Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disorder that affects a significant portion of the global population, severely impacting the quality of life and causing physical and psychological distress of patients. Oxidative stress, resulting from an imbalance between oxidation and antioxidation activities, plays a pivotal role in the pathogenesis of AD. Monitoring oxidative stress products can offer valuable insights into the development of AD and highlight essential clinical and therapeutic effects. Additionally, evidence suggests that antioxidant strategies can alleviate or avert oxidative damage induced by free radicals and offer significant promise in the treatment of AD. In addition to directly utilizing natural products and nanomaterials for antioxidant interventions, these can also be incorporated into hydrogels, which help repair the skin barrier and support the sustained release of therapeutic agents. Furthermore, microneedles provide a minimally invasive method for delivering antioxidants to the deeper layers of the skin, enhancing treatment efficacy. This review aims to summarize the role of the oxidative stress in the pathogenesis of AD, focusing in the main oxidative products (DNA, protein, and lipid oxidation products), as well as antioxidant therapeutic approaches involving natural products, nanomaterials, hydrogels, and microneedles. Understanding these biomarkers and antioxidant therapy approaches provides important insights into the management of AD.
Generation of oxidative stress products and antioxidant therapy in atopic dermatitis (AD). External stimuli trigger the production of reactive oxygen species (ROS), leading to oxidative stress and the oxidation of DNA, proteins, and lipids. This process subsequently induces skin inflammation, the release of proinflammatory cytokines, and disruption of the skin barrier. Antioxidant therapies involving natural products, nanomaterials, hydrogels, microneedles, and antioxidant enzymes help alleviate these effects and improve the condition of AD. 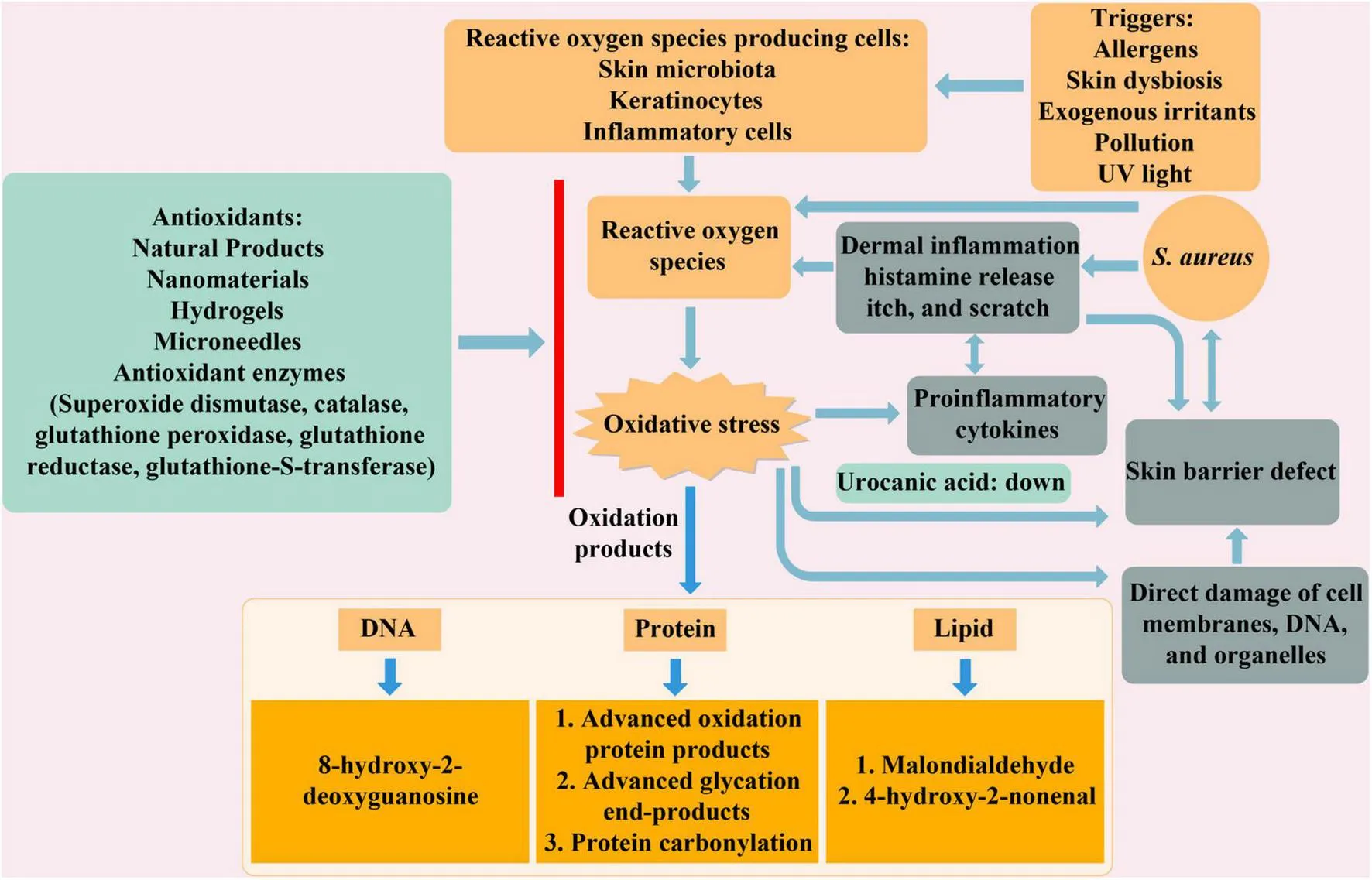
1 Introduction
Atopic dermatitis (AD), also recognized as atopic eczema (AE), presents as a chronic, recurrent, and inflammatory skin disorder (1). Affecting approximately 20% of children and up to 10% of adults worldwide (2), AD often manifests at birth or within the first years of life (3), with the majority of cases presenting before the age of two. In the past four decades, the global prevalence of AD has sharply risen, especially in developed countries (4). Predominant symptoms include skin dryness and intense pruritus, often accompanied by erythema, rash, which significantly impact mental health and quality of life (5). Otherwise, AD is associated with various comorbidities, such as food allergies (6), asthma (7), and allergic rhinitis (8).
The pathogenesis of AD is complex and not completely understood. Its multifactorial origins include genetic predisposition (9), environmental factors (10), Staphylococcus aureus colonization (11), and neurogenic inflammation (12), all contributing to the AD development. Deficiencies in epidermal barrier function and immune imbalance are intricately linked, with mutations in structural epidermal barrier proteins and immune regulatory factors playing pivotal roles in the pathogenesis of AD (13). Reduced expression of filaggrin is often associated with S. aureus colonization. S. aureus aggravates AD skin lesions (14) and induces pruritus and skin damage through the V8-PAR1 axis (15). One study has reported that filaggrin mutations are linked to infantile eczema and AD. Individuals carrying filaggrin gene mutations are not only at a higher risk of developing skin dryness on the trunk and extensor surfaces of the limbs in infants aged 3–6 months, but they also face an increased risk of developing eczema and AD (16). During the onset of AD, particularly in the acute phase, changes in the skin barrier activate a Th2-mediated immune response. Chronic skin inflammation in AD patients is caused by persistent Th2 inflammation and skin barrier disruption, accompanied by significant reactive oxygen species (ROS) production (17).
Reactive oxygen species are generated in response to different triggers such as allergens, cutaneous dysbiosis, exogenous irritants, pollutants and UV light (18). In particular, ROS are primarily derived from activated immune cells, including neutrophils and macrophages, as well as keratinocytes under oxidative stress conditions. The accumulation of ROS disrupts the balance between ROS generation and the antioxidant defense mechanisms. Eventually, the excessive oxidative stress is related to the progression of AD (19, 20). In the pathogenesis of AD, ROS derived from activated keratinocytes act in an autocrine and paracrine manner; being important mediators in the main functions of the keratinocyte: maintenance of the skin barrier function, interaction with the skin microbiome and triggering the immune response, including the recruitment of inflammatory cells from the dermis. However, elevated levels of ROS cause excessive oxidative stress and damage cellular components. Additionally, excessive ROS leads to oxidative damage to DNA and proteins, as well as lipid peroxidation of cell membranes, ultimately resulting in cell death (21). Research has indicated that biomarkers linked to oxidative stress, such as urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG), malondialdehyde (MDA), nitrite (NO2–), nitrate (NO3–), and biopyrrin, are significantly increased in AD patients (22–24). Furthermore, oxidative stress and inflammation mutually reinforce each other (25), with inflammatory cells producing ROS exacerbating oxidative stress, while ROS and oxidative stress products promote inflammatory responses.
To mitigate or avert oxidative damage induced by free radicals, the body has developed an intricate antioxidant defense mechanism. The skin, in particular, has its own antioxidant defense system that eliminates excess ROS through both enzymatic and non-enzymatic pathways, thereby maintaining redox balance and prevent the damage of ROS to cellular tissues (26). Supplementation with external antioxidants is a crucial strategy to combat oxidative stress, especially in AD patients, enhancing their ability to manage oxidative stress (27). The use of exogenous antioxidants is thus a significant component of antioxidant therapy for AD. Currently, various antioxidant methods have been formulated for the management of AD, including the use of natural product like pterostilbene (28), nanomaterial such as HC-HT-CSNPs (29), SINH-liposome-hydrogel (30), and lipid microparticles loaded with quercetin on microneedles (31), among other antioxidants.
Although ROS contribute to oxidative stress and inflammation, they are also essential for normal physiological functions. Besides direct microbial killing, ROS are also involved in immune responses and emerging as central signaling molecules in the inflammatory response (32). Excessive reduction of ROS may impair these fundamental physiological functions, potentially weakening host defense mechanisms and disrupting normal skin repair processes. Additionally, it introduces the role of antioxidants in the treatment of AD from four therapeutic approaches: natural products, nanomaterials, hydrogels, and microneedles. This review primarily analyzes oxidative stress products in AD at three levels: DNA, protein, and lipid. Additionally, it introduces the role of antioxidants in the treatment of AD from four perspectives: natural products, nanomaterials, hydrogels, and microneedles. Our study aims to explore oxidative stress markers and antioxidant treatment strategies in AD, thereby providing additional therapeutic opportunities for AD management in the future.
2 Role of oxidative stress in AD
Atopic dermatitis is a chronic inflammatory skin disease that commonly occurs in children (33). It is characterized by immune activation, epidermal hyperplasia, and defects in barrier function, reflecting potential changes in keratinocyte differentiation (34). The relationship between skin barrier changes and AD has been confirmed in the pathogenesis of the disease (35). Keratinocytes are key contributors to skin barrier function, playing a central role in the formation of the lipid bilayer and the production of filaggrin. Filaggrin is subsequently degraded into urocanic acid, an essential component of natural moisturizing factors that helps maintain skin hydration. Due to the reaction of reactive substances produced in keratinocytes to the environment and endogenous pro-oxidants, the skin has become the main target of response to oxidative stress. As the largest organ and a vital barrier separating the body’s internal environment from the external milieu, the skin is constantly exposed to a variety of external substances, leading to the generation of oxidative and inflammatory mediators (36). Uncontrolled production of ROS and cytokines results in oxidative stress and inflammation. While ROS production is a natural response to environmental changes, prolonged exposure to elevated ROS levels or oxidative stress facilitates the occurrence and exacerbation of skin diseases (37).
Oxidants encompass free radicals or any species containing unpaired electrons, including ROS. Oxidative stress assumes a critical role in AD and other dermatological conditions, evidenced by increased oxidative stress marker levels and diminished antioxidant levels in affected individuals (38). The pathogenesis of AD involves heightened ROS production, as evidenced by elevated ROS levels in skin biopsy specimens from AD patients, assessed using chemiluminescence techniques (39). The colonization of S. aureus frequently observed on the skin of AD patients, is associated with the generation of ROS through bacterial enzymes binding to the aryl hydrocarbon receptor (AHR) (40). AHR contributes to skin homeostasis by upregulating barrier-related proteins, including filaggrin (FLG), loricrin (LOR), and involucrin (IVL). However, the protective effects of AHR activation must be balanced against the antagonistic IL-13/IL-4–JAK–STAT6/STAT3 signaling pathway, which disrupts barrier integrity and promotes oxidative stress in AD. Moreover, ROS-induced high-mobility-group-protein B1 (HMGB1) secretion from keratinocytes facilitates S. aureus colonization and persistence by disrupting skin barrier integrity through the downregulation of epidermal barrier genes (41). Protein and lipid peroxidation products generated by oxidative stress in keratinocytes contribute to skin barrier dysfunction and exacerbate the progression of AD (42). Inflammatory responses and ROS production are closely intertwined (43). Inflammatory responses enhance the production of ROS, which further amplify inflammation in turn.
Beyond keratinocytes, oxidative stress also influences various immune cells. Dendritic cells exposed to ROS undergo activation, leading to the secretion of pro-inflammatory cytokines that amplify the immune response in AD (44). In the acute phase of AD, Th2-mediated immune reactions initiate the release of pro-inflammatory cytokines, further perpetuating the inflammatory cascade (45). Additionally, keratinocyte-derived cytokines can also activate Th2-mediated responses under oxidative stress conditions, contributing to skin inflammation in AD (46). In addition, oxidative stress promotes the polarization of macrophages toward a pro-inflammatory M1 phenotype, contributing to sustained inflammation (47). Oxidative stress disrupts the balance between regulatory T cells and effector T cells, leading to immune dysregulation and sustained inflammation in AD (48). Oxidative stress also plays a critical role in modulating cellular signaling events in multiple cell types involved in AD pathogenesis. ROS influence signaling pathways such as NF-κB, JAK-STAT, and MAPK, which are involved in keratinocyte function, immune cell activation, and cytokine production (49). The activation of these pathways by oxidative stress exacerbates inflammation and skin barrier dysfunction, further promoting AD progression.
3 Marker of oxidative stress in AD
Oxidative stress affects DNA, proteins, and lipids, leading to the formation of various oxidative products in AD. The major oxidative stress markers in AD are summarized in Table 1.
TABLE 1
| Study population | Type | Oxidative stress markers | References |
| Children, adult | DNA oxidation | 8-hydroxy-2′-deoxyguanosine (8-OHdG) | (22, 50, 51) |
| Children, adult | Protein oxidation | Advanced oxidation protein products (AOPPs) Advanced glycation end- products (AGEs) |
(52) |
| Children, adult | (53, 54) | ||
| Children, adult | Protein carbonylation (PC) | (55, 56) | |
| Children, adult | Lipid peroxidation | Malondialdehyde (MDA) 4-hydroxy-2-nonenal (4-HNE) |
(57- 59) |
| Children, adult | (52, 60) | ||
| Infant, children | Others | Nitric oxide (NO), nitrite and nitrate Thiol/disulfide balance Biopyrrin |
(61, 62) |
| Infant, children | (63, 64) | ||
| Children, adult | (24, 65, 66) |
Oxidative stress markers in atopic dermatitis.
3.1 DNA oxidation products-8-hydroxy-2’-deoxyguanosine
Oxidative stress-induced DNA damage can be assessed using nucleoside derivatives, which act as indicators of oxidative damage. One of the biomarkers is 8-hydroxy-2′-deoxyguanosine (8-OHdG), generated by the oxidation of the deoxyguanosine at the C-8 position (Figure 1). This biomarker is characterized with sensitive, stable and holistic biomarker of oxidative stress in vivo (67, 68). 8-OHdG, a product of damaged DNA is released into the bloodstream as a result of the action of the repair enzyme DNA glycosylase, and it is subsequently eliminated in urine (69).
FIGURE 1

Generation of DNA oxidation products. The C-8 position of guanine in DNA is susceptible to reactive oxygen species (ROS) attack and hydroxylation, generating the adduct 8-hydroxy-2′- deoxyguanosine (8-OHdG).
Detecting levels of 8-OHdG in urine can serve as a means to evaluate oxidative damage to DNA in the evaluation of AD (50, 70). Studies comparing 8-OHdG levels in the urine of AD patients and healthy controls have demonstrated positive correlations between 8-OHdG levels and dermatitis scores, indicating disease severity. Additionally, urinary 8-OHdG levels are markedly elevated in children with AD compared to controls, further supporting its utility as a biomarker for AD. A large-scale study involving 200 children diagnosed with AD found that urinary levels of 8-OHdG were considerably increased in the AD group relative to healthy controls (p < 0.001) (22). Children with chronic AD exhibited urinary 8-OHdG levels that were 1.6 times higher compared to in healthy controls, with a trend toward decreasing levels as the patients began to heal (51). However, these observations may not be exclusive to acute exacerbations of AD but could instead reflect general changes seen in inflammatory or infectious disorders (65). Moreover, investigations into psoriasis, another chronic refractory skin disease, have also detected 8-OHdG in the urine of affected individuals (71). The results showed that DNA oxidative damage also existed in psoriasis, and 8-OHdG could also be used to monitor the incidence of psoriasis, with expression levels comparable to those observed in AD.
3.2 Protein oxidation products
3.2.1 Advanced oxidation protein products
Advanced oxidation protein products (AOPPs) constitute a class of complex protein compounds composed of dimethyltyrosine, pentosidine, and carbonyl residues. AOPPs originate from oxidative stress reactions involving plasma proteins and chlorinated oxidants. The principal mechanism driving AOPPs generation involves the activated myeloperoxidase-H2O2-chloride system in neutrophils, with myeloperoxidase serving as the sole enzyme capable of generating chlorinated oxidants (Figure 2) (72, 73). Hypochlorous acid (HOCl) produced by this system is indicative of AOPPs production. In hemodialysis patients, elevated levels of AOPPs have exhibited a positive correlation with plasma myeloperoxidase activity (74) and oxidized fibrinogen was identified as a principal molecule contributing to the positive chemical reaction to AOPPs (75, 76). In 1996, the first detection of this biomarker of oxidative stress was proposed for plasma in patients with chronic uremia (77). Compared to healthy individuals, AOPPs levels in patients with advanced chronic kidney failure who had not yet undergone dialysis were nearly threefold higher.
FIGURE 2

Generation of protein oxidation products. The principal mechanism driving advanced oxidation protein products (AOPPs) generation involves the activated myeloperoxidase-H2O2-chloride system in neutrophils. AGFs are primarily generated through Maillard reaction, which is roughly divided into initial stage, intermediate stage and final stage. Protein carbonylation (PC) may occur due to direct oxidation of amino acid residues by reactive oxygen species (ROS) or non-oxidative reaction with carbonyl containing oxidized lipids.
As markers of oxygen-mediated protein damage, AOPPs have been identified as indicators of oxidative protein damage and proinflammatory mediators (78, 79). Elevated AOPPs levels have been associated with the advancement of various human diseases and their associated complications, thus serving as markers of oxidative stress across diverse pathologies. Monitoring AOPPs can help predict the development of diseases associated with oxidative stress. In addition to significantly elevated plasma AOPPs levels observed in patients with chronic uremia, similar findings have been demonstrated in other conditions such as cutaneous burns (80). In both groups of second and third degree thermal burns, AOPPs levels were reduced following treatment as a result of decreased levels of oxidative stress. Notably, ROS may contribute to oxidized protein damage within the stratum corneum, thereby disrupting barrier function and exacerbating AD (55). Currently, studies have shown that compared to healthy individuals, patients with AD and chronic urticaria exhibit elevated levels of AOPPs (52). Moreover, an association between AOPPs levels and age has been documented in patients with AD, underscoring the potential utility of AOPPs as biomarkers for disease severity and progression (52).
3.2.2 Advanced glycation end- products
Advanced glycation end-products (AGEs) and AOPPs share structural similarities and exert comparable biological effects. Their accumulation in biological systems results in analogous clinical outcomes. In the context of protein oxidation, the relationship between AGEs and the generation of AOPPs is noteworthy (75). Both AOPPs and AGEs possess similar structures that induce comparable biological effects, and their accumulation in biological systems leads to similar clinical consequences. AGEs, a diverse group of biologically active compounds, were initially identified by French researchers. They are produced through a non-enzymatic glycation process called the Maillard reaction, which uses the carbonyl groups of reducing sugars and the free amino groups of proteins as substrates (Figure 2) (81). AGEs are categorized into endogenous and exogenous sources (82). Endogenous AGEs are generated during normal physiological processes and aging, while exogenous AGEs primarily originate from dietary sources, with their content varying across different foods.
AGEs are produced in a slow and controlled process, accumulating in the body, including the skin (83). The skin is particularly reactive to changes in AGE levels. Their accumulation increases free radical production, stimulates the release of pro-inflammatory factors, and exacerbates inflammatory reactions. AGEs disrupt the dynamic balance of the skin (84), alter the normal substance composition and structure of different skin layers, impair the skin barrier function, and trigger skin issues. The skin barrier function is essential in the onset and progression of AD. Research has documented that AGEs levels are elevated in the keratinocytes of AD patients compared to healthy individuals, with severe AD patients exhibiting significantly higher levels than those with mild AD. However, no significant difference in serum AGE levels was found between typical AD patients and healthy controls (53). Pentosidine, a specific AGE, is closely associated with oxidative stress, with accelerated production observed in oxidative stress-related diseases (85). Pentosidine can serve as a marker for detecting AD (54). Urine tests in AD patients have shown significantly higher levels of pentosidine compared to the healthy controls. Pentosidine levels were significantly higher in AD patients in the acute phase, but reduced during the recovery process, mirroring trends observed for another AD biomarker, 8-OHdG. The consumption of exogenous AGEs increases the risk of developing AD (86), with pregnant women consuming high-AGEs foods potentially exposing their fetus to a higher AGEs environment, thereby increasing the likelihood of AD development.
3.2.3 Protein carbonylation
In addition to AOPPs and AGEs, protein carbonylation (PC) serves as another marker of protein oxidation in patients with AD. From a medical perspective, protein carbonylation has been emphasized as markers of protein oxidation, oxidative stress, and disease progression (52). The oxidative modification of proteins, characterized by the formation and/or introduction of carbonyl groups into proteins, represents a primary indicator of oxidative damage to proteins (87–89). PC, an irreversible oxidative modification (88), is classified into two types based on the origin of the carbonylated product (Figure 2). Consequently, changes in protein conformation following modification typically result in the loss of protein function (90). Proteins undergoing carbonylation exhibit diverse biological significance within biological systems, leading to varied biological effects (88, 91–93). PC is reflected in various diseases, including brain diseases, inflammatory diseases, autoimmune diseases, and aging (94–96), showing important connections between carbonyls in oxidized proteins, oxidative stress, and disease.
Oxidative stress plays an important role in the pathogenesis of AD, with studies demonstrating elevated levels of PC in dry skin and AD skin lesions (56). Levels of PC in the skin of AD patients are elevated and positively correlated with the severity of the disease (55). Sampling from AD patients utilizing the tape stripping method has revealed increased levels of PC in the stratum corneum, a phenomenon similarly observed in patients with psoriasis (97). The accumulation of PC in the skin contributes to transepidermal water loss and altered dermal matrix accumulation (98). ROS are implicated in inducing damage to cuticular oxidation proteins, disrupting skin barrier function, and exacerbating the development of AD.
3.3 Lipid peroxidation products
3.3.1 Malondialdehyde
Lipids are the most impacted biomolecules in oxidative stress-induced damage, and the identification of these end-products in inflammatory diseases suggest that lipid peroxidation is crucial in such diseases (99). Malondialdehyde (MDA), an important metabolite of arachidonic acid and unsaturated fatty acids possessing multiple unsaturated C-C double bonds in lipids, was the main and most widely studied compound (100) derived from lipid peroxidation following oxidative damage from oxygen radical attack (Figure 3) (101, 102). Upon exposure to oxidative stress, excessive accumulation of ROS disrupts the structure and function of the cell membrane, alters its permeability, and causes lipid peroxidation, inducing the production of MDA.
FIGURE 3

Generation of lipid oxidation products. 4-hydroxy-2-nonenal (4-HNE) and Malondialdehyde (MDA) are prevalent lipid oxidation products in atopic dermatitis (AD). Lipid oxidation is closely related to polyunsaturated fatty acids (PUFAs), typically progresses through three stages: initiation, propagation, and termination.
Malondialdehyde is produced in vivo through both enzymatic and non-enzymatic oxidation, making it a widely monitored biomarker for oxidative stress across various diseases and particularly favored for characterizing oxidative stress in AD patients (57). A likely correlation exists between serum antioxidant levels and MDA in individuals with eczema (58), with an inverse correlation between antioxidant levels and MDA levels and decreased serum levels of antioxidant vitamins in patients than in healthy persons. In children with AD, serum levels of MDA were found to be on average 0.055 units higher, while melatonin (a hormone with antioxidant activity) levels were approximately 3.05 units higher compared to controls. The increase in serum melatonin in AD might represent a compensatory mechanism to reduce skin inflammation by attempting to alleviate excessive oxidant production (59). For the antioxidant properties of quercetin, liposomes incorporating quercetin gel have demonstrated both protective and therapeutic effects on skin eczema (103). Treatment with quercetin-loaded liposomes resulted in significantly reduced skin pathological symptoms compared to untreated counterparts, accompanied by reduced levels of MDA in both liver and skin tissues.
3.3.2 4-hydroxy-2-nonenal
4-hydroxy-2-nonenal (4-HNE) is a lipid peroxide produced by polyunsaturated fatty acids (PUFAs) in response to oxidative stress (Figure 3). It belongs to the same class of lipid peroxidation products as MDA. Both 4-HNE and MDA are extensively studied markers of lipid peroxidation, with MDA recognized as a mutagenic product of lipid peroxidation and 4-HNE recognized as the most toxic product of lipid oxidation (104), worsening the damage resulting from oxidative stress (105, 106). 4-HNE is classified as an α, β-unsaturated aldehyde and possesses three functional groups: carbon-carbon double bonds, carbonyl groups and hydroxyl groups. Due to the existence of the conjugated system of C = C bonds and carbonyl groups, 4-HNE can provide partial positive charge to the carbon at position three and become efficient electrophiles, allowing it to react with essential biological molecules, including proteins and DNA (107–109). 4-HNE can also be generated through both enzymatic and non-enzymatic reactions from the breakdown of ω-6 PUFAs. However, the chemical reaction mechanism of 4-HNE formation remains unclear. Several mechanisms have been proposed over the years. The earliest suggestion was that 4-HNE was formed from PUFAs catalyzed by transition metal ions (102, 110). The formation mechanism of 4-HNE is attributed to the observation of hydroperoxides as an intermediate product.
As a secondary messenger of free radicals and growth regulators, 4-HNE participates in various pathophysiological processes and act as a bioactive marker, especially in oxidative stress related to diseases (111). Skin homeostasis plays an important role in the development of AD. Studies have found that cigarette smoke can trigger the generation of ROS (112). Exposure to cigarette smoke can affect skin homeostasis due to oxidative and inflammatory reactions, as well as inducing lipid peroxidation, leading to increased levels of 4-HNE (113). In a study examining oxidative stress markers in exhaled breath condensates of children with AD, 4-HNE levels were elevated in AD patients but did not differ significantly from those in healthy children (60). Another study found a similar phenomenon by measuring serum 4-HNE levels in AD patients, with 4-HNE concentrations comparable to those in healthy subjects.
3.4 Others
3.4.1 Nitric oxide, nitrite and nitrate
Nitric oxide (NO), a free radical gas containing unpaired electrons, is the smallest biologically active molecule produced by mammalian cells. It exhibits lipophilicity and possesses a high degree of diffusivity in tissues and cells (114). NO is primarily synthesized in organisms by nitric oxide synthase (NOS), an enzyme divided into three subtypes: inducible iNOS, endothelial eNOS, and neurogenic nNOS (115). Additionally, NO can be produced via non-canonical pathways, either through the reduction of nitrite to NO or by the sequential conversion of nitrate to nitrite and then to NO (116). With diverse biological functions, NO is essential for regulating the body in both healthy and disease states (117). In dermatology, NO is implicated in mechanisms underlying inflammatory or immune-mediated dermatoses, skin infections, skin cancers, and wound healing (118). Skin inflammation is significantly affected by NO (119). Keratinocytes, fibroblasts, and immune cells synthesize NO, and each contributing to the occurrence of inflammatory responses. The importance of NO as a mediator of skin inflammation is underscored by the delicate balance between its production and degradation, which is closely related to the onset and development of inflammatory skin diseases. Overproduction of NO has been linked to conditions such as AD and psoriasis (120–122).
Direct measurement of nitric oxide (NO) presents challenges due to its extremely short half-life in the bloodstream, typically disappearing within seconds, and its involvement in various biochemical reactions within living organisms (123, 124). NO is highly reactive owing to its unpaired electrons, capable of engaging in oxidative stress by reacting with free radicals, with the primary oxidative metabolites being nitrite and nitrate (125). A study found elevated levels of IgE, nitrite, and nitrate in the plasma of AD patients (61). Furthermore, a study involving 88 cases of AD and 12 cases of non-AD founded that serum nitrate levels not only showed a significant increase in children with AD, but also associated with the severity of the disease (62).
3.4.2 Thiol/disulfide balance
Thiols have emerged as a novel marker of oxidative stress, representing a class of organic compounds containing sulfhydryl groups. These sulfhydryl groups, commonly known as thiols, consist of sulfur and hydrogen atoms bonded to carbon atoms (126). The sulfhydryl groups within thiols provide protection against oxidative stress by scavenging ROS through enzymatic or non-enzymatic mechanisms. Thiols serve as physiological agents for neutralizing free radicals and other ROS (127). Through a series of reactions, thiols undergo modifications and react with oxidants to form disulfide bonds (128). The oxidation of thiols to disulfides is facilitated by through various processes, crucially involving three distinct mechanisms (129). Disulfide compounds formed as a result of these reactions can undergo reversible conversion to a thiol structure, thereby maintaining the dynamic balance of thiol/disulfide equilibrium (130).
Disruption of this balance has associated with the development of certain inflammatory diseases. In infants diagnosed with AD, thiols have been found to be significantly reduced compared to in comparison to healthy controls, while disulfide levels were markedly elevated, indicative of dysregulation in the thiol/disulfide balance favoring peroxidation (63). However, contrasting findings were observed in another study (64), which serum disulfide levels were observed to decrease in AD children in comparison to healthy children, leading to a reduction in the disulfide/natural thiol and disulfide/total thiol ratios.
3.4.3 Biopyrrin
Biopyrrin is a metabolite derived from the oxidation of bilirubin. Bilirubin, acting as a potent ROS scavenger, reacts with ROS, thereby exerting a robust antioxidant effect (131). Upon oxidation, bilirubin is converted into various forms of bilirubin oxidation metabolites (BOMs), comprising at least seven hydrophilic metabolites that are promptly excreted into urine because of their hydrophilic nature (132). These metabolites, including biopyrrin, can be detected by anti-bilirubin monoclonal antibody 24G7 (133, 134). A key advantage of using biopyrrin as an oxidative stress marker is its ability to provide real-time insights into the dynamic changes of oxidative stress through urine detection, thereby reflecting the oxidative stress status associated with various disease states.
Biopyrrin holds promise as a novel biomarker for AD (66). Urinary excretion of biopyrrin was markedly increased in pediatric patients experiencing acute exacerbations of AD, with levels averaging 1.8 times higher than those observed in healthy individuals (65). Bilirubin oxidation was enhanced in the diseased skin of patients with AD and levels of the oxidized metabolite biopyrrin detected in urine correlated with the severity of AD (24). Moreover, urinary biopyrrin levels were positively correlated with serum IgE and TARC/CCL17 expression. Biopyrrin expression was higher in AD lesions compared to normal skin, as detected by the 24G7 antibody. These findings highlight the potential utility of biopyrrin as a valuable biomarker for assessing oxidative stress and the severity of AD.
4 Manage oxidative stress in AD
Immunity and inflammation are pivotal in the pathogenesis of AD. Current therapeutic strategies predominantly focus on anti-inflammatory or immune-modulating agents. While the majority of patients respond favorably to topical corticosteroids (135), calcineurin inhibitors (136), and immunosuppressants (137), these treatments are often associated with significant adverse effects during long-term use (138). Oxidative stress is positively correlated with factors influencing the onset and advancement of AD. Moreover, prolonged exposure to oxidative stress impacts the condition of keratinocytes, leading to alterations in skin barrier function and cell death. Therefore, considering the use of antioxidants to mitigate AD is an essential strategy in the management of AD.
4.1 Natural products
Plants are the primary source of natural antioxidants (139), with antioxidant compounds primarily synthesized as secondary metabolites. Numerous plants and their derivatives exhibit antioxidant properties and frequently possess other significant biological activities. Due to their low toxicity, these natural antioxidants have been extensively utilized in the prevention and management of diseases related to oxidative stress (140). Plant-derived antioxidant compounds can be categorized into several groups: phenolic acids, phenolic diterpenes, flavonoids, volatile oils, carotenoids, and anthocyanins (141, 142). These compounds are abundant in herbs, spices, seeds, essential oils, fruits, and vegetables (143). Additionally, plants and foods containing vitamins and certain trace minerals contribute to the antioxidant process and constitute essential components of natural antioxidants (144, 145).
4.1.1 Natural extracts
Plants typically contain several highly active antioxidant compounds that can be extracted using various technical methods (146). The antioxidant effects of plant extracts are related to the chemical and physical properties of these compounds and operate through multiple mechanisms (147–149). The therapeutic potential of natural extracts for treating AD has been thoroughly explored through both in vivo and in vitro studies. A study involving 20 patients with mild to moderate AD demonstrated that a cream containing 100,000 IU of superoxide dismutase (SOD) and 4% plant extracts significantly alleviated AD symptoms and was effective across all phases of the disease (150). The therapeutic efficacy of this cream is attributed to the synergistic actions of SOD and the plant extracts, including antioxidant, anti-inflammatory, and additional beneficial properties. Resveratrol, a naturally occurring polyphenol abundant in grapes and berries, has shown positive therapeutic effects on skin disorders (151), potentially affecting inflammation through its antioxidant activity and free radical scavenging properties (152). Intragastric administration of resveratrol has been shown to ameliorate AD in mice induced by dinitrochlorobenzene (DNCB), by downregulating chemokine and proinflammatory factor levels and upregulating the expression of skin barrier proteins (153). Another study also demonstrated that topical formulations based on the antioxidant properties of resveratrol can reduce ROS, inhibit inflammatory responses, and improve skin barrier function (154). Analysis of the chemical composition of Lentinula edodes ethanolic extract revealed that polyphenols are the main antioxidant components, along with flavonoids, β-carotene, and lycopene. This ethanolic extract has shown to decrease serum IgE levels, downregulate the expression of inflammatory cytokines, and alleviate AD symptoms (155). Additionally, some natural extracts can exert antioxidant and anti-inflammatory effects and regulate the Nrf2/HO-1/NQO1 and NF-κB/MAPK signaling pathways to treat AD (156, 157).
4.1.2 Vitamins
Vitamins are a group of organic compounds crucial for maintaining normal physiological functions in the body and can be categorized into fat-soluble and water-soluble groups (158). Most vitamins are obtained through the daily diet. The primary sources of vitamins A, C, and E are fresh vegetables and fruits, while vitamin D is primarily biosynthesized through the skin under sunlight. These four vitamins inherently possess antioxidant properties, allowing them to function as antioxidants (159, 160).
There is a significant connection between vitamins and skin diseases. Maintaining a reasonable and stable vitamin level is crucial for preserving normal skin health (161). β-carotene (provitamin A) exhibits antioxidant and immunomodulatory effects, enhancing skin barrier function and reducing inflammation levels in hairless mice with oxazolone-induced AD (162). Vitamin C contributes to the formation of skin structure and skin antioxidation (163), ameliorating chronic inflammation and positively impacting AD. In groups supplemented with vitamin E, levels of oxidative stress markers were decreased, and a reduction in vitamin E concentration contributed to the progressing of AD in dogs (164). Another study supports that vitamin E supplementation can lower IgE levels in AD patients and improve AD symptoms (165). Vitamin D supplementation is beneficial for AD treatment and can be used to treat AD in children and dogs (166, 167). Furthermore, these vitamins can work synergistically to enhance AD treatment. For instance, the dermatitis score was lower in the group receiving both vitamin D and vitamin E compared to groups receiving either vitamin alone (168). However, there are differing views on the efficacy of vitamins in improving AD. Diets rich in antioxidant compounds can reduce the risk of AD. Taking in β-carotene and vitamin E is negatively correlated with AD, whereas vitamin C intake does not show a consistent correlation (169). Additionally, higher concentrations of vitamin C in breast milk are linked to a lower risk of atopy in infants, whereas vitamin E shows no consistent relationship with AD (170).
4.1.3 Minerals
Trace minerals, also known as trace elements, play an important role in maintaining overall nutrition and health (171), playing vital roles in the metabolism and physiological processes of the body. Several trace elements are involved in the redox reaction process. Selenium (Se) is an essential trace mineral that forms a key part of selenoproteins, which primarily exert their nutritional functions through a family of 25 selenoproteins. Se is an enzymatic antioxidant that has no antioxidant effect by itself but participates in selenoproteins as redox-active selenoenzymes to safeguard against oxidative damage (172, 173). Inhibiting iron death by regulating selenoprotein GPx4 plays a significant role in improving skin inflammation (174). Keratinocytes, which are integral to the skin barrier function, are also implicated in skin disorders such as AD. The supplementation of Se and selenoprotein SEPP1 can alleviate the oxidative stress and toxicity of 4-ClBQ-induced keratinocytes (175). Nevertheless, a 12 weeks study revealed that selenium-enriched yeast supplementation did not result in significant improvements in AD severity, indicating no substantial difference before and after supplementation (176).
Zinc, another essential nutrient for skin health, is abundantly present in the epidermis (177). Although zinc itself is not an antioxidant and is redox-inert, it contributes to oxidative defense through several mechanisms (178, 179). Decreased zinc levels have been observed in AD patients, taking zinc supplements orally may help those who are zinc deficient to manage AD (180). Zinc is commonly used as a nutritional supplement for cosmetic purposes and in the management of AD. However, caution is warranted regarding zinc concentration, as excessive intake can lead to zinc toxicity (181). The efficacy of zinc supplementation in treating AD remains controversial, with some studies suggesting no significant benefit, while others indicate potential therapeutic effects (182).
4.2 Nanomaterials
Nanotechnology has seen extensive development across various disciplines and facilitates the synthesis of nanoparticles via bottom-up and top-down strategies (183). Conventional antioxidants frequently encounter challenges, including limited permeability, poor aqueous solubility, instability, and low bioavailability (184). Consequently, nanomaterials have become a pivotal area of research dedicated to improving the efficacy of antioxidants. The utilization of nanotechnology presents a promising avenue for overcoming the limitations associated with conventional antioxidants, thereby exhibiting significant potential in the realm of antioxidant therapy. Antioxidant nanomaterials can be broadly categorized into two types: those with inherent antioxidant properties and antioxidant delivery nanomaterials. The first type includes nanomaterials that possess antioxidant properties independently, without the need for functionalization with antioxidants. The second type comprises nanomaterials that do not inherently have antioxidant properties but can be used to load and deliver antioxidants, thereby exerting antioxidant effects.
4.2.1 Nanomaterials with intrinsic antioxidant activity
Among antioxidant nanomaterials, several types possess inherent antioxidant properties, most of which are metal nanoparticles. These nanomaterials can mimic the efficacy of antioxidant enzymes like catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx). The antioxidant enzyme activity of nanomaterials is influenced by factors including size, morphology, surface modification, and composition (185). Catalase-like nanoenzymes, a category of nanomaterials with intrinsic CAT activity, operate by decomposing H2O2 into H2O and O2 (186). Superoxide radicals (O2∙–), a type of ROS generated during metabolic processes, are converted into H2O2 and O2 by SOD, using metal as a cofactor (187). GPx, the final antioxidant enzyme, catalyzes the reduction of H2O2 or organic hydrogen peroxide to H2O or alcohol in the presence of reduced glutathione (188). Cerium oxide nanoparticles (189) exhibit SOD-like activity, and their PEGylation can enhance the survival rate of keratinocytes while significantly reducing intracellular ROS levels. Cobalt oxide nanoparticles, synthesized via a one-pot method, demonstrate three enzymatic catalytic activities. These nanoparticles can protect keratinocytes from hydrogen peroxide-induced ROS and toxicity, alleviating the symptoms of AD (190). Furthermore, hematoxylin and eosin and toluidine blue staining indicated a decrease in epidermal thickness and a reduction in the number of mast cells in the treated group.
4.2.2 Antioxidant delivery nanomaterials
Most antioxidants have low bioavailability due to their inherent properties (191, 192). However, nanotechnology can enhance the antioxidant effect by preparing nanoparticles as carriers for these antioxidants, allowing for targeted and controlled release (193). Currently, a variety of antioxidant delivery systems have been developed to transport natural and synthetic antioxidants, antioxidant gases, genes, and other antioxidant compounds, thereby significantly expanding the scope of antioxidant delivery nanomaterials (194). Nanoparticles prepared with the natural polyphenol antioxidant hydroxytyrosol, hydrocortisone, and chitosan have shown significant improvement in the pathological characteristics of AD in mice. Compared to the AD group, the treatment group exhibited decreased expression levels of IgE, histamine, PGE2, VEGF-α, and AD-related Th1 and Th2 cytokines. Histological examination also demonstrated that HC-HT-CS-NPs exerted a therapeutic effect on AD (195). Additionally, HC-HT-CS-NPs demonstrated favorable safety in healthy individuals (196).
4.3 Hydrogels
Hydrogel is polymer material with a three-dimensional porous structure formed by physical or chemical crosslinking of polymer chains. Hydrogels exhibit notable hydrophilicity, enabling them to absorb water and biological fluids, and they possess excellent moisturizing and air permeability properties (197). Due to their high water content, structural similarity to natural tissues, and favorable biocompatibility, hydrogels are widely used in biomedical fields, particularly for drug delivery, facilitating controlled release and enhancing efficacy (198–200). Additionally, the physical and chemical properties of hydrogels can be easily modified to impart various functions, including antioxidant properties (201). Antioxidant hydrogels can be categorized into self-antioxidant hydrogels and those combined with antioxidant components (202).
These hydrogels have been applied in skin diseases and have shown promise as carriers for the local treatment of AD. Lignin, a polyphenol-containing substance extracted from lignocellulosic biomass, has the capability to scavenge ROS. Hydrogels prepared by crosslinking lignin with polyethylene glycol exhibit CAT and superoxide SOD enzyme-mimicking properties and possess antioxidant capacity (203). These hydrogels can treat AD by reducing skin oxidative stress. They protect HaCaT cells from oxidative stress damage caused by H2O2. In DNCB-induced AD mice, treatment with these hydrogels reduced dermatitis scores and epidermal thickness, inhibited inflammation, alleviated DNA oxidative damage, and decreased Th2 cytokine levels.
Furthermore, cerium oxide nanoparticles known for their high ROS scavenging ability, have been incorporated into hydrogels. By adjusting pH and crosslinking sodium alginate polymer with Ca2+, a CENP-sodium alginate hydrogel was prepared. This hydrogel effectively mimics CAT and SOD activity, protecting cells from oxidative stress damage. In AD mice, treatment with this hydrogel reduced epidermal thickness, decreased 8-OHdG accumulation, lowered Th2 cytokine and IgE levels, and reduced mast cell infiltration, showing its therapeutic potential for AD management (204).
4.4 Microneedles
The skin is composed of three primary layers: the epidermis, dermis, and subcutaneous tissue (205). The stratum corneum, an integral part of the skin barrier, is formed through the differentiation of keratinocytes and serves as the primary protective layer against external injury and stimulation (206). However, this physiological structure presents a challenge for transdermal drug delivery, limiting the bioavailability of drugs administered through the skin. Microneedles, ranging in length from 25 to 2,000 μm (207), offer a promising transdermal technology by piercing the stratum corneum and crossing the skin barrier to reach all layers of the skin. This technology has been widely used in the treatment of skin diseases, enabling the delivery of antioxidants into the skin or using the inherent antioxidant properties of the microneedles to address oxidative stress-related skin conditions (208–210).
In the treatment of AD, microneedles loaded with epigallocatechin gallate, a potent antioxidant, and L-ascorbic acid as a stabilizing reductant, prepared from poly-γ-glutamic acid, exhibit multiple beneficial functions. These microneedles improve DNCB-induced AD in mice through antioxidant, anti-inflammatory, and immunomodulatory effects (211). Treatment outcomes include reduced epidermal thickness, decreased mast cell infiltration, and lower levels of serum IgE and histamine. Microneedles incorporating natural polyphenols such as curcumin and gallic acid, prepared from PLGA/HA in a double-layer configuration, enable rapid treatment and long-term management of AD (212). The curcumin and gallic acid -loaded microneedles provide immediate antioxidant and anti-inflammatory effects, alleviating AD symptoms in the short term, while the embedded PLGA needle mediates the sustained release of curcumin for long-term improvement. Post-treatment, AD mice exhibited reduced dermatitis scores and improved pathological conditions, with a significant decrease in ROS levels in the lesional skin after 56 days. Additionally, a novel polydopamine nanozyme integrated with near-infrared-responsive microneedles was developed using natural dopamine. Hyaluronic acid was used for the backing layer, and hyaluronic acid methacrylate was employed for the tip, enabling antioxidant treatment of AD (213). PDA MNs + NIR treatment alleviated AD symptoms and inhibited Th2 immune-related reactions. Measurement of the DNA oxidative stress marker 8-OHdG revealed that the PDA MNs + NIR group significantly downregulated its levels.
5 Conclusion
Atopic dermatitis is a chronic inflammatory skin disorder characterized by disruptions in skin barrier integrity and immune dysregulation. The complex pathogenesis of AD involves oxidative stress, which induces several cellular damages in keratinocytes, impairs skin barrier function, and exacerbates the inflammatory response. This oxidative damage results to modifications of DNA, proteins, and lipids, culminating in the formation of various oxidation products. While several antioxidant strategies, such as the use of natural products, nanomaterials, hydrogels, and microneedles have shown promise in mitigating oxidative stress and alleviating AD symptoms, there remain knowledge gaps. Further research is required to fully elucidate the specific oxidative modifications underlying AD pathology, the precise mechanisms of action of antioxidant therapies, and the strategies for optimizing these treatments for clinical application. Addressing these gaps will be essential for developing more effective therapeutic strategies and monitoring tools to improve the management of AD.
Statements
Author contributions
YL: Writing – original draft, Writing – review and editing. JH: Conceptualization, Writing – review and editing. ZZ: Conceptualization, Writing – review and editing. YZ: Visualization, Writing – review and editing. YW: Funding acquisition, Supervision, Writing – review and editing. JS: Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Natural Science Foundation of Chongqing (CSTB2023NSCQ-MSX1081) and the National Natural Science Foundation of China (82304032).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Boguniewicz M Leung D . Atopic dermatitis: A disease of altered skin barrier and immune dysregulation.Immunol Rev. (2011) 242:233–46. 10.1111/j.1600-065X.2011.01027.x
2.
Laughter M Maymone M Mashayekhi S Arents B Karimkhani C Langan S et al The global burden of atopic dermatitis: Lessons from the global burden of disease study 1990–2017. Br J Dermatol. (2021) 184:304–9. 10.1111/bjd.19580
3.
Xiao H Gu X Huang Y Zhu W Shen M . Phototherapy for atopic dermatitis: Systematic review and network meta-analysis of randomized controlled trials.Photodermatol Photoimmunol Photomed. (2022) 38:233–40. 10.1111/phpp.12741
4.
Pezzolo E Naldi L . Epidemiology of major chronic inflammatory immune-related skin diseases in 2019.Exp Rev Clin Immunol. (2020) 16:155–66. 10.1080/1744666X.2020.1719833
5.
Mohd Kasim V Noble S Liew K Tan J Israf D Tham C . Management of atopic dermatitis via oral and topical administration of herbs in murine model: A systematic review.Front Pharmacol. (2022) 13:785782. 10.3389/fphar.2022.785782
6.
Jois S Andy-Nweye A Jungles K Tobin M Mahdavinia M . Type of food allergen is associated with atopic dermatitis severity.J Allergy Clin Immunol. (2019) 143:AB132. 10.1016/j.jaci.2018.12.400
7.
Amat F Soria A Tallon P Bourgoin-Heck M Lambert N Deschildre A et al New insights into the phenotypes of atopic dermatitis linked with allergies and asthma in children: An overview. Clin Exp Allergy. (2018) 48:919–34. 10.1111/cea.13156
8.
Knudgaard M Andreasen T Ravnborg N Bieber T Silverberg J Egeberg A et al Rhinitis prevalence and association with atopic dermatitis: A systematic review and meta-analysis. Ann Allergy Asthma Immunol. (2021) 127:49–56.e1. 10.1016/j.anai.2021.02.026
9.
Sliz E Huilaja L Pasanen A Laisk T Reimann E Mägi R et al Uniting biobank resources reveals novel genetic pathways modulating susceptibility for atopic dermatitis. J Allergy Clin Immunol. (2022) 149:1105–12.e9. 10.1016/j.jaci.2021.07.043
10.
Kantor R Silverberg J . Environmental risk factors and their role in the management of atopic dermatitis.Exp Rev Clin Immunol. (2017) 13:15–26. 10.1080/1744666X.2016.1212660
11.
Nolan S Archer N . From top to bottom: Staphylococci in atopic dermatitis.Cell Host Microbe. (2023) 31:573–5. 10.1016/j.chom.2023.03.014
12.
Sobkowiak P Langwiński W Nowakowska J Wojsyk-Banaszak I Szczepankiewicz D Jenerowicz D et al Neuroinflammatory gene expression pattern is similar between allergic rhinitis and atopic dermatitis but distinct from atopic asthma. BioMed Res Int. (2020) 2020:7196981. 10.1155/2020/7196981
13.
Tsakok T Woolf R Smith C Weidinger S Flohr C . Atopic dermatitis: The skin barrier and beyond.Br J Dermatol. (2019) 180:464–74. 10.1111/bjd.16934
14.
van Drongelen V Haisma E Out-Luiting J Nibbering P El Ghalbzouri A . Reduced filaggrin expression is accompanied by increased staphylococcus aureus colonization of epidermal skin models.Clin Exp Allergy. (2014) 44:1515–24. 10.1111/cea.12443
15.
Deng L Costa F Blake K Choi S Chandrabalan A Yousuf M et al S. aureus drives itch and scratch-induced skin damage through a V8 protease-par1 axis. Cell. (2023) 186:5375–93.e25. 10.1016/j.cell.2023.10.019
16.
Hoyer A Rehbinder E Färdig M Asad S Lødrup Carlsen K Endre K et al Filaggrin mutations in relation to skin barrier and atopic dermatitis in early infancy. Br J Dermatol. (2022) 186:544–52. 10.1111/bjd.20831
17.
Choi D Park J Choi J Piao M Suh M Lee J et al Keratinocytes-derived reactive oxygen species play an active role to induce Type 2 inflammation of the skin: A pathogenic role of reactive oxygen species at the early phase of atopic dermatitis. Ann Dermatol. (2021) 33:26–36. 10.5021/ad.2021.33.1.26
18.
Ni Q Zhang P Li Q Han Z . Oxidative stress and gut microbiome in inflammatory skin diseases.Front Cell Developmental Biol. (2022) 10:849985. 10.3389/fcell.2022.849985
19.
Corsini E Galbiati V Nikitovic D Tsatsakis A . Role of oxidative stress in chemical allergens induced skin cells activation.Food Chem Toxicol. (2013) 61:74–81. 10.1016/j.fct.2013.02.038
20.
Okayama Y . Oxidative stress in allergic and inflammatory skin diseases.Curr Drug Targets Inflammation Allergy. (2005) 4:517–9. 10.2174/1568010054526386
21.
Kim T Kim Y Jegal J Jo B Choi H Yang M . Haplopine ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice and Tnf-A/Ifn-Γ-induced inflammation in human keratinocyte.Antioxidants. (2021) 10:806. 10.3390/antiox10050806
22.
Chen P Chen C Su Y Chang W Kao W Yang C et al Associations between levels of urinary oxidative stress of 8-Ohdg and risk of atopic diseases in children. Int J Environ Res Public Health. (2020) 17:8207. 10.3390/ijerph17218207
23.
Nakai K Yoneda K Maeda R Munehiro A Fujita N Yokoi I et al Urinary biomarker of oxidative stress in patients with psoriasis vulgaris and atopic dermatitis. J Eur Acad Dermatol Venereol. (2009) 23:1405–8. 10.1111/j.1468-3083.2009.03327.x
24.
Shibama S Ugajin T Yamaguchi T Yokozeki H . Bilirubin oxidation derived from oxidative stress is associated with disease severity of atopic dermatitis in adults.Clin Exp Dermatol. (2019) 44:153–60. 10.1111/ced.13674
25.
McGarry T Biniecka M Veale D Fearon U . Hypoxia, oxidative stress and inflammation.Free Radic Biol Med. (2018) 125:15–24. 10.1016/j.freeradbiomed.2018.03.042
26.
Baek J Lee M . Oxidative stress and antioxidant strategies in dermatology.Redox Rep. (2016) 21:164–9. 10.1179/1351000215Y.0000000015
27.
da Silva Andrade R Suano de Souza FI Sanchez Aranda C Carvalho Mallozi M Cordeiro Ferreira A Lemos Neves Barreto T et al Antioxidant defense of children and adolescents with atopic dermatitis: Association with disease severity. Allergol Immunopathol. (2024) 52:65–70. 10.15586/aei.v52i1.933
28.
Bangash Y Saleem A Akhtar M Anwar F Akhtar B Sharif A et al Pterostilbene reduces the progression of atopic dermatitis via modulating inflammatory and oxidative stress biomarkers in mice. Inflammopharmacology. (2023) 31:1289–303. 10.1007/s10787-023-01214-z
29.
Siddique M Katas H Sarfraz M Chohan T Jamil A Mohd Amin M . Clinical insights into topically applied multipronged nanoparticles in subjects with atopic dermatitis.J Drug Delivery Sci Technol. (2021) 65:102744. 10.1016/j.jddst.2021.102744
30.
Chen X Wu Y Jia R Fang Y Cao K Yang X et al Antioxidant activity and the therapeutic effect of sinomenine hydrochloride-loaded liposomes-in-hydrogel on atopic dermatitis. Int J Mol Sci. (2024) 25:7676. 10.3390/ijms25147676
31.
Paleco R Vučen S Crean A Moore A Scalia S . Enhancement of the in vitro penetration of quercetin through pig skin by combined microneedles and lipid microparticles.Int J Pharm. (2014) 472:206–13. 10.1016/j.ijpharm.2014.06.010
32.
André-Lévigne D Modarressi A Pepper M Pittet-Cuénod B . Reactive oxygen species and nox enzymes are emerging as key players in cutaneous wound repair.Int J Mol Sci. (2017) 18:2149. 10.3390/ijms18102149
33.
Campanati A Bianchelli T Gesuita R Foti C Malara G Micali G et al Comorbidities and treatment patterns in adult patients with atopic dermatitis: Results from a nationwide multicenter study. Arch Dermatol Res. (2022) 314:593–603. 10.1007/s00403-021-02243-w
34.
Tintle S Shemer A Suárez-Fariñas M Fujita H Gilleaudeau P Sullivan-Whalen M et al Reversal of atopic dermatitis with narrow-band uvb phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. (2011) 128:583–93.e4. 10.1016/j.jaci.2011.05.042
35.
Das P Mounika P Yellurkar M Prasanna V Sarkar S Velayutham R et al Keratinocytes: An enigmatic factor in atopic dermatitis. Cells. (2022) 11:1683. 10.3390/cells11101683
36.
Briganti S Picardo M . Antioxidant activity, lipid peroxidation and skin diseases. What’s new.J Eur Acad Dermatol Venereol. (2003) 17:663–9. 10.1046/j.1468-3083.2003.00751.x
37.
Nakai K Tsuruta D . What are reactive oxygen species, free radicals, and oxidative stress in skin diseases?Int J Mol Sci. (2021) 22:10799. 10.3390/ijms221910799
38.
Hebert A . Oxidative stress as a treatment target in atopic dermatitis: The Role of Furfuryl palmitate in mild-to-moderate atopic dermatitis.Int J Women’s Dermatol. (2020) 6:331–3. 10.1016/j.ijwd.2020.03.042
39.
Sapuntsova S Lebed’ko O Shchetkina M Fleyshman M Kozulin E Timoshin S . Status of free-radical oxidation and proliferation processes in patients with atopic dermatitis and lichen planus.Bull Exp Biol Med. (2011) 150:690–2. 10.1007/s10517-011-1224-0
40.
Furue M . Regulation of skin barrier function via competition between Ahr axis versus Il-13/Il-4–Jak–Stat6/Stat3 axis: Pathogenic and therapeutic implications in atopic dermatitis.J Clin Med. (2020) 9:3741. 10.3390/jcm9113741
41.
Focken J Scheurer J Jäger A Schürch C Kämereit S Riel S et al Neutrophil extracellular traps Enhance S. Aureus skin colonization by oxidative stress induction and downregulation of epidermal barrier genes. Cell Rep. (2023) 42:113148. 10.1016/j.celrep.2023.113148
42.
Sticozzi C Belmonte G Pecorelli A Arezzini B Gardi C Maioli E et al Cigarette smoke affects keratinocytes Srb1 expression and localization via H2o2 production and hne protein adducts formation. PLoS One. (2012) 7:e33592. 10.1371/journal.pone.0033592
43.
Mittal M Siddiqui M Tran K Reddy S Malik A . Reactive oxygen species in inflammation and tissue injury.Antioxidants Redox Signaling. (2013) 20:1126–67. 10.1089/ars.2012.5149
44.
Yao W Chang J Sehra S Travers J Chang C Tepper R et al Altered cytokine production by dendritic cells from infants with atopic dermatitis. Clin Immunol. (2010) 137:406–14. 10.1016/j.clim.2010.09.001
45.
Bertino L Guarneri F Cannavò S Casciaro M Pioggia G Gangemi S . Oxidative stress and atopic dermatitis.Antioxidants. (2020) 9:196. 10.3390/antiox9030196
46.
Borgia F Custurone P Peterle L Pioggia G Gangemi S . Role of epithelium-derived cytokines in atopic dermatitis and psoriasis: Evidence and therapeutic perspectives.Biomolecules. (2021) 11:1843. 10.3390/biom11121843
47.
Huang Y Zhao C Zheng G Yuan Y Gong L Liu R et al Dictamnine ameliorates Dnfb-induced atopic dermatitis like skin lesions in mice by inhibiting M1 macrophage polarization and promoting autophagy. Biol Pharm Bull. (2024) 47:175–86. 10.1248/bpb.b23-00436
48.
Furue M Hashimoto-Hachiya A Tsuji G . Aryl hydrocarbon receptor in atopic dermatitis and psoriasis.Int J Mol Sci. (2019) 20:5424. 10.3390/ijms20215424
49.
Yan C Ying J Lu W Changzhi Y Qihong Q Jingzhu M et al Mir-1294 suppresses ros-dependent inflammatory response in atopic dermatitis via restraining Stat3/Nf-Kb pathway. Cell Immunol. (2022) 371:104452. 10.1016/j.cellimm.2021.104452
50.
Tsuboi H Kouda K Takeuchi H Takigawa M Masamoto Y Takeuchi M et al 8-Hydroxydeoxyguanosine in urine as an index of oxidative damage to DNA in the evaluation of atopic dermatitis. Br J Dermatol. (1998) 138:1033–5. 10.1046/j.1365-2133.1998.02273.x
51.
Omata N Tsukahara H Ito S Ohshima Y Yasutomi M Yamada A et al Increased oxidative stress in childhood atopic dermatitis. Life Sci. (2001) 69:223–8. 10.1016/S0024-3205(01)01124-9
52.
Galiniak S Mołoń M Biesiadecki M Bożek A Rachel M . The Role of oxidative stress in atopic dermatitis and chronic urticaria.Antioxidants (2022) 11:1590. 10.3390/antiox11081590
53.
Hong J Kim M Hong J Noh H Park K Lee M et al In vivo quantitative analysis of advanced glycation end products in atopic dermatitis-possible culprit for the comorbidities? Exp Dermatol. (2020) 29:1012–6. 10.1111/exd.14167
54.
Tsukahara H Shibata R Ohta N Sato S Hiraoka M Ito S et al High levels of urinary pentosidine, an advanced glycation end product, in children with acute exacerbation of atopic dermatitis: Relationship with oxidative stress. Metab Clin Exp. (2003) 52:1601–5. 10.1016/s0026-0495(03)00310-x
55.
Niwa Y Sumi H Kawahira K Terashima T Nakamura T Akamatsu H . Protein oxidative damage in the stratum corneum: Evidence for a link between environmental oxidants and the changing prevalence and nature of atopic dermatitis in Japan.Br J Dermatol. (2003) 149:248–54. 10.1046/j.1365-2133.2003.05417.x
56.
Oxford Academic. O07: Carbonylated proteins as markers of oxidative stress and their association with filaggrin genotype in atopic eczema. Br J Dermatol. (2022) 187:14. 10.1111/bjd.21093
57.
Sivaranjani N Rao S Rajeev G . Role of reactive oxygen species and antioxidants in atopic dermatitis.J Clin Diagnostic Res. (2013) 7:2683–5. 10.7860/jcdr/2013/6635.3732
58.
Amin M Liza K Sarwar M Ahmed J Adnan M Chowdhury M et al Effect of lipid peroxidation, antioxidants, macro minerals and trace elements on Eczema. Arch Dermatol Res. (2015) 307:617–23. 10.1007/s00403-015-1570-2
59.
Devadasan S Sarkar R Barman K Kaushik S . Role of serum melatonin and oxidative stress in childhood atopic dermatitis: A prospective study.Indian Dermatol Online J. (2020) 11:925–9. 10.4103/idoj.IDOJ_77_20
60.
Peroni D Bodini A Corradi M Coghi A Boner A Piacentini G . Markers of oxidative stress are increased in exhaled breath condensates of children with atopic dermatitis.Br J Dermatol. (2012) 166:839–43. 10.1111/j.1365-2133.2011.10771.x
61.
Hanusch B Sinningen K Brinkmann F Dillenhöfer S Frank M Jöckel K et al Characterization of the L-Arginine/Nitric oxide pathway and oxidative stress in pediatric patients with atopic diseases. Int J Mol Sci. (2022) 23:2136. 10.3390/ijms23042136
62.
Taniuchi S Kojima T Hara MK Yamamoto A Sasai M Takahashi H et al Increased serum nitrate levels in infants with atopic dermatitis. Allergy. (2001) 56:693–5. 10.1034/j.1398-9995.2001.00131.x
63.
Karacan G Ercan N Bostanci I Alisik M Erel OA . Novel oxidative stress marker of atopic dermatitis in infants: Thiol-disulfide balance.Arch Dermatol Res. (2020) 312:697–703. 10.1007/s00403-020-02054-5
64.
Uysal P Avcil S Neşelioğlu S Biçer C Çatal F . Association of oxidative stress and dynamic thiol-disulphide homeostasis with atopic dermatitis severity and chronicity in children: A prospective study.Clin Exp Dermatol. (2018) 43:124–30. 10.1111/ced.13219
65.
Tsukahara H Shibata R Ohshima Y Todoroki Y Sato S Ohta N et al Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci. (2003) 72:2509–16. 10.1016/s0024-3205(03)00145-0
66.
Raimondo A Serio B Lembo S . Oxidative stress in atopic dermatitis and possible biomarkers: Present and future.Indian J Dermatol. (2023) 68:657–60. 10.4103/ijd.ijd_878_22
67.
Tsukahara H Hiraoka M Kobata R Hata I Ohshima Y Jiang M et al Increased oxidative stress in rats with chronic nitric oxide depletion: Measurement of urinary 8-Hydroxy-2’-deoxyguanosine excretion. Redox Rep. Commun Free Radic Res. (2000) 5:23–8. 10.1179/rer.2000.5.1.23
68.
Graille M Wild P Sauvain J Hemmendinger M Guseva Canu I Hopf N . Urinary 8-Ohdg as a biomarker for oxidative stress: A systematic literature review and meta-analysis.Int J Mol Sci. (2020) 21:3743. 10.3390/ijms21113743
69.
Shimamoto J Kurokawa T Tanizaki H Moriwaki S . The evaluation of oxidative stress in patients with psoriasis vulgaris and atopic dermatitis by measuring the urinary level of 8-Hydroxy-2′-Deoxyguanosine.J Cutaneous Immunol Allergy. (2019) 2:163–8. 10.1002/cia2.12088
70.
Alyoussef A . Attenuation of experimentally induced atopic dermatitis in mice by sulforaphane: Effect on inflammation and apoptosis.Toxicol Mech Methods. (2022) 32:224–32. 10.1080/15376516.2021.1994076
71.
Ahmad J Cooke M Hussieni A Evans M Patel K Burd R et al Urinary thymine dimers and 8-Oxo-2′-Deoxyguanosine in psoriasis. FEBS Lett. (1999) 460:549–53. 10.1016/S0014-5793(99)01402-7
72.
Colombo G Clerici M Giustarini D Portinaro N Badalamenti S Rossi R et al A central role for intermolecular dityrosine cross-linking of fibrinogen in high molecular weight advanced oxidation protein product (Aopp) formation. Biochim Biophys Acta (2015) 1850:1–12. 10.1016/j.bbagen.2014.09.024
73.
Cao W Hou F Nie J . Aopps and the progression of kidney disease.Kidney Int Supplements. (2014) 4:102–6. 10.1038/kisup.2014.19
74.
Rodríguez-Ayala E Anderstam B Suliman M Seeberger A Heimbürger O Lindholm B et al Enhanced rage-mediated nfκb stimulation in inflamed hemodialysis patients. Atherosclerosis. (2005) 180:333–40. 10.1016/j.atherosclerosis.2004.12.007
75.
Selmeci L . Advanced oxidation protein products (Aopp): Novel uremic toxins, or components of the non-enzymatic antioxidant system of the plasma proteome?Free Radic Res. (2011) 45:1115–23. 10.3109/10715762.2011.602074
76.
Selmeci L Székely M Soós P Seres L Klinga N Geiger A et al Human blood plasma advanced oxidation protein products (Aopp) correlates with fibrinogen levels. Free Radic Res. (2006) 40:952–8. 10.1080/10715760600818789
77.
Witko-Sarsat V Friedlander M Capeillère-Blandin C Nguyen-Khoa T Nguyen A Zingraff J et al Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. (1996) 49:1304–13. 10.1038/ki.1996.186
78.
Witko-Sarsat V Gausson V Nguyen A Touam M Drüeke T Santangelo F et al Aopp-Induced activation of human neutrophil and monocyte oxidative metabolism: A potential target for N-Acetylcysteine treatment in dialysis patients. Kidney Int. (2003) 64:82–91. 10.1046/j.1523-1755.2003.00044.x
79.
Witko-Sarsat V Friedlander M Nguyen Khoa T Capeillère-Blandin C Nguyen A Canteloup S et al Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. (1998) 161:2524–32. 10.4049/jimmunol.161.5.2524
80.
Mert H Açikkol S Çalli İ Çibuk S Keskin S Mert N . Advanced oxidation protein product (Aopp) Levels in second- and third-degree thermal burns.J Burn Care Res. (2021) 42:207–11. 10.1093/jbcr/iraa127
81.
Gupta R Gupta K Sharma A Das M Ansari I Dwivedi P . Maillard reaction in food allergy: Pros and cons.Crit Rev Food Sci Nutr. (2018) 58:208–26. 10.1080/10408398.2016.1152949
82.
Song Q Liu J Dong L Wang X Zhang X . Novel advances in inhibiting advanced glycation end product formation using natural compounds.Biomed Pharmacother. (2021) 140:111750. 10.1016/j.biopha.2021.111750
83.
Koetsier M Lutgers H de Jonge C Links T Smit A Graaff R . Reference values of skin autofluorescence.Diabetes Technol Therapeutics. (2010) 12:399–403. 10.1089/dia.2009.0113
84.
Chen C Zhang J Li L Guo M He Y Dong Y et al Advanced glycation end products in the skin: Molecular mechanisms, methods of measurement, and inhibitory pathways. Front Med. (2022) 9:837222. 10.3389/fmed.2022.837222
85.
Quadros K Roza N França R Esteves A Barreto J Dominguez W et al Advanced glycation end products and bone metabolism in patients with chronic kidney disease. JBMR Plus. (2023) 7:e10727. 10.1002/jbm4.10727
86.
Smith P Masilamani M Li X Sampson H . The false alarm hypothesis: Food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins.J Allergy Clin Immunol. (2017) 139:429–37. 10.1016/j.jaci.2016.05.040
87.
Roe M Xie H Bandhakavi S Griffin T . Proteomic mapping of 4-hydroxynonenal protein modification sites by solid-phase hydrazide chemistry and mass spectrometry.Anal Chem. (2007) 79:3747–56. 10.1021/ac0617971
88.
Estévez M Díaz-Velasco S Martínez R . Protein carbonylation in food and nutrition: A concise update.Amino Acids. (2022) 54:559–73. 10.1007/s00726-021-03085-6
89.
Nyström T . Role of oxidative carbonylation in protein quality control and senescence.EMBO J. (2005) 24:1311–7. 10.1038/sj.emboj.7600599
90.
Fedorova M Bollineni R Hoffmann R . Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies.Mass Spectrometry Rev. (2014) 33:79–97. 10.1002/mas.21381
91.
Akagawa M . Protein carbonylation: Molecular mechanisms, biological implications, and analytical approaches.Free Radical Res. (2021) 55:307–20. 10.1080/10715762.2020.1851027
92.
Dalle-Donne I Rossi R Giustarini D Milzani A Colombo R . Protein carbonyl groups as biomarkers of oxidative stress.Clin Chim Acta Int J Clin Chem. (2003) 329:23–38. 10.1016/s0009-8981(03)00003-2
93.
Madian A Regnier F . Proteomic identification of carbonylated proteins and their oxidation sites.J Proteome Res. (2010) 9:3766–80. 10.1021/pr1002609
94.
Gonos E Kapetanou M Sereikaite J Bartosz G Naparło K Grzesik M et al Origin and pathophysiology of protein carbonylation, nitration and chlorination in age-related brain diseases and aging. Aging. (2018) 10:868–901. 10.18632/aging.101450
95.
Foell D Wittkowski H Roth J . Mechanisms of disease: A ‘Damp’ view of inflammatory arthritis.Nat Clin Pract Rheumatol. (2007) 3:382–90. 10.1038/ncprheum0531
96.
Mititelu R Pădureanu R Băcănoiu M Pădureanu V Docea A Calina D et al Inflammatory and oxidative stress markers-mirror tools in rheumatoid arthritis. Biomedicines. (2020) 8:125. 10.3390/biomedicines8050125
97.
Iwai I Shimadzu K Kobayashi Y Hirao T Etou T . Increased carbonyl protein level in the stratum corneum of inflammatory skin disorders: A Non-invasive approach.J Dermatol. (2010) 37:693–8. 10.1111/j.1346-8138.2010.00867.x
98.
Yamawaki Y Mizutani T Okano Y Masaki H . the impact of carbonylated proteins on the skin and potential agents to block their effects.Exp Dermatol. (2019) 28:32–7. 10.1111/exd.13821
99.
Busch C Binder C . Malondialdehyde epitopes as mediators of sterile inflammation.Biochim Biophys Acta Mol Cell Biol Lipids. (2017) 1862:398–406. 10.1016/j.bbalip.2016.06.016
100.
Cordiano R Di Gioacchino M Mangifesta R Panzera C Gangemi S Minciullo P . Malondialdehyde as a potential oxidative stress marker for allergy-oriented diseases: An update.Molecules (Basel, Switzerland). (2023) 28:5979. 10.3390/molecules28165979
101.
Del Rio D Stewart A Pellegrini NA . Review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress.Nutr Metab Cardiovasc Dis. (2005) 15:316–28. 10.1016/j.numecd.2005.05.003
102.
Ayala A Muñoz M Argüelles S . Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal.Oxidative Med Cell Longevity. (2014) 2014:360438. 10.1155/2014/360438
103.
Liu C Cheng X Wu Y Xu W Xia H Jia R et al Antioxidant activity of quercetin-containing liposomes-in-gel and its effect on prevention and treatment of cutaneous eczema. Pharmaceuticals (Basel, Switzerland). (2023) 16:84. 10.3390/ph16081184
104.
Dix T Aikens J . Mechanisms and biological relevance of lipid peroxidation initiation.Chem Res Toxicol. (1993) 6:2–18. 10.1021/tx00031a001
105.
Akagawa M Ito S Toyoda K Ishii Y Tatsuda E Shibata T et al Bispecific Abs against modified protein and DNA with oxidized lipids. Proc Natl Acad Sci U S A. (2006) 103:6160–5. 10.1073/pnas.0600865103
106.
Li Y Zhao T Li J Xia M Li Y Wang X et al Oxidative stress and 4-Hydroxy-2-Nonenal (4-Hne): Implications in the pathogenesis and treatment of aging-related diseases. J Immunol Res. (2022) 2022:2233906. 10.1155/2022/2233906
107.
Schaur R Siems W Bresgen N Eckl P . 4-Hydroxy-nonenal-a bioactive lipid peroxidation product.Biomolecules. (2015) 5:2247–337. 10.3390/biom5042247
108.
Reyes-Jiménez E Ramírez-Hernández A Santos-Álvarez J Velázquez-Enríquez J Pina-Canseco S Baltiérrez-Hoyos R et al Involvement of 4-Hydroxy-2-nonenal in the pathogenesis of pulmonary fibrosis. Mol Cell Biochem. (2021) 476:4405–19. 10.1007/s11010-021-04244-9
109.
Mustafa A Alfaqih M Al-Shboul O . The 4-Hydroxynonenal mediated oxidative damage of blood proteins and lipids involves secondary lipid peroxidation reactions.Exp Therapeutic Med. (2018) 16:2132–7. 10.3892/etm.2018.6419
110.
Spickett C . The lipid peroxidation product 4-Hydroxy-2-nonenal: Advances in chemistry and analysis.Redox Biol. (2013) 1:145–52. 10.1016/j.redox.2013.01.007
111.
Zarkovic N Zarkovic K Milkovic L Andrisic L Borovic S Waeg G et al Revealing 4-Hydroxynonenal-Histidine adducts as qualitative and quantitative biomarker of oxidative stress, lipid peroxidation and oxidative homeostasis. Free Radical Biol Med. (2013) 65:S14–5. 10.1016/j.freeradbiomed.2013.08.139
112.
Seo Y Park J Kim J Lee M . Cigarette smoke-induced reactive oxygen species formation: A concise review.Antioxidants. (2023) 12:1732. 10.3390/antiox12091732
113.
Avezov K Reznick A Aizenbud D . Oxidative damage in keratinocytes exposed to cigarette smoke and aldehydes.Toxicol Vitro. (2014) 28:485–91. 10.1016/j.tiv.2014.01.004
114.
Lancaster JRA . Tutorial on the diffusibility and reactivity of free nitric oxide.Nitric Oxide. (1997) 1:18–30. 10.1006/niox.1996.0112
115.
Lind M Hayes A Caprnda M Petrovic D Rodrigo L Kruzliak P et al Inducible nitric oxide synthase: Good or bad? Biomed Pharmacother. (2017) 93:370–5. 10.1016/j.biopha.2017.06.036
116.
Kapil V Khambata R Jones D Rathod K Primus C Massimo G et al The noncanonical pathway for in vivo nitric oxide generation: The nitrate-nitrite-nitric oxide pathway. Pharmacol Rev. (2020) 72:692. 10.1124/pr.120.019240
117.
Lundberg J Weitzberg E . Nitric oxide signaling in health and disease.Cell. (2022) 185:2853–78. 10.1016/j.cell.2022.06.010
118.
Oliver S Pham T Li Y Xu F Boyer C . More than skin deep: Using polymers to facilitate topical delivery of nitric oxide.Biomaterials Sci. (2021) 9:391–405. 10.1039/D0BM01197E
119.
Man M Wakefield J Mauro T Elias P . Regulatory role of nitric oxide in cutaneous inflammation.Inflammation. (2022) 45:949–64. 10.1007/s10753-021-01615-8
120.
Ormerod A Weller R Copeland P Benjamin N Ralston S Grabowksi P et al Detection of nitric oxide and nitric oxide synthases in psoriasis. Arch Dermatol Res. (1998) 290:3–8. 10.1007/s004030050268
121.
Wang L Xie X Ke B Huang W Jiang X He G . Recent advances on endogenous gasotransmitters in inflammatory dermatological disorders.J Adv Res. (2022) 38:261–74. 10.1016/j.jare.2021.08.012
122.
Yu L Li L . Potential biomarkers of atopic dermatitis.Front Med. (2022) 9:1028694. 10.3389/fmed.2022.1028694
123.
Thomas D Liu X Kantrow S Lancaster J . The biological lifetime of nitric oxide: Implications for the perivascular dynamics of No and O2.Proc Natl Acad Sci U S A. (2001) 98:355–60. 10.1073/pnas.98.1.355
124.
Hakim T Sugimori K Camporesi E Anderson G . Half-life of nitric oxide in aqueous solutions with and without haemoglobin.Physiol Meas. (1996) 17:267–77. 10.1088/0967-3334/17/4/004
125.
Umbrello M Dyson A Pinto B Fernandez B Simon V Feelisch M et al Short-term hypoxic vasodilation in vivo is mediated by bioactive nitric oxide metabolites, rather than free nitric oxide derived from haemoglobin-mediated nitrite reduction. J Physiol. (2014) 592:1061–75. 10.1113/jphysiol.2013.255687
126.
Erel O Neselioglu SA . Novel and automated assay for thiol/disulphide homeostasis.Clin Biochem. (2014) 47:326–32. 10.1016/j.clinbiochem.2014.09.026
127.
Turell L Radi R Alvarez B . The thiol pool in human plasma: The central contribution of albumin to redox processes.Free Radic Biol Med. (2013) 65:244–53. 10.1016/j.freeradbiomed.2013.05.050
128.
Cremers C Jakob U . Oxidant sensing by reversible disulfide bond formation.J Biol Chem. (2013) 288:26489–96. 10.1074/jbc.R113.462929
129.
Georgescu S Mitran C Mitran M Matei C Popa G Erel O et al Thiol-disulfide homeostasis in skin diseases. J Clin Med. (2022) 11:1507. 10.3390/jcm11061507
130.
Jones D Liang Y . Measuring the poise of Thiol/Disulfide couples in vivo.Free Radic Biol Med. (2009) 47:1329–38. 10.1016/j.freeradbiomed.2009.08.021
131.
Stocker R Yamamoto Y McDonagh A Glazer A Ames B . Bilirubin is an antioxidant of possible physiological importance.Science. (1987) 235:1043–6. 10.1126/science.3029864
132.
Yamaguchi T Shioji I Sugimoto A Komoda Y Nakajima H . Chemical structure of a new family of bile pigments from human urine.J Biochem. (1994) 116:298–303. 10.1093/oxfordjournals.jbchem.a124523
133.
Shimizu S Izumi Y Yamazaki M Shimizu K Yamaguchi T Nakajima H . Anti-bilirubin monoclonal antibody. I. preparation and properties of monoclonal antibodies to covalently coupled bilirubin-albumin.Biochim Biophys Acta. (1988) 967:255–60. 10.1016/0304-4165(88)90017-7
134.
Yamaguchi T Shioji I Sugimoto A Komoda Y Nakajima H . Epitope of 24g7 anti-bilirubin monoclonal antibody.Biochim Biophys Acta. (1996) 1289:110–4. 10.1016/0304-4165(95)00128-x
135.
Chiricozzi A Comberiati P D’Auria E Zuccotti G Peroni D . Topical corticosteroids for pediatric atopic dermatitis: Thoughtful tips for practice.Pharmacol Res. (2020) 158:104878. 10.1016/j.phrs.2020.104878
136.
Carr W . Topical calcineurin inhibitors for atopic dermatitis: Review and treatment recommendations.Pediatric Drugs. (2013) 15:303–10. 10.1007/s40272-013-0013-9
137.
Akhavan A Rudikoff D . The treatment of atopic dermatitis with systemic immunosuppressive agents.Clin Dermatol. (2003) 21:225–40. 10.1016/S0738-081X(02)00362-0
138.
Puar N Chovatiya R Paller A . New treatments in atopic dermatitis.Ann Allergy Asthma Immunol. (2021) 126:21–31. 10.1016/j.anai.2020.08.016
139.
Oroian M Escriche I . Antioxidants: Characterization, natural sources, extraction and analysis.Food Res Int. (2015) 74:10–36. 10.1016/j.foodres.2015.04.018
140.
Akbari B Baghaei-Yazdi N Bahmaie M Mahdavi Abhari F . The role of plant-derived natural antioxidants in reduction of oxidative stress.BioFactors. (2022) 48:611–33. 10.1002/biof.1831
141.
Chen L Teng H Xie Z Cao H Cheang W Skalicka-Woniak K et al Modifications of dietary flavonoids towards improved bioactivity: An update on structure-activity relationship. Crit Rev Food Sci Nutr. (2018) 58:513–27. 10.1080/10408398.2016.1196334
142.
Kohlert C van Rensen I März R Schindler G Graefe E Veit M . Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans.Planta Medica. (2000) 66:495–505. 10.1055/s-2000-8616
143.
Rahaman M Hossain R Herrera-Bravo J Islam M Atolani O Adeyemi O et al Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci Nutr. (2023) 11:1657–70. 10.1002/fsn3.3217
144.
Koekkoek W van Zanten A . Antioxidant vitamins and trace elements in critical illness.Nutr Clin Pract. (2016) 31:457–74. 10.1177/0884533616653832
145.
Amir Aslani B Ghobadi S . Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system.Life Sci. (2016) 146:163–73. 10.1016/j.lfs.2016.01.014
146.
Nwozo O Effiong E Aja P Awuchi C . Antioxidant, phytochemical, and therapeutic properties of medicinal plants: A review.Int J Food Properties. (2023) 26:359–88. 10.1080/10942912.2022.2157425
147.
Neha K Haider M Pathak A Yar M . Medicinal prospects of antioxidants: A review.Eur J Med Chem. (2019) 178:687–704. 10.1016/j.ejmech.2019.06.010
148.
Vo T Chu P Tuan V Te J Lee I . The promising role of antioxidant phytochemicals in the prevention and treatment of periodontal disease via the inhibition of oxidative stress pathways: Updated insights.Antioxidants. (2020) 9:1211. 10.3390/antiox9121211
149.
Chaudhary P Janmeda P Docea A Yeskaliyeva B Abdull Razis A Modu B et al Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front Chem. (2023) 11:1158198. 10.3389/fchem.2023.1158198
150.
Sgouros D Katoulis A Rigopoulos D . Novel topical agent containing superoxide dismutase 100 000 iu and 4% of plant extracts as a mono-therapy for atopic dermatitis.J Cosmetic Dermatol. (2018) 17:1069–72. 10.1111/jocd.12462
151.
Lin M Hung C Sung H Yang S Yu H Fang J . The bioactivities of resveratrol and its naturally occurring derivatives on skin.J Food Drug Analy. (2021) 29:15–38. 10.38212/2224-6614.1151
152.
Marko M Pawliczak R . Resveratrol and its derivatives in inflammatory skin disorders-atopic dermatitis and psoriasis: A review.Antioxidants. (2023) 12:1954. 10.3390/antiox12111954
153.
Shen Y Xu J. *Resveratrol exerts therapeutic effects on mice with atopic dermatitis. Wounds Compendium Clin Res Pract. (2019) 31:279–84.
154.
Wagemaker T Maia Campos P Shimizu K Kyotani D Yoshida D . Antioxidant-based topical formulations influence on the inflammatory response of Japanese skin: A clinical study using non-invasive techniques.Eur J Pharm Biopharm. (2017) 117:195–202. 10.1016/j.ejpb.2017.03.025
155.
Choi E Park Z Kim E . Chemical composition and inhibitory effect of lentinula edodes ethanolic extract on experimentally induced atopic dermatitis in vitro and in vivo.Molecules (Basel, Switzerland). (2016) 21:993. 10.3390/molecules21080993
156.
Lee J Lim J Jeon Y Yun D Lee Y Kim D . Extract of wheatgrass and aronia mixture ameliorates atopic dermatitis-related symptoms by suppressing inflammatory response and oxidative stress in vitro and in vivo.Antioxidants. (2022) 12:27. 10.3390/antiox12010027
157.
Quah Y Lee S Lee E Birhanu B Ali M Abbas M et al Cornus officinalis ethanolic extract with potential anti-allergic, anti-inflammatory, and antioxidant activities. Nutrients. (2020) 12:3317. 10.3390/nu12113317
158.
Ofoedu C Iwouno J Ofoedu E Ogueke C Igwe V Agunwah I et al Revisiting food-sourced vitamins for consumer diet and health needs: A perspective review, from vitamin classification, metabolic functions, absorption, utilization, to balancing nutritional requirements. PeerJ. (2021) 9:e11940. 10.7717/peerj.11940
159.
Didier A Stiene J Fang L Watkins D Dworkin L Creeden J . Antioxidant and anti-tumor effects of dietary vitamins a, C, and E.Antioxidants. (2023) 12:632. 10.3390/antiox12030632
160.
Wimalawansa S . Vitamin D deficiency: Effects on oxidative stress, epigenetics, gene regulation, and aging.Biology. (2019) 8:30. 10.3390/biology8020030
161.
Uzunçakmak TKÜ Altunkalem N Tüzün Y . Vitamin deficiencies/hypervitaminosis and the skin.Clin Dermatol. (2021) 39:847–57. 10.1016/j.clindermatol.2021.05.010
162.
Kake T Imai M Takahashi N . Effects of B-carotene on oxazolone-induced atopic dermatitis in hairless mice.Exp Dermatol. (2019) 28:1044–50. 10.1111/exd.14003
163.
Ponec M Weerheim A Kempenaar J Mulder A Gooris G Bouwstra J et al The Formation of competent barrier lipids in reconstructed human epidermis requires the presence of Vitamin C. J Invest Dermatol. (1997) 109:348–55. 10.1111/1523-1747.ep12336024
164.
Plevnik Kapun A Salobir J Levart A Tavčar Kalcher G Nemec Svete A Kotnik T . Vitamin E supplementation in canine atopic dermatitis: Improvement of clinical signs and effects on oxidative stress markers.Veterinary Rec. (2014) 175:560. 10.1136/vr.102547
165.
Tsoureli-Nikita E Hercogova J Lotti T Menchini G . Evaluation of dietary intake of Vitamin E in the treatment of atopic dermatitis: A study of the clinical course and evaluation of the immunoglobulin E serum levels.Int J Dermatol. (2002) 41:146–50. 10.1046/j.1365-4362.2002.01423.x
166.
Fioranelli M Roccia M Di Nardo V Aracena C Lotti T . Vitamin D supplementation for childhood atopic dermatitis.Dermatol Ther. (2016) 29:303. 10.1111/dth.12341
167.
Kotnik T . Vitamin D therapy in canine atopic dermatitis.Vet Rec. (2018) 182:403–5. 10.1136/vr.k1559
168.
Javanbakht M Keshavarz S Djalali M Siassi F Eshraghian M Firooz A et al Randomized controlled trial using Vitamins E and D supplementation in atopic dermatitis. J Dermatol Treatment. (2011) 22:144–50. 10.3109/09546630903578566
169.
Oh S Chung J Kim M Kwon S Cho B . Antioxidant nutrient intakes and corresponding biomarkers associated with the risk of atopic dermatitis in young children.Eur J Clin Nutr. (2010) 64:245–52. 10.1038/ejcn.2009.148
170.
Hoppu U Rinne M Salo-Väänänen P Lampi A Piironen V Isolauri E . Vitamin C in breast milk may reduce the risk of atopy in the infant.Eur J Clin Nutr. (2005) 59:123–8. 10.1038/sj.ejcn.1602048
171.
Islam M Akash S Jony M Alam MN Nowrin FT Rahman MM et al Exploring the potential function of trace elements in human health: A therapeutic perspective. Mol Cell Biochem. (2023) 478:2141–71. 10.1007/s11010-022-04638-3
172.
Rayman M . The importance of selenium to human health.Lancet (London, England). (2000) 356:233–41. 10.1016/s0140-6736(00)02490-9
173.
Steinbrenner H Speckmann B Klotz L . Selenoproteins: Antioxidant selenoenzymes and beyond.Arch Biochem Biophys. (2016) 595:113–9. 10.1016/j.abb.2015.06.024
174.
Arbiser J Bonner M Ward N Elsey J Rao S . Selenium unmasks protective iron armor: A possible defense against cutaneous inflammation and cancer.Biochim Biophys Acta General Sub. (2018) 1862:2518–27. 10.1016/j.bbagen.2018.05.018
175.
Xiao W Zhu Y Sarsour E Kalen A Aykin-Burns N Spitz D et al Selenoprotein P regulates 1-(4-Chlorophenyl)-Benzo-2,5-quinone-induced oxidative stress and toxicity in human keratinocytes. Free Radic Biol Med. (2013) 65:70–7. 10.1016/j.freeradbiomed.2013.06.010
176.
Fairris G Perkins P Lloyd B Hinks L Clayton B. *The effect on atopic dermatitis of supplementation with selenium and Vitamin E. Acta Dermato-Venereol. (1989) 69:359–62. 10.2340/0001555569359362
177.
Schwartz J Marsh R Draelos Z . Zinc and skin health: Overview of physiology and pharmacology.Dermatol Surg. (2005) 31:837–47. 10.1111/j.1524-4725.2005.31729
178.
Chasapis C Ntoupa P Spiliopoulou C Stefanidou M . Recent aspects of the effects of zinc on human health.Arch Toxicol. (2020) 94:1443–60. 10.1007/s00204-020-02702-9
179.
Kloubert V Rink L . Zinc as a micronutrient and its preventive role of oxidative damage in cells.Food Funct. (2015) 6:3195–204. 10.1039/C5FO00630A
180.
Kim J Yoo S Jeong M Ko J Ro Y . Hair zinc levels and the efficacy of oral zinc supplementation in patients with atopic dermatitis.Acta Dermato-Venereol. (2014) 94:558–62. 10.2340/00015555-1772
181.
Sugiura T Goto K Ito K Ueta A Fujimoto S Togari H . Chronic zinc toxicity in an infant who received zinc therapy for atopic dermatitis.Acta Paediatrica. (2005) 94:1333–5. 10.1111/j.1651-2227.2005.tb02097.x
182.
Gray N Dhana A Stein D Khumalo N . Zinc and atopic dermatitis: A systematic review and meta-analysis.J Eur Acad Dermatol Venereol. (2019) 33:1042–50. 10.1111/jdv.15524
183.
Wais U Jackson A He T Zhang H . Nanoformulation and encapsulation approaches for poorly water-soluble drug nanoparticles.Nanoscale. (2016) 8:1746–69. 10.1039/C5NR07161E
184.
Hu B Liu X Zhang C Zeng X . Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols.J Food Drug Anal. (2017) 25:3–15. 10.1016/j.jfda.2016.11.004
185.
Thao N Do H Nam N Tran N Dan T Trinh K . Antioxidant nanozymes: Mechanisms, activity manipulation, and applications.Micromachines. (2023) 14:1017. 10.3390/mi14051017
186.
Xu D Wu L Yao H Zhao L . Catalase-like nanozymes: Classification, catalytic mechanisms, and their applications.Small. (2022) 18:2203400. 10.1002/smll.202203400
187.
Zhao H Zhang R Yan X Fan K . Superoxide dismutase nanozymes: An emerging star for anti-oxidation.J Materials Chem B. (2021) 9:6939–57. 10.1039/D1TB00720C
188.
Lai Y Wang J Yue N Zhang Q Wu J Qi W et al Glutathione peroxidase-like nanozymes: Mechanism, classification, and bioapplication. Biomaterials Sci. (2023) 11:2292–316. 10.1039/D2BM01915A
189.
Singh R Karakoti A Self W Seal S Singh S . Redox-sensitive cerium oxide nanoparticles protect human keratinocytes from oxidative stress induced by glutathione depletion.Langmuir. (2016) 32:12202–11. 10.1021/acs.langmuir.6b03022
190.
Mao M Guan X Wu F Ma L . Coo nanozymes with multiple catalytic activities regulate atopic dermatitis.Nanomaterials. (2022) 12:638. 10.3390/nano12040638
191.
Morry J Ngamcherdtrakul W Yantasee W . Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles.Redox Biol. (2017) 11:240–53. 10.1016/j.redox.2016.12.011
192.
Hajhashemi V Vaseghi G Pourfarzam M Abdollahi A. *Are antioxidants helpful for disease prevention? Res Pharm Sci. (2010) 5:1–8.
193.
Puri S Mazza M Roy G England R Zhou L Nourian S et al Evolution of nanomedicine formulations for targeted delivery and controlled release. Adv Drug Delivery Rev. (2023) 200:114962. 10.1016/j.addr.2023.114962
194.
Liu Y Shi J . Antioxidative nanomaterials and biomedical applications.Nano Today. (2019) 27:146–77. 10.1016/j.nantod.2019.05.008
195.
Hussain Z Katas H Mohd Amin M Kumolosasi E . efficient immuno-modulation of Th1/Th2 biomarkers in 2,4-Dinitrofluorobenzene-induced atopic dermatitis: Nanocarrier-mediated transcutaneous co-delivery of anti-inflammatory and antioxidant drugs.PLoS One. (2014) 9:e113143. 10.1371/journal.pone.0113143
196.
Siddique M Katas H Jamil A Mohd Amin M Ng S Zulfakar M et al Potential treatment of atopic dermatitis: Tolerability and safety of cream containing nanoparticles loaded with hydrocortisone and hydroxytyrosol in human subjects. Drug Delivery Transl Res. (2019) 9:469–81. 10.1007/s13346-017-0439-7
197.
Li S Dong S Xu W Tu S Yan L Zhao C et al Antibacterial hydrogels. Adv Sc. (2018) 5:1700527. 10.1002/advs.201700527
198.
Hamedi H Moradi S Hudson S Tonelli A . Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review.Carbohydrate Polym. (2018) 199:445–60. 10.1016/j.carbpol.2018.06.114
199.
Guimarães C Ahmed R Marques A Reis R Demirci U . Engineering hydrogel-based biomedical photonics: Design, fabrication, and applications.Adv Mater. (2021) 33:e2006582. 10.1002/adma.202006582
200.
Nagula R Wairkar S . Cellulose microsponges based gel of naringenin for atopic dermatitis: Design, optimization, in vitro and in vivo investigation.Int J Biol Macromol. (2020) 164:717–25. 10.1016/j.ijbiomac.2020.07.168
201.
Liang Y He J Guo B . Functional hydrogels as wound dressing to enhance wound healing.ACS Nano. (2021) 15:12687–722. 10.1021/acsnano.1c04206
202.
Hu B Ouyang Y Zhao T Wang Z Yan Q Qian Q et al Antioxidant hydrogels: Antioxidant mechanisms, design strategies, and applications in the treatment of oxidative stress-related diseases. Adv Healthcare Materials. (2024) 13:2303817. 10.1002/adhm.202303817
203.
Trinh T Nguyen T Kim J . Lignin-based antioxidant hydrogel patch for the management of atopic dermatitis by mitigating oxidative stress in the skin.ACS Appl Mater Interfaces. (2024) 16:33135–48. 10.1021/acsami.4c05523
204.
Kim Y Choi S Kim M Nguyen T Kim J . Therapeutic hydrogel patch to treat atopic dermatitis by regulating oxidative stress.Nano Lett. (2022) 22:2038–47. 10.1021/acs.nanolett.1c04899
205.
Kim B Gao G Kim J Cho D . 3d cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin.Adv Healthc Materials. (2019) 8:1801019. 10.1002/adhm.201801019
206.
Danso M Berkers T Mieremet A Hausil F Bouwstra J . An Ex vivo human skin model for studying skin barrier repair.Exp Dermatol. (2015) 24:48–54. 10.1111/exd.12579
207.
Tuan-Mahmood T McCrudden M Torrisi B McAlister E Garland M Singh T et al Microneedles for intradermal and transdermal drug delivery. Eur J Pharm Sci. (2013) 50:623–37. 10.1016/j.ejps.2013.05.005
208.
Bergmann C Pochmann D Bergmann J Bocca F Proença I Marinho J et al The use of retinoic acid in association with microneedling in the treatment of epidermal melasma: Efficacy and oxidative stress parameters. Arch Dermatol Res. (2021) 313:695–704. 10.1007/s00403-020-02140-8
209.
Zasada M Markiewicz A Drożdż Z Mosińska P Erkiert-Polguj A Budzisz E . Preliminary randomized controlled trial of antiaging effects of l-ascorbic acid applied in combination with no-needle and microneedle mesotherapy.J Cosmetic Dermatol. (2019) 18:843–9. 10.1111/jocd.12727
210.
Chi J Sun L Cai L Fan L Shao C Shang L et al Chinese herb microneedle patch for wound healing. Bioactive Mater. (2021) 6:3507–14. 10.1016/j.bioactmat.2021.03.023
211.
Chiu Y Wu Y Hung J Chen M . Epigallocatechin Gallate/L-Ascorbic Acid–Loaded Poly-Γ-glutamate microneedles with antioxidant, anti-inflammatory, and immunomodulatory effects for the treatment of atopic dermatitis.Acta Biomater. (2021) 130:223–33. 10.1016/j.actbio.2021.05.032
212.
Chen Y Chang C Lin Y Chen M . Double-Layered Plga/Ha microneedle systems as a long-acting formulation of polyphenols for effective and long-term management of atopic dermatitis.Biomaterials Sci. (2023) 11:4995–5011. 10.1039/D3BM00182B
213.
Zhang Y Zhang X Wu X Zhao Y . Photo-responsive polydopamine nanoenzyme microneedles with oxidative stress regulation ability for atopic dermatitis treatment.Nano Today. (2024) 56:102241. 10.1016/j.nantod.2024.102241
Summary
Keywords
atopic dermatitis, skin barrier, oxidative stress, oxidation products, oxidative stress marker, antioxidant therapy
Citation
Luo Y, Hu J, Zhou Z, Zhang Y, Wu Y and Sun J (2025) Oxidative stress products and managements in atopic dermatitis. Front. Med. 12:1538194. doi: 10.3389/fmed.2025.1538194
Received
20 December 2024
Accepted
22 April 2025
Published
09 May 2025
Volume
12 - 2025
Edited by
Claudia Fiorillo, University of Florence, Italy
Reviewed by
Sabina Galiniak, University of Rzeszów, Poland
Juan Luis Santiago, Poland Universitario de Ciudad Real, Spain
Hiroki Satooka, Shiga University of Medical Science, Japan
Jie Zhang, Kunming University of Science and Technology, China
Updates
Copyright
© 2025 Luo, Hu, Zhou, Zhang, Wu and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaguang Wu, wuyaguangcq@tmmu.edu.cnJiaying Sun, sjy@cqut.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.