Abstract
Background:
Chronic atrophic gastritis (CAG) associated with Helicobacter pylori (HP) infection is a common precancerous condition characterized by mucosal atrophy and gastrointestinal symptoms. Existing treatments show limited efficacy in symptom relief and are challenged by rising antibiotic resistance. Qingyoufang decoction (QYFD), a traditional Chinese herbal formula, is recommended for patients with spleen–stomach damp–heat syndrome (SSDHS), a common subtype of HP-positive CAG.
Methods:
This real-world retrospective study enrolled 113 patients with HP-positive CAG and SSDHS from a single hospital between September 2021 and September 2024. Patients received either standard bismuth-containing quadruple therapy (n = 44) or the same regimen plus QYFD (n = 69) for 2 weeks. Clinical efficacy was evaluated through HP eradication rates, SSDHS symptom scores, serum inflammatory markers (interleukin-6 [IL-6], interleukin-6 [IL-8], and tumor necrosis factor-alpha [TNF-α]), and 6-month symptom recurrence. Kaplan–Meier analysis and Cox regression analysis were used to assess prognostic factors.
Results:
The HP eradication rate was significantly higher in the QYFD group than in the control group (92.75% vs. 79.55%, p = 0.038). The overall symptom improvement rate (cure + marked improvement + improvement) was also higher in the QYFD group (92.75%) than in the control group (79.55%, p = 0.033). In an exploratory subgroup analysis (n = 8 control, n = 13 QYFD), inflammatory cytokines (IL-6, IL-8, TNF-α) decreased in both groups, with a greater trend toward reduction in the QYFD group (p < 0.05 for all). The 6-month symptom recurrence rate was lower in the QYFD group (15.87% vs. 37.14%, p = 0.017). The Cox regression analysis identified alcohol consumption (HR = 8.681, 95% CI: 1.070–70.413, p = 0.043) and severe atrophy (HR = 26.536, 95% CI: 3.390–207.735, p = 0.002) as independent risk factors for recurrence, while QYFD treatment was a protective factor (HR = 0.318, 95% CI: 0.107–0.840, p = 0.038).
Conclusion:
QYFD, as an adjunct to standard therapy, was associated with higher HP eradication rates, better symptom improvement, and lower symptom recurrence in patients with HP-positive CAG. A small exploratory subgroup suggested a potential reduction in inflammatory cytokines, which should be interpreted with caution. Further prospective studies are warranted to confirm these findings.
Introduction
Chronic atrophic gastritis (CAG) is a challenging digestive disorder characterized by the atrophy of gastric mucosal glands, often associated with intestinal metaplasia or atypical hyperplasia (1, 2). In China, CAG is relatively prevalent, affecting approximately 20–30% of the population, with a rising incidence among younger individuals (3, 4). Although the pathogenesis of CAG is not fully understood, it is widely believed to be influenced by multiple factors, including genetic predispositions, environmental influences, pathogenic microbial infections, psychological status, and lifestyle choices (5). Among these, Helicobacter pylori (HP) infection is regarded as one of the most significant exogenous pathogenic factors (6). A meta-analysis conducted over the past decade revealed that HP-positive individuals had approximately 2.4-fold higher risk of developing CAG compared with HP-negative individuals (7). Furthermore, both HP infection and CAG are associated with an accelerated progression toward gastric cancer.
Currently, treatment for HP-positive CAG mainly relies on HP eradication therapy, gastric mucosal protectants, and proton-pump inhibitors, supplemented by lifestyle modifications such as dietary adjustment and stress management (8). However, these medications exhibit limited efficacy in these patients and may pose potential risks such as renal impairment, hypomagnesemia, and osteoporosis (9). More critically, the growing global crisis of antibiotic resistance in HP presents a formidable challenge. For instance, clarithromycin resistance has risen from 15.6% in the early 2000s to over 40% in 2020, while metronidazole resistance has increased from 58 to 78% during the same period (10). In response, contemporary clinical guidelines recommend bismuth-containing quadruple therapy as the first-line regimen, replacing the previously favored clarithromycin-based triple therapy (11). In cases of eradication failure, multiple rounds of different antibiotic combinations may be required. Nevertheless, prolonged antibiotic exposure can lead to gastrointestinal adverse effects, including diarrhea, appetite disturbances, nausea, and abdominal pain, as well as decreased drug sensitivity (12). Furthermore, the safety profile of antimicrobial therapy remains a significant concern in vulnerable populations—such as the elderly, children, and pregnant women—rendering such treatment approaches less feasible or inadvisable in these groups (13).
Effective management of patient symptoms is a critical factor in enhancing patient compliance. A recent study indicates that 56.7% of CAG patients experience one or more gastrointestinal symptoms, with dyspepsia and postprandial distress syndrome being the most prevalent, affecting over half of those symptomatic patients. Importantly, HP infection itself can cause gastrointestinal symptoms (14). However, conventional Western medical treatments—such as proton-pump inhibitors, gastric mucosal protectants, and prokinetic agents—have demonstrated limited efficacy in symptom relief. Therefore, there is an urgent need for innovative therapies, particularly non-antibiotic treatments, for HP-positive CAG patients to enhance HP eradication and alleviate associated symptoms.
Traditional Chinese medicine (TCM) has demonstrated significant success in personalizing treatment for CAG patients by considering their constitution, syndromes, and underlying etiology (15). Recently, the 2023 update of the Expert Consensus on Traditional Chinese Medicine Diagnosis and Treatment of Chronic Gastritis indicates that the spleen–stomach damp-heat syndrome (SSDHS) is the most prevalent symptom combination observed in HP-positive CAG patients (Table 1) (16). This syndrome comprises four primary symptoms: epigastric fullness, epigastric pain, heaviness of limbs, and loose stools, along with four secondary symptoms: poor appetite, a bitter taste in the mouth, halitosis, and fatigue. The guidelines advocate for adherence to the TCM principle of clearing heat and eliminating dampness. Qingyoufang, a traditional Chinese medicine decoction known for its heat-clearing and dampness-removing properties, is recommended for these patients (Table 2). Pharmacologically, QYFD combines heat-clearing and anti-inflammatory herbs (e.g., Scutellaria baicalensis and Coptis chinensis), dampness-resolving and motility-regulating herbs (e.g., Magnolia officinalis), and spleen-fortifying and mucosal-protective herbs (e.g., Atractylodes macrocephala and Codonopsis pilosula), forming a coherent adjunct to quadruple therapy for HP-positive CAG. Consequently, this study aimed to analyze the clinical efficacy and safety of Qingyoufang decoction (QYFD) in HP-positive CAG patients.
Table 1
| Symptom | None (0) | Mild | Moderate | Severe | Score |
|---|---|---|---|---|---|
| Epigastric fullness* | No sensation of fullness. | Occasional upper-abdominal fullness after meals, lasting < 1 h. | Noticeable fullness or post-meal bloating, each episode lasting 1–3 h. | Persistent, severe fullness lasting more than 3 h per episode. | |
| Epigastric pain* | No pain. | Occasional pain (dull, distending, or stabbing) resolving within 1 h. | Frequent pain (dull, distending, or stabbing), tolerable, lasting 1–3 h. | Intense abdominal pain (dull/distending/stabbing), intolerable, lasting > 3 h. | |
| Heaviness of limbs* | No sensation of heaviness. | Occasional limb heaviness resolves within 1 h. | Daily heaviness with mild impact on work, resolves in 2–3 h. | Continuous heaviness, significantly impairs work, not relieved by rest. | |
| Loose stools* | Normal stool consistency. | Soft or slightly loose stools, ≤ 3 bowel movements/day. | Loose stools, 4–5 bowel movements/day. | Mushy stools, > 6 bowel movements/day. | |
| Poor appetite# | Normal appetite. | Decreased interest in eating but maintains usual intake. | Little or no appetite; food intake reduced by ~ 1/3. | Anorexia; intake reduced by > 1/2. | |
| Bitter taste in mouth# | No bitter or sticky taste. | Occasional bitter/sticky sensation, no impact on eating. | Frequent bitter/sticky sensation, mildly affects eating. | Persistent bitter/sticky taste, significantly impairs eating. | |
| Halitosis (bad breath)# | No noticeable odor. | Subjective awareness of bad breath. | Bad breath noticeable to others. | Severe, offensively strong halitosis. | |
| Fatigue# | Normal energy level. | Mild fatigue, less talkative but able to work. | Noticeable fatigue, reduced speech and work capacity. | Extreme fatigue, strong desire to lie down; markedly impaired work ability. |
Manifestations and diagnostic criteria of SSDHS.
* = Main symptom, scoring: none = 0; mild = 2; moderate = 4; severe = 6; # = secondary symptom, scoring: none = 0; mild = 2; moderate = 4; severe = 6; diagnostic criteria for spleen–stomach damp-heat syndrome: at least two main symptoms (*) and two secondary symptoms (#) must be present concurrently.
Table 2
| No. | Herb name (English) | Herb name (Pinyin) | Dosage (g) |
|---|---|---|---|
| 1 | Codonopsis Root | Dang Shen | 15 |
| 2 | Lindera Root | Wu Yao | 10 |
| 3 | Bletilla Rhizome | Bai Ji | 6 |
| 4 | Atractylodes Rhizome | Bai Zhu (Fu-chao, bran-fried) | 12 |
| 5 | Poria | Fu Ling | 12 |
| 6 | Licorice Root | Zhi Gan Cao | 5 |
| 7 | Acorus Rhizome | Shi Chang Pu | 12 |
| 8 | Scutellaria Root | Huang Qin | 10 |
| 9 | Coptis Rhizome | Huang Lian | 5 |
| 10 | Magnolia Bark | Hou Po | 10 |
| 11 | Salvia Root | Dan Shen | 15 |
| 12 | Barley Sprout | Mai Ya (Chao, fried) | 15 |
Composition of the QYFD used in this study.
Methods
Study design
This study retrieved clinical data from patients with HP-positive CAG who visited the outpatient clinic or were hospitalized at Quzhou Traditional Chinese Medicine Hospital between September 2021 and September 2024. Patients included in this retrospective real-world study met the following criteria: (1) confirmed HP-positive status through a 13C-urea breath test analyzer or pathological examination; (2) pathological diagnosis of CAG; (3) diagnostic criteria for SSDHS were based on a standardized TCM symptom scoring system (Supplementary Table S2). The syndrome includes four primary symptoms (epigastric fullness, epigastric pain, heaviness of limbs, and loose stools) and four secondary symptoms (poor appetite, bitter taste in the mouth, halitosis, and fatigue). A diagnosis of SSDHS was confirmed when at least two primary symptoms and two secondary symptoms were present concurrently; (4) aged between 20 and 75 years; and (5) no use of acid-suppressing drugs or antibiotics within 28 days prior to enrollment. The exclusion criteria were as follows: (1) presence of other gastrointestinal diseases such as reflux esophagitis or gastrointestinal malignancies; (2) previous history of upper gastrointestinal surgery; (3) serious medical conditions such as acute liver or kidney failure or acute myocardial infarction; (4) known allergies to any drugs or antibiotics used in this study; and (5) patients who are preparing for pregnancy or are pregnant during the study period. This study received approval from the Ethics Committee of Quzhou Traditional Chinese Medicine Hospital, and all investigations were conducted in accordance with the Declaration of Helsinki (revised in 2013).
Treatment
Patients in the Western medicine group (control group) were treated for 2 weeks with a bismuth-containing quadruple regimen comprising esomeprazole enteric-coated tablets (0.02 g per dose), colloidal pectin–bismuth capsules (0.2 g per dose), amoxicillin capsules (1.0 g per dose), and furazolidone tablets (0.1 g per dose), each administered twice daily 30 min after breakfast and dinner.
Patients in the combined Chinese–Western medicine group (experimental group) received the identical quadruple regimen supplemented with QYFD—a standardized traditional Chinese medicine decoction (see Table 2 for formula details). The decoction was dispensed under sterile conditions in 200-mL bags by the Decoction Room of Quzhou Traditional Chinese Medicine Hospital and administered warm 30 min after breakfast for the same 2-week period.
Data collection and outcomes evaluations
-
General information: The patient’s gender, age, course of disease, endoscopic pathological results (atrophy and intestinal metaplasia), living habits, and common diseases were collected.
-
Modern medicine observation indicators: HP eradication rate: The 13C-urea breath test was repeated 4 weeks after drug withdrawal, and a delta over baseline (DOB) value < 4 showed that HP was considered eradicated. Inflammation level: 5 mL of fasting venous blood was collected from each patient before and after the treatment, and the levels of IL-6, IL-8, and TNF-α in serum were detected using enzyme-linked immunosorbent assay (ELISA) kits.
-
Traditional Chinese medicine observation indicators: The symptoms of CAG in patients were quantitatively scored before and after the treatment, following the Guiding Principles for Clinical Research of New Chinese Medicines. As shown in Table 1, the main and secondary symptoms of SSDHS were graded and scored using an integral method. Symptoms were categorized into four levels: none, mild, moderate, and severe, with main symptoms scored as 0, 2, 4, and 6 points and secondary symptoms scored as 0, 1, 2, and 3 points. Recovery was defined as a decrease of ≥ 95% in the total score after the treatment; marked improvement was defined as a decrease of 70–95%; effectiveness was defined as a decrease of 30–70%; and ineffectiveness was defined as a decrease of less than 30%.
-
Follow-up within 6 months: After the treatment concluded, a 6-month follow-up was conducted through a review of the hospitalization and outpatient follow-up records of the two groups of patients who exhibited improved symptoms (including recovery, marked improvement, and overall effectiveness). The recurrence or aggravation of clinical symptoms, indicated by an increase in the total score of traditional Chinese medicine (TCM) syndromes compared to the post-treatment assessment, was recorded as a positive endpoint event. Conversely, if no endpoint event occurred within the 6 months, it was classified as a cutoff event.
Statistical analysis
The normality of continuous variables was tested using the Shapiro–Wilk test. Normally distributed data were analyzed using the independent-samples t-test, whereas non-normally distributed data were analyzed using the Mann–Whitney U-test. Continuous variables were summarized as mean ± SD or median (P25, P75). Between-group differences were tested using independent t-tests (Welch’s if variances were unequal) or Mann–Whitney U-tests; within-group (pre–post) differences were tested using paired t-tests or Wilcoxon signed-rank. Categorical data were shown as percentages and analyzed using chi-squared, continuity-corrected chi-squared, or Fisher’s exact test. Ordinal data were assessed using the rank-sum test. Kaplan–Meier curves with log-rank tests were used for recurrence analysis. Cox regression analysis was performed to identify associated risk factors, with p < 0.05 considered statistically significant. The proportional hazards assumption was tested using Schoenfeld residuals. All statistical analyses were conducted using SPSS version 26.0 (IBM Corp., USA). A two-tailed test was applied for all analyses, with a significance level of α = 0.05.
Results
Patient characteristics and outcomes
This study retrospectively analyzed 387 patients diagnosed with HP-positive CAG who were admitted to Quzhou Hospital of Traditional Chinese Medicine between September 2021 and September 2024. A total of 113 patients met the inclusion criteria, with 44 receiving conventional quadruple therapy and 69 receiving a combination of QYFD and quadruple therapy. Figure 1 illustrates the detailed patient enrollment process. No statistically significant differences were observed between the two groups regarding clinical characteristics, including age, gender, disease duration, gastric mucosal atrophy, and intestinal metaplasia. Specific patient characteristics are summarized in Table 3.
Figure 1

Study flow diagram.
Table 3
| Groups | Control group (n = 44) | Experimental group (n = 69) | X2/t | p |
|---|---|---|---|---|
| Gender, n (%) | 0.183 | 0.669 | ||
| Male | 25 (56.81) | 42 (60.87) | ||
| Female | 19 (43.19) | 27 (39.13) | ||
| Age (years, mean ± SD) | 59.54 ± 6.82 | 60.33 ± 5.52 | 0.645 | 0.521 |
| Duration of illness (years, mean ± SD) | 3.86 ± 1.48 | 4.07 ± 1.40 | 0.751 | 0.455 |
| Gastric atrophy, n (%) | 0.252 | 0.882 | ||
| Mild | 12 (27.28) | 20 (28.99) | ||
| Moderate | 22 (50.00) | 36 (52.17) | ||
| Severe | 10 (22.72) | 13 (18.84) | ||
| Intestinal metaplasia, n (%) | 2.834 | 0.418 | ||
| None | 22 (50.00) | 35 (50.72) | ||
| Mild | 11 (25.00) | 18 (26.09) | ||
| Moderate | 10 (22.72) | 10 (14.50) | ||
| Severe | 1 (2.28) | 6 (8.69) | ||
| Smoking, n (%) | 22 (50.00) | 36 (52.17) | 0.051 | 0.822 |
| Alcohol consumption, n (%) | 20 (45.45) | 38 (55.07) | 0.995 | 0.319 |
| Hypertension, n (%) | 9 (20.45) | 12 (17.39) | 0.167 | 0.683 |
| Diabetes mellitus, n (%) | 4 (9.09) | 8 (11.59) | 0.012 | 0.914 |
Baseline demographic and clinical characteristics of study participants.
HP eradication rates and TCM therapeutic efficacy
Four weeks after treatment withdrawal, HP status was reassessed using the 13C-urea breath test. The HP eradication rate was 92.75% (64/69) in the experimental group and 79.55% (35/44) in the control group, with a statistically significant difference between the two groups (p = 0.038) (Table 4).
Table 4
| Groups | Control group (n = 44) | Experimental group (n = 69) | X2/Z | p |
|---|---|---|---|---|
| HP eradication rates, n (%) | 4.318 | 0.038 | ||
| Negative | 35 (79.55) | 64 (92.75) | ||
| Positive | 9 (20.45) | 5 (7.25) | ||
| TCM therapeutic efficacy, n (%) | 2.137 | 0.033 | ||
| Ineffectiveness | 9 (20.45) | 5 (7.25) | ||
| Effectiveness | 17 (38.64) | 20 (28.99) | ||
| Markedly effective | 10 (22.73) | 28 (40.58) | ||
| Recovery | 8 (18.18) | 16 (23.18) |
Comparison of HP eradication rates and TCM therapeutic efficacy between the two groups.
SSDHS-related symptom scores before and after the treatment are presented in Supplementary Table S1. Both groups showed reductions in symptom scores following treatment across all listed domains. Intra-group comparisons indicated improvements in each symptom, while between-group comparisons of post-treatment values revealed significantly lower scores in the experimental group for epigastric fullness, epigastric pain, heaviness of limbs, and fatigue.
The distribution of TCM therapeutic efficacy outcomes is summarized in Table 4. In the experimental group, 23.18% (16/69) of patients achieved recovery, 40.58% (28/69) were markedly improved, 28.99% (20/69) were effective, and 7.25% (5/69) were ineffective. In contrast, the corresponding figures in the control group were 18.18% (8/44), 22.73% (10/44), 38.64% (17/44), and 20.45% (9/44), respectively. The difference in efficacy distribution between the groups was statistically significant (p = 0.033).
Levels of inflammatory factors
Serum IL-6, IL-8, and TNF-α levels were measured in 8 patients from the control group and 13 patients from the experimental group before and after the treatment (Table 5). Baseline levels of IL-6 (22.97 ± 3.01 vs. 23.74 ± 1.99 pg/mL), IL-8 (49.06 ± 4.72 vs. 50.30 ± 5.01 pg/mL), and TNF-α (35.35 ± 3.43 vs. 33.98 ± 3.22 pg/mL) showed no significant differences between the groups (p > 0.05). After the treatment, IL-6 levels decreased to 19.50 ± 2.23 pg/mL in the control group and 14.36 ± 2.32 pg/mL in the experimental group (t = 6.748, p < 0.001). IL-8 levels declined to 43.78 ± 3.65 and 38.27 ± 3.43 pg/mL, respectively (t = 5.189, p < 0.001). TNF-α levels were reduced to 29.10 ± 3.04 and 22.82 ± 2.92 pg./mL, respectively (t = 5.025, p < 0.001).
Table 5
| Groups | Control group (n = 8) | Experimental group (n = 13) | t | p |
|---|---|---|---|---|
| IL-6 | ||||
| Before treatment | 22.97 ± 3.01 | 23.74 ± 1.99 | 0.643 | 0.533 |
| After treatment | 19.50 ± 2.23 | 14.36 ± 2.32 | 5.054 | <0.001 |
| IL-8 | ||||
| Before treatment | 49.06 ± 4.72 | 50.30 ± 5.01 | 0.571 | 0.575 |
| After treatment | 43.78 ± 3.65 | 38.27 ± 3.43 | 3.435 | 0.004 |
| TNF-α | ||||
| Before treatment | 35.35 ± 3.43 | 33.98 ± 3.22 | 0.910 | 0.388 |
| After treatment | 29.10 ± 3.04 | 22.82 ± 2.92 | 4.664 | <0.001 |
Comparison of serum inflammatory factor levels (x ± s, pg/mL) before and after treatment.
SSDHS-related symptom recurrence
Recurrence analysis was performed only among patients who achieved recovery, marked improvement, or improvement after the treatment (35 in the control group and 63 in the QYFD group). Patients with ineffective responses were not eligible for recurrence assessment, as recurrence can only occur after an initial response.
As shown in Table 6, recurrence occurred in 13 patients (37.14%) in the control group and 10 patients (15.87%) in the experimental group. The difference in recurrence rates between the groups was statistically significant (p = 0.017).
Table 6
| Groups | Recurrence (n, %) | No recurrence (n, %) | X2 | p |
|---|---|---|---|---|
| Control group (n = 35) | 13 (37.14) | 22 (62.86) | 5.667 | 0.017 |
| Experimental group (n = 63) | 10 (15.87) | 53 (84.13) |
Comparison of 6-month symptom recurrence between the two groups.
Kaplan–Meier survival analysis revealed a lower cumulative recurrence rate in the experimental group compared to the control group over the 6-month follow-up period. As shown in Figure 2, the difference between the two curves was significant based on the log-rank test (p = 0.0148).
Figure 2
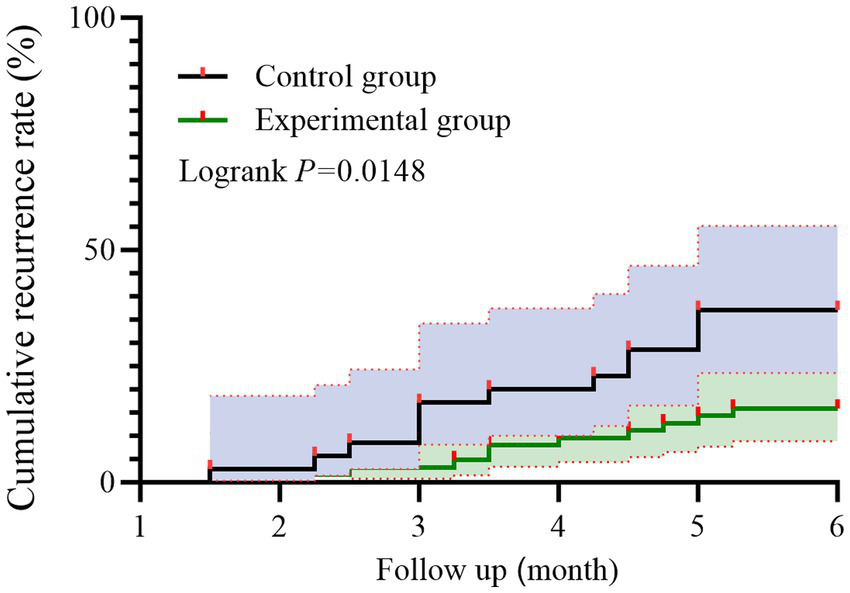
Kaplan–Meier curves of symptom recurrence in patients with HP-positive CAG. Red markers denote recurrence events; Individuals without recurrence by 6 months were censored at 6 months.
Prognostic factors for SSDHS-related symptoms
As shown in Figure 3, univariate Cox regression analysis identified that alcohol consumption (yes vs. no; HR = 19.897, 95% CI: 2.614–151.466, p = 0.004), severe atrophy (yes vs. no; HR = 26.524, 95% CI: 7.370–95.455, p < 0.001), and intestinal metaplasia (yes vs. no; HR = 5.550, 95% CI: 1.564–19.691, p = 0.008) were potential risk factors for the recurrence of SSDHS-related symptoms. In addition, the use of QYFD (yes vs. no) was associated with a reduced risk of recurrence (HR = 0.364, 95% CI: 0.129–1.023, p = 0.055).
Figure 3

Univariate analysis of prognostic factors for symptom recurrence.
As presented in Figure 4, variables with p < 0.1 in the univariate analysis were subsequently included in the multivariate Cox regression model. The results showed that alcohol consumption (HR = 8.681, 95% CI: 1.070–70.413, p = 0.043) and severe atrophy (HR = 26.536, 95% CI: 3.390–207.735, p = 0.002) remained independent risk factors. Meanwhile, treatment with QYFD was independently associated with a significantly lower risk of recurrence (HR = 0.318, 95% CI: 0.107–0.840, p = 0.038). The proportional hazards assumption was satisfied for all models.
Figure 4
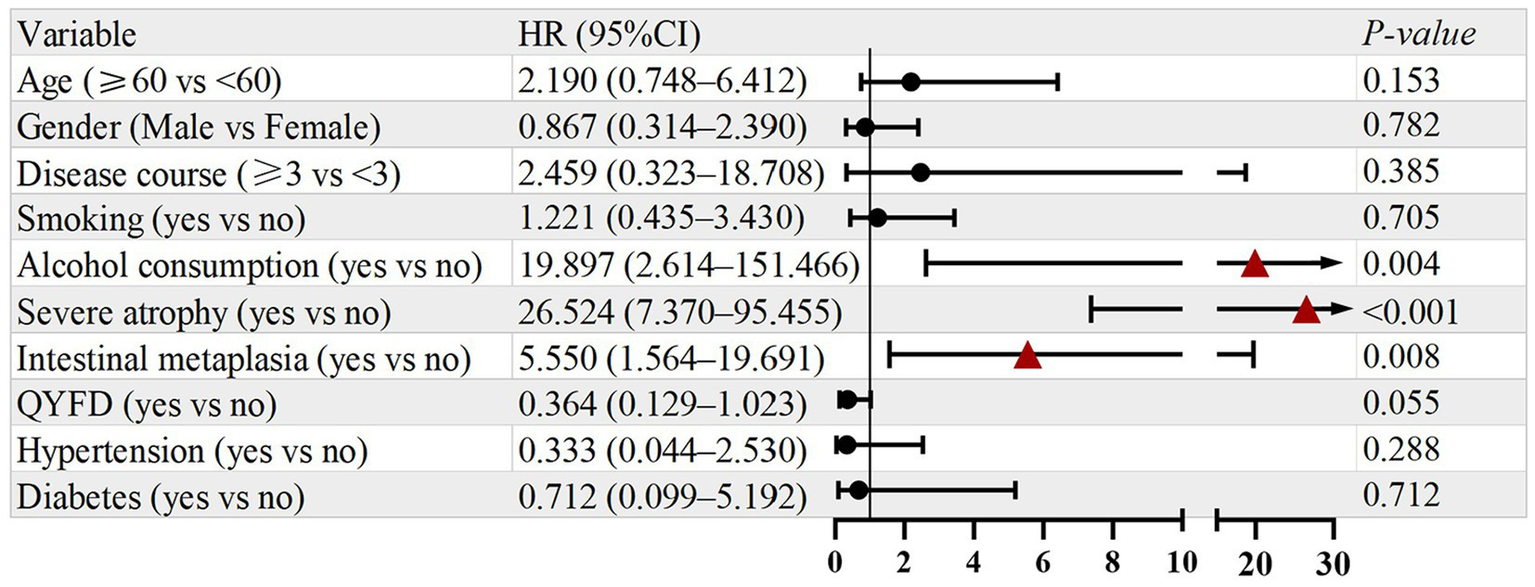
Multivariate analysis of prognostic factors for symptom recurrence.
Adverse events
During the treatment period, one patient in the control group experienced dizziness, and two patients experienced nausea, resulting in a total adverse event rate of 6.8%. In contrast, two patients in the experimental group experienced nausea, leading to a total adverse event rate of 2.9%. Post-treatment evaluations revealed no abnormalities in the blood, urine, stool, routine, or liver and kidney function tests for all patients, indicating that QYFD has a favorable safety profile.
Discussion
In this real-world retrospective study, we focused on HP-positive CAG, a patient subgroup characterized by a high prevalence and severity of gastrointestinal symptoms. Our analyses indicated that adding QYFD to standard quadruple therapy was associated with greater symptom improvement, higher HP eradication rates, and a reduced risk of symptom recurrence. An exploratory subgroup with a small sample size suggested a potential trend toward lower levels of inflammatory markers, but this observation should be interpreted with caution. These findings underscore the therapeutic value of syndrome-specific Chinese herbal interventions in managing HP-CAG, providing real-world evidence to support the integration of traditional medicine into modern clinical practice for digestive diseases.
TCM has been increasingly adopted as a non-antibiotic adjunctive approach for managing CAG and HP infection. Previous studies have demonstrated that several classical herbal formulas—including Banxia Xiexin decoction (17, 18), Moluo Dan (19), Weierkang pills (20), and Liujunzi decoction (21)—can enhance HP eradication, slow the progression of gastric mucosal atrophy, and effectively alleviate clinical symptoms. According to modern TCM theory, CAG is mainly characterized by spleen–stomach deficiency, where a hypoacidic gastric environment facilitates HP colonization and persistence. To address this pathological condition, QYFD follows the therapeutic principle of strengthening the spleen and clearing internal heat. Comprising multiple medicinal herbs, it exerts synergistic, multi-target, and multi-pathway actions that help restore physiological balance and enhance host defense. Polysaccharides from Codonopsis pilosula have been shown to alleviate spleen deficiency-related symptoms by modulating pro-inflammatory cytokines and maintaining gut microbiota homeostasis (22). In addition, active compounds such as flavonoids, saponins, alkaloids, and steroids present in Codonopsis exhibit immunomodulatory, antimicrobial, and antitumor properties (23, 24). Coptis chinensis has shown notable efficacy against HP, with berberine identified as its principal bioactive constituent. Berberine is believed to inhibit bacterial metabolism by blocking glucose oxidation and related intermediates and also to exert anti-inflammatory effects via the Toll-like receptor 4/nuclear factor kappa B (NF-κB) signaling pathway (25, 26). Additionally, berberine and Codonopsis polysaccharides have been reported to downregulate gastric mucosal pro-inflammatory cytokines (IL-6, IL-8, TNF-α), indicating immuno-inflammatory modulation (27, 28). Poria polysaccharides inhibit JAK2 and STAT3 phosphorylation, thereby improving intestinal barrier integrity and mitigating inflammation (29). Moreover, they promote the secretion of immune-stimulating mediators while suppressing immunosuppressive factors, ultimately enhancing host immune responses (30). Extracts of Acorus calamus rhizome and its bioactive components have been reported to exert antibacterial effects by modulating cell membrane permeability and lipid composition (31). These findings suggest that QYFD may contribute to restoring spleen–stomach function and immune homeostasis, reducing gastric mucosal injury, suppressing the excessive release of inflammatory mediators, and interfering with HP colonization—thereby potentially improving disease outcomes and supporting long-term management of CAG.
In China, bismuth-containing quadruple regimens based on furazolidone and amoxicillin are widely used in clinical practice due to their relatively low resistance rates and cost-effectiveness. Zhang et al. conducted a retrospective analysis of 992 patients with HP infection treated at the Second Affiliated Hospital of Zhejiang University in 2015, reporting that this regimen achieved an eradication rate of nearly 95%, with manageable side effects (32). However, subsequent data from the same hospital between 2017 and 2020, involving 16,784 patients, revealed a notable decline in efficacy, with the eradication rate decreasing to 87.6% (14,707 cases) (33). More recently, a real-world study published in 2024 further reported a reduced eradication rate of approximately 84.4% with the same regimen. These findings suggest a downward trend in the efficacy of this standard antibiotic-based therapy. In our study, the eradication rate in the control group, which received the same regimen, was only 79.55%. This may be attributed to increased antibiotic resistance and atrophy of gastric mucosal glands in the study population (34, 35). Notably, adding QYFD to the standard regimen significantly improved the eradication rate to 92.75% while maintaining good safety and tolerability. Against the backdrop of escalating antibiotic resistance, non-antibiotic adjunctive therapies such as QYFD and other traditional Chinese medicine-based approaches may offer promising clinical potential for enhancing HP eradication outcomes.
The eradication of HP significantly impacts symptom relief. A recent meta-analysis of 29 randomized controlled trials involving 6,781 HP-positive patients with functional dyspepsia demonstrated that successful HP eradication was significantly associated with symptom improvement compared to failed eradication (RR = 0.65, 95% CI: 0.52–0.82) (36). Consistently, in our study, patients who achieved HP clearance experienced varying degrees of symptom alleviation, while those who remained HP-positive after treatment reported persistent symptoms. However, HP-positive CAG presents a more complex clinical picture. In addition to HP-induced discomfort, symptoms may arise from the underlying mucosal atrophy and impaired gastric barrier and motility. Previous studies have shown that approximately 24% of CAG patients experience heartburn, 12% report reflux, and other common symptoms include postprandial fullness (7.1%) and early satiety (10.1%) (37). Currently, symptom management in CAG primarily relies on proton-pump inhibitors (PPIs) and mucosal protective agents. Nevertheless, their efficacy is limited in patients with recurrent symptoms. More importantly, prolonged PPI use has been associated with adverse outcomes such as gastric cancer, neuroendocrine tumors, chronic kidney disease, and heart failure and may even exacerbate mucosal atrophy (34, 38, 39). In this context, our results showed that among patients with successful HP eradication, those receiving QYFD treatment achieved significantly better symptom control than controls, suggesting its potential advantage in symptom relief. While existing predictive models have largely focused on assessing the risk of intestinal metaplasia, dysplasia, or psychological comorbidities in CAG, few have addressed symptom recurrence or persistence. Interestingly, a prior Mendelian randomization study has established a link between functional dyspepsia and alcohol consumption (40). Furthermore, the severity of mucosal atrophy may influence the efficacy of prokinetic agents, such as acotiamide, suggesting a physiological basis for the association between gastric mucosal status and symptom severity (41). For instance, both antral and corporal atrophies have been linked to delayed gastric emptying and impaired motility, which may contribute to symptoms such as epigastric fullness and discomfort. These findings partially support our observation that alcohol intake and severe mucosal atrophy were associated with symptom recurrence in this cohort.
This study has several limitations. First, due to the limited sample size and single-center retrospective design, the results may be subject to bias, and the relatively small number of outcome events may have reduced the precision of estimates (as reflected by wide confidence intervals in the Cox regression) and increased the risk of model overfitting, although the proportional hazards assumption was satisfied. Moreover, as a real-world retrospective study, treatment adherence could not be strictly controlled, and a small number of patients were lost to follow-up during the 6-month observation period. Second, serum inflammatory markers were not available for all patients, and HP antibiotic susceptibility was not assessed, which may have introduced additional bias. Additionally, some studies suggest that probiotics may enhance eradication when added to quadruple therapy, but probiotics were not routinely used at our center and were not evaluated in this study. The complexity of symptom recurrence renders the results susceptible to confounding factors such as immune status, HP subtypes, antibiotic sensitivity, lifestyle, and psychological stress, and the lack of a unified quantitative standard for Traditional Chinese Medicine symptom assessment may affect diagnostic consistency. Moreover, because the assessors were aware of treatment allocation, unconscious inclination toward the experimental group could not be entirely ruled out. Furthermore, the 6-month follow-up duration may be insufficient to fully capture long-term recurrence in CAG, and longer follow-up (12–24 months) would be warranted to assess sustained outcomes more comprehensively. Future research should aim to expand the sample size and adopt multicenter and prospective designs to validate these findings.
Conclusion
This real-world retrospective study examined patients with HP-positive CAG, a population at increased risk for gastrointestinal symptoms and persistent HP infection. Our analyses showed that adjunctive treatment with the traditional Chinese herbal decoction Qingyoufang (QYFD) was associated with better symptom control, higher HP eradication rates, and a lower rate of symptom recurrence compared with standard therapy alone. An exploratory analysis based on a small subgroup suggested that QYFD might be associated with reductions in pro-inflammatory cytokines (IL-6, IL-8, and TNF-α), indicating a potential but preliminary trend toward modulation of gastric mucosal inflammation. These findings suggest that QYFD may represent a promising non-antibiotic complementary approach for managing HP-positive CAG, particularly in patients with spleen–stomach damp–heat syndrome, although further prospective validation is needed.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethical Committee of Quzhou Hospital of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JW: Conceptualization, Writing – original draft. GC: Methodology, Software, Writing – original draft. DZ: Supervision, Writing – original draft. CM: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Acknowledgments
The authors would like to thank the patients for their participation and agreement to publication of the study.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1701915/full#supplementary-material
Abbreviations
CAG, Chronic atrophic gastritis; HP, Helicobacter pylori; QYFD, Qingyoufang decoction; SSDHS, Spleen–stomach damp–heat syndrome; TCM, Traditional Chinese medicine; DOB, Delta over baseline; ELISA, Enzyme-Linked Immunosorbent Assay.
References
1.
Raza M Bhatt H . Atrophic gastritisStatPearls Publishing Copyright © 2025, California: StatPearls Publishing LLC (2025).
2.
Shah SC Piazuelo MB Kuipers EJ Li D . AGA clinical practice update on the diagnosis and Management of Atrophic Gastritis: expert review. Gastroenterology. (2021) 161:e7:1325–32. doi: 10.1053/j.gastro.2021.06.078,
3.
Zhang Z Zhang X . Chronic atrophic gastritis in different ages in South China: a 10-year retrospective analysis. BMC Gastroenterol. (2023) 23:37. doi: 10.1186/s12876-023-02662-1,
4.
Du Y Bai Y Xie P Fang J Wang X Hou X et al . Chronic gastritis in China: a national multi-center survey. BMC Gastroenterol. (2014) 14:21. doi: 10.1186/1471-230X-14-21,
5.
Douda L Cyrany J Tachecí I . Early gastric cancer. Vnitrni lekarstvi. (2022) 68:371–5. doi: 10.36290/vnl.2022.077,
6.
Lim NR Chung WC . Helicobacter pylori-associated chronic atrophic gastritis and progression of gastric carcinogenesis. Korean J Gastroenterol. (2023) 82:171–9. doi: 10.4166/kjg.2023.097,
7.
Yin Y Liang H Wei N Zheng Z . Prevalence of chronic atrophic gastritis worldwide from 2010 to 2020: an updated systematic review and meta-analysis. Annals Palliative Med. (2022) 11:3697–703. doi: 10.21037/apm-21-1464,
8.
Conti CB Agnesi S Scaravaglio M Masseria P Dinelli ME Oldani M et al . Early gastric Cancer: update on prevention, diagnosis and treatment. Int J Environ Res Public Health. (2023) 20. doi: 10.3390/ijerph20032149,
9.
Zhang Y Yang Q Song B Tang W Yu F Chen H et al . Efficacy and safety of Piwei Peiyuan prescription in the treatment of chronic atrophic gastritis: a multicenter, double-blind, double-simulated, randomized, controlled clinical trial. Medicine. (2024) 103:e37981. doi: 10.1097/MD.0000000000037981,
10.
Garvey E Rhead J Suffian S Whiley D Mahmood F Bakshi N et al . High incidence of antibiotic resistance amongst isolates of Helicobacter pylori collected in Nottingham, UK, between 2001 and 2018. J Med Microbiol. (2023) 72. doi: 10.1099/jmm.0.001776,
11.
Bujanda L Nyssen OP Vaira D Saracino IM Fiorini G Lerang F et al . Antibiotic resistance prevalence and trends in patients infected with Helicobacter pylori in the period 2013-2020: results of the European registry on H. pylori management (Hp-EuReg). Antibiotics (Basel). (2021) 10. doi: 10.3390/antibiotics10091058,
12.
Poonyam P Chotivitayatarakorn P Vilaichone RK . High effective of 14-day high-dose PPI- bismuth-containing quadruple therapy with probiotics supplement for Helicobacter Pylori eradication: a double blinded-randomized placebo-controlled study. Asian Pac J Cancer Prev. (2019) 20:2859–64. doi: 10.31557/APJCP.2019.20.9.2859,
13.
Peng C Hu Y Ge ZM Zou QM Lyu NH . Diagnosis and treatment of Helicobacter pylori infections in children and elderly populations. Chronic Dis Transl Med. (2019) 5:243–51. doi: 10.1016/j.cdtm.2019.12.003,
14.
Mărginean CD Mărginean CO Meliț LE . Helicobacter pylori-related extraintestinal manifestations-myth or reality. Children (Basel). (2022) 9. doi: 10.3390/children9091352,
15.
Chen L Wei S He Y Wang X He T Zhang A et al . Treatment of chronic gastritis with traditional Chinese medicine: pharmacological activities and mechanisms. Pharmaceuticals (Basel, Switzerland). (2023) 16. doi: 10.3390/ph16091308,
16.
Wang Ping KL Yang Q Tang X . Expert consensus on traditional Chinese medicine diagnosis and treatment of chronic gastritis (2023). Chin J Tradit Chin Med. (2023) 38:5904–11.
17.
Cao Y Zheng Y Niu J Zhu C Yang D Rong F et al . Efficacy of Banxia Xiexin decoction for chronic atrophic gastritis: a systematic review and meta-analysis. PLoS One. (2020) 15:e0241202. doi: 10.1371/journal.pone.0241202,
18.
Li XH Xu JY Wang X Liao LJ Huang L Huang YQ et al . BanXiaXieXin decoction treating gastritis mice with drug-resistant helicobacter pylori and its mechanism. World J Gastroenterol. (2023) 29:2818–35. doi: 10.3748/wjg.v29.i18.2818,
19.
Tang XD Zhou LY Zhang ST Xu YQ Cui QC Li L et al . Randomized double-blind clinical trial of Moluodan () for the treatment of chronic atrophic gastritis with dysplasia. Chin J Integr Med. (2016) 22:9–18. doi: 10.1007/s11655-015-2114-5,
20.
Chen X Shen K Deng Y Mo J Ni J Hendi M et al . A randomized double-blind clinical trial of Weierkang pills for the treatment of chronic atrophic gastritis. J Clin Gastroenterol. (2023) 57:165–71. doi: 10.1097/MCG.0000000000001663,
21.
Bao Z Wu G Du J Ye Y Zheng Y Wang Y et al . The comparative efficacy and safety of 9 traditional Chinese medicines combined with standard quadruple therapy for Helicobacter pylori-associated gastritis: a systematic review and network meta-analysis. Ann Transl Med. (2022) 10:1349. doi: 10.21037/atm-22-5421,
22.
Cao L Du C Zhai X Li J Meng J Shao Y et al . Codonopsis pilosula polysaccharide improved spleen deficiency in mice by modulating gut microbiota and energy related metabolisms. Front Pharmacol. (2022) 13:862763. doi: 10.3389/fphar.2022.862763,
23.
Shi Q Chen Z Yang J Liu X Su Y Wang M et al . Review of Codonopsis Radix biological activities: a plant of traditional Chinese tonic. J Ethnopharmacol. (2024) 332:118334. doi: 10.1016/j.jep.2024.118334,
24.
Lodi RS Dong X Jiang C Sun Z Deng P Sun S et al . Antimicrobial activity and enzymatic analysis of endophytes isolated from Codonopsis pilosula. FEMS Microbiol Ecol. (2023) 99. doi: 10.1093/femsec/fiad071,
25.
Lv S Zhang Z Su X Li W Wang X Pan B et al . Qingrequzhuo capsule alleviated methionine and choline deficient diet-induced nonalcoholic steatohepatitis in mice through regulating gut microbiota, enhancing gut tight junction and inhibiting the activation of TLR4/NF-κB signaling pathway. Front Endocrinol. (2022) 13:1106875. doi: 10.3389/fendo.2022.1106875,
26.
Huang YQ Huang GR Wu MH Tang HY Huang ZS Zhou XH et al . Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: treatment of Helicobacter pylori-induced multidrug resistance. World J Gastroenterol. (2015) 21:4225–31. doi: 10.3748/wjg.v21.i14.4225,
27.
Guo H Lou Y Hou X Han Q Guo Y Li Z et al . A systematic review of the mechanism of action and potential medicinal value of codonopsis pilosula in diseases. Front Pharmacol. (2024) 15:1415147. doi: 10.3389/fphar.2024.1415147,
28.
Tong Y Liu L Wang R Yang T Wen J Wei S et al . Berberine attenuates chronic atrophic gastritis induced by MNNG and its potential mechanism. Front Pharmacol. (2021) 12:644638. doi: 10.3389/fphar.2021.644638,
29.
Lu M Yin J Xu T Dai X Liu T Zhang Y et al . Fuling-Zexie formula attenuates hyperuricemia-induced nephropathy and inhibits JAK2/STAT3 signaling and NLRP3 inflammasome activation in mice. J Ethnopharmacol. (2024) 319:117262. doi: 10.1016/j.jep.2023.117262,
30.
Ríos JL . Chemical constituents and pharmacological properties of Poria cocos. Planta Med. (2011) 77:681–91. doi: 10.1055/s-0030-1270823,
31.
Kongkham B Duraivadivel P Hariprasad P . Acorus calamus L. rhizome extract and its bioactive fraction exhibits antibacterial effect by modulating membrane permeability and fatty acid composition. J Ethnopharmacol. (2024) 331:118323. doi: 10.1016/j.jep.2024.118323,
32.
Zhang YW Hu WL Cai Y Zheng WF Du Q Kim JJ et al . Outcomes of furazolidone- and amoxicillin-based quadruple therapy for Helicobacter pylori infection and predictors of failed eradication. World J Gastroenterol. (2018) 24:4596–605. doi: 10.3748/wjg.v24.i40.4596,
33.
Wang Y Xiang Y Liao O Wu Y Li Y Du Q et al . Short-term outcomes and intermediate-term follow-up of Helicobacter pylori infection treatment for naïve patients: a retrospective observational study. BMJ Open. (2022) 12:e062096. doi: 10.1136/bmjopen-2022-062096,
34.
Abrahami D McDonald EG Schnitzer ME Barkun AN Suissa S Azoulay L . Proton pump inhibitors and risk of gastric cancer: population-based cohort study. Gut. (2022) 71:16–24. doi: 10.1136/gutjnl-2021-325097,
35.
Shao Y Lin Y Fang Z Yan J Zheng T Ye G . Analysis of Helicobacter pylori resistance in patients with different gastric diseases. Sci Rep. (2024) 14:4912. doi: 10.1038/s41598-024-55589-2,
36.
Ford AC Tsipotis E Yuan Y Leontiadis GI Moayyedi P . Efficacy of Helicobacter pylori eradication therapy for functional dyspepsia: updated systematic review and meta-analysis. Gut. (2022) 71:1967–75. doi: 10.1136/gutjnl-2021-326583,
37.
Rodriguez-Castro KI Franceschi M Noto A Miraglia C Nouvenne A Leandro G et al . Clinical manifestations of chronic atrophic gastritis. Acta Bio-Med Atenei Parmensis. (2018) 89:88–92. doi: 10.23750/abm.v89i8-S.7921,
38.
Ohori K Yano T Katano S Nagaoka R Numazawa R Yamano K et al . Independent association between use of proton pump inhibitors and muscle wasting in patients with heart failure: a single-center, ambispective, observational study. Drugs Aging. (2023) 40:731–9. doi: 10.1007/s40266-023-01035-3,
39.
Li Z Wu C Li L Wang Z Xie H He X et al . Effect of long-term proton pump inhibitor administration on gastric mucosal atrophy: a meta-analysis. Saudi J Gastroenterol. (2017) 23:222–8. doi: 10.4103/sjg.SJG_573_16,
40.
Wang Z Liu T Cao D Luo H Yang Z Kang X et al . The associations between functional dyspepsia and potential risk factors: a comprehensive Mendelian randomization study. PLoS One. (2024) 19:e0302809. doi: 10.1371/journal.pone.0302809,
41.
Miki A Yamamoto T Maruyama K Nakamura N Aoyagi H Isono A et al . Extensive gastric mucosal atrophy is a possible predictor of clinical effectiveness of acotiamide in patients with functional dyspepsia. Int J Clin Pharmacol Ther. (2017) 55:901–4. doi: 10.5414/CP203016,
Summary
Keywords
QYFD, chronic atrophic gastritis, Helicobacter pylori , clinical efficacy, adverse events
Citation
Wang J, Chen G, Zheng D and Ma C (2026) Efficacy and safety of adjunctive Chinese herbal decoction in treating Helicobacter pylori–positive chronic atrophic gastritis: a real-world retrospective study. Front. Med. 12:1701915. doi: 10.3389/fmed.2025.1701915
Received
09 September 2025
Revised
22 November 2025
Accepted
22 December 2025
Published
12 January 2026
Volume
12 - 2025
Edited by
Dan-Lucian Dumitrașcu, University of Medicine and Pharmacy Iuliu Hatieganu, Romania
Reviewed by
Hui Hui Zhao, Beijing University of Chinese Medicine, China
Md. Enamul Hoq, University of Arkansas for Medical Sciences, United States
Danish Jamil, University of Santiago de Compostela, Spain
Updates
Copyright
© 2026 Wang, Chen, Zheng and Ma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuifang Ma, 13186718582@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.