Abstract
Through the formation of covalent connections with hyaluronic acid (HA), the inter-α-trypsin inhibitor (IαI) family collaborates to preserve the stability of the extracellular matrix (ECM). The five distinct homologous heavy chains (ITIH) and one type of light chain make up the IαI family. ITIH alone or in combination with bikunin (BK) has been proven to have important impacts in a number of earlier investigations. This implies that BK and ITIH might be crucial to both physiological and pathological processes. The functions of BK and ITIH in various pathophysiological processes are discussed independently in this paper. In the meanwhile, this study offers suggestions for further research on the roles of BK and ITIH in the course of disease and summarizes the plausible mechanisms of the previous studies.
1 Introduction
Members of the ancient and distinctive inter-α-trypsin inhibitor (IαI) family have developed throughout hundreds of millions of years of vertebrate history (1). Hepatocytes are the primary source of the 225 kDa IαI family protein complexes, which are found in the blood at high concentrations of 0.15 to 0.5 mg/mL (2). Human IαI family consists of three different polypeptide chains, namely bikunin (BK), heavy chain 1 and heavy chain 2 (3). IαI family, which makes up the majority of family members in human serum, is thought to be dormant until it enters the target tissue, where it is cleaved by TNF-stimulated gene 6 protein (TSG-6). After that, heavy chains (HCs) are transferred to hyaluronic acid (HA), a significant part of the extracellular matrix (ECM), through the formation of temporary covalent bonds with TSG-6 (4). TSG-6 is essential for the interaction with HA because it facilitates two following ester exchange reactions: it binds HC1 or HC2 of IαI family covalently and then moves them to the HA fraction in this complex, where the heavy chain conjugates and releases free TSG-6 (5, 6). HCs have been found to function as structural proteins that can directly cross-link HA that is secreted (7). IαI HC forms a strong bond with HA produced by fibroblasts in culture (7). In addition, the physiological correlation of HA with members of the IαI HC family has been linked to various cell types that show HA-containing outer membranes. For example, the maturation process of oocytes and experiments in vitro culture of mesothial cells (8). In summary, the IαI family of proteins interacts with HA to maintain the stability of the ECM, which is a critical function in numerous illnesses. This work aims to provide an overview of the mechanisms underlying the occurrence and development of IαI family proteins that have been linked to current disorders. Additionally, it offers suggestions for future research on IαI family proteins.
2 The structure and function of inter-α-trypsin inhibitor family members
2.1 Bikunin
Bikunin (BK), the light chain, and five homologous heavy chains come together to form the IαI family (9) (Figure 1). There are various homologous heavy chains (ITIH), and the one that has been discovered to date has five members (ITIH1, ITIH2, ITIH3, ITIH4, and ITIH5), despite the fact that there is only one type of light chain. The α-1-microglobulin/BK precursor (AMBP) encodes the light chain and α-1 microglobulin, a member of the lipid transport protein superfamily independent of the ITI family either physically or functionally (10). The ITI light chain is referred to as “BK” because it has two tandem repeats of a Kunitz-type structural domain (11). Serum proteoglycans generated from liver are known as BK isoforms. They contain chondroitin sulfate (CS) chains, which are mainly esterified by one or two glycoproteins referred to as “heavy chains” (HC). There are three primary serum isoforms that have been extensively documented: pro-alpha-proteinase inhibitor and inter-alpha-trypsin inhibitor, which carry one and two HC, respectively, and urotrypsin inhibitor, which corresponds to BK and is connected to the CS chain (10). These complexes create the characteristic ITI protein-glycosaminoglycan-protein structure (12).
Figure 1

Composition of inter-a-trypsin inhibitors and serum-derived hyaluronan-associated proteins. The IαI family is assembled from light chain-bikunin and five homologous heavy chains.
2.2 ITIH
Apart from BK, the ITI heavy chain also serves biological purposes (13). Numerous diseases, including inflammatory reactions in local tissues, acute inflammation, tumor growth, and problems connected to psychiatry, have been linked to ITIHs, according to studies (13, 14). Subsequent investigations have also revealed that ITIHs, a main constituent of ITI, plays an important role, either by itself or in conjunction with BK (13). The von Willebrand A-type structural domains and the vault are two of the modules that make up the H chain, which can communicate with the ECM. The carboxyl group of the matching H-chain’s C-terminal aspartic acid residue and the C-6 hydroxyl group of the internal N-acetylamino galactose residue of Bk’s chondroitin sulfate component are what link the H-chain to the protein. ITIHs can undergo an ester exchange reaction to covalently conjugate to locally generated HA. Serum-derived hyaluronan-associated protein is made up of D-glucuronic acid and N-acyl-D-glucosamine, which together make up the desialylated polymer known as HA (2). ITIHs bind covalently to HA to stabilize the ECM (15). Many extracellular proteins released by cells make up the ECM, which controls intercellular communication as well as biological activities (16). Many interacting proteins and proteoglycans that control cellular activity have an impact on the stiffness or rigidity of HA (17, 18). One important element of the ECM is HA (19). Together, these molecules of the ECM—HA, proliferating cells, migration, and tumor metastasis—maintain the stability of the ECM by forming a reticular structure (20, 21). A common ECM component, HA is a high molecular weight polymer that does not need to be modified further (21). Numerous biological processes, including wound healing, differentiation, and cell motility, are facilitated by HA-binding proteins, especially membrane-bound receptors like CD44 and RHAMM (22, 23). For instance, during the healing of skin injury, synthesis is elevated. The covalent transfer of heavy chains from IαI to HA is catalyzed by TsG-6, and the resulting HC-HA complex is engaged in remodeling and inflammatory processes in both healthy and pathological contexts (24). Furthermore, HA participates in angiogenic processes. The size of HA affects its angiogenic potential: short segments of HA produced in inflammation and tissue damage are strongly angiogenic (25), while high molecular weight HA possesses vasopressor qualities (26). After degradation, HA becomes a pro-angiogenic ligand (15). For instance, it has been discovered in earlier research that HA stimulates angiogenesis in lung damage and is even linked to abnormal angiogenesis (17). Increased ITIHs protein may prevent the HA-CD44 and ECM pathways from degrading, which would have anti-angiogenic effects. This could be one of the key ways that the protein ITIHs functions as a putative oncogene.
3 Advances in the study of BK and disease
In both physiological and pathological processes, BK has pleiotropic functions. BK appears to be involved in numerous activities, according on genetic studies of mice deficient the protein. Genes related to stress, apoptosis, proteases, aging, cytokines, HA metabolism, and female ovulation processes are dysregulated when BK is absent (27, 28). Subsequent research revealed that female mice deficient the BK gene exhibit considerably lower fertility (28). This results from a malfunction in the lateral protein precursors’ ability to form compounds with hyaluronic acid in the ovarian mound before ovulation. Therefore, hyaluronan, which is necessary for mammalian ovulation and fertilization, requires the delivery of serum-derived hyaluronan-related proteins by the chondroitin sulfate part of BK (29). Concurrently, research has produced intriguing findings on the potential of the medication BK to lower the risk of preterm labor and enhance neonatal outcomes (30).
Furthermore, in pancreatitis, septic shock, and rheumatoid arthritis, it has been demonstrated that the BK core protein inhibits inflammation-associated proteases such trypsin, elastase, and fibrinolytic enzymes (31, 32). As an anti-inflammatory, BK prevents the production of cytokines that are triggered by lipopolysaccharide (LPS). Through extracellular signal-regulated kinase signaling (ERK), calcium endocytosis, and endotoxin receptors, BK suppresses the generation of cytokines. Endotoxin stimulates calcium inward flow, generates phosphorylated ERK, and activates multiple transcription factors, including nuclear factor kappa B and early growth response-1, through endotoxin receptor signaling, all of which support the development of cytokines (33). It was discovered to prevent the generation of pro-inflammatory cytokines in a number of cell types in cellular tests (3, 34). Mice injected BK showed a decrease in multiple inflammatory markers in animal models of inflammatory disorders (35). Furthermore, BK knockout mice, Bik (−/−), were used for in vitro cytokine experiments and in vivo animal models. It was discovered that Bik (−/−) mice induced higher levels of endotoxin-induced death when compared to wild-type (Wt) mice; that application of BK significantly reduced LPS-induced lethality; and that endotoxin significantly increased endotoxin-induced lethality when compared to Wt mice. BK combined with application of BK inhibited the levels of these cytokines; additionally, BK inhibited endotoxin-induced up-regulation of cytokine expression by suppressing macrophage phosphorylation of ERK1/2, JNK, and p38; this implies that BK has a major anti-inflammatory function (36). In different acute and chronic inflammatory reactions, BK is a non-invasive circulating or urine biomarker (37).
BK has been found in cancer studies to inhibit tumor cell invasion through direct inhibition of fibrinolytic activity associated with tumor cells as well as urokinase-type fibrinogen activator (UPA) expression at the gene and protein levels, potentially through inhibition of MAP kinase signaling cascades and/or CD44 dimer. By interacting with different cell types’ cartilage junction proteins and BK receptors, BK can be suppressed in order to prevent cell invasion (38). Furthermore, ovarian cancer cells’ gene expression patterns are changed by BK, which prevents tumor cell invasion (39). In animal investigations, it was discovered that the exogenous injection of BK inhibited the growth of intraperitoneal ovarian tumors as well as peritoneal disseminated metastases (40). Treatment with BK in the adjuvant setting and/or in conjunction with cytotoxic medicines to enhance therapeutic efficacy may be helpful in postponing the beginning of metastases in patients with advanced ovarian cancer (41). Patients with ovarian cancer who had preoperative BK concentrations higher than 11.5 μg/mL were shown to have a significantly better prognosis than those with lower amounts. Patients with pretreatment values of 11.5 μg/mL had 2.2-fold higher Hazard Ratios (risk of mortality) than those with concentrations of more than 11.5 μg/mL of BK. Patients in both groups had a median survival of 26 months and more than 60 months, respectively (42). This implies that BK has a critical function and importance in determining the prognosis of ovarian cancer patients. Remarkably, there has been no report of BK overexpression being linked to human pathology (1).
The above cellular and animal experiments suggest that BK has potential medical value. It also plays a significant role in mammalian ovulation and even directly affects fertility in knockout mice. Additionally, BK has important roles and functions as an anti-inflammatory protein and an anti-tumor invasive protein. These findings may lead to new ideas for diagnostic and therapeutic approaches in the future gene therapy of ovarian cancer, inflammation, and infertility. It might offer suggestions for later gene therapy treatments for ovarian cancer, inflammation, and infertility. Figure 2 presents a summary of the physiological and pathological processes that are mediated by BK.
Figure 2
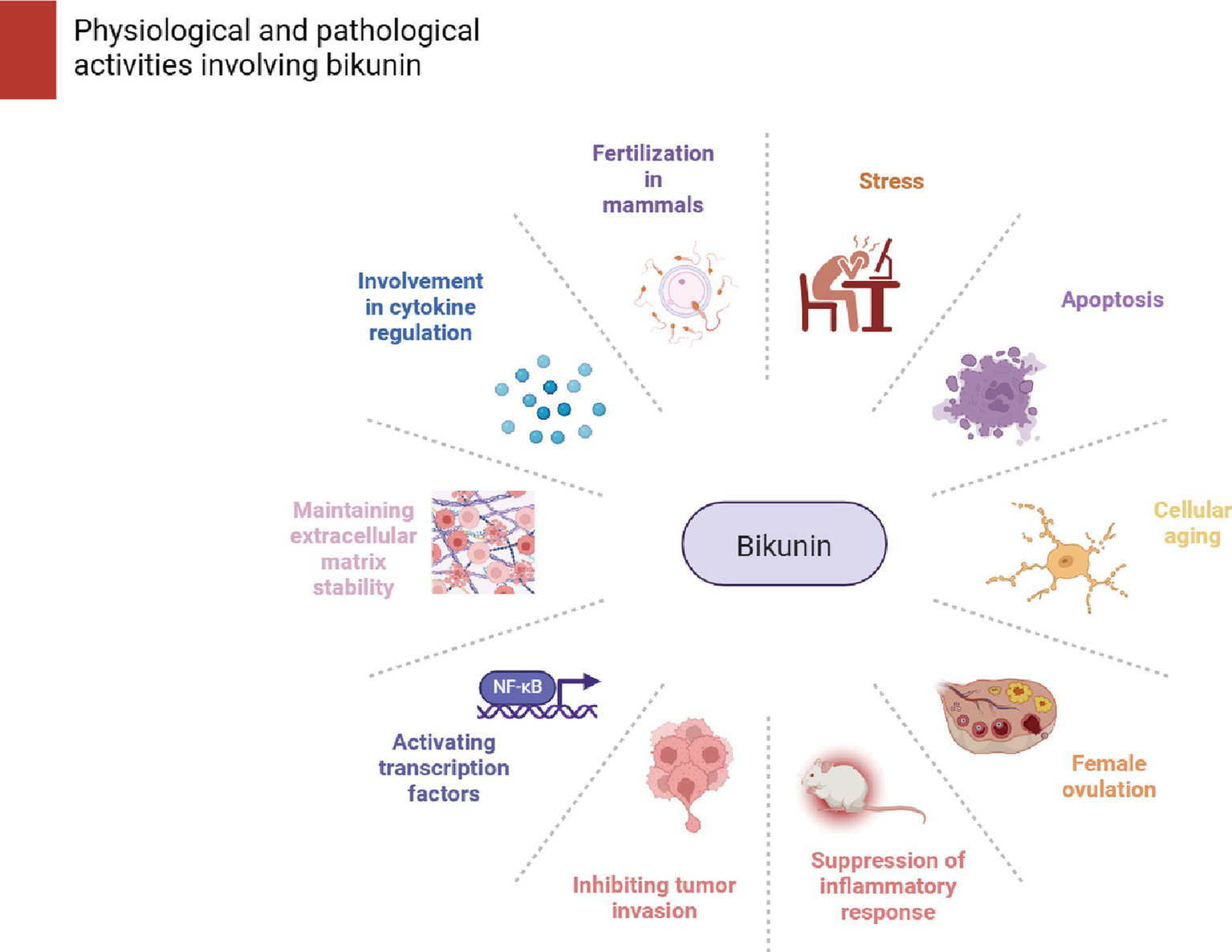
Stress, apoptosis, proteases, aging, signaling molecules, cytokines, HA metabolism, and female ovulation processes are all impacted by bakunin. Its responsibilities and functions as an anti-inflammatory and anti-tumor invasion protein are also significant.
4 Developments in the understanding of ITIH proteins and illnesses
ITIH has lately been found and thoroughly explained to be involved in several pathophysiological processes, such as inflammation and carcinogenesis (9, 43). Depending on the circumstances, these proteins may be positively or negatively regulated; nonetheless, there is compelling evidence that every member of the ITIH family is crucial to the development of tumors and cellular malignant processes (9, 44). ITIH proteins function as both pre- and anti-inflammatory acute phase proteins during inflammation, which leads to contradictory functions in the process. While the H3 chain is up-regulated and the related molecules behave as positive acute phase proteins, the H2 and BK chains are down-regulated and the associated molecules behave as negative acute phase proteins in acute inflammation, Inflammatory situations do not seem to have an impact on H1 chain (45). Strong evidence suggests that genes in the ITIH family may be tumor suppressors because these genes are highly down-regulated in a range of human solid tumors, including lung cancer, breast cancer and colon cancer (9). ITIH proteins stabilize the ECM in a way that inhibits tumor growth, which plays a significant role in carcinogenesis (46). ITIH’s covalent binding to HA and its capacity to maintain the ECM are two potential pathways. ITIH2 expression and estrogen receptor expression are substantially associated (p = 0.001) in breast cancer, according to a number of prior research. Cancer invasion and motility have been shown to be inhibited by estrogen (47). Because ITIH2 has an estrogen-binding structural domain that profoundly affects ECM integrity and may therefore be crucial for tumor growth and metastasis, there used to be a high correlation between ITIH2 expression levels and estrogen levels (48). ITIH2 is expressed in low-grade CNS cancers and normal brain tissues; however, it is not expressed in glioblastomas, especially glioblastoma multiforme, which is a highly invasive CNS tumor. This suggests that ITIH2 may have an anti-invasive function (49). The ITIH family gene has been linked in certain studies to shared genetic risk factors for schizophrenia and depression, in addition to its significant function in inflammation and malignancies. These findings imply that the ITIH family may potentially be implicated in the pathophysiology of psychiatric disorders (50). Furthermore, aberrant expression of ITIH family proteins has been found in neurodegenerative diseases (51, 52). The following table provides a summary of the roles and possible mechanisms of action of heavy chain participation (see Tables 1–5).
Table 1
| Functions | Mechanisms | References | |
|---|---|---|---|
| ITIH1 | Insulin sensitivity |
|
(16) |
| ITIH1 | Indicators for ankylosing spondylitis diagnosis | Markedly elevated in ankylosing spondylitis patients, p = 0.035 ROC distinguished between ankylosing spondylitis patients (AUC = 0.98) | (53) |
| ITIH1 | Evaluation of hepatic fibrosis | Liver fibrosis development and impaired ECM stability are linked to lower ITIH1 levels | (54) |
| ITIH1 | Control of adherence of leukocytes | Adhesion of the inflammatory HA is regulated by thrombin cleavage of ITIH1 | (55) |
| ITIH1 | Modulator of immunity | Blocks the C3 complement pathway | (56) |
| ITIH1 | Potential biomarker for hepatocellular carcinoma that is both diagnostic and predictive | Hepatocellular carcinoma patients have much lower levels of ITIH1 expression, and this downregulation is detrimental to the prognosis of these patients | (57) |
| ITIH1 | Involved in the development of hepatocellular carcinoma | By stimulating the PI3K/AKT signaling pathway and epigenetically suppressing ITIH1 transcription, KDM5C can encourage the malignant progression of hepatocellular carcinoma | (58) |
| ITIH1 | Markers for radiographic knee osteoarthritis development prediction | Shows promise for enhancing clinical practice radiographic knee osteoarthritis incidence prediction | (59) |
| ITIH1 | Potential biomarkers for ovarian cancer | Variations in expression in the serum of patients with ovarian cancer | (60) |
| ITIH1 | Biomarker for coronary heart disease risk stratification | When paired with additional proteins, ITIH1 exhibits a Lasso-logistic score that is highly effective in categorization (cross-validated area under the curve = 0.74) | (61) |
Functions and potential mechanisms of ITIH1.
Table 2
| Functions | Mechanisms | References | |
|---|---|---|---|
| ITIH2 | Increases intercellular adhesion, suppresses cell proliferation, and prevents glioblastoma invasion | Reduces the activity of the PI3K/AKT signaling pathway | (49) |
| ITIH2 | Biomarker for multiple sclerosis in children | Patients with pediatric multiple sclerosis had considerably reduced serum levels of ITIH2 protein | (62) |
| ITIH2 | Pancreatic cancer diagnostic biomarker | Area under the curve = 0.947; ROC separates pancreatic cancer from chronic pancreatitis | (63) |
| ITIH2 | Potential candidates associated with diabetic retinopathy | Comparing the protein profiles of vitreous humor in patients with idiopathic macular lentigines who are not diabetics, it was discovered that they had significantly decreased expression of ITIH2 protein | (64) |
| ITIH2 | Indicators of serum proteins for osteoarticular. Tuberculosis detection | For the diagnosis of osteoarticular tuberculosis, the AUC of ITIH2 was 0.7167 (95% CI: 0.5846–0.8487) | (65) |
| ITIH2 | Hinders the growth of mother cells in gliomas | Tumor cell contact is inhibited by gonadotropin-releasing hormone interaction | (66) |
Functions and potential mechanisms of ITIH2.
Table 3
| Functions | Mechanisms | References | |
|---|---|---|---|
| ITIH3 | Play in the development of the human brain | Differential spatiotemporal expression of ITIH3 in the developing human brain is shown by analysis of spatiotemporal histology data, which indicates that the SNP locus rs25352629 of ITIH3 is a susceptibility variable for autism | (67) |
| ITIH3 | Intrahepatic cholestasis in pregnancy: diagnostic indicators | For the diagnosis of intrahepatic cholestasis in pregnancy, the AUC of ITIH3 was 0.8163 | (68) |
| ITIH3 | Strongly correlated with a history of attempted suicide in bipolar illness and schizophrenia cases | After multiple testing was taken into account, there was a substantial correlation found between the risk allele for the SNP locus rs2239547 of ITIH3 and a history of suicide attempts | (14) |
| ITIH3 | Strongly correlated with the likelihood of getting schizophrenia | The ITIH3 SNP locus rs3617 may impact neurodevelopment and protein function, raising the risk of schizophrenia | (69) |
| ITIH3 | Potentially forecast ovarian cancer’s susceptibility to cisplatin | Platinum resistance and a poor prognosis are strongly correlated with low expression of ITIH3 protein in ovarian cancer tissues | (70) |
| ITIH3 | Unknown hereditary susceptibility to myocardial infarction | In human atherosclerotic lesions, macrophages and vascular smooth muscle cells express the ITIH3 protein | (71) |
| ITIH3 | It is a helpful biomarker for gastric cancer early detection Assesses the likelihood of gastric cancer |
According to ROC, ITIH3 has a maximum sensitivity of 96% and a maximum specificity of 66% in the identification of gastric cancer. Furthermore, patients with early gastric cancer had considerably greater plasma levels of ITIH3 than non-cancerous patients (p < 0.001) | (72, 73) |
| ITIH3 | Might not be adequate as a potent biomarker for early gastric cancer detection | Between early and advanced gastric cancer, there was no difference in expression; the AUC value was 0.65 (95% CI: 0.55–0.75) | (74) |
| ITIH3 | Enhances acute and chronic heart failure and hypertrophic cardiomyopathy. Treatment with biomarker candidate | Proteomic analysis was used to identify putative core biomarkers | (75) |
| ITIH3 | A biomarker for the detection of pancreatic cancer | In comparison to controls, expression in pancreatic cancer was 1.80 times greater | (76) |
| ITIH3 | It is a biomarker for the early detection of rheumatoid arthritis | Utilizing innovative diagnostic techniques, rheumatoid arthritis diagnosis approaches 100% sensitivity and specificity | (77) |
| ITIH3 | Contributes to the development of synovial joint disease | Significant elevation of ITIH3 in synovial joint diseases | (78) |
Functions and potential mechanisms of ITIH3.
Table 4
| Functions | Mechanisms | References | |
|---|---|---|---|
| ITIH4 | Pro-inflammatory reaction | Activation of the PGK1-ITIH4 axis causes a pro-inflammatory response | (79) |
| ITIH4 | Cholestatic liver disease biomarkers | Cholestatic liver dysfunction was associated with considerably higher plasma ITIH4 values | (80) |
| ITIH4 | Biomarkers that show how NAFLD progresses and how hepatocellular carcinoma develops as a result | Compared to patients with simple steatosis and virus-associated hepatocellular carcinoma, patients with hepatocellular carcinoma-NAFLD had considerably higher serum ITIH4 levels Following hepatectomy, patients with hepatocellular carcinoma-NAFLD who had greater serum ITIH4 levels had a worse prognosis |
(81) |
| ITIH4 | A possible therapeutic target to prevent the spread of tumors | HuH7 cell movement was markedly reduced by ITIH4 overexpression and increased by ITIH4 knockdown, respectively. Patients with hepatocellular carcinoma who had a good prognosis had tumor tissues with higher levels of ITIH4 expression than those who had a bad prognosis | (82) |
| ITIH4 | Distinguishes multisystemic and unisystemic histiocytosis of Langerhans cells | Significant variations in ITIH4 expression were found between the two illness groups by peptideomics analysis | (83) |
| ITIH4 | Participated in repeated abortions | The activation of the IL-6 signaling pathway by ITIH4(ΔN688) promotes inflammatory responses The long isoforms of ITIH4 have different functions in controlling cell invasion, migration, proliferation, and inflammatory responses |
(84) |
| ITIH4 | Beneficial for anticipating and monitoring sepsis | The expression of ITIH4 varies with the stage of sepsis | (85) |
| ITIH4 | Possible prognostic biomarker for psoriasis patients’ liver fibrosis brought on by methotrexate | Patients with psoriasis who had liver fibrosis brought on by methotrexate had abnormally high levels of it in their urine | (86) |
| ITIH4 | Early gastric cancer biomarkers | When compared to patients receiving will-care controls, patients with early gastric cancer had noticeably higher serum levels of ITIH ITIH4 expression increased during Helicobacter pylori infection |
(87, 88) |
| ITIH4 | Biomarkers for ovarian cancer | Patients with ovarian cancer had far lower ITIH4 expression levels in their urine than did controls | (89) |
| ITIH4 | Evaluation of the diagnostic and prognostic importance in individuals with hepatitis B-associated hepatocellular carcinoma and liver cirrhosis caused by the virus | Patients diagnosed with hepatocellular carcinoma had lower serum ITIH4 levels, and those with hepatitis B-associated hepatocellular carcinoma also had shorter survival times | (90) |
| ITIH4 | Associated with autoimmune disease | Prevention of neutrophil recruitment in order to reduce inflammatory autoimmunity | (91) |
| ITIH4 | Prognostic markers for aneurysmal subarachnoid hemorrhage in humans (ASAH) |
|
(92) |
| ITIH4 | Prostate cancer and prostatic hyperplasia identification | Patients with prostate cancer had considerably higher ITIH4 protein levels | (93) |
| ITIH4 | Ischemic acute stroke biomarkers | As the acute ischemic stroke condition progressed, ITIH4 eventually returned to normal | (94) |
| ITIH4 | Preventing the growth of melanoma | A finding not further explained in the text | (95) |
| ITIH4 | Monitoring of patients with postoperative breast cancer | ITH4 was substantially lower in postoperative breast cancer patients than in controls | (96) |
| ITIH4 | Accurately forecasting hyperlipidemia | C/T polymorphism of single nucleotides at IVS17 + 8 of ITIH4. Ninety percent of those missing the T allele went on to develop hypercholesterolemia. Of those with the T allele, only 10% experienced hypercholesterolemia (p < 0.0001) | (97) |
| ITIH4 | Potential Alzheimer’s disease serum biomarkers | Increased intact size of ITIH4 protein and decreased fragmentation of ITIH4; increased ITIH4 expression | (51, 52) |
| ITIH4 | Interstitial cystitis biomarkers | ITIH4 plasma concentrations were greater in the patients (p = 0.019) | (98) |
Functions and potential mechanisms of ITIH4.
Table 5
| Functions | Mechanisms | References | |
|---|---|---|---|
| ITIH5 | Indicator biomarkers for bile duct cancer diagnostics | In comparison to the control group, which included people with hepatocellular carcinoma, benign illness, chronic hepatitis B, and healthy persons, the cholangiocarcinoma group had greater serum ITIH5 levels. The AUC for ITIH5 varied between 0.839 and 0.851, indicating a significant difference between bile duct cancer and the control group | (99) |
| ITIH5 | Prevention of the development of pancreatic cancer | Prevention of the spread of pancreatic cancer | (100) |
| ITIH5 | Prevention of the development of bladder cancer | Overexpression of ITIH5 in bladder cancer cells prevented colony spreading and cell migration | (101) |
| ITIH5 | Prevention of the spread of breast cancer | ITIH5-overexpressing breast cancer cells nearly totally failed to develop lung metastasis in a mouse metastasis model | (102) |
| ITIH5 | Stops the spread of cervical cancer | Reduced the growth and invasiveness of tumor spheres to a large degree, which increased the rate of death in cervical cancer cells | (103) |
| ITIH5 | Adipokine released by adipocytes | By comparing gene expression in subcutaneous and visceral fat microarray investigations in obese and lean subjects, ITIH5 was discovered as a novel adipokine | (104) |
| ITIH5 | Involved in wound healing | The transformation of fibroblasts into myofibroblasts, which is dependent on growth factor β1, requires the interaction of ITIH5 with cell surface HA | (105) |
| ITIH5 | Involved in breast cancer development | Breast cancers exhibit a marked downregulation of ITIH5 expression. While ITIH5 expression is either consistently missing or significantly downregulated in invasive ductal carcinomas, normal breast epithelial cells express ITIH5 substantially. Both benign breast cell lines and breast cancer cell lines did not exhibit ITIH5 gene expression. Breast cancer development may be linked to the lack of ITIH5 expression | (106) |
| ITIH5 | Prevention of the development of cervical cancer | ITIH5 overexpression resulted in a marked decrease in cell migration and clone formation as well as a considerable inhibition of cervical cancer cell proliferation | (107) |
| ITIH5 | Prevents the growth of colon cancer cell | Colon cancer cells were unable to proliferate when ITIH5 was overexpressed | (108) |
| ITIH5 | Prevention of the development of pancreatic cancer | ITIH5 may obstruct many oncogenic signaling pathways, including as the PI3K/AKT pathway. Changes in cell migration and the creation of local adhesions may result from this. These alterations in the cells could be connected to ITIH5’s ability to prevent metastasis in pancreatic cancer | (109) |
| ITIH5 | Prevention of the development of non-small cell lung cancer | Lower expression of ITIH5 is linked to a worse prognosis in non-small cell lung cancer | (110) |
| ITIH5 | Responsible for the emergence of clinical metabolic abnormalities and obesity | Obese people express ITIH5 more than lean people do, and dieting-induced weight loss reduces ITIH5 expression | (111) |
| ITIH5 | Contribute to the pathophysiology of congenital megacolon | Elevated ITIH5 expression prevents cell migration and proliferation | (112) |
Functions and potential mechanisms of ITIH5.
The ITIH family has five homologous variants that are encoded by various genes. Through an overview of current research pertaining to the ITIH family, we discovered that ITIH plays a role in numerous pathophysiological mechanisms. These comprise immunological responses, tumor growth, psychological issues, and inflammation. These protein families play a role in the modification of extracellular structures necessary for cell migration and the growth of malignant tumors. ITIH family has intricate functions. Various disease processes may involve distinct heavy chain proteins. Different diseases may involve the same heavy chain protein. This implies that ITIH family may not be as accurate a diagnostic or prognostic marker for some disorders. Large-scale multicenter clinical trials are required in the future to assess if ITIH family may be used as a prognostic or diagnostic biomarker for specific illnesses. Despite the fact that numerous studies have demonstrated that the ITIH family prevents the growth of different types of solid tumors. The ITIH family is a putative oncogene, but further investigation and analysis are required, and it’s still unclear how it works.
5 Summary
To jointly preserve the stability of the ECM, members of the IαI family bind covalently to HA. Hence the characteristics play a role in several physiological and pathological processes. The IαI family contains the protein BK, which is important for mammalian ovulation and human cancer. It is also an anti-invasive protein. The ITIH family is important for inflammation, immunity, psychiatric disorders, tumorigenesis, and development. Although BK has been extensively researched, its exact mechanism of action in the pathophysiological processes involving the ITIH family is still unclear. In order to examine the possible mechanisms and offer a theoretical foundation for targeted therapy of the associated disorders, a significant amount of research is required going forward. In addition, existing studies have shown that BK and ITIH family have been found to play a role in both inflammation and tumors, but whether there is an interaction between the two remains unclear.
Statements
Author contributions
X-fZ: Investigation, Writing – original draft, Data curation. X-lZ: Writing – original draft, Data curation, Investigation. LG: Writing – original draft. Y-pB: Writing – original draft. YT: Supervision, Writing – review & editing. H-yL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Related research was funded by Huang Changming Expert Workstation in Yunnan Province (202105AF150040).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Lord MS Melrose J Day AJ Whitelock JM . The inter-alpha-trypsin inhibitor family: versatile molecules in biology and pathology. J Histochem Cytochem. (2020) 68:907–27. doi: 10.1369/0022155420940067
2.
Zhuo L Hascall VC Kimata K . Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J Biol Chem. (2004) 279:38079–82. doi: 10.1074/jbc.R300039200
3.
Fries E Blom AM . Bikunin—not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. (2000) 32:125–37. doi: 10.1016/S1357-2725(99)00125-9
4.
Jessen TE Odum L . Role of tumour necrosis factor stimulated gene 6 (TSG-6) in the coupling of inter-alpha-trypsin inhibitor to hyaluronan in human follicular fluid. Reproduction. (2003) 125:27–31. doi: 10.1530/rep.0.1250027
5.
Sanggaard KW Scavenius C Rasmussen AJ Wisniewski HG Thogersen IB Enghild JJ . The TSG-6/HC2-mediated transfer is a dynamic process shuffling heavy chains between glycosaminoglycans. J Biol Chem. (2010) 285:21988–93. doi: 10.1074/jbc.M109.041046
6.
Colon E Shytuhina A Cowman MK Band PA Sanggaard KW Enghild JJ et al . Transfer of inter-alpha-inhibitor heavy chains to hyaluronan by surface-linked hyaluronan-TSG-6 complexes. J Biol Chem. (2009) 284:2320–31. doi: 10.1074/jbc.M807183200
7.
Huang L Yoneda M Kimata K . A serum-derived hyaluronan-associated protein (SHAP) is the heavy chain of the inter alpha-trypsin inhibitor. J Biol Chem. (1993) 268:26725–30. doi: 10.1016/S0021-9258(19)74373-7
8.
Chen L Mao SJ Larsen WJ . Identification of a factor in fetal bovine serum that stabilizes the cumulus extracellular matrix. A role for a member of the inter-alpha-trypsin inhibitor family. J Biol Chem. (1992) 267:12380–6. PMID:
9.
Hamm A Veeck J Bektas N Wild PJ Hartmann A Heindrichs U et al . Frequent expression loss of inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: a systematic expression analysis. BMC Cancer. (2008) 8:25. doi: 10.1186/1471-2407-8-25
10.
Salier JP Rouet P Raguenez G Daveau M . The inter-alpha-inhibitor family: from structure to regulation. Biochem J. (1996) 315:1–9. doi: 10.1042/bj3150001
11.
Gebhard W Schreitmuller T Hochstrasser K Wachter E . Two out of the three kinds of subunits of inter-alpha-trypsin inhibitor are structurally related. Eur J Biochem. (1989) 181:571–6. doi: 10.1111/j.1432-1033.1989.tb14762.x
12.
Enghild JJ Salvesen G Thogersen IB Valnickova Z Pizzo SV Hefta SA . Presence of the protein-glycosaminoglycan-protein covalent cross-link in the inter-alpha-inhibitor-related proteinase inhibitor heavy chain 2/bikunin. J Biol Chem. (1993) 268:8711–6. doi: 10.1016/S0021-9258(18)52933-1
13.
Zhuo L Kimata K . Structure and function of inter-alpha-trypsin inhibitor heavy chains. Connect Tissue Res. (2008) 49:311–20. doi: 10.1080/03008200802325458
14.
Finseth PI Sonderby IE Djurovic S Agartz I Malt UF Melle I et al . Association analysis between suicidal behaviour and candidate genes of bipolar disorder and schizophrenia. J Affect Disord. (2014) 163:110–4. doi: 10.1016/j.jad.2013.12.018
15.
Pardue EL Ibrahim S Ramamurthi A . Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis. (2008) 4:203–14. doi: 10.4161/org.4.4.6926
16.
Kim TH Koo JH Heo MJ Han CY Kim YI Park SY et al . Overproduction of inter-alpha-trypsin inhibitor heavy chain 1 after loss of Gα13 in liver exacerbates systemic insulin resistance in mice. Sci Transl Med. (2019) 11:eaan4735. doi: 10.1126/scitranslmed.aan4735
17.
Garantziotis S Zudaire E Trempus CS Hollingsworth JW Jiang D Lancaster LH et al . Serum inter-alpha-trypsin inhibitor and matrix hyaluronan promote angiogenesis in fibrotic lung injury. Am J Respir Crit Care Med. (2008) 178:939–47. doi: 10.1164/rccm.200803-386OC
18.
Zhuo L Yoneda M Zhao M Yingsung W Yoshida N Kitagawa Y et al . Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J Biol Chem. (2001) 276:7693–6. doi: 10.1074/jbc.C000899200
19.
Chanmee T Ontong P Itano N . Hyaluronan: a modulator of the tumor microenvironment. Cancer Lett. (2016) 375:20–30. doi: 10.1016/j.canlet.2016.02.031
20.
Zhao M Yoneda M Ohashi Y Kurono S Iwata H Ohnuki Y et al . Evidence for the covalent binding of SHAP, heavy chains of inter-alpha-trypsin inhibitor, to hyaluronan. J Biol Chem. (1995) 270:26657–63. doi: 10.1074/jbc.270.44.26657
21.
Fraser JR Laurent TC Laurent UB . Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. (1997) 242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x
22.
Slevin M Krupinski J Gaffney J Matou S West D Delisser H et al . Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. (2007) 26:58–68. doi: 10.1016/j.matbio.2006.08.261
23.
Bourguignon LY . Matrix hyaluronan-activated CD44 signaling promotes keratinocyte activities and improves abnormal epidermal functions. Am J Pathol. (2014) 184:1912–9. doi: 10.1016/j.ajpath.2014.03.010
24.
Tan KT McGrouther DA Day AJ Milner CM Bayat A . Characterization of hyaluronan and TSG-6 in skin scarring: differential distribution in keloid scars, normal scars and unscarred skin. J Eur Acad Dermatol Venereol. (2011) 25:317–27. doi: 10.1111/j.1468-3083.2010.03792.x
25.
Deed R Rooney P Kumar P Norton JD Smith J Freemont AJ et al . Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer. (1997) 71:251–6. doi: 10.1002/(sici)1097-0215(19970410)71:2<251::aid-ijc21>3.0.co;2-j
26.
West DC Hampson IN Arnold F Kumar S . Angiogenesis induced by degradation products of hyaluronic acid. Science. (1985) 228:1324–6. doi: 10.1126/science.2408340
27.
Suzuki M Kobayashi H Tanaka Y Kanayama N Terao T . Reproductive failure in mice lacking inter-alpha-trypsin inhibitor (ITI)—ITI target genes in mouse ovary identified by microarray analysis. J Endocrinol. (2004) 183:29–38. doi: 10.1677/joe.1.05803
28.
Sato H Kajikawa S Kuroda S Horisawa Y Nakamura N Kaga N et al . Impaired fertility in female mice lacking urinary trypsin inhibitor. Biochem Biophys Res Commun. (2001) 281:1154–60. doi: 10.1006/bbrc.2001.4475
29.
Zhuo L Salustri A Kimata K . A physiological function of serum proteoglycan bikunin: the chondroitin sulfate moiety plays a central role. Glycoconj J. (2002) 19:241–7. doi: 10.1023/A:1025331929373
30.
Lepedda AJ De Muro P Capobianco G Formato M . Role of the small proteoglycan bikunin in human reproduction. Hormones. (2020) 19:123–33. doi: 10.1007/s42000-019-00149-x
31.
Pratt CW Pizzo SV . Mechanism of action of inter-alpha-trypsin inhibitor. Biochemistry. (1987) 26:2855–63. doi: 10.1021/bi00384a029
32.
Yingsung W Zhuo L Morgelin M Yoneda M Kida D Watanabe H et al . Molecular heterogeneity of the SHAP-hyaluronan complex. Isolation and characterization of the complex in synovial fluid from patients with rheumatoid arthritis. J Biol Chem. (2003) 278:32710–8. doi: 10.1074/jbc.M303658200
33.
Kobayashi H . Endogenous anti-inflammatory substances, inter-alpha-inhibitor and bikunin. Biol Chem. (2006) 387:1545–9. doi: 10.1515/BC.2006.192
34.
Nakamura H Abe S Shibata Y Sata M Kato S Saito H et al . Inhibition of neutrophil elastase-induced interleukin-8 gene expression by urinary trypsin inhibitor in human bronchial epithelial cells. Int Arch Allergy Immunol. (1997) 112:157–62. doi: 10.1159/000237448
35.
Futamura Y Kajikawa S Kaga N Shibutani Y . Protection against preterm delivery in mice by urinary trypsin inhibitor. Obstet Gynecol. (1999) 93:100–8. doi: 10.1016/s0029-7844(98)00396-2
36.
Wakahara K Kobayashi H Yagyu T Matsuzaki H Kondo T Kurita N et al . Bikunin suppresses lipopolysaccharide-induced lethality through down-regulation of tumor necrosis factor- alpha and interleukin-1 beta in macrophages. J Infect Dis. (2005) 191:930–8. doi: 10.1086/428134
37.
Lepedda AJ Nieddu G Cannas C Formato M . Molecular and pathobiological insights of bikunin/UTI in cancer. Mol Biol Rep. (2023) 50:1701–11. doi: 10.1007/s11033-022-08117-2
38.
Hirashima Y Kobayashi H Suzuki M Tanaka Y Kanayama N Fujie M et al . Characterization of binding properties of urinary trypsin inhibitor to cell-associated binding sites on human chondrosarcoma cell line HCS-2/8. J Biol Chem. (2001) 276:13650–6. doi: 10.1074/jbc.M009906200
39.
Suzuki M Kobayashi H Tanaka Y Hirashima Y Kanayama N Takei Y et al . Bikunin target genes in ovarian cancer cells identified by microarray analysis. J Biol Chem. (2003) 278:14640–6. doi: 10.1074/jbc.M300239200
40.
Suzuki M Kobayashi H Tanaka Y Hirashima Y Kanayama N Takei Y et al . Suppression of invasion and peritoneal carcinomatosis of ovarian cancer cell line by overexpression of bikunin. Int J Cancer. (2003) 104:289–302. doi: 10.1002/ijc.10950
41.
Kobayashi H Suzuki M Hirashima Y Terao T . The protease inhibitor bikunin, a novel anti-metastatic agent. Biol Chem. (2003) 384:749–54. doi: 10.1515/BC.2003.083
42.
Matsuzaki H Kobayashi H Yagyu T Wakahara K Kondo T Kurita N et al . Plasma bikunin as a favorable prognostic factor in ovarian cancer. J Clin Oncol. (2005) 23:1463–72. doi: 10.1200/JCO.2005.03.010
43.
Singh K Zhang LX Bendelja K Heath R Murphy S Sharma S et al . Inter-alpha inhibitor protein administration improves survival from neonatal sepsis in mice. Pediatr Res. (2010) 68:242–7. doi: 10.1203/PDR.0b013e3181e9fdf0
44.
Ohyama K Yoshimi H Aibara N Nakamura Y Miyata Y Sakai H et al . Immune complexome analysis reveals the specific and frequent presence of immune complex antigens in lung cancer patients: a pilot study. Int J Cancer. (2017) 140:370–80. doi: 10.1002/ijc.30455
45.
Daveau M Rouet P Scotte M Faye L Hiron M Lebreton JP et al . Human inter-alpha-inhibitor family in inflammation: simultaneous synthesis of positive and negative acute-phase proteins. Biochem J. (1993) 292:485–92. doi: 10.1042/bj2920485
46.
Kopylov AT Stepanov AA Malsagova KA Soni D Kushlinsky NE Enikeev DV et al . Revelation of proteomic indicators for colorectal cancer in initial stages of development. Molecules. (2020) 25:619. doi: 10.3390/molecules25030619
47.
Rochefort H Platet N Hayashido Y Derocq D Lucas A Cunat S et al . Estrogen receptor mediated inhibition of cancer cell invasion and motility: an overview. J Steroid Biochem Mol Biol. (1998) 65:163–8. doi: 10.1016/S0960-0760(98)00010-7
48.
Zhang Q Huang R Tang Q Yu Y Huang Q Chen Y et al . Leucine-rich alpha-2-glycoprotein-1 is up-regulated in colorectal cancer and is a tumor promoter. Onco Targets Ther. (2018) 11:2745–52. doi: 10.2147/OTT.S153375
49.
Werbowetski-Ogilvie TE Agar NY Waldkircher de Oliveira RM Faury D Antel JP Jabado N et al . Isolation of a natural inhibitor of human malignant glial cell invasion: inter alpha-trypsin inhibitor heavy chain 2. Cancer Res. (2006) 66:1464–72. doi: 10.1158/0008-5472.CAN-05-1913
50.
He K Wang Q Chen J Li T Li Z Li W et al . ITIH family genes confer risk to schizophrenia and major depressive disorder in the Han Chinese population. Prog Neuro-Psychopharmacol Biol Psychiatry. (2014) 51:34–8. doi: 10.1016/j.pnpbp.2013.12.004
51.
Zhang X Yu W Cao X Wang Y Zhu C Guan J . Identification of serum biomarkers in patients with Alzheimer’s disease by 2D-DIGE proteomics. Gerontology. (2022) 68:686–98. doi: 10.1159/000520961
52.
Shi X Ohta Y Liu X Shang J Morihara R Nakano Y et al . Acute anti-inflammatory markers ITIH4 and AHSG in mice brain of a novel Alzheimer’s disease model. J Alzheimers Dis. (2019) 68:1667–75. doi: 10.3233/JAD-181218
53.
Fischer R Trudgian DC Wright C Thomas G Bradbury LA Brown MA et al . Discovery of candidate serum proteomic and metabolomic biomarkers in ankylosing spondylitis. Mol Cell Proteomics. (2012) 11:M111.013904. doi: 10.1074/mcp.M111.013904
54.
Caillot F Hiron M Goria O Gueudin M Francois A Scotte M et al . Novel serum markers of fibrosis progression for the follow-up of hepatitis C virus-infected patients. Am J Pathol. (2009) 175:46–53. doi: 10.2353/ajpath.2009.080850
55.
Petrey AC de la Motte CA . Thrombin cleavage of inter-alpha-inhibitor heavy chain 1 regulates leukocyte binding to an inflammatory hyaluronan matrix. J Biol Chem. (2016) 291:24324–34. doi: 10.1074/jbc.M116.755660
56.
Briggs DC Langford-Smith AWW Birchenough HL Jowitt TA Kielty CM Enghild JJ et al . Inter-alpha-inhibitor heavy chain-1 has an integrin-like 3D structure mediating immune regulatory activities and matrix stabilization during ovulation. J Biol Chem. (2020) 295:5278–91. doi: 10.1074/jbc.RA119.011916
57.
Chang QH Mao T Tao Y Dong T Tang XX Ge GH et al . Pan-cancer analysis identifies ITIH1 as a novel prognostic indicator for hepatocellular carcinoma. Aging. (2021) 13:11096–119. doi: 10.18632/aging.202765
58.
Qian X Bao ZM Yao D Shi Y . Lysine demethylase 5C epigenetically reduces transcription of ITIH1 that results in augmented progression of liver hepatocellular carcinoma. Kaohsiung J Med Sci. (2022) 38:437–46. doi: 10.1002/kjm2.12501
59.
Lourido L Balboa-Barreiro V Ruiz-Romero C Rego-Perez I Camacho-Encina M Paz-Gonzalez R et al . A clinical model including protein biomarkers predicts radiographic knee osteoarthritis: a prospective study using data from the osteoarthritis initiative. Osteoarthr Cartil. (2021) 29:1147–54. doi: 10.1016/j.joca.2021.04.011
60.
Lin B White JT Wu J Lele S Old LJ Hood L et al . Deep depletion of abundant serum proteins reveals low-abundant proteins as potential biomarkers for human ovarian cancer. Proteomics Clin Appl. (2009) 3:853–61. doi: 10.1002/prca.200800141
61.
Anwar MA Dai DL Wilson-McManus J Smith D Francis GA Borchers CH et al . Multiplexed LC-ESI-MRM-MS-based assay for identification of coronary artery disease biomarkers in human plasma. Proteomics Clin Appl. (2019) 13:e1700111. doi: 10.1002/prca.201700111
62.
Solmaz I Kocak E Kaplan O Celebier M Anlar B . Analysis of plasma protein biomarkers in childhood onset multiple sclerosis. J Neuroimmunol. (2020) 348:577359. doi: 10.1016/j.jneuroim.2020.577359
63.
Saraswat M Joenvaara S Seppanen H Mustonen H Haglund C Renkonen R . Comparative proteomic profiling of the serum differentiates pancreatic cancer from chronic pancreatitis. Cancer Med. (2017) 6:1738–51. doi: 10.1002/cam4.1107
64.
Garcia-Ramirez M Canals F Hernandez C Colome N Ferrer C Carrasco E et al . Proteomic analysis of human vitreous fluid by fluorescence-based difference gel electrophoresis (DIGE): a new strategy for identifying potential candidates in the pathogenesis of proliferative diabetic retinopathy. Diabetologia. (2007) 50:1294–303. doi: 10.1007/s00125-007-0627-y
65.
Chen X Wang J Wang J Ye J Di P Dong C et al . Several potential serum proteomic biomarkers for diagnosis of osteoarticular tuberculosis based on mass spectrometry. Clin Chim Acta. (2023) 547:117447. doi: 10.1016/j.cca.2023.117447
66.
Tripathi PH Akhtar J Arora J Saran RK Mishra N Polisetty RV et al . Quantitative proteomic analysis of GnRH agonist treated GBM cell line LN229 revealed regulatory proteins inhibiting cancer cell proliferation. BMC Cancer. (2022) 22:133. doi: 10.1186/s12885-022-09218-8
67.
Xie X Meng H Wu H Hou F Chen Y Zhou Y et al . Integrative analyses indicate an association between ITIH3 polymorphisms with autism spectrum disorder. Sci Rep. (2020) 10:5223. doi: 10.1038/s41598-020-62189-3
68.
Chen L Li J You Y Qian Z Liu J Jiang Y et al . Secreted proteins in plasma and placenta as novel non-invasive biomarkers for intrahepatic cholestasis of pregnancy: a case-control study. Heliyon. (2023) 9:e21616. doi: 10.1016/j.heliyon.2023.e21616
69.
Li K Li Y Wang J Huo Y Huang D Li S et al . A functional missense variant in ITIH3 affects protein expression and neurodevelopment and confers schizophrenia risk in the Han Chinese population. J Genet Genomics. (2020) 47:233–48. doi: 10.1016/j.jgg.2020.04.001
70.
Liu Y Shi L Yuan C Feng Y Li M Liu H et al . Downregulation of ITIH3 contributes to cisplatin-based chemotherapy resistance in ovarian carcinoma via the Bcl-2 mediated anti-apoptosis signaling pathway. Oncol Lett. (2023) 25:61. doi: 10.3892/ol.2022.13646
71.
Ebana Y Ozaki K Inoue K Sato H Iida A Lwin H et al . A functional SNP in ITIH3 is associated with susceptibility to myocardial infarction. J Hum Genet. (2007) 52:220–9. doi: 10.1007/s10038-006-0102-5
72.
Chong PK Lee H Zhou J Liu SC Loh MC Wang TT et al . ITIH3 is a potential biomarker for early detection of gastric cancer. J Proteome Res. (2010) 9:3671–9. doi: 10.1021/pr100192h
73.
Shang F Wang Y Shi Z Deng Z Ma J . Development of a signature based on eight metastatic-related genes for prognosis of GC patients. Mol Biotechnol. (2023) 65:1796–808. doi: 10.1007/s12033-023-00671-9
74.
Uen YH Lin KY Sun DP Liao CC Hsieh MS Huang YK et al . Comparative proteomics, network analysis and post-translational modification identification reveal differential profiles of plasma Con A-bound glycoprotein biomarkers in gastric cancer. J Proteome. (2013) 83:197–213. doi: 10.1016/j.jprot.2013.03.007
75.
Chen H Tesic M Nikolic VN Pavlovic M Vucic RM Spasic A et al . Systemic biomarkers and unique pathways in different phenotypes of heart failure with preserved ejection fraction. Biomol Ther. (2022) 12:1419. doi: 10.3390/biom12101419
76.
Tonack S Jenkinson C Cox T Elliott V Jenkins RE Kitteringham NR et al . iTRAQ reveals candidate pancreatic cancer serum biomarkers: influence of obstructive jaundice on their performance. Br J Cancer. (2013) 108:1846–53. doi: 10.1038/bjc.2013.150
77.
Chen HM Tsai YH Hsu CY Wang YY Hsieh CE Chen JH et al . Peptide-coated bacteriorhodopsin-based photoelectric biosensor for detecting rheumatoid arthritis. Biosensors. (2023) 13:929. doi: 10.3390/bios13100929
78.
Zhang C Gawri R Lau YK Spruce LA Fazelinia H Jiang Z et al . Proteomics identifies novel biomarkers of synovial joint disease in a canine model of mucopolysaccharidosis I. Mol Genet Metab. (2023) 138:107371. doi: 10.1016/j.ymgme.2023.107371
79.
Park HB Choi BC Baek KH . PGK1 modulates balance between pro- and anti-inflammatory cytokines by interacting with ITI-H4. Biomed Pharmacother. (2023) 161:114437. doi: 10.1016/j.biopha.2023.114437
80.
Laursen TL Bossen L Pihl R Troldborg A Sandahl TD Hansen AG et al . Highly increased levels of inter-alpha-inhibitor heavy chain 4 (ITIH4) in autoimmune cholestatic liver diseases. J Clin Transl Hepatol. (2022) 10:796–802. doi: 10.14218/JCTH.2021.00515
81.
Nakamura N Hatano E Iguchi K Sato M Kawaguchi H Ohtsu I et al . Elevated levels of circulating ITIH4 are associated with hepatocellular carcinoma with nonalcoholic fatty liver disease: from pig model to human study. BMC Cancer. (2019) 19:621. doi: 10.1186/s12885-019-5825-8
82.
Lee EJ Yang SH Kim KJ Cha H Lee SJ Kim JH et al . Inter-alpha inhibitor H4 as a potential biomarker predicting the treatment outcomes in patients with hepatocellular carcinoma. Cancer Res Treat. (2018) 50:646–57. doi: 10.4143/crt.2016.550
83.
Murakami I Oh Y Morimoto A Sano H Kanzaki S Matsushita M et al . Acute-phase ITIH4 levels distinguish multi-system from single-system Langerhans cell histiocytosis via plasma peptidomics. Clin Proteomics. (2015) 12:16. doi: 10.1186/s12014-015-9089-2
84.
Li L Choi BC Ryoo JE Song SJ Pei CZ Lee KY et al . Opposing roles of inter-alpha-trypsin inhibitor heavy chain 4 in recurrent pregnancy loss. EBioMedicine. (2018) 37:535–46. doi: 10.1016/j.ebiom.2018.10.029
85.
Larsen JB Pihl R Aggerbeck MA Larsen KM Hvas CL Johnsen N et al . Inter-alpha-inhibitor heavy chain H4 and sepsis-related coagulation disturbances: another link between innate immunity and coagulation. Res Pract Thromb Haemost. (2023) 7:100078. doi: 10.1016/j.rpth.2023.100078
86.
van Swelm RP Laarakkers CM Kooijmans-Otero M de Jong EM Masereeuw R Russel FG . Biomarkers for methotrexate-induced liver injury: urinary protein profiling of psoriasis patients. Toxicol Lett. (2013) 221:219–24. doi: 10.1016/j.toxlet.2013.06.234
87.
Sun Y Jin J Jing H Lu Y Zhu Q Shu C et al . ITIH4 is a novel serum biomarker for early gastric cancer diagnosis. Clin Chim Acta. (2021) 523:365–73. doi: 10.1016/j.cca.2021.10.022
88.
Jing D Jin J Mei Z Zhu Q Lu Y Wang X . Effects of Helicobacter pylori infection and interleukin 6 on the expression of ITIH4 in human gastric cancer cells. Transl Cancer Res. (2020) 9:4656–65. doi: 10.21037/tcr-20-1766
89.
Abdullah-Soheimi SS Lim BK Hashim OH Shuib AS . Patients with ovarian carcinoma excrete different altered levels of urine CD59, kininogen-1 and fragments of inter-alpha-trypsin inhibitor heavy chain H4 and albumin. Proteome Sci. (2010) 8:58. doi: 10.1186/1477-5956-8-58
90.
Noh CK Kim SS Kim DK Lee HY Cho HJ Yoon SY et al . Inter-alpha-trypsin inhibitor heavy chain H4 as a diagnostic and prognostic indicator in patients with hepatitis B virus-associated hepatocellular carcinoma. Clin Biochem. (2014) 47:1257–61. doi: 10.1016/j.clinbiochem.2014.05.002
91.
Iwai T Ohyama A Osada A Nishiyama T Shimizu M Miki H et al . Role of inter-alpha-trypsin inhibitor heavy chain 4 and its citrullinated form in experimental arthritis murine models. Clin Exp Immunol. (2024) 215:302–12. doi: 10.1093/cei/uxae001
92.
Tian H Wang G Zhong Q Zhou H . Usability of serum inter-alpha-trypsin inhibitor heavy chain 4 as a biomarker for assessing severity and predicting functional outcome after human aneurysmal subarachnoid hemorrhage: a prospective observational cohort study at a single institution. Clin Chim Acta. (2024) 552:117679. doi: 10.1016/j.cca.2023.117679
93.
Jayapalan JJ Ng KL Shuib AS Razack AH Hashim OH . Urine of patients with early prostate cancer contains lower levels of light chain fragments of inter-alpha-trypsin inhibitor and saposin B but increased expression of an inter-alpha-trypsin inhibitor heavy chain 4 fragment. Electrophoresis. (2013) 34:1663–9. doi: 10.1002/elps.201200583
94.
Kashyap RS Nayak AR Deshpande PS Kabra D Purohit HJ Taori GM et al . Inter-alpha-trypsin inhibitor heavy chain 4 is a novel marker of acute ischemic stroke. Clin Chim Acta. (2009) 402:160–3. doi: 10.1016/j.cca.2009.01.009
95.
Kormosh NG Davidova TV Kopyltsov VN Serebryakova MV Kabieva AO Voyushin KE et al . Conformational changes in inter-alpha-trypsin inhibitor heavy chain 4 activate its tumor-specific activity in mice with B16 melanoma. Mol Med Rep. (2015) 12:4483–93. doi: 10.3892/mmr.2015.3961
96.
van den Broek I Sparidans RW van Winden AW Gast MC van Dulken EJ Schellens JH et al . The absolute quantification of eight inter-alpha-trypsin inhibitor heavy chain 4 (ITIH4)-derived peptides in serum from breast cancer patients. Proteomics Clin Appl. (2010) 4:931–9. doi: 10.1002/prca.201000035
97.
Fujita Y Ezura Y Emi M Sato K Takada D Iino Y et al . Hypercholesterolemia associated with splice-junction variation of inter-alpha-trypsin inhibitor heavy chain 4 (ITIH4) gene. J Hum Genet. (2004) 49:24–8. doi: 10.1007/s10038-003-0101-8
98.
Canter MP Graham CA Heit MH Blackwell LS Wilkey DW Klein JB et al . Proteomic techniques identify urine proteins that differentiate patients with interstitial cystitis from asymptomatic control subjects. Am J Obstet Gynecol. (2008) 198:e551–6. doi: 10.1016/j.ajog.2008.01.052
99.
Chen M Ma J Xie X Su M Zhao D . Serum ITIH5 as a novel diagnostic biomarker in cholangiocarcinoma. Cancer Sci. (2024) 115:1665–79. doi: 10.1111/cas.16143
100.
Sasaki K Kurahara H Young ED Natsugoe S Ijichi A Iwakuma T et al . Genome-wide in vivo RNAi screen identifies ITIH5 as a metastasis suppressor in pancreatic cancer. Clin Exp Metastasis. (2017) 34:229–39. doi: 10.1007/s10585-017-9840-3
101.
Rose M Gaisa NT Antony P Fiedler D Heidenreich A Otto W et al . Epigenetic inactivation of ITIH5 promotes bladder cancer progression and predicts early relapse of pT1 high-grade urothelial tumours. Carcinogenesis. (2014) 35:727–36. doi: 10.1093/carcin/bgt375
102.
Rose M Kloten V Noetzel E Gola L Ehling J Heide T et al . ITIH5 mediates epigenetic reprogramming of breast cancer cells. Mol Cancer. (2017) 16:44. doi: 10.1186/s12943-017-0610-2
103.
Daum AK Dittmann J Jansen L Peters S Dahmen U Heger JI et al . ITIH5 shows tumor suppressive properties in cervical cancer cells grown as multicellular tumor spheroids. Am J Transl Res. (2021) 13:10298–314. PMID:
104.
Dahlman I Elsen M Tennagels N Korn M Brockmann B Sell H et al . Functional annotation of the human fat cell secretome. Arch Physiol Biochem. (2012) 118:84–91. doi: 10.3109/13813455.2012.685745
105.
Martin J Midgley A Meran S Woods E Bowen T Phillips AO et al . Tumor necrosis factor-stimulated gene 6 (TSG-6)-mediated interactions with the inter-alpha-inhibitor heavy chain 5 facilitate tumor growth factor beta1 (TGFbeta1)-dependent fibroblast to myofibroblast differentiation. J Biol Chem. (2016) 291:13789–801. doi: 10.1074/jbc.M115.670521
106.
Himmelfarb M Klopocki E Grube S Staub E Klaman I Hinzmann B et al . ITIH5, a novel member of the inter-alpha-trypsin inhibitor heavy chain family is downregulated in breast cancer. Cancer Lett. (2004) 204:69–77. doi: 10.1016/j.canlet.2003.09.011
107.
Dittmann J Ziegfeld A Jansen L Gajda M Kloten V Dahl E et al . Gene expression analysis combined with functional genomics approach identifies ITIH5 as tumor suppressor gene in cervical carcinogenesis. Mol Carcinog. (2017) 56:1578–89. doi: 10.1002/mc.22613
108.
Kloten V Rose M Kaspar S von Stillfried S Knuchel R Dahl E . Epigenetic inactivation of the novel candidate tumor suppressor gene ITIH5 in colon cancer predicts unfavorable overall survival in the CpG island methylator phenotype. Epigenetics. (2014) 9:1290–301. doi: 10.4161/epi.32089
109.
Kosinski J Sechi A Hain J Villwock S Ha SA Hauschulz M et al . ITIH5 as a multifaceted player in pancreatic cancer suppression, impairing tyrosine kinase signaling, cell adhesion and migration. Mol Oncol. (2024) 18:1486–509. doi: 10.1002/1878-0261.13609
110.
Dotsch MM Kloten V Schlensog M Heide T Braunschweig T Veeck J et al . Low expression of ITIH5 in adenocarcinoma of the lung is associated with unfavorable patients’ outcome. Epigenetics. (2015) 10:903–12. doi: 10.1080/15592294.2015.1078049
111.
Ruhl T Sessler TM Keimes JM Beier JP Villwock S Rose M et al . ITIH5 inhibits proliferation, adipogenic differentiation, and secretion of inflammatory cytokines of human adipose stem cells-a new key in treating obesity?FASEB J. (2024) 38:e23352. doi: 10.1096/fj.202301366R
112.
Cai P Li H Huo W Zhu H Xu C Zang R et al . Aberrant expression of LncRNA-MIR31HG regulates cell migration and proliferation by affecting miR-31 and miR-31* in Hirschsprung’s disease. J Cell Biochem. (2018) 119:8195–203. doi: 10.1002/jcb.26830
Summary
Keywords
inter-α-trypsin inhibitor (IαI) family, bikunin (BK), ITIHs, hyaluronic acid (HA), extracellular matrix (ECM)
Citation
Zhang X-f, Zhang X-l, Guo L, Bai Y-p, Tian Y and Luo H-y (2024) The function of the inter-alpha-trypsin inhibitors in the development of disease. Front. Med. 11:1432224. doi: 10.3389/fmed.2024.1432224
Received
13 May 2024
Accepted
15 July 2024
Published
01 August 2024
Volume
11 - 2024
Edited by
Ian James Martins, University of Western Australia, Australia
Reviewed by
Xiaolei Li, University of Pennsylvania, United States
Xianfang Rong, IMiracle, China
Updates
Copyright
© 2024 Zhang, Zhang, Guo, Bai, Tian and Luo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Tian, 905790434@qq.comHua-you Luo, km-lhy@qq.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.