- 1McMaster University Department of Family Medicine, Hamilton, ON, Canada
- 2Skinopathy Research, North York, ON, Canada
- 3The University of Buckingham Medical School, Crewe, United Kingdom
- 4University of Toronto Temerty Faculty of Medicine, Toronto, ON, Canada
- 5Western University Faculty of Science, London, ON, Canada
- 6Scarborough Health Network Centenary Hospital, Scarborough, ON, Canada
1 Introduction
Vitiligo is a chronic skin disorder characterized by the selective loss of melanocytes, resulting in the development of depigmented patches on the skin (1). Recent epidemiological studies estimate the global prevalence of vitiligo to be approximately 0.36–0.40%, with higher prevalence in adults than children, and notable geographical variation (2, 3). Although the past decade of research has established vitiligo as a disease of autoimmune origin, the underlying pathogenic mechanisms are not fully understood, and no definitive cure currently exists (4, 5). Furthermore, new areas of research, such as the relationship between the skin and gut microbiome, are emerging; however, the data remain inconclusive due to variability in methodologies and sample sizes (6). Despite advances in therapeutic approaches, vitiligo patients often suffer from delayed diagnosis due to a scarcity of dermatological resources and expertise (7, 8). Since many vitiligo therapies are most effective in the early stages of disease (9, 10), this diagnostic gap underscores the need for innovative solutions that can enhance the accuracy and accessibility of clinical care.
In recent years, technological innovations utilizing artificial intelligence (AI) and computer vision models have begun to reshape the dermatological landscape, offering the potential to enhance diagnostic precision and support clinical decision-making (11). These tools hold particular promise for vitiligo, where the monitoring of disease progression and treatment response has historically been difficult to standardize (12). Recently, Abdolahnejad et al. proposed an AI-driven mobile application that enables patients to remotely assess and track the progression of their vitiligo (13). While this approach is encouraging, it also invites questions about clinical utility, generalizability, and readiness for integration into routine care. It is also necessary to consider the broader clinical applications of vitiligo tools, such as their potential role in psychosocial support and holistic patient care (14, 15). In this opinion paper, we place this proof-of-concept study in the context of the broader technological landscape for vitiligo, discussing both emerging and established tools, and reflecting on their potential to improve clinical outcomes and access to care. Here, we define clinical utility to mean a demonstrated, measurable benefit to patient care or workflow (e.g., improved diagnostic agreement, reduced time-to-treatment, or more reliable VASI/T-VASI change detection). We define generalizability to mean stable performance across Fitzpatrick skin types I–VI, anatomic sites, capture contexts (clinic cameras and mobile), and across care settings (specialist, primary care, and teledermatology).
2 Existing vitiligo tools
Vitiligo is primarily diagnosed through clinical examination, based on the presence of white macules with or without depigmented hairs (leukotrichia) in affected areas of the skin (1, 16). Diagnosis can be supported by Wood's lamp examination, dermoscopy, punch biopsy, and molecular testing to rule out other causes of hypopigmentation (10, 17). The most common tool used to assess vitiligo is the Wood's lamp, which emits ultraviolet (UV) light at wavelengths between 320 and 400 nm, with a peak at 365 nm (10, 18). Under the Wood's lamp, vitiligo appears as bright bluish-white patches, often with sharp demarcations (18, 19). This enables clinicians to detect subtle depigmentation in fair-skinned individuals and identify spreading vitiligo that may not be visible in natural light (17, 18). Therefore, in addition to its diagnostic value, the Wood's lamp can also be used to non-invasively monitor active disease progression and repigmentation after treatment (17, 20).
Vitiligo assessment and monitoring can also be supported by other imaging modalities, including dermoscopy and reflectance confocal microscopy (RCM) (21, 22). Though less commonly used, RCM is primarily a diagnostic technique used to visualize the epidermis and superficial dermis at near-histological resolution (23). Although RCM can also support monitoring of disease activity and treatment response, its clinical use is limited by the need for specialized equipment and training (24–26). In contrast, dermoscopy provides clinicians with an illuminated, magnified view of the epidermis and papillary dermis, enabling visualization of abnormalities in the pigmentary network, as well as perifollicular and perilesional changes (27, 28). While some dermoscopic features used to differentiate stable from unstable vitiligo are inconsistently reported in the literature (27–30), a recent study has found that dermoscopy and Wood's lamp are equally helpful, with dermoscopy demonstrating a slightly higher agreement with clinical assessments (31). However, both methods only demonstrate fair agreement (κ = 0.33 for Wood's lamp and κ = 0.40 for dermoscopy) with clinical evaluations (31). Therefore, correlating dermoscopic findings with other tools may improve diagnostic accuracy and clinical confidence (21, 22).
Traditionally, vitiligo progression and treatment response are evaluated by combining photographic documentation with clinical scoring systems (10, 17). Common systems that assess the size and extent of depigmentation include the Vitiligo Area Scoring Index (VASI), its facial (F-VASI) and total body (T-VASI) variants (32–34), the Vitiligo European Task Force assessment (VETFa) (35), and the Vitiligo Extent Score (VES) (36, 37). Although these systems are validated and generally considered reliable, they are inherently subjective and susceptible to inter-observer variability (17, 35, 38). Proper assessment can also be time-consuming in clinical practice, and dependent on the expertise of the assessor (17). Therefore, effective vitiligo management is contingent on access to clinicians with the tools and expertise to monitor the condition.
3 Artificial intelligence and vitiligo
In practice, the diagnosis and treatment of vitiligo are often limited by healthcare system constraints, cost barriers, and a shortage of specialists (7, 39). As a result, there is a growing need for data-driven technologies that can provide accurate and objective assessments for vitiligo patients. These innovations are especially important for advancing equitable care in geographically remote or underserved populations.
To address these challenges, many researchers have begun exploring artificial intelligence as a means of supporting vitiligo diagnosis and monitoring. Although machine learning and artificial intelligence have only recently gained widespread prominence, predictive artificial neural network (ANN) models for vitiligo were described as early as 2009 (40). Over the past decade, deep learning models, such as convolutional neural networks (CNNs), have become central to many high-performing dermatological AI systems (41–44). These multi-layered systems are trained on large datasets of labeled images, and can automatically extract key hierarchical features to make predictions (42, 45).
The architecture of a CNN determines how its layers are arranged and connected, and it plays a critical role in how a model processes images and generates outputs (45, 46). In the context of vitiligo, deep learning models are typically designed to either identify vitiligo (classification) or measure and quantify depigmentation (segmentation). Depending on the task, modern CNN models often adopt architectures from the ResNet, DenseNet, EfficientNet, YOLO, Inception, and U-Net families (43, 45, 46). These model families are most clinically useful when mapped to: (i) differential diagnosis (vitiligo vs. mimickers such as pityriasis alba or tinea versicolor), and (ii) burden quantification to complement VASI/T-VASI for treatment decisions. Segmentation models directly support serial burden tracking and re-pigmentation analysis, while detection models help standardize photography by localizing lesions and proposing consistent fields of view for follow-up.
For classification tasks, ResNet (42, 47), DenseNet (42, 48), EfficientNet (13, 47), and Inception (49, 50) architectures have been successfully applied by various groups to distinguish vitiligo from other skin conditions and/or healthy skin. These architectures are typically responsible for feature extraction and serve as the backbone of a CNN model. In contrast, architectures such as U-Net (51), PSPNet (52), and fully convolutional neural networks (FCNNs) (53) have been used to perform segmentation tasks for vitiligo lesions. Traditional machine learning algorithms, including K-means clustering, have also been used in combination with deep learning systems for vitiligo segmentation (13, 54). Practically, backbones support diagnosis vs. mimickers, segmentation nets support extent/re-pigmentation tracking, and detection models support standardized image capture for longitudinal care.
Architectures in the You Only Look Once (YOLO) family are object detection frameworks that can be adapted for vitiligo lesion classification and localization (52). Instead of performing classification on entire images, YOLO models can be trained to rapidly detect and draw bounding boxes around skin lesions (55). In 2023, Guo et al. proposed a hybrid deep learning model for vitiligo that integrated the YOLO v3 architecture for lesion detection and a U-Net++ architecture for segmentation (52). This system achieved a lesion detection sensitivity of 92.91% and a segmentation Jaccard index of 0.79 (52). Clinically, fast lesion localization can improve site selection for phototherapy and enable consistent recapture of vitiligo lesions across visits.
While CNN-based systems remain the foundation of most current models, recent advances in transformer-based architectures suggest new possibilities for even greater performance and generalizability (56, 57). Transformers are deep learning architectures that use self-attention mechanisms to model complex relationships within input data (57). In 2024, Zhong et al. demonstrated that shifted window (Swin) transformers outperformed CNN ResNet models in the classification of vitiligo, with top accuracies of 93.82% vs. 89.26% (58). For clinicians, these systems may translate into improved early change detection (e.g., perifollicular repigmentation) and potential “second-reader” support in uncertain cases.
Overall, deep learning systems reported in the literature consistently demonstrate strong performance, with classification accuracies ranging from 66% to 97% (47, 50) and segmentation accuracies between 93% and 97% (54, 59). Notably, the model developed by Liu et al., which reported the lowest classification accuracy (66%), was found to be non-inferior to dermatologists and outperformed both primary care physicians and nurse practitioners in diagnostic accuracy (50). These direct comparisons with healthcare professionals underscore the clinical relevance of deep learning systems for vitiligo assessment and highlight their potential to enhance the accessibility and consistency of vitiligo care.
4 AI applications for vitiligo patients
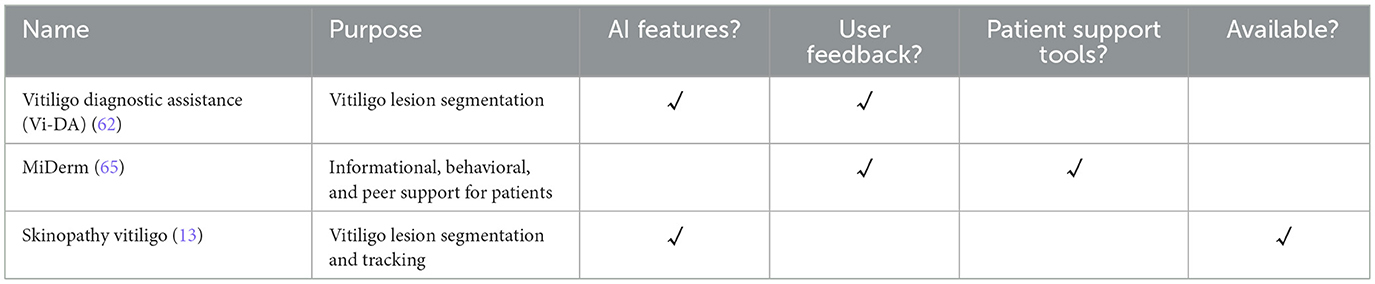
Although research into dermatological AI is rapidly advancing, few models have successfully translated into practical clinical or patient-facing applications (Table 1). This slow adoption is largely due to valid concerns around data privacy, trust, regulatory hurdles, and the need for robust external validation (60, 61). In practice, patient-facing tools can support self-monitoring, enable teledermatology triaging, and offer standardized photography to improve the reliability of detecting changes. While some mobile applications allow users to upload images and manually track their vitiligo, there are currently no widely adopted tools that leverage AI to quantitatively monitor disease progression.
One of the earliest patient-facing tools described in the literature is Vitiligo Diagnostic Assistance (Vi-DA), a prototype Android application introduced by Nugraha et al. in 2018 (62). Vi-DA was developed using traditional image processing algorithms and unsupervised machine learning for the segmentation of vitiligo lesions. The segmentation algorithm employed Fuzzy C-Means (FCM) clustering and yielded similar results to alternative segmentation models (62). However, formal performance metrics were not reported, and evaluations were limited to qualitative comparisons with images captured under normal lighting. Notably, the authors also assessed the usability of Vi-DA with vitiligo patients, who expressed generally positive feedback, but concerns about long processing times (62). Despite this early exploration, the Vi-DA application remained at the prototype stage and was never released for public use.
More recently, Abdolahnejad et al. introduced a proof-of-concept mobile application that applies deep learning techniques to detect and monitor vitiligo (13). The AI system is embedded in a mobile interface and performs classification, segmentation, and colorimetric analysis on user-submitted images. Vitiligo detection is carried out by a CNN based on the EfficientNet-B7 architecture, which achieved a reported accuracy of 95.0% (13). Following lesion identification, segmentation is performed using K-means clustering and Boundary Attention Mapping (BAM), a technique that utilizes activation maps from the CNN to refine lesion boundaries (13, 63). The system also performs colorimetric analysis on segmented regions by extracting pixel-level color data, providing users with an assessment of vitiligo severity and depigmentation. While quantitative performance metrics for segmentation and colorimetry were not reported, qualitative comparisons between AI segmentations and images taken under the Wood's lamp demonstrated close visual alignment (13).
Despite promising technical results, further large-scale clinical validation studies are needed to evaluate the generalizability of these deep learning systems. These studies can offer valuable insights into the performance of AI models across diverse patient populations, skin tones, and lighting conditions (64). As interest in AI-based tools for vitiligo continues to grow, future work should address both technical improvements and broader considerations for clinical adoption. Implementation strategies and mechanisms for clinician oversight will also be essential to support the safe and effective use of AI tools in real-world settings.
While AI-based vitiligo applications primarily focus on clinically relevant features, it is equally important to recognize the value of holistic digital tools that support patients beyond the clinic. One proposed tool is MiDerm, which is an application that provides education, self-management resources, and peer support for patients living with different skin conditions, including vitiligo (65). Although the application is currently under development, preliminary qualitative studies have highlighted the significant psychological burden associated with vitiligo and the unmet need for additional support (65). By targeting aspects of quality of life that extend beyond physical symptoms, these tools highlight the value of integrating medical and psychosocial support into digital platforms. Therefore, the most impactful patient-facing applications for vitiligo will combine clinically useful AI functionalities with holistic support features, offering a comprehensive approach to disease management.
5 Recommendations for future applications
Although promising AI tools for vitiligo are emerging, few have undergone the rigorous development, validation, and integration necessary for widespread clinical adoption. To ensure that these technologies can effectively support patient care and meet the standards of clinical practice, future efforts should prioritize strategies that address the needs and concerns of both clinicians and patients. The following recommendations highlight key considerations that may help guide the development of future vitiligo AI applications.
I. Clinical validation: large-scale clinical studies are essential to evaluate the effectiveness of AI applications in routine practice. Performance should be assessed not only in comparison to dermatologists but also across different care settings and patient demographics. Utility should be reported by task-specific endpoints (e.g., ΔVASI minimal detectable change, triage accuracy vs. dermatologist, and time-to-treatment) and generalizability should be demonstrated through pre-specified analyses across various Fitzpatrick skin types (I–VI), devices, lighting conditions, and anatomic sites. The inclusion of outcome metrics such as diagnostic accuracy, user adherence, and patient-reported satisfaction will improve credibility and clinical relevance.
II. Ethical considerations and data security: applications should adhere to stringent data protection protocols and explicitly address ethical concerns. This includes compliance with local privacy regulations, as well as transparent policies on data ownership and informed consent.
III. Scalability and integration: for clinical adoption, the application should integrate into the existing digital health infrastructure. This may include compatibility with electronic medical record (EMR) systems, interoperability with clinical workflows, and support for remote care models. Practical guidance for real-world implementation should be considered early in the development process. These would include device/lighting robustness checks, guidance for image quality controls, and EMR/teledermatology integration hooks (e.g., Fast Healthcare Interoperability Resources (FHIR) data formatting standards and VASI fields) to ensure performance transfers from research to practice.
IV. User-centered design: applications should prioritize patient usability through iterative user testing, intuitive design, and accessibility features. Incorporating structured feedback from diverse patient populations will improve engagement, adherence, and trust in the system. The evaluation of interface design alongside clinical performance is critical for real-world adoption.
6 Limitations and future directions
While AI holds significant promise for the future of dermatological care, it is essential to acknowledge the limitations of machine learning models. Data availability is the most obvious obstacle. Deep learning systems are highly dependent on the quality and diversity of the data they are trained on. However, many research groups rely on relatively small, homogenous datasets that do not reflect the broad spectrum of real-world patients. To address this gap, the Diverse Dermatology Images (DDI) dataset was created in 2022 to provide researchers with a publicly available, curated dataset with diverse skin tones (64). When three state-of-the-art dermatology models were tested on the DDI dataset, performance declined significantly—ROC-AUC scores dropped from ranges of 0.88–0.94 to 0.56–0.67 (64). Furthermore, in the context of vitiligo, even commonly used clinical tools such as the Wood's lamp have been reported to produce false-negative results in darker skin tones (12). Therefore, both the diversity of training datasets and the clinical benchmarks used for evaluation must be carefully considered during model development. Future studies should prioritize endpoints that reflect clinical utility and include stratified analyses that demonstrate generalizability across skin tones and image capture conditions.
It is also important to recognize the technical limitations of image-based detection and segmentation systems. As with clinical photography, standardized image capture procedures are essential for accurate tracking of vitiligo by machine learning models. Controlling factors such as lighting, positioning, and background contrast enable both clinicians and AI models to collect comparable images over time (66). The two-dimensional nature of images presents additional challenges, as AI systems often struggle to assess lesions on curved or complex anatomical surfaces (67). Natural artifacts, such as hair or shadows, can further interfere with model performance (67). However, emerging solutions, such as 3D imaging, image preprocessing techniques, and calibration tools, may help address these limitations (67, 68).
Ultimately, the advancement of AI for vitiligo will depend not only on technological improvements but also on ethical and inclusive implementation. Future models should be trained on large and diverse datasets that reflect variations in skin tone, age, and anatomical location. The inclusion of quality-of-life measures in patient-facing applications can also support a more holistic approach to vitiligo care. When designed with clinical and patient-centered perspectives in mind, AI has the potential to enhance diagnosis, support early intervention, and promote equitable care for individuals living with vitiligo. Realizing this potential will require close collaboration between researchers, clinicians, patients, and policymakers to ensure that AI tools are accurate, accessible, and trustworthy.
Author contributions
MP: Writing – review & editing, Writing – original draft. GF: Writing – original draft, Writing – review & editing. FP: Writing – review & editing, Writing – original draft. MK: Writing – original draft, Writing – review & editing. DC: Writing – review & editing, Writing – original draft. KK: Writing – review & editing, Writing – original draft. SH: Writing – review & editing, Writing – original draft. SL: Writing – review & editing, Writing – original draft. CH: Writing – review & editing, Writing – original draft. HC: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
DC volunteered at the company Skinopathy Research.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bergqvist C, Ezzedine K. Vitiligo: a review. Dermatol Basel Switz. (2020) 236:571–92. doi: 10.1159/000506103
2. Akl J, Lee S, Ju HJ, Parisi R, Kim JY, Jeon JJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. (2024) 9:e386–96. doi: 10.1016/S2468-2667(24)00026-4
3. Haulrig MB, Al-Sofi R, Baskaran S, Bergmann MS, Løvendorf M, Dyring-Andersen B, et al. The global epidemiology of vitiligo: a systematic review and meta-analysis of the incidence and prevalence. JEADV Clin Pract. (2024) 3:1410–9. doi: 10.1002/jvc2.526
4. Ezzedine K, Sheth V, Rodrigues M, Eleftheriadou V, Harris JE, Hamzavi IH, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. (2015) 73:883–5. doi: 10.1016/j.jaad.2015.07.039
5. Marchioro HZ, Silva de Castro CC, Fava VM, Sakiyama PH, Dellatorre G, Miot HA. Update on the pathogenesis of vitiligo. An Bras Dermatol. (2022) 97:478–90. doi: 10.1016/j.abd.2021.09.008
6. Nigro A, Osman A, Suryadevara P, Cices A. Vitiligo and the microbiome of the gut and skin: a systematic review. Arch Dermatol Res. (2025) 317:201. doi: 10.1007/s00403-024-03679-6
7. Eleftheriadou V, Delattre C, Chetty-Mhlanga S, Lee C, Girardat-Rotar L, Khan I, et al. Burden of disease and treatment patterns in patients with vitiligo: findings from a national longitudinal retrospective study in the UK. Br J Dermatol. (2024) 191:216–24. doi: 10.1093/bjd/ljae133
8. Patel K, Lopes FCPS, Sebastian KR, Jambusaria A, Ahmed AM. Assessing treatment delays for vitiligo patients: a retrospective chart review. Cutis. (2022) 109:327–9. doi: 10.12788/cutis.0531
9. Esmat SM, Bassiouny D, Hegazy R, Shalaby S, Ragab N, Ibrahim S, et al. Early localized vitiligo, a medical emergency: long-term follow-up study. Dermatol Ther. (2022) 35:e15219. doi: 10.1111/dth.15219
10. van Geel N, Speeckaert R, Taïeb A, Ezzedine K, Lim HW, Pandya AG, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the international vitiligo task force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. (2023) 37:2173–84. doi: 10.1111/jdv.19451
11. Li Pomi F, Papa V, Borgia F, Vaccaro M, Pioggia G, Gangemi S. Artificial intelligence: a snapshot of its application in chronic inflammatory and autoimmune skin diseases. Life. (2024) 14:516. doi: 10.3390/life14040516
12. Abdi P, Anthony MR, Farkouh C, Chan AR, Kooner A, Qureshi S, et al. Non-invasive skin measurement methods and diagnostics for vitiligo: a systematic review. Front Med. (2023) 10:1200963. doi: 10.3389/fmed.2023.1200963
13. Abdolahnejad M, Jeong H, Lin V, Ng T, Altaki E, Mo A, et al. Leveraging machine learning & mobile application technology for vitiligo management: a proof-of-concept. medRxiv. (2024). doi: 10.1101/2024.09.06.24313068
14. Ezzedine K, Eleftheriadou V, Jones H, Bibeau K, Kuo FI, Sturm D, et al. psychosocial effects of vitiligo: a systematic literature review. Am J Clin Dermatol. (2021) 22:757–74. doi: 10.1007/s40257-021-00631-6
15. Morrison B, Burden-Teh E, Batchelor JM, Mead E, Grindlay D, Ratib S. Quality of life in people with vitiligo: a systematic review and meta-analysis. Br J Dermatol. (2017) 177:e338–9. doi: 10.1111/bjd.15933
16. Seneschal J. Clinical features of vitiligo and social impact on quality of life. Dermatol Pract Concept. (2023) 13:e2023312S. doi: 10.5826/dpc.1304S2a312S
17. Böhm M, Schunter JA, Fritz K, Salavastru C, Dargatz S, Augustin M, et al. S1 guideline: diagnosis and therapy of vitiligo. J Dtsch Dermatol Ges J Ger Soc Dermatol. (2022) 20:365–78. doi: 10.1111/ddg.14713
18. Dyer JM, Foy VM. Revealing the unseen: a review of wood's lamp in dermatology. J Clin Aesthetic Dermatol. (2022) 15:25.
19. Klatte JL, van der Beek N, Kemperman PMJH. 100 years of wood's lamp revised. J Eur Acad Dermatol Venereol. (2015) 29:842–7. doi: 10.1111/jdv.12860
20. Wang YJ, Chang CC, Cheng KL. Wood's lamp for vitiligo disease stability and early recognition of initiative pigmentation after epidermal grafting. Int Wound J. (2017) 14:1391–4. doi: 10.1111/iwj.12800
21. Yang Y, Morriss S, Rodrigues M. Dermoscopy in vitiligo, diagnostic clues and markers of disease activity: a review of the literature. Clin Exp Dermatol. (2024) 49:969–75. doi: 10.1093/ced/llad365
22. Wang HF, Wang CY, Zhou XF, Deng XF, Huang H, Wang J, et al. A new assessment method of vitiligo by combination of dermoscopy and reflectance confocal microscopy. Clin Cosmet Investig Dermatol. (2023) 16:3615–23. doi: 10.2147/CCID.S432169
23. Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. (2018) 4:1014–23. doi: 10.1016/j.jdcr.2018.09.019
24. Lai L-g, Xu Ae. In vivo reflectance confocal microscopy imaging of vitiligo, nevus depigmentosus and nevus anemicus. Skin Res Technol. (2011) 17:404–10. doi: 10.1111/j.1600-0846.2011.00521.x
25. Kang HY, le Duff F, Passeron T, Lacour JP, Ortonne JP, Bahadoran P. A noninvasive technique, reflectance confocal microscopy, for the characterization of melanocyte loss in untreated and treated vitiligo lesions. J Am Acad Dermatol. (2010) 63:e97–100. doi: 10.1016/j.jaad.2010.02.010
26. Gu J, Xia R, Zou Y. Reflectance confocal microscopy for identification of vulvar lichen sclerosus et atrophicus and vitiligo. Am J Dermatopathol. (2022) 44:867–73. doi: 10.1097/DAD.0000000000002269
27. Suresh MS, Susmitha Divya K, Jahnavi S, Patnala G. Cross-sectional descriptive study of the utility of dermoscopy in assessing the activity and stability of vitiligo. Dermatol Pract Concept. (2024) 14:e2024258. doi: 10.5826/dpc.1404a258
28. Godínez-Chaparro JA, Roldán-Marín R, Vidaurri-de la Cruz H, Soto-Mota LA, Férez K. Dermatoscopic patterns in vitiligo. Dermatol Pract Concept. (2023) 13:e2023197. doi: 10.5826/dpc.1304a197
29. Ibrahim S, Hegazy RA, Gawdat HI, Esmat S, Mahmoud E, Rashed L, et al. Differentiating active from stable vitiligo: the role of dermoscopic findings and their relation to CXCL10. J Cosmet Dermatol. (2022) 21:4651–8. doi: 10.1111/jocd.14922
30. Kumar Jha A, Sonthalia S, Lallas A, Chaudhary RKP. Dermoscopy in vitiligo: diagnosis and beyond. Int J Dermatol. (2018) 57:50–4. doi: 10.1111/ijd.13795
31. Mohan AJ, Sarin A, Kidangazhiathmana A, Asokan N. Comparison of dermoscopy and wood's lamp in the assessment of stability of vitiligo. Indian Dermatol Online J. (2025). doi: 10.4103/idoj.idoj_728_24
32. Hamzavi I, Jain H, McLean D, Shapiro J, Zeng H, Lui H. Parametric modeling of narrowband UV-B phototherapy for vitiligo using a novel quantitative tool: the vitiligo area scoring index. Arch Dermatol. (2004) 140:677–83. doi: 10.1001/archderm.140.6.677
33. Bibeau K, Butler K, Wang M, Skaltsa K, Hamzavi IH. Psychometric evaluation of the facial and total vitiligo area scoring index instruments in the TRuE-V phase 3 studies. Dermatol Ther. (2024) 14:2223–34. doi: 10.1007/s13555-024-01223-y
34. Ezzedine K, Soliman AM, Camp HS, Ladd MK, Pokrzywinski R, Coyne KS, et al. Psychometric properties and meaningful change thresholds of the vitiligo area scoring index. JAMA Dermatol. (2025) 161:39–46. doi: 10.1001/jamadermatol.2024.4534
35. Taïeb A, Picardo M. VETF members. The definition and assessment of vitiligo: a consensus report of the vitiligo european task force. Pigment Cell Res. (2007) 20:27–35. doi: 10.1111/j.1600-0749.2006.00355.x
36. van Geel N, Lommerts J, Bekkenk M, Wolkerstorfer A, Prinsen CAC, Eleftheriadou V, et al. Development and validation of the vitiligo extent score (VES): an international collaborative initiative. J Invest Dermatol. (2016) 136:978–84. doi: 10.1016/j.jid.2015.12.040
37. Orozco-Jiménez S, Rueda-Galvis PA, Builes-Montaño CE, Arango-Salgado A. A scoping review of the extent of the clinical research on the vitiligo extent score. Arch Dermatol Res. (2025) 317:498. doi: 10.1007/s00403-024-03778-4
38. Komen L, da Graça V, Wolkerstorfer A, de Rie MA, Terwee CB, van der Veen JPW. Vitiligo area scoring index and vitiligo European task force assessment: reliable and responsive instruments to measure the degree of depigmentation in vitiligo. Br J Dermatol. (2015) 172:437–43. doi: 10.1111/bjd.13432
39. Chen T, Grau C, Suprun M, Silverberg NB. Vitiligo patients experience barriers in accessing care. Cutis. (2016) 98:385–8.
40. Cazzaniga S, Sassi F, Mercuri SR, Naldi L. Prediction of clinical response to excimer laser treatment in vitiligo by using neural network models. Dermatol Basel Switz. (2009) 219:133–7. doi: 10.1159/000225934
41. Tanvir S, Syed SA, Hussain S, Zia R, Rashid M, Zahid H. Detection of vitiligo through machine learning and computer-aided techniques: a systematic review. BioMed Res Int. (2024) 2024:3277546. doi: 10.1155/bmri/3277546
42. Zhang L, Mishra S, Zhang T, Zhang Y, Zhang D, Lv Y, et al. Design and assessment of convolutional neural network based methods for vitiligo diagnosis. Front Med. (2021) 8:754202. doi: 10.3389/fmed.2021.754202
43. Jeong HK, Park C, Henao R, Kheterpal M. Deep learning in dermatology: a systematic review of current approaches, outcomes, and limitations. JID Innov Skin Sci Mol Popul Health. (2023) 3:100150. doi: 10.1016/j.xjidi.2022.100150
44. Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. (2017) 542:115–8. doi: 10.1038/nature21056
45. Alzubaidi L, Zhang J, Humaidi AJ, Al-Dujaili A, Duan Y, Al-Shamma O, et al. Review of deep learning: concepts, CNN architectures, challenges, applications, future directions. J Big Data. (2021) 8:53. doi: 10.1186/s40537-021-00444-8
46. Khan A, Sohail A, Zahoora U, Qureshi AS. A survey of the recent architectures of deep convolutional neural networks. Artif Intell Rev. (2020) 53:5455–516. doi: 10.1007/s10462-020-09825-6
47. PC A, V AH, Suresh N, KS N, Sha A. Facial grid environment for vitiligo detection: a deep reinforcement learning perspective. In: 2024 IEEE International Conference on Interdisciplinary Approaches in Technology and Management for Social Innovation (IATMSI) (2024). p. 1–5. Available online at: https://ieeexplore.ieee.org/document/10502869 (Accessed June 15, 2025).
48. Luo W, Liu J, Huang Y, Zhao N. An effective vitiligo intelligent classification system. J Ambient Intell Humaniz Comput. (2023) 14:5479–88. doi: 10.1007/s12652-020-02357-5
49. Agrawal N, Aurelia S. Corroboration of skin diseases: melanoma, vitiligo & vascular tumor using transfer learning. In: 2021 7th Int Conf Electr Energy Syst ICEES (Chennai: IEEE) (2021). p. 590–2. doi: 10.1109/ICEES51510.2021.9383682
50. Liu Y, Jain A, Eng C, Way DH, Lee K, Bui P, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. (2020) 26:900–8. doi: 10.1038/s41591-020-0842-3
51. Low M, Huang V, Raina P. Automating vitiligo skin lesion segmentation using convolutional neural networks. In: 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI) (2020). p. 1–4. Available online at: https://ieeexplore.ieee.org/abstract/document/9098682 (Accessed Jun 15, 2025)
52. Guo L, Yang Y, Ding H, Zheng H, Yang H, Xie J, et al. A deep learning-based hybrid artificial intelligence model for the detection and severity assessment of vitiligo lesions. Ann Transl Med. (2022) 10:590. doi: 10.21037/atm-22-1738
53. Li Y, Kong AWK, Thng S. Segmenting vitiligo on clinical face images using CNN trained on synthetic and internet images. IEEE J Biomed Health Inform. (2021) 25:3082–93. doi: 10.1109/JBHI.2021.3055213
54. Khatibi T, Rezaei N, Ataei Fashtami L, Totonchi M. Proposing a novel unsupervised stack ensemble of deep and conventional image segmentation (SEDCIS) method for localizing vitiligo lesions in skin images. Skin Res Technol. (2021) 27:126–37. doi: 10.1111/srt.12920
55. Ragab MG, Abdulkadir SJ, Muneer A, Alqushaibi A, Sumiea EH, Qureshi R, et al. A comprehensive systematic review of YOLO for medical object detection (2018 to 2023). IEEE Access. (2024) 12:57815–36. doi: 10.1109/ACCESS.2024.3386826
56. Zhang L, Yin X, Liu X, Liu Z. Medical image segmentation by combining feature enhancement swin transformer and upernet. Sci Rep. (2025) 15:14565. doi: 10.1038/s41598-025-97779-6
57. Liu Z, Lin Y, Cao Y, Hu H, Wei Y, Zhang Z, et al. Swin transformer: hierarchical vision transformer using shifted windows. In: 2021 IEEE/CVF International Conference on Computer Vision (ICCV) (2021). p. 9992–10002. Available online at: https://ieeexplore.ieee.org/document/9710580 (Accessed Jun 15, 2025).
58. Zhong F, He K, Ji M, Chen J, Gao T, Li S, et al. Optimizing vitiligo diagnosis with resnet and swin transformer deep learning models: a study on performance and interpretability. Sci Rep. (2024) 14:9127. doi: 10.1038/s41598-024-59436-2
59. Hillmer D, Merhi R, Boniface K, Taieb A, Barnetche T, Seneschal J, et al. Evaluation of facial vitiligo severity with a mixed clinical and artificial intelligence approach. J Invest Dermatol. (2024) 144:351–7.e4. doi: 10.1016/j.jid.2023.07.014
60. Pantanowitz L, Hanna M, Pantanowitz J, Lennerz J, Henricks WH, Shen P, et al. Regulatory aspects of artificial intelligence and machine learning. Mod Pathol. (2024) 37:100609. doi: 10.1016/j.modpat.2024.100609
61. Martínez-Vargas E, Mora-Jiménez J, Arguedas-Chacón S, Hernández-López J, Zavaleta-Monestel E. The emerging role of artificial intelligence in dermatology: a systematic review of its clinical applications. Dermato. (2025) 5:9. doi: 10.3390/dermato5020009
62. Nugraha GA, Nurhudatiana A, Bahana R. Vi-da: vitiligo diagnostic assistance mobile application. J Phys Conf Ser. (2018) 978:012003. doi: 10.1088/1742-6596/978/1/012003
63. Ethier O, Chan HO, Abdolahnejad M, Morzycki A, Fansi Tchango A, Joshi R, et al. Using computer vision and artificial intelligence to track the healing of severe burns. J Burn Care Res. (2024) 45:700–8. doi: 10.1093/jbcr/irad197
64. Daneshjou R, Barata C, Betz-Stablein B, Celebi ME, Codella N, Combalia M, et al. Checklist for evaluation of image-based artificial intelligence reports in dermatology: clear derm consensus guidelines from the international skin imaging collaboration artificial intelligence working group. JAMA Dermatol. (2022) 158:90–6. doi: 10.1001/jamadermatol.2021.4915
65. Hewitt RM, Dale C, Purcell C, Pattinson R, Bundy C. A qualitative exploration of the prospective acceptability of the MiDerm app; a complex digital intervention for adults living with skin conditions. Br J Health Psychol. (2025) 30:e12778. doi: 10.1111/bjhp.12778
66. Van Geel N, Hamzavi I, Kohli I, Wolkerstorfer A, Lim HW, Bae JM, et al. Standardizing serial photography for assessing and monitoring vitiligo: a core set of international recommendations for essential clinical and technical specifications. J Am Acad Dermatol. (2020) 83:1639–46. doi: 10.1016/j.jaad.2019.10.055
67. Mirikharaji Z, Abhishek K, Bissoto A, Barata C, Avila S, Valle E, et al. A survey on deep learning for skin lesion segmentation. Med Image Anal. (2023) 88:102863. doi: 10.1016/j.media.2023.102863
Keywords: vitiligo, AI, technology, healthcare, dermatology
Citation: Parikh M, Fang G, Poon F, Kyeremeh M, Cruz D, Ki K, Huang S, Lin S, Hong C and Chan HO (2025) Technological advances in vitiligo management: perspectives on AI, mobile tools, and clinical utility. Front. Med. 12:1661554. doi: 10.3389/fmed.2025.1661554
Received: 07 July 2025; Accepted: 26 August 2025;
Published: 06 October 2025.
Edited by:
Yan Valle, Vitiligo Research Foundation, United StatesReviewed by:
Yolanda Gilaberte, Hospital Universitario Miguel Servet, SpainKonstantin Lomonosov, I.M. Sechenov First Moscow State Medical University, Russia
Copyright © 2025 Parikh, Fang, Poon, Kyeremeh, Cruz, Ki, Huang, Lin, Hong and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manasi Parikh, bWFuYXNpLnBhcmlraEBtZWRwb3J0YWwuY2E=
†Present Address: Daniel Cruz, King's School of Management, Economics, and Mathematics, Western University, London, ON, Canada
 Manasi Parikh
Manasi Parikh Gloria Fang
Gloria Fang Faith Poon3
Faith Poon3 Manuella Kyeremeh
Manuella Kyeremeh