Abstract
Exosomes are a type of nanoscale extracellular vesicle secreted by cells, with a diameter of approximately 30–150 nm, which carry important biological molecules such as proteins, lipids, and RNA, functioning as messengers between cells and playing a central role in cell communication. Due to their involvement in various physiological and pathological processes and their low immunogenicity and good tissue penetration, exosomes have become a research hotspot for disease diagnostic markers and drug delivery carriers. Type 2 diabetes mellitus (T2DM) is a common chronic metabolic disorder characterized primarily by high blood sugar, whose core pathogenesis includes insulin resistance and the subsequent functional deficiency of pancreatic β-cells, which can lead to various serious complications over the long term. The review systematically elaborates on the key roles of exosomes from different cell sources in regulating T2DM and its complications, focusing on how these exosomes, through their specific microRNAs (miRNAs), proteins, and other active substances they carry, act on different key targets and thereby regulate core pathological processes such as insulin signaling pathways, inflammatory responses, cell apoptosis, fibrosis, and angiogenesis. Through the review of existing evidence, we aim to reveal the complex network of exosomes as intercellular messengers and provide a solid theoretical basis for their development as new diagnostic markers and targeted therapeutic strategies.
1 Introduction
Type 2 diabetes mellitus (T2DM) is a group of metabolic diseases characterized by chronic hyperglycemia, whose core mechanisms involve insufficient insulin secretion and insulin resistance. The typical clinical manifestations are “three more and one less,” namely, increased thirst, increased appetite, increased urination, and weight loss. Currently, T2DM has become a major global public health issue with a rapidly growing prevalence rate, closely related to obesity, unhealthy lifestyles, and population aging (1). If blood sugar levels remain poorly controlled over a long period, it can lead to severe chronic complications in multiple systems and organs throughout the body. These mainly include microvascular complications, including diabetic retinopathy (DR) and diabetic neuropathy (DN), as well as macrovascular complications, including atherosclerosis, seriously endangering the quality of life and lifespan of patients (2).
In recent years, exosomes, a type of nano-sized extracellular vesicle secreted actively by cells, have attracted widespread attention in the field of metabolic disease research as a key medium for intercellular information transmission (3). These microvesicles, with diameters of approximately 30–150 nm, form a complex intercellular communication network in the body by carrying biologically active molecules such as proteins, lipids, and nucleic acids (4), thereby participating in the regulation of various physiological and pathological processes (5). The biogenesis process of exosomes is a highly coordinated intracellular event. It begins with the endosomal pathway, where the endosomal membrane buds to form multivesicular bodies (MVBs) and eventually fuses with the plasma membrane, releasing its contents into the extracellular space. This process is precisely regulated by various molecules, such as endosomal sorting complex required for transport (ESCRT) complexes, transmembrane proteins including CD9, CD63, and CD81, and Rab GTPases (6). The molecular composition of exosomes depends on the type of parent cell and its physiological and pathological state (7), enabling them not only to reflect the condition of the source cell but also to regulate the physiological functions of the receptor cell by transferring functional molecules (8). This characteristic makes exosomes an ideal source of biomarkers and a promising therapeutic tool. Exosomes show great potential as diagnostic biomarkers. Due to their stable presence in various body fluids and their contents being able to reflect changes in metabolic status dynamically, they make them ideal choices for non-invasive diagnostic tools (9). Exosomes, due to their inherent low immunogenicity, good biocompatibility, and excellent ability to penetrate biological barriers, are regarded as highly promising drug delivery systems (10). They can be used as natural therapeutic agents or engineered to become carriers of therapeutic molecules.
This review aims to systematically present the latest progress on exosomes in the research of T2DM, focusing on their dual potential as biomarkers and therapeutic agents. Through in-depth analysis of the core role of exosomes in various complications of T2DM, exploration of current challenges, and outlook for future development, we hope to provide valuable perspectives for understanding this rapidly evolving field and promote the accelerated translation of basic research on exosomes into clinical applications.
2 Biological characteristics of exosomes
Exosomes are small extracellular vesicles, typically ranging from 30 to 150 nm in diameter, released by cells via an endocytic mechanism (11). Their formation begins with endocytosis, leading to the development of endosomes, which subsequently biosynthesize MVBs (12). This process involves the invagination of a portion of the cell membrane, forming an endocytic vesicle that encapsulates cytoplasmic contents, as illustrated in Figure 1. The vesicle then fuses with the endosome, and as the endosome matures, some of it transforms into MVBs. Under specific conditions, MVBs release their vesicles into the extracellular space as exosomes (13). The release of exosomes is regulated by intracellular molecular signals and external factors, such as cellular stress, metabolic status, and intercellular interactions, which control both the frequency and volume of exosome secretion (14). Additionally, the composition of exosomes is influenced by the physiological and pathological state of the parent cell, making them potential biomarkers for early disease detection and therapeutic monitoring (15).
Figure 1
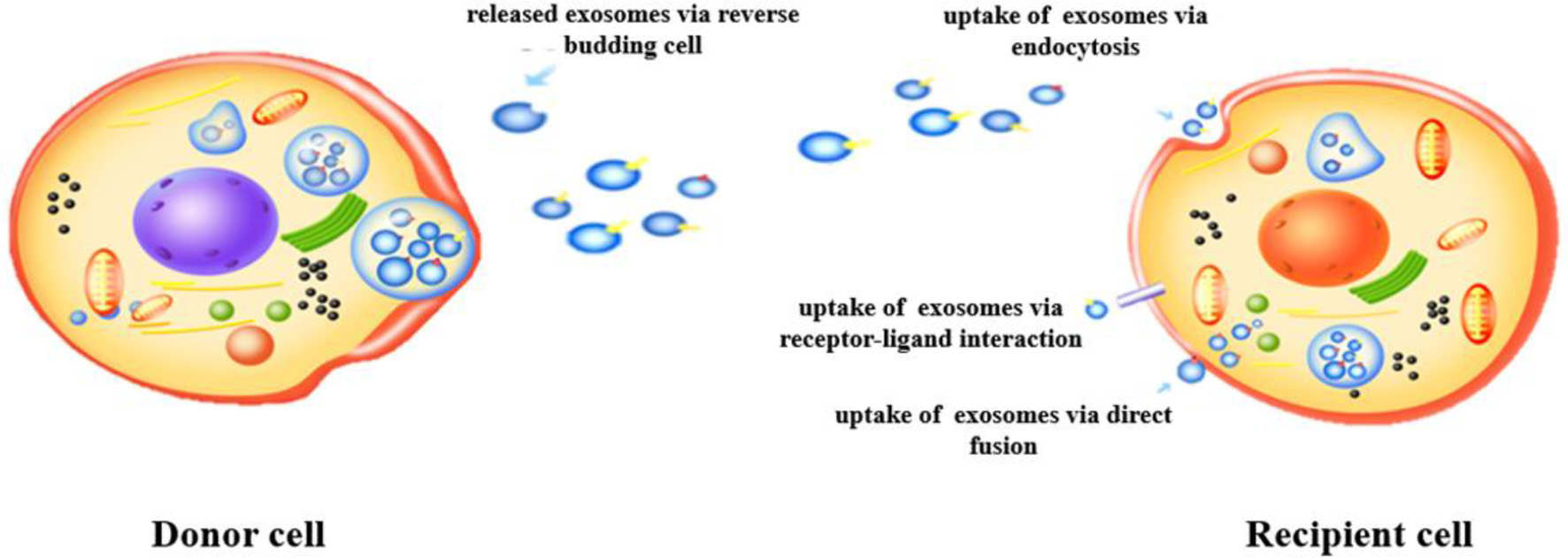
Formation and secretion of exosomes.
Exosomes contain a variety of biomolecules, including proteins, lipids, RNA (miRNA, mRNA, and lncRNA), and small amounts of DNA (16), as shown in Figure 2. These components reflect the physiological state of the parent cell and facilitate intercellular communication by transferring biological information to recipient cells, thereby influencing their functions (17). Exosomal proteins are abundant and include small GTPases, membrane transporters, kinases, and cell cycle regulators (18). Specific proteins such as CD9, CD63, and CD81 are widely recognized as exosomal markers, aiding in their isolation and purification in experimental studies (19).
Figure 2
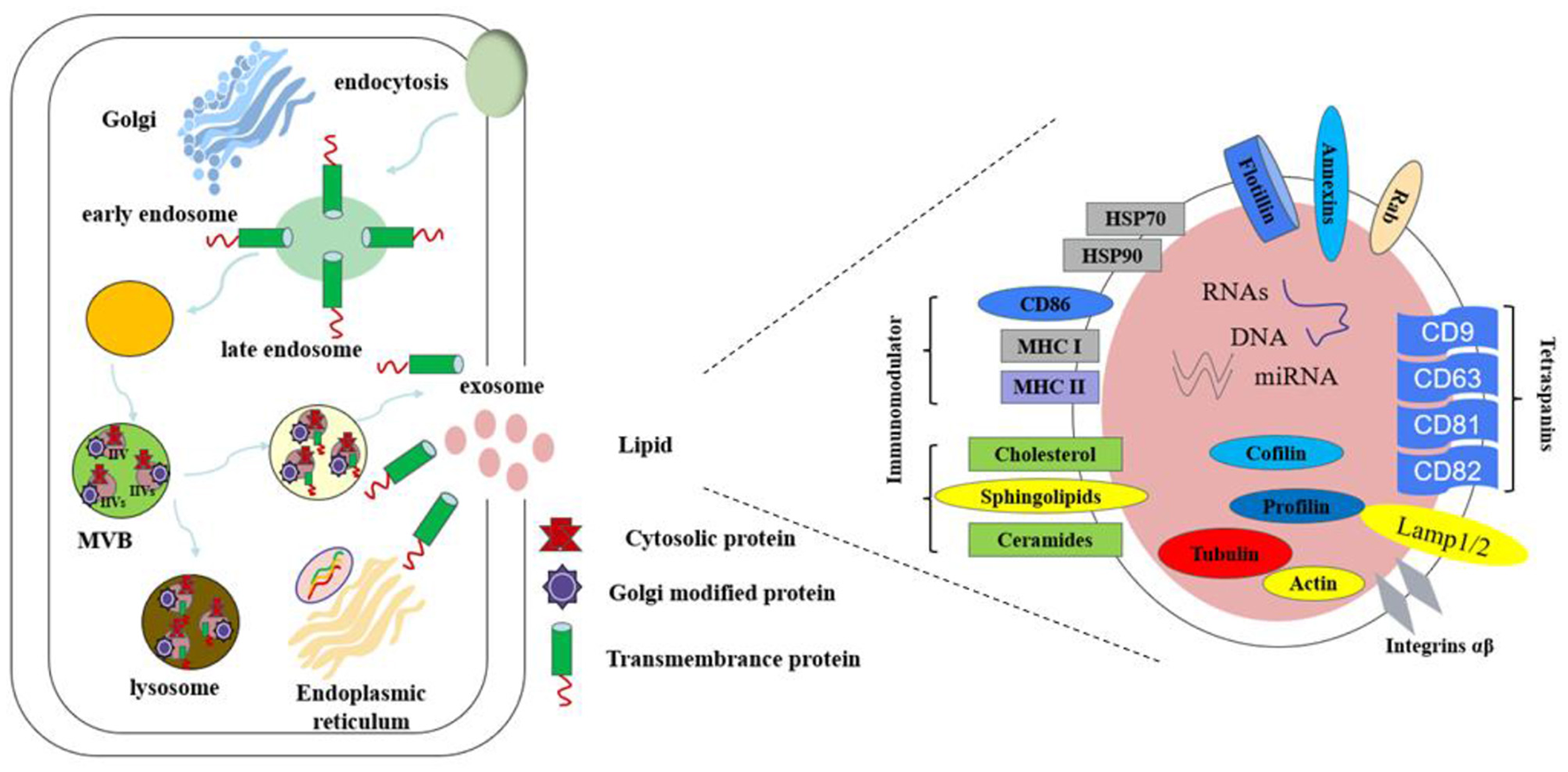
The composition of exosomes.
The exosomal membrane consists primarily of a phospholipid bilayer enriched with lipids like cholesterol and sphingolipids. Lipid composition is crucial for the formation, structural integrity, and functional properties of exosomes. Research indicates that variations in lipid content can influence exosome distribution and intercellular interactions, modulating downstream signaling pathways (20). RNA molecules, particularly miRNA, have garnered significant research interest due to their role in gene regulation. miRNAs are small RNA molecules that regulate gene expression and participate in key cellular processes such as growth, differentiation, and apoptosis (21). The ability of exosomes to carry miRNAs makes them vital in intercellular gene regulation. By transferring specific miRNAs or mRNAs, exosomes enable efficient information exchange. For example, studies have shown that the miRNA profile in exosomes from obese individuals differs markedly from that of healthy individuals, suggesting a role for exosomes in regulating glucose and lipid metabolism (22).
Although the DNA component of exosomes is relatively minor, recent studies suggest that exosomes can carry specific genomic DNA fragments that influence immune cell states and, consequently, affect systemic metabolic balance. These DNA fragments may facilitate the transfer of genetic material between cells, further enhancing the functional significance of exosomes (23). The diverse molecular cargo within exosomes highlights their potential applications in cell communication, disease signaling, and therapeutic development. Future investigations into exosome components will provide deeper insights into their mechanisms, particularly in the context of T2DM.
Exosomes not only transmit biological information but also modulate various cellular processes, as illustrated in Figure 3. They can activate signaling pathways in recipient cells, initiating diverse biological responses (24). For example, exosomes contribute to the regulation of glucose and lipid metabolism by mediating interactions among liver cells, muscle cells, and adipocytes. Furthermore, exosomes play a pivotal role in inflammatory responses, especially in chronic inflammation (25). In T2DM, high levels of free fatty acids and inflammatory mediators in obese individuals stimulate exosome production in adipose tissue, which in turn affects other cell types such as hepatocytes and myocytes, exacerbating insulin resistance (26). Understanding the mechanisms underlying exosome function under these conditions may lead to innovative therapeutic approaches.
Figure 3
Effects of exosomes on various organs of the human body.
3 The role of exosomes in T2DM and its complications in different physiological systems
3.1 Skin healing
The hyperglycemic environment in T2DM directly impairs the proliferation and migration abilities of fibroblasts and keratinocytes through mechanisms such as the polyol pathway, accumulation of advanced glycation end products (AGEs), and mitochondrial oxidative stress. At the same time, the continuous activation of inflammatory signaling pathways, such as nuclear factor kappa-B (NF-κB), leads to chronic and excessive inflammation and inhibits the expression of vascular endothelial growth factor (VEGF), thereby hindering the formation of new blood vessels. These functional dysfunctions collectively result in delayed re-epithelialization and formation of granulation tissue (27).
Multiple studies have confirmed that the exosomes secreted by mesenchymal stem cells (MSCs) extracted from various tissues, such as umbilical cord, placenta, and bone marrow, can significantly promote the healing of diabetic wounds through multiple mechanisms. Exosomes derived from MSCs can promote the upregulation of vascular endothelial growth factor receptor (VEGFR) and angiogenesis through miRNA-21-5p/miR-221-3p and a kinase transforming (AKT)/mitogen-activated protein kinase (MAPK) signaling pathways (28, 29) and also upregulate the expression of endothelial cells (ECs) tubular formation and glucose transporter by influencing the energy metabolism of vascular ECs, including cell survival ability, metabolic activity, oxidative stress, and antioxidant capacity, thereby facilitating vascular formation and glucose metabolism (30). In addition, exosomes derived from human umbilical cord mesenchymal stem cells (hUC-MSCs) can also accelerate the healing of diabetic wounds by alleviating oxidative stress damage to ECs, enhancing the regeneration of granulation tissue, and promoting angiogenesis through up-regulating the expression of VEGF and transforming growth factor β-1 (TGFβ-1) (31, 32). Liang et al. reported that exosomes derived from UCMSCs can also promote EC proliferation, migration, and angiogenesis through the miR-20b-5p/Nrf2/VEGFA axis, thereby promoting angiogenesis in diabetic ulcer wounds (33). Another study shows that the inhibitory agents derived from human placental mesenchymal stem cells (hPMSCs) from patients with gestational diabetes mellitus (GDM) can promote the proliferation and angiogenesis of ECs by targeting intercellular cell adhesion molecule-1 (ICAM-1) through miRNA-130b-3p and increase the expression of angiogenesis-related factors (34). To enhance the sustained release and bioavailability of this exosome, Varyani et al. encapsulated the exosomes derived from hPMSCs in a hydrogel. They found that compared with the exosome-only group, the wound contraction rate, new epidermal length, number of fibroblasts and blood vessels, collagen density, and antioxidant factors, including glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT), in the treatment group were significantly increased (35).
Some studies have also shown that exosomes derived from adipose-derived stem cells (ADSCs) have a significant effect in the treatment of T2DM. In contrast, they can regulate macrophage polarization, anti-inflammatory and anti-apoptotic functions to improve glucose tolerance and insulin sensitivity while conferring immune regulatory functions to ECs by delivering circular Snhg11 (36, 37). In contrast, they can regulate miR-204-3p/ homeodomain-interacting protein kinase 2 (HIPK2) and miR-1248 to promote ECs proliferation, migration, and angiogenesis and upregulate the mRNA and protein levels of pro-angiogenic factors VEGF-A, angiopoietin-1 (Angpt-1), and TGF-β, thereby improving diabetic foot ulcers (38, 39). Song et al. (40) also incorporated it into gelMA hydrogel to form a mixture and found that gelMA-HExo could enhance the migration, proliferation, and vascular regeneration potential of vascular ECs through miR-144-3p/nuclear factor erythroid 2-related factor 2 (NFE2L2)/hypoxia-inducible factor 1-α(HIF1α).
A study by Ji et al. showed that macrophage-derived exosomes induced by AGEs could impair the proliferation, migration, and tube formation of ECs through the miR-22-5p/forkhead box protein P1 (FOXP1) signaling axis, while increasing monocyte adhesion and the release of proinflammatory cytokines. It can be seen that miR-22-5p is a potential therapeutic target for diabetic skin healing, as inhibiting it can release exosomes and fibroblast growth factors, accelerating oxidative diabetic wound healing (41). Platelet-rich plasma (PRP)-derived exosomes encapsulating hydrogels inhibit fibroblast ferroptosis by upregulating FosB expression, resulting in increased cell survival, reduced oxidative stress, and elevated iron levels. At the same time, the expressions of glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (SLC7A11) are upregulated, thereby accelerating wound healing (42). Since the role of ECs in skin healing is of paramount importance, many researchers are now focusing on exosomes derived from these. They reported that exosomes derived from ECs can accelerate the healing of skin wounds in diabetic mice by promoting the expression levels of miRNA-221-3p, VEGF, CD31, and Ki67, which are vascularization-related factors and cell proliferation markers. Bioinformatics analysis indicated that miRNA-221-3p may be involved in the Advanced Glycation End product–Receptor for AGE (AGE–RAGE) signaling pathway in diabetes complications, as well as the cell cycle and p53 signaling pathways (43). Furthermore, in exosomes derived from ECs, they reprogram DNA methyltransferase 1 by promoting the expression of endothelial nitric oxide synthase (eNOS), hCAT-1, VEGF, and ICAM-1, thereby reducing the expression of exosome proteins related to vascular complications, such as Thrombospondin1, Pentraxin3, and Cystatin C (44, 45). Exosomes derived from hypoxic urine-derived stem cells and fibroblast stem cells negatively regulate the HIF-1α/VEGF/VEGFR signaling pathway through miR-486-5p and miRNA-24-3p, thereby promoting the proliferation, migration, and tubal formation of ECs (46, 47).
In recent years, engineered exosomes have gained attention due to their customization. Zhai et al. discovered that engineered exosomes containing interleukin-4 (IL-4) have a significant synergistic effect in regulating immune responses and enhancing angiogenesis. In contrast, they can increase the survival rate, migration, and tubule formation ability of endothelial cells (HUVECs), and, in contrast, they can cause macrophages to polarize toward the anti-inflammatory M2 phenotype, achieving complete repair of diabetic wounds (48). Plant-derived exosomes, due to their excellent biocompatibility and stability, can serve as potential therapeutic agents. Studies have found that mango–ginger-derived plant-derived nanovesicles (MGDNVs) can significantly promote keratinocyte migration in vitro and in vivo by inducing the expression of proteins similar to follicular protein 1 (FSTL1). It can be seen that FSTL1 is a key factor promoting migration in wound healing. The study confirmed that MGDNV can become a natural and cost-effective local alternative to traditional biological agents for the treatment of chronic wounds (49).
Exosomes, as nanoscale messengers between cells, play a crucial role in regulating the healing process of diabetic skin wounds by delivering various bioactive substances, as shown in Table 1. Their inherent advantages, such as low immunogenicity, high stability, and ease of storage and transportation, make them a highly promising new therapeutic strategy.
Table 1
| Source of exosomes | Mechanism | Signal path or receptor | References |
|---|---|---|---|
| MSCs | • Promote ischemic tissue repair and angiogenesis | AKT-MAPK/miRNA-21-5p | (28) |
| *BMSCs | • Promote the angiogenesis, cell viability, and migration | miR-221-3p | (29) |
| MSCs | • Promote cell viability, metabolic activity, and antioxidant capacity | – | (30) |
| hUC-MSCs | • Ameliorate Oxidative Stress and promote angiogenesis | – | (31) |
| hUC-MSCs | • Enhance regeneration of granulation tissue • Upregulate expression of VEGF and TGFβ-1 | – | (32) |
| UC-MSCs | • Promote cell proliferation, migration, and angiogenesis | CircHIPK3/miR-20b-5p/Nrf2/VEGFA | (33) |
| hPMSCs | • Regulate proliferation, migration, and angiogenesis | microRNA-130b-3p/ ICAM-1 | (34) |
| hPMSCs | • Anti-inflammation • Anti-oxidative stress | – | (35) |
| ADSCs | • Regulate macrophage polarization | miR-222-3p/ Bcl2l11/Bim | (36) |
| ADSCs | • Promote proliferation, migration, and angiogenesis | miR-204-3p/HIPK2 | (38) |
| ADSCs | • Upregulate pro-angiogenic factors | miR-1248 | (39) |
| ADSCs | • Enhance autophagy | miR-144-3p/NFE2L2/HIF1α | (40) |
| Macrophages | • Regulate proliferation, migration, and tube formation capabilities | miR-22-5p | (41) |
| PRP | • Inhibit fibroblast ferroptosis | FosB | (42) |
| HUVECs | • Promote vascular formation and cell proliferation | AGE-RAGE/miRNA-221-3p | (43) |
| HUVECs | • Regulate endothelial dysfunction | – | (44) |
| HUVECs | • Epigenetic reprogramming | – | (45) |
| *hUSCS | • Promote endothelial cell proliferation, migration, and tube formation | miR-486-5p/HIF-1α | (46) |
| Fibroblasts | • Improve neovascularization and healing dynamics | miRNA-24-3p/HIF-1α/VEGF/VEGFR | (47) |
| Engineered exos of IL-4 | • Regulate immunomodulatory and pro-angiogenic efficacy | – | (48) |
| MGDNVs | • Promote keratinocyte migration | FSTL1 | (49) |
| M2 macrophages | • Anti-apoptosis | miR-223 | (99) |
| *OSCC | • Promote the proliferation and migration | – | (100) |
| *SHED | • Promote angiogenesis | – | (51) |
| Skeletal muscle | • Anti-reactive oxygen species production • Promote angiogenesis | Nrf2/NF-κB | (101) |
The mechanism of kinds of exosomes regarding skin healing of T2DM.
*hUSCS, hypoxic urine-derived stem cells; OSCC, oral squamous cell carcinoma; BMSCs, bone marrow mesenchymal stem cells; SHED, stem cells from human exfoliated deciduous teeth.
3.2 Liver
Insulin resistance leads to fatty degeneration of the liver. High insulin and blood sugar levels enhance lipid synthesis and uptake in the liver, while inhibiting fatty acid oxidation, resulting in abnormal fat accumulation within liver cells and the development of non-alcoholic fatty liver disease. Additionally, chronic high blood sugar triggers oxidative stress and endoplasmic reticulum stress, driving inflammatory responses and activation of hepatic stellate cells, which jointly exacerbate liver inflammation and fibrosis (50).
Studies have shown that exosomes derived from bone marrow macrophages (BMMs) can alleviate insulin resistance in hepatocytes and enhance insulin sensitivity through miR-143-5p and miR204/Elovl6. Meanwhile, miR204-mediated interactions between adipocytes and macrophages can regulate insulin sensitivity in adipocytes (51). The exosomes secreted by ADSCs from high-sugar diet sources promote inflammation in adipocytes by inhibiting nuclear factor erythroid 2-related factor 2 (Nrf2) expression and increase the expression of NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome-related proteins (52), while the exosomes secreted by aADSCs from high-fat diet sources induce the formation of non-alcoholic fatty liver disease, lipid accumulation and inflammation (53), from which can be seen that the exosomes derived from ADSCs play a significant role in regulating liver lipid metabolism. In recent years, plant-derived exosomes have been increasingly reported. Lipid-derived exosome-like nanovesicles that are derived from orange peels can alleviate liver steatosis in T2DM by regulating lipid metabolism and the intestinal microbiota (54). Natural exosomes-like nanoparticles in mung bean (MELN) sprouts can alleviate the progression of T2DM by activating the phosphatidylinositol 3-kinase (PI3K)/Akt/glucose transporter type 4 (GLUT4)/glycogen synthase kinase-3β (GSK-3β) signaling pathway, which reduces fasting blood glucose levels, triglycerides (TG), total cholesterol (TC) and reduces the inflammatory infiltration and oxidative stress levels in liver cells, thereby improving the survival ability of liver cells (55). Dual-Carriers of Tartary Buckwheat-Derived Exosome-Like Nanovesicles (TB-ELNs) can regulate glucose metabolism in the gut-liver axis and enhance the phagocytic function of the endoplasmic reticulum, thereby reducing damage to the gastrointestinal tract (56). Exosomes derived from liver and milk can, respectively, inhibit PHLPP2 and the mammalian target of rapamycin (mTOR) in adipocytes through miR-130a-3p and miR-101-3p, thereby alleviating glucose tolerance, demonstrating that they are key players in the body's energy homeostasis (57, 58). A clinical study conducted in 2022 demonstrated that by using a mass spectrometry-based method to analyze the global proteome and phosphorylated proteome of exosomes in patients with prediabetes and T2DM, the results showed that the circulating exosomes in diabetic patients contained higher levels of specific phosphorylated kinases, such as protein kinase b-alpha (AKT1), glycogen synthase kinase 3β (GSK3B), LYN, mitogen-activated protein kinase 2 (MAP2K2), myosin light chain kinase (MYLK_, and Protein Kinase C Delta (PRKCD). Moreover, the activated kinase system may be systematically distributed throughout the body, which provides new insights into the pathobiology of T2DM (59).
Exosomes, as key carriers of intercellular communication, have shown great potential in the treatment of liver diseases in T2DM, as shown in Table 2. By carrying specific miRNAs, proteins, they precisely regulate the fate of liver cells, providing novel strategies for drug delivery and liver disease intervention. Their efficacy has been confirmed in disease models, including fatty liver and liver fibrosis.
Table 2
| Source of exosomes | Mechanism | Signal path or receptor | References |
|---|---|---|---|
| ADSCs and macrophages | • Anti-inflammation | Nrf2/miR-500a-5p | (52) |
| ADSCs | • Inhibit lipid accumulation and apoptosis | AMPK α1 | (53) |
| Tangerine peel | • Regulate lipid metabolism and intestinal microflora | – | (54) |
| Mung bean | • Anti-inflammation • Anti-oxidative stress | PI3K/Akt/GLUT4/GSK-3β | (55) |
| Dual-carriers of tartary buckwheat | • Activate the phagocytic function of the endoplasmic reticulum | – | (56) |
| Liver | • Improve glucose intolerance | PHLPP2 /miR-130a-3p | (57) |
| Milk | • Regulate proliferation | mTOR/miR-101-3p | (58) |
| Circulating extracellular | • Distribute activated kinases | OXPHOS | (59) |
| Macrophages | • Promote insulin resistance | MKP5/ miR-143-5p | (102) |
The mechanism of kinds of exosomes regarding liver lesion of T2DM.
3.3 Pancreas
The core mechanisms underlying pancreatic lesions in T2DM are the progressive β-cell failure and the formation of pancreatic amyloidosis. In the context of insulin resistance, β-cells undergo compensatory proliferation and secrete excessive insulin, eventually leading to increased endoplasmic reticulum stress and oxidative stress. Lipotoxicity and glycolipotoxicity jointly induce β-cell apoptosis, while abnormal deposition of pancreatic amylin forms pancreatic amyloid substances, further damaging the pancreatic structure (60). This vicious cycle ultimately results in a reduction in β-cell numbers and insulin secretion defects, leading to irreversible pathological changes.
Exosomes derived from MSCs, in contrast, protect β-cells from hypoxia-induced apoptosis and alleviate endoplasmic reticulum stress by regulating miR-21 and inhibiting p38 MAPK phosphorylation, significantly increasing the survival rate of β-cells (61) and, in contrast, they enhance the function and quantity of β-cells, as well as reduce random blood glucose levels, improve glucose and insulin tolerance, and increase insulin secretion, which mechanism is achieved through AKT/extracellular signal-regulated kinase (ERK) (62). Furthermore, multiple studies have shown that some miRNAs play a crucial role in protecting the pancreas. Exosomes derived from ADSCs protect β-cells function by carrying miR-138-5p and regulating the sex-determining region Y (SRY)-box transcription factor 4 (SOX4)-mediated Wnt/β-catenin pathway (63), exosomes derived from M1 macrophages inhibit β-cell insulin secretion by targeting sirtuin 2 (SIRT2) with miR-212-5p and inhibiting the Akt/GSK-3β/β-catenin pathway (64) and Pancreatic cancer-derived exosomes inhibit β-cell function and reduce insulin secretion by targeting adenylate cyclase 1 (ADCY1) and exchange protein directly activated by cAMP 2 (EPAC2) (65). Besides, exosomes derived from liver and muscle can respectively alleviate β-cell damage through AKT kinase and mTOR signaling pathways, significantly increasing their cell survival rate (66, 67).
Exosomes offer innovative therapeutic ideas for pancreatic lesions in T2DM, as shown in Table 3. These natural nanovesicles can precisely improve the function of pancreatic β-cells by delivering active components, such as miRNAs. This therapy, based on intercellular communication, can reshape the pancreatic microenvironment, opening a new therapeutic pathway to prevent the progressive failure of β-cells and reverse the progression of diabetes.
Table 3
| Source of exosomes | Mechanism | Signal path or receptor | References |
|---|---|---|---|
| MSCs | • Anti-apoptosis, • Reduce endoplasmic reticulum stress | p38 MAPK | (61) |
| MSCs | • Suppresses ferroptosis | AKT/ERK | (62) |
| ADSCs | • Anti-apoptosis • Enhance insulin sensitivity | SOX4/Wnt/β-actin/miR-138-5p | (63) |
| M1 macrophages | • Increase insulin secretion | Akt/GSK-3β/β-catenin | (64) |
| Pancreatic cancer | • Regulate β-cell dysfunction | microRNA-19a | (65) |
| Liver | • Affect insulin expression | AKT | (66) |
| Muscle | • Reduce oxidative stress • Enhance autophagy • Anti-apoptosis | Akt/mTOR | (67) |
| Macrophages | • Enhance insulin sensitivity | miR204/Elovl6 | (103) |
The mechanism of kinds of exosomes regarding pancreas lesion of T2DM.
3.4 Diabetic nephropathy
T2DM primarily causes kidney damage through metabolic and hemodynamic pathways. Long-term high blood sugar leads to the accumulation of AGEs, activation of protein kinase C, and hyperactivity of the polyol pathway, all of which jointly cause damage to the glomerular filtration barrier. At the same time, high blood sugar, in conjunction with hypertension, causes high intra-glomerular pressure and hyperfiltration, continuously activating the renin-angiotensin system, promoting inflammatory responses and fibrosis processes, ultimately leading to glomerular sclerosis and tubulointerstitial fibrosis and gradually developing into diabetic nephropathy (68).
Exosomes derived from MSCs can not only induce autophagy in glomerular endothelial cells through the mammalian target of rapamycin (mTOR) signaling pathway to improve DN (69), but also inhibit pyroptosis in proximal tubular cells of the kidney through miR-30e-5p (70), which is the first time the role of exosomes in inhibiting cell pyroptosis in T2DM has been reported. Furthermore, the exosomes derived from BMSCs alleviate DN in rats by inhibiting cell apoptosis and reducing inflammatory levels. The results of the in vivo experiments showed a significant decrease in the levels of glucose (GLU), creatinine (Cr), and blood urea nitrogen (BUN) (71). Ning et al. reported that exosomes derived from glomerular ECs themselves can also carry miR-30a-5p and regulate angiogenesis through the Notch1/VEGF signaling pathway (72). These findings suggest that exosome miR-30-5p may become a potential strategy for treating DN. Besides MSC-derived exosomes, exosomes secreted by ADSCs can also inhibit podocyte apoptosis and slow down DR by activating autophagy flux (73, 74). Studies have confirmed that the expression of miRNA-615-3p and miRNA-3147 in urine-derived exosomes is closely related to the inflammation and fibrosis of DN and is positively correlated with serum cystatin C, plasma TGF-β1, creatinine, blood urea nitrogen (BUN), protein creatinine ratio (PCR), and 24-h urine protein, and negatively correlated with eGFR and albumin (75).
In conclusion, exosomes demonstrate multi-dimensional therapeutic potential in the intervention of DR, as shown in Table 4. They can simultaneously regulate multiple pathological processes of the renal microenvironment: not only inhibiting the release of inflammatory factors and oxidative stress damage, but also maintaining the structural integrity of podocytes and blocking the activation of renal fibrosis signaling pathways. Their natural targeting properties and low immunogenicity offer new avenues for the precise treatment of diabetic nephropathy, especially their unique advantage in delaying glomerular sclerosis and improving renal function indicators.
Table 4
| Source of exosomes | Mechanism | Signal path or receptor | References |
|---|---|---|---|
| MSCs | • Regulate autophagy | mTOR | (69) |
| BMSCs | • Inhibit pyroptosis | miR-30e-5p/ ELAVL1 | (70) |
| BMSCs | • Anti-apoptosis • Anti-inflammation | – | (71) |
| *GECs | • Regulate angiogenesis | Notch1/VEGF /miR-30a-5p | (72) |
| ADSCs | • Promote autophagy flux and inhibit apoptosis | miR-486/Smad1/mTOR | (73) |
| ADSCs | • Anti-apoptosis • Anti-inflammation | USP25 | (74) |
| hUSCS | • Inhibit inflammation and fibrosis | miRNA-615-3p and miRNA-3147 | (75) |
The mechanism of kinds of exosomes regarding DN of T2DM.
*GECs, Glomerular endothelial cells.
3.5 Neurological disorders
In contrast, persistent hyperglycemia leads to activation of the polyol pathway, accumulation of advanced glycation end products, activation of the protein kinase C pathway, and oxidative stress response, all of which jointly cause metabolic disorders in neurons and Schwann cells. In contrast, high blood sugar damages microvessels, resulting in narrowing and ischemia of nerve-feeding vessels. These pathological changes ultimately lead to demyelination of peripheral nerve fibers and axonal degeneration, triggering the typical diabetic peripheral neuropathy (76).
The exosomes secreted by BMSCs can exert neuroprotective functions through multiple pathways. First, they can enhance neural conduction velocity and increase the number of epineural nerve fibers (IENFs), myelin thickness, and axon diameter in the sciatic nerve by targeting miRNAs through the Toll-like receptor 4 (TLR4)/NF-κB signaling pathway, and can also reduce and increase the markers of M1 and M2 macrophage phenotypes (77). Second, they can target and weaken the neurological dysfunction after diabetic cerebral hemorrhage through miR-129-5p, promoting neuroinflammation and functional recovery (78). Third, these exosomes can be transferred to the damaged neuronal and astrocyte regions to improve cognitive impairment caused by diabetes and restore normality (79). Additionally, a clinical study derived from adipose tissue exosomes shows that through Mendelian randomization and multicenter population studies, it was confirmed that 13 exosomal miRNAs were significantly upregulated in the adipose tissue of T2DM patients, among which the increase of miR-125a-5p was associated with Alzheimer's disease (AD), risk of amnestic mild cognitive impairment (aMCI) and reduced volume of the left hippocampus. This confirmed that the serum exosomal miR-125a-5p extracted from adipose tissue can serve as a pathogenic biomarker for cognitive impairment in T2DM patients (80).
Exosomes precisely target the core pathological links of neurological disorders regarding T2DM through multiple mechanisms, including regulating neuroinflammation, promoting synaptic remodeling, and clearing abnormal protein aggregates, as shown in Table 5. This intercellular communication carrier provides a new strategy to break through traditional treatment bottlenecks and has significant application prospects in neural regeneration and functional recovery.
Table 5
| Source of exosomes | Mechanism | Signal path or receptor | References |
|---|---|---|---|
| MSCs | • Alleviate neurovascular dysfunction • Anti-suppression of proinflammatory genes | TLR4/NF-κB | (77) |
| BMSCs | • Anti-inflammation | miR-129-5p | (78) |
| BMSCs | • improve cognitive impairment | – | (79) |
| Serum from adipose | • Serum exosomal | miR-125a-5p | (80) |
| BMSCs and *HUCBCs | • Angiogenesis, neurogenesis, vascular remodeling • Modulate inflammatory and immune responses | – | (104) |
The mechanism of kinds of exosomes regarding neurological disorders of T2DM.
*HUCBCs, human umbilical cord blood cells.
3.6 Osteoporosis
In patients with T2DM, elevated blood sugar and the accumulation of AGEs directly inhibit the function of osteoblasts and reduce bone quality. At the same time, inflammatory factors released during chronic inflammation further disrupt the balance between bone formation and bone resorption. Moreover, the combined effects of insulin resistance and oxidative stress interfere with the normal metabolism and repair capabilities of the bones, resulting in reduced bone formation, increased bone fragility, and significantly delaying the bone regeneration process (81).
In terms of bone loss, exosomes derived from damaged liver tissues are significantly enriched in the periodontal region, inducing pyroptosis in periodontal ligament cells (PDLCs). Among them, fatty acid synthase (Fasn) leads to the ectopic synthesis of fatty acids in PDLCs and activates the cleavage of gasdermin D. The results show that the deletion of Fasn in the liver can effectively alleviate the pyroptosis of PDLCs and reduce bone loss. This study reveals the organ communication mediated by exosomes in the “liver-bone” axis, providing insights for the prevention and treatment of diabetes-related bone diseases in the future (26). Salivary exosomes can inhibit local inflammatory responses through miR-25-3p, reducing periodontal alveolar bone loss by approximately 34% (82). In terms of osteogenesis, Schwann cell-derived exosomes improve bone parameters around dental implants in T2DM rats through the miR-15b-5p/Txnip signaling axis (83), BMSC-derived exosomes promote bone regeneration by regulating the miR-17-5p/mothers against decapentaplegic homolog 7 (SMAD7) axis (84) and stem cell exosomes overexpressing Smpd3 promote osteogenesis and differentiation of BMSCs derived from the jawbone by activating jawbone autophagy and inhibiting macrophage polarization and oxidative stress caused by blood glucose fluctuations (85), which collectively provide insights into the molecular mechanism of peripheral nerve regulation of bone regeneration in diabetic patients.
Exosomes, as a key medium for intercellular communication, have shown crucial potential in treating diabetic osteogenic dysplasia, as shown in Table 6. In contrast, they promote the proliferation and differentiation of osteoblasts and enhance their mineralization function. In contrast, they inhibit the activity of osteoclasts. This multi-targeted mechanism can effectively improve microcirculation disorders and inflammatory states in the context of high blood sugar, providing a novel therapeutic strategy for reversing bone loss and accelerating bone defect repair.
Table 6
| Source of exosomes | Mechanism | Signal path or receptor | References |
|---|---|---|---|
| Liver | • Reduce pyroptosis | – | (26) |
| Saliva | • Anti-inflammation | miR-25-3p | (82) |
| Schwann cells | • Anti-inflammation • Anti-oxidative stress | miR-15b-5p | (83) |
| BMSCs | • Affect bone regeneration | miR-17-5p/SMAD7 | (84) |
| BMSCs | • Promote the osteogenesis and differentiation • Activate autophagy • Inhibit macrophage polarization • Aniti-oxidative stress | – | (85) |
The mechanism of kinds of exosomes regarding osteoporosis of T2DM.
3.7 Other complications
Exosomes also have therapeutic effects on other physiological organ complications. Due to the limited research available, we will present these together. Regarding Diabetic cardiomyopathy (DCM), Lin et al. confirmed that exosomes derived from macrophages improve the formation of resistance proteins and neointimal hyperplasia through miRNA-150-5p, exerting a protective effect on the heart (86). However, Govindappa believes that macrophage exosomes significantly enhance the inflammatory and pro-fibrotic responses of mouse fibroblasts and myocardial fibrosis. Only by knocking down human antigen R (HuR) in the exosomes can the angiotensin II-induced myocardialization response be inhibited and the left ventricular function be maintained (87). A clinical study stated that they isolated serum exosomes from 147 patients with and without diabetes and found that CD14 from serum exosomes was associated with T2DM. T2DM may promote the increase of CD14 protein in exosomes, thereby increasing the susceptibility to atherosclerosis. However, the analysis of the contents of exosomes from diabetic patients is still in its early stage, so a comprehensive characterization is crucial for it to serve as a biomarker or to analyze its potential contribution to diabetic complications (88).
Exosomes also exert a promising effect against DR. Exosomes derived from MSCs carry miR-125a-5p, in contrast, they enhance the survival rate, proliferation and migration of corneal ECs. In contrast, they inhibit the endoplasmic reticulum stress induced by high glucose, thereby jointly improving diabetic keratopathy (DK) (89). Additionally, exosomes derived from adipocytes regulate the levels of apoptosis, inflammation, and oxidative stress in mouse retinal microvascular endothelial cells (mRMECs) by modulating the miR-361-5p/TRAF3 axis, making them a potential target for treating microvascular complications induced by diabetes (90). Exosomes derived from Müller cells regulate microglial cell polarization in DR by carrying lncRNAs, including miR-320-3p, miR-221-3p, and miR-574-5p, promoting the polarization of microglia to the M1 phenotype (91).
Furthermore, adipocyte exosomes carry miR-4472, which downregulates the expression level of myocyte enhancer factor 2D (MEF2D), inhibiting the glucose consumption and uptake capacity of skeletal muscle cells (92). Garlic-like exosome-like nanoparticles (GaELNs) increase the level of phosphatidylcholine through the gut–brain axis, inhibiting cGas and STING-mediated inflammation as well as the interaction between GLP-1R and the insulin pathway (93). MSCs-derived exosomes alleviate the damage of human pulmonary microvascular endothelial cells (hPMECs) induced by high glucose and lipopolysaccharide through the Nrf2/heme oxygenase 1 (HO-1) pathway, reducing the levels of inflammation and ferroptosis (94).
Therefore, exosomes derived from different cell sources also play certain therapeutic roles in the heart, lungs, retina, skeletal muscle, and intestinal microbiota, as shown in Table 7. However, current research in these areas is relatively limited. In the future, more studies can be conducted to further explore their mechanism of action.
Table 7
| Source of exosomes | Mechanism | Signal path or receptor | References |
|---|---|---|---|
| Macrophages | • Ameliorate resistin and neointimal hyperplasia formation | miRNA-150-5p | (86) |
| Macrophages | • Attenuate fibrosis and inflammation | – | (87) |
| Serum from T2DM | • Increase atherogenic index of plasma | CD14 | (88) |
| MSCs | • Regulate endoplasmic reticulum stress | miR-125a-5p | (89) |
| ADSCs | • Anti-inflammation • Anti-oxidative stress | LINC00968/miR-361-5p/TRAF3 | (90) |
| Müller Cells | • Regulate microglia polarization | miR-320-3p、miR-221-3p 和 miR-574-5p | (91) |
| ADSCs | • Inhibit glucose uptake | MEF2D/GLUT4/miR-4472 | (92) |
| Garlic | • Regulate gut bacteria via the gut-brain axis | – | (93) |
| MSCs | • Anti-inflammation • Inhibit ferroptosis | Nrf2/HO-1 | (94) |
| hUC-MSCs | • Anti-inflammation | INS/SOD1 | (105) |
The mechanism of kinds of exosomes regarding other complications of T2DM.
Exosomes, as a key medium for intercellular communication, have shown great potential in the treatment of multiple systemic complications of T2DM, as shown in Figure 4. These natural nanovesicles can precisely regulate multiple physiological system complications and organs across different tissues. Their unique natural targeting ability and low immunogenicity enable exosomes to act on multiple pathological processes simultaneously, breaking through the limitations of traditional single-target drugs. This treatment strategy, based on intercellular communication, offers a new idea for the collaborative management of T2DM complications and is expected to become an important breakthrough direction in the management of diabetes that involves multiple systems.
Figure 4
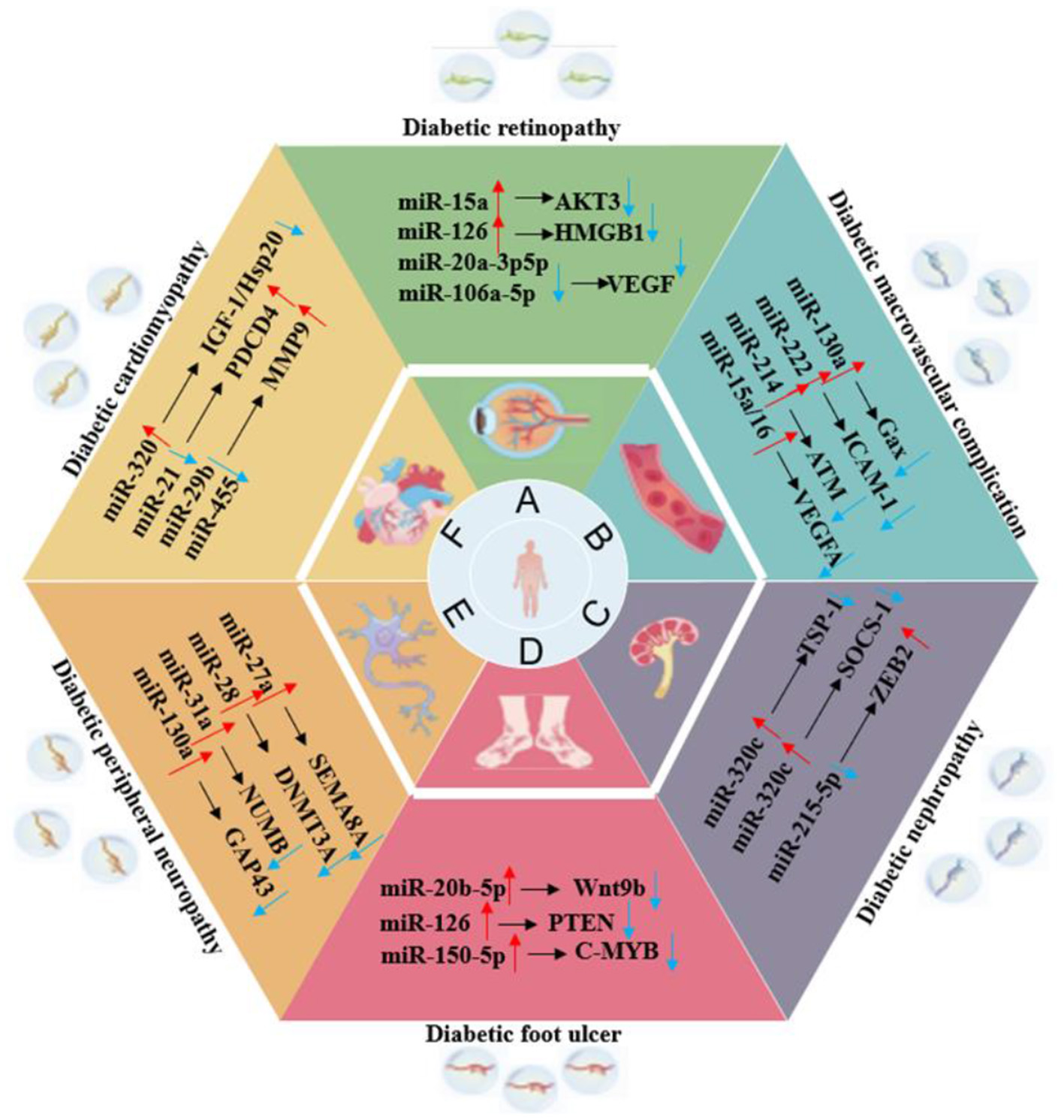
The role of exosomes in various organ complications of T2DM.
4 As the biomarker of disease
Exosomes are emerging as potential biomarkers because they reflect the physiological state of their originating cells, a characteristic that varies notably in metabolic diseases (95). This property highlights their clinical relevance for early diagnosis, disease monitoring, and evaluation of therapeutic outcomes. For example, the distinct expression of miRNAs in exosomes enables the early detection of diabetes (96), offering a more precise approach to disease management. Furthermore, regulating the production, release, and composition of exosomes presents promising avenues for therapeutic intervention in T2DM (97). A clinical trial demonstrated that miR-125a-5p exosomes derived from adipose tissue in the serum could serve as a causal biomarker for cognitive impairment in patients with T2DM (80). Such interventions hold the potential to provide personalized treatment plans tailored to the specific physiological and pathological profiles of patients.
As an emerging therapeutic approach and drug delivery system, exosomes offer significant advantages in treating diabetes and its complications. Compared to traditional treatments, such as insulin injections and oral hypoglycemic drugs, and artificial nano-drug carriers, exosomes exhibit higher biocompatibility, lower immunogenicity, and natural targeting properties, enabling them to effectively overcome biological barriers in drug delivery (80). Exosomes can also reverse immunosuppression under hyperglycemic conditions, not only delivering antibiotics for targeted sterilization but also reversing macrophage senescence and improving the microenvironment of diabetic wounds (54). Traditional antibiotic treatments often struggle to address both immunosuppression and bacterial resistance simultaneously. In contrast to synthetic nano-carriers like liposomes or polymer nanoparticles, exosomes offer superior tissue penetration and stability (98). The potential of exosomes in treating T2DM is attracting increasing attention. These small vesicles, which facilitate intercellular communication, can also function as biomarkers or drug-delivery systems.
Although exosome-based therapies for T2DM are still in the research phase, their future potential is significant. A deeper understanding of the biological properties of exosomes, their mechanisms, and their involvement in T2DM could lead to innovative therapeutic strategies in this field.
5 Limitations
Although exosome therapy shows great potential, there are still some challenges and limitations in successfully translating it into clinical practice. First, exosomes' size, contents, and functions vary greatly due to differences in cell source, cell state, and isolation methods. This high heterogeneity leads to poor reproducibility of research results and uncertainty in the stability and safety assessment of efficacy. Second, although exosomes have natural targeting properties, this targeting ability is often inefficient and non-specific. After systemic administration, the majority of exosomes are rapidly cleared by the mononuclear phagocytic system in the liver and spleen, and only a small portion can accumulate in the target tissues, which not only reduces efficacy but also may lead to off-target effects. Moreover, as a biologically active carrier, the contents of exosomes are complex and may contain oncogenic or proinflammatory molecules, posing potential safety risks. Although their immunogenicity is low, when heterologous exosomes are repeatedly injected, they may still trigger immune responses, leading to decreased efficacy or adverse reactions. These unknown safety variables are key issues that must be carefully evaluated in preclinical and future clinical studies. Third, exosomes exert their effects through a “multi-component-multi-target-multi-pathway” collaborative network, which is both an advantage and a barrier to defining them as a specific drug. Our current understanding of their exact mechanism of action, metabolic kinetics in the body, and optimal treatment dose and administration route is still very limited, making it extremely difficult to trace and control efficacy. In conclusion, the transition of exosome therapy from the laboratory to clinical practice still requires fundamental breakthroughs in production processes, safety assessment, and mechanism exploration.
6 Prospects
Exosome therapy still holds an extremely promising and exciting future, mainly manifested in the following aspects. First, engineered exosomes have the potential to achieve unprecedented, precise targeted treatment. By modifying their membrane surface, they can efficiently accumulate in specific tissues such as the damaged pancreas, kidneys, or retina. At the same time, by loading specific therapeutic molecules, they can precisely regulate key signaling pathways related to insulin resistance, cell apoptosis, or fibrosis, achieving “targeted” intervention at the root of the disease, thereby enhancing the therapeutic effect while minimizing systemic side effects. Second, exosomes' contents can serve as sensitive biomarkers for diagnosing T2DM and its different complications. In the future, the diagnostic markers of exosomes can be detected to assess the condition and be used as delivery carriers for therapeutic drugs to achieve dynamic monitoring and personalized treatment of the disease in a closed loop. Third, by using exosomes produced by the patient's own cells, highly individualized treatment plans can be developed, which effectively avoid immune rejection problems. The regenerative signals carried by these endogenous exosomes can not only protect and repair damaged β-cells and vascular endothelium, but also strongly promote the healing and regeneration of tissues.
7 Conclusions
This review systematically elaborates on how exosomes exert practical therapeutic effects in T2DM and its various complications through different key targets and signaling pathways. With their unique biological functions and engineering advantages, exosomes have opened up a highly promising new approach for the prevention and treatment of T2DM and its complications. Although there are still challenges in terms of standardization, targeting, and mechanism elucidation, with the deepening of research and the breakthrough of technical bottlenecks, it is expected to lead diabetes treatment from the traditional model to a new era of precise diagnosis and regenerative repair.
Statements
Author contributions
YL: Writing – original draft, Writing – review & editing. JW: Writing – review & editing, Investigation. YS: Investigation, Writing – review & editing. YC: Writing – review & editing, Writing – original draft.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This review was supported by Natural Science Foundation of Jilin Province (YDZJ202301ZYTS074).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Młynarska E Czarnik W Dzieża N Jedraszak W Majchrowicz G Prusinowski F et al . Type 2 diabetes mellitus: new pathogenetic mechanisms, treatment and the most important complications. Int J Mol Sci. (2025) 26:1094. doi: 10.3390/ijms26031094
2.
Strati M Moustaki M Psaltopoulou T Vryonidou A Paschou SA . Early onset type 2 diabetes mellitus: an update. Endocrine. (2024) 85:965–78. doi: 10.1007/s12020-024-03772-w
3.
Gong T Liu YT Fan J . Exosomal mediators in sepsis and inflammatory organ injury: unraveling the role of exosomes in intercellular crosstalk and organ dysfunction. Mil Med Res. (2024) 11:24. doi: 10.1186/s40779-024-00527-6
4.
Li G Zhang S Zou Y Ai H Zheng X Qian K et al . The therapeutic potential of exosomes in immunotherapy. Front Immunol. (2024) 15:1424081. doi: 10.3389/fimmu.2024.1424081
5.
Tao H Gao B . Exosomes for neurodegenerative diseases: diagnosis and targeted therapy. J Neurol. (2024) 271:3050–62. doi: 10.1007/s00415-024-12329-w
6.
Rahman E Webb WR Rao P Abu-Farsakh HN Upton AE Yu N et al . Exosomes exposed: overview systematic review on evidence versus expectation in aesthetic and regenerative medicine. Aesthetic Plast Surg. (2025) 49:557–68. doi: 10.1007/s00266-024-04276-8
7.
Li H Liu G Wang B Momeni MR . Exosomes and microRNAs as mediators of the exercise. Eur J Med Res. (2025) 30:38. doi: 10.1186/s40001-025-02273-4
8.
Batista IA Machado JC Melo SA . Advances in exosomes utilization for clinical applications in cancer. Trends Cancer. (2024) 10:947–68. doi: 10.1016/j.trecan.2024.07.010
9.
Chen Z Shang Y Ou Y Gong S Xiang X Ji X et al . Exosomes in obstructive sleep apnea-related diseases. Chin Med J. (2025) 138:2540–51. doi: 10.1097/CM9.0000000000003784
10.
Karabay AZ Barar J Hekmatshoar Y Rahbar Saadat Y . Multifaceted therapeutic potential of plant-derived exosomes: immunomodulation, anticancer, anti-aging, anti-melanogenesis, detoxification, and drug delivery. Biomolecules. (2025) 15:394. doi: 10.3390/biom15030394
11.
Wang JS Schellenberg SJ Demeros A Lin AY . Exosomes in review: a new frontier in CAR-T cell therapies. Neoplasia. (2025) 62:101147. doi: 10.1016/j.neo.2025.101147
12.
Yao T Dong X Wang X Liu X Fu L Li L . Engineering exosomes for mRNA delivery: a review. Int J Biol Macromol. (2025) 316:144662. doi: 10.1016/j.ijbiomac.2025.144662
13.
Krylova SV Feng D . The machinery of exosomes: biogenesis, release, and uptake. Int J Mol Sci. (2023) 24:1337. doi: 10.3390/ijms24021337
14.
Hessvik NP Llorente A . Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. (2018) 75:193–208. doi: 10.1007/s00018-017-2595-9
15.
Kimiz-Gebologlu I Oncel SS . Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Contr Release. (2022) 347:533–43. doi: 10.1016/j.jconrel.2022.05.027
16.
Moghassemi S Dadashzadeh A Sousa MJ Vlieghe H Yang J León-Félix CM et al . Extracellular vesicles in nanomedicine and regenerative medicine: a review over the last decade. Bioact Mater. (2024) 36:126–56. doi: 10.1016/j.bioactmat.2024.02.021
17.
Tang YT Huang YY Zheng L Qin SH Xu XP An TX et al . Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. (2017) 40:834–44. doi: 10.3892/ijmm.2017.3080
18.
Li D Wang Y Jin X Hu D Xia C Xu H et al . NK cell-derived exosomes carry miR-207 and alleviate depression-like symptoms in mice. J Neuroinflammation. (2020) 17:126. doi: 10.1186/s12974-020-01787-4
19.
Jiang X You L Zhang Z Cui X Zhong H Sun X et al . Biological properties of milk-derived extracellular vesicles and their physiological functions in infant. Front Cell Dev Biol. (2021) 9:693534. doi: 10.3389/fcell.2021.693534
20.
Zhang J Li S Li L Li M Guo C Yao J et al . Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. (2015) 13:17–24. doi: 10.1016/j.gpb.2015.02.001
21.
Li Y Tang Y Yang GY . Therapeutic application of exosomes in ischaemic stroke. Stroke Vasc Neurol. (2021) 6:483–95. doi: 10.1136/svn-2020-000419
22.
McMullan E Joladarashi D Kishore R . Unpacking exosomes: a therapeutic frontier for cardiac repair. Curr Cardiol Rep. (2025) 27:73. doi: 10.1007/s11886-025-02225-8
23.
Konaka H Kato Y Hirano T Tsujimoto K Park J Koba T et al . Secretion of mitochondrial DNA via exosomes promotes inflammation in Behçet's syndrome. EMBO J. (2023) 42:e112573. doi: 10.15252/embj.2022112573
24.
Hajialiasgary Najafabadi A Soheilifar MH Masoudi-Khoram N . Exosomes in skin photoaging: biological functions and therapeutic opportunity. Cell Commun Signal. (2024) 22:32. doi: 10.1186/s12964-023-01451-3
25.
Kok VC Yu CC . Cancer-derived exosomes: their role in cancer biology and biomarker development. Int J Nanomed. (2020) 15:8019–36. doi: 10.2147/IJN.S272378
26.
Liu J Dou G Zhao W Hu J Jiang Z Wang W et al . Exosomes derived from impaired liver aggravate alveolar bone loss via shuttle of Fasn in type 2 diabetes mellitus. Bioact Mater. (2024) 33:85–99. doi: 10.1016/j.bioactmat.2023.10.022
27.
Wang Y Jing L Lei X Ma Z Li B Shi Y et al . Umbilical cord mesenchymal stem cell-derived apoptotic extracellular vesicles ameliorate cutaneous wound healing in type 2 diabetic mice via macrophage pyroptosis inhibition. Stem Cell Res Ther. (2023) 14:257. doi: 10.1186/s13287-023-03490-6
28.
Huang C Luo W Wang Q Ye Y Fan J Lin L et al . Human mesenchymal stem cells promote ischemic repairment and angiogenesis of diabetic foot through exosome miRNA-21-5p. Stem Cell Res. (2021) 52:102235. doi: 10.1016/j.scr.2021.102235
29.
Qiu ZY Xu WC Liang ZH . Bone marrow mesenchymal stem cell-derived exosomal miR-221-3p promotes angiogenesis and wound healing in diabetes via the downregulation of forkhead box P1. Diabet Med. (2024) 41:e15386. doi: 10.1111/dme.15386
30.
Liang J Cheng S Song Q Tang Y Wang Q Chen H et al . Effect of mesenchymal stem cell-derived extracellular vesicles induced by advanced glycation end products on energy metabolism in vascular endothelial cells. Kidney Int Rep. (2025) 10:227–46. doi: 10.1016/j.ekir.2024.10.015
31.
Yan C Xv Y Lin Z Endo Y Xue H Hu Y et al . Human umbilical cord mesenchymal stem cell-derived exosomes accelerate diabetic wound healing via ameliorating oxidative stress and promoting angiogenesis. Front Bioeng Biotechnol. (2022) 10:829868. doi: 10.3389/fbioe.2022.829868
32.
Yang J Chen Z Pan D Li H Shen J . Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomed. (2020) 15:5911–26. doi: 10.2147/IJN.S249129
33.
Liang ZH Lin SS Pan NF Zhong GY Qiu ZY Kuang SJ et al . UCMSCs-derived exosomal circHIPK3 promotes ulcer wound angiogenesis of diabetes mellitus via miR-20b-5p/Nrf2/VEGFA axis. Diabet Med. (2023) 40:e14968. doi: 10.1111/dme.14968
34.
Gao Z Wang N Liu X . Human placenta mesenchymal stem cell-derived exosome shuttling microRNA-130b-3p from gestational diabetes mellitus patients targets ICAM-1 and perturbs human umbilical vein endothelial cell angiogenesis. Acta Diabetol. (2022) 59:1091–107. doi: 10.1007/s00592-022-01910-2
35.
Varyani L Ahmadpanah N Kasiri R Shahzamani S Tomraee S Jafari A et al . Human amniotic membrane hydrogel loaded with exosomes derived from human placental mesenchymal stem cells accelerate diabetic wound healing. Tissue Cell. (2024) 91:102590. doi: 10.1016/j.tice.2024.102590
36.
Xia W Liu Y Jiang X Li M Zheng S Zhang Z et al . Lean adipose tissue macrophage derived exosome confers immunoregulation to improve wound healing in diabetes, J Nanobiotechnol. (2023) 21:128. doi: 10.1186/s12951-023-01869-4
37.
Shi R Jin Y Zhao S Yuan H Shi J Zhao H . Hypoxic ADSC-derived exosomes enhance wound healing in diabetic mice via delivery of circ-Snhg11 and induction of M2-like macrophage polarization. Biomed Pharmacother. (2022) 153:113463. doi: 10.1016/j.biopha.2022.113463
38.
Huang H Zhu W Huang Z Zhao D Cao L Gao X . Adipose-derived stem cell exosome NFIC improves diabetic foot ulcers by regulating miR-204-3p/HIPK2. J Orthop Surg Res. (2023) 18:687. doi: 10.1186/s13018-023-04165-x
39.
Jian X Han J Liu X Deng Y Gao S Xiao S et al . Exosome-carried miR-1248 from adipose-derived stem cells improves angiogenesis in diabetes-associated wounds. Int J Biol Macromol. (2025) 297:139822. doi: 10.1016/j.ijbiomac.2025.139822
40.
Song J Liu J Cui C Hu H Zang N Yang M et al . Mesenchymal stromal cells ameliorate diabetes-induced muscle atrophy through exosomes by enhancing AMPK/ULK1-mediated autophagy. J Cachexia Sarcopenia Muscle. (2023) 14:915–29. doi: 10.1002/jcsm.13177
41.
Ji Y Chen H Pang L Chen C Wang S Chen J et al . AGE induced macrophage-derived exosomes induce endothelial dysfunction in diabetes via miR-22-5p/FOXP1. Cardiovasc Diabetol. (2025) 24:158. doi: 10.1186/s12933-025-02715-7
42.
Wang S Wu J Ren K Zhang Y Gao F Chen Y et al . Platelet-rich plasma-derived exosome-encapsulated hydrogels accelerate diabetic wound healing by inhibiting fibroblast ferroptosis. ACS Appl Mater Interfaces. (2025) 17:27923–36. doi: 10.1021/acsami.5c02705
43.
Xu J Bai S Cao Y Liu L Fang Y Du J et al . miRNA-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diabetes Metab Syndr Obes. (2020) 13:1259–70. doi: 10.2147/DMSO.S243549
44.
Sáez T de Vos P Kuipers J Sobrevia L Faas MM . Fetoplacental endothelial exosomes modulate high d-glucose-induced endothelial dysfunction. Placenta. (2018) 66:26–35. doi: 10.1016/j.placenta.2018.04.010
45.
Vasishta S Ammankallu S Poojary G Gomes SM Ganesh K Umakanth S et al . High glucose induces DNA methyltransferase 1 dependent epigenetic reprogramming of the endothelial exosome proteome in type 2 diabetes. Int J Biochem Cell Biol. (2024) 176:106664. doi: 10.1016/j.biocel.2024.106664
46.
Fan MH Zhang XZ Jiang YL Pi JK Zhang JY Zhang YQ et al . Exosomes from hypoxic urine-derived stem cells facilitate healing of diabetic wound by targeting SERPINE1 through miR-486-5p. Biomaterials. (2025) 314:122893. doi: 10.1016/j.biomaterials.2024.122893
47.
Chen Y Yin W Liu Z Lu G Zhang X Yang J et al . Exosomes derived from fibroblasts enhance skin wound angiogenesis by regulating HIF-1α/VEGF/VEGFR pathway. Burns Trauma. (2025) 13:tkae071. doi: 10.1093/burnst/tkae071
48.
Zhai M Tan H Xu A Wu B Xie F Lu Y et al . Immunomodulatory hydrogel loaded with PD-L1-expressing exosomes reprograms macrophages and accelerates diabetic wound healing. Biomater Adv. (2025) 176:214362. doi: 10.1016/j.bioadv.2025.214362
49.
Suresh A Ravilla J Narayanan J Sundaram GM . Mango ginger-derived exosome-like nanovesicles promotes diabetic wound healing via inducing the promigratory protein, follistatin-like 1. Int J Biol Macromol. (2025) 322:146991. doi: 10.1016/j.ijbiomac.2025.146991
50.
Tanase DM Gosav EM Costea CF Ciocoiu M Lacatusu CM Maranduca MA et al . The intricate relationship between Type 2 diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. (2020) 2020:3920196. doi: 10.1155/2020/3920196
51.
Sunartvanichkul T Arayapisit T Sangkhamanee SS Chaweewannakorn C Iwasaki K Klaihmon P et al . Stem cell-derived exosomes from human exfoliated deciduous teeth promote angiogenesis in hyperglycemic-induced human umbilical vein endothelial cells. J Appl Oral Sci. (2023) 31:e20220427. doi: 10.1590/1678-7757-2022-0427
52.
Li YZ Tian Y Yang C Liu YF Qu SL Huang L et al . Adipose tissue macrophages-derived exosomal MiR-500a-5p under high glucose promotes adipocytes inflammation by suppressing Nrf2 expression. Int J Biochem Cell Biol. (2025) 178:106713. doi: 10.1016/j.biocel.2024.106713
53.
Yan C Tian X Li J Liu D Ye D Xie Z et al . A high-fat diet attenuates AMPK α1 in adipocytes to induce exosome shedding and nonalcoholic fatty liver development in vivo. Diabetes. (2021) 70:577–88. doi: 10.2337/db20-0146
54.
Zou J Song Q Shaw PC Wu Y Zuo Z Yu R . Tangerine peel-derived exosome-like nanovesicles alleviate hepatic steatosis induced by type 2 diabetes: evidenced by regulating lipid metabolism and intestinal microflora. Int J Nanomed. (2024) 19:10023–43. doi: 10.2147/IJN.S478589
55.
He C Wang K Xia J Qian D Guo J Zhong L et al . Natural exosomes-like nanoparticles in mung bean sprouts possesses anti-diabetic effects via activation of PI3K/Akt/GLUT4/GSK-3β signaling pathway. J Nanobiotechnol. (2023) 21:349. doi: 10.1186/s12951-023-02120-w
56.
Li D Yi G Cao G Midgley AC Yang Y Yang D et al . Dual-carriers of tartary buckwheat-derived exosome-like nanovesicles synergistically regulate glucose metabolism in the intestine-liver axis. Small. (2025) 21:e2410124. doi: 10.1002/smll.202410124
57.
Wu J Dong T Chen T Sun J Luo J He J et al . Hepatic exosome-derived miR-130a-3p attenuates glucose intolerance via suppressing PHLPP2 gene in adipocyte. Metabolism. (2020) 103:154006. doi: 10.1016/j.metabol.2019.154006
58.
Zheng Z Mo J Lin F Wang J Chen J Luo H et al . Milk exosomes from gestational diabetes mellitus (GDM) and healthy parturient exhibit differential miRNAs profiles and distinct regulatory bioactivity on hepatocyte proliferation. Mol Nutr Food Res. (2023) 67:e2300005. doi: 10.1002/mnfr.202300005
59.
Nunez Lopez YO Iliuk A Petrilli AM Glass C Casu A Pratley RE . Proteomics and phosphoproteomics of circulating extracellular vesicles provide new insights into diabetes pathobiology. Int J Mol Sci. (2022) 23:5779. doi: 10.3390/ijms23105779
60.
Xourafa G Korbmacher M Roden M . Inter-organ crosstalk during development and progression of type 2 diabetes mellitus. Nat Rev Endocrinol. (2024) 20:27–49. doi: 10.1038/s41574-023-00898-1
61.
Chen J Chen J Cheng Y Fu Y Zhao H Tang M et al . Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res Ther. (2020) 11:97. doi: 10.1186/s13287-020-01610-0
62.
Xia L Yang M Zang N Song J Chen J Hu H et al . PEGylated β-cell-targeting exosomes from mesenchymal stem cells improve β cell function and quantity by suppressing NRF2-mediated ferroptosis. Int J Nanomed. (2024) 19:9575–96. doi: 10.2147/IJN.S459077
63.
Fan S Li N . Obesity-induced adipocytes promote diabetes mellitus by regulating β islet cell function through exosome miR-138-5p. Sci Rep. (2025) 15:17275. doi: 10.1038/s41598-025-01564-4
64.
Qian B Yang Y Tang N Wang J Sun P Yang N et al . M1 macrophage-derived exosomes impair beta cell insulin secretion via miR-212-5p by targeting SIRT2 and inhibiting Akt/GSK-3β/β-catenin pathway in mice. Diabetologia. (2021) 64:2037–51. doi: 10.1007/s00125-021-05489-1
65.
Pang W Yao W Dai X Zhang A Hou L Wang L et al . Pancreatic cancer-derived exosomal microRNA-19a induces β-cell dysfunction by targeting ADCY1 and EPAC2. Int J Biol Sci. (2021) 17:3622–33. doi: 10.7150/ijbs.56271
66.
Mahmoudi-Aznaveh A Tavoosidana G Najmabadi H Azizi Z Ardestani A . The liver-derived exosomes stimulate insulin gene expression in pancreatic beta cells under condition of insulin resistance. Front Endocrinol. (2023) 14:1303930. doi: 10.3389/fendo.2023.1303930
67.
Zeng XY Liu ZW Li HZ Liu JY . Muscle-derived stem cell exosomes enhance autophagy through the regulation of the mTOR signaling pathway to attenuate glucolipotoxicity-induced pancreatic β-cell injury. Curr Stem Cell Res Ther. (2025) 20:584–91. doi: 10.2174/011574888X288930240523045700
68.
Qi C Mao X Zhang Z Wu H . Classification and differential diagnosis of diabetic nephropathy. J Diabetes Res. (2017) 2017:8637138. doi: 10.1155/2017/8637138
69.
Ebrahim N Ahmed IA Hussien NI Dessouky AA Farid AS Elshazly AM et al . Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells. (2018) 7:226. doi: 10.20944/preprints201809.0153.v1
70.
Lv J Hao YN Wang XP Lu WH Xie LY Niu D . Bone marrow mesenchymal stem cell-derived exosomal miR-30e-5p ameliorates high-glucose induced renal proximal tubular cell pyroptosis by inhibiting ELAVL1. Ren Fail. (2023) 45:2177082. doi: 10.1080/0886022X.2023.2177082
71.
Liu L Zhou Y Zhao X Yang X Wan X An Z et al . Bone marrow mesenchymal stem cell-derived exosomes alleviate diabetic kidney disease in rats by inhibiting apoptosis and inflammation. Front Biosci. (2023) 28:203. doi: 10.31083/j.fbl2809203
72.
Ning Y Zhou X Wang G Zhang L Wang J . Exosome miR-30a-5p regulates glomerular endothelial cells' EndMT and angiogenesis by modulating Notch1/VEGF signaling pathway. Curr Gene Ther. (2024) 24:159–77. doi: 10.2174/0115665232258527230919071328
73.
Jin J Shi Y Gong J Zhao L Li Y He Q et al . Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. (2019) 10:95. doi: 10.1186/s13287-019-1177-1
74.
Wang X Huang S Li X Cheng H . The transfer of USP25 by exosomes of adipose tissue-derived mesenchymal stem cells ameliorates diabetic nephropathy through stabilizing SMAD7 expression. Chem Biol Drug Des. (2025) 105:e70118. doi: 10.1111/cbdd.70118
75.
Wang J Tao Y Zhao F Liu T Shen X Zhou L . Expression of urinary exosomal miRNA-615-3p and miRNA-3147 in diabetic kidney disease and their association with inflammation and fibrosis. Ren Fail. (2023) 45:2121929. doi: 10.1080/0886022X.2022.2121929
76.
Zhu J Hu Z Luo Y Liu Y Luo W Du X et al . Diabetic peripheral neuropathy: pathogenetic mechanisms and treatment. Front Endocrinol. (2023) 14:1265372. doi: 10.3389/fendo.2023.1265372
77.
Fan B Li C Szalad A Wang L Pan W Zhang R et al . Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia. (2020) 63:431–43. doi: 10.1007/s00125-019-05043-0
78.
Wang YY Li K Wang JJ Hua W Liu Q Sun YL et al . Bone marrow-derived mesenchymal stem cell-derived exosome-loaded miR-129-5p targets high-mobility group box 1 attenuates neurological-impairment after diabetic cerebral hemorrhage. World J Diabetes. (2024) 15:1979–2001. doi: 10.4239/wjd.v15.i9.1978
79.
Nakano M Nagaishi K Konari N Saito Y Chikenji T Mizue Y et al . Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep. (2016) 6:24805. doi: 10.1038/srep24805
80.
Yang S Yuan Y Zhang B Wu T Yu C Li F et al . Identification of adipose tissue-derived exosomal microRNA as a novel causal biomarker for cognitive impairment in type 2 diabetes mellitus: triangulating evidence from Mendelian randomization and multicentre population studies. Diabetes Obes Metab. (2025) 27:1265–75. doi: 10.1111/dom.16121
81.
Martiniakova M Biro R Penzes N Sarocka A Kovacova V Mondockova V et al . Links among obesity, type 2 diabetes mellitus, and osteoporosis: bone as a target. Int J Mol Sci. (2024) 25:4827. doi: 10.3390/ijms25094827
82.
Byun JS Lee HY Tian J Moon JS Choi J Lee SH et al . Effect of salivary exosomal miR-25-3p on periodontitis with insulin resistance, Front Immunol. (2021) 12:775046. doi: 10.3389/fimmu.2021.775046
83.
Wang Y Qu F Wu Y Lan K Shen Y Wu Z et al . Peripheral nerves modulate the peri-implant osteogenesis under type 2 diabetes through exosomes derived from Schwann cells via miR-15b-5p/Txnip signaling axis. J Nanobiotechnol. (2025) 23:51. doi: 10.1186/s12951-025-03160-0
84.
Li Z Zhang B Shang J Wang Y Jia L She X et al . Diabetic and nondiabetic BMSC-derived exosomes affect bone regeneration via regulating miR-17-5p/SMAD7 axis. Int Immunopharmacol. (2023) 125:111190. doi: 10.1016/j.intimp.2023.111190
85.
Wang L Yang H Zhang C Zhang Y He Y Liu Y et al . A blood glucose fluctuation-responsive delivery system promotes bone regeneration and the repair function of Smpd3-reprogrammed BMSC-derived exosomes. Int J Oral Sci. (2024) 16:65. doi: 10.1038/s41368-024-00328-6
86.
Lin CM Wang BW Pan CM Fang WJ Chua SK Shyu KG . Catalpol ameliorates hyperglycemia-stimulated resistin through macrophage-derived exosomal miRNA-150-5p in diabetic neointimal hyperplasia: micro- and macro-environmental communication. Eur J Pharmacol. (2025) 1002:177848. doi: 10.1016/j.ejphar.2025.177848
87.
Govindappa PK Patil M Garikipati V Verma SK Saheera S Narasimhan G et al . Targeting exosome-associated human antigen R attenuates fibrosis and inflammation in diabetic heart. FASEB J. (2020) 34:2238–51. doi: 10.1096/fj.201901995R
88.
Pérez-Macedonio CP Flores-Alfaro E Alarcón-Romero L Vences-Velázquez A Castro-Alarcón N Martínez-Martínez E et al . CD14 and CD26 from serum exosomes are associated with type 2 diabetes, exosomal Cystatin C and CD14 are associated with metabolic syndrome and atherogenic index of plasma. PeerJ. (2022) 10:e13656. doi: 10.7717/peerj.13656
89.
Li W He S Lin C Yang S Zhang W . Mesenchymal stem cell-derived exosomes carry miR-125a-5p to improve diabetic keratopathy by regulating endoplasmic reticulum stress. Tissue Cell. (2025) 93:102669. doi: 10.1016/j.tice.2024.102669
90.
He W Lin A Wang C . Adipocyte-derived exosomal LINC00968 promotes mouse retina microvascular endothelial cell dysfunction in a high-glucose environment by modulating the miR-361-5p/TRAF3 axis. Horm Metab Res. (2023) 55:124–35. doi: 10.1055/a-1939-7355
91.
Fu S Sun W Liu L Xiao J Xiong J Hu Y et al . Müller cells harboring exosomal lncRNA OGRU modulate microglia polarization in diabetic retinopathy by serving as miRNA sponges. Diabetes. (2024) 73:1919–34. doi: 10.2337/db23-1015
92.
Sun C Wen X Chu X Yuan F Chen Y Peng C et al . Adipocyte exosome miR-4472 inhibits glucose uptake in skeletal muscle through downregulation of MEF2D. J Diabetes Investig. (2025) 16:1382–97. doi: 10.1111/jdi.70054
93.
Sundaram K Teng Y Mu J Xu Q Xu F Sriwastva MK et al . Outer membrane vesicles released from garlic exosome-like nanoparticles (GaELNs) train gut bacteria that reverses type 2 diabetes via the gut-brain axis. Small. (2024) 20:e2308680. doi: 10.1002/smll.202308680
94.
Sun H Lu S Qu G Li J Song B . Mesenchymal stem cells-derived exosomes ameliorate high glucose and lipopolysaccharide-induced HPMECs injury through the Nrf2/HO-1 pathway. Autoimmunity. (2023) 56:2290357. doi: 10.1080/08916934.2023.2290357
95.
Hong P Yu M Tian W . Diverse RNAs in adipose-derived extracellular vesicles and their therapeutic potential. Mol Ther Nucleic Acids. (2021) 26:665–77. doi: 10.1016/j.omtn.2021.08.028
96.
Pan S Chen Y Yan J Li F Chen X Xu X et al . The emerging roles and mechanisms of exosomal non-coding RNAs in the mutual regulation between adipose tissue and other related tissues in obesity and metabolic diseases. Front Endocrinol. (2022) 13:975334. doi: 10.3389/fendo.2022.975334
97.
He X Kuang G Wu Y Ou C . Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med. (2021) 11:e468. doi: 10.1002/ctm2.468
98.
Preethi KA Selvakumar SC Sekar D . Diagnostic and therapeutic application of exosomal microRNAs inducing inflammation in type 2 diabetes mellitus. Crit Rev Immunol. (2022) 42:1–11. doi: 10.1615/CritRevImmunol.2022044927
99.
Xiong Y Chen L Liu P Yu T Lin C Yan C et al . All-in-one: multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small. (2022) 18:e2104229. doi: 10.1002/smll.202104229
100.
Zhang M Guo J Xiang K Chen J Wang C Jiang T et al . Exosomes derived from oral squamous cell carcinoma tissue accelerate diabetic wound healing. Am J Physiol Cell Physiol. (2023) 324:C1307-1307C1319. doi: 10.1152/ajpcell.00541.2022
101.
Nie Y Sato Y Garner RT Kargl C Wang C Kuang S et al . Skeletal muscle-derived exosomes regulate endothelial cell functions via reactive oxygen species-activated nuclear factor-κB signaling. Exp Physiol. (2019) 104:1262–73. doi: 10.1113/EP087396
102.
Li L Zuo H Huang X Shen T Tang W Zhang X et al . Bone marrow macrophage-derived exosomal miR-143-5p contributes to insulin resistance in hepatocytes by repressing MKP5. Cell Prolif . (2021) 54:e13140. doi: 10.1111/cpr.13140
103.
Yao Q Wang R Wang H Yuan D Yuan C . Total saponins from Panax japonicus alleviate insulin resistance via exosomal miR204/Elovl6-mediated adipocyte-macrophage crosstalk. Phytomedicine. (2025) 142:156748. doi: 10.1016/j.phymed.2025.156748
104.
Venkat P Chopp M Chen J . Cell-based and exosome therapy in diabetic stroke. Stem Cells Transl Med. (2018) 7:451–5. doi: 10.1002/sctm.18-0014
105.
Chen X An H Du Y Zhong H Zhang F Zeng X et al . hucMSC-derived exosomes targeting macrophage polarization attenuate systemic inflammation in T1DM via INS/SOD1 delivery. Stem Cell Res Ther. (2025) 16:384. doi: 10.1186/s13287-025-04521-0
Summary
Keywords
biomarkers, complications, exosomes, therapeutic effect, type 2 diabetes mellitus
Citation
Liu Y, Wang J, Sun Y and Chen Y (2025) The therapeutic effect of exosomes in type 2 diabetes mellitus and its complications. Front. Med. 12:1735392. doi: 10.3389/fmed.2025.1735392
Received
30 October 2025
Accepted
02 December 2025
Published
19 December 2025
Volume
12 - 2025
Edited by
Adegbenro Omotuyi John Fakoya, Louisiana State University Health Shreveport, United States
Reviewed by
Armel Hervé Nwabo Kamdje, University of Garoua, Cameroon
Edileia Bagatin, Universidade Federal de São Paulo, Brazil
Grażyna Nowicka, Medical University of Warsaw, Poland
Updates
Copyright
© 2025 Liu, Wang, Sun and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Chen, Jdeylyy@jlu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.