Abstract
Background:
Acute respiratory distress syndrome (ARDS) is highly heterogeneous, both in its clinical presentation and in the patient’s physiological responses to changes in mechanical ventilator settings, such as PEEP. This study investigates the clinical efficacy of a physiological model-based ventilatory decision support system (DSS) to personalize ventilator therapy in ARDS patients.
Methods:
This international, multicenter, randomized, open-label study enrolled patients with ARDS during the COVID-19 pandemic. Patients were randomized to either receive active advice from the DSS (intervention) or standard care without DSS advice (control). The primary outcome was to detect a reduction in average driving pressure between groups. Secondary outcomes included several clinically relevant measures of respiratory physiology, ventilator-free days, time from control mode to support mode, number of changes in ventilator settings per day, percentage of time in control and support mode ventilation, ventilation- and device-related adverse events, and the number of times the advice was followed.
Results:
A total of 95 patients were randomized in this study. The DSS showed no significant effect on average driving pressure between groups. However, patients in the intervention arm had a statistically improved oxygenation index when in support mode ventilation (−1.41, 95% CI: −2.76, −0.08; p = 0.0370). Additionally, the ventilatory ratio significantly improved in the intervention arm for patients in control mode ventilation (−0.63, 95% CI: −1.08, −0.17, p = 0.0068). The application of the DSS led to a significantly increased number of ventilator changes for pressure settings and respiratory frequency.
Conclusion:
The use of a physiological model-based decision support system for providing advice on mechanical ventilation in patients with COVID-19 and non-COVID-19 ARDS showed no significant difference in driving pressure as a primary outcome measure. However, the application of approximately 60% of the DSS advice led to improvements in the patient’s physiological state.
Clinical trial registration:
clinicaltrials.gov, NCT04115709.
Introduction
The clinical presentation of acute respiratory distress syndrome (ARDS) is highly heterogeneous, with varying degrees and types of abnormalities in pulmonary gas exchange and mechanics, and treatment response to ventilator adjustments (1, 2). This physiological heterogeneity is evident even within a single etiology, such as coronavirus disease 2019 (COVID-19), emphasizing the need for individualized ventilator management tailored to each patient (3–8). However, ventilator interventions should also maintain consistency by treating physiological phenotypes with similar treatment responses in a homogenous manner (9). Achieving this balance requires a strategy of personalized, yet replicable, ventilatory care grounded in a clear understanding of the patient’s real-time physiology and standardized responses to specific physiological conditions. Currently, evidence suggests that this is not always achieved, with 69% of ventilator settings failing to adhere to evidence-based lung protective ventilation strategies (10).
Decision support systems (DSS) can aid in the process of individualized ventilatory care by providing advice tailored to the patient’s specific physiological state. When these systems are designed to reflect a patient’s individual physiology, they enable care that is both personalized and consistently replicable (9). This is particularly critical in managing complex conditions such as ARDS. The Beacon Care system (Mermaid Care A/S, Nørresundby) is a physiologically-based DSS that provides recommendations for mechanical ventilation adjustments (11).
As illustrated in Figure 1 and described in detail in the electronic Supplementary material, this system integrates data from pulmonary mechanics, gas exchange, indirect calorimetry, volumetric capnography, blood acid–base balance, and pulse oximetry. It uses these inputs to calibrate mathematical models of patent physiology, creating a “digital twin” of the patient. This individualized model allows the system to offer recommendations aimed at minimizing the risk of negative outcomes, using utility theory to evaluate trade-offs (12).
Figure 1
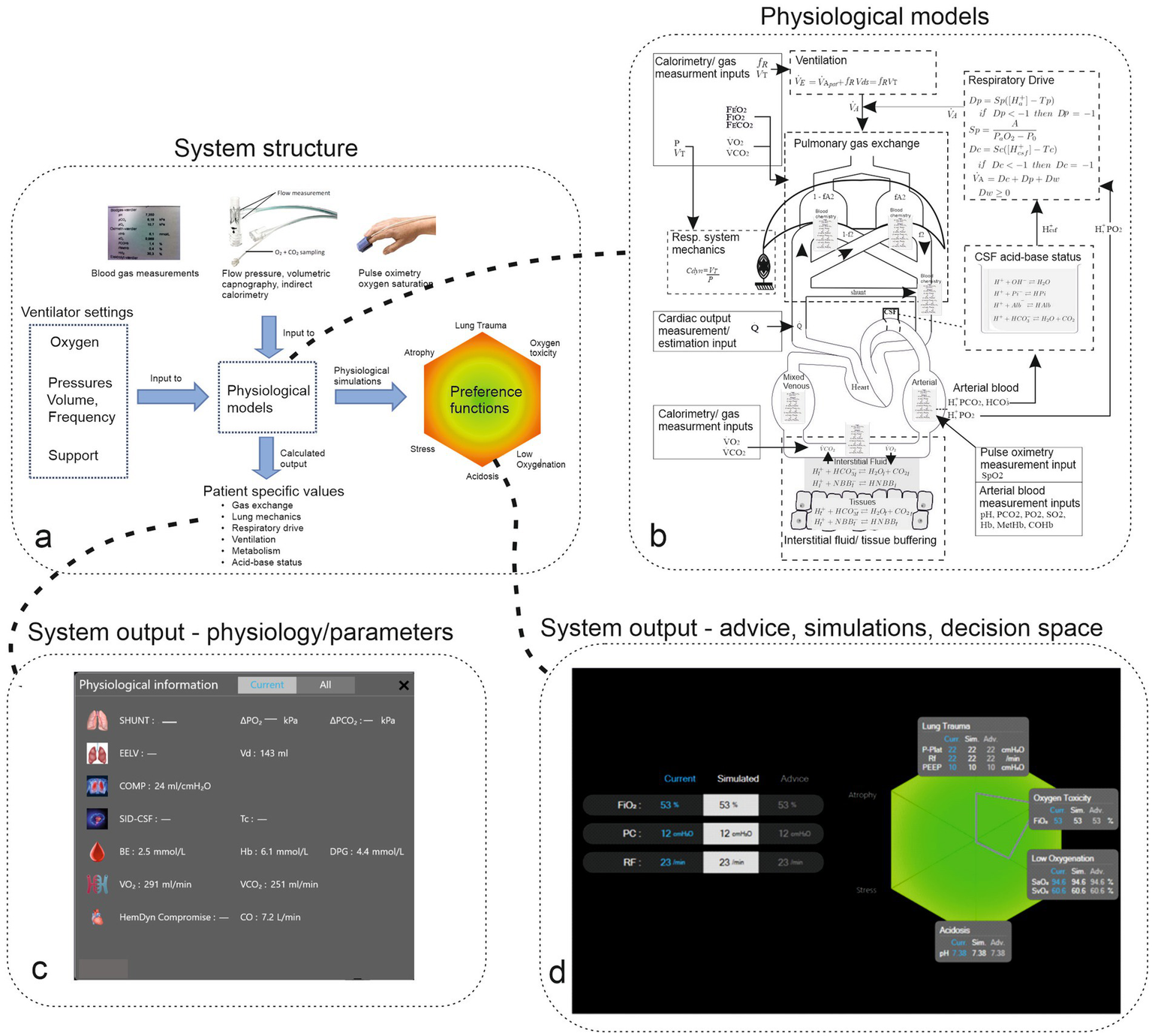
Panel (a) illustrates the structure of the system, including measurement and ventilator inputs to physiological models, which result in calculated patient-specific model parameters and physiological simulations on the color-coded decision space of outcomes. Panel (b) illustrates the complexity of the physiological model, including physiological subsystems (surrounded by dashed boxes) and measurement inputs (surrounded by solid boxes). Panel (c) illustrates the system’s output of patients’ physiological state represented as parameter values for organ systems. Panel (d) illustrates current ventilator settings and systems advice along with the patient’s resulting state, which is illustrated on the hexagon representing the decision space. Gray boxes illustrate current and simulated physiological variables. The green heagon represents a patient with a small risk of adverse effects (color green) with decision-theoretic penalty scores plotted as coordinates on the hexagon represented as a gray outline.
The model-tuning process adjusts the generic physiological models based on the patient’s data, making the system adaptable to individual variations. In addition to advice, the system can be used to analyze the patient’s physiological state and changes over time and simulate potential responses to changes in ventilator settings. This advice is presented in two formats: suggested ventilator adjustment and visual representations on a hexagon that illustrate the trade-offs involved, supported by simulations that predict the physiological impact of those adjustments.
This multi-layered presentation allows clinicians to interact with the advice according to their expertise, facilitating smooth integration into clinical practice (13) and providing detailed explanations behind each recommendation. Further details of the system and its advice-generation process are available in the electronic Supplementary material (ESM S.1). The physiological models of the system, as depicted in Figure 1, have been previously validated, including in ARDS patients both with and without COVID-19. In addition, the system has shown its ability to improve physiological outcomes during short periods of mechanical ventilation and reduce pressure support without overstressing respiratory muscles in non-ARDS patients (14, 15).
This multi-center study is the first to investigate the application of the DSS in a population of ARDS patients with varying levels of severity. The primary aim was to determine whether the use of the DSS could positively impact the physiological status of ARDS patients. Additionally, the study sought to evaluate the barriers to the system’s implementation and adoption in clinical settings.
Materials and methods
Trial design and oversight
DeVENT was a multi-center, international, randomized, controlled, allocation-concealed, open, pragmatic, superiority clinical trial enrolling patients with ARDS. In the United Kingdom, the study received ethical approval from the London South-East Research Ethics Committee (Ref: 19/LO/1606) on 15th January 2020, with protocol version 4.0 approved on 16th June 2021.
The study protocol was approved by the French Ethics Committee (Comité de Protection des Personnes Sud Mediterranee III; approved on 30th July 2020, under number 2019.12.06 ter_19.11.15.76132) and the French Medicine Agency (Agence Nationale de Sécurité du Médicament; approved on 3th July 2020, under number 2019-A02610-57-A). The study protocol was approved by the ethics committee of the Medical University of Vienna, Austria (EC No. 2056/2019) on 28th April 2020. Monitoring and oversight were provided by the Imperial Clinical Trials Unit and independent trial steering, data monitoring, and ethics committees. The study was conducted in accordance with Good Clinical Practice guidelines, local regulations, and the ethical principles outlined in the Declaration of Helsinki.
Participants
The study was conducted in three adult intensive care units (ICUs), across the United Kingdom, France, and Austria. Adult patients were eligible for inclusion if they were receiving invasive mechanical ventilation and met the criteria for ARDS as defined by the Berlin definition. These criteria included: the following: a known clinical insult leading to worsening respiratory symptoms, the presence of bilateral infiltrates on a chest radiograph consistent with pulmonary edema not fully explained by cardiac failure, and hypoxemia with a PaO2/FiO2 ratio of ≤300 mmHg (or ≤ 40 kPa). For patients placed on extracorporeal support, pre-ECMO PaO2/FiO2 ratios were used (16).
The exclusion criteria included patients younger than 18 years, absence of an arterial catheter, mechanical ventilation lasting longer than 7 days, imminent withdrawal of treatment within 24 h; a do-not-resuscitate (DNR) order, severe chronic respiratory disease requiring home ventilation and/or oxygen therapy (excluding sleep-disordered breathing), requirement of veno-arterial ECMO, and head trauma or other conditions requiring tight regulation of arterial CO2 levels. Informed consent was obtained from the patient, a personal consultee, or an independent nominated professional, with retrospective consent obtained from the patient or personal consultee when possible.
Procedures
The Beacon Care system was attached to all enrolled patients, who were then randomized to either have the system’s advice active (intervention group) or inactive (control group). For patients in the control group, data from the system and driving pressure measurements were not available to the attending physicians. Randomization was stratified by site, ECMO/non-ECMO, and COVID-19/non-COVID-19. The primary objective was to assess whether the use of the DSS affected the driving pressure applied to patients when ventilated in a controlled ventilation mode. Full details of the study protocol have been published previously (17), and the study has been registered on clinicaltrials.gov (NCT04115709). A summary of the methods is provided here, with all other clinical therapies administered according to standard practice.
In the intervention arm, the DSS was attached, and advice was activated. Advice was suspended during periods when the DSS algorithm could not function (e.g., during ECMO or airway pressure release ventilation (APRV), the only ventilation mode not supported by the DSS). Advice continued until extubation, death, or transfer but was not re-initiated on re-intubation. In the control arm, the DSS was attached, and the advice was switched off. A detailed description of the DSS in both arms is provided in the Supplementary material. The research team and nurse superusers were trained by system experts prior to the initiation of the study. Subsequently, the research team conducted regular training and refresher courses, including at-the-elbow training for all new clinical incomers, allowing the system to be operational at all beds.
Data were collected directly from the DSS or entered into an electronic case report form (SMART Trial, Copenhagen, Denmark).
Outcome measures
The primary outcome measure was average driving pressure over the period of time attached to the DSS, as illustrated in the electronic Supplementary material (ESM S2).
Secondary clinical outcome measures were as follows: (1): daily average calculated delivered pressure over time during periods of spontaneous breathing; (2) daily average calculated mechanical power over time; (3) daily average calculated oxygenation index over time; (4) daily average ventilatory ratio over time; (5) ventilator-free days at 90 days; (6) time from control mode to support mode; (7) a number of changes in ventilator settings per day; (8) a percentage of time in control mode ventilation; (9) a percentage of time in support mode ventilation; (10) total duration of mechanical ventilation; (11) tidal volume over time; (12) PEEP setting over time; (13) ventilation-related complications, e.g., pneumothorax and/or pneumomediastinum; (13) device malfunction event rate; (14) device-related adverse event rate; and (15) number of times the advice from the Beacon system is followed through the duration of the study. All outcomes were reported from the time of randomization. Measurement and/or calculation of outcomes are described in the ESM. The outcomes not reported in this article will be reported separately.
Statistical analysis
Assuming a standard deviation of 2.5 cmH2O and including a 40% dropout, 110 patients would provide 90% power to detect a 2 cmH2O reduction in driving pressure. Following the onset of COVID-19, study power was re-estimated, taking into consideration repeated measurements and estimating the intraclass correlation coefficient (ICC) of driving pressure values and the coefficient of variation (CV) for days of data collected (per patient) from available data.
From available data, using an ICC of 0.3 and CV of 0.8, 23 patients per arm allowed for a powered analysis. Driving pressure and other repeated measurements were analyzed using a mixed model approach, including a random clustering effect per patient and fixed-effect covariates for site, ECMO and COVID-19 status, duration of ventilation prior to DSS connection, duration of hospital admission prior to intubation, and number of days in non-ECMO and non-support mode.
Continuous variables were presented as mean (SD) or medians (IQR) if non-normally distributed, and appropriate log transformations were considered where analysis residuals were non-normal. Differences between treatment groups are presented with 95% confidence intervals. Categorical data were presented as numbers and percentages, and any comparisons between the two groups were performed using the Chi-squared or Fisher exact test. An intention-to-treat basis was used for the primary analysis, including all patients randomized into the study, with per-protocol analysis used as a follow-up. All statistical tests were 2-sided, and the significance was set at a p-value of <0.05. No imputation was carried out for missing data outside of that undertaken within the mixed-effects model. Similarly, no adjustments have been made for multiple testing. Analyses were performed using SAS version 9.4 software.
A per-protocol subgroup analysis was conducted at randomization based on ECMO/non-ECMO status. An additional post-hoc analysis was added to consider the absence of ECMO treatment during the whole period of DSS application. This was decided to account for the extended ECMO and ventilator durations in COVID-19 patients supported on ECMO, which were uncertain at the time of study design.
Results
Between 19th March 2020 (first patient recruitment) and 4th May 2021, 95 patients fulfilled the inclusion criteria and underwent randomization, with 47 patients allocated to control and 48 patients to the intervention arm (Figure 2). Patients were ventilated in pressure and volume-regulated mandatory modes and pressure support.
Figure 2
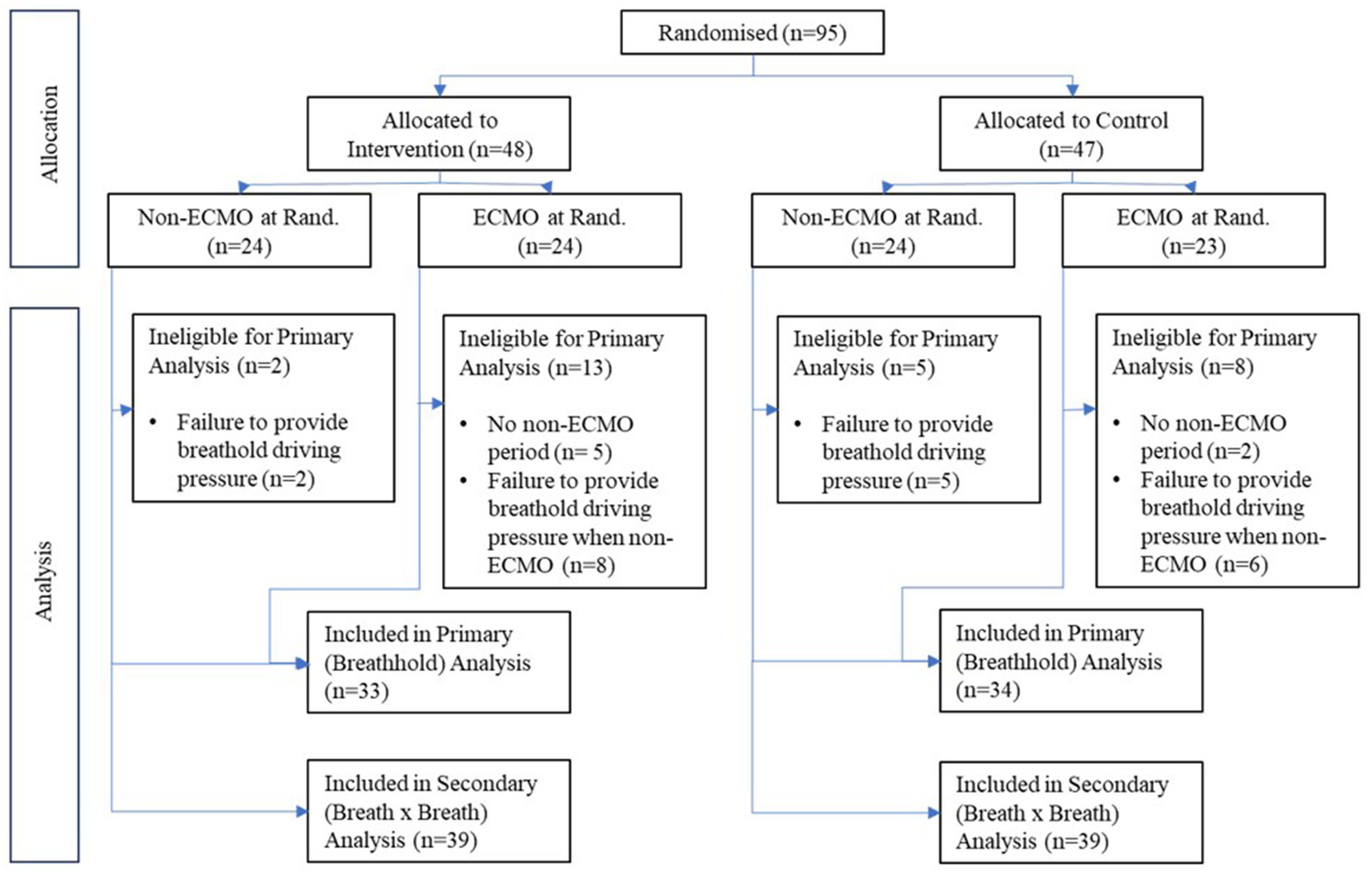
CONSORT diagram illustrating the number of patients randomized in the study and allocated to intervention and control arms and whether treated with extracorporeal membrane oxygenation (ECMO). The number of patients used in each analysis is also illustrated.
Seven patients (two control, five intervention) had no periods off ECMO during their respective follow-up periods, with an additional 21 patients (11 control, 10 intervention) unable to provide a breath-hold driving pressure reading due to either (a) being off ECMO for a minute period (often prior to death/extubation) and/or (b) being exclusively under pressure support during non-ECMO periods. As a result, primary analysis was possible in 67 patients (33 intervention, 34 control). Secondary outcomes based on continuous breath-by-breath measurements could be calculated in 78 patients.
Patient baseline demographics
Demographic data by study arm are illustrated in Table 1, and treatment groups were well matched for clinical parameters at baseline. Each arm had an almost identical distribution of ECMO and non-ECMO patients.
Table 1
| Variable | Control (N = 47) | Intervention (N = 48) | All (N = 95) | |
|---|---|---|---|---|
| Age at consent (y) | Mean (SD) | 52 (14) | 54 (16) | 53 (15) |
| Gender – Male (%) | n (%) | 30 (64) | 32 (67) | 62 (65) |
| Height (cm) | Mean (SD) | 172 (9) | 171 (9) | 172 (9) |
| Weight (kg) | Mean (SD) | 96 (30) | 98 (33) | 97 (32) |
| Body mass index (kg/m2) | Mean (SD) | 33 (10) | 33 (11) | 33 (10) |
| Predicted weight (kg) | Mean (SD) | 66 (10) | 65 (10) | 66 (10) |
| Smoking history (%) | Current smoker | 4 (9) | 0 | 4 (4) |
| Current vaper | 0 | 1 (2) | 1 (1) | |
| Ex-Smoker | 7 (15) | 8 (17) | 15 (16) | |
| Never Smoker | 14 (30) | 20 (42) | 34 (36) | |
| Unknown | 22 (47) | 19 (40) | 41 (43) | |
| Comorbidities – chronic pulmonary disease (%) | n (%) | 13 (28) | 13 (27) | 26 (27) |
| Unknown | 1 (2) | 0 | 1 (1) | |
| Comorbidities – cardiovascular disease (%) | n (%) | 23 (49) | 18 (38) | 41 (43) |
| Unknown | 1 (2) | 0 | 1 (1) | |
| Comorbidities – metabolic and endocrine | n (%) | 26 (55) | 20 (42) | 46 (48) |
| Unknown | 2 (4) | 0 | 2 (2) | |
| Comorbidities – chronic immuno-suppression | n (%) | 3 (6) | 3 (6) | 6 (6) |
| Unknown | 0 | 1 (2) | 1 (1) | |
| Comorbidities – chronic neurological disease | n (%) | 2 (4) | 1 (2) | 3 (3) |
| Unknown | 7 (15) | 2 (4) | 9 (10) | |
| Days since Ards Onset | Median [IQR] | 2 [1–5] | 1 [1–3] | 2 [1–4] |
Patient demographics at randomization.
Outcomes
There was no statistically significant difference in the primary outcome variable, with values of driving pressure (Table 2; Figure 3) measured from either breath hold (−0.34 cmH2O with intervention, 95% CI: −2.22, 1.54 cmH2O; p = 0.72) or continuous measures (−0.23 cmH2O, 95% CI: −2.1, 1.67 cmH2O; p = 0.81) being not statistically different between groups. However, the intervention arm appeared to have some tendency toward tighter interquartile ranges (Figure 3).
Table 2
| Control | Intervention | |||||
|---|---|---|---|---|---|---|
| n | mean (SD) median [IQR] | n | mean (SD) median [IQR] | Effect [95% CI] | p | |
| Primary outcome measure | ||||||
| Breath hold driving pressure (cmH2O) | 34 | 13.71 (4.20) | 33 | 13.78 (3.62) | −0.34 [−2.22, 1.54] | 0.72 |
| Secondary outcome measures | ||||||
| Breath by breath driving pressure (cmH2O) | 39 | 16.33 (4.66) | 39 | 16.54 (4.89) | −0.23 [−2.13, 1.67] | 0.81 |
| Breath by breath mechanical power (J/min) | 41 | 24.64 (8.45) | 39 | 25.80 (7.54) | −2.23 [−3.06, 2.60] | 0.87 |
| Oxygenation index – mandatory modes | 26 | 11.10 (5.70) | 30 | 11.58 (5.61) | 0.33 [−2.34, 3.00] | 0.81 |
| Oxygenation index – support modes | 24 | 7.69 (3.49) | 25 | 6.60 (2.39) | −1.41 [−2.74, −0.08] | 0.0370 |
| Ventilatory ratio – mandatory modes | 26 | 2.66 (0.82) | 31 | 2.42 (0.59) | −0.31 [−0.67, 0.05] | 0.09 |
| Ventilatory ratio – support modes | 25 | 2.55 (0.65) | 25 | 2.56 (0.72) | −0.13 [−0.47, 0.22] | 0.48 |
| Average ventilator free days at 90 daysƮ | 47 | 49 [0–73] | 48 | 37.5 [0–69] | 0.0 [−20.0, 0.0] | 0.27 |
| Time from control to support ~ | 34 | 2.35 (3.13) | 32 | 2.42 (2.98) | −0.17 [−1.58, 1.25] | 0.81 |
| Average daily settings change (PEEP) Ʈ | 44 | 1.11 [0.54–1.84] | 42 | 1.27 [0.47–2.20] | 0.14 [−0.29, 0.58] | 0.52 |
| Average daily settings change (FiO2) Ʈ | 44 | 7.50 [4.42–10.62] | 42 | 8.27 [4.32–11.7] | 1.01 [−1.07, 3.32] | 0.29 |
| Average daily settings change (PS) Ʈ | 43 | 2.65 [0.44–5.66] | 41 | 1.23 [0.24–2.70] | −0.84 [−2.10, 0.00] | 0.06 |
| Average daily settings change (PC) Ʈ | 44 | 0.66 [0–2.36] | 42 | 2.25 [0–4.86] | 0.96 [0.00, 2.15] | 0.0246 |
| Average daily settings change (RF) Ʈ | 44 | 0.94 [0–2.02] | 42 | 2.31 [0.95–4.96] | 1.30 [0.61, 2.30] | 0.0004 |
| % in control mode~ | 44 | 55.9 (36.4) | 42 | 50.3 (32.9) | −6.27 [−20.9, 8.33] | 0.40 |
| % in support mode~ | 44 | 31.3 (32.0) | 42 | 42.1 (31.1) | 11.6 [−1.81, 25.0] | 0.09 |
| Duration of mechanical ventilation.~ | 44 | 10.52 (9.18) | 42 | 13.80 (13.40) | 3.04 [−1.35, 7.43] | 0.18 |
| Tidal volume measured control | 28 | 531 (113) | 33 | 520 (132) | −10.6 [−74.4, 53.1] | 0.74 |
| Tidal volume measured support | 27 | 644 (142) | 29 | 648 (189) | −42.82 [−125.0, 39.4] | 0.31 |
| PEEP setting | 37 | 9.85 (2.38) | 37 | 9.88 (2.30) | 0.37 [−0.68, 1.42] | 0.49 |
| Subjects with device related adverse events Ǫ | 47 | 0 (0%) | 48 | 1 (2%) | 1.00 | |
| Device-related adverse event rate (per day) Ʈ | 47 | 0 [0–0] | 48 | 0 [0–0] | 0.0 [0.0, 0.0] | 0.32 |
| Subjects with re-intubation events Ǫ | 47 | 2 (2%) | 48 | 2 (2%) | 1.00 | |
| percentage time disconnected prior to reintubationƮ | 47 | 0 [0–0] | 48 | 0 [0–0] | 0.0 [0.0, 0.0] | 0.98 |
Primary and secondary outcomes, all patients.
The table shows the mean (SD) or median [IQR] of primary and secondary outcome variables by treatment group alongside the treatment effect derived from the mixed-effects model. The model incorporates fixed effects for COVID-19 and Site and also adjusted for continuous variables (1) Duration of ventilation prior to connection, (2) duration of hospitalization prior to intubation, and (3) the number of days non-ECMO and non-support and has a random effect accounting for clustering per subject. ~ No clustering effect was included for these variables, as data was provided as an overall average per subject. *p < 0.05, **p < 0.01, ***p < 0.001. Ʈ Median [Q1-Q3] presented. Effect represents the difference in median via Hodges-Lehmann estimate and distribution-free confidence interval. p- value from the Wilcoxon Test. Ǫ Frequencies presented as n (%). p- values to assess differences in event frequencies derived from Fisher’s exact test. PEEP, Positive end-expiratory pressure; FiO2, fraction inspired oxygen; PS, pressure support; PC, pressure control; RF, respiratory frequency.
Figure 3
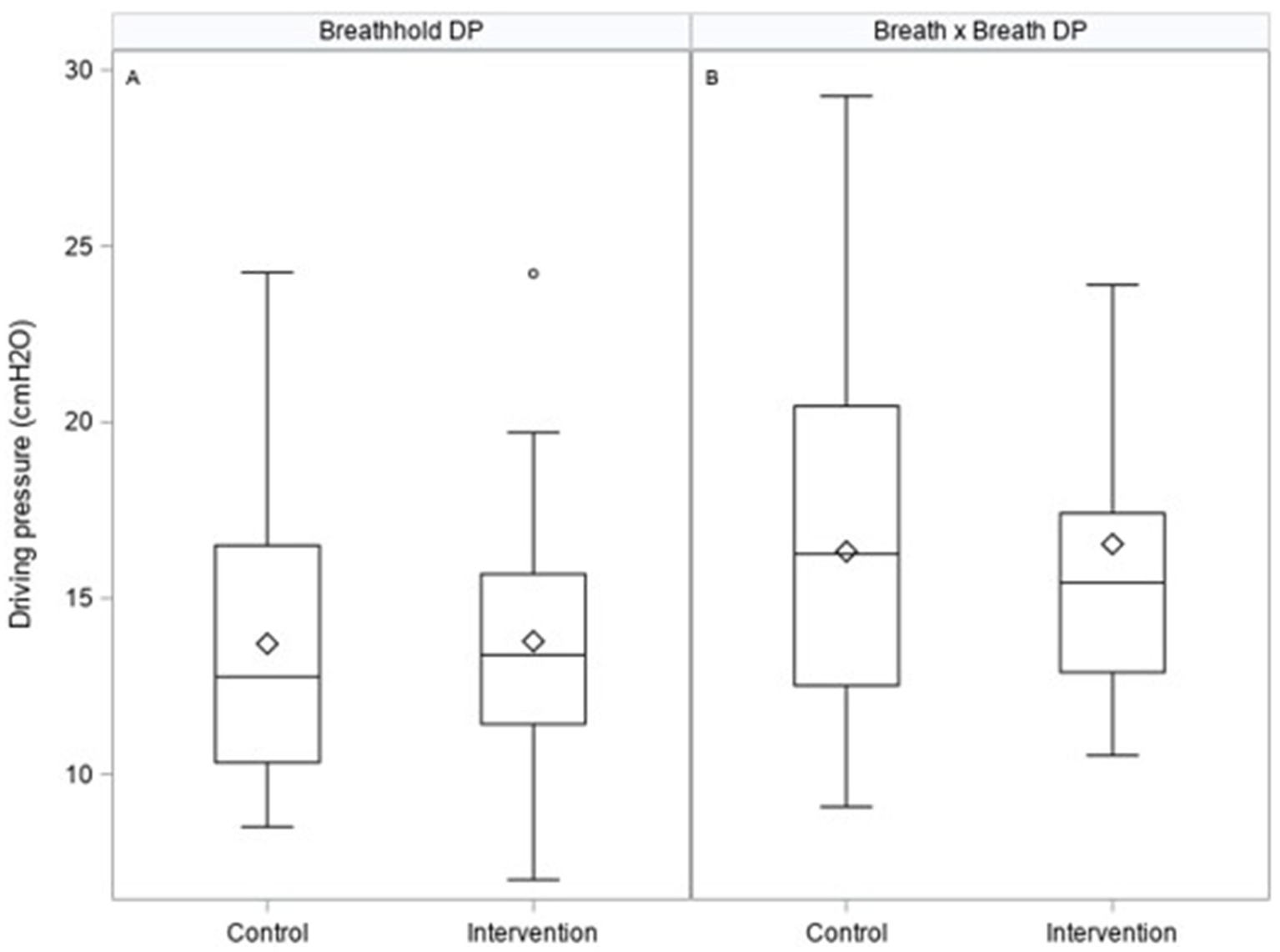
Box plots illustrating primary outcome measures of driving pressures on the control and intervention arm measured from breath holds (A) or breath by breath (B) as calculated in the ESM.
Patients in the intervention arm had statistically improved oxygenation index when in support mode ventilation (−1.41, 95% CI: −2.76, −0.08; p = 0.0370). For the subsequent post-hoc sub-group analysis of non-ECMO patients (Table 3), the oxygenation index was improved in the intervention arm for patients in support mode (−2.60, 95% CI:-4.13, −1.08; p = 0.0010), with controlled mandatory ventilation showing a numerical improvement although this did not reach statistical significance (−2.66, 95% CI -5.38, 0.06; p = 0.06). The ventilatory ratio was also significantly improved in the intervention arm for non-ECMO patients in control mode ventilation (−0.63, 95% CI: −1.08, −0.17, p = 0.0068), although this effect was reduced when extended to the full study population (−0.31, 95% CI -0.67, 0.05; p = 0.09). There is a tendency for patients in the intervention arm to spend a greater proportion of ventilator time in pressure support mode in comparison to mandatory modes (11.6, 95% CI, −1.8, 25.0, p = 0.09).
Table 3
| Control | Intervention | |||||
|---|---|---|---|---|---|---|
| n | mean (SD) | n | mean (SD) | Effect [95% CI] | p | |
| Pressure, oxygenation, and ventilation | ||||||
| Breath hold driving pressure (cmH2O) | 19 | 14.79 (4.82) | 17 | 13.64 (3.57) | −1.44 [−3.98, 1.11] | 0.27 |
| Secondary outcome measures | ||||||
| Breath by breath driving pressure (cmH2O) | 22 | 17.61 (4.97) | 17 | 18.04 (5.85) | −0.4 [−3.55, 2.27] | 0.67 |
| Breath by breath mechanical power (J/min) | 23 | 26.91 (8.88) | 17 | 27.88 (8.71) | −1.22 [−5.08, 2.63] | 0.53 |
| Oxygenation index – mandatory modes | 17 | 13.14 (5.89) | 14 | 12.05 (5.81) | −2.66 [−5.38, 0.06] | 0.06 |
| Oxygenation index – support modes | 12 | 9.11 (6.08) | 11 | 6.08 (2.13) | −2.60 [−4.13, −1.08] | 0.0010 |
| Ventilatory ratio – mandatory modes | 17 | 2.72 (0.85) | 14 | 2.24 (0.52) | −0.63 [−1.08, −0.17] | 0.0068 |
| Ventilatory ratio – support modes | 13 | 2.64 (0.1) | 11 | 2.27 (0.50) | −0.13 [−0.47, 0.22] | 0.48 |
| Average ventilator free days at 90 daysƮ | 23 | 19 [9–29] | 19 | 16 [9–27] | 0.0 [−13.0, 12.0] | 0.89 |
| Time from control to support ~ | 17 | 3.28 (3.94) | 15 | 3.88 (3.45) | 0.32 [−2.45, 3.09] | 0.82 |
| Average daily settings change (PEEP) Ʈ | 23 | 1.41 [0.83–3.20] | 19 | 1.37 [1.05–2.20] | −0.16 [−1.20, 0.55] | 0.69 |
| Average daily settings change (FiO2) Ʈ | 21 | 8.51 [3.16–11.65] | 16 | 9.30 [3.02–11.63] | 0.12 [−3.83, 3.21] | 0.92 |
| Average daily settings change (PS) Ʈ | 23 | 2.10 [0.20–5.66] | 18 | 2.06 [1.00–3.06] | −0.22 [−2.10, 1.00] | 0.64 |
| Average daily settings change (PC) Ʈ | 23 | 1.17 [0–1.96] | 19 | 2.31 [0–5.56] | 0.26 [−0.48, 2.41] | 0.37 |
| Average daily settings change (RF) Ʈ | 20 | 1.40 [0.70–2.27] | 19 | 2.33 [0.96–4.24] | 0.92 [0.00, 2.22] | 0.06 |
| % in Control Mode~ | 23 | 63.2 (32.8) | 19 | 49.3 (30.2) | −13.39 [−32.82, 6.05] | 0.17 |
| % in Support Mode~ | 23 | 29.0 (29.6) | 19 | 41.8 (30.4) | 12.75 [−5.70, 31.2] | 0.17 |
| Duration of mechanical ventilation~ | 23 | 13.07 (9.24) | 19 | 15.13 (9.45) | 0.99 [−4.71, 6.70] | 0.73 |
| Tidal volume measured control | 17 | 517 (130) | 14 | 499 (113) | 27.1 [−50.5, 104.8] | 0.49 |
| Tidal volume measured support | 13 | 658 (168) | 11 | 621 (140) | −7.90 [−121.2, 105.4] | 0.89 |
| PEEP setting | 21 | 10.31 (2.61) | 16 | 10.20 (2.93) | 0.35 [−1.07, 1.76] | 0.63 |
| Subjects with device related adverse eventsǪ | 23 | 0 (0%) | 19 | 1 (5%) | 0.45 | |
| Device-related adverse event rate (per day)Ʈ | 23 | 0 [0–0] | 19 | 0 [0–0] | 0.0 [0.0, 0.0] | 0.27 |
| Subjects with Re-intubation events Ǫ | 23 | 2 (9%) | 19 | 2 (11%) | 1.00 | |
| percentage time disconnected prior to reintubationƮ | 23 | 0 [0–0] | 19 | 0 [0–0] | 0.0 [0.0, 0.0] | 0.84 |
Primary and secondary outcomes, non-ECMO patients only.
Post-hoc analysis exclusively looked at patients who were never placed on ECMO. The table displays the mean (SD) or median [IQR] of primary and secondary outcome variables by treatment group alongside the treatment effect derived from the mixed-effects model. The model incorporates fixed effects for COVID-19 and Site and also adjusted for continuous variables (1) Duration of Ventilation prior to connection, (2) duration of hospitalization prior to intubation, and (3) the number of days non-ECMO and non-support and has a random effect accounting for clustering per subject. ~ No clustering effect was included for these variables, as data were provided as an overall average per subject. *p < 0.05, **p < 0.01, ***p < 0.001. Ʈ Median [Q1-Q3] presented. Effect represents the difference in median via Hodges-Lehmann estimate and distribution-free confidence interval. p- value from Wilcoxon Test. Ǫ Frequencies presented as n (%). p- values to assess differences in event frequencies derived from the Fisher’s exact test. PEEP, Positive end-expiratory pressure; FiO2, fraction inspired oxygen; PS, pressure support; PC, pressure control; RF, respiratory frequency.
Safety data are presented in Table 4 and Figure 4. No significant differences were observed in median time-to-death (control vs. intervention: 19 (15–59) vs. 19.5 (10–36) days; p = 0.64).
Table 4
| Control | Intervention | Comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| Deaths | Days to event | Deaths | Days to event | |||||
| n | n (%) | Med. [Q1-Q3] | n | n (%) | Med. [Q1-Q3] | Δ [95% CI] | p- value | |
| Death – all patients | 47 | 12 (26) | 19.0 [15–59] | 48 | 18 (38) | 19.5 [10–36] | −1.5 [−18.0, 14.0] | 0.64 |
| Death – non-ECMO patients | 23 | 9 (39) | 19.0 [18–22] | 19 | 7 (37) | 16.0 [6–21] | −6.0 [−44.0, 5.0] | 0.20 |
| n | Mean | Median | n | Mean | Median | Δ [95% CI] | p- value | |
| % time below SpO2 88% | 44 | 12.0 | 8.7 | 42 | 9.3 | 5.2 | −0.98 [−5.07, 1.51] | 0.44 |
| % time FetCO2 > 7 kPa, mandatory | 32 | 16.5 | 4.8 | 38 | 9.6 | 0.6 | −0.05 [−7.96, 0.00] | 0.19 |
| % time FetCO2 > 7 kPa, support | 32 | 16.7 | 0.1 | 34 | 7.4 | 0.0 | 0.00 [−1.06, 0.00] | 0.06 |
| % time RF < 12, support | 34 | 9.6 | 1.1 | 35 | 3.9 | 0.0 | −0.20 [−1.58, 0.00] * | 0.0167 |
| % time RSBI >100, support | 32 | 1.0 | 0.0 | 34 | 0.8 | 0.0 | 0.00 [0.00, 0.00] | 0.78 |
Safety analysis showing a 90-day mortality rate and key ventilation parameters.
Δ [95% CI] represents the difference in medians and a distribution-free 95% CI via Hodges-Lehmann estimate and distribution-free confidence interval. p- value from Wilcoxon Test. *p < 0.05; **p < 0.01; ***p < 0.001. SpO2, pulse oximetry oxygen saturation; FetCO2, end-tidal carbon dioxide fraction; RF, respiratory frequency; RSBI, rapid shallow breathing index.
Figure 4
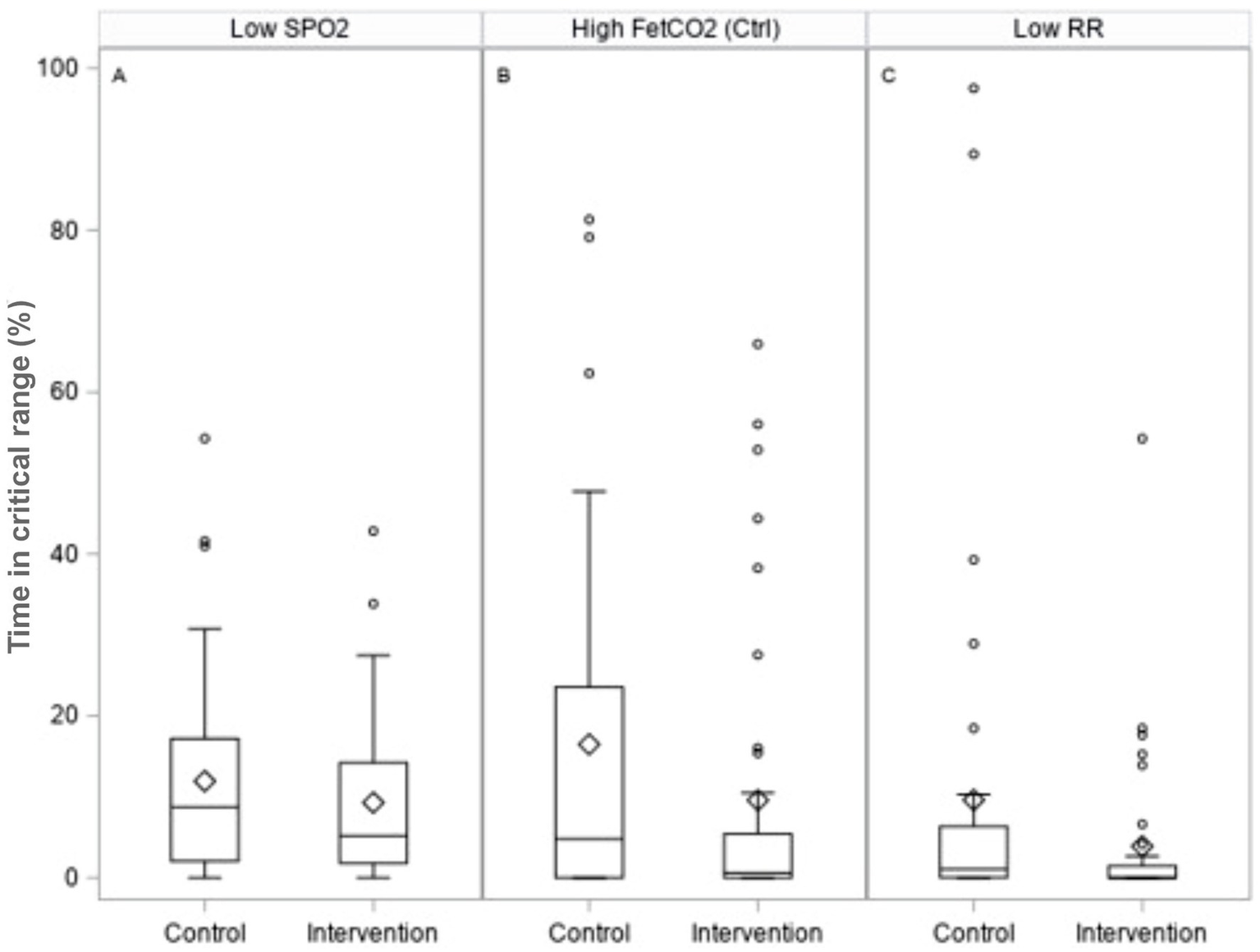
Box plots illustrating safety measures for the percentage time spent: A - below SpO2 values of 88%; B - above end-tidal CO2 values of 7 kPa in control ventilation; and C - below the respiratory rate of 12 breaths per minute in pressure support ventilation.
We observed no significant difference in mortality between the intervention arm (38% vs. 26%) and the subset of patients not receiving ECMO (37% vs. 39%). There were no differences between ventilation-related complications, device malfunction rates, or device-related adverse events. No significant difference was seen in the time spent or incidence of hypoxemia or hypercapnia in patients ventilated on control mode between groups. In support mode, in terms of per-patient percentage of their respective DSS application, significantly less time was spent at very low respiratory rates (< 12 breaths per minute) in the intervention arm (difference in medians −0.2, 95% CI -1.6, 0.0; p = 0.0167), and at high CO2 levels (>7 kPa), although this was not statistically significant (difference in medians −0.0, 95% CI -1.1, 0.0; p = 0.06; Figure 4; Table 4).
The application of the DSS resulted in a significantly increased number of ventilator changes for pressure settings during control mode ventilation (control vs. intervention: 0.65 vs. 2.25 changes per day; p < 0.05) and respiratory frequency (control vs. intervention: 0.94 vs. 2.31 changes per day; p < 0.001). Table 5 illustrates the uptake of advice over a 2-h window following the advice presentation, where advice was to change ventilator settings to those different from current values. Indeed, only 44% of advice was given to change pressure support, and 66% for FIO2 was actioned. The advice was often not followed, meaning that no ventilator changes were made during the two-hour window following the advice presentation. Of note, changes in ventilator settings in the opposite direction to the advice presented within the two-hour window were minimal.
Table 5
| Advice Followed (%) | Advice Ignored (%) | Changes made which were opposite to the advice suggested (%) | ||
|---|---|---|---|---|
| n | Median [Q1-Q3] | Median [Q1-Q3] | Median [Q1-Q3] | |
| FiO2, PEEP | 34 | 65.7 [40.0–72.7] | 25.0 [12.5–40.0] | 12.1 [0.0–0.0] |
| PC, RF (mandatory) | 19 | 60.0 [0.0–71.4] | 33.3 [13.3–83.3] | 0.0 [0.0–14.3] |
| VT, RF (mandatory) | 7 | 61.5 [14.3–83.3] | 50.0 [9.5–66.5] | 16.7 [4.8–50.0] |
| PS | 21 | 43.8 [0–60.0] | 39.6 [0.0–54.5] | 0.0 [0.0–10.0] |
Application of system’s advice – intervention arm only.
The table presents medians and quartiles of how the advice was implemented following Beacon recommendations. Advice recommended by Beacon was either followed, ignored (with no change made to ventilator settings), or different from what the clinician advised. FiO2, fraction inspired oxygen; PEEP, Positive end-expiratory pressure; PC, pressure control; RF, respiratory frequency; PS, pressure support.
No other significant differences were seen in secondary endpoints assessing physiological measurements or ventilation duration.
Discussion
This study was designed to evaluate this decision support system in patients with ARDS receiving mechanical ventilation prior to COVID-19 onset and repurposed for implementation during the pandemic under extremely challenging circumstances, with the inclusion of patients with ARDS from both COVID-19 and non-COVID-19 etiologies. This DSS is a novel, open-loop system providing advice from physiological mathematical models individualized by automatically tuning models to the patient’s data at the bedside. These systems aid rather than replace clinical expertise and provide personalized, adapted therapy as the models learn from changes in patient state and exemplars of recently described systems for the future (18).
No significant differences were seen in driving pressure, the study’s primary outcome; however, patients in the intervention arm tended to have tighter regulation of driving pressure than those in standard care. This may be important as it is perhaps more crucial to reduce the incidence of high levels of driving pressure than to modify the median value delivered, as values below 15cmH2O may reflect little increased risk for the patient (19).
Application of DSS advice showed optimization of physiological state under certain conditions. Significant improvement in oxygenation index was seen without increased incidence of hypoxemia, consistent with appropriate use of FiO2. A significantly improved ventilatory ratio was seen for non-ECMO patients under controlled ventilation modes.
We observed a significantly greater number of changes in pressure control (2.25 vs. 0.66) and respiratory frequency settings (2.31 vs. 0.94) in the intervention arm, but this was not significant in non-ECMO patients (2.31 vs. 1.17 and 2.33 vs. 1.30, respectively), and no significant changes in the number of pressure support setting changes. Support mode ventilation was delivered at significantly reduced time spent with a respiratory rate of less than 12 breaths/min, values below which have been shown previously to be associated with ventilatory over assistance (20). Furthermore, there was a trend toward a reduction in the percentage time spent at end-tidal carbon dioxide levels greater than 7 kPa (p = 0.06), alongside non-significant tendencies for pressure support reduction of about 1-2cmH2O. No significant detrimental effects were observed by the application of the advice.
Despite the challenges of implementing a complex, “open loop” DSS intervention during pandemic conditions, the data indicate that advice was applied approximately 60% of the time, with the exception of advice on pressure settings, for which advice was applied a median of 44%. However, despite a significant proportion of advice being either ignored or not seen, optimization of gas exchange still occurred. This may reflect the system requesting small changes in ventilatory parameters, which may be at odds with purely clinician-made changes. This lack of adoption may be enhanced during the pandemic surges and contrasts with other studies evaluating the application of the system in short, controlled periods (14, 15). Importantly, for advice on pressure settings in either mandatory or support modes, clinicians tended not to make changes different from the advice (as represented by a median change count of zero within both mode settings). For FiO2, clinicians made settings in the opposite direction a median of 12% of the time. The lack of implementation of a higher percentage of advice places limitations on the interpretation of these results, illustrating that improvements may be made when the advice is used as an augmentation of current care rather than a replacement. If such a system were to be used in a closed-loop context, analysis of the reasons for differences in opinions would be necessary. Nonetheless, this system also enables an understanding of the frequency of ventilator changes that are made and the implementation space for such a device.
Other decision support systems and closed-loop control systems for mechanical ventilation have been evaluated in prospective studies, but few in relation to the management of ARDS and none with a detailed physiological model of the individual patient. Notably, East et al. showed physiological efficacy through significant improvement in morbidity scores in 200 ARDS patients randomized to decision support advice based on empirical rules rather than physiological models in a non-commercial system (21). The most widely applied and evaluated routine commercial tools, SmartCare (Dräger Medical) and Intelli-Vent Adaptive Support Ventilation (INTELLiVENT-ASV) or its predecessor ASV (Hamilton Medical), apply closed-loop automation rather than open-loop advice. SmartCare provides control during support mode ventilation and has been shown in some studies to significantly reduce weaning duration in patients after evaluation of weaning readiness (22, 23). ASV and INTELLiVENT-ASV control the patient through all phases of ventilation, and studies have shown significant reductions in weaning time (24, 25) and total ventilator time (26, 27). However, these have been in fast-track cardiac patients or patients with COPD, but patients with ARDS have been excluded. A single retrospective study with Intelli-Vent ASV has shown a significant reduction in driving pressure in 51 COVID-19-ARDS patients 2 h after conversion to INTELLiVENT-ASV, suggesting potential for improvement in care without evidence provided by prospective evaluation (28). Hence, such novel technologies using personalized approaches may lead to improvements in weaning (29).
Titration of ventilator settings in the acute phase based on pressure-volume curves and applying optimal PEEP and driving pressure settings are associated with better outcomes in ARDS (30, 31). However, it remains uncertain whether this benefit is due to optimized PEEP or the use of low tidal volume ventilation (32). Indeed, within the current era of ARDS, lung protective ventilation strategies remain a standard of care in the specialist center involved in this study and may explain why no effect on driving pressure was apparent. Furthermore, the differences in pathophysiology between COVID-19-ARDS and non-COVID-19 ARDS may have played a role in how the system could influence reductions in driving pressure and may explain why differences were observed in composite measures such as oxygenation index and ventilatory ratio (33–36).
There are several limitations to this study. The primary challenge was conducting the research during the COVID-19 pandemic, which created a less-than-ideal environment for the evaluation and implementation of a new device. This likely contributed to the high percentage of advice being ignored by clinicians. In addition, two of the study sites were ECMO centers, and many of the enrolled patients required ECMO support, which introduced greater heterogeneity in the patient cohort. The device in this study measures oxygen consumption and carbon dioxide production at the mouth using indirect calorimetry and incorporates these data into its advice calculations. However, it does not account for the gas exchange occurring through ECMO, making its use contraindicated during ECMO periods.
Adapting the device to account for ECMO gas exchange would, in the future, enable the calculation of advice during ECMO periods. The evaluation of differences in driving pressure in patients who underwent a few days of non-ECMO ventilation, followed by a prolonged ECMO period, and then another period of non-ECMO ventilation, may have resulted in comparisons biased by the duration of ECMO. Notably, our sensitivity analysis showed that improvements in physiological status were more pronounced in the non-ECMO group.
Conclusion
This study is the first evaluation of a physiological model-based DDS for guiding mechanical ventilation in patients with both COVID-19 and non-COVID-19 ARDS. The results showed no significant difference in driving pressure as the primary outcome. However, the application of approximately 60% of the system’s advice led to improvements in the physiological state. The study also demonstrated greater homogeneity in ventilatory management, and the system proved safe to implement in patients with ARDS, even during pandemic conditions. Further studies assesing the implementation of such a DSS would help determine its clinical significance and/or health economic benefits.
Group member of DeVENT Study Group
Statements
Data availability statement
The datasets presented in this article are not readily available because the datasets generated during the current study are not publicly available due to privacy issues but may be available from the corresponding author on reasonable request conditional upon local law relating to confidentiality and anonymization. Requests to access the datasets should be directed to sr@hst.aau.dk.
Ethics statement
The studies involving humans were approved in the United Kingdom, and the study received ethical approval from the London South-East Research Ethics Committee (Ref: 19/LO/1606) on 15 January 2020, with protocol version 4.0 approved on 16 June 2021. The study protocol was approved by the French Ethics Committee (Comité de Protection des Personnes Sud Mediterranee III; approved on July 30, 2020, under number 2019.12.06 ter_19.11.15.76132) and Medicine Agency (Agence Nationale de Sécurité du Médicament; approved on July 3, 2020, under number 2019-A02610-57-A). The study protocol was approved by the ethics committee of the Medical University of Vienna, Austria (EC No. 2056/2019) on 28 April 2020. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
BP: Writing – original draft, Writing – review & editing. SM: Writing – review & editing. NJ: Writing – review & editing. RH: Writing – review & editing. SP: Writing – review & editing. TL: Writing – review & editing. MA: Writing – review & editing. EF: Writing – review & editing. IA: Writing – review & editing. DM: Writing – review & editing. MT: Writing – review & editing. TS: Writing – review & editing. DK: Writing – review & editing. MJ: Writing – review & editing. PS: Writing – review & editing. SR: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The European Commission Horizon 2020 Fast Track to Innovation Program provided for all research costs for the DeVENT study (Reference EU project 853850 -ECMO-BIOMARKER). The funder had no role in the trial design, collection, management, analysis, or interpretation of data, writing of reports, or submission for publication.
Conflict of interest
SR and DK have previously been, but are no longer, shareholders of Mermaid Care A/S, who manufactured the decision support system presented here.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1473629/full#supplementary-material
References
1.
Panwar R Madotto F Laffey JG van Haren FM . Compliance phenotypes in early acute respiratory distress syndrome before the COVID-19 pandemic. Am J Respir Crit Care Med. (2020) 202:1244–52. doi: 10.1164/rccm.202005-2046OC
2.
Karbing DS Panigada M Bottino N Spinelli E Protti A Rees SE et al . Changes in shunt, ventilation/perfusion mismatch, and lung aeration with PEEP in patients with ARDS: a prospective single-arm interventional study. Crit Care. (2020) 24:111. doi: 10.1186/s13054-020-2834-6
3.
Gattinoni L Coppola S Cressoni M Busana M Rossi S Chiumello D . Covid-19 does not Lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. (2020) 201:1299–300. doi: 10.1164/rccm.202003-0817LE
4.
Scaramuzzo G Karbing DS Fogagnolo A Mauri T Spinelli E Mari M et al . Heterogeneity of ventilation/perfusion mismatch at different levels of PEEP and in mechanical phenotypes of COVID-19 ARDS. Respir Care. (2022) 68:188–98. doi: 10.4187/respcare.10242
5.
Pelosi P Ball L Barbas CSV Bellomo R Burns KEA Einav S et al . Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care. (2021) 25:250. doi: 10.1186/s13054-021-03686-3
6.
Patel BV Haar S Handslip R Auepanwiriyakul C Lee TM-L Patel S et al . Natural history, trajectory, and management of mechanically ventilated COVID-19 patients in the United Kingdom. Intens Care Med. (2021) 47:549–65. doi: 10.1007/s00134-021-06389-z
7.
Constantin J-M Jabaudon M Lefrant J-Y Jaber S Quenot J-P Langeron O et al . Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. (2019) 7:870–80. doi: 10.1016/S2213-2600(19)30138-9
8.
Calfee CS Delucchi K Parsons PE Thompson BT Ware LB Matthay MA et al . Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. (2014) 2:611–20. doi: 10.1016/S2213-2600(14)70097-9
9.
Morris AH Stagg B Lanspa M Orme J Clemmer TP Weaver LK et al . Enabling a learning healthcare system with automated computer protocols that produce replicable and personalized clinician actions. J Am Méd Inform Assoc. (2021) 28:1330–44. doi: 10.1093/jamia/ocaa294
10.
Needham DM Colantuoni E Mendez-Tellez PA Dinglas VD Sevransky JE Himmelfarb CRD et al . Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. (2012) 344:e2124. doi: 10.1136/bmj.e2124
11.
Rees SE Karbing DS . Determining the appropriate model complexity for patient-specific advice on mechanical ventilation. Biomed Tech. (2017) 62:183–98. doi: 10.1515/bmt-2016-0061
12.
Rees SE Spadaro S Corte FD Dey N Brohus JB Scaramuzzo G et al . Transparent decision support for mechanical ventilation using visualization of clinical preferences. Biomed Eng Online. (2022) 21:5. doi: 10.1186/s12938-021-00974-5
13.
Murali M Ni M Karbing DS Rees SE Komorowski M Marshall D et al . Clinical practice, decision-making, and use of clinical decision support systems in invasive mechanical ventilation: a narrative review. Br J Anaesth. (2024) 133:164–77. doi: 10.1016/j.bja.2024.03.011
14.
Karbing DS Spadaro S Dey N Ragazzi R Marangoni E Corte FD et al . An open-loop, physiologic model–based decision support system can provide appropriate ventilator settings. Crit Care Med. (2018) 46:e642–8. doi: 10.1097/CCM.0000000000003133
15.
Spadaro S Karbing DS Corte FD Mauri T Moro F Gioia A et al . An open-loop, physiological model based decision support system can reduce pressure support while acting to preserve respiratory muscle function. J Crit Care. (2018) 48:407–13. doi: 10.1016/j.jcrc.2018.10.003
16.
Ranieri VM Rubenfeld GD Thompson BT Ferguson ND Caldwell E Fan E et al . Acute respiratory distress syndrome: the Berlin definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
17.
Patel B Mumby S Johnson N Falaschetti E Hansen J Adcock I et al . Decision support system to evaluate ventilation in the acute respiratory distress syndrome (DeVENT study)—trial protocol. Trials. (2022) 23:47. doi: 10.1186/s13063-021-05967-2
18.
Cecconi M . Reflections of an intensivist in 2050: three decades of clinical practice, research, and human connection. Crit Care. (2023) 27:391. doi: 10.1186/s13054-023-04674-5
19.
Amato MBP Meade MO Slutsky AS Brochard L Costa ELV Schoenfeld DA et al . Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. (2015) 372:747–55. doi: 10.1056/NEJMsa1410639
20.
Pletsch-Assuncao R Pereira MC Ferreira JG Cardenas LZ De AALP De Carvalho CRR et al . Accuracy of invasive and noninvasive parameters for diagnosing Ventilatory Overassistance during pressure support ventilation*. Crit Care Med. (2018) 46:411–7. doi: 10.1097/CCM.0000000000002871
21.
East TD Heermann LK Bradshaw RL Lugo A Sailors RM Ershler L et al . Efficacy of computerized decision support for mechanical ventilation: results of a prospective multi-center randomized trial. Proc AMIA Symp. (1999) 1:251–5. PMID:
22.
Lellouche F Mancebo J Jolliet P Roeseler J Schortgen F Dojat M et al . A multicenter randomized trial of computer-driven Protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med. (2006) 174:894–900. doi: 10.1164/rccm.200511-1780OC
23.
Burns KEA Meade MO Lessard MR Hand L Zhou Q Keenan SP et al . Wean earlier and automatically with new technology (the WEAN study). A multicenter, pilot randomized controlled trial. Am J Respir Crit Care Med. (2013) 187:1203–11. doi: 10.1164/rccm.201206-1026OC
24.
Sulzer CF Chioléro R Chassot P-G Mueller XM Revelly J-P . Adaptive support ventilation for fast tracheal Extubation after cardiac surgery. Anesthesiology. (2001) 95:1339–45. doi: 10.1097/00000542-200112000-00010
25.
Gruber PC Gomersall CD Leung P Joynt GM Ng SK Ho K et al . Randomized controlled trial comparing adaptive-support ventilation with pressure-regulated volume-controlled ventilation with automode in weaning patients after cardiac surgery. Anesthesiology. (2008) 109:81–7. doi: 10.1097/ALN.0b013e31817881fc
26.
Kirakli C Naz I Ediboglu O Tatar D Budak A Tellioglu E . A randomized controlled trial comparing the ventilation duration between adaptive support ventilation and pressure assist/control ventilation in medical patients in the ICU. Chest. (2015) 147:1503–9. doi: 10.1378/chest.14-2599
27.
Kirakli C Ozdemir I Ucar ZZ Cimen P Kepil S Ozkan SA . Adaptive support ventilation for faster weaning in COPD: a randomised controlled trial. Eur Respir J. (2011) 38:774–80. doi: 10.1183/09031936.00081510
28.
Buiteman-Kruizinga LA Mkadmi HE Neto AS Kruizinga MD Botta M Schultz MJ et al . Effect of INTELLiVENT-ASV versus conventional ventilation on ventilation intensity in patients with COVID-19 ARDS—an observational study. J Clin Med. (2021) 10:5409. doi: 10.3390/jcm10225409
29.
Rose L Schultz MJ Cardwell CR Jouvet P McAuley DF Blackwood B . Automated versus non-automated weaning for reducing the duration of mechanical ventilation for critically ill adults and children: a cochrane systematic review and meta-analysis. Crit Care. (2015) 19:48. doi: 10.1186/s13054-015-0755-6
30.
Amato MB Barbas CS Medeiros DM de Schettino GP Filho GL Kairalla RA et al . Beneficial effects of the “open lung approach” with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am J Respir Crit Care Med. (1995) 152:1835–46. doi: 10.1164/ajrccm.152.6.8520744
31.
Amato MBP Barbas CSV Medeiros DM Magaldi RB Schettino GP Lorenzi-Filho G et al . Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. (1998) 338:347–54. doi: 10.1056/NEJM199802053380602
32.
Network ARDS Brower RG Matthay MA Morris A Schoenfeld D Thompson BT et al . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and acute respiratory distress syndrome. N Engl J Med. (2000) 342:1301–8. doi: 10.1056/NEJM200005043421801
33.
Patel BV Arachchillage DJ Ridge CA Bianchi P Doyle JF Garfield B et al . Pulmonary Angiopathy in severe COVID-19: physiologic, imaging and hematologic observations. Am J Resp Crit Care. (2020) 202:690–9. doi: 10.1164/rccm.202004-1412oc
34.
Tsolaki VS Zakynthinos GE Mantzarlis KD Deskata KV Papadonta M-EE Gerovasileiou ES et al . Driving pressure in COVID-19 acute respiratory distress syndrome is associated with respiratory distress duration before intubation. Am J Respir Crit Care Med. (2021) 204:478–81. doi: 10.1164/rccm.202101-0234LE
35.
Pozzi T Collino F Brusatori S Romitti F Busana M Moerer O et al . Specific respiratory system compliance in COVID-19 and non–COVID–19 acute respiratory distress syndrome. Am J Respir Crit Care Med. (2023) 208:328–30. doi: 10.1164/rccm.202302-0223LE
36.
Bos LDJ Sjoding M Sinha P Bhavani SV Lyons PG Bewley AF et al . Longitudinal respiratory subphenotypes in patients with COVID-19-related acute respiratory distress syndrome: results from three observational cohorts. Lancet Respir Med. (2021) 9:1377–86. doi: 10.1016/S2213-2600(21)00365-9
Summary
Keywords
ARDS, mechanical ventilation, clinical decision support, respiratory mechanics, driving pressure
Citation
Patel BV, Mumby S, Johnson N, Handslip R, Patel S, Lee T, Andersen MS, Falaschetti E, Adcock IM, McAuley DF, Takata M, Staudinger T, Karbing DS, Jabaudon M, Schellongowski P, Rees SE and On behalf of the DeVENT Study Group (2024) A randomized control trial evaluating the advice of a physiological-model/digital twin-based decision support system on mechanical ventilation in patients with acute respiratory distress syndrome. Front. Med. 11:1473629. doi: 10.3389/fmed.2024.1473629
Received
02 August 2024
Accepted
14 October 2024
Published
30 October 2024
Volume
11 - 2024
Edited by
Patricia R. M. Rocco, Federal University of Rio de Janeiro, Brazil
Reviewed by
Claude E. Guérin, Hospices Civils de Lyon, France
Tobias Becher, University Medical Center Schleswig-Holstein, Germany
Updates
Copyright
© 2024 Patel, Mumby, Johnson, Handslip, Patel, Lee, Andersen, Falaschetti, Adcock, McAuley, Takata, Staudinger, Karbing, Jabaudon, Schellongowski and Rees.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brijesh V. Patel, brijesh.patel@imperial.ac.ukStephen E. Rees, sr@hst.aau.dk
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.