Abstract
Background:
The aim of this study was to examine the prognostic significance of serum albumin-to-creatinine ratio (ACR) in critically ill patients with sepsis.
Methods:
This retrospective study analyzed sepsis cases admitted to the Affiliated Hospital of Jiangsu University between January 2015 and November 2023. The patients were divided into four groups based on their ACR upon admission to the intensive care unit (ICU). Laboratory data were collected at the time of ICU admission, and the primary outcome measure was in-hospital all-cause mortality. Kaplan–Meier survival curves were generated to illustrate the differences in 30−/60-day mortality among the various groups. Multivariate Cox regression models and restricted cubic splines (RCS) were utilized to explore the association between ACR and all-cause mortality in sepsis patients. Subgroup analyses were conducted to examine the impact of other covariates on the relationship between ACR and all-cause mortality.
Results:
A total of 1,123 eligible patients were included in the study, with a median ACR of 0.169. The in-hospital mortality rate was 33.7%, the ICU mortality rate was 31.9%, and the 30-day mortality rate was 28.1%. Kaplan–Meier survival analysis demonstrated that patients with higher ACR had a significantly lower risk of 30−/60-day mortality (log-rank p < 0.001). Multivariable Cox proportional hazards analyses revealed that ACR was an independent predictor of in-hospital death (HR: 0.454, 95% CI 0.271–0.761, p = 0.003), ICU death (HR: 0.498, 95% CI 0.293–0.847, p = 0.010), and 30-day death (HR: 0.399, 95% CI 0.218–0.730, p = 0.003). For each 1-unit increase in ACR, there was a 1.203-fold decrease in the risk of death during the hospital stay. The RCS curve illustrated a non-linear negative correlation between ACR and in-hospital mortality (p for non-linear =0.018), ICU mortality (p for non-linear =0.005), and 30-day mortality (p for non-linear =0.006). Sensitivity analysis indicated consistent effect sizes and directions in different subgroups, confirming the stability of the results.
Conclusion:
Low ACR levels were identified as independent risk factors associated with increased in-hospital, ICU, and 30-day mortality in sepsis patients. ACR can serve as a significant predictor of the clinical outcome of sepsis.
Introduction
Sepsis is a life-threatening condition characterized by organ dysfunction resulting from an imbalanced immune response to infection (1). In 2017, the World Health Organization (WHO) recognized sepsis as a global health priority (2), and the Global Burden of Disease (GBD) project estimated that nearly 50 million cases of sepsis and 11 million sepsis-related deaths occurred worldwide in the same year (3). It was worth noting that sepsis remained a significant reason for ICU admissions in low and middle-income countries, where mortality rates as high as 80% had been reported (4). Furthermore, sepsis survivors faced ongoing challenges, with a 15% mortality rate within the first year after discharge and 6–8% in the subsequent 5 years (5, 6). Early identification and intervention for sepsis patients at higher risk of mortality were crucial for improving prognosis (7). Therefore, early risk stratification played a vital role in helping clinicians determine appropriate treatment strategies and long-term management approaches.
Many biomarkers had been utilized in predicting the prognosis of sepsis patients, including Pentraxin (PTX-3), mid-regional pro adrenomedullin (MR-proADM), and soluble tumor necrosis factor receptor type 1 (sTNFR1) (8–10). However, these tools were either expensive or not readily accessible. It was well-known that inflammation and oxidative stress played crucial roles in the pathogenesis of sepsis (11, 12). Serum albumin (Alb), a significant plasma protein, possessed important physiological functions such as immune regulation, endothelial stability, inflammation response, and antioxidant effects (13). There was mounting evidence indicating a close association between serum Alb levels and infectious diseases like sepsis, COVID-19, and acute pancreatitis (14–16). Nevertheless, it should be noted that the patient’s nutritional status or chronic inflammation can also impact albumin levels, which may introduce limitations when relying solely on albumin levels for prediction (17). Furthermore, serum creatinine, an indicator of renal function, was linked to oxidative stress, endothelial function, and inflammation (18, 19). Elevated levels of serum creatinine upon admission could aid in predicting the onset of adverse events in sepsis patients (20). However, it was important to consider that abnormal creatinine levels may also result from chronic kidney disease. Therefore, relying solely on creatinine prediction may not guarantee reliable outcomes. Remarkably, the serum albumin-to-creatinine ratio (ACR), a novel composite ratio, had emerged as an easily obtainable parameter that had shown promising predictive utility in assessing adverse outcomes among patients with ST-elevation myocardial infarction (STEMI), heart transplantation, and diabetes (21–23). To the best of our knowledge, the effectiveness of serum ACR as a predictive marker had not been investigated in sepsis patients. Hence, the main objective of this retrospective study was to analyze the correlation between serum ACR and prognosis in critically ill sepsis patients.
Methods
Study design and setting
This retrospective study included adult patients (aged ≥ 18 years) who were admitted to the ICU of the Affiliated Hospital of Jiangsu University between January 2015 and November 2023. The study protocol underwent review by the ethical review board of the Affiliated Hospital of Jiangsu University (No. KY2023K1007). Written informed consent was obtained from all participating patients.
Study population
Patients were included in this study if they met the following criteria: a diagnosis of sepsis based on the sepsis 3.0 diagnostic criteria, which included infection and a Sequential Organ Failure Assessment (SOFA) score of ≥2 (24). If a patient had multiple admission records, data from their first admission were used. Patients were excluded if they met any of the following conditions: being under 18 years old, staying in the ICU for less than 24 h, or having chronic kidney disease or hepatic cirrhosis. Ultimately, a total of 1,123 patients were included in the final analysis. The detailed inclusion and exclusion process was depicted in Figure 1.
Figure 1

Flow of included patients through the trial. ACR, albumin-to-creatinine ratio; ICU, Intensive Care Unit.
Data collection
The potential variables for this study were extracted from the electronic medical record system. The following basic characteristics of all patients were collected upon admission to the hospital: age, gender, body mass index (BMI), smoking history, hypertension, diabetes, coronary artery disease, chronic obstructive pulmonary disease (COPD), and cerebral infarction. The following laboratory test parameters were collected at the day of ICU admission: white blood cell (WBC), neutrophil (Neu), lymphocyte (Lym), monocyte (Mon), hemoglobin (Hb), platelet (PLT), C-reactive protein (CRP), total bilirubin (Tbil), alanine transaminase (ALT), aspartate aminotransferase (AST), albumin (Alb), glucose, creatinine, blood urea nitrogen (BUN), uric acid, D-dimer, potassium, and lactate. We selected the first set of parameters if the variables included were measured more than once on the day of ICU admission. Additionally, the Acute Physiology and Chronic Health Evaluation II (APACHE II) score and SOFA score were calculated within the first 24 h of ICU admission. The treatments received, including continuous renal replacement therapy (CRRT), vasoactive drugs, and invasive ventilation, were also recorded. The follow-up duration was defined as the period from ICU admission until death or discharge. The serum albumin-to-creatinine ratio at presentation was calculated as follows: serum albumin (g/L)/serum creatinine (μmol/L). Subsequently, the patients were categorized into four groups based on their ACR quartile range: Quartile 1 group (ACR ≤ 0.169, ≤ 25th), Quartile 2 group (0.169 < ACR ≤ 0.299, 25th-50th), Quartile 3 group (0.299 < ACR ≤ 0.463, 50th–75th), and Quartile 4 group (ACR > 0.463, > 75th).
Primary outcome and secondary outcomes
The primary outcome of the current study was all-cause mortality occurring during the hospital stay. The secondary endpoints included mortality specifically within the ICU and mortality within 30 days following admission to the ICU.
Statistical analysis
The statistical analysis was conducted using SPSS version 26.0, GraphPad Prism 10.0, and R software version 4.1.3. Statistical significance was defined as p < 0.05. Continuous variables were presented as mean ± standard deviation (mean ± SD) for normally distributed data, or median (25th and 75th) for non-normally distributed data. Categorical variables were expressed as numbers and percentages. The Mann–Whitney U test or Student’s t-test was employed to compare continuous variables, while the chi-square test was used for categorical variables. To visualize the time to the occurrence of 30−/60-day mortality, Kaplan–Meier analysis was performed, and the log-rank test was used to compare differences between the four groups based on the ACR. Cox proportional hazard models were employed to calculate the hazard ratio (HR) and 95% confidence interval (CI) to explore the association between ACR and 30-day, ICU, and in-hospital mortality. Three Cox proportional hazards regression models were constructed to evaluate the independent association of ACR with the primary endpoint. Model 1 was an unadjusted model with no variables adjusted. Model 2 was adjusted for age, gender, BMI, smoking, hypertension, and diabetes. Model 3 was adjusted for age, gender, BMI, smoking, hypertension, diabetes, WBC, Neu, Lym, CRP, ALT, AST, glucose, APACHE II score, and SOFA score. ACR was included in the models as either a continuous variable or a categorical variable. Moreover, restricted cubic spline (RCS) regression with three knots (10th, 50th, and 90th percentiles) was applied to assess the potential non-linear association between ACR and 30-day, ICU, and in-hospital mortality. Subgroup analysis was conducted based on age, gender, BMI, smoking, hypertension, diabetes, and SOFA score. p values for interaction were calculated to investigate the effect of each subgroup on the outcome.
Results
Baseline characteristics
The baseline clinical and biochemical characteristics of the 1,123 individuals were assessed in this study, with 707 (63.0%) being males (Table 1). The median levels of Alb, creatinine, and ACR were determined to be 28.2 g/L (IQR: 24.2–33.2), 92.6 μmol/L (IQR: 63.7–153.1), and 0.300 (IQR: 0.169–0.463), respectively. The in-hospital, ICU, and 30-day mortality rates were observed to be 33.7, 31.9, and 28.1%, respectively. It was noted that patients with higher ACR levels exhibited a lower proportion of males and coronary artery disease, but a higher prevalence of COPD. Furthermore, these patients demonstrated elevated levels of Lym, PLT, Hb, and Alb, along with reduced levels of WBC, Neu, CRP, Tbil, ALT, AST, glucose, creatinine, BUN, uric acid, D-dimer, potassium, and lactate. Notably, they also displayed a lower severity of illness as measured by APACHE II score and SOFA score, as well as a decreased utilization of CRRT and vasoactive drugs.
Table 1
| Variables | Overall | Q1 group (ACR ≤ 0.169) | Q2 group (0.169 < ACR ≤ 0.299) | Q3 group (0.299 < ACR ≤ 0.463) | Q4 group (ACR > 0.463) | p-value |
|---|---|---|---|---|---|---|
| N | 1,123 | 281 | 281 | 281 | 280 | |
| Age, years | 75 (65–84) | 75 (67–83) | 76 (66–85) | 77 (66–86) | 73 (61–83) | 0.096 |
| Male, n (%) | 707 (63.0) | 187 (66.5) | 174 (61.9) | 192 (68.3) | 154 (55.0) | 0.005 |
| BMI, kg/m2 | 22.49 (20.08–25.21) | 22.41 (20.10–24.50) | 22.60 (19.96–25.64) | 22.83 (20.42–25.39) | 22.49 (16.70–24.89) | 0.188 |
| Smoking, n (%) | 229 (20.4) | 57 (20.3) | 55 (19.6) | 65 (23.1) | 52 (18.6) | 0.579 |
| Comorbidities, n (%) | ||||||
| Hypertension | 579 (51.3) | 152 (54.1) | 153 (54.4) | 146 (52.0) | 128 (45.7) | 0.138 |
| Diabetes | 309 (27.5) | 93 (33.1) | 75 (26.7) | 75 (26.7) | 66 (23.6) | 0.080 |
| Coronary artery disease | 116 (10.3) | 42 (14.9) | 30 (10.7) | 25 (8.9) | 19 (6.8) | 0.012 |
| COPD | 87 (7.7) | 15 (5.3) | 18 (6.4) | 22 (7.8) | 32 (11.4) | 0.040 |
| Cerebral infarction | 161 (14.3) | 37 (13.2) | 43 (15.3) | 35 (12.5) | 46 (16.4) | 0.506 |
| Laboratory tests | ||||||
| WBC *109 /L | 11.4 (7.4–17.1) | 13.1 (7.9–20.0) | 11.9 (6.8–17.1) | 10.7 (7.5–16.8) | 10.6 (7.4–15.0) | 0.004 |
| Neu *109 /L | 10.1 (6.3–15.5) | 12.2 (6.8–18.5) | 10.5 (6.0–15.7) | 9.5 (6.3–15.5) | 9.3 (6.2–13.5) | <0.001 |
| Lym *109 /L | 0.6 (0.3–0.9) | 0.5 (0.3–0.8) | 0.5 (0.3–0.9) | 0.6 (0.4–0.9) | 0.6 (0.4–1.0) | <0.001 |
| Mon *109 /L | 0.4 (0.2–0.7) | 0.4 (0.2–0.7) | 0.4 (0.2–0.7) | 0.4 (0.2–0.7) | 0.4 (0.2–0.6) | 0.598 |
| Hb, g/dL | 115 (97–130) | 109 (89–128) | 112 (97–129) | 117 (100–131) | 118 (101–133) | 0.001 |
| PLT *109 /L | 149 (95–214) | 114 (67–172) | 146 (92–206) | 160 (109–223) | 186 (130–251) | <0.001 |
| CRP, mg/L | 104.2 (42.0–163.2) | 144.5 (90.0–214.7) | 112.0 (50.9–161.8) | 91.5 (33.4–154.8) | 72.1 (21.3–116.2) | <0.001 |
| Tbil, μmol/L | 17.4 (10.9–28.2) | 20.2 (12.4–34.4) | 18.6 (11.4–32.2) | 16.0 (10.6–23.8) | 15.3 (9.0–23.6) | <0.001 |
| ALT, U/L | 32.0 (21.0–56.0) | 40.0 (23.0–78.0) | 32.0 (22.0–59.5) | 29.0 (20.0–45.0) | 30.0 (20.0–51.0) | <0.001 |
| AST, U/L | 38.1 (23.9–73.0) | 55.9 (29.0–146.0) | 41.0 (24.0–84.5) | 34.5 (22.1–59.0) | 31.0 (22.0–60.0) | <0.001 |
| Alb, g/L | 28.2 (24.2–33.2) | 24.6 (21.1–28.8) | 27.2 (23.8–31.8) | 29.5 (25.2–33.7) | 32.0 (28.5–36.3) | <0.001 |
| Glucose, mmol/L | 8.2 (6.6–11.8) | 9.0 (6.8–13.9) | 8.3 (6.6–12.2) | 8.1 (6.5–11.4) | 7.7 (6.4–10.4) | 0.003 |
| Creatinine, μmol/L | 92.6 (63.7–153.1) | 224.0 (169.8–344.8) | 119.9 (101.5–136.8) | 76.1 (65.9–88.9) | 52.4 (44.1–62.4) | <0.001 |
| BUN, mmol/L | 8.89 (6.04–13.95) | 17.83 (12.06–24.20) | 10.01 (7.67–14.43) | 7.03 (5.48–9.48) | 5.70 (4.30–7.70) | <0.001 |
| Uric acid, μmol/L | 286.9 (192.3–411.7) | 441.3 (327.5–561.7) | 311.1 (255.0–429.4) | 256.0 (169.4–327.1) | 189.3 (135.6–251.4) | <0.001 |
| D-dimer, mg/L | 4.2 (2.1–8.4) | 6.2 (3.2–12.7) | 5.6 (2.9–9.6) | 3.9 (2.1–7.1) | 2.6 (1.5–5.1) | <0.001 |
| Potassium, mmol/L | 3.7 (3.3–4.2) | 3.9 (3.5–4.7) | 3.6 (3.2–4.2) | 3.7 (3.3–4.1) | 3.6 (3.3–3.9) | <0.001 |
| Lactate, mmol/L | 2.1 (1.4–3.6) | 2.9 (1.9–5.3) | 2.4 (1.7–4.0) | 1.9 (1.3–2.9) | 1.7 (1.2–2.4) | <0.001 |
| ACR | 0.300 (0.169–0.463) | 0.118 (0.077–0.145) | 0.230 (0.202–0.268) | 0.379 (0.340–0.419) | 0.597 (0.517–0.724) | <0.001 |
| Severity scoring | ||||||
| APACHE II score | 25 (19–30) | 28 (22–33) | 25 (19–30) | 25 (19–29) | 23 (18–28) | <0.001 |
| SOFA score | 12 (10–14) | 13 (11–15) | 12 (10–14) | 12 (9–14) | 11 (9–14) | <0.001 |
| Treatments | ||||||
| CRRT, n (%) | 78 (6.9) | 61 (21.7) | 14 (5.0) | 0 (0.0) | 3 (1.1) | <0.001 |
| Vasoactive drug, n (%) | 748 (66.6) | 235 (83.6) | 214 (76.2) | 163 (58.0) | 136 (48.6) | <0.001 |
| Invasive ventilation, n (%) | 752 (67.0) | 189 (67.3) | 192 (68.3) | 196 (69.8) | 175 (62.5) | 0.289 |
| Endpoints | ||||||
| 30-day mortality, n (%) | 316 (28.1) | 123 (43.8) | 76 (27.0) | 67 (23.8) | 50 (17.9) | <0.001 |
| 60-day mortality, n (%) | 375 (33.4) | 135 (48.0) | 97 (34.5) | 80 (28.5) | 63 (22.5) | <0.001 |
| Length of ICU stay, days | 6 (3–12) | 7 (4–13) | 5 (3–11) | 5 (3–11) | 5 (2–13) | <0.001 |
| Length of hospital stay, days | 16 (11–25) | 18 (9–26) | 15 (10.25) | 17 (12–25) | 16 (11–26) | 0.289 |
| ICU mortality, n (%) | 358 (31.9) | 128 (45.6) | 92 (32.7) | 75 (26.7) | 63 (22.5) | <0.001 |
| Hospital mortality, n (%) | 379 (33.7) | 135 (48.0) | 97 (34.5) | 82 (29.2) | 65 (23.2) | <0.001 |
Characteristics and outcomes of participants categorized by ACR.
ACR, albumin-to-creatinine ratio; BMI, body mass index; COPD, chronic obstructive pulmonary disease; WBC, white blood cell count; Neu, neutrophil; Lym, lymphocyte; Mon, monocyte; Hb, hemoglobin; PLT, platelet; CRP, C-reactive protein; Tbil, total bilirubin; ALT, alanine transaminase; AST, aspartate aminotransferase; Alb, albumin; BUN, blood urea nitroge; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; CRRT, continuous renal replacement therapy; ICU, Intensive Care Unit.
As ACR levels increased, there was a gradual decrease observed in the ICU length of stay (7 days vs. 5 days vs. 5 days vs. 5 days, p < 0.001), 30-day mortality rate (43.8% vs. 27.0% vs. 23.8% vs. 17.9%, p < 0.001), 60-day mortality rate (48.0% vs. 34.5% vs. 28.5% vs. 22.5%, p < 0.001), ICU mortality rate (45.6% vs. 32.7% vs. 26.7% vs. 22.5%, p < 0.001), and hospital mortality rate (48.0% vs. 34.5% vs. 29.2% vs. 23.2%, p < 0.001). Moreover, there was a notable decreasing trend in hospital mortality (p for trend <0.001), ICU mortality (p for trend <0.001), and 30-day mortality rates as ACR levels increased (p for trend <0.001) (Figures 2A–C).
Figure 2

(A) The prevalence of hospital mortality ratio among different quartiles of ACR. (B) The prevalence of ICU mortality ratio among different quartiles of ACR. (C) The prevalence of 30-day mortality ratio among different quartiles of ACR. ACR quartiles: Q1 group (ACR ≤ 0.169); Q2 group (0.169 < ACR ≤ 0.299); Q3 group (0.299 < ACR ≤ 0.463); Q4 group (ACR > 0.463). ACR, albumin-to-creatinine ratio; ICU, Intensive Care Unit.
ACR and in-hospital, ICU, and 30-day mortality
The levels of ACR in the survivor group were found to be significantly higher than those in the non-survivor group (0.335 vs. 0.226, p < 0.001). The distribution of ACR, stratified by the mortality status of all-cause in-hospital death, ICU death, and 30-day death, was depicted in Supplementary Figure S1. Kaplan–Meier analysis in Figure 3 illustrated the cumulative incidence of 30−/60-day mortality based on ACR levels. Patients with higher ACR demonstrated a lower risk of 30−/60-day mortality compared to those with lower ACR levels (log-rank p < 0.001 for both).
Figure 3

Kaplan–Meier curves showing cumulative probability of all-cause mortality according to groups at 30 days (A), and 60 days (B). ACR quartiles: Q1 group (ACR ≤ 0.169); Q2 group (0.169 < ACR ≤ 0.299); Q3 group (0.299 < ACR ≤ 0.463); Q4 group (ACR > 0.463).
To identify prognostic predictors in patients with sepsis, we performed univariate Cox regression analysis. Variables that showed significance in the univariate analysis (p < 0.05) or were potentially associated with clinical outcomes were included as independent variables for Cox regression analysis (Supplementary Table S1). In three multivariable Cox regression analysis models, both the absolute change rate (ACR) increased by 1 unit or 1 SD were significantly and negatively correlated with all-cause in-hospital death, ICU death, and 30-day death (Table 2). When considering ACR as a nominal variable, patients in the higher quartile of ACR were significantly associated with a lower risk of hospital death in the three established Cox proportional hazards models compared to subjects in the lowest quartile. In Model 1, ACR was negatively associated with hospital death [HR (95%CI): 0.474 (0.353–0.638); p < 0.001]. After adjusting for confounding factors, the negative association still remained in Model 2 [HR (95%CI): 0.457 (0.339–0.616); p < 0.001] and Model 3 [HR (95%CI): 0.635 (0.461–0.875); p = 0.005]. The test for trends across tertiles of ACR for the risk of hospital death was statistically significant (Figure 4). Similar results were observed in the multivariate Cox proportional risk analysis of ACR and ICU mortality and 30-day mortality (Table 2). Furthermore, we found a nonlinear negative correlation between these variables, with the HR curve for in-hospital mortality (p for non-linear =0.018), ICU mortality (p for non-linear =0.005), and 30-day mortality (p for non-linear =0.006) first declining sharply as ACR increased and then leveling off (Figure 5).
Table 2
| Variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | P for trend | HR (95% CI) | p-value | P for trend | HR (95% CI) | p-value | P for trend | |
| Hospital mortality | |||||||||
| Per Unit increase | 0.271 (0.164–0.448) | <0.001 | 0.252 (0.151–0.419) | <0.001 | 0.454 (0.271–0.761) | 0.003 | |||
| Per SD increase | 0.726 (0.642–0.821) | <0.001 | 0.713 (0.629–0.808) | <0.001 | 0.824 (0.726–0.935) | 0.003 | |||
| Quartilea | <0.001 | <0.001 | 0.005 | ||||||
| Q1 group | Ref | Ref | Ref | ||||||
| Q2 group | 0.787 (0.606–1.021) | 0.072 | 0.758 (0.583–0.986) | 0.039 | 0.903 (0.688–1.186) | 0.464 | |||
| Q3 group | 0.597 (0.454–0.786) | <0.001 | 0.582 (0.442–0.767) | <0.001 | 0.798 (0.594–1.072) | 0.134 | |||
| Q4 group | 0.474 (0.353–0.638) | <0.001 | 0.457 (0.339–0.616) | <0.001 | 0.635 (0.461–0.875) | 0.005 | |||
| ICU mortality | |||||||||
| Per Unit increase | 0.283 (0.169–0.474) | <0.001 | 0.265 (0.157–0.447) | <0.001 | 0.498 (0.293–0.847) | 0.010 | |||
| Per SD increase | 0.734 (0.647–0.833) | <0.001 | 0.722 (0.635–0.821) | <0.001 | 0.843 (0.740–0.960) | 0.010 | |||
| Quartilea | <0.001 | <0.001 | 0.009 | ||||||
| Q1 group | Ref | Ref | Ref | ||||||
| Q2 group | 0.781 (0.597–1.022) | 0.071 | 0.755 (0.576–0.989) | 0.041 | 0.907 (0.686–1.201) | 0.496 | |||
| Q3 group | 0.573 (0.431–0.762) | <0.001 | 0.557 (0.419–0.742) | <0.001 | 0.772 (0.569–1.049) | 0.098 | |||
| Q4 group | 0.486 (0.359–0.657) | <0.001 | 0.471 (0.347–0.638) | <0.001 | 0.665 (0.479–0.923) | 0.015 | |||
| 30-day mortality | |||||||||
| Per Unit increase | 0.208 (0.116–0.373) | <0.001 | 0.198 (0.110–0.358) | <0.001 | 0.399 (0.218–0.730) | 0.003 | |||
| Per SD increase | 0.680 (0.589–0.785) | <0.001 | 0.673 (0.582–0.778) | <0.001 | 0.798 (0.689–0.926) | 0.003 | |||
| Quartilea | <0.001 | <0.001 | 0.002 | ||||||
| Q1 group | Ref | Ref | Ref | ||||||
| Q2 group | 0.655 (0.492–0.872) | 0.004 | 0.637 (0.477–0.850) | 0.002 | 0.753 (0.559–1.014) | 0.062 | |||
| Q3 group | 0.536 (0.398–0.721) | <0.001 | 0.529 (0.393–0.713) | <0.001 | 0.709 (0.515–0.975) | 0.035 | |||
| Q4 group | 0.406 (0.292–0.564) | <0.001 | 0.396 (0.285–0.552) | <0.001 | 0.571 (0.401–0.814) | 0.002 | |||
Cox proportional hazard ratios (HR) for all-cause mortality.
ACR: Q1 group (ACR ≤ 0.169); Q2 group (0.169 < ACR ≤ 0.299); Q3 group (0.299 < ACR ≤ 0.463); Q4 group (ACR > 0.463).
Model 1: unadjusted.
Model 2: adjusted for age, gender, BMI, Smoking, hypertension, and diabetes.
Model 3: adjusted for age, gender, BMI, Smoking, hypertension, diabetes, WBC, Neu, Lym, CRP, ALT, AST, glucose, APACHE II score, and SOFA score.
ACR, albumin-to-creatinine ratio; BMI, body mass index; WBC, white blood cell; Neu, neutrophil; Lym, lymphocyte; CRP, C-reactive protein; ALT, alanine transaminase; AST, aspartate aminotransferase; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; ICU, Intensive Care Unit.
Figure 4

(A–C) Hazard ratios (95% CIs) for hospital/ICU/30-day mortality according to ACR quartiles after adjusting for age, gender, BMI, Smoking, hypertension, diabetes, WBC, Neu, Lym, CRP, ALT, AST, glucose, APACHE II score, and SOFA score. Error bars indicate 95% CIs. The first quartile is the reference. ACR quartiles: Q1 group (ACR ≤ 0.169); Q2 group (0.169 < ACR ≤ 0.299); Q3 group (0.299 < ACR ≤ 0.463); Q4 group (ACR > 0.463). ACR, albumin-to-creatinine ratio; BMI, body mass index; WBC, white blood cell; Neu, neutrophil; Lym, lymphocyte; CRP, C-reactive protein; ALT, alanine transaminase; AST, aspartate aminotransferase; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; ICU, Intensive Care Unit.
Figure 5
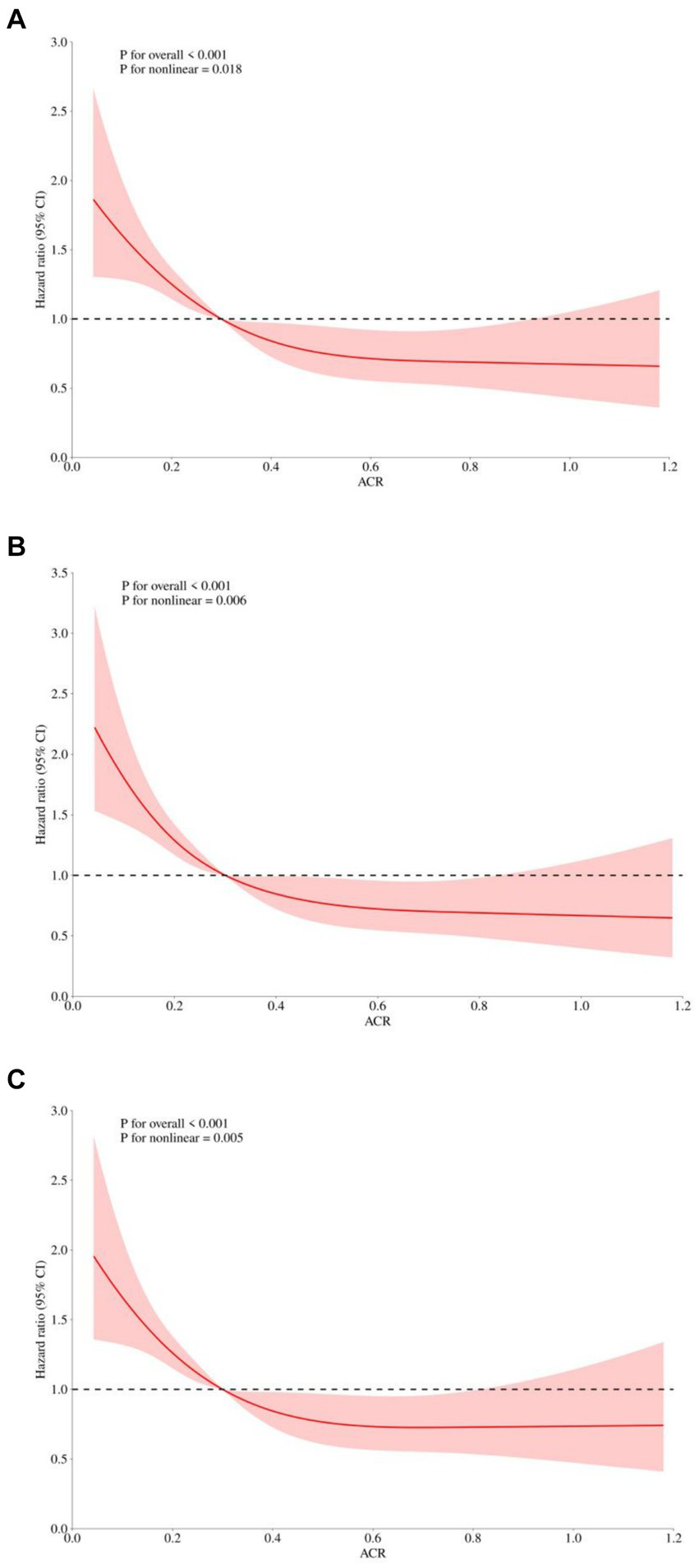
Restricted cubic spline regression analysis of ACR with in hospital all-cause mortality. Heavy central lines represent the estimated adjusted hazard ratios, with shaded ribbons denoting 95% confidence intervals. The horizontal dotted lines represent the hazard ratio of 1.0. (A) Restricted cubic spline for hospital mortality. (B) Restricted cubic spline for ICU mortality. (C) Restricted cubic spline for 30-day mortality. ACR, albumin-to-creatinine ratio; ICU, Intensive Care Unit.
Subgroup analysis
To further explore whether ACR remained an independent prognostic factor in specific subgroups of patients with sepsis, we conducted an exploratory subgroup analysis based on age (≤65 or > 65), gender (male or female), BMI (≤22.49 or > 22.49), smoking (yes or no), hypertension (yes or no), diabetes (yes or no), and SOFA score (≤12 or > 12). The associations between ACR and hospital mortality, ICU mortality, and 30-day mortality were generally consistent across the subgroups (Tables 3–5). We did not observe a significant interaction between ACR and age, gender, BMI, smoking, hypertension, diabetes, or SOFA score (all p-values for interaction >0.05). This indicated that ACR was an independent prognostic factor.
Table 3
| Subgroups | No. hospital mortality/No. patients | HR (95% CI) | p-value | P for interaction |
|---|---|---|---|---|
| Age | 0.836 | |||
| >65 | 315/840 | 0.281 (0.162–0.490) | <0.001 | |
| ≤65 | 64/283 | 0.233 (0.068–0.798) | 0.020 | |
| Gender | 0.604 | |||
| Male | 251/707 | 0.236 (0.122–0.454) | <0.001 | |
| Female | 128/416 | 0.317 (0.143–0.704) | 0.005 | |
| BMI | 0.833 | |||
| >22.49 | 150/549 | 0.284 (0.128–0.628) | 0.002 | |
| ≤22.49 | 229/574 | 0.257 (0.134–0.493) | <0.001 | |
| Smoking | 0.428 | |||
| Yes | 88/230 | 0.175 (0.055–0.559) | 0.003 | |
| No | 291/893 | 0.300 (0.172–0.523) | <0.001 | |
| Hypertension | 0.760 | |||
| Yes | 211/579 | 0.237 (0.122–0.462) | <0.001 | |
| No | 168/544 | 0.286 (0.131–0.625) | 0.002 | |
| Diabetes | 0.093 | |||
| Yes | 111/309 | 0.135 (0.049–0.370) | <0.001 | |
| No | 268/814 | 0.363 (0.203–0.651) | 0.001 | |
| SOFA | 0.822 | |||
| >12 | 221/488 | 0.355 (0.189–0.667) | 0.001 | |
| ≤12 | 158/635 | 0.301 (0.137–0.662) | 0.003 |
Subgroup analysis regarding the influence of different ACR in the hospital mortality.
ACR, albumin-to-creatinine ratio; BMI, body mass index; SOFA, Sequential Organ Failure Assessment.
Table 4
| Subgroups | No. ICU mortality/No. patients | HR (95% CI) | p-value | P for interaction |
|---|---|---|---|---|
| Age | 0.802 | |||
| >65 | 297/840 | 0.294 (0.166–0.519) | <0.001 | |
| ≤65 | 61/283 | 0.237 (0.068–0.830) | 0.024 | |
| Gender | ||||
| Male | 238/707 | 0.231 (0.117–0.454) | <0.001 | 0.397 |
| Female | 120/416 | 0.367 (0.164–0.821) | 0.015 | |
| BMI | ||||
| >22.49 | 142/549 | 0.277 (0.122–0.630) | 0.002 | 0.969 |
| ≤22.49 | 216/574 | 0.280 (0.144–0.544) | <0.001 | |
| Smoking | ||||
| Yes | 85/230 | 0.160 (0.049–0.524) | 0.002 | 0.293 |
| No | 273/893 | 0.324 (0.183–0.572) | <0.001 | |
| Hypertension | ||||
| Yes | 199/579 | 0.248 (0.125–0.491) | <0.001 | 0.787 |
| No | 159/544 | 0.299 (0.135–0.664) | 0.003 | |
| Diabetes | ||||
| Yes | 104/309 | 0.121 (0.042–0.352) | <0.001 | 0.051 |
| No | 254/814 | 0.398 (0.220–0.719) | 0.002 | |
| SOFA | ||||
| >12 | 210/488 | 0.368 (0.193–0.701) | 0.002 | 0.843 |
| ≤12 | 148/635 | 0.324 (0.144–0.728) | 0.006 | |
Subgroup analysis regarding the influence of different ACR in the ICU mortality.
ACR, albumin-to-creatinine ratio; BMI, body mass index; SOFA, Sequential Organ Failure Assessment; ICU, Intensive Care Unit.
Table 5
| Subgroups | No. ICU mortality/No. patients | HR (95% CI) | p-value | P for interaction |
|---|---|---|---|---|
| Age | 0.448 | |||
| >65 | 266/840 | 0.236 (0.125–0.446) | <0.001 | |
| ≤65 | 50/283 | 0.121 (0.027–0.541) | 0.006 | |
| Gender | 0.290 | |||
| Male | 202/707 | 0.155 (0.070–0.341) | <0.001 | |
| Female | 114/416 | 0.289 (0.121–0.690) | 0.005 | |
| BMI | 0.712 | |||
| >22.49 | 124/549 | 0.239 (0.095–0.601) | 0.002 | |
| ≤22.49 | 192/574 | 0.188 (0.088–0.400) | <0.001 | |
| Smoking | 0.701 | |||
| Yes | 78/230 | 0.165 (0.049–0.556) | 0.004 | |
| No | 238/893 | 0.220 (0.113–0.428) | <0.001 | |
| Hypertension | 0.603 | |||
| Yes | 173/579 | 0.237 (0.110–0.511) | <0.001 | |
| No | 143/544 | 0.176 (0.071–0.436) | <0.001 | |
| Diabetes | 0.403 | |||
| Yes | 90/309 | 0.137 (0.044–0.431) | 0.001 | |
| No | 226/814 | 0.246 (0.124–0.487) | <0.001 | |
| SOFA | 0.524 | |||
| >12 | 188/488 | 0.314 (0.153–0.644) | 0.002 | |
| ≤12 | 128/635 | 0.219 (0.087–0.553) | 0.001 |
Subgroup analysis regarding the influence of different ACR in the 30-day mortality.
ACR, albumin-to-creatinine ratio; BMI, body mass index; SOFA, Sequential Organ Failure Assessment.
Discussion
This study represented the first investigation into the correlation between ACR and clinical outcomes in critically ill patients with sepsis. The key findings presented suggested that a decrease in ACR may serve as an independent risk factor contributing to in-hospital mortality among sepsis patients. This conclusion was consistent with previous observations of ICU and 30-day mortality rates in sepsis patients, demonstrating a reliable association. Importantly, this relationship remained significant even after adjusting for various clinical and laboratory variables. Additionally, the RCS curve analysis revealed a negative and non-linear correlation between ACR levels and the risk of in-hospital, ICU, and 30-day mortality. Given its cost-effectiveness and widespread availability in clinical settings, ACR had the potential to serve as a valuable prognostic indicator for critically ill patients with sepsis.
The precise mechanisms underlying the relationship between ACR and the risk of adverse outcomes in sepsis patients had yet to be fully elucidated. However, it was known that inflammation and oxidative stress played significant roles in the onset and progression of sepsis (11, 12). Alb, a commonly measured parameter in clinical laboratories, had a profound impact on various physiological processes (13). Previous studies had indicated that decreased synthesis and increased breakdown of serum Alb were associated with inflammation (25). Inflammatory states can lead to increased microvascular permeability, altering the distribution of Alb between the intra- and extravascular compartments and resulting in reduced serum Alb levels in critically ill patients (26). Arnau-Barrés et al. (27) had shown that serum Alb level < 2.6 g/dL can be used as a prognosis factor for 30-day mortality among elderly patients with sepsis. Cao et al. (28) demonstrated that Alb level was associated with 28-day, 60-day, 180-day and 1-year mortality in sepsis patients. Kumar et al. (29) also confirmed that hypoalbuminemia may serve as an indicator of severity and adverse prognosis in sepsis. Furthermore, decreased serum Alb had been linked to the promotion of oxidative stress, further increasing the risk of mortality in sepsis patients (30, 31).
Early onset of multiple organ damage was a well-known predictor of in-hospital and long-term mortality in sepsis patients, with acute kidney injury (AKI) being a common occurrence (32, 33). Sepsis-induced AKI was associated with an elevated risk of subsequent chronic kidney disease and was linked to increased morbidity and mortality rates in sepsis cases (34, 35). Elevated levels of serum creatinine, an indicator of acute renal impairment, played a crucial role in assessing oxidative stress and inflammatory status, all of which directly impacted the prognosis of sepsis (36). Research conducted by Xiao et al. (20) demonstrated that increased serum creatinine levels were prevalent among sepsis patients and were associated with 28-day mortality. Additionally, these elevated levels served as an independent risk factor for higher mortality rates. Vanmassenhove et al. (37) suggested that changes in serum creatinine within the first 24 h of admission were associated with mortality, with an increase of 0.3 mg/mL in creatinine demonstrating predictive value for sepsis-related death. Therefore, serum creatinine, as a biomarker of AKI, had the potential to reflect the severity of sepsis and aided in the identification of high-risk patients.
Currently, the diagnosis of sepsis using a combination of multiple biochemical markers was a highly researched topic (38–40). While the association between serum Alb and creatinine levels and sepsis had been supported by previous scientific evidence, there had been controversies regarding the factors that influenced their concentrations, such as nutritional status, chronic inflammation, and chronic kidney disease (17). Taking these factors into consideration, we propose using the ratio of serum Alb to serum creatinine to assess the relationship between ACR and clinical outcomes in sepsis. ACR was a reproducible and cost-effective parameter that can be easily collected during routine clinical management and had been frequently used to reflect the body’s inflammatory state and oxidative stress (21). Therefore, this investigation enriches the recent evidence that ACR level can be used to predict renal outcomes in type 2 diabetic and prediabetic subjects (41). Liu et al. (42) demonstrated that ACR can serve as a useful biomarker for predicting all-cause mortality and adverse outcomes in a cohort of 2,250 patients with early-phase acute myocardial infarction (AMI) in the emergency department (ED). Shen et al. (22) discovered that preoperative ACR levels were significant independent predictors of postoperative respiratory complications, renal complications, liver injury, infections, and in-hospital death in patients undergoing heart transplantation. Additionally, a study by Turkyilmaz et al. (43) revealed that ACR was an independent predictor of in-hospital mortality, contrast-induced nephropathy, congestive heart failure, and stent thrombosis at 30 days among patients with ST-segment elevation myocardial infarction (STEMI). However, no research had been conducted to investigate the association between ACR and the risk of poor outcomes in patients with sepsis. Therefore, our study represented the first evaluation of the predictive potential of ACR for all-cause mortality in sepsis patients, and confirmed that ACR was a promising indicator that independently predicted in-hospital, ICU, and 30-day mortality in sepsis patients. We also explored the joint association of ACR with in-hospital, ICU, and 30-day mortality among different subgroups. The results remained consistent in sensitivity analysis and subgroup analysis, indicating that the predictive value of ACR for all-cause mortality was applicable to almost all populations. In summary, these findings suggested that ACR was a readily available and objective hematological biomarker for systemic inflammation.
Apart from inflammation, several other potential mechanisms may contribute to the pathophysiology of sepsis: (1) Sepsis stimulated endothelial cells to produce nitric oxide, which led to vasodilation and a loss of autoregulation, resulting in endothelial dysfunction. This dysfunction can easily trigger microthrombus formation and capillary obstruction, leading to insufficient microcirculatory perfusion in the local renal tissue. Consequently, this prolonged the exposure of renal tubular epithelial cells to inflammatory mediators, leading to cellular damage (44). Existing studies had confirmed that biomarkers of endothelial dysfunction, such as the microalbuminuria to creatinine ratio and adrenomedullin, can serve as indicators for predicting the prognosis of septic patients (45–47). (2) Patients with sepsis were in a state of high catabolism and hypermetabolism, which resulted in the excessive consumption of energy. This increased the metabolism and breakdown of fats and proteins, subsequently leading to a decrease in Alb levels (48, 49). The ACR leveraged early laboratory observations that creatinine levels were positively associated with sepsis, while albumin levels were negatively associated. This ratio enhanced the predictive potential for sepsis.
However, there were some limitations to this study that should be taken into consideration. Firstly, our study was a retrospective study conducted at a single center, which limited our ability to establish a causal relationship between ACR and sepsis compared to prospective studies. Consequently, our findings may lack some persuasive power. Secondly, while repeated measurements of serum Alb and creatinine levels during hospitalization can enhance risk assessment in sepsis patients, we only measured ACR once upon admission. Therefore, the potential variations in ACR over the course of hospitalization and its predictive value for adverse events were not explored in this study. Thirdly, the sample size in our study was relatively small, and the follow-up period was relatively short, particularly during subgroup analysis. Lastly, since our study population consisted solely of adults from China, it was necessary to conduct further research to determine whether the association between ACR and poor outcomes extended to other demographic groups, including minors and individuals from different countries. In future studies, we intend to conduct more comprehensive laboratory investigations with larger participant cohorts and consider additional confounding factors to delve deeper into the underlying mechanisms.
Conclusion
In summary, our study revealed that a low ACR was correlated with a higher risk of all-cause mortality in sepsis patients. Assessing the ACR could serve as a useful tool for stratifying individuals with sepsis at a heightened risk of unfavorable outcomes. This valuable information can aid healthcare professionals in implementing timely early interventions and developing effective clinical plans to improve patient outcomes.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Affiliated Hospital of Jiangsu University (No. KY2023K1007). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZH: Writing – review & editing, Writing – original draft, Supervision, Conceptualization. CS: Writing – review & editing, Conceptualization. JZ: Writing – original draft, Supervision, Formal analysis, Data curation, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thanks all participants in the Affiliated Hospital of Jiangsu University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1436533/full#supplementary-material
References
1.
Evans L Rhodes A Alhazzani W Antonelli M Coopersmith CM French C et al . Surviving Sepsis campaign: international guidelines for management of Sepsis and Septic Shock 2021. Crit Care Med. (2021) 49:e1063–143. doi: 10.1097/CCM.0000000000005337
2.
Reinhart K Daniels R Kissoon N Machado FR Schachter RD Finfer S . recognizing sepsis as a global health priority-a WHO resolution. N Engl J Med. (2017) 377:414–7. doi: 10.1056/NEJMp1707170
3.
Rudd KE Johnson SC Agesa KM Shackelford KA Tsoi D Kievlan DR et al . Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
4.
Schultz MJ Dünser MW Dondorp AM Adhikari NKJ Iyer S Kwizera A et al . Current challenges in the management of sepsis in ICUs in resource-poor settings and suggestions for the future. Intensive Care Med. (2017) 43:612–24. doi: 10.1007/s00134-017-4750-z
5.
Angus DC Opal S . Immunosuppression and secondary infection in Sepsis: part, not all, of the story. JAMA. (2016) 315:1457–9. doi: 10.1001/jama.2016.2762
6.
Prescott HC Osterholzer JJ Langa KM Angus DC Iwashyna TJ . Late mortality after sepsis: propensity matched cohort study. BMJ. (2016) 353:i2375. doi: 10.1136/bmj.i2375
7.
Hou N Li M He L Xie B Wang L Zhang R et al . Predicting 30-days mortality for MIMIC-III patients with sepsis-3: a machine learning approach using XGboost. J Transl Med. (2020) 18:462. doi: 10.1186/s12967-020-02620-5
8.
Caironi P Masson S Mauri T Bottazzi B Leone R Magnoli M et al . Pentraxin 3 in patients with severe sepsis or shock: the ALBIOS trial. Eur J Clin Investig. (2017) 47:73–83. doi: 10.1111/eci.12704
9.
Barichello T Generoso JS Singer M Dal-Pizzol F . Biomarkers for sepsis: more than just fever and leukocytosis-a narrative review. Crit Care. (2022) 26:14. doi: 10.1186/s13054-021-03862-5
10.
Anderson BJ Calfee CS Liu KD Reilly JP Kangelaris KN Shashaty MGS et al . Plasma sTNFR1 and IL8 for prognostic enrichment in sepsis trials: a prospective cohort study. Crit Care. (2019) 23:400. doi: 10.1186/s13054-019-2684-2
11.
Parrish WR Gallowitsch-Puerta M Czura CJ Tracey KJ . Experimental therapeutic strategies for severe sepsis: mediators and mechanisms. Ann N Y Acad Sci. (2008) 1144:210–36. doi: 10.1196/annals.1418.011
12.
Thoppil J Mehta P Bartels B Sharma D Farrar JD . Impact of norepinephrine on immunity and oxidative metabolism in sepsis. Front Immunol. (2023) 14:1271098. doi: 10.3389/fimmu.2023.1271098
13.
Spinella R Sawhney R Jalan R . Albumin in chronic liver disease: structure, functions and therapeutic implications. Hepatol Int. (2016) 10:124–32. doi: 10.1007/s12072-015-9665-6
14.
Sheng S Zhang YH Ma HK Huang Y . Albumin levels predict mortality in sepsis patients with acute kidney injury undergoing continuous renal replacement therapy: a secondary analysis based on a retrospective cohort study. BMC Nephrol. (2022) 23:52. doi: 10.1186/s12882-021-02629-y
15.
Xie C Wang S Zhou J Tong L Shao C . Albumin level as an independent predictive factor for adverse outcomes in COVID-19 patients: a retrospective cohort study. J Infect Dev Ctries. (2022) 16:1696–702. doi: 10.3855/jidc.16880
16.
Liu Q Zheng HL Wu MM Wang QZ Yan SJ Wang M et al . Association between lactate-to-albumin ratio and 28-days all-cause mortality in patients with acute pancreatitis: a retrospective analysis of the MIMIC-IV database. Front Immunol. (2022) 13:1076121. doi: 10.3389/fimmu.2022.1076121
17.
Eckart A Struja T Kutz A Baumgartner A Baumgartner T Zurfluh S et al . Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. (2020) 133:713–722.e7. doi: 10.1016/j.amjmed.2019.10.031
18.
Shulman NB Ford CE Hall WD Blaufox MD Simon D Langford HG et al . Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The hypertension detection and follow-up program cooperative group. Hypertension. (1989) 13:I80–93. doi: 10.1161/01.hyp.13.5_suppl.i80
19.
Yamaguchi J Kasanuki H Ishii Y Yagi M Nagashima M Fujii S et al . Serum creatinine on admission predicts long-term mortality in acute myocardial infarction patients undergoing successful primary angioplasty: data from the Heart Institute of Japan Acute Myocardial Infarction (HIJAMI) registry. Circ J. (2007) 71:1354–9. doi: 10.1253/circj.71.1354
20.
Xiao Y Yan X Shen L Wang Q Li F Yang D et al . Evaluation of qSOFA score, and conjugated bilirubin and creatinine levels for predicting 28-day mortality in patients with sepsis. Exp Ther Med. (2022) 24:447. doi: 10.3892/etm.2022.11374
21.
Huang X Liu Y Zhong C Lin Z Zheng B . Association between serum albumin-to-creatinine ratio and clinical outcomes among patients with ST-elevation myocardial infarction after percutaneous coronary intervention: a secondary analysis based on dryad databases. Front Cardiovasc Med. (2023) 10:1191167. doi: 10.3389/fcvm.2023.1191167
22.
Shen Q Yao D Zhao Y Qian X Zheng Y Xu L et al . Elevated serum albumin-to-creatinine ratio as a protective factor on outcomes after heart transplantation. Front Cardiovasc Med. (2023) 10:1210278. doi: 10.3389/fcvm.2023.1210278
23.
Gerstein HC Ramasundarahettige C Avezum A Basile J Conget I Cushman WC et al . A novel kidney disease index reflecting both the albumin-to-creatinine ratio and estimated glomerular filtration rate, predicted cardiovascular and kidney outcomes in type 2 diabetes. Cardiovasc Diabetol. (2022) 21:158. doi: 10.1186/s12933-022-01594-6
24.
Yang WS Kang HD Jung SK Lee YJ Oh SH Kim YJ et al . A mortality analysis of septic shock, vasoplegic shock and cryptic shock classified by the third international consensus definitions (Sepsis-3). Clin Respir J. (2020) 14:857–63. doi: 10.1111/crj.13218
25.
Don BR Kaysen G . Serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
26.
Soeters PB Wolfe RR Shenkin A . Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. (2019) 43:181–93. doi: 10.1002/jpen.1451
27.
Wischmeyer PE . Nutrition therapy in Sepsis. Crit Care Clin. (2018) 34:107–25. doi: 10.1016/j.ccc.2017.08.008
28.
Frenkel A Novack V Bichovsky Y Klein M Dreiher J . Serum albumin levels as a predictor of mortality in patients with Sepsis: a multicenter study. Isr Med Assoc J. (2022) 24:454–9. PMID:
29.
Kendall H Abreu E Cheng AL . Serum albumin trend is a predictor of mortality in ICU patients with Sepsis. Biol Res Nurs. (2019) 21:237–44. doi: 10.1177/1099800419827600
30.
Taverna M Marie AL Mira JP Guidet B . Specific antioxidant properties of human serum albumin. Ann Intensive Care. (2013) 3:4. doi: 10.1186/2110-5820-3-4
31.
Huet O Obata R Aubron C Spraul-Davit A Charpentier J Laplace C et al . Plasma-induced endothelial oxidative stress is related to the severity of septic shock. Crit Care Med. (2007) 35:821–6. doi: 10.1097/01.CCM.0000257464.79067.AF
32.
Kofman N Margolis G Gal-Oz A Letourneau-Shesaf S Keren G Rozenbaum Z et al . Long-term renal outcomes and mortality following renal injury among myocardial infarction patients treated by primary percutaneous intervention. Coron Artery Dis. (2019) 30:87–92. doi: 10.1097/MCA.0000000000000678
33.
Khoury S Margolis G Ravid D Rozenbaum Z Keren G Shacham Y . Outcomes of early and reversible renal impairment in patients with ST segment elevation myocardial infarction undergoing percutaneous coronary intervention. Eur Heart J Acute Cardiovasc Care. (2020) 9:684–9. doi: 10.1177/2048872618808456
34.
Bellomo R Kellum JA Ronco C Wald R Martensson J Maiden M et al . Acute kidney injury in sepsis. Intensive Care Med. (2017) 43:816–28. doi: 10.1007/s00134-017-4755-7
35.
Alobaidi R Basu RK Goldstein SL Bagshaw SM . Sepsis-associated acute kidney injury. Semin Nephrol. (2015) 35:2–11. doi: 10.1016/j.semnephrol.2015.01.002
36.
Ronco C Bellomo R Kellum JA . Acute kidney injury. Lancet. (2019) 394:1949–64. doi: 10.1016/S0140-6736(19)32563-2
37.
Vanmassenhove J Lameire N Dhondt A Vanholder R Van Biesen W . Prognostic robustness of serum creatinine based AKI definitions in patients with sepsis: a prospective cohort study. BMC Nephrol. (2015) 16:112. doi: 10.1186/s12882-015-0107-4
38.
Wang Y Gao S Hong L Hou T Liu H Li M et al . Prognostic impact of blood urea nitrogen to albumin ratio on patients with sepsis: a retrospective cohort study. Sci Rep. (2023) 13:10013. doi: 10.1038/s41598-023-37127-8
39.
Zhou X Fu S Wu Y Guo Z Dian W Sun H et al . C-reactive protein-to-albumin ratio as a biomarker in patients with sepsis: a novel LASSO-COX based prognostic nomogram. Sci Rep. (2023) 13:15309. doi: 10.1038/s41598-023-42601-4
40.
Hu C He Y Li J Zhang C Hu Q Li W et al . Association between neutrophil percentage-to-albumin ratio and 28-day mortality in Chinese patients with sepsis. J Int Med Res. (2023) 51:3000605231178512. doi: 10.1177/03000605231178512
41.
Sanchez RA Sanchez MJ Ramirez AJ . Renal function, albumin-creatinine ratio and pulse wave velocity predict silent coronary artery disease and renal outcome in type 2 diabetic and prediabetic subjects. Curr Hypertens Rev. (2021) 17:131–6. doi: 10.2174/1573402116999201210194817
42.
Liu H Zhang J Yu J Li D Jia Y Cheng Y et al . Prognostic value of serum albumin-to-creatinine ratio in patients with acute myocardial infarction: results from the retrospective evaluation of acute chest pain study. Medicine (Baltimore). (2020) 99:e22049. doi: 10.1097/MD.0000000000022049
43.
Turkyilmaz E Ozkalayci F Birdal O Karagoz A Tanboga IH Tanalp AC et al . Serum albumin to creatinine ratio and short-term clinical outcomes in patients with ST-elevation myocardial infarction. Angiology. (2022) 73:809–17. doi: 10.1177/00033197221089423
44.
Peerapornratana S Manrique-Caballero CL Gómez H Kellum JA . Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. (2019) 96:1083–99. doi: 10.1016/j.kint.2019.05.026
45.
Guan HL Liu H Hu XY Abdul M Dai MS Gao X et al . Urinary albumin creatinine ratio associated with postoperative delirium in elderly patients undergoing elective non-cardiac surgery: a prospective observational study. CNS Neurosci Ther. (2022) 28:521–30. doi: 10.1111/cns.13717
46.
Sachdev A Raheja K Gupta N Chugh P . Association of Urinary Albumin:creatinine ratio with outcome of children with Sepsis. Indian J Crit Care Med. (2020) 24:465–72. doi: 10.5005/jp-journals-10071-23463
47.
Spoto S Basili S Cangemi R Yuste JR Lucena F Romiti GF et al . A focus on the pathophysiology of adrenomedullin expression: endothelitis and organ damage in severe viral and bacterial infections. Cells. (2024) 13:892. doi: 10.3390/cells13110892
48.
Englert JA Rogers AJ . Metabolism, metabolomics, and nutritional support of patients with Sepsis. Clin Chest Med. (2016) 37:321–31. doi: 10.1016/j.ccm.2016.01.011
49.
Norbury WB Jeschke MG Herndon DN . Metabolism modulators in sepsis: propranolol. Crit Care Med. (2007) 35:S616–20. doi: 10.1097/01.CCM.0000278599.30298.80
Summary
Keywords
sepsis, albumin, creatinine, albumin-to-creatinine ratio, mortality
Citation
Hu Z, Song C and Zhang J (2024) Elevated serum albumin-to-creatinine ratio as a protective factor on clinical outcomes among critically ill patients with sepsis: a retrospective study. Front. Med. 11:1436533. doi: 10.3389/fmed.2024.1436533
Received
30 May 2024
Accepted
06 September 2024
Published
19 September 2024
Volume
11 - 2024
Edited by
Silvia Spoto, Fondazione Policlinico Universitario Campus Bio-Medico, Italy
Reviewed by
Giorgio D’Avanzo, Campus Bio-Medico University, Italy
Marta Fogolari, Campus Bio-Medico University, Italy
Updates
Copyright
© 2024 Hu, Song and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhui Zhang, 894080423@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.