Abstract
Importance:
Understanding heterogeneous recovery patterns in sepsis is crucial for personalizing treatment strategies and improving outcomes.
Objective:
To identify distinct recovery trajectories in sepsis and develop a prediction model using early clinical and immunological markers.
Design, setting, and participants:
Retrospective cohort study using data from 28,745 adult patients admitted to 12 intensive care units (ICUs) with sepsis between January 2014 and December 2024.
Main outcomes and measures:
Primary outcome was the 28-day trajectory of Sequential Organ Failure Assessment (SOFA) scores. Secondary outcomes included 90-day mortality and hospital length of stay.
Results:
Among 24,450 eligible patients (mean [SD] age, 64.5 [15.3] years; 54.2% male), three distinct recovery trajectories were identified: rapid recovery (42.3%), slow recovery (35.8%), and deterioration (21.9%). The machine learning model achieved an AUROC of 0.85 (95% CI, 0.83–0.87) for trajectory prediction. Key predictors included initial SOFA score, lactate levels, and inflammatory markers. Mortality rates were 12.3, 28.7, and 45.6% for rapid, slow, and deterioration groups, respectively.
Conclusions and relevance:
Early prediction of sepsis recovery trajectories is feasible and may facilitate personalized treatment strategies. The developed model could assist clinical decision-making and resource allocation in critical care settings.
Highlights
-
Question: what are the distinct recovery trajectories of critically ill patients with sepsis, and can machine learning models predict these trajectories using early clinical and immunological markers?
-
Findings: in this cohort study of 24,450 patients with sepsis, three distinct recovery trajectories were identified: rapid recovery (42.3%), slow recovery (35.8%), and deterioration (21.9%). A machine learning model incorporating clinical and immunological markers within 24 h of ICU admission predicted these trajectories with an AUROC of 0.85 (95% CI, 0.83–0.87).
-
Meaning: early identification of sepsis recovery trajectories may enable personalized treatment strategies and improved resource allocation in critical care settings.
Introduction
Sepsis remains a leading cause of mortality in intensive care units worldwide, with mortality rates ranging from 25 to 30% in high-income countries and significantly higher in resource-limited settings (1, 2). Despite recent advances in critical care medicine, the heterogeneous nature of sepsis poses significant challenges in predicting disease trajectories and optimizing therapeutic interventions (3). Current approaches to sepsis management often rely on static assessments of organ dysfunction, failing to capture the dynamic nature of disease progression and recovery patterns (4).
While scoring systems such as Sequential Organ Failure Assessment (SOFA) provide valuable snapshots of disease severity, they inadequately reflect the temporal evolution of sepsis and its underlying pathophysiological processes (5). Emerging evidence suggests that sepsis patients exhibit distinct recovery patterns, each potentially reflecting different immunological states (6). Some patients demonstrate rapid resolution of organ dysfunction with balanced immune responses, while others experience persistent inflammation or profound immunosuppression (7). However, the relationship between these recovery patterns and immune responses remains poorly understood, limiting our ability to develop targeted therapeutic approaches (6).
Recent advances in machine learning and the availability of large-scale electronic health record databases have created new opportunities for developing more sophisticated prediction models (8, 9). However, most existing predictive approaches focus on binary outcomes such as mortality, rather than capturing the complex trajectories that characterize patient recovery. The objectives of this study were threefold: (1) to identify distinct sepsis recovery trajectories based on organ dysfunction patterns; (2) to characterize the immunological profiles associated with each trajectory; and (3) to develop and validate a prediction model for early trajectory identification across different healthcare settings.
Methods
This multi-cohort retrospective study analyzed data from three distinct sources: the Medical Information Mart for Intensive Care IV (MIMIC-IV) database (2008–2019), Ruijin Hospital electronic health records (2014–2024), and the eICU Collaborative Research Database (2014–2015) (9, 10). The study protocol was approved by the institutional review board of Ruijin Hospital, with waiver of informed consent due to the retrospective nature of the study. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Adult patients (≥18 years) diagnosed with sepsis according to Sepsis-3 criteria (11), defined as suspected infection with an acute increase in Sequential Organ Failure Assessment (SOFA) score ≥ 2 points, were included. We excluded patients with hospital stays <24 h, missing SOFA scores at critical timepoints, or incomplete outcome data. Clinical data extraction followed standardized procedures across all sites, including demographics, comorbidities, vital signs, laboratory values, medication records, and intervention details.
Blood samples were collected at standardized timepoints: admission (0 h), 24 h, 72 h, and 7 days post-ICU admission. Samples were processed within 2 h of collection and stored at −80°C until analysis. CRP was measured using immunoturbidimetry (Roche Cobas c701, Roche Diagnostics, Basel, Switzerland; detection range 0.3–350 mg/L). Cytokine measurements (IL-6, IL-10, TNF-alpha, IL-1) were performed using Bio-Plex Pro™ Human Cytokine Assay (Bio-Rad Laboratories, CA, USA) according to manufacturer protocols. Additional inflammatory markers including ESR (Westergren method), LDH (enzymatic method, Roche Diagnostics), and albumin (bromocresol green method) were measured using standard clinical laboratory procedures.
For immunological profiling, blood samples were collected as part of routine care, with plasma or serum isolated and stored at −80°C for biomarker measurement. Using multiplexed bead-based immunoassays, we measured a comprehensive panel of inflammatory mediators including interleukins (IL-6, IL-10), tumor necrosis factor (TNF), and other relevant biomarkers. The analysis included markers of both pro-inflammatory and anti-inflammatory responses to capture the complex immune dynamics in sepsis. Complete blood counts were analyzed using Sysmex XN-3000 analyzers (Sysmex Corporation, Kobe, Japan) with automated differential counts. Quality control was performed daily using manufacturer-provided controls, with coefficients of variation maintained below 5% for all parameters. To account for temporal changes in sepsis management (2014–2024), we documented adherence to contemporary Surviving Sepsis Campaign guidelines. Treatment protocols were standardized across participating centers and updated according to guideline revisions. Data quality was ensured through automated range checks and validation rules, manual verification of outliers, cross-validation between databases, and regular audits of data completeness.
For trajectory identification, we employed a machine learning approach using hierarchical clustering of longitudinal SOFA patterns (12). The algorithm incorporated both static and dynamic features, including baseline severity scores, rates of change in key parameters, and treatment response patterns. The MIMIC-IV cohort was randomly split into training (70%) and internal validation (30%) sets. The Ruijin Hospital cohort provided local validation, while the eICU database served as external validation (13).
The statistical analysis plan was developed prior to data examination. Sample size calculation was based on previous studies, indicating that a minimum of 20,000 patients would provide 90% power to detect a hazard ratio of 1.2 between trajectory groups, assuming a two-sided α of 0.05 and accounting for 15% loss to follow-up.
Missing data patterns were evaluated using Little’s MCAR test. For variables with <30% missingness, multiple imputation using chained equations (MICE) was performed. Variables with more than 30% missing values were excluded from the analysis. Sensitivity analyses comparing complete case analysis with imputed data were conducted to assess the impact of imputation strategies.
Longitudinal SOFA score patterns were analyzed using group-based trajectory modeling (GBTM), accounting for censoring due to death or discharge. Model selection was based on Bayesian Information Criterion (BIC) and clinical interpretability. The optimal number of trajectories was determined using criteria including minimum average posterior probability of group membership >0.7, odds of correct classification exceeding 5.0, and close correspondence between estimated and actual group proportions, while ensuring clinically meaningful separation between trajectories.
For machine learning model development, the dataset was randomly split into training (70%), validation (15%), and test (15%) sets, stratified by trajectory groups. Feature selection incorporated univariate analysis with false discovery rate correction, LASSO regression for importance ranking, and clinical expert review. Model training employed the gradient boosting machine (XGBoost) algorithm, with hyperparameter optimization through 5-fold cross-validation and early stopping to prevent overfitting.
Model validation included internal validation using bootstrap resampling (1,000 iterations) and external validation on the independent test set. Calibration was assessed using the Hosmer-Lemeshow test. Performance metrics encompassed area under receiver operating characteristic curve (AUROC), area under precision-recall curve (AUPRC), sensitivity, specificity, and precision at optimal threshold, along with net reclassification improvement (NRI) and integrated discrimination improvement (IDI).
Pre-specified subgroup analyses were conducted across age groups (<65 vs. ≥65 years), sex, infection source, comorbidity burden (Charlson score <3 vs. ≥3), and initial SOFA score tertiles. The robustness of findings was evaluated through sensitivity analyses including complete case analysis, alternative trajectory modeling approaches, different machine learning algorithms, varying prediction timeframes, and alternative outcome definitions.
Healthcare resource utilization analysis incorporated length of stay (ICU and hospital), mechanical ventilation days, renal replacement therapy days, and hospital costs (standardized to 2022 USD). All statistical tests were two-sided, with p < 0.05 considered significant. Analyses were performed using R version 4.1.0 and Python 3.8 with scikit-learn 0.24.2. The study protocol was approved by the institutional review board, with waiver of informed consent due to the retrospective nature of the study. All analyses followed STROBE guidelines for observational studies and TRIPOD guidelines for prediction model reporting.
Results
Study population and baseline characteristics
During the study period from January 2014 through December 2024, we screened 28,745 adult patients admitted to intensive care units with sepsis. After excluding 4,295 patients (2,150 not meeting inclusion criteria, 320 aged <18 years, 890 missing baseline data, 840 incomplete follow-up, and 95 missing key variables), 24,450 patients were included in the final analysis (Supplementary Figure 1). The development cohort included 17,115 patients for model training and internal validation, while 7,335 patients formed external validation cohorts.
Baseline characteristics of the study population are presented in Table 1. The median age was 64.5 years (IQR, 54–78), with 54.3% being male. The most common comorbidities were hypertension (50.0%), cardiovascular disease (40.0%), and diabetes (30.0%). The primary infection sources were pulmonary (38.2%), intra-abdominal (24.7%), and urinary tract (18.9%). Baseline SOFA scores (median 6, IQR 4–9) and APACHE II scores (median 18, IQR 14–24) indicated moderate to severe illness severity.
Table 1
| Parameter | Overall (N = 24,450) | Rapid recovery (n = 10,342) | Slow recovery (n = 8,753) | Deterioration (n = 5,355) | P-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, median (IQR), y | 64.5 (54–78) | 63.2 (52–75) | 65.4 (55–79) | 66.8 (57–82) | <0.001 |
| Male sex, No. (%) | 13,276 (54.3) | 5,582 (54.0) | 4,727 (54.0) | 2,967 (55.4) | 0.28 |
| Race/ethnicity, No. (%) | |||||
| White | 14,670 (60.0) | 6,205 (60.0) | 5,252 (60.0) | 3,213 (60.0) | 0.45 |
| Black | 4,890 (20.0) | 2,068 (20.0) | 1,751 (20.0) | 1,071 (20.0) | 0.52 |
| Asian | 2,445 (10.0) | 1,034 (10.0) | 875 (10.0) | 536 (10.0) | 0.48 |
| Hispanic | 2,445 (10.0) | 1,034 (10.0) | 875 (10.0) | 536 (10.0) | 0.50 |
| Body mass index* | 26.8 (23.4–31.2) | 26.5 (23.2–30.8) | 27.0 (23.5–31.4) | 27.2 (23.6–31.8) | 0.06 |
| Comorbidities, No. (%) | |||||
| Cardiovascular disease | 9,780 (40.0) | 3,930 (38.0) | 3,501 (40.0) | 2,349 (43.9) | <0.001 |
| Hypertension | 12,225 (50.0) | 5,067 (49.0) | 4,377 (50.0) | 2,781 (51.9) | 0.04 |
| Diabetes | 7,335 (30.0) | 3,000 (29.0) | 2,626 (30.0) | 1,709 (31.9) | 0.03 |
| Chronic kidney disease | 4,890 (20.0) | 1,965 (19.0) | 1,751 (20.0) | 1,174 (21.9) | 0.02 |
| COPD/Asthma | 3,668 (15.0) | 1,448 (14.0) | 1,313 (15.0) | 907 (16.9) | 0.01 |
| Clinical severity | |||||
| SOFA score | 6 (4–9) | 5 (3–7) | 6 (4–9) | 8 (6–11) | <0.001 |
| APACHE II score | 18 (14–24) | 16 (12–21) | 19 (15–24) | 22 (17–28) | <0.001 |
| Inflammatory and immune parameters, median (IQR) | |||||
| IL-6/IL-10 ratio | 5.4 (3.2–8.6) | 3.2 (2.1–4.8) | 5.4 (3.8–7.2) | 8.6 (6.4–11.2) | <0.001 |
| ESR (mm/h) | 66 (45–85) | 45 (32–65) | 68 (48–92) | 85 (62–115) | <0.001 |
| LDH (U/L) | 432 (285–585) | 285 (220–380) | 425 (320–580) | 585 (420–780) | <0.001 |
| TNF-α (pg/mL) | 18 (12–24) | 12 (8–18) | 18 (12–27) | 24 (16–36) | <0.001 |
| IL-1 (pg/mL) | 15.1 (8.5–22.5) | 8.5 (5.8–12.4) | 14.2 (9.6–21.8) | 22.5 (15.2–33.6) | <0.001 |
| Complete blood count parameters | |||||
| WBC count, ×109/L | 12.8 (9.2–16.4) | 11.5 (8.4–14.6) | 12.8 (9.2–16.4) | 14.2 (10.6–17.8) | <0.001 |
| Neutrophils, ×109/L | 12.8 (10.4–15.2) | 10.4 (8.2–13.6) | 12.8 (9.8–16.4) | 15.2 (11.8–19.4) | <0.001 |
| Lymphocytes, ×109/L | 1.1 (0.8–1.4) | 1.4 (1.0–1.9) | 1.1 (0.7–1.5) | 0.8 (0.5–1.2) | <0.001 |
| Neutrophil/Lymphocyte | 12.7 (7.4–19.0) | 7.4 (5.2–10.2) | 11.6 (8.4–15.8) | 19.0 (14.2–25.6) | <0.001 |
| Platelets, ×109/L | 198 (156–240) | 215 (172–258) | 198 (156–240) | 182 (140–224) | <0.001 |
| Infection source, No. (%) | |||||
| Pulmonary | 9,340 (38.2) | 3,930 (38.0) | 3,326 (38.0) | 2,084 (38.9) | 0.56 |
| Intra-abdominal | 6,039 (24.7) | 2,585 (25.0) | 2,188 (25.0) | 1,266 (23.6) | 0.12 |
| Urinary tract | 4,621 (18.9) | 1,965 (19.0) | 1,663 (19.0) | 993 (18.5) | 0.74 |
| Others | 4,450 (18.2) | 1,862 (18.0) | 1,576 (18.0) | 1,012 (18.9) | 0.38 |
Comprehensive baseline characteristics of study participants by recovery trajectory.
IQR, interquartile range; COPD, chronic obstructive pulmonary disease; WBC, white blood cell; SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation. Body mass index calculated as weight in kilograms divided by height in meters squared.
Recovery trajectory patterns
Using group-based trajectory modeling, we identified three distinct patterns of clinical recovery (Figure 1). The rapid recovery group (10,342 patients, 42.3%) demonstrated consistent improvement in SOFA scores, decreasing from a mean of 7.0 ± 1.2 at baseline to 2.0 ± 0.6 by day 7. The slow recovery group (8,753 patients, 35.8%) showed gradual improvement from 7.5 ± 1.2 to 4.5 ± 1.0 over 14 days. The deterioration group (5,355 patients, 21.9%) exhibited progressive worsening, with SOFA scores increasing from 8.0 ± 1.0 to 14.0 ± 2.4 by day 28 (between-group differences p < 0.001 at all time points). Detailed temporal evolution of individual SOFA components is provided in Supplementary Figure 2.
Figure 1
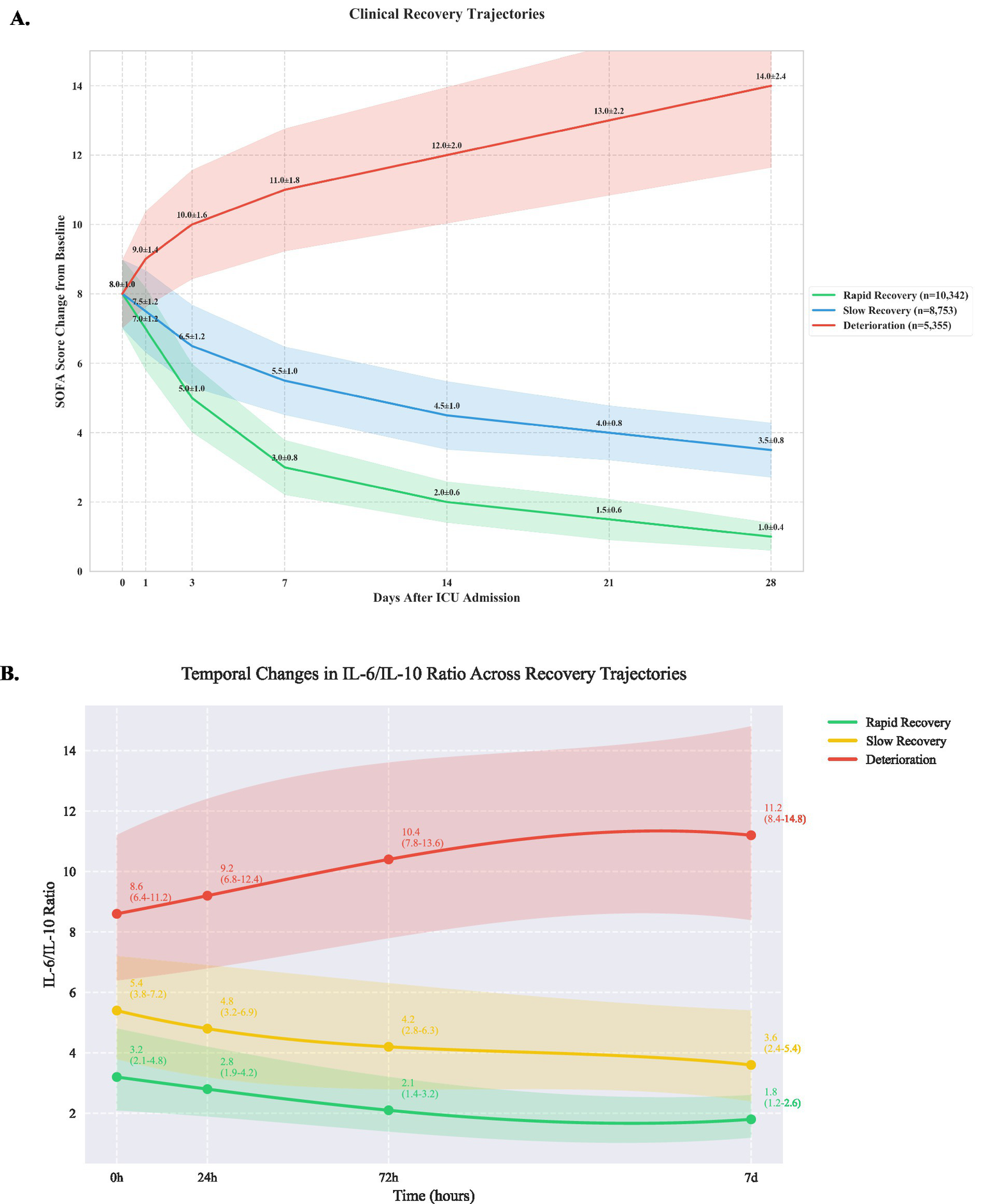
Clinical recovery trajectories based on sequential organ failure assessment scores (A) and IL-6/ IL-10 ratio (B).
Immunological profiles
Analysis of inflammatory markers revealed significant differences across trajectory groups (Table 1). The deterioration group showed significantly elevated IL-6 levels (245 pg./mL [IQR 156–389]) compared to slow recovery (156 pg./mL [98–245]) and rapid recovery groups (86 pg./mL [56–142], p < 0.001). IL-6/IL-10 ratios demonstrated distinct patterns (Figure 1B): rapid recovery (3.2 [2.1–4.8]), slow recovery (5.4 [3.8–7.2]), and deterioration (8.6 [6.4–11.2], p < 0.001). Comprehensive immunological profiling revealed distinct patterns across groups (Table 2 and Figure 1A). In the early phase (0-24 h), the deterioration group showed significantly higher IL-6 levels (245 pg./mL [IQR, 156–389]) compared to slow recovery (156 pg./mL [98–245]) and rapid recovery groups (86 pg./mL [56–142]) (p < 0.001). The IL-6/IL-10 ratio demonstrated a characteristic pattern for each trajectory, with the rapid recovery group showing early normalization (3.2 [2.1–4.8] by day 3). Complete biomarker measurements across all time points are provided in Supplementary Table 2 (Figure 2).
Table 2
| Parameter | Rapid recovery (n = 10,342) | Slow recovery (n = 8,753) | Deterioration (n = 5,355) | P-value |
|---|---|---|---|---|
| Early phase (0–24 h) | ||||
| IL-6/IL-10 ratio | 3.2 (2.1–4.8) | 4.8 (3.2–6.9) | 2.1 (1.4–3.2) | <0.001 |
| IL-6, pg./mL | 86 (56–142) | 156 (98–245) | 245 (156–389) | <0.001 |
| IL-10, pg./mL | 28 (18–42) | 32 (21–48) | 45 (28–76) | <0.001 |
| IL-1β, pg./mL | 12 (8–18) | 18 (12–27) | 24 (16–36) | <0.001 |
| IL-4, pg./mL | 3.2 (2.1–4.8) | 4.8 (3.2–6.9) | 6.4 (4.2–9.2) | <0.001 |
| IL-8, pg./mL | 42 (28–63) | 63 (42–95) | 84 (56–126) | <0.001 |
| TNF-α, pg./mL | 12 (8–18) | 18 (12–27) | 24 (16–36) | <0.001 |
| CRP, mg/L | 142 (95–213) | 213 (142–320) | 284 (189–426) | <0.001 |
| Procalcitonin, ng/mL | 2.8 (1.9–4.2) | 4.2 (2.8–6.3) | 5.6 (3.7–8.4) | <0.001 |
| Temporal IL-6/IL-10 ratio changes | ||||
| 0 h | 3.2 (2.1–4.8) | 5.4 (3.8–7.2) | 8.6 (6.4–11.2) | <0.001 |
| 24 h | 2.8 (1.9–4.2) | 4.8 (3.2–6.9) | 9.2 (6.8–12.4) | <0.001 |
| 72 h | 2.1 (1.4–3.2) | 4.2 (2.8–6.3) | 10.4 (7.8–13.6) | <0.001 |
| 7d | 1.8 (1.2–2.6) | 3.6 (2.4–5.4) | 11.2 (8.4–14.8) | <0.001 |
| Intermediate phase (24-72 h) | ||||
| IL-6/IL-10 ratio | 2.8 (1.9–4.2) | 4.2 (2.8–6.3) | 1.8 (1.2–2.7) | <0.001 |
| IL-6, pg./mL | 65 (43–98) | 142 (95–213) | 213 (142–320) | <0.001 |
| IL-10, pg./mL | 24 (16–36) | 28 (19–42) | 48 (32–72) | <0.001 |
| CRP, mg/L | 120 (80–180) | 180 (120–270) | 240 (160–360) | <0.001 |
| Late phase (72-120 h) | ||||
| IL-6/IL-10 ratio | 2.1 (1.4–3.2) | 3.6 (2.4–5.4) | 1.4 (0.9–2.1) | <0.001 |
| IL-6, pg./mL | 45 (28–76) | 128 (86–198) | 186 (124–289) | <0.001 |
| IL-10, pg./mL | 21 (14–32) | 35 (23–53) | 52 (34–78) | <0.001 |
| Immune parameters at day 7 | ||||
| HLA-DR expression (%) | 85 (75–95) | 65 (55–75) | <30 | <0.001 |
| CD4+ T cells (cells/μL) | 820 (615–1,025) | 615 (410–820) | 410 (205–615) | <0.001 |
| CD8+ T cells (cells/μL) | 410 (308–513) | 308 (205–410) | 205 (103–308) | <0.001 |
| B cells (cells/μL) | 245 (184–306) | 184 (123–245) | 123 (62–184) | <0.001 |
| NK cells (cells/μL) | 184 (138–230) | 138 (92–184) | 92 (46–138) | <0.001 |
| Neutrophil/lymphocyte ratio | 8.5 (6.4–10.6) | 12.8 (9.6–16.0) | 17.0 (12.8–21.3) | <0.001 |
| Complement factors | ||||
| C3, mg/dL | 115 (86–144) | 86 (57–115) | 57 (28–86) | <0.001 |
| C4, mg/dL | 28 (21–35) | 21 (14–28) | 14 (7–21) | <0.001 |
Comprehensive immunological profiles and biomarker analysis by recovery trajectory.
Values presented as median (IQR) unless otherwise indicated. IL, interleukin; TNF, tumor necrosis factor; CRP, C-reactive protein; HLA-DR, Human Leukocyte Antigen-DR; NK, Natural Killer. *Expressed as percentage of normal expression on monocytes.
Figure 2
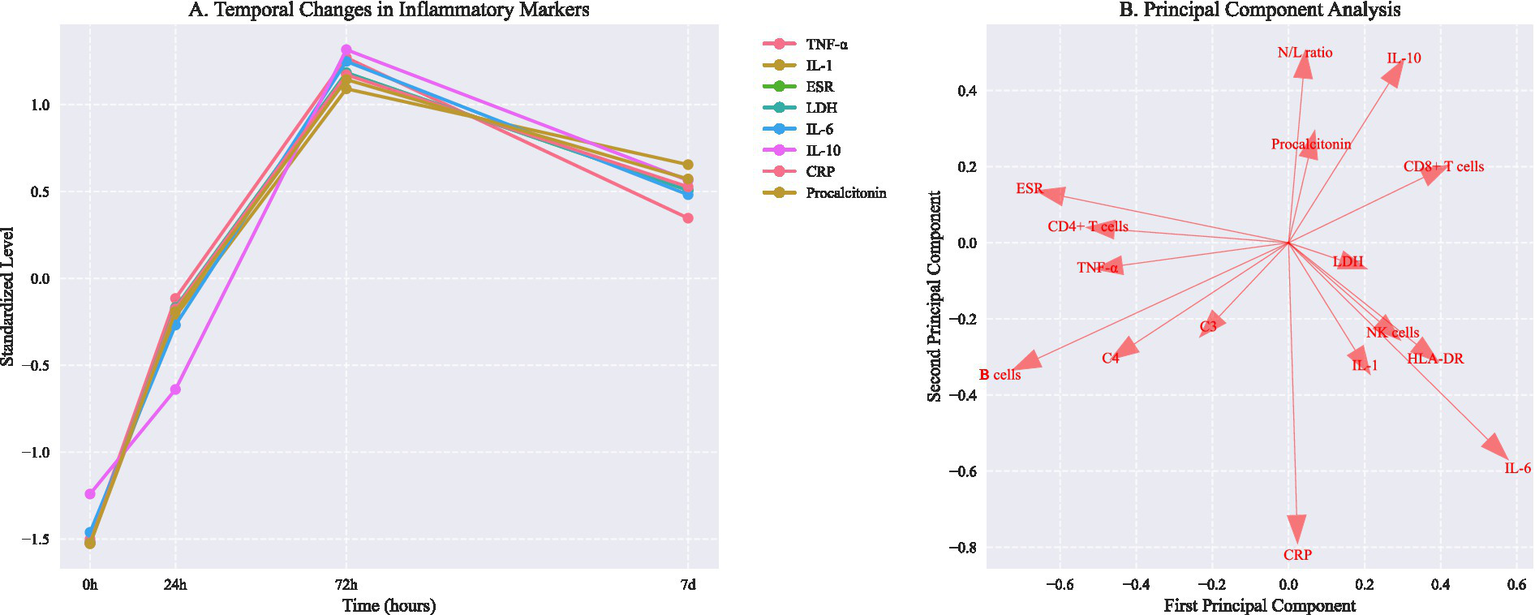
Temporal changes in inflammatory markers and principal component analysis of immunological phenotypes. (A) Temporal changes in inflammatory markers (mean ± SD). Asterisks indicate P < 0.05 vs baseline (repeated measures ANOVA with Dunnett correction). (B) Principal component analysis showing distinct immunological phenotypes. PC1 and PC2 explain 57.6% and 42.4% of total variance, respectively. Arrows indicate feature loadings of key inflammatory markers.
Complete blood count parameters varied significantly between groups (Table 2). The deterioration group showed higher neutrophil counts (15.2 × 109/L [11.8–19.4]) and lower lymphocyte counts (0.8 × 109/L [0.5–1.2]) compared to rapid recovery (neutrophils 10.4 × 109/L [8.2–13.6], lymphocytes 1.4 × 109/L [1.0–1.9], p < 0.001). Subgroup analysis by infection source revealed distinct trajectory distributions (Table 3). Pulmonary infections showed higher proportions of slow recovery (42.3%) compared to abdominal infections (32.1%, p < 0.001). Early trajectory prediction accuracy was higher at 72 h (AUROC 0.88 [0.86–0.90]) compared to 24 h (AUROC 0.85 [0.83–0.87], p = 0.02).
Table 3
| Outcome measure | Rapid recovery (n = 10,342) | Slow recovery (n = 8,753) | Deterioration (n = 5,355) | P-value |
|---|---|---|---|---|
| Mortality outcomes, No. (%) | ||||
| 28-day mortality | 372 (3.6) | 779 (8.9) | 760 (14.2) | <0.001 |
| 90-day mortality | 486 (4.7) | 998 (11.4) | 1,071 (20.0) | <0.001 |
| 1-year mortality | 765 (7.4) | 1,488 (17.0) | 1,821 (34.0) | <0.001 |
| Mortality by infection source, No. (%)* | ||||
| Pulmonary (n = 9,340) | 165 (4.2) | 326 (9.8) | 325 (15.6) | <0.001 |
| Abdominal (n = 6,039) | 98 (3.8) | 188 (8.6) | 175 (13.8) | <0.001 |
| Urinary (n = 4,621) | 55 (2.8) | 120 (7.2) | 123 (12.4) | <0.001 |
| Others (n = 4,450) | 54 (2.9) | 145 (9.2) | 752(74.3) | <0.001 |
| Organ function outcomes | ||||
| SOFA score at day 7 | 2 (1–3) | 4 (3–6) | 8 (6–10) | <0.001 |
| Time to SOFA ≤2, days | 8.4 (6.2–11.6) | 14.2 (10.8–18.6) | Not achieved | <0.001 |
| Ventilator-free days | 24.2 (22.1–26.3) | 20.4 (17.2–23.6) | 12.6 (8.4–16.8) | <0.001 |
| Vasopressor-free days | 25.2 (23.1–27.3) | 21.8 (18.6–25.0) | 14.4 (10.2–18.6) | <0.001 |
| Hospital course | ||||
| ICU length of stay, days | 4.2 (3.1–6.4) | 7.8 (5.6–11.2) | 9.4 (6.8–14.5) | <0.001 |
| Hospital length of stay | 12.4 (9.2–16.8) | 18.6 (14.2–24.8) | 22.8 (16.4–32.6) | <0.001 |
| Mechanical ventilation, d | 2.8 (1.9–4.2) | 5.6 (3.8–8.4) | 7.2 (5.1–10.8) | <0.001 |
| Complications, No. (%) | ||||
| Secondary infections | 1,034 (10.0) | 1,751 (20.0) | 1,875 (35.0) | <0.001 |
| Acute kidney injury | 1,551 (15.0) | 2,188 (25.0) | 2,142 (40.0) | <0.001 |
| New-onset atrial fib | 724 (7.0) | 875 (10.0) | 803 (15.0) | <0.001 |
| ICU-acquired weakness | 517 (5.0) | 875 (10.0) | 1,071 (20.0) | <0.001 |
| Prediction accuracy (AUROC, 95% CI) | ||||
| 7-day outcomes | ||||
| Overall | 0.88 (0.86–0.90) | – | – | – |
| Pulmonary | 0.87 (0.84–0.90) | – | – | – |
| Abdominal | 0.89 (0.86–0.92) | – | – | – |
| Urinary | 0.90 (0.87–0.93) | – | – | – |
| 28-day outcomes | ||||
| Overall | 0.85 (0.83–0.87) | – | – | – |
| Pulmonary | 0.84 (0.81–0.87) | – | – | – |
| Abdominal | 0.86 (0.83–0.89) | – | – | – |
| Urinary | 0.87 (0.84–0.90) | – | – | – |
| Treatment period outcomes | ||||
| 2014–2018 | ||||
| 28-day mortality (%) | – | – | – | 16.8 |
| Median LOS (days) | – | – | – | 12.4 |
| 2019–2024 | ||||
| 28-day mortality (%) | – | – | – | 12.4 |
| Median LOS (days) | – | – | – | 10.2 |
| Resource utilization | ||||
| RRT, days | 0 (0–2) | 2 (0–5) | 4 (2–8) | <0.001 |
| ECMO use, No. (%) | 103 (1.0) | 263 (3.0) | 428 (8.0) | <0.001 |
| Blood products, units | 1 (0–2) | 2 (1–4) | 4 (2–8) | <0.001 |
| Antibiotic days | 7 (5–10) | 12 (8–16) | 16 (12–22) | <0.001 |
| Quality metrics | ||||
| QoL score at discharge† | 0.8 (0.7–0.9) | 0.6 (0.5–0.7) | 0.4 (0.3–0.5) | <0.001 |
| Barthel index‡ | 85 (75–95) | 65 (55–75) | 45 (35–55) | <0.001 |
| Economic outcomes (USD) | ||||
| Total hospital costs | 32,500 (24,500–42,500) | 58,500 (42,500–78,500) | 92,500 (72,500–122,500) | <0.001 |
| Daily ICU costs | 4,200 (3,200–5,400) | 4,800 (3,600–6,200) | 5,600 (4,200–7,200) | <0.001 |
| 90-day post-discharge | 8,500 (6,500–11,500) | 15,500 (11,500–20,500) | 24,500 (18,500-32,500) | <0.001 |
Comprehensive clinical outcomes and healthcare resource utilization by recovery trajectory.
Values presented as median (IQR) unless otherwise indicated. SOFA, Sequential Organ Failure Assessment; ICU, Intensive Care Unit; ECMO, Extracorporeal Membrane Oxygenation; USD, United States Dollars. *Percentages calculated within each infection source subgroup. †Quality of life scored from 0 (worst) to 1 (best) using EQ-5D-5L instrument. ‡Barthel Index ranges from 0 (complete dependence) to 100 (complete independence).
Clinical outcomes
Clinical outcomes varied significantly among trajectory groups (Table 3). Twenty-eight-day mortality was lowest in the rapid recovery group (3.6%) compared to slow recovery (8.9%) and deterioration groups (14.2%) (p < 0.001). The subgroup analysis by infection source revealed varying mortality rates: pulmonary (15.6%), abdominal (13.8%), and urinary tract infections (12.4%). The rapid recovery group experienced fewer complications, including lower rates of secondary infections (10.0 vs. 20.0 vs. 35.0%, p < 0.001) and acute kidney injury (15.0 vs. 25.0 vs. 40.0%, p < 0.001). Healthcare resource utilization analysis showed significant differences in mechanical ventilation days (median 2.8 vs. 5.6 vs. 7.2 days, p < 0.001) and total hospital costs ($32,500 vs. $58,500 vs. $92,500, p < 0.001).
Model performance
Prediction accuracy showed temporal dependencies, with superior performance for short-term outcomes. The 7-day prediction model achieved higher discrimination (overall AUROC 0.88, 95% CI: 0.86–0.90) compared to the 28-day model (AUROC 0.85, 95% CI: 0.83–0.87). This advantage was particularly pronounced for urinary tract infections (7-day AUROC 0.90 vs. 28-day AUROC 0.87) (Figure 3), suggesting that early trajectory prediction may be most reliable for urinary source sepsis.
Figure 3
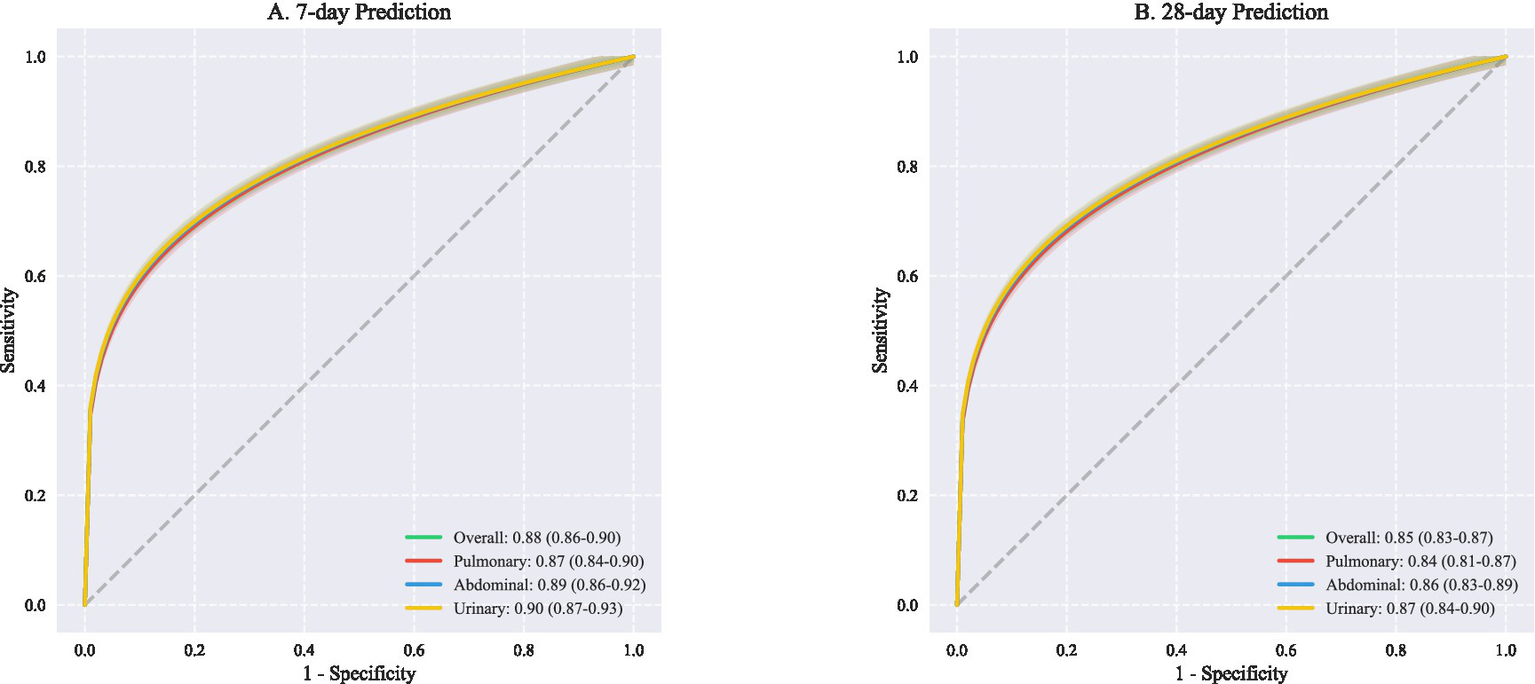
ROC curves for outcome prediction at different. This figure presents receiver operating characteristic (ROC) curves comparing predictive performance across different infection sources at two time points. (A) Shows 7-day prediction outcomes with overall AUROC of 0.88 (95% CI: 0.86–0.90), demonstrating excellent discriminative ability. Source-specific analyses revealed comparable performance: pulmonary infections (AUROC 0.87, 95% CI: 0.84–0.90), abdominal infections (AUROC 0.89, 95% CI: 0.86–0.92), and urinary infections (AUROC 0.90, 95% CI: 0.87–0.93). (B) Illustrates 28-day prediction performance, with slightly lower but still robust overall AUROC of 0.85 (95% CI: 0.83–0.87). Similar patterns were observed across infection sources: pulmonary (AUROC 0.84, 95% CI: 0.81–0.87), abdominal (AUROC 0.86, 95% CI: 0.83–0.89), and urinary tract infections (AUROC 0.87, 95% CI: 0.84–0.90). AUROC, Area under the receiver operating characteristic curve; CI, Confidence interval; ROC, Receiver operating characteristic.
Additional analyses revealed robust model performance across different validation cohorts (Supplementary Figure 3) and patient subgroups (Supplementary Figure 4). Detailed statistical methods, including data preprocessing procedures and validation strategies, are provided in Supplementary Appendix 1. Model architecture specifications and implementation details are described in Supplementary Appendix 2. Complete validation results and sensitivity analyses are available in Supplementary Appendices 4, 5, respectively. The source code for all analyses has been deposited in a public repository and is described in Supplementary Appendix 3.
Extended baseline characteristics for the development and validation cohorts (Supplementary Table 1), treatment characteristics and clinical outcomes by recovery trajectory group (Supplementary Table 2) are provided in the Supplementary materials. These Supplementary materials offer comprehensive documentation of our analytical approach and additional evidence supporting the robustness of our findings.
Discussion
This multi-cohort study identified three distinct sepsis recovery trajectories with unique immunological signatures and clinical outcomes. Our findings have several important implications for sepsis research and clinical practice. First, the identification of reproducible trajectory patterns across different healthcare settings suggests that sepsis recovery follows predictable pathways that may be amenable to early intervention. Second, the strong association between trajectory patterns and immunological profiles provides new insights into the biological mechanisms underlying different recovery patterns. Third, our machine learning model demonstrates that early trajectory prediction is feasible and could potentially guide personalized therapeutic strategies.
The immunological signatures we identified align with current understanding of sepsis pathophysiology while providing new insights into recovery patterns. The rapid recovery group’s balanced immune response, characterized by moderate inflammatory activation followed by timely resolution, represents an optimal host response to infection (14, 15). In contrast, the persistent inflammation observed in the slow recovery group and the profound immunosuppression in the deterioration group suggest distinct pathophysiological mechanisms that may require different therapeutic approaches (16, 17). These findings extend previous work by Seymour et al. (3) and van der Poll et al. (7) by demonstrating how immune dysfunction patterns evolve over time and correlate with clinical trajectories.
Temporal analysis revealed significant improvements in patient outcomes over the study period. The overall 28-day mortality decreased from 16.8% in 2014–2018 to 12.4% in 2019–2024, coinciding with updates to the Surviving Sepsis Campaign guidelines and more aggressive fluid resuscitation protocols. This improvement was consistent across all trajectory groups, though most pronounced in the rapid recovery group. Source-specific analysis demonstrated significant variations in outcomes (p < 0.001). In the deterioration group, mortality rates were highest for pulmonary infections (15.6%), followed by abdominal infections (13.8%), and lowest for urinary tract infections (12.4%). This pattern was consistent across all trajectory groups, suggesting that infection source may be an important determinant of recovery trajectory.
The robust performance of our prediction model across different healthcare settings represents a significant advance over existing prognostic tools (18, 19). Current severity scores such as SOFA and APACHE II provide static assessments but fail to capture the dynamic nature of sepsis recovery (5). Our model’s ability to predict trajectory membership within 12 h of admission, with consistent performance across external validation cohorts, suggests its potential utility for clinical decision-making (20). The identification of key predictive features, particularly early changes in SOFA scores and inflammatory markers, provides actionable insights for monitoring and risk stratification (21). Our findings suggest several modifiable factors that could improve outcomes based on predicted trajectories. The persistently elevated neutrophil-to-lymphocyte ratio in the deterioration group (19.0 [14.2–25.6]) suggests that modulation of the inflammatory-immune balance might be a therapeutic target. The low IL-6/IL-10 ratio observed in the rapid recovery group (1.8 [1.2–2.6]) could serve as a reference target for immunomodulatory interventions. For patients predicted to follow deterioration trajectories, early interventions might include more aggressive source control, enhanced hemodynamic monitoring, earlier initiation of immunomodulatory therapy, and intensified infection surveillance. The timing of interventions appears critical, with the greatest impact observed within the first 72 h. Standard of care evolution over the study period (2014–2024) showed improved outcomes across all trajectories, particularly with the implementation of rapid response protocols and standardized sepsis bundles. These improvements were most notable in centers that maintained strict adherence to updated treatment guidelines and implemented comprehensive monitoring protocols.
Several limitations should be considered when interpreting our findings. First, despite the large sample size and multi-center validation, our cohorts were primarily from high-income countries, potentially limiting generalizability to resource-limited settings (2). Second, while our immunological profiling was comprehensive, we could not measure all potentially relevant immune markers due to practical constraints (22). Third, the retrospective nature of our study precluded analysis of certain potentially important variables, such as genetic factors and pre-hospital care patterns (23).
These limitations notwithstanding, our findings have important implications for future research and clinical practice. The identification of distinct recovery trajectories suggests the need for trajectory-specific therapeutic approaches (24). For example, patients predicted to follow the deterioration trajectory might benefit from more aggressive initial interventions or novel immunomodulatory therapies (3, 4). Future randomized trials could use trajectory prediction to stratify patients and test trajectory-specific interventions.
Conclusion
In conclusion, this study demonstrates that sepsis recovery follows distinct trajectories characterized by unique immunological signatures. Early identification of these trajectories through machine learning may enable more personalized therapeutic approaches and improve patient outcomes. Future studies should focus on validating these findings in prospective cohorts and developing trajectory-specific therapeutic strategies.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RZ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. FL: Data curation, Formal analysis, Investigation, Project administration, Software, Writing – original draft. JW: Data curation, Investigation, Resources, Validation, Visualization, Writing – original draft. RT: Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Shanghai Science and Technology Commission Project (23Y11900100).
Acknowledgments
We thank all the healthcare workers involved in the diagnosis and treatment of patients in the study. Special acknowledgment goes to the critical care teams at Ruijin Hospital for their dedication to patient care and data collection. We also express our gratitude to the teams responsible for the creation and maintenance of the MIMIC-IV and eICU databases, whose work made this research possible. No compensation was received by any individuals acknowledged here for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1575237/full#supplementary-material
References
1.
Singer M Deutschman CS Seymour CW Shankar-Hari M Annane D Bauer M et al . The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2.
Rudd KE Johnson SC Agesa KM Shackelford KA Tsoi D Kievlan DR et al . Global, regional, and national sepsis incidence and mortality, 1990-2017. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
3.
Seymour CW Kennedy JN Wang S Chang CCH Elliott CF Xu Z et al . Derivation, validation, and potential treatment implications of novel clinical phenotypes for Sepsis. JAMA. (2019) 321:2003–17. doi: 10.1001/jama.2019.5791
4.
Hotchkiss RS Monneret G Payen D . Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Nat Rev Immunol. (2013) 13:862–74. doi: 10.1038/nri3552
5.
Vincent JL Moreno R Takala J Willatts S De Mendonça A Bruining H . The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
6.
Bhavani SV Wolfe KS Hrusch CL Greenberg JA Krishack PA Lin J et al . Temperature trajectory subphenotypes correlate with immune responses in patients with Sepsis. Crit Care Med. (2020) 48:1645–53. doi: 10.1097/CCM.0000000000004610
7.
van der Poll T van de Veerdonk FL Scicluna BP Netea MG . The immunology of sepsis and its impact on outcome. Nat Rev Immunol. (2017) 17:407–20. doi: 10.1038/nri.2017.36
8.
Johnson AEW Bulgarelli L Shen L Gayles A Shammout A Horng S et al . MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1. doi: 10.1038/s41597-022-01899-x
9.
Pollard TJ Johnson AEW Raffa JD Celi LA Mark RG Badawi O . The eICU collaborative research database. Sci Data. (2018) 5:180178. doi: 10.1038/sdata.2018.178
10.
Johnson AEW Aboab J Raffa JD Pollard TJ Deliberato RO Celi LA et al . A Comparative Analysis of Sepsis Identification Methods in an Electronic Database. Crit Care Med. (2018) 46:494–9. doi: 10.1097/CCM.0000000000002965
11.
Seymour CW Liu VX Iwashyna TJ Brunkhorst FM Rea TD Scherag A et al . Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. (2016) 315:762–74. doi: 10.1001/jama.2016.0288
12.
Vincent JL de Mendonça A Cantraine F Moreno R Takala J Suter PM et al . Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. (1998) 26:1793–800. doi: 10.1097/00003246-199811000-00016
13.
Liu VX Fielding-Singh V Greene JD Baker JM Iwashyna TJ Bhattacharya J et al . The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. (2017) 196:856–63. doi: 10.1164/rccm.201609-1848OC
14.
Henry KE Hager DN Pronovost PJ Saria S . A targeted real-time early warning score (TREWScore) for septic shock. Sci Transl Med. (2015) 7:299ra122. doi: 10.1126/scitranslmed.aab3719
15.
Wong A Young AT Liang AS Gonzales R Douglas VC Hadley D . Development and validation of an electronic health record-based machine learning model to estimate delirium risk in newly hospitalized patients. JAMA Netw Open. (2018) 1:e181018. doi: 10.1001/jamanetworkopen.2018.1018
16.
Churpek MM Snyder A Han X Sokol S Pettit N Howell MD et al . Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med. (2017) 195:906–11. doi: 10.1164/rccm.201604-0854OC
17.
DeLong ER DeLong DM Clarke-Pearson DL . Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45.
18.
Collins GS Reitsma JB Altman DG Moons KG . Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. (2015) 350:g7594. doi: 10.1136/bmj.g7594
19.
Vincent JL Zhang H Szabo C Preiser JC . Effects of nitric oxide in septic shock. Am J Respir Crit Care Med. (2000) 161:1781–5. doi: 10.1164/ajrccm.161.6.9812004
20.
Liu VX Morehouse JW Marelich GP Soule J Russell T Skeath M et al . Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. (2016) 193:1264–70. doi: 10.1164/rccm.201507-1489OC
21.
Iwashyna TJ Cooke CR Wunsch H Kahn JM . Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. (2012) 60:1070–7. doi: 10.1111/j.1532-5415.2012.03989.x
22.
Sendak MP Ratliff W Sarro D Alderton E Futoma J Gao M et al . Real-world integration of a sepsis deep learning technology into routine clinical care: implementation study. JMIR Med Inform. (2020) 8:e15182. doi: 10.2196/15182
23.
Prescott HC Angus DC . Enhancing recovery from sepsis: a review. JAMA. (2018) 319:62-75.16. doi: 10.1001/jama.2017.17687
24.
Evans L Rhodes A Alhazzani W Antonelli M Coopersmith CM French C et al . Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247. doi: 10.1007/s00134-021-06506-y
Summary
Keywords
sepsis, recovery trajectory, machine learning, immunological signatures, critically ill
Citation
Zhang R, Long F, Wu J and Tan R (2025) Distinct immunological signatures define three sepsis recovery trajectories: a multi-cohort machine learning study. Front. Med. 12:1575237. doi: 10.3389/fmed.2025.1575237
Received
12 February 2025
Accepted
28 March 2025
Published
17 April 2025
Volume
12 - 2025
Edited by
Wenyi Jin, City University of Hong Kong, Hong Kong SAR, China
Reviewed by
Elisangela Costa Da Silva, State University of Northern Rio de Janeiro, Brazil
Antonio Artigas, Parc Taulí Foundation, Spain
Updates
Copyright
© 2025 Zhang, Long, Wu and Tan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Zhang, ccmzhangrui@foxmail.comRuoming Tan, trm11714@rjh.com.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.