Abstract
Background:
Elderly patients undergoing painless gastrointestinal endoscopy are at increased risk for sedation-related adverse events (SRAEs) because of their greater physiological vulnerability and higher likelihood of comorbidities. Risk stratification before endoscopy may improve perioperative safety and individualize sedation and management.
Objective:
This study aimed to develop and internally validate a Firth-penalized multivariable logistic regression model and nomogram to predict SRAEs in elderly patients undergoing painless gastrointestinal endoscopy.
Methods:
Prospective data from 520 patients at least 60 years old who underwent painless gastrointestinal endoscopy between April 2023 and June 2024 at our medical center were randomly divided into a training set (n = 364) and validation set (n = 156). SRAEs were defined as intraoperative hypotension or hypoxemia, and independent predictors of SRAEs in the training set were identified through Firth’s penalized multivariable logistic regression. A nomogram to predict risk of SRAEs was developed using R software and tested against the validation set. Its performance was assessed in terms of receiver operating characteristic curves, calibration plots, and decision curve analysis.
Results:
In the training set, hypotension and hypoxemia occurred in 39.0 and 33.5% of patients, respectively, the incidence of SRAEs was 45.6%. The independent predictors were older age, history of snoring, frailty, preexisting hypertension, chronic obstructive pulmonary disease, prolonged fasting before the procedure, and higher initial dose of etomidate-propofol. Conversely, regular physical activity was a protective factor. The nomogram built from the training set discriminated between people in the validation set who experienced SRAEs or not with an area under the curve of 0.92 (95% CI, 0.86–0.97), it showed good calibration in the Hosmer–Lemeshow test (P = 0.63), and decision curve analysis demonstrated clinical utility across a wide range of threshold probabilities (7–93%).
Conclusion:
A predictive model based on readily available clinical variables can accurately estimate SRAE risk in elderly patients undergoing painless gastrointestinal endoscopy. The model may be useful for individualizing sedation and patient management.
Clinical trial registration:
https://www.chictr.org.cn/showproj.html?proj=188331, identifier ChiCTR2300069816.
1 Introduction
Endoscopy has become a widely used clinical tool for the early detection of gastrointestinal malignancies, and a growing number of patients opt for sedation during the procedure to increase diagnostic accuracy and comfort (1). However, sedation increases the risk of hypotension and hypoxemia, and it can lead in rare cases to life-threatening cardiocerebrovascular events (2, 3). For example, the most frequently used sedative for painless gastrointestinal endoscopy is propofol, which causes hypotension in up to 36% of patients and hypoxemia in up to 21% of patients. The risk of these sedation-related adverse events (SRAEs) is likely to be higher among elderly than younger patients (4), because elderly are more likely to be experiencing respiratory depression, upper airway obstruction, decreased chest wall compliance (2, 3, 5), reduced cardiopulmonary reserve, impaired hepatic and renal function, and multiple chronic comorbidities. Therefore, early identification of elderly patients at high risk of SRAEs during painless gastrointestinal endoscopy is critical for individualizing peri-sedation management and improving patient safety.
Although studies have described models to predict risk of intra-procedural hypotension and other SRAEs (6, 7), the literature on SRAEs has not focused on elderly or taken into account variables from throughout the sedation process. Multivariable prediction models that integrate pre-procedural characteristics, intra-procedural management, and sedation-related factors may help refine risk stratification in this vulnerable population. Therefore, we took comprehensive account of peri-procedural variables and developed a Firth-penalized multivariable logistic regression prediction model and nomogram to estimate the risk of SRAEs in elderly patients undergoing painless gastrointestinal endoscopy, which we validated internally. This model is intended to support clinical decision-making and improve anesthetic care for elderly patients at high risk of sedation-related complications.
2 Materials and methods
2.1 Patients
This was a prospective, single-center, observational study conducted at Wenjiang District People’s Hospital of Chengdu. The study protocol was approved by the institutional ethics committee (approval EC-Research-2023–003), and the trial was prospectively registered in the Chinese Clinical Trial Registry (registration ChiCTR2300069816, registration date: March 27, 2023). Informed consent was obtained from all participants prior to enrollment.
Data were collected from a consecutive series of patients at least 60 years old who underwent painless gastrointestinal endoscopy between April 2023 and June 2024 at our hospital and who had an American Society of Anesthesiologists (ASA) physical status I–III at baseline. The case report form (CRF) used in this study was specifically developed for this project to collect demographic, clinical, anesthetic, and hemodynamic data of elderly patients undergoing painless gastrointestinal endoscopy. The CRF has not been previously published elsewhere. An English version of the CRF is provided as Supplementary File 1. Patients were excluded if they (1) were known to be allergic or hypersensitive to sedative or anesthetic agents; (2) were suffering from gastric retention or acute upper gastrointestinal bleeding involving excessive gastric content; (3) had a history of epilepsy, psychiatric disorders, hearing impairment, dementia, or other conditions affecting their compliance in the study; (4) experienced hemodynamic shock or instability before endoscopy; (5) had severe hepatic or renal insufficiency before endoscopy; (6) had systolic blood pressure > 160 mmHg or < 90 mmHg or diastolic blood pressure > 100 mmHg or < 60 mmHg before endoscopy; (7) had peripheral oxygen saturation (SpO2) < 90% with room air before endoscopy; or (8) received medications other than sedatives, such as norepinephrine spray, during endoscopy.
2.2 Minimal sample size
The planned sample size was determined a priori based on events-per-variable (EPV) (8, 9) considerations for multivariable logistic regression and the anticipated incidence of SRAEs in elderly patients undergoing painless gastrointestinal endoscopy. Assuming an SRAE incidence of approximately 30% in this high-risk population and a maximum of eight predictors in the final model, and adopting a conservative threshold of 15 events per variable, we required at least 120 SRAE events, corresponding to about 400 analyzable patients. Allowing for an anticipated 20% dropout or exclusion rate, the target enrollment was therefore set at a minimum of 500 elderly patients.
2.3 Sedation and management
Before endoscopy, all patients were evaluated at our anesthesia outpatient clinic, during which they provided written informed consent to undergo the procedure and to participate in our study. Patients fasted for at least 8 h and abstained from liquids for at least 4 h before the procedure. Patients scheduled for colonoscopy also received oral sodium phosphate solution and simethicone emulsion for bowel cleansing. In the procedure room, patients were positioned in the left lateral decubitus position, intravenous access was established via the right dorsal hand vein, and lactated Ringer’s solution was infused. Heart rate, SpO2 and non-invasive blood pressure on the left upper arm were monitored continuously. Oxygen was administered via nasal cannula at 2–3 L/min. Endoscopic procedures were performed by qualified endoscopists under the supervision of board-certified anesthesiologists, in accordance with standard diagnostic and therapeutic protocols.
Anesthesia was induced using intravenous sufentanil citrate at 0.05 μg/kg, followed by administration of 1% lidocaine at 0.3 mg/kg after 1.5–2 min, which was administered solely to attenuate the injection pain of the subsequent etomidate–propofol mixture, then slow injection of 0.2 mL/kg of an etomidate-propofol mixture that had been prepared by combining 10 mL propofol with 10 mL etomidate. Anesthesia was maintained by administering an additional 0.07 mL/kg of the etomidate-propofol mixture every 5 min. If the patient exhibited body movement or cough reflex during the procedure, additional etomidate-propofol mixture was given as needed at a dose of 0.07 mL/kg.
Intraoperative hypotension was treated with intravenous ephedrine 3–6 mg, while sinus bradycardia, defined as a heart rate below 50 bpm, was treated with intravenous atropine 0.3–0.5 mg. When SpO2 fell below 90%, hypoxemia was treated initially with manual chin lifting; if this proved ineffective, endoscopy was paused and oxygen was delivered under positive pressure via facemask until SpO2 ≥ 90%. After the procedure, patients were transferred to the post-anesthesia care unit, from which they were discharged in the company of a family member once they met the criteria routinely used at our hospital.
2.4 Definition of SRAEs
SRAEs in this study were defined as either hypotension or hypoxemia occurring between the initiation of anesthesia induction and patient discharge from the procedure room. Hypotension was defined as a decrease in systolic blood pressure by at least 20% from the level before anesthesia induction (10). Hypoxemia was defined as SpO2 of 75–89% for < 60 s, and severe hypoxemia is defined as SpO2 < 75% at any time (11).
2.5 Definition of potential risk factors of SRAEs
Potential risk factors of intraoperative SRAEs were extracted from the literature and our clinical experience and integrated into a standardized data collection form. When patients arrived in the waiting area before sedation and endoscopy, trained investigators conducted face-to-face interviews to obtain baseline information about these potential risk factors as well as demographic and clinical characteristics. The collected data included the following: sex, age, body mass index, physical status on the American Society of Anesthesiologists scale, smoking and alcohol history, physical activity level, history of snoring, frailty status, comorbidities, fasting time before the procedure, and type of endoscopic procedure. Smoking meant daily consumption of ≥ 10 cigarettes within 2 weeks before the procedure. Alcohol consumption meant drinking any type of alcohol at least twice per week during the 2 weeks prior to the procedure. Regular physical activity was defined as exercising for at least 30 min at least 3 times per week at moderate intensity (e.g., brisk walking) during the previous month (12). Snoring severity was graded on the following 4-point scale (13): grade 0, no snoring; grade 1, snoring without apnea or related symptoms; grade 2, loud snoring with symptoms (e.g., daytime fatigue) but no apnea; grade 3, snoring with apnea and evidence of end-organ damage. Frailty was assessed using the Fried Frailty Phenotype, and the scores were categorized as follows (14): 0 points, robust; 1–2 points, pre-frail; ≥ 3 points, frail. Prolonged fasting before the procedure was defined as an interval of more than 20 h between the last oral intake and the start of endoscopy. The following perioperative vital signs were recorded before anesthesia and immediately before endoscope insertion: systolic and diastolic blood pressure, mean arterial pressure, heart rate, and SpO2. Intraoperative adverse events included hypotension, hypoxemia, patient movement, sinus bradycardia, and repeated coughing, which was defined as ≥ 3 cough episodes during intubation. Patient discomfort immediately after the procedure was also documented.
2.6 Development and validation of a nomogram to predict SRAEs
The dataset was randomly divided into a training set (70%) and a validation set (30%) using a computer-generated allocation sequence, and patients were assigned to a group who experienced at least one SRAE or not. Data were compared between the two groups using SPSS 25.0 (IBM, Armonk, NY, United States). Continuous variables with a normal distribution were reported as mean ± standard deviation, and intergroup differences were assessed for significance using the independent-samples t-test. Continuous variables with a skewed distribution were reported as median and interquartile range (IQR), and intergroup differences were assessed using the Mann–Whitney U test. Categorical variables were reported as n (%), and differences were assessed using the χ2 test. Differences that were associated with two-sided P < 0.05 were considered statistically significant.
Variables differing significantly between groups in the training set at the significance level P < 0.05 were entered into Firth’s penalized multivariable logistic regression to identify independent risk factors of SRAEs, which were incorporated into a predictive nomogram constructed in R 4.2.0, The model was internally validated in the validation cohort using bootstrap resampling (1,000 iterations). The model’s performance in both training and validation sets was evaluated in terms of the area under the receiver operating characteristic curve (AUC), calibration plots, the Hosmer–Lemeshow goodness-of-fit test, and clinical utility based on decision curve analysis. Where appropriate, results were reported together with their associated 95% confidence interval (CI).
3 Results
Of the 578 elderly patients who underwent sedation-assisted gastrointestinal endoscopy at our hospital during the enrollment period, 58 (10.0%) were excluded because their medical data were incomplete or because they were lost to follow-up. The remaining 520, who were 68 ± 6.5 years old (range, 60–93 years) and 266 of whom were men, were included in the final analysis (Figure 1). Just over half the sample underwent both gastroscopy and colonoscopy (286, 55.0%), while a smaller proportion underwent only gastroscopy (174, 33.5%) and even fewer underwent only colonoscopy (60, 11.5%). Intraoperative hypotension occurred in 201 patients (38.7%), while intraoperative hypoxemia occurred in 166 (31.9%). Endotracheal intubation was not required during the study.
FIGURE 1

Flowchart of patient enrollment, allocation and analysis. SRAE, sedation-related adverse event.
3.1 Identification of risk factors for SRAEs
Patients were randomly assigned in a 7:3 ratio to either the training set (n = 364) or the validation set (n = 156), and the two sets did not differ significantly in any of the baseline clinicodemographic characteristics that we examined (Table 1). In contrast, several baseline variables differed significantly between patients who experienced at least one SRAE or not within the training and validation sets (Table 2). The variables that differed significantly in the training set were coded (Table 3) and entered into Firth-penalized multivariable logistic regression which identified the following independent risk factors for SRAEs (Table 4): advanced age, history of snoring, frailty, preoperative hypertension, chronic obstructive pulmonary disease, prolonged fasting before the procedure, and increased initial dose of etomidate-propofol. Conversely, regular physical activity was found to be a protective factor.
TABLE 1
| Characteristic | Training set (n = 364) |
Validation set (n = 156) |
t/χ2/z | P |
|---|---|---|---|---|
| Sex | 0.529 | 0.467 | ||
| Men | 190 (52.2) | 76 (48.7) | ||
| Women | 174 (47.8) | 80 (51.3) | ||
| Age, yr | 68 ± 7 | 68 ± 6 | 0.734 | 0.511 |
| Body mass index, kg/m2 | 23.2 ± 2.8 | 23.7 ± 3.5 | 1.572 | 0.117 |
| Status on the American Society of Anesthesiologists scale | 1.467 | 0.690 | ||
| I | 25 (6.9) | 9 (5.8) | ||
| II | 250 (68.7) | 102 (65.4) | ||
| III | 89 (24.5) | 45 (28.9) | ||
| Smoking | 54 (14.8) | 16 (10.3) | 1.965 | 0.161 |
| Alcohol consumption | 94 (25.8) | 41 (26.3) | 0.012 | 0.913 |
| Regular physical activity | 262 (72.0) | 106 (68.0) | 0.857 | 0.355 |
| History of snoring | 6.449 | 0.092 | ||
| None | 204 (56.0) | 95 (60.9) | ||
| Mild (grade 1) | 104 (28.6) | 29 (18.6) | ||
| Moderate (grade 2) | 38 (10.4) | 21 (13.5) | ||
| Severe (grade 3) | 18 (5.0) | 11 (7.1) | ||
| Frailty | 2.361 | 0.307 | ||
| Robust | 131 (36.0) | 48 (30.8) | ||
| Pre-frail | 182 (50.0) | 79 (50.6) | ||
| Frail | 51 (14.0) | 29 (18.6) | ||
| Comorbidities | ||||
| Hypertension | 119 (32.7) | 58 (37.2) | 0.979 | 0.322 |
| Coronary artery disease | 57 (15.7) | 24 (15.4) | 0.006 | 0.937 |
| COPD | 152 (41.8) | 59 (37.8) | 0.702 | 0.402 |
| Renal insufficiency | 34 (9.3) | 15 (9.6) | 0.010 | 0.922 |
| Diabetes mellitus | 55 (15.1) | 20 (12.8) | 0.464 | 0.496 |
| Fasting time before procedure, h | 17(14.0,18.0) | 17(16.0,18.0) | 1.430 | 0.153 |
| Endoscopy type | 1.671 | 0.434 | ||
| Gastroscopy | 121 (33.2) | 53 (34.0) | ||
| Colonoscopy | 38 (10.4) | 22 (14.1) | ||
| Both | 205 (56.3) | 81 (51.9) | ||
| Sufentanil dosage, μg | 2.3 ± 0.7 | 2.2 ± 0.6 | 0.482 | 0.630 |
| Initial EP mixture, mL | 12 ± 1.9 | 11.9 ± 2.1 | 0.476 | 0.634 |
| Change from baseline (%) in | ||||
| Systolic blood pressure | 17.0 (11.0, 23.0) | 18.0 (12.0, 22.0) | −1.274 | 0.203 |
| Diastolic blood pressure | 18.0 (10.0, 25.0) | 18(11.0, 24.0) | −0.860 | 0.390 |
| Heart rate | 0.0 (13.0) | 0.0 (9.2) | −0.317 | 0.752 |
| Hypotension | 142 (39.0) | 59 (37.8) | 0.065 | 0.798 |
| Hypoxemia | 122 (33.5) | 44 (28.2) | 1.418 | 0.234 |
| Body movement | 21 (5.8) | 11 (7.1) | 0.311 | 0.577 |
| Coughing | 11 (3.0) | 4 (2.6) | 0.082 | 0.775 |
Baseline characteristics of patients in the training and validation sets.
The values are descriptive statistics for the entire training and validation cohorts. Values are n (%), mean ± SD, or median (interquartile range), unless otherwise noted. COPD, chronic obstructive pulmonary disease; EP, etomidate-propofol.
TABLE 2
| Characteristic | Training set | P | Validation set | P | ||
|---|---|---|---|---|---|---|
| No SRAE (n = 198) |
SRAE (n = 166) |
No SRAE n = 95 |
SRAE n = 61 |
|||
| Sex | 0.548 | 0.701 | ||||
| Men | 100(50.5) | 90(54.2) | 44(46.3) | 31(50.8) | ||
| Women | 98(49.5) | 76(45.8) | 51(53.7) | 30(49.2) | ||
| Age, yr | 67.9 ± 5.6 | 69.3 ± 7.2 | 0.033 | 65.8 ± 5.9 | 68.7 ± 6.6 | 0.006 |
| Body mass index, kg/m2 | 22.8 ± 2.8 | 23.5 ± 2.6 | 0.014 | 22.8 ± 3.8 | 23.8 ± 3.3 | 0.084 |
| Status on the American Society of Anesthesiologists scale | 0.014 | 0.012 | ||||
| I | 12(6.0) | 13(7.8) | 3(3.2) | 6(9.8) | ||
| II | 116(58.6) | 134(80.7) | 57(60.0) | 45(73.8) | ||
| III | 70(35.3) | 19(11.4) | 35(36.8) | 10(16.4) | ||
| Smoking | 2(1.0) | 52(30.7) | < 0.001 | 2(2.1) | 14(23.0) | < 0.001 |
| Alcohol consumption | 38(19.2) | 56(33.7) | 0.002 | 38(40.0) | 5(8.2) | < 0.001 |
| Regular physical activity | 189(95.5) | 73(44.0) | < 0.001 | 87(91.6) | 19(31.1) | < 0.001 |
| History of snoring | < 0.001 | <0.001 | ||||
| None | 133(67.2) | 64(38.6) | 71(74.7) | 28(45.9) | ||
| Mild (grade 1) | 63(31.8) | 49(29.5) | 24(25.3) | 15(24.6) | ||
| Moderate (grade 2) | 2(1.0) | 35(21.1) | 0(0.0) | 11(18.0) | ||
| Severe (grade 3) | 0(0.00) | 18(10.9) | 0(0.0) | 7(11.5) | ||
| Frailty | < 0.001 | <0.001 | ||||
| Robust (0 points) | 81(41.0) | 51(30.7) | 40(42.1) | 19(31.2) | ||
| Pre-frail | 117(59.1) | 64(38.6) | 52(54.7) | 16(26.2) | ||
| Frail | 0(0.00%) | 51(30.7) | 3(3.2) | 26(42.6) | ||
| Comorbidities | ||||||
| Hypertension | 54(27.3) | 65(38.6) | 0.029 | 36(37.9) | 18(29.5) | 0.367 |
| Coronary artery disease | 16(9.1) | 36(23.5) | < 0.001 | 17(17.9) | 7(11.5) | 0.608 |
| COPD | 30(15.2) | 122(73.5) | <0.001 | 15(15.8) | 44(72.1) | < 0.001 |
| Renal insufficiency | 21(12.1) | 12(7.8) | 0.241 | 9(11.6) | 6(11.5) | 0.454 |
| Diabetes mellitus | 30(15.2) | 25(15.7) | 0.543 | 10(10.5) | 10(16.4) | 0.409 |
| Fasting time before procedure, h | 17(16.0,18.0) | 17(15.0,19.0) | 0.02 | 17(16.0,19.0) | 17(13.0,18.0) | 0.01 |
| Endoscopy type | 0.514 | 0.92 | ||||
| Gastroscopy | 66(38.4) | 53(25.9) | 34(35.8) | 20(32.8) | ||
| Colonoscopy | 20(10.1) | 17(10.2) | 13(13.7) | 9(14.8) | ||
| Both | 112(51.5) | 96(63.8) | 48(50.5) | 32(52.5) | ||
| Sufentanil dosage, μg | 2.2 ± 0.5 | 2.4 ± 0.7 | 0.001 | 2.1 ± 0.5 | 2.3 ± 0.7 | 0.125 |
| Initial EP mixture, mL | 12.0 ± 1.5 | 12.3 ± 1.9 | 0.037 | 11.9 ± 1.3 | 12.4 ± 1.8 | 0.081 |
| Change from baseline in (%) | ||||||
| Systolic blood pressure | 17 (10.5, 23.5) | 17 (11.0, 23.0) | 0.534 | 18 (14.0,23.0) | 17 (13.0,22.0) | 0.513 |
| Diastolic blood pressure | 17 (10.0,24.0) | 19 (11.0, 26.0) | 0.185 | 18 (12.0, 24.0) | 18 (12.0,26.0) | 0.893 |
| Heart rate | 1 (−9.0,5.0) | −1.0 (−9.0, 5.0) | 0.093 | 0 (−5.0,3.0) | 0(−5.0,7.0) | 0.437 |
Comparison of baseline characteristics and perioperative variables between patients who experienced at least one SRAE or not in the training and validation sets.
ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; EP, etomidate-propofol; SRAE, sedation-related adverse event.
TABLE 3
| Variable | Name | Description |
|---|---|---|
| Sex | X1 | Male = 1, Female = 2 |
| Age, yr | X2 | 60–69 = 1, 70–79 = 2, 80 + = 3 |
| Body mass index, kg/m2 | X3 | <24 = 1, 24–28 = 2, > 28 = 3 |
| ASA classification | X4 | I-II = 1, III = 2 |
| Smoking | X5 | No = 0, Yes = 1 |
| Alcohol consumption | X6 | No = 0, Yes = 1 |
| Regular physical activity | X7 | No exercise = 0, Regular exercise = 1 |
| History of snoring | X8 | None = 0, Mild = 1, Moderate = 2, Severe = 3 |
| Frailty status | X9 | Robust = 0, Pre-frail = 1, Frail = 2 |
| Hypertension | X10 | None = 0, Stage 1 = 1, Stage 2 = 2 |
| Coronary artery disease | X11 | No = 0, Yes = 1 |
| COPD | X12 | No = 0, Yes = 1 |
| Renal insufficiency | X13 | No = 0, Yes = 1 |
| Diabetes mellitus | X14 | No = 0, Yes = 1 |
| Fasting time before procedure | X15 | ≤ 20 h = 0, > 20 h = 1 |
| Endoscopy type | X16 | Gastroscopy = 0, Colonoscopy = 1, Both = 2 |
| Sufentanil dosage, μg | X17 | 1–3 μg = 0, 4–5 μg = 1 |
| Initial EP dose, mL | X18 | ≤8 mL = 0, 9–15 mL = 1, > 15 mL = 2 |
Definitions of variables in logistic regression.
ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; EP, etomidate-propofol.
TABLE 4
| Variable | B | Wald | P | Exp (B) | 95% confidence interval |
|---|---|---|---|---|---|
| Age | 1.56 | 9.52 | 0.01 | 4.76 | 1.66–8.66 |
| Regular physical activity | −2.35 | 5.02 | 0.02 | 0.09 | 0.04–0.38 |
| History of snoring | 1.76 | 12.40 | 0.001 | 5.83 | 1.80–7.29 |
| Frailty | 1.92 | 4.28 | 0.03 | 6.84 | 2.33–9.50 |
| Hypertension | 1.32 | 5.76 | 0.02 | 3.74 | 1.81–6.10 |
| Chronic obstructive pulmonary disease | 0.80 | 4.98 | 0.02 | 2.23 | 1.33–7.21 |
| Fasting time before procedure | 1.40 | 6.32 | 0.003 | 4.04 | 3.31–10.32 |
| Initial etomidate-propofol dose | 2.21 | 8.85 | 0.007 | 9.14 | 3.94–13.73 |
| Constant | −5.732 | 23.72 | 0.001 | 0.001 | Not applicable |
Firth-penalized multivariable logistic regression to identify risk factors of sedation-related adverse events in the training set.
OR and 95% CI were estimated using Firth’s penalized-likelihood logistic regression to reduce small-sample bias and potential quasi-complete separation.
3.2 Development and internal validation of a nomogram to predict SRAEs
A nomogram was constructed based on the eight independent risk factors of SRAEs identified through Firth-penalized multivariable logistic regression (Figure 2). ROC analysis demonstrated excellent discriminative ability of the Firth-penalized logistic regression model (Figure 3). In the training set, the area under the ROC curve (AUC) was 0.96 (95% CI, 0.93–0.98). In the independent validation set, the AUC remained high at 0.92 (95% CI, 0.86–0.97), indicating only a modest decrease in performance. These findings suggest that the nomogram provides strong and stable discrimination between patients with and without SRAEs in both the development and validation cohorts.
FIGURE 2

Nomogram to predict sedation-related adverse events in elderly patients undergoing painless gastrointestinal endoscopy. The nomogram incorporates eight independent risk factors identified through multivariable logistic regression of data in the training set. COPD, chronic obstructive pulmonary disease.
FIGURE 3

Receiver operating characteristic curves to assess the ability of the nomogram to predict sedation-related adverse events in the training set (blue) or validation set (orange). The areas under the curves and corresponding 95% confidence intervals are shown at the lower right. The diagonal dotted line represents a non-discriminative model (AUC = 0.5).
Next, calibration plots showed good agreement between the predicted and observed probabilities of SRAEs in both the training and validation sets (Figure 4). In the training set, the bootstrapped calibration curve nearly overlapped the 45° ideal line across the full range of predicted risks, indicating minimal optimism and excellent calibration. In the validation set, the calibration curve also closely followed the ideal line, with only slight deviations at intermediate predicted probabilities, suggesting that the Firth-penalized logistic regression model provides reliable absolute risk estimates in an independent cohort. The Hosmer–Lemeshow test showed P = 0.41 in the training set and P = 0.63 in the validation set, indicating good agreement between the predicted and observed probabilities.
FIGURE 4

Calibration curves of the nomogram when applied to the (A) training set or (B) validation set. The diagonal dotted line represents perfect prediction.
3.3 Clinical utility of the nomogram
Decision curves indicated that the nomogram showed good clinical utility across a broad range of threshold probabilities, whether in the training set (5–96%) or validation set (7–93%) (Figure 5).
FIGURE 5
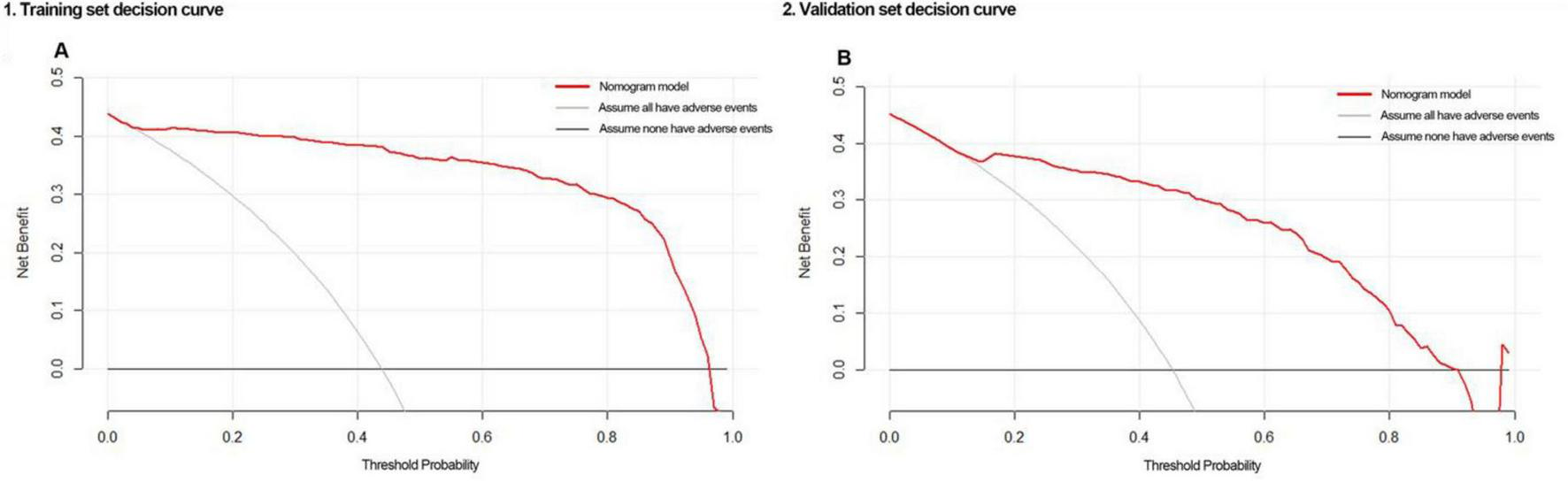
Decision curves of the nomogram when applied to the (A) training set or (B) validation set.
4 Discussion
In this study, perioperative data from 520 elderly patients undergoing painless gastrointestinal endoscopy were used to develop a nomogram to predict SRAEs based on eight independent risk factors, which performed well in internal validation and showed clinical utility across a broad range of threshold probabilities. This model may help identify individuals at high risk of SRAEs and personalize perioperative procedures. Because the nomogram incorporates the initial etomidate–propofol dose, it is best interpreted as an intra-procedural risk to assess the ability ment tool rather than a purely pre-procedural screening model. In practice, this model can help inform the choice of sedation strategy, further refine risk stratification, and guide subsequent intra-procedural management.
Anesthesia in our study was induced with intravenous sufentanil citrate 0.05 μg/kg and lidocaine 0.3 mg/kg, followed by a slow bolus of a 1:1 etomidate–propofol mixture (0.2 mL/kg) and top-up doses of 0.07 mL/kg every 5 min as needed. This regimen corresponds to deep sedation without tracheal intubation and is widely used in China because etomidate provides relatively stable hemodynamics (15), whereas propofol increases risk of hypotension and respiratory depression (16, 17) but offers rapid onset and recovery. However, our observed incidences of intraoperative hypotension (38.7%) and hypoxemia (31.9%) indicate that this protocol is not risk-free in elderly patients with multiple comorbidities. Despite standardized treatment of hypotension with intravenous ephedrine (3–6 mg) and of sinus bradycardia with atropine (0.3–0.5 mg), clinically relevant hemodynamic and respiratory events remained common in this vulnerable population. Compared with previous studies of propofol-based deep sedation in mixed-age adults undergoing gastrointestinal endoscopy, where hypotension and hypoxemia are typically reported in up to approximately 36 and 20% of patients (2, 3), respectively, our rates are at the upper end of these ranges. This likely reflects the higher baseline risk in our cohort, which consisted exclusively of patients ≥ 60 years with a substantial burden of hypertension and chronic obstructive pulmonary disease and relatively long fasting times. Importantly, we identified a higher initial etomidate–propofol dose as an independent predictor of SRAEs, highlighting that the choice and dosing of sedative agents are modifiable risk factors. These findings suggest that, in elderly patients, more conservative dosing strategies and careful titration of etomidate–propofol, or the use of alternative sedative regimens, should be considered to reduce the risk of hypotension and hypoxemia.
The independent predictors of SRAEs in our sample are consistent with age-related decline. As individuals grow older, arteriosclerosis reduces vascular elasticity and the autonomic regulatory system becomes weaker, increasing risk of intraoperative hypotension. Frailty is associated with lower physiological reserves across multiple systems, rendering patients more vulnerable to the hemodynamic stress of anesthesia induction (18, 19). For example, frailty may reduce oxygenation (20), increasing the risk of intraoperative hypoxemia. Indeed, hypotension and hypoxemia were more frequent among our frail patients than among non-frail patients, raising the possibility that conventional anesthesia protocols may fail to meet the perfusion and oxygenation demands of this population. Future research should explore this in detail.
Longer fasting time before endoscopy was associated with higher risk of SRAEs in our sample. Elderly patients may be more sensitive to fluid imbalance than younger individuals (21), and the hypovolemia arising from extended fasting may exacerbate the already strong variations in blood pressure and heart rate that older patients experience during gastroscopy (22). It may be necessary to tailor fluid management strategies for elderly undergoing gastroscopy, which should be explored in future work.
History of snoring predicted intraoperative hypoxemia in our sample, consistent with the known association between such a history and risk of airway obstruction during anesthesia due to mandibular relaxation, posterior tongue displacement or upper airway collapse (23, 24). Chronic obstructive pulmonary disease also predicted intraoperative hypoxemia in our sample, probably reflecting that compromised alveolar function, ventilation–perfusion mismatch, chronic inflammation, and airway hyperresponsiveness in COPD can further impair oxygenation during anesthesia (25).
Regular physical activity protected against SRAEs in our sample. Moderate exercise is known to enhance cardiopulmonary function, improve oxygen uptake and use, delay age-related organ decline, and promote metabolic efficiency. Exercise-based interventions can improve postoperative outcomes and reduce risk of complications in elderly with gastrointestinal malignancies (26). Our study extends these findings to the context of sedation for painless endoscopy. Exercise and potentially other lifestyle factors may be important to consider when stratifying patients by SRAE risk and when planning their sedation before the procedure and their management afterward.
Our study substantially extends the literature on SRAEs by focusing on elderly and defining a composite endpoint of either hypotension or hypoxemia, rather than only one SRAE (27). It considered factors related to lifestyle (e.g., physical activity, snoring), physiological status (frailty), and the perioperative experience (preoperative fasting time, initial dose of etomidate-propofol) when predicting SRAE risk. Our finding that fasting time before the procedure appeared in the final nomogram underscores the importance of proper preparation of elderly patients before the procedure.
At the same time, our findings should be interpreted with caution because they are derived from a relatively small, single-center cohort. All patients were elderly Chinese individuals treated at Wenjiang District People’s Hospital of Chengdu, an urban–suburban setting with a relatively high prevalence of hypertension and chronic obstructive pulmonary disease, which may limit the generalizability of our results to other regions and ethnicities. The incidences of intraoperative hypotension (38.7%) and hypoxemia (31.9%) in this cohort are at the upper end of the ranges reported in mixed-age populations undergoing propofol-based sedation for gastrointestinal endoscopy [hypotension up to ∼36% and hypoxemia around 20% (2, 3)]. This likely reflects the inclusion of only patients ≥ 60 years, the higher burden of cardiovascular and respiratory comorbidities, and relatively long fasting times (median 17 h). Thus, although the identified predictors of SRAEs—such as age, snoring, frailty, hypertension, COPD, and prolonged fasting—are biologically plausible and probably relevant across settings, the absolute risk estimates from our nomogram should be externally validated and, if necessary, recalibrated in other hospitals, regions, and ethnic groups before routine clinical use. In addition, although our model showed excellent internal discrimination, the very high AUC values and large odds ratios for some predictors raise concerns about overfitting or quasi-complete separation. We therefore refitted the model using Firth’s penalized-likelihood logistic regression, which produced attenuated but still significant effects for key predictors and more conservative estimates, although residual overfitting cannot be excluded. Furthermore, we categorized several continuous predictors such as age and BMI using fixed cutoffs to improve clinical interpretability and to maintain model stability with a relatively modest number of events. This approach may have led to some loss of information and reduced statistical power, and future multicenter studies should consider modeling these predictors as continuous variables using flexible, data-driven approaches (e.g., splines) to better capture potential non-linear relationships.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Ethics Committee of Wenjiang District People’s Hospital of Chengdu. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LX: Funding acquisition, Writing – original draft, Supervision, Writing – review & editing. QY: Writing – original draft, Formal analysis, Data curation, Writing – review & editing. HL: Resources, Funding acquisition, Supervision, Writing – review & editing. QL: Resources, Funding acquisition, Writing – review & editing. HZ: Writing – review & editing, Writing – original draft.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by the Health Commission of Sichuan Province Medical Science and Technology Program (24QNMP067), the Medical Research Subject of Chengdu Health Commission (2024083 and 2022037), and the Medical Research Project of Sichuan Medical Association (S2024086).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1713720/full#supplementary-material
References
1.
Zhou S Zhu Z Dai W Qi S Tian W Zhang Y et al National survey on sedation for gastrointestinal endoscopy in 2758 Chinese hospitals. Br J Anaesth. (2021) 127:56–64. 10.1016/j.bja.2021.01.028
2.
Sneyd J Absalom A Barends C Jones J . Hypotension during propofol sedation for colonoscopy: a retrospective exploratory analysis and meta-analysis.Br J Anaesth. (2022) 128:610–22. 10.1016/j.bja.2021.10.044
3.
Wang L Zhang Y Han D Wei M Zhang J Cheng X et al Effect of high flow nasal cannula oxygenation on incidence of hypoxia during sedated gastrointestinal endoscopy in patients with obesity: multicentre randomised controlled trial. BMJ. (2025) 388:e080795. 10.1136/bmj-2024-080795
4.
Shimizu H Homma Y Norii T . Incidence of adverse events among elderly vs non-elderly patients during procedural sedation and analgesia with propofol.Am J Emerg Med. (2021) 44:411–4. 10.1016/j.ajem.2020.04.094
5.
Qadeer M Lopez A Dumot J Vargo J . Hypoxemia during moderate sedation for gastrointestinal endoscopy: causes and associations.Digestion. (2011) 84:37–45. 10.1159/000321621
6.
Geng W Jia D Wang Y Jin S Ren Y Liang D et al A prediction model for hypoxemia during routine sedation for gastrointestinal endoscopy. Clinics. (2018) 73:e513. 10.6061/clinics/2018/e513
7.
Wang Z Ma J Liu X Gao J . Development and validation of a predictive model for PACU hypotension in elderly patients undergoing sedated gastrointestinal endoscopy.Aging Clin Exp Res. (2024) 36:149. 10.1007/s40520-024-02807-6
8.
Peduzzi P Concato J Kemper E Holford T Feinstein AR . A simulation study of the number of events per variable in logistic regression analysis.J Clin Epidemiol. (1996) 49:1373–9. 10.1016/s0895-4356(96)00236-3
9.
Vittinghoff E McCulloch C . Relaxing the rule of ten events per variable in logistic and Cox regression.Am J Epidemiol. (2007) 165:710–8. 10.1093/aje/kwk052
10.
Liu X Gao Z Jiang Y Tuo X He S Xu F et al Comparison of low-frequency or high-frequency electrical acupoint stimulation on hypotension after spinal anesthesia in parturients: a prospective randomized controlled clinical trial. J Integr Complement Med. (2024) 30:770–5. 10.1089/jicm.2023.0610
11.
Shao L Liu S Liu F Zou Y Hou H Tian M et al Comparison of two supplement oxygen methods during gastroscopy with intravenous propofol anesthesia in obese patients: study protocol for a randomized controlled trial. Trials. (2018) 19:602. 10.1186/s13063-018-2994-8
12.
Duan Y Lu G . A randomized controlled trial to determine the impact of resistance training versus aerobic training on the management of FGF-21 and related physiological variables in obese men with type 2 diabetes mellitus.J Sports Sci Med. (2024) 23:495–503. 10.52082/jssm.2024.495
13.
Netzer N Stoohs R Netzer C Clark K Strohl K . Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome.Ann Intern Med. (1999) 131:485–91. 10.7326/0003-4819-131-7-199910050-00002
14.
Geladari E Alexopoulos T Vasilieva L Tenta R Mani I Sevastianos V et al Evaluation of five screening tools in detecting physical frailty in cirrhosis and their prognostic role. J Clin Med. (2024) 13:5169. 10.3390/jcm13175169
15.
Zheng Z Su Y Fan X Zhang W Li J Xue S . BIS feedback closed-loop target-controlled infusion of propofol or etomidate in elderly patients with spinal surgery.Am J Transl Res. (2023) 15:1231–8.
16.
Gan T Bertoch T Habib A Yan P Zhou R Lai Y et al Comparison of the efficacy of HSK3486 and propofol for induction of general anesthesia in adults: a multicenter, randomized, double-blind, controlled, phase 3 noninferiority trial. Anesthesiology. (2024) 140:690–700. 10.1097/ALN.0000000000004886
17.
Barbosa E Espírito Santo P Baraldo S Meine G . Remimazolam versus propofol for sedation in gastrointestinal endoscopic procedures: a systematic review and meta-analysis.Br J Anaesth. (2024) 132:1219–29. 10.1016/j.bja.2024.02.005
18.
Daum N Hoff L Spies C Pohrt A Bald A Langer N et al Influence of frailty status on the incidence of intraoperative hypotensive events in elective surgery: hypo-frail, a single-centre retrospective cohort study. Br J Anaesth. (2025) 135:40–7. 10.1016/j.bja.2024.10.050
19.
Schnetz M Zakaria L Ahuja S Khanna A . Frailty and intraoperative hypotension: is the risk significant and modifiable?Br J Anaesth. (2025) 135:1–4. 10.1016/j.bja.2025.05.002
20.
Verduri A Clini E Carter B Hewitt J . Influence of frailty on cardiovascular events and mortality in patients with Chronic obstructive pulmonary disease (COPD): study protocol for a multicentre European observational study.PLoS One. (2024) 19:e0300945. 10.1371/journal.pone.0300945
21.
Mladinov D Isaza E Gosling A Clark A Kukreja J Brzezinski M . Perioperative fluid management.Clin Geriatr Med. (2025) 41:83–99. 10.1016/j.cger.2024.03.008
22.
Deng R Wu J Xu K Sun F Chang F . The impact of early gastroscopy examination on cardiovascular event-related indices in elderly patients with acute upper gastrointestinal bleeding.Medicine. (2024) 103:e37378. 10.1097/MD.0000000000037378
23.
Seet E Waseem R Chan M Wang C Liao V Suen C et al Characteristics of patients with unrecognized sleep apnea requiring postoperative oxygen therapy. J Pers Med. (2022) 12:1543. 10.3390/jpm12101543
24.
Kandasamy G Almeleebia T . A prospective study on obstructive sleep apnea, clinical profile and polysomnographic variables.J Pers Med. (2023) 13:919. 10.3390/jpm13060919
25.
Owens R Derom E Ambrosino N . Supplemental oxygen and noninvasive ventilation.Eur Respir Rev. (2023) 32:220159. 10.1183/16000617.0159-2022
26.
Hamad A Zhang H Zhang Y Shen C Fa P Huang H et al Understanding the mechanism behind preoperative exercise therapy in patients with gastrointestinal cancers: a prospective randomized clinical trial. BMC Sports Sci Med Rehabil. (2025) 17:50. 10.1186/s13102-025-01094-6
27.
Zheng L Wu X Gu W Wang R Wang J He H et al Development and validation of a hypoxemia prediction model in middle-aged and elderly outpatients undergoing painless gastroscopy. Sci Rep. (2025) 15:17965. 10.1038/s41598-025-02540-8
Summary
Keywords
sedation, gastrointestinal endoscopy, elderly patients, hypotension, hypoxemia
Citation
Xu L, Yin Q, Liu H, Liu Q and Zhang H (2026) A nomogram for predicting sedation-related adverse events in elderly patients undergoing painless gastrointestinal endoscopy. Front. Med. 12:1713720. doi: 10.3389/fmed.2025.1713720
Received
26 September 2025
Revised
25 November 2025
Accepted
19 December 2025
Published
12 January 2026
Volume
12 - 2025
Edited by
Somchai Amornyotin, Mahidol University, Thailand
Reviewed by
John Sieh Dumbuya, Affiliated Hospital of Guangdong Medical University, China
Gawel Solowski, Bingöl University, Türkiye
Updates
Copyright
© 2026 Xu, Yin, Liu, Liu and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyan Zhang, 13708206443@163.comQian Liu, 396277067@qq.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.