Abstract
Background:
Chronic hepatitis B virus (HBV) infection remains a major global health challenge. Peginterferon-α (PEG-IFN-α) and nucleos(t)ide analogs (NAs) are standard therapies used to suppress HBV replication. Although HBsAg clearance and seroconversion are ideal therapeutic endpoints, whether PEG-IFN-α combined with NAs offers superior efficacy compared with PEG-IFN-α monotherapy remains controversial.
Methods:
Controlled clinical trials published between 2000 and 2025 that compared PEG-IFN-α plus NA combination therapy with PEG-IFN-α monotherapy in patients with chronic hepatitis B, with treatment duration ≥ 48 weeks, were included. The primary outcomes were HBsAg clearance and seroconversion.
Results:
Eleven trials involving 2,439 patients were identified and analyzed. After 48 weeks of treatment, the HBsAg clearance rate was significantly higher in the combination therapy group than in the monotherapy group [odds ratio (OR) = 1.59, 95% confidence interval (CI): 1.01–2.52, P = 0.05]. However, no significant difference in HBsAg clearance rates was observed between the two groups at 24 weeks of post-treatment follow-up (OR = 1.33, 95% CI: 0.76–2.33, P = 0.31). Likewise, no significant differences were found in HBsAg seroconversion rates after 48 weeks of treatment or at 24 weeks post-treatment follow-up (OR = 1.77, 95% CI: 0.94–3.32, P = 0.08; and OR = 1.42, 95% CI: 0.88–2.28, P = 0.15, respectively).
Conclusion:
PEG-IFN-α promotes HBsAg clearance and seroconversion. Combination therapy with PEG-IFN-α plus NAs yields higher short-term clearance than monotherapy; however, sustained benefits appear to require prolonged treatment.
1 Introduction
Chronic hepatitis B virus (HBV) infection remains a major global public health concern, affecting millions of individuals worldwide (1, 2). HBV reactivation can lead to liver injury and clinically manifests as hepatitis (3, 4). Antiviral therapy is recognized as the first-line treatment for chronic hepatitis B (CHB) (5). Although hepatitis B e-antigen (HBeAg) seroconversion is considered an important treatment endpoint in HBeAg-positive CHB (5, 6), the clearance and seroconversion of hepatitis B surface antigen (HBsAg) represent the outcomes most closely associated with a functional cure (7, 8). Current therapeutic options for CHB primarily include interferon-based therapy and oral nucleos(t)ide analogs (NAs). Peginterferon-α (PEG-IFN-α) is widely used to suppress HBV replication and reduce liver inflammation and fibrosis progression (9, 10). Although both PEG-IFN-α and NAs are established first-line therapies, international guidelines recommend PEG-IFN-α for appropriately selected patients because of its finite treatment duration and potential to induce durable immunologic control (11, 12).
Several clinical trials have examined whether combining PEG-IFN-α with NAs offers superior efficacy compared with PEG-IFN-α monotherapy (8, 13, 14). However, evidence regarding the benefit of combination therapy for enhancing HBsAg clearance or seroconversion remains inconsistent. In line with current international guidelines (e.g., EASL, AASLD), PEG-IFN-α or NAs are recommended as monotherapy for most patients with chronic hepatitis B, rather than as first-line combination therapy, based on considerations of efficacy, safety, and cost-effectiveness. Nevertheless, the potential of combination strategies to improve rates of functional cure remains a subject of ongoing debate among experts. Therefore, in this study, we conducted a systematic review and meta-analysis of controlled clinical trials to evaluate whether combination therapy with PEG-IFN-α plus NAs provides additional benefit over PEG-IFN-α monotherapy in achieving HBsAg clearance or seroconversion in patients with CHB.
2 Materials and methods
2.1 Literature search and study design
Eligible trials published between January 1995 and March 29, 2025, were identified through systematic searches of the following electronic databases: PubMed/Medline, EMBASE, the Cochrane Library, ClinicalKey and Web of Science databases. Discrepancies in study selection were resolved through discussion among the reviewers. The search strategy used combinations of the following keywords: “HBsAg,” “chronic hepatitis B,” “hepatitis B,” “HBV,” “peginterferon,” and “pegylated interferon.” In addition, the reference lists of relevant articles and conference proceedings were manually searched to identify any additional eligible studies. Studies were categorized into two intervention groups: PEG-IFN-α plus NA combination therapy and PEG-IFN-α monotherapy. Data were extracted according to predefined efficacy outcomes and study characteristics, and any data discrepancies were resolved by consensus among the reviewers. Separate meta-analyses were conducted for each outcome of interest.
2.2 Inclusion and exclusion criteria
We included trials that compared PEG-IFN-α plus NA combination therapy with PEG-IFN-α monotherapy therapy in patients with CHB. Two reviewers independently screened and assessed all retrieved records for eligibility. Studies were included if they met all of the following criteria: (1) Published in English; (2) Controlled clinical trial design; (3) Study population consisting of CHB patients; (4) Intervention comparing PEG-IFN-α plus NA combination therapy with PEG-IFN-α monotherapy; (5) Availability of valid outcome data, either directly reported or derivable; (6) In cases of duplicate publications, only the report with the highest methodological quality was included; (7) Reported outcomes included HBsAg clearance or seroconversion.
Studies were excluded if they met any of the following criteria: (1) Compared conventional IFN-α plus NA with conventional IFN-α monotherapy; (2) Preclinical studies involving animals, cell lines, or other non-human models; (3) Included patients with liver transplantation or co-infection with hepatitis C, hepatitis D, or human immunodeficiency virus; (4) Included patients with a history of alcohol or drug abuse, hepatocellular carcinoma, decompensated liver disease, or severe medical or psychiatric illness; (5) Concurrent use of corticosteroids or immunosuppressive agents; (6) Did not report outcomes related to HBsAg clearance or seroconversion; (7) Reported outcomes unclearly or incompletely; (8) Duplicate publications or lack of accessible full text. When necessary, study investigators were contacted to obtain missing data. The methodological quality of each included study was assessed using the Jadad quality scale, with scores ≥ 3 considered high quality (9, 10). The literature search was restricted to studies published in English.
2.3 Outcome measures
The primary outcomes were HBsAg clearance and seroconversion. HBsAg clearance was defined as the complete loss of HBsAg from the serum. HBsAg seroconversion was defined as HBsAg loss accompanied by the presence of anti-HBs antibodies. Secondary outcomes included virological and serological responses (HBeAg loss and HBeAg seroconversion), the proportion of patients achieving HBV DNA levels < 400 copies/mL, and biochemical response, defined as normalization of alanine aminotransferase (ALT).
2.4 Statistical analysis
This meta-analysis evaluated differences in treatment efficacy between PEG-IFN-α monotherapy and PEG-IFN-α plus NA combination therapy in CHB patients. Statistical analyses and forest plot construction were performed using Review Manager (RevMan) software (version 5.4.1) (15). Pooled outcomes included HBsAg clearance and seroconversion, HBeAg clearance and seroconversion, the proportion of patients receiving HBV DNA < 400 copies/mL, and ALT normalization at 48 and 72 weeks. Effect sizes were summarized using odds ratio (OR) with corresponding 95% confidence interval (95% CI). Fixed- or random-effects models were selected according to the level of heterogeneity.
Statistical heterogeneity across studies was assessed using the Chi-square test and quantified with the I2 statistic, which ranges from 0 to 100%. Heterogeneity was considered significant when P < 0.10. A fixed-effects model was applied when heterogeneity was low (I2 < 50%, P ≥ 0.05); otherwise, a random-effect model was used (I2 ≥ 50%, P < 0.05).
Potential publication bias for the primary outcomes was evaluated using funnel plots and further examined with Egger’s and Begg’s tests using StataMP (version 18.0). The risk of bias for each included study was assessed and categorized as low, high, or unclear according to established methodological criteria (16–18).
3 Results
3.1 Study selection and characteristics
The study selection process is illustrated in Figure 1. A total of 1,268 articles were initially identified. Of these, 593 potentially relevant controlled clinical trials evaluating PEG-IFN-α-based therapy for chronic HBV infection underwent detailed screening. After removing 26 duplicate records, 85 studies were excluded based on a lack of full text or irrelevance according to titles and abstracts. Of the remaining 14 articles, 3 were excluded because they did not clearly report HBsAg clearance or seroconversion outcomes or their methodological quality was insufficient for inclusion in a meta-analysis. Ultimately, 11 studies met all predefined criteria and were included in the final analysis. Table 1 summarizes the main characteristics of the included studies, which collectively enrolled 2,758 patients. Nine studies (involving 2,546 patients) were multicenter, double-blind controlled trials. Based on the Jadad scale, seven randomized controlled trials (RCTs) scored between 4 and 6 points, indicating high methodological quality; the remaining two non-RCTs also scored 4-6. Among the included studies, four reported both HBsAg clearance and seroconversion outcomes (13, 14, 19–22), three reported only HBsAg clearance (23–25), and two reported only HBsAg seroconversion (26, 27). All patients received treatment for 48 weeks, followed by a 24-week post-treatment follow-up period. Ten studies evaluated concurrent combination therapy, while one study assessed sequential therapy (23). All studies were available as full-text publications in English.
FIGURE 1

Literature search and data extraction.
TABLE 1
| Study (year) | Study type | Treatment type | n | Treatment period | Follow-up period | Jadad score | Patient characteristics | Naive or experienced | NA type | Post-treatment hepatic flare reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Boglione L., 2013 (20) | Non-RCT | PEG-IFN-α PEG-IFN-α plus NA |
40 | 48 weeks | 24 weeks | 4 | HBeAg +, high HBV DNA, non-D genotypes, adults | Naive | Entecavir | Not specified |
| Bunyamin D., 2004 (27) | RCT | PEG-IFN-α PEG-IFN-α plus NA |
182 | 48 weeks | 24 weeks | 6 | Children, HBeAg +, elevated ALT, CHB | Naive | Lamivudine | Moderate flare only in monotherapy group |
| Erik H., 2008 (23) | Non-RCT | PEG-IFN-α PEG-IFN-α plus NA |
172 | 48 weeks | 24 weeks | 5 | HBeAg +, adults, mixed genotypes, from prior RCT | Both (prior study included naive and experienced) | Lamivudine | Not specified |
| George K., 2005 (26) | RCT | PEG-IFN-α PEG-IFN-α plus NA |
542 | 48 weeks | 24 weeks | 5 | HBeAg +, mostly Asian, adults, high HBV DNA | Both (prior therapy allowed) | Lamivudine | ALT elevations reported, no significant difference between groups |
| Harry L., 2005 (19) | RCT | PEG-IFN-α PEG-IFN-α plus NA |
266 | 48 weeks | 24 weeks | 5 | HBeAg +, adults, mixed genotypes, elevated ALT | Both (21% IFN-experienced) | Lamivudine | Hepatitis flare reported as serious adverse event in both groups |
| Marcellin P., 2004 (22) | RCT | PEG-IFN-α PEG-IFN-α plus NA |
356 | 48 weeks | 24 weeks | 5 | HBeAg-negative, adults, mixed race, bridging fibrosis/cirrhosis ∼27% | Both (prior lamivudine 4–8%, prior interferon 6–10%) | Lamivudine | ALT elevations during and after treatment reported |
| Marcellin P., 2016 (21) | RCT | PEG-IFN-α PEG-IFN-α plus NA |
555 | 48 weeks | 24 weeks | 5 | HBeAg-positive and negative, non-cirrhotic, adults, mixed genotypes | Naive for interferon and nucleotide analogs (prior nucleoside allowed if stopped > 24 weeks) | Tenofovir disoproxil fumarate | ALT elevations and hepatic flares reported, with details |
| Martijn J., 2006 (24) | RCT | PEG-IFN-α PEG-IFN-α plus NA |
266 | 48 weeks | 24 weeks | 5 | HBeAg +, adults, mixed genotypes, elevated ALT | Both (21% IFN-experienced, 12% lamivudine-experienced) | Lamivudine | ALT flares reported, but not specifically post-treatment |
| Paola P., 2009 (25) | RCT | PEG-IFN-α PEG-IFN-α plus NA |
60 | 48 weeks | 24 weeks | 4 | HBeAg-negative, compensated CHB, median age 48, 67% male, mean ALT 3.3x ULN, HBV DNA 5.8 log10 IU/mL | Both (prior IFN 8.3%, prior NA 18.3%) | Adefovir dipivoxil | Not specifically reported |
| Jiang-Shan Lian, 2022 (14) | RCT | PEG-IFN-α PEG-IFN-α plus NA |
181 | 48 weeks | 24 weeks | 6 | Details not fully available (assumed HBeAg-positive or negative adults) | Not specified | Not specified | Not specified |
| Yan Xu 2017 (13) | RCT | PEG-IFN-α PEG-IFN-α plus NA |
138 | 48 weeks | 24 weeks | 6 | Details not fully available (assumed HBeAg-positive or negative adults) | Not specified | Not specified | Not specified |
Characteristics of the studies included in the meta-analysis.
3.2 Primary outcomes: HBsAg clearance and seroconversion with PEG-IFN-α plus NA combination therapy versus PEG-IFN-α monotherapy
At the end of treatment, the rates of HBsAg clearance and seroconversion were 6.4 and 4.9%, respectively, in the PEG-IFN-α plus NA combination group, compared to 4.4 and 2.9% in the PEG-IFN-α monotherapy group. The corresponding forest plots are shown in Figure 2. Because heterogeneity was low, all analyses applied a fixed-effect model. Pooled analysis of seven studies demonstrated that comparison therapy significantly increased the HBsAg clearance rate after 48 weeks of treatment compared to monotherapy (OR = 1.59, 95% CI: 1.01–2.52, P = 0.05, I2 = 0%) (Figure 2a). In contrast, no significant differences was observed in HBsAg seroconversion at week 48 (OR = 1.77, 95% CI: 0.94–3.32, P = 0.08, I2 = 0%) (Figure 2b).
FIGURE 2

Analysis of HBsAg clearance (a) and HBsAg seroconversion (b) between PEG-IFN-α+NA combination and PEG-IFN-α monotherapy after 48 weeks treatment (overall trials).
A fixed-effect model was similarly used for the 24-week post-treatment follow-up analyses. During follow-up, the rates of HBsAg clearance and seroconversion were 5.3 and 5.0% in the combination therapy group and 4.1 and 3.5% in the monotherapy group, respectively. Pooled analysis of four studies showed no significant difference in HBsAg clearance rates at 24 weeks post-treatment (OR = 1.33, 95% CI: 0.76–2.33, P = 0.31, I2 = 0%) (Figure 3a) or in HBsAg seroconversion (OR = 1.42, 95% CI: 0.88–2.28, P = 0.15, I2 = 0%) (Figure 3b).
FIGURE 3

Analysis of HBsAg clearance (a) and HBsAg seroconversion (b) between PEG-IFN-α+NA combination and PEG-IFN-α monotherapy after 24 weeks follow-up (overall trials).
3.3 Secondary outcomes: HBeAg serological response
HBeAg serological response included both HBeAg loss and HBeAg seroconversion in CHB patients treated with PEG-IFN-α plus NA combination therapy or PEG-IFN-α monotherapy. Comparative analyses at different time points are shown in Figures 4, 5. As presented in Figure 4, there were no significant differences between the combination therapy and monotherapy groups in HBeAg loss after 48 weeks of treatment (OR = 1.39, 95% CI: 0.95–2.02, P = 0.09, I2 = 64%) (Figure 4a). Similarly, no significant difference was observed for HBeAg seroconversion at week 48 (OR = 1.44, 95% CI: 0.97–2.15, P = 0.07, I2 = 62%) (Figure 4b). At the 24-week post-treatment follow-up (Figure 5), the two groups also showed no significant differences in HBeAg loss (OR = 0.95, 95% CI: 0.75–1.22, P = 0.70, I2 = 16%) (Figure 5a) or in HBsAg seroconversion (OR = 0.98, 95% CI: 0.77–1.25, P = 0.88, I2 = 29%) (Figure 5b).
FIGURE 4

Analysis of HBeAg clearance (a) and HBeAg seroconversion (b) between PEG-IFN-α+NA combination and PEG-IFN-α monotherapy after 48 weeks treatment (overall trials).
FIGURE 5
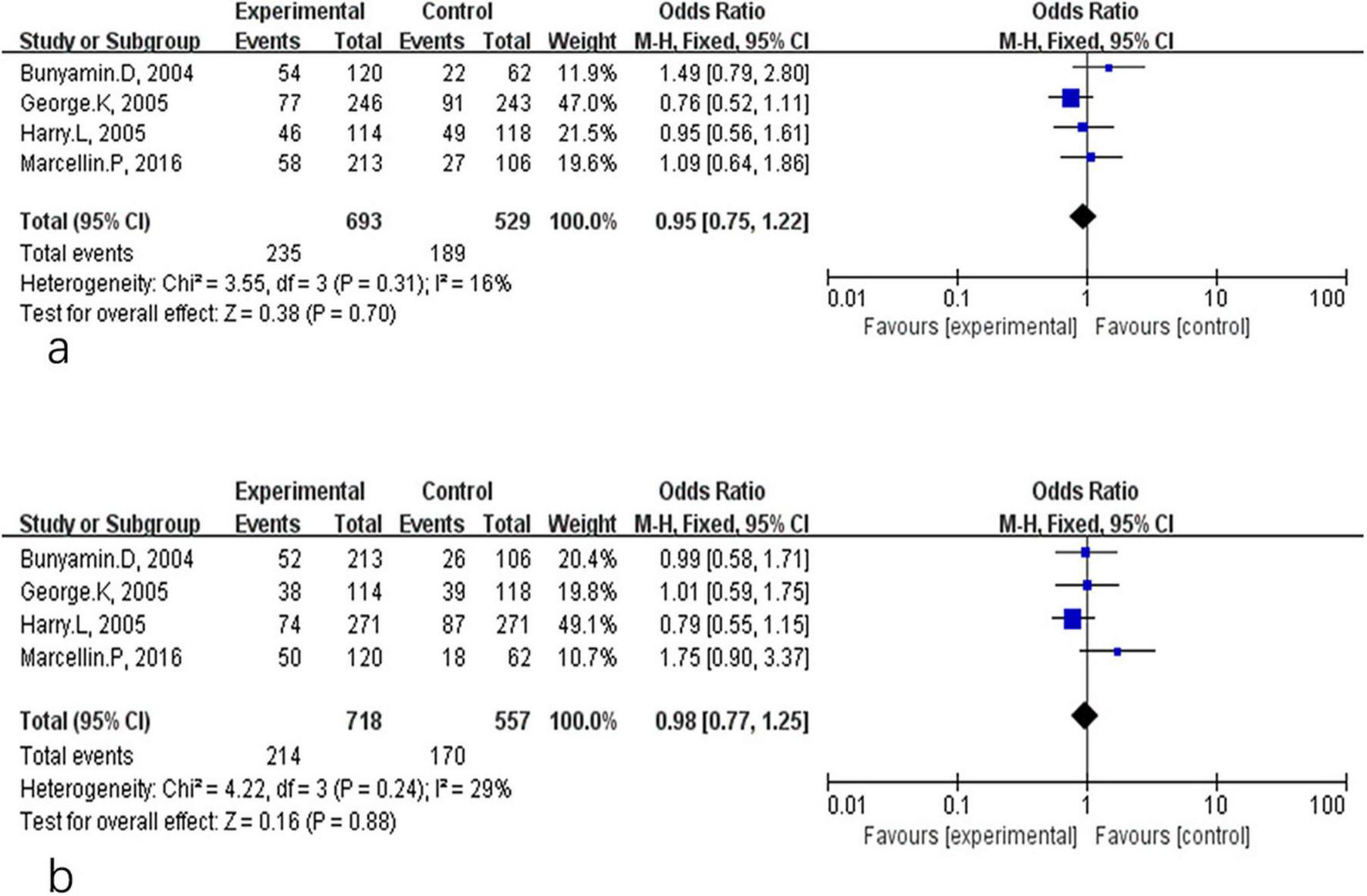
Analysis of HBeAg clearance (a) and HBeAg seroconversion (b) between PEG-IFN-α+NA combination and PEG-IFN-α monotherapy after 24 weeks follow-up (overall trials).
3.4 Secondary outcomes: HBV DNA < 400 copies/mL
Figure 6 compares the proportion of patients achieving HBV DNA < 400 copies/mL in the two treatment groups. A significant difference favoring combination therapy was observed at the end of treatment (OR = 5.79, 95% CI: 3.08–10.90, P < 0.01, I2 = 88%) (Figure 6a). However, this benefit was not sustained during the 24-week follow-up period, and no significant difference was found between groups (OR = 1.99, 95% CI: 0.95–4.18, P = 0.07, I2 = 84%) (Figure 6b).
FIGURE 6
![Two forest plots showing meta-analysis results. Panel (a) indicates odds ratios for eight studies comparing experimental and control groups. The overall effect favors the experimental group with an odds ratio of 5.79, confidence interval [3.08, 10.90], and significant heterogeneity (I-squared = 88%). Panel (b) also presents odds ratios for eight studies with a slightly lower overall effect favoring the experimental group, odds ratio 1.99, confidence interval [0.95, 4.18], and heterogeneity at 84%. Blue squares and lines represent study-specific odds ratios and confidence intervals.](https://www.frontiersin.org/files/Articles/1716225/xml-images/fmed-12-1716225-g006.webp)
Analysis of HBV DNA < 400 copies/mL rate between PEG-IFN-α+NA combination and PEG-IFN-α monotherapy after 48 weeks treatment (a) and after 24 weeks follow-up (overall trials) (b).
3.5 Secondary outcomes: biochemical response (ALT normalization)
Figure 7 summarizes the biochemical response, defined as ALT normalization. After 48 weeks of treatment, combination therapy was associated with a significantly higher frequency of ALT normalization compared with monotherapy (OR = 1.95, 95% CI: 1.27–3.01, P < 0.01, I2 = 73%) (Figure 7a). However, the difference was no longer significant at the 24-week follow-up (OR = 1.17, 95% CI: 0.92–1.50, P = 0.19, I2 = 0%) (Figure 7b).
FIGURE 7

Analysis of ALT normalization rate between PEG-IFN-α+NA combination and PEG-IFN-α monotherapy after 48 weeks treatment (overall trials) (a) and after 24 weeks follow-up (overall trials) (b).
3.6 Publication bias
Potential publication bias was assessed using funnel plots for the primary outcomes. No significant publication bias was detected for HBsAg clearance and seroconversion when comparing PEG-IFN-α plus NA combination therapy to PEG-IFN-α monotherapy. The funnel plots showed no appreciable asymmetry for HBsAg clearance (Figure 8a) or HBsAg seroconversion (Figure 8b) at week 48. Similarly, no evidence of publication bias was observed for HBsAg clearance (Figure 8c) or HBsAg seroconversion (Figure 8d) at the 24-week follow-up. Further quantitative assessment confirmed these findings. For HBsAg clearance at week 48, Begg’s test (P = 0.368) and Egger’s test (P = 0.082) indicated no significant publication bias (Figure 9a). For HBsAg seroconversion, Begg’s test (P = 0.462) and Egger’s test (P = 0.489) also showed no evidence of significant bias (Figure 9b). A leave-one-out sensitivity analysis was conducted to evaluate the robustness of the pooled estimates for HBsAg clearance (Figure 9a) and seroconversion (Figure 9b) at week 48. Removal of any single study did not materially alter the overall effect estimates, demonstrating that the primary findings of this meta-analysis are stable and reliable. The overall risk of bias for the included studies, evaluated using the Cochrane Collaboration’s risk of bias tool, is presented in Figure 10.
FIGURE 8

Assessment of publication bias for PEG-IFN-α+NA combination versus PEG-IFN-α monotherapy on HBsAg clearance and HBsAg seroconversion after 48 weeks treatment (a,b), and after 24 weeks follow-up (overall trials) (c,d).
FIGURE 9

Sensitivity analysis, Egger’s test and Begg’s test to examine the risk of primary outcome potential publication bias for PEG-IFN-α+NA combination versus PEG-IFN-α monotherapy on HBsAg clearance (a) and HBsAg seroconversion (b) after 48 weeks treatment.
FIGURE 10

Risk of bias in all included studies was assessed using the Cochrane Collaboration’s tool. (a) Risk of bias graph: each risk of bias item was presented as percentages in all included studies. (b) Risk of bias summary: each risk of bias item was presented in each included study.
4 Discussion
Our study includes several high-quality trials comparing PEG-IFN-α plus NA combination therapy with PEG-IFN-α monotherapy for the treatment of CHB, with a primary focus on HBsAg clearance and seroconversion. By pooling data from all relevant clinical controlled trials, this meta-analysis aims to clarify the ongoing debate regarding the optimal antiviral treatment strategy. However, the limited number of well-controlled trials remains a constraint for drawing definitive conclusions. Numerous studies have evaluated both PEG-IFN-α plus NA combination therapy and PEG-IFN-α monotherapy for their ability to prevent HBV reactivation and achieve virological, serological, and biochemical responses (19–27). Although both regimens demonstrate clinical efficacy, their comparative effectiveness remains uncertain.
Mechanistically, NAs act primarily as HBV DNA polymerase inhibitors (28, 29), whereas PEG-IFN-α suppresses HBV replication by promoting post-transcriptional degradation of HBV RNA and inducing the expression of antiviral proteins (24, 30, 31). NAs generally require long-term, sometimes indefinite, administration to maintain viral suppression, which carries a risk of antiviral resistance (32). In contrast, PEG-IFN-α therapy involves inconvenient subcutaneous administration and is associated with significant adverse effects (33). Given these limitations, combining PEG-IFN-α with an NA is recommended in clinical guidelines for CHB management (19, 20), as this strategy may enhance antiviral efficacy and reduce the risk of drug resistance by targeting HBV through complementary mechanisms (21, 23). Several studies have reported that combination therapy provides superior virological and biochemical responses compared to monotherapy. Some RCTs have demonstrated that combination therapy can achieve greater reductions in HBV DNA levels (24, 27), and higher rates of HBsAg and HBeAg clearance compared to PEG-IFN-α monotherapy (20, 23). However, other clinical trials have reported comparable therapeutic outcomes between the two regimens (19, 20, 27). Contributing to ongoing debate over whether combination therapy offers a meaningful advantage in routine clinical practice. To address this controversy and provide more precise estimates of treatment effects, we conducted this meta-analysis to systematically compare the efficacy of PEG-IFN-α alone versus PEG-IFN-α plus NA combination therapy in patients with CHB.
In our analysis of the primary outcomes, PEG-IFN-α plus NA combination therapy resulted in significantly higher HBsAg clearance rates compared to PEG-IFN-α monotherapy (P = 0.05) after 48 weeks of treatment. However, no significant differences were observed between the two groups in terms of HBsAg seroconversion after 48 weeks and HBsAg clearance or seroconversion at 24 weeks post-treatment follow-up (20–22). Regarding secondary outcomes, no significant differences were identified in serological responses, including HBeAg loss and HBeAg seroconversion, between the combination and monotherapy groups (24). Notably, patients receiving PEG-IFN-α plus NA achieved significantly greater HBV DNA suppression (P < 0.01) and higher rates of ALT normalization (P < 0.01) after 48 weeks of treatment compared to those receiving PEG-IFN-α monotherapy (13). We acknowledge the substantial heterogeneity observed for this outcome (I2 = 88%). Major sources likely include variability in patient populations, differences in combination treatment regimens, and inconsistencies in assay sensitivity across studies. These factors collectively limit the interpretability and generalizability of the effect-pooled estimate. Taken together, these findings indicate that PEG-IFN-α plus NA combination therapy provides a modest but clinically meaningful advantage in CHB patients in terms of HBsAg clearance, virological response (HBV DNA < 400 copies/mL), and biochemical response (ALT normalization). This suggests that combination therapy may be a preferable option for selected patients. Overall, our results support the notion that combining PEG-IFN-α with an NA enhances HBsAg clearance, ALT normalization, and the control of both clinical and virological HBV reactivation.
The improved outcomes with combination therapy likely reflect the complementary mechanisms of action of the two agents. PEG-IFN-α enhances host immune responses against HBV, while nucleos(t)ide analogs provide sustained suppression of viral replication (21, 27). This dual action may promote virus-specific immune responses, leading to higher rates of HBsAg clearance, biochemical normalization, and virological control. However, it is important to note that only a limited proportion of patients maintained these benefits after treatment cessation (21). In our analysis, patients receiving PEG-IFN-α plus NA for 48 weeks achieved greater reductions in HBV DNA levels and higher ALT normalization rates compared to PEG-IFN-α monotherapy. Whether extending combination therapy beyond 48 weeks could further improve sustained HBV DNA suppression remains an open question and warrants evaluation in future studies (25). Nevertheless, our long-term follow-up analyses did not reveal significant differences in HBsAg clearance or seroconversion, HBeAg clearance or seroconversion, or HBV DNA suppression between the two groups after treatment discontinuation. For instance, at 24 weeks post-treatment, the HBsAg clearance rate among patients receiving PEG-IFN-α plus NA was not significantly higher than among those receiving PEG-IFN-α monotherapy (20). This suggests that while combination therapy may increase HBsAg clearance and ALT normalization at the end of treatment, sustaining these benefits long-term remains challenging. By the end of 48 weeks, patients treated with the combination regimen achieved higher rates of HBsAg seroconversion compared to monotherapy (27), likely reflecting the distinct antiviral mechanisms of the two agents. However, no significant differences were observed in ALT normalization, HBsAg loss, or HBV DNA clearance rates at 24 weeks post-treatment. One potential explanation for this virological “breakthrough” is premature treatment discontinuation, highlighting the need for further investigation in larger, long-term RCTs. For example, in a multicenter randomized trial conducted by Paola Piccolo et al., only one patient (3.3%) in the combination therapy group achieved HBsAg clearance at 24 weeks post-treatment (25).
Overall, our meta-analysis provides direct evidence that PEG-IFN-α plus NA combination therapy, through complementary mechanisms, produces significantly higher rates of HBsAg loss compared to PEG-IFN-α monotherapy after 48 weeks of treatment. Additionally, the combination improves ALT normalization and HBV DNA suppression. Nevertheless, no significant differences were observed in serological outcomes, including HBeAg loss and seroconversion, between the two groups at any time point. These findings suggest that, although combination therapy can improve certain virological and biochemical endpoints, PEG-IFN-α monotherapy remains an effective option, particularly in settings where cost considerations are important. Our analysis demonstrated that the combination regimen significantly improved HBsAg clearance at 48 weeks; however, this advantage was not sustained during follow-up. Whether extending PEG-IFN-α plus NA therapy could further enhance functional cure rates remains uncertain and should be explored in future longer-duration trials. Any incremental benefit from prolonged treatment must also be balanced against the increased cost, adverse effects, and overall treatment burden.
Importantly, the therapeutic benefit of PEG-IFN-α is not uniform across all patient populations. Growing evidence indicates that its addition is most effective in selected subgroups, particularly in individuals with low baseline HBsAg levels. This includes patients receiving long-term NA therapy who have achieved virological suppression but remain uncured. For these individuals, adding or switching to PEG-IFN-α may help overcome immune tolerance and increase the likelihood of HBsAg loss or seroconversion. This meta-analysis has several limitations. Significant heterogeneity existed across studies, resulting from differences in patient characteristics, NA regimens, and study designs, which prevented more refined subgroup analyses, including evaluation of hepatic flare. The absence of individual patient data, especially baseline HBsAg levels, restricted assessment of key predictors of response. Furthermore, the uniform 48-week treatment duration and relatively short follow-up period limit conclusions regarding long-term outcomes and the efficacy of de novo combination therapy.
Statements
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SZ: Writing – original draft, Writing – review & editing. ZY: Conceptualization, Writing – original draft, Writing – review & editing. YW: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. XC: Writing – original draft, Writing – review & editing. CQ: Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Lok ASF . Toward a functional cure for hepatitis B.Gut Liver. (2024) 18:593–601. 10.5009/gnl240023
2.
Jun A Bau S Kim JS Phillips SJ . Overview of chronic hepatitis B management.Nurse Pract. (2025) 50:7–13. 10.1097/01.NPR.0000000000000246
3.
Vutien P Nguyen MH . HBV reactivation in immunosuppressed patients: screening, prevention, and management including solid organ transplant recipients.Viruses. (2025) 17:388. 10.3390/v17030388
4.
Kuo MH Ko PH Wang ST Tseng CW . Incidence of HBV reactivation in psoriasis patients undergoing cytokine inhibitor therapy: a single-center study and systematic review with a meta-analysis.Viruses. (2024) 17:42. 10.3390/v17010042
5.
Fan R Zhao S Niu J Ma H Xie Q Yang S et al High accuracy model for HBsAg loss based on longitudinal trajectories of serum qHBsAg throughout long-term antiviral therapy. Gut. (2024) 73:1725–36. 10.1136/gutjnl-2024-332182
6.
Liu T Shi Y Wu J Qin L Qi Y . Predictive value of serum HBV RNA on HBeAg seroconversion in treated chronic hepatitis B patients.Eur J Gastroenterol Hepatol. (2025) 37:738–44. 10.1097/MEG.0000000000002946
7.
Yin S Wan Y Issa R Zhu Y Xu X Liu J et al The presence of baseline HBsAb-Specific B cells can predict HBsAg or HBeAg seroconversion of chronic hepatitis B on treatment. Emerg Microbes Infect. (2023) 12:2259003. 10.1080/22221751.2023.2259003
8.
Xu ZY Dai ZS Gong GZ Zhang M . C-X-C chemokine receptor type 5+CD8+ T cells as immune regulators in hepatitis Be antigen-positive chronic hepatitis B under interferon-alpha treatment.World J Gastroenterol. (2025) 31:99833. 10.3748/wjg.v31.i3.99833
9.
Wu F Lu R Liu Y Wang Y Tian Y Li Y et al Efficacy and safety of peginterferon alpha monotherapy in Chinese inactive chronic hepatitis B virus carriers. Liver Int. (2021) 41:2032–45. 10.1111/liv.14897
10.
Zoulim F Chen PJ Dandri M Kennedy PT Seeger C . Hepatitis B virus DNA integration: implications for diagnostics, therapy, and outcome.J Hepatol. (2024) 81:1087–99. 10.1016/j.jhep.2024.06.037
11.
Wong GL . Updated Guidelines for the Prevention and Management of Chronic Hepatitis B-World Health Organization 2024 Compared With China 2022 HBV Guidelines.J Viral Hepat. (2024) 31(Suppl 2):13–22. 10.1111/jvh.14032
12.
European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. (2025) 83:502–83. 10.1016/j.jhep.2025.03.018
13.
Xu Y Wang X Liu Z Zhou C Qi W Jiao J et al Addition of nucleoside analogues to peg-IFNα-2a enhances virological response in chronic hepatitis B patients without early response to peg-IFNα-2a: a randomized controlled trial. BMC Gastroenterol. (2017) 17:102. 10.1186/s12876-017-0657-y
14.
Lian J Kuang W Jia H Lu Y Zhang X Ye C et al Pegylated interferon-α-2b combined with tenofovir disoproxil fumarate, granulocyte-macrophage colony-stimulating factor, and hepatitis B vaccine treatment for naïve HBeAg-positive chronic hepatitis B patients: a prospective, multicenter, randomized controlled study. J Med Virol. (2022) 94:5475–83. 10.1002/jmv.28003
15.
Sakamoto K Ogawa K Tamura K Honjo M Funamizu N Takada Y . Prognostic role of the intrahepatic lymphatic system in liver cancer.Cancers. (2023) 15:2142. 10.3390/cancers15072142
16.
Pourahmad R Saleki K Zoghi S Hajibeygi R Ghorani H Javanbakht A et al Percutaneous transluminal angioplasty and stenting (PTAS) in patients with symptomatic intracranial vertebrobasilar artery stenosis (IVBS). Stroke Vasc Neurol. (2025) 10:e003224. 10.1136/svn-2024-003224
17.
Phua QS Lu L Harding M Poonnoose SI Jukes A To MS . Systematic analysis of publication bias in neurosurgery meta-analyses.Neurosurgery. (2022) 90:262–9. 10.1227/NEU.0000000000001788
18.
Barker TH Habibi N Aromataris E Stone JC Leonardi-Bee J Sears K et al The revised JBI critical appraisal tool for the assessment of risk of bias for quasi-experimental studies. JBI Evid Synth. (2024) 22:378–88. 10.11124/JBIES-23-00268
19.
Janssen HL van Zonneveld M Senturk H Zeuzem S Akarca US Cakaloglu Y et al Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. (2005) 365:123–9. 10.1016/S0140-6736(05)17701-0
20.
Boglione L D’Avolio A Cariti G Milia MG Simiele M De Nicolò A et al Sequential therapy with entecavir and PEG-INF in patients affected by chronic hepatitis B and high levels of HBV-DNA with non-D genotypes. J Viral Hepat. (2013) 20:e11–9. 10.1111/jvh.12018
21.
Marcellin P Ahn SH Ma X Caruntu FA Tak WY Elkashab M et al Combination of tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. (2016) 150:134–44.e10. 10.1053/j.gastro.2015.09.043
22.
Marcellin P Lau GK Bonino F Farci P Hadziyannis S Jin R et al Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. (2004) 351:1206–17. 10.1056/NEJMoa040431
23.
Buster EH Flink HJ Cakaloglu Y Simon K Trojan J Tabak F et al Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology. (2008) 135:459–67. 10.1053/j.gastro.2008.05.031
24.
ter Borg MJ van Zonneveld M Zeuzem S Senturk H Akarca US Simon C et al Patterns of viral decline during PEG-interferon alpha-2b therapy in HBeAg-positive chronic hepatitis B: relation to treatment response. Hepatology. (2006) 44:721–7. 10.1002/hep.21302
25.
Piccolo P Lenci I Demelia L Bandiera F Piras MR Antonucci G et al A randomized controlled trial of pegylated interferon-alpha2a plus adefovir dipivoxil for hepatitis B e antigen-negative chronic hepatitis B. Antivir Ther. (2009) 14:1165–74. 10.3851/IMP1466
26.
Lau GK Piratvisuth T Luo KX Marcellin P Thongsawat S Cooksley G et al Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. (2005) 352:2682–95. 10.1056/NEJMoa043470
27.
Dikici B Ozgenc F Kalayci AG Targan S Ozkan T Selimoglu A et al Current therapeutic approaches in childhood chronic hepatitis B infection: a multicenter study. J Gastroenterol Hepatol. (2004) 19:127–33. 10.1111/j.1440-1746.2004.03209.x
28.
Jeng WJ Papatheodoridis GV Lok ASF . Hepatitis B.Lancet. (2023) 401:1039–52. 10.1016/S0140-6736(22)01468-4
29.
Wong GLH Gane E Lok ASF . How to achieve functional cure of HBV: stopping NUCs, adding interferon or new drug development?J Hepatol. (2022) 76:1249–62. 10.1016/j.jhep.2021.11.024
30.
Zhao Q Liu H Tang L Wang F Tolufashe G Chang J et al Mechanism of interferon alpha therapy for chronic hepatitis B and potential approaches to improve its therapeutic efficacy. Antiviral Res. (2024) 221:105782. 10.1016/j.antiviral.2023.105782
31.
Huang D Yuan Z Wu D Yuan W Chang J Chen Y et al HBV antigen-guided switching strategy From Nucleos(t)ide analogue to interferon: avoid virologic breakthrough and improve functional cure. J Med Virol. (2024) 96:e70021. 10.1002/jmv.70021
32.
Hirode G Hansen BE Chen CH Su TH Wong GLH Seto WK et al Limited sustained remission after nucleos(t)ide analog withdrawal: results from a large, global, multiethnic cohort of patients with chronic hepatitis B (RETRACT-B Study). Am J Gastroenterol. (2024) 119:1849–56. 10.14309/ajg.0000000000002759
33.
Wu F Wang Y Cui D Tian Y Lu R Liu C et al Short-Term Peg-IFN α-2b Re-treatment induced a high functional cure rate in patients with HBsAg Recurrence after Stopping Peg-IFN α-Based Regimens. J Clin Med. (2023) 12:361. 10.3390/jcm12010361
Summary
Keywords
chronic hepatitis B, HBsAg seroconversion, HBsAg clearance, nucleos(t)ide analogues, peginterferon
Citation
Zhu S, Yang Z, Wang Y, Cai X and Qin C (2026) A meta-analysis of controlled clinical trials comparing peginterferon-α plus nucleos(t)ide analogs versus peginterferon-α monotherapy for HBsAg clearance or seroconversion in patients with chronic hepatitis B. Front. Med. 12:1716225. doi: 10.3389/fmed.2025.1716225
Received
16 October 2025
Revised
10 December 2025
Accepted
15 December 2025
Published
08 January 2026
Volume
12 - 2025
Edited by
Diego Ripamonti, Papa Giovanni XXIII Hospital, Italy
Reviewed by
Wanyu Deng, Shangrao Normal University, China
Pınar Korkmaz, Kutahya Health Sciences University, Türkiye
Updates
Copyright
© 2026 Zhu, Yang, Wang, Cai and Qin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaochao Qin, qinchaonannong@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.