Abstract
Objective:
The objective of this study was to investigate whether colchicine could safely and effectively reduce inflammatory marker levels and improve the prognosis in patients with heart failure with preserved ejection fraction (HFpEF).
Methods:
This study enrolled patients diagnosed with HFpEF. Venous blood samples were collected to assess the levels of inflammatory markers such as IL-6, IL-8, and TNF-α. If these markers were elevated, patients were treated with colchicine 0.5 mg once daily. Inflammatory markers were reassessed after 2 weeks of treatment in the outpatient department. The primary endpoint was the change in inflammatory factor levels before and after colchicine treatment. Secondary outcomes included assessments of anxiety (HAMA), depression (HAMD), and quality of life (KCCQ) after 1 month of colchicine treatment. The clinical trial was registered at Chinese Clinical Trail Registry (ChiCTR2500103522).
Results:
A total of 126 patients were included. The serum inflammatory markers most notably elevated in the HFpEF cohort were IL-8 and IL-6, with IL-8 showing the most significant increase. After 2 weeks of colchicine treatment, serum levels of inflammatory markers decreased significantly. IL-8 levels decreased by −28.65 (95% CI: −54.20 to −11.47, p = 0.0010), a 65.5% reduction (95% CI: −82.0% to −45.0%). IL-6 levels decreased by −1.925 (95% CI: −4.510 to −0.29, p = 0.0028), reflecting a 30.5% reduction (95% CI: −54.0% to −5.0%). After 1 month of colchicine treatment, patients’ overall quality of life improved significantly. Anxiety (HAMA) decreased by −1 (95% CI: −1.0 to −1.0, p < 0.0001), depression (HAMD) decreased by −1 (95% CI: −1.0 to 0.0, p < 0.0001), and quality of life (KCCQ) increased by 10 points (95% CI: 9.0 to 12.0, p < 0.0001).
Conclusion:
This study is the first to explore colchicine as an anti-inflammatory treatment for HFpEF. Our results indicate that colchicine can safely and effectively reduce inflammatory markers such as IL-8 and IL-6. After 1 month of treatment, significant improvements were observed in anxiety, depression, and quality of life. These findings support colchicine’s potential as a therapeutic option for managing inflammation and improving outcomes in patients with HFpEF.
1 Introduction
Heart failure (HF) is a multifactorial clinical syndrome resulting from structural or functional impairments of the heart, and it represents the final stage of various cardiac diseases (1). Among the different types of heart failure, heart failure with preserved ejection fraction (HFpEF) accounts for at least 50% of all cases, and its prevalence is steadily increasing, making it the most common form of heart failure (2, 3). Emerging evidence indicates that multiple inflammatory mediators, including IL-1β, IL-6, TNF-α, and others, along with their associated signaling pathways, play pivotal roles in myocardial remodeling, endothelial dysfunction, and diastolic impairment in HFpEF, with elevated circulating levels correlating closely with disease severity, clinical manifestations, and adverse prognosis (4–8).
Colchicine is a well-established anti-inflammatory agent whose primary mechanisms of action include disruption of microtubule function, inhibition of selectin expression, reduction of neutrophil–platelet interactions, and suppression of NLRP3 inflammasome activation, thereby downregulating the release of inflammatory cytokines such as IL-1β and IL-6 (9, 10). Clinically, colchicine has established applications in gout, familial Mediterranean fever, and other inflammatory cardiovascular conditions. In addition, low-dose colchicine is commonly used for the prevention of recurrent pericarditis and as secondary prevention in patients with coronary artery disease, demonstrating favorable safety and efficacy in large clinical trials. Common adverse effects include mild gastrointestinal symptoms and liver function abnormalities, with rare occurrences of myelosuppression; caution is advised when used at high doses or in patients with impaired renal function (11).
Given the pivotal role of inflammation in the pathogenesis of HFpEF and the limited evidence regarding the anti-inflammatory potential of colchicine in this population, this study proposes the novel use of colchicine as an anti-inflammatory therapy for HFpEF. We comprehensively evaluated multiple cytokines related to inflammatory pathways to explore whether colchicine can safely and effectively reduce systemic inflammation and improve short-term clinical outcomes in these patients.
2 Methods
2.1 Trial design
This was a single-center, single-arm, prospective interventional study conducted at the First Affiliated Hospital of Hebei Medical University. A total of 126 patients with HFpEF were enrolled from June to August 2025. The study included measurements of inflammatory markers in the venous blood of patients, and if elevated levels of these markers were detected, patients received colchicine 0.5 mg daily. Follow-up assessments were performed accordingly. The study protocol and data analysis were approved by the Ethics Committee of the First Affiliated Hospital of Hebei Medical University in accordance with the Declaration of Helsinki (Approval number: 2025-072-01). Written informed consent was obtained from all participants prior to enrollment. The clinical trial was registered at Chinese Clinical Trail Registry (ChiCTR2500103522).
2.2 Trial population
Inclusion criteria were as follows:
-
Diagnosed with HFpEF: The diagnosis was based on hemodynamic measurements obtained through left heart catheterization, which is considered the gold standard for HFpEF diagnosis (12). Resting left ventricular end-diastolic pressure (LVEDP) > 15 mmHg, or clinical diagnosis was further supported by a straight leg raising test, with a LVEDP ≥19 mmHg when LVEDP was measured to be >10 mmHg but ≤15 mmHg at rest.
-
Elevated inflammatory markers: Patients with one or more inflammatory markers above the normal range were included.
Exclusion criteria included:
-
Echocardiographic evidence of an ejection fraction <50%.
-
Patients under 18 years of age.
-
Evidence of myocardial ischemia on coronary angiography that required interventional therapy.
-
Congenital heart disease, restrictive cardiomyopathy, pulmonary arterial hypertension.
-
Severe hepatic or renal dysfunction, advanced malignancy, autoimmune disease, multi-organ failure, or any other condition that might interfere with the study outcomes.
2.3 Trial procedures
Eligible participants were administered colchicine at a dose of 0.5 mg once daily. Inflammatory marker levels were reassessed after 2 weeks of treatment at the outpatient clinic. Additionally, quality of life assessments were performed before and after 1 month of treatment.
2.4 Cytokine measurements
Venous blood samples were collected at baseline and after 2 weeks of treatment, with cytokine levels measured on the same day. All assays were performed using cytokine detection kits based on magnetic particle luminescence (Su, 20,220,256) according to the manufacturer’s instructions. Each sample was measured in duplicate, and the mean value was used for analysis. Standard curves were generated for each assay, and intra- and inter-assay coefficients of variation were strictly maintained below 10%. Negative and positive controls were included to ensure the accuracy and reproducibility of the measurements.
2.5 Trial endpoints
The primary efficacy endpoint was the change in inflammatory factor concentrations from baseline to 2 weeks. Exploratory endpoints included the assessment of patient symptoms using the Hamilton Anxiety Scale (HAMA), the Hamilton Depression Scale (HAMD), and the Kansas City Cardiomyopathy Questionnaire (KCCQ). Safety endpoints of particular interest included the evaluation of gastrointestinal reactions, liver function, and renal function.
2.6 Statistical analysis
Statistical analyses were performed using SPSS 26.0 software. Quantitative data that were normally distributed were expressed as mean ± standard deviation (SD), while non-normal data were presented as median (first, third quartile). Paired t-tests were used for comparisons of data between baseline and follow-up. Statistical significance was set at p < 0.05.
3 Results
3.1 Baseline characteristics
During the study period, 365 subjects underwent left heart catheterization and inflammatory marker assessment at the First Hospital of Hebei Medical University, of whom 126 met the inclusion criteria (Figure 1). The baseline clinical characteristics of the study population are summarized in Table 1.
Figure 1

The flowchart shows the enrolment pathway in this study.
Table 1
| Characteristics | Overall |
|---|---|
| Age, mean (SD), y | 60.45 (±10.20) |
| Male, No. (%) | 65 (60%) |
| BMI, mean (SD), (Kg/m2) | 25.59 (±3.39) |
| SBP (mmHg) | 128 (122, 144) |
| DBP (mmHg) | 79.5 (72.25, 88.75) |
| Hypertension, No. (%) | 68 (63%) |
| Diabetes, No. (%) | 21 (19%) |
| Dyslipidemia, No. (%) | 56 (52%) |
| Atrial fibrillation, No. (%) | 10 (9%) |
| Other types of arrhythmia, No. (%) | 23 (21%) |
| Treatment | |
| Diuretic agent, No. (%) | 29 (27%) |
| MRA, No. (%) | 33 (31%) |
| ACEI/ARB, No. (%) | 4 (4%) |
| Beta-blocker, No. (%) | 66 (61%) |
| CCB, No. (%) | 25 (23%) |
| ARNI, No. (%) | 38 (35%) |
Baseline characteristics of the patients.
BMI, Body Mass Index; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; MRA, Mineralocorticoid Receptor Antagonists; ACEI, Angiotensin-Converting Enzyme Inhibitors; ARB, Angiotensin II Receptor Blockers; CCB, Calcium Channel Blockers; ARNI, Angiotensin Receptor-Neprilysin Inhibit.
Among the enrolled HFpEF patients, IL-8 and IL-6 were the primary inflammatory markers analyzed. Both cytokines showed elevated levels in a majority of patients, with IL-8 demonstrating the most pronounced increase (Figure 2; Supplementary Table 1).
Figure 2
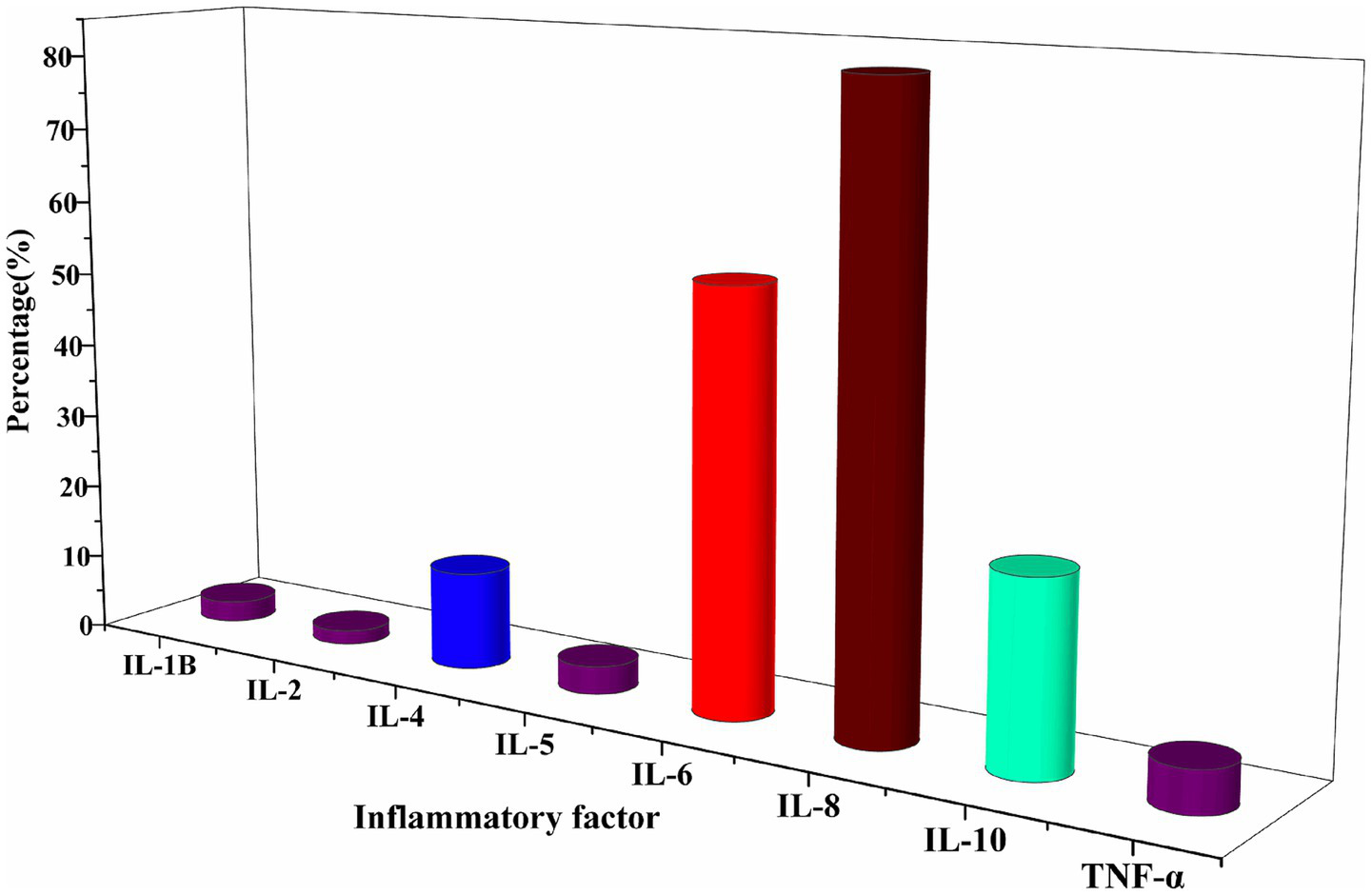
Characterised by elevated levels of inflammatory factors at baseline. The primary inflammatory markers assessed were IL-8 and IL-6.
3.2 Primary efficacy outcome
After 2 weeks of colchicine treatment, a reduction in serum inflammatory factors was observed (Figure 3). Specifically, the concentration of IL-8 decreased from baseline by −28.65 (95% CI: −54.20 to −11.47, p = 0.0010), representing a percentage reduction of 65.5% (95% CI: −82.0% to −45.0%). Similarly, IL-6 levels decreased by −1.925 (95% CI: −4.510 to −0.29, p = 0.0028), reflecting a percentage change of −30.5% (95% CI: −54.0% to −5.0%; Table 2; Supplementary Figure 1).
Figure 3
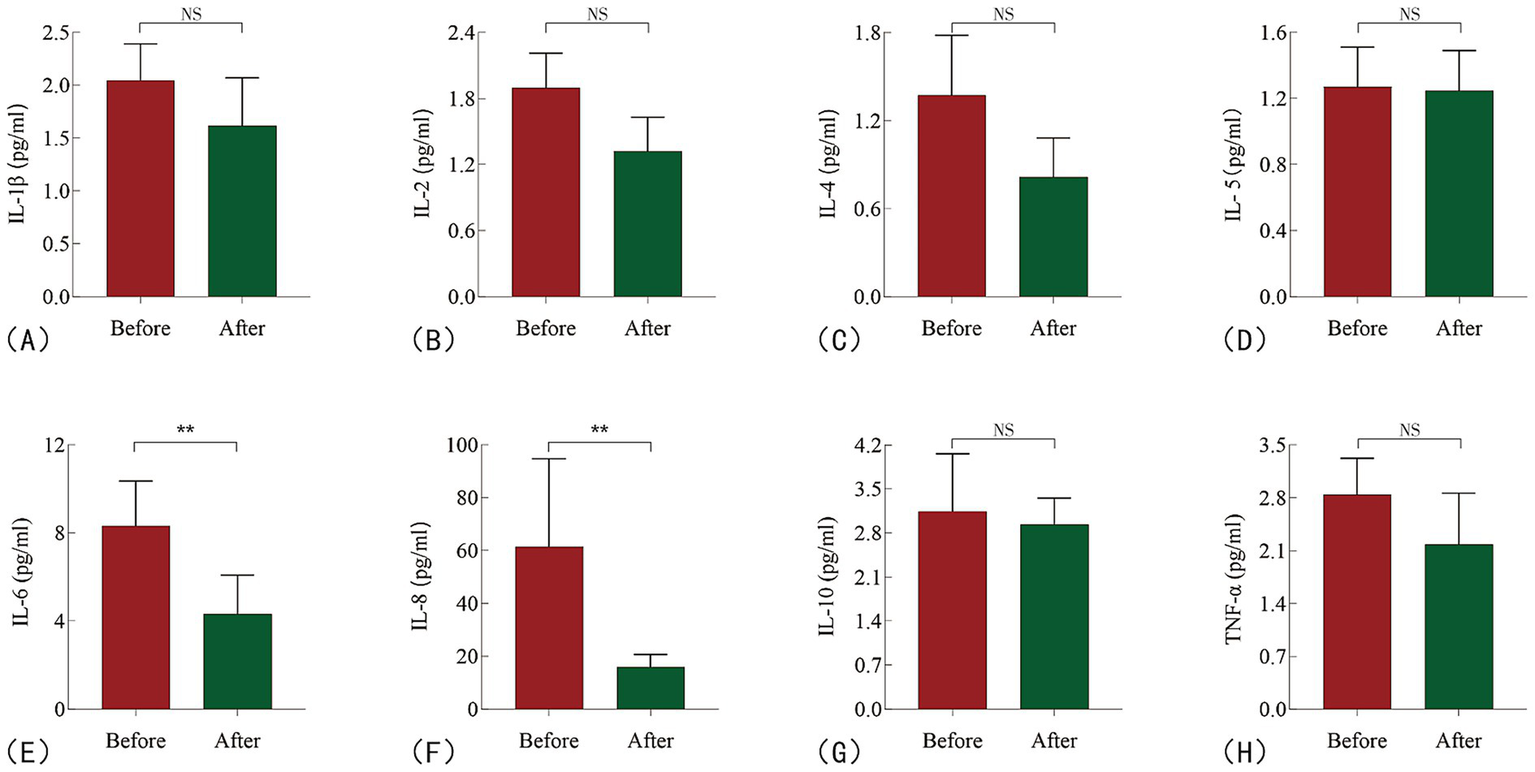
Changes in inflammatory cytokine levels in HFpEF patients before and after treatment. (A) IL-1β; (B) IL-2; (C) IL-4; (D) IL-5; (E) IL-6; (F) IL-8; (G) IL-10; (H) TNF-α. NS: no sense; **P < 0.05.
Table 2
| Inflammatory factor | Before-treatment (median) | After-treatment (median) | Changing value | Percentage change | p value (Wilcoxon test) |
|---|---|---|---|---|---|
| IL-1B | 2.045 (1.285, 2.605) (95%CI: 1.520 ~ 2.390) |
1.615 (1.083, 3.000) (95%CI: 1.320 ~ 2.070) |
−0.135 (−1.618, 1.288) (95%CI: −0.690 ~ 0.480) |
−10.5% (−62.5% 89.25%) (95%CI: −38.00% ~ 30.00%) |
0.7285 |
| IL-2 | 1.895 (1.043, 2.550) (95%CI: 1.460 ~ 2.210) |
1.320 (0.7825, 2.543) (95%CI: 1.050 ~ 1.630) |
−0.380 (−1.405, 0.735) (95%CI: −0.860 ~ 0.220) |
−20.5% (−62.75%, 46%) (95%CI: −45.00% ~ 18.00%) |
0.0787 |
| IL-4 | 1.370 (0.8675, 2.463) (95%CI: 1.080 ~ 1.780) |
0.815 (0.3900, 1.395) (95%CI: 0.60 ~ 1.080) |
−0.345 (−1.495, 0.5525) (95%CI: −1.040 ~ 0.01) |
−34.5% (−78%, 69%) (95%CI: −58% ~ 1.0%) |
0.0936 |
| IL-5 | 1.270 (0.8850, 1.715) (95%CI: 1.110 ~ 1.510) |
1.245 (0.6125, 1.635) (95%CI: 1.080 ~ 1.490) |
−0.120 (−0.735, 0.530) (95%CI: −0.450 ~ 0.230) |
−12% (−48.25%, 50.75%) (95%CI: −31.0% ~ 22.0%) |
0.4534 |
| IL-6 | 8.310 (3.965, 14.26) (95%CI: 5.340 ~ 10.35) |
4.310 (2.563, 7.723) (95%CI: 3.520 ~ 6.080) |
−1.925 (−10.25, 1.163) (95%CI:-4.510 ~ −0.29) |
−30.5% (−73.75%, 28.25%) (95%CI: −54.0% ~ −5.0%) |
0.0028 |
| IL-8 | 61.51 (30.71, 189.6) (95%CI: 43.95 ~ 94.74) |
15.92 (6.038, 53.74) (95%CI: 10.93 ~ 20.68) |
−28.65 (−149.8, 7.163) (95%CI: −54.20 ~ −11.47) |
−65.5% (−95%, 27%) (95%CI: −82.0% ~ −45.0%) |
0.0010 |
| IL-10 | 3.140 (2.383, 4.678) (95%CI: 2.640 ~ 4.060) |
2.935 (1.988, 4.208) (95%CI: 2.270 ~ 3.350) |
−0.57 (−1.565, 1.305) (95%CI: −1.070 ~ 0.770) |
−14.5% (−48%, 53%) (95%CI: −37.0% ~ 32.0%) |
0.3346 |
| TNF-α | 2.840 (2.045, 3.720) (95%CI: 2.310 ~ 3.320) |
2.180 (1.400, 3.315) (95%CI: 1.730 ~ 2.860) |
−0.29 (−1.575, 0.665) (95%CI: −1.030 ~ 0.09) |
−14% (−47%, 33.5%) (95%CI: −36.0% ~ 4.0%) |
0.1262 |
Changes in the levels of inflammatory factors before and after treatment with colchicine application.
3.3 Secondary efficacy outcomes
For secondary endpoints, a significant improvement in patients’ overall quality of life was observed after 1 month of colchicine treatment, as compared to baseline (Figure 4). Specifically, the HAMA showed a change of −1 (95% CI: −1.0 to −1.0, p < 0.0001), with a percentage change of −25% (95% CI: −32.0% to −20.0%). Similarly, the HAMD exhibited a change of −1 (95% CI: −1.0 to 0.0, p < 0.0001), with a percentage change of −17% (95% CI: −25.0 to 0.0%). The KCCQ showed a change of 10 (95% CI: 9.0 to 12.0, p < 0.0001), with a percentage change of 14.5% (95% CI: 12.0 to 17.0%; Table 3; Supplementary Figure 2).
Figure 4
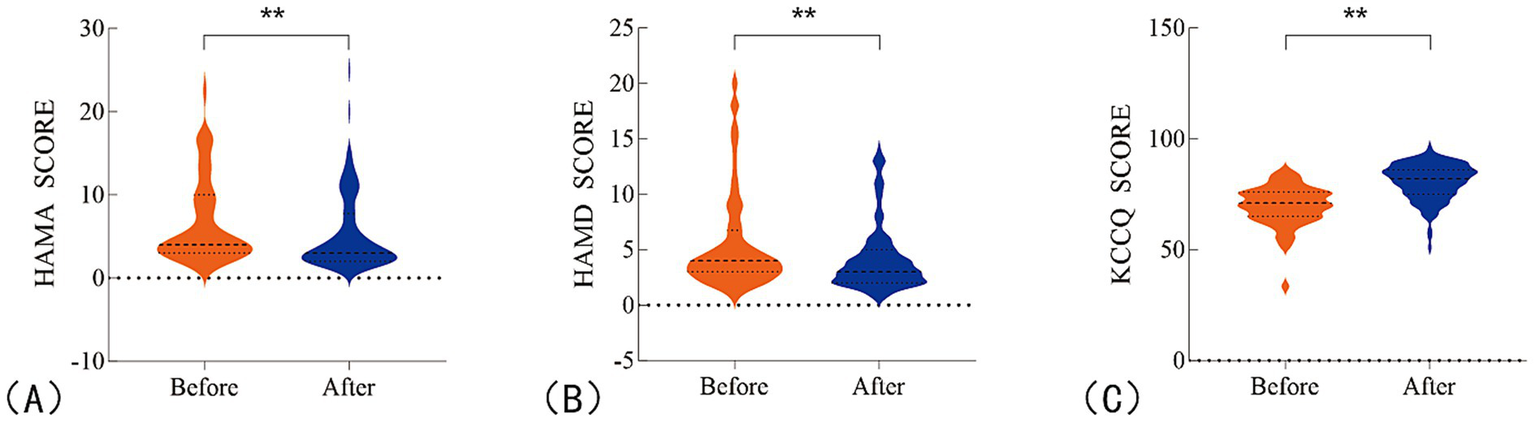
Changes in patients’ overall quality of life before and after treatment. (A) HAMA scores for anxiety; (B) HAMD scores for depression; (C) KCCQ scores for quality of life. **P <0.05.
Table 3
| Scale | Before –treatment (median) | After –treatment (median) | Descending value | Percentage of descend | p value (Wilcoxon test) |
|---|---|---|---|---|---|
| HAMA | 4.000 (3.000, 10.000) (95%CI: 4.0 ~ 5.0) |
3.000 (2.000, 7.750) (95%CI: 3.0 ~ 4.0) |
−1 (−2, 0) (95%CI: −1.0 ~ −1.0) |
−25% (−39.5%, 0%) (95%CI: −32.0% ~ 20.0%) |
<0.0001 |
| HAMD | 4.000 (3.000, 6.750) (95%CI: 3.0 ~ 4.0) |
3.000 (2.000, 5.000) (95%CI: 3.0 ~ 4.0) |
−1 (−1, 0) (95%CI: −1.0 ~ 0.0) |
−17% (−33%, 0%) (95%CI: −25.0% ~ 0.0%) |
<0.0001 |
| KCCQ | 71.00 (65.00, 76.00) (95%CI: 69.0 ~ 72.0) |
82.00 (75.00, 86.00) (95%CI: 80.0 ~ 84.0) |
10 (6.25, 14.75) (95%CI: 9.0 ~ 12.0) |
14.5% (9%, 21.75%) (95%CI: 12.0% ~ 17.0%) |
<0.0001 |
Changes in quality of life before and after treatment with colchicine application.
HAMA, Hamilton Anxiety Scale; HAMD, Hamilton Depression Scale; KCCQ, Kansas City Cardiomyopathy Questionnaire.
3.4 Safety outcome
During the colchicine treatment period, the incidence of adverse reactions was observed to be 4.1%. Importantly, these adverse reactions were mild, with no serious adverse events reported. The most common side effects were gastrointestinal in nature, occurring in four patients, and primarily manifested as diarrhea and flatulence. Additionally, three patients experienced transient renal function abnormalities, characterized by a decrease in estimated glomerular filtration rate (eGFR) of more than 30 mL/min/1.73m2 from baseline. However, these patients did not show any clinical symptoms, and renal disease-related tests did not indicate any significant abnormalities. After appropriate dose adjustment, renal function returned to normal values in all affected patients.
No liver function impairment was observed in any of the participants throughout the study (Table 4). These findings suggest that colchicine is both safe and well-tolerated when used for the treatment of HFpEF, with adverse reactions being limited to mild gastrointestinal and transient renal changes, all of which were reversible with proper management.
Table 4
| Adverse reaction | Number of cases |
|---|---|
| Gastrointestinal manifestations | 4 |
| Diarrhea | 2 |
| Nausea | 0 |
| Flatulence | 2 |
| Gastrointestinal hemorrhage | 0 |
| Hepatotoxicity (AST or ALT > 3 times normal) | 0 |
| Nephrotoxicity (a ≥ 30% decline in eGFR) | 0 |
Safety endpoints of colchicine application.
AST, Aspartate aminotransferase; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate.
4 Discussion
Our study demonstrated that inflammatory factors, particularly IL-8 and IL-6, were elevated in patients with early-stage HFpEF, consistent with findings from the Patrick study, which reported increased levels of IL-8, IL-6, and MCP-1 in the blood of 181 HFpEF patients (13–15). These findings further substantiate the role of inflammatory mediators in the pathogenesis of HFpEF and highlight the potential for targeting inflammation as part of the therapeutic approach (16–18).
Left ventricular diastolic dysfunction remains the hallmark pathophysiological feature of HFpEF. Previous research has shown that titin, a key regulator of myocardial passive tension, plays a central role in this dysfunction (19). Specifically, the transition of titin from the compliant N2BA isotype to the stiffer N2B isotype has been implicated in the development of diastolic dysfunction (20, 21). Our study suggests that IL-8, through binding to the CXCR1 and CXCR2 receptors on myocardial cells, may activate the phosphoinositide-specific phospholipase C pathway, leading to the activation of protein kinase C (PKC) (22, 23). This cascade ultimately results in the phosphorylation of titin, decreasing the N2BA: N2B ratio, reducing cardiomyocyte compliance, and contributing to left ventricular diastolic dysfunction (24). Colchicine, an anti-inflammatory agent, appears to mitigate this pathway by inhibiting IL-8 secretion, reducing titin phosphorylation, and restoring the balance of the N2BA: N2B ratio, thus improving myocardial stiffness and alleviating diastolic dysfunction (25).
Currently, only two randomized clinical trials have explored the effect of colchicine on heart failure. The study by Deftereos et al. showed that colchicine could reduce C-reactive protein (CRP) and IL-6 levels in patients with chronic stable heart failure but did not affect cardiac function or reduce hospital readmissions (26). Similarly, the COLICA trial by Pascual-Figal et al. found that colchicine reduced inflammatory markers such as CRP and IL-6 in patients with acute heart failure, but it did not significantly lower NT-proBNP levels, which is basically consistent with our research results (Supplementary Figure 3). Our study aligns with these findings, as we observed no significant difference in BNP levels before and after colchicine treatment, confirming that colchicine’s anti-inflammatory effects do not directly translate into immediate changes in cardiac biomarkers such as BNP (Supplementary Table 2) (27).
This study is the first to investigate the effects of colchicine on inflammatory responses in HFpEF patients. Colchicine significantly reduced IL-6 (~30%) and IL-8 (65%) levels, suggesting potential efficacy in modulating HFpEF-related inflammatory pathways. Assessment using HAMA, HAMD, and KCCQ indicated improvements in anxiety, depression, and cardiac-related quality of life (HAMA decreased by 25%, HAMD by 17%, and KCCQ increased by 15%), suggesting potential benefits beyond anti-inflammatory effects. Low-dose colchicine (0.5 mg daily) was well tolerated, with no serious adverse events or treatment discontinuations observed, indicating a favorable risk–benefit profile. These findings support colchicine as a potential anti-inflammatory intervention that may also improve quality of life in patients with HFpEF.
The safety data from this study indicate that colchicine was generally well tolerated, with a low incidence of adverse events. We specifically monitored gastrointestinal adverse events, including diarrhea, nausea, bloating, gastrointestinal bleeding, as well as liver and kidney function. The most common adverse effects observed were mild gastrointestinal symptoms, such as diarrhea and bloating. Based on the literature and clinical practice, the predefined discontinuation criteria included severe gastrointestinal discomfort, liver function abnormalities (ALT/AST ≥ 3 × upper limit of normal), and a decline in glomerular filtration rate (≥30%). In this study, no liver or kidney dysfunction was observed, and treatment did not lead to an increased discontinuation rate, even among elderly patients. These findings indicate that low-dose colchicine (0.5 mg daily) has a favorable safety profile and tolerability, making it a promising therapeutic option for patients with HFpEF.
4.1 Limitations
Despite these encouraging findings, several limitations of this study should be acknowledged. As a single-arm trial without a control group, definitive conclusions regarding the efficacy of colchicine in patients with HFpEF cannot be drawn. Future multi-center, randomized, double-blind, placebo-controlled trials are warranted to confirm the precise therapeutic effects of colchicine in this population. A subset of patients included in this study had mild to moderate coronary atherosclerosis (stenosis <50%) without evidence of acute ischemia or an indication for coronary intervention. Because coronary artery disease itself involves inflammatory processes, it may have influenced inflammatory biomarkers. Future studies should further exclude or stratify patients with coronary artery disease to better clarify its potential impact on the observed outcomes. Furthermore, although short-term improvements in symptoms and inflammatory markers were observed, assessments of quality of life and symptom burden relied primarily on total scores from HAMA, HAMD, and KCCQ, lacking detailed domain-specific analysis; thus, it remains unclear which aspects of psychological or functional status were most affected. Long-term follow-up is needed to evaluate the sustained impact of colchicine on HFpEF progression, patient-reported outcomes, and overall clinical prognosis. Future studies should also explore individualized treatment regimens based on patient characteristics to maximize therapeutic benefits while ensuring safety.
5 Conclusion
In conclusion, this study is the first to explore the use of colchicine for anti-inflammatory treatment in HFpEF patients. Our findings indicate that colchicine can safely and effectively reduce inflammatory factors such as IL-8 and IL-6. In addition, colchicine treatment was associated with improvements in patients’ quality of life and symptoms, as assessed by validated clinical scales. Further research is warranted to confirm these findings and to explore the underlying mechanisms, providing a potential therapeutic option for HFpEF.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the First Affiliated Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. XZ: Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. LL: Data curation, Supervision, Writing – original draft, Writing – review & editing. YW: Data curation, Supervision, Writing – original draft, Writing – review & editing. QZ: Data curation, Supervision, Writing – original draft, Writing – review & editing. YZ: Supervision, Writing – original draft, Writing – review & editing. YH: Data curation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CL: Conceptualization, Data curation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was funded by the Project of Investigating the Efficacy and Molecular Mechanisms of Glucocorticoid Therapy in Heart Failure with Diuretic Resistance (Grant No. ZF2025057) and the Natural Science Foundation of Hebei Province (Grant No. H2024206415).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1702293/full#supplementary-material
References
1.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Böhm M et al . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368,
2.
Campbell P Rutten FH Lee MM Hawkins NM Petrie MC . Heart failure with preserved ejection fraction: everything the clinician needs to know. Lancet. (2024) 403:1083–92. doi: 10.1016/S0140-6736(23)02756-3,
3.
Trullàs JC Pérez-Calvo JI Conde-Martel A Llàcer Iborra P Suárez Pedreira I Ormaechea G et al . Epidemiología de la insuficiencia cardiaca con fracción de eyección preservada: resultados del Registro RICA. Med Clín. (2021) 157:1–9. doi: 10.1016/j.medcli.2020.05.059,
4.
DeBerge M Shah SJ Wilsbacher L Thorp EB . Macrophages in heart failure with reduced versus preserved ejection fraction. Trends Mol Med. (2019) 25:328–40. doi: 10.1016/j.molmed.2019.01.002,
5.
Liu H Magaye R Kaye DM Wang BH . Heart failure with preserved ejection fraction: the role of inflammation. Eur J Pharmacol. (2024) 980:176858. doi: 10.1016/j.ejphar.2024.176858,
6.
Ovchinnikov AG Arefieva TI Potekhina AV Filatova AY Ageev FT Boytsov SА . The molecular and cellular mechanisms associated with a microvascular inflammation in the pathogenesis of heart failure with preserved ejection fraction. Acta Nat. (2020) 12:40–51. doi: 10.32607/actanaturae.11154,
7.
Zhao Z Qi D Zhang Z Du X Zhang F Ma R et al . Prognostic value of inflammatory cytokines in predicting hospital readmissions in heart failure with preserved ejection fraction. J Inflamm Res. (2024) 17:3003–12. doi: 10.2147/JIR.S459989,
8.
Song B Zhang H Zhou B . Effects of dapagliflozin on myocardial remodeling, inflammatory factors, and cardiac events in heart failure with preserved ejection fraction. Naunyn Schmiedeberg's Arch Pharmacol. (2024) 397:629. doi: 10.1007/s00210-023-02590-7,
9.
Dalbeth N Lauterio TJ Wolfe HR . Mechanism of action of colchicine in the treatment of gout. Clin Ther. (2014) 36:1465–79. doi: 10.1016/j.clinthera.2014.07.017,
10.
Hamed EFA Taha AA Ghany SMA Al-Attar A-SR Fawzy EM . Colchicine: a dual therapeutic target for Trichinellosis. Acta Parasitol. (2025) 70:42. doi: 10.1007/s11686-024-00979-9,
11.
Libby P . Inflammation in atherosclerosis—no longer a theory. Clin Chem. (2021) 67:131–42. doi: 10.1093/clinchem/hvaa275,
12.
Van De Bovenkamp AA Wijkstra N Oosterveer FPT Vonk Noordegraaf A Bogaard HJ Van Rossum AC et al . The value of passive leg raise during right heart catheterization in diagnosing heart failure with preserved ejection fraction. Circ Heart Fail. (2022) 15:e008935. doi: 10.1161/CIRCHEARTFAILURE.121.008935,
13.
Seropian IM El-Diasty M El-Sherbini AH González GE Rabinovich GA . Central role of Galectin-3 at the cross-roads of cardiac inflammation and fibrosis: implications for heart failure and transplantation. Cytokine Growth Factor Rev. (2024) 80:47–58. doi: 10.1016/j.cytogfr.2024.10.002,
14.
Gu Q Wang B Zhao H Wang W Wang P Deng Y . LncRNA promoted inflammatory response in ischemic heart failure through regulation of miR-455-3p/TRAF6 axis. Inflamm Res. (2020) 69:667–81. doi: 10.1007/s00011-020-01348-8,
15.
Mezzaroma E Abbate A Toldo S . The inflammasome in heart failure. Curr Opin Phys. (2021) 19:105–12. doi: 10.1016/j.cophys.2020.09.013,
16.
Collier P Watson CJ Voon V Phelan D Jan A Mak G et al . Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure?Eur J Heart Fail. (2011) 13:1087–95. doi: 10.1093/eurjhf/hfr079,
17.
Kim D-G Cho S Park S Kim GR Ko K-Y Kim SE et al . Predisposing risk factors affecting reversibility of left ventricular diastolic filling pattern in patients with preserved ejection fraction. Yonsei Med J. (2025) 66:1–8. doi: 10.3349/ymj.2023.0410,
18.
Zheng Y Nie Z Zhang Y Guo Z . The association between heart failure and systemic inflammatory response index: a cross-sectional study. J Natl Med Assoc. (2024) 116:662–72. doi: 10.1016/j.jnma.2024.10.007,
19.
Hullon D Subeh GK Volkova Y Janiec K Trach A Mnevets R . The role of glucagon-like peptide-1 receptor (GLP-1R) agonists in enhancing endothelial function: a potential avenue for improving heart failure with preserved ejection fraction (HFpEF). Cardiovasc Diabetol. (2025) 24:70. doi: 10.1186/s12933-025-02607-w,
20.
Zile MR Baicu CF Ikonomidis J Stroud RE Nietert PJ Bradshaw AD et al . Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. (2015) 131:1247–59. doi: 10.1161/CIRCULATIONAHA.114.013215,
21.
Ross L Patel S Stevens W Burns A Prior D La Gerche A et al . The clinical implications of left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol. (2022) 40:1986–92. doi: 10.55563/clinexprheumatol/irc0ih,
22.
Schraufstatter IU Chung J Burger M . IL-8 activates endothelial cell CXCR1 and CXCR2 through rho and Rac signaling pathways. Am J Physiol-Lung Cell Mol Physiol. (2001) 280:L1094–103. doi: 10.1152/ajplung.2001.280.6.L1094,
23.
Xv Y Feng Y Lin J . CXCR1 and CXCR2 are potential neutrophil extracellular trap-related treatment targets in ulcerative colitis: insights from mendelian randomization, colocalization and transcriptomic analysis. Front Immunol. (2024) 15:1425363. doi: 10.3389/fimmu.2024.1425363,
24.
Makarenko I Opitz CA Leake MC Neagoe C Kulke M Gwathmey JK et al . Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. (2004) 95:708–16. doi: 10.1161/01.RES.0000143901.37063.2f,
25.
Borbély A Falcao-Pires I Van Heerebeek L Hamdani N Édes I Gavina C et al . Hypophosphorylation of the stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. (2009) 104:780–6. doi: 10.1161/CIRCRESAHA.108.193326,
26.
Deftereos S Giannopoulos G Panagopoulou V Bouras G Raisakis K Kossyvakis C et al . Anti-inflammatory treatment with colchicine in stable chronic heart failure. JACC Heart Fail. (2014) 2:131–7. doi: 10.1016/j.jchf.2013.11.006,
27.
Pascual-Figal D Núñez J Pérez-Martínez MT González-Juanatey JR Taibo-Urquia M Llàcer-Iborra P et al . Colchicine in acutely decompensated heart failure: the COLICA trial. Eur Heart J. (2024) 45:4826–36. doi: 10.1093/eurheartj/ehae538,
Summary
Keywords
colchicine, heart failure with preserved ejection fraction, inflammatory cytokines, observational pilot study, symptoms
Citation
Shi M, Zhang X, Li L, Wang Y, Zhao Q, Zhen Y, Huang Y and Liu C (2026) Colchicine reduces inflammatory cytokines and improves symptoms in HFpEF: an observational pilot study. Front. Med. 12:1702293. doi: 10.3389/fmed.2025.1702293
Received
09 September 2025
Revised
26 November 2025
Accepted
15 December 2025
Published
16 January 2026
Volume
12 - 2025
Edited by
Youhua Wang, Shanghai University of Traditional Chinese Medicine, China
Reviewed by
Ricardo Adrian Nugraha, Universitas Airlangga—Dr. Soetomo General Hospital, Indonesia
Phillip Suwalski, Charité Universitätsmedizin Berlin, Germany
Updates
Copyright
© 2026 Shi, Zhang, Li, Wang, Zhao, Zhen, Huang and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Liu, liuchao@hebmu.edu.cnYaomeng Huang, 59203728@hebmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.