Abstract
Objective:
Xuebijing injection (XBJ) has been widely recognized in the treatment of sepsis, however, inadequate information regarding XBJ's optimal dosage and frequency suffice. We aimed to assess the effectiveness of various doses and administration frequencies in patients with sepsis using a network meta-analysis (NMA) to offer therapeutic prescription guidance.
Methods:
We examined eight databases for 1,765 randomized controlled trials published before July 2024, organized the literature using NoteExpress software and extracted data using Microsoft Excel software. The literature's quality was assessed using the risk of bias evaluation approach endorsed by the Cochrane Collaboration. The analysis was conducted by NMA inside a frequency-based framework.
Results:
Forty-three qualifying studies were included in the analysis, including 5,818 participants. Regarding the enhancement of 28-day mortality, 50 Milliliter (ml)-tie in die (tid) exhibited optimal efficacy, 100 ml-tid demonstrated superior efficacy in ameliorating APACHE II scores, 50 ml-bis in die (bid) proved more effective in enhancing the activated partial thromboplastin time (APTT), while 100 ml-quaque die (qd) significantly improved C-reactive protein (CRP) levels. Additional findings are displayed in net league tables, forest plots, and funnel plot.
Conclusions:
A daily dose of 100 ml of XBJ was associated with improvement in APTT and CRP levels in patients with sepsis, a daily dose of 150 ml may decrease 28-day mortality; while XBJ with a single-day dose of 300 ml is more effective at improving the APACHE II score, higher dosages correlated with improved prognosis in these patients compared to other doses.
1 Introduction
Sepsis is a critical condition characterized by organ dysfunction resulting from the body's aberrant response to infection (1). As global awareness of sepsis has increased, it is estimated that 6–9 million individuals worldwide develop it annually (2). In the United States, Sepsis is the primary cause of mortality among patients with critical sickness, resulting in about 210,000 fatalities per year (3). Patients recovering from sepsis are frequently readmitted due to organ dysfunction (4) and the emergence of new symptoms (5). Sepsis-related rehospitalization constitutes 12.2% of all hospital readmissions in the United States (6). A study from China reports that the morbidity rate of sepsis in the Intensive Care Unit (ICU) was 20.6%, the mortality rate was 35.5%, and the mortality rate for severe sepsis exceeded 50% (7). The management of sepsis presents certain challenges. The potential for substantial advancements in early prevention, pharmacological treatment, lifesaving measures, and rehabilitation to reduce the current high rates of morbidity, mortality, and rehospitalization is a critical issue that should be addressed to enhance critical care, emergency medicine, and preventive medicine in future (8).
Research has shown that certain Chinese herbal medicines can influence inflammation and the immune response. Additionally, the combination of herbal medicines with antibiotics has been found to decrease the prevalence of drug-resistant bacteria and the incidence of multiple organ dysfunction syndrome (MODS). In the Chinese treatment guidelines and expert consensus (9–11). for sepsis management, herbal medicines are recommended as complementary to conventional sepsis treatment, with XueBiJing injection (XBJ) being a widely used injectable product authorized in China since 2004 for the treatment of Sepsis and MODS. XBJ was formulated from a blend of Carthamus tinctorius flowers (Honghua), Paeonia lactiflora roots (Chishao), Salvia miltiorrhiza roots (Danshen), Ligusticum chuanxiong rhizomes (Chuanxiong), and Angelica sinensis roots (Danggui) (12). Several meta-analyses (MA) have shown that combining XBJ with the standard treatment for sepsis can further reduce mortality by 28 days, reduce complication rates, and enhance patient prognosis (13–15).
Nonetheless, in clinical applications and associated studies, the dosage and frequency of XBJ differs (16, 17), and no study has clarified the optimal dosage and frequency of XBJ for sepsis treatment to enhance patient prognosis and reduce mortality. This study presents preliminary findings on the appropriate dosage and frequency of XBJ using network meta-analysis (NMA), aiming to guide doctors and data support for future research on XBJ as an adjunctive therapy for sepsis, as shown in Figure 1.
Figure 1

Cognitive process diagram.
2 Methods
This study adheres to the PRISMA-2020 statement (18). The trial was not registered, and a protocol was not established.
2.1 Data sources and search strategy
We conducted a comprehensive search across many academic databases, including the China National Knowledge Infrastructure (CNKI), WanFang Medical Database, China Science and Technology Journal Database (VIP), Chinese Biomedical Literature Database (CBM), PubMed, Embase, Web of Science, and Cochrane Library. The literature search included studies that compared XBJ with placebo or Standard drug therapy (STDT) in randomized controlled trials (RCTs), with publications collected up to July 2024. In addition, we conducted a thorough manual examination of the pertinent MAs, reviews, pooled analyses, and reference lists of the included studies to ensure that no significant information was inadvertently excluded. A comprehensive literature search encompassing the topic “Sepsis” was equally performed. Specifically, we focused on the RCTs that investigated the efficacy and safety of XBJ.
2.2 Study selection and selection criteria
Two investigators independently performed the literature search, and in cases of disagreement, a group discussion was held to reach a consensus. Inclusion criteria were as follows: (1) compliance with an RCT, (2) the participants should be diagnosed with sepsis, based on “The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) (1),” and (3) no restrictions were placed on gender, age, geographic location, ethnicity, race, duration of disease. Exclusion criteria included: (1) non-RCTs, such as animal experimentations, reviews, systematic evaluations, case reports, or conference abstracts, (2) non-septic patients or subjects with conditions other than sepsis, and (3) duplicate publications, plagiarism, studies with unextractable or controversial data.
2.3 Data extraction and quality assessment
Four authors (XP Q, RW J, Z H, and Q Z) screened and extracted data using NoteExpress software for literature screening and management. The software excluded duplicates, animal experiments, MAs, case reports, and other irrelevant materials. Subsequently, two authors reviewed the titles and abstracts to identify compliant studies and non-compliant studies were excluded. Thereafter, the literature was acquired and comprehensively examined to encompass studies that satisfied the specified criteria. In instances of disagreement, a neutral third party was involved to facilitate resolution. The extracted data included the first author, publication year, country, ethnicity, sample size, duration of treatment, application of anti-heart failure drugs or glucose-lowering pharmaceuticals, outcomes, and methods of randomization and blinding.
The quality of the studies was assessed using the Cochrane Collaboration's risk-of-bias evaluation approach. To construct the Literature Quality Assessment Form, the RevMan 5.4 software was utilized. Any disagreements between RW J and XP Q were reevaluated and discussed until a consensus was reached.
2.4 Data synthesis and analysis
NMA is a collective term for indirect and mixed treatment comparisons, and one key advantage is its ability to quantitatively compare various interventions for a given disease. Additionally, it allows the integration of evidence from both direct and indirect comparisons. This enabled the ranking of interventions based on their superiority or inferiority in terms of specific outcomes, thereby facilitating the identification of the optimal option. The majority of NMA was conducted using frequency-based or Bayesian methods. Following the process of extracting data and evaluating the quality of the studies that were included, the collected data were subjected to frequency-based NMA using Stata15 software. Network plots were generated using the same software to summarize the evidence from the direct and indirect comparisons. Additionally, the surface under the cumulative ranking (SUCRA) was computed to rank the interventions for each outcome, assigning scores ranging from 0 to 100%. The forest plot displays the findings of the studies included in the analysis, comparing them with a placebo group across five different outcome measures. Publication bias was evaluated by visually examining the funnel plots of the five outcomes. The net league table (inverted triangle plot) graphically presents the results of mixed evidence reporting for pairwise comparisons of different interventions, with a statistically significant difference (P < 0.05) between the experimental and control groups when the 95% confidence interval (CI) did not include a value of 1. The same software was used to conduct sensitivity analyses.
3 Results
3.1 Literature screening results
Overall, 1,765 studies were identified. After excluding 756, duplicates 1,009 studies remained. Of these, 43 studies met the inclusion criteria. Detailed information regarding the literature screening process is shown in Figure 2.
Figure 2

Study selection process.
3.2 Study characteristics
The baseline information of all included studies is presented in Supplementary Appendix S1. The treatment duration ranged from a minimum of 3 days (19, 20) to a maximum of 2 weeks (21–27). Seven studies used a 12 h dosing interval, classified as “bid” (16, 19, 20, 28–31), while three studies used an 8 h interval and were included as “tid” (19, 22, 32). The largest sample size among the studies was 1,817 patients (16), and the smallest sample size was 62 patients (33). This study included 59% male and 38% female participants, although some studies did not report the exact breakdown of male and female participants (34). The treatment group received 10 interventions that varied in dosage and frequency. Control group interventions included placebo and standard drug therapy (STDT). The primary endpoints of the studies were assessed using four outcome metrics, with detailed data for each outcome metric shown in Table 1.
Table 1
| Study | Treatments | 28-d mortality (r/n) | APACHE II score (Mean ±SD) | APTT (Mean ±SD) | CRP (Mean ±SD) |
|---|---|---|---|---|---|
| Cheng et al. (19) | 50 ml-bid | NA | 12.41 ± 5.13 | NA | NA |
| PLA | 17.03 ± 6.82 | ||||
| Chen et al. (60) | 100 ml-tid (3d) | NA | NA | NA | 115.07 ± 13.86 |
| 100 ml-bid (3d) | 120.37 ± 30.43 | ||||
| STDT (3d) | 129.30 ± 19.96 | ||||
| 100 ml-tid (7d) | 93.43 ± 10.41 | ||||
| 100 ml-bid (7d) | 102.03 ± 13.98 | ||||
| STDT (7d) | 109.47 ± 7.08 | ||||
| Dai et al. (35) | 100 ml-bid | NA | 12.57 ± 1.57 | 23.46 ± 2.31 | 85.47 ± 12.34 |
| STDT | 17.23 ± 2.03 | 28.12 ± 2.65 | 100.38 ± 15.32 | ||
| Dong et al. (33) | 100 ml-bid | NA | 11.23 ± 2.17 | NA | NA |
| STDT | 14.45 ± 3.24 | ||||
| Dou et al. (36) | 100 ml-bid | 10/45 | NA | NA | NA |
| STDT | 12/46 | ||||
| Gong et al. (37) | 50 ml-bid | 1/42 | 6.62 ± 2.91 | 30.95 ± 8.48 | NA |
| STDT | 1/40 | 12.87 ± 4.54 | 42.25 ± 7.73 | ||
| Ji et al. (38) | 100 ml-tid | NA | 6.03 ± 1.55 | 34.16 ± 6.38 | 10.73 ± 3.85 |
| STDT | 9.46 ± 2.58 | 37.33 ± 6.86 | 14.98 ± 5.64 | ||
| Ji et al. (39) | 50 ml-bid | NA | 13.25 ± 1.36 | NA | NA |
| STDT | 15.13 ± 2.11 | ||||
| Jia and Bao (20) | 100 ml-bid (7d) | NA | 13.63 ± 2.64 | NA | 35.64 ± 11.67 |
| STDT (7d) | 15.46 ± 3.75 | 90.65 ± 16.57 | |||
| 100 ml-bid (3d) | NA | 68.59 ± 17.23 | |||
| STDT (3d) | 102.43 ± 18.42 | ||||
| Jiang et al. (40) | 50 ml-bid | 12/95 | 9.48 ± 2.85 | NA | NA |
| PLA | 15/95 | 11.9 ± 3.32 | |||
| Jiang et al. (28) | 60 ml-bid | NA | 10.7 ± 2.6 | NA | NA |
| STDT | 13.8 ± 1.3 | ||||
| Jiang (48) | 50 ml-bid | NA | NA | 30.36 ± 5.34 | NA |
| PLA | 45.83 ± 6.71 | ||||
| Li et al. (21) | 100 ml-bid | NA | NA | 35.56 ± 5.56 | NA |
| STDT | 25.53 ± 3.83 | ||||
| Liu (41) | 100 ml-qd | 9/34 | 13.81 ± 7.53 | NA | 78.85 ± 48.78 |
| STDT | 13/30 | 15.36 ± 8.74 | 96.59 ± 81.01 | ||
| Liu et al. (16) | 100 ml-bid | 165/878 | NA | NA | NA |
| PLA | 230/882 | ||||
| Liu et al. (29) | 50 ml-bid | NA | 13 ± 2.5 | 30.5 ± 6.4 | NA |
| STDT | 15.2 ± 3.7 | 34.7 ± 6.2 | |||
| Lu et al. (50) | 50 ml-bid | NA | 8.42 ± 3.11 | NA | NA |
| STDT | 11.1 ± 2.85 | ||||
| Ma et al. (51) | 50 ml-bid | NA | NA | 32.18 ± 6.31 | NA |
| STDT | 36.49 ± 6.78 | ||||
| Ming et al. (52) | 300 ml-qd | NA | NA | NA | 16.15 ± 3.12 |
| STDT | 28.65 ± 4.52 | ||||
| Pu et al. (22) | 50 ml-tid (7 d) | NA | 13.01 ± 2.61 | NA | NA |
| STDT (7 d) | 14.15 ± 2.66 | ||||
| 50 ml-tid (14 d) | 5/45 | 11.22 ± 2.15 | |||
| STDT (14 d) | 13/45 | 12.28 ± 2.73 | |||
| Shao et al. (53) | 50 ml-bid | NA | 9.4 ±5.4 | NA | NA |
| STDT | 14.8 ± 5.7 | ||||
| Shao et al. (32) | 100 ml-bid | NA | NA | 26.26 ± 5.86 | NA |
| STDT | 38.05 ± 8.56 | ||||
| Shen et al. (23) | 100 ml-bid | NA | NA | NA | 32.39 ± 6.25 |
| STDT | 41.28 ± 7.39 | ||||
| Shi et al. (42) | 100 ml-qd | NA | 8.29 ± 5.62 | NA | NA |
| STDT | 11.39 ± 6.13 | ||||
| Song et al. (54) | 50 ml-bid | 1/47 | 10.4 ± 1.1 | NA | NA |
| STDT | 3/47 | 15.3 ± 1.4 | |||
| Su et al. (43) | 100 ml-bid | NA | NA | NA | 53.7 ± 18.8 |
| STDT | 91.3 ± 32.8 | ||||
| Sun and Yang (30) | 100 ml-bid | NA | NA | 35.5 ± 1.11 | NA |
| STDT | 39.82 ± 0.48 | ||||
| Sun (44) | 50 ml-bid | NA | 6.29 ± 1.71 | NA | NA |
| STDT | 8.35 ± 1.82 | ||||
| Wang et al. (24) | 100 ml-qd (7 d) | NA | NA | NA | 71.22 ± 40.75 |
| STDT (7 d) | 105.68 ± 47.31 | ||||
| 100 ml-qd (14d) | 37.0 ± 33.47 | ||||
| STDT (14d) | 76.47 ± 59.04 | ||||
| Wang et al. (55) | 100 ml-qd | NA | 13.61 ± 7.62 | NA | NA |
| STDT | 16.34 ± 8.7 | ||||
| Wang et al. (45) | 100 ml-bid | NA | 8.58 ± 1.64 | NA | NA |
| PLA | 10.02 ± 1.98 | ||||
| Wu et al. (25) | 50 ml-bid | NA | NA | NA | 77.1 ± 24.9 |
| STDT | 97.5 ± 27.6 | ||||
| Qiao (31) | 50 ml-bid | NA | 11.3 ± 2.8 | NA | NA |
| STDT | 13.6 ± 3.1 | ||||
| Xing et al. (56) | 100 ml-bid (7 d) | NA | 8.93 ± 5.5 | NA | NA |
| STDT (7 d) | 12.99 ± 6.03 | ||||
| 100 ml-bid (10 d) | 9/33 | 8.12 ± 4.36 | |||
| STDT (10 d) | 16/30 | 12.49 ± 5.97 | |||
| Xu et al. (26) | 50 ml-bid | NA | NA | NA | 28.5 ± 20.0 |
| STDT | 45.4 ± 19.6 | ||||
| Yang (49) | 50 ml-bid | NA | 12.92 ± 3.13 | 31.98 ± 6.98 | NA |
| STDT | 16.17 ± 4.89 | 42.19 ± 7.12 | |||
| Yin and Li (57) | 100 ml-bid | 18/88 | NA | NA | NA |
| STDT | 29/83 | ||||
| Zhang and Pei (46) | 100 ml-bid | NA | NA | 29.41 ± 4.72 | NA |
| STDT | 32.64 ± 3.61 | ||||
| Zhang et al. (34) | 50 ml-bid | NA | NA | 39.47 ± 4.73 | NA |
| STDT | 42.75 ± 5.24 | ||||
| Zhang (27) | 100 ml-bid | NA | 8.72 ± 6.23 | NA | 27.38 ± 22.13 |
| STDT | 11.59 ± 5.66 | 35.76 ± 24.66 | |||
| Zhang et al. (59) | 50 ml-bid | NA | 12.5 ± 3.16 | 37.88 ± 3.95 | NA |
| STDT | 15.63 ± 2.79 | 40.12 ± 4.08 | |||
| Zhang et al. (47) | 50 ml-bid (7d) | NA | 14.02 ± 5.71 | NA | NA |
| STDT (7d) | 17.42 ± 6.23 | ||||
| 50 ml-bid (10d) | 11.06 ± 4.02 | ||||
| STDT (10d) | 13.19 ± 4.17 | ||||
| Zhu et al. (58) | 100 ml-bid | 12/33 | NA | NA | NA |
| PLA | 17/33 |
Baseline information for data analysis of included studies.
3.3 Study quality assessment
The quality of included studies was assessed using three categories: high, low, and unclear risk-of-bias. Considered factors included: (1) Selection bias- generation of randomized sequences: 19 studies used the random number table method (19, 20, 29, 33–47) one study used drawing lots (48), which was rated as “low bias,” one research used the order of admission time (49), which was rated as “high bias”, 20 studies mentioned randomization (16, 22–28, 30–32, 34, 47, 50–56), incapable of detecting if it was classified as “low bias” or “high bias,” and was rated as “unclear,” (2) Allocation concealment: none of the documents mentioned whether or not the allocation scheme was concealed, and it was rated as “unclear,” (3) Performance bias—investigator and subject blinding: two studies (16, 21) mentioned blinding and were rated as “low bias,” the remaining studies did not describe blinding and were rated as “unclear,” (4) Measurement bias—blinding of study outcomes: none of the papers mentioned whether blinding was conducted on the outcomes, and they were rated as “unclear,” (5) Attrition bias—totality of outcome data: the research outcomes of the four articles contain missing data (16, 30, 33, 46) were rated as “high bias,” other literatures did not include discharges, withdrawals due to adverse effects, etc., the rating was “low bias,” (6) Reporting bias—selective or insufficient reporting of outcome indicators: all outcome indicators of all studies were reported in total, and they were rated as “low bias,” (7) Other bias—the existence of other bias: none of the studies mentioned the existence of other bias, and it was rated as “low bias.” Refer to Figure 3.
Figure 3

Risk of bias graph.
3.4 Data synthesis and analysis
Global and local inconsistency tests were conducted on the four outcome studies using Stata15 software. A P > 0.05 indicated inconsistency between the direct and indirect comparisons, and the data were analyzed using the consistency model. The P-value for the local inconsistency test was computed using node-splitting analysis. If the resulting P-value exceeded 0.05, this indicated statistical consistency between the direct and indirect evidence. The data displayed in Table 2 suggest an inconsistency between the direct and indirect comparisons for the cardiovascular mortality outcomes. The P-values obtained from both the global and local inconsistency tests for all remaining outcome indicators were greater than the significance level of 0.05, indicating that there was no inconsistency between direct and indirect comparisons.
Table 2
| Outcomes | Global inconsistency (P-value) | Local inconsistency (P-value) |
|---|---|---|
| 28-d mortality | 0.6201 | > 0.1 |
| APACHE II score | 0.2154 | > 0.1 |
| APTT | 0.6403 | > 0.1 |
| CRP | 0.1313 | > 0.1 |
The global inconsistency test and local inconsistency test for four outcomes.
APTT, activated partial thromboplastin time; CRP, C-reactive protein.
3.5 Network meta-analysis results
3.5.1 The 28-day mortality
The (9 RCTs, 2,646 patients) network evidence plot for 28-day mortality is shown in Figure 4A. All studies were two-arm, comprising nine studies (16, 22, 36, 40, 41, 54, 56–58). A smaller area under the curve (AUC) indicated greater efficacy in reducing 28-day mortality, as illustrated in the SUCRA plot (Figure 5A). The SUCRA table (Table 3) presents the percentage of AUC for the seven interventions. Analysis of the plot and table indicates that the efficacy of XBJ in reducing 28-day mortality is ranked as follows: STDT > PLA > 50 Milliliter (ml)-bis in die (bid) > 100 ml- bid > 100 ml-quaque die (qd) > 200 ml-bid > 50 ml-ter in die (tid). The 50 ml-tid dosage demonstrated greater efficacy in reducing 28-day mortality than other dosages, while STDT proved to be the least effective among the interventions assessed.
Figure 4
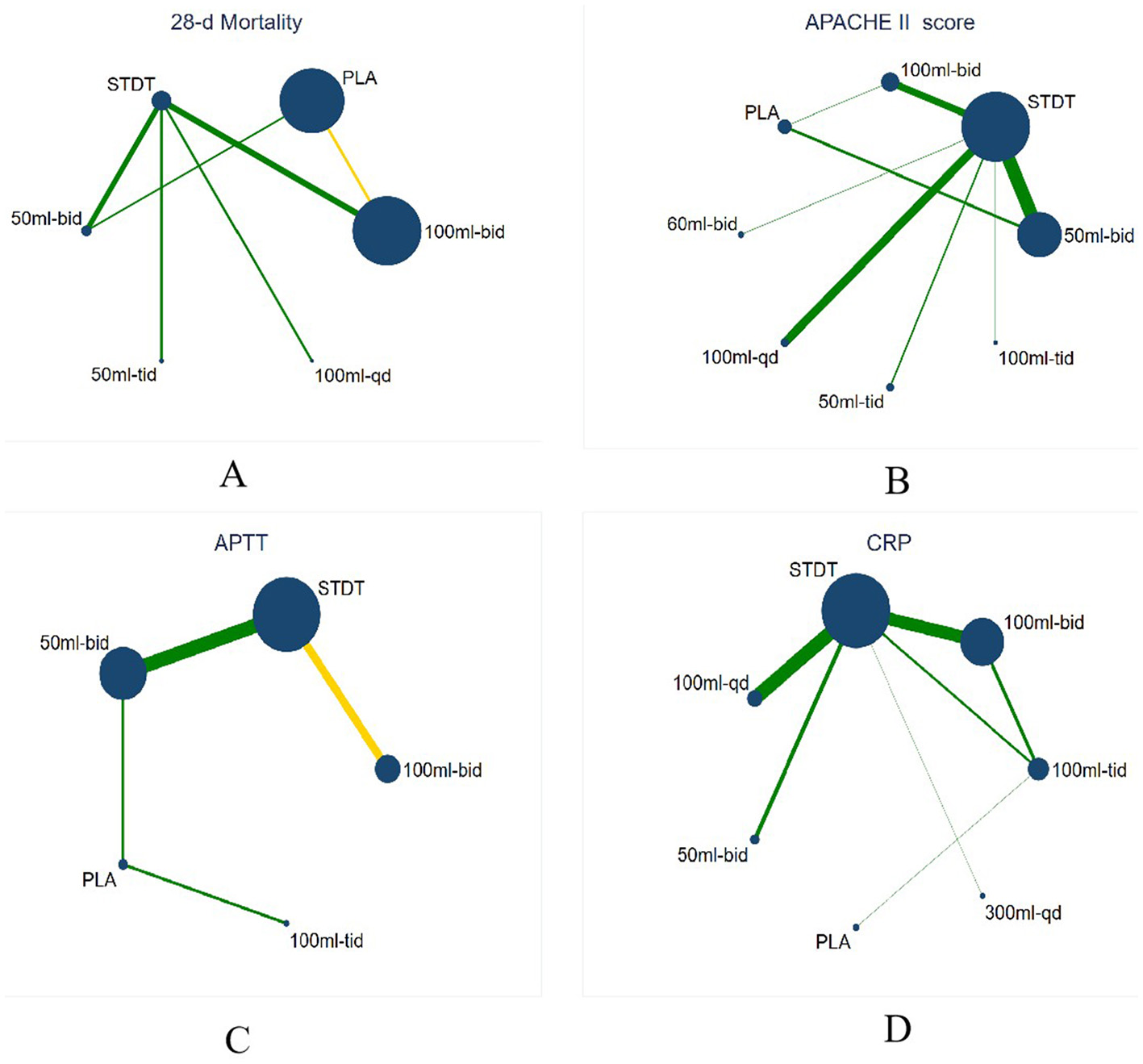
Network evidence plot. The size of the dots indicates the sample size, the larger the point, the larger the sample size, the thickness of the edges indicates the number of studies, and the yellow edges indicate that the study was blinded. (A) Network evidence plot for 28-d mortality, (B) Network evidence plot for APACHE II score, (C) Network evidence plot for APTT, (D) Network evidence plot for CRP.
Figure 5

The SUCRA of the estimated probability among the treatments. (A) Network evidence plot for 28-d mortality, (B) Network evidence plot for APACHE II score, (C) Network evidence plot for APTT, (D) Network evidence plot for CRP.
Table 3
| SUCRA (%) | ||||
|---|---|---|---|---|
| Treatments | Outcomes | |||
| 28-d mortality | APACHE II score | APTT | CRP | |
| 50 ml-bid | 44.7 | 26.2 | 7.1 | 48.3 |
| 60 ml-bid | NA | 31.7 | NA | NA |
| 50 ml-tid | 17.1 | 74.3 | NA | NA |
| 100 ml-qd | 37.8 | 43.2 | NA | 16.1 |
| 100 ml-bid | 34.2 | 25.5 | 29.1 | 39.8 |
| 100 ml-tid | NA | 24.6 | 70.9 | 39.9 |
| 300 ml-qd | NA | NA | NA | 61.9 |
| STDT | 93.2 | 95.8 | 53.1 | 93.1 |
| PLA | 73 | 78.7 | 89.7 | 50.9 |
The SUCRA.
3.5.2 APACHE II score
The network evidence plot graded by APACHE II (25 RCTs, 2,200 patients) is illustrated in Figure 4B, featuring eight interventions. Three research with four arms were included (22, 47, 56), while the remaining 22 studies were all two-arm studies (19, 20, 22, 25–29, 33, 35, 37–42, 44, 50, 53–55, 59). A smaller AUC indicated a more favorable outcome for enhancing the APACHE II score, as illustrated in the SUCRA plot (Figure 5B). The SUCRA table (Table 3) presents the percentage of AUC for the eight interventions. By integrating the plot and the table, it can be inferred that the efficacy of XBJ in enhancing the APACHE II score is rated as follows: STDT > PLA > 50 ml-tid > 100 ml-qd > 60 ml-bid > 50 ml-bid > 100 ml-bid > 100 ml-tid. The 100 ml-tid was more effective in enhancing the APACHE II score than the other intervention dosages, whereas STDT was the least beneficial.
3.5.3 APTT
The network evidence plot for activated partial thromboplastin time (APTT; 13 RCTs and 1,458 patients) is illustrated in Figure 4C, which includes five interventions. The literature included 13 studies (21, 29, 30, 32, 34, 35, 37, 38, 46, 48, 49, 51, 59), all of which were two-arm studies. A reduced AUC correlated with a more favorable outcome for the enhancement of APTT, as illustrated in the SUCRA plot (Figure 5C). The SUCRA table of AUC (Table 3) presents the percentage of the AUC for the five interventions. By integrating the plot and table, it can be inferred that the effectiveness of XBJ in enhancing APTT had been graded as follows: PLA > 100 mL tid > STDT > 100 ml-bid > 50 ml-bid. The 50 ml-bid dosages showed superior efficacy in enhancing APTT compared to other intervention dosages, whereas PLA exhibited the least efficacy.
3.5.4 C-reactive proteins
The network evidence plot for C-reactive protein (CRP) based on 12 RCTs (889 patients) is presented in Figure 4D, with seven therapies included. The literature comprised 12 papers featuring nine two-arm studies (23, 25–27, 35, 38, 41, 43, 52) based on treatment duration, along with one six-arm research (60) and two four-arm studies (20, 24). A reduced AUC indicated superior efficacy in enhancing the CRP levels, as illustrated in the SUCRA plot (Figure 5D). The SUCRA table (Table 3) illustrates the percentage of the AUC for the seven interventions. By combining the plot and table, it can be inferred that the effectiveness of XBJ in enhancing CRP levels was rated in the following order: STDT > 300 ml-qd > PLA > 50 ml-bid > 100 ml-tid > 100 ml-bid > 100 ml-qd. The efficacy of 100 ml-qd in enhancing CRP was higher than that of the other intervention doses, whereas STDT exhibited the least efficacy.
A net league table of the four outcomes illustrates the results of the two-by-two comparisons between the 10 interventions shown in Figures 6A–D, with a 95% Cl excluding one indicating statistical significance. ORs of <1 indicated a preference for the intervention measures defined in the columns, and a smaller OR in the two-by-two comparisons suggested better efficacy. The forest plot in Figures 7A–D shows a comparison of the four outcomes with the placebo. A smaller OR indicated a more favorable efficacy of the intervention compared to a placebo. The OR for each intervention aligned with the findings reported in the net league table (Figure 6).
Figure 6

Netleague table of the four outcome indicators sorted by SUCRA values. Any statistically significant findings are highlighted in bold font. (A) Network evidence plot for 28-d mortality, (B) Network evidence plot for APACHE II score, (C) Network evidence plot for APTT, (D) Network evidence plot for CRP.
Figure 7

The forest plots depicting the four outcomes in comparison to the placebo were generated via frequency-based methods. (A) Network evidence plot for 28-d mortality, (B) Network evidence plot for APACHE II score, (C) Network evidence plot for APTT, (D) Network evidence plot for CRP.
3.6 Publication bias
Publication bias was assessed using funnel plots generated from the four outcomes measures using Stata15 software. As shown in Figures 8A–D, the comparatively corrected funnel plots for the four outcomes demonstrated a relatively symmetrical distribution. This suggests that there was no substantial evidence of publication bias across all studies incorporated in the NMA. The detection of publication bias in the funnel plot involves a degree of subjectivity; therefore, we employed the Egger method, which identifies publication bias when the P-value is less than 0.05. Each of the four outcome indicators underwent the Egger test, resulting in a P-value of 0.218 for 28-day mortality, 0.929 for APACHE II scores, and 0.831 for APTT, indicating the absence of publication bias in all three outcome indicators. The correlation coefficient was 0.929, and the P value for APTT was 0.831, signifying the absence of publication bias for all three outcome measures. The P-value for CRP was 0.002, suggesting the presence of publication bias. We evaluated the stability of the aggregated results using the trim and fill analysis for CRP as an outcome measure. The P-value from the random-effects model employing this method was also 0.002, with a 95% confidence interval of (0, 5.496), which included 1, indicating that the results remained statistically insignificant and that there was no reversal of findings. This suggests that publication bias had minimal impact on the results, and the combined findings were relatively robust.
Figure 8

A funnel plot for evaluating bias risk. (A) Network evidence plot for 28-d mortality, (B) Network evidence plot for APACHE II score, (C) Network evidence plot for APTT, (D) Network evidence plot for CRP.
4 Discussion
Traditional Chinese medicine has evolved over the past millennia, with extensive clinical practice demonstrating its significant efficacy. The integration of Chinese medicine with Western medical practices has enhanced treatment outcomes, mitigating the adverse reactions associated with Western medicine, and bolsters patient immunity. Many proprietary Chinese medicines have been used in the treatment of sepsis, such as ShenFu injection (SF) for infectious shock, ShengMeiFang (SMF) for sepsis with deficiency of qi and yin, Xuan Bai Cheng Qi Tang (XBCQ) for sepsis with acute respiratory distress syndrome (ARDS), and Qing Wen Bai DuTang (QWBD) for sepsis with toxin-heat internalized sepsis. Furthermore, XBJ is a traditional Chinese medicine formulation used extensively in clinical adjunctive therapy for various disorders. In addition to its application in the adjuvant treatment of sepsis, numerous evidence-based studies have indicated that this injection enhances clinical efficacy and decreases mortality rates in the management of severe pneumonia (61), acute pancreatitis (62), novel coronavirus infections (63), and other conditions. The high morbidity, mortality, and rehospitalization rates associated with sepsis have become a significant medical concern in global healthcare. During the pharmacological management of sepsis, an increase in antibiotic use is correlated with an increase in the prevalence of drug-resistant and pan-resistant bacteria. This phenomenon diminishes the body's immune response, compromises its defense against pathogen invasion, significantly elevates the risk of re-infection, and contributes to an increased mortality rate associated with the disease. This study aimed to identify the optimal dose and frequency of XBJ administration to enhance the clinical efficacy of supplemental sepsis treatment.
This review included 43 RCTs, including 5,818 participants, evaluating 10 treatments with varying dosages and frequencies. The MA results indicated that XBJ at any specified doses and frequencies significantly improved the 28-day mortality, APACHE II score, APTT, and CRP levels compared to placebo and STDT. Numerous published MAs have shown that XBJ improves 28-day mortality, APACHE II scores (13, 15, 64), and CRP levels (15), corroborating the findings of this study. Furthermore, various animal experiments and network pharmacology studies have revealed that XBJ enhances coagulation function in patients with sepsis (65). Additionally, an MA revealed that XBJ not only elevated platelet levels in patients with sepsis but also reduced APTT and PT (66). thereby offering empirical support for the findings of APTT reduction in this study. A certain NMA report revealed that 50 ml-tid of XBJ was superior to other dosages in enhancing 28-day mortality, 100 ml-tid was more effective than alternative doses in improving APACHE II scores, 50 ml-bid was more efficacious than other doses in enhancing APTT, and 100 ml-qd significantly improved CRP levels. The administration of 100 ml of XBJ was more effective in enhancing APTT and CRP levels, whereas a larger daily dosage was more effective in decreasing clinical mortality (150 ml) and improving the prognosis of patients with sepsis (300 ml).
This study reviewed several prominent conventional databases and ultimately identified 43 RCTs, of which 41 were published in Chinese. Some Chinese studies lacked detailed descriptions of randomization and blinding, whereas others acknowledged these methods but failed to describe in detail their implementations. This inconsistency results in an elevated risk of bias, potentially affecting the outcomes and introducing a degree of precision bias in interpreting the results. Furthermore, the baseline characteristics in the literature were inadequately reported, for instance, while symptomatic treatment or conventional medication was referenced, the specific medications used were not disclosed. Additionally, some studies omitted data without providing explanations for the participant's dropouts. The literature indicated that a brief treatment regimen may also influence outcomes. Egger's test indicated the absence of publication bias in the outcomes of 28-day mortality, APACHE II score, and APTT. Furthermore, the findings related to the trim and fill analysis demonstrated that CRP publishing bias did not significantly influence the results, and the combined outcomes were robust.
Given that the literature lacks detailed information on the medications employed in conventional treatment, and considering that the enhancement of outcome indicators may be influenced by conventional medication, potentially skewing the interpretation of results, the SUCRA values derived from the final NMA should be interpreted with caution and should not be generalized. A single-day administration of 100 ml is most effective in enhancing APTT and CRP levels, while a 150 ml dose is superior for reducing 28-day mortality. A 300 ml dose effectively improves the APACHE II Score. Therefore, in clinical practice, the total single-day dosage of XBJ should be maintained between 100 ml and 300 ml. Furthermore, most studies are conducted over a duration of 7 days, after which varying doses and frequencies (totaling between 100 and 300 ml) are administered based on the patient's condition, yielding improved efficacy and reduced costs in clinical settings.
5 Conclusion
In conclusion, XBJ enhanced the 28-day mortality, APACHE II score, APTT, and CRP levels in patients with sepsis. The total dose of XBJ that enhanced APTT and CRP levels was 100 ml, and the application of 50 ml-tid and 100 ml-tid further improved the 28-day mortality and APACHE II scores. Consequently, these findings offer a reference for the clinical application of XBJ injections. This outcome requires validation through randomized controlled trials with substantial sample sizes and comprehensive experimental designs. This study provides a reference value for the therapeutic application of XBJ and offers evidence-based medical support for its clinical investigation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
GL: Data curation, Formal analysis, Methodology, Software, Writing – original draft. XQ: Software, Writing – review & editing, Data curation. RJ: Writing – review & editing, Data curation, Software. YW: Writing – review & editing, Methodology. ZQ: Data curation, Writing – review & editing, Software. HZ: Data curation, Writing – review & editing, Software. RM: Supervision, Writing – review & editing. JX: Supervision, Writing – review & editing. JW: Supervision, Writing – review & editing. XX: Supervision, Writing – review & editing. CY: Supervision, Writing – review & editing. XW: Supervision, Writing – review & editing. LG: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Clinical Research Award of the First Affiliated Hospital of Xi'an Jiaotong University, China (No. XJTU1AF-CRF-2022-016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1577414/full#supplementary-material
References
1.
Singer M Deutschman CS Seymour CW Shankar-Hari M Annane D et al . The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315:801–10. 10.1001/jama.2016.0287
2.
Fleischmann C Scherag A Adhikari NK Hartog CS Tsaganos T Schlattmann P et al . International forum of acute care trialists. Assessment of global incidence and mortality of hospital-treated sepsis current estimates and limitations. Am J Respir Crit Care Med. (2016) 193:259–72. 10.1164/rccm.201504-0781OC
3.
Dugar S Choudhary C Duggal A . Sepsis and septic shock: guideline-based management. Cleve Clin J Med. (2020) 87:53–64. 10.3949/ccjm.87a.18143
4.
Shah FA Pike F Alvarez K Angus D Newman AB Lopez O et al . Bidirectional relationship between cognitive function and pneumonia. Am JRespir Crit Care Med. (2013) 188:586–92. 10.1164/rccm.201212-2154OC
5.
Huang C Daniels R Lembo A . Sepsis survivors' satisfaction with support services during and after their hospitalization [abstract]. Crit Care Med. (2016) 44:425. 10.1097/01.ccm.0000510071.45969.89
6.
Rocheteau P Chatre L Briand D Mebarki M Jouvion G Bardon J et al . Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun. (2015) 6:10145. 10.1038/ncomms10145
7.
Xie J Wang H Kang Y Zhou L Liu Z et al . Chinese epidemiological study of sepsis (CHESS) study investigators. The epidemiology of sepsis in Chinese ICUs: a national cross-sectional survey. Crit Care Med. (2020) 48:e209–18. 10.1097/CCM.0000000000004155
8.
Giamarellos-Bourboulis EJ Aschenbrenner AC Bauer M Bock C Calandra T Gat-Viks I et al . The pathophysiology of sepsis and precision-medicine-based immunotherapy. Nat Immunol. (2024) 25:19–28. 10.1038/s41590-023-01660-5
9.
Zhao GZ Chen RB Li B Guo YH Xie YM Liao X et al . Clinical practice guideline on traditional Chinese medicine therapy alone or combined with antibiotics for sepsis. Ann Transl Med. (2019) 7:122. 10.21037/atm.2018.12.23
10.
Yu XZ Yao YM Zhou RB . Chinese guidelines for emergency management of sepsis and septic shock. Clin Emerg. (2018) 38:741–56. 10.3969/j.issn.1002-1949.2018.09.001
11.
Wang LJ Chi YF . Chinese emergency medicine expert consensus on diagnosis and treatment of sepsis complicated with disseminated intravascular coagulation. Care Med. (2017) 29:577–80. 10.3760/cma.j.issn.2095-4352.2017.07.001
12.
Cheng C Yu X . Research progress in Chinese herbal medicines for treatment of sepsis: pharmacological action, phytochemistry, and pharmacokinetics. Int J Mol Sci. (2021) 22:11078. 10.3390/ijms222011078
13.
Li C Wang P Zhang L Li M Lei X Liu S et al . Efficacy and safety of Xuebijing injection (a Chinese patent) for sepsis: a meta-analysis of randomized controlled trials. J Ethnopharmacol. (2018) 224:512–21. 10.1016/j.jep.2018.05.043
14.
Chen G Gao Y Jiang Y Yang F Li S Tan D et al . Efficacy and safety of Xuebijing injection combined with ulinastatin as adjunctive therapy on sepsis: a systematic review and meta-analysis. Front Pharmacol. (2018) 9:743. 10.3389/fphar.2018.00743
15.
Wu Y Zhang J Qi L . Clinical efficacy and safety of Xuebijing injection on sepsis: a meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2020) 32:691–5. 10.3760/cma.j.cn121430-20200427-00475
16.
Liu S Yao C Xie J Liu H Wang H Lin Z et al . Effect of an herbal-based injection on 28-day mortality in patients with sepsis: the EXIT-SEP randomized clinical trial. JAMA Intern Med. (2023) 183:647–55. 10.1001/jamainternmed.2023.0780
17.
Zhang H Wei L Zhao G Liu S Zhang Z Zhang J et al . Protective effect of Xuebijing injection on myocardial injury in patients with sepsis: a randomized clinical trial. J Tradit Chin Med. (2016) 36:706–10. 10.1016/S0254-6272(17)30003-1
18.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71
19.
Cheng X Li G Qin H . Effects of different doses of Xuebijing injection on inflammatory indexes and alternative treatment and prognosis in patients with sepsis. Chin J TCM WM Crit. (2023) 30:132–5. 10.3969/j.issn.1008-9691.2023.02.002
20.
Jia HD Bao SH . Effect of Xuebijing injection on inflammatory factors and high-sensitivity C-reactive protein in patients with sepsis Pract Prev Med. (2014) 21:609–11. 10.3969/j.issn.1006-3110.2014.05.038
21.
Li B Chen GB Ge L . Study on effect of Xuebijing Injection on serum tumor necrosis factor-α and blood coagulation in patients with septic septic collision with septic shock. Liaoning J Tradit Chin Med. (2018) 45:977–9. 10.13192/j.issn.1000-1719.2018.05.026
22.
Pu Q Liu LL Ju PH Hua W . Clinical effects of Xuebijing injection in treating sepsis and its impacts on the levels of serum thyroid hormone. Western Journal of Traditional Chinese Medicine. (2020) 33:85–8. 10.12174/j.issn.1004-6852.2020.06.22
23.
Shen Y Li M Jiang Y Yu Q Chen T . Effects of Xuebijing injection on the lung function and inflammatory factors of patients with sepsis-induced lung injury. Progress in Modern Biomedicine. (2017) 17:6684–7,25. 10.13241/j.cnki.pmb.2017.34.017
24.
Wang DH Xu JH Gao L . Serum level of C reactive protein and incidence of sepsis in patients with severe burn treated with Xuebijing injection. China Pharmacy. (2009) 20:1662–3.
25.
Wu HB Sun XC Huang H Guan CM . Injection treatment greatly affects serum biochemical parameters of patients with severe sepsis. Chin J Mod Med. (2015) 25:83–6.
26.
Xu JF Li YL Yang LJ Cao YY Liang RS Ye SY . Clinical observation of Xuebijing in the treatment of capillary leak syndrome in patients with sepsis. J New Chin Med. (2016) 48:139–40. 10.13457/j.cnki.jncm.2016.05.053
27.
Zhang CL . Clinical observation of sepsis disease adjunctive treated with Xuebijing injection. Chin J Exp Clin Infect Dis (Electronic Edition). (2014) 360–2. 10.3877/cma.j.issn.1674-1358.2013.03.014
28.
Jiang HB Ma MY Lao ML . Effect of Xuebijing injection on urine albumin creatinine ratio in septic patients. Chin J Basic Med Tradit Chin Med. (2014) 20:979–81.
29.
Liu XF Li WF Zhao L Lin ZF . Clinical study of Xuebijing injection on protection of organ function of patients with severe Sepsis in Intensive care unit. Chin J TCM WM Crit Care. (2010) 17:20–3.
30.
Sun B Yang ZP . Effect of Xuebijing on the prevention and treatment of sepsis combined with DIC in plateau areas. Chin J Prim Med Pharm. (2007) 14:1190–2. 10.3760/cma.j.issn.1008-6706.2007.07.069
31.
Qiao X . Effects of Xuebijing injection on hemodynamics and inflammatory factors in patients with septic shock. Mod J Integr Tradit Chin West Med. (2016) 25:3459–61. 10.3969/j.issn.1008-8849.2016.31.014
32.
Shao J Li W Shi HQ . Observation on the therapeutic effect of Xuebijing injection in the adjuvant treatment of septic shock in the elderly. People's Mil Surg. 55:144–6.
33.
Dong YY Peng LB Huang YF Chen Y Li DZ Zhang ZJ . Effect of Xuebijing injection on the expression of MYO. CysC and KIM-1 in sepsis patients. JETCM. (2018) 7:40–3.
34.
Zhang PP Wang QS Li ZJ Wang DQ Li YP . Effects of Xuebijing injection on blood coagulation in patients with sepsis. Chin J TCM WM Crit Care. (2014) 21:198–200. 10.3969/j.issn.1008-9691.2014.03.010
35.
Dai QC Na L Hui Z Miao XY Shen HL Zhang XW . The efficacy of Xuebijing injection in the treatment of severe sepsis and its effect on serum biochemical. J Gerontol. (2016) 36:2989–91. 10.3969/j.issn.1005-9202.2016.12.078
36.
Dou ZM Yin C Liu J Li B . Effect of Xuebijing injection on microcirculation in patients with sepsis. J Clin Med. (2018) 15:196–9. 10.3969/j.issn.1672-6170.2018.03.061
37.
Gong RY Tie MH Gong WY Shen L Pang YC Ma J . Intervention effect of Xuebijing injection on coagulation function of patients with severe sepsis. Chin J Integr Tradit West Med Intensive Crit Care. (2018) 25:254–6. 10.3969/j.issn.1008-9691.2018.03.008
38.
Ji XZ Hu QF Zhang YS . Application of Xuebijing injection in patients with severe acute pancreatitis complicated with sepsis. Hainan Med. (2018) 29:3321–4. 10.3969/j.issn.1003-6350.2018.23.021
39.
Ji P Liang M Yang C Tong L . The efficacy of Xuebijing injection in treating post-burn sepsis and its protective effect on kidney. Mod J Tradit Chin West Med. (2016) 25:3831–3. 10.3969/j.issn.1008-8849.2016.34
40.
Jiang WS Zhou YH Wang YH Chen CY . Effect of Xuebijing injection on inflammatory stress response and myocardial function in patients with Sepsis of EICU. Shaanxi Journal of Traditional Chinese Medicine. (2020) 41:502–23. 10.3969/j.issn.1000-7369.2020.04.030
41.
Liu DK . Clinical study on the efficacy of Xuebijing injection in the treatment of severe sepsis and its effect on serum IL-6 and IL-10 levels. J New Chin Med. (2013) 45:31–3. 10.13457/j.cnki.jncm.2013.01.087
42.
Shi W Gou Pei XJ Zhang LD . Clinical analysis of Xuebijing injection in the treatment of sepsis. Guid J Tradit Chin Med Pharmacol. (2017) 23:102–4.
43.
Su W Yang Z Wang SR Liu JY . Effect of Xuebijing injection on inflammatory response and cellular immune function in patients with severe sepsis. Chin J Postgrad Med. (2012) 35:29–32. 10.3760/cma.j.issn.1673-4904.2012.01.009
44.
Sun TZ . Preventive effect of Xuebijing injection on sepsis in patients with severe burn and its effect on inflammatory factors. Hebei Med. (2019) 25:969–72. 10.3969/j.issn.1006-6233.2019.06.022
45.
Wang YZ Li Y Bai K Gao HB Zhang JJ . Effect of Xuebijing injection on lactate clearance rate and lactate area in patients with sepsis and the analysis of outcome. Pract Pharm Clin Rem. (2019) 22:397–400. 10.14053/j.cnki.ppcr.201904014
46.
Zhang LD Pei XJ . Effects of Xuebijing parenteral solution on blood coagulation function and blood gas analysis in severe sepsis patients. Chin J Crit Care Med. (2012) 32:1088–91. 10.3969/j.issn.1002-1949.2012.12.007
47.
Zhang H Wei LY Wei LY Zhao G Liu SZ Yang YJ et al . Effect of Xuebijing injection on procalcitonin in severe sepsis patients. Chin J Prim Med Pharm. (2017) 24:664–7. 10.3760/cma.j.issn.1008-6706.2017.05.006
48.
Jiang YS . Analysis of the effect of Xuebijing injection on coagulation function and prognosis of severe sepsis. Chin J Crit Care Med. (2017) 37:306–7. 10.3969/j.issn.1002-1949.2017.z1.232
49.
Yang XN . Effect of Xuebijing injection on coagulation function in patients with sepsis. Jilin Med J. (2020) 41: 1827–9.
50.
Lu RH Hou M Li S Guan W . Effect of Xuebijing on serum chitinase protein 40 and sE-selectin levels in patients with sepsis. Chin J Gerontol. (2018) 38:173–5. 10.3969/j.issn.1005-9202.2018.09.050
51.
Ma T Zhang L Li X . Effect of Xuebijing on organ function and inflammatory factors in patients with severe sepsis. Hebei Med. (2016) 22:552–5. 10.3969/j.issn.1006-6233.2016.04.009
52.
Ming ZQ Yu LM Lv YX Wang LW Lv SM . Observe on the anti-inflammatory action of XueBijing injection on sepsis illness. Mod J Integr Tradit Chin West Med. (2007) 16:731–2. 10.3969/j.issn.1008-8849.2007.06.006
53.
Shao M Liu B Wang JQ Tao XG Zhou SS Jin K et al . Effect of Xuebijing injection on T helper 17 and CD4+CD25+regulatory T cells in patients with sepsis. Chin Crit Care Med. (2011) 23:430–4. 10.3760/cma.j.issn.1003-0603.2011.07.014
54.
Song XJ Li WY Fu YJ Gu W . Clinical efficacy of Xuebijing in the treatment of sepsis and its effect on the ratio of neutrophil count to lymphocyte count. Chin J TCM WM Crit Care. (2021) 28:690–2. 10.3969/j.issn.1008-9691.2021.06.010
55.
Wang X Lyu H Chen MQ Lu J Cheng L Zhou HQ et al . A clinical research on renal protective effect of Xuebijing injection in patients with sepsis. Zhonghua wei zhong bing ji jiu yi xue. (2015) 27:371–4.
56.
Xing J Wang N Zhang Y . Effects of Xuebijing injection on cardiac function and prognosis in patients with septic multiple organ dysfunction syndrome. Chin J TCM WM Crit Care. (2011) 18:359–62. 10.3969/j.issn.1008-9691.2011.06.014
57.
Yin Q Li C . Treatment effects of Xuebijing injection in severe septic patients with disseminated intravascular coagulation. Evid Based Complement Alternat Med. (2014) 2014:949254. 10.1155/2014/949254
58.
Zhu RY Zhang D Lan L Xia WF Zou HD Zhou QS . Observation on the therapeutic effect of Xuebijing Injection in the treatment of patients with sepsis. Tradit Chin Med J. (2014) 13:44–6.
59.
Zhang R Zhang GF Chen J . Impact of Xuebijing injection on coagulation function and prognosis of patients with severe sepsis. J Chengdu Med Coll. (2016) 11:48–51. 10.3969/j.issn.1674-2257.2016.01.011
60.
Chen M Zhou WJ Che ZQ Ma L Zhao B Shao JW et al . Effect of Xuebijing injection on coagulation function in emergency patients with severe sepsis. OccupHealth & Emerg Rescue. (2023) 41:421–25,35. 10.16369/j.oher.issn.1007-1326.2023.04.006
61.
Song Y Yao C Yao Y Han H Zhao X Yu K et al . XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit Care Med. (2019) 47:e735–43. 10.1097/CCM.0000000000003842
62.
Zhu F Yin S Zhou L Li Z Yan H Zhong Y et al . (2022). Chinese herbal medicine Xuebijing injection for acute pancreatitis: An overview of systematic reviews. Front Pharmacol. 13:883729. 10.3389/fphar.2022.883729
63.
Ni L Chen L Huang X Han C Xu J Zhong Y et al . Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm Sin B. (2020) 10:1149–62. 10.1016/j.apsb.2020.06.009
64.
Tian S Qin D Ye Y Yang H Chen S Liu T et al . Scientific evidence of Xuebijing injection in the treatment of sepsis. Evid Based Complement Alternat Med. (2021) 2021:6879278. 10.1155/2021/6879278
65.
Zhou W Lai X Wang X Yao X Wang W Li S . Network pharmacology to explore the anti-inflammatory mechanism of Xuebijing in the treatment of sepsis. Phytomedicine. (2021) 85:153543. 10.1016/j.phymed.2021.153543
66.
Hou SY Feng XH Lin CL Tan YF . Efficacy of Xuebijing for coagulopathy in patients with sepsis. Saudi Med J. (2015) 36:164–9. 10.15537/smj.2015.2.9895
Summary
Keywords
sepsis, Chinese herbal medicines, Xuebijing injection, network meta-analysis, randomized controlled trial
Citation
Liu G, Qu X, Jiang R, Wu Y, Qin Z, Zhang H, Ma R, Xue J, Wang J, Xu X, Yan C, Wei X and Guo L (2025) Effects of different dosages/frequency of Xuebijing injection (a Chinese patent) for sepsis: a network meta-analysis of randomized controlled trials. Front. Med. 12:1577414. doi: 10.3389/fmed.2025.1577414
Received
15 February 2025
Accepted
24 April 2025
Published
22 May 2025
Volume
12 - 2025
Edited by
Zhongheng Zhang, Sir Run Run Shaw Hospital, China
Reviewed by
Yongsheng Chen, Jinan University, China
Yuxiang Fei, China Pharmaceutical University, China
Updates
Copyright
© 2025 Liu, Qu, Jiang, Wu, Qin, Zhang, Ma, Xue, Wang, Xu, Yan, Wei and Guo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Litao Guo glt02@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.