Abstract
Objective:
Growing evidence suggests that probiotics may offer therapeutic benefits for asthma, attracting increasing scientific attention. To clarify their effects in pediatric patients with asthma, we conducted a systematic review and meta-analysis using trial sequential analysis (TSA).
Methods:
A comprehensive search of five electronic databases was performed for studies published until August 30, 2025. Data on study characteristics, outcomes, and risk of bias were extracted. The meta-analysis was conducted using RevMan 5.3, and TSA was performed using TSA 0.9.5.10 beta. Dichotomous outcomes were expressed as risk ratios, and continuous outcomes as mean difference or standardized mean difference (SMD). Publication bias was assessed using funnel plots and the certainty of the evidence was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation approach.
Results:
Six randomized controlled trials (RCTs) involving 731 pediatric patients with asthma were included. The pooled analysis indicated that probiotics significantly reduced interleukin-4 (IL-4) levels [SMD –0.66, 95% confidence intervals (CI) –1.24 to –0.08, P = 0.03] and significantly increased those of interferon-γ (INF-γ) (SMD 1.78, 95% CI 0.13–3.44, P = 0.03). However, probiotics did not significantly affect daytime or nighttime asthma symptom scores, forced expiratory volume in 1 s, forced vital capacity, peak expiratory flow, or tumor necrosis factor-α levels (P ≥ 0.05). TSA did not confirm the conclusiveness of the findings for IL-4 and INF-γ, indicating insufficient evidence to support their clinical significance. Additionally, the funnel plots suggested potential publication bias for these outcomes. The certainty of the evidence for all outcomes was rated as very low.
Conclusion:
Although probiotics showed statistically significant effects on IL-4 and INF-γ, the TSA results revealed that neither outcome crossed the monitoring boundaries, indicating that the evidence remains insufficient. Moreover, the very low certainty of the evidence and the fact that all included RCTs were conducted in China suggest that external validity is limited. In view of these uncertainties, the available evidence does not support the routine use of probiotics as an adjunctive therapy in pediatric asthma, and further large-scale, multicenter, RCTs are warranted.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO/view/CRD420251174081, identifier CRD420251174081.
1 Introduction
Asthma is a heterogeneous disease characterized by chronic airway inflammation, airway hyperresponsiveness, and reversible airflow limitations that typically begins early in life (1). Epidemiological studies indicate that approximately 300 million people worldwide suffer from asthma, with children being the most affected group (2). This condition commonly manifests as recurrent wheezing, shortness of breath, chest tightness, and coughing (3). During severe attacks, patients may exhibit restlessness, orthopnea, shoulder elevation during breathing, the “three-concave sign,” nasal flaring, and cyanosis of the lips. Asthma not only interferes with the daily activities and learning of pediatric patients, but can also be life-threatening in severe cases (4). Inadequate or irregular treatment of these patients may lead to poor symptom control, decline in pulmonary function, and increased risk of developing chronic respiratory diseases in adulthood (5). The long-term goals of managing pediatric patients with asthma are to control symptoms, prevent exacerbations, improve lung function, and minimize adverse drug reactions (6). Current treatment strategies primarily include pharmacological interventions such as β2-adrenergic agonists and inhaled corticosteroids, as well as non-pharmacological measures including lifestyle modification and exercise (5, 7). In pediatric patients with asthma and concomitant allergic diseases, antihistamines can be administered when necessary (8). Although the effectiveness of these therapies in terms of symptom relief has notably improved, a subset of pediatric patients with asthma still experience suboptimal responses and remain at risk of severe exacerbations (9). Therefore, it is crucial to explore safe and effective adjunctive therapies to further improve clinical outcomes and quality of life in these patients.
The gut microbiota, composed of bacteria, fungi, and other microorganisms residing in the gastrointestinal tract, plays an essential role in maintaining human health, particularly during early life (10, 11). Increasing evidence highlights its relevance for pediatric patients with asthma through the gut–lung axis, a bidirectional network in which microbial metabolites and immune cells circulate between the intestine and the respiratory tract (12, 13). The gut microbiota modulates airway immune responses via metabolites such as short-chain fatty acids and immune cell trafficking (13, 14). Compared to healthy children, those at risk of asthma have significantly reduced relative abundances of genera such as Lachnospira, Veillonella, Faecalibacterium, and Roth (15). Subsequent research demonstrated that the proportions of Bifidobacterium and Megasphaera are significantly lower in pediatric patients with asthma than in healthy controls (16). This dysbiosis may compromise the integrity of the intestinal epithelial barrier, facilitating the systemic translocation of pro-inflammatory molecules such as lipopolysaccharides and thus exacerbating pulmonary inflammation (17, 18). Additionally, gut microbiota imbalance may disrupt the T helper (Th)1/Th2 ratio as well as that of T regulatory (Treg)/Th17, leading to allergic inflammation and asthma symptoms (19). Collectively, these findings highlight the gut microbiota as a critical regulator of pediatric asthma pathogenesis via the gut-lung axis, and suggest that targeting microbial composition could represent a promising avenue for preventive or therapeutic strategies.
However, previous studies have reported conflicting findings regarding whether probiotics can improve the prognosis of pediatric patients with asthma. A meta-analysis by Xie et al. (20) reported that probiotics significantly reduced fractional exhaled nitric oxide and the severity of asthma symptoms, and increased the Childhood Asthma Control Test (CACT) score, although no effects were observed on the forced expiratory volume in 1 s (FEV1) or on the FEV1/forced vital capacity (FVC) ratio. Another meta-analysis by Lin et al. (21) found that probiotics reduced the frequency of asthma exacerbation, decreased interleukin-4 (IL-4) levels, and increased those of interferon-γ (IFN-γ), but did not significantly affect CACT scores, daytime or nighttime asthma symptoms, FEV1, or peak expiratory flow (PEF). Similarly, Liu et al. (22) reported that probiotics significantly reduced the risk of acute asthma attacks and improved FEV1/FVC but had no significant effect on FEV1. These inconsistencies reveal an ongoing controversy regarding the effects of probiotics on various clinical symptoms, inflammatory responses, and pulmonary function outcomes in pediatric patients with asthma.
Notably, the studies by Xie et al. (20) and Lin et al. (21) included pediatric patients with recurrent wheezing and a first-degree family history of atopic diseases. The heterogeneity of the study populations may have introduced additional confounding factors and reduced the precision of the pooled estimates. Moreover, Xie et al. (20) and Liu et al. (22) included non-randomized controlled trials, which further increased methodological heterogeneity and undermined the reliability and precision of their findings. To address these limitations, we conducted a more rigorous meta-analysis restricted to randomized controlled trials (RCTs) involving pediatric patients with a confirmed diagnosis of asthma, and incorporating trial sequential analysis (TSA). Individuals with recurrent wheezing and other high-risk populations were excluded from the study, effectively reducing participant-related clinical heterogeneity. In addition, unlike previous meta-analyses, the present study is the result of a comprehensive search in both international and Chinese databases, with the Chinese literature restricted to the Chinese Science Citation Database (CSCD) to enhance the rigor of the evidence base. Furthermore, compared with previous studies, the present study incorporated additional inflammation markers such as tumor necrosis factor-α (TNF-α) and other cytokines, as well as lung function parameters including FVC, to more comprehensively assess the potential effects of probiotics on asthma-related symptoms, inflammatory responses, and pulmonary function in pediatric patients with asthma. By adopting these measures for the study, we aimed to provide more robust and precise evidence regarding the potential role of probiotics as an adjunctive therapy for pediatric patients with asthma.
2 Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and was registered in the PROSPERO database (CRD420251174081). The primary objective of this study was to evaluate the effects of probiotics in pediatric patients with asthma.
2.1 Inclusion and exclusion criteria
Inclusion criteria: (i) Participants: Pediatric patients aged 0–18 years diagnosed with asthma, without restrictions on sex, ethnicity, or disease duration. (ii) Interventions: Probiotics supplementation administered either alone or in combination with standard pharmacological therapy for asthma. (iii) Comparisons: Standard pharmacological therapy for asthma alone or placebo. (iv) Outcomes: asthma-related outcomes including scores for daytime and nighttime symptoms; Pulmonary function outcomes including FEV1, FVC and PEF; Inflammatory biomarkers including TNF-α, IL-4 and IFN-γ. (v) Study design: RCTs only.
The exclusion criteria were as follows: (i) Duplicate publications or retracted articles; (ii) Publications with full text unavailable or with incomplete data; and (iii) Publications containing evident statistical errors.
2.2 Literature search strategy
A comprehensive literature search was performed across five electronic databases: The CSCD, PubMed, Embase, Web of Science, and ClinicalTrials.gov. The first four databases were used to identify published studies, whereas ClinicalTrials.gov was searched for gray literature. The search strategy combined Medical Subject Headings (MeSH) and free-text terms in the title or abstract, including: [(Asthma OR Asthmas) AND (Probiotic OR Probiotics OR Bifidobacterium OR Bifidobacteria OR Bacillus bifida OR Yeast OR Saccharomyces cerevisiae OR Saccharomyces italicus OR Saccharomyces oviformis OR S cerevisiae OR S. cerevisiae OR Saccharomyces uvarum var melibiosus OR Candida robusta OR Saccharomyces capensis OR Lactobacillus acidophilus OR Lactobacillus amylovorus OR Lactobacill OR Lactic acid bacteria OR Clostridium butyricum OR Bacillus OR Natto Bacteria OR Streptococcus thermophiles OR Enterococcus)]. The search covered all available records from the inception of each database to August 30, 2025, without language restrictions. Additionally, the reference lists of relevant reviews and included studies were manually screened to ensure comprehensive coverage. The search strategy and results for each database are shown in Supplementary Table 1.
2.3 Study selection process
All retrieved records were imported into Endnote X9 software, where duplicates were initially removed automatically and subsequently verified manually to enhance methodological rigor. Two independent reviewers then systematically screened the titles and abstracts in strict accordance with the predefined inclusion and exclusion criteria. The full-text version of all articles considered as potentially eligible were obtained and thoroughly assessed by both reviewers. Any discrepancies were resolved through discussion, and when a consensus could not be reached, a third reviewer was consulted to ensure objectivity. The entire study selection process was comprehensively documented and presented in a visual manner through the PRISMA flow diagram.
2.4 Data extraction
Data were extracted independently by two reviewers using a standardized form. The information retrieved included authorship, publication year, sample size, participant demographics, intervention and control characteristics. To prevent bias from duplicate or overlapping cohorts, all included studies were carefully cross-checked for similarities in research groups, study sites, and recruitment periods. When overlapping datasets were identified, only the study with the most complete data, longest follow-up, or largest sample size was retained to ensure independence of the observations. For outcomes with missing or incomplete data, appropriate statistical procedures were applied. When standard deviations were not explicitly reported, they were derived from standard errors, confidence intervals, or interquartile ranges, in accordance with the Cochrane Handbook. These steps ensured consistency in data extraction and minimized the risk of bias related to duplicate datasets or incomplete reporting.
2.5 Risk of bias assessment
The methodological quality of the included trials was independently evaluated by two reviewers using the Cochrane Risk of Bias tool, covering sequence generation, allocation concealment, blinding procedures, incomplete outcome data, selective reporting, and other potential sources of bias. Domains were rated as low, high, or unclear risk, with an overall study-level judgment. Discrepancies were resolved through consensus or third-party adjudication.
2.6 Data synthesis and statistical analysis
Meta-analyses were performed using the Review Manager software (version 5.3). Risk ratios (RR) with 95% confidence intervals (CI) were calculated for dichotomous outcomes, whereas the mean difference (MD) or standardized mean difference (SMD) with 95% CI was used for continuous outcomes, depending on the measurement tools. Heterogeneity was assessed with Cochran’s Q-test and the I2 statistic, with P ≥ 0.10 and I2 < 50% indicating low heterogeneity; otherwise, heterogeneity was considered high. Fixed-effects models were applied when heterogeneity was low, and random-effects models were used in the remaining cases. In cases in which substantial heterogeneity was detected for a given outcome and more than three studies were available, sensitivity and exploratory subgroup analyses were conducted to identify potential sources of heterogeneity. Subgroup analyses were conducted based on key clinical variables including average age, disease duration, treatment duration, probiotic type, and probiotic dosage.
Additionally, TSA was performed using the TSA software (version 0.9.5.10 beta) to control for random errors and assess the reliability of cumulative evidence. The required information size was calculated based on the anticipated effect size estimated from the observed MD. The MD and standard deviation were automatically computed by the software from the extracted data, with a two-sided type I error of 5% and a power of 80%. TSA monitoring boundaries were applied to determine whether the cumulative evidence was sufficient for definitive conclusions; crossing the boundary indicated conclusive results, whereas failure to cross suggested the need for further studies.
2.7 Publication bias
Publication bias was assessed using funnel plots, in which the MD or SMD were plotted on the x-axis against their standard errors on the y-axis. The distribution symmetry of the plotted studies was visually inspected to provide an initial indication of potential publication bias or small-study effects. When the number of included studies was ≥ 10, the Egger test was applied to quantitatively assess publication bias; otherwise, visual inspection of the funnel plot was used as the primary evaluation method.
2.8 Certainty of evidence
The certainty of evidence for each outcome was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system, which accounts for the risk of bias, inconsistency, indirectness, imprecision, and publication bias. Outcomes were categorized as high, moderate, low, or very low quality of evidence, guiding confidence in the pooled estimates and subsequent recommendations.
3 Results
3.1 Study selection
The comprehensive literature search across various databases yielded a total of 3,847 records: 886 from PubMed, 1,414 from Embase, 1,455 from Web of Science, 81 from CSCD, and 11 from other sources. After eliminating 2,077 duplicate entries, 1,770 records were retained for screening based on the titles and abstracts. This initial screening led to the exclusion of 1,744 studies that were deemed irrelevant. The full texts of the remaining articles were then evaluated for eligibility, resulting in the exclusion of 20 studies: Five were excluded because they were not RCTs, two did not meet the intervention standards, and 13 failed to meet the outcome requirements. Ultimately, six studies were included in this meta-analysis (23–28). A detailed flow diagram is presented in Figure 1.
FIGURE 1

Literature screening process. CSCD, Chinese Science Citation Database.
3.2 Basic characteristics of included studies
The meta-analysis included six RCTs with a total of 731 participants (23–28). These studies were published between 2010 and 2022. All studies were conducted in China. The male ratio ranged from 48.91% to 60.87%. The mean age ranged from 6.36 to 11.2 years. Two studies utilized probiotics formulations containing a single strain, whereas four used multi-strain preparations. The treatment duration varied from 2 to 12 weeks. Additional characteristics of the included studies are summarized in Table 1.
TABLE 1
| Author name | Sample size | Age (years) | Male (%) | Disease duration (months) | Intervention | Treatment duration (weeks) |
|---|---|---|---|---|---|---|
| Ding R 2022 (27) | 75 | 6.36 ± 4.54 | 58.67% | / | 6.0 × 106 CFU/d Bifidobacterium spp., 6.0 × 106 CFU/d Lactobacillus acidophilus, 6.0 × 106 CFU/d Enterococcus faecium, 6.0 × 105 CFU/d Bacillus cereus + Budesonide suspension 0.25 mg Bid |
2 |
| 75 | 6.89 ± 5.12 | 52.00% | / | Budesonide suspension, 0.25 mg Bid | ||
| Zhang PH 2017 (26) | 94 | 7.0 ± 3.3 | 60.64% | 18.4 ± 4.5 | 2.6 × 109 CFU/d Saccharomyces boulardii CNCM I-745 + GINA regimen treatment |
4 |
| 90 | 6.8 ± 3.9 | 61.11% | 18.0 ± 4.9 | GINA regimen treatment | ||
| Xia QX 2022 (25) | 46 | 6.84 ± 2.71 | 52.17% | 28.62 ± 9.34 | 2.0 × 107 CFU/d Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis + Budesonide suspension, 1 mg Bid + Terbutaline sulfate solution for nebulized inhalation, > 20 kg 5 mg/ < 20 kg 2.5 mg Bid + Ipratropium bromide solution for inhalation, 250 μg Bid. After symptom relief, switch to Budesonide suspension 0.5 mg Bid |
4 |
| 46 | 6.53 ± 2.59 | 45.65% | 28.21 ± 9.20 | Budesonide suspension, 1 mg Bid + Terbutaline sulfate solution for nebulized inhalation, > 20 kg 5 mg/ < 20 kg 2.5 mg Bid + Ipratropium bromide solution for inhalation, 250 μg Bid. After symptom relief, switch to Budesonide suspension 0.5 mg Bid |
||
| Zhen XG 2018 (24) | 27 | 11.2 ± 1.8 | 55.56% | / | 2.0 × 107 CFU/d Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis + Budesonide suspension 1.0 mg Bid |
6 |
| 26 | 10.9 ± 1.5 | 50.00% | / | Budesonide suspension 1.0 mg Bid | ||
| Huang CF 2018 (28) | 38 | 7.68 ± 2.21 | 57.89% | / | 2.0 × 109CFU/d Lactobacillus paracasei GMNL-133 | 12 |
| 38 | 7.37 ± 2.34 | 63.16% | / | 2.0 × 109 CFU/d Lactobacillus fermentum GM-090 | 12 | |
| 36 | 7.00 ± 1.79 | 52.78% | / | 2.0 × 109CFU/d Lactobacillus paracasei GMNL-133 + 2.0 × 109 CFU/d Lactobacillus fermentum GM-090 |
12 | |
| 35 | 7.86 ± 2.50 | 51.43% | / | Placebo Qd | ||
| Chen YS 2010 (23) | 49 | 8.1 ± 3.0 | 57.10% | / | 4 × 109 CFU/d Lactobacillus gasseri PM-A0005 | 8 |
| 56 | 9.4 ± 4.1 | 57.10% | / | Placebo Bid |
Basic characteristics of included studies.
3.3 Risk of bias
All six included trials adopted computer-generated random sequence, indicating a low risk of bias in the random sequence generation. Only two studies (23, 28) explicitly reported allocation concealment and placebo-controlled design, and were therefore judged to be at low risk for allocation concealment and blinding of participants and personnel. As the primary outcomes were assessed using objective measures, the risk of bias from the outcome assessment was also low. The attrition rates were below 20% in all studies, suggesting a low risk of attrition bias. Furthermore, all trials had pre-specified outcomes, with no evidence of selective reporting or other biases. Collectively, the risk of bias across all domains was low (Figure 2).
FIGURE 2
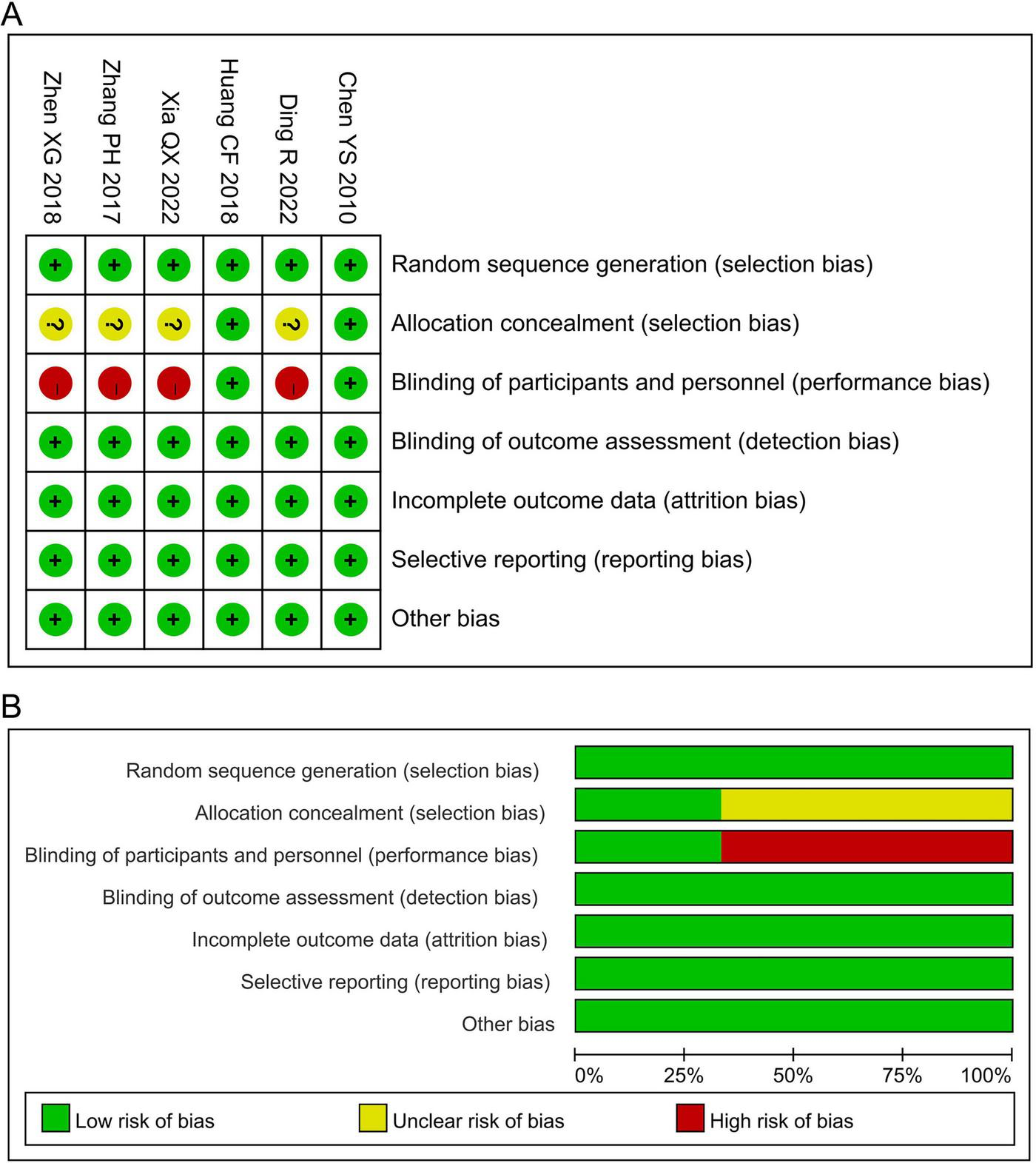
Risk assessment of bias. (A) Risk of bias summary; (B) risk of bias graph.
3.4 Meta-analysis
3.4.1 Asthma-related symptoms
3.4.1.1 Asthma daytime symptom score
The meta-analysis for asthma daytime symptom score included two studies involving 158 participants. Cochran’s Q-test and the I2 statistic indicated high heterogeneity (P = 0.02; I2 = 80%). The pooled results indicated no statistically significant difference in asthma daytime symptom score between the probiotics and non-probiotics groups (MD –0.38, 95% CI –0.86 to 0.11, P = 0.13; Figure 3A).
FIGURE 3
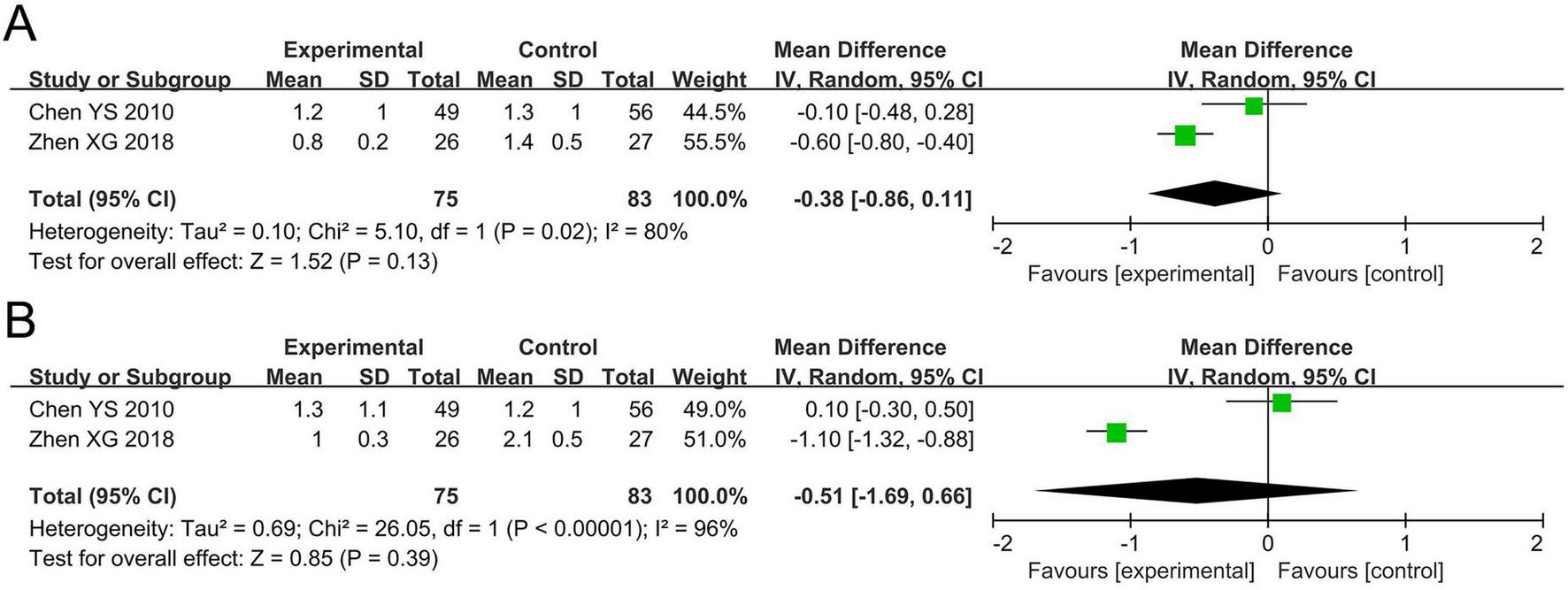
Forest plots of the meta-analysis on asthma-related symptoms: (A) Asthma daytime symptom score; (B) asthma nighttime symptom score. CI, confidence interval; IV, inverse variance; MD, mean difference; SD, standard deviation. Each horizontal bar represents an individual study, with the size of the square in the middle of the bar indicating the study weight. The length of the horizontal bar corresponds to the study’s CI, the solid line denotes the vertical line at the null value, and the diamond symbol indicates the pooled effect size.
3.4.1.2 Asthma nighttime symptom score
The meta-analysis for asthma nighttime symptom score included two studies involving 158 participants. The Cochran’s Q-test and the I2 statistic indicated high heterogeneity (P < 0.00001; I2 = 96%). The pooled results indicated no statistically significant difference in asthma nighttime symptom score between the probiotics and non-probiotics groups (MD –0.51, 95% CI –1.69 to 0.66, P = 0.39; Figure 3B).
3.4.2 Pulmonary function
3.4.2.1 FEV1
The meta-analysis for FEV1 included three studies involving 381 participants. Cochran’s Q-test and the I2 statistic indicated high heterogeneity (P < 0.00001; I2 = 94%). The pooled results demonstrated no statistically significant difference in FEV1 between the probiotics and non-probiotics groups (MD 0.44 L, 95% CI –0.03 to 0.90, P = 0.07; Figure 4A).
FIGURE 4
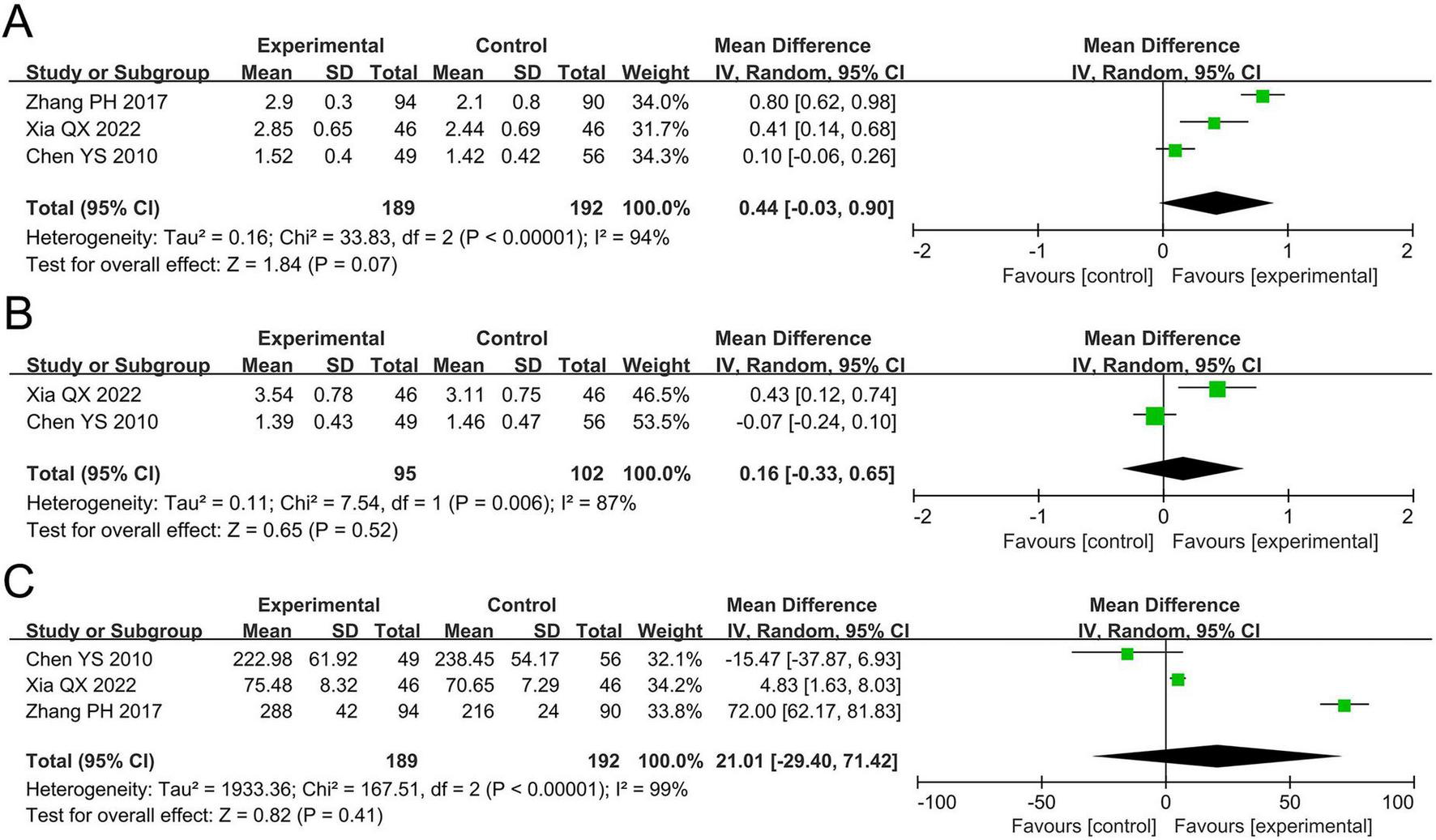
Forest plots of the meta-analysis on pulmonary function: (A) FEV1; (B) FVC; (C) PEF. FEV1, forced expiratory volume in one second; FVC, forced vital capacity; PEF, peak expiratory flow; CI, confidence interval; IV, inverse variance; MD, mean difference; SD, standard deviation. Each horizontal bar represents an individual study, with the size of the square in the middle of the bar indicating the study weight. The length of the horizontal bar corresponds to the study’s CI, the solid line denotes the vertical line at the null value, and the diamond symbol indicates the pooled effect size.
3.4.2.2 FVC
The meta-analysis for FVC included two studies involving 197 participants. Cochran’s Q-test and the I2 statistic indicated high heterogeneity (P = 0.006; I2 = 87%). The pooled results revealed no statistically significant difference in FVC between the two groups (MD 0.16 L, 95% CI –0.33 to 0.65, P = 0.52; Figure 4B).
3.4.2.3 PEF
The meta-analysis for PEF included three studies involving 381 participants. Cochran’s Q-test and the I2 statistic indicated high heterogeneity (P < 0.00001; I2 = 99%). The pooled results revealed no statistically significant difference in PEF between the two groups (MD 21.01 L/min, 95% CI –29.40 to 71.42, P = 0.41; Figure 4C).
3.4.3 Inflammatory biomarkers
3.4.3.1 TNF-α
The meta-analysis for TNF-α levels included two studies involving 297 participants. Cochran’s Q-test and the I2 statistic indicated high heterogeneity (P < 0.00001; I2 = 99%). The pooled results revealed no statistically significant difference in TNF-α levels between the two groups (SMD –1.21, 95% CI –3.60 to 1.18, P = 0.32; Figure 5A).
FIGURE 5
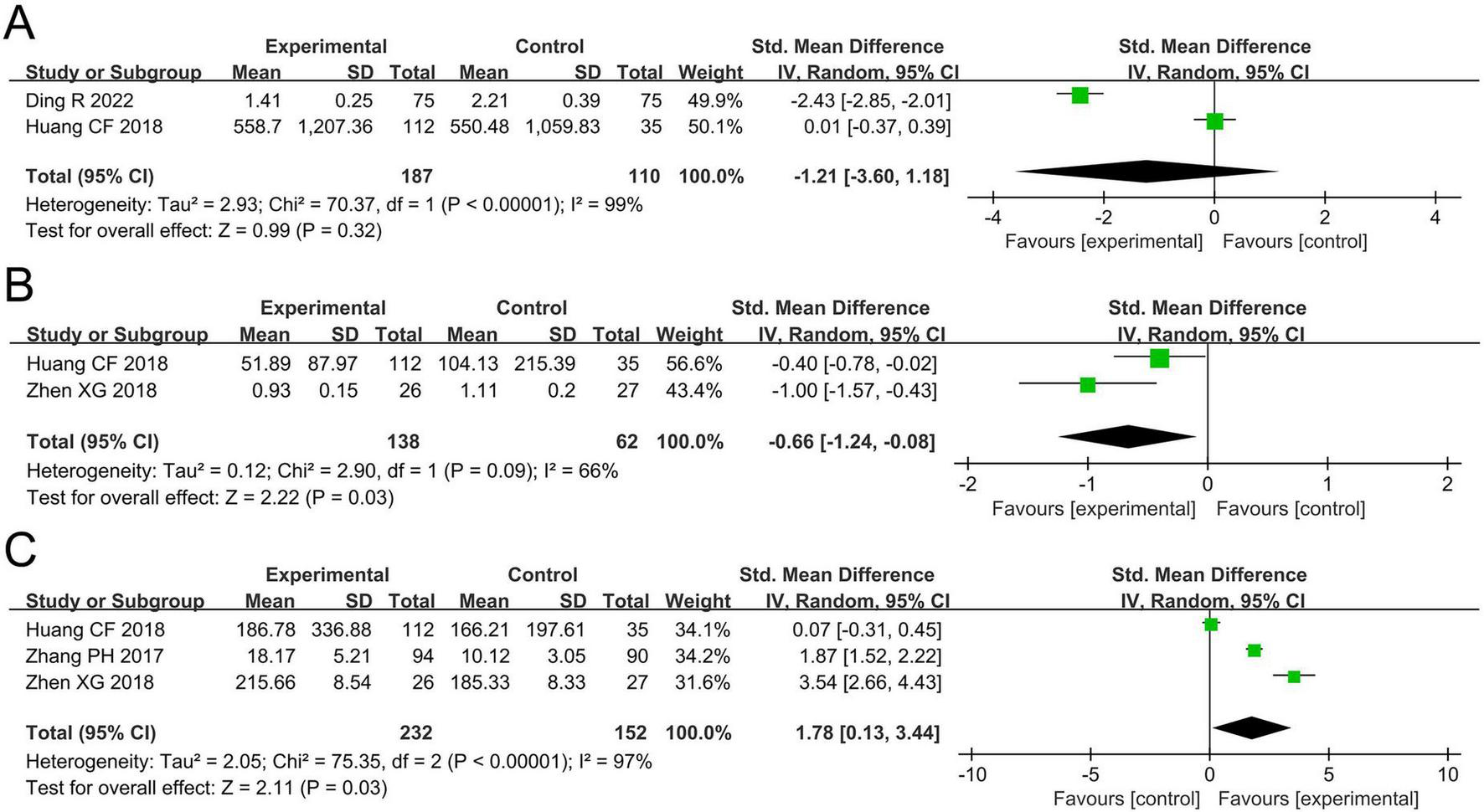
Forest plots of the meta-analysis on inflammatory biomarkers: (A) TNF-α; (B) IL-4; (C) IFN-γ. TNF-α, tumor necrosis factor-alpha; IL-4, interleukin-4; IFN-γ, interferon-gamma; CI, confidence interval; IV, inverse variance; SMD, standardized mean difference; SD, standard deviation. Each horizontal bar represents an individual study, with the size of the square in the middle of the bar indicating the study weight. The length of the horizontal bar corresponds to the study’s CI, the solid line denotes the vertical line at the null value, and the diamond symbol indicates the pooled effect size.
3.4.3.2 IL-4
The meta-analysis for IL-4 levels included two studies involving 200 participants. Cochran’s Q-test and the I2 statistic indicated high heterogeneity (P = 0.09; I2 = 66%). The pooled results demonstrated a significant reduction in IL-4 levels in the probiotics group compared to the non-probiotics group (SMD –0.66, 95% CI –1.24 to –0.08, P = 0.03; Figure 5B).
3.4.3.3 IFN-γ
The meta-analysis for IFN-γ levels included three studies involving 384 participants. Cochran’s Q-test and the I2 statistic indicated high heterogeneity (P < 0.00001; I2 = 97%). The pooled results revealed a significant increase in IFN-γ levels in the probiotics group compared to the non-probiotics group (SMD 1.78, 95% CI 0.13–3.44, P = 0.03; Figure 5C).
3.4.4 Safety outcomes
Among the included studies, only two studies (23, 27) reported safety outcomes, with both indicating that no adverse events related to probiotics use occurred during the study period. However, the current evidence is insufficient to conclusively establish the safety of probiotics in pediatric patients with asthma.
3.5 TSA
TSA was conducted to evaluate the reliability and validity of the meta-analysis results for IL-4 and IFN-γ, and to minimize the risk of false positives, as illustrated in Figures 6A,B. The analysis was performed using a two-sided type I error of 5% and a statistical power of 80%. The anticipated effect sizes were estimated based on the observed MD (–12.91 for IL-4 and 19.15 for IFN-γ). TSA calculated the required information size (RIS) (1,000 for IL-4 and 1,274 for IFN-γ) and generated cumulative Z-curves. The analysis revealed that the Z-value curve for IL-4 and IFN-γ did not cross the monitoring boundaries. This implies that the result for IL-4 and IFN-γ needs further verification through additional studies with a similar design.
FIGURE 6
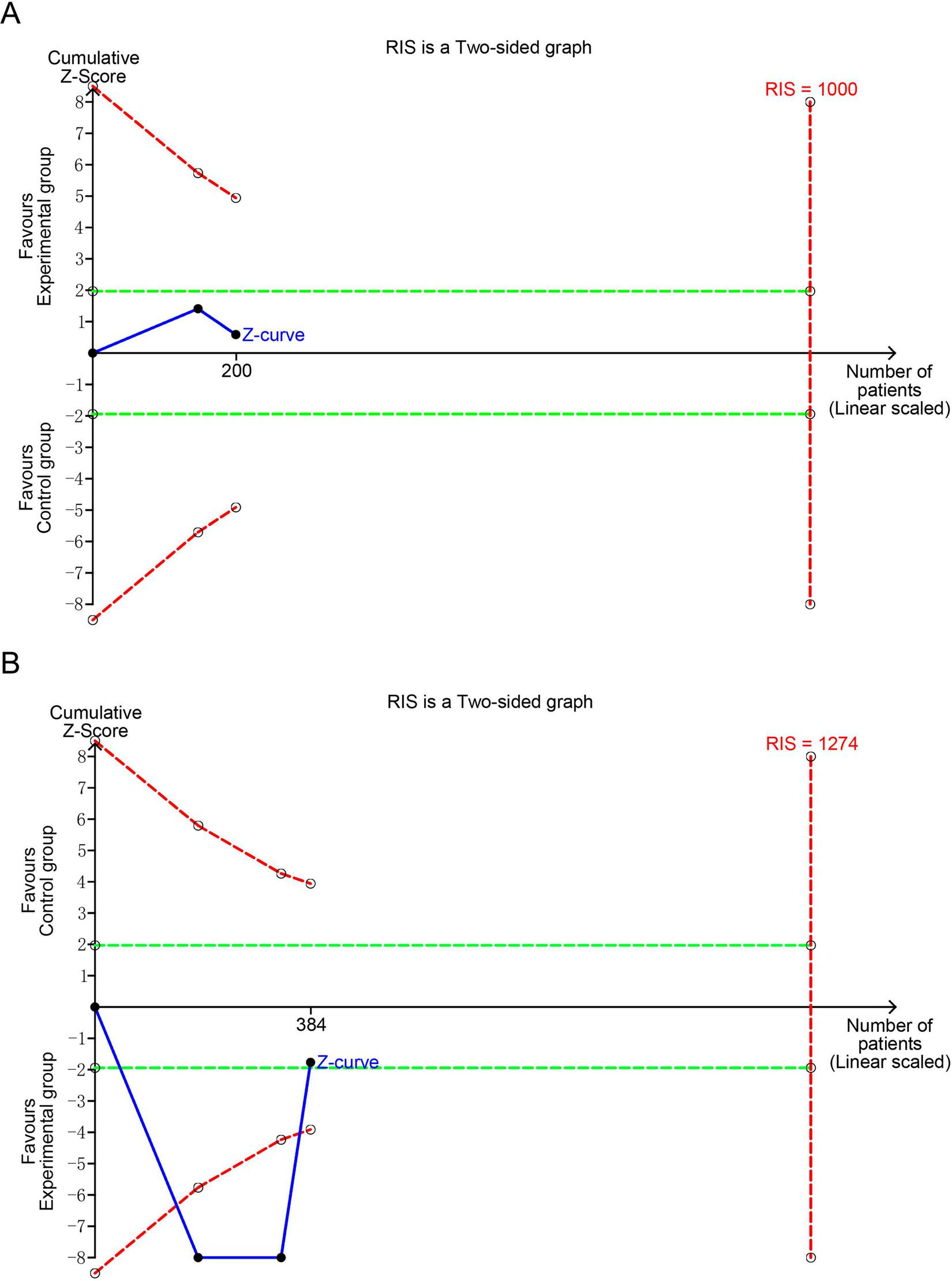
Trial sequential analyses of positive outcomes: (A) IL-4; (B) IFN-γ. IL-4, interleukin-4; IFN-γ, interferon-γ; RIS, required information size. The blue curve represents the Z-curve, while the red curves above and below represent the trial sequential monitoring boundaries, the dashed green line represents the conventional level of statistical significance, the red vertical line represents the required information size, and the solid dot represents the included study. If the Z-curve does not cross the RIS boundary, it indicates that the outcome lacks conclusive evidence.
3.6 Publication bias
Publication bias was examined using funnel plots for asthma daytime and nighttime symptom scores, FEV1, FVC, PEF, and the levels of TNF-α, IL-4, and IFN-γ (Figure 7). The analysis revealed no significant publication bias for the nighttime symptom score, FEV1, PEF, and TNF-α levels, while there was publication bias for the daytime symptom score, FVC, and the levels of IL-4, and IFN-γ. However, the Egger test to assess publication bias was not conducted owing to the insufficient number of included studies, as fewer than 10 were available for analysis. The effectiveness of Egger’s test is significantly reduced with such a small sample size, which may lead to unreliable results.
FIGURE 7
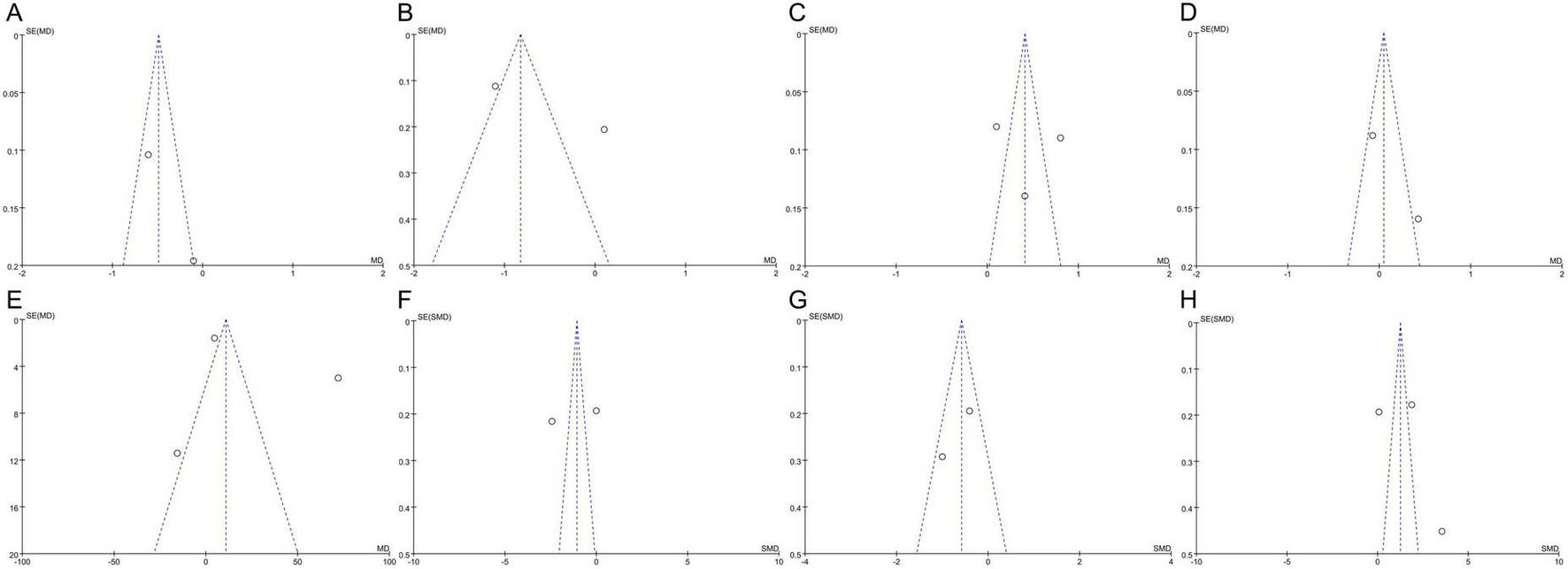
Funnel plots of publication bias: (A) Asthma daytime symptom score; (B) asthma nighttime symptom score; (C) FEV1; (D) FVC; (E) PEF; (F) TNF-α; (G) IL-4; (H) IFN-γ. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PEF, peak expiratory flow; TNF-α, tumor necrosis factor-alpha; IL-4, interleukin-4; IFN-γ, interferon-gamma; MD, mean difference; SMD, standardized mean difference; SE, standard error. The circles represent the effect size and standard error of each individual study, while the central vertical dashed line indicates the pooled effect estimate of the meta-analysis, and the two diagonal dashed lines denote the 95% confidence limits.
3.7 Certainty of evidence
According to the GRADE system, the certainty of evidence for the asthma daytime and nighttime symptom scores, FEV1, FVC, PEF, and the levels of TNF-α, IFN-γ, and IL-4 was classified as very low, underscoring the need for additional rigorously designed studies to validate these findings (Table 2).
TABLE 2
| Outcome | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | MD (95% CI) | Certainty of evidence |
|---|---|---|---|---|---|---|---|
| Asthma daytime symptom score | Serious(a) | Serious(b) | None | Serious(c) | Suspected(d) | –0.38 (–0.86, 0.11) | Very Low |
| Asthma nighttime symptom score | Serious(a) | Serious(b) | None | Serious(c) | None | –0.51 (–1.69, 0.66) | Very low |
| FEV1 | Serious(a) | Serious(b) | None | Serious(c) | None | 0.44 (–0.03, 0.90) | Very low |
| FVC | Serious(a) | Serious(b) | None | Serious(c) | Suspected(d) | 0.16 (–0.33, 0.65) | Very low |
| PEF | Serious(a) | Serious(b) | None | Serious(c) | None | 21.01 (–29.40, 71.42) | Very low |
| TNF-α | Serious(a) | Serious(b) | None | Serious(c) | None | –1.21 (–3.60, 1.18) | Very low |
| IL-4 | Serious(a) | Serious(b) | None | Serious(c) | Suspected(d) | –0.66 (–1.24, –0.08) | Very low |
| IFN-γ | Serious(a) | Serious(b) | None | Serious(c) | Suspected(d) | 1.78 (0.13, 3.44) | Very low |
Certainty of evidence.
MD, mean difference; CI, confidence interval; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PEF, peak expiratory flow; TNF-α, tumor necrosis factor-alpha; IL-4, interleukin-4; IFN-γ, interferon-gamma. (a)At least one included study has a potential risk of bias. (b)There is high or extremely high heterogeneity. (c)The broad confidence interval decreases credibility. (d)The funnel plot suggests potential publication bias.
4 Discussion
Compared to previous meta-analyses, our study provides new insights and methodological improvements. Unlike earlier analyses, we excluded studies involving pediatric patients with recurrent wheezing and a first-degree family history of atopic disease to minimize potential confounding factors. This strategy allowed us to more accurately assess the true effects of probiotics on asthma rather than merely on wheezing episodes. Our findings indicated that probiotic supplementation significantly reduced IL-4 levels and increased those of IFN-γ in pediatric patients with asthma. However, no significant effects were found on asthma daytime or nighttime symptom scores, FEV1, FVC, PEF, or TNF-α levels. Notably, TSA results indicated that these findings were inconclusive, implying that the true benefits of probiotics remain uncertain. This uncertainty is further reflected in the overall quality of evidence for the efficacy of probiotics in asthma treatment, which was classified as “very low.” Consequently, the current data do not provide sufficient support for the widespread use of probiotics as an adjunctive treatment for asthma. Clinicians should exercise caution when considering probiotics for asthma management because their clinical benefits remains uncertain.
Regarding asthma-related symptoms, no significant differences were observed between the probiotics and the control groups in terms of daytime or nighttime asthma symptom scores. Interestingly, two of the included studies (23, 24) reported significant reductions in both daytime and nighttime symptom scores in the probiotics group compared to the control group. We speculate that this inconsistency may stem from both clinical and statistical heterogeneity. First, the two studies reporting asthma-related outcomes differed in terms of participants and interventions. Zhen et al. (24) included pediatric patients with asthma aged 1–14 years, among whom only 54.71% had a history of allergic diseases, while Chen et al. (23) enrolled pediatric patients with asthma aged 6–12 years with mild-to-moderate persistent asthma lasting longer than a year, all of whom had concomitant allergic rhinitis. Zhen et al. (24) used a multi-strain probiotics preparation containing a combination of live Bifidobacterium, Lactobacillus, and Enterococcus, whereas Chen et al. (23) used a single-strain formulation containing Lactobacillus gasseri PM-A0005. Second, when the analysis model was switched to a fixed-effect model, significant differences emerged for both daytime (MD –0.49, 95% CI –0.67 to –0.31, P < 0.00001) and nighttime symptom scores (MD –0.82, 95% CI –1.02 to –0.63, P < 0.00001). However, despite the statistical significance, the effect sizes were small (0.49 and 0.82, respectively), suggesting that probiotics have negligible clinical benefits on asthma-related symptoms. Additionally, Kardani et al. (29) reported that probiotics combined with immunotherapy showed no significant improvement in the Asthma Control Test score compared to immunotherapy alone, further supporting the ineffectiveness of probiotics in improving asthma-related symptoms. Collectively, the available evidence indicates that probiotics offer minimal, if any, benefits in the alleviation of asthma symptoms in pediatric patients.
Regarding pulmonary function, pediatric patients with asthma typically exhibit obstructive ventilatory dysfunction characterized by decreased FEV1, FEV1/FVC, and PEF values (30). Pulmonary function not only reflects the degree of control over asthma symptoms but also correlates closely with prognosis (31). The results of this meta-analysis showed no significant differences in FEV1, FVC, or PEF between the probiotics and control groups. Similar findings were reported by Xie et al. (20), who observed no improvement in FEV1, and by Lin et al. (21), who found no significant benefit in either FEV1 or PEF. However, Liu et al. (32) found that although probiotics did not significantly affect FEV1, they markedly increased the FEV1/FVC ratio. These conflicting findings may result from additional confounding factors. Specifically, both Xie et al. (20) and Lin et al. (21) included a study by Rose et al. (33) involving pediatric patients with recurrent wheezing and a first-degree family history of atopic diseases, while Xie et al. (20) and Liu et al. (22) included in the analysis a non-randomized controlled trial reported by Moura et al. (34). The inclusion of participants with recurrent wheezing and of studies with non-randomized designs may have introduced confounding effects and biased the pooled results. In contrast, we excluded these studies from our analysis, thereby reducing potential confounding factors. The results suggested that probiotics exert no significant impact on pulmonary function in pediatric patients with asthma. Moreover, Chen et al. (23) found no significant difference in the change of MEF 25–75% between the probiotics and control groups (P = 0.211), indicating that probiotics do not appear to improve small airway function in pediatric patients with asthma. Collectively, these findings suggest that probiotics have no meaningful clinical effect on lung function in pediatric asthma. However, due to the limited sample size and short duration of the intervention in the included studies, the impact of probiotics on pulmonary function remains uncertain and warrants further investigation through large-scale RCTs.
TNF-α plays a critical role in the regulation of inflammatory responses and induction of airway hyperresponsiveness (35, 36). IL-4, a key driver of Th2-type inflammation, is central to airway inflammation and remodeling in asthma (37, 38). In contrast, IFN-γ serves as a hallmark cytokine of Th1-type immune responses and can suppress asthma-related inflammation (39). Our meta-analysis demonstrated that probiotics significantly reduced IL-4 levels (SMD –0.66, 95% CI –1.24 to –0.08) and increased those of IFN-γ (SMD 1.78, 95% CI 0.13–3.44), while showing no significant effect on TNF-α levels. These findings are consistent with those reported by Lin et al. (21), who also observed that probiotics decreased IL-4 levels and increased IFN-γ levels in pediatric patients with asthma. From a mechanistic perspective, these results suggest that probiotics may help re-balance immune function in pediatric asthma. The reduction in IL-4 levels indicates suppression of Th2-mediated allergic inflammation, while the increase in IFN-γ levels reflects potential enhancement of Th1-type responses. In terms of effect size, the IL-4 reduction represents a moderate effect, whereas the increase in IFN-γ levels corresponds to a large effect. Together, these changes imply that probiotics can help rebalance immune function by suppressing Th2-type responses and enhancing Th1-type responses in pediatric patients with asthma. Notably, although the variations in IL-4 and IFN-γ levels showed statistical significance in the pooled analysis, the SMD reflects a standardized statistical effect rather than a clinically meaningful one. The wide confidence intervals and high heterogeneity further complicate the interpretation of these results. Taken together, while probiotics may show potential for immune modulation, current evidence does not support their widespread clinical application in pediatric asthma. The clinical efficacy of probiotics, particularly on the regulation of inflammatory cytokine levels, remains unclear.
The limited efficacy of probiotics can be attributed to strain-specific characteristics and host-related immunological barriers. Probiotics exhibit strain-dependent survival and persistence in the gastrointestinal tract (40). During mucosal inflammation, disrupted tight junctions and increased antimicrobial peptide secretion create a hostile environment that impairs probiotic adhesion and survival, thereby reducing their ability to modulate immune responses (41). Probiotics are further constrained by colonization resistance driven by host-associated microbiota and immune factors, which can prevent their interaction with epithelial or immune cells (42). Moreover, in the context of epithelial barrier dysfunction, probiotics are unable to effectively reinforce barrier integrity or induce tolerogenic signaling, limiting their capacity to suppress pro-inflammatory pathways (43). Therefore, large-scale multicenter RCTs are needed to better assess the role of probiotics in asthma management and address the uncertainties raised by TSA analysis.
Marked heterogeneity was detected across several outcomes (I2 > 80%), prompting the use of a random-effects model. This inconsistency likely reflects substantial clinical variation in probiotic interventions, including differences in strain composition (single-strain versus multi-strain), dosage (3.1 × 10 to 10 × 10 CFU/day), and treatment duration (2–12 weeks), as well as variations in participant characteristics such as age (ranging from 1 to 18 years) and the presence of comorbid atopic conditions. Given that most of the outcomes considered were reported in only two or three trials, subgroup analyses to further explore the sources of heterogeneity were not feasible. Consequently, the high heterogeneity observed in this study limits the robustness and generalizability of the findings. To address this issue, future research should include large-scale RCTs with standardized probiotic strains, dosages, and treatment durations, clearly defined participant characteristics, and consistent outcome measures to reduce clinical and methodological heterogeneity.
Regarding safety, only Ding et al. (27) and Chen et al. (23) explicitly reported no adverse events related to the administration of probiotics to pediatric patients with asthma, whereas other studies did not mention any safety outcomes. Therefore, we did not perform a meta-analysis on safety data. Although Hassanzad et al. (44) reported one case of vomiting leading to treatment withdrawal among 51 pediatric patients with asthma receiving synbiotic treatment, this isolated finding does not substantially strengthen the evidence for potential safety concerns. Moreover, the incomplete reporting of adverse events and the short duration of the interventions in the included trials further constrain the ability to detect rare or delayed probiotic-related complications. Taken together, the available evidence is insufficient to draw reliable conclusions regarding the safety of probiotics in in pediatric patients with asthma, and the findings should be interpreted with caution. Larger, well-designed, multicenter RCTs with standardized and comprehensive adverse event monitoring are required to provide a more definitive assessment of the safety profile of probiotics in this patient subpopulation.
This meta-analysis has several limitations. First, the diagnostic criteria used across the included studies varied, encompassing the Global Initiative for Asthma guidelines, the 2016 Guidelines for the Diagnosis and Prevention of Childhood Bronchial Asthma (45), and the 2008 Guidelines for the Diagnosis and Prevention of Childhood Bronchial Asthma (46), which may have introduced clinical heterogeneity to the analysis. Second, only six clinical trials involving a total of 731 patients were included, which may have reduced the precision and statistical power of the pooled estimates. Third, all studies were conducted in China. This geographic concentration substantially limits the external validity of the findings, as the results primarily reflect outcomes in Chinese pediatric patients with asthma and may not necessarily be applicable to patients in other regions, healthcare systems, or ethnic groups. Fourth, the intervention duration in the included studies ranged from 2 to 12 weeks, and therefore the long-term effects of probiotics on pediatric asthma could not be properly analyzed. Considering these limitations, further research is warranted. Future studies should be designed as large-scale, multicenter, double-blind RCTs with a particular focus on evaluating the effects of probiotics on airway inflammation and pulmonary function to provide stronger evidence for their clinical application in pediatric asthma. In addition, stratified analyses based on probiotics strains, dosage, and treatment durations are needed to establish standardized therapeutic protocols for probiotics use in pediatric patients with asthma.
5 Conclusion
Although probiotics appeared to influence IL-4 and IFN-γ levels, the very low certainty of the evidence, inconclusive TSA results, and the fact that all included RCTs were conducted in China limit the external validity and clinical reliability of the findings of this meta-analysis. Furthermore, the incomplete reporting of safety outcomes prevents a definitive assessment of the safety profile of probiotics in pediatric patients with asthma. Therefore, the available evidence does not support the routine use of probiotics as adjunctive therapy in these patients. Large-scale, multicenter, double-blind, RCTs are needed to clarify the potential role of probiotics in asthma management and provide more robust evidence for their clinical application.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directe d to the corresponding authors.
Author contributions
GH: Data curation, Formal analysis, Methodology, Writing – original draft. KL: Data curation, Formal analysis, Writing – original draft. YY: Data curation, Formal analysis, Writing – original draft. XY: Writing – original draft. FL: Writing – review & editing. MW: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by Hunan Province World-Class Discipline Cultivation Program in 2022 (4912-0005001010), and the Key Project of Hunan Provincial Administration of Traditional Chinese Medicine (A2024027).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1730100/full#supplementary-material
References
1.
Shipp C Gergen P Gern J Matsui E Guilbert T . Asthma management in children.J Allergy Clin Immunol Pract. (2023) 11:9–18. 10.1016/j.jaip.2022.10.031
2.
Jayasooriya S Devereux G Soriano J Singh N Masekela R Mortimer K et al Asthma: epidemiology, risk factors, and opportunities for prevention and treatment. Lancet Respir Med. (2025) 13:725–38. 10.1016/S2213-2600(24)00383-7
3.
Chung K Dixey P Abubakar-Waziri H Bhavsar P Patel P Guo S et al Characteristics, phenotypes, mechanisms and management of severe asthma. Chin Med J. (2022) 135:1141–55. 10.1097/CM9.0000000000001990
4.
Chen C Li H Xing X Guan H Zhang J Zhao J . Effect of TRPV1 gene mutation on bronchial asthma in children before and after treatment.Allergy Asthma Proc. (2015) 36:e29–36. 10.2500/aap.2015.36.3828
5.
Pijnenburg M Frey U De Jongste J Saglani S . Childhood asthma: pathogenesis and phenotypes.Eur Respir J. (2022) 59:2100731. 10.1183/13993003.00731-2021
6.
GINA. GINA-2024-Strategy-Report-24_05_22_WMS.pdf. (2025). Available online at: https://ginasthma.org/wp-content/uploads/2024/05/GINA-2024-Strategy-Report-24_05_22_WMS.pdf(accessed October 15, 2025).
7.
Lu K Forno E . Exercise and lifestyle changes in pediatric asthma.Curr Opin Pulm Med. (2020) 26:103–11. 10.1097/MCP.0000000000000636
8.
Xue X Wang Q Yang X Tu H Deng Z Kong D et al Effect of bilastine on chronic urticaria: a systematic review and meta-analysis. Int Arch Allergy Immunol. (2023) 184:176–85. 10.1159/000527181
9.
Papadopoulos N Bacharier L Jackson D Deschildre A Phipatanakul W Szefler S et al Type 2 inflammation and asthma in children: a narrative review. J Allergy Clin Immunol Pract. (2024) 12:2310–24. 10.1016/j.jaip.2024.06.010
10.
Pantazi A Mihai C Balasa A Chisnoiu T Lupu A Frecus C et al Relationship between gut microbiota and allergies in children: a literature review. Nutrients. (2023) 15:2529. 10.3390/nu15112529
11.
Xue X Yang X Shi X Deng Z . Efficacy of probiotics in pediatric atopic dermatitis: a systematic review and meta-analysis.Clin Transl Allergy. (2023) 13:e12283. 10.1002/clt2.12283
12.
Hufnagl K Pali-Schöll I Roth-Walter F Jensen-Jarolim E . Dysbiosis of the gut and lung microbiome has a role in asthma.Semin Immunopathol. (2020) 42:75–93. 10.1007/s00281-019-00775-y
13.
Lv J Zhang Y Liu S Wang R Zhao J . Gut-lung axis in allergic asthma: microbiota-driven immune dysregulation and therapeutic strategies.Front Pharmacol. (2025) 16:1617546. 10.3389/fphar.2025.1617546
14.
Yang Z Mao W Wang J Yin L . The gut-lung axis in asthma: microbiota-driven mechanisms and therapeutic perspectives.Front Microbiol. (2025) 16:1680521. 10.3389/fmicb.2025.1680521
15.
Arrieta M Stiemsma L Dimitriu P Thorson L Russell S Yurist-Doutsch S et al Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. (2015) 7:307ra152. 10.1126/scitranslmed.aab2271
16.
Mo M Wang J Gu H Zhou J Zheng C Zheng Q et al Intestinal microbes-based analysis of immune mechanism of childhood asthma. Cell Mol Biol. (2022) 68:70–80. 10.14715/cmb/2022.68.2.11
17.
Guo H Han Y Rong X Shen Z Shen H Kong L et al Alleviation of allergic asthma by rosmarinic acid via gut-lung axis. Phytomedicine. (2024) 126:155470. 10.1016/j.phymed.2024.155470
18.
Zhang M Qin Z Huang C Liang B Zhang X Sun W . The gut microbiota modulates airway inflammation in allergic asthma through the gut-lung axis related immune modulation: a review.Biomol Biomed. (2025) 25:727–38. 10.17305/bb.2024.11280
19.
Zhou Y Wang T Zhao X Wang J Wang Q . Plasma metabolites and gut microbiota are associated with t cell imbalance in BALB/c model of eosinophilic asthma.Front Pharmacol. (2022) 13:819747. 10.3389/fphar.2022.819747
20.
Xie Q Yuan J Wang Y . Treating asthma patients with probiotics: a systematic review and meta-analysis.Nutr Hosp. (2023) 40:829–38. 10.20960/nh.04360
21.
Lin J Zhang Y He C Dai J . Probiotics supplementation in children with asthma: a systematic review and meta-analysis.J Paediatr Child Health. (2018) 54:953–61. 10.1111/jpc.14126
22.
Liu Y Zhang Y Li Y Zhang X Xie L Liu H . Probiotics for children with asthma: a systematic review and meta-analysis.Front Pediatr. (2025) 13:1577152. 10.3389/fped.2025.1577152
23.
Chen Y Jan R Lin Y Chen H Wang J . Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis.Pediatr Pulmonol. (2010) 45:1111–20. 10.1002/ppul.21296
24.
Zhen X Guo Y Zhang YH Qian HQ Shen ZB Feng RS et al Effect of probiotics in the control and immune regulation of bronchial asthma in children. Chinese J Pract Pediatrics. (2018) 33:812–4. 10.19538/j.ek2018100617
25.
Xia Q Lu X Fang J . Efficacy of probiotics combined with Budesonide in children with bronchial asthma and its effects on peripheral blood CD4+ and CD8+ cell levels.Chinese J Microecol. (2022) 34:451–4. 10.13381/j.cnki.cjm.202204014
26.
Zhang P Chen X Chen B Li H . Study on asthmatic children serum cytokine levels and saccharomyces boulardii intervention.Chinese J Clin Pharmacol Therapeutics. (2017) 22:332–6.
27.
Ding R Zhang Z Lu C . Microecological preparations improving immune function in children with allergic asthma.Chinese J Microecol. (2022) 34:57–61. 10.13381/j.cnki.cjm.202201010
28.
Huang C Chie W Wang I . Efficacy of lactobacillus administration in school-age children with asthma: a randomized, placebo-controlled trial.Nutrients. (2018) 10:1678. 10.3390/nu10111678
29.
Astrid Kristina Kardani AKK . The effect of house dust mite immunotherapy, probiotic and nigella sativa in the number of th17 cell and asthma control test score.IOSR-JDMS. (2013) 6:37–47. 10.9790/0853-0643747
30.
Gopalakrishna Mithra CA Ratageri VH . Pulmonary function tests in childhood asthma: which indices are better for assessment of severity?Indian J Pediatr. (2023) 90:566–71. 10.1007/s12098-022-04258-1
31.
McGeachie M Yates K Zhou X Guo F Sternberg A Van Natta M et al Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. (2016) 374:1842–52. 10.1056/NEJMoa1513737
32.
Liu C Makrinioti H Saglani S Bowman M Lin L Camargo C et al Microbial dysbiosis and childhood asthma development: integrated role of the airway and gut microbiome, environmental exposures, and host metabolic and immune response. Front Immunol. (2022) 13:1028209. 10.3389/fimmu.2022.1028209
33.
Rose M Stieglitz F Köksal A Schubert R Schulze J Zielen S . Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk.Clin Exp Allergy. (2010) 40:1398–405. 10.1111/j.1365-2222.2010.03560.x
34.
Moura J Moura I Gaspar G Mendes G Faria B Jentzsch N et al The use of probiotics as a supplementary therapy in the treatment of patients with asthma: a pilot study and implications. Clinics. (2019) 74:e950. 10.6061/clinics/2019/e950
35.
Xue K Ruan L Hu J Fu Z Tian D Zou W . Panax notoginseng saponin R1 modulates TNF-α/NF-κB signaling and attenuates allergic airway inflammation in asthma.Int Immunopharmacol. (2020) 88:106860. 10.1016/j.intimp.2020.106860
36.
Lin R Gu Q Lee L . Hypersensitivity of vagal pulmonary afferents induced by tumor necrosis factor alpha in mice.Front Physiol. (2017) 8:411. 10.3389/fphys.2017.00411
37.
Sahnoon L Bajbouj K Mahboub B Hamoudi R Hamid Q . Targeting IL-13 and IL-4 in asthma: therapeutic implications on airway remodeling in severe asthma.Clin Rev Allergy Immunol. (2025) 68:44. 10.1007/s12016-025-09045-2
38.
Feng Y Chen S Xiong L Chang C Wu W Chen D et al Cadherin-26 amplifies airway epithelial IL-4 receptor signaling in asthma. Am J Respir Cell Mol Biol. (2022) 67:539–49. 10.1165/rcmb.2021-0109OC
39.
Song L Luan B Xu Q Wang X . Effect of TLR7 gene expression mediating NF-κB signaling pathway on the pathogenesis of bronchial asthma in mice and the intervention role of IFN-γ.Eur Rev Med Pharmacol Sci. (2021) 25:866–79. 10.26355/eurrev_202101_24655
40.
Kumari A Bhawal S Kapila S Kapila R . Strain-specific effects of probiotic Lactobacilli on mRNA expression of epigenetic modifiers in intestinal epithelial cells.Arch Microbiol. (2022) 204:411. 10.1007/s00203-022-03027-0
41.
Ohland C Macnaughton W . Probiotic bacteria and intestinal epithelial barrier function.Am J Physiol Gastrointest Liver Physiol. (2010) 298:G807–19. 10.1152/ajpgi.00243.2009
42.
Zmora N Zilberman-Schapira G Suez J Mor U Dori-Bachash M Bashiardes S et al Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. (2018) 174:1388–405.e21. 10.1016/j.cell.2018.08.041.
43.
Zheng Y Zhang Z Tang P Wu Y Zhang A Li D et al Probiotics fortify intestinal barrier function: a systematic review and meta-analysis of randomized trials. Front Immunol. (2023) 14:1143548. 10.3389/fimmu.2023.1143548
44.
Hassanzad M Maleki Mostashari K Ghaffaripour H Emami H Rahimi Limouei S Velayati A . Synbiotics and treatment of asthma: a double-blinded, randomized, placebo-controlled clinical trial.Galen Med J. (2019) 8:e1350. 10.31661/gmj.v8i0.1350
45.
The Subspecialty Group of Respiratory Diseases, The Society of Pediatrics, Chinese Medical Association and The Editorial Board. Guideline for the diagnosis and optimal management of asthma in children. Chin J Pediatr. (2016) 54:167–81. 10.3760/cma.j.issn.0578-1310.2016.03.003
46.
The Subspecialty Group of Respiratory Diseases, The Society of Pediatrics, Chinese Medical Association and The Editorial Board, ChenAHLiCCZhaoDYet alGuideline for the diagnosis and optimal management of asthma in children.Chin J Pediatr. (2008) 46:745–53. 10.3760/cma.j.issn.0578-1310.2008.10.106
Summary
Keywords
asthma, meta-analysis, pediatric populations, probiotics, systematic review
Citation
Hu G, Liao K, Yu Y, Yang X, Li F and Wang M (2026) Efficacy and safety of probiotics in the treatment of pediatric asthma: a systematic review and meta-analysis. Front. Med. 12:1730100. doi: 10.3389/fmed.2025.1730100
Received
24 October 2025
Revised
26 November 2025
Accepted
05 December 2025
Published
05 January 2026
Volume
12 - 2025
Edited by
Francisco Epelde, Parc Taulí Foundation, Spain
Reviewed by
Chris Kyriakopoulos, University Hospital of Ioannina, Greece
Xiali Xue, Peking University Third Hospital, China
Updates
Copyright
© 2026 Hu, Liao, Yu, Yang, Li and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Li, 827521109@qq.comMengqing Wang, wmqwmq2009@sina.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.