Abstract
Introduction:
Automated radiolabeling of gallium-68-labeled experimental radiopharmaceuticals is crucial for ensuring high reproducibility and regulatory compliance in clinical settings. FAP-2286, a promising DOTA-pseudopeptide targeting the tumor microenvironment, has demonstrated superior tumor retention compared to quinoline-based analogs, making it an attractive theranostic agent. This study aimed to optimize and automate the preparation of [68Ga]Ga-FAP-2286 on the GAIA® synthesizer, ensuring high radiochemical purity (RCP) and radiochemical yield (RCY).
Methods:
Manual radiolabeling assays were initially performed to identify optimal reaction conditions, varying buffer, antioxidant, vector amount, heating time, and purification methods. The selected conditions were then adapted to an automated protocol using a GAIA® module. A strong cation exchange (SCX) cartridge for 68Ga pre-concentration and a solid-phase extraction (SPE) step for final purification were included in the process. RCY, RCP, and stability over 4 h were assessed using radio-HPLC and radio-TLC. Additionally, the applicability of the optimized automated method was evaluated for 3BP-3940, a structurally related pseudopeptide.
Results:
Initial optimization studies identified sodium acetate buffer 0.1 M with methionine as an antioxidant, 25 μg of FAP-2286, and a 4-min heating time as the best manual radiolabeling conditions, achieving a RCP > 98%. In the automated synthesis, adjustments were made, including doubling the vector amount and extending heating to 9 min, resulting over three test-batches in a moderate RCY of 59.85 ± 3.73% and a RCP just over 94% up to 4 h after the end of synthesis. Importantly, the method was successfully transposed to [68Ga]Ga-3BP-3940, yielding better RCY (75.62 ± 11.76%), RCP and stability profiles (> 95.95% over 4 h).
Conclusion:
This study established a robust, automated protocol for the synthesis of [68Ga]Ga-FAP-2286, ensuring high purity, reproducibility, and compatibility with clinical applications. The method’s successful adaptation to 3BP-3940 highlights its versatility for such radiopharmaceuticals, supporting the broader implementation of automated theranostic agent production in nuclear medicine.
1 Introduction
Positron emission tomography (PET) imaging is now an established and essential tool in oncology, helping in diagnosis, staging and assessment of treatment response in many types of cancer (1). Beyond its more common clinical applications, PET imaging is also a highly dynamic field of research and development (2, 3), particularly with the increasing availability of gallium-68 (68Ga) (4). The widespread use of 68Ga is largely attributed to the convenience of its generator-based production and the possibility of radiolabeling a wide variety of targeting molecules, provided that they are functionalized with an appropriate chelating agent, e.g., 2,2′,2″,2″-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl) tetraacetic acid (DOTA), 2-(4,7-bis (carboxymethyl)-1,4,7-triazonan-1-yl) pentanedioic acid (NODAGA), or 2,2′,2″-(1,4,7-triazacyclononane-1,4,7-triyl) triacetic acid (NOTA) (5). As a result, such a framework tends to facilitate the rapid translation of novel radiopharmaceuticals into clinical applications.
In recent years, there has been growing interest in targeting the tumor microenvironment, particularly through fibroblast activation protein (FAP) inhibitors (FAPIs) (6). The first generation of FAPI molecules, sharing a quinoline moiety and a glycine-cyanoproline motif, was developed as PET imaging probes radiolabeled with 68Ga (7–9). Among these compounds, FAPI-04 and FAPI-46 are widely used in clinical practice, notably for cancers where the non-specific but highly sensitive [18F] FDG radiopharmaceutical fails to provide satisfactory imaging results (10, 11). A second generation of FAPI compounds has since been developed, featuring a pseudopeptide structure composed of a seven-amino acid sequence cyclized via reaction with 1,3,5-tris (bromomethyl) benzene to form a mesityl cyclic core, subsequently functionalized with a DOTA chelator (Figure 1). The first of these pseudopeptide FAPI compounds to be used in humans for PET imaging was FAP-2286 (rofapitide tetraxetan) (12). After an earlier study demonstrated its potent affinity for human FAP protein and its effective binding in vitro (IC50 from 1.3 to 2.2 nM) (13, 14), it was suggested that [68Ga]Ga-FAP-2286 was superior to [18F]FDG for detecting lesions in selected cancers, such as gastric, pancreatic, and hepatic tumors (particularly intrahepatic cholangiocarcinomas) (15). Subsequent studies have consolidated these results (16, 17), extending the potential applications of [68Ga]Ga-FAP-2286 to other tumor types such as urothelial (18, 19) and lung cancers (20). Shortly afterward, a compound directly related to FAP-2286 called 3BP-3940 was studied in clinical settings and also displayed excellent properties as a molecular PET imaging agent, including a remarkably high tumor-to-background ratio and minimal renal accumulation (21, 22). Importantly, a major advantage of anti-FAP pseudopeptide derivatives over quinoline compounds is their higher intratumoral retention (23, 24), making them suitable as theranostic vectors—enabling PET imaging when radiolabeled with 68Ga and therapeutic applications when radiolabeled with 177Lu, for example. Consequently, several studies have reported on the use and efficacy of [177Lu]Lu-FAP-2286 in various cancers (25–27), with case reports further supporting these findings (28–33). The FAP-2286 vector associated with the 68Ga/177Lu theranostic pair is currently being investigated in the LuMIERE phase 1/2 trial to evaluate its safety, pharmacokinetics, and preliminary efficacy in patients with selected advanced solid tumors (NCT04939610) (34).
FIGURE 1

Chemical structure of the vector molecules FAP-2286 and 3BP-3940.
The automation of radiopharmaceuticals production has played an increasing role in ensuring consistent and reproducible radiolabeling processes (35). While automation has long been established in the industrial production of fluorine-18-labeled compounds (36), the past two decades have seen a proliferation of synthesizers designed for nuclear medicine departments. Initially semi-automated, these systems have now evolved into fully automated platforms compatible with radiolabeling using various isotopes, including radiometals such as 68Ga (37, 38). Moreover, the well-documented chemistry and on-site availability of 68Ga make it particularly suited for the automated synthesis of experimental 68Ga-labeled radiopharmaceuticals using customized protocols (39, 40). By carefully selecting the nature and concentration of reaction components, radiolabeling conditions can be finely tuned to one specific vector molecule.
In this context, we investigated the optimization of 68Ga-radiolabeling conditions for FAP-2286 through a systematic screening of reaction buffers, antioxidant compounds, vector amounts, heating times, and purification methods. Following a series of manual radiolabeling experiments, the optimal conditions for [68Ga]Ga-FAP-2286 synthesis were implemented on a specific automated synthesis module (GAIA®, Elysia Raytest). Additionally, we explored the applicability of these optimized conditions to the radiolabeling of 3BP-3940, a pseudopeptidic compound structurally related to FAP-2286.
2 Materials and methods
2.1 Reagents and equipment
All reagents used for radiolabeling were of the highest available purity and sourced from Merck (Germany). Pharmaceutical-grade water for injection (WFI; Eau pour prép. injectables 10 mL PROAMP®, Aguettan, France; 100 mL Ecoflac, B. Braun, France) and 0.9% sodium chloride solution (Chlorure de sodium PROAMP® 0.9% 10 mL, Aguettan, France) were employed in the process. Ethanol absolute (≥ 99.8%, Ph. Eur. grade, VWR, United States), was also used. The radiolabeling optimization experiments were carried out with non-GMP grade FAP-2286 (MedChem Express, NJ, United States). A stock solution of 1 mg/mL pseudopeptide in WFI was prepared, aliquoted into 25 μg/25 μL fractions using Eppendorf Protein LoBind Tubes (1.5 mL), and stored at −20°C for up to 3 months. Gallium-68 was eluted in 0.1 N hydrochloric acid as [68Ga]GaCl3 from a pharmaceutical-grade 68Ge/68Ga generator (GALLI AD® 1.85 GBq, Ire Elit, Belgium). Consecutive elutions were spaced by a minimum of 4 h and did not exceed a 24-h interval. The manual preparation of [68Ga]Ga-FAP-2286 took place in a shielded cell (MEDI 2000, LemerPax, France), where both the 68Ge/68Ga generator and a dry bath (Zinsser Analytic, Germany) were housed. Automated preparation of [68Ga]Ga-FAP-2286 and [68Ga]Ga-3BP-3940 was performed on a GAIA® synthesizer (Elysia-Raytest, Germany) in a shielded, GMP ISO 5 cell with laminar airflow (MEDI 9000 Research 4R, LemerPax, France) where the synthesis module and a second GALLI AD® generator were positioned.
2.2 Manual radiolabeling assays for the study of reaction conditions
For each radiolabeling condition tested, three identical reactions were run simultaneously. Typically, aliquots of vector (1 mg/mL, i.e., 0.68 μmol/L, 12.5–50 μL) contained in 1.5 mL Eppendorf vials were warmed to room temperature and diluted in 375 μL of buffer solution. Depending on the conditions tested, 25 μL of antioxidant compound solution were also added. In the shielded cell, the 68Ga generator was eluted into a bulk vial to obtain ∼1 mL of 68Ga3+ solution that was not further purified. Then, 267 μL (∼120 MBq) of this gallium solution were added to each Eppendorf of the triplicate. The reaction mixtures were heated in a 95°C water bath for 4, 8, or 12 min. After the reaction, the Eppendorf tubes were allowed to cool for 5 min, after which a sample was taken from each crude mixture for quality controls. During the study of reaction conditions, quality controls only included radiochemical purity (RCP) determination by radio-HPLC and pH check by indicator strip.
The radiolabeling conditions tested were essentially inspired by protocols found in the literature, or chosen to facilitate a logical, comprehensive discussion of the results (e.g., for selected buffer concentrations) (Table 1). Each triplicate varied by only a single parameter. Buffer solutions were prepared extemporaneously as 5 mL stock solutions. Importantly, the pH of these solutions was finely adjusted with ultrapure 37% HCl, so that a mixture of 375 μL buffer and 267 μL 0.1 M HCl (mimicking the 68Ga-eluate) would reach a pH of 3.6–3.8, ideal for such radiolabeling reaction (41). For each buffer and mixture, pH value was measured using a recently calibrated Vario® pH meter (WTW®, Xylem, United States) equipped with a SenTix® 41 pH electrode (WTW®, Xylem, United States).
TABLE 1
| Buffer solution tested | References |
| Sodium acetate 0.1 M | (50) |
| Sodium acetate 0.5 M | (51) |
| Sodium acetate 1.5 M | (82) |
| Ammonium acetate 0.1 M | (83) |
| Ammonium acetate 0.5 M | (84) |
| Ammonium acetate 1.5 M | (85) |
| Sodium formate 0.5 M | (75) |
| Sodium formate 1.5 M | (86) |
| HEPES 0.5 M | (87) |
| HEPES 1.5 M | (88, 89) |
| Antioxidant compound tested | References |
| Ascorbic acid 12 mg/mL | (52) |
| Gentisic acid 16 mg/mL | (63) |
| Methionine 10 mg/mL | (53) |
Buffer solutions and antioxidant compounds tested for the preparation of [68Ga]Ga-FAP-2286.
Antioxidant compound solutions, i.e., ascorbic acid 14 mg/mL (79.5 mM), gensitic acid 16 mg/mL (103.8 mM) and methionine 10 mg/mL (67 mM) (Table 1), were also freshly prepared as 10 mL stock solutions. For experiments involving the addition of one of these antioxidant compounds, the stability of the radiolabeling product was monitored by radio-HPLC over 4 h.
Three different amounts of vector molecule were used in the radiolabeling tests (12.5, 25, or 50 μg, i.e., 8.5, 17, or 34 nmol) in order to identify the lowest amount of FAP-2286 required to achieve good RCP. Similarly, heating times of 4, 8, and 12 min were tested to optimize preparation duration.
Finally, four solid-phase extraction cartridge models (i.e., Sep-Pak® Plus Short C18, Oasis HLB Plus Short, Strata-X, Sep-Pak Accell Plus CM Plus Short) were each tested for final purification on a radiolabeling triplicate. For the cartridges concerned, washing was performed with 4 mL WFI after deposition of the crude reaction medium, and elution was performed with 1.5 mL 60% ethanol.
2.3 Application of the best reaction conditions to an automated radiolabeling protocol
The best radiolabeling conditions resulting from manual experiments were transposed to a custom automated preparation method on the GAIA® module. This system uses sterile, single-use tubing sets with 3 ramps (named A, B and C from left to right) of 5 manifolds each (numbered from 1 to 5, from top to bottom or left to right), and relies on a peristaltic pump to transfer liquids into the fluidic system. First, the cassette was assembled as shown in Figure 2. Specifically, a strong cation exchange (SCX) cartridge (Bond Elut SCX, 100 mg, 1 mL, 40 μm, Agilent) with an appropriate Luer adapter was used to connect position A2 to position B1 horizontal. Likewise, a solid phase extraction cartridge (either a Sep-Pak C18 Plus Short cartridge or an Oasis HLB Plus Short cartridge) was used to connect position B5 horizontal to position C2 after appropriate manual preconditioning of the cartridge with 5 mL of absolute ethanol and 5 mL of WFI. The other reagents were then connected to the manifolds, i.e., a mixture of 170 μL sodium acetate 0.8 M and 1.03 mL methionine 10 mg/mL solubilizing 50 μg vector in B3, 12.8 mL methionine 1 mg/mL in 0.9% NaCl for formulation in B4, 2.3 mL 60% ethanol for solid phase extraction (SPE) elution in vertical B5, 0.4 mL 5 M NaCl in 0.13 N HCl for SCX elution in horizontal C1, and a 500 mL bag of WFI in C4.
FIGURE 2

(A) Cassette-based scheme of the GAIA® synthesizer for [68Ga]Ga-FAP-2286. (B) Photograph of the set-up on the GAIA® module.
Once initiated, automated radiolabeling proceeds as follows: first, the system is purged with filtered air to remove any residual liquid from the SPE cartridge. Next, an integrity test of the tubing set is performed by pumping filtered air into the system, raising the pressure in the kit above 1,500 mbar. Once the system is sealed, the test is considered successful if the pressure drop does not exceed 400 mbar, allowing the sequence to proceed. The buffer solution and antioxidant mixture, which solubilizes the vector, is then transferred to the reaction vial. Meanwhile, the SCX and C18 cartridges are conditioned with WFI, and the system is purged with filtered air. At this stage, the generator can be eluted, passing the 68Ga-solution in 0.1 N HCl through the SCX cartridge, where 68Ga3+ ions are retained. Approximately 950 MBq were involved in the automated radiolabeling process at the time of elution from the generator. After washing with WFI, the SCX cartridge is eluted with 0.4 mL of NaCl-saturated solution in 0.13 N HCl, directing the activity to the reaction vial. The reaction vial, maintained at 60°C up to this point, is then heated to 95°C for 9 min to facilitate radiolabeling. The radiolabeled product retained on the SPE cartridge is eluted in four successive fractions of 60% ethanol (total volume: 2.3 mL), alternating with NaCl plus methionine fractions. The product solution is eluted into the product vial through a 0.22 μ sterile filter. The final product is formulated by adding the remaining 1 mg/mL methionine solution in saline (total volume: 12.8 mL), after which the terminal vial containing the product can be removed. Finally, the system performs an automated bubble point integrity test on the 0.22 μm end filter. The sequential steps of this protocol are summarized in Figure 3.
FIGURE 3
![Flowchart of the synthesis process for Gallium-68 ([68Ga]) labeled FAP-2286. The left side lists 15 step-by-step procedures for the synthesis, starting with tubing set preparation and ending with final product vial release. The right side illustrates the setup, including equipment like the generator, SCX and SPE cartridges, reaction medium, and final product vial. Key stages include reaction medium preparation and washing processes, detailing specific conditions like temperatures and solution concentrations.](https://www.frontiersin.org/files/Articles/1628158/xml-images/fmed-12-1628158-g003.webp)
Flow chart for the automated [68Ga]Ga-FAP-2286 production process.
To determine the radiochemical yield (RCY) of the automated synthesis, radioactivity measurements were made in a calibrated ionization chamber (CRC®-25R, Capintec, United States). These measurements included the final product vial, reaction vial, waste vial, purification cartridge, and terminal filter, with activity values adjusted to the radiolabeling endpoint for accurate yield calculation. RCY is the ratio of the activity in the terminal vial to the sum of the activities of all the elements in the system, weighted by the RCP and all decay-corrected to the end of synthesis (EoS) time.
To assess the feasibility of adapting this automated radiolabeling method for another 68Ga-labeled pseudopeptide, the previously described protocol was applied to produce three test batches of (68Ga) Ga-3BP-3940, synthesized using a non-GMP grade vector (MedChem Express, NJ, United States). All parameters used in these three syntheses were identical to those employed in the automated preparation of [68Ga]Ga-FAP-2286.
2.4 Quality controls
For each radiolabeling reaction product, a RCP analysis was performed by radio-HPLC, using a Nexera X3 station (Shimadzu, Japan) supplied with HPLC-grade solvents. The apparatus included a solvent degasser (DGU-405), a solvent pump (LC40D), an autosampler (SIL-40) set at 20 μL injection volume, a column oven (CTO-40S) set at 30°C, a UV detector (SPD-40 190–700 nm) set at 254 and 280 nm and a radioactivity detector (GABI Nova with mid-energy probe and 2 × 5 μL flow cell) connected in series. A C18 ACE® Equivalence™ column (3.0 × 150 mm, 110 Å pore size and 3 μm particles size) was used as the stationary phase. The flow rate was 0.6 mL/min and the mobile phase gradient was programmed with 0.1% TFA in water (line A) to 0.1% TFA in acetonitrile (line B) as follow: 0–1 min 95/5 A/B; 1–8 min linear gradient from 95/5 A/B to 60/40 A/B; 8–9 min 60/40 A/B; 9–10 min linear gradient from 60/40 A/B to 95/5 A/B; 10–12 min 95/5 A/B. RCP was calculated using the dedicated software (Gina X, Elysia Raytest, Germany) by spectra integration and comparison of areas under peaks.
During the radiolabeling assays, pH of the reaction products was controlled using either 2-zones Rota pH 1–11 indicator paper (VWR, PA, United States) or MQuant® pH 2.5–4.5 indicator strips (Merk, NJ, Unites States).
Specific quality controls were performed only on test batches produced via the automated process:
-
•
Radio-TLC analyses used a two-strip iTLC-SG system inspired from [68Ga]Ga-edotreotide summary of product characteristics (42), with aqueous ammonium acetate 1 M in methanol (1:1 mixture) (conditions A) and aqueous sodium citrate 0.1 M pH 5 (conditions B) as mobile phases. Measurement of the percentages of radioactivity at the origin and at the solvent front was carried out using a radio-TLC scanner (miniGITA® Star, Elysia-Raytest, Germany). The corresponding acquisition software (TLC Control v.2.30, Raytest, Germany) and analysis software (GINA Star TLC™ v.6.0, Elysia-Raytest, Germany) were used for data analysis. Under conditions A, Rf values of 0.0–0.2 for 68Ga-impurities and 0.8–1.0 for [68Ga]Ga-FAP-2286 were expected. Under conditions B, Rf values of 0.0–0.2 for [68Ga]Ga-FAP-2286 and 0.8–1.0 for free 68Ga were expected.
-
•
Gamma spectrometry was conducted on a low-activity sample (around 100 kBq in 1 mL) from each validation batch of [68Ga]Ga-FAP-2286 using a Hidex AMG® gamma counter (LabLogic, United Kingdom). Identification focused on the 511 and 1,077 keV peaks of annihilation photons.
-
•
The half-life was confirmed by performing multiple measurements over approximately 1 h. Expected values ranged between 61 and 75 min, with a theoretical reference of 67.71 min (43).
-
•
To evaluate radionuclide purity, the same samples used for radionuclide identity testing were reanalyzed by gamma counting after 48 h of decay. This measurement allowed the identification of any residual 68Ga activity resulting from 68Ge breakthrough or other radionuclide impurities with long half-life. Residual radioactivity after 48 h was expected to remain below 0.001% of the initial activity recorded in each sample.
The RCP of the test batches was assessed by radio-HPLC for up to 4 h post-preparation as described above.
2.5 Statistical analysis
Student’s t-test was used to compare triplicate RCP or RCY values obtained under two different reaction conditions. For each triplicate tested, normal distribution of data was confirmed by a Shapiro-Wilk test. The p-value was used to estimate statistical significance, with p ≤ 0.05 considered significant. For stability tests, the relationship between variation in RCP and time was estimated by regression analysis.
3 Results
3.1 Selection of optimal radiolabeling conditions
The general process for manual radiolabeling described above allowed the efficient screening of 17 different radiolabeling conditions and four purification methods, representing more than 60 individual radiolabeling reactions.
The radio-HPLC RCP values measured from different reaction buffers are summarized in Figure 4, and suggest that the preparation of [68Ga] Ga-FAP-2286 allows the use of a variety of buffer types and molarities. Indeed, 8 of the 10 buffers tested led to a RCP > 80% without terminal purification. This was particularly the case for sodium formate, for which molarity did not seem to have a significant influence (RCP = 88.76 ± 0.79% at 0.5 M; RCP = 89.17 ± 2.19% at 1.5 M, p = 0.778). The HEPES buffer produced comparable or even slightly improved results at the low concentration (RCP = 92.56 ± 3.34% at 0.5 M; RCP = 86.70 ± 4.72% at 1.5 M). This molecule, which belongs to Good’s buffers (44, 45), is particularly well suited for the preparation of 68Ga-radiopharmaceuticals due to its weak complexing properties and excellent pH control (46, 47). However, regulatory restrictions in final radiopharmaceuticals formulations make its use inadvisable whenever possible (48, 49). Excellent RCP values were achieved with acetate buffers at low concentrations (sodium acetate 0.1 M: 90.89 ± 0.69%; ammonium acetate 0.1 M: 92.73 ± 1.53%), while an increase in molarity seemed to be unfavorable to the good complexation of gallium by the DOTA-pseudopeptide (sodium acetate 1.5 M: 73.39 ± 4.39%, p = 0.024; ammonium acetate 1.5 M: 82.90 ± 1.93%, p = 0.023). Overall, as the RCP values obtained with low-molarity acetate buffers were not significantly different from each other (p = 0.161), and in view of the efficiency of sodium acetate in numerous other 68Ga-radiolabeling protocols (50–58), it was selected for the following assays.
FIGURE 4

Mean RCP values (determined by radio-HPLC) for [68Ga]Ga-FAP-2286 using buffers of different types and molarities.
Among the three antioxidant compounds tested (Figure 5A), ascorbic acid at 79.5 mM (∼2.9 mM in the reaction volume) slightly reduced RCP (88.08 ± 1.46%), though not significantly (p = 0.061) when added to the radiolabeling mixture. The difference in RCP between radiolabeling with 0.1 M sodium acetate alone and with the same buffer supplemented with gentisic acid (∼3.8 mM in the reaction volume) was significantly unfavorable for this antioxidant (RCP = 78.80 ± 3.41%, p = 0.022). Conversely, adding methionine (∼2.4 mM in the reaction medium) to the radiolabeling reaction significantly improved purity, as measured by radio-HPLC (RCP = 94.65 ± 0.53%, p = 0.002). Both methionine and ascorbic acid were highly effective in maintaining RCP over 4 h (slope of regression line not significantly different from zero, p = 0.23 and 0.20, respectively) (Figure 5B). In the absence of an antioxidant compound, the purity of the radiolabeled product gradually decreased over time (p = 0.017; RCP at 4 h = 81.88 ± 0.65%). Notably, this decline was even more pronounced in the presence of gentisic acid (p = 0.025; RCP at 4 h = 69.68 ± 8.88%). In view of the above results, the subsequent radiolabeling tests were carried out in the presence of methionine in the reaction medium. Interestingly, modifying the reaction medium to significantly increase the amount and concentration of the antioxidant agent allowed for further optimization of the radiolabeling conditions. This was achieved by combining 60 μL of 0.8 M sodium acetate (final concentration ∼0.1 M) with 365 μL of 10 mg/mL methionine (final concentration ∼8.6 mg/mL) in a total reaction volume of 692 μL. Under these conditions, which were used for the subsequent assays, the average RCP reached 98.12 ± 0.72%.
FIGURE 5
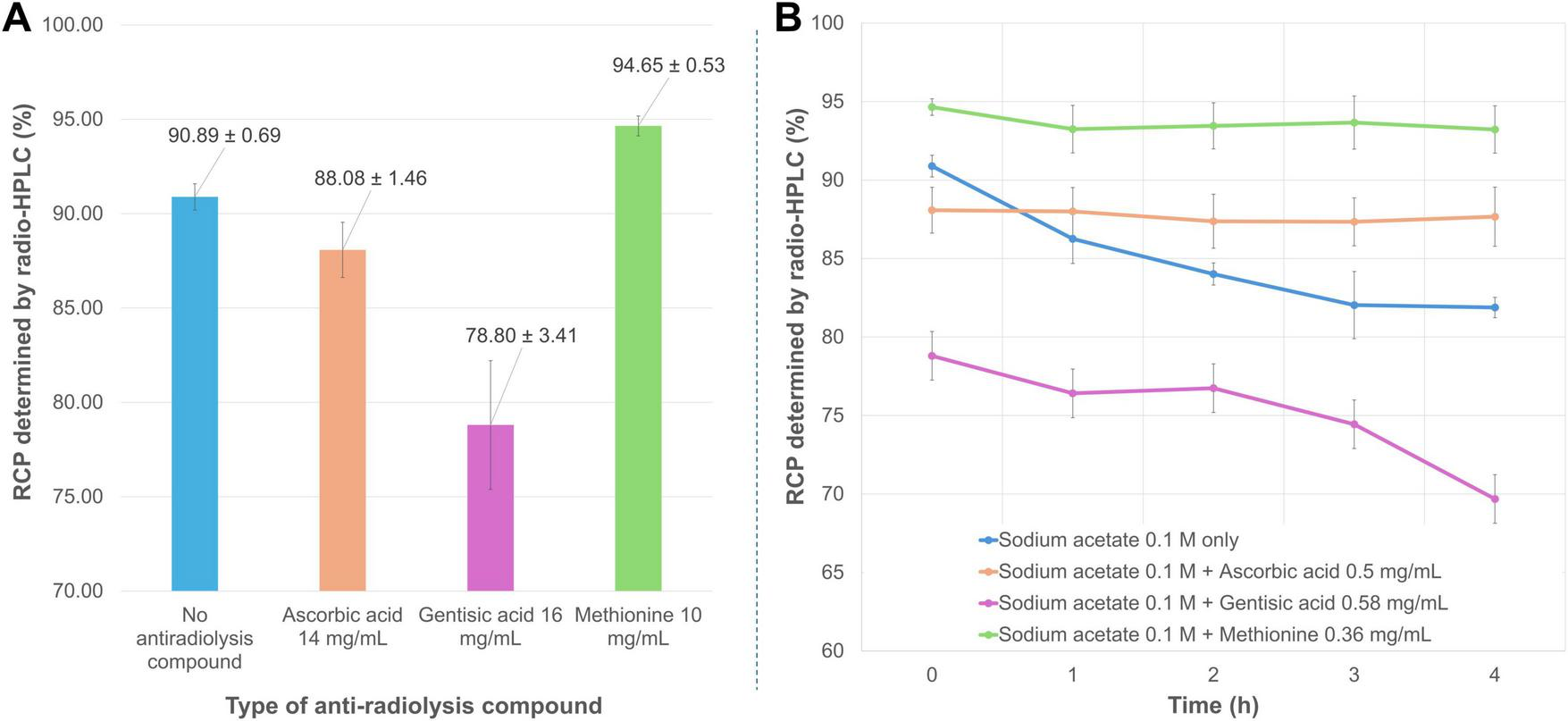
(A) Mean RCP values (determined by radio-HPLC) for [68Ga]Ga-FAP-2286 prepared in the presence of different antioxidant compounds. (B) Time course of mean RCP (determined by radio-HPLC) for [68Ga]Ga-FAP-2286 in the presence of different antioxidant compounds.
Studying the amount of vector molecule involved in a radiolabeling reaction is crucial, as reducing this quantity can increase the molar activity of the radiopharmaceutical preparation (i.e., the amount of vectorized radioactivity per mol of vector molecule). Conversely, increasing the vector amount may enhance the complexation of 68Ga3+, following the rationale of the law of mass action. For FAP-2286, reducing the pseudopeptide quantity by half from 25 μg to 12.5 μg (i.e., from 17 nmol to 8.5 nmol) significantly decreased the purity of the radiolabeling product (RCP = 93.51 ± 1.82% vs. 98.12 ± 0.72%, p = 0.015). Conversely, doubling the amount of FAP-2286 from 25 μg to 50 μg (i.e., from 17 nmol to 34 nmol) did not significantly enhance gallium incorporation (RCP = 98.60 ± 0.12% vs. 98.12 ± 0.72%, p = 0.32) (Figure 6). Consequently, the 25 μg (17 nmol) amount of FAP-2286 was retained, allowing both a high RCP and acceptable specific activity of 7 MBq/nmol.
FIGURE 6

Mean RCP values (determined by radio-HPLC) for [68Ga]Ga-FAP-2286 depending on the amount of vector molecule used for radiolabeling.
Given the short physical half-life of gallium-68, optimizing the heating time required for radiolabeling is also an important consideration. As observed in the study of vector amounts, changing from an 8 to a 12-min reaction time for [68Ga]Ga-FAP-2286 preparation did not significantly improve the purity of the final product (RCP = 98.30 ± 0.21% vs. 98.12 ± 0.72%, p = 0.7). However, halving the heating time from 8 to 4 min resulted in comparable RCP values (98.63 ± 0.02% vs. 98.12 ± 0.72%, p = 0.289) (Figure 7). Consequently, a reduced reaction time of 4 min was adopted to shorten the preparation process.
FIGURE 7

Mean RCP values (determined by radio-HPLC) for [68Ga]Ga-FAP-2286 depending on the heating time of the reaction.
Finally, four SPE cartridges were tested for potential implementation as a terminal purification step in the automated [68Ga]Ga-FAP-2286 synthesis sequence. Most rely on a “bind and elute” approach, where the crude reaction mixture is loaded onto the stationary phase, small polar impurities are removed by rinsing with WFI, and the product of interest is eluted with an ethanol solution. The CM cartridge is an exception, as its weak cation-exchange properties are designed to retain unreacted 68Ga3+ while allowing the radiolabeled product to pass through, eliminating the need for an elution step. As shown in Figure 8, all tested purification methods yielded products with good to excellent RCP, ranging from approximately 93 to 98%. However, the Strata-X cartridge (bearing N-vinylpyrrolidone moieties) showed low and variable recovery of the loaded activity, averaging 23.63 ± 12.17%. Similarly, the CM cartridge achieved an average recovery of only 63.7 ± 11.8%. In contrast, apolar-phase cartridges commonly used for 68Ga-radiopharmaceutical purification, such as C18 and hydrophilic-lipophilic balanced (HLB) cartridges (bearing divinylbenzene-co-N-vinylpyrrolidone moieties), yielded recoveries of approximately 75%. No statistically significant differences were observed between the RCP and recovery values obtained with C18 and OASIS HLB cartridges (p = 0.716 and p = 0.646, respectively), suggesting that either modality could be considered for the terminal purification of [68Ga]Ga-FAP-2286. However, it is important to note that this purification step results in the loss of approximately one-quarter of the activity at EoS.
FIGURE 8

Mean RCP values (determined by radio-HPLC) and mean recovery (%) for [68Ga]Ga-FAP-2286 depending on the cartridge used for post-synthesis purification.
Overall, the optimal radiolabeling conditions included 0.1 M sodium acetate buffer, a high concentration of methionine as an antioxidant agent, 25 μg of FAP-2286, a heating time of 4 min, and terminal purification using a C18 or HLB cartridge.
3.2 Automated [68Ga]Ga-FAP-2286 preparation protocol on GAIA® module
The fully automated synthesis of [68Ga]Ga-FAP-2286 using the GAIA® module was completed in around 24 min, from initiation to transfer of the radiolabeled compound into the final product vial. The initial process relied on two sequential “bind and elute” steps (one for concentrating the 68Ga-eluate and another for purifying the radiolabeled product) with a 4-min heating phase in between to allow radiolabeling. The activity of the eluate at the time of elution was around 950 MBq. The average molar activity achieved under these conditions was approximately 14.5 MBq/nmol. Notably, incorporating an SCX cartridge minimizes the impact of eluate volume and generator model, potentially enabling the use of eluates from multiple 68Ge/68Ga generators within a single synthesis (56, 59–61). However, this additional enrichment step of the 68Ga eluate required a methodical readjustment of the quantities of buffer solution used in the reaction, in order to correctly control the pH (the volume of saturated sodium chloride pH 1 used to elute the SCX cartridge was maintained at 0.4 mL in all tests). Successive tests have shown that a reaction pH of 3.8 can be achieved using 170 μL of 0.8 M sodium acetate supplemented with 1.03 mL methionine 10 mg/mL. Nevertheless, a significant part of the activity involved in the reaction was found either retained on the SPE cartridge (whether C18 or HLB) or in the waste vial. To address this issue and ensure complete complexation of the entire 68Ga amount in radiolabeling, the pseudopeptide concentration was doubled, using 50 μg of FAP-2286 in the automated reaction compared to 25 μg in manual assays. Additionally, the heating time was extended to 9 min to optimize the reaction. To ensure complete elution of the SPE cartridge, the volume of ethanol 60% used during terminal purification was increased by 50%, from 1.5 to 2.3 mL. Similarly, the volume of methionine 1 mg/mL in saline was increased proportionally, from 8.6 to 12.8 mL, resulting in a final volume of 15.1 mL and ensuring an ethanol concentration below 9.2% in the terminal formulation.
After the identification of this reliable, secondarily optimized automated protocol, it was implemented for the production of three test batches of [68Ga]Ga-FAP-2286. The radiopharmaceutical was obtained with an average RCY of 59.85 ± 3.73% and good purity (RCP > 95%, both in radio-TLC and radio-HPLC, Figure 9). Nevertheless, two radioimpurity peaks were systematically found just before the peak of interest in radio-HPLC, with a significant impact on RCP. As expected, each test synthesis resulted in a clear, colorless final product, with the 68Ga radioelement identified by the energy of its gamma photons (peaks at 0.511 and 1.077 MeV) and its half-life (ranging from 61 to 75 min). As anticipated with the use of a pharmaceutical-grade 68Ge/68Ga generator (62), the radionuclidic purity of the three test batches of [68Ga]Ga-FAP-2286 exceeded 99.999%, further enhanced by the final solid-phase purification step. Mean activity at EoS for the three test batches was 517.3 ± 17.8 MBq using a 3-month-old generator. Since the automated synthesis process and the reagents used (buffer and antioxidant) are GMP-compliant, using a pharmaceutical-grade vector would allow the resulting [68Ga]Ga-FAP-2286 to be used in a clinical setting.
FIGURE 9

Representative radio-TLC [(A) aqueous ammonium acetate 1 M in methanol (1:1); (B) aqueous sodium citrate 0.1 M pH 5] and radio-HPLC (C) spectra for [68Ga]Ga-FAP-2286 produced via automated method.
3.3 Transposition of the automated radiolabeling protocol to 3BP-3940
The automated preparation method described above was applied to the synthesis of [68Ga]Ga-3BP-3940, another DOTA-pseudopeptide targeting fibroblast activating protein. This transposition was motivated by the high structural homology between FAP-2286 and 3BP-3940, with the latter differing only by the presence of a urea motif replacing an amide function. Three test preparations of 68Ga-labeled 3BP-3940 were completed, with extensive quality control providing excellent results, surpassing those obtained for [68Ga]Ga-FAP-2286. Specifically, mean RCP was 97.74 ± 1.48% and 97.59 ± 0.93% in radio-TLC and radio-HPLC, respectively. The final formulations displayed a mean activity at EoS of 606.7 ± 44.7 MBq and remained stable over 4 h (RCP > 95.95% in radio-HPLC over this period). The full results of the quality controls performed on the two sets of test batch triplicates are presented in Table 2.
TABLE 2
| Test | [68Ga]Ga-FAP-2286 (n = 3) | [68Ga]Ga-3BP-3940 (n = 3) |
| Appearance | Clear, colorless solution | Clear, colorless solution |
| Identification | ||
| Energy of gamma photons (MeV) | 0.511 and 1.077 | 0.511 and 1.077 |
| Half-life (min) | 68.16 ± 2.71 | 68.89 ± 1.87 |
| pH | 6 | 6 |
| Radionuclidic purity | ||
| (68Ga) Gallium (%) | 99.99998332 ± 5.46 × 10–6 | 99.99995589 ± 3.02 × 10–5 |
| (68Ge) Germanium and other γ-emitting impurities (%) | 1.67 × 10–5 ± 5.46 × 10–6 | 4.41 × 10–5 ± 3.02 × 10–5 |
| Radiochemical purity at EoS | ||
| [68Ga]Ga-FAP inhibitor (HPLC) | 95.21 ± 0.22 | 97.59 ± 0.93 |
| [68Ga]gallium impurities (HPLC) | 4.79 ± 0.22 | 2.41 ± 0.94 |
| [68Ga]Ga-FAP inhibitor (TLC) | 96.88 ± 0.71 | 97.74 ± 1.48 |
| [68Ga]gallium impurities (TLC) | 3.12 ± 0.71 | 2.26 ± 1.48 |
| Filter integrity test (mbar) | > 3,500 | >3,500 |
| Volume activity at EoS (MBq/mL)* | 25.6 ± 0.88 | 30.0 ± 2.21 |
| Specific activity at EoS (MBq/μg) | 9.85 ± 0.35 | 11.85 ± 0.98 |
| Molar activity at EoS (GBq/μmol) | 14.49 ± 0.52 | 17.44 ± 1.44 |
| Radiochemical yield (based on RCP determined by HPLC) |
59.85 ± 3.73 | 75.62 ± 11.76 |
| Stability over 4 h (HPLC) | ≥ 94.98% | ≥95.95% |
Summary of products specifications for the test batches of [68Ga]Ga-FAP-2286 et [68Ga]Ga-3BP-3940.
*Calculated with total theoretical volume of 15.2 mL.
4 Discussion
FAPI pseudopeptides, such as FAP-2286 and 3BP-3940, are emerging as leading theranostic agents for targeting the tumor microenvironment. Their cyclic pseudopeptide structure provides excellent plasma stability, while their affinity for human FAP reaches nanomolar levels (KD = 1.1 nM for FAP-2286) (14). Preliminary evaluations suggest that these compounds offer several advantages over quinoline derivatives such as FAPI-04 and FAPI-46, particularly for targeted radionuclide therapy. However, regarding their diagnostic applications, there are few reports detailing the preparation conditions for [68Ga]Ga-FAP-2286 and [68Ga]Ga-3BP-3940, whereas the literature on [68Ga]Ga-FAPI-46 production is more extensive (52, 59–61, 63–69).
An early report on the preparation of [68Ga]Ga-FAP-2286 used a mixture of 1.0 M ammonium acetate and 0.125 M ascorbic acid (4:1) at pH 4.0, already suggesting the interest of an acetate buffer with added antioxidant compound on the overall fate of the reaction (14). Additionally, radiocomplex purification was performed using an OASIS HLB cartridge; however, the preparation process was carried out manually. For the first clinical use of FAP-2286, reported by Baum et al. (25), 68Ga-radiotracer preparation was carried out on a synthesis module (Modular-Lab PharmTracer, Eckert & Ziegler). Notably, this configuration allowed to involve up to four generator eluates in the preparation process, achieving an overall activity of up to 2.6 GBq using an SCX cartridge. In relation to these high activities, 150 μg of FAP-2286 were involved in the reaction, leading to maximum specific activities of around 17 MBq/μg. Other radiolabeling reagents were 1 M sodium acetate buffer supplemented with 5 mg of L-ascorbic acid and 1.2 mg of L-methionine, for a total reaction volume of approximately 3.1 mL. These first automated conditions tended to confirm the value of acetate buffers, as well as the benefits of L-methionine in preventing the formation of oxidation byproducts during the preparation of [68Ga]Ga-FAP-2286. Several other models of synthesizers were used for the 68Ga-radiolabeling of FAP-2286, such as iQS® (ITM Pharma Solutions GmbH) (19) or GRP® (Scintomics Molecular, Applied Theranostics Technologies GmBH) (16), the latter employing conditions previously used for the manual preparation of [68Ga]Ga-FAP-2286, namely 1 M ammonium acetate and 200 μL of 0.125 M sodium ascorbate. Recently, a detailed report on the automated synthesis of [68Ga]Ga-FAP-2286 on a GRP®-3V module was proposed by Hörmann et al., describing an efficient method using HEPES 1.5 M as a buffer (70). As the European Pharmacopoeia classifies HEPES in radiopharmaceutical preparations as an impurity, a maximum quantity of 500 μg per injected volume is permitted in the final formulation. To verify compliance with this limit, chromatographic methods such as TLC (71, 72) or, less commonly, HPLC (73, 74) are recommended. However, this additional quality control step extends the time between radiopharmaceutical production and patient administration. Therefore, despite the excellent buffering properties of HEPES for 68Ga-radiolabeling, we opted for low-molarity sodium acetate, with its volume finely adjusted to achieve a pH close to 3.8 after adding 0.4 mL of HCl 0.13 M used to elute the SCX cartridge. Notably, precise control of the buffer volume and molarity during radiolabeling eliminates the need for prior pH adjustment with 30% ultrapure HCl, as is the case here.
Transposing radiolabeling conditions optimized for manual reactions to an automated process often requires adjustments (39), as the best approach would be to study automated radiolabeling conditions directly at the synthesizer scale (63, 75). Nevertheless, the screening of a large number of reaction conditions becomes all the more difficult. In our case, slight adjustments were made to the conditions identified during manual synthesis to suit the fluidic process. In particular, 50 μg of vector was used to enhance reaction completion. These quantities align with several literature protocols, notably for the preparation of [68Ga]Ga-3BP-3940 (21, 76, 77). For FAP-2286, Baum’s team reports using 150 μg of pseudopeptide per reaction, which should be considered in conjunction with the combination of eluates from up to four 68Ge/68Ga generators for a single radiolabeling. This protocol involves a Modular-Lab PharmTracer automaton (Eckert and Ziegler). Under these conditions, molar activities ranged from 11.8 to 25.5 MBq/nmol at elution time (25). Other procedures use 40 μg (17) or even 25 μg of FAP-2286, reaching 20.4–40.8 MBq/nmol and 54.4–65.3 MBq/nmol at elution time, respectively (15). However, the corresponding automated sequences do not include pre-purification of the eluate on SCX cartridges, making these methods less complex. Synthesis using 40 μg vector was performed on an iQS 68Ga-fluidic labeling module (ITM Pharma Solutions GmbH). It is worth noting that no protocol for the preparation of [68Ga]Ga-3BP-3940 involving pre-treatment of the gallium eluate with a SCX cartridge has yet been reported, and only two have been described for [68Ga]Ga-FAP-2286 (25, 70). In all cases, precise pH control within the target range is essential for successful radiolabeling. The heating time of 4 min, sufficient in manual assays to achieve very good RCP, was extended to 9 min, which is still slightly shorter than most reaction times reported in the literature. Indeed, the preparation of [68Ga]Ga-FAP-2286 usually requires 10 min (15, 17, 19), or even 15 min heating (14, 78). Only Hörmann et al. describe a reaction time of 6 min at 125°C on a Scintomics GRP-3V module (70). However, given the thermosensitivity of 3BP-3940 demonstrated by Greifenstein’s team (21, 76, 77), such a high temperature was not considered. Instead, a longer radiolabeling time of 9 min at 95°C was preferred. Importantly, no significant side product formation was observed under these conditions, either with FAP-2286 or with 3BP-3940. Similarly, increasing the elution volume from the terminal SPE cartridge aligns with the literature, where reported protocols (when specified) typically indicate a final volume of 15 mL (14, 15, 78) to 17 mL (70) for [68Ga]Ga-FAP-2286 preparations. Interestingly, [68Ga]Ga-3BP-3940 appears to be more easily eluted from an HLB cartridge using just 0.5 mL of 100% ethanol, enabling a final preparation volume of 10.5 mL (21). Nevertheless, to maintain a single radiolabeling protocol compatible with both pseudopeptides, the use of 2.3 mL of 60% ethanol and 12.8 mL of saline with methionine 1 mg/mL was deemed preferable. This final SPE purification step results in some activity loss on the cartridge and extends the preparation process. However, it ensures the highest purity of the radiolabeled product while also allowing control over the final formulation, especially through the removal of the reaction buffer.
Overall, the method presented here, developed through a thorough study of radiolabeling conditions in manual tests, provides a single turnkey solution for preparing [68Ga]Ga-FAP-2286 and [68Ga]Ga-3BP-3940 on a GAIA® module. Although this step complicates the process and is likely to have an impact on RCY, the use of a SCX cartridge ensures compatibility with various 68Ge/68Ga generator models and allows for the potential integration of multiple generators in a single synthesis. This approach enables higher terminal activity, facilitating the management of a larger number of patients. Given the theranostic potential of emerging new-generation FAPI radiopharmaceuticals (79) and the growing innovation in the field of anti-FAP pseudopeptides (80, 81), the demand for PET imaging of the tumor microenvironment is expected to increase significantly.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the author, without undue reservation.
Author contributions
MA: Data curation, Investigation, Writing – original draft. JT: Investigation, Writing – review & editing. SR: Writing – review & editing. LR: Conceptualization, Methodology, Writing – review & editing. CF: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Yasmine Soualy and Jeanne Chillard for their help in preparing the automated radiolabeling reactions presented in this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1628158/full#supplementary-material
References
1.
Schwenck J Sonanini D Cotton J Rammensee H La Fougère C Zender L et al Advances in PET imaging of cancer. Nat Rev Cancer. (2023) 23:474–90. 10.1038/s41568-023-00576-4
2.
Adler S Seidel J Choyke P . Advances in preclinical PET.Semin Nucl Med. (2022) 52:382–402. 10.1053/j.semnuclmed.2022.02.002
3.
Ninatti G Pini C Lazar A Gelardi F . The wings of progress: technological and radiopharmaceutical innovations in nuclear medicine.Eur J Nucl Med Mol Imaging. (2024) 51:3815–21. 10.1007/s00259-024-06913-5
4.
Kleynhans J Ebenhan T Sathekge M . Expanding role for gallium-68 PET imaging in oncology.Semin Nucl Med. (2024) 54:778–91. 10.1053/j.semnuclmed.2024.06.001
5.
Spang P Herrmann C Roesch F . Bifunctional gallium-68 chelators: past, present, and future.Semin Nucl Med. (2016) 46:373–94. 10.1053/j.semnuclmed.2016.04.003
6.
Fouillet J Torchio J Rubira L Fersing C . Unveiling the tumor microenvironment through fibroblast activation protein targeting in diagnostic nuclear medicine: a didactic review on biological rationales and key imaging agents.Biology. (2024) 13:967. 10.3390/biology13120967
7.
Loktev A Lindner T Mier W Debus J Altmann A Jäger D et al A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med. (2018) 59:1423–9. 10.2967/jnumed.118.210435
8.
Lindner T Loktev A Altmann A Giesel F Kratochwil C Debus J et al Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. (2018) 59:1415–22. 10.2967/jnumed.118.210443
9.
Loktev A Lindner T Burger E Altmann A Giesel F Kratochwil C et al Development of fibroblast activation protein–targeted radiotracers with improved tumor retention. J Nucl Med. (2019) 60:1421–9. 10.2967/jnumed.118.224469
10.
Dong Y Zhou H Alhaskawi A Wang Z Lai J Yao C et al The superiority of fibroblast activation protein inhibitor (FAPI) PET/CT versus FDG PET/CT in the diagnosis of various malignancies. Cancers. (2023) 15:1193. 10.3390/cancers15041193
11.
Guglielmo P Alongi P Baratto L Abenavoli E Buschiazzo A Celesti G et al Head-to-head comparison of FDG and radiolabeled FAPI PET: a systematic review of the literature. Life. (2023) 13:1821. 10.3390/life13091821
12.
Kline B Aggarwal R Flavell R Heath C Li Y Ippisch R et al First-in-human evaluation of 68Ga-FAP-2286, a fibroblast activation protein targeted radioligand. J Nucl Med. (2022) 63:2279.
13.
Zboralski D Osterkamp F Simmons A Bredenbeck A Schumann A Paschke M et al Preclinical evaluation of FAP-2286, a peptide-targeted radionuclide therapy (PTRT) to fibroblast activation protein alpha (FAP) (Abstract 571P). Ann Oncol. (2020) 31:S488. 10.1016/j.annonc.2020.08.685
14.
Zboralski D Hoehne A Bredenbeck A Schumann A Nguyen M Schneider E et al Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur J Nucl Med Mol Imaging. (2022) 49:3651–67. 10.1007/s00259-022-05842-5
15.
Pang Y Zhao L Meng T Xu W Lin Q Wu H et al PET imaging of fibroblast activation protein in various types of cancer using 68Ga-FAP-2286: comparison with 18F-FDG and 68Ga-FAPI-46 in a single-center, prospective study. J Nucl Med. (2023) 64:386–94. 10.2967/jnumed.122.264544
16.
Banihashemian S Divband G Pirayesh E Nikkholgh B Amini H Shahrnoy A et al [68Ga]Ga-FAP-2286, a novel promising theragnostic approach for PET/CT imaging in patients with various type of metastatic cancers. Eur J Nucl Med Mol Imaging. (2024) 51:1981–8. 10.1007/s00259-024-06635-8
17.
Kline B Yadav S Seo Y Ippisch R Castillo J Aggarwal R et al 68 Ga-FAP-2286 PET of solid tumors: biodistribution, dosimetry, and comparison with 18 F-FDG. J Nucl Med. (2024) 65:938–43. 10.2967/jnumed.123.267281
18.
Escobar D Koshkin V Kline B Chou J Meng M Friedlander T et al The use of 68Ga-FAP-2286 PET imaging in patients with localized bladder cancer (Abstract PD09-12). J Urol. (2023) 209:e245. 10.1097/JU.0000000000003240.12
19.
Koshkin V Kumar V Kline B Escobar D Aslam M Cooperberg M et al Initial experience with 68 Ga-FAP-2286 PET imaging in patients with urothelial cancer. J Nucl Med. (2024) 65:199–205. 10.2967/jnumed.123.266390
20.
Xiang F Zhang Y Tan X Zhang J Li T Yan Y et al Comparison of 68Ga-FAP-2286 and 18F-FDG PET/CT in the diagnosis of advanced lung cancer. Front Oncol. (2024) 14:1413771. 10.3389/fonc.2024.1413771
21.
Greifenstein L Gunkel A Hoehne A Osterkamp F Smerling C Landvogt C et al 3BP-3940, a highly potent FAP-targeting peptide for theranostics - production, validation and first in human experience with Ga-68 and Lu-177. iScience. (2023) 26:108541. 10.1016/j.isci.2023.108541
22.
Jakobsson V Zhang J Greifenstein L Huang J Chen X Baum R . First-in-human study of a novel radiolabeled fibroblast activating protein (FAP)-targeted peptide 68Ga-FAP-3BP-3940 for PET/CT imaging in patients with solid tumors.J Nucl Med. (2023) 64:1615.
23.
Millul J Koepke L Haridas G Sparrer K Mansi R Fani M . Head-to-head comparison of different classes of FAP radioligands designed to increase tumor residence time: monomer, dimer, albumin binders, and small molecules vs peptides.Eur J Nucl Med Mol Imaging. (2023) 50:3050–61. 10.1007/s00259-023-06272-7
24.
Zboralski D Hoehne A Bredenbeck A Paschke M Von Hacht J Xiao J et al Comparative biodistribution and radiotherapeutic efficacy of the fibroblast activation protein (FAP)-targeting agents FAP-2286 and FAPI-46 (Abstract 3317). Cancer Res. (2022) 82:3317. 10.1158/1538-7445.AM2022-3317
25.
Baum R Schuchardt C Singh A Chantadisai M Robiller F Zhang J et al Feasibility, biodistribution, and preliminary dosimetry in peptide-targeted radionuclide therapy of diverse adenocarcinomas using 177Lu-FAP-2286: first-in-humans results. J Nucl Med. (2022) 63:415–23. 10.2967/jnumed.120.259192
26.
Banihashemian S Akbari M Pirayesh E Divband G Abolhosseini Shahrnoy A Nami R et al Feasibility and therapeutic potential of [177Lu]Lu-FAPI-2286 in patients with advanced metastatic sarcoma. Eur J Nucl Med Mol Imaging. (2024) 52:237–46. 10.1007/s00259-024-06795-7
27.
Xie Y Ma J Tang W Zhang Y Zhang C Chen Y . Efficacy and safety evaluation of 177Lu-FAP-2286 in the treatment of advanced lung cancer.Clin Nucl Med. (2024) 49:830–7. 10.1097/RLU.0000000000005297
28.
Banihashemian S Akbari M Norouzi G Nikkholgh B Amini H Divband G et al The complete metabolic/molecular response to chemotherapy combined with [177Lu]Lu-FAP-2286 in metastatic breast cancer. Eur J Nucl Med Mol Imaging. (2024) 51:4185–7. 10.1007/s00259-024-06817-4
29.
Rao Z Zhang Y Liu L Wang M Zhang C . [177Lu]Lu-FAP-2286 therapy in a case of right lung squamous cell carcinoma with systemic metastases.Eur J Nucl Med Mol Imaging. (2023) 50:1266–7. 10.1007/s00259-022-06048-5
30.
Yang H Liu H Zhang Y Zhang Y Chen Y . Metastatic lung adenocarcinoma received combined 177Lu-FAP-2286 radiation therapy and targeted therapy.Clin Nucl Med. (2024) 49:569–71. 10.1097/RLU.0000000000005169
31.
Wan Z Wang W Chen Y Zheng W Huang Z . 177Lu-FAP-2286 therapy in a patient with metastatic rhabdoid meningioma.Clin Nucl Med. (2024) 49:879–81. 10.1097/RLU.0000000000005258
32.
Yang H Liu H Li H Zhang Y Chen Y . 177Lu-FAP-2286 therapy in a metastatic bone malignant solitary fibrous tumor.Clin Nucl Med. (2022) 49:472–4. 10.1097/RLU.0000000000005168
33.
Li L Yang J Peng D Zhang Y Chen Y . 177 Lu-FAP-2286 therapy in a case of recurrent bladder cancer with multiple metastatic lesions.Clin Nucl Med. (2023) 48:1012–4. 10.1097/RLU.0000000000004865
34.
McConathy J Menda Y Rodon J Goenka A Moy R Morse S et al LuMIERE: a phase I/II study evaluating safety, dosimetry, and preliminary activity of [177Lu]Lu-FAP-2286 in patients with advanced solid tumors. Ann Oncol. (2024) 35:S526. 10.1016/j.annonc.2024.08.737
35.
Bruton L Scott P . Automated synthesis modules for PET radiochemistry. In: <snm>Scott P</gnm>, <snm>Kilbourn M</gnm> editors. Handbook of radiopharmaceuticals.Hoboken, NJ: Wiley (2020). p. 437–56. 10.1002/9781119500575.ch13
36.
Olberg E Hjelstuen K Labeling O . Strategies of peptides with 18f for positron emission tomography.CTMC. (2010) 10:1669–79. 10.2174/156802610793176747
37.
Boschi S Malizia C Lodi F . Overview and perspectives on automation strategies in 68ga radiopharmaceutical preparations. In: <snm>Baum R</gnm>, <snm>Rösch F</gnm> editors. Theranostics, Gallium-68, and Other Radionuclides. Recent Results in Cancer Research.Berlin: Springer Berlin Heidelberg (2013). p. 17–31. 10.1007/978-3-642-27994-2_2
38.
Boschi S Lodi F Malizia C Cicoria G Marengo M . Automation synthesis modules review.Appl Radiat Isotopes. (2013) 76:38–45. 10.1016/j.apradiso.2012.09.010
39.
Nelson B Andersson J Wuest F Spreckelmeyer S . Good practices for 68Ga radiopharmaceutical production.EJNMMI Radiopharm Chem. (2022) 7:27. 10.1186/s41181-022-00180-1
40.
Vis R Lavalaye J van de Garde EM . GMP-compliant 68Ga radiolabelling in a conventional small-scale radiopharmacy: a feasible approach for routine clinical use.EJNMMI Res. (2015) 5:27. 10.1186/s13550-015-0105-3
41.
Mueller D Breeman W Klette I Gottschaldt M Odparlik A Baehre M et al Radiolabeling of DOTA-like conjugated peptides with generator-produced 68Ga and using NaCl-based cationic elution method. Nat Protoc. (2016) 11:1057–66. 10.1038/nprot.2016.060
42.
European Directorate for the Quality of Medicines & Healthcare [EDQM]. Gallium (68Ga) edotreotide injection. Eur Pharmacopoeia 110. (2022) 2482:1274–6.
43.
McCutchan E . Nuclear data sheets for A = 68.Nuclear Data Sheets. (2012) 113:1735–870. 10.1016/j.nds.2012.06.002
44.
Good N Winget G Winter W Connolly T Izawa S Singh R . Hydrogen ion buffers for biological research.Biochemistry. (1966) 5:467–77. 10.1021/Bi00866a011
45.
Pielak G . Buffers, especially the good kind.Biochemistry. (2021) 60:3436–40. 10.1021/acs.biochem.1c00200
46.
Brom M Joosten L Oyen W Gotthardt M Boerman O . Improved labelling of DTPA- and DOTA-conjugated peptides and antibodies with 111In in HEPES and MES buffer.EJNMMI Res. (2012) 2:4. 10.1186/2191-219X-2-4
47.
Martins A Prata M Rodrigues S Geraldes C Riss P Amor-Coarasa A et al Spectroscopic, radiochemical, and theoretical studies of the Ga3+-N-2-hydroxyethyl piperazine-N’-2-ethanesulfonic acid (HEPES buffer) system: evidence for the formation of Ga3+ - HEPES complexes in (68) Ga labeling reactions. Contrast Media Mol Imaging. (2013) 8:265–73. 10.1002/cmmi.1517
48.
Le Roux J Kleynhans J Rubow S . The use of HEPES-buffer in the production of gallium-68 radiopharmaceuticals – time to reconsider strict pharmacopoeial limits?EJNMMI Radiopharm Chem. (2021) 6:15. 10.1186/s41181-021-00129-w
49.
Guleria M Pallavi K Gujarathi P Das T . Evaluation of acute intravenous toxicity of HEPES: is good’s buffer good and safe enough for clinical utilization in nuclear medicine?Nucl Med Biol. (2024) 132–133:108895. 10.1016/j.nucmedbio.2024.108895
50.
Fuscaldi L Sobral D Durante A Mendonça F Miranda A da Cunha M et al Standardization of the [68Ga]Ga-PSMA-11 radiolabeling protocol in an automatic synthesis module: assessments for PET imaging of prostate cancer. Pharmaceuticals (Basel). (2021) 14:385. 10.3390/ph14050385
51.
Bauwens M Chekol R Vanbilloen H Bormans G Verbruggen A . Optimal buffer choice of the radiosynthesis of 68Ga–Dotatoc for clinical application.Nucl Med Commun. (2010) 31:753–8. 10.1097/MNM.0b013e32833acb99
52.
Da Pieve C Costa Braga M Turton D Valla F Cakmak P Plate K et al New fully automated preparation of high apparent molar activity 68Ga-FAPI-46 on a Trasis AiO platform. Molecules. (2022) 27:675. 10.3390/molecules27030675
53.
Haskali M . Automated preparation of clinical grade [68Ga]Ga-DOTA-CP04, a cholecystokinin-2 receptor agonist, using iPHASE MultiSyn synthesis platform.EJNMMI Radiopharm Chem. (2019) 4:23. 10.1186/s41181-019-0067-2
54.
Bartoli F Elsinga P Nazario L Zana A Galbiati A Millul J et al Automated radiosynthesis, preliminary in vitro/in vivo characterization of OncoFAP-based radiopharmaceuticals for cancer imaging and therapy. Pharmaceuticals. (2022) 15:958. 10.3390/ph15080958
55.
Reverchon J Khayi F Roger M Moreau A Kryza D . Optimization of the radiosynthesis of [68Ga]Ga-PSMA-11 using a Trasis MiniAiO synthesizer: do we need to heat and purify?Nucl Med Commun. (2020) 41:977–85. 10.1097/MNM.0000000000001233
56.
Durieux F Dekyndt B Legrand J Rogeau A Malek E Semah F et al Optimization of automated radiosynthesis of gallium-68-labeled PSMA11 with two 68Ge/68Ga generators: fractional elution or prepurification? Pharmaceuticals (Basel). (2023) 16:1544. 10.3390/ph16111544
57.
Wang Y Shao G Wu J Cui C Zang S Qiu F et al Preparation of 68 Ga-PSMA-11 with a synthesis module for micro PET-CT imaging of PSMA expression during prostate cancer progression. Contrast Media Mol Imaging. (2018) 2018:1–9. 10.1155/2018/8046541
58.
Wichmann C Ackermann U Poniger S Young K Nguyen B Chan G et al Automated radiosynthesis of [68Ga]Ga-PSMA-11 and [177Lu]Lu-PSMA-617 on the iPHASE MultiSyn module for clinical applications. J Labelled Compounds Radiopharm. (2021) 64:140–6. 10.1002/jlcr.3889
59.
Plhak E Pichler C Dittmann-Schnabel B Gößnitzer E Aigner R Stanzel S et al Automated synthesis of [68Ga]Ga-FAPI-46 on a scintomics GRP synthesizer. Pharmaceuticals. (2023) 16:1138. 10.3390/ph16081138
60.
Morandeau L Ioppolo J Alvarez de Eulate E Mohamed S Cullen D Asad AH et al An automated radiosynthesis of [68Ga]Ga-FAPI-46 for routine clinical use. JoVE. (2024) 207:66708. 10.3791/66708
61.
Paty L Degueldre S Provost C Schmitt C Trump L Fouque J et al Development of a versatile [68Ga]Ga-FAPI-46 automated synthesis suitable to multi-elutions of germanium-68/gallium-68 generators. Front Chem. (2024) 12:1411312. 10.3389/fchem.2024.1411312
62.
European Directorate for the Quality of Medicines & Healthcare [EDQM]. Gallium (68Ga) chloride solution for radiolabelling. Eur Pharmacopoeia 110. (2013) 2464:1273–4.
63.
Rubira L Donzé C Fouillet J Algudo B Kotzki P Deshayes E et al [68Ga]Ga-FAPI-46 synthesis on a GAIA® module system: thorough study of the automated radiolabeling reaction conditions. Appl Radiat Isotopes. (2024) 206:111211. 10.1016/j.apradiso.2024.111211
64.
Spreckelmeyer S Balzer M Poetzsch S Brenner W . Fully-automated production of [68Ga]Ga-FAPI-46 for clinical application.EJNMMI Radiopharm Chem. (2020) 5:31. 10.1186/s41181-020-00112-x
65.
Boonkawin N Chotipanich C . The first radiolabeled 68Ga-FAPI-46 for clinical PET applications using a fully automated iQS-TS synthesis system in Thailand.J Chulabhorn Royal Acad. (2020) 3:180–8.
66.
Nader M Valla D Vriamont C Masset J Pacelli A Herrmann K et al [68Ga]/[90Y]FAPI-46: automated production and analytical validation of a theranostic pair. Nucl Med Biol. (2022) 110–111:37–44. 10.1016/j.nucmedbio.2022.04.010
67.
Alfteimi A Lützen U Helm A Jüptner M Marx M Zhao Y et al Automated synthesis of [68Ga]Ga-FAPI-46 without pre-purification of the generator eluate on three common synthesis modules and two generator types. EJNMMI Radiopharm Chem. (2022) 7:20. 10.1186/s41181-022-00172-1
68.
Rubira L Torchio J Fouillet J Vanney J Fersing C . GMP-compliant automated radiolabeling and quality controls of [68Ga]Ga-FAPI-46 for fibroblast activation protein-targeted PET imaging in clinical settings.Chem Pharm Bull. (2024) 72:1014–23. 10.1248/cpb.c24-00531
69.
Brusa I Cabitza V Emiliani S Malizia C Fortunati E Zanoni L et al Optimization and scale-up of [68Ga]Ga-FAPI-46 production on a Modular-Lab PharmTracer platform for clinical application. Nucl Med Biol. (2025) 140–141:108974. 10.1016/j.nucmedbio.2024.108974
70.
Hörmann A Schweighofer-Zwink G Rendl G Türk K Nadeje S Haas K et al [68Ga]Ga-FAP-2286—synthesis, quality control and comparison with [18F]FDG PET/CT in a patient with suspected cholangiocellular carcinoma. Pharmaceuticals. (2024) 17:1141. 10.3390/ph17091141
71.
European Directorate for the Quality of Medicines & Healthcare [EDQM]. Gallium (68Ga) PSMA-11 injection. Eur Pharmacopoeia 110. (2021) 3044:1276–7.
72.
Pfaff S Nehring T Pichler V Cardinale J Mitterhauser M Hacker M et al Development and evaluation of a rapid analysis for HEPES determination in 68Ga-radiotracers. EJNMMI Res. (2018) 8:95. 10.1186/s13550-018-0449-6
73.
Antunes I Franssen G Zijlma R Laverman P Boersma H Elsinga P . New sensitive method for HEPES quantification in 68Ga-radiopharmaceuticals.EJNMMI Radiopharm Chem. (2020) 5:12. 10.1186/s41181-020-00093-x
74.
Migliari S Scarlattei M Baldari G Silva C Ruffini LA . Specific HPLC method to determine residual HEPES in [68Ga]Ga-radiopharmaceuticals: development and validation.Molecules. (2022) 27:4477. 10.3390/molecules27144477
75.
Souche C Fouillet J Rubira L Donzé C Sallé A Dromard Y et al Towards optimal automated68 Ga-radiolabeling conditions of the DOTA-bisphosphonate BPAMD without pre-purification of the generator eluate. Labelled Comp Radiopharm. (2024) 67:441–53. 10.1002/jlcr.4128
76.
Greifenstein L Martin M Kramer C Klega A Mueller C Landvogt C et al Radiolabeling of 3BP-3940 with 68Ga, 90Y, 177Lu and 225Ac for imaging and peptide targeted radiotherapy (PTRT). J Nucl Med. (2022) 63:2532.
77.
Greifenstein L Gunkel A Hoehne A Osterkamp F Smerling C Landvogt C et al A highly potent FAP-targeting peptide for theranostics: production, validation and first in human experience with Ga-68 and Lu-177 3BP-3940. J Nucl Med. (2024) 65:242079. 10.1016/j.isci.2023.108541
78.
Mueller D Fuchs A Leshch Y Schulze P Singh A Kulkarni H et al Radiolabeling and stability of FAP- seeking radiopharmaceuticals for radio-molecular imaging and therapy. J Nucl Med. (2020) 61:1129.
79.
Baum R Novruzov E Zhao T Greifenstein L Jakobsson V Perrone E et al Radiomolecular theranostics with fibroblast-activation-protein inhibitors and peptides. Semin Nucl Med. (2024) 54:537–56. 10.1053/j.semnuclmed.2024.05.010
80.
Wang W Wang R Huang M Tian R . Preclinical Evaluation of 68Ga/177Lu labeled novel FAP-targeted peptide for tumor radionuclide imaging and therapy.J Nucl Med. (2024) 65:242425. 10.2967/jnumed.124.268689
81.
Wang R Huang M Wang W Li M Wang Y Tian R . Preclinical evaluation of 68 Ga/177 Lu-labeled FAP-targeted peptide for tumor radiopharmaceutical imaging and therapy.J Nucl Med. (2025) 66:250–6. 10.2967/jnumed.124.268689
82.
Satpati D Shinto A Kamaleshwaran K Sane S Banerjee S . Convenient preparation of [68Ga]DKFZ-PSMA-11 using a robust single-vial kit and demonstration of its clinical efficacy.Mol Imaging Biol. (2016) 18:420–7. 10.1007/s11307-016-0943-z
83.
Daniel T Balouzet Ravinet C Clerc J Batista R Mouraeff Y . Automated synthesis and quality control of [68Ga]Ga-PentixaFor using the Gaia/Luna Elysia-Raytest module for CXCR4 PET imaging.EJNMMI Radiopharm Chem. (2023) 8:4. 10.1186/s41181-023-00187-2
84.
Meisenheimer M Kürpig S Essler M Eppard E . Ethanol effects on 68Ga-radiolabelling efficacy and radiolysis in automated synthesis utilizing NaCl post-processing.EJNMMI Radiopharm Chem. (2019) 4:26. 10.1186/s41181-019-0076-1
85.
Mueller D Klette I Baum R . Combined cationic-anionic pre-purification of 68Ga for the synthesis and clinical application of 68Ga-BPAMD.J Nucl Med. (2013) 54:1190.
86.
Asti M Iori M Capponi P Rubagotti S Fraternali A Versari A . Development of a simple kit-based method for preparation of pharmaceutical-grade 68Ga-DOTATOC.Nucl Med Commun. (2015) 36:502–10. 10.1097/MNM.0000000000000275
87.
Fonseca A Sereno J Almeida S Ferreira H Hrynchak I Falcão A et al Unveiling the potential of copper-61 vs. gallium-68 for SSTR PET imaging. Eur J Nucl Med Mol Imaging. (2025) 52:2671–84. 10.1007/s00259-025-07116-2
88.
Martin R . Cationic eluate pretreatment for automated synthesis of [68Ga]CPCR4.2.Nucl Med Biol. (2014) 41:84–9. 10.1016/j.nucmedbio.2013.09.002
89.
Sammartano A Migliari S Scarlattei M Baldari G Ruffini L . Synthesis, validation and quality controls of [68Ga]-DOTA- pentixafor for PET imaging of chemokine receptor CXCR4 expression.Acta Biomed. (2020) 91:e2020097. 10.23750/abm.v91i4.9106
Summary
Keywords
radiopharmaceuticals, automated radiolabeling, gallium-68, FAP-2286, PET imaging, tumor microenvironment
Citation
Ammour M, Torchio J, Renaud SC, Rubira L and Fersing C (2025) Specific reaction conditions for efficient automated 68Ga-radiolabeling of the FAP-2286 pseudopeptide on a GAIA® synthesizer. Front. Med. 12:1628158. doi: 10.3389/fmed.2025.1628158
Received
14 May 2025
Accepted
10 July 2025
Published
22 July 2025
Volume
12 - 2025
Edited by
Sipho Mdanda, Nuclear Medicine Research Infrastructure, South Africa
Reviewed by
Pardeep Kumar, National Institute of Mental Health and Neurosciences, India
Cathryn Driver, South African Nuclear Energy Corporation, South Africa
Updates
Copyright
© 2025 Ammour, Torchio, Renaud, Rubira and Fersing.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cyril Fersing, cyril.fersing@icm.unicancer.fr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.