Abstract
Osteoarthritis (OA), a chronic degenerative joint disease, arises from a confluence of factors including aging, mechanical injury, and obesity. Autophagy, a fundamental cellular process involving the degradation and recycling of cellular components, plays a critical role in chondrocyte homeostasis and survival under stress. Non-coding RNAs (ncRNAs), a diverse class of RNA molecules with no protein-coding potential, exert significant influence on gene expression through post-transcriptional and epigenetic mechanisms. Growing evidence suggests a crucial interplay between ncRNAs, autophagy, and OA pathogenesis. This review summarizes the multifaceted role of autophagy in OA chondrocytes and delves into the regulatory mechanisms of ncRNAs on OA-associated autophagy, aiming to elucidate the intricate pathological network underlying OA development and identify novel therapeutic targets.
1 Introduction
Osteoarthritis (OA), a chronic degenerative joint disease, is characterized by progressive cartilage loss, synovial inflammation, osteophyte formation, and subchondral bone sclerosis (1, 2). Primarily affecting knees, hips, hands, and spine, OA is a global health burden, affecting over 500 million individuals worldwide and ranking as the fourth leading cause of disability (3). A global survey projected that by 2021, over 22% of adults over 40 would be afflicted with knee OA. OA pathogenesis is multifactorial, encompassing aging, obesity, mechanical injury, and chronic inflammation (4). Cartilage homeostasis, maintained by chondrocytes, is crucial for joint health. Aging significantly impacts this homeostasis through mechanisms such as cellular senescence, leading to SASP production, mitochondrial dysfunction, and increased inflammation (5). These age-related changes, alongside obesity, sex, and joint trauma, contribute to the disruption of cartilage homeostasis and the development of OA (6). Currently, effective treatments for OA are limited, and end-stage disease often necessitates joint replacement surgery. However, artificial joints have a finite lifespan and carry inherent risks, resulting in substantial medical and healthcare costs (7, 8).
Regardless of the initial etiology of OA, epigenetic alterations, including changes in gene expression and transcription factor activity, significantly impact the expression of genes crucial for cartilage homeostasis (9). Non-coding RNAs (ncRNAs), such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), are a diverse class of RNA molecules that regulate gene expression at the post-transcriptional and epigenetic levels (10). These ncRNAs have emerged as key players in various biological processes, including cell proliferation, differentiation, apoptosis, and autophagy. Growing evidence suggests that dysregulation of ncRNA expression profiles contributes significantly to chondrocyte senescence, hypertrophy, apoptosis, and extracellular matrix (ECM) degradation, ultimately driving OA pathogenesis (11). Consequently, ncRNAs hold considerable promise as diagnostic biomarkers and therapeutic targets for OA (12, 13).
Autophagy, an essential cellular process for degrading and recycling intracellular components, plays a crucial role in maintaining cellular homeostasis under diverse stress conditions, including nutrient deprivation, oxidative stress, infection, and hypoxia (14). Age-related decline in autophagic activity, characterized by decreased levels of autophagy markers such as LC3, Beclin1, and ULK1, contributes to the accumulation of cellular debris and impaired cellular function in various tissues, including heart, kidney, bone, cartilage, and skeletal muscle (15). In OA, a chronic degenerative joint disease, autophagy dysfunction plays a critical role. Aging, a major risk factor for OA, is associated with impaired autophagy. Specifically, mTOR, a key negative regulator of autophagy, is upregulated in OA cartilage, inhibiting autophagic flux and exacerbating cartilage degeneration (16–18). Furthermore, studies in mice with ATG5 deficiency have demonstrated that autophagy plays a protective role in cartilage by mitigating OA progression, including fibrosis and proteoglycan loss (19). While these findings highlight the significance of autophagy in OA pathogenesis, the precise molecular mechanisms underlying its role remain to be fully elucidated.
Numerous studies have established a strong association between ncRNAs and autophagy (20). Recognizing the pivotal roles of both ncRNAs and autophagy in OA, this review comprehensively examines the intricate role of autophagy in OA pathogenesis (21). Furthermore, we delve into the regulatory influence of ncRNAs, particularly miRNAs, lncRNAs, and circRNAs, on autophagy. By elucidating the intricate interplay within the ncRNA-autophagy axis, we aim to gain deeper insights into the mechanisms that govern cartilage homeostasis and contribute to the development or inhibition of OA, ultimately paving the way for novel diagnostic and therapeutic strategies.
2 OA pathogenesis
While traditionally viewed as primarily a disease of articular cartilage, OA is now recognized as a multifaceted condition affecting all joint structures, including cartilage, subchondral bone, synovium, and other joint capsule tissues (Figure 1).
Figure 1

Structural features of a healthy knee joint and pathological alterations in osteoarthritis (OA). A normal knee joint is formed by two articulating bones covered with smooth articular cartilage and enclosed by a capsule lined with synovial tissue. Aging, obesity, and mechanical stress are major contributors to OA onset. These factors trigger elevated production of pro-inflammatory mediators such as IL-1β, IL-6, TNF-α, and NF-κB within cartilage and synovium, suppressing anabolic markers (COL2, ACAN, TGF-β) while enhancing catabolic enzymes (ADAMTS, MMPs) and inflammatory molecules (COX-2, PGE-2). The resulting imbalance leads to progressive cartilage erosion, synovial inflammation, osteophyte formation, and joint degeneration. This figure was generated and edited using Adobe Illustrator (Adobe Inc., United States).
2.1 Cartilage and chondrocytes
Articular cartilage, composed primarily of water and extracellular matrix (ECM), is synthesized exclusively by chondrocytes through the secretion of collagen type II, aggrecan, and other proteoglycans (22).
In healthy cartilage, chondrocytes exhibit limited mitotic activity, maintaining minimal ECM synthesis (23). This low turnover rate is maintained by the pericellular matrix, which acts as a protective barrier, isolating chondrocytes from the interterritorial matrix and limiting their interaction with ECM components (24). However, in OA, chondrocytes undergo a phenotypic shift. Their repair capacity diminishes, and the delicate balance between matrix synthesis and degradation tilts toward catabolism (25). This is characterized by downregulation of matrix components, including proteoglycans and collagen type II, and upregulation of matrix-degrading enzymes such as MMP13 (26).
In the early stages of OA, elevated matrix synthesis in chondrocytes indicates unsuccessful attempts at repair (27). Damage to the pericellular matrix exposes chondrocytes to surface receptors, including integrins and discoidin structural domain receptors in the interdomain matrix, ultimately resulting in chondrocyte dysfunction (28). Initially, chondrocytes undergo hypertrophic activation, leading to the production of various inflammatory mediators. The actions of these pro-inflammatory factors are crucial in the early stages of OA (29). Interleukin-1 β (IL-1β) is a key inflammatory mediator in OA, driving autocrine secretion and the production of other inflammatory cytokines, such as IL-6 and tumor necrosis factor-α (TNF-α) (30). The interaction of TNF-α with its receptor, in synergy with IL-1β, activates nuclear factor kappa-B (NF-κB) signaling, promoting the expression of adhesion molecules and further increasing the production of pro-inflammatory mediators (31). As OA progresses, there is a heightened inflammatory response and ongoing secretion of degradative enzymes like ADAMTS5, MMP3, and MMP13, leading to the depletion of proteoglycans and destruction of the collagen network, which contributes to the irreversible nature of OA (32). Chondrocyte apoptosis is a defining event in the final stages of cartilage destruction, exacerbating the catabolism of the cartilage matrix (33). The detachment of cartilage fragments creates a gap between the bone and calcified cartilage, and these free fragments perpetuate the inflammatory cycle, ultimately resulting in lesions in the subchondral bone and synovial tissues (34). This leads to osteosclerosis, thickening of the synovial membrane, and reduced synthesis of synovial molecules, including lubricin and hyaluronic acid.
2.2 Subchondral bone
Healthy subchondral bone is composed of two main structures: the subchondral plate and subchondral trabeculae. The subchondral plate is a thin cortical layer situated beneath the calcified cartilage, with a porous region beneath it. The subchondral trabeculae lie beneath the plate and play a crucial role in providing structural support, absorbing mechanical stress, and facilitating the supply of nutrients (35, 36).
Subchondral bone remodeling, characterized by an imbalance between bone formation and resorption driven by aberrant osteoblast–osteoclast coupling, is a hallmark of OA (37). The subchondral bone microarchitecture undergoes significant changes throughout OA progression. Early-stage OA is characterized by increased bone turnover, with elevated bone resorption leading to decreased bone mass (38). This manifests as thinning and increased porosity of the subchondral bone plate, along with reduced trabecular number and thickness. In contrast, advanced OA is characterized by a shift toward increased bone formation, resulting in subchondral bone sclerosis (39). This is evidenced by thickening of the subchondral plate, increased trabecular number and thickness, and decreased trabecular spacing (40).
Despite increased subchondral bone volume, alterations in bone composition, such as an elevated carbonate apatite-to-hydroxyapatite ratio and decreased collagen content, compromise bone mineralization and reduce its elastic modulus (41). This weakens the subchondral bone, diminishing its ability to cushion stress, thereby subjecting it to increased mechanical loading. This vicious cycle of increased stress and bone remodeling exacerbates the progression of OA (42). Concomitant with these changes, fibrovascular invasion through micro-fissures in the subchondral bone disrupts the bone-cartilage interface, facilitating abnormal communication between these tissues (43). This aberrant crosstalk accelerates cartilage degeneration, leading to upward displacement of the tidemark and further aggravating subchondral bone loading and remodeling.
2.3 Synovial tissue
The synovium, a specialized connective tissue lining the joint cavity, comprises two layers: an intimal layer containing fibroblast-like and macrophage-like synoviocytes, and a subintimal layer consisting of fibrous connective tissue, blood vessels, and a limited number of immune cells (44). This critical structure plays a vital role in maintaining joint homeostasis. Fibroblast-like synoviocytes are responsible for producing essential components of synovial fluid, including hyaluronic acid and lubricin, which nourish the avascular articular cartilage and facilitate smooth joint movement. Macrophage-like synoviocytes contribute to joint health by clearing debris and combating infections within the synovial fluid (45).
OA synovium exhibits characteristic pathological features, including hyperplasia, fibrosis, and increased vascularity. This inflammatory environment is characterized by significant infiltration of immune cells, such as T cells, B cells, and macrophages, contributing to the formation of a dense inflammatory mass that further degrades articular cartilage and subchondral bone (46). Synovial hyperplasia, driven by excessive production of pro-inflammatory cytokines like TNF-α and IL-1, is characterized by increased fibroblast-like synoviocyte proliferation and inhibited apoptosis. Furthermore, these cells contribute to inflammation by phagocytosing cartilage wear particles and releasing inflammatory mediators such as IL-6, nitric oxide, and prostaglandin E2 (47). Pathological vascular proliferation within the synovium exacerbates inflammation. Macrophage-like cells and fibroblasts contribute to this process by producing vascular endothelial growth factor (VEGF), which increases endothelial permeability and promotes plasma extravasation (48).
3 The definition and process of autophagy
3.1 Definition of autophagy
The term “autophagy” is derived from the Greek words “auto,” meaning self, and “phagy,” meaning to eat or consume (49). Autophagy is a fundamental process in eukaryotic evolution in which a portion of the cytoplasm is enclosed by a double-membrane vesicle known as the autophagosome, which is then transported to the lysosome for degradation (50). Initially, autophagy was viewed primarily as a cellular waste disposal mechanism, but over time, it became clear that constitutive autophagy plays a critical role in eliminating damaged organelles, misfolded or long-lived proteins, and other cellular debris, while also maintaining basal energy homeostasis (51). In contrast, adaptive autophagy mobilizes intracellular components to meet energy demands during various stress conditions such as nutrient deprivation, hypoxia, and other forms of cellular stress (52). This adaptive form of autophagy may have evolved from an ancient survival mechanism in primitive unicellular organisms, enabling them to provide nutrients during energy shortages (53). Overall, autophagy is a key metabolic process that regulates the energy balance within organisms.
3.2 Process of autophagy
As a cellular response to stress, autophagy proceeds through distinct stages: initiation, nucleation, elongation, fusion, and degradation of cytoplasmic components within autophagosomes (Figure 2).
Figure 2
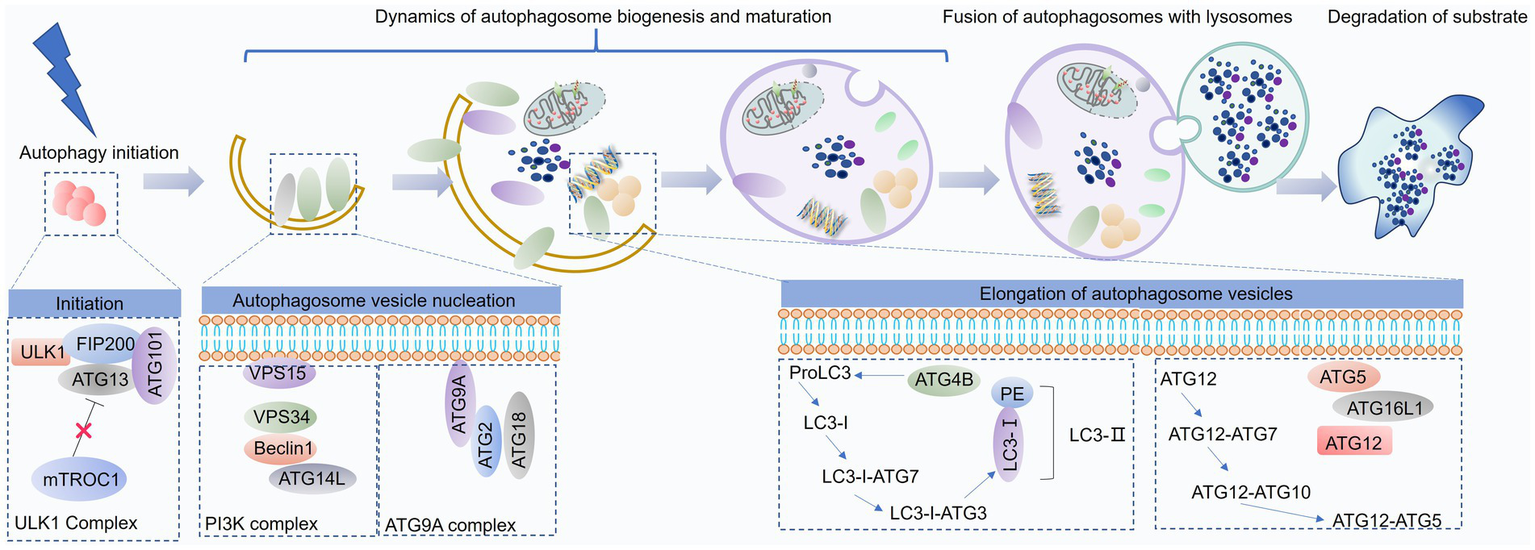
Overview of the autophagy process. Autophagy is a dynamic catabolic pathway comprising several sequential stages: initiation, formation of a double-membrane isolation vesicle, elongation and maturation of the autophagosome, its fusion with lysosomes, and subsequent degradation of the enclosed components. This figure was generated and edited using Adobe Illustrator (Adobe Inc., United States).
The ULK1 complex, comprising ULK1, ATG13, FIP200, and ATG101, recruits the Beclin1-Vps34 complex (consisting of Beclin1, Vps34, Vps15, and ATG14L) to the sites of autophagosome formation. This recruitment is facilitated by Ambra1, which, upon phosphorylation by ULK1, binds to TRAF6, acting as an E3 ubiquitin ligase to stabilize ULK1 through K63-linked ubiquitination (54). Furthermore, ULK1 directly phosphorylates Beclin1, enhancing the activity of the Vps34 complex (55). The combined activity of these complexes leads to the accumulation of phosphatidylinositol 3-phosphate (PI3P) at the nascent autophagosome membrane. This PI3P enrichment subsequently recruits key autophagy proteins, such as WD-repeat domain phosphoinositide-interacting protein (WIPI) and DFCP1, driving autophagosome formation and expansion (56).
Autophagosome elongation and maturation are facilitated by two ubiquitin-like conjugation systems: the ATG12-ATG5-ATG16L complex and the LC3-phosphatidylethanolamine (PE) conjugation system (57). The ATG12-ATG5-ATG16L complex forms through the conjugation of ATG12 to ATG5, catalyzed by ATG7 and ATG10. This complex then promotes the lipidation of LC3, a key step in autophagosome membrane expansion (58). LC3 undergoes proteolytic cleavage by ATG4 to generate LC3-I, which is subsequently conjugated to PE by the concerted action of ATG7, ATG3, and the ATG12-ATG5-ATG16L complex. This lipidated form of LC3, LC3-II, associates with the autophagosome membrane, driving its maturation (59).
Following their formation, autophagosomes undergo microtubule-dependent trafficking toward lysosomes, where they subsequently fuse. This fusion process, orchestrated by the Rab-SNARE protein machinery and other autophagy-related proteins, facilitates the delivery of autophagosomal cargo to the lysosomal lumen for degradation by lysosomal hydrolases (60).
4 Autophagy in OA
4.1 Autophagy in cartilage and chondrocyte
Chondrocyte function is critical for maintaining cartilage integrity as they synthesize and remodel the ECM (61). However, chondrocytes are susceptible to various stressors, including aging and mechanical injury, leading to inflammation, oxidative stress, and ultimately, disruptions in cartilage homeostasis and the development of OA. Autophagy, a fundamental cellular process for maintaining homeostasis, plays a crucial role in regulating chondrocyte survival and function (Figure 3) (62). While essential for cellular health, autophagy can also have detrimental effects. Excessive autophagy has been implicated in chondrocyte damage and death (63). This highlights the dual role of autophagy in chondrocyte function, where a delicate balance is crucial for maintaining cartilage health. In the following sections, we will delve deeper into the multifaceted role of autophagy in chondrocytes and its implications for OA pathogenesis (64).
Figure 3
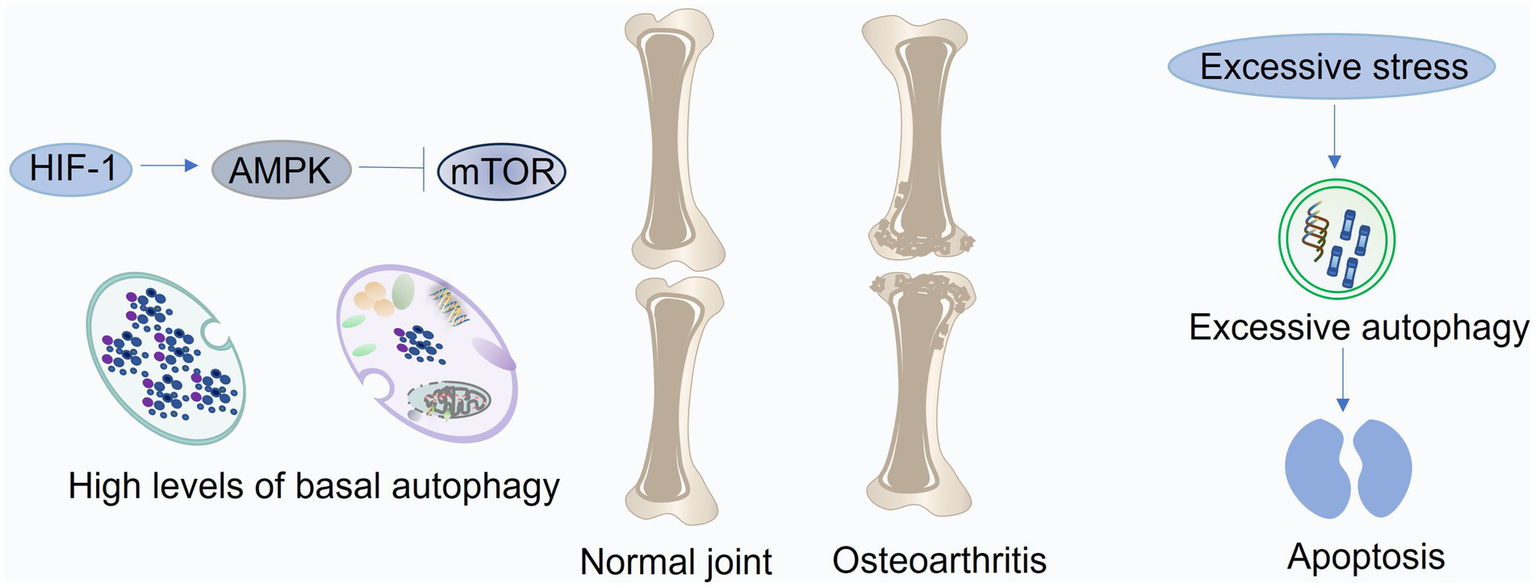
Roles of autophagy in cartilage and chondrocytes. In healthy articular cartilage, autophagy is primarily regulated through the HIF-1/AMPK/mTOR pathway to maintain chondrocyte homeostasis under hypoxic conditions. During the early phase of osteoarthritis (OA), autophagy is transiently enhanced in response to oxidative stress triggered by aging or mechanical injury. As OA progresses, persistent oxidative and inflammatory stress surpasses the compensatory capacity of autophagy, leading to its suppression and resulting in disrupted balance between cartilage anabolism and catabolism. Moreover, excessive autophagy induced by prolonged stress can aggravate chondrocyte apoptosis and cartilage degeneration. This figure was generated and edited using Adobe Illustrator (Adobe Inc., United States).
4.1.1 Protective autophagy in cartilage and chondrocyte
4.1.1.1 Autophagy and hypoxia
Articular cartilage, an avascular tissue, relies on diffusion for nutrient and oxygen supply from the subchondral bone and synovial fluid (65). This results in a steep oxygen gradient within the cartilage, with oxygen concentrations ranging from approximately 6% at the surface to 1% in the deeper layers. Chondrocytes, adapted to this hypoxic environment, exhibit a strong dependence on hypoxia-inducible factors (HIFs) for their survival and function. HIF-1, expressed in both normal and OA cartilage, promotes chondrocyte phenotype and viability, stimulates matrix synthesis, and supports metabolic adaptation to hypoxia (66). In contrast, HIF-2, predominantly expressed in highly differentiated chondrocytes, drives catabolic processes, including the upregulation of MMPs, ADAMTSs, nitric oxide synthase-2, and prostaglandin-endoperoxide synthase-2, ultimately contributing to cartilage degradation (67). Furthermore, chondrocytes in the hypoxic cartilage environment exhibit increased basal autophagy, which is essential for maintaining cellular homeostasis and promoting collagen synthesis. HIF-1 positively regulates autophagy, while HIF-2 exerts an inhibitory effect (68). This interplay between HIFs and autophagy highlights their crucial roles in chondrocyte function and cartilage health. Conversely, HIF-1 silencing impairs cellular autophagy, leading to MMP13 accumulation and mitochondrial damage (69). The HIF-1-AMPK-mTOR axis serves as a key regulator of autophagy in chondrocytes under hypoxic conditions. Hypoxia stimulates HIF-1 activity, which in turn enhances glycolysis and activates AMPK, leading to subsequent mTOR inhibition and autophagy initiation. This coordinated response ensures chondrocyte viability and facilitates cell cycle progression within the hypoxic environment (70). Interestingly, HIF-2 knockdown elicits a pronounced increase in autophagy in chondrocytes of mouse growth plates. This elevated autophagy is associated with decreased activity of the Akt–mTOR pathway and reduced Bcl-2 expression (71).
4.1.1.2 Autophagy and aging
Autophagy plays a crucial role in chondrocyte differentiation, promoting growth plate maturation and hypertrophy. While robust autophagy is observed in the superficial zone of healthy adult cartilage, its activity declines in aged cartilage (72). Interestingly, autophagy may initially serve as a protective mechanism against early degenerative changes in OA, as evidenced by transient increases in ULK1, Beclin1, and LC3 expression. However, as OA progresses, autophagy activity diminishes, particularly in the superficial zone (73). In advanced stages, autophagy defects become evident across the entire cartilage layer, ultimately contributing to chondrocyte death (74). These findings suggest an age-dependent decline in autophagic activity, paralleling chondrocyte degeneration and preceding cartilage damage (75). Indeed, ATG5-deficient mice exhibit premature chondrocyte senescence and accelerated OA development, potentially linked to cysteoaspartase-dependent apoptosis. In young GFP-LC3 transgenic mice, LC3 and ATG5 expression was most prominent in superficial and middle zone chondrocytes (76). Conversely, senile mice exhibited a significant reduction in autophagosome numbers within chondrocytes, alongside a marked decrease in vesicle abundance in the subchondral bone. Concomitantly, impaired autophagy exacerbated OA-like changes in the synovium (77). This evidence suggests that autophagy defects contribute to early senescent changes in cartilage, ultimately leading to aberrant matrix synthesis, catabolic gene expression, and ECM breakdown. Furthermore, a recent study reported that fibroblast-like synoviocyte senescence, characterized by impaired autophagy and upregulated SASP factors, exacerbates OA progression (78). Notably, restoring autophagy in these senescent cells reversed the senescence phenotype, suppressed SASP secretion, and mitigated cartilage destruction by inhibiting GATA4 (79).
4.1.1.3 Autophagy and mechanical injury
Acute or prolonged abnormal mechanical loading compromises chondrocyte regenerative capacity, contributing to the development of degenerative lesions and the onset of OA (80). Mechanical stress triggers the activation of multiple inflammatory and oxidative stress pathways, driving joint inflammation through the upregulation of cartilage-degrading enzymes, ultimately leading to chondrocyte apoptosis, ECM degradation, and aberrant subchondral bone remodeling (81). Experimental studies have demonstrated that mechanical injury inhibits autophagy. For instance, 500 ms of mechanical shock induced chondrocyte apoptosis and sulfated glycosaminoglycan loss in a time-dependent manner, accompanied by a significant decrease in ULK1, Beclin1, and LC3 expression in the superficial cartilage at 48 h post-injury (82). Notably, exogenous autophagy activation mitigated the chondrocyte damage induced by mechanical injury (83).
Conversely, appropriate mechanical loading exerts beneficial effects on chondrocytes, alleviating senescence and inflammation, regulating autophagy, and promoting cartilage repair (84). In a rat model of DMM, treadmill exercise inhibited excessive autophagy by activating the PI3K-Akt–mTOR pathway, thereby mitigating cartilage inflammation and degeneration (85). Furthermore, in another mouse OA model, appropriate mechanical loading enhanced autophagy levels in cartilage, as evidenced by increased LC3 expression and decreased p62 levels, ultimately promoting chondrocyte regeneration and facilitating cartilage repair (86).
4.1.1.4 Autophagy and inflammation
In OA, chondrocytes encounter a milieu of stressors, including mechanical injury, cellular senescence, and exposure to inflammatory cytokines and degradation products (87). These stressors activate various signaling pathways, leading to aberrant gene expression, including increased production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. These cytokines, in turn, stimulate the expression of MMPs and ADAMTSs, driving cartilage matrix degradation (88). Chondrocytes themselves contribute to the inflammatory milieu by expressing receptors for various pro-inflammatory factors and chemokines, acting as both sources and targets of inflammatory mediators within the joint (89). Furthermore, inflammation within the synovium and subchondral bone significantly contributes to cartilage degeneration in OA (90). These inflammatory factors have been shown to exacerbate OA by inhibiting autophagy and promoting chondrocyte senescence (91). IL-1β-induced inflammation in chondrocytes significantly activates the PI3K-Akt–mTOR signaling pathway, inhibiting autophagy and promoting cell cycle arrest, characterized by G1 phase blockade and reduced S phase progression (92). Conversely, inhibiting the PI3K-Akt–mTOR pathway enhances autophagy and attenuates inflammation in chondrocytes from OA rats. Furthermore, PPARγ deficiency leads to increased expression of inflammatory markers, such as inducible nitric oxide synthase and cyclooxygenase-2, accompanied by elevated mTOR expression and suppressed autophagy. This exacerbates inflammation within the cartilage and drives OA progression (93). The mTOR pathway also plays a crucial role in regulating synovitis. Inhibition of mTOR activates autophagy, suppressing osteoclastogenesis and IL-1 expression in the synovium, thereby protecting cartilage from erosion (94). Additionally, the MAPK/NF-κB pathway contributes to the suppression of autophagy in OA. Recent studies have highlighted the involvement of epigenetic modifications in regulating autophagy and inflammation in OA (95). Specifically, methyltransferase-like 3 stabilizes Bcl-2 mRNA through the m6A/Yth m6A RNA-binding protein 1 axis, thereby inhibiting Beclin1-mediated autophagy and exacerbating chondrocyte apoptosis and subchondral bone degeneration in vivo (96).
4.1.1.5 Autophagy and oxidative stress
Oxidative stress arises from an imbalance between reactive oxygen species (ROS) production and antioxidant defenses within the mitochondria (95). In OA, this imbalance is exacerbated by a disruption in redox homeostasis, particularly involving superoxide dismutase 2 (97). In OA chondrocytes, stimuli such as mechanical injury and senescence activate cytokine receptors and toll-like receptors (TLRs), leading to increased mitochondrial ROS production and decreased expression of antioxidant enzymes, including superoxide dismutase 1 and 2 (98, 99). This oxidative environment promotes the modification of intracellular and extracellular components, inhibits collagen synthesis, and activates MMPs and ADAMTSs, driving cartilage degradation (100). Furthermore, degradation products stimulate the activation of NADPH oxidase and inducible nitric oxide synthase, creating a vicious cycle of inflammation and ROS production, further exacerbating joint degeneration (101).
Autophagy exhibits a biphasic response in OA. While elevated in the early stages, likely as a protective mechanism against oxidative stress, autophagy activity declines in the mid-late stages of the disease (102). The accumulation of p62, a marker of impaired autophagy, in OA chondrocytes exposed to oxidative stress promotes chondrocyte apoptosis. Alginate treatment effectively restores autophagic flux by targeting BNIP3, a key regulator of autophagy, thereby mitigating oxidative stress-induced damage (103). Furthermore, TGFβ1 pretreatment enhances LC3-II expression in H2O2-treated chondrocytes via forkhead box O1, mitigating oxidative stress-induced cell death (104). Additionally, glabridin, a natural antioxidant, inhibits mTOR signaling, thereby activating autophagy and protecting chondrocytes from oxidative damage (105).
4.1.2 Detrimental autophagy in cartilage and chondrocyte
These findings mentioned above suggest that autophagy plays a crucial but complex role in OA pathogenesis. While impaired autophagy contributes to OA progression in aging and disease models, restoring appropriate autophagic flux can mitigate inflammation, oxidative stress, and other pathological hallmarks of OA. However, excessive autophagy activation can also have detrimental effects, highlighting the need for a delicate balance in autophagy activity for optimal cartilage health (106).
Mitofusin 2, a key regulator of autophagy and inflammation, significantly disrupts autophagic homeostasis when overexpressed. This overexpression inhibits the PI3K/AKT/mTOR pathway, leading to excessive autophagy, increased ROS production, and subsequent inflammation and cartilage degeneration (107). While Lee et al. (108) observed increased autophagy and autophagic cell death in OA chondrocytes, characterized by elevated LC3 and Beclin-1 expression in the mid-upper zone, Caramés et al. (109) reported decreased expression of these autophagy markers in the superficial zone of OA cartilage, potentially due to chondrocyte loss in this region. However, prolonged exposure to acidic conditions (pH 6.0 for 48 h) activates Acid-sensitive ion channel 1a, inducing chondrocyte senescence through sustained autophagy activation (110). This highlights that excessive autophagy can have detrimental effects, contributing to cellular dysfunction. Oxidative DNA damage can also disrupt autophagic flux. In response to acute oxidative stress, healthy chondrocytes exhibit a transient increase in autophagy activity, followed by a return to basal levels within 24 h. In contrast, OA chondrocytes exhibit sustained elevated autophagy levels, likely due to mitochondrial dysfunction and increased ROS production (111). This persistent oxidative stress in OA chondrocytes leads to the formation of a pre-autophagic pool, impairing the ability of these cells to appropriately modulate autophagy in response to further oxidative stress. This dysregulated autophagy can ultimately drive chondrocyte death (112).
Autophagy plays a multifaceted role in OA, responding to a variety of stressors, including aging, hypoxia, mechanical loading, inflammation, and oxidative stress. While autophagy can initially serve as a protective mechanism against these stressors, mitigating cellular damage, excessive or prolonged activation can have detrimental consequences (113). For example, mitochondrial dysfunction induced by oxidative stress can lead to spatiotemporal dysregulation of autophagy, resulting in excessive autophagic flux and ultimately, chondrocyte death (114). Similarly, prolonged exposure to stress can overwhelm cellular compensatory mechanisms, leading to sustained high levels of autophagy and subsequent cell death. These findings underscore the complex and dynamic nature of autophagy in OA, highlighting the critical importance of maintaining a delicate balance between beneficial and detrimental autophagic activity (115). The precise impact of autophagy likely depends on the type, intensity, and duration of the stress stimulus. While research has implicated autophagy in OA pathogenesis, further investigation is needed to define the precise threshold between beneficial and detrimental autophagy in chondrocytes under various pathological conditions (116). Despite these limitations, autophagy holds significant promise as a therapeutic target for OA. However, therapeutic interventions should be carefully designed to modulate autophagy activity within a therapeutic window, avoiding both insufficient and excessive autophagy (117).
4.2 Autophagy in subchondral bone
Subchondral bone remodeling plays a critical role in OA pathogenesis. Early OA is characterized by increased bone resorption, leading to decreased bone volume. In contrast, late-stage OA is characterized by excessive bone formation and sclerosis. These alterations in bone metabolism highlight the potential of targeting key signaling molecules in osteoblasts and osteoclasts as therapeutic strategies for OA (118). Autophagy plays a crucial role in bone metabolism, influencing osteoblast activity, differentiation, and mineralization. Similarly, autophagy regulates osteoclast differentiation and maturation (119). The RANK/RANKL/OPG signaling pathway governs subchondral bone remodeling. In DMM-induced OA, decreased bone volume and mineral density are associated with increased RANKL expression and enhanced osteoclast formation. Notably, rapamycin-induced autophagy activation inhibits osteoclastogenesis through the RANK/RANKL/OPG axis, suggesting a potential therapeutic avenue for OA treatment (120). Early OA is characterized by alterations in subchondral bone structure, including changes in osteoblast phenotype and mineralization. Defective autophagy contributes to impaired osteoblast mineralization in early OA, while enhanced autophagy restores osteoblast function and alleviates subchondral bone thinning (121). In late-stage OA, 15-lipoxygenase-1, a pro-inflammatory lipid mediator, is upregulated in subchondral osteoblasts. This upregulation inhibits autophagy through mTORC1 activation, while simultaneously stimulating osteoblast activity, leading to increased expression of osteoblastic markers such as RUNX2, COL1, and OCN. This aberrant osteoblast activity contributes to abnormal bone remodeling and exacerbates OA (122). Furthermore, 15-lipoxygenase-1-mediated mTORC1 activation promotes AMPK phosphorylation, further suppressing autophagy and stimulating TGF-β1 expression, which enhances osteoblast proliferation and differentiation, ultimately contributing to abnormal ECM synthesis and mineralization within the subchondral bone. While these findings highlight the crucial role of autophagy in regulating subchondral bone remodeling in OA, further research is needed to fully elucidate the mechanisms underlying autophagy regulation of osteoblast and osteoclast function in this context (122).
4.3 Autophagy in synovium
Synovial inflammation plays a critical role in OA pathogenesis. With aging, fibroblast-like synoviocytes (FLS) undergo senescence, characterized by increased secretion of inflammatory cytokines, MMPs, and senescence-associated secretory proteins (SASPs), thereby contributing to cartilage destruction (123). Previous studies have demonstrated that FLS senescence in OA is closely associated with impaired autophagy. Restoring autophagic flux effectively mitigates FLS senescence and SASP secretion, suggesting a crucial role for autophagy in attenuating OA progression. Interestingly, Delco et al. (124) reported that excessive m6A RNA methylation, mediated by the methyltransferase METTL3, inhibits autophagy in OA-FLS by destabilizing ATG7 mRNA. Silencing METTL3 enhances autophagic flux, suppresses GATA4 expression, and attenuates FLS senescence, demonstrating a therapeutic potential for targeting m6A methylation in OA (125). While these findings highlight the importance of autophagy in regulating synovial inflammation, further research is needed to fully elucidate the underlying mechanisms and explore potential therapeutic strategies (126).
5 The mechanism of ncRNA-mediated autophagy in OA
Numerous studies have highlighted the intricate interplay between ncRNAs and autophagy in age-related diseases, including OA (127). These ncRNAs can be broadly classified based on their ability to either promote or inhibit autophagic flux. While the pivotal roles of both autophagy and ncRNAs in OA pathogenesis are well-established, the precise molecular mechanisms underlying their crosstalk remain largely unexplored (128). Further research is warranted to elucidate the intricate relationship between ncRNA dysregulation and autophagy deficiency in the context of OA.
5.1 MiRNAs and autophagy in OA
MiRNAs are small, single-stranded RNA molecules that regulate gene expression by binding to the 3′ untranslated regions (UTRs) of target mRNAs (129). This regulatory network is complex, with each miRNA capable of targeting multiple genes and multiple miRNAs potentially originating from a single mRNA transcript. MiRNAs exert profound influence on diverse cellular processes, including development, proliferation, differentiation, cell cycle regulation, and autophagy (130). Numerous studies have demonstrated the ability of miRNAs to directly regulate autophagy at various stages, including initiation, vesicle formation, and autophagosome-lysosome fusion. Furthermore, miRNAs can indirectly modulate autophagy by targeting upstream signaling pathways, such as the mTOR, sirtuin, and AMPK pathways (131). The following sections will discuss the role and mechanisms of miRNA-mediated autophagy regulation in OA (Figure 4; Table 1).
Figure 4
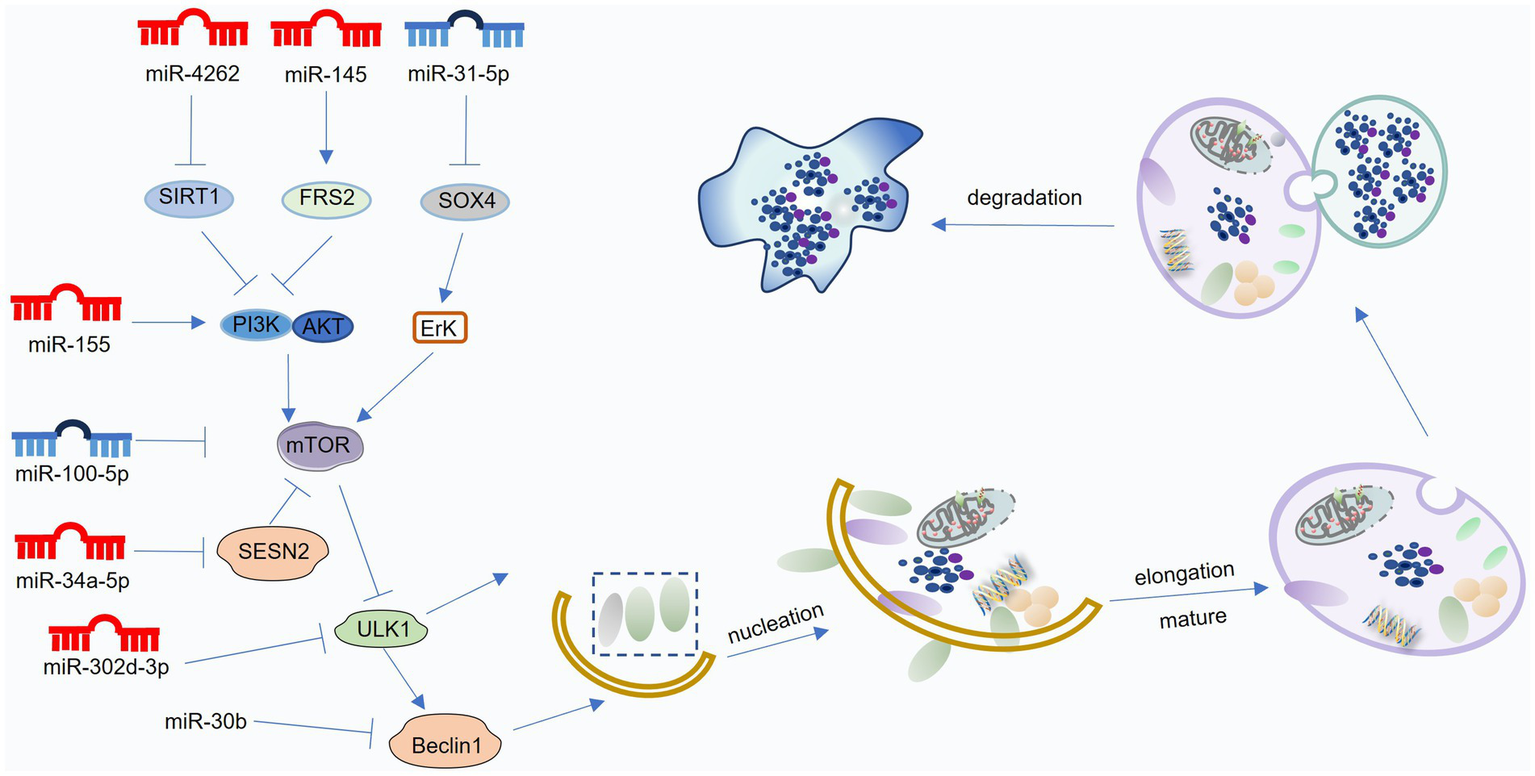
Mechanisms of miRNA-mediated regulation of autophagy in osteoarthritis (OA). MiRNAs modulate various stages of the autophagic process—including initiation, vesicle formation and elongation, autolysosome maturation, and degradation—by targeting key regulators such as mTOR, Beclin1, ATG proteins, and LC3. Blue miRNAs denote those that enhance autophagy and protect against OA progression, whereas red miRNAs indicate those that suppress autophagy and contribute to OA development. This figure was generated and edited using Adobe Illustrator (Adobe Inc., United States).
Table 1
| Model | MicroRNA expression | Targeting pathway | Effect on autophagy | Effect on cartilage or chondrocyte | Ref. |
|---|---|---|---|---|---|
| Human OA chondrocytes | miR-335–5p ↓ | mTOR ↓ | Promote | Inflammation ↓ cell viability ↑ |
(203) |
| Human OA/C20A4 cell | miR-145 ↓ | FRS2 PI3K-Akt–mTOR ↓ |
Promote | Promote | (204) |
| OA model IL-1β stimulated chondrocytes |
miR-31–5p ↓ | SOX4 ↓ ERK-mTORC1 ↓ |
Promote | proliferation↑ apoptosis ↓ | (205) |
| Hypoxia-induced chondrocytes | miR-146a ↑ | Bcl-2 ↓ | Promote | ECM degeneration ↓ | (206) |
| Hypoxia-induced primary chondrocytes | miR-146a ↑ | Traf6-IRAK1-Bcl-2↓ | Promote | ECM degeneration ↓ | (207) |
| DMM induced OA | miR-17–5p ↓ | P62 ↓ | Promote | cartilage injury ↓ | (208) |
| IL-1β stimulated primary chondrocytes | miR-766–3p ↓ | AIFM1 ↓ | Promote | Apoptosis ↓ ECM ↑ |
(209) |
| Human primary chondrocytes | miR-140–5p/149 ↓ | FUT1 ↓ | Promote | Apoptosis ↓ | (210) |
| Human primary chondrocytes | miR-155 ↑ | mTOR ↑ | Inhibit | Apoptosis ↑ | (211) |
| Polychlorinated biphenyls reduced OA | miR-155 ↑ | RICTOR-Akt- mTOR ↑ |
Inhibit | cartilage matrix ↓ | (212) |
| Developmental dysplasia of the hip reduced OA | miR-34a-5p ↑ | SESN2 ↓, mTOR ↑ | Inhibit | Proliferation ↓ cell migration ↓ |
(213) |
| TNF-α stimulated chondrocytes | miR-4262 ↑ | SIRT1 ↓ | Inhibit | Apoptosis ↑ | (214) |
| CHON-001 cell | miR-302d-3p ↑ | ULK1 ↓ | Inhibit | Inflammation ↑ | (215) |
| DMM-induced OA | miR-375 ↑ | ATG2b ↓ | Inhibit | Apoptosis ↑ | (216) |
| TNF-α induced ATDC5 cells | miR-30b ↑ | BECN1, ATG5 ↓ | Inhibit | Apoptosis ↑ | (217) |
| chondrocytes | miR-20a ↑ | ATG7 ↓ | Inhibit | chondrogenic differentiation ↓ | (218) |
| chondrocytes | miR-20a ↑ | ATG7 ↓ | Inhibit | chondrogenic differentiation ↓ | (219) |
| Human OA chondrocytes | miR-20 ↑ | ATG10 ↓ | Inhibit | Proliferation ↓ | (220) |
| ACLT induced OA | miR-128a ↑ | ATG12 ↓ | Inhibit | Apoptosis ↑ ECM degradation ↑ |
(221) |
| miR-378 transgenic (TG) mice | miR-378 ↑ | ATG2a ↓ | Inhibit | BMSCs chondrogenesis ↓ | (222) |
| Dexamethasone reduced OA | miR-1912–3p↑ | CTSD ↓ | Inhibit | MMP3, MMP13, ADAMTS5 ↑ cartilage matrix ↓ |
(223) |
| ACLT induced OA | miR-146a-5p ↑ | NUMB ↓ | Inhibit | Apoptosis ↑ | (224) |
MiRNAs mediated autophagy on OA.
5.1.1 Positive miRNAs to suppress OA pathogenesis
Emerging evidence suggests that miRNAs can exert protective effects in OA by promoting autophagy. A common mechanism involves targeting the mTOR signaling pathway. Several miRNAs, including miR-335-5p, activate the AMPK-mTOR pathway, leading to mTOR inhibition and subsequent autophagy activation (132). MiR-145 targets FRS2, inhibiting PI3K/Akt/mTOR signaling, thereby promoting autophagy and mitigating oxidative stress (133). MiR-31-5p negatively regulates SOX4, inhibiting mTORC1 activation through the ERK pathway, ultimately enhancing autophagy and promoting chondrocyte survival (134).
Beyond mTOR, other autophagy-related proteins, such as Bcl-2 and p62, are also subject to miRNA regulation. The Bcl-2 pathway plays a crucial role in miRNA-mediated autophagy regulation, particularly under hypoxic conditions. Zhang et al. (135) demonstrated that HIF-1α upregulates miR-146a expression in response to hypoxia. MiR-146a, in turn, inhibits Bcl-2, promoting autophagy and enhancing chondrocyte survival. Furthermore, miR-146a directly targets TRAF6 and IRAK1, key mediators of the NF-κB pathway, thereby attenuating inflammation (136). Another example is miR-145, which targets FRS2, inhibiting PI3K/Akt/mTOR signaling and promoting autophagy. MiR-31-5p, downregulated in OA, targets SOX4. This downregulation relieves the inhibitory effect of SOX4 on mTORC1, promoting autophagy and enhancing chondrocyte survival (137). These findings highlight the intricate interplay between miRNAs, autophagy, and cellular signaling pathways in the context of OA. Hypoxia-induced miR-146a promotes chondrocyte autophagy by inhibiting Bcl-2 expression through the TRAF6-IRAK1 pathway, independent of SMAD-4 (138). P62/SQSTM1, a key autophagy receptor, plays a critical role in autophagosome formation by transporting ubiquitinated proteins to the lysosome for degradation. MiR-17-5p directly targets and downregulates p62 expression. In the DMM-induced OA model, decreased p62 levels correlated with reduced miR-17-5p expression, consistent with its downregulation in IL-1β-treated SW1353 chondrosarcoma cells (139). MiR-17-5p overexpression significantly suppressed p62 expression in SW1353 cells, leading to enhanced autophagic activity. Furthermore, p62 knockdown partially reversed the inhibitory effect of miR-17-5p inhibition on LC3 expression, suggesting a crucial role for p62 in regulating autophagy in response to miR-17-5p modulation (140).
Beyond directly targeting autophagy-related proteins, miRNAs can also regulate autophagy through indirect mechanisms. For example, Wang et al. (141) demonstrated that miR-140-5p/149 directly targets fucosyltransferase 1 (FUT1) in human primary chondrocytes. Overexpression of miR-140-5p/149 inhibits FUT1 expression, leading to enhanced autophagy, increased cell proliferation, and reduced apoptosis. Conversely, FUT1 knockdown rescued the inhibitory effects of miR-140-5p/149 inhibition on autophagy. These findings suggest that the miR-140-5p/149-FUT1 axis plays a crucial role in regulating autophagy and chondrocyte homeostasis, highlighting its potential as a novel therapeutic target for OA.
5.1.2 Negative miRNAs to promote OA pathogenesis
Numerous studies have implicated miRNAs in OA pathogenesis through their ability to inhibit autophagy. MiR-155 targets the PI3K-Akt–mTOR pathway, suppressing autophagy by inhibiting ULK1, FOXO3, ATG14, ATG5, ATG3, and LC3 expression (142). Additionally, miR-155 directly targets RICTOR, a key component of the mTORC2 complex, further activating the Akt/mTOR signaling pathway, leading to impaired autophagy and exacerbated OA (143). MiR-34a-5p targets SESN2, a negative regulator of mTOR signaling. Upregulation of miR-34a-5p in OA cartilage suppresses SESN2 expression, leading to increased mTOR activity and impaired autophagy (144). Conversely, miR-34a-5p knockdown enhances SESN2 expression and autophagy activity. Besides, MiR-4262 targets SIRT1, a key regulator of cellular metabolism. In TNF-α-treated chondrocytes, miR-4262 overexpression inhibits SIRT1 expression, leading to increased PI3K/Akt/mTOR signaling and subsequent suppression of autophagy (145). Conversely, SIRT1 overexpression inhibits PI3K/Akt/mTOR signaling, promoting autophagy and mitigating the detrimental effects of miR-4262 overexpression, such as apoptosis and impaired ECM synthesis (146).
MiRNAs can exert detrimental effects in OA by targeting key autophagy proteins. MiR-378 directly targets ATG2a, a crucial protein involved in autophagosome formation. In miR-378 transgenic mice, downregulated ATG2a expression leads to impaired autophagic vesicle formation and contributes to OA development (147). MiR-375 directly targets ATG2b, inhibiting autophagosome membrane closure. This miR-375-mediated inhibition of ATG2b exacerbates endoplasmic reticulum stress and promotes chondrocyte apoptosis (148). MiR-30b directly targets Beclin1 and ATG5, inhibiting autophagy initiation and elongation, respectively. MiR-30b overexpression in chondrocytes promotes apoptosis and inhibits autophagy, likely contributing to OA pathogenesis (149). Additionally, MiR-20a targets ATG7, a key protein involved in LC3 lipidation. MiR-20a overexpression in ATDC-5 cells downregulates ATG7, leading to impaired autophagosome formation and reduced chondrogenesis (150). MiR-128a targets ATG12, inhibiting LC3 lipidation and autophagosome formation. Additionally, miR-128a overexpression is associated with H3K27 hypomethylation and enhancer of zeste homolog 2 inactivation, further contributing to impaired autophagy and exacerbated OA progression (151). These findings demonstrate that miRNAs can regulate autophagy at multiple levels, including initiation, elongation, and maturation, by targeting key autophagy-related proteins such as Beclin1, ATG5, ATG7, and ATG12. Dysregulation of these miRNAs can contribute to OA pathogenesis by disrupting autophagic flux and promoting chondrocyte dysfunction (152).
MiRNAs can indirectly inhibit autophagy by targeting factors that regulate autophagic flux. MiR-1912-3p targets CTSD, a lysosomal protease crucial for autophagic degradation. In a model of fetal dexamethasone exposure, miR-1912-3p downregulates CTSD expression, impairing autophagy and increasing offspring susceptibility to OA (153). Besides, MiR-146a-5p targets NUMB, a negative regulator of autophagy. Upregulation of miR-146a-5p in OA cartilage inhibits NUMB expression, leading to decreased autophagy, increased apoptosis, and exacerbated OA progression (154).
5.2 LncRNAs and autophagy in OA
lncRNAs are non-coding RNA transcripts exceeding 200 nucleotides in length with limited or no protein-coding potential (155). LncRNAs exert diverse regulatory functions, including chromatin modification, mRNA splicing, and translational control. Furthermore, lncRNAs interact with miRNAs through several mechanisms. For example, LncRNAs can act as ceRNAs, competing with mRNAs for miRNA binding, thereby influencing gene expression (156). Besidesm, some lncRNAs serve as precursors for miRNAs, such as npc83 and npc521, which generate miR-869a and miR-160c, respectively (157). LncRNAs can also be direct targets of miRNAs, leading to their degradation. In OA, lncRNAs primarily function as ceRNAs, regulating gene expression by modulating miRNA activity. These lncRNAs influence various cellular processes, including inflammation, proliferation, apoptosis, and autophagy, ultimately impacting cartilage homeostasis (158). Numerous lncRNAs have been implicated in regulating autophagy in OA, either by directly targeting autophagy-related genes or by acting as miRNA sponges (Figure 5; Table 2).
Figure 5
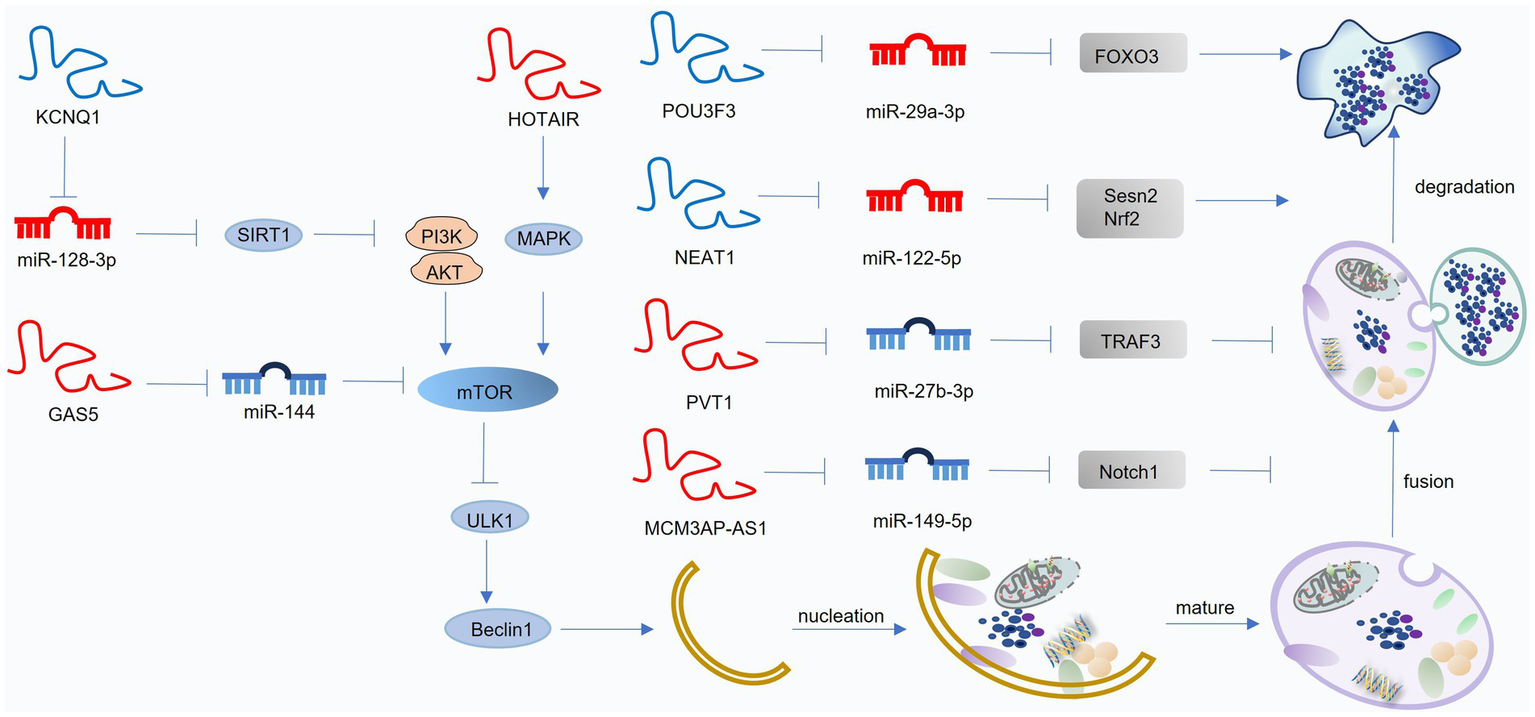
Mechanisms of lncRNA-mediated regulation of autophagy in osteoarthritis (OA). LncRNAs mainly act as competing endogenous RNAs (ceRNAs) that interact with miRNAs to modulate autophagy-related pathways. Blue lncRNAs indicate those that activate autophagy and suppress OA progression, while red lncRNAs denote those that inhibit autophagy and facilitate OA development. This figure was generated and edited using Adobe Illustrator (Adobe Inc., United States).
Table 2
| Model | LncRNA expression | Targeted miRNAs | Targeting pathway | Effect on autophagy | Effect on cartilage or chondrocyte | Ref. |
|---|---|---|---|---|---|---|
| Human OA cartilage | lncRNA POU3F3 ↓ | miR-29a-3p | FOXO3 ↑ | Promote | inflammation ↓ apoptosis ↓ | (225) |
| Human OA cartilage, DMM induced OA chondrocyte |
lncRNA NEAT1 ↓ | miR-122–5p | Sesn2, Nrf2 ↑ | Promote | apoptosis ↓ proliferation ↑ |
(226) |
| HFD induced OA | lncRNA KCNQ1 ↓ | miR-128–3p | SIRT1 ↑ | Promote | inflammation ↓ apoptosis ↓ |
(227) |
| DMM induced OA | lncRNA HOTAIR ↑ | / | p38MAPK ↑ | Inhibit | inflammation ↑ ECM degeneration ↑ |
(228) |
| Human OA cartilage chondrocyte | lncRNA HOTAIR ↑ | miR-130a-3p | / | Inhibit | apoptosis ↑ | (229) |
| DMM induced OA | lncRNA GAS5 ↑ | miR-144 | mTOR ↑ | Inhibit | apoptosis ↑ | (230) |
| Human OA cartilage | lncRNA GAS5 ↑ | miR-21 | LC3B ↓ | Inhibit | apoptosis ↑ | (231) |
| Human OA cartilage, IL-1β induced C28/I2 | lncRNA PVT1 ↑ | miR-27b-3p | TRAF3 ↑ | Inhibit | inflammation ↑ apoptosis ↑ | (232) |
| Human OA cartilage | lncRNA | miR-149–5p | Notch1 ↑ | Inhibit | ECM degeneration ↑ | (233) |
| Human OA synoviocytes | lncRNA PCGEM1 ↑ | miR-770 | / | Promote | apoptosis ↓ proliferation ↑ | (234) |
LncRNAs mediated autophagy on OA.
5.2.1 Positive lncRNAs to suppress OA pathogenesis
Several lncRNAs exert protective effects in OA by promoting autophagy through their miRNA sponging activity. For example, downregulated in OA cartilage, lncRNA POU3F31 acts as a ceRNA for miR-29a-3p, thereby increasing FOXO3 expression and promoting autophagy. This, in turn, inhibits inflammation and apoptosis in chondrocytes (159). Downregulated in HFD-induced OA, lncRNA KCNQ1OT1 acts as a ceRNA for miR-34a-5p. Upregulation of lncRNA KCNQ1OT1, for example, by salbutamol B treatment, inhibits miR-34a-5p activity, leading to increased SIRT1 expression, enhanced autophagy, and reduced inflammation in chondrocytes (160).
5.2.2 Negative lncRNAs to promote OA pathogenesis
Conversely, some lncRNAs contribute to OA progression by inhibiting autophagy. For instance, lncRNA HOTAIR inhibits the p38MAPK pathway, suppressing autophagy and enhancing inflammation (161). Additionally, HOTAIR acts as a ceRNA for miR-130a-3p, further inhibiting autophagy and promoting apoptosis by downregulating miR-130a-3p-mediated suppression of the mTOR pathway (162). Upregulated in OA, lncRNA GAS5 acts as a ceRNA for miR-144. Silencing of lncRNA GAS5 increases miR-144 expression, inhibiting mTOR signaling and enhancing autophagy, ultimately protecting chondrocytes from apoptosis (163). Moreover, LncRNA GAS5 acts as a ceRNA for miR-21, inhibiting its activity. This leads to enhanced autophagy by preventing miR-21-mediated suppression of autophagy complex formation, ultimately mitigating apoptosis and ECM degradation (164). Besides, Upregulated in OA, lncRNA PVT1 acts as a ceRNA for miR-27b-3p, promoting TRAF3 expression and inhibiting autophagy. This contributes to increased inflammation and apoptosis (165). Upregulated by SOX4, lncRNA MCM3AP-AS1 acts as a ceRNA for miR-149-5p, promoting Notch1 expression. This dysregulated axis contributes to impaired autophagy and exacerbated ECM degradation in OA (166).
Beyond chondrocyte dysfunction, synovial inflammation plays a critical role in OA pathogenesis. Activated synoviocytes secrete pro-inflammatory cytokines, such as IL-1β and TNF-α, which stimulate the production of matrix metalloproteinases (MMPs) and other degradative enzymes, leading to cartilage destruction (167). LncRNA PCGEM is upregulated in OA synoviocytes. By acting as a ceRNA for miR-770, lncRNA PCGEM promotes autophagy, inhibits apoptosis, and stimulates proliferation in synoviocytes, thereby exacerbating OA pathology (168).
5.3 CircRNAs and autophagy in OA
CircRNAs are a class of endogenous, single-stranded, non-coding RNAs characterized by a covalently closed-loop structure formed through back-splicing. This unique structure confers enhanced stability to circRNAs compared to linear RNAs. CircRNAs exert their biological functions through various mechanisms. Firstly, CircRNAs can act as competing endogenous RNAs (ceRNAs), sequestering miRNAs and thereby preventing miRNA-mediated gene silencing. Secondly, CircRNAs can modulate gene transcription. Thirdly, CircRNAs can interact with RNA-binding proteins. Fourthly, Some circRNAs have been shown to possess protein-coding potential. Dysregulated circRNA expression patterns have been observed in OA (169). CircRNAs contribute to OA pathogenesis by modulating various cellular processes, including chondrocyte proliferation and apoptosis, cartilage erosion, and inflammation. Furthermore, circRNAs play a crucial role in regulating autophagy in OA, primarily through their ceRNA activity (Figure 6; Table 3).
Figure 6
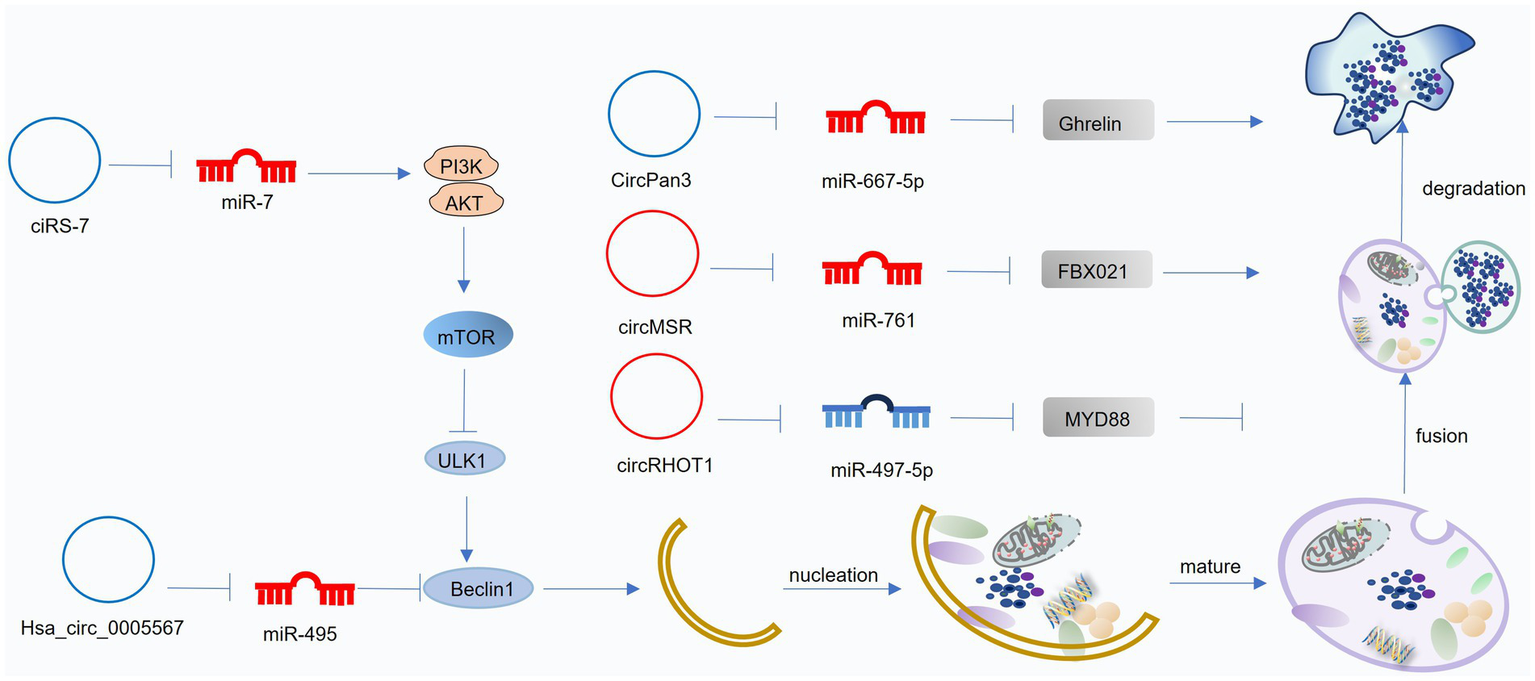
Mechanisms of circRNA-mediated regulation of autophagy in osteoarthritis (OA). CircRNAs modulate autophagy by acting as competing endogenous RNAs (ceRNAs) that influence downstream miRNA activity. Blue circRNAs indicate those enhancing autophagy and alleviating OA progression, whereas red circRNAs represent those suppressing autophagy and accelerating OA pathogenesis. This figure was generated and edited using Adobe Illustrator (Adobe Inc., United States).
Table 3
| Model | CircRNA expression | Targeted MiRNAs | Targeting pathway | Effect on autophagy | Effect on cartilage or chondrocyte | Ref. |
|---|---|---|---|---|---|---|
| DMM induced OA | circFOXO3 ↓ | / | FOXO3↑ | Promote | apoptosis ↓ ECM synthesis ↑ |
(235) |
| Human OA cartilage, DMM induced OA | ciRS-7 ↓ | miR-7 | PI3K-Akt–mTOR↓ | Promote | ECM synthesis ↑ ECM degradation ↓ |
(236) |
| IL-1β-inducd chondrocytes | hsa_circ_0005567 ↓ | miR-495 | ATG14↑ | Promote | apoptosis ↓ | (237) |
| DMM induced OA | CircPan3 ↓ | miR-667–5p | Growth-releasing peptide↑ | Promote | ADAMTS5, MMP13 ↓ Col2, ACAN ↑ |
(238) |
| IL-1β-inducd CHON-001 cells | hsa_circ_0037658 ↑ | / | / | Inhibit | MMP13↑, ACANA ↓ | (239) |
| Human OA cartilage | CircRNA-MSR ↑ | miR-761 | FBXO21↑ | FBXO21↑ | proliferation ↓ | (240) |
| ACLT induced OA | circRHOT1 ↑ | miR-142–5p | CCND1↑ | Inhibit | apoptosis↑ proliferation ↓ | (241) |
| IL-1β-induced human chondrocytes | circMELK ↑ | miR-497–5p | Myeloid differentiation factor 88↑ NF-κB↑ |
Inhibit | inflammation↑ apoptosis ↑ | (242) |
CircRNAs mediated autophagy in OA.
5.3.1 Positive circRNAs to suppress OA pathogenesis
Several circRNAs have been implicated in regulating autophagy and influencing OA pathogenesis. circFOXO3 acts as a sponge for its parental gene, FOXO3, inhibiting PI3K-Akt signaling and promoting autophagy. This, in turn, enhances collagen synthesis, suppresses apoptosis, and reduces ECM degradation in OA (170). CiRS-7 acts as a ceRNA for miR-7. Downregulation of CiRS-7 in OA leads to increased miR-7 activity, inhibiting autophagy and promoting cartilage degeneration. Conversely, miR-7 inhibition attenuates OA progression (171). hsa_circ_0005567 acts as a ceRNA for miR-495, promoting ATG14 expression and enhancing autophagy. circPan3 acts as a ceRNA for miR-667-5p, promoting the expression of growth-releasing peptide, which in turn activates autophagy and facilitates ECM repair (172).
5.3.2 Negative circRNAs to promote OA pathogenesis
Several circRNAs contribute to OA pathogenesis by inhibiting autophagy. Upregulated in OA, hsa_circ_0037658 inhibits autophagy in chondrocytes. Silencing of hsa_circ_0037658 promotes autophagy, reduces MMP13 expression, and enhances collagen synthesis, mitigating OA progression (173). Upregulated in OA, hsa_circ_0037658 inhibits autophagy in chondrocytes. Silencing of hsa_circ_0037658 promotes autophagy, reduces MMP13 expression, and enhances collagen synthesis, mitigating OA progression (174). circRNA-MSR acts as a ceRNA for miR-761, leading to increased FBXO21 expression and impaired autophagy in OA (175). circRHOT1 acts as a ceRNA for miR-142-5p, promoting CCND1 expression and inhibiting autophagy. This contributes to chondrocyte apoptosis and OA progression (176). Upregulated in OA chondrocytes, circMELK acts as a ceRNA for miR-497-5p, promoting myeloid differentiation factor 88 (MyD88) expression and activating the NF-κB pathway. This leads to impaired autophagy, increased inflammation, and enhanced chondrocyte apoptosis (177). These findings demonstrate the diverse mechanisms by which circRNAs can regulate autophagy and contribute to OA pathogenesis.
This study categorized ncRNAs involved in OA autophagy into two groups: those that promote autophagy (positive regulators) and those that inhibit autophagy (negative regulators) (178). While the miRNA-autophagy network has been extensively explored, with miRNAs directly regulating various stages of autophagy, our understanding of the role of lncRNAs and circRNAs in this context is still evolving. Further research is needed to elucidate the intricate regulatory networks between these RNAs and autophagy in OA. Current OA models, such as DMM, ACTL, and inflammatory models induced by IL-1β or TNF-α, have identified numerous ncRNAs with altered expression levels (Tables 1–3). These ncRNAs may serve as valuable biomarkers for OA diagnosis and treatment. However, future studies should investigate ncRNA expression patterns in a broader range of OA models, including those associated with obesity, aging, and drug-induced injury, to gain a more comprehensive understanding of the ncRNA landscape in OA.
While the studies discussed above primarily focus on the role of ncRNAs in modulating basal or moderately elevated autophagy, it is crucial to acknowledge their potential involvement in regulating excessive autophagy, which can also contribute to OA pathogenesis (179). miR-27a targets PI3K, inhibiting the PI3K/Akt/mTOR pathway and promoting excessive autophagy, ultimately leading to chondrocyte death. In the SDF-1-induced OA model, miR-146a-5p targets CXCR4, inhibiting SDF-1/CXCR4 signaling and attenuating excessive autophagy. This lncRNA may contribute to the regulation of excessive autophagy, potentially through interactions with the miR-34a-5p/SYVN axis. Further research is warranted to identify additional ncRNAs that regulate excessive autophagy and to elucidate the underlying mechanisms contributing to OA pathogenesis.
6 Innovative approaches for targeting ncRNA-regulated autophagy in the treatment of OA
The extensive involvement of ncRNAs in regulating autophagy in OA suggests their significant potential as novel therapeutic targets. While research has primarily focused on ncRNAs that modulate basal or moderate levels of autophagy, the role of ncRNAs in regulating excessive autophagy, which can also contribute to OA pathogenesis, warrants further investigation.
6.1 Natural products
Current OA management primarily relies on pharmacological approaches, with NSAIDs, analgesics, and glucocorticosteroids constituting the mainstay of treatment. While these agents effectively alleviate OA symptoms, they are not without limitations. Long-term use of NSAIDs and glucocorticosteroids can lead to adverse effects on various organ systems, including the gastrointestinal and cardiovascular systems. Topical formulations, while minimizing systemic side effects, often exhibit poor penetration and require frequent application (180).
Given the increasing prevalence of drug-related side effects, natural products are emerging as promising alternatives for OA treatment. Their diverse chemical structures and generally low toxicity profile offer significant advantages (181). Many plant extracts, such as those derived from frankincense and Angelica dahurica, have demonstrated potent anti-inflammatory and antioxidant properties, effectively alleviating OA pain and dysfunction compared to conventional analgesics and glucosamine (182). Recent studies have highlighted the ability of natural products to modulate autophagy, a key cellular process implicated in OA pathogenesis. Propolis, a natural resin produced by bees, possesses a wide range of pharmacological properties, including antioxidant and anti-inflammatory effects. These beneficial effects are attributed to its rich polyphenol content, making it a promising candidate for natural anti-aging therapies (183). A recent study investigated the impact of ethanol extract of propolis (EEP) on miRNA expression in IL-1β-stimulated chondrocytes. Among five miRNAs known to regulate autophagy (miR-19a, miR-125b, miR-181a, miR-185, and miR-335), EEP treatment significantly downregulated miR-125b and upregulated miR-185, suggesting a potential role for these miRNAs in mediating the anti-inflammatory and autophagy-modulating effects of propolis in OA (184). Further investigation is needed to validate these findings and translate these preclinical observations into clinical applications for OA treatment.
6.2 Gene therapy
Gene therapy holds significant promise as a novel therapeutic approach for OA. The avascular nature of articular cartilage makes intra-articular injection an attractive route for delivering therapeutic nucleic acids, circumventing limitations associated with systemic drug delivery (185). Studies have demonstrated the therapeutic potential of intra-articularly injected miRNAs, such as miR-181a-5p and miR-34a-5p, in OA (186). However, the clinical translation of miRNA-based therapies faces challenges. Natural miRNAs exhibit limited stability within the joint environment, readily degrading due to enzymatic activity. Furthermore, efficient cellular uptake of these molecules remains a significant hurdle. Therefore, the development of effective gene delivery vectors is crucial for successful miRNA-based OA therapies. These vectors should protect miRNAs from degradation and facilitate their efficient delivery to target cells within the joint.
Nanoparticle-based delivery systems offer a promising approach for enhancing the efficacy and stability of miRNA therapeutics in OA. Chen et al. (187) demonstrated the efficacy of G5-AHP, a multifunctional polymeric nanoparticle, in delivering miR-224-5p to chondrocytes. Compared to conventional transfection methods, G5-AHP-mediated delivery significantly improved cellular uptake of miR-224-5p, leading to enhanced autophagy, reduced apoptosis, and improved ECM synthesis. Furthermore, intra-articular injection of G5-AHP-miR-224-5p nanoparticles in an OA mouse model significantly alleviated disease progression, as evidenced by reduced joint space narrowing, bone sclerosis, and synovial inflammation (188). These findings highlight the potential of nanoparticle-based delivery systems to overcome the limitations of traditional miRNA therapies and provide a promising avenue for the development of effective OA treatments.
Extracellular vesicles (EVs), including exosomes, microvesicles, and apoptotic bodies, are naturally occurring, membrane-bound vesicles released by various cell types (189). Compared to synthetic delivery systems like liposomes and polymeric nanoparticles, EVs offer several advantages, including high biocompatibility, low immunogenicity, and enhanced stability (190). Studies have shown that MSC-derived EVs exert therapeutic effects in OA by promoting cartilage regeneration. For example, MSC-derived exosomes from infrapatellar fat pads (IPFP-MSC-Exos) have been shown to reduce cartilage degradation and promote ECM synthesis in DMM-induced OA. Mechanistically, these exosomes deliver miR-100-5p, which inhibits mTOR signaling and promotes autophagy in chondrocytes (191). These EVs contain lncRNA NEAT1, which acts as a ceRNA for miR-122-5p. By downregulating miR-122-5p, NEAT1 increases the expression of its target gene, sestrin 2, activating the Nrf2 pathway and promoting autophagy in chondrocytes (192). These findings highlight the therapeutic potential of EVs in OA by delivering bioactive molecules, such as miRNAs and lncRNAs, to modulate autophagy and promote cartilage repair.
7 Conclusion and perspectives
Autophagy plays a crucial role in maintaining chondrocyte homeostasis. Under physiological conditions, autophagy serves as a protective mechanism, clearing cellular debris, mitigating oxidative stress, and promoting cellular survival. However, excessive autophagy can also contribute to chondrocyte death and OA progression. Therefore, therapeutic strategies targeting autophagy in OA should carefully consider the specific stage and context of the disease. Enhancing autophagy may be beneficial in situations where autophagy is impaired, such as in early stages of OA. Conversely, strategies to inhibit excessive autophagy may be necessary in later stages where autophagy contributes to chondrocyte death. A deeper understanding of the complex regulatory mechanisms governing autophagy in OA is crucial for developing effective therapeutic interventions.
Emerging evidence indicates that traditional Chinese medicine (TCM), including specific herbal components and manual therapies, can modulate autophagy-related pathways involved in the pathogenesis of OA. For instance, icariin, a flavonoid derived from Epimedium species, has been shown to alleviate OA in rat models by activating autophagy and suppressing the PI3K/AKT/mTOR signaling pathway, thereby enhancing chondrocyte viability and preserving cartilage integrity (193). In addition to herbal compounds, TCM-based manual therapy such as Tuina has been demonstrated in vivo to attenuate cartilage injury and inflammatory responses while regulating chondrocyte autophagy and apoptosis through the PI3K/AKT/mTOR pathway in experimental OA models (194). Beyond these interventions, accumulating evidence supports the autophagy-modulating effects of additional bioactive compounds derived from traditional Chinese herbal medicine. Oroxin B (OB), a flavonoid with documented anti-inflammatory properties, has been shown to alleviate chondrocyte injury by suppressing inflammatory signaling, inhibiting PI3K/AKT/mTOR activity, and promoting autophagy, suggesting its therapeutic potential in OA management (195). Similarly, Lou et al. demonstrated that glabridin (Gla), an isoprenylated isoflavone isolated from Glycyrrhiza glabra, effectively preserves extracellular matrix (ECM) homeostasis and maintains autophagic flux via modulation of the PI3K/AKT/FOXO3A signaling axis, highlighting a dual regulatory strategy for OA treatment (196). Moreover, Yang et al. reported that the Gubi Zhitong formula exerts protective effects against OA in both in vitro and in vivo models by regulating BNIP3L-mediated mitophagy, underscoring the importance of mitochondrial quality control in chondrocyte homeostasis (197).
Emerging evidence highlights a complex interplay between ncRNAs and autophagy in the pathogenesis of OA. While miRNAs have been extensively studied in this context, our understanding of the role of lncRNAs and circRNAs in regulating autophagy in OA remains limited. MiRNAs exert significant influence over autophagy by directly targeting key autophagy genes, including components of the mTOR pathway, ULK1, Beclin1, and ATG proteins, thereby modulating various stages of the autophagic process (198). LncRNAs and circRNAs primarily function as ceRNAs, indirectly influencing autophagy by regulating miRNA activity. However, the role of these ncRNAs in regulating both basal and excessive autophagy in OA requires further investigation. Furthermore, while the impact of altered autophagy levels on OA pathogenesis has been extensively studied, the specific role of ncRNAs in modulating excessive autophagy remains relatively unexplored (199). A comprehensive understanding of the complex interplay between ncRNAs and autophagy is crucial for developing novel therapeutic strategies for OA.
The therapeutic application of the ncRNA-autophagy axis in OA is still in its early stages. While promising, current research primarily focuses on preclinical models, with limited clinical translation. Compounds derived from natural sources offer potential therapeutic benefits due to their generally low toxicity profiles (200). However, challenges remain, including potential for systemic side effects and limitations in bioavailability. Gene therapy approaches, such as the use of gene delivery vectors to target specific ncRNAs, hold significant promise. However, optimizing delivery systems to ensure efficient and safe delivery of therapeutic agents to the target site within the joint remains a critical challenge. Key considerations include biocompatibility, biodegradability, and minimizing off-target effects. Further research is crucial to address these challenges and translate the promising preclinical findings into effective clinical therapies for OA (201).
OA pathogenesis is a complex process involving multiple factors, including chondrocyte senescence, inflammation, oxidative stress, and autophagy. While ncRNAs play a crucial role in regulating autophagy, they represent only one facet of the intricate network of factors contributing to OA (202). Furthermore, the interplay between ncRNAs and autophagy is multifaceted. A single study may not fully capture the complex interactions within this network. Therefore, a comprehensive understanding of OA pathogenesis, including the roles of various cellular processes and the intricate regulatory mechanisms involving ncRNAs, is crucial for the development of effective ncRNA-based therapies.
Statements
Author contributions
BW: Supervision, Writing – review & editing, Writing – original draft, Visualization. ZW: Methodology, Project administration, Writing – review & editing, Writing – original draft.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by 2025 Open Fund Project of the Provincial Scientific Research Platform of Hefei Normal University (2025KYPT63).
Acknowledgments
The authors thank everyone who helped with this work.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Kachroo U Livingston A Vinod E Sathishkumar S Boopalan P . Comparison of electrophysiological properties and gene expression between human chondrocytes and chondroprogenitors derived from normal and osteoarthritic cartilage. Cartilage. (2020) 11:374–84. doi: 10.1177/1947603518796140,
2.
Mok SW Fu SC Cheuk YC Chu IM Chan KM Qin L et al . Intra-articular delivery of quercetin using Thermosensitive hydrogel attenuate cartilage degradation in an osteoarthritis rat model. Cartilage. (2020) 11:490–9. doi: 10.1177/1947603518796550,
3.
Tang Y Wang Z Cao J Tu Y . Bone-brain crosstalk in osteoarthritis: pathophysiology and interventions. Trends Mol Med. (2025) 31:281–95. doi: 10.1016/j.molmed.2024.09.006,
4.
Ogura T Ackermann J Barbieri Mestriner A Merkely G Gomoll AH . Minimal clinically important differences and substantial clinical benefit in patient-reported outcome measures after autologous chondrocyte implantation. Cartilage. (2020) 11:412–22. doi: 10.1177/1947603518799839,
5.
Tsuyuguchi Y Nakasa T Ishikawa M Miyaki S Matsushita R Kanemitsu M et al . The benefit of minced cartilage over isolated chondrocytes in Atelocollagen gel on chondrocyte proliferation and migration. Cartilage. (2021) 12:93–101. doi: 10.1177/1947603518805205,
6.
Zhang Y Li J Liu J Gao Y Li K Zhao X et al . Ferroptosis in osteoarthritis: towards novel therapeutic strategy. Cell Prolif. (2025) 58:e13779. doi: 10.1111/cpr.13779,
7.
Lee B Parvizi J Bramlet D Romness DW Guermazi A Noh M et al . Results of a phase II study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1. J Knee Surg. (2020) 33:167–72. doi: 10.1055/s-0038-1676803,
8.
Chen Z Li C Qian YH Fu Y Feng ZM . Enhancement of autophagy flux by isopsoralen ameliorates interleukin-1β-stimulated apoptosis in rat chondrocytes. J Asian Nat Prod Res. (2020) 22:179–92. doi: 10.1080/10286020.2018.1537265,
9.
Carballo CB Coelho TRP de Holanda Afonso RC Faria JCO Alves T Monte SM et al . Osteoarthritic synovial fluid and TGF-β1 induce Interleukin-18 in articular chondrocytes. Cartilage. (2020) 11:385–94. doi: 10.1177/1947603518796149,
10.
Ackermann J Ogura T Duerr RA Barbieri Mestriner A Gomoll AH . Preoperative mental health has a stronger association with baseline self-assessed knee scores than defect morphology in patients undergoing cartilage repair. Cartilage. (2020) 11:309–15. doi: 10.1177/1947603518783484,
11.
Shen K Zhou H Zuo Q Gu Y Cheng J Yan K et al . GATD3A-deficiency-induced mitochondrial dysfunction facilitates senescence of fibroblast-like synoviocytes and osteoarthritis progression. Nat Commun. (2024) 15:10923. doi: 10.1038/s41467-024-55335-2,
12.
Hamasaki M Terkawi MA Onodera T Homan K Iwasaki N . A novel cartilage fragments stimulation model revealed that macrophage inflammatory response causes an upregulation of catabolic factors of chondrocytes In vitro. Cartilage. (2021) 12:354–61. doi: 10.1177/1947603519828426,
13.
Wagner G Lehmann C Bode C Miosge N Schubert A . High mobility group box 1 protein in osteoarthritic knee tissue and Chondrogenic progenitor cells: An ex vivo and In vitro study. Cartilage. (2021) 12:484–95. doi: 10.1177/1947603519835897,
14.
Liu Y Li X Jin A . Rapamycin inhibits Nf-ΚB activation by autophagy to reduce catabolism in human chondrocytes. J Investigative Surg: Official J Acad Surg Res. (2020) 33:861–73. doi: 10.1080/08941939.2019.1574321,
15.
Yang H Wen Y Zhang M Liu Q Zhang H Zhang J et al . MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint. Autophagy. (2020) 16:271–88. doi: 10.1080/15548627.2019.1606647,
16.
Hu P Du J Zhang S Wang T Li J Chen G et al . Oral Administration of Strontium Gluconate Effectively Reduces Articular Cartilage Degeneration through Enhanced Anabolic Activity of chondrocytes and Chondrogenetic differentiation of mesenchymal stromal cells. Biol Trace Elem Res. (2020) 193:422–33. doi: 10.1007/s12011-019-01711-9,
17.
Liao FX Huang F Ma WG Qin KP Xu PF Wu YF et al . The new role of Sirtuin1 in human osteoarthritis chondrocytes by regulating autophagy. Cartilage. (2021) 13:1237s–48s. doi: 10.1177/1947603519847736,
18.
Wang L Yin J Yang B Qu C Lei J Han J et al . Serious selenium deficiency in the serum of patients with Kashin-Beck disease and the effect of Nano-selenium on their chondrocytes. Biol Trace Elem Res. (2020) 194:96–104. doi: 10.1007/s12011-019-01759-7,
19.
Xiao L Liu C Wang B Fei W Mu Y Xu L et al . Targeting Discoidin domain receptor 2 for the development of disease-modifying osteoarthritis drugs. Cartilage. (2021) 13:1285s–91s. doi: 10.1177/1947603519852401,
20.
Yoon TH Jung M Choi CH Kim HS Lee YH Choi YS et al . Arthroscopic gel-type autologous chondrocyte implantation presents histologic evidence of regenerating hyaline-like cartilage in the knee with articular cartilage defect. Knee Surg, Sports Traumatol, Arthroscopy: Official J ESSKA. (2020) 28:941–51. doi: 10.1007/s00167-019-05572-6,
21.
Mei Z Yilamu K Ni W Shen P Pan N Chen H et al . Chondrocyte fatty acid oxidation drives osteoarthritis via SOX9 degradation and epigenetic regulation. Nat Commun. (2025) 16:4892. doi: 10.1038/s41467-025-60037-4,
22.
Chen K Zhu H Zheng MQ Dong QR . LncRNA MEG3 inhibits the degradation of the extracellular matrix of chondrocytes in osteoarthritis via targeting miR-93/TGFBR2 Axis. Cartilage. (2021) 13:1274s–84s. doi: 10.1177/1947603519855759,
23.
Martínez-Calleja A Cruz R Miranda-Sánchez M Fragoso-Soriano R Vega-López MA Kouri JB . Latexin expression correlated with mineralization of articular cartilage during progression of post-traumatic osteoarthritis in a rat model. Histol Histopathol. (2020) 35:269–78. doi: 10.14670/HH-18-151,
24.
Suzuki Y Hasegawa M Matsui Y Unno H Iino T Yoshida T et al . Intra-articular injection of rebamipide prevents articular cartilage degeneration in murine post-traumatic osteoarthritis models. Mod Rheumatol. (2020) 30:765–72. doi: 10.1080/14397595.2019.1641912,
25.
Hegde DD Hadya Ananda K Vavachan N . Effectiveness of quadriceps strength training in adults with knee osteoarthritis: a systematized review. Musculoskeletal Care. (2025) 23:e70134. doi: 10.1002/msc.70134,
26.
Yu Z Zhang H Jia G Hu P Huang J Cong Z et al . Effects of MiR-34a on the proliferation and apoptosis of osteoarthritis chondrocytes via the ERK1/2 pathway. Panminerva Med. (2021) 63:241–2. doi: 10.23736/S0031-0808.19.03688-7,
27.
Gao K Zhu W Li H Ma D Liu W Yu W et al . Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod Rheumatol. (2020) 30:758–64. doi: 10.1080/14397595.2019.1651445,
28.
Fiegen A Leland DP Bernard CD Krych AJ Barlow JD Dahm DL et al . Articular cartilage defects of the Glenohumeral joint: a systematic review of treatment options and outcomes. Cartilage. (2021) 13:401s–13s. doi: 10.1177/1947603519870858,
29.
Sanchez C Lambert C Dubuc JE Bertrand J Pap T Henrotin Y . Syndecan-4 is increased in osteoarthritic knee, but not hip or shoulder, articular hypertrophic chondrocytes. Cartilage. (2021) 13:862s–71s. doi: 10.1177/1947603519870855,
30.
Brown SB Hornyak JA Jungels RR Shah YY Yarmola EG Allen KD et al . Characterization of Post-traumatic osteoarthritis in rats following anterior cruciate ligament rupture by non-invasive knee injury (NIKI). J Orthop Res. (2020) 38:356–67. doi: 10.1002/jor.24470,
31.
Stiffel V Rundle CH Sheng MH Das S Lau KW . A mouse noninvasive Intraarticular Tibial plateau compression loading-induced injury model of posttraumatic osteoarthritis. Calcif Tissue Int. (2020) 106:158–71. doi: 10.1007/s00223-019-00614-0,
32.
Balbinot G Schuch CP Nascimento PSD Lanferdini FJ Casanova M Baroni BM et al . Photobiomodulation therapy partially restores cartilage integrity and reduces chronic pain behavior in a rat model of osteoarthritis: involvement of spinal glial modulation. Cartilage. (2021) 13:1309s–21s. doi: 10.1177/1947603519876338,
33.
Takada S Nakamura E Sabanai K Tsukamoto M Otomo H Kanoh S et al . Attenuation of Post-traumatic osteoarthritis after anterior cruciate ligament injury via inhibition of hedgehog signaling. J Orthop Res. (2020) 38:609–19. doi: 10.1002/jor.24494,
34.
Hatano M Sasabuchi Y Okada A Kimura Y Ishikura H Tanaka T et al . Osteoarthritis risk associated with romosozumab compared with teriparatide in individuals with osteoporosis: a target trial emulation study. Ann Rheum Dis. (2025) 84:1938–47. doi: 10.1016/j.ard.2025.06.2124,
35.
Ding LB Li Y Liu GY Li TH Li F Guan J et al . Long non-coding RNA PVT1, a molecular sponge of miR-26b, is involved in the progression of hyperglycemia-induced collagen degradation in human chondrocytes by targeting CTGF/TGF-β signal ways. Innate Immun. (2020) 26:204–14. doi: 10.1177/1753425919881778,
36.
Zhou Y Lu H Deng L Lin CH Pennington Klein K Wu M . HMGB2 is associated with pressure loading in chondrocytes of temporomandibular joint: In vitro and in vivo study. Cytokine. (2020) 126:154875. doi: 10.1016/j.cyto.2019.154875,
37.
He B Jiang D . HOTAIR-induced apoptosis is mediated by sponging miR-130a-3p to repress chondrocyte autophagy in knee osteoarthritis. Cell Biol Int. (2020) 44:524–35. doi: 10.1002/cbin.11253,
38.
Hiruthyaswamy SP Bose A Upadhyay A Raha T Bhattacharjee S Singha I et al . Molecular signaling pathways in osteoarthritis and biomaterials for cartilage regeneration: a review. Bioengineered. (2025) 16:2501880. doi: 10.1080/21655979.2025.2501880,
39.
Wu Y Hou M Deng Y Xia X Liu Y Yu J et al . Swimming exercise induces redox-lipid crosstalk to ameliorate osteoarthritis progression. Redox Biol. (2025) 81:103535. doi: 10.1016/j.redox.2025.103535,
40.
Ma D He J He D . Chamazulene reverses osteoarthritic inflammation through regulation of matrix metalloproteinases (MMPs) and NF-kβ pathway in in-vitro and in-vivo models. Biosci Biotechnol Biochem. (2020) 84:402–10. doi: 10.1080/09168451.2019.1682511,
41.
Chen C Zhu Z Hu N Liang X Huang W . Leonurine hydrochloride suppresses inflammatory responses and ameliorates cartilage degradation in osteoarthritis via NF-κB signaling pathway. Inflammation. (2020) 43:146–54. doi: 10.1007/s10753-019-01104-z,
42.
Lin M Zhang C Li H Li K Gou S He X et al . Pyroptosis for osteoarthritis treatment: insights into cellular and molecular interactions inflammatory. Front Immunol. (2025) 16:1556990. doi: 10.3389/fimmu.2025.1556990,
43.
Blanco FJ June RK 2nd . Cartilage metabolism, mitochondria, and osteoarthritis. J Am Acad Orthop Surg. (2020) 28:e242–4. doi: 10.5435/JAAOS-D-19-00442,
44.
Sueishi T Akasaki Y Goto N Kurakazu I Toya M Kuwahara M et al . GRK5 inhibition attenuates cartilage degradation via decreased NF-κB signaling. Arthritis Rheumatol (Hoboken, NJ). (2020) 72:620–31. doi: 10.1002/art.41152,
45.
Liang R Zhao J Li B Cai P Loh XJ Xu C et al . Implantable and degradable antioxidant poly(ε-caprolactone)-lignin nanofiber membrane for effective osteoarthritis treatment. Biomaterials. (2020) 230:119601. doi: 10.1016/j.biomaterials.2019.119601,
46.
Wei W He S Wang Z Dong J Xiang D Li Y et al . LINC01534 promotes the aberrant metabolic dysfunction and inflammation in IL-1β-simulated osteoarthritic chondrocytes by targeting miR-140-5p. Cartilage. (2021) 13:898s–907s. doi: 10.1177/1947603519888787,
47.
Cheng BF Lian JJ Yang HJ Wang L Yu HH Bi JJ et al . Neural cell adhesion molecule regulates chondrocyte hypertrophy in chondrogenic differentiation and experimental osteoarthritis. Stem Cells Transl Med. (2020) 9:273–83. doi: 10.1002/sctm.19-0190,
48.
Contentin R Demoor M Concari M Desancé M Audigié F Branly T et al . Comparison of the Chondrogenic potential of mesenchymal stem cells derived from bone marrow and umbilical cord blood intended for cartilage tissue engineering. Stem Cell Rev Rep. (2020) 16:126–43. doi: 10.1007/s12015-019-09914-2,
49.
Harrell CR Markovic BS Fellabaum C Arsenijevic N Djonov V Volarevic V . The role of interleukin 1 receptor antagonist in mesenchymal stem cell-based tissue repair and regeneration. BioFactors (Oxford, England). (2020) 46:263–75. doi: 10.1002/biof.1587,
50.
Geng Y Chen J Alahdal M Chang C Duan L Zhu W et al . Intra-articular injection of hUC-MSCs expressing miR-140-5p induces cartilage self-repairing in the rat osteoarthritis. J Bone Miner Metab. (2020) 38:277–88. doi: 10.1007/s00774-019-01055-3,
51.
Yu F Zhu C Wu W . Senile osteoarthritis regulated by the gut microbiota: from mechanisms to treatments. Int J Mol Sci. (2025) 26:505. doi: 10.3390/ijms26041505,
52.
Wu S Yi Z Ling M Liu S Sun Z Guo X . DR4-associated death receptor signal promotes cartilage damage in patients with Kashin-Beck disease. Cartilage. (2021) 13:789s–96s. doi: 10.1177/1947603519886626,
53.
Tsuchiya S Ohashi Y Ishizuka S Ishiguro N O'Rourke DP Knudson CB et al . Suppression of murine osteoarthritis by 4-methylumbelliferone. J Orthop Res. (2020) 38:1122–31. doi: 10.1002/jor.24541,
54.
Zhao X Huang P Li G Feng Y Zhendong L Zhou C et al . Overexpression of Pitx1 attenuates the senescence of chondrocytes from osteoarthritis degeneration cartilage-a self-controlled model for studying the etiology and treatment of osteoarthritis. Bone. (2020) 131:115177. doi: 10.1016/j.bone.2019.115177,
55.
Si HB Yang TM Li L Tian M Zhou L Li DP et al . miR-140 attenuates the progression of early-stage osteoarthritis by retarding chondrocyte senescence. Molecular Therapy Nucleic Acids. (2020) 19:15–30. doi: 10.1016/j.omtn.2019.10.032,
56.
Papathanasiou I Balis C Trachana V Mourmoura E Tsezou A . The synergistic function of miR-140-5p and miR-146a on TLR4-mediated cytokine secretion in osteoarthritic chondrocytes. Biochem Biophys Res Commun. (2020) 522:783–91. doi: 10.1016/j.bbrc.2019.11.168,
57.
Cheng F Hu H Sun K Yan F Geng Y . miR-455-3p enhances chondrocytes apoptosis and inflammation by targeting COL2A1 in the in vitro osteoarthritis model. Biosci Biotechnol Biochem. (2020) 84:695–702. doi: 10.1080/09168451.2019.1690974,
58.
Tian X Teng J Chen J . New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy. (2021) 17:2680–2688. doi: 10.1080/15548627.2020.1823124
59.
Huang X You Y Xi Y Ni B Chu X Zhang R et al . P-Coumaric acid attenuates IL-1β-induced inflammatory responses and cellular senescence in rat chondrocytes. Inflammation. (2020) 43:619–28. doi: 10.1007/s10753-019-01142-7,
60.
Tu Y Ma T Wen T Yang T Xue L Cai M et al . MicroRNA-377-3p alleviates IL-1β-caused chondrocyte apoptosis and cartilage degradation in osteoarthritis in part by downregulating ITGA6. Biochem Biophys Res Commun. (2020) 523:46–53. doi: 10.1016/j.bbrc.2019.11.186,
61.
Ng J Little CB Woods S Whittle S Lee FY Gronthos S et al . Stem cell-directed therapies for osteoarthritis: the promise and the practice. Stem Cells (Dayton, Ohio). (2020) 38:477–86. doi: 10.1002/stem.3139,
62.
Schivo S Khurana S Govindaraj K Scholma J Kerkhofs J Zhong L et al . ECHO, the executable CHOndrocyte: a computational model to study articular chondrocytes in health and disease. Cell Signal. (2020) 68:109471. doi: 10.1016/j.cellsig.2019.109471,
63.
Bartell LR Fortier LA Bonassar LJ Szeto HH Cohen I Delco ML . Mitoprotective therapy prevents rapid, strain-dependent mitochondrial dysfunction after articular cartilage injury. J Orthop Res. (2020) 38:1257–67. doi: 10.1002/jor.24567,
64.
Liu Y Da W Xu MJ Xiao CX Deng T Zhou SL et al . Single-cell transcriptomics reveals novel chondrocyte and osteoblast subtypes and their role in knee osteoarthritis pathogenesis. Signal Transduct Target Ther. (2025) 10:40. doi: 10.1038/s41392-025-02136-8,
65.
Xiang A Deng H Cheng K Liu H Lin L Qu X et al . Laser photobiomodulation for cartilage defect in animal models of knee osteoarthritis: a systematic review and meta-analysis. Lasers Med Sci. (2020) 35:789–96. doi: 10.1007/s10103-019-02937-8,
66.
Attur M Zhou H Samuels J Krasnokutsky S Yau M Scher JU et al . Interleukin 1 receptor antagonist (IL1RN) gene variants predict radiographic severity of knee osteoarthritis and risk of incident disease. Ann Rheum Dis. (2020) 79:400–7. doi: 10.1136/annrheumdis-2019-216055,
67.
Dai C Jia J Kot A Liu X Liu L Jiang M et al . Selective inhibition of progesterone receptor in osteochondral progenitor cells, but not in mature chondrocytes, modulated subchondral bone structures. Bone. (2020) 132:115196. doi: 10.1016/j.bone.2019.115196,
68.
Ma W Wang X Wang C Gong M Ren P . Up-regulation of P21-activated kinase 1 in osteoarthritis chondrocytes is responsible for osteoarthritic cartilage destruction. Biosci Rep. (2020) 40:1017. doi: 10.1042/BSR20191017,
69.
Liu F Liu X Yang Y Sun Z Deng S Jiang Z et al . NEAT1/miR-193a-3p/SOX5 axis regulates cartilage matrix degradation in human osteoarthritis. Cell Biol Int. (2020) 44:947–57. doi: 10.1002/cbin.11291,
70.
Xie X Tan Y Xiao H Wen Y Magdalou J Chen L et al . Subchondral bone dysplasia mediates susceptibility to osteoarthritis in female adult offspring rats induced by prenatal caffeine exposure. Toxicol Lett. (2020) 321:122–30. doi: 10.1016/j.toxlet.2019.12.026,
71.
Zou X Xu H Qian W . Macrophage polarization in the osteoarthritis pathogenesis and treatment. Orthop Surg. (2025) 17:22–35. doi: 10.1111/os.14302,
72.
Li Z Li Y . Effect of growth hormone releasing hormone on chondrocytes of osteoarthritis. Korean J Intern Med. (2022) 37:222–9. doi: 10.3904/kjim.2018.399,
73.
Xiang W Zhang T Li B Li S Zhang B Fang S et al . Senescent macrophages induce ferroptosis in skeletal muscle and accelerate osteoarthritis-related muscle atrophy. Nature Aging. (2025) 5:1295–316. doi: 10.1038/s43587-025-00907-0,
74.
Ji Q Qiao X Liu Y Wang D Yan J . Silencing of long-chain non-coding RNA GAS5 in osteoarthritic chondrocytes is mediated by targeting the miR-34a/Bcl-2 axis. Mol Med Rep. (2020) 21:1310–9. doi: 10.3892/mmr.2019.10900
75.
Guo Y Tian L Du X Deng Z . MiR-203 regulates estrogen receptor α and cartilage degradation in IL-1β-stimulated chondrocytes. J Bone Miner Metab. (2020) 38:346–56. doi: 10.1007/s00774-019-01062-4,
76.
Radbakhsh S Najar M Merimi M Benderdour M Fernandes JC Martel-Pelletier J et al . RNA modifications in osteoarthritis: Epitranscriptomic insights into pathogenesis and therapeutic targets. Int J Mol Sci. (2025) 26:955. doi: 10.3390/ijms26104955,
77.
Chu J Yan B Zhang J Peng L Ao X Zheng Z et al . Casticin attenuates osteoarthritis-related cartilage degeneration by inhibiting the ROS-mediated NF-κB signaling pathway in vitro and in vivo. Inflammation. (2020) 43:810–20. doi: 10.1007/s10753-019-01167-y,
78.
Duan ZX Tu C Liu Q Li SQ Li YH Xie P et al . Adiponectin receptor agonist AdipoRon attenuates calcification of osteoarthritis chondrocytes by promoting autophagy. J Cell Biochem. (2020) 121:3333–44. doi: 10.1002/jcb.29605,
79.
Shao QQ Zhang SN Li XZ . Pathogenesis of osteoarthritis and treatment with traditional Chinese medicine: new perspectives on platelet aggregation. Int Immunopharmacol. (2025) 167:115665. doi: 10.1016/j.intimp.2025.115665,
80.
Zhang G Gu M Xu Y Wu Z . A comprehensive analysis on the effects of 1,25(OH)2D3 on primary chondrocytes cultured from patients with osteoarthritis. Gene. (2020) 730:144322. doi: 10.1016/j.gene.2019.144322,
81.
Shamrock AG Wolf BR Ortiz SF Duchman KR Bollier MJ Carender CN et al . Preoperative validation of the patient-reported outcomes measurement information system in patients with articular cartilage defects of the knee. Arthroscopy: J Arthroscopic & Related Surg: Official Pub Arthroscopy Association of North America and the International Arthroscopy Association. (2020) 36:516–20. doi: 10.1016/j.arthro.2019.08.043,
82.
Sperry MM Yu YH Kartha S Ghimire P Welch RL Winkelstein BA et al . Intra-articular etanercept attenuates pain and hypoxia from TMJ loading in the rat. J Orthop Res. (2020) 38:1316–26. doi: 10.1002/jor.24581,
83.
Kim D Ansari MM Ghosh M Heo Y Choi KC Son YO . Implications of obesity-mediated cellular dysfunction and adipocytokine signaling pathways in the pathogenesis of osteoarthritis. Mol Asp Med. (2025) 103:101361. doi: 10.1016/j.mam.2025.101361,
84.
Sun P Wu Y Li X Jia Y . miR-142-5p protects against osteoarthritis through competing with lncRNA XIST. J Gene Med. (2020) 22:e3158. doi: 10.1002/jgm.3158,
85.
Du X Chen Y Zhang Q Lin J Yu Y Pan Z et al . Ezh2 ameliorates osteoarthritis by activating TNFSF13B. J Bone Mineral Res: Official J American Society for Bone and Mineral Res. (2020) 35:956–65. doi: 10.1002/jbmr.3952,
86.
Cai J Chen Z Yang X Cai JW Chen L Chen S et al . Role of mitochondrial damage-associated molecular patterns in osteoarthritis: from pathogenesis, diagnosis, and prognosis to therapeutics. Pharmacol Res. (2025) 219:107865. doi: 10.1016/j.phrs.2025.107865,
87.
Huang K Cai H . The role of chondrocyte senescence in osteoarthritis pathogenesis and therapeutic implications. Exp Gerontol. (2025) 208:112828. doi: 10.1016/j.exger.2025.112828,
88.
Li ZZ Wang F Liu S Li H Wang Y . Ablation of PKM2 ameliorated ER stress-induced apoptosis and associated inflammation response in IL-1β-treated chondrocytes via blocking Rspo2-mediated Wnt/β-catenin signaling. J Cell Biochem. (2020) 121:4204–13. doi: 10.1002/jcb.29611,
89.
Liu Z Zhang H Wang H Wei L Niu L . Magnolol alleviates IL-1β-induced dysfunction of chondrocytes through repression of SIRT1/AMPK/PGC-1α signaling pathway. J Interferon Cytokine Res: Official J Int Society for Interferon and Cytokine Res. (2020) 40:145–51. doi: 10.1089/jir.2019.0139,
90.
Ackermann J Mestriner AB VanArsdale C Gomoll AH . Prior surgery negatively affects cell culture identity in patients undergoing autologous chondrocyte implantation. Am J Sports Med. (2020) 48:635–41. doi: 10.1177/0363546519897051,
91.
Zhang X Cai D Zhou F Yu J Wu X Yu D et al . Targeting downstream subcellular YAP activity as a function of matrix stiffness with Verteporfin-encapsulated chitosan microsphere attenuates osteoarthritis. Biomaterials. (2020) 232:119724. doi: 10.1016/j.biomaterials.2019.119724,
92.
Shu Z Miao X Tang T Zhan P Zeng L Jiang Y . The GSK-3β/β-catenin signaling pathway is involved in HMGB1-induced chondrocyte apoptosis and cartilage matrix degradation. Int J Mol Med. (2020) 45:769–78. doi: 10.3892/ijmm.2020.4460,
93.
Zheng W Ding B Li X Liu D Yokota H Zhang P . Knee loading repairs osteoporotic osteoarthritis by relieving abnormal remodeling of subchondral bone via Wnt/β-catenin signaling. FASEB J. (2020) 34:3399–412. doi: 10.1096/fj.201902117R,
94.
Sun JL Yan JF Li J Wang WR Yu SB Zhang HY et al . Conditional deletion of Adrb2 in mesenchymal stem cells attenuates osteoarthritis-like defects in temporomandibular joint. Bone. (2020) 133:115229. doi: 10.1016/j.bone.2020.115229,
95.
Xu C Jiang T Ni S Chen C Li C Zhuang C et al . FSTL1 promotes nitric oxide-induced chondrocyte apoptosis via activating the SAPK/JNK/caspase3 signaling pathway. Gene. (2020) 732:144339. doi: 10.1016/j.gene.2020.144339,
96.
Wu Z Wang X Yang Y Xia C Lou L Fei W et al . The role of FoxO3a in the pathogenesis of osteoarthritis and its therapeutic applications. Front Immunol. (2025) 16:1650194. doi: 10.3389/fimmu.2025.1650194,
97.
Yao J Long H Zhao J Zhong G Li J . Nifedipine inhibits oxidative stress and ameliorates osteoarthritis by activating the nuclear factor erythroid-2-related factor 2 pathway. Life Sci. (2020) 253:117292. doi: 10.1016/j.lfs.2020.117292,
98.
Kotelsky A Carrier JS Aggouras A Richards MS Buckley MR . Evidence that reduction in volume protects in situ articular chondrocytes from mechanical impact. Connect Tissue Res. (2020) 61:360–74. doi: 10.1080/03008207.2020.1711746,
99.
Xie SC Yang L Shu T Liu Q Wang W . miR-149-5p mitigates tumor necrosis factor-α-induced chondrocyte apoptosis by inhibiting TRADD. Arch Med Sci AMS. (2024) 20:602–11. doi: 10.5114/aoms.2020.92324
100.
Abbasi Pashaki P Rahim F Habibi Roudkenar M Razavi-Toosi S Ebrahimi A . MicroRNA tough decoy knockdowns miR-195 and represses hypertrophy in chondrocytes. Appl Biochem Biotechnol. (2020) 191:1056–71. doi: 10.1007/s12010-020-03229-6,
101.
Xi Y Huang X Tan G Chu X Zhang R Ma X et al . Protective effects of Erdosteine on interleukin-1β-stimulated inflammation via inhibiting the activation of MAPK, NF-κB, and Wnt/β-catenin signaling pathways in rat osteoarthritis. Eur J Pharmacol. (2020) 873:172925. doi: 10.1016/j.ejphar.2020.172925,
102.
Uddin SMZ Komatsu DE . Therapeutic potential low-intensity pulsed ultrasound for osteoarthritis: pre-clinical and clinical perspectives. Ultrasound Med Biol. (2020) 46:909–20. doi: 10.1016/j.ultrasmedbio.2019.12.007,
103.
Huang J Chen C Liang C Luo P Xia G Zhang L et al . Dysregulation of the Wnt signaling pathway and synovial stem cell dysfunction in osteoarthritis development. Stem Cells Dev. (2020) 29:401–13. doi: 10.1089/scd.2019.0260,
104.
Kim KI Lee MC Lee JH Moon YW Lee WS Lee HJ et al . Clinical efficacy and safety of the intra-articular injection of autologous adipose-derived mesenchymal stem cells for knee osteoarthritis: a phase III, randomized, double-blind, placebo-controlled trial. Am J Sports Med. (2023) 51:2243–53. doi: 10.1177/03635465231179223,
105.
Guan M Zhu Y Liao B Tan Q Qi H Zhang B et al . Low-intensity pulsed ultrasound inhibits VEGFA expression in chondrocytes and protects against cartilage degeneration in experimental osteoarthritis. FEBS Open Bio. (2020) 10:434–43. doi: 10.1002/2211-5463.12801,
106.
Huang J Wang L Zhou J Dai T Zhu W Wang T et al . Unveiling the ageing-related genes in diagnosing osteoarthritis with metabolic syndrome by integrated bioinformatics analysis and machine learning. Artificial Cells, Nanomed Biotechnol. (2025) 53:57–68. doi: 10.1080/21691401.2025.2471762,
107.
Song SY Hong J Go S Lim S Sohn HS Kang M et al . Interleukin-4 gene transfection and spheroid formation potentiate therapeutic efficacy of mesenchymal stem cells for osteoarthritis. Adv Healthc Mater. (2020) 9:e1901612. doi: 10.1002/adhm.201901612,
108.
Lee KI Choi S Matsuzaki T Alvarez-Garcia O Olmer M Grogan SP et al . FOXO1 and FOXO3 transcription factors have unique functions in meniscus development and homeostasis during aging and osteoarthritis. Proc Natl Acad Sci USA. (2020) 117:3135–43. doi: 10.1073/pnas.1918673117,
109.
Wang X Chen J Xu H Wu J Xie S . Identification of a novel diagnostic biomarker for osteoarthritis associated with chromatin regulators based on bioinformatics and experiments. J Orthop Surg Res. (2025) 20:1047. doi: 10.1186/s13018-025-06493-6,
110.
Riegger J Huber-Lang M Brenner RE . Crucial role of the terminal complement complex in chondrocyte death and hypertrophy after cartilage trauma. Osteoarthr Cartil. (2020) 28:685–97. doi: 10.1016/j.joca.2020.01.004,
111.
Zhao C Kong K Liu P Chen X Rong K Zhang P et al . Regulating obesity-induced osteoarthritis by targeting p53-FOXO3, osteoclast ferroptosis, and mesenchymal stem cell adipogenesis. Nat Commun. (2025) 16:4532. doi: 10.1038/s41467-025-59883-z,
112.
Moulin D Sellam J Berenbaum F Guicheux J Boutet MA . The role of the immune system in osteoarthritis: mechanisms, challenges and future directions. Nat Rev Rheumatol. (2025) 21:221–36. doi: 10.1038/s41584-025-01223-y,
113.
Qin Y Di J Guo Z Chen S Xiang C . Association of organs-crosstalk with the pathogenesis of osteoarthritis: cartilage as a key player. Front Endocrinol. (2025) 16:1593658. doi: 10.3389/fendo.2025.1593658,
114.
Jeon JH Yun BG Lim MJ Kim SJ Lim MH Lim JY et al . Rapid cartilage regeneration of spheroids composed of human nasal septum-derived chondrocyte in rat Osteochondral defect model. Tissue Eng Regenerative Med. (2020) 17:81–90. doi: 10.1007/s13770-019-00231-w,
115.
Xiao Z Wang J Chen S Feng Y . Autophagy promotion enhances the protective effect of Morroniside on human OA chondrocyte. Biosci Biotechnol Biochem. (2020) 84:989–96. doi: 10.1080/09168451.2020.1717925,
116.
Zhao M Song L Liu Z Li Y Wang Y . Necroptosis and its role in the pathogenesis and treatment of temporomandibular joint osteoarthritis: a narrative review. Arch Oral Biol. (2025) 176:106300. doi: 10.1016/j.archoralbio.2025.106300,
117.
Rajendran K Murthy NS Frick MA Tao S Unger MD LaVallee KT et al . Quantitative knee arthrography in a large animal model of osteoarthritis using photon-counting detector CT. Investig Radiol. (2020) 55:349–56. doi: 10.1097/RLI.0000000000000648,
118.
Sun MM Beier F . Liver X receptor activation regulates genes involved in lipid homeostasis in developing chondrocytes. Osteoarthritis Cartilage Open. (2020) 2:100030. doi: 10.1016/j.ocarto.2020.100030,
119.
Song JS Hong KT Kim NM Jung JY Park HS Lee SH et al . Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: two-year follow-up. Regenerative Therapy. (2020) 14:32–9. doi: 10.1016/j.reth.2019.10.003,
120.
Miyaji N Nishida K Tanaka T Araki D Kanzaki N Hoshino Y et al . Inhibition of knee osteoarthritis progression in mice by administering SRT2014, an activator of silent information regulator 2 Ortholog 1. Cartilage. (2021) 13:1356s–66s. doi: 10.1177/1947603519900795,
121.
Suyasa IK Lestari AAW Setiawan I . Recombinant platelet-derived growth factor-BB and hyaluronic acid stimulates knee cartilage regeneration by forming higher chondrocytes count and lower YKL-40 level in rats model. J Clinical Orthopaedics Trauma. (2020) 11:S76–s79. doi: 10.1016/j.jcot.2019.01.010,
122.
Pezzanite L Chow L Soontararak S Phillips J Goodrich L Dow S . Amikacin induces rapid dose-dependent apoptotic cell death in equine chondrocytes and synovial cells in vitro. Equine Vet J. (2020) 52:715–24. doi: 10.1111/evj.13243,
123.
Jiang L Xu K Li J Zhou X Xu L Wu Z et al . Nesfatin-1 suppresses interleukin-1β-induced inflammation, apoptosis, and cartilage matrix destruction in chondrocytes and ameliorates osteoarthritis in rats. Aging (Albany NY). (2020) 12:1760–77. doi: 10.18632/aging.102711,
124.
Delco ML Goodale M Talts JF Pownder SL Koff MF Miller AD et al . Integrin α10β1-selected mesenchymal stem cells mitigate the progression of osteoarthritis in an equine Talar impact model. Am J Sports Med. (2020) 48:612–23. doi: 10.1177/0363546519899087,
125.
Can VC Locke IC Kaneva MK Kerrigan MJP Merlino F De Pascale C et al . Novel anti-inflammatory and chondroprotective effects of the human melanocortin MC1 receptor agonist BMS-470539 dihydrochloride and human melanocortin MC3 receptor agonist PG-990 on lipopolysaccharide activated chondrocytes. Eur J Pharmacol. (2020) 872:172971. doi: 10.1016/j.ejphar.2020.172971,
126.
Tian W Dong SS Jiang F Zhang JQ Wang C He CY et al . Genetic transcriptional regulation profiling of cartilage reveals pathogenesis of osteoarthritis. EBioMedicine. (2025) 117:105821. doi: 10.1016/j.ebiom.2025.105821,
127.
Li H Chen J Li B Fang X . The protective effects of dulaglutide against advanced glycation end products (AGEs)-induced degradation of type II collagen and aggrecan in human SW1353 chondrocytes. Chem Biol Interact. (2020) 322:108968. doi: 10.1016/j.cbi.2020.108968
128.
Cui L Han Y Dong Z . miR-106a mimics the nuclear factor-κB signalling pathway by targeting DR6 in rats with osteoarthritis. Archives Medical Sci: AMS. (2024) 20:302–8. doi: 10.5114/aoms.2020.92831,
129.
Yang J Song X Feng Y Liu N Fu Z Wu J et al . Natural ingredients-derived antioxidants attenuate H(2)O(2)-induced oxidative stress and have chondroprotective effects on human osteoarthritic chondrocytes via Keap1/Nrf2 pathway. Free Radic Biol Med. (2020) 152:854–64. doi: 10.1016/j.freeradbiomed.2020.01.185,
130.
Zhang M Wang Z Li B Sun F Chen A Gong M . Identification of microRNA-363-3p as an essential regulator of chondrocyte apoptosis in osteoarthritis by targeting NRF1 through the p53-signaling pathway. Mol Med Rep. (2020) 21:1077–88. doi: 10.3892/mmr.2020.10940,
131.
He B Wu F Li X Liu Y Fan L Li H . Mitochondrial dependent pathway is involved in the protective effects of carboxymethylated chitosan on nitric oxide-induced apoptosis in chondrocytes. BMC Complementary Med Therapies. (2020) 20:23. doi: 10.1186/s12906-019-2808-x,
132.
López-Senra E Casal-Beiroa P López-Álvarez M Serra J González P Valcarcel J et al . Impact of prevalence ratios of chondroitin sulfate (CS)- 4 and −6 isomers derived from marine sources in cell proliferation and Chondrogenic differentiation processes. Mar Drugs. (2020) 18:94. doi: 10.3390/md18020094,
133.
Li Y Nie J Jiang P . Oleanolic acid mitigates interleukin-1β-induced chondrocyte dysfunction by regulating miR-148-3p-modulated FGF2 expression. J Gene Med. (2020) 22:e3169. doi: 10.1002/jgm.3169,
134.
Li H Kang L Wu R Li C Zhang Q Zhong R et al . MiR-378-mediated glycolytic metabolism enriches the Pax7(hi) subpopulation of satellite cells. Cell Regen (Lond). (2022) 11:11. doi: 10.1186/s13619-022-00112-z,
135.
Ruscitto A Morel MM Shawber CJ Reeve G Lecholop MK Bonthius D et al . Evidence of vasculature and chondrocyte to osteoblast transdifferentiation in craniofacial synovial joints: implications for osteoarthritis diagnosis and therapy. FASEB J. (2020) 34:4445–61. doi: 10.1096/fj.201902287R,
136.
Lu W He Z Shi J Wang Z Wu W Liu J et al . AMD3100 attenuates post-traumatic osteoarthritis by maintaining transforming growth factor-β1-induced expression of tissue inhibitor of metalloproteinase-3 via the phosphatidylinositol 3-kinase/Akt pathway. Front Pharmacol. (2019) 10:1554. doi: 10.3389/fphar.2019.01554,
137.
Lee SA Park BR Moon SM Hong JH Kim DK Kim CS . Chondroprotective effect of cynaroside in IL-1β-induced primary rat chondrocytes and organ explants via NF-κB and MAPK signaling inhibition. Oxidative Med Cell Longev. (2020) 2020:9358080. doi: 10.1155/2020/9358080
138.
Sirong S Yang C Taoran T Songhang L Shiyu L Yuxin Z et al . Effects of tetrahedral framework nucleic acid/wogonin complexes on osteoarthritis. Bone Research. (2020) 8:6. doi: 10.1038/s41413-019-0077-4,
139.
Yang G Wang Y Chen Y Huang R . UFL1 attenuates IL-1β-induced inflammatory response in human osteoarthritis chondrocytes. Int Immunopharmacol. (2020) 81:106278. doi: 10.1016/j.intimp.2020.106278,
140.
Riff AJ Huddleston HP Cole BJ Yanke AB . Autologous chondrocyte implantation and Osteochondral allograft transplantation render comparable outcomes in the setting of failed marrow stimulation. Am J Sports Med. (2020) 48:861–70. doi: 10.1177/0363546520902434,
141.
Zhou Y Ming J Deng M Li Y Li B Li J et al . Berberine-mediated up-regulation of surfactant protein D facilitates cartilage repair by modulating immune responses via the inhibition of TLR4/NF-ĸB signaling. Pharmacol Res. (2020) 155:104690. doi: 10.1016/j.phrs.2020.104690,
142.
Lin Z Lin C Fu C Lu H Jin H Chen Q et al . The protective effect of Ellagic acid (EA) in osteoarthritis: an in vitro and in vivo study. Biomed Pharmacother. (2020) 125:109845. doi: 10.1016/j.biopha.2020.109845,
143.
Park HJ Lee CK Song SH Yun JH Lee A Park HJ . Highly bioavailable curcumin powder suppresses articular cartilage damage in rats with mono-iodoacetate (MIA)-induced osteoarthritis. Food Sci Biotechnol. (2020) 29:251–63. doi: 10.1007/s10068-019-00679-5,
144.
Li L Wei X Wang D Lv Z Geng X Li P et al . Positive effects of a young systemic environment and high growth differentiation factor 11 levels on chondrocyte proliferation and cartilage matrix synthesis in old mice. Arthritis Rheumatol (Hoboken, NJ). (2020) 72:1123–33. doi: 10.1002/art.41230,
145.
Shin HJ Park H Shin N Kwon HH Yin Y Hwang JA et al . p47phox siRNA-loaded PLGA nanoparticles suppress ROS/oxidative stress-induced chondrocyte damage in osteoarthritis. Polymers. (2020) 12:443. doi: 10.3390/polym12020443,
146.
Gatenholm B Lindahl C Brittberg M Simonsson S . Collagen 2A type B induction after 3D bioprinting chondrocytes In situ into osteoarthritic chondral Tibial lesion. Cartilage. (2021) 13:1755s–69s. doi: 10.1177/1947603520903788,
147.
Dicks A Wu CL Steward N Adkar SS Gersbach CA Guilak F . Prospective isolation of chondroprogenitors from human iPSCs based on cell surface markers identified using a CRISPR-Cas9-generated reporter. Stem Cell Res Ther. (2020) 11:66. doi: 10.1186/s13287-020-01597-8,
148.
Yang F Huang R Ma H Zhao X Wang G . miRNA-411 regulates chondrocyte autophagy in osteoarthritis by targeting hypoxia-inducible factor 1 alpha (HIF-1α). Med Sci Monit. (2020) 26:e921155. doi: 10.12659/MSM.921155,
149.
Jiang RH Xu JJ Zhu DC Li JF Zhang CX Lin N et al . Glycyrrhizin inhibits osteoarthritis development through suppressing the PI3K/AKT/NF-κB signaling pathway in vivo and in vitro. Food Funct. (2020) 11:2126–36. doi: 10.1039/C9FO02241D,
150.
Gao YH Zhao CW Liu B Dong N Ding L Li YR et al . Corrigendum to "An update on the association between metabolic syndrome and osteoarthritis and on the potential role of leptin in osteoarthritis" [cytokine 129 (2020) 155043]. Cytokine. (2025) 190:156923. doi: 10.1016/j.cyto.2025.156923,
151.
Yang YZ Li JD Zhang JG Zhang K Zhang AR Li PP et al . Mechanism of action and new developments in the study of curcumin in the treatment of osteoarthritis: a narrative review. Inflammopharmacology. (2025) 33:929–40. doi: 10.1007/s10787-025-01665-6,
152.
Jo SY Domowicz MS Henry JG Schwartz NB . The role of Dot1l in prenatal and postnatal murine chondrocytes and trabecular bone. JBMR Plus. (2020) 4:e10254. doi: 10.1002/jbm4.10254,
153.
An Y Wan G Tao J Cui M Zhou Q Hou W . Down-regulation of microRNA-203a suppresses IL-1β-induced inflammation and cartilage degradation in human chondrocytes through Smad3 signaling. Biosci Rep. (2020) 40:723. doi: 10.1042/BSR20192723,
154.
Silva JC Han X Silva TP Xia K Mikael PE Cabral JMS et al . Glycosaminoglycan remodeling during chondrogenic differentiation of human bone marrow−/synovial-derived mesenchymal stem/stromal cells under normoxia and hypoxia. Glycoconj J. (2020) 37:345–60. doi: 10.1007/s10719-020-09911-5,
155.
Li H Ding X Terkeltaub R Lin H Zhang Y Zhou B et al . Exploration of metformin as novel therapy for osteoarthritis: preventing cartilage degeneration and reducing pain behavior. Arthritis Res Ther. (2020) 22:34. doi: 10.1186/s13075-020-2129-y,
156.
Sun X Wang S Tian J Li M . Role of long non-coding RNAs in cartilage development and osteoarthritis pathogenesis. Eur J Med Res. (2025) 30:900. doi: 10.1186/s40001-025-03173-3,
157.
Hong G . Enoxolone suppresses apoptosis in chondrocytes and progression of osteoarthritis via modulating the ERK1/2 signaling pathway. Arch Med Sci AMS. (2024) 20:947–61. doi: 10.5114/aoms.2020.93211
158.
Moqbel SAA He Y Xu L Ma C Ran J Xu K et al . Rat chondrocyte inflammation and osteoarthritis are ameliorated by madecassoside. Oxidative Med Cell Longev. (2020) 2020:7540197. doi: 10.1155/2020/7540197
159.
Anderson-Baron M Kunze M Mulet-Sierra A Adesida AB . Effect of cell seeding density on matrix-forming capacity of meniscus fibrochondrocytes and nasal chondrocytes in meniscus tissue engineering. FASEB J. (2020) 34:5538–51. doi: 10.1096/fj.201902559R,
160.
Tang S Zhou W Zhong X Xu J Huang H Zheng X et al . Arctigenin prevents the progression of osteoarthritis by targeting PI3K/Akt/NF-κB axis: In vitro and in vivo studies. J Cell Mol Med. (2020) 24:4183–93. doi: 10.1111/jcmm.15079,
161.
Shi FL Ren LX . Up-regulated miR-374a-3p relieves lipopolysaccharides induced injury in CHON-001 cells via regulating wingless-type MMTV integration site family member 5B. Mol Cell Probes. (2020) 51:101541. doi: 10.1016/j.mcp.2020.101541,
162.
Scotece M Koskinen-Kolasa A Pemmari A Leppänen T Hämäläinen M Moilanen T et al . Novel adipokine associated with OA: retinol binding protein 4 (RBP4) is produced by cartilage and is correlated with MMPs in osteoarthritis patients. Inflammation Res: Official J European Histamine Res Society. (2020) 69:415–21. doi: 10.1007/s00011-020-01326-0,
163.
Hafsia N Forien M Renaudin F Delacour D Reboul P Van Lent P et al . Galectin 3 deficiency alters chondrocyte primary cilium formation and exacerbates cartilage destruction via mitochondrial apoptosis. Int J Mol Sci. (2020) 21:486. doi: 10.3390/ijms21041486,
164.
Zhang W Zhang C Hu C Luo C Zhong B Yu X . Circular RNA-CDR1as acts as the sponge of microRNA-641 to promote osteoarthritis progression. J Inflammation (London, England). (2020) 17:8. doi: 10.1186/s12950-020-0234-y,
165.
Yang B Li X Fu C Cai W Meng B Qu Y et al . Extracellular vesicles in osteoarthritis of peripheral joint and temporomandibular joint. Front Endocrinol. (2023) 14:1158744. doi: 10.3389/fendo.2023.1158744,
166.
Peng K Li Y Lu C Hu S . ABIN-1 protects chondrocytes from lipopolysaccharide-induced inflammatory injury through the inactivation of NF-κB signalling. Clin Exp Pharmacol Physiol. (2020) 47:1212–20. doi: 10.1111/1440-1681.13291,
167.
Kang X Qian Z Liu J Feng D Li H Zhang Z et al . Neuropeptide Y acts directly on cartilage homeostasis and exacerbates progression of osteoarthritis through NPY2R. J Bone Miner Res. (2020) 35:1375–84. doi: 10.1002/jbmr.3991,
168.
Ren T Wei P Song Q Ye Z Wang Y Huang L . MiR-140-3p ameliorates the progression of osteoarthritis via targeting CXCR4. Biol Pharm Bull. (2020) 43:810–6. doi: 10.1248/bpb.b19-00959,
169.
Zhang G Zhang H You W Tang X Li X Gong Z . Therapeutic effect of resveratrol in the treatment of osteoarthritis via the MALAT1/miR-9/NF-κB signaling pathway. Exp Ther Med. (2020) 19:2343–52. doi: 10.3892/etm.2020.8471,
170.
Riegger J Brenner RE . Pathomechanisms of posttraumatic osteoarthritis: chondrocyte behavior and fate in a precarious environment. Int J Mol Sci. (2020) 21:560. doi: 10.3390/ijms21051560,
171.
Tarricone E Elia R Mattiuzzo E Faggian A Pozzuoli A Ruggieri P et al . The viability and anti-inflammatory effects of hyaluronic acid-Chitlac-Tracimolone Acetonide- β-Cyclodextrin complex on human chondrocytes. Cartilage. (2021) 13:920s–4s. doi: 10.1177/1947603520908658,
172.
Feng K Chen H Xu C . Chondro-protective effects of celastrol on osteoarthritis through autophagy activation and NF-κB signaling pathway inhibition. Inflamm Res. (2020) 69:385–400. doi: 10.1007/s00011-020-01327-z,
173.
Jeon HJ Yoon KA An ES Kang TW Sim YB Ahn J et al . Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells combined with cartilage acellular matrix mediated via bone Morphogenic protein 6 in a rabbit model of articular cruciate ligament transection. Stem Cell Rev Rep. (2020) 16:596–611. doi: 10.1007/s12015-020-09958-9,
174.
Cai Z Hong M Xu L Yang K Li C Sun T et al . Prevent action of magnoflorine with hyaluronic acid gel from cartilage degeneration in anterior cruciate ligament transection induced osteoarthritis. Biomed Pharmacother. (2020) 126:109733. doi: 10.1016/j.biopha.2019.109733,
175.
Baba S Motomura G Ikemura S Sonoda K Kubo Y Utsunomiya T et al . Femoral head fracture similar to slipped capital femoral epiphysis in an elderly woman with antecedent hip osteoarthritis after subchondral insufficiency fracture: a case report. J Orthop Sci. (2020) 25:533–6. doi: 10.1016/j.jos.2017.09.009,
176.
Nakagawa Y Muneta T Watanabe T Horie M Nakamura T Otabe K et al . Arthroscopic centralization achieved good clinical improvements and radiographic outcomes in a rugby player with osteoarthritis after subtotal lateral meniscectomy: a case report. J Orthop Sci. (2020) 25:537–43. doi: 10.1016/j.jos.2017.09.011,
177.
Kloek PT Bossen PT de Vries H de Bakker DHP Veenhof PT Dekker JP . Physiotherapists' experiences with a blended osteoarthritis intervention: a mixed methods study. Physiother Theory Pract. (2020) 36:572–9. doi: 10.1080/09593985.2018.1489926
178.
Skármeta ND Araneda LD Araya CD . Destructive psoriatic arthritis of the temporomandibular joint: a clinical case, an overview of the pathophysiology and its differential diagnoses. Cranio: J Craniomandibular Prac. (2020) 38:201–7. doi: 10.1080/08869634.2018.1484575,
179.
Lee SH Lee OS Kim ST Lee YS . Revisiting arthroscopic partial meniscectomy for degenerative tears in knees with mild or no osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin J Sport Med. (2020) 30:195–202. doi: 10.1097/JSM.0000000000000585
180.
Hevesi M Sanders TL Pareek A Milbrandt TA Levy BA Stuart MJ et al . Osteochondritis Dissecans in the knee of skeletally immature patients: rates of persistent pain, osteoarthritis, and arthroplasty at mean 14-years' follow-up. Cartilage. (2020) 11:291–9. doi: 10.1177/1947603518786545,
181.
Ngarmukos S Scaramuzza S Theerawattanapong N Tanavalee A Honsawek S . Circulating and synovial fluid heat shock protein 70 are correlated with severity in knee osteoarthritis. Cartilage. (2020) 11:323–8. doi: 10.1177/1947603518790075,
182.
Flynn C Hurtig M Lamoure E Cummins E Roati V Lowerison M et al . Modeling and staging of osteoarthritis progression using serial CT imaging and arthroscopy. Cartilage. (2020) 11:338–47. doi: 10.1177/1947603518789997,
183.
Melugin HP Desai VS Levy BA Tanaka Y Horibe S Nakamura N et al . Osteochondritis Dissecans of the knee: short-term outcomes of a hybrid technique to restore a partially salvageable progeny fragment. Cartilage. (2020) 11:300–8. doi: 10.1177/1947603518796132,
184.
Rai MF Sandell LJ Barrack TN Cai L Tycksen ED Tang SY et al . A microarray study of articular cartilage in relation to obesity and severity of knee osteoarthritis. Cartilage. (2020) 11:458–72. doi: 10.1177/1947603518796122,
185.
Macías-Hernández SI Zepeda-Borbón ER Lara-Vázquez BI Cuevas-Quintero NM Morones-Alba JD Cruz-Medina E et al . Prevalence of clinical and radiological osteoarthritis in knee, hip, and hand in an urban adult population of Mexico City. Reumatol Clin. (2020) 16:156–60. doi: 10.1016/j.reuma.2018.06.001,
186.
Van de Vyver A Clockaerts S van de Lest CHA Wei W Verhaar J Van Osch G et al . Synovial fluid fatty acid profiles differ between osteoarthritis and healthy patients. Cartilage. (2020) 11:473–8. doi: 10.1177/1947603518798891,
187.
Peeler J Leiter J MacDonald P . Effect of body weight-supported exercise on symptoms of knee osteoarthritis: a follow-up investigation. Clin J Sport Med: Official J Canadian Acad Sport Med. (2020) 30:e178–85. doi: 10.1097/JSM.0000000000000668,
188.
Hart HF Collins NJ Ackland DC Crossley KM . Is self-reported knee stability associated with symptoms, function, and quality of life in people with knee osteoarthritis after anterior cruciate ligament reconstruction?Clin J Sport Med. (2020) 30:e134–8. doi: 10.1097/JSM.0000000000000674
189.
Malahias MA Roumeliotis L Nikolaou VS Chronopoulos E Sourlas I Babis GC . Platelet-rich plasma versus corticosteroid intra-articular injections for the treatment of Trapeziometacarpal arthritis: a prospective randomized controlled clinical trial. Cartilage. (2021) 12:51–61. doi: 10.1177/1947603518805230,
190.
Saltzman BM Redondo ML Beer A Cotter EJ Frank RM Yanke AB et al . Wide variation in methodology in level I and II studies on cartilage repair: a systematic review of available clinical trials comparing patient demographics, treatment means, and outcomes reporting. Cartilage. (2021) 12:7–23. doi: 10.1177/1947603518809398,
191.
Chinzei N Kanzaki N Matsushita T Matsumoto T Hayashi S Hoshino Y et al . Total ankle arthroplasty with total talar prosthesis for talar osteonecrosis with ankle osteoarthritis: a case report. J Orthop Sci. (2021) 26:725–30. doi: 10.1016/j.jos.2018.10.006,
192.
Neelapala YVR Bhagat M Shah P . Hip muscle strengthening for knee osteoarthritis: a systematic review of literature. J Geriatr Phys Ther. (2001) 43:89–98. doi: 10.1519/JPT.0000000000000214
193.
Tang Y Li Y Xin D Chen L Xiong Z Yu X . Icariin alleviates osteoarthritis by regulating autophagy of chondrocytes by mediating PI3K/AKT/mTOR signaling. Bioengineered. (2021) 12:2984–99. doi: 10.1080/21655979.2021.1943602,
194.
Wang Z Xu H Wang Z Wang Y Diao J Chen J et al . Traditional Chinese manual therapy (Tuina) improves knee osteoarthritis by regulating chondrocyte autophagy and apoptosis via the PI3K/AKT/mTOR pathway: An in vivo rat experiment and machine learning study. J Inflamm Res. (2024) 17:6501–19. doi: 10.2147/JIR.S488023,
195.
Lu R He Z Zhang W Wang Y Cheng P Lv Z et al . Oroxin B alleviates osteoarthritis through anti-inflammation and inhibition of PI3K/AKT/mTOR signaling pathway and enhancement of autophagy. Front Endocrinol. (2022) 13:1060721. doi: 10.3389/fendo.2022.1060721,
196.
Lou L Zhu Z Xu L Wu Z Wang X Yuan L et al . Glabridin mitigates osteoarthritis progression through modulation of the PI3K/AKT/FOXO3A autophagy axis. Phytomed: Int J Phytotherapy Phytopharmacol. (2025) 148:157359. doi: 10.1016/j.phymed.2025.157359,
197.
Yang J Zhou Z Ding X He R Li A Wei Y et al . Gubi Zhitong formula alleviates osteoarthritis in vitro and in vivo via regulating BNIP3L-mediated mitophagy. Phytomed: Int J Phytotherapy Phytopharmacol. (2024) 128:155279. doi: 10.1016/j.phymed.2023.155279,
198.
Resende VAC Neto AC Nunes C Andrade R Espregueira-Mendes J Lopes S . Higher age, female gender, osteoarthritis and blood transfusion protect against periprosthetic joint infection in total hip or knee arthroplasties: a systematic review and meta-analysis. Knee Surg, Sports Traumatology, Arthroscopy: Official J ESSKA. (2021) 29:8–43. doi: 10.1007/s00167-018-5231-9,
199.
Mostafaee N Negahban H Shaterzadeh Yazdi MJ Goharpey S Mehravar M Pirayeh N . Responsiveness of a Persian version of knee injury and osteoarthritis outcome score and Tegner activity scale in athletes with anterior cruciate ligament reconstruction following physiotherapy treatment. Physiother Theory Pract. (2020) 36:1019–26. doi: 10.1080/09593985.2018.1548672,
200.
Jacob B Jüllig M Middleditch M Payne L Broom N Sarojini V et al . Protein levels and microstructural changes in localized regions of early cartilage degeneration compared with adjacent intact cartilage. Cartilage. (2021) 12:192–210. doi: 10.1177/1947603518809401,
201.
Kim CW Lee CR Gwak HC Kim JH Kwon YU Kim DY . The effects of surgical technique in Total knee arthroplasty for Varus osteoarthritic knee on the rotational alignment of femoral component: gap balancing technique versus measured resection technique. J Knee Surg. (2020) 33:144–51. doi: 10.1055/s-0038-1676766,
202.
Doiron-Cadrin P Kairy D Vendittoli PA Lowry V Poitras S Desmeules F . Feasibility and preliminary effects of a tele-prehabilitation program and an in-person prehablitation program compared to usual care for total hip or knee arthroplasty candidates: a pilot randomized controlled trial. Disabil Rehabil. (2020) 42:989–98. doi: 10.1080/09638288.2018.1515992,
203.
Xie H Lu Y Pan J Zeng H Zhang Z Yin J et al . MiR-335-5p escaped from circKIAA0586 adsorption contributes to mechanical overloading-induced cartilage degeneration by targeting lymphoid-specific helicase. Research (Washington, DC). (2025) 9:0694. doi: 10.34133/research.0694
204.
Hong Y Feng Z Ge Y Xi Y Zhang B Wu J et al . MiR-145-enriched BMSCs-derived exosomes ameliorate neurogenic erectile dysfunction in aged rats via TGFBR2 inhibition. Regen Ther. (2025) 29:455–65. doi: 10.1016/j.reth.2025.04.004,
205.
Zhao L Li X Gao M Liu L Ma B Liu X et al . M6A modified miR-31-5p suppresses M1 macrophage polarization and autoimmune dry eye by targeting P2RX7. Adv Sci (Weinh). (2025) 12:e2415341. doi: 10.1002/advs.202415341,
206.
Song S Shi K Fan M Wen X Li J Guo Y et al . Clostridium butyricum and its metabolites regulate macrophage polarization through miR-146a to antagonize gouty arthritis. J Adv Res. (2025). doi: 10.1016/j.jare.2025.05.036,
207.
Chen HR Huang HS Zeng SY Sun YF Chen L Lai SY et al . miR-146a-5p targets IRAK1/TRAF6 to promote bacillus Calmette-Guérin survival by exosome-mediated autocrine actions. Mol Immunol. (2025) 185:105–15. doi: 10.1016/j.molimm.2025.07.012,
208.
Xian Y Sun Y Wang L Lin L Wu Z Zhang K et al . Plasma Exosomal miR-17-5p regulates macrophage polarization by targeting Bcl11b in Sepsis-induced lung injury. J Inflamm Res. (2025) 18:10651–68. doi: 10.2147/JIR.S524742,
209.
Kim HE Lee JY Son GY Park JY Kim KB Choi CM et al . Nitazoxanide modulates mitochondrial function and inflammatory metabolism in chondrocytes from patients with osteoarthritis via AMPK/mTORC1 signaling. Antioxidants (Basel). (2025) 14. doi: 10.3390/antiox14050512,
210.
Wang Z Hu J Pan Y Shan Y Jiang L Qi X et al . miR-140-5p/miR-149 affects chondrocyte proliferation, apoptosis, and autophagy by targeting FUT1 in osteoarthritis. Inflammation. (2018) 41:959–71. doi: 10.1007/s10753-018-0750-6,
211.
Chen T Ehnert S Tendulkar G Zhu S Arnscheidt C Aspera-Werz RH et al . Primary human chondrocytes affected by cigarette smoke-therapeutic challenges. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21051901,
212.
Gan D Tao C Jin X Wu X Yan Q Zhong Y et al . Piezo1 activation accelerates osteoarthritis progression and the targeted therapy effect of artemisinin. J Adv Res. (2024) 62:105–17. doi: 10.1016/j.jare.2023.09.040,
213.
Feng WJ Wang H Shen C Zhu JF Chen XD . Severe cartilage degeneration in patients with developmental dysplasia of the hip. IUBMB Life. (2017) 69:179–87. doi: 10.1002/iub.1606,
214.
Wu J Wu J Xiang W Gong Y Feng D Fang S et al . Engineering exosomes derived from TNF-α preconditioned IPFP-MSCs enhance both yield and therapeutic efficacy for osteoarthritis. J Nanobiotechnol. (2024) 22:555. doi: 10.1186/s12951-024-02795-9,
215.
Cai W Wu A Lin Z Cao W Pathak JL Jaspers RT et al . S-propargyl-cysteine attenuates temporomandibular joint osteoarthritis by regulating macrophage polarization via inhibition of JAK/STAT signaling. Mol Med. (2025) 31:128. doi: 10.1186/s10020-025-01186-6,
216.
Sun K Zhang X Hou L Lu F Liu H Zheng Z et al . TRPM2-mediated feed-forward loop promotes chondrocyte damage in osteoarthritis via calcium-cGAS-STING-NF-κB pathway. J Adv Res. (2025) 75:213–27. doi: 10.1016/j.jare.2024.11.007,
217.
Collins JA Kim CJ Coleman A Little A Perez MM Clarke EJ et al . Cartilage-specific Sirt6 deficiency represses IGF-1 and enhances osteoarthritis severity in mice. Ann Rheum Dis. (2023) 82:1464–73. doi: 10.1136/ard-2023-224385,
218.
Tang H Gong X Dai J Gu J Dong Z Xu Y et al . The IRF1/GBP5 axis promotes osteoarthritis progression by activating chondrocyte pyroptosis. J orthopaedic translation. (2024) 44:47–59. doi: 10.1016/j.jot.2023.11.005,
219.
Cao H Chen M Cui X Liu Y Liu Y Deng S et al . Cell-free osteoarthritis treatment with sustained-release of chondrocyte-targeting exosomes from umbilical cord-derived mesenchymal stem cells to rejuvenate aging chondrocytes. ACS Nano. (2023) 17:13358–76. doi: 10.1021/acsnano.3c01612,
220.
Li B Guan G Mei L Jiao K Li H . Pathological mechanism of chondrocytes and the surrounding environment during osteoarthritis of temporomandibular joint. J Cell Mol Med. (2021) 25:4902–11. doi: 10.1111/jcmm.16514,
221.
Liu L Zhang W Liu T Tan Y Chen C Zhao J et al . The physiological metabolite α-ketoglutarate ameliorates osteoarthritis by regulating mitophagy and oxidative stress. Redox Biol. (2023) 62:102663. doi: 10.1016/j.redox.2023.102663,
222.
Zeng P Han W Li C Li H Zhu D Zhang Y et al . MiR-378 attenuates muscle regeneration by delaying satellite cell activation and differentiation in mice. Acta Biochim Biophys Sin Shanghai. (2016) 48:833–9. doi: 10.1093/abbs/gmw077,
223.
Feng L Yang Z Li Y Pan Q Zhang X Wu X et al . MicroRNA-378 contributes to osteoarthritis by regulating chondrocyte autophagy and bone marrow mesenchymal stem cell chondrogenesis. Molecular Therapy Nucleic acids. (2022) 28:328–41. doi: 10.1016/j.omtn.2022.03.016,
224.
Feng L Zhang JF Shi L Yang ZM Wu TY Wang HX et al . MicroRNA-378 suppressed Osteogenesis of MSCs and impaired bone formation via inactivating Wnt/β-catenin signaling. Molecular therapy Nucleic acids. (2020) 21:1017–28. doi: 10.1016/j.omtn.2020.07.018,
225.
Dönges L Damle A Mainardi A Bock T Schönenberger M Martin I et al . Engineered human osteoarthritic cartilage organoids. Biomaterials. (2024) 308:122549. doi: 10.1016/j.biomaterials.2024.122549,
226.
Zheng Z Shang X Sun K Hou Y Zhang X Xu J et al . P21 resists ferroptosis in osteoarthritic chondrocytes by regulating GPX4 protein stability. Free Radic Biol Med. (2024) 212:336–48. doi: 10.1016/j.freeradbiomed.2023.12.047,
227.
Ansari MY Novak K Haqqi TM . ERK1/2-mediated activation of DRP1 regulates mitochondrial dynamics and apoptosis in chondrocytes. Osteoarthr Cartil. (2022) 30:315–28. doi: 10.1016/j.joca.2021.11.003,
228.
Matsuoka K Bakiri L Bilban M Toegel S Haschemi A Yuan H et al . Metabolic rewiring controlled by c-Fos governs cartilage integrity in osteoarthritis. Ann Rheum Dis. (2023) 82:1227–39. doi: 10.1136/ard-2023-224002,
229.
Charlier E Deroyer C Ciregia F Malaise O Neuville S Plener Z et al . Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem Pharmacol. (2019) 165:49–65. doi: 10.1016/j.bcp.2019.02.036,
230.
Ji Q Qiao X Liu Y Wang D . Expression of long-chain noncoding RNA GAS5 in osteoarthritis and its effect on apoptosis and autophagy of osteoarthritis chondrocytes. Histol Histopathol. (2021) 36:475–84. doi: 10.14670/HH-18-312,
231.
Swahn H Li K Duffy T Olmer M D'Lima DD Mondala TS et al . Senescent cell population with ZEB1 transcription factor as its main regulator promotes osteoarthritis in cartilage and meniscus. Ann Rheum Dis. (2023) 82:403–15. doi: 10.1136/ard-2022-223227,
232.
Xu K Meng Z Xian XM Deng MH Meng QG Fang W et al . LncRNA PVT1 induces chondrocyte apoptosis through upregulation of TNF-α in synoviocytes by sponging miR-211-3p. Mol Cell Probes. (2020) 52:101560. doi: 10.1016/j.mcp.2020.101560,
233.
Xu F Hu QF Li J Shi CJ Luo JW Tian WC et al . SOX4-activated lncRNA MCM3AP-AS1 aggravates osteoarthritis progression by modulating miR-149-5p/Notch1 signaling. Cytokine. (2022) 152:155805. doi: 10.1016/j.cyto.2022.155805,
234.
Su Y Gu X Zheng Q Zhu L Lu J Li L . LncRNA PCGEM1 in human cancers: functions, mechanisms and promising clinical utility. Front Oncol. (2022) 12:847745. doi: 10.3389/fonc.2022.847745,
235.
Shi M Sun M Wang C Shen Y Wang Y Yan S . Therapeutic potential of POU3F3, a novel Long non-coding RNA, alleviates the pathogenesis of osteoarthritis by regulating the miR-29a- 3p/FOXO3 Axis. Curr Gene Ther. (2022) 22:427–38. doi: 10.2174/1566523222666220309150722,
236.
Chen Z Zhao C Liu P Huang H Zhang S Wang X . Anti-apoptosis and autophagy effects of melatonin protect rat chondrocytes against oxidative stress via regulation of AMPK/Foxo3 pathways. Cartilage. (2021) 13:1041s–53s. doi: 10.1177/19476035211038748,
237.
Zhang R Huang L Jin M Kou H Ma J Zhang W et al . Curcumin controls the senescence of adipose-derived mesenchymal stem cells via the FoxO3/autophagy signaling pathway to enhance joint homeostasis for osteoarthritis therapy. Stem Cell Res Ther. (2025). doi: 10.1186/s13287-025-04836-y,
238.
Zhang H Lin C Zeng C Wang Z Wang H Lu J et al . Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann Rheum Dis. (2018) 77:1524–34. doi: 10.1136/annrheumdis-2018-213450,
239.
Chen X Gong W Shao X Shi T Zhang L Dong J et al . METTL3-mediated m(6)a modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann Rheum Dis. (2022) 81:87–99. doi: 10.1136/annrheumdis-2021-221091,
240.
Fang G Wen X Jiang Z Du X Liu R Zhang C et al . FUNDC1/PFKP-mediated mitophagy induced by KD025 ameliorates cartilage degeneration in osteoarthritis. Mol Ther. (2023) 31:3594–612. doi: 10.1016/j.ymthe.2023.10.016,
241.
Xu K He Y Moqbel SAA Zhou X Wu L Bao J . SIRT3 ameliorates osteoarthritis via regulating chondrocyte autophagy and apoptosis through the PI3K/Akt/mTOR pathway. Int J Biol Macromol. (2021) 175:351–60. doi: 10.1016/j.ijbiomac.2021.02.029,
242.
Guan Z Jin X Guan Z Liu S Tao K Luo L . The gut microbiota metabolite capsiate regulate SLC2A1 expression by targeting HIF-1α to inhibit knee osteoarthritis-induced ferroptosis. Aging Cell. (2023) 22:e13807. doi: 10.1111/acel.13807,
Summary
Keywords
autophagy, non-coding RNAs, osteoarthritis, pathological network, therapeutic targets
Citation
Wang B and Wang Z (2026) Mechanistic insights into non-coding RNAs regulate autophagy in chondrocytes and their contribution to osteoarthritis. Front. Med. 12:1735826. doi: 10.3389/fmed.2025.1735826
Received
30 October 2025
Revised
15 December 2025
Accepted
17 December 2025
Published
02 February 2026
Volume
12 - 2025
Edited by
Antonella Fioravanti, ISMH (International Society of Medical Hydrology and Climatology), United Kingdom
Reviewed by
Marcin Tomsia, Medical University of Silesia in Katowice, Poland
Jian Liu, Anhui University of Chinese Medicine, China
Updates
Copyright
© 2026 Wang and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Wang, 18756938320@163.com; lijuanzhan@sina.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.