Abstract
Objective:
Chronic obstructive pulmonary disease (COPD) is one of the most important causes of death in the world, and its core is chronic inflammation. Antioxidants play a positive role in the onset and prognosis of chronic respiratory diseases. In maintaining human health, the composite dietary antioxidant index (CDAI) plays an important function. Therefore, the purpose of the current study was to investigate the relationship between CDAI and all-cause and cancer mortality in individuals with COPD.
Methods:
A prospective cohort study was conducted by investigating NHANES data between 1999–2018. The study included people who satisfied the inclusion and exclusion criteria. In this study, the association between CDAI and all-cause and cancer mortality was investigated using weighted Cox regression. The relationship between them is illustrated by drawing constrained cubic spline curves (RCS). Finally, subgroup analysis is used to further verify.
Results:
The study included 1,534 participants. CDAI was associated with COPD patients mortality, and after adjusting for multiple factors, we observed a 5% reduction in the risk of all-cause mortality (HR = 0.95, 95% CI: 0.92–0.97) was associated with a 9% lower risk of cancer mortality for each one-unit increase in CDAI (HR = 0.91, 95% CI: 0.85–0.98). After adjusting for multiple factors, high CDAI was associated with a reduced risk of mortality, with patients in the high CDAI group having 35% lower all-cause mortality than those in the low CDAI group (HR = 0.65, 95% CI: 0.50–0.85), the high CDAI group had a 61% lower risk of cancer mortality (HR = 0.39,95% CI: 0.23–0.68). Subgroup analysis and sensitivity analysis showed a consistent association between CDAI and COPD mortality.
Conclusion:
Our study highlights the inverse association between CDAI and all-cause and cancer mortality in patients with COPD. Further prospective studies are needed to confirm the role of CDAI in mortality risk in patients with COPD.
1 Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive, irreversible decline in lung function and persistent airflow limitation. It is the third leading cause of disability-adjusted life years (DALYs) globally (1). The prevalence of COPD is approximately 10.3% among individuals aged 30 to 79 years (1). COPD is characterized by chronic inflammation that begins in the airways and, as the disease progresses, can gradually affect other organs, eventually becoming a systemic condition (2). COPD primarily affects individuals over the age of 40. As people age and are exposed to harmful substances such as tobacco smoke, the risk of developing various malignancies, including lung cancer, increases (3). Given the limited effectiveness of pharmacological treatments for COPD, it is essential to identify modifiable lifestyle factors that can mitigate the risk of developing the disease (4).
Recent research highlights reactive oxygen species (ROS) as crucial in oxidative stress. Overproduction of ROS is linked to chronic inflammation, COPD, and various malignancies. Under physiological conditions, antioxidants typically regulate ROS levels. However, antioxidants can be obtained both in vivo and ex vivo (5). Malnutrition and an antioxidant deficit might intensify the body’s reaction to oxidative stress molecules, causing tissue damage (6). The Composite Dietary Antioxidant Index (CDAI) is a novel measure that aggregates various antioxidants, including vitamins, minerals, and phytochemicals, to reflect the overall antioxidant capacity of the diet (7). This index aims to provide a comprehensive view of dietary antioxidant intake, crucial for understanding its potential health benefits. As these antioxidants are incorporated, the CDAI value increases, indicating a higher antioxidant capacity of the diet. The composite dietary antioxidant index (CDAI) is a valid method for evaluating an individual’s antioxidant consumption. It also concentrates on carotenoids including zinc, selenium, and vitamins A, C, and E (8). A higher CDAI score reflects greater antioxidant capacity.
Currently, a substantial body of research utilizes the CDAI to analyze the risk relationships between antioxidant-rich diets and common diseases (9). Although existing studies suggest that certain dietary nutrients can prevent airway inflammation in the general population, research on the relationship between CDAI and mortality in COPD patients is lacking. To investigate the relationship between them, we used community population data from the National Health and Nutrition Examination Survey (NHANES).
2 Materials and methods
2.1 Data source and study design
We conducted a study using data from the Centers for Disease Control and Prevention’s 1999–2018 National Health and Nutrition Examination Survey. Based on changes to the updated Helsinki Declaration, the research protocol was authorized by the National Center for Health Statistics Research Ethics Review Board. Informed consent was provided by NHANES participants. A complete description of the study can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
We selected a cohort of COPD patients (n = 2,244) from the 1999–2018 NHANES data. Exclusion criteria: (1) excluded missing follow-up data (n = 9); (2) excluded patients <40 years (n = 89); and (3) excluded patients with covariate missing value (n = 612). Finally, a total of 1,534 COPD patients were analyzed (Figure 1).
Figure 1

Flowchart of participant selection.
2.2 Outcome assessment
All-cause mortality was calculated by combining NHANES data with NDI records as of December 31, 2019. ICD-10 codes C00-C97 were used to reflect cancer fatalities.
2.3 Factors of exposure
The CDAI is predominantly affected by dietary factors. Intake of antioxidants, micronutrients, and total energy was calculated using the US Department of Agriculture’s Dietary Research Food and Nutrition Database (7). Using the questionnaire survey, we assessed each participant’s intake of dietary supplements in the past month, including dose, frequency, and number of doses. To calculate the CDAI, we standardized the intake of six specific dietary vitamins and minerals by subtracting their global average and dividing by the global standard deviation (10). We then computed the CDAI by summing the standardized intake of these vitamins and minerals as described below.
2.4 Covariates
The relevant covariates were obtained from questionnaires. Age (40–64, ≥65), sex (male, female), race (white, other), health insurance (yes, no), educational level (<high school, high school diploma, >High school), poverty income ratio (PIR) (< 1.3, 1.3–3.5, > 3.5), body mass index (BMI) (< 25, 25–30, > 30), smoking status (never, fomer, now), alcohol consumption status (yes, no), hypertension (yes, no), diabetes (borderline, yes, no) and COPD (survival, death) were used as categorical variables. There are two categories for alcohol consumption status: never drank (that is, consuming less than 12 drinks in a lifetime) and drank (that is, consuming 12 or more drinks in a lifetime) (11). Smoking status is classified as never smokers (having smoked less than 100 cigarettes), past smokers (not smoking at the moment but having smoked more than 100 cigarettes), or current smokers (having smoked more than 100 cigarettes and smoking daily or sometimes) (12). The PIR is the family income divided by the relevant poverty threshold for the survey year (13). Participants self-reported their diabetes and hypertension diagnoses using questionnaires.
2.5 Statistical analyses
R version 4.3.3 was used for the statistical analyses. The intricate stratified sampling and sample weights were taken into consideration in all statistical studies. For categorical variables, differences between groups were analyzed using chi-square tests. The “maxstat” package’s maximum selected rank statistics method was used to determine the ideal CDAI cutoff point linked to survival outcomes (14, 15). Using weighted Cox regression analysis, the relationship between CDAI and cancer and all-cause death in individuals with COPD was evaluated. Model 1 adjusted for sex, age, and race; Model 2 further adjusted for education, smoking, poverty-income ratio (PIR), insurance, and alcohol consumption; Model 3 included additional adjustments for BMI, diabetes, and hypertension. To examine the association and interactions between CDAI and mortality, subgroup analyses were conducted based on age, sex, race, BMI, and smoking status. Furthermore, the possible dose–response association between CDAI and cancer and all-cause mortality in individuals with COPD was visualized using restricted cubic spline (RCS) models. Finally, by removing deaths that occurred within two years of the baseline evaluation, sensitivity analyses were performed to evaluate the reliability of the primary findings.
3 Results
3.1 Characteristics of the study population
This study comprised 1,534 COPD patients in total. According to Table 1, white males aged 40–64 who have a high level of education, smoke, consume alcohol, and have hypertension are more likely to develop COPD. The optimal CDAI cutoff value (1.19) was determined according to MSRSM (Figure 2), and participants were divided into low CDAI group (Q1: CDAI <1.19) and high CDAI group (Q2: CDAI ≥1.19). Patients with low CDAI levels had more COPD deaths than those with high CDAI levels, suggesting that CDAI may be linked to the mortality risk in COPD patients.
Table 1
| Variable | Total | CDAI | p value | |
|---|---|---|---|---|
| Q1 | Q2 | |||
| Sex, n (%) | 0.48 | |||
| Female | 664 (49.00) | 488 (49.99) | 176 (46.94) | |
| Male | 870 (51.00) | 624 (50.01) | 246 (53.06) | |
| Age, years, n (%) | 0.51 | |||
| 40–64 | 753 (58.85) | 539 (58.01) | 214 (60.62) | |
| ≥65 | 781 (41.15) | 573 (41.99) | 208 (39.38) | |
| Race, n (%) | 0.06 | |||
| White | 1,030 (84.19) | 732 (82.81) | 298 (87.10) | |
| Others | 504 (15.81) | 380 (17.19) | 124 (12.90) | |
| Educational level, n (%) | < 0.001 | |||
| < High school | 486 (22.78) | 392 (27.10) | 94 (13.71) | |
| High school diploma | 376 (25.38) | 263 (23.41) | 113 (29.54) | |
| > High school | 672 (51.84) | 457 (49.49) | 215 (56.76) | |
| BMI, kg/m2, n (%) | 0.78 | |||
| < 25 | 428 (26.40) | 309 (25.93) | 119 (27.40) | |
| 25–30 | 517 (33.49) | 374 (33.04) | 143 (34.43) | |
| > 30 | 589 (40.11) | 429 (41.03) | 160 (38.17) | |
| PIR, n (%) | < 0.001 | |||
| < 1.3 | 548 (26.14) | 423 (29.05) | 125 (20.01) | |
| 1.3–3.5 | 584 (36.60) | 431 (38.46) | 153 (32.71) | |
| > 3.5 | 402 (37.26) | 258 (32.50) | 144 (47.27) | |
| Health insurance, n (%) | 0.86 | |||
| No | 143 (9.36) | 108 (9.50) | 35 (9.08) | |
| Yes | 1,391 (90.64) | 1,004 (90.50) | 387 (90.92) | |
| Smoking status, n (%) | 0.14 | |||
| Former | 759 (48.19) | 542 (45.83) | 217 (53.16) | |
| Never | 241 (17.38) | 174 (17.55) | 67 (17.01) | |
| Now | 534 (34.43) | 396 (36.62) | 138 (29.83) | |
| Alcohol consumption status, n (%) | 0.01 | |||
| No | 112 (5.95) | 92 (7.15) | 20 (3.43) | |
| Yes | 1,422 (94.05) | 1,020 (92.85) | 402 (96.57) | |
| Diabetes, n (%) | 0.33 | |||
| Borderline | 53 (3.15) | 36 (2.68) | 17 (4.14) | |
| No | 1,155 (79.35) | 826 (78.68) | 329 (80.76) | |
| Yes | 326 (17.50) | 250 (18.64) | 76 (15.10) | |
| Hypertension, n (%) | 0.26 | |||
| No | 650 (48.29) | 474 (46.80) | 176 (51.42) | |
| Yes | 884 (51.71) | 638 (53.20) | 246 (48.58) | |
| COPD, n (%) | < 0.0001 | |||
| Survival | 932 (70.15) | 642 (66.03) | 290 (78.82) | |
| Death | 602 (29.85) | 470 (33.97) | 132 (21.18) | |
Characteristic of participants.
The p-value was calculated by the weighted linear regression model. % for categorical variables; Q1: CDAI < 1.19, Q2: CDAI ≥ 1.19. BMI, body mass index; PIR, poverty income ratio; CDAI, composite dietary antioxidant index.
Figure 2
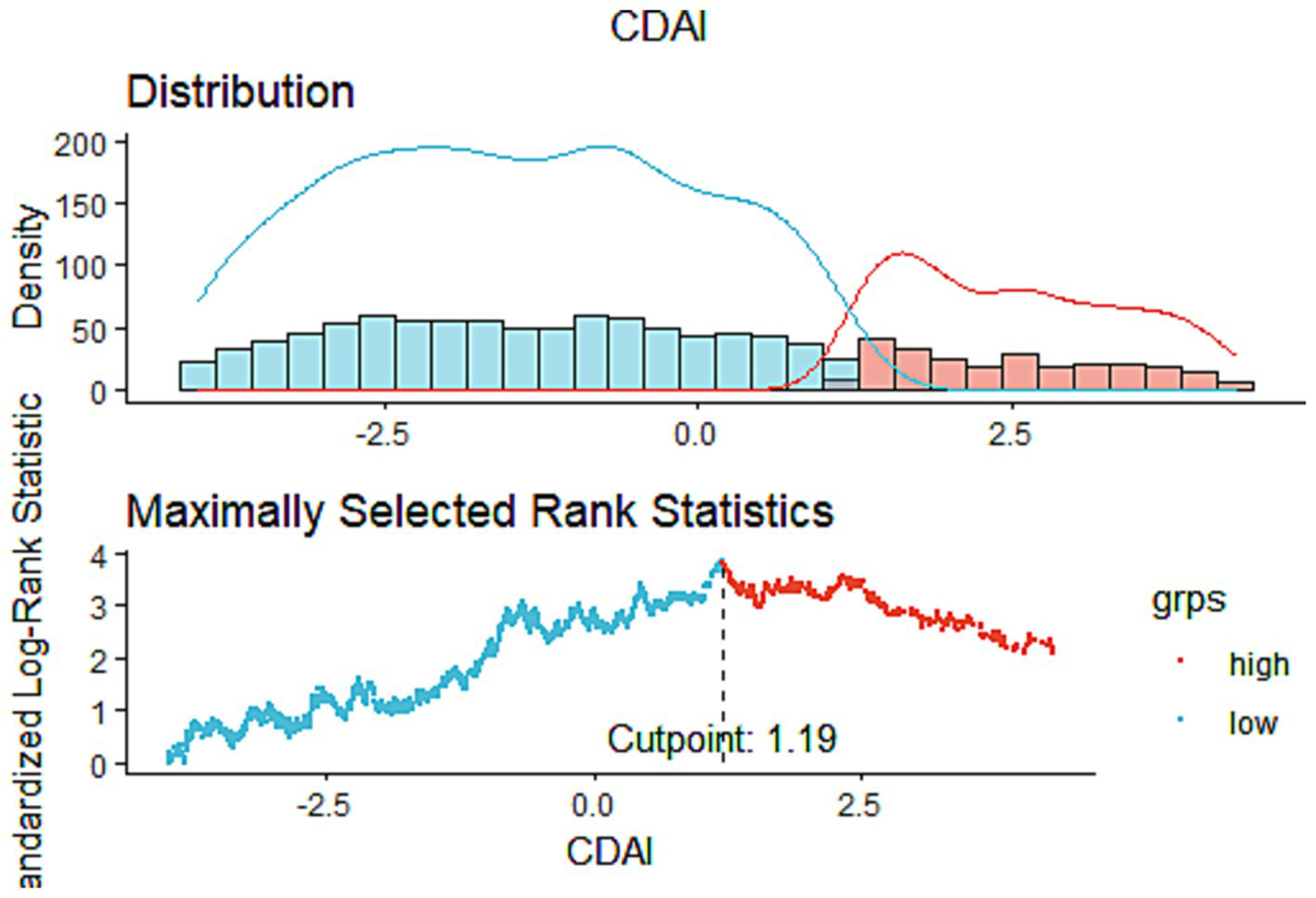
The cutoff point was calculated using the maximally selected rank statistics based on the “maxstat” package.
3.2 Association of CDAI with all-cause mortality in COPD
During a median follow-up period of 92 months, 602 out of the 1,534 COPD patients died of all-cause mortality. In Model 1, we observed that for each unit increase in CDAI, the risk of all-cause mortality decreased by 7% (HR = 0.93, 95% CI: 0.91–0.96). After adjusting for multiple factors, each additional unit increase in CDAI was associated with a 5% reduction in all-cause mortality risk (Model 2, HR = 0.95, 95% CI: 0.92–0.98; Model 3, HR = 0.95, 95% CI: 0.92–0.97) (Table 2). When CDAI was categorized into high CDAI group and low CDAI group, Model 1, we found that COPD patients in the high CDAI group had a 41% lower risk of all-cause mortality compared to those in the low CDAI group (HR = 0.59, 95% CI: 0.46–0.77). The association between high CDAI and lower mortality risk remained in Models 2 and 3 even after controlling for several variables. COPD patients in the high CDAI group had a 35% lower probability of dying from all causes than those in the low CDAI group (Model 2: HR = 0.65, 95% CI: 0.50–0.85; Model 3: HR = 0.65, 95% CI: 0.50–0.85) (Table 2).
Table 2
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| All-cause mortality | ||||||
| CDAI | 0.93 (0.91,0.96) | <0.0001 | 0.95 (0.92,0.98) | <0.001 | 0.95 (0.92,0.97) | <0.001 |
| CDAI category | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.59 (0.46,0.77) | <0.0001 | 0.65 (0.50,0.85) | 0.002 | 0.65 (0.49,0.85) | 0.002 |
The relationships between CDAI and all-cause mortality in COPD.
Data were present as odds ratio (OR) and 95% confidence interval (95%CI). Model 1, Sex, age, and race were adjusted. Model 2, Sex, age, race, education, smoking, PIR, insurance, and drinking were adjusted. Model 3, Sex, age, race, education, smoking, PIR, insurance, drinking, BMI, diabetes, and hypertension were adjusted. Ref, reference; PIR, poverty income ratio; BMI, body mass index; CDAI, composite dietary antioxidant index. Q1: CDAI < 1.19, Q2: CDAI ≥ 1.19.
Using restricted cubic splines (RCS), it was found that CDAI and mortality from all causes were linearly associated (P for overall = 0.0007, P for non-linearity =0.1559) (Figure 3A).
Figure 3
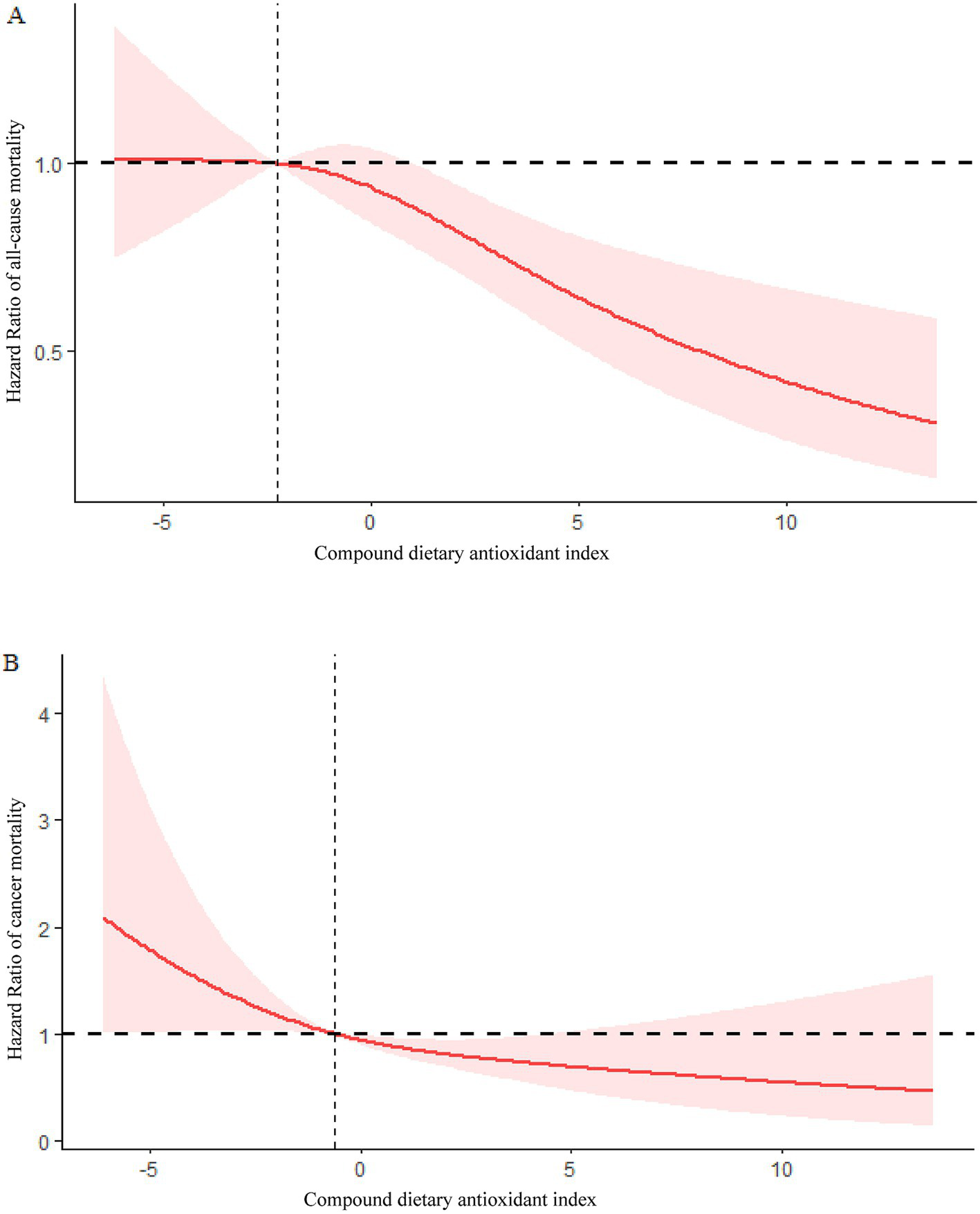
(A) Association between CDAI and risk of COPD all-cause mortality in the 1999–2018 NHANES survey. (B) Association between CDAI and risk of COPD cancer mortality in the 1999–2018 NHANES survey.
Through subgroup analyses based on age, sex, race, BMI, and smoking status, we further investigated the relationship between CDAI values and all-cause mortality in individuals with COPD. The findings showed no significant interactions across subgroups and that the association between CDAI and all-cause mortality among COPD patients remained constant (p > 0.05) (Table 3).
Table 3
| Character | Q1 | Q2 | P | P for interaction |
|---|---|---|---|---|
| Sex, n (%) | 0.07 | |||
| Female | Ref | 0.78 (0.52,1.16) | 0.22 | |
| Male | Ref | 0.44 (0.30,0.66) | <0.0001 | |
| Age, years, n (%) | 0.81 | |||
| 40–64 | Ref | 0.63 (0.36,1.08) | 0.09 | |
| ≥65 | Ref | 0.58 (0.42,0.81) | 0.001 | |
| Race, n (%) | 0.09 | |||
| White | Ref | 0.53 (0.39,0.71) | <0.0001 | |
| Others | Ref | 1.00 (0.56,1.79) | 1 | |
| BMI, kg/m2, n (%) | 0.23 | |||
| < 25 | Ref | 0.57 (0.36,0.92) | 0.02 | |
| 25–30 | Ref | 0.40 (0.23,0.68) | <0.001 | |
| > 30 | Ref | 0.76 (0.47,1.24) | 0.28 | |
| Smoking status, n (%) | 0.06 | |||
| Former | Ref | 0.43 (0.30,0.62) | <0.0001 | |
| Never | Ref | 0.58 (0.28,1.21) | 0.15 | |
| Now | Ref | 0.87 (0.54,1.40) | 0.57 | |
Subgroup analysis of CDAI and all-cause mortality in COPD.
BMI, body mass index; 95% CI, 95% confidence interval; OR, odds ratio; Ref: Reference.
3.3 Association of CDAI with cancer mortality in COPD
A total of 1,083 COPD patients were included in the analysis of the association between CDAI and cancer mortality, with 151 patients dying from cancer. Further Cox regression analysis also indicated a significant association between CDAI and cancer mortality. In Model 1, we observed that for each unit increase in CDAI, the risk of cancer mortality decreased by 9% (HR = 0.91, 95% CI: 0.84–0.98). Following additional correction for several variables, every unit rise in CDAI was linked to an 8% (Model 2: HR = 0.92, 95% CI: 0.85–0.99) and 9% (Model 3: HR = 0.91, 95% CI: 0.85–0.98) reduction in cancer mortality risk (Table 4). Model 1 showed that the high CDAI group had a 63% lower risk of cancer mortality when CDAI was handled as a categorical variable (HR = 0.37, 95% CI: 0.22–0.63) compared to the low CDAI group. In Model 2, these statistical associations remained significant (HR = 0.41, 95% CI: 0.24–0.70). In Model 3, the relationship between high CDAI and reduced mortality risk persisted, with cancer mortality risk in the high CDAI group being reduced by 61% compared to the low CDAI group (HR = 0.39, 95% CI: 0.23–0.68) (Table 4).
Table 4
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Cancer mortality | ||||||
| CDAI | 0.91 (0.84,0.98) | 0.01 | 0.92 (0.85,0.99) | 0.03 | 0.91 (0.85,0.98) | 0.01 |
| CDAI category | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.37 (0.22,0.63) | <0.001 | 0.41 (0.24,0.70) | 0.001 | 0.39 (0.23,0.68) | <0.001 |
The relationships between CDAI and cancer mortality in COPD.
Data were present as odds ratio (OR) and 95% confidence interval (95%CI). Model 1, Sex, age, and race were adjusted. Model 2, Sex, age, race, education, smoking, PIR, insurance, and drinking were adjusted. Model 3, Sex, age, race, education, smoking, PIR, insurance, drinking, BMI, diabetes, and hypertension were adjusted. Ref, reference; PIR, poverty income ratio; BMI, body mass index; CDAI, composite dietary antioxidant index. Q1: CDAI < 1.19, Q2: CDAI ≥ 1.19.
Based on RCS analysis, CDAI and cancer mortality were linearly associated in COPD patients (P for overall = 0.0145, P for non-linearity = 0.3948) (Figure 3B).
Furthermore, subgroup analyses by age, sex, race, BMI, and smoking status showed no significant interactions between subgroups and that the association between CDAI and cancer mortality in COPD patients remained constant (p > 0.05) (Table 5).
Table 5
| Character | Q1 | Q2 | P | P for interaction |
|---|---|---|---|---|
| Sex, n (%) | 0.28 | |||
| Female | Ref | 0.58 (0.24,1.39) | 0.22 | |
| Male | Ref | 0.30 (0.16,0.56) | <0.001 | |
| Age, years, n (%) | 0.87 | |||
| 40–64 | Ref | 0.36 (0.15,0.84) | 0.02 | |
| ≥65 | Ref | 0.36 (0.15,0.84) | 0.02 | |
| Race, n (%) | 0.27 | |||
| White | Ref | 0.36 (0.20,0.66) | <0.001 | |
| Others | Ref | 0.72 (0.32,1.61) | 0.42 | |
| BMI, kg/m2, n (%) | 0.16 | |||
| < 25 | Ref | 0.55 (0.23,1.31) | 0.17 | |
| 25–30 | Ref | 0.16 (0.06,0.42) | <0.001 | |
| > 30 | Ref | 0.47(0.21,1.04) | 0.06 | |
| Smoking status, n (%) | 0.52 | |||
| Former | Ref | 0.29 (0.15,0.59) | <0.001 | |
| Never | Ref | 0.55 (0.26,1.17) | 0.12 | |
| Now | Ref | 0.42 (0.07,2.54) | 0.35 | |
Subgroup analysis of CDAI and cancer mortality in COPD.
BMI, body mass index; 95% CI, 95% confidence interval; OR, odds ratio; Ref: Reference.
3.4 Sensitivity analyses
In the sensitivity analyses, after excluding COPD patients who died within two years of the start of follow-up, the results regarding the associations between CDAI and both all-cause mortality and cancer mortality in COPD patients remained unchanged (Table 6).
Table 6
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| All-cause mortality | ||||||
| CDAI | 0.94 (0.89,0.98) | 0.004 | 0.94(0.90,0.98) | 0.01 | 0.93 (0.89,0.98) | 0.003 |
| CDAI category | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.61 (0.42,0.90) | 0.01 | 0.63 (0.43,0.92) | 0.02 | 0.62 (0.42,0.92) | 0.02 |
| Cancer mortality | ||||||
| CDAI | 0.87 (0.78,0.97) | 0.01 | 0.85 (0.74, 0.96) | 0.01 | 0.83 (0.73, 0.95) | 0.01 |
| CDAI category | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.22 (0.08,0.56) | 0.002 | 0.17 (0.06, 0.49) | 0.001 | 0.16 (0.05, 0.48) | 0.001 |
Sensitivity analysis of CDAI and all-cause and cancer mortality in COPD.
Data were present as odds ratio (OR) and 95% confidence interval (95%CI). Model 1, Sex, age, and race were adjusted. Model 2, Sex, age, race, education, smoking, PIR, insurance, and drinking were adjusted. Model 3, Sex, age, race, education, smoking, PIR, insurance, drinking, BMI, Diabetes, and Hypertension were adjusted. Ref, reference; PIR, poverty income ratio; BMI, body mass index; CDAI, composite dietary antioxidant index. Q1: CDAI < 1.19, Q2: CDAI ≥ 1.19.
4 Discussion
Data from the NHANES were analyzed from 1999 to 2018 to examine the potential associations between CDAI and both all-cause and cancer mortality in US adults with COPD over the age of 40. We discovered that, in this cohort, CDAI was negatively correlated with the chances of both cancer and all-cause mortality after controlling for all confounding variables. Patients with COPD who scored higher on the CDAI were less likely to die from cancer and all causes. Additionally, stratified analyses revealed no variables that significantly affected the outcomes, suggesting that the association between CDAI and cancer and all-cause death is constant across subgroups. These findings confirm CDAI’s potential predictive role as a predictor of mortality in COPD patients.
As an innovative multidimensional tool, the CDAI systematically integrates concentrations of redox-active nutrients including water-soluble vitamins, trace elements, and polyphenolic compounds, thereby enabling standardized assessment of total dietary antioxidant defense capacity across populations (7). This concept is supported by numerous studies that have explored the relationship between dietary antioxidants and health outcomes. CDAI is a valuable tool for evaluating the relationship between a diet rich in antioxidants and various diseases. Studies have shown that a higher CDAI is associated with a reduced risk of developing multiple diseases, including hypertension, cancer, and obesity (16). The index assesses the intake of multiple dietary antioxidants, providing a comprehensive measure of antioxidant consumption and its potential health benefits. These studies suggest that an increase in the CDAI score is associated with a reduced likelihood of certain diseases. For instance, one study showed a favorable correlation between forced expiratory volume in one second (FEV1) and vitamin C, vitamin E, and total carotenoids (8). This indicates that CDAI may lower the risk of COPD by improving lung function (17). Another cross-sectional study observed that higher CDAI values are linked to a lower risk of all-cause and cancer-specific mortality in cancer survivors (3). According to our findings, modulating the CDAI can reduce COPD patients’ mortality due to all causes and cancer-specific causes. The association between antioxidant-rich diets and COPD remains robust even when age, sex, smoking status, and ethnicity are taken into account. This suggests the possibility of using antioxidant-rich diets as a primary prevention strategy. While our study primarily investigated dietary antioxidants in COPD progression, it is important to acknowledge the potential interplay of other modifiable lifestyle factors. Previous studies have demonstrated that physical activity levels are independently associated with reduced COPD exacerbations, and adherence to inhaled medications significantly improves lung function decline (18). Additionally, overall diet quality may exert synergistic effects beyond isolated antioxidants (19). The mechanism by which CDAI reduces the risk of COPD death remains unclear. One possibility is that circulating antioxidants may have direct effects on lung function. Alternatively, dietary antioxidants might lower COPD risk by reducing lung inflammation through the modulation of inflammatory markers like alkaline phosphatase and C-reactive protein (20).
Oxidative stress refers to a condition characterized by an elevated level of exogenous reactive oxygen and nitrogen species within the organism, leading to indiscriminate reactions with cellular components and resulting in oxidative damage, including DNA strand breaks, protein denaturation, and lipid peroxidation (21). This imbalance disrupts the homeostasis of the antioxidant defense system in the body, thereby triggering or accelerating inflammatory processes. Pro-inflammatory cytokines, reactive oxygen species, and oxidatively modified molecules produced during the inflammatory response further exacerbate oxidative stress, creating a vicious cycle (22). The body needs antioxidants to keep the balance between harmful reactive oxygen species and beneficial antioxidants. Research suggests that increasing antioxidant intake through diet can lower the prevalence of respiratory conditions (23). Additionally, oxidative stress-related inflammation and a reduction in endogenous antioxidant enzymes are also important mechanisms in the pathogenesis of certain malignancies (24). Multiple studies have shown that a diet rich in antioxidants has significant preventive and alleviating effects on COPD (25). This diet improves lung function by reducing the generation of free radicals and mitigating inflammation associated with oxidative stress, thereby lowering the incidence of COPD and slowing its progression. Antioxidants can neutralize reactive oxygen species in the body, inhibit oxidative damage, and maintain the structure and function of cells.
The pathogenesis of COPD is complex, and the role of CDAI in systemic inflammation may influence the risk of COPD through multiple mechanisms. Dietary fiber regulates the gut microbiome, promoting the production of anti-inflammatory metabolic products such as short-chain fatty acids, which help alleviate systemic inflammation (26). Additionally, dietary fiber can enhance immune function, improve resistance to oxidative stress, reduce the incidence of pulmonary inflammation, and regulate glucose and lipid metabolism, thereby decreasing the risk of metabolic diseases associated with COPD (27). Experimental evidence suggests that antioxidants modulate NF-κB signaling—a key pathway driving pro-inflammatory cytokine production in COPD lungs (28). By suppressing NF-κB activation, antioxidants may reduce neutrophil infiltration and mucus hypersecretion (29). In addition, animal models demonstrate that dietary antioxidants accelerate alveolar repair by promoting epithelial cell proliferation and inhibiting fibroblast-to-myofibroblast transition (30). These mechanisms collectively suggest that systemic antioxidants exert both protective and regenerative effects on lung tissue. The inverse association between dietary fiber intake and the risk of COPD mortality can be explained by these processes taken together, underscoring the potential benefit of nutritional interventions in lowering the risk of COPD-related death. Research has shown that higher dietary fiber intake is linked to lower levels of pro-inflammatory mediators, such as IL-6 and CRP (31). Furthermore, by controlling the innate immune system through the gut-liver-lung axis, dietary fiber may lower the risk of COPD (32). Vitamins A and α-tocopherol strengthen the antioxidant qualities of vitamins A, C, and E by reducing lipid peroxidation and neutralizing free radicals (33). A cross-sectional study found that adequate intake of vitamin A can delay the progression of COPD by improving lung function, thereby enhancing patients’ quality of life and reducing mortality rates (34). Similar to vitamin A, vitamin C maintains epithelial barrier integrity and affects immune cell function, thereby modulating cellular redox reactions (35). An experimental study on lung repair after tobacco-induced emphysema examined the impact of vitamin C supplementation in mice. Compared to the control group, the vitamin C supplementation group showed reduced activities of catalase, superoxide dismutase, and matrix metalloproteinases, along with lower levels of tumor necrosis factor-α, suggesting less lung tissue damage (36). Additionally, some studies have found a close association between vitamin C levels and all-cause mortality in patients with COPD (37). Additionally, Luo et al. found that vitamin C may serve as a protective factor against malignancies, including lung cancer. An increase of 100 mg/day in daily vitamin C intake correlates with a 7% decrease in the likelihood of lung cancer development (38). Vitamin E helps prevent lipid peroxidation. Studies have shown that increasing vitamin E intake is associated with a reduced prevalence of COPD (39). This data is consistent with a large cross-sectional investigation by Liu et al. that found a negative relationship between the incidence of COPD and vitamin E intake (40). The mechanism by which vitamin E reduces the risk of COPD is not fully understood. However, it is hypothesized that vitamin E may negatively regulate the epidermal growth factor receptor/mitogen-activated protein kinase (EGFR/MAPK) axis and inhibit cyclooxygenase 2 (COX2)-mediated nuclear translocation of phosphorylated STAT3. This regulation may help alleviate inflammation, apoptosis, and ROS in lung tissue caused by cigarette smoking, ultimately reducing the risk of acute exacerbation of COPD (41). Furthermore, intracellular antioxidant enzymes require zinc and selenium as cofactors. The respiratory system is supported by their ability to reduce inflammation and promote tissue repair (42). There is a link between the deficiency of these minerals and impaired respiratory function, as well as an increased chance of respiratory infection. Carotenoids, antioxidant pigments found in various foods, have been shown to protect the respiratory system by reducing the risk of asthma and COPD (43). Astaxanthin (AXT) is a natural carotenoid known for its antioxidant and anti-inflammatory properties. Research indicates that AXT can alleviate cigarette-induced emphysema through a SIRT1-dependent mechanism (44). After binding with SIRT1, AXT promotes the deacetylation of both SIRT1 and NRF2, leading to the production of antioxidant enzymes that reduce oxidative stress. Additionally, it inhibits the transcriptional activity of p65 NF-κB, thereby suppressing inflammatory responses (45). Another study has shown that AXT can improve the redox balance in cancer cells by targeting signaling molecules within tumor-associated signaling pathways, thereby inducing apoptosis and slowing the onset and progression of tumors (46). Therefore, increasing the intake of CDAI can help reduce all-cause mortality and cancer mortality in COPD patients.
This study shows notable strengths in several dimensions. First of all, this is the first time that the association between CDAI and the mortality rates from all causes and cancer in patients with COPD has been investigated using a large-scale, nationally representative dataset. Significantly, the results of the study are robust when based on a nationally representative sample for weighted analysis. Secondly, the sample size of this study is large and representative of the whole country.
Although our study has several limitations, these limitations should be considered carefully when interpreting and evaluating the results. Firstly, the NHANES database’s self-reported surveys serve as the main source of data, which could introduce memory bias and compromise data accuracy. Secondly, the process of sample selection excluded data with incomplete covariables, a practice that potentially omitted certain populations, thereby affecting the representativeness of the sample and the completeness of the analysis results. Moreover, although multivariate analysis was implemented to control for many known and potential confounding factors, there remain unaddressed potential residual confounders, particularly those that are difficult to measure. Given these limitations, future research should be based on large-scale longitudinal studies to enhance the validation of the findings. Furthermore, a more thorough knowledge of the mechanisms behind the association between CDAI and the mortality rates from all causes and cancer in individuals with COPD requires extensive basic study. Through these further studies, we can expect to obtain more solid scientific evidence, thereby contributing to more precise strategies for the prevention and management of chronic diseases.
5 Conclusion
In conclusion, we found that higher CDAI scores were negatively associated with all-cause mortality and cancer-specific mortality in patients with COPD.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The National Center for Health Statistics Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WL: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. JB: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft. YG: Methodology, Software, Visualization, Writing – original draft. YF: Methodology, Software, Visualization, Writing – original draft. QH: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing. ZD: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by a funded research project of the Zigong First People's Hospital (Zigong Academy of Medical Sciences) (grant nos. 2023YLWS21, 2023YKY07, ZGYKY22KF001, and ZGYKY22KF005).
Acknowledgments
We would like to thank the US Centers for Disease Control and Prevention for collecting the data and making the data available to the public.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Collaborators USBoD Mokdad AH Ballestros K Echko M Glenn S Olsen HE et al . The State of US Health, 1990-2016: Burden of Diseases, Injuries, and Risk Factors Among. JAMA. (2018) 319:1444–72. doi: 10.1001/jama.2018.0158
2.
Venkatesan P . GOLD COPD report: 2025 update. Lancet Respir Med. (2025) 13:e7–8. doi: 10.1016/S2213-2600(24)00413-2
3.
Tan Z Meng Y Li L Wu Y Liu C Dong W et al . Association of Dietary Fiber, composite dietary antioxidant index and risk of death in tumor survivors: National Health and nutrition examination survey 2001-2018. Nutrients. (2023) 15:2968. doi: 10.3390/nu15132968
4.
Janjua S Pike KC Carr R Coles A Fortescue R Batavia M . Interventions to improve adherence to pharmacological therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. (2021) 2021:CD013381. doi: 10.1002/14651858.CD013381.pub2
5.
Wu D Wang H Wang W Qing C Zhang W Gao X et al . Association between composite dietary antioxidant index and handgrip strength in American adults: Data from National Health and nutrition examination survey (NHANES, 2011-2014). Front Nutr. (2023) 10:1147869. doi: 10.3389/fnut.2023.1147869
6.
Liu Z Ren Z Zhang J Chuang CC Kandaswamy E Zhou T et al . Role of ROS and Nutritional antioxidants in human diseases. Front Physiol. (2018) 9:477. doi: 10.3389/fphys.2018.00477
7.
Xiong B Wang J He R Qu G . Composite dietary antioxidant index and sleep health: a new insight from cross-sectional study. BMC Public Health. (2024) 24:609. doi: 10.1186/s12889-024-18047-2
8.
Wright ME Mayne ST Stolzenberg-Solomon RZ Li Z Pietinen P Taylor PR et al . Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. (2004) 160:68–76. doi: 10.1093/aje/kwh173
9.
Kweon S Kim Y Jang MJ Kim Y Kim K Choi S et al . Data resource profile: the Korea National Health and nutrition examination survey (KNHANES). Int J Epidemiol. (2014) 43:69–77. doi: 10.1093/ije/dyt228
10.
Zhou H Li T Li J Zheng D Yang J Zhuang X . Linear association of compound dietary antioxidant index with hyperlipidemia: a cross-sectional study. Front Nutr. (2024) 11:1365580. doi: 10.3389/fnut.2024.1365580
11.
Zeng G You D Ye L Wu Y Shi H Lin J et al . N-3 PUFA poor seafood consumption is associated with higher risk of gout, whereas n-3 PUFA rich seafood is not: NHANES 2007-2016. Front Nutr. (2023) 10:1075877. doi: 10.3389/fnut.2023.1075877
12.
Kang H Lee JP Choi K . Exposure to phthalates and environmental phenols in association with chronic kidney disease (CKD) among the general US population participating in multi-cycle NHANES (2005-2016). Sci Total Environ. (2021) 791:148343. doi: 10.1016/j.scitotenv.2021.148343
13.
Chen Z Li W Tang Y Zhou P He Q Deng Z . The neutrophil-lymphocyte ratio predicts all-cause and cardiovascular mortality among United States adults with COPD: results from NHANES 1999-2018. Front Med (Lausanne). (2024) 11:1443749. doi: 10.3389/fmed.2024.1443749
14.
Seckinger A Meissner T Moreaux J Depeweg D Hillengass J Hose K et al . Clinical and prognostic role of annexin A2 in multiple myeloma. Blood. (2012) 120:1087–94. doi: 10.1182/blood-2012-03-415588
15.
Zhang L Chen S Wang W Wang Y Liang Y . Inflammatory and nutritional scoring system for predicting prognosis in patients with newly diagnosed multiple myeloma. J Inflamm Res. (2023) 16:7–17. doi: 10.2147/JIR.S390279
16.
Ma Y Griffith JA Chasan-Taber L Olendzki BC Jackson E Stanek EJ et al . Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. (2006) 83:760–6. doi: 10.1093/ajcn/83.4.760
17.
Hu G Cassano PA . Antioxidant nutrients and pulmonary function: the third National Health and nutrition examination survey (NHANES III). Am J Epidemiol. (2000) 151:975–81. doi: 10.1093/oxfordjournals.aje.a010141
18.
Cheng SWM McKeough Z Alison J Dennis S Hamer M Stamatakis E . Associations of total and type-specific physical activity with mortality in chronic obstructive pulmonary disease: a population-based cohort study. BMC Public Health. (2018) 18:268. doi: 10.1186/s12889-018-5167-5
19.
English LK Ard JD Bailey RL Bates M Bazzano LA Boushey CJ et al . Evaluation of dietary patterns and all-cause mortality: a systematic review. JAMA Netw Open. (2021) 4:e2122277. doi: 10.1001/jamanetworkopen.2021.22277
20.
Liu Z Li J Chen T Zhao X Chen Q Xiao L et al . Association between dietary antioxidant levels and chronic obstructive pulmonary disease: a mediation analysis of inflammatory factors. Front Immunol. (2023) 14:1310399. doi: 10.3389/fimmu.2023.1310399
21.
Liu W Wang J Wang M Hou H Ding X Ma L et al . Oxidative stress factors mediate the association between Life's essential 8 and accelerated phenotypic aging: NHANES 2005-2018. J Gerontol A Biol Sci Med Sci. (2024) 79:240. doi: 10.1093/gerona/glad240
22.
Antus B Kardos Z . Oxidative stress in COPD: molecular background and clinical monitoring. Curr Med Chem. (2015) 22:627–50. doi: 10.2174/092986732205150112104411
23.
Salo PM Mendy A Wilkerson J Molsberry SA Feinstein L London SJ et al . Serum antioxidant vitamins and respiratory morbidity and mortality: a pooled analysis. Respir Res. (2022) 23:150. doi: 10.1186/s12931-022-02059-w
24.
Yu YC Paragomi P Wang R Jin A Schoen RE Sheng LT et al . Composite dietary antioxidant index and the risk of colorectal cancer: findings from the Singapore Chinese health study. Int J Cancer. (2022) 150:1599–608. doi: 10.1002/ijc.33925
25.
Ferrari CK Percario S Silva JC da Silva Torres EA . An apple plus a Brazil nut a day keeps the doctors away: antioxidant capacity of foods and their health benefits. Curr Pharm Des. (2016) 22:189–95. doi: 10.2174/1381612822666151117122715
26.
Sonnenburg JL Backhed F . Diet-microbiota interactions as moderators of human metabolism. Nature. (2016) 535:56–64. doi: 10.1038/nature18846
27.
Blaak EE Canfora EE Theis S Frost G Groen AK Mithieux G et al . Short chain fatty acids in human gut and metabolic health. Benef Microbes. (2020) 11:411–55. doi: 10.3920/BM2020.0057
28.
Scoditti E Massaro M Garbarino S Toraldo DM . Role of diet in chronic obstructive pulmonary disease prevention and treatment. Nutrients. (2019) 11:1357. doi: 10.3390/nu11061357
29.
Sanap A Bhonde R Joshi K . Conditioned medium of adipose derived mesenchymal stem cells reverse insulin resistance through downregulation of stress induced serine kinases. Eur J Pharmacol. (2020) 881:173215. doi: 10.1016/j.ejphar.2020.173215
30.
Noe J Petrusca D Rush N Deng P VanDemark M Berdyshev E et al . CFTR regulation of intracellular pH and ceramides is required for lung endothelial cell apoptosis. Am J Respir Cell Mol Biol. (2009) 41:314–23. doi: 10.1165/rcmb.2008-0264OC
31.
Ma Y Hebert JR Li W Bertone-Johnson ER Olendzki B Pagoto SL et al . Association between dietary fiber and markers of systemic inflammation in the Women's Health Initiative observational study. Nutrition. (2008) 24:941–9. doi: 10.1016/j.nut.2008.04.005
32.
Young RP Hopkins RJ Marsland B . The gut-liver-lung Axis. Modulation of the innate immune response and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. (2016) 54:161–9. doi: 10.1165/rcmb.2015-0250PS
33.
Bishopp A Sathyamurthy R Manney S Webbster C Krishna MT Mansur AH . Biomarkers of oxidative stress and antioxidants in severe asthma: a prospective case-control study. Ann Allergy Asthma Immunol. (2017) 118:445–51. doi: 10.1016/j.anai.2017.02.004
34.
Shen T Bimali M Faramawi M Orloff MS . Consumption of vitamin K and vitamin a are associated with reduced risk of developing emphysema: NHANES 2007-2016. Front Nutr. (2020) 7:47. doi: 10.3389/fnut.2020.00047
35.
Carr AC , Maggini S. Vitamin C and immune function. Nutrients(2017) 9:1211. doi: 10.3390/nu9111211
36.
Valenca SS Bezerra FS Romana-Souza B Paiva RO Costa AM Porto LC . Supplementation with vitamins C and E improves mouse lung repair. J Nutr Biochem. (2008) 19:604–11. doi: 10.1016/j.jnutbio.2007.08.004
37.
Bates CJ Hamer M Mishra GD . Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and nutrition survey of people aged 65 years and over. Br J Nutr. (2011) 105:123–32. doi: 10.1017/S0007114510003053
38.
Luo J Shen L Zheng D . Association between vitamin C intake and lung cancer: a dose-response meta-analysis. Sci Rep. (2014) 4:6161. doi: 10.1038/srep06161
39.
Agler AH Kurth T Gaziano JM Buring JE Cassano PA . Randomised vitamin E supplementation and risk of chronic lung disease in the Women's health study. Thorax. (2011) 66:320–5. doi: 10.1136/thx.2010.155028
40.
Liu Z Su Y Chen Q Xiao L Zhao X Wang F et al . Association of Dietary intake of vitamin E with chronic obstructive pulmonary disease events in US adults: a cross-sectional study of NHANES 2013-2018. Front Nutr. (2023) 10:1124648. doi: 10.3389/fnut.2023.1124648
41.
Zhao H Gong J Li L Zhi S Yang G Li P et al . Vitamin E relieves chronic obstructive pulmonary disease by inhibiting COX2-mediated p-STAT3 nuclear translocation through the EGFR/MAPK signaling pathway. Lab Investig. (2022) 102:272–80. doi: 10.1038/s41374-021-00652-z
42.
Riccioni G D'Orazio N . The role of selenium, zinc and antioxidant vitamin supplementation in the treatment of bronchial asthma: adjuvant therapy or not?Expert Opin Investig Drugs. (2005) 14:1145–55. doi: 10.1517/13543784.14.9.1145
43.
Manochkumar J Singh A Efferth T Ramamoorthy S . Untapping the protective role of carotenoids against respiratory diseases. Phytomedicine. (2022) 104:154286. doi: 10.1016/j.phymed.2022.154286
44.
Deng M Tong R Bian Y Hou G . Astaxanthin attenuates cigarette smoking-induced oxidative stress and inflammation in a sirtuin 1-dependent manner. Biomed Pharmacother. (2023) 159:114230. doi: 10.1016/j.biopha.2023.114230
45.
Patel S Khan H Majumdar A . Crosstalk between Sirtuins and Nrf2: SIRT1 activators as emerging treatment for diabetic neuropathy. Metab Brain Dis. (2022) 37:2181–95. doi: 10.1007/s11011-022-00956-z
46.
Kavitha K Kowshik J Kishore TK Baba AB Nagini S . Astaxanthin inhibits NF-kappaB and Wnt/beta-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim Biophys Acta. (2013) 1830:4433–44. doi: 10.1016/j.bbagen.2013.05.032
Summary
Keywords
COPD, CDAI, all-cause mortality, cancer mortality, NHANES
Citation
Li W, Bai J, Ge Y, Fan Y, Huang Q and Deng Z (2025) Association between compound dietary antioxidant index and all-cause and cancer mortality in patients with chronic obstructive pulmonary disease: results from NHANES 1999–2018. Front. Med. 12:1544841. doi: 10.3389/fmed.2025.1544841
Received
19 December 2024
Accepted
10 March 2025
Published
21 March 2025
Volume
12 - 2025
Edited by
Kurtis Francis Budden, The University of Newcastle, Australia
Reviewed by
Fedor Malykhin, Stavropol State Medical University, Russia
Georgios Spyropoulos, 424 General Military Hospital, Greece
Vrushali Chimankar, Royal Prince Alfred Hospital, Australia
Updates
Copyright
© 2025 Li, Bai, Ge, Fan, Huang and Deng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Huang, hq15196767955@163.comZhiping Deng, Zgsdzp1016@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.