Abstract
Background:
Although dose reduction is the guideline-recommended tapering strategy for roxadustat to maintain target hemoglobin levels in peritoneal dialysis (PD) patients during the maintenance phase, reducing the administration frequency represents another potential clinical approach. However, the specific efficacy of this strategy in sustaining target hemoglobin levels remains unclear.
Methods:
This was a retrospective cohort study conducted from 1 January 2021 to 31 December 2024, enrolling PD patients from three dialysis centers in South China. Participants who achieved target hemoglobin levels were stratified into two groups based on roxadustat tapering strategies: The dose-reduction group, in which the per-dose amount was gradually decreased while maintaining the administration frequency, and the frequency-reduction group, in which the administration frequency was reduced without changing the per-dose amount. During a 12-month follow-up period, with assessments conducted every 3 months, mean hemoglobin levels, hemoglobin target attainment rates, and hemoglobin variability—evaluated using the residual standard deviation (RSD) method—were compared between the two tapering strategies. The association between hemoglobin target non-attainment and tapering strategies was analyzed using Cox proportional hazards models.
Results:
Among the 402 PD patients included in the analysis, the mean age was 45.3 ± 13.8 years and 55.5% were male. No significant difference was observed in the hemoglobin change trend and mean hemoglobin levels between the two groups throughout the follow-up period. Compared to the dose-reduction group, the patients who reduced dosing frequency demonstrated significantly higher hemoglobin target attainment rates at months 3 (64.1% vs. 40.4%), 6 (55.6% vs. 43.2%), and 12 (49.2% vs. 36.6%; all p < 0.05). In addition, the patients in this group exhibited a lower mean Res-SD value (12.3 vs. 15.5; p < 0.05). Moreover, the frequency-reduction strategy was associated with a significantly lower risk of hemoglobin target non-attainment (adjusted hazards ratio [HR] 0.64, 95% CI 0.50–0.82; p < 0.001).
Conclusion:
Compared to the dose-reduction group, the frequency-reduction group showed higher hemoglobin target attainment rates, lower hemoglobin variability, and a reduced risk of hemoglobin target non-attainment. The frequency-reduction strategy appears to be a potential tapering approach for peritoneal dialysis (PD) patients.
Introduction
Chronic kidney disease (CKD) is one of the significant contributors to the global health burden, affecting more than 10% of the world’s population (1). Renal anemia, a common complication of CKD, has an incidence exceeding 50% in patients with Stage 5 CKD (2), with prevalence rates surpassing 50% in non-dialysis and 90% in dialysis-dependent populations (3). Evidence indicates that renal anemia not only impairs quality of life but also accelerates CKD progression (4–6). Despite treatment with iron supplementation and erythropoiesis-stimulating agents (ESAs), a significant proportion of patients with CKD struggle to maintain hemoglobin levels within the KDIGO guideline-recommended target range (110–130 g/L) (7). Even after temporary anemia correction, hemoglobin levels in these patients often show greater variability—fluctuating more widely around the target range compared to healthy individuals (8). Such variability has been linked to increased risks of hospitalization, cardiovascular events, and mortality in some reports (9–12). These fluctuations are attributable to blood loss during hemodialysis, inflammatory conditions, endogenous erythropoietin (EPO) resistance, and modifications in the dosing of exogenous erythropoietic agents.
In this context, roxadustat has emerged as a groundbreaking therapeutic alternative for renal anemia. As an oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI), roxadustat mimics the body’s physiological response to hypoxia, thereby enhancing endogenous erythropoietin (EPO) production and improving iron metabolism (13). Clinically, roxadustat has been proven to remain equally effective in patients with EPO resistance or underlying inflammatory conditions (14). Notably, recent evidence suggests that roxadustat induces lower hemoglobin variability compared to erythropoiesis-stimulating agents (ESAs) in hemodialysis patients (15).
Since its approval, roxadustat dosage optimization has been a clinical priority. Prior studies have extensively explored optimal initial strategies—including dose and frequency—to achieve hemoglobin correction in both dialysis-dependent and non-dialysis-dependent CKD populations (16–20). However, only a few studies have been conducted on tapering strategies during maintenance therapy. Current guidelines predominantly recommend dose reduction as the exclusive tapering strategy after achieving hemoglobin targets (21). Given that both dose magnitude and administration frequency are adjustable during clinical therapy (19, 20), frequency reduction might be a potential tapering strategy during the maintenance phase. To explore this possibility, we conducted a multi-center retrospective cohort study comparing dose-reduction and frequency-reduction strategies in terms of longitudinal hemoglobin control among peritoneal dialysis (PD) patients who had achieved target levels.
Methods
Study population
This multi-center retrospective cohort study was conducted across three dialysis centers in Southern China—Nanfang Hospital, the First People’s Hospital of Foshan, and Shunde Hospital of Southern Medical University—from 1 January 2021 to 31 December 2024. The initial cohort comprised 642 adult patients on maintenance peritoneal dialysis (PD) who had received roxadustat for ≥ 4 weeks and achieved target hemoglobin levels (110–130 g/L). After applying the exclusion criteria, 227 patients were excluded for the following reasons: (i) follow-up duration less than 12 months (98), (ii) concomitant use of exogenous erythropoietin (8), (iii) active hemorrhagic disorder (7), and (iv) clinically significant infection (23). Among the 516 eligible participants, 88 patients underwent adjustments in both roxadustat dose and frequency, while 18 maintained unchanged regimens. Consequently, 402 patients were included in the final cohort analysis (Figure 1).
Figure 1

Flowchart of patient selection into the study cohort. EPO, erythropoietin.
Inclusion criteria
-
Patients aged > 18 years.
-
CKD patients on PD for more than 3 months.
-
Patients diagnosed with renal anemia and treated with roxadustat for ≥ 4 weeks.
-
Patients with hemoglobin levels within the range of 110–130 g/L (inclusive).
-
Patients with body weight between 45 and 100 kg (inclusive).
Exclusion criteria
-
Evidence of any clinically significant infection.
-
Concomitant use of exogenous erythropoietin.
-
History of malignancy.
-
Active hemorrhagic disorder.
-
Anemia due to causes other than chronic kidney disease.
-
Blood transfusion within 12 weeks prior to baseline.
-
Pregnancy.
-
Life expectancy less than 12 months.
Data collection and measurement
Baseline demographic characteristics (age, sex, and body mass index [BMI]) were collected from medical records. Systolic blood pressure (SBP), diastolic blood pressure (DBP), laboratory parameters (including blood hemoglobin, serum creatinine, serum albumin, total cholesterol, C-reactive protein, and serum ferritin), and PD-related characteristics (dialysate glucose concentration [GLUC], 24-h ultrafiltration volume, 24-h urine volume, and weekly total Kt/V) were collected at baseline and at each follow-up visit. All biochemical parameters were measured using standardized, automated methods.
Conventional weekly total Kt/V was measured using standard methods (22).
The hemoglobin target achievement rate was defined as the percentage of patients whose hemoglobin levels were maintained within the target range of 110–130 g/L at every measurement, in accordance with the KDIGO guidelines.
A patient was considered to have achieved the hemoglobin target status for the subsequent 3 months if the hemoglobin level met the criterion of 110–130 g/L at a given follow-up visit. Time to hemoglobin target non-attainment was defined as the cumulative duration (in months) from baseline until the hemoglobin level first fell outside the target range.
The standard deviation of residual hemoglobin (Res-SD) was used to assess hemoglobin variability (23).
Res-SD: Res-SD was defined as the square root of the sum of the squared differences between the observed hemoglobin values and the linear regression-predicted values, divided by the number of observations. The formula is as follows:
Treatment methods
In both patient groups, the starting dose of roxadustat was 100 mg three times per week for patients weighing 45–60 kg and 120 mg three times per week for patients weighing ≥ 60 kg. Dose-reduction group: The dose was adjusted using a predefined gradient (250 mg, 200 mg, 150 mg, 120 mg, 100 mg, 70 mg, 50 mg, 40 mg, and 20 mg) based on changes in hemoglobin levels to maintain the target range. Frequency-reduction group: The administration frequency was adjusted using a predefined gradient (3 times weekly, 2 times weekly, or 1 time weekly) based on changes in hemoglobin levels to maintain the target range. All patients were required to follow the dose-adjustment rule according to the hemoglobin response outlined in the study by Chen et al. (21). The specific adjustment regimen is detailed in Supplementary Table 1. Oral and intravenous iron supplementation was permitted, and blood transfusion therapy was allowed when hemoglobin levels were below 60 g/L.
Statistics
Means ± SDs or medians (interquartile range) for continuous variables and proportions for categorical variables were calculated. Differences in baseline characteristics between the dose-reduction group and the frequency-reduction group were compared using t-tests, Kruskal–Wallis tests, or chi-squared tests, respectively. Comparisons of continuous measurements within each group were performed using repeated measures ANOVA. The association between tapering strategies and hemoglobin target non-attainment was evaluated using Cox proportional hazards models (hazards ratio [HR] and 95% confidence interval [95% CI]), without or with adjustments for age, sex, history of diabetes mellitus, PD vintage, mean daily roxadustat exposure and baseline BMI, arterial pressure, 24-h urine volume, weekly total Kt/V score, phosphorus, intact parathyroid hormone (iPTH), serum albumin, C-reactive protein, and ferritin. Moreover, we further divided the patients into subgroups based on sex (male vs. female), age (< 45 vs. ≥ 45 years), BMI (< 24 vs. ≥ 24 kg/m2), mean serum albumin level (< 35 vs. ≥ 35 g/L), mean serum ferritin level (< 200 vs. ≥ 200 ng/mL), history of diabetes mellitus (yes vs. no), PD vintage (< 14 vs. ≥ 14 month), and clinical center (clinical center 1, 2, 3). Potential effect modifiers in the relationship between tapering strategies and hemoglobin target non-attainment were evaluated using subgroup analysis, and their interactions were assessed.
A two-tailed p-value of < 0.05 was considered statistically significant in all the analyses. SPSS software package version 22.0 and R software were used for all data analyses.
Results
Participant characteristics
As shown in the flowchart (Figure 1), a total of 402 PD patients were included in the final analyses. Among them, the mean age was 45.3 ± 13.8 years, 55.5% were male, and the mean baseline SBP and DBP were 136.2 ± 17.2 and 87.1 ± 10.4 mmHg, respectively. The patients in the frequency-reduction group were more likely to have a longer PD vintage and a lower prevalence of diabetes. Detailed baseline characteristics are presented in Table 1.
Table 1
| Characteristic | Total | Dose-reduction | Frequency-reduction | p-value |
|---|---|---|---|---|
| N | 402 | 215 | 187 | |
| Male, No. (%) | 223 (55.5) | 117 (54.4) | 106 (56.7) | 0.649 |
| Age, y | 45.3 ± 13.8 | 45.8 ± 13.1 | 44.7 ± 14.6 | 0.327 |
| BMI | 22.6 ± 3.9 | 22.3 ± 3.7 | 23.0 ± 4.1 | 0.216 |
| Diabetes mellitus | 64 (15.9) | 43 (20.0) | 21 (11.2) | 0.017 |
| SBP, mmHg | 136.2 ± 17.2 | 136.6 ± 17.1 | 135.7 ± 14.6 | 0.222 |
| DBP, mmHg | 87.1 ± 10.4 | 87.2 ± 10.3 | 87.0 ± 10.4 | 0.668 |
| Iron supplementation, No. (%) | 203 (50.4) | 111 (51.6) | 92 (49.1) | 0.119 |
| Baseline laboratory examinations | ||||
| Phosphorus, mmol/L | 1.6 ± 0.5 | 1.6 ± 0.5 | 1.6 ± 0.5 | 0.959 |
| Blood hemoglobin, g/L | 115.3 ± 5.6 | 116.2 ± 6.0 | 114.4 ± 5.1 | 0.133 |
| Serum albumin, g/L | 37.0 ± 6.1 | 36.4 ± 6.2 | 37.5 ± 5.9 | 0.138 |
| Serum creatinine, μmol/L | 925.6 ± 322.3 | 912.8 ± 346.2 | 940.2 ± 292.8 | 0.201 |
| CRP, mg/L | 4.3 ± 8.2 | 3.7 ± 5.8 | 5.0 ± 10.2 | 0.493 |
| iPTH, pg/mL | 421.8 ± 405.2 | 421.9 ± 431.3 | 421.8 ± 374.1 | 0.999 |
| Ferritin, ng/mL | 224.3 ± 228.0 | 211.9 ± 210.5 | 238.2 ± 246.0 | 0.409 |
| Transferrin saturation, % | 28.12 ± 16.16 | 28.07 ± 16.02 | 28.16 ± 16.32 | 0.960 |
| Total cholesterol, mmol/L | 4.0 (3.3, 4.9) | 3.9 (3.2, 4.8) | 4.0 (3.3, 5.0) | 0.353 |
| Baseline PD characteristics | ||||
| PD vintage, mo | 28.6 ± 26.7 | 27.9 ± 27.8 | 29.4 ± 25.1 | <0.001 |
| 24-h UF volume, ml/d | 350.0 (200.0, 612.5) | 425.0 (312.5, 637.5) | 312.5 (200.0, 600.0) | 0.342 |
| 24-h Urine volume, ml/d | 600.0 (350.0, 900.0) | 400.0 (212.5, 712.5) | 600.0 (400.0, 900.0) | 0.145 |
| Dialysate GLUC, % | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 0.222 |
| Total Kt/V score | 2.1 ± 0.2 | 2.1 ± 0.1 | 2.1 ± 0.2 | 0.548 |
| Cause of ESRD | ||||
| Primary glomerular disease, No. (%) | 232 (57.7) | 122 (56.7) | 110 (58.8) | |
| Hypertensive nephropathy, No. (%) | 20 (4.9) | 11 (5.11) | 9 (4.8) | |
| Diabetic nephropathy, No. (%) | 20 (4.9) | 12 (5.5) | 8 (4.2) | |
| Other, No. (%) | 110 (27.3) | 50 (23.2) | 60 (32.0) | |
Demographic, clinical, and laboratory characteristics of the patients at baseline.
Continuous variables are presented as mean ± SD or IQR (25th, 75th), while categorical variables are presented as No. (%). p-values were calculated to compare differences between the two groups using t-test, Kruskal–Wallis test, or chi-squared tests for continuous and categorical variables, respectively.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; iPTH, intact parathyroid hormone; ESRD, end-stage renal disease; CRP, C-reactive protein; PD, peritoneal dialysis; UF, ultrafiltration.Bold values indicate statistical significance at p < 0.05.
Mean hemoglobin levels and hemoglobin target attainment rates
We first analyzed the mean hemoglobin levels and hemoglobin target attainment rates during the follow-up period. As shown in Figures 2, 3, the mean hemoglobin levels were 116.2 ± 5.8, 120.2 ± 19.2, 114.5 ± 16.9, 115.5 ± 14.3, and 117.1 ± 20.2 in the dose-reduction group and 114.3 ± 5.0, 117.0 ± 14.2, 115.1 ± 14.3, 113.7 ± 15.1, and 116.1 ± 16.4 in the frequency-reduction group at baseline and at months 3, 6, 9, and 12, respectively. Overall, there was no difference in the changing trend of hemoglobin (p = 0.14) or in hemoglobin levels at each visit (all p > 0.05). The patients in the frequency-reduction group had significant higher hemoglobin target attainment rates at months 3, 6, and 12 compared to those in the dose-reduction group (64.1% vs. 40.4%, p < 0.05; 55.6% vs. 43.2%, p < 0.05; 49.2% vs. 36.6%, p < 0.05).
Figure 2

Mean hemoglobin levels in the dose-reduction and frequency-reduction groups during the follow-up period. p for trend compared the hemoglobin change trend.
Figure 3

Hemoglobin target attainment rates in the dose-reduction and frequency-reduction groups at baseline and each follow-up visit. Target hemoglobin levels were 110–130 g/L.
Hemoglobin variability
We next calculated the Res-SD among the patients. The patients in the dose-reduction group had a mean Res-SD value of 15.5 (95% CI 14.6–16.4), which was significantly greater than that of the frequency-reduction group (12.3, 95% CI 11.5–13.2, p < 0.001; Figure 4).
Figure 4

Res-SD scores in the dose-reduction and frequency-reduction groups. Res-SD was calculated by fitting a regression line to each patient’s hemoglobin measurements and then computing the square root of the sum of the squared vertical deviations between each actual hemoglobin value and its corresponding linear regression-predicted value. Res-SD, Standard deviation of residual hemoglobin.
Association between tapering strategies and hemoglobin target non-attainment
We also analyzed the association between tapering strategies and hemoglobin target non-attainment using a Cox regression model. As shown in Table 2, the frequency-reduction group was associated with a lower risk of hemoglobin target non-attainment compared to the dose-reduction group, with an HR of 0.65 (95% CI 0.52–0.80, p < 0.001). After adjustment for age, sex, history of diabetes mellitus, PD vintage, mean daily roxadustat exposure and baseline BMI, arterial pressure, 24-h urine volume, weekly total Kt/V score, phosphorus, iPTH, serum albumin, C-reactive protein, and ferritin, the risk of hemoglobin target non-attainment remained lower in the frequency-reduction group, with a HR of 0.64 (95% CI 0.50–0.82, p < 0.001). A similar trend was found in the Kaplan–Meier curve using the log-rank test (Supplementary Figure 1).
Table 2
| Hemoglobin target non-attainment, mo | Crude modela | Adjusted modelb | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Male | 0.95 (0.77, 1.18) | 0.664 | 1.02 (0.80, 1.30) | 0.847 |
| Age, y | 1.00 (0.99, 1.00) | 0.394 | 1.00 (0.99, 1.00) | 0.270 |
| BMI | 0.99 (0.96, 1.02) | 0.437 | 0.99 (0.96, 1.02) | 0.538 |
| Mean arterial pressure, mmHg | 1.00 (0.99, 1.01) | 0.519 | 0.99 (0.98, 1.01) | 0.325 |
| Diabetes mellitus | 0.93 (0.69, 1.24) | 0.603 | 0.86 (0.62, 1.18) | 0.343 |
| PD vintage, mo | 1.00 (0.99, 1.00) | 0.355 | 1.00 (0.99, 1.00) | 0.749 |
| Mean daily roxadustat exposure, mg/d | 1.01 (1.00, 1.02) | 0.142 | 1.00 (0.99, 1.01) | 0.876 |
| Phosphorus, mmol/L | 1.06 (0.84, 1.33) | 0.628 | 1.03 (0.80, 1.34) | 0.797 |
| iPTH, pg/mL | 1.00 (1.00, 1.00) | 0.929 | 1.00 (1.00, 1.00) | 0.841 |
| Serum albumin, g/L | 1.00 (0.98, 1.01) | 0.676 | 1.00 (0.98, 1.02) | 0.661 |
| CRP, mg/L | 1.00 (0.99, 1.01) | 0.993 | 1.01 (0.99, 1.02) | 0.393 |
| Serum ferritin, ng/mL | 1.00 (1.00, 1.00) | 0.681 | 1.00 (1.00, 1.00) | 0.813 |
| 24-h UV, ml/d | 1.00 (1.00, 1.00) | 0.185 | 1.00 (1.00, 1.00) | 0.670 |
| Total Kt/V score | 1.18 (0.42, 3.29) | 0.757 | 1.04 (0.32, 3.26) | 0.973 |
| Groups | ||||
| Dose-reduction group | REF | REF | ||
| Frequency-reduction group | 0.65 (0.52, 0.80) | < 0.001 | 0.64 (0.50, 0.82) | < 0.001 |
Association between hemoglobin target non-attainment and tapering strategies.
BMI, body mass index; CRP, C-reactive protein; iPTH, intact parathyroid hormone; UV, urine volume.
Crude model: No covariates were adjusted.
Adjusted model: Adjusted for age, sex, history of diabetes mellitus, PD vintage, mean daily roxadustat exposure and baseline BMI, arterial pressure, 24-h urine volume, weekly total Kt/V score, phosphorus, iPTH, serum albumin, CRP, and ferritin.Bold values indicate statistical significance at p < 0.05.
Stratified analyses
To better understand other possible influencing factors in the relationship between tapering strategies and hemoglobin target non-attainment, we conducted additional exploratory subgroup analyses. None of the variables, including sex (male vs. female), age (< 45 vs. ≥ 45 years), BMI (< 24 vs. ≥ 24 kg/m2), mean serum albumin level (< 35 vs. ≥ 35 g/L), mean serum ferritin level (< 200 vs. ≥ 200 ng/mL), history of diabetes mellitus (yes vs. no), PD vintage (< 14 vs. ≥ 14 month), and clinical center (clinical center 1,2,3) significantly modified the association between tapering strategies and hemoglobin target non-attainment (all p-interactions > 0.05) (Figure 5).
Figure 5
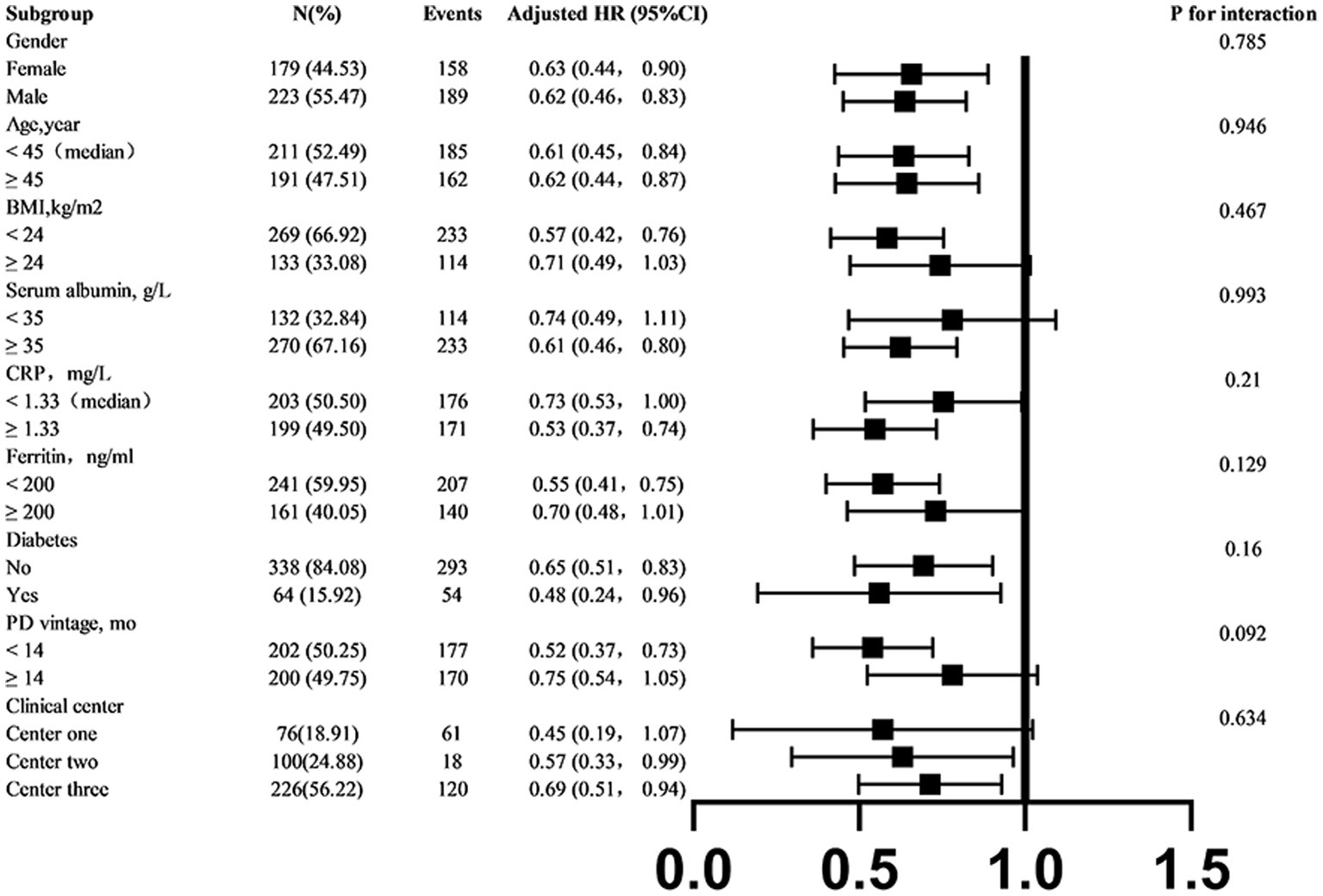
Subgroup analyses of the association between tapering strategies and hemoglobin target non-attainment. If a variable was not used for stratification, analyses were adjusted for age, sex, history of diabetes mellitus, PD vintage, clinical center, baseline arterial pressure, BMI, total Kt/V score, 24-h urine volume, serum albumin, CRP, and serum ferritin. BMI, body mass index; CRP, C-reactive protein.
Discussion
In this multi-center retrospective study, the patients receiving the roxadustat frequency-reduction strategy exhibited higher hemoglobin target attainment rates, lower mean Res-SD values, and a reduced risk of hemoglobin target non-attainment compared to those in the dose-reduction group. These findings support frequency reduction as a potential tapering strategy for roxadustat in PD patients.
Roxadustat is increasingly used in clinical practice for anemia management in CKD patients, demonstrating benefits including enhanced iron metabolism, attenuated inflammation, reduced ESA resistance, oral administration convenience, and broad insurance coverage (16, 24–26). Within our center, adoption was significantly higher among PD patients, with approximately 70% receiving oral roxadustat compared to fewer than 20% continuing ESA injections. This disparity may be attributable to the standardized 1–3 month follow-up intervals for the PD population.
Current guidelines in China recommend initiating roxadustat at 100 mg (45–60 kg) or 120 mg (≥ 60 kg), administered thrice weekly (TIW) for dialysis-dependent patients. During the maintenance phase, target hemoglobin levels are maintained through stepwise dose reductions based on hemoglobin dynamics while retaining the TIW frequency (21). The rationale for this regimen is primarily based on pharmacokinetic studies: endogenous erythropoietin (EPO) peaks within 24 h post-dose and returns to baseline within 48 h after discontinuation, necessitating TIW dosing to sustain effective erythropoietic activity (27). However, the cascade of cellular signals drives the proliferation and differentiation of erythroid progenitor cells, leading to a stable lifespan of mature red blood cells and a sustained increase in hemoglobin levels (more than 60 days) (27). This provides a pharmacological basis for frequency reduction after anemia correction. A few trials have explored the feasibility of adopting a reduced fixed frequency of roxadustat after hemoglobin reached target levels (19, 20, 28). In a phase II study conducted in Japan in a non-dialysis-dependent population, patients were re-randomized into groups receiving roxadustat either TIW or once weekly after achieving target hemoglobin levels. No difference was observed in subsequent mean hemoglobin levels between the groups. Taken together, frequency reduction might be a potential tapering strategy in clinical practice.
In our study, nearly half of the patients (46.5%) adopted the frequency reduction strategy. Compared to the dose-reduction group, the patients in the frequency-reduction group exhibited higher hemoglobin target attainment rates at months 3, 6, and 12 and lower mean Res-SD values after calculation, along with similar mean hemoglobin levels and trends during subsequent follow-up visits. These results suggest the feasibility of the frequency reduction tapering strategy in peritoneal dialysis populations. Moreover, the frequency-reduction strategy seemed to be better for hemoglobin stability. Further analysis revealed that the dose-reduction group exhibited a higher mean roxadustat dose and a higher proportion of patients with hemoglobin > 130 g/L, while the proportions of patients with hemoglobin < 110 g/L were comparable at months 3, 6, 9, and 12 (Supplementary Figure 2). These findings suggest that relatively higher drug exposure might cause lower hemoglobin stability. Same protocol designs in non-dialysis CKD (29) and PD (30) populations also exhibited comparable efficacy, with significantly lower hemoglobin variability observed in patients receiving low-dose roxadustat compared to standard-dose therapy for anemia correction.
Roxadustat is commonly available in two tablet strengths: 20 mg and 50 mg. However, the 50 mg tablet was the only formulation stocked in many centers, which often precluded precise small dose reductions (e.g., from 100 mg to 70 mg). Consequently, physicians were sometimes compelled to make larger dose adjustments (e.g., from 100 mg to 50 mg). Due to concerns that such significant reductions could induce hemoglobin fluctuations, some clinicians preferred to reduce the administration frequency (e.g., from three times per week to twice per week) as a more manageable alternative. In addition, for patients with sub-optimal medication adherence or those seeking to reduce the administration frequency, physicians might prefer the frequency-reduction strategy to simplify the regimen. Furthermore, after achieving target hemoglobin levels, some physicians were hesitant to discontinue the drug completely due to concerns about potential hemoglobin decline, opting instead to reduce the dosing frequency to maintain a lower level of drug exposure. Therefore, both frequency- and dose-reduction strategies were applied in clinical practice. In our data, the patients in the frequency-reduction group exhibited higher hemoglobin target attainment rates and lower mean Res-SD values while maintaining similar mean hemoglobin levels and trends compared to the dose-reduction group. Therefore, in situations where the 20 mg tablet is unavailable or for patients who wish to reduce the administration frequency, the frequency-reduction strategy might be a potentially feasible alternative method. In other situations, both tapering strategies might be suitable.
Our study has several limitations that should be considered when interpreting the findings. First, as a retrospective cohort study, the design is inherently observational and cannot establish causal relationships between variables. Second, although we adjusted for a broad set of covariates in the analysis, residual confounding may persist due to unmeasured factors such as physicians’ individualized prescribing preferences, undocumented patient comorbidities, variations in medication adherence, and differences in iron dosage and administration patterns. Third, the frequency of hemoglobin monitoring in our study population may have been insufficient to capture more dynamic fluctuations, potentially affecting the accuracy of the conclusions. Fourth, the direct dose reduction of roxadustat from 100 mg to 50 mg, as observed in some of our clinical centers, may affect the validity of our conclusions. Finally, the study participants were recruited exclusively from southern China, which may limit the generalizability of the findings to other geographic or ethnic populations. Further validation in broader and more diverse cohorts is warranted.
Conclusion
Collectively, our retrospective cohort study provides clinical evidence supporting the roxadustat frequency-reduction strategy for maintaining hemoglobin stability in PD patients, indicating its potential feasibility in this population. Further high-quality clinical studies are warranted to confirm these findings.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of Nanfang Hospital, Guangzhou, China (ethics number NFEC-2019-223). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XM: Methodology, Writing – review & editing, Writing – original draft, Data curation, Visualization. GL: Data curation, Writing – review & editing. YF: Writing – review & editing, Data curation. LZ: Writing – review & editing, Data curation. YL: Writing – review & editing, Data curation. TZ: Writing – review & editing, Data curation. XZ: Writing – review & editing, Data curation. XS: Writing – review & editing, Data curation. XD: Writing – review & editing. JA: Writing – review & editing, Supervision, Funding acquisition, Resources.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was funded by the Nature and Science Foundation of China (grant number 82370744 to JA), the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (grant number 2017J013 to JA).
Acknowledgments
We gratefully acknowledge all the doctors and nurses at the three centers for their efforts in patient follow-up and for maintaining detailed medical records. We also thank the Program of Introducing Talents of Discipline to Universities, 111 Plan (D18005 to FFH), and the Key Technologies R&D Program of Guangdong Province (2023B1111030004 to FFH) for their generous support.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1708916/full#supplementary-material
SUPPLEMENTARY FIGURE 1Kaplan-Meier survival analyses of the hemoglobin target non-attainment.
SUPPLEMENTARY FIGURE 2Comparison of roxadustat dose between the dose-reduction group and the frequency-reduction group. p for trend compared roxadustat change trend.
References
1.
Zaimi M Grapsa E . Current therapeutic approach of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. (2024) 28:671–89. doi: 10.1111/1744-9987.14177,
2.
Portoles J Martin L Broseta JJ Cases A . Anemia in chronic kidney disease: from pathophysiology and current treatments, to future agents. Front Med. (2021) 8:642296. doi: 10.3389/fmed.2021.642296,
3.
Evans M Bower H Cockburn E Jacobson SH Barany P Carrero J . Contemporary management of anaemia, erythropoietin resistance and cardiovascular risk in patients with advanced chronic kidney disease: a nationwide analysis. Clin Kidney J. (2020) 13:821–7. doi: 10.1093/ckj/sfaa054,
4.
van Haalen H Jackson J Spinowitz B Milligan G Moon R . Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: analysis of multinational real-world data. BMC Nephrol. (2020) 21:88. doi: 10.1186/s12882-020-01746-4,
5.
Hoshino J Muenz D Zee J Sukul N Speyer E Guedes M et al . Associations of hemoglobin levels with health-related quality of life, physical activity, and clinical outcomes in persons with stage 3-5 nondialysis ckd. J Ren Nutr. (2020) 30:404–14. doi: 10.1053/j.jrn.2019.11.003,
6.
Akizawa T Okumura H Alexandre AF Fukushima A Kiyabu G Dorey J . Burden of anemia in chronic kidney disease patients in Japan: a literature review. Ther Apher Dial. (2018) 22:444–56. doi: 10.1111/1744-9987.12712,
7.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. (2024) 105:S117–314. doi: 10.1016/j.kint.2023.10.018
8.
Cases-Amenos A Martinez-Castelao A Fort-Ros J Bonal-Bastons J Ruiz MP Valles-Prats M et al . Prevalence of anaemia and its clinical management in patients with stages 3-5 chronic kidney disease not on dialysis in Catalonia: micenas i study. Nefrologia. (2014) 34:189–98. doi: 10.3265/Nefrologia.pre2013.Dec.12261
9.
Bal Z Demirci BG Karakose S Tutal E Erkmen Uyar M Acar NO et al . Factors influencing hemoglobin variability and its association with mortality in hemodialysis patients. ScientificWorldJournal. (2018) 2018:8065691. doi: 10.1155/2018/8065691,
10.
Kuragano T Matsumura O Matsuda A Hara T Kiyomoto H Murata T et al . Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int. (2014) 86:845–54. doi: 10.1038/ki.2014.114,
11.
Lee WJ Choi S Park SM Lee G Chang J Oh YH et al . Association of hemoglobin variability with the risk of cardiovascular disease: a nationally representative retrospective cohort study from South Korea. Sci Rep. (2023) 13:2148. doi: 10.1038/s41598-023-28029-w,
12.
Kuragano T Okami S Tanaka-Mizuno S Uenaka H Kimura T Ishida Y et al . Anemia treatment, hemoglobin variability, and clinical events in patients with nondialysis-dependent ckd in Japan. Kidney360. (2023) 4:e1223–35. doi: 10.34067/KID.0000000000000204,
13.
Yap DYH McMahon LP Hao C Hu N Okada H Suzuki Y et al . Recommendations by the Asian Pacific Society of Nephrology (APSN) on the appropriate use of HIF-PH inhibitors. Nephrology (Carlton). (2021) 26:105–18. doi: 10.1111/nep.13835,
14.
Fishbane S El-Shahawy MA Pecoits-Filho R Van BP Houser MT Frison L et al . Roxadustat for treating anemia in patients with ckd not on dialysis: results from a randomized phase 3 study. J Am Soc Nephrol. (2021) 32:737–55. doi: 10.1681/ASN.2020081150,
15.
Wang X Zhu N Zeng W Wang P . Hemoglobin variability in patients receiving epo and roxadustat during maintenance hemodialysis: a self-control study. Eur Rev Med Pharmacol Sci. (2024) 28:303–9. doi: 10.26355/eurrev_202401_34917,
16.
Akizawa T Yamaguchi Y Otsuka T Reusch M . A phase 3, multicenter, randomized, two-arm, open-label study of intermittent oral dosing of roxadustat for the treatment of anemia in japanese erythropoiesis-stimulating agent-naive chronic kidney disease patients not on dialysis. Nephron Clin Pract. (2020) 144:372–82. doi: 10.1159/000508100,
17.
Provenzano R Besarab A Wright S Dua S Zeig S Nguyen P et al . Roxadustat (fg-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis. (2016) 67:912–24. doi: 10.1053/j.ajkd.2015.12.020,
18.
Fishbane S Pollock CA El-Shahawy M Escudero ET Rastogi A Van BP et al . Roxadustat versus epoetin alfa for treating anemia in patients with chronic kidney disease on dialysis: results from the randomized phase 3 rockies study. J Am Soc Nephrol. (2022) 33:850–66. doi: 10.1681/ASN.2020111638,
19.
Besarab A Provenzano R Hertel J Zabaneh R Klaus SJ Lee T et al . Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (fg-4592) to treat anemia in nondialysis-dependent chronic kidney disease (ndd-ckd) patients. Nephrol Dial Transplant. (2015) 30:1665–73. doi: 10.1093/ndt/gfv302,
20.
Provenzano R Besarab A Sun CH Diamond SA Durham JH Cangiano JL et al . Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (fg-4592) for the treatment of anemia in patients with ckd. Clin J Am Soc Nephrol. (2016) 11:982–91. doi: 10.2215/CJN.06890615,
21.
Chen N Hao C Liu B Lin H Wang C Xing C et al . Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. (2019) 381:1011–22. doi: 10.1056/NEJMoa1901713,
22.
National kidney foundation . Nkf-doqi clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis. (1997) 30:S67–S136. doi: 10.1016/s0272-6386(97)70028-3
23.
Brunelli SM Lynch KE Ankers ED Joffe MM Yang W Thadhani RI et al . Association of hemoglobin variability and mortality among contemporary incident hemodialysis patients. Clin J Am Soc Nephrol. (2008) 3:1733–40. doi: 10.2215/CJN.02390508,
24.
Wu H Cheng H Wang C Yao L Qin S Zuo L et al . Roxadustat and oral iron absorption in chinese patients with anemia of chronic kidney disease: a randomized, open-label, phase 4 study (Altai). Adv Ther. (2024) 41:1168–83. doi: 10.1007/s12325-023-02741-5,
25.
Akizawa T Yamaguchi Y Majikawa Y Reusch M . Factors affecting the doses of roxadustat vs darbepoetin alfa for anemia treatment in hemodialysis patients. Ther Apher Dial. (2021) 25:575–85. doi: 10.1111/1744-9987.13609,
26.
Liu J Li S Yang F Li T Li R Waheed Y et al . A retrospective study on the efficacy of roxadustat in peritoneal dialysis patients with erythropoietin hyporesponsiveness. Korean J Intern Med. (2024) 39:488–500. doi: 10.3904/kjim.2023.520,
27.
Czock D Keller F . Clinical pharmacokinetics and pharmacodynamics of roxadustat. Clin Pharmacokinet. (2022) 61:347–62. doi: 10.1007/s40262-021-01095-x,
28.
Akizawa T Iwasaki M Otsuka T Reusch M Misumi T . Roxadustat treatment of chronic kidney disease-associated anemia in japanese patients not on dialysis: a phase 2, randomized, double-blind, placebo-controlled trial. Adv Ther. (2019) 36:1438–54. doi: 10.1007/s12325-019-00943-4,
29.
Yang Z Ma T Xu X Fu G Zhao J Xu Y et al . Randomized study on the efficacy of standard versus low roxadustat dose for anemia in patients on peritoneal dialysis. Kidney Int Rep. (2022) 7:455–64. doi: 10.1016/j.ekir.2021.12.025,
30.
Li P Sun X Zhang L Lin H Wang N Li Y et al . Randomized trial of lower-dose roxadustat efficacy and safety in non-dialysis-dependent ckd-associated anemia. Kidney Int Rep. (2025) 10:1050–62. doi: 10.1016/j.ekir.2025.01.027,
Summary
Keywords
dose reduction, frequency reduction, hemoglobin stability, peritoneal dialysis, roxadustat tapering strategies
Citation
Mi X, Lin G, Fang Y, Zhu L, Lin Y, Zhang T, Zhong X, Su X, Dou X and Ai J (2026) Frequency-reduction strategy of roxadustat in patients undergoing peritoneal dialysis: a multi-center retrospective cohort study. Front. Med. 12:1708916. doi: 10.3389/fmed.2025.1708916
Received
19 September 2025
Revised
09 December 2025
Accepted
15 December 2025
Published
14 January 2026
Volume
12 - 2025
Edited by
Daqing Hong, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, China
Reviewed by
Guisen Li, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, China
Savas Ozturk, Istanbul University, Türkiye
Updates
Copyright
© 2026 Mi, Lin, Fang, Zhu, Lin, Zhang, Zhong, Su, Dou and Ai.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Ai, aij1980@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.