Abstract
Osteoarthritis (OA) is the most common chronic joint disease, characterized by whole-joint degenerative disease with cartilage degeneration as the primary pathogenesis. It is also a major cause of disability and increased social costs, particularly among the elderly. With the aging population and increasing obesity rates, the incidence of OA increases annually. The main symptoms include joint pain and loss of joint function, which severely impact the quality of life and daily activities of patients. Despite numerous treatments attempted over the past few decades, the long-term treatments have been disappointing. The main challenge lies in the very low bioavailability of drugs within the joint cavity, Therefore, the development of a therapeutic approach with cartilage targeting and efficient bioavailability is the key point to address OA. This paper summarizes the latest research on the use of PLGA in drug delivery for the treatment of OA, which provides an important foundation and a more comprehensive perspective for the subsequent drug treatment of joint diseases. We hope this will lead to more accurate and effective treatment plans for arthritis patients and promote the continuous advancement of the medical field.
1 Introduction
OA, mainly characterized by degeneration of articular cartilage and osteophyte formation (Figure 1A), accompanied by degeneration of cartilage tissue, subchondral bone and synovium (Figure 1B), is a common chronic joint disease (1, 2). In addition to age, gender, and genetics, the causes of OA include abnormal increases in joint loading caused by factors such as obesity and occupation (3–5), disruption of mechanical balance due to fractures, meniscal injuries, and ligament tears (3) (Figure 1C), as well as damage to the infrapatellar fat pad (6, 7). With the aggravation of population aging and degeneration of the cartilage and meniscus, the incidence rate is increasing year by year, which not only brings great pain to patients themselves (Figure 1E), but also causes great social and economic burden (8). Patients often present with joint pain, swelling, and stiffness, eventually leading to chronic pain and physical disability (9, 10). However, the treatment of OA mainly focuses on relieving symptoms, divided into conservative treatment and surgical treatment. Conservative treatment mainly through physical therapy and drugs to alleviate the pain symptoms of OA, and surgical treatment in addition to joint replacement, other treatment is still difficult to prevent cartilage destruction (11, 12).
Figure 1

(A) Deformation of OA in the distal and proximal interphalangeal joints, plain radiograph of an osteoarthritic hip joint and MRI of an osteoarthritic knee. Reproduced from Bijlsma et al. (117). (B) Schematic representation of healthy knee joint structure and pathological changes of knee OA. Reproduced from Mao et al. (13). (C) Changes and interactions of different tissue structures in OA disease. Reproduced from Bijlsma et al. (117). (D) Signaling pathways and structural changes in the development of OA. Reproduced from Glyn-Jones et al. (89). (E) The cause of pain in OA within a biopsychosocial model. Reproduced from Hunter et al. (10).
In the face of the huge medical demand, more and more research tries new treatment methods, including new drug development, intra-articular injection and the exploration of gene signal pathways (10) (Figure 1D). These studies mainly hope to prolong the effect of drugs, more accurate drug targeting and less treatment side effects (13). With the rapid development of biomaterials, we have focused on the great potential of nanoparticles (NPs) in the treatment of joint inflammation (14, 15). NPs refer to solid colloidal particles made of natural or synthetic polymer materials in the order of nanometer size (0.1–100 nm). The controllable size gives the feasibility of direct intra-articular injection (16–18). NPs are a promising cargo delivery system, which can bind drugs on the surface or substrate to protect drugs from enzymatic degradation, improve their permeability in cartilage matrix, and regulate the pharmacokinetics of drugs. And the NPs composed of biocompatible and biodegradable materials can achieve controlled and continuous drug release (19–21). This paper reviews the wide application of PLGA NPs in the treatment of OA in the past year, and explores the progress and challenges of PLGA in inflammatory arthritis based on cartilage degradation.
2 Overview of PLGA material
Poly(D,L-lactic-co-glycolic acid) (PLGA) is a biodegradable functional polymer organic compound composed of random polymerization of two monomers poly(lactic acid) (PLA) and poly (glycolic acid) (PGA) (22, 23). PLA degradation rate is slow, and PGA degradation rate is fast, so adjust the proportion of the two can regulate the mechanical properties and degradation time of polymer. We summarize the key data of recent studies cited in this review in Table 1, where the lactic acid/glycolic acid (LA/GA) ratio is shown to exert a significant regulatory effect on the degradation and drug release processes. At the same time, the polymer exhibits good biocompatibility, biodegradability, good encapsulation and film forming properties, and has been authorized by the food and drug administration for drug delivery system (24). At present, there are many preparation methods of PLGA particles, including: emulsion solvent volatilization method, microfluidic technology, spray drying technology, nanoprecipitation and phase separation, etc. (22, 25–27).
Table 1
| Polymer | Composition | Degradation kinetics | Drug release profile | Reference |
|---|---|---|---|---|
| PLGA | LA:GA (50:50) + LA:GA (75:25) | 1 month: moderate rupture | Burst release: 18–22% (24 h); Cumulative release: 78% (28d) |
Sun et al. (35) |
| PLGA | LA:GA (50:50) (MW: 30–60 kDa) | 28d: complete degradation | Burst release: 31.64% (24 h); Cumulative release: 95.45% (28d) |
Wei et al. (37) |
| PLGA-F127 | PLGA (50:50) (MW:100 kDa) + Pluronic F127 | 120d: complete degradation | Release onset: 60d; total duration: 4 months |
Seon et al. (79) |
| PLGA | LA:GA (50:50) | 28d: pore expansion | Burst release: 28.3% (24 h); Cumulative release: 85% (28d) |
Zhu et al. (86) |
| PLGA | LA:GA (75:25) (MW: 20 kDa) | >34d: in cartilage | Burst release: <3%; Cartilage retention: >34d |
Deng et al. (28) |
PLGA degradation kinetics and drug release profiles of different LA/GA ratios.
The study demonstrates that varying LA/GA ratios in PLGA significantly influence particle degradation and drug release. When the LA/GA ratio was adjusted from 50:50 to 75:25, the degradation duration extended to 34 days with a 24-h burst release rate below 3%, which correlates with the increased LA content that slows particle degradation (28). In early-stage osteoarthritis (OA), where cartilage matrix integrity remains relatively high, the 75:25 LA/GA ratio enables sustained drug release through gradual penetration of the cartilage surface. However, in advanced OA characterized by cartilage matrix breakdown, the 50:50 PLGA formulation demonstrates accelerated degradation and rapid drug delivery through the compromised cartilage matrix.
3 The use of PLGA in OA
For the special anatomical structure of the knee joint, oral drugs are difficult to reach and form an effective local drug concentration, while the drugs injected in the arthrosis are quickly removed from the synovial fluid through the relevant lymphatic vessels and vasculature. To overcome these limitations, many NP-based drug delivery systems have been developed, such as PLGA-NPs delivering small-molecule drugs or endogenous growth factors (29, 30). The latest research have tried to explore the injectable in situ molding implants build intra-articular drug library. Sustainable release disease-modifying OA drug can improve OA. The sealing PLGA in original molding implant can stabilize for weeks and releasing drug can inhibit collagenase. However, such implants after formation exist certain cytotoxicity. Researchers may need to further modify to solve the problem of drug toxicity, but the method of implant treatment OA may be an innovative idea (31).
3.1 PLGA NPs delivering small-molecule drugs
Curcumin (CUR), as a natural polyphenolic compound, has potent assimilative, antioxidant, anti-inflammatory and anti-rheumatic properties. But the therapeutic efficiency of CUR is greatly limited due to its low water solubility and limited oral bioavailability (32, 33). One study on the preparation of PLGA NPs (CURNPs) containing curcumin for knee OA in rats, showed CURNPs inhibited the upregulation of several inflammatory factors, including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), and significantly retained type II collagen in articular cartilage. This is mainly achieved by inhibiting NF-κB pathway. Meanwhile, the radiographic and histological lesions of OA were significantly reduced (34). Sun et al. (35) prepared meloxicam-loaded PLGA microspheres (MLX-MS) by the emulsification-solvent evaporation method. An orthogonal test design was employed to optimize the formulation. Dynamic light scattering was used to measure the average particle size, which was controlled between 100 and 110 μm with a span of 0.5–0.6. The Fourier Transform Infrared Spectroscopy and X-ray Powder Diffraction were simultaneously utilized to confirm that there were no alterations in the drug during the encapsulation process. In the OA model of rats, various inflammatory factors including IL-1β, IL-6, and TNF-α were successfully inhibited, and the meloxicam exhibited a long-term sustained-release pattern. Meanwhile, compared to oral administration, local injection of MLX-MS significantly increased the elimination half-life and time to peak concentration in the plasma. Intra-articular injection of MLX-MS significantly reduced drug distribution in the gastrointestinal tract and allowed the drug for better penetration of the drug into the inflamed area. Zhu et al. (36) found that the small molecule drug salicin (SA) is important for the progression of OA, clarifying mechanisms by RNA sequencing, molecular docking and drug affinity-response target stability analysis in vitro. SA directly binds to IRE1α and occupies the IRE1α phosphorylation site, preventing IRE1α phosphorylation, and regulates IRE1α-mediated ER stress via IRE 1α-IκBα-p65. Injection of PLGA particles containing SA in the OA rats significantly improved OA progression. A large number of studies have shown that resveratrol (RSV) showed protective effects on articular cartilage through various mechanisms, including anti-inflammatory and anti-apoptosis, or regulation of signaling pathways or active factors. But resveratrol has the problems of poor chemical stability, poor water solubility and low bioavailability. Wei et al. (37) prepared RSV-PLGA NPs via the incorporation of RSV into PLGA. Using the good biocompatibility and stable drug release performance of PLGA, PLGA NPs significantly inhibited cartilage cell apoptosis and promoted glycosaminoglycan (GAG) synthesis and maintained continuous drug release for 35 days after a single injection in, supporting the superiority of intra-injection.
Previous studies have found that HDACi prevents the disruption of the extracellular matrix, and this destruction is induced by inflammatory factors (38–40). And, HDACi suppresses cartilage destruction and articular cartilage degeneration. So, Ye et al. (41) proposed that PLGA microcapsule delivered Chidamide to treat OA. Phenotype-associated genes of ECM were retained and increased in HDACi treated OA chondrocytes, and decreased expression of catabolism-related genes. The rat OA model later confirmed that Chidamide significantly reduced osteophyte formation on the medial side of the tibial plateau and effectively prevented the remodeling of the inferior tibial bone.
Rapamycin is also a well-known immunomodulator and antibiotic that has been used in a variety of clinical treatments and can delay the progression of OA in mouse models (42). Rapamycin induces cellular autophagy by inhibiting ribosomal protein S6 phosphorylation and affecting the mTOR signaling pathway, while reducing cellular senescence by increasing the levels of Nrf2 (42–44). Dhanabalan et al. (45) loaded rapamycin in PLGA particles, Rapamycin-loaded PLGA microparticles (RMPs) induce cellular autophagy in primary articular chondrocytes of OA patients, preventing senescence and continuous production of sulfated glycosaminoglycans. At the same time, the RMPs was retained in the mouse for up to 35 days, and PLGA particles with higher molecular weight can show longer residence time in the joint, significantly reducing the frequency of injection, which is beneficial to improve patient compliance in clinical practice.
Post-traumatic OA (PTOA) often begins with joint injuries including anterior cruciate ligament rupture, meniscus injury, and joint dislocation, along with progressive deterioration of the articular cartilage and subchondral bone (46). The particularity of the disease is that the inflammation is initiated at the molecular and cellular levels immediately after joint injury. Proinflammatory factors, including: IL-1β, IL-6, and TNFα, are rapidly induced (47). Subsequently the matrix-degrading enzymes such as matrix metalloproteinases, collagenase, and cathepsin are further induced (48). These factors cause irreversible damage to the surrounding tissues, eventually leading to OA (49).
The CDK 9 inhibitor Flavpiridol is a potent drug that prevents the acute inflammatory response and activation of catabolic pathways in cartilage, it has been shown that Flavpiridol suppressed the expression of inducible nitric oxide synthase and inflammatory mediator genes under proinflammatory stimuli. So, Sangsuwan et al. (50) prepared PLGA microspheres loaded with Flavpiridol to prevent PTOA by early reducing inflammation in rat knee joints, and found that the drug has less accumulation in the liver and kidney and is a potential PTOA candidate (Figure 2). Meanwhile, some studies applied PLGA microsphere loaded with Flavpiridol in the OA models of rabbit and horse. It more truly reflected the actual effect of the drug-loaded microsphere in large mammals, which also showed good slow-release lubrication, anti-inflammatory analgesia and articular cartilage protection (51, 52). Kim et al. (53) prepared NPs of two different materials for the delivery of rebamipide, demonstrating significant therapeutic effects both in vivo and in vitro. This study further showed the therapeutic potential of NP drug delivery systems within the unique anatomical structure of the joint cavity.
Figure 2
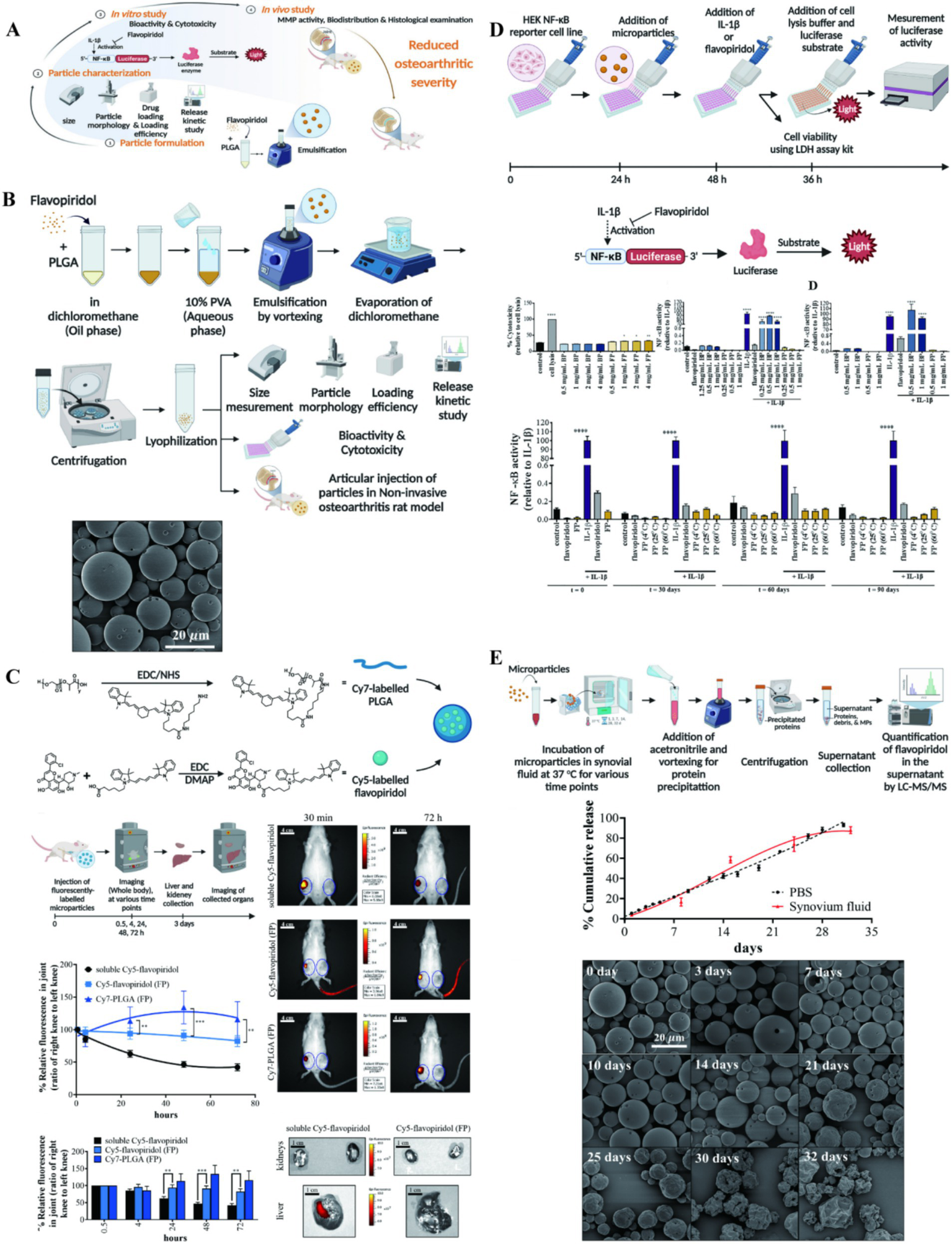
(A) The illustration on preparation and application of PLGA particle. (B) Preparation and characterization of flavopiridol-loaded PLGA particles. (C) Biodistribution of Flavopiridol-loaded microparticles (FPs) following intra-articular injection in rats. (D) NF-κB activity of FPs. (E) Release kinetic of FPs. Reproduced from Sangsuwan et al. (50).
However, the hydrophobicity of drugs and molecular size significantly influence encapsulation efficiency in PLGA. As a hydrophobic polymer, PLGA demonstrates enhanced compatibility with hydrophobic drugs, thereby improving encapsulation efficiency. Additionally, drug molecular size plays a crucial role in PLGA loading and release kinetics: smaller molecular weight drugs diffuse more easily into PLGA but risk premature release during initial stages, while larger molecular weight drugs exhibit lower encapsulation rates due to steric hindrance effects, though they deliver slower release rates. These factors may warrant further investigation in future research.
3.2 PLGA NPs delivering the endogenous components
Melatonin is an endogenous hormone secreted by the pineal gland, performing circadian regulation simultaneously with a potent antioxidant capacity. This hormone has great potential in osteoporosis, atherosclerosis, and diabetes, and has been reported for the treatment of OA (54–58). It is well known that ROS and TLR mediated cascade of inflammatory response OA has a critical role. Inhibition of the innate immune response and generation of reactive oxygen species are potential targets for treating OA. Liang et al. (59) packaged melatonin in PLGA by oil-in-water method and grafted type II collagenase in the surface. A nano-delivery system loaded with melatonin was prepared. Researchers evaluated the behavior of this system in cartilage and the therapeutic efficacy in mouse OA models, confirming that melatonin can protect chondrocytes by clearing ROS and inhibiting the TLR2/4-MyD88-NFκB pathway, and prevent degeneration of knee cartilage and remodeling of subchondral bone in early OA (Figure 3). The small molecule drugs and endogenous components delivered by PLGA and experimental studies on animal OA are outlined in Table 2.
Figure 3
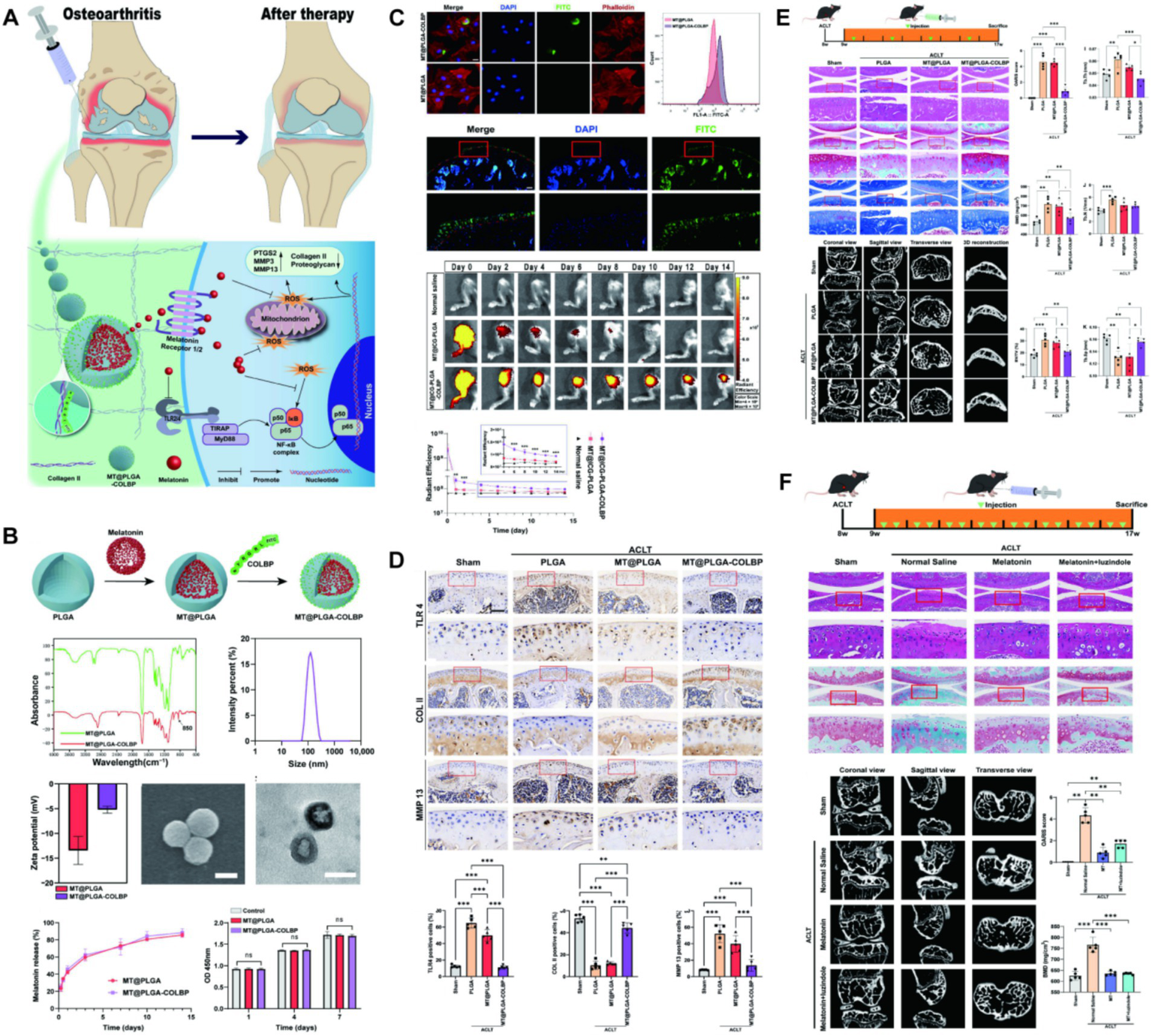
(A) The mechanism of melatonin-loaded nano-delivery system (MT@PLGA-COLBP) with cartilage-targeting effect for OA therapy. (B) Synthesis and characterization of MT@PLGA-COLBP NPs. (C) MT@PLGA-COLBP NPs target chondrocytes. (D) MT@PLGA-COLBP improves the protein expression of immune response and cartilage matrix in vivo. (E) Therapeutic effect of intra-articular injection of MT@PLGA-COLBP NPs on early OA mice. (F) Intra-articular injection of melatonin delays the development of OA in mice. Reproduced from Liang et al. (59).
Table 2
| Carriers | Drugs/components | Damage | Induction | Animal | References |
|---|---|---|---|---|---|
| PLGA NPs | Curcumin | KOA | MIA injection | Rats | Curcumin NPs (34) |
| PLGA microspheres | Meloxicam | KOA | MIA injection | Rats | PLGA microspheres loaded with meloxicam (35) |
| PLGA | Salicin | KOA | ACLT | Rats | SA-loaded PLGA (36) |
| PLGA NPs | Resveratrol | KOA | DMM | Rats | PLGA resveratrol sustained-release NPs (37) |
| PLGA microcapsules | Chidamide | KOA | ACLT | Rats | Intra-articular Histone Deacetylase Inhibitor Microcarrier (41) |
| PLGA microparticles | Rapamycin | KOA | DMM | Mice | Intra-articular injection of rapamycin microparticles (45) |
| PLGA microspheres | Flavpiridol | KOA | ACLT | Rats | Intra-articular Injection of Flavopiridol-loaded Microparticles (50) |
| PLGA NPs | Melatonin | KOA | ACLT | Mice | Preparation of Melatonin-Loaded NPs (59) |
| PLGA microspheres | Nanofat | KOA | DMM | Rats | Nanofat functionalized injectable super-lubricating microfluidic microspheres (64) |
| PLGA NPs | P16INK4A-siRNA | KOA | PMMx | Mice | p16INK4a-siRNA NPs (68) |
PLGA NPs delivering small-molecule drugs and endogenous components.
PLGA, Poly(D,L-lactic-co-glycolic acid); NPs, Nanoparticles; KOA, Knee Osteoarthritis; MIA, Monosodium Iodoacetate; ACLT, Anterior Cruciate Ligament Transection; DMM, Destabilization of Medial Meniscus; PMMx, Partial Medial Meniscectomy.
Nanofat (NF) is an injectable sticky extract rich in lipids, growth factors and stem cells, and NF has been successfully used in scar repair, vascular regeneration and cartilage defect repair (60–62). However, it is difficult to apply to precise transplantation due to the low mobility and low biological activity of NF (63). To overcome these limitations, Han et al. (64) prepared three-dimensional PLGA porous microspheres using microfluidic techniques. Combining NF into PLGA porous microspheres (PMs) through Schiff base condensation and noncovalent binding (PMs@NF). The construct loaded a large amount of biologically active NF, increased the local cytokine concentration secreted by stem cells. At the same time, it has targeted adhesion to the surface of cartilage, which can achieve accurate delivery. The structure not only strengthened the surface lubrication of articular cartilage, but also increased the expression of cartilage synthetic substances (Figure 4). PMs@NF downregulated the expression of genes involved in cartilage catabolic enzymes, inflammation and pain, significantly reducing osteophyte formation in arthritic rats. NF also activates the intracellular PI3K/Akt signaling pathway, promotes the proliferation and matrix synthesis of chondrocytes, and ultimately improves the progression of OA. Cellular senescence is an important factor in the pathogenesis of OA. Chondrocytes produce a senescence-related secretory phenotype (SASP), including inflammatory cytokines and matrix remodeling regulating metalloproteinases, causing chronic inflammation and ultimately leading to OA (65, 66). The cell cycle inhibitor P16INK4A protein has an important role in cellular senescence. Chondrocytes positive for the cell cycle inhibitor P16INK4A protein will secrete large amounts of inflammatory cytokines. These inflammatory factors often accumulate in the tissues and promote tissue degeneration through persistent chronic inflammation and extracellular matrix remodeling (67). However, the role of cell cycle inhibitor P16INK4A protein in OA and its inhibitory effect have not been defined, Park et al. (68) found that a marked increase in the cell cycle inhibitor P16INK4A protein in synovial and articular chondrocytes from OA patients. They used PLGA NPs to deliver the cell cycle inhibitor P16INK4A protein-siRNA. This NPs can significantly reduce the levels of TNF-α, IL-1β, and IL-6. In the mouse model of OA, NPs mainly focused on the synovial membrane, preferentially reducing the cell cycle inhibitor P16INK4A protein in synovial cells, reducing synovial inflammation and relieving joint pain. The authors were combined with previous studies (69, 70), speculating that fibroblasts in the synovial membrane may be new and exciting targets for OA therapy, promise as a potential drug to slow the progression of OA.
Figure 4
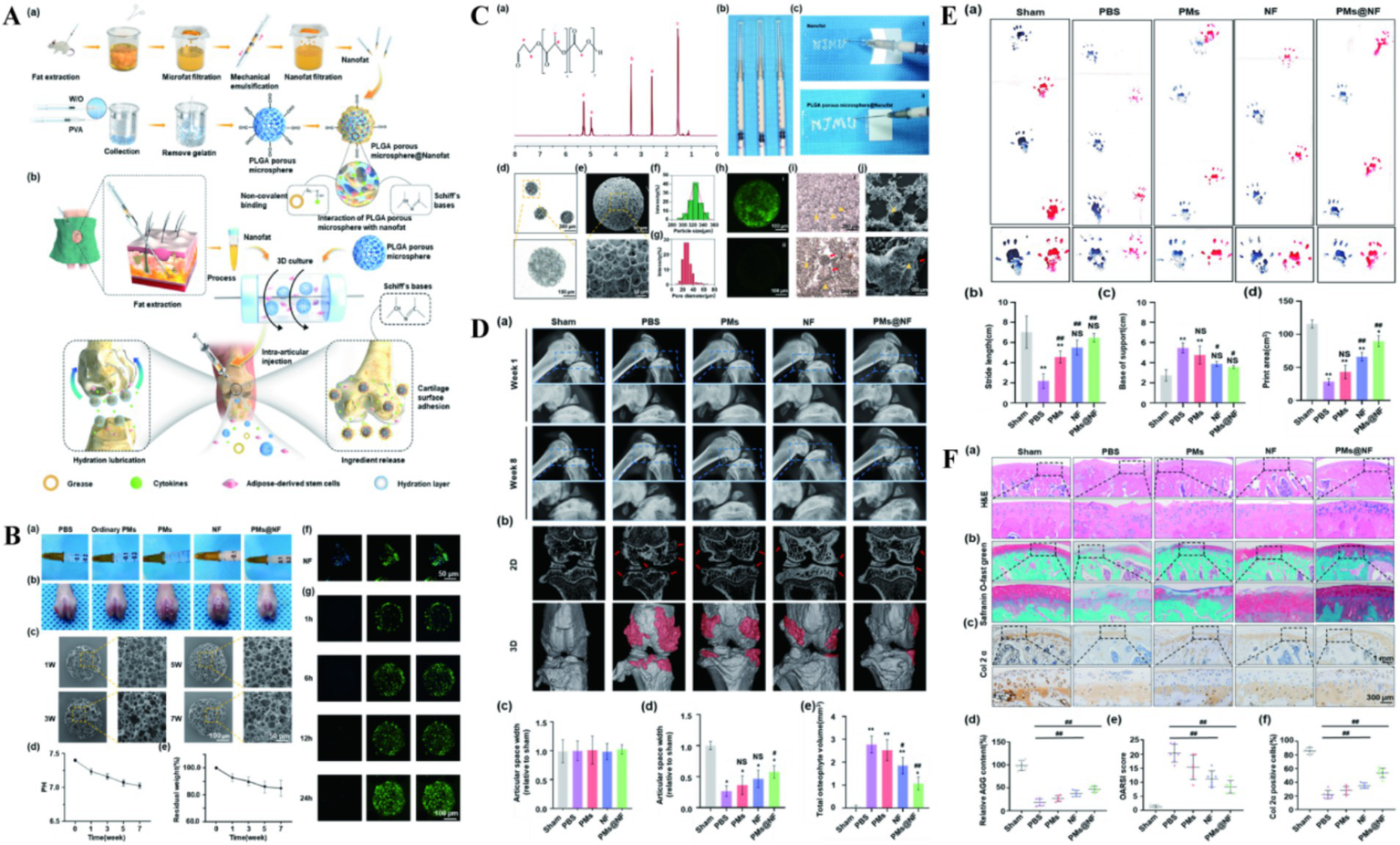
(A) The illustration preparation of NF, PMs and PMs@NF and the clinical application of multifunctional microfluidic PMs@NF. (B) Intra articular injection, in vitro degradation analysis and Calcein AM/DAPI staining of PMs@NF. (C) Characterization of NF functionalized injectable super-lubricating microfluidic PMs. (D) The performance of super-lubricating PMs@NF treat OA model in vivo. (E) Footprints collection of rats 8 weeks postoperatively. (F) Super-lubricating PMs@NF protects cartilage from invariance. Reproduced from Han et al. (64).
3.3 PLGA-based hybrid systems for combinatorial therapy
Ketorolac, as a non-steroidal anti-inflammatory drug for OA, is injected into the joint to inhibit the synthesis of prostaglandins and exerts antipyretic, analgesic and anti-inflammatory effects (71, 72). However, the drug has a short half-life in vivo and requires frequent injection, causing poor patient compliance. So, the researchers prepared a variety of ketorolac particles by different types of polymer materials to increase the slow-release properties of the drug, including: polymethacrylate, ethyl cellulose, chitosan, polycaprolactone, PLGA and the blend of two polymers, such as chitosan/gelatin (71, 73). Through modulation of two polymers, polymer blends can have customized drug release and improved physicochemical properties. In the study conducted by Wongrakpanich et al. (74), using two emulsification techniques, probe ultrasonication (PS) and high-speed agitation (HSS), prepared PLGA and PLA mixed polymer particles by water-in-oil-in-water (w/o/w) double emulsion solvent evaporation. Two emulsification techniques prepared drug-loaded microspheres with different particle size ranges. These microspheres delivered ketorolac with hyaluronic acid to treat OA. Found that the PS particles exhibited higher drug release within 24 h, whereas the HSS particles exhibited sustained release for more than 35 days. The combination of the two preparations as an alternative to OA treatment required only a monthly use.
Currently for OA in chronic inflammatory diseases and rheumatoid arthritis in autoimmune diseases, injecting corticosteroids into the joint is still an effective way to control pain and reduce inflammatory responses (75, 76). Triamcinolone sustained-release injections based on PLGA microsphere have been developed, however this injection has a stronger toxic effect on cells than dexamethasone at high doses (77, 78). Thus, Seon et al. (79) prepared self-assembled microspheres for microprecipitation reactions, loaded with dexamethasone, using PLGA and Pluronic®F-127 (F127). The in-situ implant within the joint cavity is formed by utilizing the characteristic of F127 solution to transition from sol to gel when it is above the lower critical solution temperature. By comparing the drug release between PLGA-F127 microspheres and PLGA-only microspheres, it was found that the in-situ implant did not exhibit initial burst release, but started releasing the drug after being retained for 60 days. The entire drug release time can reach up to 4 months. Compared to other drug delivery systems, PLGA-F127-MS demonstrates potential as a novel sustained-release drug delivery system.
In OA studies, abnormal subchondral bone remodeling is the main phenotypic feature of early stage of OA. Osteoclast activation, and locally elevated transforming growth factor (TGF)-β1 exacerbates early subchondral bone loss. They induce vascularization and hypomineralization in osteoblasts, leading to sclerosis during OA progression (80–82). TGF-β may be a potential therapeutic target for treating OA. Pirfenidone (PFD) is a pyridine-like small-molecule TGFβ1-3 inhibitor, which can specifically inhibit the TGFβ signaling pathway to exert anti-fibrotic and anti-inflammatory effects. This drug has been used clinically for treating various pathological fibrosis including pulmonary fibrosis and liver fibrosis (83). Some studies have demonstrated the effectiveness of oral PFD for OA, but long-term, high-dose oral PFD caused a wide range of side effects (84, 85). Zhu et al. (86) controlled the local concentration of PFD by preparing PLGA microsphere loaded with PFD. This microsphere combined with hyaluronic acid solution allowed sustained release PFD in the joint cavity, preventing subchondral bone loss in early OA and subchondral bone sclerosis in late OA. Meanwhile, this combination alleviated synovial inflammation and pain-related behavioral changes and achieved the disease-modifying effect of early OA.
Between the formation and resorption of bone and cartilage, there are a variety of cells that can influence OA progression, including mesenchymal stem cells (MSCs), chondrocytes, and osteoclasts (87, 88). Magnesium, as a skeletal system element, has an important role in the maintenance of skeletal and cartilage health processes. It has been shown to effectively stabilize nucleic acids and enzymes to exert therapeutic effects on a variety of cells in OA progression (89). The latest research utilized magnesium treatment to chondrocytes in the simulate environment of OA. It was found that AKT phosphorylation was activated, and the apoptotic markers cleaved calpain I and BAX/BCL2 were significantly reduced at the protein level, resulting in a significant decrease in the rate of cell apoptosis. Meanwhile, the phosphorylation level of AKT in osteoclasts was significantly decreased, and transcriptome sequencing analysis revealed significant changes in the PI3K/Akt pathway. Researchers have prepared stearic acid (SA) modified PLGA microspheres loaded with nano-magnesium oxide—MgO&SA@PLGA (90). This microsphere provided a suitable concentration of Mg2, which not only promoted MSC proliferation and chondrogenic differentiation but also effectively inhibited osteogenic differentiation. The treatment demonstrated significant relief effects in the rat. This study provided further support for the use of magnesium in the treatment of OA-related issues in cartilage and subchondral bone by activating the AKT signaling pathway.
Kartogenin (KGN) is a hydrophobic small molecule drug that can significantly promote chondrogenic differentiation of bone marrow mesenchymal stem cells and can induce cartilage regeneration in OA (91–94). However, KGN, as a small molecule, has poor water solubility, and its therapeutic effect is not efficient when used alone. Therefore, the use of nanocarriers may be beneficial for drug therapy and intracellular drug delivery. In the early stage of OA, irregular partial defects occur in articular cartilage, causing progressive destruction of OA. Zhang et al. (95) prepared PLGA microspheres uniformly loaded with KGN using microfluidic technology (KGN@PLGA NP). Subsequently, the PLGA microspheres were modified with dopamine to form a dopamine coating KGN@PLGA NP. Finally, the E7 recruiting peptide was non-covalently bound to the KGN@PLGA NP to prepare an injectable multifunctional PLGA microsphere for repairing partial defects in cartilage. The PDA coating could enhance the adhesion ability of PLGA microspheres, while the E7 peptide could recruit endogenous stem cells. After being injected into the joint cavity, the multifunctional microspheres could adhere to the damaged cartilage matrix and release the E7 peptide to recruit stem cells to the lesion area. With the degradation of PLGA, the release of KGN induced osteogenic differentiation of stem cells. Ultimately, the cartilage surface in the treatment group became smooth and the GAG content returned to normal, indicating the microspheres could promote the repair of cartilage injuries. New research has shown that the use of phenol-rich PDA can effectively scavenge ROS, reduce acute inflammatory responses, and inhibit the enhancement of osteoclasts (Figure 5). PLGA/polydopamine-based core/shell NPs loaded with KGN can significantly induce cartilage synthesis in vitro, and simultaneously effectively protect cartilage and subchondral bone in OA models of rats (96). Bai et al. (97) utilized nanomaterials as carriers for stem cell expansion, attempting to treat OA through stem cell tissue engineering. Firstly, PLGA porous microspheres loaded with KGN were prepared by an emulsification method. These microspheres were then anchored with chitosan (CS) using an amidation reaction to prepare PLGA-CS@KGN porous microspheres, which were subsequently cocultured with mesenchymal stem cells. It was found that the system has the ability to carry high cell densities of 1 × 104 mm−3 and can protect MSCs by controlling their release, migration, and proliferation in an inflammatory microenvironment. This system also provided prospects for the treatment of OA through stem cell tissue engineering.
Figure 5
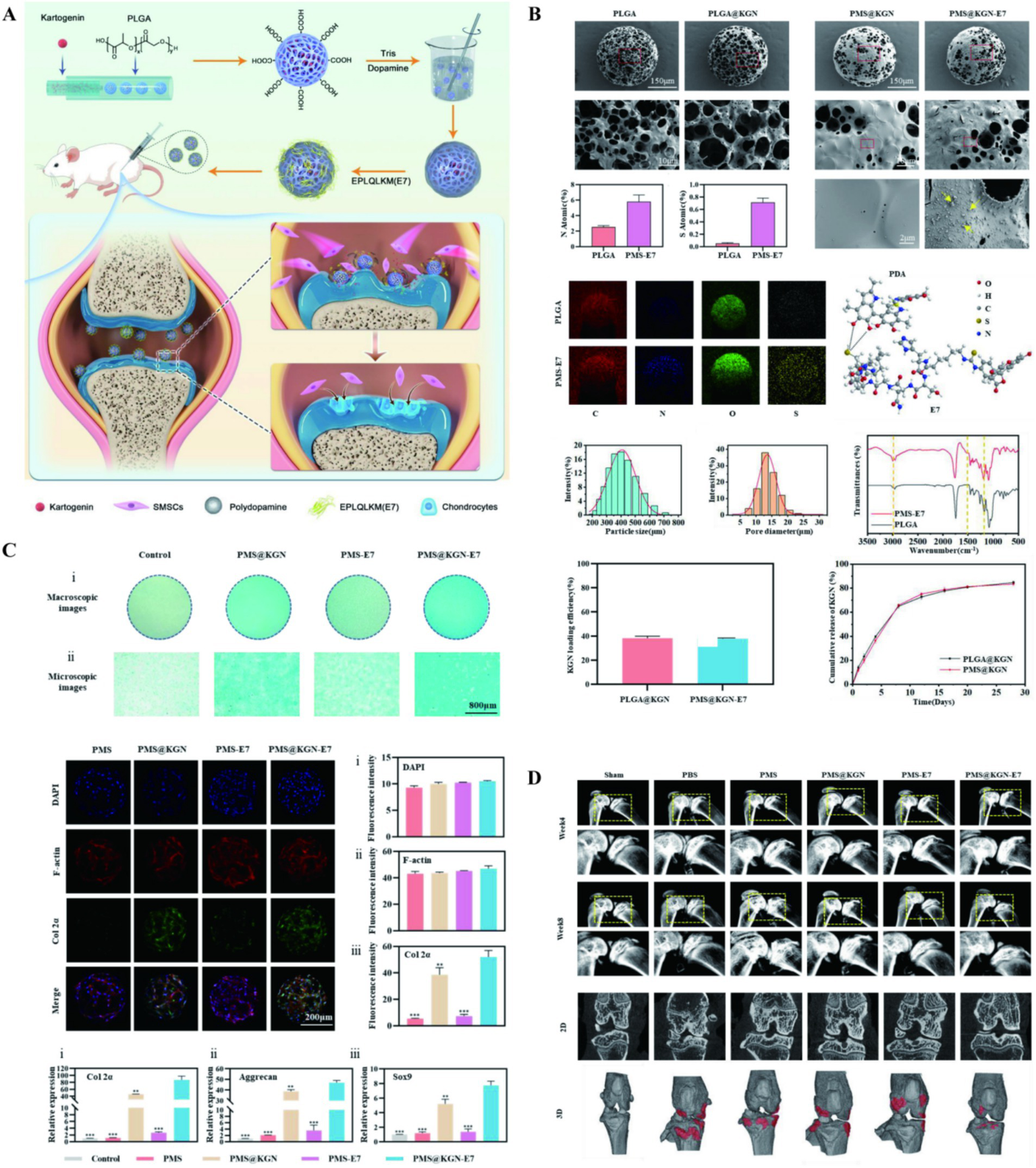
(A) The illustration on preparation and application of multifunctional cartilage repair microspheres. (B) Morphological and characterization of microspheres. (C) Microspheres promote chondrogenic differentiation. (D) Microspheres treatment and morphological assessment. Reproduced from Zhang et al. (95).
Besides delivering drugs, the use of NPs to deliver endogenous components for regulating the progression of OA is also a promising therapeutic approach. MSCs play a leading role in tissue engineering and regeneration. They primarily regulate the local microenvironment by secreting bioactive molecules and possess multidirectional differentiation capabilities, enabling them to renew and differentiate into various lineage cells such as fat, bone, cartilage, tendons, and skin (98). MSCs have been widely used in preclinical research. However, the application of conditioned media during cell culture in vitro still requires extensive cell isolation and maintenance, and there are risks of spontaneous behavior and property changes, including cell contamination, infection transmission, and malignant tumors (98–100). Meanwhile, the main executors of therapeutic effects are paracrine factors secreted by stem cells in tissue engineering regeneration. Therefore, Shah et al. (101) selected growth factors of great significance to chondrogenesis, including insulin-like growth factor, TGF-β1, fibroblast growth factor-18, and human growth hormone. They encapsulated the recombinant proteins of these growth factors in PLGA to construct a synthetic artificial stem cell system. The system constructed exhibited significant anti-inflammatory and chondroprotective effects in vitro, and alleviated cartilage degeneration and improved the biomechanical properties of articular cartilage in vivo, verifying the possibility of a new therapeutic strategy for OA.
MicroRNA (miRNA) is a class of non-coding single-stranded RNA molecules, approximately 22 nucleotides in length, encoded by endogenous genes and involved in post-transcriptional gene expression regulation in both plants and animals (102, 103). Several important microRNAs have been identified to play crucial roles in the progression of OA, including as regulators of pro-inflammatory and matrix-degrading mediators (102). Among them, miR-140 is specifically expressed in human articular chondrocytes, and dysregulation of miR-140 can promote chondrocyte inflammation and lead to degenerative lesions. Especially, downregulation of miR-140 can cause excessive activation of ADAMTS-5 signaling, leading to the loss of proteoglycan and type II collagen in chondrocytes (104, 105). Zhao et al. (106) aimed to slow the progression of OA by delivering recombinant miR-140. Firstly, a cartilage-targeting peptide (CAP)-modified poly(vinyl amine) (PVAm)-PLGA copolymer was prepared. And then it was formed into spherical NPs with r-miR-140 (CPPNPs). The introduction of PLGA significantly improved the mechanical properties and stability of CPPNPs. Meanwhile, CAP endowed the NPs with cartilage targeting ability, enabling CPPNPs to exhibit significant permeability and accumulation in cartilage and subchondral bone, thereby overcoming the two major biological barriers of cartilage: avascularity and high ECM density. In the mouse model, CPPNPs treatment significantly reduced cartilage degeneration and synovial inflammation, providing a new foundation for delivering RNA to treat OA by overcoming cartilage barriers.
However, with the progression of OA, the increase in cartilage damage leads to the gradual loss of cartilage components, weakening the abilities of passive targeting and active targeting of single ligands. Therefore, compared to targeting a single component of cartilage, a delivery system with multiple affinity peptide ligands is more capable of overcoming the difficulties of the extracellular matrix barrier of chondrocytes and cartilage targeting (107–109). Deng et al. (28) inspired by chondrocyte-matrix interactions, utilized naturally derived chondrocyte membranes (CMs) as a mean of enhancing the specificity and binding capacity of drug delivery. Firstly, PLGA particles loaded with the Wnt pathway inhibitor adavivint were prepared, and then naturally derived chondrocyte membranes were coated on the surface of the PLGA particles for modification. It was found that NPs coated with chondrocyte membranes could be preferentially taken up by chondrocytes, mainly through membrane protein-targeted recognition and clathrin-mediated endocytosis, as well as micropinocytosis. Meanwhile, these CM-NPs could overcome the extracellular matrix barrier of chondrocytes, penetrate into the cartilage matrix, and remain there for over 34 days, effectively protecting articular cartilage and alleviating the progression of OA. The combination of natural cell membranes with synthetic NPs could disguise the NPs as endogenous cells, effectively avoiding clearance by immune cells and extending the duration of treatment. The cell membrane on the surface can also exert corresponding biological effects, making it an OA treatment strategy with almost no side effects (110). Previous studies have shown that MSCs can promote cartilage proliferation and may restart the chondrocyte cycle. Human synovial CD90-positive MSCs may be involved in cartilage repair in OA (111, 112). Li et al. (113) utilized cytochalasin B to stimulate CD90 + MSCs to secrete microvesicles (CD90@MV) and prepared PLGA NPs encapsulated with triamcinolone acetonide (TA) within these microvesicles (T-CD90@NP). It was found that the membrane proteins of CD90@MV were similar to those of CD90 + MSCs, and their bioactivity was comparable to that of CD90 + MSCs in inducing cartilage proliferation. T-CD90@NP demonstrated significant cartilage repair and anti-inflammatory capabilities in OA models of rats and rabbits, capable of inducing cartilage to restart the cell cycle and reducing chondrocyte apoptosis. Bioinformatics analysis and mRNA sequencing confirmed that T-CD90@NP mainly reduced cell apoptosis through the FOXO pathway and regulated inflammation by affecting M2 macrophage polarization through IL-10. The study of PLGA with other materials co-delivering drugs for OA model treatment is outlined in Table 3.
Table 3
| Carriers | Components | Preparation | References |
|---|---|---|---|
| PLGA and PLA | Ketorolac | The w/o/w double emulsification solvent evaporation | Ketorolac-Loaded PLGA-/PLA-Based Microparticles (74) |
| PLGA and Pluronic®F-127 | Dexamethasone | Microemulsion | Self-Assembled PLGA-Pluronic F127 Microsphere (79) |
| PLGA microparticles and HA solution | PFD | The o/w emulsification solvent evaporation | Intra-articular sustained-release of PFD (86) |
| SA modified PLGA microspheres | MgO | N/a | Engineered MgO NPs (90) |
| PLGA/PDA-PEG-E7 | KGN | Microfluidic technology | Intra-Articular Injection of PLGA/Polydopamine Core−Shell NP (96) |
| PLGA-CS | KGN/BMSCs | Emulsification solvent evaporation | Stem cells expansion vector via bio-adhesive porous microspheres (97) |
| PLGA | IGF1, TGF-β1, FGF-18, and HGH | The w/o/w double emulsification solvent evaporation | The synthetic artificial stem cell system (101) |
| CAP-PVAm-PLGA | R-miR-140 | N/a | Co-polymer carrier with dual advantages of cartilage-penetrating and targeting (106) |
| CM-NPs | Adavivint | Extrusion | Chondrocyte membrane–coated NPs (28) |
| CD90@NP | Triamcinolone acetonide | Water bath ultrasound | Triamcinolone acetonide-loaded NPs (113) |
PLGA with other materials.
PLGA, Poly(D,L-lactic-co-glycolic acid); PFD, Pirfenidone; KGN, Kartogenin.
4 Challenges and prospects
Due to its excellent biocompatibility and biodegradability, PLGA has proven to be an excellent carrier for controlled drug, peptide, and protein delivery (21, 114). Many studies utilizing PLGA particles for OA treatment have achieved significant results in vivo or in vitro experiments (115, 116). PLGA particles show great potential in preclinical models of OA. However, the systems and methods used for evaluation are mainly based on lower mammalian models such as mice and rabbits (29).
There are still many shortcomings in the research of PLGA delivery system. Rodent knee joints feature smaller volumes and simpler anatomical structures, enabling rapid and uniform drug distribution within the cavity. In contrast, human knee joint cavities are more complex and contain larger volumes, resulting in significant variations in drug delivery patterns. Additionally, the significantly thicker articular cartilage in humans imposes higher penetration requirements for drug particles, potentially necessitating adjustments to the LA/GA ratio or the introduction of targeted delivery strategies. Furthermore, rodents exhibit denser vascular and lymphatic networks in synovial tissues, coupled with faster clearance rates within the joint cavity, presenting new challenges for particle metabolism and product accumulation in human joints. To address these challenges, experimental validation using large animal models serves as a crucial translational bridge.
Given the repeated drug injections in osteoarthritis patients, the degradation of PLGA into lactic acid and glycolic acid within joints may alter the microenvironment. Therefore, we propose regulating the degradation rate of PLGA to ensure the production and rate of metabolites remain within the metabolic tolerance threshold of the joint microenvironment. Additionally, repeated injections should not activate immune cells. Future studies could further mitigate potential effects by introducing neutralizing components or extending the degradation cycle.
The rapid release of drugs from PLGA carriers primarily results from free drug dissolution. Surface modification strategies are employed to regulate drug distribution through physical or chemical modifications. These include PDA surface coatings that reduce direct drug exposure and bind to drugs via π–π bonds, as well as chondrokinin peptide modifications that form a hydration layer on the carrier surface to slow drug diffusion into synovial fluid. Another method involves multi-layer coating techniques: HA coatings create gel-like barriers while chitosan/sodium alginate coatings generate electrostatic attraction. By modifying the composition and main chain structure of PLGA copolymers, drug release kinetics can be optimized at the source. For instance, blending PLGA with Pluronic F127/PCL block copolymers enables sustained drug delivery.
Conventional single-target PLGA delivery systems struggle to address the complex pathological process of OA involving cartilage degradation, synovial inflammation, and subchondral bone remodeling. Multi-target synergistic delivery, leveraging the controlled release properties of PLGA carriers, enables precise regulation of active components targeting OA tissue lesions. The next-generation PLGA delivery systems could integrate anti-inflammatory drugs with RNA or growth factors to achieve coordinated modulation of synovial cartilage or subchondral bone. Long-term validation in large animal models would better align with the clinical manifestations of OA.
Therefore, we need to establish a drug prioritization framework centered on disease-modifying effects, emphasizing therapeutic interventions that go beyond symptom relief. This approach will prove valuable for OA treatment. The strategy comprises three key components: (1) PLGA composite systems targeting cartilage repair and multi-pathological mechanisms (including inflammation suppression, matrix degradation inhibition, and subchondral bone protection), focusing on disease-modifying effects; (2) PLGA gene delivery systems targeting core signaling pathways of cellular senescence and apoptosis; (3) PLGA small-molecule delivery systems providing long-term anti-inflammatory action and mild cartilage protection, primarily aimed at symptom management.
Critical barriers remain to further advance the clinical translation of this material, with two core bottlenecks: large-scale Good Manufacturing Practice (GMP) production of the drug delivery system and long-term safety and efficacy assessment.
First, there is a significant gap between small-scale laboratory preparation and large-scale GMP production. Current research mainly relies on small-batch fabrication techniques, such as microfluidics or emulsification-solvent evaporation. These methods exhibit excellent performance in particle size control and drug loading efficiency. However, during GMP scale-up production, insufficient homogenization pressure or improper surfactant concentration can compromise the size stability of particles. Meanwhile, subtle variations in monomer purity and polymerization conditions across production batches may alter the in vivo drug release cycle of the system, thereby impairing therapeutic efficacy. Second, the innovative composite systems developed in many studies require multi-step modification and detection, which can be precisely controlled in small-scale laboratory research. In contrast, during large-scale GMP production, each modification step demands strict quality control and aseptic processing—including verification of particle targeting and functionality, as well as aseptic handling and storage of particles. This significantly increases production costs and process complexity.
At present, the hybrid system has key value in application prospect. For instance, the core of GMP compliance for PLGA/PDA hybrid systems lies in the controllable and stable PDA coating process. Selecting microfluidic technology to control the coefficient of variation in PDA coating thickness may ensure batch stability. In collagen-mixed systems, standardization of collagen raw materials and compatibility of composite processes require consideration. Commercially available recombinant collagen could be adopted to avoid batch variations from animal sources, while developing sterilization processes that preserve collagen activity. For cartilage cell membrane hybrid systems, limitations mainly stem from cell membrane sourcing and standardized extraction/quality control systems. Future efforts might involve establishing standardized cell banks, using flow cytometry for membrane protein quality control, and developing long-term storage solutions for cartilage membranes under GMP conditions. The GMP transformation of hybrid systems should focus on three core aspects: raw materials, processes, and quality control. By controlling batch variations in raw materials and reducing manual operations, clinical applications can be progressively achieved.
Meanwhile, conducting stability testing and long-term storage studies on PLGA delivery systems prior to clinical application holds translational value. The testing focuses on the physical, chemical, and biological stability of the delivery system to ensure structural integrity of the PLGA carrier and stable drug release kinetics. It also verifies that the drug degradation and its resulting PLGA decomposition products will not affect the microenvironment of the joint cavity. Ultimately, validation must confirm that the PLGA delivery system maintains activity in vivo and remains stable even after prolonged storage, thereby meeting clinical requirements.
Finally, research on the long-term in vivo safety and efficacy of this drug delivery system remains inadequate. Current animal studies have short evaluation periods and primarily use young individuals, whereas clinical OA patients are mainly elderly, and OA treatment may last for several years. Consequently, clinical OA treatment may require repeated intra-articular injections. Long-term injection and degradation of PLGA may lead to changes in the pH of synovial fluid, and long-term accumulation of particles in articular cartilage or subchondral bone may exacerbate metabolic changes in osteocytes. Additionally, elderly individuals often have impaired renal and hepatic function, resulting in reduced clearance rates of degradation products and a subsequent increase in the risk of systemic toxicity. Although PLGA-based composite systems with other materials may offer advantages in targeting and functionality for OA treatment, the immunogenic risks associated with long-term in vivo exposure to these components also warrant attention.
The elderly and metabolically impaired populations constitute the primary group affected by OA. The joint microenvironment in these groups shows significant differences compared to young, healthy animals. Therefore, evaluating the repair efficacy and safety of OA animal models is essential, while assessing the inflammatory regulation and microenvironment compatibility of PLGA formulations in metabolically impaired models holds practical value. We advocate establishing comparative evaluation systems between aged/metabolically impaired animals and their younger counterparts in future research. This approach is crucial for advancing the clinical translation of such systems.
Although there are still many problems to be solved in the treatment of human OA using NPs, we have seen that NPs exhibit good effects in drug delivery, tissue engineering, and gene therapy, providing new ideas for disease treatment. The PLGA particles discussed in this review also demonstrate great potential in OA treatment. Their unique properties make them powerful tools for new therapeutic methods and treatments, including loading small molecule drugs, recombinant proteins, and gene delivery, as well as hybrid modifications with different materials or endogenous biological components. Future research should align with the development of PLGA systems, including the development of GMP compliant manufacturing technologies, the establishment of unified quality testing standards for finished products, and the conduct of long-term safety and efficacy studies in aged animal models.
The combination of NPs with gene therapy may be a new direction for OA therapy. Such as the binding of exosomes to PLGA NPs, which can carry an siRNA targeting key genes of OA. Exosomes also have good biocompatibility and targeting properties. Targeted treatment of cell membrane camouflage is also of concern, which can not only deceive immune cells but also target cell and tissue repair. NPs combined with immune regulation also have great potential, inhibiting the inflammatory response to provide a favorable microenvironment for cartilage repair. In the future, multi-dimensional integration of these mechanisms may be needed to further prevent the development of OA. Therefore, we present a perspective on the clinical translation of this drug delivery system (Figure 6).
Figure 6
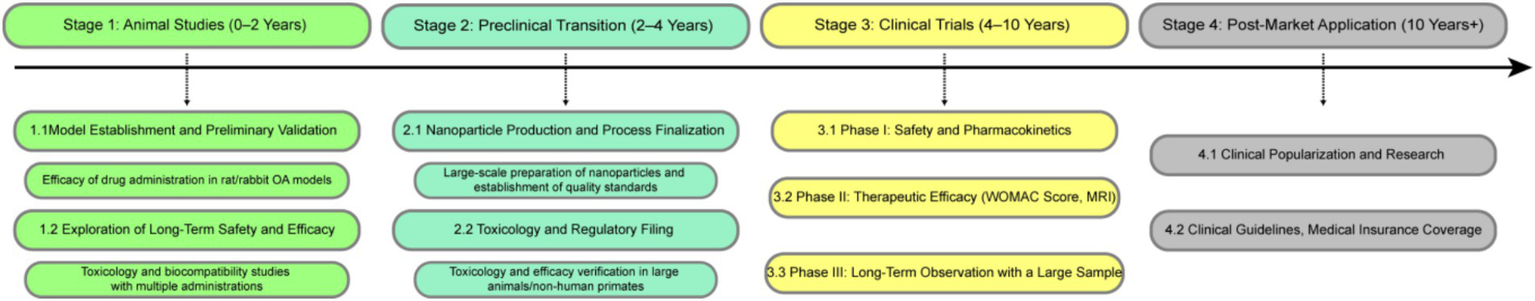
Proposed clinical research roadmap of PLGA delivery systems for OA therapy.
Beyond these aspects, combined therapies demonstrate high efficacy in OA clinical practice. Non-pharmacological interventions enhance joint function and modulate the local microenvironment, thereby supporting the therapeutic effects of PLGA delivery systems. Physical therapy regulates blood flow, tissue permeability, and cellular activity in the joint area through physical energy modulation, which enhances the efficacy of PLGA-delivered medications. In conservative OA treatment, strength training improves joint stability and cartilage nutrition supply, while PLGA’s sustained delivery provides continuous protection for cartilage during physical activity. Mechanical decompression devices reduce joint load, helping minimize inflammation and cartilage damage caused by excessive wear, creating a critical window for cartilage repair. Therefore, future research should build upon the PLGA studies outlined in this review and further validate the long-term effectiveness of combined therapies in large animal models.
We should establish a comprehensive evaluation system for standardized preclinical protocols, including dose adjustments tailored to PLGA’s inherent properties and animal models across small animals, large animals, and humans. The trial duration should be structured into distinct disease progression phases: acute phase, chronic phase, and long-term follow-up. Simultaneously, multiple biomarkers within the body must undergo safety and efficacy testing, encompassing both safety biomarkers and therapeutic biomarkers, along with imaging and functional metrics. These established standards may provide new insights for future research.
We believe that with the advancements in material engineering and drug delivery technology, the PLGA NP delivery system will receive more attention and exploration in scientific research, further promoting clinical studies on PLGA NPs in OA treatment. Therefore, the summary of this review is beneficial for readers to understand the latest research findings and corresponding challenges of PLGA NPs used in OA treatment.
Statements
Author contributions
KY: Conceptualization, Formal analysis, Project administration, Writing – original draft, Writing – review & editing. YY: Data curation, Investigation, Methodology, Resources, Supervision, Visualization, Writing – original draft. L-MG: Conceptualization, Data curation, Investigation, Project administration, Supervision, Writing – original draft. W-DL: Investigation, Project administration, Resources, Validation, Writing – review & editing. C-HD: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing. F-XX: Data curation, Methodology, Project administration, Writing – review & editing. FL: Conceptualization, Investigation, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. X-XJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Yunnan Province Science and Technology Department-Kunming Medical University Basic Research Joint Special Project (202401AY070001-087).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Sharma L . Osteoarthritis of the knee. N Engl J Med. (2021) 384:51–9. doi: 10.1056/NEJMcp1903768
2.
Eccleston A . Cartilage regeneration for osteoarthritis. Nat Rev Drug Discov. (2023) 22:96. doi: 10.1038/d41573-022-00215-x
3.
Olivotto E Trisolino G Belluzzi E Lazzaro A Strazzari A Pozzuoli A et al . Macroscopic synovial inflammation correlates with symptoms and cartilage lesions in patients undergoing arthroscopic partial meniscectomy: a clinical study. J Clin Med. (2022) 11:4330. doi: 10.3390/jcm11154330
4.
Kim M Kim J Lee I . Mediating effect of lower extremity muscle on the relationship between obesity and osteoarthritis in middle-aged and elderly women in Korea: based on the 2009-2011 Korea national health and nutrition examination survey. Epidemiol Health. (2024) 46:e2024027. doi: 10.4178/epih.e2024027
5.
Lee S Kim T-N Kim S-H Kim Y-G Lee C-K Moon H-B et al . Obesity, metabolic abnormality, and knee osteoarthritis: a cross-sectional study in Korean women. Mod Rheumatol. (2015) 25:292–7. doi: 10.3109/14397595.2014.939393
6.
Chen B Sun Y Xu G Jiang J Zhang W Wu C et al . Role of crosstalk between synovial cells and chondrocytes in osteoarthritis (review). Exp Ther Med. (2024) 27:201. doi: 10.3892/etm.2024.12490
7.
Macchi V Stocco E Stecco C Belluzzi E Favero M Porzionato A et al . The infrapatellar fat pad and the synovial membrane: an anatomo-functional unit. J Anat. (2018) 233:146–54. doi: 10.1111/joa.12820
8.
Pigeolet M Jayaram A Park KB Meara JG . Osteoarthritis in 2020 and beyond. Lancet. (2021) 397:1059–60. doi: 10.1016/S0140-6736(21)00208-7
9.
Long H Liu Q Yin H Wang K Diao N Zhang Y et al . Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the global burden of disease study 2019. Arthritis Rheumatol. (2022) 74:1172–83. doi: 10.1002/art.42089
10.
Hunter DJ Bierma-Zeinstra S . Osteoarthritis. Lancet. (2019) 393:1745–59. doi: 10.1016/S0140-6736(19)30417-9
11.
Bennell KL Paterson KL Metcalf BR Duong V Eyles J Kasza J et al . Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. (2021) 326:2021–30. doi: 10.1001/jama.2021.19415
12.
Messier SP Beavers DP Queen K Mihalko SL Miller GD Losina E et al . Effect of diet and exercise on knee pain in patients with osteoarthritis and overweight or obesity: a randomized clinical trial. JAMA. (2022) 328:2242–51. doi: 10.1001/jama.2022.21893
13.
Mao L Wu W Wang M Guo J Li H Zhang S et al . Targeted treatment for osteoarthritis: drugs and delivery system. Drug Deliv. (2021) 28:1861–76. doi: 10.1080/10717544.2021.1971798
14.
Zhan M Sun H Wang Z Li G Yang R Mignani S et al . Nanoparticle-mediated multiple modulation of bone microenvironment to tackle osteoarthritis. ACS Nano. (2024) 18:10625–41. doi: 10.1021/acsnano.4c00909
15.
Kelsey R . Nanoparticle-based Pazopanib shows promise in OA. Nat Rev Rheumatol. (2024) 20:253. doi: 10.1038/s41584-024-01104-w
16.
Hong C Alser O Gebran A He Y Joo W Kokoroskos N et al . Modulating nanoparticle size to understand factors affecting hemostatic efficacy and maximize survival in a lethal inferior vena cava injury model. ACS Nano. (2022) 16:2494–510. doi: 10.1021/acsnano.1c09108
17.
Hoshyar N Gray S Han H Bao G . The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine. (2016) 11:673–92. doi: 10.2217/nnm.16.5
18.
Banerjee A Qi J Gogoi R Wong J Mitragotri S . Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release. (2016) 238:176–85. doi: 10.1016/j.jconrel.2016.07.051
19.
Mitchell MJ Billingsley MM Haley RM Wechsler ME Peppas NA Langer R . Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. (2021) 20:101–24. doi: 10.1038/s41573-020-0090-8
20.
Kou L Bhutia YD Yao Q He Z Sun J Ganapathy V . Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front Pharmacol. (2018) 9:27. doi: 10.3389/fphar.2018.00027
21.
Blanco E Shen H Ferrari M . Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. (2015) 33:941–51. doi: 10.1038/nbt.3330
22.
Ding D Zhu Q . Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater Sci Eng C. (2018) 92:1041–60. doi: 10.1016/j.msec.2017.12.036
23.
Kumar L Kukreti G Rana R Chaurasia H Sharma A Sharma N et al . Poly(lactic-co-glycolic) acid (PLGA) nanoparticles and transdermal drug delivery: an overview. Curr Pharm Des. (2023) 29:2940–53. doi: 10.2174/0113816128275385231027054743
24.
Stromberg ZR Lisa Phipps M Magurudeniya HD Pedersen CA Rajale T Sheehan CJ et al . Formulation of stabilizer-free, nontoxic PLGA and elastin-PLGA nanoparticle delivery systems. Int J Pharm. (2021) 597:120340. doi: 10.1016/j.ijpharm.2021.120340
25.
Gentile P Chiono V Carmagnola I Hatton P . An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. (2014) 15:3640–59. doi: 10.3390/ijms15033640
26.
Makadia HK Siegel SJ . Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. (2011) 3:1377–97. doi: 10.3390/polym3031377
27.
Galindo-Rodriguez S Allémann E Fessi H Doelker E . Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm Res. (2004) 21:1428–39. doi: 10.1023/B:PHAM.0000036917.75634.be
28.
Deng R Zhao R Zhang Z Chen Y Yang M Lin Y et al . Chondrocyte membrane–coated nanoparticles promote drug retention and halt cartilage damage in rat and canine osteoarthritis. Sci Transl Med. (2024) 16:eadh9751. doi: 10.1126/scitranslmed.adh9751
29.
Zhang M Hu W Cai C Wu Y Li J Dong S . Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater Today Bio. (2022) 14:100223. doi: 10.1016/j.mtbio.2022.100223
30.
Puricelli C Gigliotti CL Stoppa I Sacchetti S Pantham D Scomparin A et al . Use of poly lactic-co-glycolic acid nano and micro particles in the delivery of drugs modulating different phases of inflammation. Pharmaceutics. (2023) 15:1772. doi: 10.3390/pharmaceutics15061772
31.
Elder SH Ross MK Nicaise AJ Miller IN Breland AN Hood ARS . Development of in situ forming implants for controlled delivery of Punicalagin. Int J Pharm. (2024) 652:123842. doi: 10.1016/j.ijpharm.2024.123842
32.
Wang J Ma J Gu J-H Wang F-Y Shang X-S Tao H-R et al . Regulation of type II collagen, matrix metalloproteinase-13 and cell proliferation by interleukin-1β is mediated by curcumin via inhibition of NF-κB signaling in rat chondrocytes. Mol Med Rep. (2017) 16:1837–45. doi: 10.3892/mmr.2017.6771
33.
Shen L Liu C-C An C-Y Ji H-F . How does curcumin work with poor bioavailability? Clues from experimental and theoretical studies. Sci Rep. (2016) 6:20872. doi: 10.1038/srep20872
34.
Hamdalla HM Ahmed RR Galaly SR Naguib IA Alghamdi BS Ahmed OM et al . Ameliorative effect of curcumin nanoparticles against monosodium iodoacetate-induced knee osteoarthritis in rats. Mediat Inflamm. (2022) 2022:1–14. doi: 10.1155/2022/8353472
35.
Sun Z Gu X Hao T Liu J Gao R Li Y et al . Intra-articular injection PLGA blends sustained-release microspheres loaded with meloxicam: preparation, optimization, evaluation in vitro and in vivo. Drug Deliv. (2022) 29:3317–27. doi: 10.1080/10717544.2022.2144545
36.
Zhu Z Gao S Chen C Xu W Xiao P Chen Z et al . The natural product Salicin alleviates osteoarthritis progression by binding to IRE1α and inhibiting endoplasmic reticulum stress through the IRE1α-IκBα-P65 signaling pathway. Exp Mol Med. (2022) 54:1927–39. doi: 10.1038/s12276-022-00879-w
37.
Wei L Pan Q Teng J Zhang H Qin N . Intra-articular administration of PLGA resveratrol sustained-release nanoparticles attenuates the development of rat osteoarthritis. Mater Today Bio. (2024) 24:100884. doi: 10.1016/j.mtbio.2023.100884
38.
Ohzono H Hu Y Nagira K Kanaya H Okubo N Olmer M et al . Targeting FoxO transcription factors with HDAC inhibitors for the treatment of osteoarthritis. Ann Rheum Dis. (2023) 82:262–71. doi: 10.1136/ard-2021-221269
39.
Cai D Yin S Yang J Jiang Q Cao W . Histone deacetylase inhibition activates Nrf2 and protects against osteoarthritis. Arthritis Res Ther. (2015) 17:269. doi: 10.1186/s13075-015-0774-3
40.
Zhang H Ji L Yang Y Zhang X Gang Y Bai L . The role of HDACs and HDACi in cartilage and osteoarthritis. Front Cell Dev Biol. (2020) 8:560117. doi: 10.3389/fcell.2020.560117
41.
Ye J Deng R Wang X Song S Xu X Zhang J-Y et al . Intra-articular histone deacetylase inhibitor microcarrier delivery to reduce osteoarthritis. Nano Lett. (2023) 23:10832–40. doi: 10.1021/acs.nanolett.3c03037
42.
Caramés B Hasegawa A Taniguchi N Miyaki S Blanco FJ Lotz M . Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. (2012) 71:575–81. doi: 10.1136/annrheumdis-2011-200557
43.
Takayama K Kawakami Y Kobayashi M Greco N Cummins JH Matsushita T et al . Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res Ther. (2014) 16:482. doi: 10.1186/s13075-014-0482-4
44.
Wang R Yu Z Sunchu B Shoaf J Dang I Zhao S et al . Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell. (2017) 16:564–74. doi: 10.1111/acel.12587
45.
Dhanabalan KM Dravid AA Agarwal S Sharath RK Padmanabhan AK Agarwal R . PLGA intra-articular injection of rapamycin microparticles prevent senescence and effectively treat osteoarthritis. Bioeng Transl Med. (2023) 8:e10298. doi: 10.1002/btm2.10298
46.
Fontanella CG Belluzzi E Pozzuoli A Scioni M Olivotto E Reale D et al . Exploring anatomo-morphometric characteristics of infrapatellar, suprapatellar fat pad, and knee ligaments in osteoarthritis compared to post-traumatic lesions. Biomedicine. (2022) 10:1369. doi: 10.3390/biomedicines10061369
47.
Miller RE Miller RJ Malfait A-M . Osteoarthritis joint pain: the cytokine connection. Cytokine. (2014) 70:185–93. doi: 10.1016/j.cyto.2014.06.019
48.
Christiansen BA Anderson MJ Lee CA Williams JC Yik JHN Haudenschild DR . Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthr Cartil. (2012) 20:773–82. doi: 10.1016/j.joca.2012.04.014
49.
Rose BJ Kooyman DL . A tale of two joints: the role of matrix metalloproteases in cartilage biology. Dis Markers. (2016) 2016:1–7. doi: 10.1155/2016/4895050
50.
Sangsuwan R Yik JHN Owen M Liu G-Y Haudenschild DR Lewis JS . Intra-articular injection of flavopiridol-loaded microparticles for treatment of post-traumatic osteoarthritis. Acta Biomater. (2022) 149:347–58. doi: 10.1016/j.actbio.2022.06.042
51.
Chen Z Wen D . Flavopiridol-loaded lubricative microspheres for osteoarthritis treatment in rabbit. Mater Express. (2024) 14:734–9. doi: 10.1166/mex.2024.2674
52.
Katzman SA Cissell D Leale D Perez-Nogues M Hall MD Bloom G et al . Intra-articular injection of an extended-release flavopiridol formulation represents a potential alternative to other intra-articular medications for treating equine joint disease. Am J Vet Res. (2024) 85:1–9. doi: 10.2460/ajvr.24.03.0057
53.
Kim SE Choi SJ Park K Kim H-J Song GG Jung JH . Intra-articular injection of rebamipide-loaded nanoparticles attenuate disease progression and joint destruction in osteoarthritis rat model: a pilot study. Cartilage. (2022) 13:194760352110692. doi: 10.1177/19476035211069250
54.
Hirayama J Hattori A Takahashi A Furusawa Y Tabuchi Y Shibata M et al . Physiological consequences of space flight, including abnormal bone metabolism, space radiation injury, and circadian clock dysregulation: implications of melatonin use and regulation as a countermeasure. J Pineal Res. (2023) 74:e12834. doi: 10.1111/jpi.12834
55.
Yang J Tang Q Zeng Y . Melatonin: potential avenue for treating iron overload disorders. Ageing Res Rev. (2022) 81:101717. doi: 10.1016/j.arr.2022.101717
56.
Qin K Tang H Ren Y Yang D Li Y Huang W et al . Melatonin promotes sirtuin 1 expression and inhibits IRE1α–XBP1S–CHOP to reduce endoplasmic reticulum stress–mediated apoptosis in chondrocytes. Front Pharmacol. (2022) 13:940629. doi: 10.3389/fphar.2022.940629
57.
Zhao M Song X Chen H Ma T Tang J Wang X et al . Melatonin prevents chondrocyte matrix degradation in rats with experimentally induced osteoarthritis by inhibiting nuclear factor-κB via SIRT1. Nutrients. (2022) 14:3966. doi: 10.3390/nu14193966
58.
Maity J Dey T Banerjee A Chattopadhyay A Das AR Bandyopadhyay D . Melatonin ameliorates myocardial infarction in obese diabetic individuals: the possible involvement of macrophage apoptotic factors. J Pineal Res. (2023) 74:e12847. doi: 10.1111/jpi.12847
59.
Liang H Yan Y Sun W Ma X Su Z Liu Z et al . Preparation of melatonin-loaded nanoparticles with targeting and sustained release function and their application in osteoarthritis. Int J Mol Sci. (2023) 24:8740. doi: 10.3390/ijms24108740
60.
Yang C Yu Y Wang X Wang Q Shang L . Cellular fluidic-based vascular networks for tissue engineering. Eng Regen. (2021) 2:171–4. doi: 10.1016/j.engreg.2021.09.006
61.
Li Q Zhao F Li Z Duan X Cheng J Zhang J et al . Autologous fractionated adipose tissue as a natural biomaterial and novel one-step stem cell therapy for repairing articular cartilage defects. Front Cell Dev Biol. (2020) 8:694. doi: 10.3389/fcell.2020.00694
62.
Bora P Majumdar AS . Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. (2017) 8:145. doi: 10.1186/s13287-017-0598-y
63.
Van Nieuwenhove I Tytgat L Ryx M Blondeel P Stillaert F Thienpont H et al . Soft tissue fillers for adipose tissue regeneration: from hydrogel development toward clinical applications. Acta Biomater. (2017) 63:37–49. doi: 10.1016/j.actbio.2017.09.026
64.
Han Z Bai L Zhou J Qian Y Tang Y Han Q et al . Nanofat functionalized injectable super-lubricating microfluidic microspheres for treatment of osteoarthritis. Biomaterials. (2022) 285:121545. doi: 10.1016/j.biomaterials.2022.121545
65.
Loeser RF Collins JA Diekman BO . Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. (2016) 12:412–20. doi: 10.1038/nrrheum.2016.65
66.
McCulloch K Litherland GJ Rai TS . Cellular senescence in osteoarthritis pathology. Aging Cell. (2017) 16:210–8. doi: 10.1111/acel.12562
67.
Toh WS Brittberg M Farr J Foldager CB Gomoll AH Hui JHP et al . Cellular senescence in aging and osteoarthritis: implications for cartilage repair. Acta Orthop. (2016) 87:6–14. doi: 10.1080/17453674.2016.1235087
68.
Park H Lee H-R Shin HJ Park JA Joo Y Kim SM et al . p16INK4a-siRNA nanoparticles attenuate cartilage degeneration in osteoarthritis by inhibiting inflammation in fibroblast-like synoviocytes. Biomater Sci. (2022) 10:3223–35. doi: 10.1039/D1BM01941D
69.
Jeon OH Kim C Laberge R-M Demaria M Rathod S Vasserot AP et al . Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. (2017) 23:775–81. doi: 10.1038/nm.4324
70.
Diekman BO Sessions GA Collins JA Knecht AK Strum SL Mitin NK et al . Expression of p16INK4a is a biomarker of chondrocyte aging but does not cause osteoarthritis. Aging Cell. (2018) 17:e12771. doi: 10.1111/acel.12771
71.
Park KD Kim TK Bae BW Ahn J Lee WY Park Y . Ultrasound guided intra-articular ketorolac versus corticosteroid injection in osteoarthritis of the hip: a retrospective comparative study. Skeletal Radiol. (2015) 44:1333–40. doi: 10.1007/s00256-015-2174-9
72.
Xu J Qu Y Li H Zhu A Jiang T Chong Z et al . Effect of intra-articular ketorolac versus corticosteroid injection for knee osteoarthritis: a retrospective comparative study. Orthop J Sports Med. (2020) 8:2325967120911126. doi: 10.1177/2325967120911126
73.
Nagda CD Chotai NP Nagda DC Patel SB Patel UL . Preparation and characterization of spray-dried mucoadhesive microspheres of ketorolac for nasal administration. Curr Drug Deliv. (2012) 9:205–18. doi: 10.2174/156720112800234503
74.
Wongrakpanich A Khunkitchai N Achayawat Y Suksiriworapong J . Ketorolac-loaded PLGA-/PLA-based microparticles stabilized by hyaluronic acid: effects of formulation composition and emulsification technique on particle characteristics and drug release behaviors. Polymers. (2023) 15:266. doi: 10.3390/polym15020266
75.
Knezevic NN Jovanovic F Voronov D Candido KD . Do corticosteroids still have a place in the treatment of chronic pain?Front Pharmacol. (2018) 9:1229. doi: 10.3389/fphar.2018.01229
76.
Law TY Nguyen C Frank RM Rosas S McCormick F . Current concepts on the use of corticosteroid injections for knee osteoarthritis. Phys Sportsmed. (2015) 43:269–73. doi: 10.1080/00913847.2015.1017440
77.
Bodick N Lufkin J Willwerth C Kumar A Bolognese J Schoonmaker C et al . An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg. (2015) 97:877–88. doi: 10.2106/JBJS.N.00918
78.
Conaghan P Strand V Hunter D Kraus VB Berenbaum F Katz NP et al . An intra-articular, extended release formulation of triamcinolone (FX006) affords clinically relevant improvements in pain and function of knee osteoarthritis: post-hoc pooled analyses of 3 randomized controlled trials. Osteoarthr Cartil. (2017) 25:S432–3. doi: 10.1016/j.joca.2017.02.747
79.
Seon S Li Y Lee S Jeon YS Kang DS Ryu DJ . Self-assembled PLGA-Pluronic F127 microsphere for sustained drug release for osteoarthritis. Pharmaceuticals. (2024) 17:471. doi: 10.3390/ph17040471
80.
Geurts J Patel A Hirschmann MT Pagenstert GI Müller-Gerbl M Valderrabano V et al . Elevated marrow inflammatory cells and osteoclasts in subchondral osteosclerosis in human knee osteoarthritis. J Orthop Res. (2016) 34:262–9. doi: 10.1002/jor.23009
81.
Muratovic D Findlay DM Cicuttini FM Wluka AE Lee YR Edwards S et al . Bone marrow lesions in knee osteoarthritis: regional differences in tibial subchondral bone microstructure and their association with cartilage degeneration. Osteoarthr Cartil. (2019) 27:1653–62. doi: 10.1016/j.joca.2019.07.004
82.
Muratovic D Findlay DM Quarrington RD Cao X Solomon LB Atkins GJ et al . Elevated levels of active transforming growth factor Β1 in the subchondral bone relate spatially to cartilage loss and impaired bone quality in human knee osteoarthritis. Osteoarthr Cartil. (2022) 30:896–907. doi: 10.1016/j.joca.2022.03.004
83.
Ruwanpura SM Thomas BJ Bardin PG . Pirfenidone: molecular mechanisms and potential clinical applications in lung disease. Am J Respir Cell Mol Biol. (2020) 62:413–22. doi: 10.1165/rcmb.2019-0328TR
84.
Margaritopoulos GA Trachalaki A Wells AU Vasarmidi E Bibaki E Papastratigakis G et al . Pirfenidone improves survival in IPF: results from a real-life study. BMC Pulm Med. (2018) 18:177. doi: 10.1186/s12890-018-0736-z
85.
Noble PW Albera C Bradford WZ Costabel U Glassberg MK Kardatzke D et al . Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. (2011) 377:1760–9. doi: 10.1016/S0140-6736(11)60405-4
86.
Zhu X Cao M Li K Chan Y-T Chan H-F Mak Y-W et al . Intra-articular sustained-release of Pirfenidone as a disease-modifying treatment for early osteoarthritis. Bioact Mater. (2024) 39:255–72. doi: 10.1016/j.bioactmat.2024.05.028
87.
Li Y Zhao S Li S Ge Y Wang R Zheng L et al . Surface engineering of biodegradable magnesium alloys for enhanced orthopedic implants. Small. (2019) 15:1904486. doi: 10.1002/smll.201904486
88.
Xu J Hu P Zhang X Chen J Wang J Zhang J et al . Magnesium implantation or supplementation ameliorates bone disorder in CFTR-mutant mice through an ATF4-dependent Wnt/β-catenin signaling. Bioact Mater. (2022) 8:95–108. doi: 10.1016/j.bioactmat.2021.06.034
89.
Glyn-Jones S Palmer AJR Agricola R Price AJ Vincent TL Weinans H et al . Osteoarthritis. Lancet. (2015) 386:376–87. doi: 10.1016/S0140-6736(14)60802-3
90.
Zheng L Zhao S Li Y Xu J Yan W Guo B et al . Engineered MgO nanoparticles for cartilage-bone synergistic therapy. Sci Adv. (2024) 10:eadk6084. doi: 10.1126/sciadv.adk6084
91.
Jiang Z Zhang Z Li S Lin S Yuan H . Magnetically guided intracartilaginous delivery of kartogenin improves stem cell-targeted degenerative arthritis therapy. Int J Nanomedicine. (2022) 17:5511–24. doi: 10.2147/IJN.S381815
92.
Zeng W-N Zhang Y Wang D Zeng Y-P Yang H Li J et al . Intra-articular injection of kartogenin-enhanced bone marrow–derived mesenchymal stem cells in the treatment of knee osteoarthritis in a rat model. Am J Sports Med. (2021) 49:2795–809. doi: 10.1177/03635465211023183
93.
Hou M Zhang Y Zhou X Liu T Yang H Chen X et al . Kartogenin prevents cartilage degradation and alleviates osteoarthritis progression in mice via the miR-146a/NRF2 Axis. Cell Death Dis. (2021) 12:483. doi: 10.1038/s41419-021-03765-x
94.
Liu H Liu P . Kartogenin promotes the BMSCs chondrogenic differentiation in osteoarthritis by down-regulation of miR-145-5p targeting Smad4 pathway. Tissue Eng Regen Med. (2021) 18:989–1000. doi: 10.1007/s13770-021-00390-9
95.
Zhang X Bai L Zhou J Gao H Chen Q Cui W et al . Injectable microspheres adhering to the cartilage matrix promote rapid reconstruction of partial-thickness cartilage defects. Acta Biomater. (2024) 179:220–33. doi: 10.1016/j.actbio.2024.03.021
96.
Zong L Wang Q Sun H Wu Q Xu Y Yang H et al . Intra-articular injection of PLGA/Polydopamine core–shell nanoparticle attenuates osteoarthritis progression. ACS Appl Mater Interfaces. (2024) 16:21450–62. doi: 10.1021/acsami.3c18464
97.
Bai L Han Q Han Z Zhang X Zhao J Ruan H et al . Stem cells expansion vector via bioadhesive porous microspheres for accelerating articular cartilage regeneration. Adv Healthc Mater. (2024) 13:2302327. doi: 10.1002/adhm.202302327
98.
Turinetto V Vitale E Giachino C . Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci. (2016) 17:1164. doi: 10.3390/ijms17071164
99.
Laurencin CT McClinton A . Regenerative cell-based therapies: cutting edge, bleeding edge, and off the edge. Regen Eng Transl Med. (2020) 6:78–89. doi: 10.1007/s40883-020-00147-1
100.
Lukomska B Stanaszek L Zuba-Surma E Legosz P Sarzynska S Drela K . Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. (2019) 2019:1–10. doi: 10.1155/2019/9628536
101.
Shah S Esdaille CJ Bhattacharjee M Kan H-M Laurencin CT . The synthetic artificial stem cell (SASC): shifting the paradigm of cell therapy in regenerative engineering. Proc Natl Acad Sci. (2022) 119:e2116865118. doi: 10.1073/pnas.2116865118
102.
Vicente R Noël D Pers Y-M Apparailly F Jorgensen C . Deregulation and therapeutic potential of microRNAs in arthritic diseases. Nat Rev Rheumatol. (2016) 12:211–20. doi: 10.1038/nrrheum.2015.162
103.
Li Y Ji R-R . Gene therapy for chronic pain management. Cell Rep Med. (2024) 5:101756. doi: 10.1016/j.xcrm.2024.101756
104.
Li X Zhilei Z Tang G Zheng C Yang G . MiR-29a and MiR-140 protect chondrocytes against the anti-proliferation and cell matrix signaling changes by IL-1β. Mol Cells. (2016) 39:103–10. doi: 10.14348/molcells.2016.2179
105.
Liang Y Duan L Xiong J Zhu W Liu Q Wang D et al . E2 regulates MMP-13 via targeting miR-140 in IL-1β-induced extracellular matrix degradation in human chondrocytes. Arthritis Res Ther. (2016) 18:105. doi: 10.1186/s13075-016-0997-y
106.
Zhao Y Deng X Tan S Zhang J Han J Wang X et al . Co-polymer carrier with dual advantages of cartilage-penetrating and targeting improves delivery and efficacy of MicroRNA treatment of osteoarthritis. Adv Healthc Mater. (2023) 12:e2202143. doi: 10.1002/adhm.202202143
107.
Rahimi M Charmi G Matyjaszewski K Banquy X Pietrasik J . Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. (2021) 123:31–50. doi: 10.1016/j.actbio.2021.01.003
108.
Tryfonidou MA De Vries G Hennink WE Creemers LB . “Old drugs, new tricks” – local controlled drug release systems for treatment of degenerative joint disease. Adv Drug Deliv Rev. (2020) 160:170–85. doi: 10.1016/j.addr.2020.10.012
109.
Li X Dai B Guo J Zheng L Guo Q Peng J et al . Nanoparticle–cartilage interaction: pathology-based intra-articular drug delivery for osteoarthritis therapy. Nano-Micro Lett. (2021) 13:149. doi: 10.1007/s40820-021-00670-y
110.
Wu H-H Zhou Y Tabata Y Gao J-Q . Mesenchymal stem cell-based drug delivery strategy: from cells to biomimetic. J Control Release. (2019) 294:102–13. doi: 10.1016/j.jconrel.2018.12.019
111.
Lu J Shen X Sun X Yin H Yang S Lu C et al . Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration. Theranostics. (2018) 8:5039–58. doi: 10.7150/thno.26981
112.
Neybecker P Henrionnet C Pape E Mainard D Galois L Loeuille D et al . In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem Cell Res Ther. (2018) 9:329. doi: 10.1186/s13287-018-1071-2
113.
Li Y Tu Q Xie D Chen S Gao K Xu X et al . Triamcinolone Acetonide-loaded nanoparticles encapsulated by CD90+ MCSs-derived microvesicles drive anti-inflammatory properties and promote cartilage regeneration after osteoarthritis. J Nanobiotechnol. (2022) 20:150. doi: 10.1186/s12951-022-01367-z
114.
Park K Skidmore S Hadar J Garner J Park H Otte A et al . Injectable, long-acting PLGA formulations: analyzing PLGA and understanding microparticle formation. J Control Release. (2019) 304:125–34. doi: 10.1016/j.jconrel.2019.05.003
115.
Wan W Lin Y Shih P Bow Y Cui Q Chang Y et al . An in situ depot for continuous evolution of gaseous H 2 mediated by a magnesium passivation/activation cycle for treating osteoarthritis. Angew Chem Int Ed. (2018) 57:9875–9. doi: 10.1002/anie.201806159
116.
Morille M Toupet K Montero-Menei CN Jorgensen C Noël D . PLGA-based microcarriers induce mesenchymal stem cell chondrogenesis and stimulate cartilage repair in osteoarthritis. Biomaterials. (2016) 88:60–9. doi: 10.1016/j.biomaterials.2016.02.022
117.
Bijlsma JW Berenbaum F Lafeber FP . Osteoarthritis: an update with relevance for clinical practice. Lancet. (2011) 377:2115–26. doi: 10.1016/S0140-6736(11)60243-2
Summary
Keywords
OA, PLGA, nanoparticle, drug, cartilage
Citation
Yang K, Yuan Y, Guo L-M, Luo W-D, Dai C-H, Xia F-X, Lin F and Jiang X-X (2025) Advanced nanoparticles for osteoarthritis: a review. Front. Med. 12:1651659. doi: 10.3389/fmed.2025.1651659
Received
22 June 2025
Accepted
29 September 2025
Published
10 October 2025
Volume
12 - 2025
Edited by
Sadiq Umar, University of Illinois Chicago, United States
Reviewed by
Sharanabasava V. Ganachari, KLE Technological University, India
Xiao Likang, Henan Provincial People's Hospital, China
Updates
Copyright
© 2025 Yang, Yuan, Guo, Luo, Dai, Xia, Lin and Jiang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Lin, licence197@qq.comXing-Xiong Jiang, jxx650011@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.