Abstract
Background:
The geriatric population, especially individuals over 65 years old with comorbidities classified by the ASA (American Society of Anesthesiologists) grading system, requires careful sedation management during flexible bronchoscopy (FB) to reduce the heightened risks of complications. Hypoxemia is a particularly critical concern in this demographic, leading to considerable morbidity, mortality, and increased healthcare costs. This study focuses on comparing the incidence of sedation-related hypoxemia and other adverse events between remimazolam besylate and propofol during FB procedures, aiming to enhance patient safety and optimize sedation practices in this vulnerable population.
Methods:
This prospective observational cohort study compared the incidence of hypoxemia and sedation-related adverse events between remimazolam besylate and propofol in 69 elderly patients (ASA I-III). Rigorous inclusion/exclusion criteria, clinical monitoring, and alongside comprehensive monitoring of clinical parameters and statistical analyses to ensure the validity of the results.
Results:
Hypoxemia occurred in 44.90% overall, with significantly lower incidence in remimazolam besylate cohort (29.42% vs. 60.00%; OR = 2.10, 95% CI 1.18–3.74, p = 0.017). Recovery to full alertness was prolonged with remimazolam (median 15[12.5–20] vs. 8[5.5–10] min; p < 0.001). A trend toward reduced hypotension was observed (17.65% vs. 37.14%, p = 0.0699), with no other significant safety differences.
Conclusion:
Remimazolam besylate demonstrates superior safety for elderly FB sedation, significantly reducing hypoxemia risk and accelerating recovery. These findings support its preferential use in geriatric sedation protocols, warranting further investigation to optimize clinical implementation strategies.
Introduction
The geriatric population undergoing invasive procedures faces heightened sedation risks due to age-related physiological decline, particularly in cardiopulmonary compensatory mechanisms. This vulnerability is exacerbated during flexible bronchoscopy (FB), where sedation-induced respiratory depression frequently precipitates hypoxemia-a critical complication associated with increased morbidity and healthcare costs (1–5).
Current sedation protocols utilizing propofol demonstrate suboptimal safety profiles in elderly patients, with documented risks of hypotension (15–30% incidence) and oxygen desaturation events (6, 7). Emerging evidence from gastrointestinal endoscopy trials suggests remimazolam besylate, a novel ultra-short-acting benzodiazepine, may offer superior hemodynamic stability and reduced hypoxemia rates (8.7% vs. 24.1% vs. propofol) (8–12). However, critical knowledge gaps persist regarding its comparative efficacy in FB procedures specifically targeting elderly populations.
This prospective observational cohort study systematically evaluates hypoxemia incidence between remimazolam besylate and propofol in ASA I-III patients aged ≥65 undergoing FB. Through rigorous patient selection and multidimensional safety monitoring, we aim to establish evidence-based recommendations for geriatric sedation optimization.
Materials and methods
This prospective observational cohort study was approved by the Ethics Committee of the Fourth Affiliated Hospital of Zhejiang University Medical College (YiWu, People’s Republic of China) (No: K2023055). The trial was registered with the Clinical Trial Registry in the 05/05/2023(No: ChiCTR2300071137). The period of enrolment was from May 2023 to March 2024. All patients gave written informed consent before enrolment.
The study recruited elderly participants aged 65 years and above who were undergoing FB and were classified under American Society of Anesthesiologists (ASA) grades I–III. The exclusion criteria encompassed the following: (1) ASA IV or Class V; (2) Body Mass Index (BMI) exceeding 35; (3) severe chronic obstructive pulmonary disease (COPD); (4) respiratory failure; (5) oxygen saturation (SPO2) below 90% when breathing room air; (6) lung function capacity of less than 15 mL/kg, with forced expiratory volume in 1 s (FEV1) less than 1,000 mL or FEV1/forced vital capacity (FVC) ratio below 35%; (7) Mallampati score greater than 4; and (8) known allergies to the study medication. Comprehensive information regarding the inclusion and exclusion criteria can be obtained from Figure 1.
Figure 1

The flowchart showing the number of patients at each phase of the study.
Following the acquisition of informed consent, initial data collection was conducted for all participants, which included measurements of weight, vital signs, and Modified Observer Assessment of Alertness/Sedation (MOAA/S) scores. Prior to the procedure, an intravenous catheter of either 18 or 20-gauge was placed in the upper extremity. Participants enrolled in the study received a 2% lidocaine solution (10 mL) via atomization 30 min before sedation, with all endoscopic procedures conducted at the endoscopy center. Each patient underwent 5 min of preoxygenation using 100% oxygen at a flow rate of 6 L/min delivered through a facemask prior to the procedure. Monitoring protocols for sedated patients adhered to the American Society of Anaesthesiologists (ASA) guidelines, which included measuring blood pressure, heart rate (HR), pulse oxygen saturation, and end-tidal carbon dioxide levels using a Cardio cap device (Mindray). The exposed group received remimazolam besylate and non-exposed group received propofol. Analgesia was provided with 0.5 μg/kg fentanyl following the infusion of 500 mL of a 0.9% lactated Ringer’s solution. Subsequently, either 0.15 mg/kg of remimazolam besylate or 1 mg/kg of propofol was given according to group assignment. A skilled endoscopist performed bronchoscopy when the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score dropped to ≤1. Lidocaine (100 mg of a 2% solution) was applied to the main airway during bronchoscopy. Sedation was sustained at a MOAA/S score of ≤1 throughout the procedure through the continuous infusion of 1 mg/kg/h of remimazolam besylate in the exposed group or propofol (4–6 mg/kg/h) in the non-exposed group. Supplementary doses of 2.5 mg of remimazolam besylate or 0.5 mg/kg of propofol were administered to address instances of coughing or body movement. The administration of medications was concluded upon completing the procedure, and oxygen supplementation was maintained at a flow rate of 6 L/min until the patient showed full alertness, which was confirmed by achieving a MOAA/S score of 5 in three consecutive evaluations.
Respiratory safety monitoring
In instances of prolonged hypoxemia (SPO2 < 90%) lasting over 10 s, interventions such as elevating the mandibular angle and applying gentle thoracic compression were implemented. If hypoxemia persisted (SPO2 < 80% without spontaneous respiration), the bronchoscope was retracted, and manual ventilation was initiated until SPO2 levels increased above 95%. To ensure hemodynamic stability, vasoactive agents like phenylephrine, ephedrine, atropine, urapidil, and esmolol were used, maintaining parameters within a 20% deviation from baseline. After the bronchoscope was removed, patients were transferred to the Post-Anesthesia Care Unit (PACU) for recovery, where their MOAA/S scores were evaluated at five-minute intervals until full alertness was achieved. No pharmacological reversal agents (e.g., flumazenil) were administered. Remimazolam besylate’s rapid esterase-mediated metabolism ensures prompt recovery, and predefined rescue measures (e.g., manual ventilation) were prioritized to manage adverse events per protocol.
Throughout the study period, adverse events such as hypoxemia, hypotension, and bradycardia were closely monitored according to established criteria. Specifically, hypoxemia (13) was characterized by an SPO2 level falling below 90% for more than 30 s, while severe hypoxemia was defined as an SPO2 level dipping below 80% at any point.
The primary aim of the study was to investigate the frequency of hypoxemia, defined as SPO2 < 90% for more than 30 s, while secondary objectives included assessing severe hypoxemia (14) (Spo2 < 80%) and duration of full alertness (15) (MOAA/S score 5 for three consecutive occurrences).
Statistical analysis
All analyses were performed using R 4.2.2 and MSTATA1 with two-tailed α = 0.05. Continuous variables were assessed for normality via the Kolmogorov–Smirnov test, reported as mean ± SD (normal distribution) or median [IQR] (non-normal). Group comparisons utilized Student’s t-test (parametric) or Mann–Whitney U test (non-parametric), while categorical variables were analyzed with Pearson’s χ2 test, multivariate logistic regression analysis for identifying independent factors associated with hypoxemia, and the significance level (α = 0.05).
Results
A total of 118 patients underwent initial assessment, of whom 46 were subsequently excluded from the study. Among the excluded patients, 38 did not meet the inclusion criteria. This included 10 cases of severe chronic obstructive pulmonary disease (COPD), 5 cases with a body mass index (BMI) greater than 35, 8 cases with a peripheral capillary oxygen saturation (SpO₂) below 90%, 9 cases with a forced expiratory volume in 1 s (FEV1) below 1,000 mL, and 6 cases with a Mallampati score of grade 3–4. Additionally, 8 patients declined to provide informed consent.” Ultimately, 72 patients were included in the study, but 3 were later excluded due to surgical requirements necessitating a modification in anesthesia approach. This led to a final analysis involving 69 patients. The participant flow diagram is presented in Figure 1.
The demographic and clinical characteristics of the patients are presented in Table 1, demonstrating that there were no significant differences in the general characteristics such as age, weight, height, BMI, ASA score, and types of surgery between the two groups (p > 0.05).
Table 1
| Characteristics | Exposed (N = 34) | Non-exposed (N = 35) | All (N = 69) | p-value |
|---|---|---|---|---|
| Sex, n (%) | 0.83 | |||
| Male | 27 (79.41%) | 26 (74.29%) | 53 (76.81%) | |
| Female | 7 (20.59%) | 9 (25.71%) | 16 (23.19%) | |
| Age, y | 73.32 (5.15) | 71.37 (5.76) | 72.33 (5.51) | 0.14 |
| BMI, (kg/m2) | 22.74 (3.50) | 23.31 (2.79) | 23.03 (3.15) | 0.46 |
| Smoking, n (%) | 16 (47.06%) | 20 (57.14%) | 36 (52.17%) | 0.55 |
| Drink, n (%) | 10 (29.41%) | 11 (31.43%) | 21 (30.43%) | >0.99 |
| History of diabetes, n (%) | 5 (14.71%) | 7 (20.00%) | 12 (17.39%) | 0.79 |
| History of hypertension, n (%) | 12 (35.29%) | 13 (37.14%) | 25 (36.23%) | >0.99 |
| ASA physical status | >0.99 | |||
| II, n (%) | 14 (41.18%) | 14 (40.00%) | 28 (40.58%) | |
| III, n (%) | 20 (58.82%) | 21 (60.00%) | 41 (59.42%) | |
| Type of surgery, n (%) | 0.88 | |||
| BAL | 18(52.94%) | 16(45.71%) | 34(49.28%) | |
| Except BAL | 16(47.06%) | 19 (54.29%) | 35(50.72%) | |
| Procedure time, (min) | 12.50[10.00–28.75] | 15.00 [14.00–20.00] | 15.00[10.00–20.00] | 0.44 |
Patient characteristics and clinical results (n = 76).
Data were presented as number (percentage) for categorical variables, mean (SD) or median (interquartile range) for continuous variables. ASD was used to evaluate the balance of baseline information between groups. ASA, American Society of Anesthesiologists; ASD, absolute standardized difference; BMI, body mass index; SD, standard deviation; BAL, indicates bronchoalveolar lavage; Except BAL include EBUS and TBB; EBUS, endobronchial ultrasound; TBB, transbronchial biopsy.
The study revealed an overall incidence of hypoxemia at 44.90%, with a lower prevalence observed in the exposed group compared to the non-exposed group (29.42% vs. 60.00%, OR 2.10, 95% CI 1.18–3.74, p = 0.017) prior to controlling for potential confounding variables. Similarly, the prevalence of severe hypoxemia was found to be 24.64%, with a lower occurrence in the exposed group compared to the non-exposed group (11.76% vs. 37.14%, OR 2.10, 95% CI 1.18–3.74, p = 0.017) before adjusting for confounding factors (Figure 2).
Figure 2
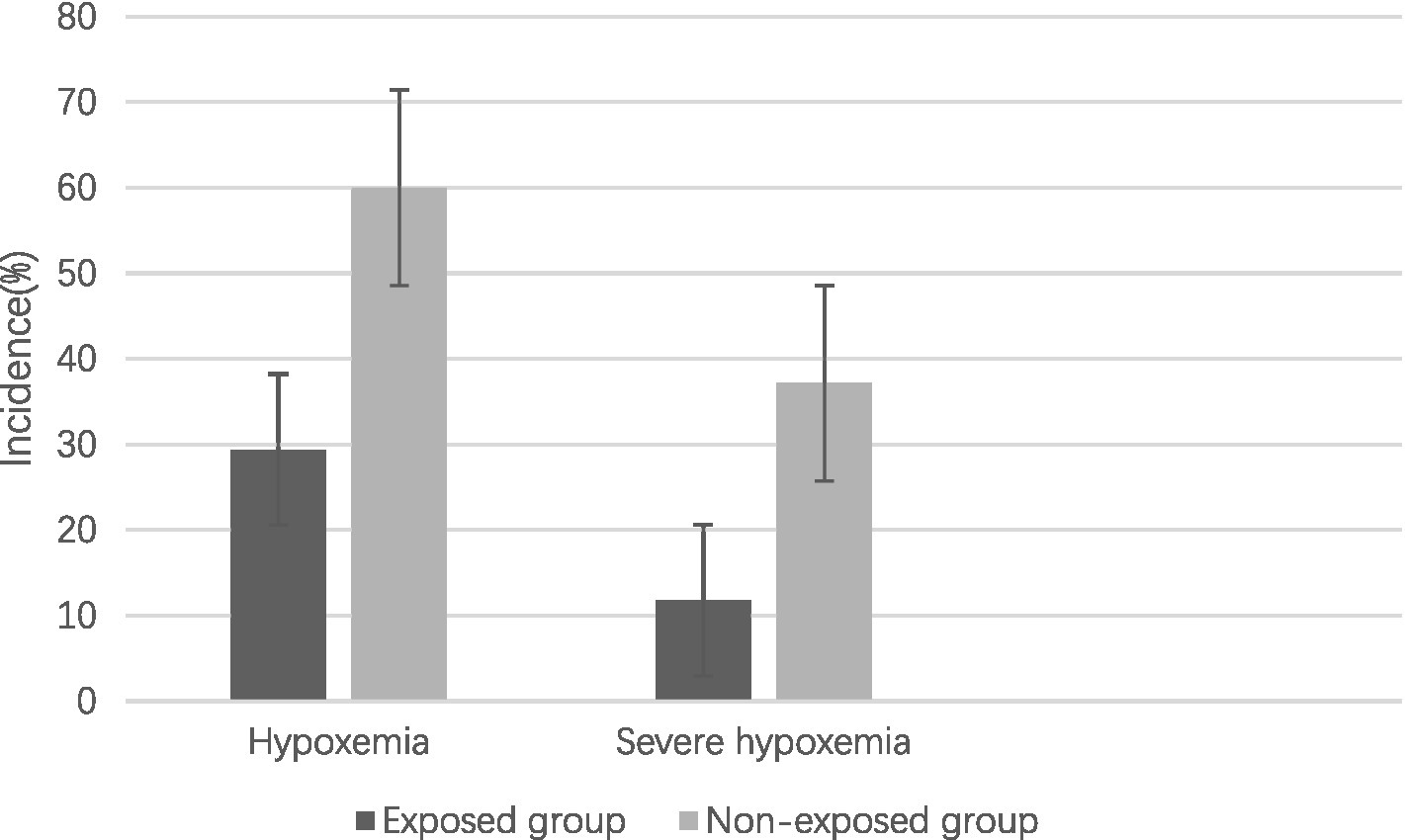
Incidence of hypoxemia and severe hypoxemia.
No statistically significant differences were observed in the occurrence of hypotension, bradycardia, or hypopnea between the two groups as indicated in Table 2. However, the incidence of hypotension was found to be 17.65%, with a lower occurrence observed in the exposed group compared to the non-exposed group (17.65% vs. 37.14%, p = 0.0699). Although the lack of statistical significance may be attributed to the small sample size, there is a trend suggesting potential clinical significance.
Table 2
| Adverse events, n (%) | Exposed (N = 34) | Unexposed (N = 35) | P value overall |
|---|---|---|---|
| Hypotension | 6(17.65%) | 13(37.14%) | 0.0699 |
| Bradycardia | 1(2.94%) | 1(2.86%) | 0.9834 |
| Hypopnea | 13(38.23%) | 18(51.43%) | 0.2707 |
Overall comparisons of adverse events after procedure.
Values were presented as number (percentage). A Pearson χ2 test or a Fisher exact was used to evaluate the associations between 2 groups.
On the contrary, the fully alert time (MOAA/S score 5 for consecutive 3 times) was found to be significantly greater in the exposed group [15(12.5, 20) min] compared to the non-exposed group [8(5.5, 10) min] (p < 0.001), as illustrated in Figure 3.
Figure 3
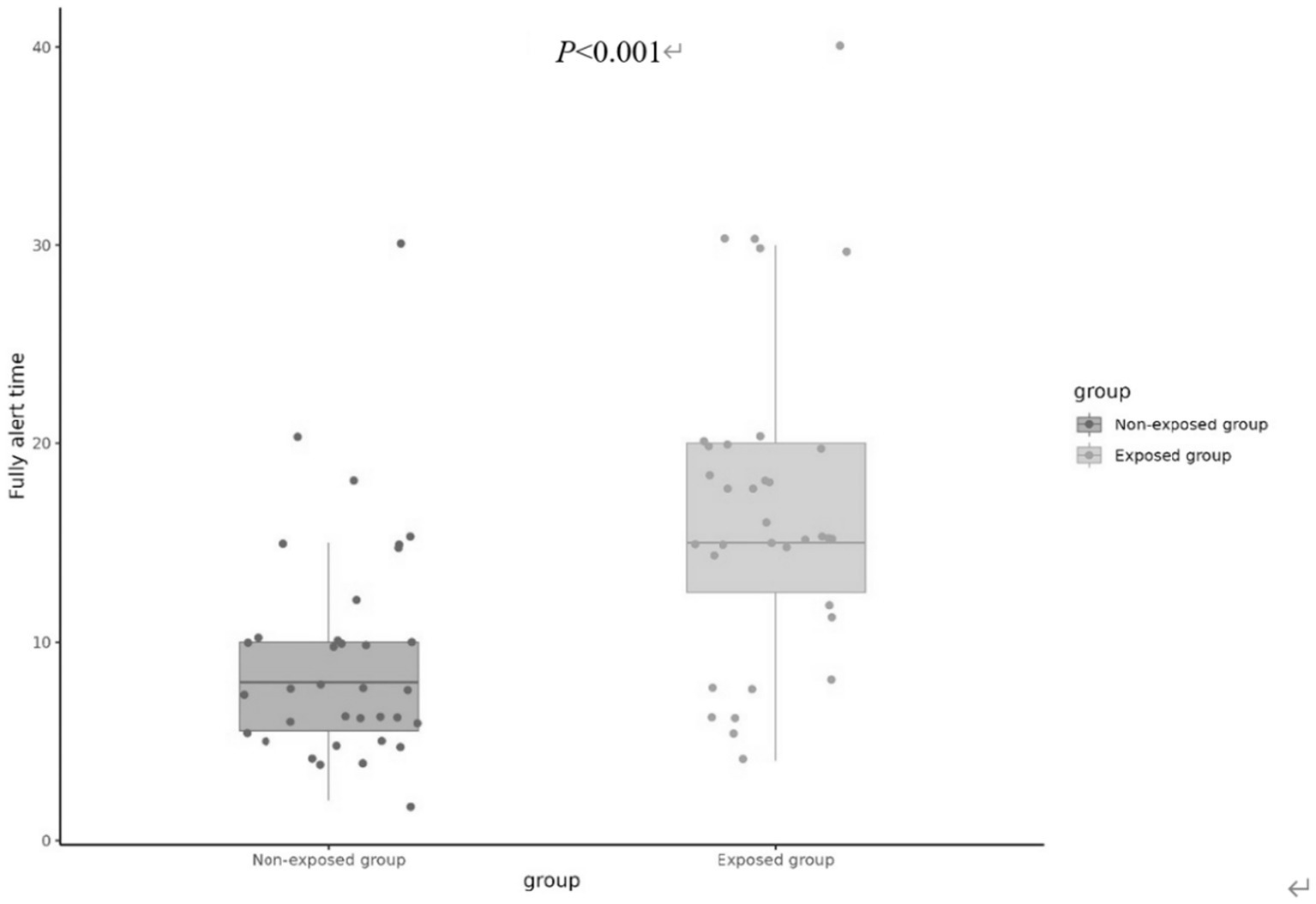
The fully alert time of two groups.
In the analysis of perioperative factors and hypoxemia through multivariate logistic regression analysis, two significant and independent factors were identified as being correlated with the occurrence of hypoxemia (Table 3).
Table 3
| Variables | P-value | OR (95%CI) |
|---|---|---|
| Group | ||
| Exposed group | 0.012* | 0.28 (0.10 ~ 0.76) |
| Sex | ||
| Female | 0.642 | 1.30 (0.43 ~ 4.00) |
| BMI | 0.233 | 1.10 (0.94 ~ 1.29) |
| Age, y | 0.445 | 0.97 (0.89 ~ 1.06) |
| Procedure time | 0.539 | 0.99 (0.94 ~ 1.03) |
| Type of surgery | ||
| BAL | 0.537 | 0.37 (0.14 ~ 0.98) |
| Smoking | ||
| Yes | 0.045* | 0.37 (0.14 ~ 0.98) |
| Drink | ||
| Yes | 0.204 | 0.50 (0.17 ~ 1.46) |
| History of hypertension | ||
| Yes | 0.166 | 2.02 (0.75 ~ 5.47) |
| History of diabetes | ||
| Yes | 0.698 | 1.28 (0.37 ~ 4.45) |
| ASA | ||
| III grade | 0.775 | 1.15 (0.44 ~ 3.03) |
Univariable logistic regression models for estimating the risk of hypoxemia.
BAL, indicates bronchoalveolar lavage; OR, odds ratio, CI, confidence interval. *p < 0.05, **p < 0.01, ***p < 0.001.
In the multivariate analysis of these factors, remimazolam besylate exposure exhibited a lower risk of hypoxemia in comparison to propofol (OR 0.16, 95% CI 0.04–0.64, p = 0.010). Additionally, smoking was found to be associated with a reduced risk of hypoxemia when compared to non-smoking (OR 0.008, 95% CI 0.01–0.51, p = 0.008), as indicated in Figure 4.
Figure 4
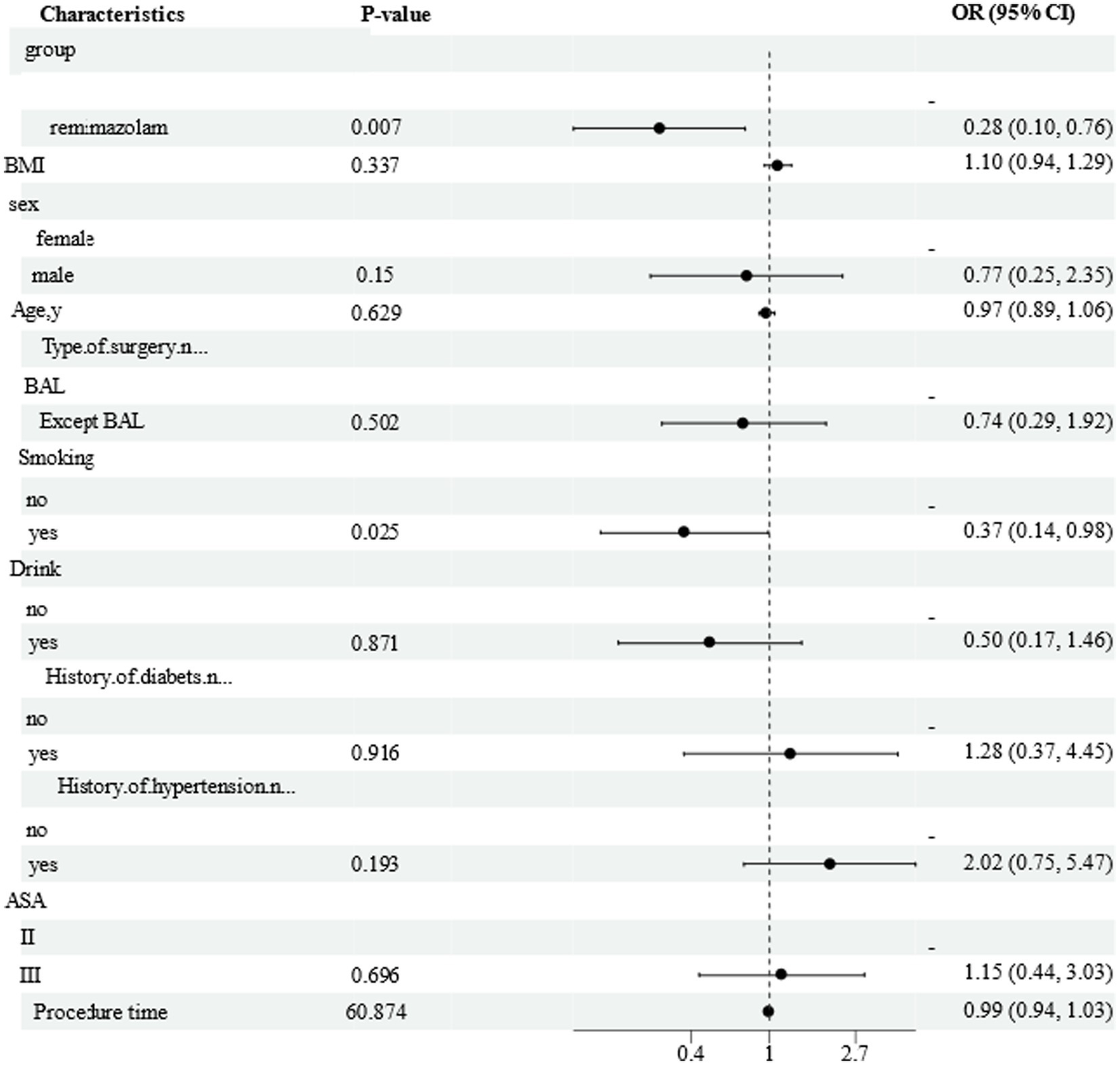
Forest plot of adjusted regression model for factors associated with hypoxemia. Figures show the reference value and OR (95%CI) for the levels of each variable; OR, odds radio.
Discussion
Hypoxemia, characterized by abnormally low arterial oxygen levels (16), remains a critical risk for elderly patients undergoing sedation during flexible bronchoscopy (FB). This study revealed a significantly lower incidence of hypoxemia in the remimazolam besylate group compared to the propofol cohort (29.42% vs. 60.00%, p = 0.017), alongside prolonged recovery to full alertness (median 15[12.5–20] min vs. 8[5.5–10] min, p < 0.001). These findings align with prior research demonstrating remimazolam besylate’s favorable safety profile in procedural sedation (2, 17, 18), but extend its validation to elderly FB patients-a population historically underrepresented in such studies.
The observed reduction in hypoxemia incidence may stem from remimazolam besylate’s unique pharmacokinetics. Unlike propofol, which suppresses respiratory drive through GABA receptor potentiation (19), remimazolam besylate combines rapid esterase metabolism with selective α1-subunit binding (19, 20), potentially mitigating respiratory depression. This mechanism likely contributed to the lower rates of severe hypoxemia (11.76% vs. 37.14%, p = 0.017) and hypotension trends (17.65% vs. 37.14%, p = 0.0699) observed in our cohort.
These results resonate with earlier trials comparing remimazolam besylate to propofol in colonoscopy patients (5), where hypotension rates were halved (23.71% vs. 51.05%). However, our study uniquely highlights its efficacy in elderly patients with ASA I-III comorbidities-a demographic at heightened risk for sedation-related complications. The logistic regression further identified remimazolam besylate exposure as an independent protective factor against hypoxemia (OR 0.16, 95% CI 0.04–0.64, p = 0.010), reinforcing its clinical utility in this high-risk population.
The innovation of this study lies in its comparative analysis of remimazolam besylate and propofol regarding hypoxemia incidence during FB in elderly patients-a population often excluded from sedation trials (17, 18). While previous research focused on colonoscopy or general anesthesia (21, 22), our findings directly address the unmet need for optimized sedation protocols in geriatric bronchoscopy. This distinction is critical, as FB imposes unique respiratory challenges due to airway instrumentation and reduced functional reserve in elderly patients (2).
Notably, smoking emerged as a protective factor against hypoxemia (OR 0.008, 95% CI 0.01–0.51, p = 0.008), though this paradoxical finding requires cautious interpretation. The gender imbalance (76.81% male) and associated smoking prevalence (52.17%) may confound this association, necessitating further investigation into potential physiological mechanisms or selection biases.
Our findings align with growing evidence favoring deep sedation for complex endoscopic procedures (23), where reduced patient movement enhances procedural success. Despite concerns that deeper sedation increases hypoxemia risk (24), remimazolam besylate demonstrated superior safety, suggesting its pharmacokinetic advantages may offset traditional risk profiles.
Study limitations include the observational design and small sample size, which limit causal inferences and generalizability. Future multicenter RCTs with extended follow-ups are needed to validate these findings. Furthermore, further studies to explore the impacts of different sedative drugs on multiple aspects of the cardiovascular and nervous systems of elderly patients. Also, study how to optimize the drug combination and administration plan to better balance the sedation effect and reduce adverse reactions, especially for elderly patients with multiple comorbidities. Nevertheless, the consistency of our results with prior trials strengthens the argument for remimazolam besylate as a first-line sedative in elderly FB patients.
In summary, this study provides compelling evidence that remimazolam besylate is associated with a significantly reduced incidence of hypoxemia compared to propofol in elderly patients undergoing flexible bronchoscopy. These findings underscore the potential of remimazolam besylate as a safer sedation option, advocating for a re-evaluation of current sedation practices in this vulnerable population. The implications of this research extend beyond individual patient outcomes, suggesting that adopting remimazolam besylate could enhance overall clinical protocols, reduce healthcare burdens associated with sedation-related complications, and ultimately improve patient care in geriatric medicine.
Conclusion
This study establishes, for the first time, that remimazolam besylate reduces hypoxemia incidence by over 50% compared to propofol in elderly patients undergoing FB-a high-risk population historically underrepresented in sedation research. By directly comparing these agents in a geriatric cohort with comorbidities, we demonstrate remimazolam’s dual advantages: superior respiratory safety (29.42% vs. 60.00% hypoxemia, p = 0.017) and hemodynamic stability, despite deeper sedation requirements. These findings redefine sedation paradigms for airway procedures in aging populations, addressing a critical gap in evidence-based protocols. Our results provide actionable insights for prioritizing its use in geriatric bronchoscopy to mitigate morbidity risks and optimize post-procedural recovery.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Fourth Affiliated Hospital Zhejiang University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DM: Writing – review & editing, Investigation, Methodology. LL: Investigation, Methodology, Writing – review & editing. FH: Methodology, Writing – review & editing, Supervision, Visualization. JX: Conceptualization, Formal analysis, Methodology, Writing – review & editing. CZ: Visualization, Writing – review & editing, Conceptualization, Data curation, Funding acquisition, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Medical Scientific Research Foundation of Zhejiang Province, China (2022KY876) and Zhejiang Provincial Department of Education Project (2021–116).
Acknowledgments
The authors would like to acknowledge all of the staff who assisted this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1.
Zhang W Wang JL Fu S Zhou JM Zhu YJ Cai SN et al . Incidence of oxygen desaturation using a high-flow nasal cannula versus a facemask during flexible bronchoscopy in patients at risk of hypoxemia: a randomised controlled trial. BMC Pulm Med. (2022) 22:389. doi: 10.1186/s12890-022-02188-4
2.
Chhajed PN Glanville AR . Management of hypoxemia during flexible bronchoscopy. Clin Chest Med. (2003) 24:511–6. doi: 10.1016/s0272-5231(03)00050-9
3.
Lu Z Zhou N Li Y Yang L Hao W . Up-down determination of the 90% effective dose (ED90) of remimazolam besylate for anesthesia induction. Ann Palliat Med. (2022) 11:568–73. doi: 10.21037/apm-22-89
4.
Tan DD Gu J Li J Yu WQ Liu DX Zhao LJ et al . The effective doses of remimazolam besylate in the procedural sedation of endoscopic retrograde cholangiopancreatography. Ibrain. (2023) 9:290–7. doi: 10.1002/ibra.12072
5.
Chen S Wang J Xu X Huang Y Xue S Wu A et al . The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res. (2020) 12:4594–603. PMID:
6.
Cao Y Chi P Zhou C Lv W Quan Z Xue FS . Remimazolam Tosilate sedation with adjuvant Sufentanil in Chinese patients with liver cirrhosis undergoing gastroscopy: a randomized controlled study. Med Sci Monit Int Med J Exp Clin Res. (2022) 28:e936580. doi: 10.12659/MSM.936580
7.
Chang Y Huang YT Chi KY Huang YT . Remimazolam versus propofol for procedural sedation: a meta-analysis of randomized controlled trials. PeerJ. (2023) 11:e15495. doi: 10.7717/peerj.15495
8.
Pastis NJ Yarmus LB Schippers F Ostroff R Chen A Akulian J et al . Safety and efficacy of Remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. (2019) 155:137–46. doi: 10.1016/j.chest.2018.09.015
9.
Rex DK Bhandari R Desta T MP DM Schaeffer C Etzkorn K . A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. (2018) 88:427–437.e6. doi: 10.1016/j.gie.2018.04.2351
10.
Chen SH Yuan TM Zhang J Bai H Tian M Pan CX et al . Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, phase III trial. J Gastroenterol Hepatol. (2021) 36:474–81. doi: 10.1111/jgh.15188
11.
Doi M Morita K Takeda J Sakamoto A Yamakage M Suzuki T . Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. (2020) 34:543–53. doi: 10.1007/s00540-020-02788-6
12.
Xu C He L Ren J Zhou J Guo H Chen N . Efficacy and safety of remimazolam besylate combined with alfentanil in painless gastroscopy: a randomized, single-blind, parallel controlled study. Contrast Media Mol Imaging. (2022) 2022:7102293. doi: 10.1155/2022/7102293
13.
Conway A Sutherland J . Depth of anaesthesia monitoring during procedural sedation and analgesia: a systematic review and meta-analysis. Int J Nurs Stud. (2016) 63:201–12. doi: 10.1016/j.ijnurstu.2016.05.004
14.
Frat JP Ricard JD Coudroy R Robert R Ragot S Thille AW et al . Preoxygenation with non-invasive ventilation versus high-flow nasal cannula oxygen therapy for intubation of patients with acute hypoxaemic respiratory failure in ICU: the prospective randomised controlled FLORALI-2 study protocol. BMJ Open. (2017) 7:e018611. doi: 10.1136/bmjopen-2017-018611
15.
Antonik LJ Goldwater DR Kilpatrick GJ Tilbrook GS Borkett KM . A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. (2012) 115:274–83. doi: 10.1213/ANE.0b013e31823f0c28
16.
Henig NR Pierson DJ . Mechanisms of hypoxemia. Respir Care Clin N Am. (2000) 6:501–21. doi: 10.1016/s1078-5337(05)70087-3
17.
Choe JW Chung MJ Park SW Oh D Han SY Yang MJ et al . Safety and efficacy of remimazolam versus propofol during EUS: a multicenter randomized controlled study. Gastrointest Endosc. (2024) 100:183–191.e1. doi: 10.1016/j.gie.2024.04.001
18.
Ahmer W Imtiaz S Alam DM Ahmed K Sajid B Yousuf J et al . Remimazolam versus propofol for sedation in gastrointestinal endoscopy and colonoscopy within elderly patients: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. (2024) 80:493–503. doi: 10.1007/s00228-024-03624-6
19.
Masui K . Remimazolam: its clinical pharmacology and evolving role in anesthesia and sedation practice. Curr Opin Anaesthesiol. (2024) 37:344–51. doi: 10.1097/ACO.0000000000001384
20.
Intzilaki CV Davodi J Vilmann P Møller AM . The clinical role of remimazolam: protocol for a scoping review. Acta Anaesthesiol Scand. (2024) 68:956–9. doi: 10.1111/aas.14421
21.
Godoroja-Diarto D Constantin A Moldovan C Rusu E Sorbello M . Efficacy and safety of deep sedation and anaesthesia for complex endoscopic procedures-a narrative review. Diagn Basel Switz. (2022) 12:1523. doi: 10.3390/diagnostics12071523
22.
Lu K Wei S Ling W Wei Y Ran X Huang H et al . Remimazolam versus propofol for deep sedation/anaesthesia in upper gastrointestinal endoscopy in elderly patients: a multicenter, randomized controlled trial. J Clin Pharm Ther. (2022) 47:2230–6. doi: 10.1111/jcpt.13797
23.
Yong SH Lee SH Oh SI Keum JS Kim KN Park MS et al . Malignant thoracic lymph node classification with deep convolutional neural networks on real-time endobronchial ultrasound (EBUS) images. Transl Lung Cancer Res. (2022) 11:14–23. doi: 10.21037/tlcr-21-870
24.
Hoshijima H Higuchi H Sato Boku A Shibuya M Morimoto Y Fujisawa T et al . Patient satisfaction with deep versus light/moderate sedation for non-surgical procedures: a systematic review and meta-analysis. Medicine. (2021) 100:e27176. doi: 10.1097/MD.0000000000027176
Summary
Keywords
remimazolam besylate, fiberoptic bronchoscopy, elderly, deep sedation, hypoxemia
Citation
Ma D, Li L, Han F, Xu J and Zhang C (2025) Evaluation of deep sedation effects and respiratory aspects of remimazolam besylate in elderly patients undergoing fiberoptic bronchoscopy. Front. Med. 12:1543866. doi: 10.3389/fmed.2025.1543866
Received
10 January 2025
Accepted
13 March 2025
Published
28 March 2025
Volume
12 - 2025
Edited by
Somchai Amornyotin, Mahidol University, Thailand
Reviewed by
Marna Eliana Sakalem, State University of Londrina, Brazil
Takehito Sato, Nagoya University Hospital, Japan
Updates
Copyright
© 2025 Ma, Li, Han, Xu and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Zhang, 8021006@zju.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.