North, Brazil between January and March 2016 | 106 | 28 | 26.42 | 24 | 22.64 | 6/8 |
Characteristics of the included studies.
The absolute frequencies of paucibacillary cases of the disease in each study were kept in bold.
Figure 2
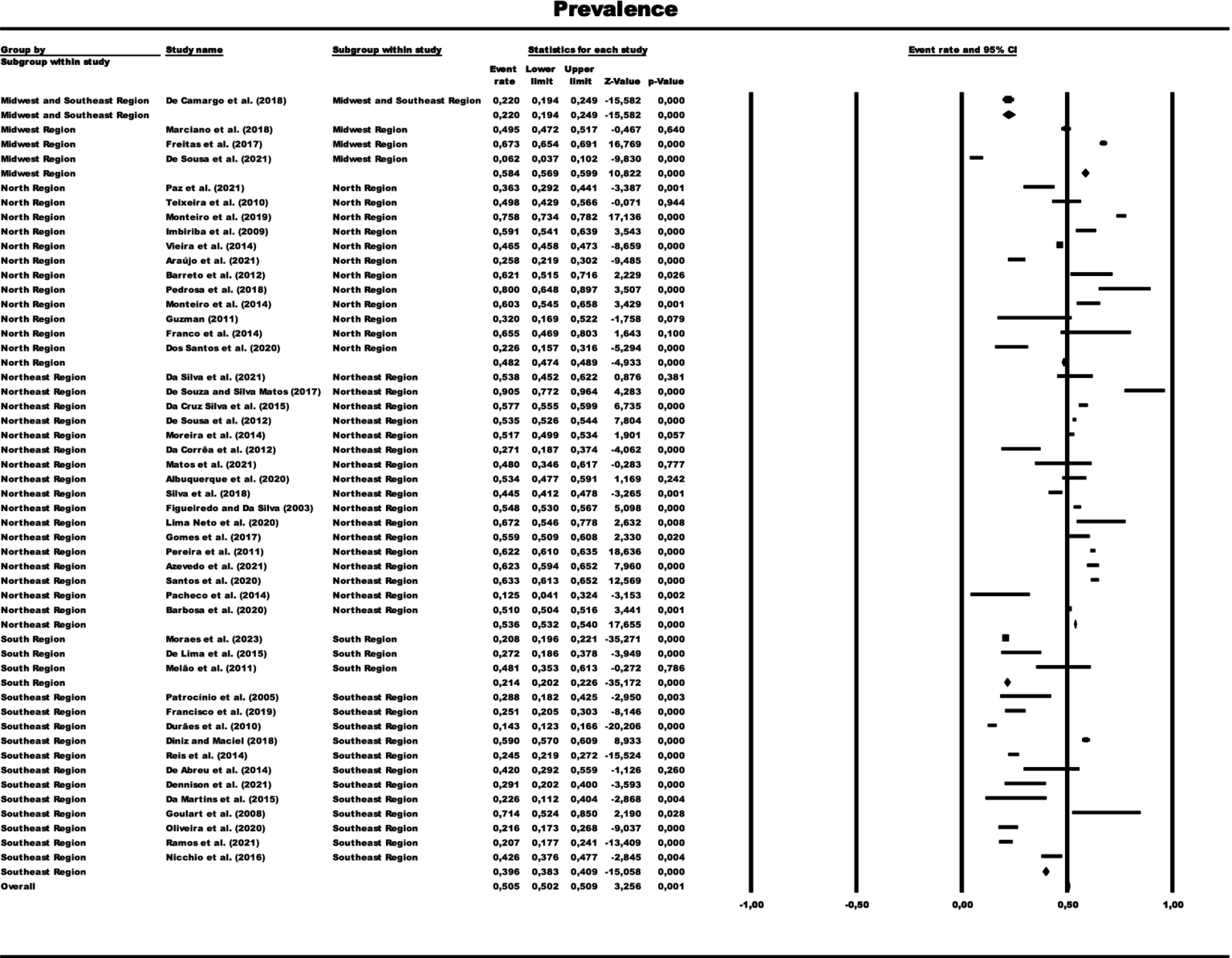
Forest graph representing the prevalence of paucibacillary cases by total sample grouped by subgroups of Brazilian regions. The size of each square corresponds to the weight of the related research in the meta-analysis, representing the Odds Ratio (OR) of each study on the map. The 95% confidence intervals (CI) for OR from each study are shown as horizontal lines. Numbers in bold indicate the overall OR and 95% CI, as well as the total frequency of cases and controls.
3.3 Results of meta-analysis and about the publication bias
In the generalized analysis of 48 studies, the paucibacillary prevalence rate was 50.5% or 0.505 (95% CI = 0.502–0.509). The disparities between the analyzes showed statistically significant differences (Q-value 4302.681; df 47; I2 98.905), meaning that the results had considerable variance between the studies, with heterogeneity evidenced by the high value of I2 (98.905). This analysis demonstrated the Midwest region, with a frequency of 58.4% or 0.584 (95% CI = 0.569–0.599), the Northeast region 53.6% or 0.536 (95% CI = 0.532–0.540), the North region 48.2% or 0.482 (95% CI = 0.474–0.489), Southeast region 39.6% or 0.396 (95% CI = 0.383–0.409), South region 21.4% or 0.214 (95% CI = 0.202–0.226) and finally, a study evaluated two regions (Midwest and Southeast) with 22% (95% CI = 0.194–0.249). A thematic map of these estimated percentage values in Brazilian regions was characterized in Figure 3. Despite the asymmetric distribution of the funnel graph (Figure 4), as it is a proportion-type meta-analysis, when applying the Begg (Kendall Tau p = 0.35) and Egger (p = 0.20) tests resulted in low publication bias.
Figure 3
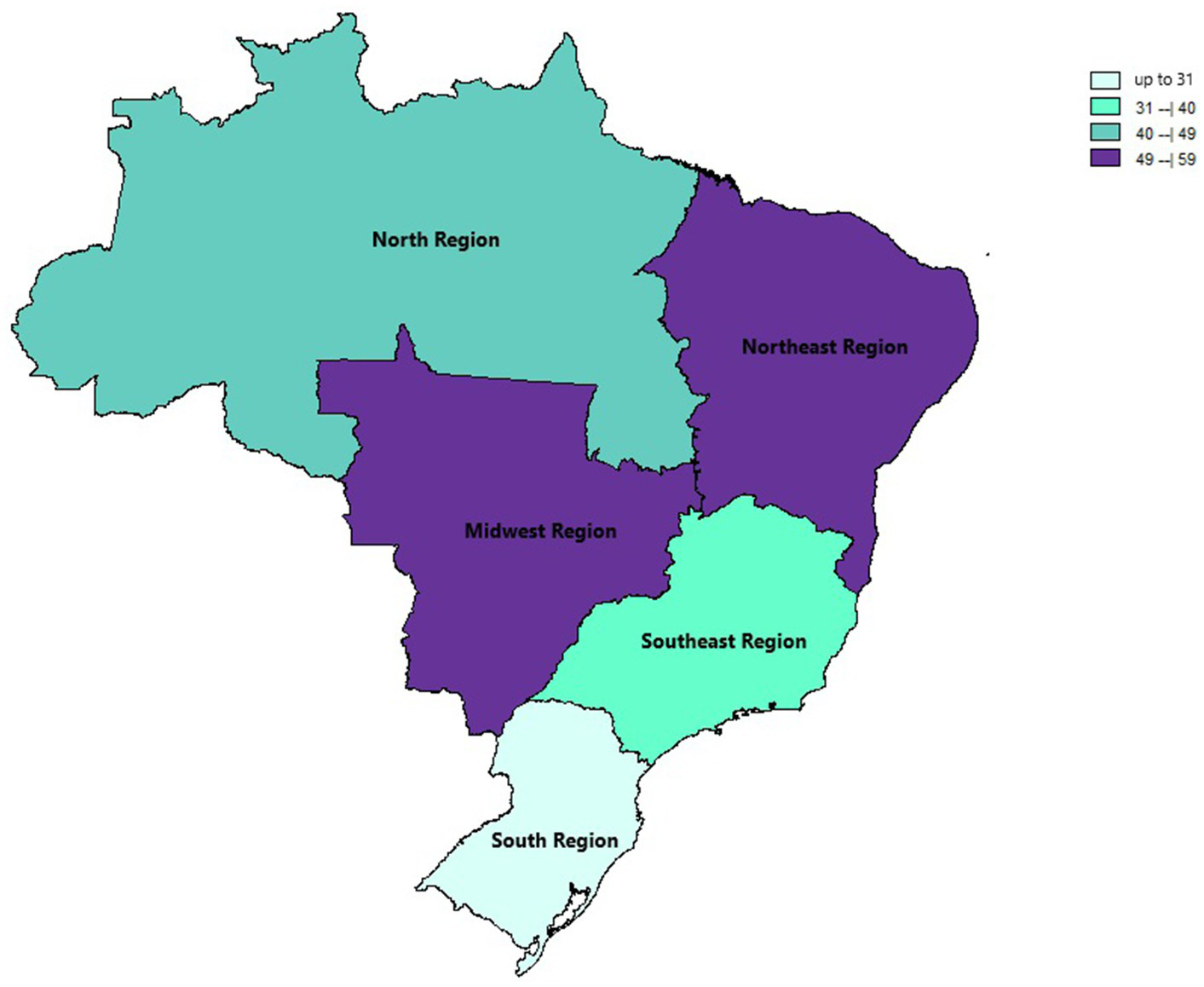
Thematic map of the estimated percentage value (%) of paucibacillary cases with national distribution over the years of this review based on official regional groupings.
Figure 4
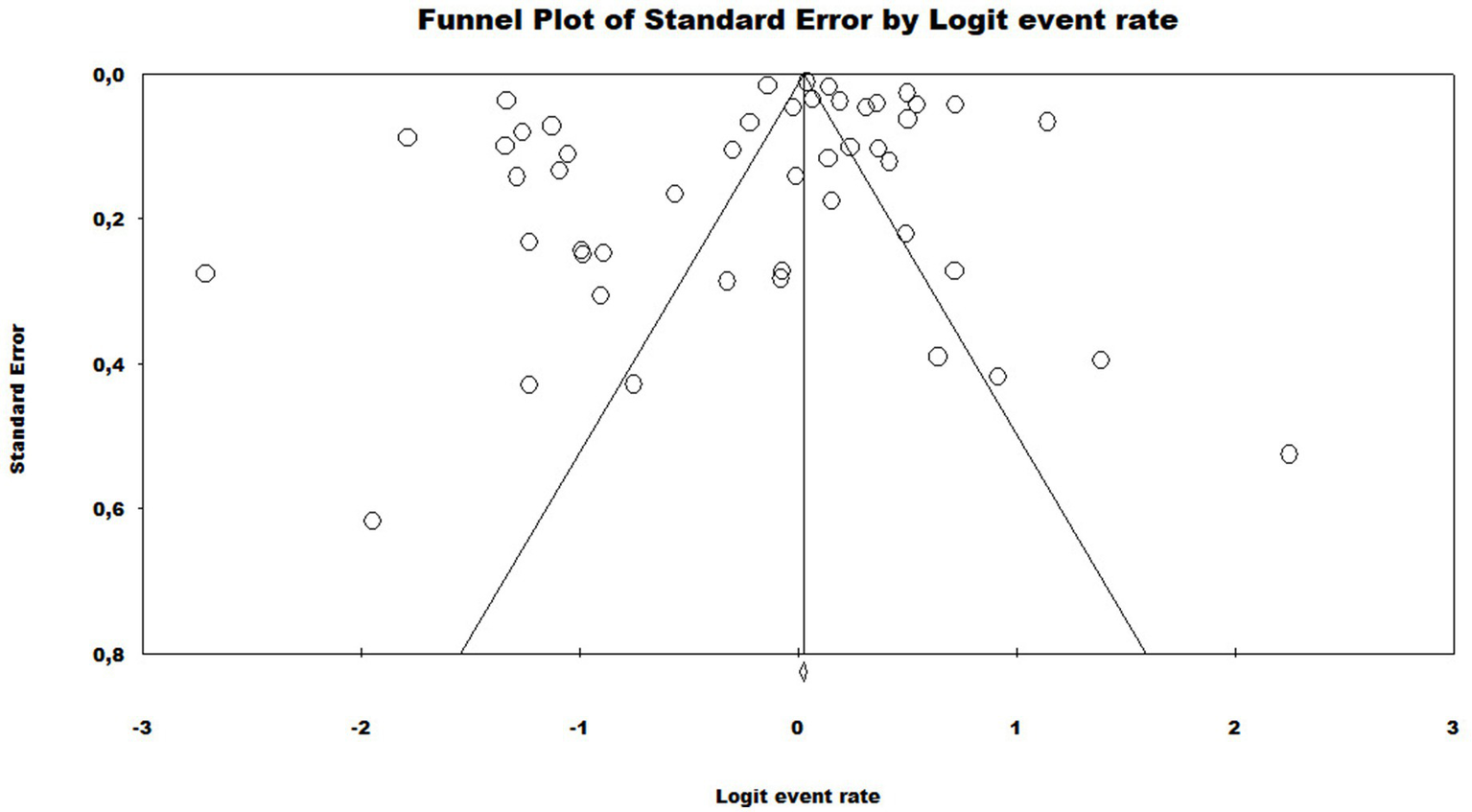
Funnel plot to assess heterogeneity between the studies included in the meta-analysis. Included studies are represented by circles, which should be evenly distributed around the overall effect to resemble an inverted funnel. The narrowest area of the funnel corresponds to the most precise studies, located closest to the real value. The standard error, displayed on the Y axis of the graph as a measure of dispersion, is influenced by the sample size of each study.
3.4 Meta-regression of studies included by year of publication
The results of the meta-regression when using the moderator year of publication of the studies to verify the origin of the bias, the results showed that it had a significant association with the presence of heterogeneity in the findings of the average rates over the years (Q = 21.05; df = 12; p = 0.0497). The possible origin of the high heterogeneity of the data presented in the meta-analysis was found with the moderator “year of publication” in the years 2010 and 2023, indicating that there may be specific factors associated with these years of studies detecting leprosy in the Brazilian population that influence the high heterogeneity found (Figures 5, 6).
Figure 5
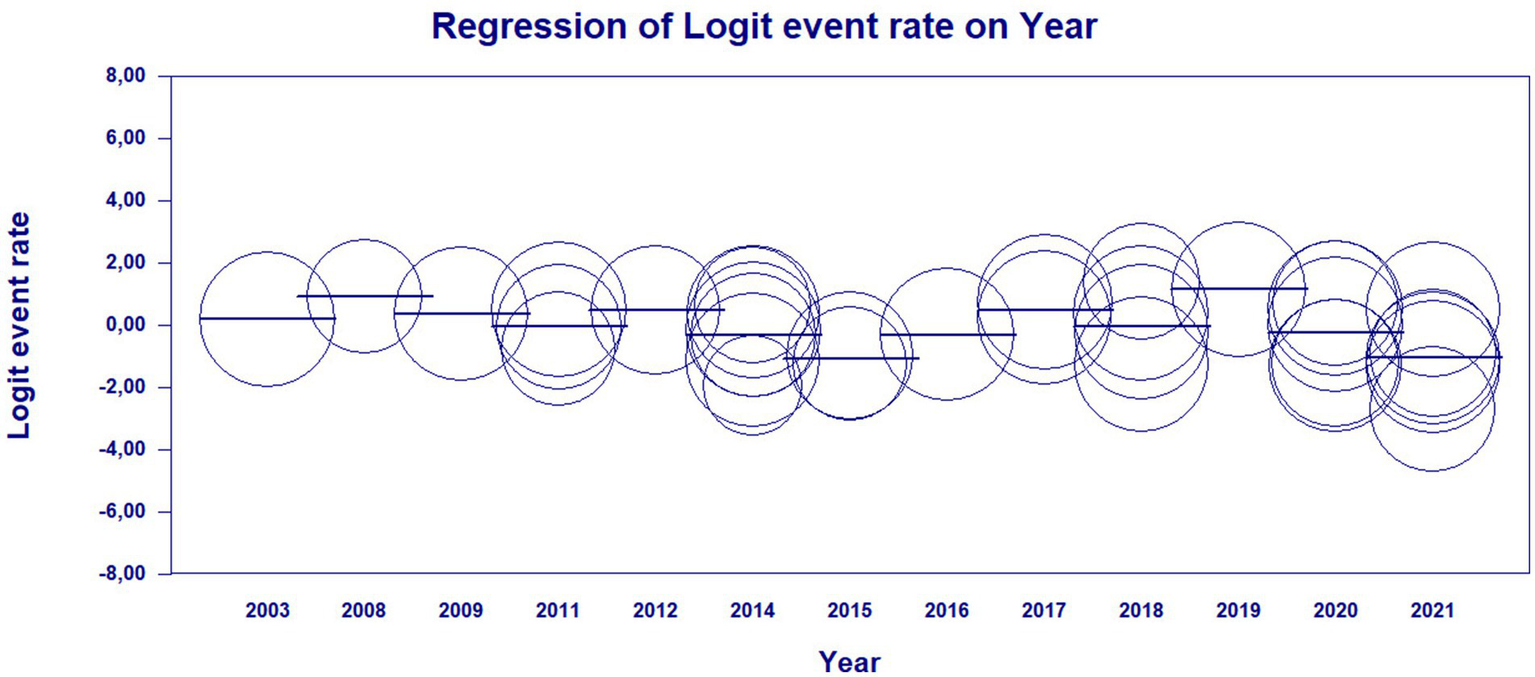
Scatterplot of meta-regression of event rate by covariate, year of study publication. Bubble size is inversely associated with study variance. The solid line reflects the linear regression.
Figure 6
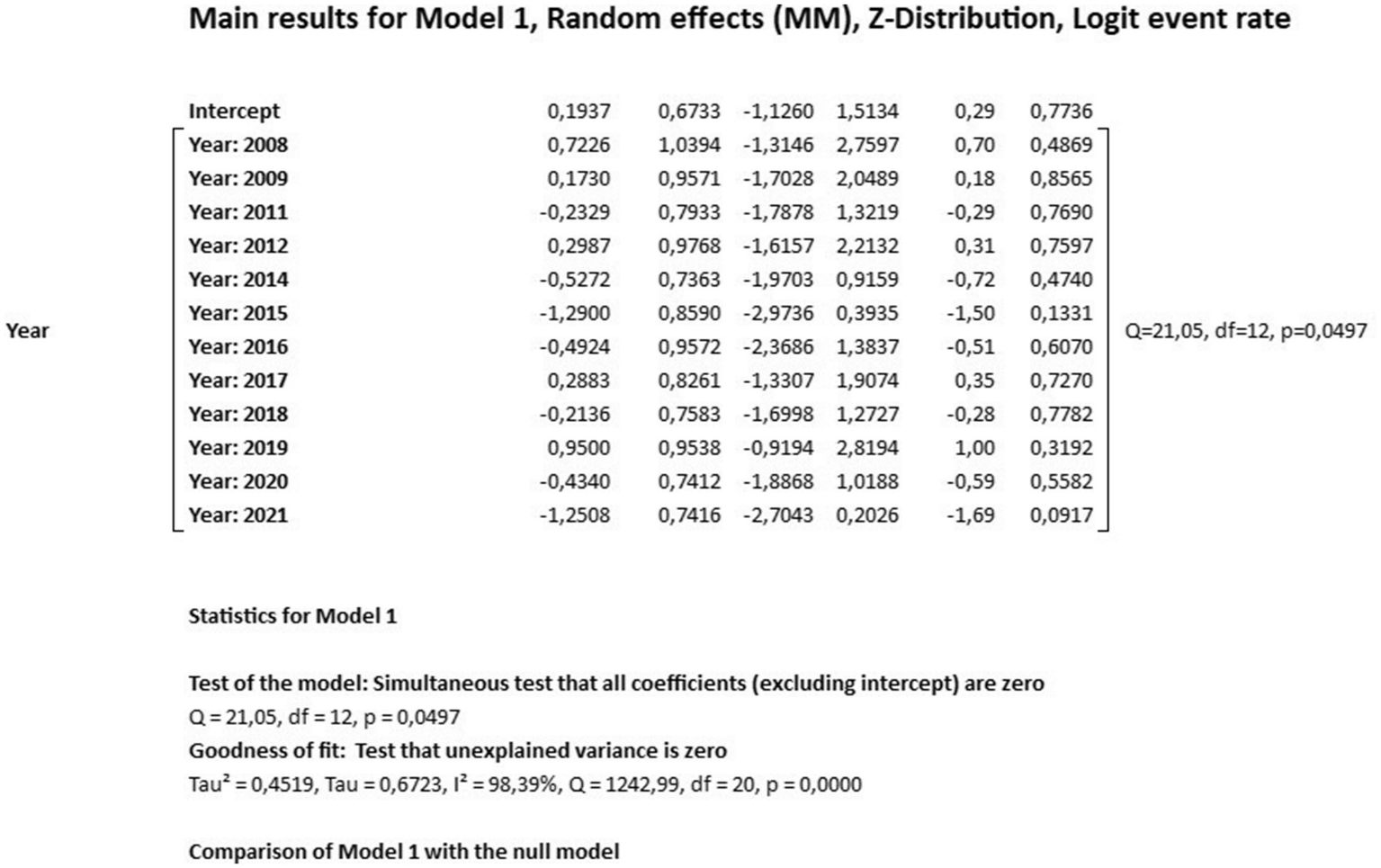
Main results for model, randomized effect, Z distribution and logit event rate.
4 Discussion
This study is among the first systematic attempts to estimate the prevalence of paucibacillary cases in Brazil in the last 20 years. Forty eight articles published between 2003 and October 2023 were analyzed. No systematic review studies on the prevalence of paucibacillary cases in Brazil were identified. In this study, the prevalence of paucibacillary cases was estimated at 50.5%, with a 95% confidence interval ranging from 50.2 to 50.9%, which indicates a relatively close consistency in the proportion of PB leprosy cases in the studies analyzed, with this prevalence being within the specific range and contributing to the understanding of the distribution of PB cases in the studied population. This variation can be influenced by several factors, such as differences in geographic regions, populations analyzed, ages analyzed, data collection methods, among others (20, 21).
The measure of identifying leprosy early is crucial in preventing the transmission of the disease, as well as avoiding the most serious forms of neural disabilities, contributing to the achievement of the global strategy developed by the WHO that aims to interrupt transmission and eliminate cases, which indigenous people with a zero leprosy incidence rate (3, 22).
Research indicates that Brazil exhibits a diverse distribution of leprosy cases, with varying proportions of MB and PB classifications across different regions (23–26). For instance, a study highlighted that only 28% of cases were classified as paucibacillary, indicating a predominance of multibacillary cases (27). This suggests that the prevalence of PB cases is relatively low, particularly in hyperendemic areas. Therefore, this present meta-analysis does not align with the pre-existing literature, considering that only the South Region (characterized by having states with low endemicity) among all the other analyses had a percentage similar to that previously recorded (28). The causes behind these discrepancies in the detection rate of this meta-analysis in comparison with other Brazilian studies already published may arise, for example, from a methodological nature, considering that this systematic review encompassed a broader range of studies over a 20-year period, potentially capturing more recent data and variations in reporting practices. The inclusion of diverse epidemiological study designs (case–control, cohort, cross-sectional) may have provided a more comprehensive picture of leprosy epidemiology. The heightened awareness and improved diagnostic capabilities in recent years may have led to more cases being identified as PB, especially in areas previously underreported. This could explain the higher prevalence rate compared to earlier studies (29).
When comparing the results of this meta-analysis with studies from other countries with similar leprosy burdens, several patterns emerge. In case of India, the country with the highest leprosy burden, it has reported varying prevalence rates of PB cases, often around 50% in certain regions. This similarity suggests that Brazil’s epidemiological landscape may reflect broader trends observed in high-burden countries (30). In countries like Nigeria and Mozambique, studies have indicated that PB cases comprise a significant portion of reported leprosy cases, often due to similar socioeconomic and healthcare challenges (31, 32). The prevalence rates can be comparable to those found in Brazil, highlighting the need for targeted public health strategies. Countries such as Indonesia and Myanmar have shown similar trends in PB prevalence, often influenced by local healthcare infrastructure and public health initiatives. The importance of early detection and treatment in reducing the burden of leprosy is a common theme across these regions (33, 34).
Brazil is still a country with a high leprosy endemicity, with a prevalence >1 per 10,000 inhabitants, representing around 94% of cases among Latin American countries. The epidemiological bulletins show the detection of new cases regarding the clinical form, a greater frequency in the prevalence coefficients of multibacillary cases, with the year 2022 representing 80.2% MB, other studies also brought results similar to the predominance of MB cases, showing late detection and active transmission of leprosy. In 2020 there was a significant reduction in new cases of leprosy compared to 2019, this decrease is probably related to the reduction in detection during the COVID-19 pandemic that affected health services around the world (35–37).Furthermore, a meta-analysis that obtained a high proportion of MB cases in several countries, including Brazil, highlighted the importance of improving the monitoring and treatment of individuals at risk of developing the most severe forms (38). The active search for contacts in these cases should be an important measure for early diagnosis, with household contacts being crucial, since there is a greater risk of developing the disease due to prolonged exposure to untreated patients. However, there is still a lack of studies regarding asymptomatic M. leprae infection, and more research is needed on this topic to facilitate diagnosis in the early stages and detect individuals infected by the bacillus (39).
As it is a slowly evolving disease, there is still no gold standard for diagnosing leprosy. A study that investigated the effectiveness of qPCR in diagnosing PB reports the difficulty and challenges that even experienced specialists encounter in interpreting the clinical differential diagnosis. and histopathological in PB cases, which may be one of the factors responsible for numerous late diagnoses of the disease (12).
Butlin and Lockwood, in a study that evaluated changes in the proportions of PB leprosy cases using global data published by the WHO in a period from 1985 to 2017, demonstrated the continuous decrease in PB cases over the years, in which until 2003 the paucibacillary form was more frequent than the multibacillary clinical form, which may be linked to significant changes in the correct classification of the patient, since therapeutic protocols and guidelines recommend that in case of doubts about the PB and MB operational classification, it can be classified as multibacillary (40). The authors highlight the need to improve the accuracy of the categorization of clinical forms, with more investigations being necessary in the validation of operational forms by national control programs (3).
When analyzing the Brazilian regions, it demonstrated that the Midwest region had the highest frequency (58.4%), followed by the Northeast (53.6%), North (48.2%), Southeast (39.6%) regions. and finally, the South region (21.4%). Similar data were found in an epidemiological study from 2005 to 2015, which obtained the highest leprosy prevalence coefficients in the Midwest, North and Northeast regions, being related to regional socioeconomic inequalities, regarding the development and quality of life of the population between regions of Brazil (41, 42).
This variation in the prevalence of paucibacillary infections highlights the Midwest region with the highest rate, while the South region has a notably lower rate, highlighting disparities between regions that contribute to the persistence of the disease. The vast territorial extension of Brazil, socioeconomic inequalities between regions and the gap in tackling social determinants were identified as important factors responsible for this discrepancy, in which the economically less favored regions stand out as being the most endemic for leprosy (43, 44).
The geographical distribution of leprosy in Brazil is notably uneven, with higher rates of new case detection in the Northern, Northeastern, and Midwest regions. For example, the states of Mato Grosso and Tocantins have reported extremely high new case detection rates (NCDR) of 82.03 and 69.88 per 100,000 inhabitants, respectively (45). This spatial variation points to the influence of socio-economic factors, healthcare access, and environmental conditions that contribute to the epidemiology of leprosy.
The implications of this geographical heterogeneity are profound for subgroup analyses. It necessitates tailored public health interventions that consider local epidemiological patterns. For instance, high-risk areas may require intensified screening and treatment programs, while regions with lower incidence rates might focus on surveillance and education to prevent outbreaks (46). Moreover, understanding the factors that contribute to the higher prevalence of MB cases in certain regions can inform targeted strategies to address the underlying causes of transmission. As a measure of the patient’s capacity to eradicate bacillus, the ratio of multibacillary to paucibacillary instances represents transmission of leprosy (47).
For example, factors such as population density, socioeconomic status, and healthcare infrastructure can significantly affect the distribution of leprosy cases, influencing both detection and classification. Regions with higher levels of poverty often experience worse health outcomes, including higher rates of leprosy. The Northeast region, for instance, has historically faced economic challenges that correlate with increased disease prevalence (48). Lower educational attainment can lead to a lack of awareness about leprosy symptoms and transmission, resulting in delayed diagnosis and treatment. Educational disparities are often pronounced in poorer regions (49). Inadequate housing and sanitation are more prevalent in economically disadvantaged areas, facilitating the spread of infectious diseases like leprosy (50). Access to healthcare services is often limited in rural and impoverished areas. Regions with fewer healthcare facilities may struggle to provide timely diagnosis and treatment for leprosy, affecting the classification of cases as PB or MB (51). Furthermore, robust public health infrastructure and effective public health interventions are crucial for effective disease surveillance and response, so that national and local health policies that prioritize leprosy control can significantly impact prevalence rates (52).
The high heterogeneity presented in the meta-analysis can be justified by the differences and variability in the characteristics of the studies, which may be related to inadequate study design, limitation of the number of samples and also the weakness in the standardization of controls. The meta-regression analyzes showed a significant association between the year of publication of the studies and the heterogeneity of the results, highlighting the years 2010 and 2023 with periods of possible specific influences, which highlights the importance of more studies to investigate the temporality of the leprosy.
Among the studies analyzed in this meta-analysis, an increase in paucibacillary cases was observed in studies that investigated the population under 15 years of age. It is known that detection rates in this age group indicate recent infection, resulting in active transmission of M. leprae between contacts, with early detection especially in endemic locations having significant relevance (53–58). An incompleteness of information was observed in some studies, in which PB and MB data did not correspond to the total number of diagnosed cases, with the total number of diagnosed cases being greater than the sum of PB and MB data, with this information in the studies being represented as “ignored” or “not filled in.” Completing the information is essential for understanding the real epidemiological situation so that actions related to the disease can be planned (59, 60).
The limitations of this study included (I) the search methodology carried out, through the specific descriptors applied; (II) the high heterogeneity of the studies, probably justified due to the period of the studies, differences in the quality of the data, which may have affected the precision of the results; (III) possible information and selection biases, sample representativeness; and (IV) there may be omissions in the included studies.
More studies are needed to investigate the prevalence of the paucibacillary operational classification, which may be crucial for reducing transmission, with treatment of paucibacillary as it has a low bacillary load, and early diagnosis to prevent worsening due to late diagnosis of leprosy. and greater community transmission. The quality of paucibacillary diagnosis and treatment can influence the reduction of active transmission, as it will prevent the evolution of low numbers of bacilli to high bacilli, the multibacillary which is the most important in the transmission of leprosy, so improving paucibacillary surveillance is a strategy effective in reducing the incidence of leprosy, and achieving the goals established by WHO and MS agreements in the coming years in the country (61).
5 Conclusion
This systematic review and meta-analysis provided comprehensive information on the prevalence of paucibacillary cases in Brazil from 2003 to 2023, using data from 48 published studies. The average prevalence rate in the Brazilian population was estimated at 50.5%, which indicates a relatively homogeneous distribution of the PB form of leprosy in the sample studies. The analysis of paucibacillary cases in Brazil is marked by significant geographical heterogeneity, which has critical implications for public health strategies. Addressing these disparities through targeted interventions and localized analyses can enhance the effectiveness of leprosy control efforts in the country. The ongoing monitoring of case classifications and regional trends will be essential in adapting strategies to combat this public health challenge effectively. In this sense, this research can contribute by filling the gap and providing new and relevant data for understanding the prevalence of paucibacillary leprosy in Brazil, in addition to providing important information for the formulation of social policies with public health strategies and guidance for future research on the theme.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
BB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. AR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. AB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. MS: Formal analysis, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. DS: Conceptualization, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KL: Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. LL: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1401685/full#supplementary-material
References
1.
Talhari C Talhari S Penna GO . Clinical aspects of leprosy. Clin Dermatol. (2015) 33:26–37. doi: 10.1016/j.clindermatol.2014.07.002
2.
Deps P Collin SM . Mycobacterium lepromatosis as a second agent of Hansen’s disease. Front Microbiol. (2021) 12:698588. doi: 10.3389/fmicb.2021.698588
3.
Towards Zero Leprosy . (2021). Global leprosy (Hansen’s disease) strategy 2021–2030. Available at:https://www.who.int/publications/i/item/9789290228509; https://www.who.int/publications-detail-redirect/9789290228509 (Accessed March 15, 2024).
4.
White C Franco-Paredes C . Leprosy in the 21st century. Clin Microbiol Rev. (2015) 28:80–94. doi: 10.1128/CMR.00079-13
5.
Steinmann P Reed SG Mirza F Hollingsworth TD Richardus JH . Innovative tools and approaches to end the transmission of Mycobacterium leprae. Lancet Infect Dis. (2017) 17:e298–305. doi: 10.1016/S1473-3099(17)30314-6
6.
Sakae TM de Almeida Pescador M Magajewski FRL . Análise de tendência histórica da evolução da hanseníase em Santa Catarina no período de 2001-2015. Arquiv Catarin Med. (2018) 47:141–58.
7.
Moschella SL . An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. (2004) 51:417–26. doi: 10.1016/j.jaad.2003.11.072
8.
Marciano LHSC De Belone AFF Rosa PS Coelho NMB Ghidella CC Nardi SMT et al . Epidemiological and geographical characterization of leprosy in a Brazilian hyperendemic municipality. Cad Saúde Públ. (2018) 34:e00197216. doi: 10.1590/0102-311X00197216
9.
Francisco LL da Silva CFG Paschoal VDA Nardi SMT . Estimativa da prevalência oculta da hanseníase em município do interior do Estado de São Paulo. Arch Health Sci. (2019) 26:89–93. doi: 10.17696/2318-3691.26.2.2019.1643
10.
Stafin I Guedes VR Mendes SUR . Diagnóstico precoce de hanseníase e ações estratégicas para a sua detecção. Rev Patol Tocant. (2018) 5:67–73. doi: 10.20873/uft.2446-6492.2018v5n2p67
11.
Eichelmann K González SG Salas-Alanis JC Ocampo-Candiani J . Leprosy. An update: definition, pathogenesis, classification, diagnosis, and treatment. Actas Dermosifiliogr. (2013) 104:554–63. doi: 10.1016/j.adengl.2012.03.028
12.
Barbieri RR Manta FSN Moreira SJM Sales AM Nery JAC Nascimento LPR et al . Quantitative polymerase chain reaction in paucibacillary leprosy diagnosis: a follow-up study. PLoS Negl Trop Dis. (2019) 13:e0007147. doi: 10.1371/journal.pntd.0007147
13.
Dharmawan Y Fuady A Korfage IJ Richardus JH . Delayed detection of leprosy cases: a systematic review of healthcare-related factors. PLoS Negl Trop Dis. (2022) 16:e0010756. doi: 10.1371/journal.pntd.0010756
14.
Amorim de Souza E Fuentes Ferreira A Heukelbach J Nzundu Boigny R Alencar CH Novaes Ramos A . Epidemiology and spatiotemporal patterns of leprosy detection in the state of Bahia, Brazilian northeast region, 2001–2014. Trop Med Infect Dis. (2018) 3:79. doi: 10.3390/tropicalmed3030079
15.
Duthie MS Pena MT Khandhar AP Picone A MacMIllen Z Truman RW et al . Development of LepReact, a defined skin test for paucibacillary leprosy and low-level M. leprae infection. Appl Microbiol Biotechnol. (2020) 104:3971–9. doi: 10.1007/s00253-020-10505-2
16.
Alavi Foumani A Yaghubi Kalurazi T Mohammadzadeh Rostami F Sedigh Ebrahim-Saraie H Nazari-Alam A Halaji M . Epidemiology of Pseudomonas aeruginosa in cystic fibrosis patients in Iran: a systematic review and meta-analysis. Infez Med. (2020) 28:314–21. PMID:
17.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18.
Stroup DF . Meta-analysis of observational studies in EpidemiologyA proposal for reporting. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
19.
Aromataris E Munn Z . JBI manual for evidence synthesisJoanna Briggs Institute. Adelaide, Australia (2020).
20.
Penna MLF De MLVDR Penna GO . The epidemiological behaviour of leprosy in Brazil. Lepr Rev. (2009) 80:332–44. doi: 10.47276/lr.80.3.332
21.
Barreto JG Bisanzio D Frade MAC Moraes TMP Gobbo AR de Souza Guimarães L et al . Spatial epidemiology and serologic cohorts increase the early detection of leprosy. BMC Infect Dis. (2015) 15:1–9. doi: 10.1186/s12879-015-1254-8
22.
Carvalho APM Coelho-Fabri ACO Lanza FM Lopes FN Lana FCF . Integration of leprosy control activities from the perspective of health professionals. J Nurs UFPE. (2015) 9:114–20.
23.
Da Corrêa RGCF De Aquino DMC De Caldas AJM Amaral DKCR França FS Mesquita ERRBP-L . Epidemiological, clinical, and operational aspects of leprosy patients assisted at a referral service in the state of Maranhão, Brazil. Rev Soc Bras Med Trop. (2012) 45:89–94. doi: 10.1590/S0037-86822012000100017
24.
Martins RJ Carloni MEOG Moimaz SAS Garbin CAS Garbin AJÍ . Sociodemographic and epidemiological profile of leprosy patients in an endemic region in Brazil. Rev Soc Bras Med Trop. (2016) 49:777–80. doi: 10.1590/0037-8682-0069-2016
25.
Tavares AMR . Epidemiological profile of leprosy in the state of Mato Grosso: descriptive study. Einstein. (2021) 19:eAO5622. doi: 10.31744/einstein_journal/2021AO5622
26.
dos Reis Ferreira TC Monteiro LV Santos APMK da Costa KA Torres LS Falcão LB . Clinical and epidemiological profile of patients with leprosy from the specialized reference unit in sanitary dermatology DR. Marcelo Cândia. Rev CPAQV Centro Pesq Avan Qual Vida. (2020) 12:2
27.
Santos ÁN Costa AKAN De Souza JÉR Alves KAN De Oliveira KPMM Pereira ZB . Epidemiological profile and tendency of leprosy in people younger than 15 years. Rev Esc Enferm USP. (2020) 54:e03659. doi: 10.1590/s1980-220x2019016803659
28.
Moraes PCD Eidt LM Koehler A Ransan LG Scrofeneker ML . Epidemiological characteristics of leprosy from 2000 to 2019 in a state with low endemicity in southern Brazil. An Bras Dermatol. (2023) 98:602–10. doi: 10.1016/j.abd.2022.08.009
29.
Andrade V Ignotti E . Secular trends of new leprosy cases diagnosed in Brazil during 1987-2006. Indian J Lepr. (2008) 80:31–8. PMID:
30.
Rao PN Suneetha S . Current situation of leprosy in India and its future implications. Indian Dermatol Online J. (2018) 9:83–9. doi: 10.4103/idoj.IDOJ_282_17
31.
Daniel O Adejumo O Oritogun K Omosebi O Kuye J Akang G et al . Spatial distribution of leprosy in Nigeria. Lepr Rev. (2016) 87:476–85. doi: 10.47276/lr.87.4.476
32.
De Ribeiro GC Dos Lages DS Lages ATC De Souza VB Marega A Lana FCF et al . Profile of the leprosy endemic in the district of Murrupula, Nampula, Mozambique: a population-based study. Rev Soc Bras Med Trop. (2023) 56:e0321–2022. doi: 10.1590/0037-8682-0321-2022
33.
Dharmawan Y Korfage IJ Abqari U Widjanarko B Richardus JH . Measuring leprosy case detection delay and associated factors in Indonesia: a community-based study. BMC Infect Dis. (2023) 23:555. doi: 10.1186/s12879-023-08552-x
34.
Matsuoka M Roa RIL Budiawan T Kyaw K Chae G-T . Genotypic analysis of Mycobacterium leprae isolates from Japan and other Asian countries reveals a global transmission pattern of leprosy. FEMS Microbiol Lett. (2006) 261:150–4. doi: 10.1111/j.1574-6968.2006.00346.x
35.
Meima A Richardus JH Habbema JDF . Trends in leprosy case detection worldwide since 1985. Lepr Rev. (2004) 75:19–33. doi: 10.47276/lr.75.1.19
36.
Pescarini JM Teixeira CSS Da SNB Sanchez MN Da NMS Rodrigues LC et al . Epidemiological characteristics and temporal trends of new leprosy cases in Brazil: 2006 to 2017. Cad Saude Publica. (2021) 37:e00130020. doi: 10.1590/0102-311x00130020
37.
De Lima LV Pavinati G Silva IGP De Moura DR De Gil NLM Magnabosco GT . Temporal trend, distribution and spatial autocorrelation of leprosy in Brazil: ecological study, 2011 to 2021. Rev Bras Epidemiol. (2022) 25:e220040. doi: 10.1590/1980-549720220040
38.
Yang J Li X Sun Y Zhang L Jin G Li G et al . Global epidemiology of leprosy from 2010 to 2020: A systematic review and meta-analysis of the proportion of sex, type, grade 2 deformity and age. Pathog Glob Health. (2022) 116:467–76. doi: 10.1080/20477724.2022.2057722
39.
Glennie M Gardner K Dowden M Currie BJ . Active case detection methods for crusted scabies and leprosy: a systematic review. PLoS Negl Trop Dis. (2021) 15:e0009577. doi: 10.1371/journal.pntd.0009577
40.
Butlin CR Lockwood DNJ . Changing proportions of paucibacillary leprosy cases in global leprosy case notification. LEPROSY. (2020) 91:255–61. doi: 10.47276/lr.91.3.255
41.
Bakker MI Hatta M Kwenang A Van Mosseveld P Faber WR Klatser PR et al . Risk factors for developing leprosy--a population-based cohort study in Indonesia. Lepr Rev. (2006) 77:48–61. doi: 10.47276/lr.77.1.48
42.
Moschioni C De Antunes CM Grossi MAF Lambertucci JR . Risk factors for physical disability at diagnosis of 19,283 new cases of leprosy. Rev Soc Bras Med Trop. (2010) 43:19–22. doi: 10.1590/s0037-86822010000100005
43.
Niitsuma ENA De Bueno IC Arantes EO Carvalho APM Xavier Junior GF Da Fernandes GR et al . Factors associated with the development of leprosy in contacts: a systematic review and meta-analysis. Rev Bras Epidemiol. (2021) 24:e210039. doi: 10.1590/1980-549720210039
44.
Ribeiro MD Silva JC Oliveira S . Estudo epidemiológico da hanseníase no Brasil: reflexão sobre as metas de eliminação. Rev Panam Salud Publica. (2018) 42:1–7. doi: 10.26633/RPSP.2018.42
45.
Blok DJ Crump RE Sundaresh R Ndeffo-Mbah M Galvani AP Porco TC et al . Forecasting the new case detection rate of leprosy in four states of Brazil: a comparison of modelling approaches. Epidemics. (2017) 18:92–100. doi: 10.1016/j.epidem.2017.01.005
46.
Lockwood DNJ Suneetha S . Leprosy: too complex a disease for a simple elimination paradigm. Bull World Health Organ. (2005) 83:230–5. PMID:
47.
Duarte-Cunha M Marcelo da Cunha G Souza-Santos R . Geographical heterogeneity in the analysis of factors associated with leprosy in an endemic area of Brazil: are we eliminating the disease?BMC Infect Dis. (2015) 15:196. doi: 10.1186/s12879-015-0924-x
48.
Nery JS Ramond A Pescarini JM Alves A Strina A Ichihara MY et al . Socioeconomic determinants of leprosy new case detection in the 100 million Brazilian cohort: a population-based linkage study. Lancet Glob Health. (2019) 7:e1226–36. doi: 10.1016/S2214-109X(19)30260-8
49.
Alves CRP Ribeiro MMF Melo EM Araújo MG . Teaching of leprosy: current challenges. An Bras Dermatol. (2014) 89:454–9. doi: 10.1590/abd1806-4841.20142444
50.
Prakoeswa FRS Ilhami AZ Luthfia R Putri AS Soebono H Husada D et al . Correlation analysis between household hygiene and sanitation and nutritional status and female leprosy in Gresik regency. Dermatol Res Pract. (2020) 2020:4379825–7. doi: 10.1155/2020/4379825
51.
da Mártires GS dos Lima GLS Gomes DE do Lessa AC da Souza CSM Ignotti E et al . Quality of healthcare services to reduce leprosy in Brazil: a trend analysis from 2001 to 2020. Rev Bras Epidemiol. (2024) 27:e240034. doi: 10.1590/1980-549720240034
52.
Awofeso N . Leprosy: international public health policies and public health eras. Admin Sci. (2011) 1:32–44. doi: 10.3390/admsci1010032
53.
De Souza CDF Silva Matos T . Characterization of leprosy in children under 15 years in an important municipality of North-Eastern Brazil. Mundo Saúde. (2017) 41:130–7. doi: 10.15343/0104-7809.20174102130137
54.
Pedrosa VL Dias LC Galban E Leturiondo A Palheta J Santos M et al . Leprosy among schoolchildren in the Amazon region: A cross-sectional study of active search and possible source of infection by contact tracing. PLoS Negl Trop Dis. (2018) 12:6261. doi: 10.1371/journal.pntd.0006261
55.
De Lima AS Pinto KC Bona MPS De Mattos SML Hoffmann MP Mulinari-Brenner FA et al . Leprosy in a University Hospital in Southern Brazil. An Bras Dermatol. (2015) 90:654–9. doi: 10.1590/abd1806-4841.20153959
56.
Lima Neto PM Silva ARD Santos LHD Lima RJCP Tauil PL Gonçalves EDGDR . Leprosy in children under 15 years of age in a municipality in northeastern Brazil: evolutionary aspects from 2003 to 2015. Rev Soc Bras Med Trop. (2020) 53:e20200515. doi: 10.1590/0037-8682-0515-2020
57.
Goulart IMB Bernardes Souza DO Marques CR Pimenta VL Gonçalves MA Goulart LR . Risk and protective factors for leprosy development determined by epidemiological surveillance of household contacts. Clin Vaccine Immunol. (2008) 15:101–5. doi: 10.1128/CVI.00372-07
58.
de Moraes PC Eidt LM Koehler A Pagani DM Scroferneker ML . Epidemiological characteristics and trends of leprosy in children and adolescents under 15 years old in a low-endemic state in southern Brazil. Rev Inst Med Trop São Paulo. 63:e80. doi: 10.1590/S1678-9946202163080
59.
Barreto JG Guimarães LDS Frade MAC Rosa PS Salgado CG . High rates of undiagnosed leprosy and subclinical infection amongst school children in the Amazon region. Mem Inst Oswaldo Cruz. (2012) 107:60–7. doi: 10.1590/S0074-02762012000900011
60.
Nicchio MVC Araujo S Martins LC Pinheiro AV Pereira DC Borges A et al . Spatial and temporal epidemiology of Mycobacterium leprae infection among leprosy patients and household contacts of an endemic region in Southeast Brazil. Acta Trop. (2016) 163:38–45. doi: 10.1016/j.actatropica.2016.07.019
61.
Walker SL Lockwood DNJ . The clinical and immunological features of leprosy. Br Med Bull. (2006) 77-78:103–21. doi: 10.1093/bmb/ldl010
62.
Paz JLP Do Silvestre MPSCA Moura LS Furlaneto IP Rodrigues YC Lima KVB et al . Association of the polymorphism of the vitamin D receptor gene (VDR) with the risk of leprosy in the Brazilian Amazon. Biosci Rep. (2021) 41:BSR20204102. doi: 10.1042/BSR20204102
63.
Teixeira MAG Silveira VMD França ERD . Características epidemiológicas e clínicas das reações hansênicas em indivíduos paucibacilares e multibacilares, atendidos em dois centros de referência para hanseníase, na Cidade de Recife, Estado de Pernambuco. Rev Soc Bras Med Trop. (2010) 43:287–92. doi: 10.1590/S0037-86822010000300015
64.
Da Silva VS Braga IO Palácio MAV Takenami I . Cenário epidemiológico da hanseníase e diferenças por sexo. Rev Soc Bras Clín Méd. (2021) 19:74–81.
65.
Patrocínio LG Goulart IMB Goulart LR Patrocínio JA Ferreira FR Fleury RN . Detection of Mycobacterium leprae in nasal mucosa biopsies by the polymerase chain reaction. FEMS Immunol Med Microbiol. (2005) 44:311–6. doi: 10.1016/j.femsim.2005.01.002
66.
Da Cruz Silva MEG De Souza CDF SPC S Da Costa FM Carmo RFD . Epidemiological aspects of leprosy in Juazeiro-BA, from 2002 to 2012. An Bras Dermatol. (2015) 90:799–805. doi: 10.1590/abd1806-4841.201533963
67.
De Sousa MWG Silva DC Carneiro LR Almino MLBF Da CALF . Epidemiological profile of leprosy in the Brazilian state of Piauí between 2003 and 2008. An Bras Dermatol. (2012) 87:389–95. doi: 10.1590/S0365-05962012000300006
68.
Moreira SC Batos CJDC Tawil L . Epidemiological situation of leprosy in Salvador from 2001 to 2009. An Bras Dermatol. (2014) 89:107–17. doi: 10.1590/abd1806-4841.20142175
69.
Matos TS De Moura JC De Fernandes TRM De Souza CDF . Epidemiological, neurofunctional profile and prevalence of factors associated with the occurrence of physical disabilities due to leprosy in a reference center in northeast Brasil: a sectional study. Rev Assoc Med Bras. (2021) 67:19–25. doi: 10.1590/1806-9282.67.01.20200139
70.
Albuquerque ARD Silva JVDM Barreto EDO Fraga CADC Santos WOD Silva MSMD et al . Epidemiological, temporal and spatial dynamics of leprosy in a municipality in northeastern Brazil (2008-2017): an ecological study. Rev Soc Bras Med Trop. (2020) 53:e20200246. doi: 10.1590/0037-8682-0246-2020
71.
Durães SMB Guedes LS De Cunha MD Magnanini MMF De Oliveira MLWDR . Estudo epidemiológico de 107 focos familiares de hanseníase no município de Duque de Caxias - Rio de Janeiro, Brasil. An Bras Dermatol. (2010) 85:339–45. doi: 10.1590/S0365-05962010000300007
72.
Silva ARD Lima Neto PM Santos LHD Lima RJCP Tauil PL Gonçalves EDGDR . Factors associated with leprosy in a municipality of the pre-Amazon region, state of Maranhão, Brazil. Rev Soc Bras Med Trop. (2018) 51:789–94. doi: 10.1590/0037-8682-0038-2018
73.
Monteiro LD Mello FRM Miranda TP Heukelbach J . Hanseníase em menores de 15 anos no estado do Tocantins, Brasil, 2001-2012: padrão epidemiológico e tendência temporal. Rev Bras Epidemiol. (2019) 22:e190047. doi: 10.1590/1980-549720190047
74.
Imbiriba ENB Da Silva Neto AL De Souza WV Pedrosa V Da Cunha MG Garnelo L . Desigualdade social, crescimento urbano e hanseníase em Manaus: abordagem espacial. Rev Saúde Públ. (2009) 43:656–65. doi: 10.1590/S0034-89102009005000046
75.
Vieira GDD Aragoso I Carvalho RMB Sousa CMD . Hanseníase em Rondônia: incidência e características dos casos notificados, 2001 a 2012. Epidemiol Serv Saúde. (2014) 23:269–75. doi: 10.5123/S1679-49742014000200008
76.
Araújo FA Abreu LC Laporta GZ Santos VS Moreira JGV Grumach AS . Hanseniasis in the municipality of Western Amazon (acre, Brazil): are we far from the goal of the World Health Organization?Braz J Infect Dis. (2021) 25:101042. doi: 10.1016/j.bjid.2020.11.007
77.
Figueiredo IA Da Silva AAM . Aumento na detecção de casos de hanseníase em São Luís, Maranhão, Brasil, de 1993 a 1998: a endemia está em expansão?Cad Saúde Públ. (2003) 19:439–45. doi: 10.1590/S0102-311X2003000200010
78.
Diniz LM Maciel LB . Leprosy: clinical and epidemiological study in patients above 60 years in Espírito Santo state - Brazil. An Bras Dermatol. (2018) 93:824–8. doi: 10.1590/abd1806-4841.20187092
79.
Monteiro LD Alencar CH Barbosa JC Novaes CCBS De Silva RCP Heukelbach J . Limited activity and social participation after hospital discharge from leprosy treatment in a hyperendemic area in North Brazil. Rev Bras Epidemiol. (2014) 17:91–104. doi: 10.1590/1415-790X201400010008ENG
80.
Reis EM Araujo S Lobato J Neves AF Costa AV Gonçalves MA et al . Mycobacterium leprae DNA in peripheral blood may indicate a bacilli migration route and high-risk for leprosy onset. Clin Microbiol Infect. (2014) 20:447–52. doi: 10.1111/1469-0691.12349
81.
De Abreu MAMM Roselino AM Enokihara M Nonogaki S Prestes-Carneiro LE Weckx LLM et al . Mycobacterium leprae is identified in the oral mucosa from paucibacillary and multibacillary leprosy patients. Clin Microbiol Infect. (2014) 20:59–64. doi: 10.1111/1469-0691.12190
82.
Dennison CL De Oliveira LB Fraga LADO RS EL Ferreira JA Clennon JA et al . Mycobacterium leprae–helminth co-infections and vitamin D deficiency as potential risk factors for leprosy: a case–control study in South-Eastern Brazil. Int J Infect Dis. (2021) 105:261–6. doi: 10.1016/j.ijid.2021.02.048
83.
Da Martins ACC Miranda A De Oliveira MLW-R Bührer-Sékula S Martinez A . Nasal mucosa study of leprosy contacts with positive serology for the phenolic glycolipid 1 antigen. Braz J Otorhinolaryngol. (2015) 76:579–87. doi: 10.1590/S1808-86942010000500008
84.
Guzman HO . Clinical and epidemiological profile of leprosy cases reported in Assis Brazil, acre, in the period 2003 to 2010Hansen International (2011) Available at:https://periodicos.saude.sp.gov.br/hansenologia/article/view/35112. (Accessed March 02, 2024).
85.
Franco MCA Macedo GMM De Menezes BQ Octávio F Neto MJ Franco ACA . Perfil de casos e fatores de risco para hanseníase, em menores de quinze anos, em município hiperendêmico da região norte do Brasil: Revista Paraense de Medicina. (2014).
86.
Gomes AVS De Loiola TMM De Araújo OD Nogueira LT De Araújo TME . Epidemiological profile of leprosy in a hyperendemic municipality in northeastern Brazil. Rev Cuba Enf. (2017) 33:124–37. doi: 10.26694/reufpi.v11i1.2608
87.
Pereira EVE Nogueira LT Machado HADS Lima LAN Ramos CHM . Perfil epidemiológico da hanseníase no município de Teresina, no período de 2001-2008. An Bras Dermatol. (2011) 86:235–40. doi: 10.1590/S0365-05962011000200005
88.
Melão S Blanco LFDO Mounzer N Veronezi CCD De Simões PWT . Epidemiological profile of leprosy patients in the extreme south of Santa Catarina between 2001 and 2007. Rev Soc Bras Med Trop. (2011) 44:79–84. doi: 10.1590/s0037-86822011000100018
89.
Azevedo YP Da Bispo VA De Oliveira RI Gondim BB Dos Santos SD Da Natividade MS et al . Perfil Epidemiológico e distribuição espacial da hanseníase em Paulo Afonso, Bahia. Rev Baia Enferm. (2021) 35. doi: 10.18471/rbe.v35.37805
90.
Freitas BHBMD Cortela DDCB Ferreira SMB . Perfil sociodemográfico, clínico e epidemiológico da hanseníase em menores de quinze anos, Mato Grosso, Brasil. Hansenol Int. (2017) 42:12–8. doi: 10.47878/hi.2017.v42.34969
91.
De Camargo RM Da Silva WL Medeiros P De Belone AFF Latini ACP . Polymorphisms in the TGFB1 and IL2RA genes are associated with clinical forms of leprosy in Brazilian population. Mem Inst Oswaldo Cruz. (2018) 113:e180274. doi: 10.1590/0074-02760180274
92.
Pacheco MAB Aires MLL Seixas ES . Prevalência e controle de hanseníase: pesquisa em uma ocupação urbana de São Luís, Maranhão, Brasil. Rev Bras Med Fam Comun. (2014) 9:23–30. doi: 10.5712/rbmfc9(30)690
93.
De Sousa PP De Sousa ALM Turchi MD . Reviewing the therapeutic management of leprosy in primary care: demand case series referred to a University Hospital in the Midwest region of Brazil. An Bras Dermatol. (2021) 96:301–8. doi: 10.1016/j.abd.2020.09.005
94.
Oliveira LRP Martins LM da Souza RC Scheidegger De Castro Y Nascimento LS Da Silva JA et al . Serological evidence of toxoplasma gondii infection as potential risk for the development of lepromatous leprosy in an endemic area for both neglected tropical diseases in Brazil. Infect Dis Poverty. (2020) 9:19. doi: 10.1186/s40249-020-0636-3
95.
Ramos ACV Alonso JB Berra TZ Alves LS Martoreli Júnior JF Santos FLD et al . Social inequalities and their association with the leprosy burden in a Brazilian city of low endemicity: an ecological study. Acta Trop. (2021) 218:105884. doi: 10.1016/j.actatropica.2021.105884
96.
Barbosa CC Bonfim CVD Brito CMGD Souza WVD Melo MFDO Medeiros ZMD . Spatial analysis of epidemiological and quality indicators of health services for leprosy in hyperendemic areas in northeastern Brazil. Rev Inst Med trop S Paulo. (2020) 62:e93. doi: 10.1590/s1678-9946202062093
97.
Dos Santos EC Silvestre MDPSCA Paz JLP Machado RLD Lima LNGC . Study of TNF-α, IFN-γ, TGF-β, IL-6, and IL-10 gene polymorphism in individuals from the leprosy-endemic area in the Brazilian Amazon. J Interf Cytokine Res. (2021) 41:125–31. doi: 10.1089/jir.2018.0162
Summary
Keywords
Brazil, leprosy, paucibacillary, Mycobacterium leprae , epidemiology
Citation
Brito Gonçalves BE, Raiol AMP, Brito AVC, Silva MJA, Sardinha DM, Lima KVB and Lima LNGC (2024) Prevalence of paucibacillary cases of leprosy in Brazil: a 20-year systematic review and meta-analysis. Front. Med. 11:1401685. doi: 10.3389/fmed.2024.1401685
Received
15 March 2024
Accepted
11 October 2024
Published
13 November 2024
Volume
11 - 2024
Edited by
Fuqiu Li, Second Affiliated Hospital of Jilin University, China
Reviewed by
Mircea Ioan Popa, Carol Davila University of Medicine and Pharmacy, Romania
Hanlin Zhang, Peking Union Medical College Hospital (CAMS), China
Updates
Copyright
© 2024 Brito Gonçalves, Raiol, Brito, Silva, Sardinha, Lima and Lima.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniele Melo Sardinha, danielle-vianna20@hotmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.