Abstract
Chronic postsurgical pain (CPSP) in older adults is a multifactorial condition shaped by biological, psychological, and social determinants. This review synthesizes current evidence regarding the underlying mechanisms, clinical assessment, and management strategies for CPSP in the older adults. Key findings emphasize age related changes in pain processing, significant challenges in pain evaluation particularly among those with cognitive impairment, and the limited effectiveness of conventional analgesic therapies. Multimodal and personalized strategies, such as regional anesthesia, tailored pharmacologic interventions, and integrative approaches including acupuncture, demonstrate potential in reducing the incidence and burden of CPSP. Implementing structured assessment protocols and comprehensive geriatric care models is crucial for enhancing postoperative quality of life. Future initiatives should focus on integrating predictive tools, long term monitoring systems, and interdisciplinary collaboration to improve pain related outcomes in this vulnerable population.
1 Introduction
Chronic postsurgical pain (CPSP), defined as pain lasting over 3 months after surgery unrelated to prior conditions (1), is a growing concern, especially among the rapidly increasing older population (2, 3). This population is experiencing a rising incidence of surgical interventions and demonstrates a heightened vulnerability to postoperative complications, including chronic pain, which poses distinct management challenges for older adults (4–6). The physiological and metabolic characteristics inherent to older patients result in pain perception and response that differ significantly from those of younger individuals, thereby exacerbating the complexities of pain management. Furthermore, the management of pain in older patients is further complicated by the frequent presence of cognitive disorders and multiple chronic conditions (such as osteoarthritis, diabetic neuropathy, cardiovascular diseases, and frailty syndrome), which often coexist with pain (7).
The management of chronic pain in older patients requires a comprehensive approach that integrates biological, psychological, and social dimensions. An optimal strategy for pain management involves a multi-modal treatment plan that emphasizes interdisciplinary collaboration to improve patients’ quality of life and autonomy (8). It is particularly important to identify and assess pain in patients with cognitive impairments, as these individuals may face challenges in effectively communicating their pain (7).
Although a variety of interventions exist for managing CPSP, traditional therapeutic approaches frequently demonstrate limited efficacy and can lead to significant side effects, particularly in the older adults. This challenge is compounded in older adults by complexities of pharmacotherapy administration, including drug interactions, polypharmacy, and altered drug metabolism, often resulting in suboptimal pain control and heightened risk of adverse reactions (9). Given the rising frequency of surgeries among the older adults and the substantial burden of CPSP, a thorough understanding of this condition is crucial. However, the limitations of current strategies highlight a critical need to investigate safer and more effective alternatives, specifically emphasizing personalized, multi-modal pain management programs for this vulnerable population. Consequently, this paper aims to provide a review and synthesis of recent research on CPSP in the older adults, focusing on its epidemiology, underlying mechanisms, assessment methods, treatment, and prevention. By critically analyzing current research challenges and identifying gaps, this study seeks to inform future academic investigations and clinical practices. The ultimate goal is to advance the development and implementation of effective strategies, thereby improving postoperative quality of life for older patients and reducing the strain on healthcare resources.
2 Epidemiology of CPSP in the older adults
2.1 The growing trend of the older population and its impact on the healthcare system
As the global trend of population aging intensifies, the number of older individuals is increasing rapidly, and the health challenges they encounter are becoming more pronounced. According to United Nations projections, the global population of individuals aged 60 and over is expected to reach 2.1 billion by 2050, representing a significant demographic shift. This trend presents an unprecedented challenge to the global healthcare system. The demographic shift toward an aging population is anticipated to significantly increase healthcare utilization and associated costs. Age-related conditions, particularly chronic diseases, are key drivers of this heightened demand for health services. A survey conducted by Lily et al. specifically examining chronic pain demonstrated a significant correlation with increased hospitalization frequency (IRR 1.10, 95% CI: 1.01, 1.31), reflecting its substantial impact on acute medical resource utilization (10). This finding aligns with broader evidence from developed nations, where chronic pain consistently contributes to elevated health service usage patterns (10).
2.2 Prevalence and epidemiological characteristics of CPSP in the older adults
Chronic postsurgical pain substantially affects the quality of postoperative recovery in older patients. Research conducted a 15 years ago estimated that over 19 million (11) (19 million = 37.36% of 51 million surgeries) older individuals undergo surgical procedures annually in the United States, with 10%–60% experiencing CPSP (12–14). A study by Gary et al. similarly reported a 40% (OR 4.73, 95% CI 1.24, 18.09) incidence of CPSP among older frail patients after surgery (15). The research elevated incidence rate can be partially attributed to age-related degeneration of the nervous system, alterations in pain perception, and a diminished capacity for postoperative recovery in older adults (16). Furthermore, older patients frequently suffer from chronic conditions such as arthritis and diabetes, which can influence the transmission and perception of pain signals through intricate mechanisms, thereby elevating the risk of developing CPSP. Epidemiological research has indicated that older women are more susceptible to CPSP compared to their male counterparts, a disparity potentially attributable to physiological and metabolic differences, as well as lifestyle factors (3). Evidence suggests that female gender may be an independent risk factor for chronic postoperative pain following thoracoscopic surgery (17). This association is mediated through multiple mechanisms. Biologically, sex-based variations exist in hormone levels, the activity of key nociceptive receptors (including N-methyl-D-aspartate and P2×3 receptors), the distribution of μ/κ opioid receptor subtypes within endogenous pain modulation pathways, and sexually dimorphic neuroanatomy and neural processing (18). Psychologically, females exhibit heightened pain awareness and expressiveness, resulting in increased pain reporting (19). Additionally, it is important to acknowledge that regional cultural variations and disparities in medical resources also contribute to variations in CPSP statistics (20).
3 Age-related mechanistic vulnerabilities in pain processing
3.1 Biological aging and pain sensitivity
Chronic postsurgical pain is more prevalent among older patients, attributed to a range of biological factors. Research suggests that age-related physiological changes, such as nervous system degeneration, immune function decline, and the presence of chronic diseases, may heighten the risk of developing postoperative chronic pain (21). Furthermore, older patients frequently present with multiple comorbidities such as osteoarthritis, diabetes mellitus with neuropathic complications, and chronic kidney disease, which can influence pain perception and processing, thereby exacerbating postoperative pain (22). Pain catastrophizing refers to a cognitive distortion characterized by the amplification of and persistent rumination on pain sensations (23). This tendency leads to an exaggerated perception of threat-related components within the pain experience, thereby influencing self-reported pain intensity and pain-associated psychological states (24). Consequently, the interaction between pain catastrophizing and negative emotions may exacerbate distress, anger, fear, frustration, or anxiety. These emotional responses play a critical role in the context of chronic pain and the overall management of pain (24).
Biological factors encompass genetic predispositions and alterations in biomarkers. Specific gene variants have been associated with pain sensitivity and the development of chronic pain (25).
The transition from acute to chronic pain involves highly complex pathological mechanisms, prominently featuring peripheral and central sensitization phenomena (26). Peripheral sensitization denotes an enhanced responsiveness and reduced activation threshold of nociceptive neurons within peripheral tissues to stimulation of their receptive fields (27). Central sensitization, conversely, refers to an increased responsiveness of nociceptive neurons within the central nervous system to both normal and subthreshold afferent input (27).
Surgical tissue injury plays a fundamental role in the development of CPSP, underpinning significant neuroplastic alterations within peripheral and central sensory neural circuits. Nociceptive input into the spinal cord dorsal horn (SCDH) triggers the release of the neurotransmitter glutamate. Glutamate subsequently acts on specific postsynaptic receptors, including α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and the critically involved N-methyl-D-aspartate receptors (NMDARs). Sustained and intense glutamate release in the SCDH, driven by persistent peripheral afferent barrage following surgical nerve injury, leads to NMDAR activation. This receptor activation facilitates synaptic plasticity and, under conditions of severe or prolonged stimulation, can contribute to neurotoxicity and neuronal apoptosis (28).
As a direct consequence of these sensitization processes, patients developing CPSP frequently exhibit characteristic clinical manifestations early in the postoperative period, including hyperalgesia (heightened pain response to noxious stimuli), allodynia (pain elicited by normally innocuous stimuli), and dysesthesias (unpleasant abnormal sensations) (26).
Aging is associated with a decline in the organism’s capacity to accurately identify noxious signals, while pain tolerance remains static or may even diminish. Paradoxically, upon detection of a noxious stimulus, an exaggerated pain response often occurs. Consequently, older individuals experiencing acute pain are highly susceptible to its rapid progression to severe, often intractable, pain states (29).
The mechanisms of peripheral and central sensitization, common in chronic pain, may develop more subtly in older adults due to reduced neuroplasticity with age. Most evidence comes from mixed-age groups or preclinical studies, with few direct neurobiological studies on older CPSP patients, highlighting a key area for future research.
3.2 Inflammaging and immune dysregulation
The mechanisms of inflammation and immune regulation in chronic pain are especially critical in geriatric patients due to the substantial alterations in immune function associated with aging. Post-operative inflammation has the potential to result in persistent pain. Increasing evidence indicates that inflammatory mediators associated with the nuclear factor κB pathway are involved in pain processes within central and peripheral nervous systems, including neurons and glial cells (particularly astrocytes, which are a major type of central glial cell) (30, 31). Interleukin-6, a cytokine associated with adverse outcomes in older adults, is implicated in both pain perception and the development of pathological pain (32, 33). However, research has demonstrated a significant correlation between increased circulating levels of pro-inflammatory cytokines (IL-8, IL-10, IL-18) and chemokines [e.g., C-C motif chemokine ligand 2 (CCL2), C-X-C motif chemokine ligand 10 (CXCL10)] and the severity of pain across various pain syndromes. CCL2, also known as monocyte chemoattractant protein 1 (MCP-1), recruits monocytes and microglia to the site of nerve injury or inflammation, activating neuroinflammatory cascades that promote central sensitization. CXCL10 enhances nociceptive signaling by binding to CXCR3 receptors on neurons, increasing neuronal excitability (34–36). The role of nuclear factor κB as a mediator in the regulation of post-surgical pain remains to be elucidated. Clinical studies suggest that plasma concentrations of inflammatory mediators generally change following surgery and may increase immediately thereafter (37–39). Substance P in neurogenic inflammation has been extensively validated in multiple studies. By binding to its receptor, neuropeptide-1 receptor (NK1R), substance P promotes the activation of inflammatory cells and the release of chemokines, thereby exacerbating inflammatory responses (40). In facial pain studies, CGRP (calcitonin gene-related peptide) amplifies pain by upregulating nitric oxide synthase expression, a process involving the p38 signaling pathway. This mechanism may also apply to postoperative pain, as it is frequently accompanied by inflammatory responses, and CGRP has been shown to contribute to inflammation-mediated pain (41). Postoperative inflammatory responses can play a critical role in the development of CPSP. In older individuals, the inflammatory response is frequently characterized by a low-grade chronic inflammatory state, which may result in an amplified postoperative inflammatory response. This heightened response can adversely affect wound healing and contribute to the induction of chronic pain.
Furthermore, immune dysregulation in the older adults often disrupts the equilibrium between pro-inflammatory and anti-inflammatory responses, potentially exacerbating the experience of chronic pain (42). Regulatory T cells (Treg cells) play a vital role in maintaining immune tolerance and modulating inflammatory responses. Following spinal cord injury, Treg cells attenuate neuropathic pain by suppressing neuroinflammation (43). This immunomodulation occurs primarily through Treg-mediated release of inhibitory cytokines and functional regulation of other immune cells. However, experimental depletion of Treg cells exacerbates mechanical allodynia (hypersensitivity to innocuous mechanical stimuli) and significantly alters systemic cytokine profiles (44). Emerging research suggests that specific probiotics have the potential to mitigate chronic pain symptoms by modulating the immune response, thereby presenting a novel strategy for managing postoperative pain in the older population (45). Aging induces modifications in immune system functionality, marked by changes in cytokine concentrations and immune cell activity. These immune alterations may affect the neurological milieu at the postoperative wound site, potentially facilitating chronic pain through complex neuro-immune interactions.
Mitochondrial dysfunction and oxidative stress are increasingly implicated in CPSP pathogenesis. Evidence indicates that mitochondrial impairment drives reactive oxygen species (ROS) accumulation, subsequently inducing neuronal pyroptosis – an inflammatory programmed cell death process that critically contributes to CPSP (46). Specifically, downregulation of mitofusin-2 (Mfn2) associates with mitochondrial dysfunction and ROS overproduction. These pathological changes promote pyroptosis in spinal GABAergic neurons, thereby facilitating chronic pain development (46).
Age-related reductions in peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) expression further contribute to pain chronification. Studies demonstrate that diminished PGC-1α levels cause aberrant neuronal dynamics within the primary somatosensory cortex (S1), exacerbating nociceptive behaviors following neural injury (47). This mechanism likely underlies the heightened vulnerability to chronic pain observed in older populations.
The concept of “inflammaging” is key to understanding CPSP in the older adults, but most studies haven’t analyzed cytokine levels by age. To validate the link between perioperative inflammation and CPSP in older patients, large, long-term studies are needed.
3.3 Genetic and epigenetic factors in aging
Genetic factors significantly influence individual susceptibility to chronic pain in the older population. Specific gene variants can impact pain sensitivity, inflammatory responses, and the adaptation of the nervous system.
Genes associated with pain perception are crucial in the transmission and perception of pain. Notably, the A118G polymorphism in the OPRM1 gene has been correlated with variations in pain severity and opioid consumption (48). Furthermore, gene variants may contribute to individual differences in pain perception, which are intricately linked to the underlying biological mechanisms of pain (49).
Genes related to inflammation and immunity have been implicated in chronic pain susceptibility. Research indicates that polymorphisms in specific immune-related genes are associated with an increased risk of chronic pain (50). Variations in specific cytokine genes–including those encoding tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and interleukin-10 (IL-10)–can affect an individual’s pain response and resilience (51). These genetic variations not only impact pain perception but may also influence the duration of chronic pain by modulating inflammatory responses and neural adaptation.
Genome-wide association studies (GWAS) demonstrate significant genetic overlap between chronic pain and major depressive disorder, with neuroticism acting as a key modulator (52). Current evidence suggests that depression comorbid with chronic pain may constitute a distinct genetic subtype of depression. This underscores the necessity of integrating personality traits and stress-related factors when investigating the genetic architecture of complex heterogeneous phenotypes. Genetic predispositions and environmental exposures interact bidirectionally to amplify or mitigate pain symptoms. Research showed that nerve injury induces upregulated expression of epigenetic regulators such as chromodomain Y-like protein (CDYL) in peripheral sensory neurons. CDYL-mediated transcriptional repression of the potassium channel gene Kcnb1 enhances neuronal hyperexcitability and promotes pain sensitization (53). These gene-environment interactions likely contribute significantly to chronic postsurgical pain development in geriatric patients.
Epigenetic regulation offers a promising avenue for understanding chronic pain in aging, but current knowledge is mostly based on preclinical models or non-surgical neuropathic pain studies. Translational research on epigenetic markers in blood or tissue from older surgical patients is necessary for biomarker discovery and understanding mechanisms.
In summary, the development of CPSP in older adults involves complex biological interactions linked to aging. Current understanding often relies on data from younger individuals or preclinical models, highlighting a gap in research specific to older surgical patients. Table 1 outlines the main mechanisms, emphasizing the urgent need for studies focused on the unique aspects of aging related to postsurgical pain.
TABLE 1
| Mechanism/pathway | Age-related changes | Evidence in general CPSP | Evidence in older adults | Key knowledge gaps |
|---|---|---|---|---|
| Central sensitization | Reduced inhibitory neurotransmission; altered NMDA receptor function | Strong (human/animal studies) (26, 28) | Limited (extrapolated from mixed-age cohorts) | Whether aging accelerates or dampens central sensitization post-surgery |
| Peripheral sensitization | Slower nerve regeneration; decreased ion channel expression | Strong (human/animal studies) (26, 27) | Moderate (inferred from neuropathic pain studies) | Role of age-related axon degeneration in CPSP persistence |
| Neuroinflammation (glial activation) | Increased baseline pro-inflammatory cytokines | Strong (animal models) (30, 31) | Limited direct evidence | Temporal dynamics of neuroinflammation in aged surgical populations |
| Immune dysregulation (Treg/cytokines) | Immunosenescence; altered Treg function | Emerging (animal models) (43, 44) | Scarce | How immunosenescence modulates CPSP risk and recovery |
| Mitochondrial dysfunction/oxidative stress | Decreased PGC-1α; increased ROS | Emerging (animal models) (46) | Indirect (associated with frailty) | Causal link between mitochondrial health and CPSP in older adults |
| Epigenetic regulation (e.g., CDYL) | Age-related epigenetic drift | Emerging (animal models) (53) | No direct human studies | Whether age-specific epigenetic signatures predict CPSP |
| Genetic polymorphisms (e.g., OPRM1) | Polymorphism frequency may vary with age | Moderate (mixed-age GWAS) (48, 52) | Limited age-stratified analyses | Gene-environment interactions in older surgical patients |
Key mechanisms of CPSP in older adults: evidence levels and knowledge gaps.
3.4 The biopsychosocial model in geriatric pain
The Biopsychosocial Model (BPS Model) offers a comprehensive framework for understanding and managing chronic pain in older adults. This model underscores that diseases and symptoms are influenced not only by biological factors but also by the interplay of psychological and social factors (54). Its significance in the study of chronic pain among the older adults lies in its ability to elucidate the complexity of pain and to inform multidimensional treatment approaches (Figure 1).
FIGURE 1

Summarizing the BPS model and its factors.
Psychological factors are crucial in the experience and management of chronic pain among older adults. Current research suggests that individuals exposed to highly stressful, threatening, or ineffective environments may develop increased sensitivity and hyper-reactive responses, including pain (55). Depression and anxiety are often considered emotional responses to the aversive nature of the sensory experience associated with chronic pain. Epidemiological evidence indicates that depression and anxiety may precede and increase the risk of the onset, severity, or persistence of chronic pain (56–58). Research has demonstrated that anxiety and depression are prevalent among the older adults, potentially intensifying their subjective perception of pain and prolonging its duration (59). An anxious state may result in an increased focus on pain, whereas depression may diminish pain tolerance; both conditions can exacerbate the sensation of pain by influencing neurotransmitter activity. These negative psychological states can adversely affect patients’ functional levels and quality of life, thereby impeding their recovery process. Research suggests that psychological risk factors may significantly impact patients’ pain coping strategies, potentially contributing to the development of chronic postoperative pain (60). A retrospective cohort analysis of 14,1466 patients demonstrated that individuals developing CPSP had a higher risk of subsequent depression compared to those without CPSP (adjusted HR = 1.41; 95% CI 1.35–1.48; p < 0.0001), confirming CPSP as a significant independent predictor of incident depression (61). Therefore, prioritizing mental health is essential in the management of chronic postoperative pain among older patients.
In addition to the nuanced effects of psychological factors on chronic pain, it is crucial to examine the intricate role of social influences. Global surgical volume estimates indicate 187.2–281.2 million major procedures were performed in 2004 (approximately 1 surgery per 25 people globally) (62). By 2012, this increased to 266.2–359.5 million procedures, reflecting an absolute growth of 33.6% over 8 years (62). CPSP develops in 10%–50% of surgical patients, with 2%–10% experiencing severe pain (62). The continuous increase in population is also a potential reason for the growth of patients suffering from CPSP. Additionally, recent systematic reviews have demonstrated that low educational attainment and socioeconomic status are consistent predictors of the prevalence and severity of chronic pain, as well as associated disabilities and unfavorable surgical outcomes (63, 64). Socioeconomic factors, including poverty, unemployment, and social isolation, are associated with the global severity of chronic pain (65–67). In a cross-sectional survey, a greater number of people living in remote areas and belonging to minority ethnic groups suffered from chronic pain (1.46, CI 95%, 1.30–1.65; p < 0.001). Emma et al.’s systematic review of 41 studies (spanning 17 countries, n = 2,161,617) demonstrated significantly elevated chronic pain prevalence and severity among immigrant and ethnic minority populations globally, revealing structural health inequities in pain burden distribution (63).
Chronic postsurgical pain in oldrer patients emerges from a complex interplay of biological, psychological, and social determinants. Biologically, age-related neuroimmune degeneration, genetic susceptibilities, inflammatory dysregulation, and comorbid disease burden collectively heighten nociceptive sensitivity and impede pain resolution. Psychologically, pre-existing affective disorders, maladaptive stress responses, and pain catastrophizing behaviors significantly amplify pain perception, impair coping mechanisms, and prolong recovery trajectories. Socially, structural inequities, including socioeconomic deprivation, limited education, cultural marginalization, and inadequate support systems, correlate with increased pain severity, functional disability, and suboptimal treatment outcomes. Critically, these domains exhibit bidirectional interactions: neuroinflammation may exacerbate depressive symptoms, while socioeconomic stressors can potentiate physiological stress responses. This multifactorial framework underscores the necessity of multidimensional risk assessment in older surgical populations. Effective CPSP mitigation requires integrated strategies addressing biomarker profiling, psychological resilience building, and social determinant optimization to enable personalized intervention paradigms.
The complex interactions between age-related physiological decline, surgical stress, and pain chronification can be conceptualized through an integrated pathophysiological framework (Figure 2). This model illustrates how baseline vulnerabilities established by neurodegeneration, immunosenescence, and metabolic alterations interact with the acute stressors of surgery (tissue injury, nerve damage, inflammation) to initiate and amplify the core mechanisms of CPSP (peripheral/central sensitization, neuroimmune inflammation).
FIGURE 2
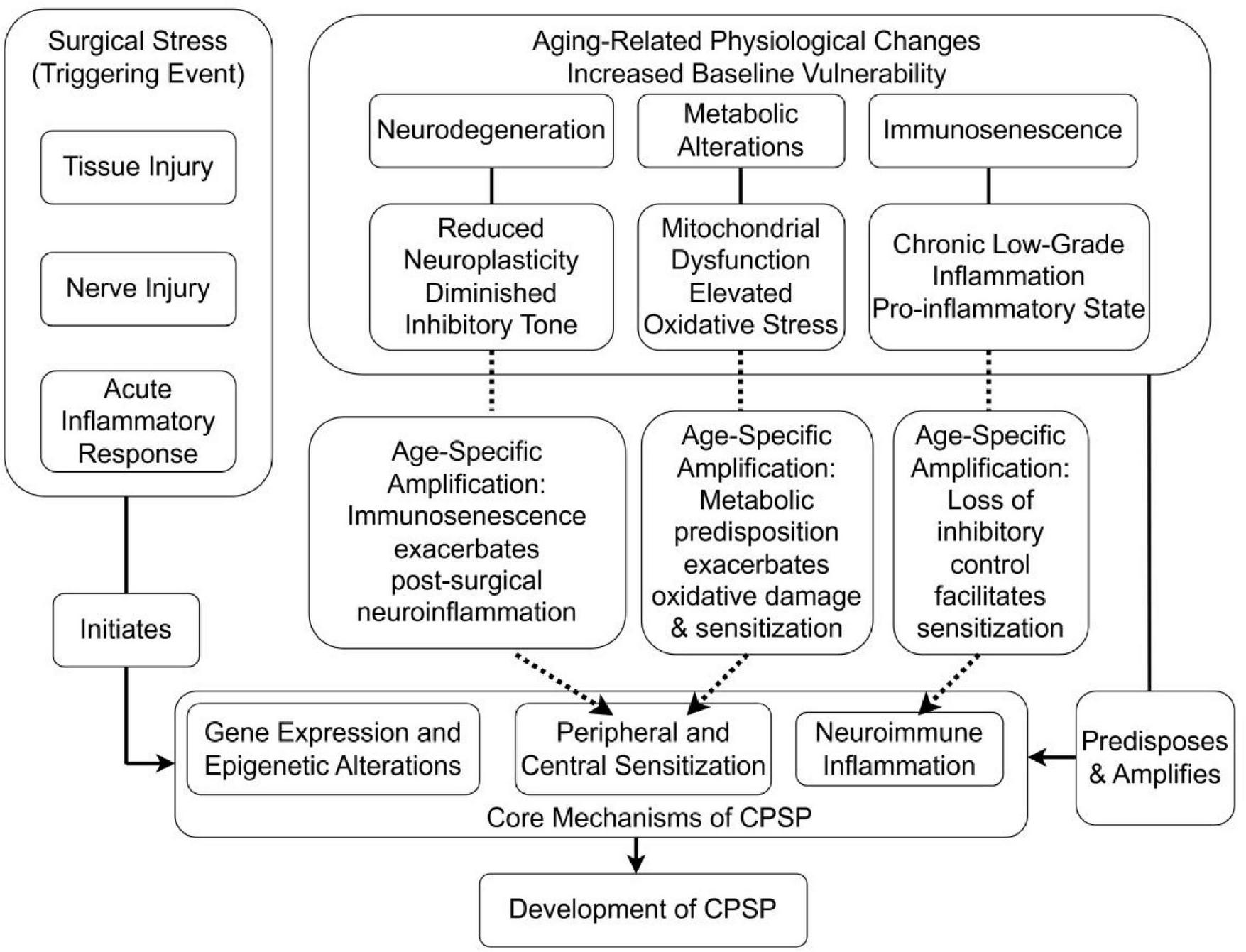
Integrated model of interactions between aging processes and CPSP development.
These mechanistic insights inform the development of targeted assessment approaches that account for age-related changes in pain expression and perception within the surgical context.
4 Surgical context and perioperative considerations
4.1 Surgical stress and neuroimmune interactions
Surgical tissue injury initiates a cascade of neuroimmune responses that can lead to persistent pain states. Post-operative inflammation has the potential to result in persistent pain. Clinical studies suggest that plasma concentrations of inflammatory mediators generally change following surgery and may increase immediately thereafter (37–39). Postoperative inflammatory responses can play a critical role in the development of CPSP. This heightened response can adversely affect wound healing and contribute to the induction of chronic pain.
Mitochondrial dysfunction and oxidative stress are increasingly implicated in CPSP pathogenesis. Evidence indicates that mitochondrial impairment drives reactive oxygen species (ROS) accumulation, subsequently inducing neuronal pyroptosis – an inflammatory programmed cell death process that critically contributes to CPSP (46).
4.2 Procedure-specific risk profiling
Older patients are subjected to a diverse array of surgical interventions, each presenting distinct risks for the development of postoperative chronic pain. The incidence and severity of CPSP are frequently correlated with the surgical site and the invasiveness of the procedure. Thoracic surgeries, especially those involving the lungs and pleura, are notably associated with a high incidence of chronic pain, which adversely affects the quality of life (68). A prospective cohort study examining open-heart surgery identified preoperative chest pain and elevated immediate postoperative pain scores as significant predictors of chronic pain 6 months following the procedure (69). A retrospective study on chronic pain after donor nephrectomy found that 33% of patients reported experiencing chronic pain (70). These findings suggest that both the nature of the surgical procedure and the patient’s preoperative condition play crucial roles in the development of CPSP. As the prevalence of complex surgical procedures among older adults continues to rise, it becomes imperative to elucidate the relationship between various surgical types and the risk of CPSP in order to develop customized pain management strategies. Comprehensive pre-operative evaluations and meticulous post-operative care are essential to mitigating the incidence of CPSP and enhancing the postoperative quality of life for older patients. Table 2 presents the incidence rates of CPSP following several common surgical procedures (13, 26, 71–73).
TABLE 2
| Type of surgery | Chronic pain up to 12 months (%) | Incidence of all cpsp (%) |
|---|---|---|
| Thoracotomy | 41.2 | 5–71 |
| Sternotomy | 27 | 7–50 |
| Knee arthroplasty | 18.4 | 13–44 |
| Hip arthroplasty | 28 | 27 |
| Inguinal hernia surgery | 29.7 | 5–63 |
| Mastectomy | 43–56 (breast cancer surgery) | 11–57 |
| Gynecologic surgery | 15–40 | Not reported |
| Lower limb amputation | 75 | 30–85 |
| Cesarean section | Not reported | 6–55 |
| Cholecystectomy | Not reported | 3–56 |
| Craniotomy | Not reported | 7–65 |
| Dental surgery | Not reported | 5–13 |
| Abdominal surgery (bowel and colorectal) | Not reported | 17–21 |
The incidence of CPSP in different types of surgery.
Understanding the surgical context and age-related vulnerabilities provides the foundation for developing comprehensive assessment strategies tailored to older adults.
5 Clinical assessment and diagnostic approaches
5.1 Predictive modeling for CPSP risk stratification
Numerous studies have been undertaken to develop and validate prediction models for CPSP in older patients, with the objective of identifying individuals at high risk and implementing preventive strategies. A retrospective cohort study involving 577 older patients (aged 65 years and older) who underwent thoracic surgery reported a CPSP incidence rate of 26.9%. The analysis identified that variables including age over 75, body mass index, intraoperative blood loss, length of hospital stay, and preoperative neutrophil count are associated with CPSP. The bootstrap stepwise model demonstrated an area under the curve (AUC) of 0.66 (95% CI, 0.61–0.71) in the observational cohort and 0.64 (95% CI, 0.59–0.69) in the validation cohort (74). The calibration curve indicated strong agreement between predicted and observed risks of chronic postsurgical pain in older adults.
Additionally, a separate prospective study has validated a CPSP risk model that is predicated on six clinical predictors. They applied the generalized linear mixed model generated by the development study (75), which integrates variables including the type of surgery, patient age, physical and mental health status, as well as preoperative pain at both the surgical site and other locations. The findings indicate that this model is capable of effectively identifying approximately 70% of patients at risk for CPSP across diverse patient populations (76).
These studies suggest that prediction models for CPSP in older patients assist clinicians in identifying individuals at high risk and in developing personalized pain management strategies, thereby enhancing postoperative quality of life (74, 76). However, several limitations are evident in these studies. Firstly, the generalizability of these models to surgical procedures not encompassed by the inclusion criteria necessitates further validation. Secondly, certain procedures analyzed demonstrated sex specificity; for instance, the study conducted by Montes et al. exclusively included male patients undergoing hernia repair or thoracotomy (76). Additionally, the retrospective nature of the study design precluded the evaluation of psychological status. Future investigations employing more advanced machine learning techniques could potentially better capture the non-linear interactions among clinical, psychosocial, and genetic factors, thereby achieving greater predictive accuracy compared to traditional statistical models.
5.2 Comprehensive geriatric pain assessment
Subjective pain assessment primarily depends on patient self-reports and constitutes a critical component of pain evaluation. Commonly employed instruments include the Visual Analog Scale (VAS), the Numeric Rating Scale (NRS), and the Facial Expression Scale (FPS). These tools necessitate a certain level of communicative and cognitive ability from patients. However, older adults may encounter challenges in accurately articulating their pain due to cognitive impairments or language expression difficulties. Consequently, the selection and application of assessment tools must be carefully tailored to accommodate the specific circumstances of each individual. Consequently, observational pain tools (OPTs) have been devised to bridge this gap. Empirical studies suggest that instruments such as the Pain Assessment in Advanced Dementia (PAINAD) and the Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC) are efficacious in assessing pain among cognitively impaired older adults (77, 78). The PAINAD and PACSLAC scales differ mainly in their methods and target groups (Table 3). PAINAD is ideal for quick pain identification and management, while PACSLAC offers greater detection accuracy through detailed behavioral observation, making it better for long-term monitoring (79, 80). These tools integrate multiple behavioral indicators, including facial expressions and body movements, to facilitate a more precise evaluation of pain in situations where self-reporting is not possible.
TABLE 3
| Feature | PAINAD | PACSLAC |
|---|---|---|
| Primary objective | To provide a rapid and efficient instrument for pain identification and initial management. | To enable a comprehensive and detailed detection of pain behaviors for longitudinal monitoring. |
| Target population | Primarily designed for individuals with advanced dementia. | Applicable to a broader range of seniors with various communication limitations. |
| Assessment domains | Evaluates five core behavioral indicators: 1) Breathing 2) Negative vocalization 3) Facial expression 4) Body language 5) Consolability |
Assesses a wide spectrum of behaviors across four categories: 1)Facial expressions 2)Activity/body movements 3)Social/personality factors 4)Physiological indicators/eating/sleeping changes |
| Typical use case | Clinical quick-check during routine rounds or in response to sudden behavioral changes. Ideal for fast-paced environments like acute care settings. | In-depth evaluation in stable, long-term care settings (e.g., nursing homes) for establishing a baseline and tracking pain over time. |
| Time required | Very brief (approximately 1–2 min to administer). | More time-consuming due to its detailed nature (approximately 5–10 min). |
| Key strength | Speed and practicality. Its simplicity facilitates high-frequency use and quick decision-making by frontline clinicians. | Comprehensiveness and accuracy. Its detail-oriented design minimizes the risk of missing subtle pain cues, enhancing detection reliability. |
| Primary limitation | May overlook subtle or complex pain presentations due to its brevity. | Its length can be a barrier to implementation in time-constrained clinical environments. |
Comparison of pain assessment tools for older adults with cognitive impairment: PAINAD vs. PACSLAC.
The selection between PAINAD and PACSLAC is not a matter of superiority but of contextual appropriateness. The PAINAD scale is optimized for rapid clinical utility, whereas the PACSLAC is engineered for detailed observational accuracy, making it better suited for long-term care planning and comprehensive assessment. Data from these studies (77, 78). PAINAD, Pain Assessment in Advanced Dementia; PACSLAC, Pain Assessment Checklist for Seniors with Limited Ability to Communicate.
5.2.1 Special considerations for geriatric pain assessment
Pain assessment in older adults requires tailored approaches accounting for cognitive, sensory, and functional limitations: (1) cognitively intact older people: standard self-report tools (NRS, VAS) remain valid, though may require larger formats and verbal reinforcement. (2) Mild-moderate cognitive impairment: behavioral observation tools (PAINAD, PACSLAC) supplemented with surrogate reporting from caregivers. (3) Advanced dementia: multimodal assessment combining behavioral observation, physiological monitoring, and trial of analgesic interventions.
Challenges in CPSP-specific assessment: (1) distinguishing new surgical pain from pre-existing chronic pain conditions. (2) Accounting for atypical pain presentations (e.g., silent ischemia, painless pathologies). (3) Integrating functional impact measures (Activities of Daily Living, mobility scales). (4) Incorporating frailty assessment (Clinical Frailty Scale) for risk stratification.
Objective physiological indicators offer an alternative method for pain assessment, particularly in patients with non-verbal or cognitive impairments. Research indicates that physiological signals, such as heart rate variability, skin conductance, and facial electromyography, can be effectively employed to evaluate pain levels (81). These indicators have the potential to augment subjective evaluations, thereby providing a more comprehensive understanding of the patient’s pain experience. For instance, a study investigating the application of wearable technology to monitor patients’ physiological responses post-surgery demonstrated the feasibility of incorporating these objective measures into standard pain assessment protocols (81). Aside from that, the researchers examined the correlation between physiological measures and subjective pain scores, discovering that although a relationship exists, the outcomes of the two assessments may not always align. This disparity underscores the significance of employing multimodal pain assessment approaches in the geriatric population (81, 82).
Research has demonstrated that these devices have the potential to outperform traditional subjective pain assessment methods by monitoring functional clinical indicators, thereby providing more objective outcomes. Advances in modular wearable technology, for instance, now permit the tracking of various health parameters, including aerobic capacity, physical activity, stress levels, and sleep quality. These objective metrics can assist in the management of chronic conditions and offer a more comprehensive evaluation of pain and functional status (83). Furthermore, research on postoperative recovery highlights the promise of wearable sensors. A comparative study of patients undergoing uniportal endoscopic versus open lumbar surgery indicated that those utilizing wearable sensors experienced a more rapid recovery of mobility and benefited from continuous physiological assessment. This methodology not only facilitates the creation of individualized rehabilitation protocols but also supports timely interventions throughout the recovery process (84).
In summary, the selection of tools for assessing postoperative chronic pain in older adults should encompass both subjective and objective metrics. Integrating observational pain assessment instruments with physiological indicators enables healthcare professionals to enhance the precision and efficacy of pain management strategies within this demographic.
5.3 Diagnostic challenges and differential diagnosis
Diagnosing CPSP in older adults presents a significant challenge due to the multifaceted nature of pain and the concomitant effects of various health conditions. Older patients frequently experience multiple comorbidities, such as osteoarthritis, neuropathic pain, and other chronic pain syndromes. These comorbidities can obscure or exacerbate postoperative pain, complicating both pain assessment and diagnosis. Consequently, differentiating between pain directly attributable to surgical intervention and pain originating from pre-existing conditions becomes increasingly complex.
Cognitive impairments can impede patients’ capacity to effectively communicate their pain levels, resulting in inadequate pain assessment and management. Research indicates that older patients with cognitive impairments are less likely to utilize standardized tools for pain assessment, often leading to delays in receiving appropriate pain interventions (85). Insufficient pain management can exacerbate cognitive decline, elevate the risk of psychosis, and further complicate clinical presentations. Moreover, cognitive dysfunction may affect a patient’s perception of pain and the effectiveness of pain management strategies. Individuals with dementia may demonstrate an atypical response to pain, potentially resulting in misdiagnosis or inappropriate treatment (86).
Chronic postsurgical pain is frequently underdiagnosed or subject to delayed diagnosis in resource-constrained settings and long-term care facilities. The limited availability of diagnostic tools and technologies in these environments constitutes a significant impediment. Advanced diagnostic modalities, such as computed tomography (CT) or magnetic resonance imaging (MRI), are often inaccessible or prohibitively expensive, thereby exacerbating the challenges associated with the precise identification of chronic postsurgical pain (87). Consequently, healthcare providers in these settings are often compelled to depend exclusively on comprehensive history-taking and physical examination for initial assessments, which may elevate the risk of misdiagnosis or overlooked cases.
The diagnostic complexities associated with postoperative chronic pain in older adults are multifaceted, involving the interplay of comorbidities and cognitive impairments. Overcoming these challenges necessitates a multidisciplinary approach that prioritizes comprehensive evaluation and individualized pain management strategies, thereby enhancing outcomes in the older adult population (Figure 3).
FIGURE 3
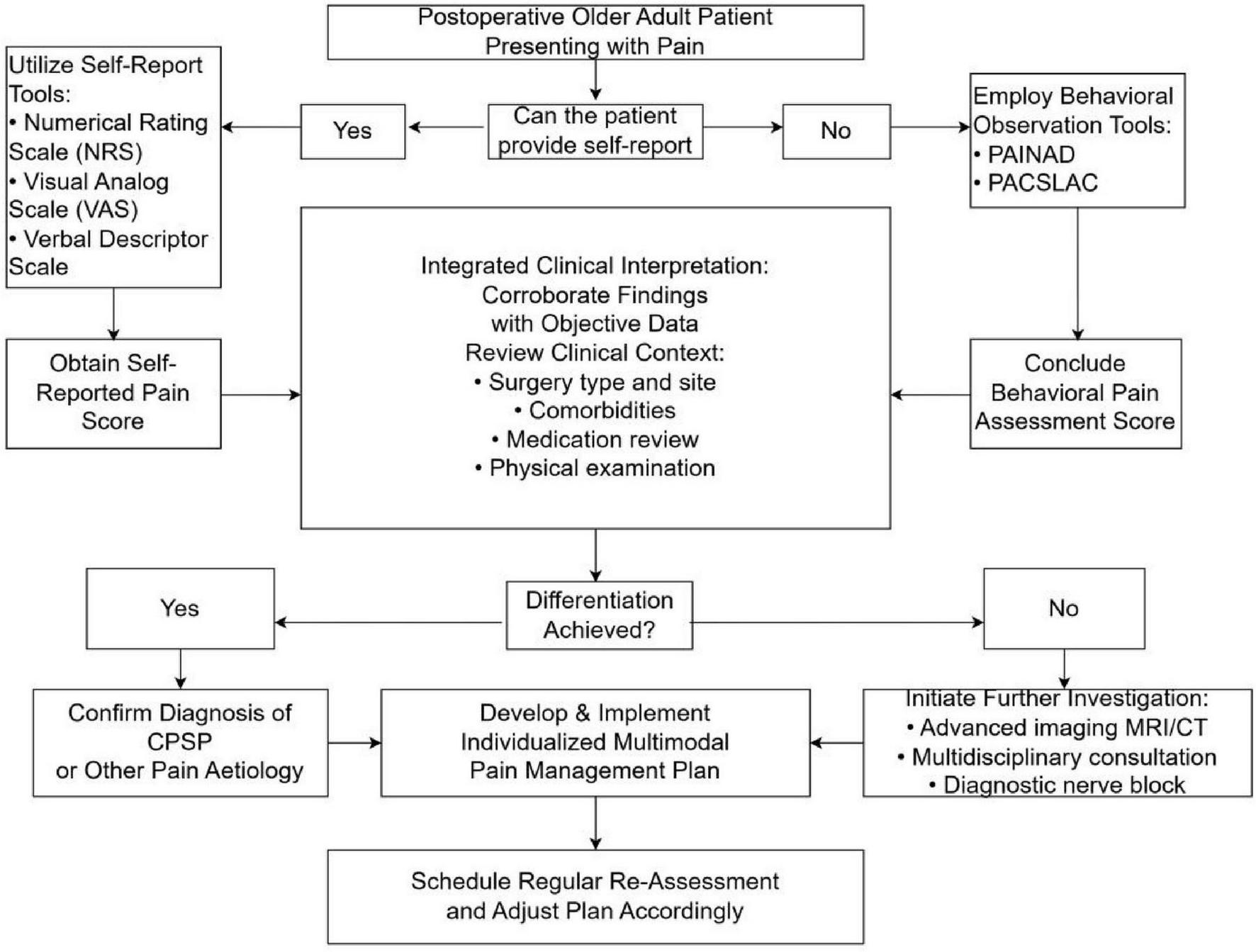
Clinical evaluation flowchart for postsurgical pain in older adults.
Accurate assessment provides the foundation for implementing personalized management strategies that address the multifactorial nature of CPSP in older adults.
6 Management and prevention strategies
6.1 Multimodal analgesia and regional techniques
At present, there are no effective pharmacological interventions or preventative measures available to mitigate CPSP in the older adults, owing to the myriad factors influencing its onset. Nonetheless, it is imperative for healthcare professionals to endeavor to minimize the incidence of CPSP and to provide appropriate attention and care to affected individuals. Multimodal analgesia is defined as two or more analgesic modes targeting different receptors along the pain pathway to improve analgesia while reducing side effects (88). Recent research indicates that multimodal analgesia may offer potential benefits in reducing CPSP in patients, with regional block techniques or epidural block techniques serving as the cornerstone of this approach. Research suggests that regional blockade can attenuate nociceptive nerve impulses, modulate glial cell signaling, and diminish neuronal synaptic plasticity (89, 90). Concurrently, local anesthetics possess intrinsic properties that contribute to the reduction of sensitization and exhibit anti-inflammatory effects. A retrospective study demonstrated that patients who underwent general anesthesia exhibited a heightened risk of developing CPSP within 6 months following surgery, in comparison to those who received regional anesthesia (3). Evidence suggests that regional anesthesia is effective in mitigating the incidence of CPSP after procedures such as hysterectomy, cesarean section, and total knee replacement surgeries (91–93). Moreover, a recent observational study suggested that the use of regional blocks may reduce postoperative opioid consumption, especially in patients with higher pain thresholds (94). The administration of elevated intraoperative opioid doses, especially remifentanil, may lead to intensified acute postoperative pain and increased analgesic consumption, potentially attributable to opioid-induced hyperalgesia (95). Employing regional block techniques can mitigate intraoperative opioid requirements and prevent the heightened pain sensitivity associated with high-dose opioid administration, thereby offering a more advantageous strategy for the prevention of CPSP in older patients.
Regional block constitutes a critical component of multimodal analgesia, with pharmacological agents also playing an indispensable role. Numerous drugs can counteract the receptors within pain pathways that are activated by surgical stress. These primarily include local anesthetics, NMDA receptor antagonists, Non-steroidal anti-inflammatory drugs (NSAIDs), and anticonvulsants, which target various channels or receptors and exert effects on both peripheral and central regions (96). Similar to regional blocks, these pharmacological agents aim to inhibit the activation of peripheral neurons, mitigate detrimental stimulation of the central nervous system, and ultimately achieve effective pain control.
6.2 Pharmacological management with geriatric considerations
6.2.1 Lidocaine infusion therapy
Drawing upon evidence from multiple randomized controlled trials and retrospective studies, lidocaine, a local anesthetic, emerges as a potentially valuable alternative for the management of chronic neuropathic pain in select patient populations. The continuous infusion of lidocaine during the perioperative period has been shown to extend pain control by attenuating the inflammatory response, diminishing the perception of neuropathic pain, and reducing central sensitization (97). The mechanism of action may extend beyond the inhibition of voltage-gated sodium channels (VGSCs) to encompass effects on hyperpolarization-activated cyclic nucleotide-gated channels, transient receptor potential ion channels, and specific G-protein-coupled receptors (GPCRs) (97, 98). Harriet’s systematic review on lidocaine examined 12 randomized controlled trials with patients averaging over 60 years old, including seven in surgical settings (99). These seven studies reported no adverse effects from lidocaine. However, a weight-based dosing of 2 mg/min for patients under 70 kg and 3 mg/min for those 70 kg or more is discouraged in the older adults due to inconsistent plasma concentrations, potentially affecting analgesic effectiveness and increasing toxicity risk (99). Two distinct lidocaine dosing regimens were utilized across these studies. The first protocol entailed a continuous intravenous infusion at a rate of 1 mg/kg/h, which was initiated at the conclusion of surgery and sustained for a duration of 24 h (100). The second regimen comprised an intravenous bolus dose of 1.5–2 mg/kg, administered either at the induction of anesthesia or 30 min prior to the commencement of surgery. This was followed by a continuous intraoperative infusion at a rate of 1.5–3 mg/kg/h. The infusion was discontinued at various time points, including 30 min before the completion of skin closure (101), at the conclusion of wound closure (102), or 1–2 h post-suturing (103–106). Intravenous administration of lidocaine has demonstrated benefits in breast and abdominal surgeries, with a recent meta-analysis suggesting that its continuous infusion reduces the incidence of chronic pain 3–6 months following surgery (97, 107).
6.2.2 Ketamine and NMDA antagonism
Ketamine is the most extensively studied NMDA receptor antagonist for pain management, as evidenced by numerous systematic reviews and meta-analyses. Research suggests that ketamine may be effective as an adjunctive therapy for both acute and non-cancer chronic pain, although its mechanism of action in chronic postoperative pain remains inadequately understood (108–110). A cochrane database system review demonstrated that the administration of intravenous ketamine at least 24 h following surgery resulted in a reduction of chronic pain at both 3 and 6 months postoperatively (109). This analysis encompassed 14 randomized controlled trials examining the perioperative administration of ketamine. In the majority of studies, ketamine was administered as a pre-operative bolus ranging from 200 to 500 μg/kg, followed by an intraoperative infusion of 50–300 μg/kg/h, or in some cases, no intraoperative dosing was applied. Esketamine, the S-enantiomer of ketamine, demonstrated more potent antidepressant effects and a more favorable side-effect profile in comparison to ketamine (111). Several randomized controlled trials have validated the safety of administering intraoperative esketamine at doses below 0.5 mg/kg/h, in conjunction with postoperative analgesia using 0.72 mg/kg of esketamine (112, 113). A systematic review demonstrated that the administration of intravenous ketamine during perioperative thoracotomy was associated with a reduction in the incidence of acute pain, however, the evidence supporting its efficacy in preventing CPSP remains limited (114). A recent systematic review and meta-analysis provides further evidence supporting the potential of ketamine in both preventing and treating CPSP (115). The authors propose that ketamine may mitigate the risk of CPSP by modulating affective and mood-related disorders associated with pain chronification. Consequently, further research is required to address existing limitations, including the need for larger sample sizes, diverse population studies, and variations in administration timing and routes (108, 116).
6.2.3 Adjuvant medications
Additional pharmacological agents encompass anticonvulsants and NSAIDs. Within the category of anticonvulsants, gabapentin and pregabalin are prominent examples. Earlier studies suggested that gabapentin and pregabalin could potentially reduce the risk of chronic postoperative pain (117). However, recent meta-analyses have found no evidence to support the efficacy of these drugs in preventing chronic postoperative pain when used perioperatively (118, 119). NSAIDs are effective in alleviating acute pain and play a crucial role in multimodal analgesia. However, it is noteworthy that randomized controlled trials have yet to demonstrate a substantial impact of these medications on the mitigation of chronic postoperative pain (116, 120). In the context of older patients, especially those with an elevated risk of bleeding, it is advisable to administer NSAIDs at reduced dosages with prolonged intervals between doses (121). Chang’s review identified two primary findings. Firstly, restricting NSAID use to the postoperative period, without pre- or intraoperative administration, and maintaining it at the lowest effective dose for a brief duration, did not elevate the risk of surgical bleeding complications (122). Secondly, the short-term use of NSAIDs for postoperative analgesia did not seem to increase the risk of acute kidney injury in patients with normal baseline renal function (122). Moreover, the current literature does not provide conclusive evidence regarding the clinically significant adverse effects of short-term NSAID administration in patients with pre-existing renal impairment (122).
6.3 Integrative and non-pharmacological approaches
Acupuncture, a traditional Chinese therapeutic practice, has garnered increasing attention in recent years for its efficacy in the prevention and management of postoperative pain. Acupuncture modulates pain through several neurochemical mechanisms. It activates opioid peptide neurons in the brain and spinal cord, enhances the expression of endogenous opioid peptides, and upregulates opioid receptors within cerebral nuclei. Additionally, acupuncture promotes central 5-hydroxytryptamine (5-HT) synthesis while inhibiting peripheral 5-HT release, resulting in suppressed pain transmission and reduced pain sensitization (123, 124). Furthermore, acupuncture aids in regulating pain conduction by helping to maintain the balance between excitatory neurotransmitters, such as glutamate and aspartate, along with their receptors, and inhibitory neurotransmitters, including γ-aminobutyric acid (GABA) and glycine, and their corresponding receptors (125). A randomized controlled trial investigating acupuncture for neuropathic pain following breast cancer surgery reported significant clinical improvements. Patients in the acupuncture group received 18 sessions over 8 weeks. Compared with the control group, they demonstrated a significantly greater reduction in Brief Pain Inventory-Short Form (BPI-SF) scores (−1.1 ± 1.7 vs. 0.3 ± 1.5; p = 0.03) (126). Empirical research suggests that acupuncture substantially mitigates postoperative pain. A systematic review and meta-analysis found that acupuncture may be more effective than pharmacological treatments in reducing pain related to lumbar disk herniation, and was associated with fewer adverse events (127). A study examining the efficacy of acupuncture for chronic pain within a military cohort demonstrated significant improvements in pain scores among patients utilizing electrostimulation acupuncture (128). Acupuncture not only mitigates pain but also potentially exerts a beneficial influence on psychological disorders associated with chronic pain. Research suggests a bidirectional relationship between chronic pain and psychological disorders. As a psychosomatic intervention, acupuncture may effectively alleviate psychological issues resulting from chronic pain (129). In the context of postoperative pain management, acupuncture has shown promise in aiding patients to more effectively manage pain and facilitate recovery. Although acupuncture has demonstrated potential in the management of postoperative pain, there is a paucity of research regarding its efficacy in CPSP. This underscores the need for additional high-quality randomized controlled trials to substantiate its effectiveness and safety.
6.4 Multidisciplinary geriatric pain management
Effective CPSP management in older adults requires a multidisciplinary approach that addresses the unique physiological, psychological, and social needs of this population. This includes: (1) geriatrician-led comorbidity optimization. (2) Pain specialist-guided medication management. (3) Physical therapist-supervised functional restoration. (4) Occupational therapist-directed activity modification. (5) Social worker-supported resource navigation. (6) Pharmacist-conducted medication reconciliation.
Special Considerations for Frail Older Adults: (1) polypharmacy management through systematic deprescribing. (2) Functional status preservation through early mobilization. (3) Cognitive and mood disorder screening and management. (4) Social support system assessment and caregiver education.
Table 4 illustrates the perioperative risk factors, assessment, prevention, and treatment strategies for chronic postsurgical pain in the older population (16, 130).
TABLE 4
| Timing | Risk factor | Assessment | Prevention and treatment |
|---|---|---|---|
| Pre-operative | Comorbidities; Psychosocial factors: decreased immune function, anxiety, depression, gene mutation, etc. Type of surgery |
Pain questionnaire survey; Psychological and functional assessment; Prediction model |
Pain management education; Preemptive analgesia |
| Intra-operative | Surgical stimulation intensity; Nerve injury; High doses of opioids |
Surgical quality assessment; Intraoperative medication evaluation |
Personalized multimodal analgesia; Regional or intraspinal block; Reducing opioid use |
| Post-operative | Comorbidities: cognitive impairment, etc. Peripheral and central sensitization; Psychosocial factors: inflammatory response, economic burden, living alone, education level, depression, anxiety, etc. |
Subjective scale evaluation and objective physiological index comprehensive evaluation; Multidisciplinary collaborative evaluation |
Postoperative nursing guidance; Pain anticipation management; Multidisciplinary cooperation; Developing personalized pain control strategies |
Perioperative risk factors, assessment, prevention and treatment of CPSP in older adults.
7 Conclusion
Chronic postsurgical pain in older adults is a multifactorial condition influenced by biological, psychological, and social determinants. This review synthesizes evidence underscoring the necessity of an integrated, interdisciplinary approach to effectively address CPSP in the aging population. Key components of such an approach include collaboration among geriatricians, pain specialists, anesthesiologists, psychologists, physical therapists, and social workers to deliver personalized, multimodal care.
Moving forward, several actionable strategies are recommended. First, policy initiatives should advocate for the integration of CPSP management into standard geriatric care protocols, ensuring that pain assessment and prevention are routine in perioperative care. Second, the implementation of structured, long-term follow-up systems utilizing telehealth and wearable technology for remote pain monitoring can enhance postoperative surveillance and facilitate timely interventions. Additionally, clinical practice should prioritize the use of validated assessment tools tailored to older adults, especially those with cognitive impairments, and embrace multimodal analgesic regimens that minimize opioid exposure.
Technological innovations, including electronic health record integrations and mobile health applications, offer promising avenues for improving pain tracking and patient engagement. Further research should focus on elucidating the molecular mechanisms of CPSP, validating predictive models incorporating genetic and psychosocial variables, and conducting large-scale randomized trials to evaluate targeted interventions.
This review highlights the critical need to reconceptualize CPSP management through a geriatric-focused lens, yet it is limited by the heterogeneity of existing studies and a reliance on observational data. Despite these constraints, this work contributes to the growing discourse on geriatric pain management by providing a comprehensive framework that bridges clinical evidence with practical strategies aimed at improving outcomes in older surgical patients.
Statements
Author contributions
JZ: Writing – review & editing, Writing – original draft. TY: Investigation, Writing – review & editing, Data curation, Formal analysis. JL: Writing – review & editing, Supervision.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This manuscript was supported by the research project of Sichuan Gerontology Society under grant no. 24SCLN094.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
ISAP Classification of Chronic Pain. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Washington, DC: ISAP (1986).
2.
Etzioni DA Liu JH Maggard MA Ko CY . The aging population and its impact on the surgery workforce.Ann Surg. (2003) 238:170–7. 10.1097/01.SLA.0000081085.98792.3d
3.
Jin J Zhang T Xiong X Chen H Jiang Y He S . A prospective study of chronic postsurgical pain in elderly patients: incidence, characteristics and risk factors.BMC Geriatr. (2023) 23:289. 10.1186/s12877-023-04006-w
4.
Hermans E van Schaik PM Prins HA Ernst MF Dautzenberg PJ Bosscha K . Outcome of colonic surgery in elderly patients with colon cancer.J Oncol. (2010) 2010:865908. 10.1155/2010/865908
5.
Soleman J Ullmann M Greuter L Ebel F Guzman R . Mortality and outcome in elderly patients undergoing emergent or elective cranial surgery.World Neurosurg. (2021) 146:e575–89. 10.1016/j.wneu.2020.10.138
6.
Schug SA Bruce J . [Risk stratification for the development of chronic postsurgical pain].Schmerz. (2018) 32:471–6. 10.1007/s00482-018-0332-4
7.
Pinter G Stromer W Donnerer J Geyrhofer S Leeb B Mitrovic N et al [Pain treatment in old age: special features and recommendations]. Zeitschrift fur Gerontologie und Geriatrie. (2021) 54:605–10. 10.1007/s00391-020-01766-7
8.
Gosch M Pils K Venkat S Singler K . [Aspects of multimodal pain therapy in old age].Zeitschrift fur Gerontologie und Geriatrie. (2021) 54:823–32. 10.1007/s00391-021-01952-1
9.
Prommer E Ficek B . Management of pain in the elderly at the end of life.Drugs Aging. (2012) 29:285–305. 10.2165/11599210-000000000-00000
10.
Mohamed Zaki LR Hairi NN . Chronic pain and pattern of health care utilization among Malaysian elderly population: National Health and Morbidity Survey III (NHMS III, 2006).Maturitas. (2014) 79:435–41. 10.1016/j.maturitas.2014.08.014
11.
Statistics NCFH. National Hospital Discharge Survey: Number of all-listed Procedures for Discharges from Short-Stay Hospitals, by Procedure Category and age: United States. (2010). Available online at: https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf [Accessed March 1, 2020].
12.
Macrae WA . Chronic post-surgical pain: 10 years on.Br J Anaesth. (2008) 101:77–86. 10.1093/bja/aen099
13.
Kehlet H Jensen TS Woolf CJ . Persistent postsurgical pain: risk factors and prevention.Lancet. (2006) 367:1618–25. 10.1016/S0140-6736(06)68700-X
14.
Wang L Guyatt GH Kennedy SA Romerosa B Kwon HY Kaushal A et al Predictors of persistent pain after breast cancer surgery: a systematic review and meta-analysis of observational studies. CMAJ. (2016) 188:E352–61. 10.1503/cmaj.151276
15.
Esses GJ Liu X Lin HM Khelemsky Y Deiner S . Preoperative frailty and its association with postsurgical pain in an older patient cohort.Reg Anesth Pain Med. (2019) [Online ahead of print.]. 10.1136/rapm-2018-100247.
16.
Esses G Deiner S Ko F Khelemsky Y . Chronic post-surgical pain in the frail older adult.Drugs Aging. (2020) 37:321–9. 10.1007/s40266-020-00761-2
17.
Zhang Y Zhou R Hou B Tang S Hao J Gu X et al Incidence and risk factors for chronic postsurgical pain following video-assisted thoracoscopic surgery: a retrospective study. BMC Surg. (2022) 22:76. 10.1186/s12893-022-01522-1
18.
Fiorelli S Cioffi L Menna C Ibrahim M De Blasi RA Rendina EA et al Chronic pain after lung resection: risk factors, neuropathic pain, and quality of life. J Pain Symptom Manage. (2020) 60:326–35. 10.1016/j.jpainsymman.2020.03.012
19.
Wandner LD Scipio CD Hirsh AT Torres CA Robinson ME . The perception of pain in others: how gender, race, and age influence pain expectations.J Pain. (2012) 13:220–7. 10.1016/j.jpain.2011.10.014
20.
Liu X Ben Liu Q . Superior medical resources or geographic proximity? The joint effects of regional medical resource disparity, geographic distance, and cultural differences on online medical consultation.Soc Sci Med. (2024) 350:116911. 10.1016/j.socscimed.2024.116911
21.
Boerboom SL de Haes A Vd Wetering L Aarts EO Janssen IMC Geurts JW et al Preperitoneal bupivacaine infiltration reduces postoperative opioid consumption, acute pain, and chronic postsurgical pain after bariatric surgery: a randomized controlled trial. Obes Surg. (2018) 28:3102–10. 10.1007/s11695-018-3341-6
22.
Glette M Landmark T Jensen MP Woodhouse A Butler S Borchgrevink PC et al Catastrophizing, solicitous responses from significant others, and function in individuals with neuropathic pain, osteoarthritis, or spinal pain in the general population. J Pain. (2018) 19:983–95. 10.1016/j.jpain.2018.03.010
23.
Gilliam WP Craner JR Morrison EJ Sperry JA . The mediating effects of the different dimensions of pain catastrophizing on outcomes in an interdisciplinary pain rehabilitation program.Clin J Pain. (2017) 33:443–51. 10.1097/AJP.0000000000000419
24.
Delgado-Gallén S Soler MD Cabello-Toscano M Abellaneda-Pérez K Solana-Sánchez J España-Irla G et al Brain system segregation and pain catastrophizing in chronic pain progression. Front Neurosci. (2023) 17:1148176. 10.3389/fnins.2023.1148176
25.
Boye Larsen D Laursen M Simonsen O Arendt-Nielsen L Petersen KK . The association between sleep quality, preoperative risk factors for chronic postoperative pain and postoperative pain intensity 12 months after knee and hip arthroplasty.Br J Pain. (2021) 15:486–96. 10.1177/20494637211005803
26.
Rosenberger DC Pogatzki-Zahn EM . Chronic post-surgical pain - update on incidence, risk factors and preventive treatment options.BJA Educ. (2022) 22:190–6. 10.1016/j.bjae.2021.11.008
27.
International Association for the Study of Pain [IASP]. Terminology [Internet]. Washington, DC: IASP (2011).
28.
Glare P Aubrey KR Myles PS . Transition from acute to chronic pain after surgery.Lancet. (2019) 393:1537–46. 10.1016/S0140-6736(19)30352-6
29.
Yiling J Juying J . Research advances on the correlation between frailty and chronic pain in the elderly.Chin J Geriatr. (2023) 42:587–91. 10.3760/cma.j.issn.0254-9026.2023.05.019
30.
Haddad JJ . On the enigma of pain and hyperalgesia: a molecular perspective.Biochem Biophys Res Commun. (2007) 353:217–24. 10.1016/j.bbrc.2006.12.032
31.
Watkins LR Maier SF Goehler LE . Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states.Pain. (1995) 63:289–302. 10.1016/0304-3959(95)00186-7
32.
Zhou YQ Liu Z Liu ZH Chen SP Li M Shahveranov A et al Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. (2016) 13:141. 10.1186/s12974-016-0607-6
33.
De Jongh RF Vissers KC Meert TF Booij LHDJ De Deyne CS Heylen RJ . The role of interleukin-6 in nociception and pain.Anesth Analg. (2003) 96:1096–103. 10.1213/01.ANE.0000055362.56604.78
34.
DeVon HA Piano MR Rosenfeld AG Hoppensteadt DA . The association of pain with protein inflammatory biomarkers: a review of the literature.Nurs Res. (2014) 63:51–62. 10.1097/NNR.0000000000000013
35.
Lu J Shi Y Li Y Niu Z Wu S Luo C et al Chemokine CCL2 mediates neuroglial crosstalk and drives chronic pain pathogenesis. Neurosci Bull. (2025) 41:2296–321. 10.1007/s12264-025-01519-9
36.
Bonfante HL Almeida CS Abramo C Grunewald STF Levy RA Teixeira HC . CCL2, CXCL8, CXCL9 and CXCL10 serum levels increase with age but are not altered by treatment with hydroxychloroquine in patients with osteoarthritis of the knees.Int J Rheum Dis. (2017) 20:1958–64. 10.1111/1756-185X.12589
37.
Kudoh A Katagai H Takazawa T . Antidepressant treatment for chronic depressed patients should not be discontinued prior to anesthesia.Can J Anaesth. (2002) 49:132–6. 10.1007/BF03020484
38.
Reikeras O Borgen P Reseland JE Lyngstadaas SP . Changes in serum cytokines in response to musculoskeletal surgical trauma.BMC Res Notes. (2014) 7:128. 10.1186/1756-0500-7-128
39.
Desborough JP . The stress response to trauma and surgery.Br J Anaesth. (2000) 85:109–17. 10.1093/bja/85.1.109
40.
Gherardini J Uchida Y Hardman JA Chéret J Mace K Bertolini M et al Tissue-resident macrophages can be generated de novo in adult human skin from resident progenitor cells during substance P-mediated neurogenic inflammation ex vivo. PLoS One. (2020) 15:e0227817. 10.1371/journal.pone.0227817
41.
Xie S Gao Z Zhang J Xing C Dong Y Wang L et al Monoclonal antibody targeting CGRP relieves cisplatin-induced neuropathic pain by attenuating neuroinflammation. Neurotox Res. (2024) 42:8. 10.1007/s12640-023-00685-w
42.
Tang CL Lian Z Ding FR Liang J Li XY . Schistosoma-related molecules as a new strategy to combat type 1 diabetes through immune regulation.Parasitol Int. (2024) 98:102818. 10.1016/j.parint.2023.102818
43.
Zhang C Li Y Yu Y Li Z Xu X Talifu Z et al Impact of inflammation and Treg cell regulation on neuropathic pain in spinal cord injury: mechanisms and therapeutic prospects. Front Immunol. (2024) 15:1334828. 10.3389/fimmu.2024.1334828
44.
Lees JG Duffy SS Perera CJ Moalem-Taylor G . Depletion of Foxp3+ regulatory T cells increases severity of mechanical allodynia and significantly alters systemic cytokine levels following peripheral nerve injury.Cytokine. (2015) 71:207–14. 10.1016/j.cyto.2014.10.028
45.
Nowak M Tardivel S Nguyen-Khoa T Abreu S Allaoui F Fournier N et al Mycophenolate mofetil and rapamycin induce apoptosis in the human monocytic U937 cell line through two different pathways. J Cell Biochem. (2017) 118:3480–7. 10.1002/jcb.26007
46.
Hu Y He X Zang H Chen Y Li L Liu T et al Downregulation of Mfn2 contributes to chronic postsurgical pain via inducing the pyroptosis of GABAergic neurons in the spinal cord. CNS Neurosci Ther. (2025) 31:e70508. 10.1111/cns.70508
47.
Wu X Yang L Li Z Gu C Jin K Luo A et al Aging-associated decrease of PGC-1α promotes pain chronification. Aging Cell. (2024) 23:e14177. 10.1111/acel.14177
48.
Curatolo M . Common biological modulators of acute pain: an overview within the AAAPT Project (ACTTION-APS-AAPM Acute Pain Taxonomy).Pain Med. (2020) 21:2394–400. 10.1093/pm/pnaa207
49.
Shi Q Cleeland CS Klepstad P Miaskowski C Pedersen NL . Biological pathways and genetic variables involved in pain.Qual Life Res. (2010) 19:1407–17. 10.1007/s11136-010-9738-x
50.
Mittal R Robalino G Gerring R Chan B Yan D Grati M et al Immunity genes and susceptibility to otitis media: a comprehensive review. J Genet Genomics. (2014) 41:567–81. 10.1016/j.jgg.2014.10.003
51.
Liu B Sen HN Nussenblatt R . Susceptibility genes and pharmacogenetics in ocular inflammatory disorders.Ocul Immunol Inflamm. (2012) 20:315–23. 10.3109/09273948.2012.710706
52.
Krause S Torok D Bagdy G Juhasz G Gonda X . Genome-wide by trait interaction analyses with neuroticism reveal chronic pain-associated depression as a distinct genetic subtype.Transl Psychiatry. (2025) 15:108. 10.1038/s41398-025-03331-5
53.
Sun ZW Waybright JM Beldar S Chen L Foley CA Norris-Drouin JL et al Cdyl deficiency brakes neuronal excitability and nociception through promoting kcnb1 transcription in peripheral sensory neurons. Adv Sci. (2022) 9:e2104317. 10.1002/advs.202104317
54.
Sullivan MD Sturgeon JA Lumley MA Ballantyne JC . Reconsidering Fordyce’s classic article, “Pain and suffering: what is the unit?” to help make our model of chronic pain truly biopsychosocial.Pain. (2023) 164:271–9. 10.1097/j.pain.0000000000002748
55.
Edwards RR Dworkin RH Sullivan MD Turk DC Wasan AD . The role of psychosocial processes in the development and maintenance of chronic pain.J Pain. (2016) 17(9 Suppl):T70–92. 10.1016/j.jpain.2016.01.001
56.
Gureje O Von Korff M Simon GE Gater R . Persistent pain and well-being: a World Health Organization Study in Primary Care.JAMA. (1998) 280:147–51. 10.1001/jama.280.2.147
57.
Hooten WM . Chronic pain and mental health disorders: shared neural mechanisms, Epidemiology, and Treatment.Mayo Clin Proc. (2016) 91:955–70. 10.1016/j.mayocp.2016.04.029
58.
Stevans JM Delitto A Khoja SS Patterson CG Smith CN Schneider MJ et al Risk factors associated with transition from acute to chronic low back pain in us patients seeking primary care. JAMA Netw Open. (2021) 4:e2037371. 10.1001/jamanetworkopen.2020.37371
59.
Hong W Mo F Zhang Z Huang M Wei X . Nicotinamide mononucleotide: a promising molecule for therapy of diverse diseases by targeting NAD+ Metabolism.Front Cell Dev Biol. (2020) 8:246. 10.3389/fcell.2020.00246
60.
Giusti EM Manna C Varallo G Cattivelli R Manzoni GM Gabrielli S et al The predictive role of executive functions and psychological factors on chronic pain after orthopaedic surgery: a longitudinal cohort study. Brain Sci. (2020) 10:685. 10.3390/brainsci10100685
61.
Sun M Wang X Lu Z Yang Y Lv S Miao M et al Chronic postsurgical pain increases postoperative depression risk. J Epidemiol Community Health. (2025) 79:515–21. 10.1136/jech-2024-222761
62.
Ladjević N Milinić M Jovanović V Jovičić J Likić I Ladjević N . Chronic postoperative pain.Acta Clin Croat. (2023) 62(Suppl4):88–96. 10.20471/acc.2023.62.s4.13
63.
Karran EL Grant AR Moseley GL . Low back pain and the social determinants of health: a systematic review and narrative synthesis.Pain. (2020) 161:2476–93. 10.1097/j.pain.0000000000001944
64.
Yap ZL Summers SJ Grant AR Moseley GL Karran EL . The role of the social determinants of health in outcomes of surgery for low back pain: a systematic review and narrative synthesis.Spine J. (2022) 22:793–809. 10.1016/j.spinee.2021.11.013
65.
Inoue S Kobayashi F Nishihara M Arai YC Ikemoto T Kawai T et al Chronic pain in the japanese community–prevalence, characteristics and impact on quality of life. PLoS One. (2015) 10:e0129262. 10.1371/journal.pone.0129262
66.
Matthews E Muldoon M O’Keeffe N McCarthy KF . Social deprivation and paediatric chronic pain referrals in Ireland: a cross-sectional study.Scand J Pain. (2021) 21:597–605. 10.1515/sjpain-2021-0031
67.
Wong WS Fielding R . Prevalence and characteristics of chronic pain in the general population of Hong Kong.J Pain. (2011) 12:236–45. 10.1016/j.jpain.2010.07.004
68.
Clephas PRD Hoeks SE Singh PM Guay CS Trivella M Klimek M et al Prognostic factors for chronic post-surgical pain after lung and pleural surgery: a systematic review with meta-analysis, meta-regression and trial sequential analysis. Anaesthesia. (2023) 78:1005–19. 10.1111/anae.16009
69.
Kampe S Geismann B Weinreich G Stamatis G Ebmeyer U Gerbershagen HJ . The influence of type of anesthesia, perioperative pain, and preoperative health status on chronic pain six months after thoracotomy-a prospective cohort study.Pain Med. (2017) 18:2208–13. 10.1093/pm/pnw230
70.
Owen M Lorgelly P Serpell M . Chronic pain following donor nephrectomy–a study of the incidence, nature and impact of chronic post-nephrectomy pain.Eur J Pain. (2010) 14:732–4. 10.1016/j.ejpain.2009.11.013
71.
Steyaert A Lavand’homme P . Prevention and treatment of chronic postsurgical pain: a narrative review.Drugs. (2018) 78:339–54. 10.1007/s40265-018-0866-x
72.
Schug SA Bruce J . Risk stratification for the development of chronic postsurgical pain.Pain Rep. (2017) 2:e627. 10.1097/PR9.0000000000000627
73.
Lopes A Seligman Menezes M Antonio Moreira de Barros G . Chronic postoperative pain: ubiquitous and scarcely appraised: narrative review.Braz J Anesthesiol. (2021) 71:649–55. 10.1016/j.bjane.2020.10.014
74.
Wu XD Zeng FF Yu XX Yang PP Wu JP Xv P et al Development and validation of a prediction model for chronic post-surgical pain after thoracic surgery in elderly patients: a retrospective cohort study. J Pain Res. (2022) 15:3079–91. 10.2147/JPR.S368295
75.
Montes A Roca G Sabate S Lao JI Navarro A Cantillo J et al Genetic and clinical factors associated with chronic postsurgical pain after hernia repair, hysterectomy, and thoracotomy: a two-year multicenter cohort study. Anesthesiology. (2015) 122:1123–41. 10.1097/ALN.0000000000000611
76.
Montes A Roca G Cantillo J Sabate S Gendolcat Study Group. Presurgical risk model for chronic postsurgical pain based on 6 clinical predictors: a prospective external validation. Pain. (2020) 161:2611–8. 10.1097/j.pain.0000000000001945
77.
Liu JY Briggs M Closs SJ . The psychometric qualities of four observational pain tools (OPTs) for the assessment of pain in elderly people with osteoarthritic pain.J Pain Symptom Manage. (2010) 40:582–98. 10.1016/j.jpainsymman.2010.02.022
78.
Natavio T McQuillen E Dietrich MS Wells N Rhoten BA Vallerand AH et al A Comparison of the Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC) and Pain Assessment in Advanced Dementia Scale (PAINAD). Pain Manag Nurs. (2020) 21:502–9. 10.1016/j.pmn.2020.04.001
79.
Guasconi M Marchioni M Miedico M Brusca A Guarnaccia G Bolzoni M et al Validity and reliability of the Italian version of painad for postoperative pain assessment in geriatric patients with proximal femur fractures. Int J Orthop Trauma Nurs. (2025) 57:101181. 10.1016/j.ijotn.2025.101181
80.
Cheung G Choi P . The use of the Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC) by caregivers in dementia care.N Z Med J. (2008) 121:21–9.
81.
Kasaeyan Naeini E Jiang M Syrjälä E Calderon MD Mieronkoski R Zheng K et al Prospective study evaluating a pain assessment tool in a postoperative environment: protocol for algorithm testing and enhancement. JMIR Res Protoc. (2020) 9:e17783. 10.2196/17783
82.
Dualé C Pereira B Abbal B Julien H Rat P Schoeffler P et al The algoplus score to assess acute postoperative pain in elderly patients-a pilot observational study. Pain Manag Nurs. (2015) 16:890–9. 10.1016/j.pmn.2015.07.002
83.
Chang JL Nguyen P Ruan QZ Pak DJ Robinson CL Dominguez M et al The potential of wearable, modular devices in monitoring functional clinical metrics in patients suffering from chronic pain. Curr Pain Headache Rep. (2025) 29:46. 10.1007/s11916-025-01367-2
84.
Ma A Mobbs RJ Maharaj MM . Patient recovery following uniportal endoscopic vs open lumbar spine surgery: objective analysis of postoperative mobility and gait patterns using wearable sensors.Int J Spine Surg. (2025) 19:39–48. 10.14444/8718
85.
Jones J Sim TF Parsons R Hughes J . Influence of cognitive impairment on pain assessment and management in the emergency department: a retrospective cross-sectional study.Emerg Med Australas. (2019) 31:989–96. 10.1111/1742-6723.13294
86.
Cantón-Habas V Carrera-González MDP Moreno-Casbas MT Quesada-Gómez JM Rich-Ruiz M . Correlation between biomarkers of pain in saliva and PAINAD scale in elderly people with cognitive impairment and inability to communicate: descriptive study protocol.BMJ Open. (2019) 9:e032927. 10.1136/bmjopen-2019-032927
87.
Adesina SA Amole IO Adefokun IG Adegoke AO Ano-Edward GH Eyesan SU . Intramuscular lipomas posing diagnostic and pre-operative counselling challenges in a low-resource setting: a case series.Int J Surg Case Rep. (2024) 122:110093. 10.1016/j.ijscr.2024.110093
88.
Kehlet H Dahl JB . The value of “multimodal” or “balanced analgesia” in postoperative pain treatment.Anesth Analg. (1993) 77:1048–56. 10.1213/00000539-199311000-00030
89.
Andreae MH Andreae DA . Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis.Br J Anaesth. (2013) 111:711–20. 10.1093/bja/aet213
90.
Weinstein EJ Levene JL Cohen MS Andreae DA Chao JY Johnson M et al Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev. (2018) 4:CD007105. 10.1002/14651858.CD007105.pub3
91.
Brandsborg B Nikolajsen L Hansen CT Kehlet H Jensen TS . Risk factors for chronic pain after hysterectomy: a nationwide questionnaire and database study.Anesthesiology. (2007) 106:1003–12. 10.1097/01.anes.0000265161.39932.e8
92.
Nikolajsen L Sørensen HC Jensen TS Kehlet H . Chronic pain following Caesarean section.Acta Anaesthesiol Scand. (2004) 48:111–6. 10.1111/j.1399-6576.2004.00271.x
93.
Sciberras SC Vella AP Vella B Spiteri J Mizzi C Borg-Xuereb K et al A randomized, controlled trial on the effect of anesthesia on chronic pain after total knee arthroplasty. Pain Manag. (2022) 12:711–23. 10.2217/pmt-2021-0081
94.
Zinboonyahgoon N Vlassakov K Lirk P Spivey T King T Dominici L et al Benefit of regional anaesthesia on postoperative pain following mastectomy: the influence of catastrophising. Br J Anaesth. (2019) 123:e293–302. 10.1016/j.bja.2019.01.041
95.
de Hoogd S Ahlers SJ van Dongen EP van de Garde EM Hamilton-Ter Brake TA Dahan A et al Is intraoperative remifentanil associated with acute or chronic postoperative pain after prolonged surgery? An update of the literature. Clin J Pain. (2016) 32:726–35. 10.1097/AJP.0000000000000317
96.
Katz J Seltzer Z . Transition from acute to chronic postsurgical pain: risk factors and protective factors.Expert Rev Neurother. (2009) 9:723–44. 10.1586/ern.09.20
97.
Hermanns H Hollmann MW Stevens MF Lirk P Brandenburger T Piegeler T et al Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. (2019) 123:335–49. 10.1016/j.bja.2019.06.014
98.
Reddi D Curran N . Chronic pain after surgery: pathophysiology, risk factors and prevention.Postgrad Med J. (2014) 90:222–7; quiz 226. 10.1136/postgradmedj-2013-132215.
99.
Daykin H . The efficacy and safety of intravenous lidocaine for analgesia in the older adult: a literature review.Br J Pain. (2017) 11:23–31. 10.1177/2049463716676205
100.
Harvey KP Adair JD Isho M Robinson R . Can intravenous lidocaine decrease postsurgical ileus and shorten hospital stay in elective bowel surgery? A pilot study and literature review.Am J Surg. (2009) 198:231–6. 10.1016/j.amjsurg.2008.10.015
101.
Kuo CP Jao SW Chen KM Wong CS Yeh CC Sheen MJ et al Comparison of the effects of thoracic epidural analgesia and i.v. infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. Br J Anaesth. (2006) 97:640–6. 10.1093/bja/ael217
102.
Staikou C Avramidou A Ayiomamitis GD Vrakas S Argyra E . Effects of intravenous versus epidural lidocaine infusion on pain intensity and bowel function after major large bowel surgery: a double-blind randomized controlled trial.J Gastrointest Surg. (2014) 18:2155–62. 10.1007/s11605-014-2659-1
103.
Koppert W Weigand M Neumann F Sittl R Schuettler J Schmelz M et al Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg. (2004) 98:1050–5. 10.1213/01.ANE.0000104582.71710.EE
104.
Martin F Cherif K Gentili ME Enel D Abe E Alvarez JC et al Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesthesiology. (2008) 109:118–23. 10.1097/ALN.0b013e31817b5a9b
105.
Groudine SB Fisher HA Kaufman RP Patel MK Wilkins LJ Mehta SA et al Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg. (1998) 86:235–9. 10.1097/00000539-199802000-00003
106.
Insler SR O’Connor M Samonte AF Bazaral MG . Lidocaine and the inhibition of postoperative pain in coronary artery bypass patients.J Cardiothorac Vasc Anesth. (1995) 9:541–6. 10.1016/s1053-0770(05)80138-7
107.
Bailey M Corcoran T Schug S Toner A . Perioperative lidocaine infusions for the prevention of chronic postsurgical pain: a systematic review and meta-analysis of efficacy and safety.Pain. (2018) 159:1696–704. 10.1097/j.pain.0000000000001273
108.
Cohen SP Bhatia A Buvanendran A Schwenk ES Wasan AD Hurley RW et al Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. (2018) 43:521–46. 10.1097/AAP.0000000000000808
109.
Chaparro LE Smith SA Moore RA Wiffen PJ Gilron I . Pharmacotherapy for the prevention of chronic pain after surgery in adults.Cochrane Database Syst Rev. (2013) 2013:CD008307. 10.1002/14651858.CD008307.pub2
110.
Klatt E Zumbrunn T Bandschapp O Girard T Ruppen W . Intra- and postoperative intravenous ketamine does not prevent chronic pain: a systematic review and meta-analysis.Scand J Pain. (2015) 7:42–54. 10.1016/j.sjpain.2014.12.005
111.
Hou L Miao J Meng H Liu X Wang D Tan Y et al Sirtuin type 1 mediates the antidepressant effect of S-Ketamine in a chronic unpredictable stress model. Front Psychiatry. (2022) 13:855810. 10.3389/fpsyt.2022.855810
112.
Zhao Y Ye C He Y Bao M Liu Y Yan M . Effect of esketamine for patient-controlled intravenous analgesia on postoperative sleep disturbance in the elderly after total hip or knee arthroplasty: a prospective, randomized, double-blind, and controlled trial.J Arthroplasty. (2025) 41:81–9. 10.1016/j.arth.2025.06.009
113.
Wang Y Ma B Wang C Wang Y Liu A Hang L . The influence of low-dose s-ketamine on postoperative delirium and cognitive function in older adults undergoing thoracic surgery.J Cardiothorac Surg. (2024) 19:324. 10.1186/s13019-024-02811-x
114.
Moyse DW Kaye AD Diaz JH Qadri MY Lindsay D Pyati S . Perioperative ketamine administration for thoracotomy pain.Pain Physician. (2017) 20:173–84.
115.
Willis DE Goldstein PA . Targeting affective mood disorders with ketamine to prevent chronic postsurgical pain.Front Pain Res. (2022) 3:872696. 10.3389/fpain.2022.872696
116.
Richebé P Capdevila X Rivat C . Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations.Anesthesiology. (2018) 129:590–607. 10.1097/ALN.0000000000002238
117.
Clarke H Soneji N Ko DT Yun L Wijeysundera DN . Rates and risk factors for prolonged opioid use after major surgery: population based cohort study.BMJ. (2014) 348:g1251. 10.1136/bmj.g1251
118.
Martinez V Pichard X Fletcher D . Perioperative pregabalin administration does not prevent chronic postoperative pain: systematic review with a meta-analysis of randomized trials.Pain. (2017) 158:775–83. 10.1097/j.pain.0000000000000838
119.
Verret M Lauzier F Zarychanski R Perron C Savard X Pinard AM et al Perioperative use of gabapentinoids for the management of postoperative acute pain: a systematic review and meta-analysis. Anesthesiology. (2020) 133:265–79. 10.1097/ALN.0000000000003428
120.
Thapa P Euasobhon P . Chronic postsurgical pain: current evidence for prevention and management.Korean J Pain. (2018) 31:155–73. 10.3344/kjp.2018.31.3.155
121.
Falzone E Hoffmann C Keita H . Postoperative analgesia in elderly patients.Drugs Aging. (2013) 30:81–90. 10.1007/s40266-012-0047-7
122.
Chang RW Tompkins DM Cohn SM . Are NSAIDs Safe? Assessing the risk-benefit profile of nonsteroidal anti-inflammatory drug use in postoperative pain management.Am Surg. (2021) 87:872–9. 10.1177/0003134820952834
123.
Chen T Zhang WW Chu YX Wang YQ . Acupuncture for pain management: molecular mechanisms of action.Am J Chin Med. (2020) 48:793–811. 10.1142/S0192415X20500408
124.
Zhao ZQ . Neural mechanism underlying acupuncture analgesia.Prog Neurobiol. (2008) 85:355–75. 10.1016/j.pneurobio.2008.05.004
125.
Chen Y Li D Li N Loh P Guo Y Hu X et al Role of nerve signal transduction and neuroimmune crosstalk in mediating the analgesic effects of acupuncture for neuropathic pain. Front Neurol. (2023) 14:1093849. 10.3389/fneur.2023.1093849
126.
Lu W Giobbie-Hurder A Freedman RA Shin IH Lin NU Partridge AH et al Acupuncture for chemotherapy-induced peripheral neuropathy in breast cancer survivors: a randomized controlled pilot trial. Oncologist. (2020) 25:310–8. 10.1634/theoncologist.2019-0489
127.
Zhang W Liu H Le X Song K Yang F Cui Z et al Acupuncture for postoperative pain of lumbar disc herniation: a systematic review and meta-analysis. Medicine. (2022) 101:e32016. 10.1097/MD.0000000000032016
128.
Plunkett A Beltran T Haley C Kurihara C McCoart A Chen L et al Acupuncture for the treatment of chronic pain in the military population: factors associated with treatment outcomes. Clin J Pain. (2017) 33:939–43. 10.1097/AJP.0000000000000518
129.
Lin LL Li HP Yang JW Hao XW Yan SY Wang LQ et al Acupuncture for psychological disorders caused by chronic pain: a review and future directions. Front Neurosci. (2020) 14:626497. 10.3389/fnins.2020.626497
130.
Chen YK Boden KA Schreiber KL . The role of regional anaesthesia and multimodal analgesia in the prevention of chronic postoperative pain: a narrative review.Anaesthesia. (2021) 76(Suppl 1):8–17. 10.1111/anae.15256
Summary
Keywords
chronic postoperative pain, older adults, pain mechanism, prevention, treatment
Citation
Zeng J, Yang T and Li J (2026) Chronic post-surgical pain in older adults: mechanisms, assessment, and management strategies. Front. Med. 12:1719477. doi: 10.3389/fmed.2025.1719477
Received
06 October 2025
Revised
02 December 2025
Accepted
19 December 2025
Published
12 January 2026
Volume
12 - 2025
Edited by
Marios Kyriazis, National Gerontology Center, Cyprus
Reviewed by
Lesly Spring Valdivia Torres, National Scientific and Technical Research Council (CONICET), Argentina
Mario García Domínguez, Universidad de Oviedo Mieres, Spain
Updates
Copyright
© 2026 Zeng, Yang and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiyu Zeng, 13629017775@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.