Abstract
Background:
Primary hyperparathyroidism (PHPT) during pregnancy is rare, with an estimated prevalence of <1% among pregnant women. While often asymptomatic, it may lead to serious maternal complications (nephrolithiasis, pancreatitis, preeclampsia) and adverse fetal outcomes (growth restriction, miscarriage, preterm delivery, stillbirth). Surgical parathyroidectomy in the second trimester remains the gold standard treatment, but may be unfeasible due to gestational age, contraindications, or patient refusal. In such situations, minimally invasive techniques such as microwave ablation (MWA) have emerged as potential alternatives, though their use in pregnancy is extremely rare, with only one case previously reported.
Case:
We report the case of a 38-year-old woman in her second trimester, with a history of multiple miscarriages and no living children, referred for hypercalcemia discovered on routine screening. Laboratory tests revealed corrected serum calcium of 11.54 mg/dL (85–105), elevated intact parathyroid hormone (iPTH) at 100.3 pg./mL (15–65), and marked hypercalciuria. Cervical ultrasound demonstrated a vascularized hypoechoic lesion posterior to the left thyroid lobe, consistent with a parathyroid adenoma, further confirmed by fine-needle aspiration with PTH washout (2075 pg./mL). After refusal of surgery, ultrasound-guided MWA was performed under local anesthesia with hydrodissection to protect adjacent structures.
Results:
Within 1 h, iPTH dropped to 16.8 pg./mL and serum calcium normalized to 10.0 mg/dL, remaining stable throughout pregnancy. Symptoms resolved within days. Obstetric monitoring confirmed healthy fetal development. At 38 weeks, the patient delivered a healthy infant by cesarean section. No maternal or fetal complications occurred.
Conclusion:
This case represents only the second reported use of MWA for PHPT during pregnancy, demonstrating its feasibility, efficacy, and safety in a highly selected patient. MWA may provide a valuable minimally invasive alternative when surgery is contraindicated or refused, though further evidence is needed to define its role in this unique clinical context.
Introduction
Primary hyperparathyroidism (PHPT) during pregnancy is an uncommon endocrine disorder, with an estimated prevalence of less than 1% among pregnant women (1, 2). Although often asymptomatic, it can be associated with significant maternal complications, including nephrolithiasis, pancreatitis, and preeclampsia, as well as adverse fetal outcomes such as intrauterine growth restriction, preterm delivery, and stillbirth (3–6). The condition is most frequently caused by a single parathyroid adenoma, with multigland disease and parathyroid carcinoma being rare in this population (7).
Surgical removal of the adenoma, preferably during the second trimester, remains the gold standard treatment for symptomatic or severe disease, as it is the only curative option (8). In some urgent cases, such as acute pancreatitis related to primary hyperparathyroidism, surgery may be required outside this optimal period (9). However, surgery may not be feasible in certain clinical scenarios, including advanced gestational age, contraindications to general anesthesia, or patient refusal (10). In such situations, medical management can be attempted but is often limited by efficacy and safety concerns (11).
In recent years, minimally invasive image-guided thermal ablation techniques, including radiofrequency ablation (RFA) and microwave ablation (MWA), have emerged as potential alternatives to surgery for selected patients with PHPT (12–14), though their use during pregnancy remains extremely rare and poorly documented (15). We report the case of a 38-year-old pregnant woman with symptomatic PHPT successfully treated with ultrasound-guided MWA of a parathyroid adenoma, highlighting the potential role of this technique in carefully selected cases.
This case is also notable given the patient’s advanced maternal age and history of recurrent miscarriages, making the pregnancy especially precious and the therapeutic decision particularly delicate.
Case presentation
A 38-year-old pregnant woman in her second trimester was referred by her gynecologist for evaluation of hypercalcemia discovered incidentally during routine blood tests. She had a history of multiple unexplained miscarriages and no living children. Her presenting symptoms included persistent fatigue, exaggerated pregnancy-related nausea and vomiting, and poor response to symptomatic treatment.
Initial laboratory evaluation revealed a markedly elevated corrected serum calcium level of 115.4 mg/L (2.89 mmol/L) (reference range: 85–105 mg/L [2.12–2.62 mmol/L]), an inappropriately high parathyroid hormone (iPTH) level of 100.3 pg./mL (15–65), and marked hypercalciuria at 558 mg/24 h, with a vitamin D level at the lower end of the normal range. She also had a history of Hashimoto’s thyroiditis, for which she was receiving levothyroxine replacement therapy.
Cervical ultrasound demonstrated a large hypoechoic, oblong lesion located posterior to the left superior thyroid lobe, measuring 26.1 × 13.3 × 10.1 mm (volume: 1.82 mL) (Figures 1A,B). The lesion was highly vascularized, with a clearly identifiable feeding artery (Figures 1C,D), strongly suggestive of a parathyroid adenoma. Although sestamibi scintigraphy can exceptionally be performed with reduced radioisotope doses in selected cases, we deliberately avoided any radiation exposure during pregnancy. Instead, the parathyroid origin of the lesion was reliably confirmed by fine-needle aspiration with in situ PTH washout, yielding a markedly elevated PTH concentration of 2075 pg./mL, thus providing a non-radiative and highly specific diagnostic confirmation.
Figure 1

(A,B) Large hypoechoic oblong lesion at the posterior aspect of the upper pole of the left thyroid lobe. (C,D) Clearly identifiable feeding artery. (E) Posterior hydrodissection with glucose serum. (F,G) Pronounced hypoechogenicity with complete absence of vascularization at the end of the procedure. White arrow: parathyroid adenoma; yellow arrow: thyroiditis; red arrow: glucose serum.
Due to the unavailability of genetic testing in our region, multiple endocrine neoplasia (MEN) could not be formally excluded at the genetic level. Therefore, MEN was assessed through a thorough clinical and biochemical evaluation. A detailed family history and clinical assessment revealed no findings suggestive of MEN. Biochemical testing included serum calcitonin measurement, methoxylated derivatives, and a comprehensive pituitary hormonal profile, all of which were within normal ranges. Nevertheless, the absence of genetic testing represents a limitation of this case.
In the absence of a clearly identified cause of recurrent miscarriages, primary hyperparathyroidism could be considered a possible contributing factor, although a formal causal relationship cannot be established.
Management
Initial treatment included intravenous hydration, which slightly reduced calcium levels to 112–113 mg/L (2.80–2.82 mmol/L) (reference range: 85–105 mg/L [2.12–2.62 mmol/L]) without symptomatic improvement. Surgery was proposed as the standard treatment, but the patient strongly declined due to anxiety about fetal risk. Given the urgency, her refusal of surgery, and favorable anatomical factors, we proposed a minimally invasive option: microwave ablation (MWA).
Procedure
The patient was placed in a supine position with mild neck extension. An experienced anesthesiologist was present for continuous monitoring. During the ablation phase, the patient was asked to repeatedly count out loud to allow real-time assessment of voice integrity and early detection of any recurrent laryngeal nerve irritation.
Under local anesthesia with 2% lidocaine, hydrodissection using chilled 5% dextrose in water (D5W) was performed to create a > 5 mm safety margin between the adenoma and critical structures (esophagus, common carotid artery, and presumed recurrent laryngeal nerve path) (Figure 1E).
A 17-gauge, 10 cm microwave antenna with a 3.5 mm active tip was used. The antenna was introduced laterally to avoid traversing the thyroid. Ablation was initiated at the feeding artery of the adenoma to minimize intratumoral bleeding, followed by targeted zone-by-zone ablation. The entire procedure was performed under continuous ultrasound guidance. Power output was set at 25 watts throughout the procedure. Total procedure time was 23 min, with an ablation time of 3 min. Hydrodissection was repeated several times due to the adenoma’s volume, using a total of 120 mL of injected fluid.
Technical difficulty was increased due to the coexisting thyroiditis, which reduced echogenic contrast between the hypoechoic adenoma and thyroid tissue (Figures 1A,B). However, post-procedural imaging confirmed marked hypoechogenicity and complete loss of vascularity of the ablated lesion (Figures 1F,G).
The patient remained under observation for 1 h with neck cooling and was discharged without discomfort or complications.
Results
One hour after completion of the procedure, iPTH dropped markedly to 16.8 pg./mL (15–65) (Figure 2A), with a concomitant normalization of serum calcium at 100.3 mg/L (2.51 mmol/L) (Figure 2B). One week later, iPTH had slightly increased but remained within the normal range at 54 pg./mL, while serum calcium remained stable at 100 mg/L (2.50 mmol/L). One month later, both PTH (51–54 pg./mL) and serum calcium (~100 mg/L [2.50 mmol/L]) remained stable, and this biochemical normalization persisted throughout the subsequent follow-up until delivery (Figure 2).
Figure 2
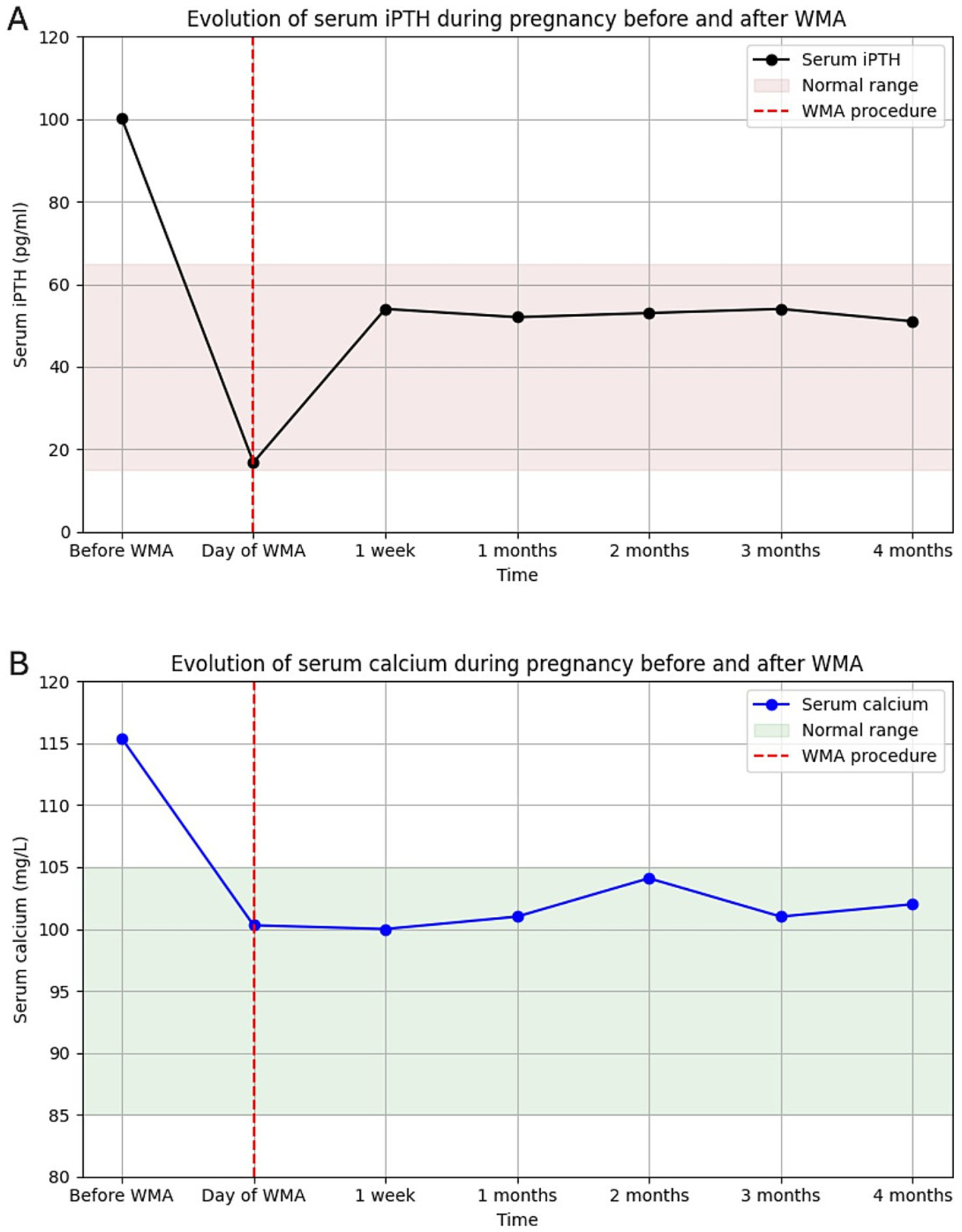
(A) Evolution of intact PTH and (B) corrected serum calcium from diagnosis to delivery after microwave ablation. Both normalized rapidly and remained stable.
Urinary calcium also showed a considerable decrease after ablation and, when measured once during the third trimester, remained at the upper limit of normal for gestational age (401 mg/24 h).
Clinically, symptoms improved by the evening of the procedure and resolved completely within 3 days. Obstetric follow-up the following day confirmed a healthy fetus.
The remainder of the pregnancy progressed smoothly without complications. At 38 weeks of gestation, the patient gave birth by cesarean section to a healthy baby boy. Although neonatal follow-up data are not available in this report, postpartum maternal and neonatal monitoring is recommended in cases of maternal primary hyperparathyroidism due to potential complications.
Discussion
Primary hyperparathyroidism (PHPT) during pregnancy is uncommon and often underdiagnosed, yet it can have significant maternal and fetal consequences, particularly when hypercalcemia is marked (1–3). Maternal complications include nephrolithiasis, pancreatitis, hyperemesis gravidarum, preeclampsia, and hypercalcemic crisis, while fetal complications range from intrauterine growth restriction to miscarriage, preterm delivery, and stillbirth (4–7). Early recognition and appropriate management are therefore crucial.
Management of PHPT in pregnancy is challenging. Conservative measures, such as hydration and a low-calcium diet, are often insufficient, while pharmacologic options such as calcitonin or cinacalcet have limited safety data in pregnancy (8–12, 16–18). Parathyroidectomy remains the standard of care and is generally recommended in the second trimester, when the risk to both mother and fetus is lowest (13, 14). In some urgent cases, such as acute pancreatitis related to primary hyperparathyroidism, surgery may be required outside this optimal period (9). However, surgery is not always feasible due to gestational timing, comorbidities, or patient preference.
Minimally invasive techniques such as thermal ablation have emerged as alternatives in selected patients. Both radiofrequency ablation and microwave ablation (MWA) have shown efficacy for parathyroid adenomas in nonpregnant populations (15, 19–24). Reports of their use in pregnancy are extremely scarce, with only one prior case of MWA for PHPT during pregnancy published (15). Our case adds to this limited evidence, demonstrating that MWA can achieve rapid and sustained normalization of calcium and PTH levels while being well tolerated and safe for the fetus.
In our patient, biochemical normalization occurred within hours and was maintained throughout pregnancy. Symptoms improved quickly, and obstetric follow-up confirmed healthy fetal development. The outcome was favorable, with the delivery of a healthy infant at 38 weeks. This observation suggests that MWA may represent a valuable therapeutic option in highly selected pregnant women when surgery is not possible, although further studies are needed to confirm its safety and efficacy.
This case presented specific clinical considerations due to the patient’s age (38 years), history of multiple miscarriages, and lack of living children. Given these factors and the ongoing pregnancy, careful consideration of maternal and fetal safety was required. A minimally invasive approach under local anesthesia was chosen to manage hypercalcemia while minimizing risks to the fetus.
Limitations
This report has several limitations. It describes a single case with a relatively short follow-up, which limits the generalizability of the findings. Genetic testing for multiple endocrine neoplasia could not be performed due to resource constraints. Neonatal data were also limited, and the procedure was conducted by a single experienced operator. Therefore, while the outcome was favorable, further studies and longer follow-up are required to confirm the safety and reproducibility of microwave ablation for parathyroid adenomas during pregnancy.
Conclusion
This case illustrates that microwave ablation may represent a feasible minimally invasive parties option for managing primary hyperparathyroidism during pregnancy in highly selected situations where surgery is contraindicated or declined. The procedure achieved rapid biochemical normalization, symptom relief, and favorable maternal and fetal outcomes in this individual case. However, given the exceptional nature of such situations and the lack of long-term follow-up data, no general conclusions can be drawn regarding its routine use. Further accumulation of clinical experience and multicenter collaboration are needed to better define the safety profile, efficacy, and potential indications of thermal ablation in this rare and challenging context.
Learning points:
-
Primary hyperparathyroidism in pregnancy, although rare, can lead to significant maternal and fetal morbidity if not promptly recognized and managed.
-
Parathyroidectomy in the second trimester remains the standard treatment, but it may not always be possible due to gestational timing, comorbidities, or patient preference.
-
Minimally invasive techniques, such as microwave ablation, may represent a safe and effective alternative in highly selected cases.
-
Individualized decision-making is essential, especially in precious pregnancies, where both maternal and fetal safety must be carefully balanced.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethical review and approval was not required for this study in accordance with local legislation and institutional requirements, as no institutional review board or ethics committee exists in our setting. Written informed consent was obtained from the patient for the publication of this case report and any potentially identifiable images or data included herein. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AB: Writing – original draft.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was used in the creation of this manuscript. Artificial intelligence tools (ChatGPT, OpenAI) were used exclusively to assist with the English translation and language editing of the manuscript, as English is not my native language. All scientific content, data analysis, interpretation, and conclusions were entirely developed by the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Norman J Politz D Politz L . Hyperparathyroidism during pregnancy and the effect of rising calcium on pregnancy loss: a call for earlier intervention. Clin Endocrinol. (2009) 71:104–9. doi: 10.1111/j.1365-2265.2008.03495.x,
2.
Schnatz PF Curry SL . Primary hyperparathyroidism in pregnancy: evidence-based management. Obstet Gynecol Surv. (2002) 57:365–76. doi: 10.1097/00006254-200206000-00022,
3.
Som M Stroup JS . Primary hyperparathyroidism and pregnancy. Proc (Bayl Univ Med Cent). (2011) 24:220–3. doi: 10.1080/08998280.2011.11928719,
4.
Hirsh D Avi L Toledano Y Hod M Kovatz S Chen R et al . Pregnancy outcome in women with primary hyperparathyroidism. J Clin Endocrinol Metab. (2009) 94:3394–8.
5.
Norman J Politz D Politz L . Hyperparathyroidism during pregnancy: maternal and fetal outcomes. Endocr Pract. (2012) 18:949–52.
6.
Paakkonen M Vehviläinen J Leino R Laine T Kantola I Valtonen H et al . Maternal and perinatal outcome of primary hyperparathyroidism in pregnancy: a multicenter study. Endocr Connect. (2022) 11:e220044
7.
Castillo JJ Jimenez JJ Bockorny B Glezerman IG . Parathyroid carcinoma during pregnancy. Endocr Pract. (2008) 14:1020–4.
8.
McMullen T Learoyd D Williams DC Sywak M Sidhu S Delbridge LW . Hyperparathyroidism in pregnancy: options for localization and surgical therapy. World J Surg. (2010) 34:1811–6. doi: 10.1007/s00268-010-0569-2,
9.
Augustin G Lai Q Cigrovski BM . Primary hyperparathyroidism-induced acute pancreatitis in pregnancy: a systematic review with a diagnostic-treatment algorithm. World J Gastroenterol. (2024) 30:3755–65. doi: 10.3748/wjg.v30.i32.3755,
10.
Som M Stroup JS . Management of hyperparathyroidism during pregnancy. Proc (Bayl Univ Med Cent). (2011) 24:220–3. doi: 10.1080/08998280.2011.11928719,
11.
Kovacs CS . Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev. (2016) 96:449–547. doi: 10.1152/physrev.00027.2015,
12.
Hu KQ Li X Liang K Liang P . Ultrasound-guided microwave ablation for primary hyperparathyroidism: safety and efficacy in a multicenter study of 105 patients. Int J Hyperth. (2020) 37:549–55.
13.
Liu F Yu X Liang P Wang Y . Ultrasound-guided percutaneous microwave ablation of parathyroid adenoma: preliminary results in 20 patients. Cardiovasc Intervent Radiol. (2019) 42:245–51.
14.
Lim CY Cho SJ Baek SM Baek JH Kim C . Radiofrequency ablation versus parathyroidectomy for primary hyperparathyroidism: a systematic review and meta-analysis. Endocrinol Metab (Seoul). (2021) 36:1–9.
15.
Wei Y He Y Chen Q Zeng M Sun H . Microwave ablation of parathyroid adenoma in pregnancy: a case report. J Clin Ultrasound. (2022) 50:236–9.
16.
Kovacs CS . Calcitonin in pregnancy and lactation. Endocrine. (2010) 37:6–17.
17.
Stathopoulos IP Liakou CG Katsalira A Trovas G Papaioannou N Lyritis GP et al . The use of bisphosphonates in women prior to or during pregnancy and lactation. Hormones (Athens). (2011) 10:280–91. doi: 10.14310/horm.2002.1319,
18.
Green SB Catherino WH Brewer JI . Skeletal anomalies in neonates exposed to bisphosphonates in utero. Am J Obstet Gynecol. (2005) 192:1452–6.
19.
Malekar-Raikar S Sinnott BP . Primary hyperparathyroidism in pregnancy—a case series and review. Arch Gynecol Obstet. (2011) 2011:1–6. doi: 10.1155/2011/520516,
20.
Kovacs CS Kronenberg HM . Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. (1997) 18:832–72. doi: 10.1210/edrv.18.6.0319,
21.
Patel J Maldonado M Bhanot R Vokes T . Primary hyperparathyroidism in pregnancy treated with cinacalcet. Endocr Pract. (2008) 14:476–9.
22.
Horjus C Groot I Telting D van Setten P van Sorge A Kovacs CS et al . Cinacalcet in pregnancy: a novel treatment for primary hyperparathyroidism?J Clin Endocrinol Metab. (2009) 94:3394–7.
23.
Zhou W Zhan W Qi H Chen Y Fan W Yang J et al . High-intensity focused ultrasound versus radiofrequency ablation for benign thyroid nodules: a multicenter study. Int J Hyperth. (2019) 36:244–50.
24.
Lim HK Cho SJ Baek JH Lee KD Son CW Baek SM et al . Efficacy and safety of thermal ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. (2021) 22:269–78.
Summary
Keywords
hypercalcemia, microwave ablation (MW ablation), parathyroid adenoma, pregnancy, primary hyperparathyroidism (pHPT)
Citation
Bey A (2026) Primary hyperparathyroidism in pregnancy successfully treated with microwave ablation: a case report. Front. Med. 12:1688249. doi: 10.3389/fmed.2025.1688249
Received
18 August 2025
Revised
15 December 2025
Accepted
17 December 2025
Published
23 January 2026
Volume
12 - 2025
Edited by
Leonardo Rossi, University of Pisa, Italy
Reviewed by
Goran Augustin, University of Zagreb, Croatia
Thang Viet Luong, University of Tasmania, Australia
Updates
Copyright
© 2026 Bey.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abderrahim Bey, ezzianesamia1951@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.