Abstract
Olmsted syndrome (OS) is a rare disorder characterized by a mutilating palmoplantar keratoderma and periorificial keratotic plaques, but which shows considerable clinical heterogeneity. Recently, transient receptor potential vanilloid 3 (TRPV3) mutations associated with autosomal dominant or recessive OS have been reported. Here we describe a classically OS case with definitive diagnosis of OS based on clinical features and a genetic assay. Genetic analysis revealed heterozygous variants in the TRPV3 gene using whole-exome sequencing of case-parents’ trios. This mutation was not identified in his mother. Notably, a previously unreported heterozygous frameshift mutation, c.1964 T > C (p.L655P), was identified in exon 15 of the TRPV3 gene in this patient and his father. Additionally, the patient was effectively managed with oral erlotinib at a daily dose of 75 mg. After 3 months of treatment, most plantar lesions resolved, and the pain experienced was mildly alleviated. No significant adverse effects were observed in this case during treatment. In addition, we review the OS literature regarding TRPV3 gene mutations.
Introduction
Olmsted Syndrome (OS) is a rare genodermatosis classically characterized bilateral progressive mutilating palmoplanter keratoderma (PPK) and periorificial keratotic plaque that gradually progress over time (1). This syndrome was first described in 1927 by Olmsted (2). The disease usually begins at birth or in early childhood, and the clinical presentation is quite heterogeneous. The most suggestive symptoms associate PPK with pseudoainhum and periorificial keratotic plaques (3, 4). Uncommon cutaneous manifestations such as nail dystrophy, leukokeratosis of the oral mucosa, and hyperhidrosis or hypohidrosis of the palmoplantar region may also be observed in association with OS (5). OS affects both genders, with a higher prevalence in males. Genetic testing provides the most reliable method for accurately diagnosing OS, although only a limited number of cases with gene mutations have been identified since its initial detection. Whole-exome sequencing has revealed pathogenic mutations in transient receptor potential vanilloid 3 (TRPV3), a gene responsible for encoding a particular type of ion channel, in individuals with autosomal-dominant OS (6, 7). Mutations in PERP (TP53 apoptosis effector) have also been reported to cause OS (8). Cheong et al. performed comparative exome sequencing and Sanger sequencing to identify a G-to-A transition at position c.573 in the TRPV3 gene, producing the missense mutation p.Gly573Ser in the proband (9). Subsequent studies have found a series of mutation sites, including p.Leu673Phe (10), p.Trp692Cys (11), p.Gly573Cys (12), and p.Gly568Val (13). In recent years, an increasing number of mutation sites have been identified, indicating the genetic heterogeneity of OS inheritance. Notably, Zhong et al. identified five previously unreported variations of the TRPV3 gene: R416Q, R416W, L655P, W692S, and L694P (14). Of particular interest is the L673F variant, which has been associated with olfactory sensory (OS) dysfunction without the presence of periorificial keratoderma, thereby categorizing it as an atypical form of OS. Present therapeutic interventions for OS are frequently inadequate and may provide temporary symptomatic relief. In a study by Greco et al., three patients with OS exhibited resolution of TRPV3 mutation-associated PPK with erlotinib hydrochloride treatment (15). In 2022, Kathleen et al. reported an another case of erlotinib-induced remission of pain and PPK in a patient with TRPV3 mutation-associated OS (16). However, erlotinib-induced remission of PPK in the Chinese patient with TRPV3 mutation-associated OS has not been reported yet.
In this study, we report a case of OS in which patients present with PPK and skin peeling on the extremities. Initially, the characteristic lesion of the proband’s plantar was examined histopathologically and ultrastructurally. Additionally, we screened TRPV3, which was previously indicated in the pathogenesis of genodermatosis with mutilating palmoplantar keratoderma. The patient’s symptoms were effectively improved by oral erlotinib at a daily dose of 75 mg. To our knowledge, this is the first report of a Chinese patient with OS harboring a missense p.L655P mutation in TRPV3.
Case report
A 31-year-old male presented to our outpatient clinic with symptoms including symmetrical focal PPK, intermittent episodes of warmth-induced pain and itching, sclerodactyly-like features, mild pseudoainhum, and skin peeling on the extremities (Figures 1A,B). The patient’s illness lasted for about 8 years. He presented with mild keratosis and peeling, and thick yellow-brown, fissure hyperkeratic plaques on the pressure sites of the plantar (Figures 1C,D). The hyperkeratotic patches were focal in nature, predominantly localized in patches at pressure points, without spreading beyond these areas. The patient suffered from recurrent episodes of painful plaques on his feet. Each episode was marked by intense and debilitating pain that did not improve with various pain medications. The severity of the pain prevented the patient from walking and engaging in sports activities. There were no signs of hyperkeratosis around the mouth or abnormalities in hair and nail structure. Systemic examination did not reveal any abnormalities. His father manifested with focal keratotic plaques, cone-shaped fingers, and desquamation on the palms (Figures 2A–D). Intriguingly, the symptoms of his father were much milder than those in the case. However, symptoms such as: acral hyperalgesia, severe itching, or photophobia, were absent in the two patients. The parents were allegedly nonconsanguineous.
Figure 1

Clinical presentation of the case. The patient presented with symmetric, focal palmoplantar keratoderma and obvious pseudoainhum on the weight-bearing areas of both feet (A,C). Palm region keratinous scale was observed in toe interdigital, desquamation and warmth in the extremities (B,D).
Figure 2

Clinical presentation of the patient’s father. His father yellow showed thickened and dry hard, hypertrophic keratotic patches, clear erythema on both feet (A,B). Cone-shaped fingers and desquamation on the palms were observed on both hands (C,D).
The histopathologic findings of plantar skin lesion showed psoriasiform hyperplasia with compact hyperkeratosis, acanthosis, hypogranulosis, and parakeratosis of “corn-like” pattern in the stratum corneum with vesicular degeneration on the corneum and epidermis (Figures 3A,B). Next, we used whole-exome sequencing (including mitochondria) to analyze the patient’s family genes. TRPV3 sequencing results revealed an identical heterozygous mutation c.1964 T > C (p.L655P) in the patient and his father (Figure 4A). Additionally, the mutation was not detected in the patient’s mother and wife (Figure 4B). This mutation is the 1964th base of the cDNA changes from T to C, resulting in the substitution of leucine for proline at amino acid position 655 (p.L655P). Based on the genetic findings, this familial case was consistent with an autosomal- dominant trait of inheritance.
Figure 3

Histopathologic and ultrastructural features of the patient’s skin lesion. Hematoxylin and eosin (H&E) staining of the skin lesion revealed psoriasiform hyperplasia characterized by dense hyperkeratosis (A), thickening of the skin layers (acanthosis), notable parakeratosis in the upper skin layers, reduced granular layer (hypogranulosis), and vesicular degeneration (B). Scale bar: A, ×50; B, ×100.
Figure 4

Result of DNA sequencing of TRPV3 mutation in the patients’ family. TRPV3 gene sequencing results revealed a identical heterozygous mutation c.1964 T > C (p.L655P) in this case and his father (A). The patient’s mother and wife did not exhibit mutations at the same gene loci (B).
Following informed consent and exclusion of contraindications, the patient received treatment with a combination of erlotinib and oral acitretin at a daily dose of 75 mg, considering his weight of 65 kg. During the treatment process, there were no significant severe side effects reported, only mild stomach discomfort and a slight burning sensation on the skin of the right forearm (Table 1). Within 3 months, significant improvement was noted, and subsequent examinations showed only mild redness and slight hyperkeratosis on both plantar surfaces after 5 months of treatment (Figure 5). The patient tolerated the medication effectively, with pain almost entirely alleviated and only intermittent flares. Notably, no severe adverse reactions were observed throughout the treatment course.
Table 1
| Adverse effect | Severity | Description |
|---|---|---|
| Burning, tingling, numbness or pain in the skin | Mild | The patient reported a mild burning sensation on the skin of the right forearm on the first day after taking the medication. |
| Stomach discomfort, upset, or pain | Mild | After the first oral administration of the medication, the patient reported mild discomfort in the stomach. |
| Cough or hoarseness | None | |
| Difficult or labored breathing | None | |
| Fever or chills | None | |
| Lower back or side pain | None | |
| Painful or difficult urination | None | |
| Stabbing chest pain | None | |
| Tightness in the chest | None | |
| Severe chest pain | None | |
| Slurred speech | None |
The common adverse effect of erlotinib in the case.
Figure 5
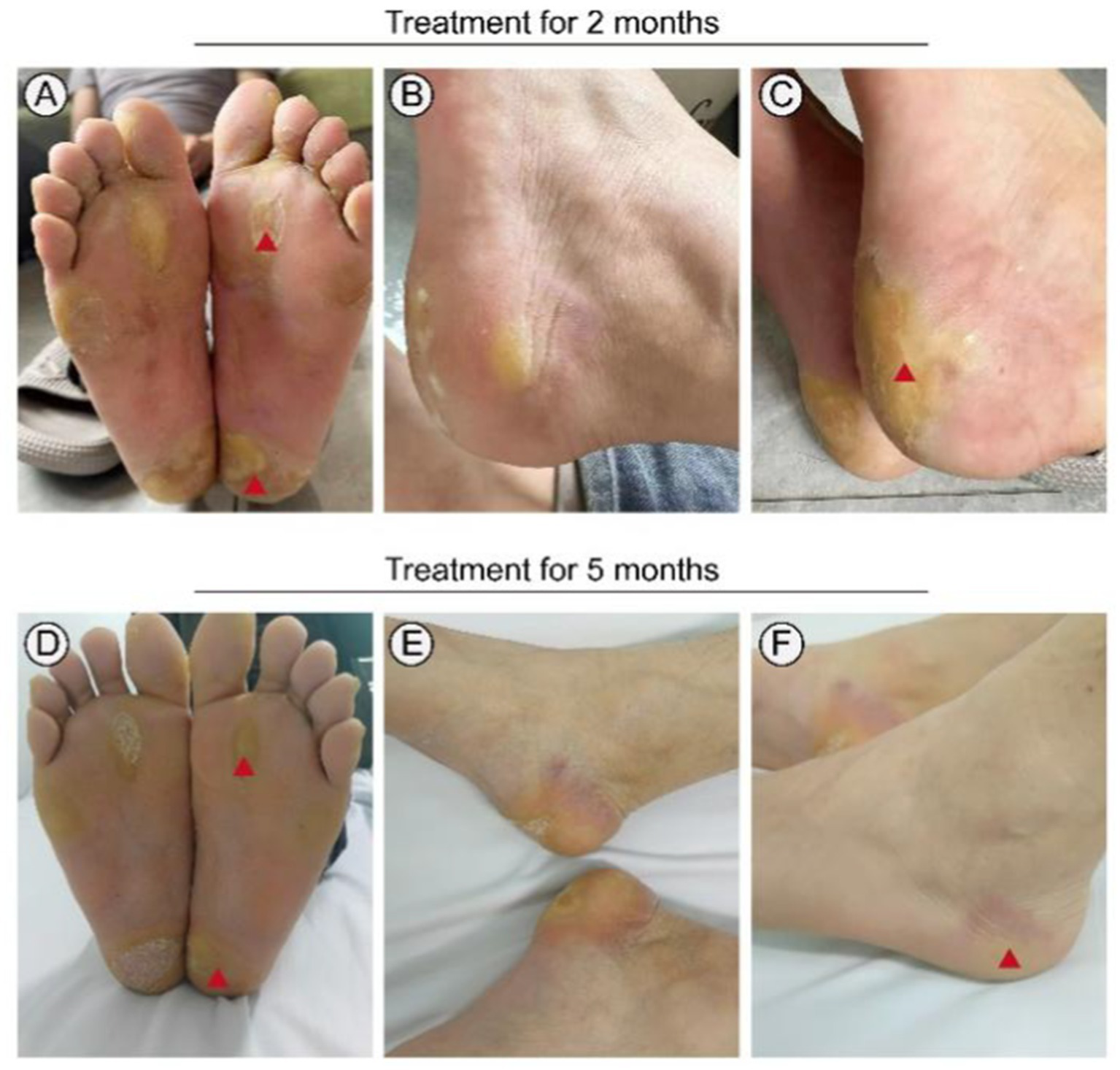
Treatment of this patient with erlotinib. Focal palmoplantar keratoderma and pseudoainhum were effectively relieved following therapy with erlotinib 75 mg for 3 months (A–C). Plantar surface of both feet showing mild erythema and subtle hyperkeratosis following therapy with erlotinib 75 mg for 5 months (D–F). Red triangle: Symptoms significantly improved.
Discussion
In this study, we present a case report of a patient with TRPV3 mutation-associated OS, who responded well to a novel use of erlotinib to treat pain and PPK. OS is an extremely rare hereditary skin disease, known for nearly a century, yet with fewer than a hundred reported cases, the prevalence remains unclear. The disease primarily manifests in the months following birth, with rare cases occurring in adulthood. OS is a genetically characterized by ulcerative palmoplantar keratosis, transgredient PPK resulting in flexural deformities and spontaneous amputation of the fingers or toes, usually accompanied by periorificial keratosis and hair loss (8). Notably, similar papules and plaques may appear over neck, axilla, cubital fossa, inguinal region and gluteal region (17). OS is observed in both sexes, although male cases are more frequent. Here we presented a typical familial case of OS with PPK, painful plaques on his feet, and cone-shaped hands. Similar OS symptoms were reported by Ning et al. (7). In addition to the sclerodactyly-like appearance and tapering fingers, pseudoainhum presented as a significant clinical feature in the current case. Literature suggests pseudoainhum may lead to damaging flexion contractures of the fingers (18). It is essential to consider Vohwinkel keratoderma, Huriez syndrome, and Mal de Meleda as differential diagnoses in cases of palmoplantar keratoderma. Pain and itching can vary in intensity, sometimes being severe. The patients in this study experienced intense pain in the weight-bearing area of the foot, significantly impacting their mobility and daily activities.
Most of reported OS cases are sporadic, with a few familial cases demonstrating various modes of inheritance (19, 20). TRPV3 represents the first identified pathogenic gene for OS and is an autosomal dominant gene (6). TRPV3 belongs to a large family of calcium-permeable transient receptor potential ion channel membrane proteins that contain six transmembrane domains (21). TRPV3 is the primary identified causative gene linked to OS, inherited in an autosomal dominant pattern. To date, around 38 cases of OS linked to TRPV3 mutations have been recorded, encompassing about 21 unique mutation sites. He et al. discovered that mutations in TRPV3 disrupt the balance between keratinocyte proliferation and differentiation processes (22). Keratinocytes in the epidermis play a crucial role in forming the protective outer layer of the skin. Proper TRPV3 function is vital for skin development. Mice lacking TRPV3 exhibit thermal hypersensitivity, impaired skin barrier formation, and abnormal hair growth (23, 24). Recent findings of various mutations in human TRPV3 associated with Olmsted Syndrome further emphasize the significance of TRPV3 activity in the development of keratinocytes (14, 15, 25). The patient in this study possessed a mutation in the TRPV3 gene identified as M1: c.1964 T > C: p.L655P. M1 indicates a heterozygous missense mutation, involving a change from thymine (T) to cytosine (C) at the 1964th position of the cDNA, resulting in the substitution of leucine with proline at the 655th codon. Sanger sequencing results validated the presence of the mutation in the index case, demonstrating a heterozygous genetic state.
Considering the presence of scleodactyly-like appearance and tapered fingers in his father, we speculate that it may be a special type of OS associated with TRPV3 mutation. TRPV3 has been associated with skin inflammation and wound healing, suggesting that abnormal activation of the TRPV3 ion channel may contribute to the sclerodactyly-like appearance and tapered fingers phenotype (26, 27). Furthermore, TRPV3 plays a vital role in itch and pain perception, regulating skin homeostasis, inflammation, and wound healing (21). Moqrich et al. found that mice without TRPV3 struggle with sensing room temperature and responding to sudden heat, but their other senses are not affected (28). The patient in this study experienced recurring episodes of painful foot plaques, impeding walking and athletic activities due to the severity of the pain. Keratinocytes with TRPV3 channels in the skin are not directly connected to sensory nerve endings. It’s suggested that certain small molecules may act as messengers between these cells and nerve endings, facilitating signal transmission. When TRPV3-positive skin cells are exposed to heat or TRPV3 stimulants, they release substances like PGE2, ATP, nitric oxide, interleukin-1α, and transforming growth factor-β (29, 30). These substances sensitize and activate the nerve endings in the skin, completing signal transmission and causing specific effects. This process could lead to an accumulation of inflammatory factors at the site due to increased TRPV3 expression, potentially contributing to peripheral neuropathy.
To elucidate the phenotypic spectrum of oral submucous fibrosis (OS), we conducted a comprehensive review of the clinical features associated with cases possessing known genetic underpinnings. Mutations in the TRPV3 gene have been identified at seven distinct amino acid residues, specifically: p.Trp521, p.Gly568, p.Gly573, p.Gln580, p.Met672, p.Leu673, and p.Trp692, the majority of which are classified as autosomal-dominant missense mutations (6, 11, 31–33). Notably, the Gly573Cys, Gly573Ser, and Trp692Gly mutations exhibit gain-of-function properties and are associated with a pronounced clinical phenotype characterized by palmoplantar keratosis, periorificial keratotic plaques, alopecia, and symptoms such as warm-induced pain and itching (7). Overall, the disease severity displays substantial variability among patients harboring different TRPV3 mutations, indicating a likely genotype–phenotype correlation linked to specific amino acid substitutions.
Mutations in the TRPV3 gene can lead to diverse clinical presentations, resulting in significant variability among patients. In this case, both the patient and his father share the same mutation site, presenting with pronounced pain and localized severe keratinization on the sides of the metatarsals. However, the father also displays finger deformities, albeit milder compared to typical OS manifestations. Zhong et al. examined nine unrelated OS cases with differing clinical severities associated with TRPV3 gene mutations to explore the relationship between genotype and phenotype (14).
The TRPV3 channel interacts with and regulates epidermal growth factor receptor (EGFR) signaling. The TRPV3 channel functions as a non-selective cation channel with a notable affinity for Ca2+. In response to various stimuli, it modulates the opening of Ca2+ ion channels within cells, thereby influencing cellular behavior. Studies have revealed that Olmsted Syndrome’s pathogenesis arises from acquired functional mutations in the TRPV3 gene, causing heightened Ca2+ influx into keratinocytes. The increased calcium influx leads to the production of signaling molecules like TGF-α through “trans-activation.” This triggers EGFR phosphorylation, initiating the TGF-α/EGFR pathway that influences keratinocyte differentiation, causing cell death and severe skin keratinization (34). Erlotinib, a first-generation EGFR inhibitor, blocks the EGFR pathway by binding to the EGFR tyrosine kinase domain, preventing downstream signaling by ATP and the EGFR tyrosine kinase domain. In the present study, oral erlotinib was used to treat OS. After 3 months treatment of initiating erlotinib 75 mg, the PPK and pain were improved. Greco et al. have also shown that erlotinib improved pain and resolved PPK in OS patients with TRPV3 mutations (15). Several case studies involving 12 patients, mostly children, demonstrated the effectiveness of oral erlotinib in OS treatment, highlighting its potential as a promising therapy (16, 35, 36).
This study is subject to certain limitations, such as the lack of a control group and a limited sample size. While the initial findings showed promise, larger-scale studies with extended follow-up durations are necessary to evaluate comprehensive clinical benefits, long-term safety profiles, disease progression post-treatment cessation, and responses in individuals with OS featuring diverse mutations. Furthermore, the absence of standardized assessment tools for OS posed a challenge in this investigation. Finally, this study did not conduct long-term follow-up on the patients and their families after treatment, particularly lacking data on the localized histological analysis of the skin following effective treatment.
In conclusion, we present a case study of a patient with OS dysfunction associated with a TRPV3 mutation, specifically highlighting the positive outcomes observed with the administration of erlotinib for managing pain and PPK. Erlotinib, as a targeted therapeutic agent, demonstrated notable efficacy in alleviating the symptoms associated with these conditions. Additionally, we identified heterozygous frameshift mutation, c.1964 T > C (p.L655P), in exon 15 of the TRPV3 gene, which was found in both the patient and his father. These findings suggest that targeted therapies like erlotinib may not only provide symptomatic relief but also address the underlying genetic factors contributing to this debilitating condition.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Department of Dermatology, First Hospital of Shanxi Medical University, Taiyuan, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Writing – original draft. MG: Writing – original draft. DY: Writing – original draft. JW: Writing – original draft. HC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Duchatelet S Hovnanian A . Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. (2015) 10:33. doi: 10.1186/s13023-015-0246-5
2.
Olmsted H . Keratodermia palmaris et plantaris congenitalis: report of a case showing associated lesions of unusual location. Am J Dis Child. (1927) 33:757–64. doi: 10.1001/archpedi.1927.04130170055008
3.
Tao J Huang CZ Yu NW Wu Y Liu YQ Li Y et al . Olmsted syndrome: a case report and review of literature. Int J Dermatol. (2008) 47:432–7. doi: 10.1111/j.1365-4632.2008.03595.x
4.
Frias-Iniesta J Sanchez-Pedreño P Martinez-Escribano JA Jimenez-Martinez A . Olmsted syndrome: report of a new case. Br J Dermatol. (1997) 136:935–8. doi: 10.1046/j.1365-2133.1997.01840.x
5.
Mevorah B Goldberg I Sprecher E Bergman R Metzker A Luria R et al . Olmsted syndrome: mutilating palmoplantar keratoderma with periorificial keratotic plaques. J Am Acad Dermatol. (2005) 53:S266–72. doi: 10.1016/j.jaad.2005.03.036
6.
Lin Z Chen Q Lee M Cao X Zhang J Ma D et al . Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. (2012) 90:558–64. doi: 10.1016/j.ajhg.2012.02.006
7.
Ni C Yan M Zhang J Cheng R Liang J Deng D et al . A novel mutation in TRPV3 gene causes atypical familial Olmsted syndrome. Sci Rep. (2016) 6:21815. doi: 10.1038/srep21815
8.
Duchatelet S Boyden LM Ishida-Yamamoto A Zhou J Guibbal L Hu R et al . Mutations in PERP cause dominant and recessive keratoderma. J Invest Dermatol. (2019) 139:380–90. doi: 10.1016/j.jid.2018.08.026
9.
Lai-Cheong JE Sethuraman G Ramam M Stone K Simpson MA McGrath JA . Recurrent heterozygous missense mutation, p.Gly 573Ser, in the TRPV3 gene in an Indian boy with sporadic Olmsted syndrome. Br J Dermatol. (2012) 167:440–2. doi: 10.1111/j.1365-2133.2012.11115.x
10.
Duchatelet S Pruvost S de Veer S Fraitag S Nitschké P Bole-Feysot C et al . A new TRPV3 missense mutation in a patient with Olmsted syndrome and erythromelalgia. JAMA Dermatol. (2014) 150:303–6. doi: 10.1001/jamadermatol.2013.8709
11.
Kariminejad A Barzegar M Abdollahimajd F Pramanik R McGrath JA . Olmsted syndrome in an Iranian boy with a new de novo mutation in TRPV3. Clin Exp Dermatol. (2014) 39:492–5. doi: 10.1111/ced.12318
12.
Wilson NJ Cole C Milstone LM Kiszewski AE Hansen CD O'Toole EA et al . Expanding the phenotypic Spectrum of Olmsted syndrome. J Invest Dermatol. (2015) 135:2879–83. doi: 10.1038/jid.2015.217
13.
Nagai H Takaoka Y Sugano A Nakamachi Y Kawano S Nishigori C . Identification of a heterozygous p.Gly568Val missense mutation in the TRPV3 gene in a Japanese patient with Olmsted syndrome: in silico analysis of TRPV3. J Dermatol. (2017) 44:1059–62. doi: 10.1111/1346-8138.13844
14.
Zhong W Hu L Cao X Zhao J Zhang X Lee M et al . Genotype–phenotype correlation of TRPV3-related Olmsted syndrome. J Invest Dermatol. (2021) 141:545–54. doi: 10.1016/j.jid.2020.06.035
15.
Greco C Leclerc-Mercier S Chaumon S Doz F Hadj-Rabia S Molina T et al . Use of epidermal growth factor receptor inhibitor Erlotinib to treat palmoplantar keratoderma in patients with Olmsted syndrome caused by TRPV3 mutations. JAMA Dermatol. (2020) 156:191–5. doi: 10.1001/jamadermatol.2019.4126
16.
Spitz KE Chu L Lawley LP . Treatment of TRPV3 mutation-associated Olmsted syndrome with erlotinib. JAAD Case Rep. (2022) 25:83–5. doi: 10.1016/j.jdcr.2022.05.029
17.
Perry HO Su WP . Olmsted syndrome. Semin Dermatol. (1995) 14:145–51. doi: 10.1016/s1085-5629(05)80011-2
18.
Tharini GK Hema N Jayakumar S Parveen B . Olmsted syndrome: report of two cases. Indian J Dermatol. (2011) 56:591–3. doi: 10.4103/0019-5154.87166
19.
Yaghoobi R Omidian M Sina N Abtahian SA Panahi-Bazaz MR . Olmsted syndrome in an Iranian family: report of two new cases. Arch Iran Med. (2007) 10:246–9. PMID:
20.
Ogawa F Udono M Murota H Shimizu K Takahashi H Ishida-Yamamoto A et al . Olmsted syndrome with squamous cell carcinoma of extremities and adenocarcinoma of the lung: failure to detect loricrin gene mutation. Eur J Dermatol. (2003) 13:524–8. PMID:
21.
Nilius B Bíró T Owsianik G . TRPV3: time to decipher a poorly understood family member!J Physiol. (2014) 592:295–304. doi: 10.1113/jphysiol.2013.255968
22.
He Y Zeng K Zhang X Chen Q Wu J Li H et al . A gain-of-function mutation in TRPV3 causes focal palmoplantar keratoderma in a Chinese family. J Invest Dermatol. (2015) 135:907–9. doi: 10.1038/jid.2014.429
23.
Radtke C Sinis N Sauter M Jahn S Kraushaar U Guenther E et al . TRPV channel expression in human skin and possible role in thermally induced cell death. J Burn Care Res. (2011) 32:150–9. doi: 10.1097/BCR.0b013e318203350c
24.
Schneider MR Werner S Paus R Wolf E . Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol. (2008) 173:14–24. doi: 10.2353/ajpath.2008.070942
25.
Jain A Sahu RP Goswami C . Olmsted syndrome causing point mutants of TRPV3 (G568C and G568D) show defects in intracellular ca (2+)-mobilization and induce lysosomal defects. Biochem Biophys Res Commun. (2022) 628:32–9. doi: 10.1016/j.bbrc.2022.08.026
26.
Steinhoff M Bíró T . A TR(I)P to pruritus research: role of TRPV3 in inflammation and itch [published correction appears in J Invest Dermatol. 2010 Mar;130(3):908]. J Invest Dermatol. (2009) 129:531–5. doi: 10.1038/jid.2008.440
27.
Yang P Zhu MX . Trpv3. In: Mammalian Transient Receptor Potential (TRP) Cation Channels: Volume I (2014). 273–91.
28.
Moqrich A Hwang SW Earley TJ Petrus MJ Murray AN Spencer KS et al . Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. (2005) 307:1468–72. doi: 10.1126/science.1108609
29.
Huang SM Lee H Chung MK Park U Yu YY Bradshaw HB et al . Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. (2008) 28:13727–37. doi: 10.1523/jneurosci.5741-07.2008
30.
Miyamoto T Petrus MJ Dubin AE Patapoutian A . TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat Commun. (2011) 2:369. doi: 10.1038/ncomms1371
31.
Eytan O Fuchs-Telem D Mevorach B Indelman M Bergman R Sarig O et al . Olmsted syndrome caused by a homozygous recessive mutation in TRPV3. J Invest Dermatol. (2014) 134:1752–4. doi: 10.1038/jid.2014.37
32.
Duchatelet S Guibbal L de Veer S Fraitag S Nitschké P Zarhrate M et al . Olmsted syndrome with erythromelalgia caused by recessive transient receptor potential vanilloid 3 mutations. Br J Dermatol. (2014) 171:675–8. doi: 10.1111/bjd.12951
33.
Danso-Abeam D Zhang J Dooley J Staats KA Van Eyck L Van Brussel T et al . Olmsted syndrome: exploration of the immunological phenotype. Orphanet J Rare Dis. (2013) 8:79. doi: 10.1186/1750-1172-8-79
34.
Zhang A Duchatelet S Lakdawala N Tower RL Diamond C Marathe K et al . Targeted inhibition of the epidermal growth factor receptor and mammalian target of rapamycin signaling pathways in Olmsted syndrome. JAMA Dermatol. (2020) 156:196–200. doi: 10.1001/jamadermatol.2019.4141
35.
Noakes R . Olmsted syndrome: response to erlotinib therapy and genotype/phenotype correlation. Australas J Dermatol. (2021) 62:445–6. doi: 10.1111/ajd.13663
36.
Butala S Phan S Siegel DH Carlberg V Paller AS . Two for two: dual therapy with erlotinib and acitretin for twins with severe keratoderma in Olmsted syndrome. Pediatr Dermatol. (2023) 40:735–7. doi: 10.1111/pde.15264
Summary
Keywords
Olmsted syndrome, erlotinib, TRPV3 gene, missense mutation, case report
Citation
Zhang J, Guo M, Yuan D, Wei J and Cui H (2025) Erlotinib therapy for Olmsted syndrome with p.L655P missense mutation in the TRPV3 gene: a case report. Front. Med. 12:1512673. doi: 10.3389/fmed.2025.1512673
Received
17 October 2024
Accepted
02 January 2025
Published
24 January 2025
Volume
12 - 2025
Edited by
Chandan Goswami, National Institute of Science Education and Research (NISER), India
Reviewed by
Jianqiang Yang, The First People’s Hospital of Huzhou, China
Yangyang Hao, The First People’s Hospital of Huzhou, China
Updates
Copyright
© 2025 Zhang, Guo, Yuan, Wei and Cui.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongzhou Cui, cuihongzhou@sxmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.