Abstract
Objective:
This study aimed to investigate the correlation between anticoagulant initiation time and thrombolysis at 30 days after anticoagulation therapy in patients with pulmonary embolism (PE).
Methods:
Patients with PE who were hospitalized in the Department of Respiratory Medicine were enrolled. Based on computed tomography pulmonary angiography (CTPA) performed 30 days after anticoagulation therapy, patients were categorized into the complete thrombolysis group (n = 112) and the incomplete thrombolysis group (n = 93). A retrospective analysis was conducted on risk factors, clinical characteristics, and auxiliary examinations. Spearman's correlation analysis was used to assess the association between anticoagulant initiation time and thrombolysis at 30 days. Receiver operating characteristic (ROC) curve analysis was applied to determine the optimal cut-off point for anticoagulation initiation time in PE.
Results:
The proportion of patients with complete thrombolysis at 30 days was 54.63% (112 of 205). Spearman's correlation analysis indicated a significant negative correlation between anticoagulant initiation time and thrombolysis at 30 days (Rs = −0.411, P < 0.001). ROC curve analysis showed that anticoagulant initiation time predicted thrombolysis at 30 days (AUC: 0.736, 95% CI: 0.668–0.804, P < 0.001). The Youden index was 0.071, and the optimal cut-off point for anticoagulant initiation time was <6.5 days, which was associated with the best therapeutic effect.
Conclusion:
Anticoagulant initiation time, PAOI, DVT, and malignant tumors were negatively correlated with thrombolysis at 30 days after anticoagulation therapy. Thrombus absorption within 1 month was significantly improved when anticoagulant therapy was initiated within 6.5 days.
1 Introduction
Pulmonary embolism (PE) is the third leading cause of death from cardiovascular disease, following myocardial infarction and stroke (1, 2). Recent studies have shown that the incidence of PE in hospitalized patients in China in 2021 was 14.19 per 100,000, with a slightly higher incidence in men than in women (3). Risk stratification using hemodynamic parameters, biomarkers of myocardial injury, and markers of right ventricular dysfunction assessed by echocardiography or computed tomography is strongly recommended after confirmation of PE, followed by appropriate treatment (4–6). For patients with low-risk PE, anticoagulant therapy may be administered in the outpatient setting, while patients at high risk may require thrombolytic therapy (7, 8).
2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism (9), and 2025 Chinese Society of Cardiology (CSC) guidelines for the diagnosis and management of PE recommend anticoagulant therapy for at least 3 months, with continuation determined by the degree of thrombus absorption (10). Factors influencing thrombolytic absorption include systemic thrombolytic agents, catheter-directed thrombolysis, and interventional therapies in patients with high-risk PE, which may accelerate thrombus resolution (11–13).
In clinical practice, earlier initiation of anticoagulation has been observed to result in better thrombolytic outcomes. However, few studies have investigated the optimal timing of anticoagulant initiation. In this study, we performed a retrospective case–control analysis to evaluate the correlation between anticoagulation initiation time and thrombolysis at 30 days in patients with PE, aiming to provide evidence to guide clinical practice.
2 Materials and methods
2.1 Study subjects
This study retrospectively collected patients with PE, in the Department of Respiratory and Critical Care Medicine, Shanghai Sixth People's Hospital, between September 1, 2018 and January 30, 2025, were included. The inclusion criteria followed the diagnostic guidelines outlined in the “2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with ERS” issued by ESC (9).
Inclusion criteria: (1) Age ≥18 years; (2) first-time diagnosis of PE confirmed by computed tomography pulmonary angiography (CTPA); (3) disease duration < 30 days; (4) no contraindications to anticoagulation therapy; (5) completion of at least 90 days of treatment and follow-up; (6) CTPA performed before anticoagulation therapy and repeated at 30 and 90 days after treatment.
Exclusion criteria: Patients were excluded if they: (1) refused anticoagulation therapy; (2) had severe heart disease, advanced malignant tumor, or an expected survival time < 2 weeks; (3) had other types of embolism such as fat embolism or chronic pulmonary embolism; (4) received thrombolytic therapy; (5) presented with clinically significant thrombocytopenia (platelet count < 75 × 109/L); (6) had coagulation disorders or bleeding tendencies that contraindicated anticoagulation therapy according to the treating physician; (7) were initially diagnosed with PE at another hospital before referral; (8) had incomplete medical records; (9) could not undergo CTPA due to obesity, severe renal insufficiency, or contrast allergy; (10) had contraindications to direct oral anticoagulants (DOACs), such as chronic kidney disease with creatinine clearance < 30 mg/dL, cirrhosis (Child–Pugh class B or C), alanine aminotransferase more than twice the upper normal limit, age < 18 years, or pregnancy.
This study was a single center retrospective design. A total of 861 patients with first-ever PE were initially enrolled. After excluding patients with incomplete clinical data (113 cases), absence of CTPA review within 30 days of anticoagulant therapy (416 cases), lack of follow-up at 90 days (124 cases), chronic pulmonary embolism (1 case), and thrombolytic therapy (2 cases), 205 patients were finally included. Based on CTPA findings at 30 days, patients were categorized into the complete thrombolysis group (112 cases) and the partial thrombolysis group (93 cases) (Figure 1).
Figure 1
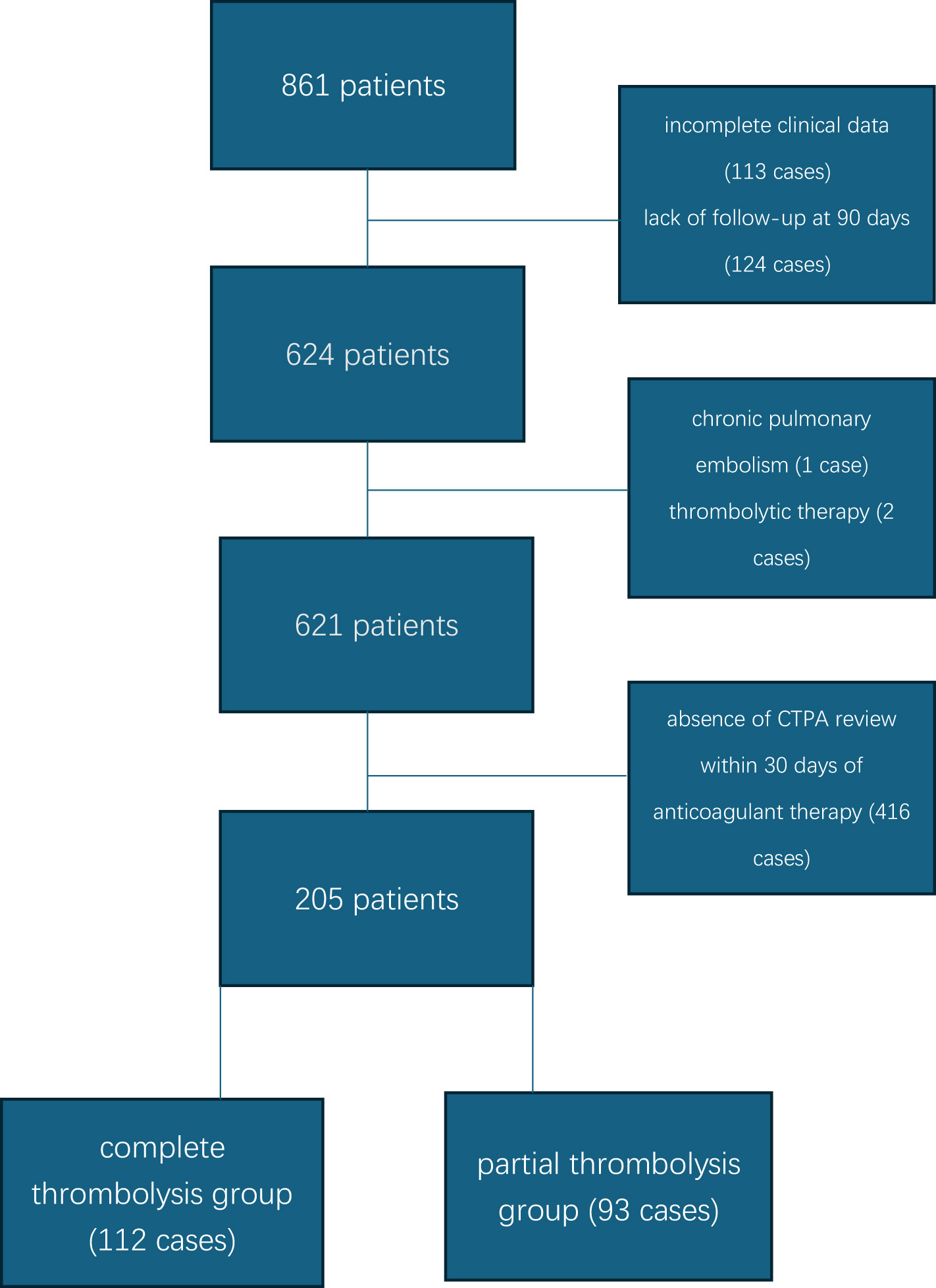
Specific flowchart.
This study was approved by the Ethics Committee of Shanghai Sixth People's Hospital, Shanghai Jiao Tong University. All procedures involving human participants were conducted in accordance with the ethical standards of the institutional and national research committees, as well as the principles outlined in the 1964 Helsinki Declaration and its subsequent amendments or comparable ethical standards. Due to the retrospective nature of the study, informed consent was waived [No.: 2023-KY-138(K)].
2.2 Data collection
The following data were retrospectively collected and analyzed: gender, age, body mass index (BMI), smoking history, underlying diseases (hypertension, stroke, diabetes, recent fracture within the past 4 weeks, recent fixation or surgery, malignancy), major symptoms (chest pain, dyspnea, etc.), arterial blood gas analysis, plasma D-dimer (D-D), N-terminal pro-brain natriuretic peptide (NT-proBNP), cardiac troponin I (cTNI), electrocardiographic findings, pulmonary artery systolic pressure (PASP; measured via echocardiography), lower extremity venous ultrasound findings, thrombus location, pulmonary hypertension (defined as PASP > 40 mmHg), pulmonary vascular obstruction index (PAOI) (14), CSC-defined PE risk categories (low risk, intermediate-low risk, intermediate-high risk, high risk), simplified Pulmonary Embolism Severity Index (sPESI; scores of 0, 1, 2, ≥3) (15), bleeding manifestations, treatment regimens, and outcomes.
2.3 Treatment
Patients with PE received anticoagulant therapy in accordance with the “2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with ERS” (9).
Following PE diagnosis, anticoagulation was initiated. In 77 patients, warfarin therapy was administered as follows: subcutaneous low-molecular-weight heparin (100 IU/kg every 12 h) was started, and oral warfarin (2.5 mg) was introduced 24–48 h later. Both agents were used concurrently for 4–5 days. When the prothrombin time (PT) and international normalized ratio (INR) reached 2.0–3.0 for two consecutive days, low-molecular-weight heparin was discontinued, while warfarin was continued orally. Warfarin dosage was adjusted as follows: increased by 0.625 mg if INR < 2.0, and reduced or discontinued if INR > 3.0.
In 128 patients, rivaroxaban was used (15 mg orally twice daily for 3 weeks, followed by 20 mg once daily for 9 weeks). All patients received anticoagulant therapy for 3 months. None experienced adverse events such as subcutaneous hematoma, gastrointestinal bleeding, or intracranial hemorrhage.
CTPA was repeated 90 days after anticoagulant therapy to evaluate thrombus resolution in patients of the partial thrombolysis group. In the complete thrombolysis group, serum D-dimer was measured on day 90. If D-dimer was positive, CTPA was performed to confirm the presence of pulmonary thrombosis. If D-dimer was negative, pulmonary thromboembolism was excluded.
2.4 Statistical analysis
Statistical analyses were performed using SPSS version 17.0. The Mann–Whitney U-test was used for intergroup comparisons. Normally distributed data were expressed as mean ± standard deviation (Mean ± SD) and compared using the t-test. Categorical variables were presented as frequencies (%) and compared using the χ2 test. Logistic regression analysis was conducted, with thrombolysis at 30 days after anticoagulation therapy (±) as the dependent variable, and gender, age, risk factors, comorbidities, arterial blood gas parameters, D-dimer, cTNI, and NT-proBNP as independent variables to identify predictors of thrombolysis. Spearman's correlation analysis was applied to examine the association between anticoagulant initiation time and thrombolysis at 30 days. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off point for anticoagulation initiation time in PE. A p-value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics
A total of 205 patients with PE, including 89 males and 116 females aged 22–94 years, were included. CTPA was repeated 30 days after initiation of anticoagulant therapy. Based on thrombolysis status, patients were classified into the complete thrombolysis group (112 cases) and the incomplete thrombolysis group (93 cases). The overall rate of thrombolysis at 30 days was 54.63%.
Among the 77 patients treated with warfarin, 44 achieved complete thrombolysis and 33 had incomplete thrombolysis, yielding a thrombolysis rate of 57.14%. Among the 128 patients treated with rivaroxaban, 68 achieved complete thrombolysis and 60 had incomplete thrombolysis, yielding a thrombolysis rate of 53.12%. Comparison between the rivaroxaban and warfarin groups showed no significant difference (χ2 = 0.313, P = 0.576). Gingival bleeding occurred in three patients receiving warfarin and three patients receiving rivaroxaban. No cases of hemoptysis, subcutaneous hematoma, gastrointestinal bleeding, or intracranial hemorrhage were reported. All patients completed anticoagulant therapy, survived, and were discharged. After 3 months, only one patient in the incomplete thrombolysis group had residual pulmonary thrombus. Detailed results are presented in Tables 1–6.
Table 1
| General Information | A group (n = 112 cases) | B group (n = 93 cases) | F/χ2value | P-value |
|---|---|---|---|---|
| Male/Female (cases) | 50/62 | 39/55 | 0.152 | 0.697 |
| Age (years) | 69.20 ± 11.31 | 70.72 ± 13.49 | 1.168 | 0.281 |
| BMI (kg/m2) | 20.76 ± 2.887 | 20.99 ± 2.927 | 0.095 | 0.759 |
| Smoking history (cases) | 44 (39.28%) | 30 (32.25%) | 1.088 | 0.297 |
| Symptom duration (days) | 4.87 ± 2.191 | 7.51 ± 3.242 | 15.529 | 0.001* |
| Oral contraceptive use (cases) | 0 | 0 | – | – |
| Long-haul travel history (cases) | 0 | 0 | – | – |
| Atrial fibrillation (cases) | 7 (6.25%) | 3 (3.22%) | 0.947 | 0.324 |
| Hypertension (cases) | 56 (50.0%) | 50 (53.26%) | 0.288 | 0.591 |
| Diabetes mellitus (cases) | 22 (19.6%) | 18 (19.35%) | 0.003 | 0.959 |
| Malignant tumor (cases) | 4 (3.57%) | 18 (19.35%) | 13.212 | < 0.001* |
| Surgery or trauma (cases) | 19 (16.9%) | 18 (19.35%) | 0.196 | 0.658 |
| Stroke history (cases) | 6 (5.35%) | 7 (7.52%) | 0.403 | 0.526 |
| Cough | 24 (21.4%) | 28 (30.1%) | 2.022 | 0.155 |
| Fever | 17 (15.17%) | 19 (20.43%) | 0.968 | 0.325 |
| Chest pain | 23 (20.53%) | 16 (17.2%) | 0.366 | 0.545 |
| Chest distress | 44 (39.28%) | 35 (37.63%) | 0.058 | 0.809 |
| SBP at admission, (mmHg) | 130.45 ± 13.54 | 133.45 ± 14.32 | 0.134 | 0.715 |
| DBP at admission, (mmHg) | 80.7 ± 11.09 | 79.22 ± 10.95 | 0.002 | 0.961 |
| Heart rate at admission, (bpm) | 91.15 ± 20.04 | 88.58 ± 19.15 | 0.520 | 0.472 |
Comparison of basic information (mean ± SD) (n, %).
* P < 0.05.
BMI, body mass index; SBP, Systolic blood pressure; DBP, diastolic blood pressure; bpm, beats per min;
Table 2
| General information | A group (n = 112 cases) | B group (n = 93 cases) | F/χ2 value | P-value |
|---|---|---|---|---|
| sPESI Class (cases) | 2.192 | 0.534 | ||
| 0 | 34 (30.35%) | 25 (26.88%) | ||
| 1 | 49 (43.75%) | 44 (47.31%) | ||
| 2 | 24 (21.42%) | 16 (17.2%) | ||
| ≥3 | 5 (4.46%) | 8 (8.6%) | ||
| cTNI (ug/L) | 0.0827 ± 0.166 | 0.066 ± 0.129 | 2.326 | 0.129 |
| Pro-BNP (ng/L) | 601.51 ± 796.35 | 589.88 ± 632.96 | 0.805 | 0.371 |
| PaO2 (mmHg) | 77.32 ± 17.74 | 81.13 ± 17.60 | 1.273 | 0.261 |
| PaCO2 (mmHg) | 37.56 ± 5.85 | 36.86 ± 5.43 | 0.014 | 0.904 |
| D-Dimer (mg/L) | 6.95 ± 5.07 | 6.96 ± 6.07 | 1.701 | 0.194 |
| PASP (mmHg) | 29.29 ± 11.24 | 28.01 ± 8.89 | 2.424 | 0121 |
| PVOI (%) | 25.49 ± 8.90 | 31.58 ± 11.22 | 15.65 | < 0.001* |
| CTS-defined risk PE | 3.657 | 0.161 | ||
| Low-risk patients | 51 (45.53%) | 37 (39.78%) | ||
| Intermediate-low-risk patients | 45 (40.17%) | 48 (51.61%) | ||
| Intermediate-high-risk patients | 17 (15.17%) | 8 (8.6%) | ||
| DVT (cases) | 19 (16.9%) | 39 (41.93%) | 15.617 | < 0.001* |
| PH (cases) | 10 (8.92%) | 7 (7.52%) | 1.318 | 0.187 |
| Minor bleeding (cases) | 3 (2.67%) | 3 (3.22%) | 0.054 | 0.817 |
| Hospitalization duration (days) | 10.59 ± 3.43 | 10.70 ± 4.00 | 0.045 | 0.832 |
| 90-day mortality (cases) | 0 | 0 | – | – |
Comparison of laboratory examinations (mean ± SD) (n, %).
* P < 0.05.
TNI, troponin I; BNP, B-type natriuretic peptide; PVOI, pulmonary vascular obstruction index; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; DVT, deep vein thrombosis; PASP, pulmonary artery systolic pressure; PH, pulmonary hypertension; sPESI, simplified pulmonary embolism severity index.
Table 3
| General information | A group (n = 44 cases) | B group (n = 33 cases) | F/χ2-value | P-value |
|---|---|---|---|---|
| Male/female (cases) | 14/30 | 10/23 | 2.203 | 0.138 |
| Age (years) | 67.34 ± 9.852 | 70.0 ± 12.62 | 1.944 | 0.167 |
| BMI (kg/m2) | 20.32 ± 2.84 | 21.53 ± 3.068 | 0.119 | 0.731 |
| Smoking history (cases) | 15 (34.09%) | 10 (30.3%) | 0.123 | 0.725 |
| Symptom duration (days) | 4.75 ± 2.334 | 7.48 ± 3.474 | 6.474 | 0.013* |
| Oral contraceptive use (cases) | 0 | 0 | – | – |
| Long-haul travel history (cases) | 0 | 0 | – | – |
| Atrial fibrillation (cases) | 3 (6.81%) | 1 (3.03%) | 0.549 | 0.459 |
| Hypertension (cases) | 26 (59.09%) | 17 (51.51%) | 0.439 | 0.508 |
| Diabetes mellitus (cases) | 7 (15.9%) | 7 (21.21%) | 0.356 | 0.550 |
| Malignant tumor (cases) | 1 (2.27%) | 9 (27.27%) | 10.429 | 0.001* |
| Surgery or trauma (cases) | 7 (15.9%) | 6 (18.18%) | 0.069 | 0.792 |
| Stroke history (cases) | 2 (4.54%) | 2 (6.06%) | 0.088 | 0.767 |
| Cough | 10 (22.72%) | 11 (33.3%) | 1.069 | 0.301 |
| Fever | 10 (22.72%) | 11 (33.3%) | 0.716 | 0.397 |
| Chest pain | 9 (20.45%) | 7 (21.21%) | 0.007 | 0.935 |
| Chest distress | 23 (52.27%) | 11 (33.3%) | 2.743 | 0.098 |
| SBP at admission, (mmHg) | 130.45 ± 13.54 | 133.45 ± 14.32 | 0.134 | 0.715 |
| DBP at admission, (mmHg) | 75.59 ± 10.23 | 74.52 ± 8.74 | 0.925 | 0.339 |
| Heart rate at admission, (bpm) | 86.55 ± 18.64 | 88.85 ± 18.44 | 0.095 | 0.759 |
Comparison of basic information (mean ± SD) (n, %) (Warfarin group).
* P < 0.05.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; bpm, beats per minute.
Table 4
| General information | A group (n = 44 cases) | B group (n = 33 cases) | F/χ2 value | P-value |
|---|---|---|---|---|
| sPESI Class (cases) | 3.631 | 0.304 | ||
| 0 | 16 (36.36%) | 9 (27.27%) | ||
| 1 | 21 (47.72%) | 13 (39.39%) | ||
| 2 | 6 (13.63%) | 8 (24.24%) | ||
| ≥3 | 1 (2.27%) | 3 (9.09%) | ||
| cTNI (ug/L) | 0.0636 ± 0.117 | 0.0681 ± 0.157 | 0.447 | 0.506 |
| Pro-BNP (ng/L) | 531.18 ± 679.06 | 442.30 ± 603.39 | 1.014 | 0.317 |
| PaO2 (mmHg) | 77.186 ± 19.768 | 79.08 ± 17.20 | 0.002 | 0.962 |
| PaCO2 (mmHg) | 38.30 ± 6.40 | 36.17 ± 6.12 | 0.086 | 0.770 |
| D-Dimer (mg/L) | 6.69 ± 4.92 | 6.186 ± 4.59 | 0.026 | 0.873 |
| PASP (mmHg) | 28.82 ± 9.61 | 29.09 ± 10.99 | 0.233 | 0.631 |
| PVOI (%) | 23.125 ± 8.76 | 31.894 ± 12.516 | 9.44 | 0.003* |
| CTS-defined risk PE | 0.379 | 0.927 | ||
| Low-risk patients | 23 (52.25%) | 18 (54.5%) | ||
| Intermediate-low-risk patients | 15 (34.09%) | 12 (36.36%) | ||
| Intermediate-high-risk patients | 6 (13.63%) | 3 (9.09%) | ||
| DVT (cases) | 7 (15.9%) | 14 (42.42%) | 6.684 | 0.010* |
| PH (cases) | 5 (11.36%) | 4 (12.12%) | 0.01 | 0.918 |
| Minor bleeding (cases) | 2 (4.54%) | 1 (3.03%) | 0.116 | 0.734 |
| Hospitalization duration (days) | 9.86 ± 3.001 | 9.94 ± 2.263 | 1.804 | 0.183 |
| 90-day mortality (cases) | 0 | 0 | – | – |
Comparison of laboratory examinations (mean ± SD) (n, %) (Warfarin group)
* P < 0.05.
TNI, troponin I; BNP, B-type natriuretic peptide; PVOI, pulmonary vascular obstruction index; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; DVT, deep vein thrombosis; PASP, pulmonary artery systolic pressure; PH, pulmonary hypertension; sPESI, simplified pulmonary embolism severity index.
Table 5
| General information | A group (n = 68 cases) | B group (n = 60cases) | F/χ2-value | P-value |
|---|---|---|---|---|
| Male/female (cases) | 36/33 | 23/37 | 2.477 | 0.116 |
| Age (years) | 70.40 ± 12.08 | 71.12 ± 14.03 | 0.328 | 0.568 |
| BMI (kg/m2) | 21.04 ± 2.90 | 20.70 ± 2.83 | 0.607 | 0.437 |
| Smoking history (cases) | 29 (42.64%) | 20 (33.33%) | 1.17 | 0.279 |
| Symptom duration (days) | 4.94 ± 2.108 | 7.52 ± 3.138 | 5.047 | 0.026* |
| Oral contraceptive use (cases) | 0 | 0 | – | – |
| Long-haul travel history (cases) | 0 | 0 | – | – |
| Atrial fibrillation (cases) | 4 (5.88%) | 2 (3.33%) | 0.464 | 0.496 |
| Hypertension (cases) | 30 (44.11%) | 33 (55.0%) | 1.51 | 0.219 |
| Diabetes mellitus (cases) | 15 (22.05%) | 11 (18.33%) | 0.320 | 0.572 |
| Malignant tumor (cases) | 3 (4.41%) | 9 (15.0%) | 4.206 | 0.040* |
| Surgery or trauma (cases) | 12 (17.64%) | 13 (21.66%) | 0.328 | 0.567 |
| Stroke history (cases) | 4 (5.88%) | 5 (8.33%) | 0.464 | 0.496 |
| Cough | 14 (20.58%) | 17 (28.33%) | 1.042 | 0.307 |
| Fever | 12 (17.64%) | 13 (21.66%) | 0.328 | 0.567 |
| Chest pain | 14 (20.58%) | 9 (15.0%) | 0.675 | 0.411 |
| Chest distress | 21 (30.88%) | 24 (40.0%) | 1.162 | 0.281 |
| SBP at admission, (mmHg) | 130.22 ± 12.64 | 134.82 ± 14.42 | 1.104 | 0.295 |
| DBP at admission, (mmHg) | 84.0 ± 10.41 | 81.80 ± 11.24 | 0.573 | 0.450 |
| Heart rate at admission, (bpm) | 94.13 ± 20.48 | 88.43 ± 19.68 | 0.938 | 0.335 |
Comparison of basic information (mean ± SD) (n, %) (Rivaroxaban group).
* P < 0.05.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; bpm, beats per minute.
Table 6
| General information | A group (n = 68 cases) | B group (n = 60 cases) | F/χ2 value | P-value |
|---|---|---|---|---|
| sPESI Class (cases) | 3.742 | 0.291 | ||
| 0 | 18 (26.47%) | 16 (26.67%) | ||
| 1 | 28 (41.17%) | 31 (51.66%) | ||
| 2 | 18 (26.47%) | 8 (13.33%) | ||
| ≥3 | 4 (5.88%) | 5 (8.33%) | ||
| cTNI (ug/L) | 0.095 ± 0.191 | 0.065 ± 0.113 | 5.921 | 0.016* |
| Pro-BNP (ng/L) | 647.01 ± 865.65 | 671.05 ± 639.05 | 0.701 | 0.404 |
| PaO2 (mmHg) | 77.41 ± 16.46 | 82.25 ± 17.86 | 2.207 | 0.140 |
| PaCO2 (mmHg) | 37.07 ± 5.467 | 37.25 ± 5.01 | 0.045 | 0.833 |
| D-Dimer (mg/L) | 7.124 ± 5.192 | 7.389 ± 6.74 | 1.718 | 0.192 |
| PASP (mmHg) | 29.60 ± 12.23 | 27.82 ± 7.969 | 5.369 | 0.121 |
| PVOI (%) | 27.022 ± 8.716 | 31.417 ± 10.55 | 5.184 | 0.024* |
| CTS-defined risk PE | 3.701 | 0.157 | ||
| Low-risk patients | 27 (39.7%) | 19 (31.67%) | ||
| Intermediate-low-risk patients | 30 (44.11%) | 36 (60.0%) | ||
| Intermediate-high-risk patients | 11 (16.17%) | 5 (8.33%) | ||
| DVT (cases) | 12 (17.64%) | 25 (41.67%) | 8.949 | 0.003* |
| PH (cases) | 9 (13.23%) | 6 (10.0%) | 0.322 | 0.570 |
| Minor bleeding (cases) | 1 (1.47%) | 2 (3.33%) | 0.483 | 0.487 |
| Hospitalization duration (days) | 11.06 ± 3.636 | 11.12 ± 4.658 | 0.808 | 0.371 |
| 90-day mortality (cases) | 0 | 0 | - | - |
Comparison of laboratory examinations (mean ± SD) (n, %) (Rivaroxaban group).
* P < 0.05.
TNI, troponin I; BNP, B-type natriuretic peptide; PVOI, pulmonary vascular obstruction index; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; DVT, deep vein thrombosis; PASP, pulmonary artery systolic pressure; PH, pulmonary hypertension; sPESI, simplified pulmonary embolism severity index.
3.2 Univariate and multivariate logistic regression
Univariate logistic regression analysis revealed that thrombolysis at 30 days after anticoagulation therapy was associated with malignant tumor (F = 13.212, P < 0.001), deep vein thrombosis (DVT) (F = 15.617, P < 0.001), PAOI (F = 15.659, P < 0.001), and anticoagulant initiation time (F = 10.529, P = 0.001). Variables with statistical significance in univariate analysis were included in multivariate logistic regression. The results indicated that thrombolysis at 30 days after anticoagulation therapy was independently associated with malignant tumors (B = −2.253, Wald = 9.934, OR = 0.105, 95% CI: 0.026–0.427, P = 0.002), anticoagulant initiation time (B = −0.434, Wald = 29.009, OR = 0.648, 95% CI: 0.553–0.759, P < 0.001), DVT (B = −1.465, Wald = 13.339, OR = 0.231, 95% CI: 0.105–0.507, P < 0.001), and PAOI (B = −0.059, Wald = 10.338, OR = 0.942, 95% CI: 0.909–0.977, P = 0.001).
In the rivaroxaban group, univariate logistic regression analyses were conducted to evaluate the associations between clinical variables and thrombolysis at 30 days after anticoagulation therapy in patients with PE. The results indicated that thrombolysis at 30 days after anticoagulation therapy was associated with DVT (F = 8.949, P = 0.003), PAOI (F = 5.184, P = 0.024), TNI (F = 5.921, P = 0.016), PASP (F = 5.369, P = 0.022), anticoagulant initiation time (F = 5.047, P = 0.026), and malignancy (F = 4.206, P = 0.040). Multivariate logistic regression analysis was performed for variables that showed statistically significant associations in the univariate analysis. It revealed that thrombolysis at 30 days after anticoagulation therapy was independently associated with PAOI (B = −0.057, Wald = 5.506, OR = 0.945, 95% CI: 0.901–0.991, P = 0.019), malignancy (B = −1.906, Wald = 4.259, OR = 0.149, 95% CI: 0.024–0.909, P = 0.039), anticoagulant initiation time (B = −0.441, Wald = 16.428, OR = 0.644, 95% CI: 0.520–0.797, P < 0.001), and DVT (B = −1.287, Wald = 6.954, OR = 0.276, 95% CI: 0.106–0.719, P = 0.008).
In the warfarin group, univariate logistic regression analyses were conducted to evaluate the associations between clinical variables and thrombolysis at 30 days after anticoagulation therapy in patients with PE. The results indicated that thrombolysis at 30 days after anticoagulation therapy was associated with malignancy (F = 6.474, P = 0.011), DVT (F = 6.684, P = 0.010), PAOI (F = 9.440, P = 0.003), and anticoagulant initiation time (F = 6.474, P = 0.013). Multivariate logistic regression analysis was performed for variables that showed statistically significant associations in the univariate analysis. It revealed that thrombolysis at 30 days after anticoagulation therapy was independently associated with anticoagulant initiation time (B = −0.427, Wald = 11.346, OR = 0.653, 95% CI: 0.509–0.837, P = 0.001), malignancy (B = −3.780, Wald = 5.183, OR = 0.023, 95% CI: 0.001–0.591, P = 0.023), PAOI (B = −0.074, Wald = 5.237, OR = 0.929, 95% CI: 0.872–0.989, P = 0.022), and DVT (B = −1.662, Wald = 4.991, OR = 0.190, 95% CI: 0.044–0.816, P = 0.025).
3.3 Spearman correlation analysis
A weak negative correlation was observed between anticoagulant initiation time and thrombolysis at 30 days after anticoagulation therapy in patients with PE (Rs = −0.411, P < 0.001). Additionally, a weak negative correlation was found between PAOI and thrombus disappearance at 30 days (Rs = −0.286, P < 0.001).
In the rivaroxaban group, a weak negative correlation was noted between anticoagulant initiation time and thrombolysis at 30 days after anticoagulation therapy (Rs = −0.425, P < 0.001), as well as between PAOI and thrombus disappearance at 30 days (Rs = −0.228, P < 0.001).
In the warfarin group, a weak negative correlation was observed between anticoagulant initiation time and thrombolysis at 30 days after anticoagulation therapy (Rs = −0.390, P < 0.001), and between PAOI and thrombus disappearance at 30 days (Rs = −0.360, P = 0.001).
3.4 ROC curve analysis
ROC curve analysis demonstrated that anticoagulant initiation time predicted thrombolysis at 30 days after anticoagulation therapy (AUC: 0.736, 95% CI: 0.668–0.804, P < 0.001). The Youden index was 0.071, and an anticoagulation initiation time of < 6.5 days yielded the most favorable therapeutic effect (Figure 2).
Figure 2
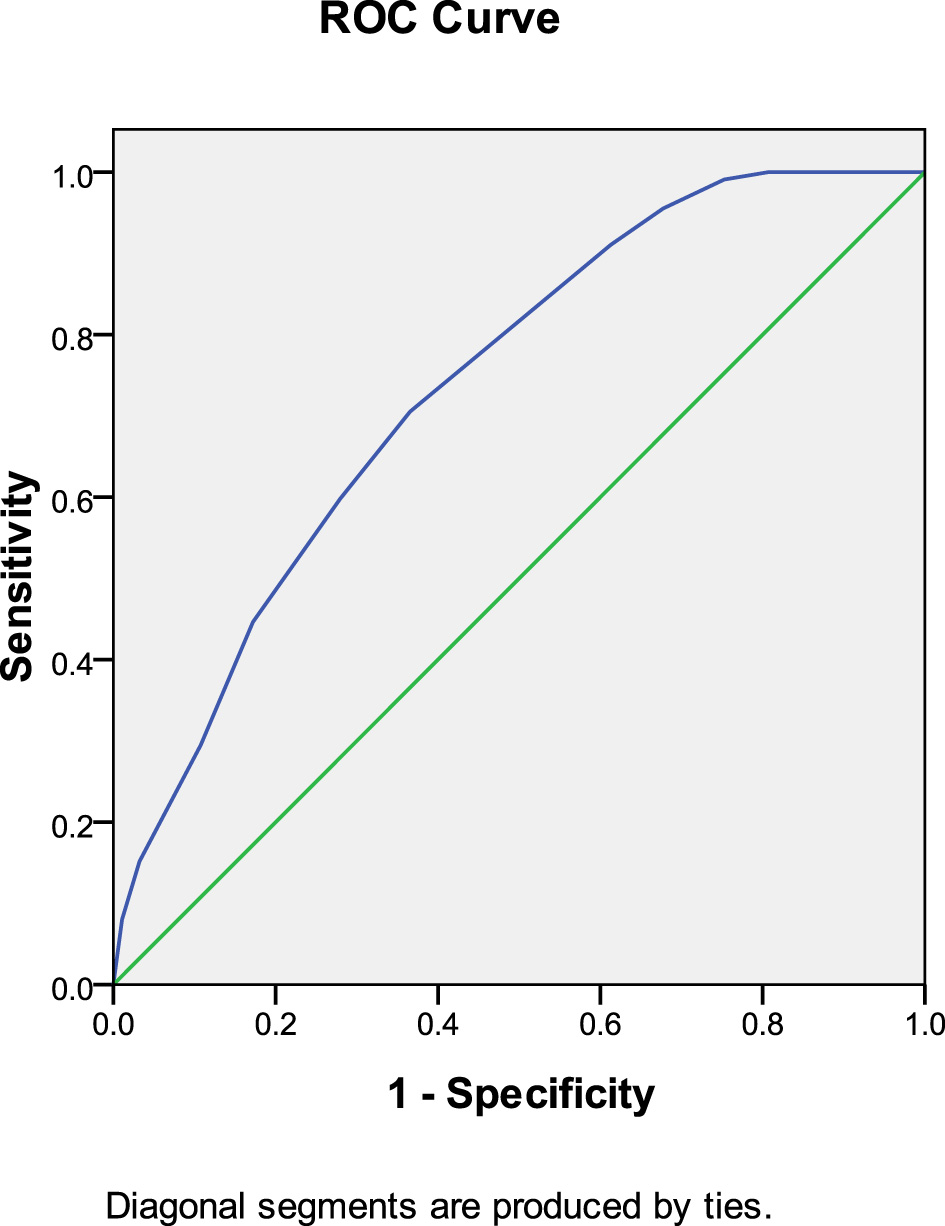
Anticoagulant initiation time predicted thrombolysis at 30 days after anticoagulation therapy.
In the rivaroxaban group, anticoagulant initiation time predicted thrombolysis at 30 days after anticoagulation therapy (AUC: 0.743, 95% CI: 0.657–0.829, P < 0.001). The Youden index was 0.024, and an anticoagulation initiation time of < 5.5 days provided the best therapeutic outcome (Figure 3).
Figure 3
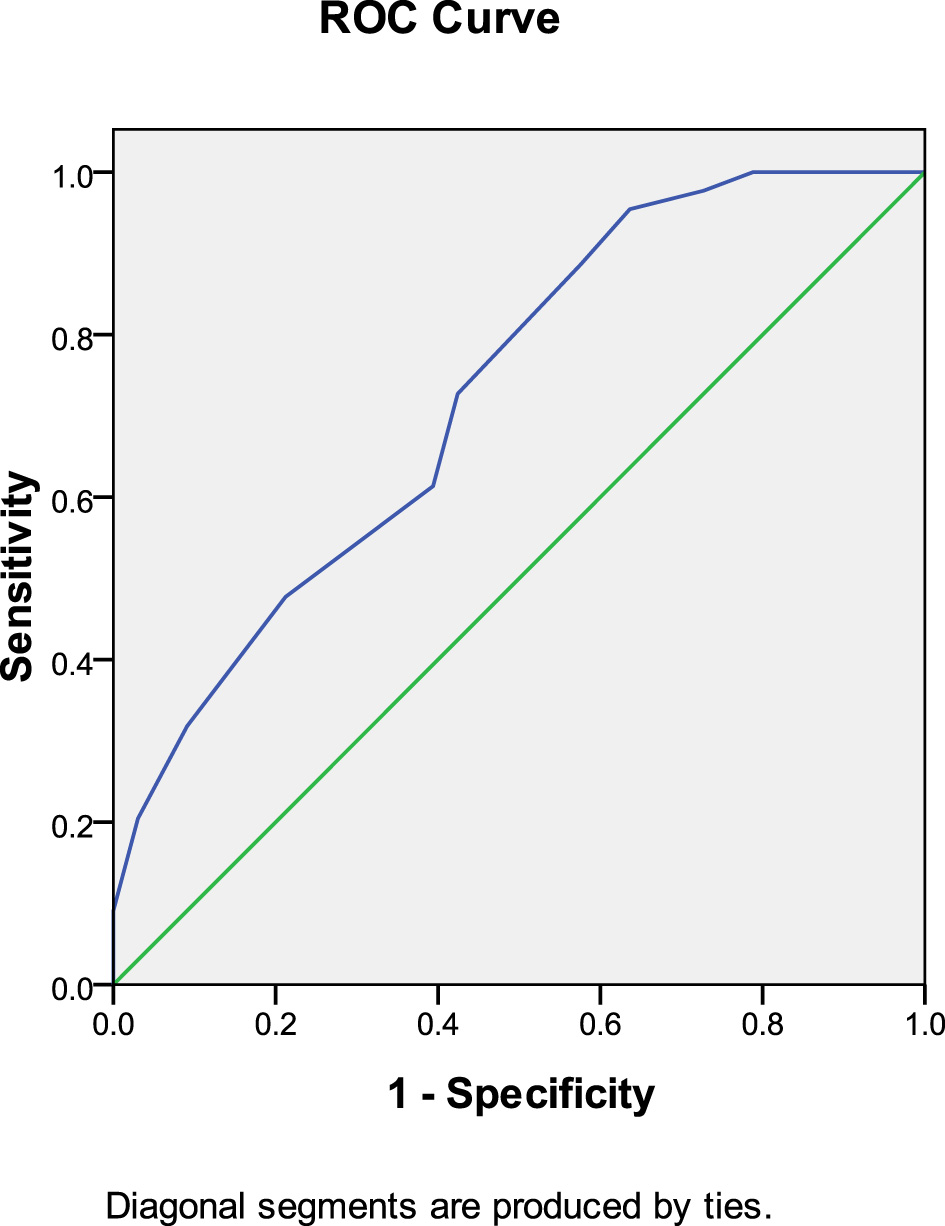
Anticoagulant initiation time predicted thrombolysis at 30 days after anticoagulation therapy (rivaroxaban group).
In the warfarin group, anticoagulant initiation time predicted thrombolysis at 30 days after anticoagulation therapy (AUC: 0.726, 95% CI: 0.612–0.840, P = 0.001). The Youden index was 0.591, and an anticoagulation initiation time of < 8.5 days produced the most favorable therapeutic effect (Figure 4).
Figure 4
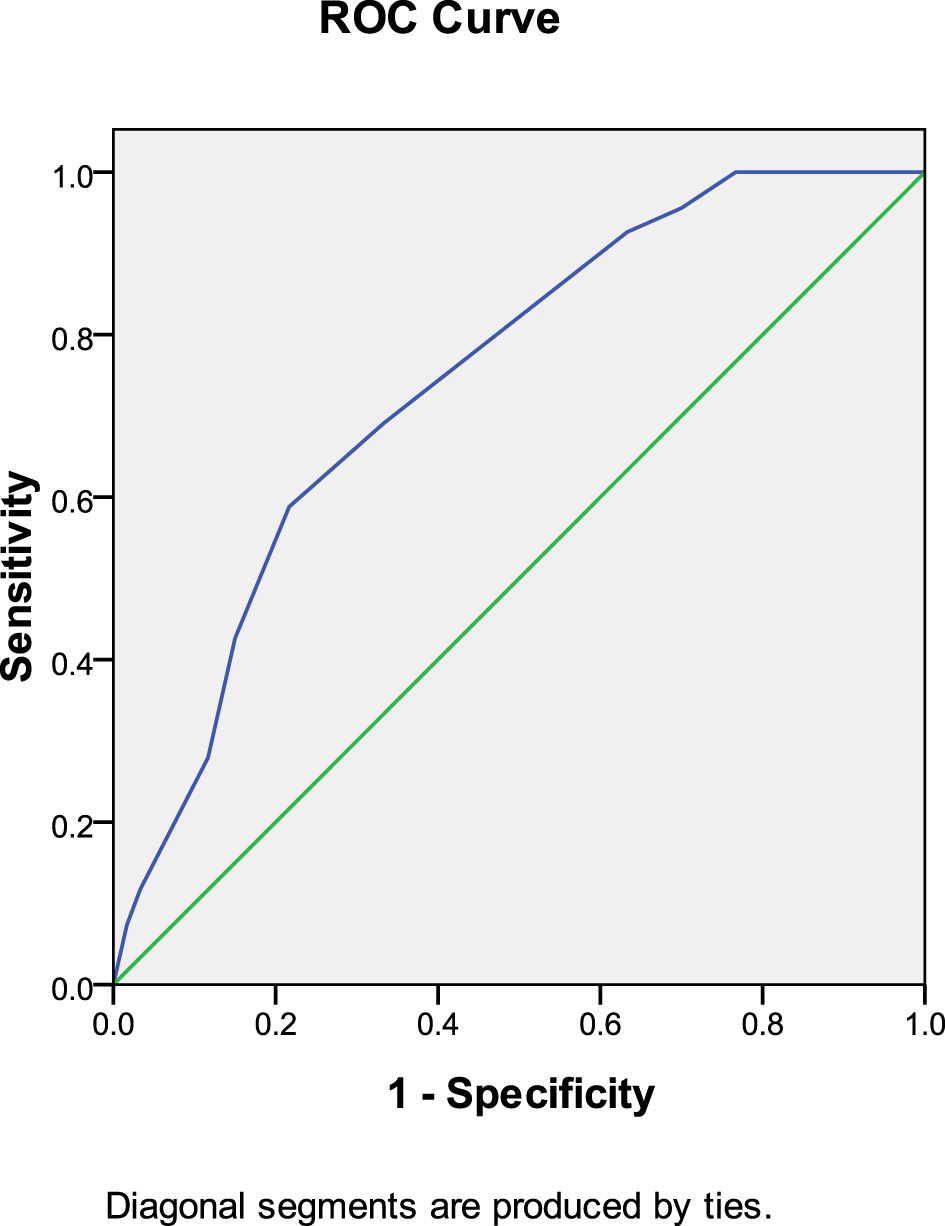
Anticoagulant initiation time predicted thrombolysis at 30 days after anticoagulation therapy (warfarin group).
4 Discussion
The ESC guidelines for the diagnosis and management of PE (2019) strongly recommend anticoagulant therapy for 3–6 months, after which the decision to extend treatment should be based on the degree of thrombolysis and absorption (9). However, in most patients with PE, the thrombus is almost completely absorbed within this period, making it difficult to assess the therapeutic effect in time. Repeated CTPA at 30 days after treatment can provide timely evaluation of thrombus dissolution and absorption. For thrombi that are not completely dissolved, interventional therapy can be implemented promptly to prevent thrombus organization and the development of chronic thrombosis. In patients with completely resolved PE, anticoagulant drugs may be adjusted to prophylactic doses according to transient VTE risk factors. Therefore, CTPA re-examination was performed at 30 days after treatment in this study.
The results showed that the rate of complete thrombus dissolution and absorption was 54.63% at 30 days after anticoagulant therapy, indicating that a high proportion of thrombi were dissolved within this period. However, there was no significant difference between the warfarin and rivaroxaban groups. These results were consistent with previous studies (16–19).
Multiple factors may influence thrombus dissolution and absorption. Jervan et al. (20) found that increased thrombus burden at diagnosis (OR 2.08, 95% CI: 1.06–4.06, P = 0.032) and PTE without clear predisposing factors (OR 2.25, 95% CI: 1.13–4.48, P = 0.021) were associated with an increased risk of residual pulmonary defects. Wang et al. (21) demonstrated that independent factors associated with residual thrombosis included unprovoked PE (OR 0.231, 95% CI: 0.062–0.861) and acute fibrinogen level (OR 1.958, 95% CI: 1.282–2.911). Fang et al. (22) reported that malignancy may affect thrombus absorption. Yu et al. (23) showed that thrombus density ≥61.8 HU and thrombus volume ≥14.0 cm3 were critical thresholds for predicting PE resolution. Kato et al. (24) identified a history of venous thromboembolism or active malignancy as risk factors for residual thrombus. An et al. (25) found that higher PVOI was associated with residual thrombus (P = 0.004) and recurrence (P = 0.03).
In the above studies, the observation time point was mostly 3–6 months after anticoagulant therapy. In contrast, the present study evaluated the thrombolytic effect at 1 month after anticoagulant therapy. Logistic regression analysis demonstrated that anticoagulant initiation time, malignancy, DVT, and PAOI were significantly correlated with pulmonary thrombolysis.
There have been few studies on the correlation between anticoagulant initiation time and thrombolysis, and no comparisons have been made regarding the initiation times of different anticoagulants. Aranda et al. (26) reported that COPD [OR, 3.22 (1.35–7.71); p = 0.009], secondary PE [OR, 2.02 (1.08–3.79); p = 0.028], anticoagulant initiation within 7 days [OR, 2.42 (1.22–4.78); p = 0.011], and a Qanadli score < 16% [OR, 2.12 (1.03–4.37); p = 0.043] were associated with thrombolysis. In another study, Aranda C et al. (27) found that in idiopathic PE, a delay of more than 7 days between symptom onset and diagnosis was associated with residual thrombus. Spearman correlation analysis demonstrated a negative correlation between anticoagulant initiation time and pulmonary thrombolysis (Rs = −0.411, p < 0.001). ROC curve analysis indicated that anticoagulant initiation within 6.5 days after symptom onset was associated with a more favorable thrombolytic effect within 1 month. The average anticoagulation initiation time was 8.5 days for warfarin and 5.5 days for rivaroxaban. The possible reasons for this are as follows: (1) rivaroxaban has been widely adopted in recent years, and with increased clinical awareness of PE, earlier and more rapid diagnoses have been achieved, leading to significantly shorter anticoagulant initiation times and improved treatment efficacy; (2) earlier initiation of anticoagulation allows treatment of relatively fresh thrombi, thereby accelerating thrombus dissolution and improving therapeutic outcomes. Mansueto et al. (28) observed histologically that platelet aggregation occurred within 1 h after thrombus formation by analyzing autopsy specimens of pulmonary thrombosis. Lymphocytes appeared between 1 and 24 h after thrombus formation. Between 48 and 72 h, fibroblasts were observed at the thrombus periphery, and after 72 h, numerous fibroblasts and collagen fibers were present within the thrombus. Therefore, earlier initiation of anticoagulation yields a more favorable therapeutic effect.
In this study, we found that patients in the incomplete thrombolysis group may have been influenced by the following factors: (1) some patients exhibited a larger PAOI, often associated with a substantial thrombus burden in the main pulmonary artery. In addition, some patients presented with DVT, resulting in a greater thrombus load, and consequently, thrombus dissolution and absorption proceeded relatively slowly during the 30 days following anticoagulation; (2) some patients had malignant tumors, and cancer-related thrombus could not be excluded, which may have further hindered thrombus dissolution and absorption. In this cohort, one patient still presented with thrombosis 90 days after anticoagulant therapy, raising the possibility of tumor thrombus; (3) some patients were elderly. In these patients, age-related declines in systemic and vascular function likely impaired thrombus dissolution and absorption.
However, this study has certain limitations. First, its retrospective design at a single center limits the representativeness of the sample. According to PE guidelines, CTPA should be performed at 3–6 months after anticoagulant therapy, whereas in this study, CTPA was performed at 30 days, resulting in a relatively lower detection rate. Second, PAOI was chosen as the index for evaluating thrombus occlusion, which does not precisely quantify thrombus volume. This may have affected the assessment of thrombus dissolution and absorption after anticoagulation therapy. Third, in accordance with PE guidelines, CTPA was used as the primary diagnostic tool in this study. Nevertheless, the sensitivity of CTPA for detecting small distal thrombi is lower than that of radionuclide pulmonary ventilation/perfusion (V/Q) imaging, which may have influenced the results of this study.
5 Conclusions
In summary, anticoagulant initiation time, malignant tumors, DVT, and PAOI were factors influencing thrombus dissolution and absorption. When anticoagulation was initiated within 6.5 days, thrombi were largely absorbed within 1 month. However, when anticoagulant initiation time exceeded 6.5 days, thrombus absorption after anticoagulation was delayed. Therefore, early diagnosis and early initiation of anticoagulation are essential for achieving optimal thrombolytic outcomes.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Shanghai Sixth People's Hospital, Shanghai Jiao Tong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YZ: Investigation, Conceptualization, Validation, Writing – original draft, Supervision. DY: Funding acquisition, Software, Investigation, Writing – review & editing, Validation, Data curation, Supervision. YL: Supervision, Data curation, Validation, Software, Investigation, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received support from the Shanghai Sixth People's Hospital Affiliated to Jiao Tong University School of Medicine Hospital-level project Fund (grant number: ynhg202317).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Bikdeli B Chatterjee S Desai NR Kirtane AJ Desai MM Bracken MB et al . Inferior vena cava filters to prevent pulmonary embolism: systematic review and meta-analysis. J Am Coll Cardiol. (2017) 70:1587–97. doi: 10.1016/j.jacc.2017.07.775
2.
Asmar S Michael G Gallo V Weinberg MD . The Role of IVC Filters in the management of acute pulmonary embolism. J ClinMed. (2024) 13:1494. doi: 10.3390/jcm13051494
3.
Zhen K Tao Y Xia L Wang S Gao Q Wang D et al . Epidemiology of pulmonary embolism in China, 2021: a nationwide hospital-based study. Lancet Reg Health West Pac. (2024) 54:101258. doi: 10.1016/j.lanwpc.2025.101472
4.
Ye W Chen X Li X Guo X Gu W . Oxygenation index and NT-proBNPas predictors of pulmonary hypertension and ventilation perfusion mismatch inacute pulmonary embolism. Front Cardiovasc Med. (2023) 10:1090805. doi: 10.3389/fcvm.2023.1090805
5.
Duffett L Castellucci LA Forgie MA . Pulmonary embolism: update on management and controversies. BMJ. (2020) 370:m2177. doi: 10.1136/bmj.m2177
6.
Roy PM Penaloza A Hugli O Klok FA Arnoux A Elias A et al . Triaging acute pulmonary embolism for home treatment by hestia or simplified PESI criteria: the HOME-PE randomized trial. Eur Heart J. (2021) 42:3146–57. doi: 10.1093/eurheartj/ehab373
7.
Jiménez D Bikdeli B Barrios D Quezada A Del Toro J Vidal G et al . Epidemiology, patterns of care and mortality for patients with hemodynamically unstable acute symptomatic pulmonary embolism. Int J Cardiol. (2018) 269:327–33. doi: 10.1016/j.ijcard.2018.07.059
8.
Piazza G . Advanced management of intermediate- and high-risk pulmonary embolism: JACC focus seminar. J Am Coll Cardiol. (2020) 76:2117–27. doi: 10.1016/j.jacc.2020.05.028
9.
Konstantinides SV Meyer G Becattini C Bueno H Geersing GJ Harjola VP et al . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society. Eur Heart J. (2020) 41:543–603. doi: 10.1093/eurheartj/ehz405
10.
Chinese Chinese Society of Cardiology Chinese Chinese Medical Association; Editorial Board of Chinese Journal of Cardiology . Guidelines for the diagnosis and treatment of acute pulmonary embolism 2025. Chin J Cardiol. (2025) 53:587–619. doi: 10.3760/cma.j.cn112148-20250225-00140
11.
Zhang RS Maqsood MH Sharp ASP Postelnicu R Sethi SS Greco A et al . Efficacy and safety of anticoagulation, catheter-directed thrombolysis, or systemic thrombolysis in acute pulmonary embolism. JACC Cardiovasc Interv. (2023) 16:2644–51. doi: 10.1016/j.jcin.2023.07.042
12.
Bing S Rui C . A comparison of the efficacy and safety between anticoagulation alone and combined with catheter-directed thrombolysis for treatment of pulmonary embolism on outcome: A systematic review and meta-analysis. Perfusion (2023). doi: 10.1177/02676591231211753
13.
Zhang RS Ho AM Elbaum L Greco AA Hall S Postelnicu R et al . Quality and rapidity of anticoagulation in patients with acute pulmonary embolism undergoing mechanical thrombectomy. Am Heart J. (2024) 267:91–4. doi: 10.1016/j.ahj.2023.10.001
14.
Qanadli SD El Hajjam M Vieillard-Baron A Joseph T Mesurolle B Oliva VL et al . New CT indexto quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. Am J Roentgenol. (2001) 176:1415–20. doi: 10.2214/ajr.176.6.1761415
15.
Konstantinides SV Meyer G . The 2019 ESC Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism. Eur Heart J. (2020) 41:543–603. doi: 10.1093/eurheartj/ehz405
16.
Schulman S Kearon C Kakkar AK Mismetti P Schellong S Eriksson H et al . Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. (2009) 361:2342–52. doi: 10.1056/NEJMoa0906598
17.
EINSTEIN–PE Investigators Büller HR Prins MH Lensin AW Decousus H Jacobson BF et al . Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. (2012) 366:1287–97. doi: 10.1056/NEJMoa1113572
18.
Agnelli G Buller HR Cohen A Curto M Gallus AS Johnson M et al . Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. (2013) 369:799–808. doi: 10.1056/NEJMoa1302507
19.
Hokusai-VTE Investigators Büller R Décousus H Grosso MA Mercuri M Middeldorp S et al .Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. (2013) 369:1406–15. doi: 10.1056/NEJMoa1306638
20.
Jervan Ø Dhayyat A Gleditsch J Haukeland-Parker S Tavoly M Klok FA et al . Demographic, clinical, and echocardiographic factors associated with residual perfusion defects beyond six months after pulmonary embolism. Thromb Res. (2023) 229:7–14. doi: 10.1016/j.thromres.2023.06.004
21.
Wang J Xu M Sun N Cheng Z Sui J . Factors associating with the presence of residual thrombosis after 3-month treatment of acute pulmonary embolism. J Thromb. (2018) 45:27–35. doi: 10.1007/s11239-017-1561-6
22.
Fang A Mayorga-Carlin M Han P Cassady S John T LaRocco A et al . Risk factors and treatment interventions associated with incomplete thrombus resolution and pulmonary hypertension after pulmonary embolism. J Vasc Surg Venous Lymphat Disord. (2024) 12:101665. doi: 10.1016/j.jvsv.2023.08.006
23.
Yu MH Jung JH Kim T Kim DK . Thrombus volume and Hounsfield unit density as a predictor of pulmonary embolism in patients with deep vein thrombosis. Acta Radiol. (2023) 64:2198–204. doi: 10.1177/02841851231165921
24.
Kato S Shimada YJ Friedmann P Kashan G Husk G Bergmann SR . Identification of residual risk factors for the development of venous thromboembolism in medical inpatients receiving subcutaneous heparin therapy for prophylaxis. Coron Artery Dis. (2012) 23:294–7. doi: 10.1097/MCA.0b013e328352e510
25.
An J Sun B Ji Y Zhang Z Zhai Z Wang C . D-dimer is a predictor of clot resolution in patients with pulmonary thromboembolism: A retrospective cohort study. Clin Respir J. (2020) 14:549–56. doi: 10.1111/crj.13167
26.
Aranda C Gonzalez P Gagliardi L Peralta L Jimenez A . Prognostic factors of clot resolution on follow-up computed tomography angiography and recurrence after a first acute pulmonary embolism. Clin Respir J. (2021) 15:949–55. doi: 10.1111/crj.13386
27.
Aranda C Peralta L Gagliardi L López A Jiménez Á Herreros B . A significant decrease in D-dimer concentration within one month of anticoagulation therapy as a predictor of both complete recanalization and risk of recurrence after initial pulmonary embolism. Thromb Res. (2021) 202:31–5. doi: 10.1016/j.thromres.2021.02.033
28.
Mansueto G Costa D Capasso E Varavallo F Brunitto G Caserta R et al . The dating of thrombus organization incases of pulmonary embolism: an autopsystudy. BMC Cardiovasc Disord. (2019) 19:250. doi: 10.1186/s12872-019-1219-8
Summary
Keywords
pulmonary embolism, pulmonary artery obstruction index, anticoagulation, D-dimer, thrombolysis
Citation
Zhou Y, Yang D and Liu Y (2025) Correlation between anticoagulant initiation time and thrombolysis at 30 days after anticoagulation therapy in patients with pulmonary embolism. Front. Med. 12:1690899. doi: 10.3389/fmed.2025.1690899
Received
22 August 2025
Accepted
27 October 2025
Published
18 November 2025
Volume
12 - 2025
Edited by
Wei Peng, Chengdu University of Traditional Chinese Medicine, China
Reviewed by
Daniele Mengato, University Hospital of Padua, Italy
Polina Kuznetsova, Research Center of Neurology, Russia
Updates
Copyright
© 2025 Zhou, Yang and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Liu, liuyi830401@126.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.