Abstract
Background:
Intravenous immunoglobulin (IVIg) is widely used to treat primary immunodeficiency, chronic inflammatory demyelinating polyneuropathy, immune thrombocytopenia, and other disorders. Although effective in maintaining IgG trough levels and reducing infections, its safety profile requires further characterization.
Methods:
A large-scale pharmacovigilance study was conducted using the U.S. FDA Adverse Event Reporting System (FAERS) from Q1 2004 to Q4 2024. Four disproportionality methods—reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and multi-item gamma Poisson shrinker (MGPS)—were applied to detect adverse event signals. Weibull modeling was used to assess temporal risk patterns.
Results:
A total of 76,138 IVIg-associated reports were identified. Common events included infusion-site reactions (swelling, erythema, pain), infections (upper respiratory tract infection, bronchitis, pneumonia, influenza, urinary tract infection), and systemic reactions (pyrexia, chills, hypersensitivity, headache, asthenia, nausea, vomiting). Several novel potential safety signals emerged, including blood pressure–related events (hypertension and hypotension), weight changes (loss and gain), and falls.
Conclusion:
Real-world FAERS data confirm the established tolerability of IVIg while highlighting rare but clinically important safety signals, particularly hemolytic anemia and aseptic meningitis. These findings warrant further clinical investigation to optimize monitoring and promote safer therapeutic use.
Background
Intravenous immunoglobulin (IVIg) is a core treatment for primary immunodeficiency (PID), chronic inflammatory demyelinating polyneuropathy (CIDP), immune thrombocytopenia (ITP), and many other diseases. Since receiving market approval, IVIg has been indicated for conditions such as PID and chronic ITP and has demonstrated strong effectiveness in real-world studies (1, 2). Retrospective multicentre cohorts further confirm that IVIg can maintain ideal IgG trough levels and significantly reduce infection rates in both PID and secondary immunodeficiency populations (3). Randomized controlled trials and open-label studies consistently demonstrate that IVIg is typically well-tolerated; common adverse events (AEs), such as headache, fever, chills, and mild skin reactions, typically occur at rates < 20% (4, 5).
However, high-dose or rapid infusion of IVIg can induce rare but potentially fatal hemolytic anemia, especially in patients with blood types A, B, or AB (6, 7). Large case series of transplant recipients and children with Kawasaki disease suggest that non-O blood type, cumulative dose, and high titers of anti-A/B isoagglutinins in the product are major risk factors (6). Analyses of large spontaneous reporting databases have reinforced this understanding: global AE data show a higher reporting rate of IVIg-related hemolysis than those of similar products, implicating high-titer anti-A/B antibodies as a key mechanism (8).
The FDA Adverse Event Reporting System (FAERS) contains over 28 million spontaneous reports submitted by healthcare professionals, patients, and the pharmaceutical industry (9, 10). FAERS can rapidly identify potential safety signals using disproportionality methods such as the reporting odds ratio (ROR) and proportional reporting ratio (PRR) (11). Previous FAERS-based pharmacovigilance studies have identified novel or rare toxicities of biologics, including unexpected respiratory and endocrine events with anti-IL-5 monoclonal antibodies (e.g., asthmatic crisis, adrenal insufficiency) and newly detected safety signals such as alopecia and sepsis associated with ocrelizumab in multiple sclerosis (12, 13). But a systematic FAERS analysis specifically for IVIg is unavailable.
Accordingly, this study aimed to utilize FAERS data from 2004 to 2024 to comprehensively describe the spectrum, signal strength, and temporal trends of IVIg-related AEs, with special focus on serious events such as hemolytic anemia, to provide evidence for clinical risk assessment and individualized therapy.
Materials and methods
Data source and processing
FDA Adverse Event Reporting System data from Q1 of 2004 to Q4 of 2024 were collected. Only reports where IVIg was listed as the primary suspected drug were included. Data extraction and preprocessing were performed using R software (version 4.3.2). The initial dataset comprised 15,942,054 reports. After removing 2,204,454 duplicate records per FDA guidelines, key fields such as PRIMARYID, CASEID, and FDA_DATE were extracted. For reports sharing the same CASEID, only the entry with the latest FDA_DATE was retained; if both CASEID and FDA_DATE were identical, the record with the highest PRIMARYID was retained. AEs were coded using Medical Dictionary for Regulatory Activities (MedDRA; version 27.1) preferred terms (PTs) and organized into system organ classes (SOCs).
Statistical analysis
To identify potential associations between IVIg and AEs, disproportionality analysis was conducted. This key pharmacovigilance tool uses a 2 × 2 contingency table to compare observed frequencies between exposed and non-exposed populations (Supplementary Table 1). Four disproportionality methods were applied to detect drug–AE signals: ROR, PRR, Bayesian confidence propagation neural network (BCPNN), and multi-item gamma Poisson shrinker (MGPS). An advantage of the ROR is its capacity to adjust for bias, particularly in situations where event counts are low (14). PRR offers higher specificity than that of ROR (15). BCPNN is particularly effective at integrating multi-source data and performing cross-validation (16). MGPS can detect signals from rare events (17). The formulas and threshold criteria for these four algorithms are presented in Supplementary Table 2. Statistical analyses were performed using R software. Higher values indicate stronger signal strength, suggesting a higher consistency between the drug and the occurrence of AEs. Subgroup analyses were conducted by repeating the disproportionality analyses within strata defined by sex (male vs. female), age (<18, 18–65, 65–85, >85 years), body weight (<50, 50–100, >100 kg), and reporter type (healthcare professionals vs. non-professionals). For time-to-onset analyses, we fitted a Weibull distribution to the onset-time data using maximum likelihood estimation, obtaining the shape (β) and scale (α) parameters with 95% confidence intervals. The AE was deemed a potential signal only if it surpassed the positive thresholds in all four disproportionality methods (ROR, PRR, BCPNN, and MGPS).
Results
Basic characteristics of IVIg-related AEs
Figure 1 illustrates the identification of IVIg-related AEs from the FAERS database. A total of 76,138 IVIg-related AE reports were identified in FAERS. Overall, women accounted for approximately 58% of reports and adults aged 18–65 years formed the largest age group. The top three countries submitting AE reports were the United States, China, and Japan (Table 1). The annual number of reports attained its peak in 2015, thereafter showing a decrease (Figure 2).
FIGURE 1

A flowchart illustrates the process of adverse event analysis for intravenous immunoglobulin (IVIg) using the FDA Adverse Event Reporting System database. PS, primary suspect drug.
TABLE 1
| ID | Count | Percentage |
|---|---|---|
| Overall | 76,138 | – |
| Sex | ||
| F | 44,185 | 58.00% |
| M | 24,912 | 32.70% |
| Missing | 7,041 | 9.20% |
| Weight | ||
| <50 kg | 4,579 | 6.00% |
| 50–100 kg | 3,924 | 5.20% |
| >100 kg | 21,882 | 28.70% |
| Missing | 45,753 | 60.10% |
| Age | ||
| <18 | 6,153 | 8.10% |
| >85 | 509 | 0.70% |
| 18–65 | 24,761 | 32.50% |
| 65–85 | 10,966 | 14.40% |
| Missing | 33,749 | 44.30% |
| Role | ||
| CN | 22,592 | 29.70% |
| HP | 14,264 | 18.70% |
| LW | 32 | 0.01% |
| MD | 12,279 | 16.10% |
| OT | 13,868 | 18.20% |
| PH | 10,234 | 13.40% |
Basic information table.
CN, consumer; HP, other health professional; LW, lawyer; MD, physician; OT, other; PH, pharmacist.
FIGURE 2
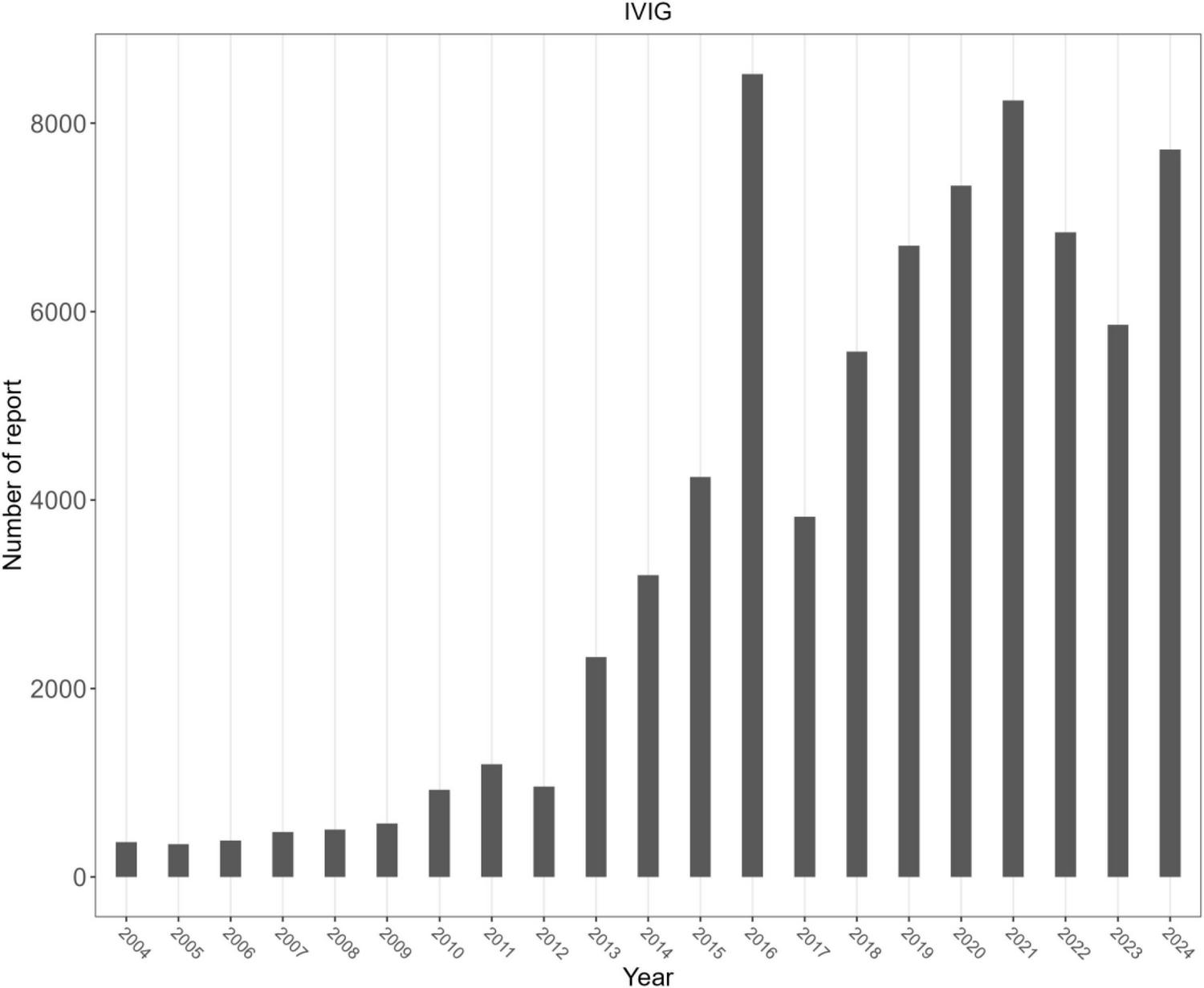
Number of reports per year.
Signal detection at the SOC level
Figure 3 depicts the proportions of the 27 SOC categories associated with IVIg. A summary of the key results is presented in Table 2, while detailed signal strengths for each SOC category are provided in Supplementary Table 3. The most frequently reported category was “General disorders and administration site conditions,” whereas “Infections and infestations” had the strongest signal. Several other SOCs also revealed significant signal detection results, including “Skin and subcutaneous tissue disorders,” “Respiratory, thoracic and mediastinal disorders,” “Vascular disorders,” and “Immune system disorders.” Categories where the lower limit of the 95% confidence interval for ROR was <1 including “Injury,” “Poisoning and procedural complications,” “Nervous system disorders,” “Gastrointestinal disorders,” “Investigations,” and “Musculoskeletal and connective tissue disorders,” did not reach signal-positive.
FIGURE 3
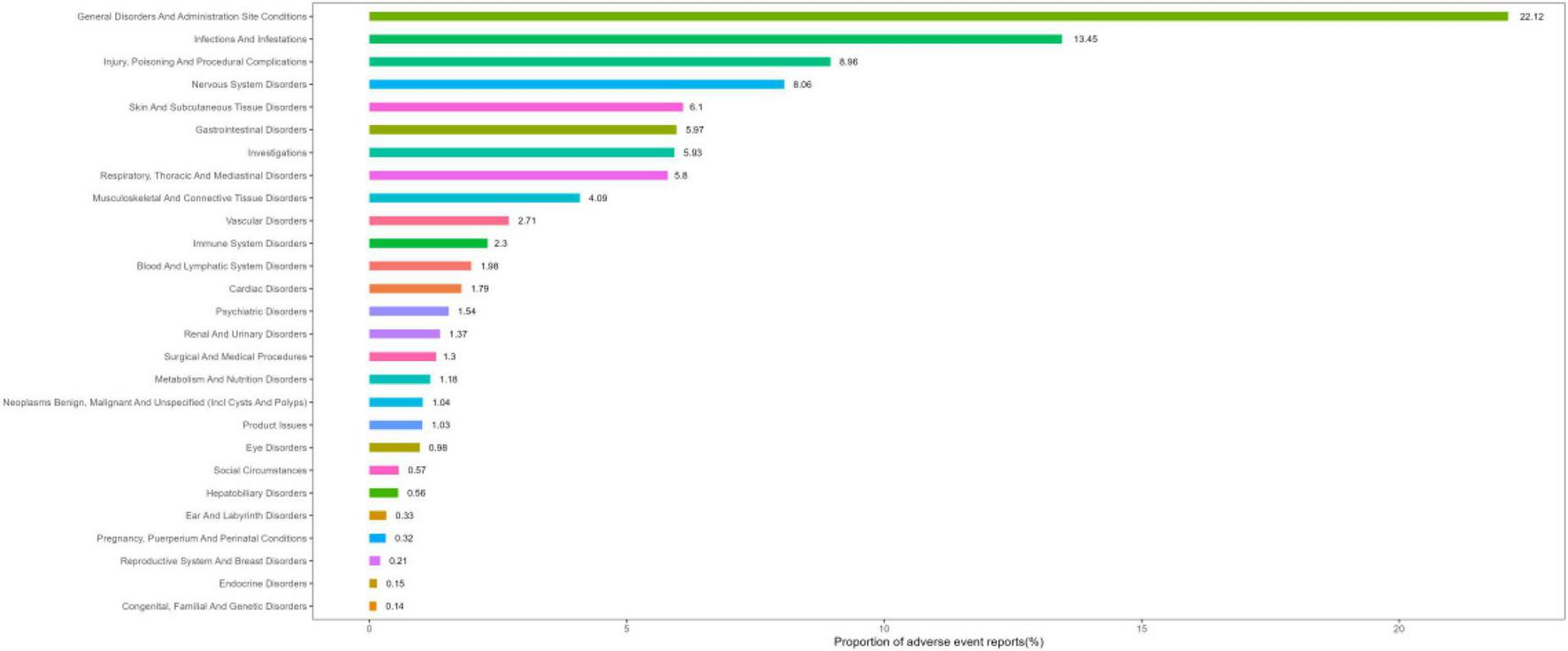
System organ class (SOC) ranking based on percentage of reports.
TABLE 2
| SOC | Number of reports | ROR (95% Cl) | PRR(X2) | EBGM (EBGM05) | IC (IC025) |
|---|---|---|---|---|---|
| General disorders and administration site conditions | 62,849 | 1.35 (1.33–1.36) | 1.27 (4314.92) | 1.27 (1.26) | 0.34 (0.33) |
| Infections and infestations | 38,226 | 2.84 (2.81–2.87) | 2.59 (38930.02) | 2.57 (2.54) | 1.36 (1.35) |
| Injury, poisoning and procedural complications | 25,465 | 0.85 (0.84–0.86) | 0.86 (608.59) | 0.86 (0.85) | −0.21 (−0.23) |
| Nervous system disorders | 22,891 | 0.95 (0.93–0.96) | 0.95 (64.66) | 0.95 (0.94) | −0.07 (−0.09) |
| Skin and subcutaneous tissue disorders | 17,342 | 1.14 (1.12–1.16) | 1.13 (283.24) | 1.13 (1.11) | 0.18 (0.16) |
| Gastrointestinal disorders | 16,957 | 0.68 (0.67–0.69) | 0.7 (2338.7) | 0.7 (0.69) | −0.51 (−0.53) |
| Investigations | 16,856 | 0.96 (0.95–0.98) | 0.97 (21.35) | 0.97 (0.95) | -0.05 (-0.07) |
| Respiratory, thoracic and mediastinal disorders | 16,478 | 1.25 (1.23–1.27) | 1.23 (763.58) | 1.23 (1.21) | 0.3 (0.28) |
| Musculoskeletal and connective tissue disorders | 11,612 | 0.78 (0.76–0.79) | 0.79 (711.67) | 0.79 (0.77) | −0.35 (−0.37) |
| Vascular disorders | 7,695 | 1.28 (1.25–1.31) | 1.27 (454.53) | 1.27 (1.24) | 0.35 (0.31) |
| Immune system disorders | 6,523 | 2.11 (2.06–2.16) | 2.08 (3682.99) | 2.07 (2.02) | 1.05 (1.02) |
| Blood and lymphatic system disorders | 5,625 | 1.17 (1.14–1.21) | 1.17 (142.22) | 1.17 (1.14) | 0.23 (0.19) |
Signal strength of intravenous immunoglobulin (IVIg) adverse events (AEs) across SOC in the FDA Adverse Event Reporting System (FAERS) database.
ROR, reporting odds ratio; PRR, proportional reporting ratio; EBGM, empirical Bayesian geometric mean; EBGM05, the lower limit of the 95% CI of EBGM; IC, information component; IC025, the lower limit of the 95% CI of the IC; CI, confidence interval; SOC, system organ class.
Signal detection at the PT level
All IVIg-related AEs were ranked according to their frequency of occurrence. The key findings are summarized in Table 3, while the full list of preferred terms is provided in Supplementary Table 4. The top 50 PTs were mainly concentrated in three areas: infusion site events (e.g., infusion site swelling, infusion site erythema, infusion site extravasation, infusion site pain), infection events (e.g., upper respiratory tract infection, bronchitis, pneumonia, influenza, urinary tract infection), and systemic events (e.g., pyrexia, chills, hypersensitivity, headache, asthenia, nausea, vomiting).
TABLE 3
| PT | Number of reports | ROR (95% Cl) | PRR (X2) | EBGM (EBGM05) | IC (IC025) |
|---|---|---|---|---|---|
| Headache | 7,622 | 2.71 (2.65–2.77) | 2.66 (7885.04) | 2.64 (2.58) | 1.4 (1.37) |
| Pyrexia | 4,800 | 3.07 (2.98–3.16) | 3.03 (6468.77) | 3 (2.91) | 1.58 (1.54) |
| Sinusitis | 4,268 | 9.62 (9.33–9.92) | 9.49 (30951.74) | 9.09 (8.82) | 3.18 (3.14) |
| No adverse event | 3,331 | 4.27 (4.13–4.42) | 4.23 (8076.23) | 4.17 (4.02) | 2.06 (2.01) |
| Pneumonia | 3,278 | 2.13 (2.05–2.2) | 2.11 (1913.88) | 2.1 (2.03) | 1.07 (1.02) |
| Chills | 3,081 | 5.84 (5.63–6.05) | 5.78 (11862.88) | 5.65 (5.45) | 2.5 (2.44) |
| Urticaria | 2,816 | 3.85 (3.71–4) | 3.82 (5769.2) | 3.77 (3.63) | 1.91 (1.86) |
| Infusion related reaction | 2,717 | 9.94 (9.56–10.33) | 9.85 (20588.52) | 9.43 (9.07) | 3.24 (3.18) |
| Covid-19 | 2,259 | 2.82 (2.71–2.94) | 2.81 (2601.46) | 2.78 (2.67) | 1.48 (1.41) |
| Infection | 2,121 | 3.34 (3.2–3.49) | 3.33 (3398.67) | 3.29 (3.15) | 1.72 (1.65) |
| Infusion site pain | 1,805 | 40.91 (38.88–43.05) | 40.66 (57735.7) | 33.79 (32.11) | 5.08 (4.98) |
| Infusion site erythema | 1,703 | 71.09 (67.24–75.15) | 70.67 (85739.46) | 52.06 (49.25) | 5.7 (5.58) |
| Infusion site swelling | 1,612 | 100.78 (94.88–107.03) | 100.21 (104401.78) | 66.41 (62.53) | 6.05 (5.91) |
| Bronchitis | 1,558 | 4.55 (4.33–4.79) | 4.53 (4197.6) | 4.45 (4.23) | 2.15 (2.08) |
| Migraine | 1,317 | 3.14 (2.97–3.31) | 3.13 (1877.89) | 3.09 (2.93) | 1.63 (1.55) |
| Illness | 1,154 | 3.16 (2.98–3.35) | 3.15 (1667.78) | 3.11 (2.94) | 1.64 (1.55) |
| Upper respiratory tract infection | 1,100 | 5.39 (5.08–5.73) | 5.37 (3813.88) | 5.26 (4.95) | 2.39 (2.3) |
Top 50 frequency of adverse events at the preferred term (PT) level.
ROR, reporting odds ratio; PRR, proportional reporting ratio; EBGM, empirical Bayesian geometric mean; EBGM05, the lower limit of the 95% CI of EBGM; IC, information component; IC025, the lower limit of the 95% CI of the IC; CI, confidence interval; PT, preferred term.
Subgroup analysis
In the subgroup analysis, several differences emerged. Sex: Pyrexia and headache were commonly reported in both men and women; however, men exhibited a higher incidence of chills and urticaria, whereas women were predisposed to sinusitis and pneumonia. Age: Hemolytic anemia and hemorrhage were more frequently observed in individuals <18 years of age. Weight: Patients weighing >50 kg had higher incidence of urinary tract infection and upper respiratory tract infection, whereas those <50 kg were more susceptible to ear infections. Reporter Type: Healthcare professionals tended to report more serious systemic conditions, such as aseptic meningitis, serum sickness, and chronic inflammatory demyelinating polyradiculoneuropathy. In contrast, reports from non-professionals (patients and caregivers) primarily involved infectious complications and functional impairments. These differences reflect the distinct perspectives of medical professionals and patients or families regarding disease perception and clinical priorities (Supplementary Tables 5–15).
Time-to-onset and Weibull distribution analysis
Intravenous immunoglobulin-related AEs most frequently occurred within the first month of treatment. The time-to-onset distribution is shown in Figure 4. The median time to onset was 66 days (interquartile range: 7–523 days), and the Weibull analysis showed an early-failure type (shape parameter β 0.47 [95% CI 0.46–0.47]; scale parameter α 189.41 [95% CI 182.16–196.66]).
FIGURE 4
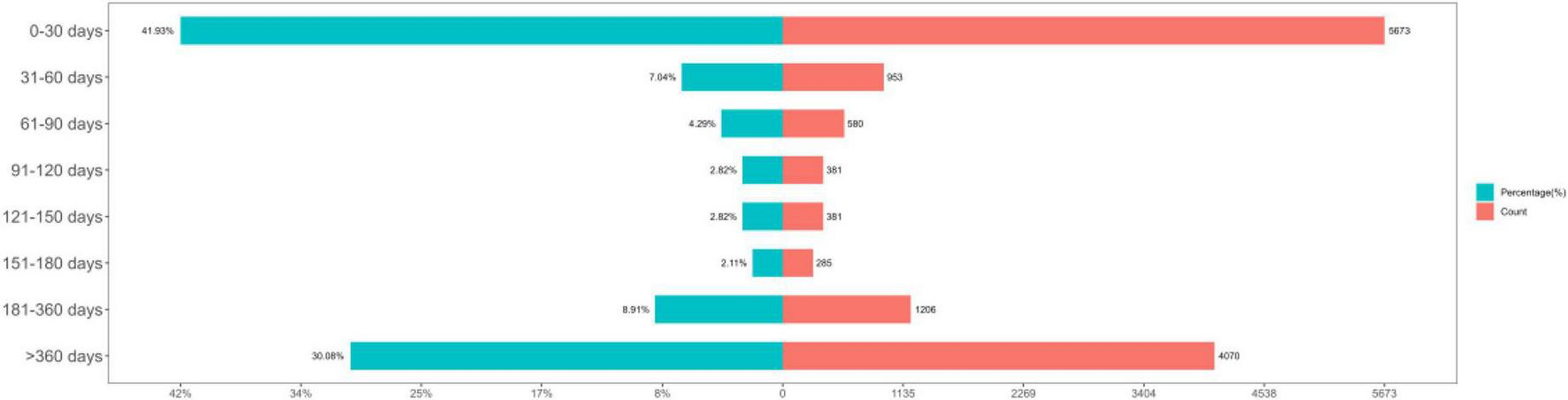
Time-to-onset of adverse events (AEs).
Sensitivity analysis
Intravenous immunoglobulinis frequently used in combination with several other drugs, such as Hizentra, Diphenhydramine, Acetaminophen, Gammagard liquid and Human immunoglobulin G. After excluding reports involving combinations with these six drugs, we included 18,996 reports, which encompassed 4,480 adverse event reports. The adverse reactions that still met the positive criteria (Supplementary Table 16).
Discussion
In this large pharmacovigilance study based on 76,138 FAERS reports, we systematically characterized the safety profile of IVIg. At the SOC level, “general disorders and administration site conditions” was the most frequently reported category, whereas “infections and infestations” showed the strongest association signal. Infusion-site reactions and systemic infusion-related symptoms (e.g., headache, pyrexia, chills) dominated the PT-level spectrum, and this pattern was broadly consistent across sex, age, weight, and reporter subgroups as well as in the sensitivity analysis excluding common co-medications. This pattern is consistent with IVIg’s clinical applications and known risks (18, 19).
Among the AEs detected in our FAERS analysis, hemolytic anemia emerged as a particularly prominent signal, with a high reporting odds ratio (ROR) and consistent detection across multiple subgroups (age, weight). This finding was unexpected, as it reinforces the known risk of hemolysis in high-dose IVIg therapy, but also highlights a particularly strong signal in certain patient populations. Hemolytic anemia is one of the most serious hematologic complications of IVIg treatment, primarily caused by anti-A and anti-B isoagglutinins present in IVIg products (20). The risk of IVIg-induced hemolysis is strongly associated with blood type: non-O blood types, particularly AB, have a significantly increased risk. High-dose IVIg therapy further increases the risk, particularly in patients with underlying inflammatory conditions (21). Patients with inflammatory diseases, such as Kawasaki disease, have elevated hemolysis rates, possibly owing to enhanced macrophage activation in inflammatory states (22).
Our study also found significant signals for vascular disorders and renal/urinary disorders, suggesting that IVIg-associated kidney injury warrants attention. The mechanisms of IVIg-induced acute kidney injury (AKI) are complex and include osmotic nephrosis, intravascular thrombosis, and immune-mediated injury (23). Osmotic nephrosis is primarily caused by stabilizers in IVIg formulations, such as sucrose or mannitol, which accumulate in renal tubular cells and lead to vacuolization and renal dysfunction (24). Infusion speed and total dose are critical contributors to AKI risk.
For patients with pre-existing renal insufficiency, comprehensive pre-treatment evaluation is advised, including assessment of baseline renal function and hydration status, and the selection of IVIg products free of sucrose or mannitol. During treatment, close monitoring of serum creatinine levels, blood urea nitrogen, and urine analysis is essential, with dose or infusion speed adjustments—or even treatment suspension—if necessary (23).
Intravenous immunoglobulin-induced aseptic meningitis is a rare but important neurologic AE, occurring in approximately 0.42%–0.60% of cases (25). Affected patients typically develop headache, fever, nuchal rigidity, and nausea or vomiting within 24–48 h after administration of IVIg infusion (26). The exact mechanism remains unclear, but may involve type III or type IV hypersensitivity reactions, direct toxicity, or immune complex deposition (27). Cerebrospinal fluid analysis typically reveals lymphocytic pleocytosis, mild protein elevation, normal glucose levels, and negative bacterial cultures (28). Clinically, IVIg-associated aseptic meningitis must be distinguished from infectious meningitis. IVIg-induced meningitis is self-limiting and resolves within 5–7 days after discontinuation of IVIg therapy without the need for specific treatment, although supportive care may be required (29). For patients who must continue IVIg therapy, premedication or switching to an alternative IVIg formulation can be considered.
Skin and subcutaneous tissue disorders also showed significant signals in this study. Common manifestations include rash, pruritus, urticaria, and angioedema. These reactions are typically mild to moderate in severity and may involve histamine release, complement activation, or allergic mechanisms (19).
Thromboembolic events are a well-recognized major concern in IVIg therapy, and the FDA has issued a black box warning to clinicians regarding this risk (30). Thrombosis related to IVIg is believed to result from IgG aggregates that activate platelets, increase blood viscosity, and promote a hypercoagulable state (31). Risk factors include advanced age, history of thrombosis, prolonged immobility, cardiac disease, and malignancy. To prevent thromboembolic complications, clinicians should evaluate patients’ thrombotic risk factors prior to treatment, avoid rapid infusion rates, ensure adequate hydration, and consider prophylactic anticoagulation in high-risk patients (32).
This study utilized FAERS data and applied four disproportionality methods (ROR, PRR, BCPNN, and MGPS) to enhance the sensitivity and specificity of signal detection (33, 34). FAERS, as one of the largest spontaneous reporting systems globally, plays a crucial role in post-marketing safety surveillance. However, spontaneous reports are subject to inherent limitations, including underreporting, selective reporting bias, incomplete information, and duplication (35). Therefore, our findings indicate potential safety signals but do not establish definitive causal relationships. Additionally, differences in reporting practices and healthcare systems across countries may influence the generalizability of our results (36).
This study had several limitations. First, FAERS is a voluntary reporting system that depends on healthcare professionals, patients, and pharmaceutical manufacturers, which may introduce selection bias, including under-reporting, duplicate entries, and incomplete data. Although duplicate records were removed in accordance with FDA guidelines, some missing or inconsistent information may persist. Second, FAERS lacks critical details of IVIg therapy, such as infusion rate, blood type matching, and anti-A/B isoagglutinin titers, which are important confounding variables closely linked to severe adverse reactions such as hemolytic anemia. Third, the database does not capture information on IVIg treatment cycles, dosing intervals, or prior exposure, which are essential factors that may influence the occurrence of adverse events. Additionally, notable regional bias exists, as the majority of reports originate from the United States; this may limit the applicability of our findings to populations with different ethnic, genetic, and disease backgrounds. Variations in clinical practice patterns, reporting habits, and regulatory requirements across regions may also influence the completeness and representativeness of AE reporting. These inherent limitations highlight the need for large-scale prospective cohort studies and randomized controlled trials to validate the safety signals identified in this study and to provide stronger evidence for the individualized and safe use of IVIg.
Conclusion
Using FAERS data from Q1 of 2004 to Q4 of 2024, we performed large-scale, multi-index disproportionality analyses to systematically map the overall distribution, signal strength, and temporal evolution of IVIg-related AEs. The results indicate that the common adverse reactions of IVIg are consistent with those reported in previous clinical trials and package inserts (predominantly headache, fever, chills, and skin reactions). Importantly, our analysis provided further evidence that hemolytic anemia and aseptic meningitis are rare but clinically significant adverse reactions to IVIg. In addition, previously recognized risks such as thromboembolic events and acute kidney injury remain important clinical considerations during IVIg therapy. These findings enhance our understanding of the potential safety risks associated with IVIg and provide evidence-based guidance for clinicians in pre-treatment risk assessment, infusion protocol selection, and risk management.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
Ethical approval was not required for the studies involving humans because ethical approval was not required for this study because it was based entirely on publicly available, de-identified data from the U.S. FDA Adverse Event Reporting System (FAERS). The database contains no personally identifiable information, and all analyses were conducted in accordance with relevant data protection and privacy regulations. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because written informed consent was not required because this study used publicly accessible, anonymized data from the FDA Adverse Event Reporting System (FAERS). No individual patient identifiers were included, and all data were analyzed in aggregate form in compliance with ethical and legal standards for secondary data use. Written informed consent was not obtained from the minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article because Written informed consent for publication was not obtained because this study did not involve any individual patient data, images, or identifiable personal information. All data were derived from the publicly available, de-identified FDA Adverse Event Reporting System (FAERS) database, which does not require consent for publication.
Author contributions
ZL: Investigation, Data curation, Software, Writing – original draft, Visualization, Methodology, Conceptualization, Writing – review & editing, Project administration, Validation, Formal analysis, Supervision. XL: Supervision, Writing – review & editing. YG: Writing – review & editing, Supervision. GS: Writing – review & editing, Funding acquisition, Supervision. YZ: Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was funded by the National Natural Science Foundation of China (82260914, 82260798, and 82460802).
Acknowledgments
We thank the US food and drug administration (FDA) for this study provide FAERS number according to the library.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1724196/full#supplementary-material
References
1.
McCormack PL . Immune Globulin (Human) 10 % liquid: a review of its use in primary immunodeficiency disorders.BioDrugs. (2013) 27:393–400. 10.1007/s40259-013-0044-3
2.
Léger J-M De Bleecker JL Sommer C Robberecht W Saarela M Kamienowski J et al Efficacy and Safety of Privigen(®) in Patients with Chronic Inflammatory Demyelinating Polyneuropathy: results of a Prospective, Single-Arm, Open-Label Phase Iii Study (the Prima Study). J Peripher Nerv Syst. (2013) 18:130–40. 10.1111/jns5.12017
3.
Sleasman JW Duff CM Dunaway T Rojavin MA Stein MR . Tolerability of a New 10% liquid immunoglobulin for intravenous use, privigen, at different infusion rates.J Clin Immunol. (2010) 30:442–8. 10.1007/s10875-010-9373-x
4.
Dorsey MJ Ho V Mabudian M Soler-Palacín P Domínguez-Pinilla N Rishi R et al Clinical experience with an L-Proline–Stabilized 10 %Intravenous Immunoglobulin (Privigen®): Real-Life Effectiveness and Tolerability. J Clin Immunol. (2014) 34:804–12. 10.1007/s10875-014-0070-z
5.
Stein MR Nelson RP Church JA Wasserman RL Borte M Vermylen C et al Safety and efficacy of privigen, a Novel 10% liquid immunoglobulin preparation for intravenous use, in patients with primary immunodeficiencies. J Clin Immunol. (2009) 29:137–44. 10.1007/s10875-008-9231-2
6.
Kahwaji J Barker E Pepkowitz S Klapper E Villicana R Peng A et al Acute hemolysis after high-dose intravenous immunoglobulin therapy in highly Hla sensitized patients. Clin J Am Soc Nephrol. (2009) 4:1993–7. 10.2215/CJN.04540709
7.
Christensen RD Ilstrup SJ Baer VL Lambert DK . Increased hemolysis after administering intravenous immunoglobulin to a neonate with erythroblastosis fetalis due to rh hemolytic disease.Transfusion. (2015) 55:1365–6. 10.1111/trf.13104
8.
Shebl A Gabriel S Van Dinther K Hubsch A Lawo J-P Hoefferer L et al Isoagglutinin reduction in intravenous immunoglobulin (Igpro10, Privigen) by specific immunoaffinity chromatography reduces its reporting rates of hemolytic reactions: an analysis of spontaneous adverse event reports. Transfusion. (2020) 60:1278–86. 10.1111/trf.15846
9.
Ko M Oh JM Kim I-W . Drug repositioning prediction for psoriasis using the adverse event reporting database.Front Med. (2023) 10:1159453. 10.3389/fmed.2023.1159453
10.
Al-Yafeai Z Sondhi M Vadlamudi K Vyas R Nadeem D Alawadi M et al Novel anti-psoriasis agent-associated cardiotoxicity, analysis of the fda adverse event reporting system (Faers). Int J Cardiol. (2024) 402:131819. 10.1016/j.ijcard.2024.131819
11.
Thompson DL Welsh K Alimchandani M . Adverse event reporting to Us Food and Drug administration and risk evaluation and mitigation strategies.Mol Ther. (2023) 31:918. 10.1016/j.ymthe.2023.03.003
12.
Ma H-Y Ma Y-B Ye P-J Bai R . A retrospective pharmacovigilance study of ocrelizumab in multiple sclerosis: analysis of the FDA adverse event reporting system (Faers) Database.Medicine. (2025) 104:e44883. 10.1097/MD.0000000000044883
13.
Zou S-P Yang H-Y Ouyang M Cheng Q Shi X Sun M-H . Post-Marketing Safety of Anti-Il-5 Monoclonal Antibodies (Mabs): An Analysis of the Fda Adverse Event Reporting System (Faers).Expert Opin Drug Saf. (2023) 23:353–62. 10.1080/14740338.2023.2251382
14.
Khouri C Nguyen T Revol B Lepelley M Pariente A Roustit M et al Leveraging the variability of pharmacovigilance disproportionality analyses to improve signal detection performances. Front Pharmacol. (2021) 12:668765. 10.3389/fphar.2021.668765
15.
Han H Bu X Wang X Chen S Tian N Jin J et al Novel insights into post-marketing aes associated with leuprorelin: a comprehensive analysis utilizing the faers database. Heliyon. (2024) 10:e34969. 10.1016/j.heliyon.2024.e34969
16.
Tada K Maruo K Isogawa N Yamaguchi Y Gosho M . Borrowing external information to improve bayesian confidence propagation neural network.Eur J Clin Pharmacol. (2020) 76:1311–9. 10.1007/s00228-020-02909-w
17.
Xia L Li K Li Y An Z Song Q Wang L et al Comparison of statistical signal detection methods in adverse events following immunization - China, 2011-2015. China CDC Wkly. (2024) 6:350–6. 10.46234/ccdcw2024.066
18.
Côté J Chaloult-Lavoie M Poulin É Hayes LA Singbo MNU Ouellet P et al Incidence of adverse events related to intravenous immunoglobulin therapy in children. Transfusion. (2025) 65:88–99. 10.1111/trf.18083
19.
Guo Y Tian X Wang X Xiao Z . Adverse effects of immunoglobulin therapy.Front Immunol. (2018) 9:1299. 10.3389/fimmu.2018.01299
20.
Bruggeman CW Nagelkerke SQ Lau W Manlhiot C de Haas M van Bruggen R et al Treatment-associated hemolysis in kawasaki disease: association with blood-group antibody titers in ivig products. Blood Adv. (2020) 4:3416–26. 10.1182/bloodadvances.2020002253
21.
Berg R Shebl A Kimber MC Abraham M Schreiber GB . Hemolytic events associated with intravenous immune globulin therapy: a qualitative analysis of 263 cases reported to four manufacturers between 2003 and 2012.Transfusion. (2015) 55(Suppl 2):S36–46. 10.1111/trf.13198
22.
Cheon EJ Oh JS . Hemolytic anemia associated with intravenous immunoglobulin in Kawasaki Disease.BMC Pediatr. (2024) 24:69. 10.1186/s12887-024-04546-z
23.
Kobayashi RH Rigas MT . Immune globulin therapy and kidney disease: overview and screening, monitoring, and management recommendations.Am J Health Syst Pharm. (2022) 79:1415–23. 10.1093/ajhp/zxac139
24.
Bollée G Anglicheau D Loupy A Zuber J Patey N Mac Gregor D et al High-dosage intravenous immunoglobulin-associated macrovacuoles are associated with chronic tubulointerstitial lesion worsening in renal transplant recipients. Clin J Am Soc Nephrol. (2008) 3:1461–8. 10.2215/CJN.00500108
25.
Bharath V Eckert K Kang M Chin-Yee IH Hsia CC . Incidence and natural history of intravenous immunoglobulin-induced aseptic meningitis: a retrospective review at a single tertiary care center.Transfusion. (2015) 55:2597–605. 10.1111/trf.13200
26.
Kretowska-Grunwald A Krawczuk-Rybak M Sawicka-Zukowska M . Intravenous immunoglobulin-induced aseptic meningitis-a narrative review of the diagnostic process, pathogenesis, preventative measures and treatment.J Clin Med. (2022) 11:3571. 10.3390/jcm11133571
27.
Vickery SB Roach JK Parsons C Vickery PB . Possible levetiracetam-induced aseptic meningitis versus viral meningitis: case report and literature review.Nurse Pract. (2022) 47:32–7. 10.1097/01.NPR.0000843216.79693.7e
28.
Graça L Alves J Nuak J Sarmento A . Immunoglobulin-induced aseptic meningitis: a case report.BMC Neurol. (2018) 18:97. 10.1186/s12883-018-1102-8
29.
De Felice ELT Toti GF Gatti B Gualtieri R Camozzi P Lava SAG et al Acute aseptic meningitis temporally associated with intravenous polyclonal immunoglobulin therapy: a systematic review. Clin Rev Allergy Immunol. (2024) 66:241–9. 10.1007/s12016-024-08989-1
30.
Kanack AJ Jones CG Singh B Leger RR Splinter NP Heikal NM et al Off-the-shelf cryopreserved platelets for the detection of hit and vitt antibodies. Blood. (2022) 140:2722–9. 10.1182/blood.2022017283
31.
Lee YJ Shin JU Lee J Kim K Kim WS Ahn JS et al A case of deep vein thrombosis and pulmonary thromboembolism after intravenous immunoglobulin therapy. J Korean Med Sci. (2007) 22:758–61. 10.3346/jkms.2007.22.4.758
32.
Aggarwal R Schessl J Charles-Schoeman C Bata-Csörgő Z Dimachkie MM Griger Z et al Safety and tolerability of intravenous immunoglobulin in patients with active dermatomyositis: results from the randomised, placebo-controlled proderm study. Arthritis Res Ther. (2024) 26:27. 10.1186/s13075-023-03232-2
33.
Dhodapkar MM Ross JS Ramachandran R . Spontaneous reporting of post-market safety signals: What evidence should support regulatory action?BMJ. (2022) 379:o2409. 10.1136/bmj.o2409
34.
Zhang H Zhou Z Wang J Wang S Ren J Zhang M et al Adverse drug reaction assessment of pembrolizumab in cervical cancer treatment: a real-world pharmacovigilance study using the faers database. Front Immunol. (2025) 16:1582050. 10.3389/fimmu.2025.1582050
35.
Alomar M Tawfiq AM Hassan N Palaian S . Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: current status, challenges and the future.Ther Adv Drug Saf. (2020) 11:2042098620938595. 10.1177/2042098620938595
36.
Sharrar RG Dieck GS . Monitoring product safety in the postmarketing environment.Ther Adv Drug Saf. (2013) 4:211–9. 10.1177/2042098613490780
Summary
Keywords
intravenous immunoglobulin, drug adverse events, FAERS database, pharmacovigilance, drug safety signal detection, disproportionality analysis
Citation
Lu Z, Lu X, Gao Y, Shang G and Zeng Y (2026) Characteristics of adverse events and clinical risks of intravenous immunoglobulin: a pharmacovigilance study based on FDA Adverse Event Reporting System (FAERS). Front. Med. 12:1724196. doi: 10.3389/fmed.2025.1724196
Received
13 October 2025
Revised
23 November 2025
Accepted
28 November 2025
Published
07 January 2026
Volume
12 - 2025
Edited by
Reza Rastmanesh, American Physical Society, United States
Reviewed by
Frits Lekkerkerker, Consultant, Amsterdam, Netherlands
Aurélie Bobet, Hôpitaux Universitaires Henri Mondor, France
Updates
Copyright
© 2026 Lu, Lu, Gao, Shang and Zeng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangbin Shang, shanggb@jxutcm.edu.cnYingjian Zeng, 13431762390@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.