Abstract
Background:
Inadequate intraoperative cerebral oxygen supply is one of the inciting causes of postoperative cognitive disturbances. Numerous studies have investigated the association between regional cerebral oxygen saturation (rScO2) monitoring and postoperative cognitive dysfunction. However, results are inconsistent, owing to differences in surgery type, patient population, and monitoring protocols. Therefore, we conducted a meta-analysis to comprehensively evaluate the association between rScO2 monitoring and the incidence of postoperative neurocognitive disorders.
Methods:
A comprehensive literature search was conducted across multiple databases from their inception to June 2025 to identify randomized controlled trials (RCTs) that compared the impact of rScO2 monitoring versus no monitoring on cognitive function. The primary outcome was the incidence of perioperative neurocognitive disorders (PNDs). Secondary outcomes were the incidences of postoperative cognitive dysfunction (POCD) and postoperative delirium (POD), as well as the economic indicators of the number needed to treat (NNT) and cost–benefit ratio (CBR).
Results:
A total of 28 RCTs were included. Overall, we found that intraoperative rScO2 monitoring significantly reduced the incidence risk of PND (relative risk [RR] = 0.47, 95% confidence interval [CI]: 0.41, 0.54), POCD (RR = 0.47, 95% CI: 0.39, 0.57), and POD (RR = 0.45, 95% CI: 0.35, 0.57). Subgroup analyses based on surgery type (cardiac, orthopedic, abdominal, and others) demonstrated consistent protective effects of monitoring. Sensitivity analyses using leave-one-out analysis, excluding Chinese-language publications, low-quality studies, and studies with a baseline rScO2 < 80%, confirmed the robustness of results. The economic evaluation showed that rScO2 monitoring is both clinically beneficial and cost-effective, as reflected in the low NNT values and favorable CBRs, which indicated that the cost of prevention is substantially lower than that of managing complications.
Conclusion:
Intraoperative rScO2 monitoring significantly reduces the incidence of PND, including POCD and POD. Consistent protective effects were observed across a wide range of surgery types, demonstrating its broad clinical applicability. Furthermore, its favorable cost–benefit profile demonstrated that the prevention of neurocognitive complications has a substantially lower cost than the estimated economic burden of managing these complications. Widespread adoption of rScO2 monitoring is recommended to improve postoperative cognitive outcomes.
Introduction
In modern anesthesiology, perioperative neurocognitive disorders (PND) have become an important clinical concern. The term PND was introduced in 2018 to replace the previous term, postoperative cognitive dysfunction (POCD), and now encompasses both POCD and postoperative delirium (POD) (1). We have divided PND into POCD and POD in this study according to the varied follow-up durations and content. POCD is a common central nervous system complication that is characterized by various symptoms, such as confusion, anxiety, personality changes, and memory impairment, which profoundly affect postoperative recovery and quality of life (2). By contrast, POD is an acute and fluctuating mental state characterized by inattention, disorganized thinking, and altered consciousness, and occurs without prior neurocognitive disorders or coma (3). PND not only prolongs hospital stays and increases the risk of other postoperative complications but also raises mortality rates and negatively impacts long-term quality of life. Thus, identifying approaches to reduce the incidence of PND is crucial.
The exact cause of PND remains unclear, although studies have suggested a potential association with general anesthesia (4). Recently, Glumac et al. (5) conducted a 4-year follow-up study and reported that a single preoperative administration of dexamethasone significantly alleviates the inflammatory response caused by cardiac surgery and reduces the incidence and severity of postoperative cognitive impairment. This indicates a possible link between inflammation and POCD, with inflammatory responses likely playing a key role in the development of PND. Similar mechanisms have been explored in previous meta-analyses (6). Cerebral oxygen saturation is a critical indicator of the brain’s oxygen supply–demand balance (7). Intraoperative factors, such as excessive intraoperative blood loss, hypoxemia, and hypotension, can lead to inadequate cerebral perfusion, causing decreased regional cerebral oxygen saturation (rScO2), which can disrupt cerebral oxygen metabolism and trigger neurological complications. A recent study by Colak et al. found that prolonged desaturation of rScO2 is an important predictor of cognitive decline (8). During surgery, the effects of anesthesia on cerebral oxygen balance may contribute significantly to an increased risk of developing postoperative neurological complications (9). Real-time monitoring of rScO2 provides immediate information on the brain’s oxygen supply and is crucial for preventing PND. Compared with other monitoring techniques, rScO2 measurement is simpler and more practical in clinical settings. By monitoring rScO2, anesthesiologists can better manage anesthetic depth and hemodynamics, which enables timely interventions that can reduce the incidence of PND.
Although numerous studies have investigated the role of rScO2 monitoring in different surgeries, findings are inconsistent. Therefore, this meta-analysis aimed to evaluate the impact of intraoperative rScO2 monitoring on the incidence of PND, POCD, and POD. Additionally, we aimed to determine the effectiveness of rScO2 monitoring in improving postoperative cognitive outcomes and assess its economic benefits via cost-effectiveness analysis.
Methods
This study was performed according to the statement on preferred reporting items for systematic reviews and meta-analyses (S1 PRISMA Checklist in Supplementary material) (10).
Inclusion and exclusion criteria
Inclusion criteria for studies were as follows: (1) adult patients undergoing surgery under general or spinal anesthesia, American Society of Anesthesiologists classification I–III; (2) use of rScO2 monitoring during surgery; (3) neuropsychological testing performed preoperatively and 1 week postoperatively to assess the occurrence of PND; and (4) studies with a clearly defined sample size. Exclusion criteria were as follows: (1) studies lacking original data or with incomplete information, preventing data extraction; (2) patients with pre-existing conditions that could affect postoperative cognitive function, such as dementia, stroke, other central nervous system diseases, a history of alcohol or drug abuse, multiple injuries, or traumatic brain injuries; (3) patients unable to undergo normal verbal communication because of language barriers or other reasons, which could interfere with pre- and postoperative neuropsychological testing; and (4) secondary studies such as meta-analyses, case reports, reviews, abstracts, letters, and non-original research studies. Strict inclusion and exclusion criteria were applied to ensure homogeneity among study subjects and reproducibility of results. This allowed for a reliable evaluation of the impact of rScO2 monitoring on postoperative cognitive function.
Search strategy
The study subjects were patients undergoing general or spinal anesthesia, regardless of surgery type. A comprehensive search was conducted using the following databases: CNKI, Wanfang, VIP, PubMed, Web of Science, Cochrane, and Embase. The search aimed to identify all randomized controlled trials (RCTs) that met the inclusion criteria, covering studies from database inception to June 2025 to ensure the inclusion of the latest findings. Only human studies were included; animal studies were excluded. The search terms were as follows: cerebral oxygen saturation, rScO2, near-infrared spectroscopy (NIRS), perioperative neurocognitive disorders, postoperative cognitive dysfunction, and postoperative delirium.
Data extraction
During the data extraction phase, a data extraction form was designed. Two researchers (JQ and DG) independently screened all articles according to the inclusion and exclusion criteria, and subsequently extracted relevant data for statistical analysis. If the two researchers had a disagreement that could not be resolved, a third researcher (JL) re-extracted the data for further analysis. All extracted data were cross-checked and confirmed by all three researchers (JQ, DG, and JL) to ensure accuracy. If there were any ambiguities or disputes regarding the data in any article, the original authors were contacted to obtain accurate original data. Extracted information included: first author, publication year, sample size, event count in the experimental and control groups, surgery type, assessment methods, and primary outcome measures.
Outcome measures and quality assessment
The primary outcome was the incidence of PND, which was used to evaluate the incidence of PND across different surgical groups. To assess the risk of bias in the included studies, we used the risk bias assessment tool Risk of Bias 2 (11) (RoB 2), as recommended by Cochrane for conducting a systematic review of RCTs. Each assessment result was classified as “low risk,” “some concern,” or “high risk.” The assessment work was completed independently by two researchers (JQ and DG). If there were any inconsistencies, the final assessment result was determined following discussion with a third researcher (JL).
Statistical analysis
Statistical analyses were conducted using relative risk (RR) with corresponding 95% confidence intervals (CI). Heterogeneity among studies was assessed using the Q test and the I2 statistic (12). A random-effects model was used for all analyses for two main reasons: (1) the Q test is characterized by low statistical power for between-study heterogeneity, which is especially relevant when few studies are available; and (2) the random-effects model is a more conservative choice when heterogeneity is present, whereas it reduces to the fixed effects model when heterogeneity is absent. p < 0.05 was considered statistically significant. Subgroup analyses were conducted to assess the effects of the intervention in different surgical categories, including cardiac surgery, orthopedic surgery, abdominal surgery, and other types of surgery. Supplementary subgroup analyses considered cognitive assessment tools, follow-up periods, and intervention thresholds for rScO2. Sensitivity analysis was performed using the following four steps: (1) one-by-one elimination method, whereby one study was eliminated at a time to observe changes in effect size; (2) exclusion of Chinese-language publications to assess potential language bias; (3) exclusion of low-quality studies (classified as “high risk” or “some concerns” using the RoB 2 tool) to explore their impact on the overall results; and (4) exclusion of studies in which the intervention was applied only when intraoperative rScO2 dropped to less than 80% of baseline to evaluate the impact of delayed or reactive interventions and ensure consistency in the monitoring thresholds across studies. Publication bias was analyzed using Begg’s funnel plot (13) and Egger’s test (14) when more than 10 studies were available. If publication bias was detected, the “trim-and-fill method” was used to adjust the results (15). The quality of evidence for each outcome was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework (16), which allows transparent and systematic ratings of evidence quality based on various factors, such as risk of bias, inconsistency, indirectness, imprecision, and other considerations. To further evaluate the clinical and economic benefits of rScO2 monitoring in preventing PND, two additional metrics were calculated: (1) number needed to treat (NNT) for therapeutic effect (17), which was defined as the reciprocal of the absolute difference in the incidence of PND between the intervention and control groups, calculated using the following formula: NNT = 1/attributable risk reduction (ARR); ARR represents the difference in the incidence of PND between the two groups; and (2) cost–benefit ratio (CBR) (18), which was calculated as the ratio of the total intervention cost required to prevent one case of PND to the economic loss that could have been avoided for that case using the formula, CBR = (NNT × monitoring cost per patient) / total cost reduction for each case of neurocognitive-related complications. All statistical analyses were performed using Stata version 18.0 (Stata Corporation, College Station, TX, United States).
Results
Literature search results
Our search of the databases and registers identified 1,509 articles. After removing 552 duplicates, 957 records remained. Following title and abstract review, 902 records were excluded as irrelevant, and 55 records advanced to full-text evaluation. Of these, 28 articles were excluded because of inappropriate study design or content (n = 22), incomplete data (n = 1), or ineligible populations (n = 5). An additional three reports from other sources were assessed, of which two were excluded. Ultimately, 28 articles were included in the meta-analysis (8, 19–45) (Figure 1), comprising 2,824 patients, of whom 1,401 patients were in the experimental group and 1,423 were in the control group. The evaluation of study quality using the RoB2 tool is provided in Supplementary Table 1. The proportion of each methodological quality item is presented in Figure 2, and the methodological quality assessment of the included studies is shown in Figure 3.
Figure 1

Flow diagram for searching included articles.
Figure 2
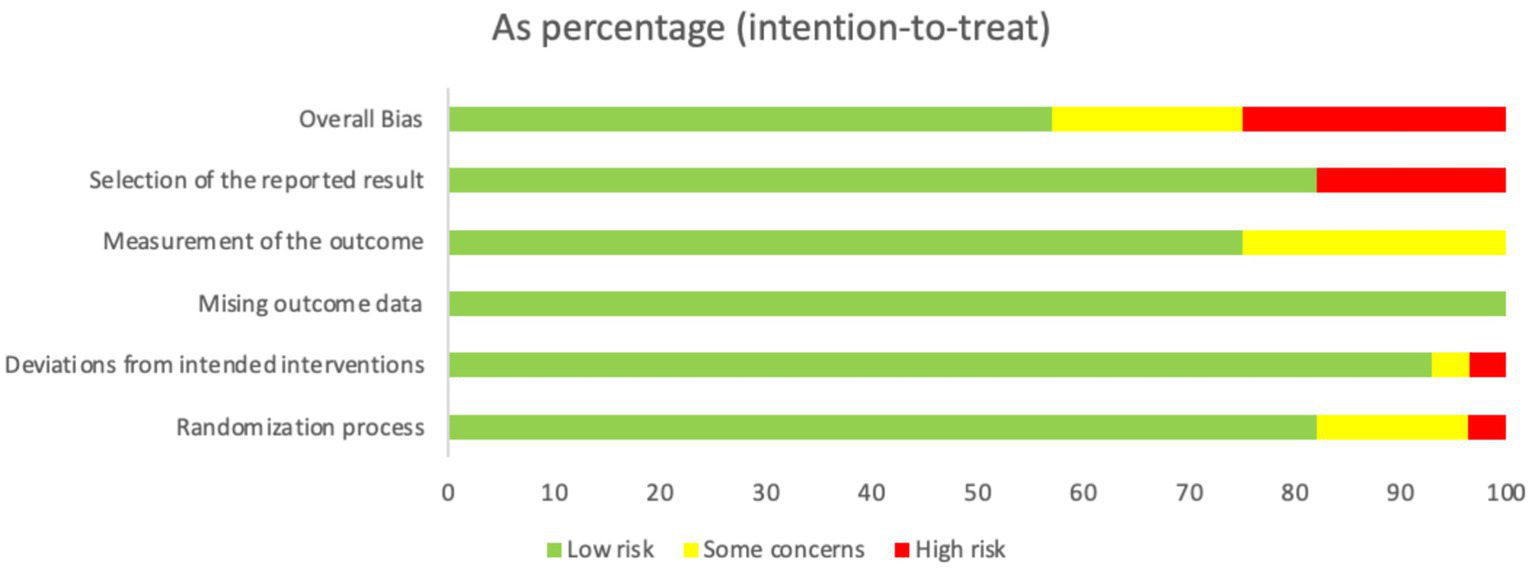
The proportion of each methodological quality item.
Figure 3
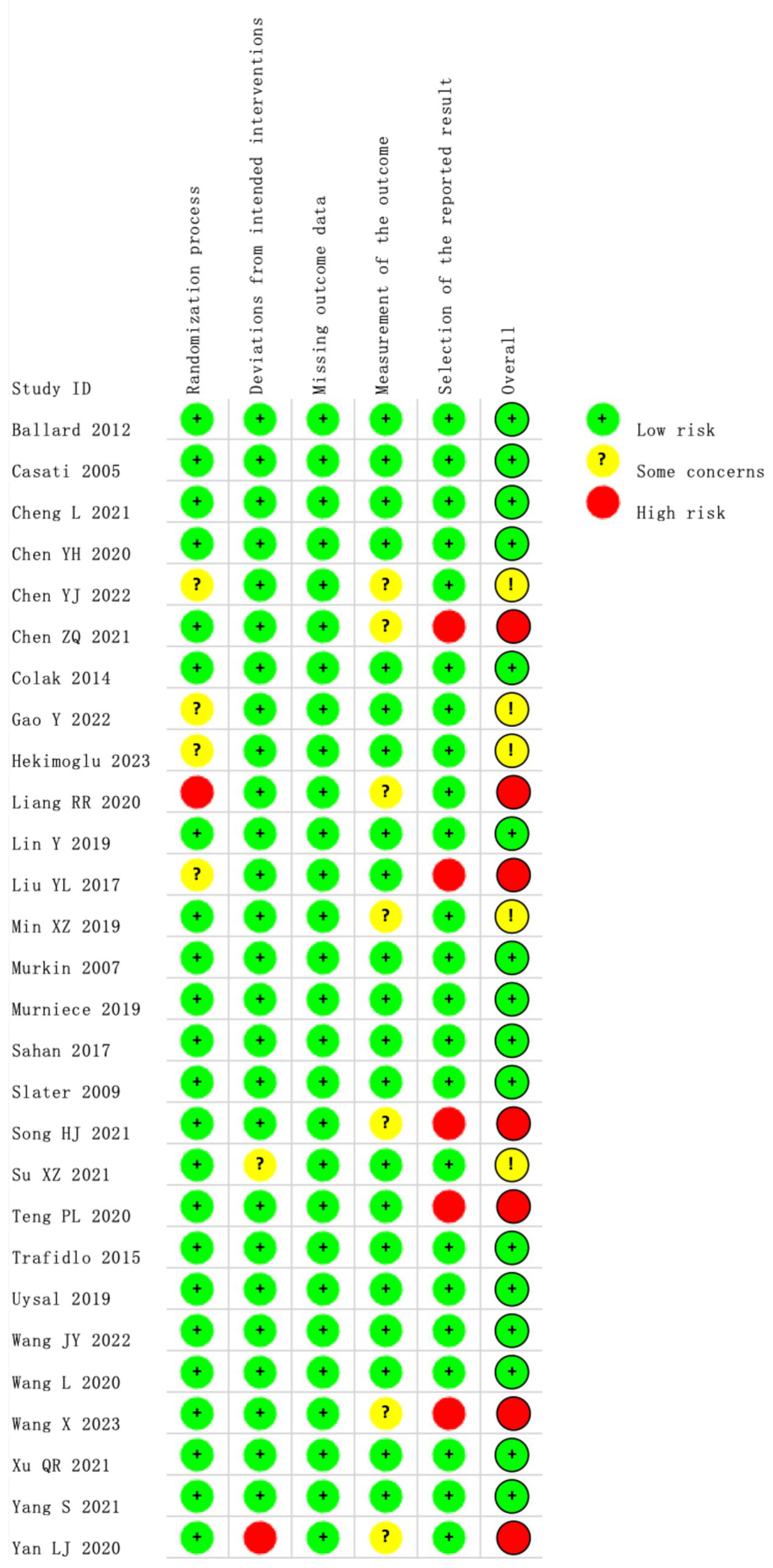
The methodological quality assessment.
Characteristics of included studies
There were no significant differences in the baseline characteristics of the patients across the included studies, which included age, sex, body mass index, and other baseline variables (Table 1). Postoperative follow-ups were conducted to assess cognitive function indicators. PNDs encompass all neurocognitive disorders related to surgery and anesthesia and include various cognitive problems that may arise during the perioperative period. PND can manifest as short-term or long-term cognitive impairment at different postoperative stages and aims to reflect the broad spectrum of cognitive dysfunctions during the perioperative period, with a particular focus on two common postoperative cognitive issues: POD and POCD. POCD is a persistent cognitive impairment that affects long-term recovery, whereas POD is an acute, early postoperative cognitive disorder with short-lived and fluctuating symptoms.
Table 1
| The first author (Year) | Experimental group | Control group | Surgical type | Outcome (time point) | Evaluation | Main baseline characteristics (age, sex, BMI) | rScO₂ intervention threshold (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Sample size | Incidence (%) | Sample Size | Incidence (%) | ||||||
| Wang L (19) (2020) | 60 | 11 (18.3%) | 60 | 22 (36.7%) | Orthopedics surgery | POD (7 day) | CAM | No significant difference | >90% |
| Cheng L (20) (2021) | 37 | 2 (5.4%) | 39 | 10 (25.6%) | Abdominal surgery | POD (3 day) | CAM | No significant difference | >65% |
| Chen YH (21) (2020) | 30 | 1 (3.3%) | 30 | 8 (26.7%) | Orthopedics surgery | POD (7 day) | CAM | No significant difference | >80% |
| Yan LJ (22) (2020) | 53 | 10 (18.9%) | 54 | 24 (44.4%) | Other surgeries | POD (3 day) | CAM | No significant difference | >80% |
| Teng PL (23) (2020) | 30 | 7 (23.3%) | 30 | 16 (53.3%) | Other surgeries | POD (5 day) | CAM | No significant difference | >90% |
| Xu QR (24) (2021) | 59 | 9 (15.3%) | 58 | 17 (29.3%) | Orthopedics surgery | POCD (7 day) | MoCA | No significant difference | >90% |
| Chen ZQ (25) (2021) | 32 | 4 (12.5%) | 32 | 28 (87.5%) | Other surgeries | POCD (4 day) | MMSE | No significant difference | >89% |
| Su XZ (26) (2021) | 28 | 5 (17.9%) | 29 | 13 (44.8%) | Other surgeries | POCD (5 day) | MoCA | No significant difference | >87% |
| Wang JY (27) (2022) | 78 | 8 (10.3%) | 81 | 28 (34.6%) | Other surgeries | POD (7 day) | CAM | No significant difference | >80% |
| Ballard (28) (2012) | 34 | 6 (17.6%) | 38 | 23 (60.5%) | Orthopedics surgery | POCD (3 month) | MMSE | No significant difference | NR |
| Colak (8) (2014) | 88 | 25 (28.4%) | 93 | 48 (51.6%) | Cardiac surgery | POCD (7 day) | MMSE | No significant difference | >80% |
| Murkin (29) (2007) | 100 | 11 (11%) | 100 | 18 (18%) | Cardiac surgery | POCD (1 month) | MMSE | No significant difference | >75% |
| Slater (30) (2009) | 19 | 7 (37%) | 21 | 10 (45%) | Cardiac surgery | POD (7 day) | DRS | No significant difference | >80% |
| Trafidlo (31) (2015) | 13 | 2 (15.4%) | 30 | 12 (40%) | Orthopedics surgery | POCD (1 month) | MMSE | No significant difference | >80% |
| Murniece (32) (2019) | 23 | 3 (13%) | 11 | 4 (36.4%) | Orthopedics surgery | POCD (2 day) | MoCA | No significant difference | >80% |
| Uysal (33) (2019) | 59 | 2 (3.4%) | 66 | 7 (10.6%) | Cardiac surgery | POCD (3 month) | MMSE | No significant difference | >60% |
| Chen YJ (34) (2022) | 30 | 6 (20%) | 30 | 22 (73.3%) | Orthopedics surgery | POCD (7 day) | MMSE | No significant difference | >80% |
| Gao Y (35) (2022) | 38 | 3 (7.9%) | 37 | 11 (29.7%) | Cardiac surgery | POD (2 day) | DRS | No significant difference | >80% |
| Min XZ (36) (2019) | 30 | 5 (16.7%) | 30 | 13 (43.3%) | Cardiac surgery | POCD (7 day) | MMSE | No significant difference | >89% |
| Wang X (37) (2023) | 53 | 4 (7.5%) | 53 | 8 (15.1%) | Orthopedics surgery | POCD (7 day) | MMSE | No significant difference | >80% |
| Sahan (38) (2017) | 19 | 7 (36.8%) | 21 | 9 (42.9%) | Cardiac surgery | POCD (7 day) | MMSE | No significant difference | >80% |
| Yang S (39) (2021) | 12 | 2 (16.7%) | 14 | 4 (28.6%) | Orthopedics surgery | POCD (7 day) | MoCA | No significant difference | >80% |
| Liang RR (40) (2020) | 34 | 7 (20.6%) | 21 | 10 (47.6%) | Abdominal surgery | POCD (7 day) | MoCA | No significant difference | >85% |
| Liu YL (41) (2017) | 137 | 20 (14.6%) | 131 | 37 (28.2%) | Abdominal surgery | POD (7 day) | CAM-ICU | No significant difference | >80% |
| Lin Y (42) (2019) | 63 | 1 (1.6%) | 74 | 2 (2.7%) | Cardiac surgery | POD (7 day) | CAM-ICU | No significant difference | >70% |
| Song HJ (43) (2021) | 30 | 2 (6.7%) | 30 | 8 (26.7%) | Orthopedics surgery | POCD (7 day) | MoCA | No significant difference | >55% |
| Casati (44) (2005) | 56 | 20 (35.7%) | 66 | 36 (54.5%) | Abdominal surgery | POCD (7 day) | MMSE | No significant difference | >75% |
| Hekimoglu (45) (2023) | 50 | 19 (38%) | 50 | 41 (82%) | Other surgeries | POCD (5 day) | MMSE | No significant difference | >80% |
Basic characteristics of included studies.
When cognitive function assessment tools (such as MMSE and MoCA) are used on the 4th day after the operation and later, the results are classified as POCD. The results are classified as POD only when a definite delirium screening tool such as CAM or CAM-ICU is used within 7 days after the operation. Although some studies used MMSE/MoCA to assess cognitive status in the early postoperative period, due to the characteristics of the tools not being suitable for diagnosing POD, they were uniformly classified under the category of POCD.
Intervention thresholds refer to the criteria used in each study to initiate intraoperative measures in response to rScO₂ desaturation.
POCD: Postoperative cognitive dysfunction refers to a decline in learning ability, memory, executive function, attention, and other cognitive functions in patients after surgery and anesthesia compared to their preoperative state.
POD: Postoperative delirium is an acute cognitive impairment characterized primarily by memory disturbances and impaired consciousness. It typically occurs in the early postoperative period, within 1 to 3 days after surgery, and generally lasts only a few days.
NR: not reported.
The commonly used methods to assess PND (Postoperative Neurocognitive Disorders) include the Mini-Mental State Examination [MMSE (54)], the Montreal Cognitive Assessment [MoCA (55)], and the Confusion Assessment Method for the ICU [CAM-ICU (56)] and the Delirium Rating Scale [DRS (57)].
The commonly used methods to assess PND (Postoperative Neurocognitive Disorders) include the Mini-Mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA), and the Confusion Assessment Method for the ICU (CAM-ICU).
The MoCA scale is designed to address the characteristics of mild cognitive impairment, with a focus on memory impairment as the main manifestation. It includes an extended delayed recall time and adjusts the scoring weight accordingly, making it more targeted to the specific features of mild cognitive impairment.
The Confusion Assessment Method (CAM) is used as a clinical aid in diagnosing delirium in the elderly. It has a high sensitivity (94–100%) and specificity (90–95%). The assessment is quick, easy to administer, and simple to operate, with good reliability and validity.
The Delirium Rating Scale (DRS), especially its revised version DRS-R-98, is a validated instrument used to evaluate the severity and symptom profile of delirium. It assesses multiple cognitive and behavioral domains, and is frequently employed in both clinical and research settings to quantify delirium severity and monitor changes over time.
PND, POD, and POCD reflect varying degrees and durations of the impact of surgery and anesthesia on cognitive function, highlighting the need for appropriate monitoring and management at different postoperative stages, and therefore we performed analyses for PND, POCD, and POD separately. This allowed us to examine the characteristics of cognitive dysfunction at different postoperative stages.
Meta-analysis results
Perioperative neurocognitive disorders
All 28 included studies reported PNDs. Overall, the experimental group that underwent intraoperative rScO2 monitoring had a significantly lower incidence risk of PND (RR = 0.47, 95% CI: 0.41, 0.54; I2 = 2.8%, Ph = 0.422; Figure 4) than the control group without monitoring. We then conducted subgroup analyses according to the type of surgery, which included cardiac surgery, orthopedic surgery, abdominal surgery, and other types of surgery. We found that the following subgroups of the experimental group that underwent rScO2 monitoring had a significantly reduced risk of postoperative PND: cardiac surgery group (RR = 0.57, 95% CI: 0.44, 0.73; I2 = 0.0%, Ph = 0.699); orthopedic surgery group (RR = 0.39, 95% CI: 0.29, 0.52; I2 = 0.0%, Ph = 0.861); abdominal surgery group (RR = 0.56, 95% CI: 0.38, 0.82; I2 = 32.3%, Ph = 0.219); other surgery group (RR = 0.38, 95% CI: 0.28, 0.51; I2 = 17.2%, Ph = 0.302). Other subgroup analyses were presented in Supplementary Table 2. A significant portion of the included studies originated from China, which may introduce geographic and population bias. To address this concern, we conducted a subgroup analysis excluding all Chinese-language articles. The results showed that compared with the control group without intraoperative rScO2 monitoring, the experimental group exhibited a significantly lower risk of PND (RR = 0.54, 95% CI: 0.45, 0.65; I2 = 0.5%, Ph = 0.441; Figure 5). The stability of these findings suggested that our results can be generalized to broader and more diverse patient populations. After excluding studies in which intraoperative interventions were applied only when rScO2 dropped below 80% of baseline, the risk of PND in the experimental group remained significantly lower than that in the control group without intraoperative rScO2 monitoring (RR = 0.45, 95% CI: 0.39, 0.53; I2 = 0.0%, Ph = 0.577; Figure 6). The sensitivity analysis using the one-by-one elimination method revealed no changes (Figure 7). Moreover, when 12 low-quality studies (22, 23, 25, 26, 34–37, 40, 41, 43, 45) were excluded, there was also no change in the results (RR = 0.54, 95% CI: 0.44, 0.65; I2 = 0.9%, Ph = 0.442; Figure 8), which further indicated that the pooled results were robust and reliable. The distribution of the funnel plot and the result of Egger’s (p = 0.012) and Begg’s tests (p = 0.023) indicated the presence of publication bias. Using the trim-and-fill method for adjustment (Figure 9), the pooled effect remained robust (RR = 0.38, 95% CI: 0.28, 0.51) without change in the direction of the result. The GRADE assessment for PND is shown in Supplementary Figure 1.
Figure 4
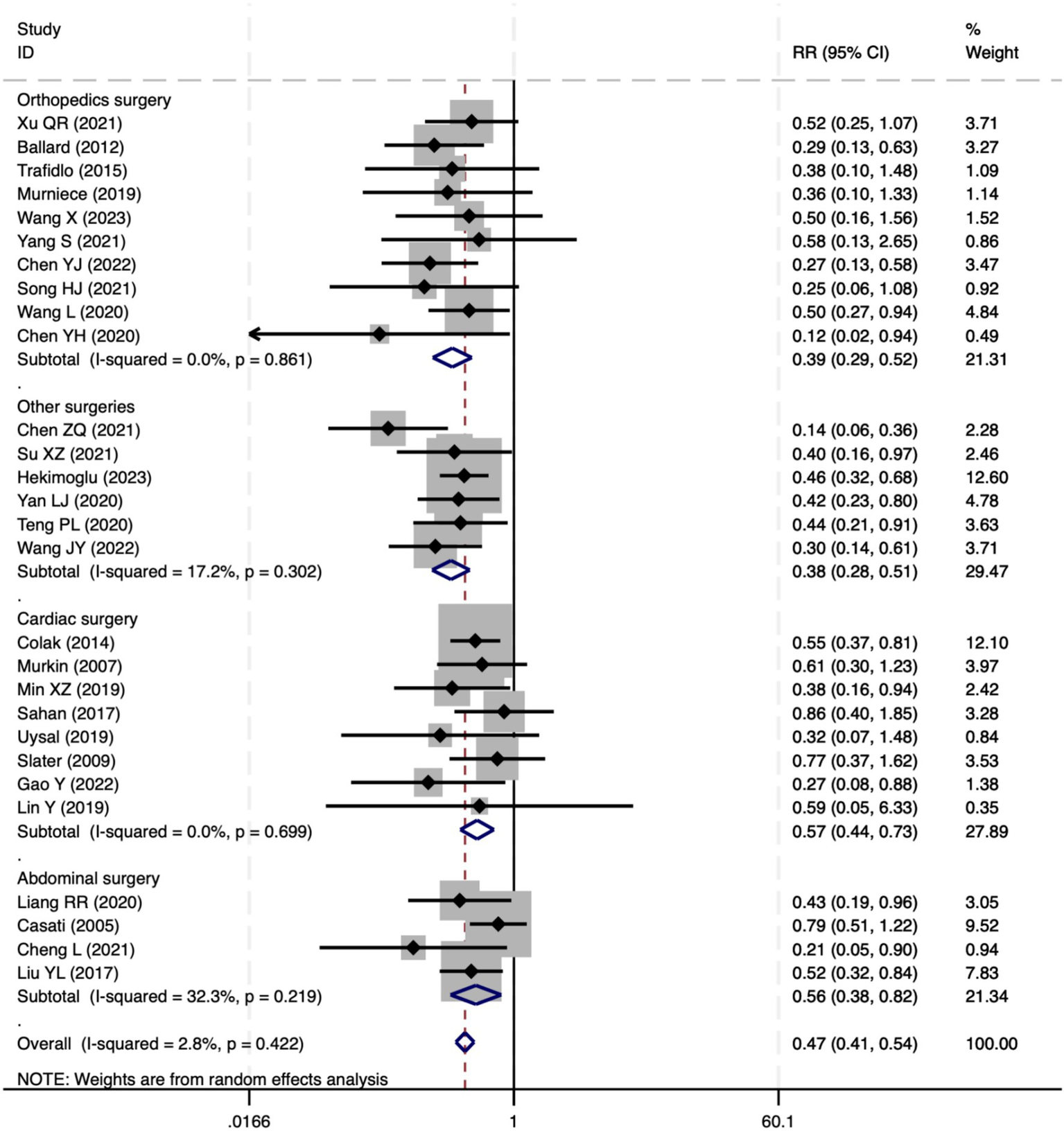
The forest plot of the impact of cerebral oxygen saturation on PND.
Figure 5
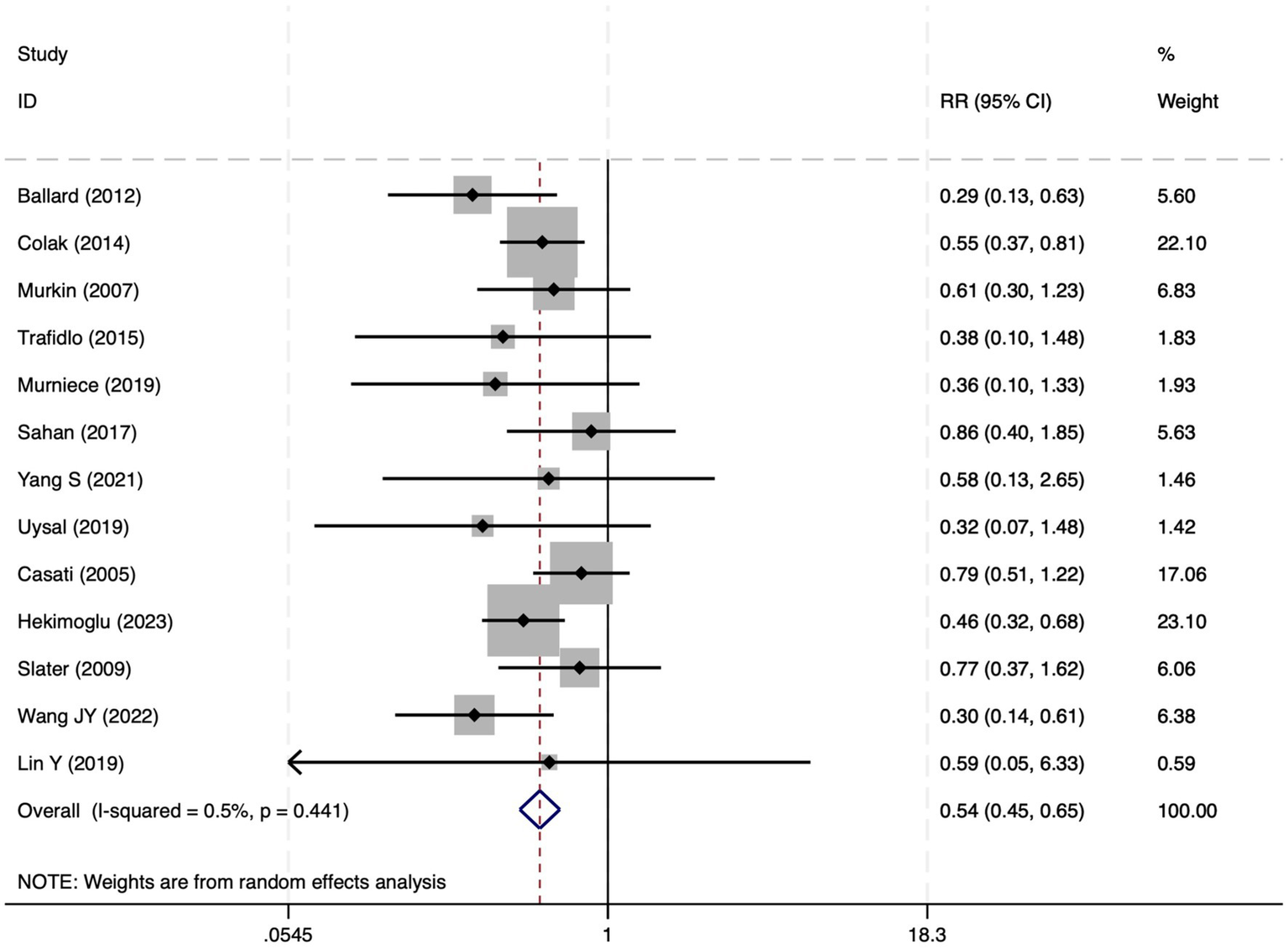
The forest plot of the impact of cerebral oxygen saturation on PND after excluding Chinese-language studies.
Figure 6
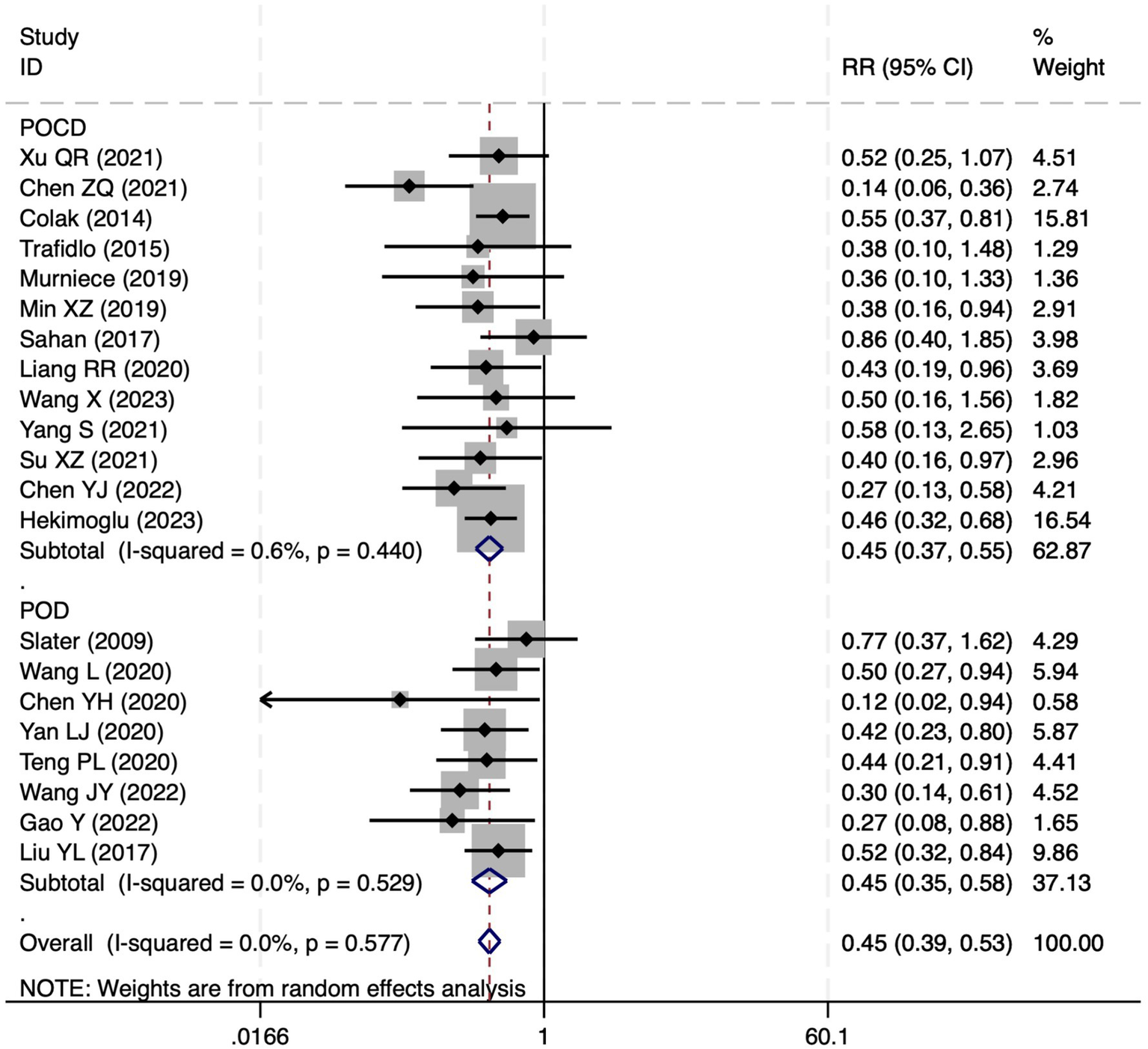
Forest plot after excluding studies where rScO2 monitoring was applied only when the baseline was below 80%.
Figure 7
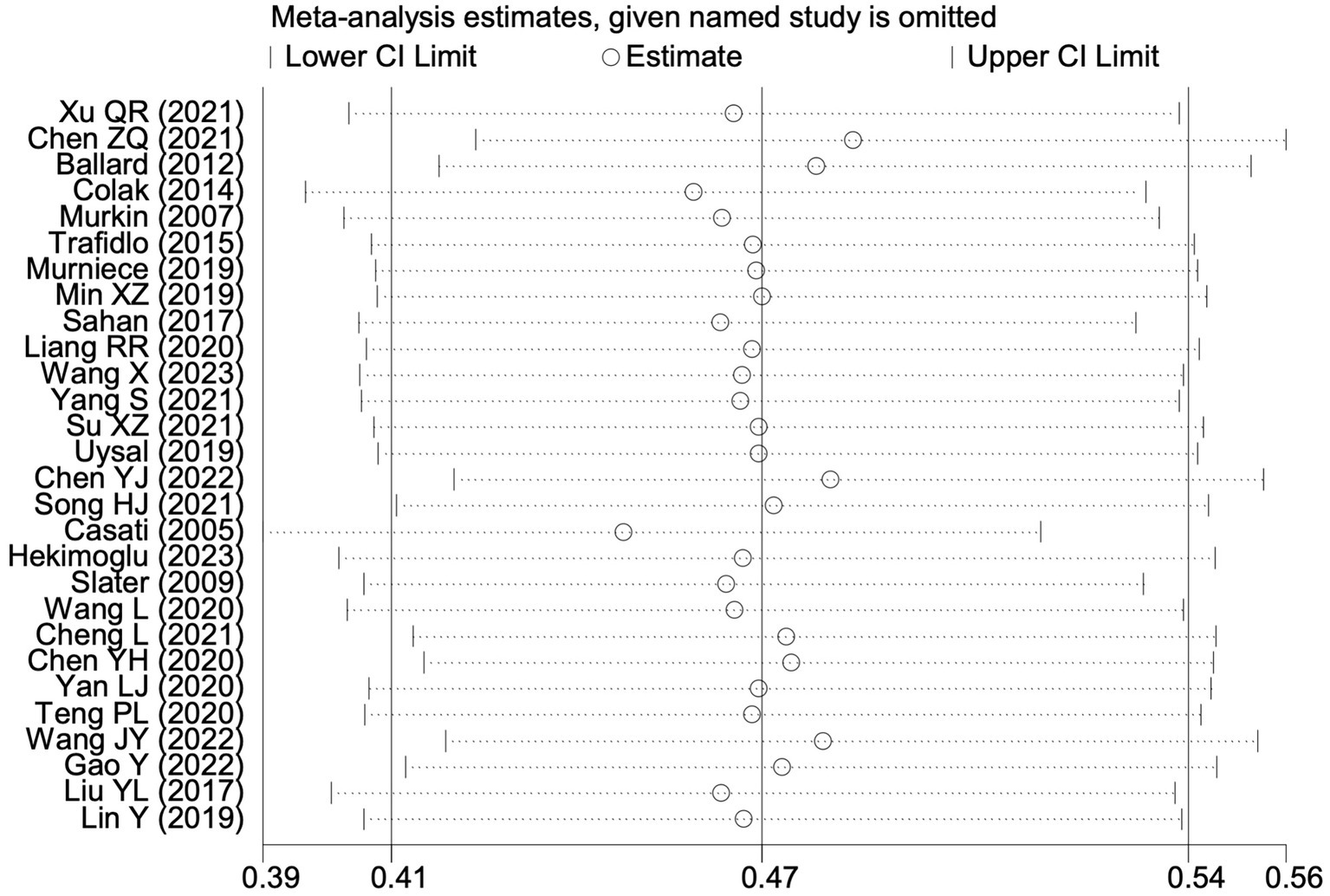
Sensitivity analysis of the effect of intraoperative cerebral oximetry monitoring on the incidence of PND using the leave-one-out method.
Figure 8
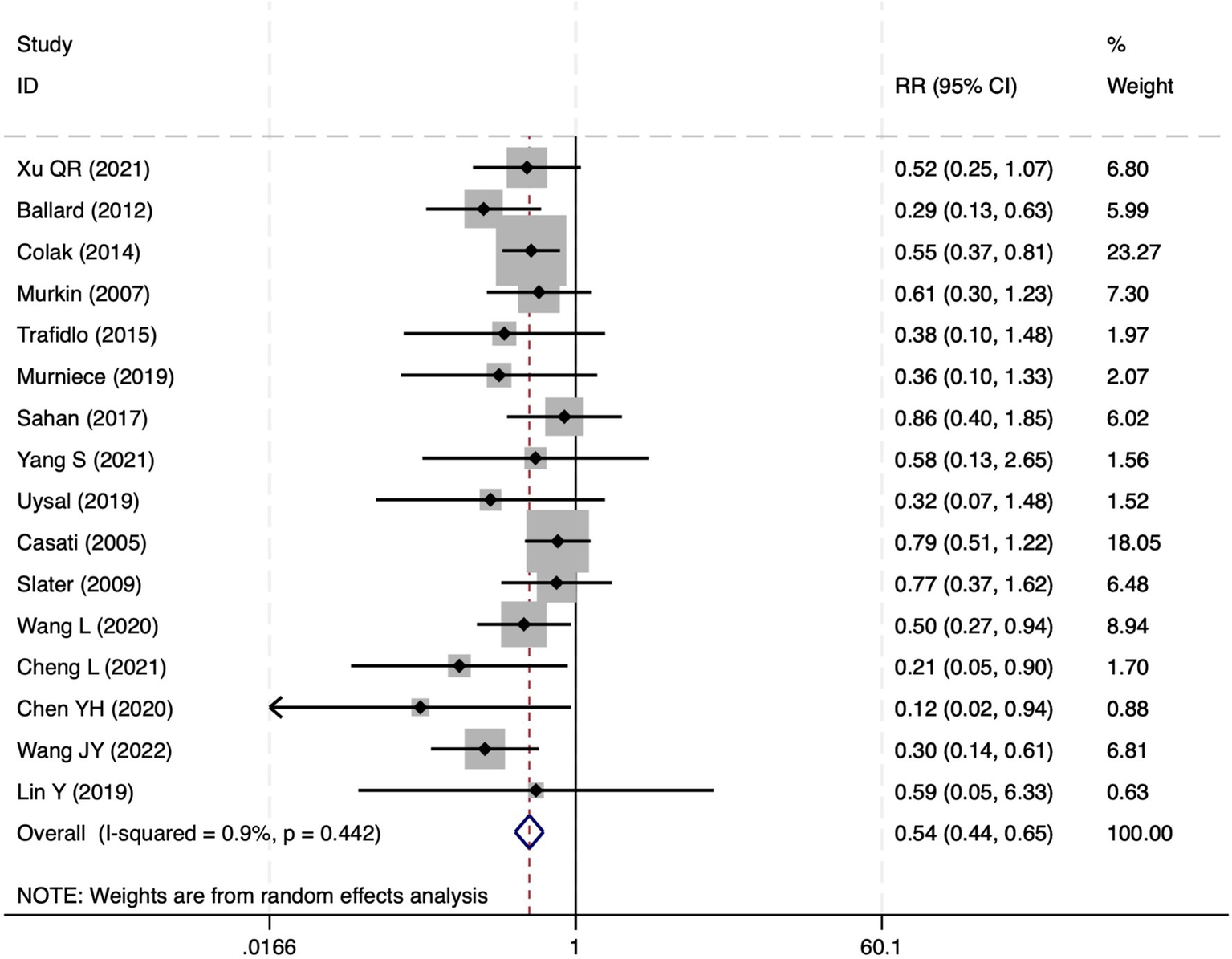
The forest plot of the impact of cerebral oxygen saturation on PND after excluding low-quality studies.
Figure 9
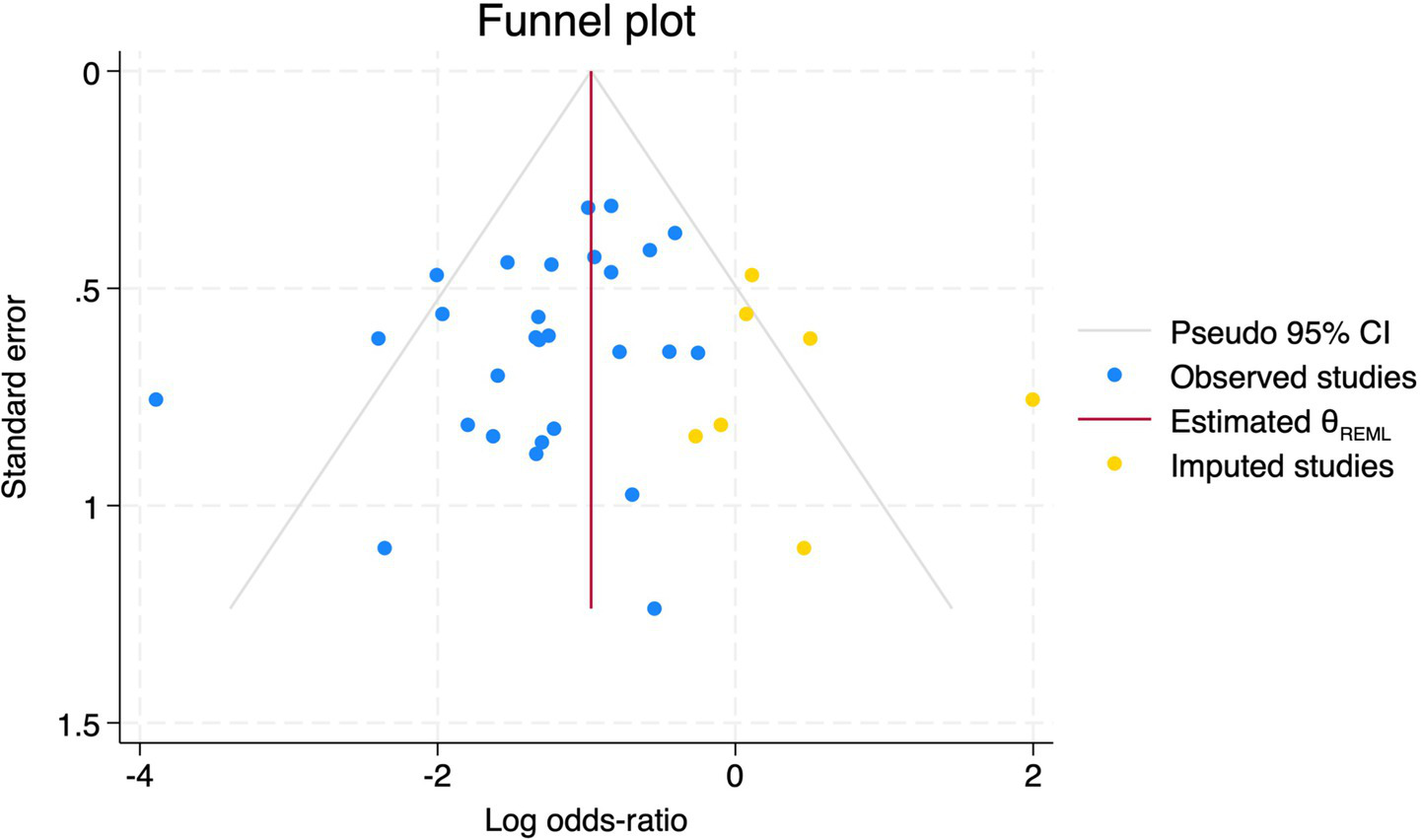
Trim and fill method for PND.
Postoperative cognitive dysfunction
Among the 28 included studies, 18 reported on POCD. Overall, a lower incidence risk of POCD was observed (RR = 0.47, 95% CI: 0.39, 0.57; I2 = 16.3%, Ph = 0.259; Figure 10) in the intervention group that underwent intraoperative rScO2 monitoring than in the control group that did not undergo monitoring. The subgroup analysis results by surgery type showed that rScO2 monitoring tended to reduce POCD risk across surgery types, especially in cardiac and orthopedic surgeries, where the effect is more significant and consistent cardiac surgery group (RR = 0.56, 95% CI: 0.42, 0.75; I2 = 0.0%, Ph = 0.654); orthopedic surgery group (RR = 0.37, 95% CI: 0.26, 0.52; I2 = 0.0%, Ph = 0.906); abdominal surgery group (RR = 0.64, 95% CI: 0.37, 1.12; I2 = 39.4%, Ph = 0.199); other surgery group (RR = 0.33, 95% CI: 0.17, 0.64; I2 = 62.4%, Ph = 0.070). Other subgroup analyses were presented in Supplementary Table 2. The subgroup analysis excluding all Chinese-language articles showed that compared with the control group without intraoperative rScO2 monitoring, the experimental group exhibited a significantly lower risk of POCD (RR = 0.55, 95% CI: 0.45, 0.67; I2 = 0.0%, Ph = 0.487; Figure 11). After excluding studies in which intraoperative interventions were applied only when rScO2 dropped below 80% of baseline, the risk of POCD in the experimental group remained significantly lower than that in the control group without intraoperative rScO2 monitoring (RR = 0.45, 95% CI: 0.37, 0.55; I2 = 0.6%, Ph = 0.440; Figure 6). The sensitivity analysis did not change the above results (Figure 12). Additionally, when eight low-quality studies (25, 26, 34, 36, 37, 40, 43, 45) were excluded, the results did not change (RR = 0.58, 95% CI: 0.46, 0.72; I2 = 0.0%, Ph = 0.582; Figure 13), which indicated that the pooled results were robust and reliable. Although the Egger’s (p = 0.070) and Begg’s tests (p = 0.081) did not indicate significant publication bias, the funnel plot (Figure 14) showed a slight asymmetry. Therefore, we performed a trim-and-fill analysis. The analysis did not impute any missing studies, which suggested minimal risk of publication bias and confirmed the robustness of the pooled results. The GRADE assessment for POCD is shown in Supplementary Figure 1.
Figure 10
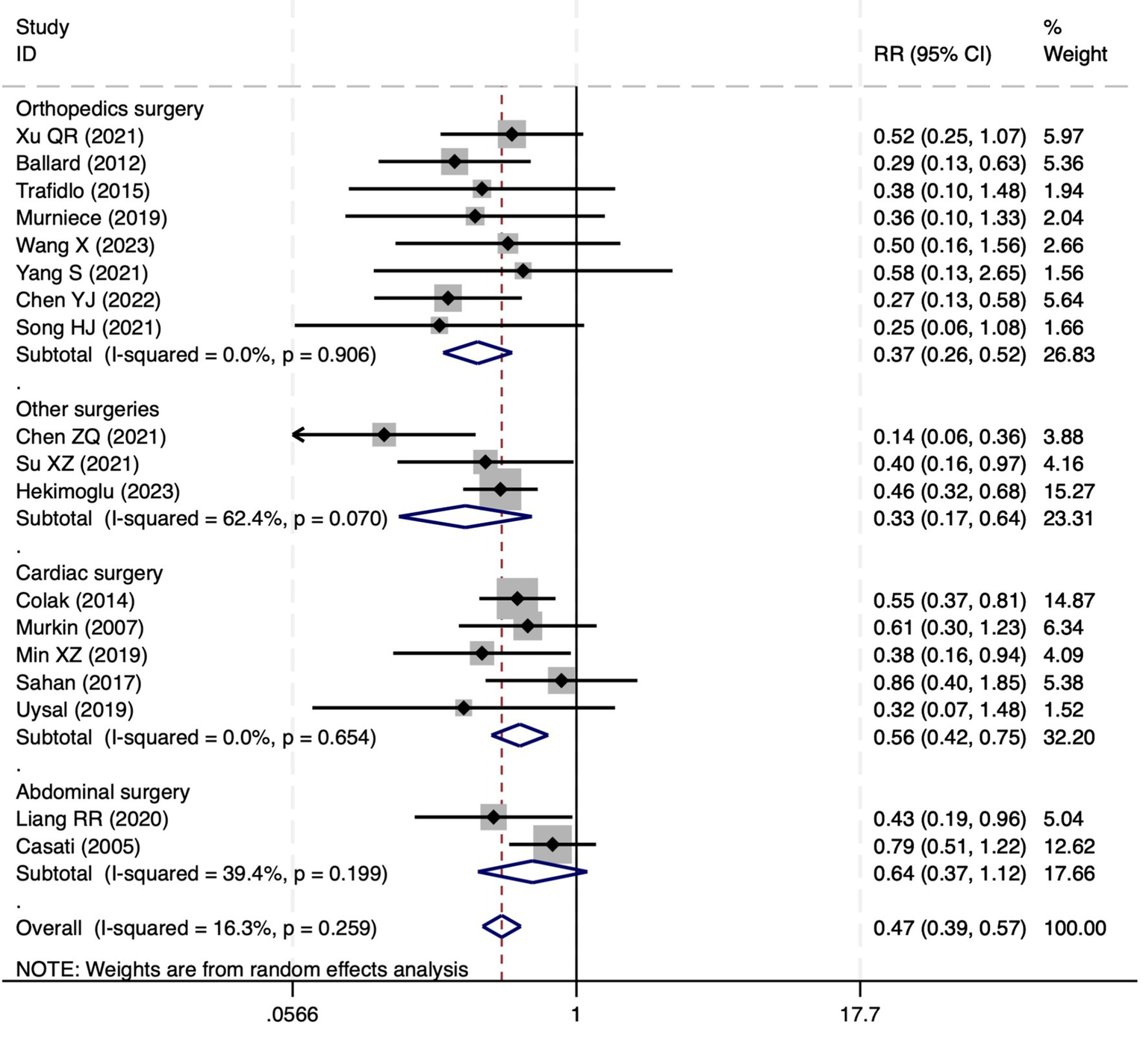
The forest plot of the impact of cerebral oxygen saturation on POCD.
Figure 11
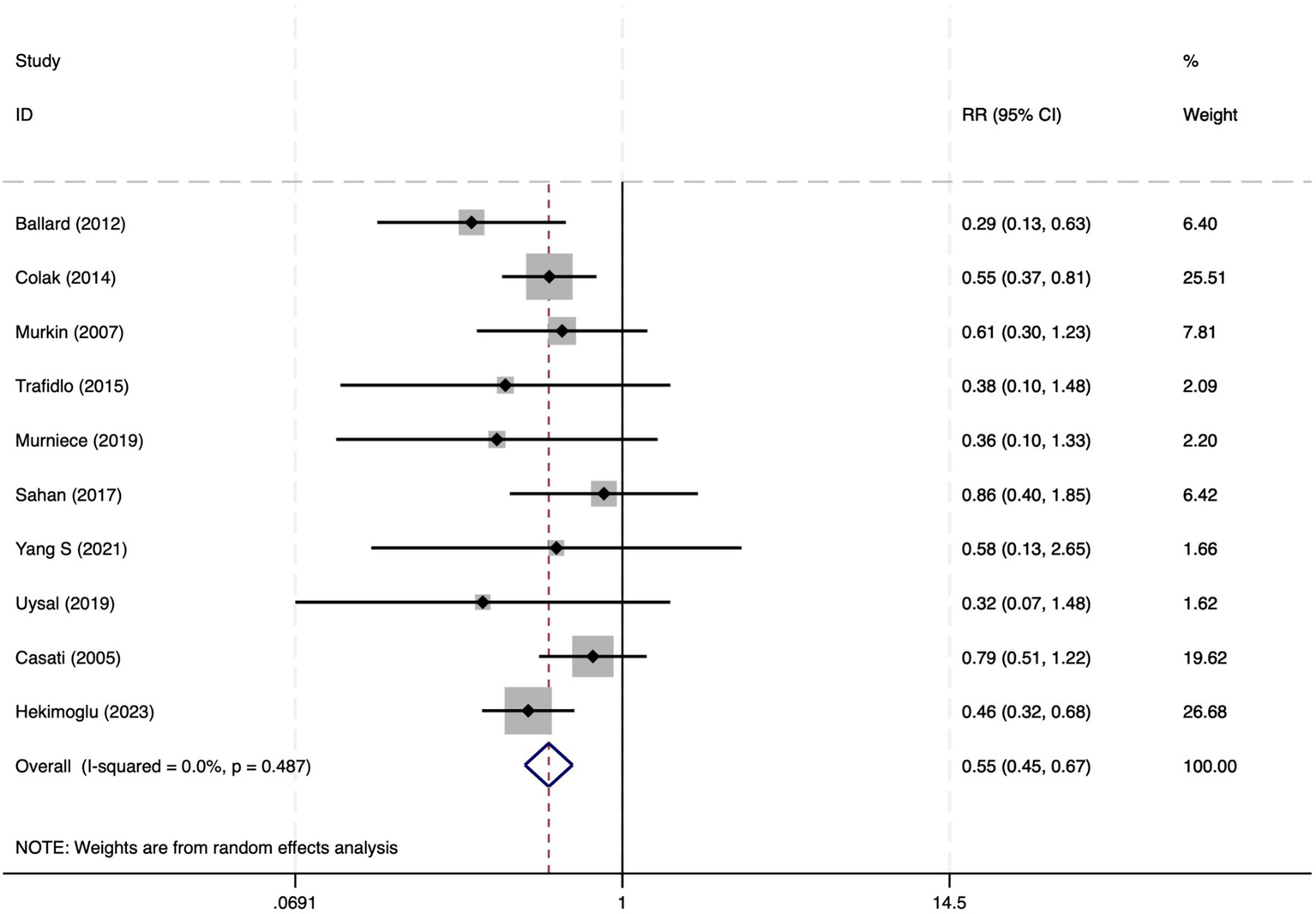
The forest plot of the impact of cerebral oxygen saturation on POCD after excluding Chinese-language studies.
Figure 12
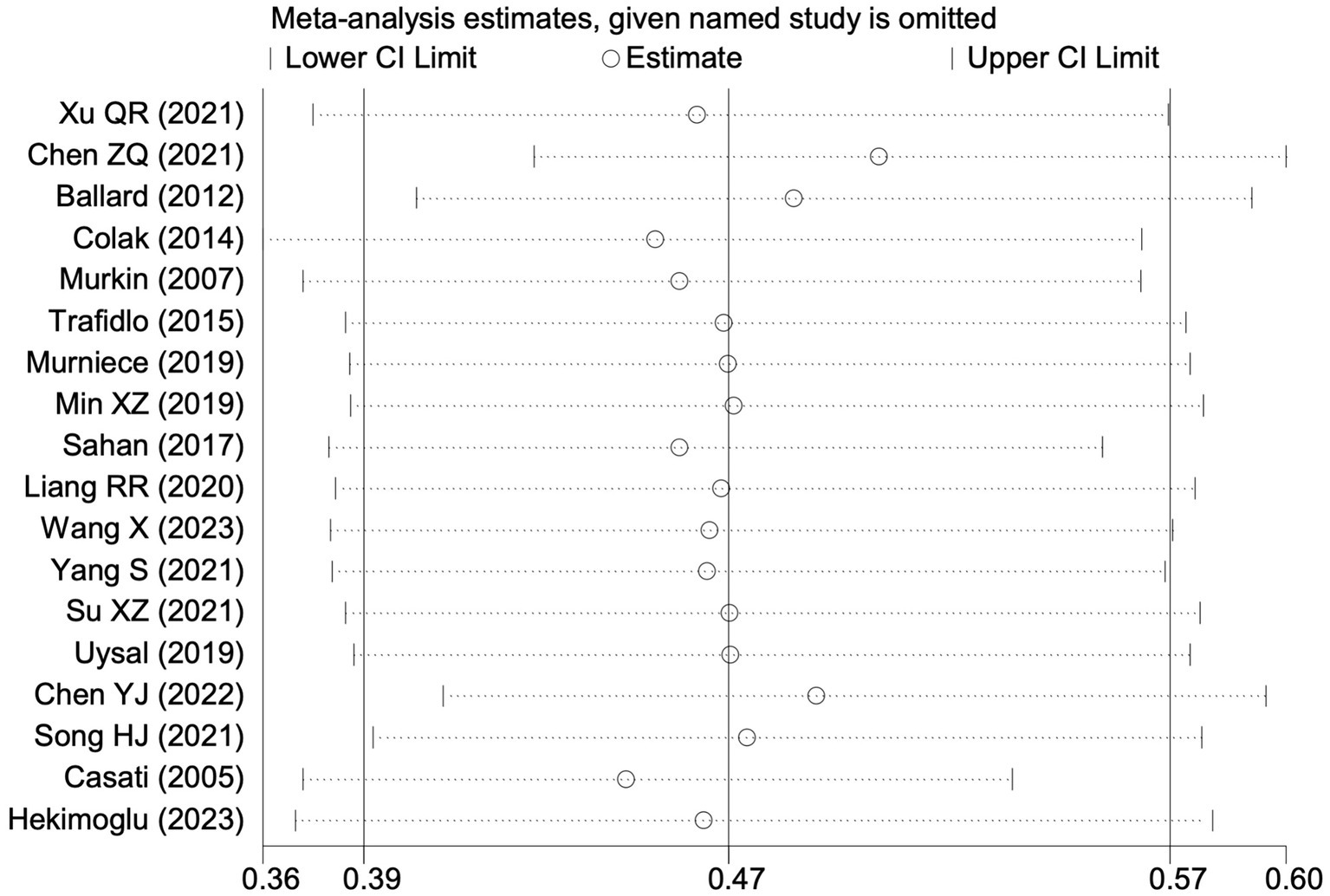
Sensitivity analysis of the effect of intraoperative cerebral oximetry monitoring on the incidence of POCD using the leave-one-out method.
Figure 13
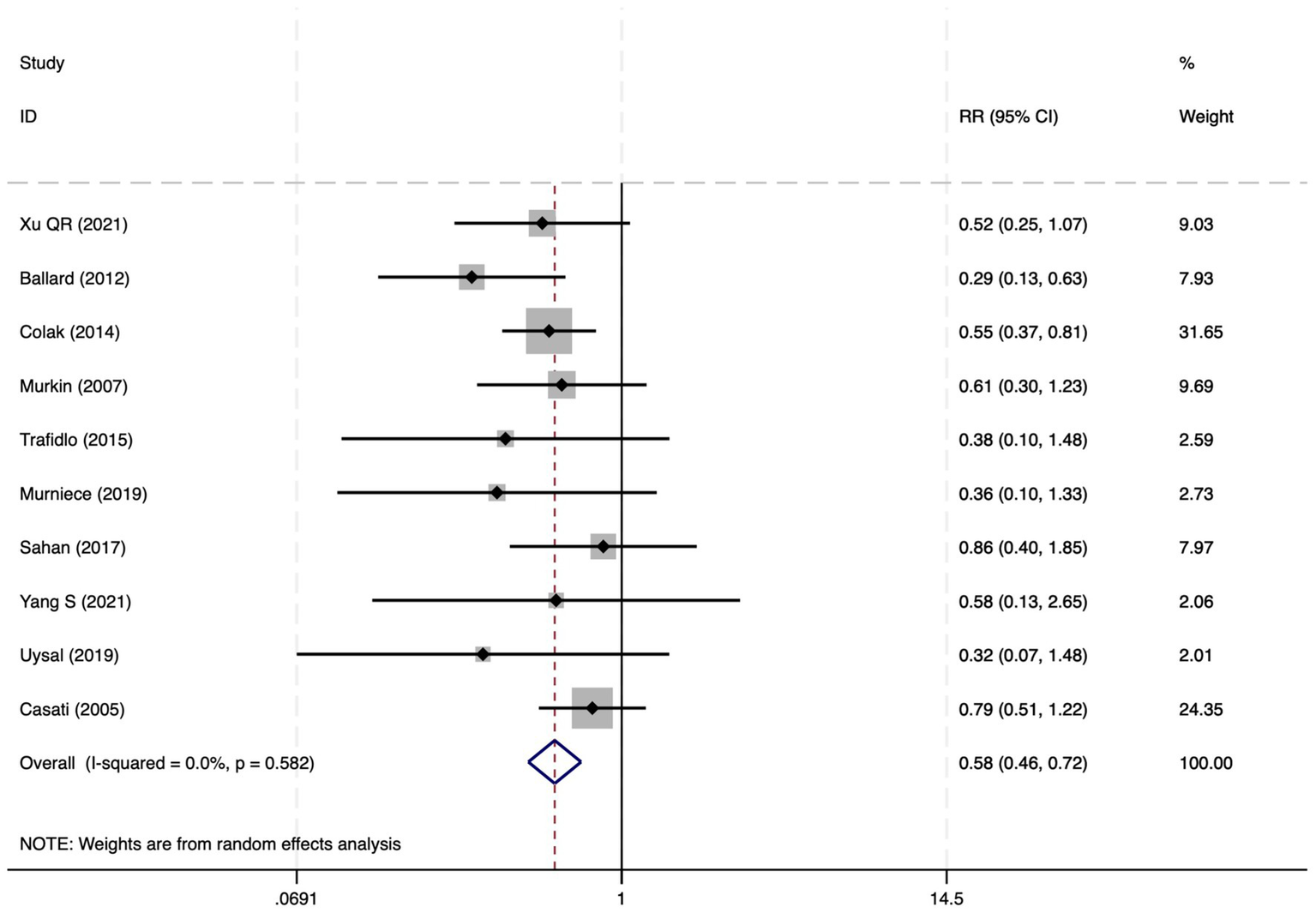
The forest plot of the impact of cerebral oxygen saturation on POCD after excluding low-quality studies.
Figure 14
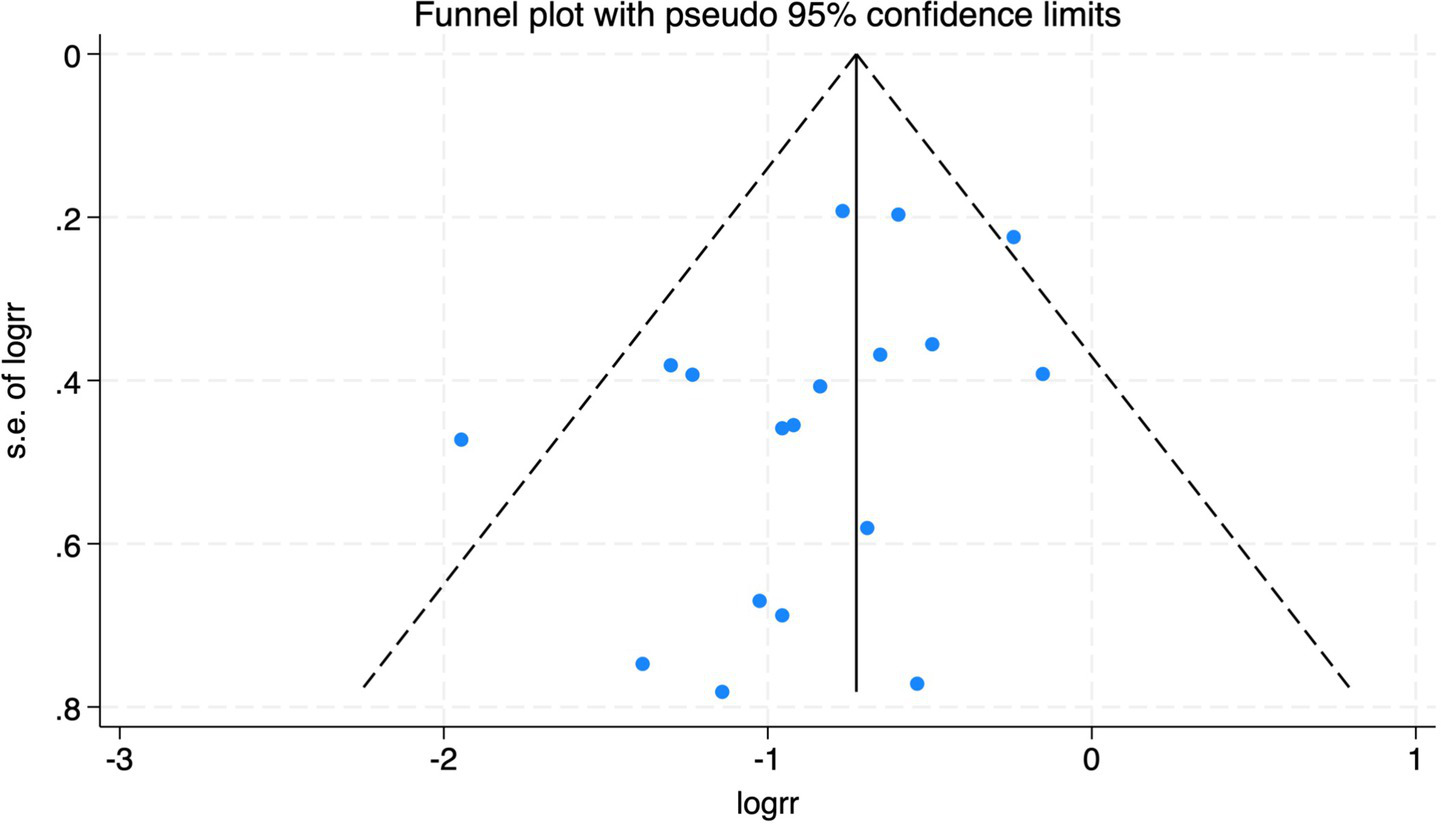
The funnel plot of the impact of cerebral oxygen saturation on POCD.
Postoperative delirium
Of the 28 included studies, 10 reported on POD. Overall, a lower incidence risk of POD was observed (RR = 0.45, 95% CI: 0.35, 0.57; I2 = 0.0%, Ph = 0.618; Figure 15) in the intervention group that underwent intraoperative rScO2 monitoring than in the control group that did not undergo monitoring. In addition, there was a trend of monitoring reducing the risk of POD across different surgery types, particularly abdominal (RR = 0.43, 95% CI: 0.21, 0.87; I2 = 24.2%, Ph = 0.251; Figure 15) and other surgeries (RR = 0.38, 95% CI: 0.26, 0.57; I2 = 0.0%, Ph = 0.701; Figure 15), which were significant. Other subgroup analyses were presented in Supplementary Table 2. When all Chinese-language articles were excluded, only three studies remained. When we compared the control and monitoring groups, intraoperative rScO2 monitoring showed a trend toward a reduced risk of POD (RR = 0.48, 95% CI: 0.23, 1.01; I2 = 40.0%, Ph = 0.189; Figure 16), although this did not reach significance. After excluding studies in which intraoperative interventions were applied only when rScO2 dropped below 80% of baseline, the risk of POD in the experimental group remained significantly lower than that in the control group without intraoperative rScO2 monitoring (RR = 0.45, 95% CI: 0.35, 0.58; I2 = 0.0%, Ph = 0.529; Figure 6). The sensitivity analysis did not change the above results (Figure 17). Moreover, when four low-quality studies (22, 23, 35, 41) were excluded, the results did not change (RR = 0.43, 95% CI: 0.28, 0.66; I2 = 17.8%, Ph = 0.299; Figure 18), which further indicated that the pooled results were robust and reliable. Although the Egger’s (p = 0.121) and Begg’s tests (p = 0.210) did not indicate significant publication bias, the funnel plot (Figure 19) showed a slight asymmetry. Therefore, we performed a trim-and-fill analysis (Figure 20). The pooled effect remained robust (RR = 0.33, 95% CI: 0.24, 0.46) without no change in the direction of the result. The GRADE assessment for POD is shown in Supplementary Figure 1.
Figure 15

The forest plot of the impact of cerebral oxygen saturation on POD.
Figure 16
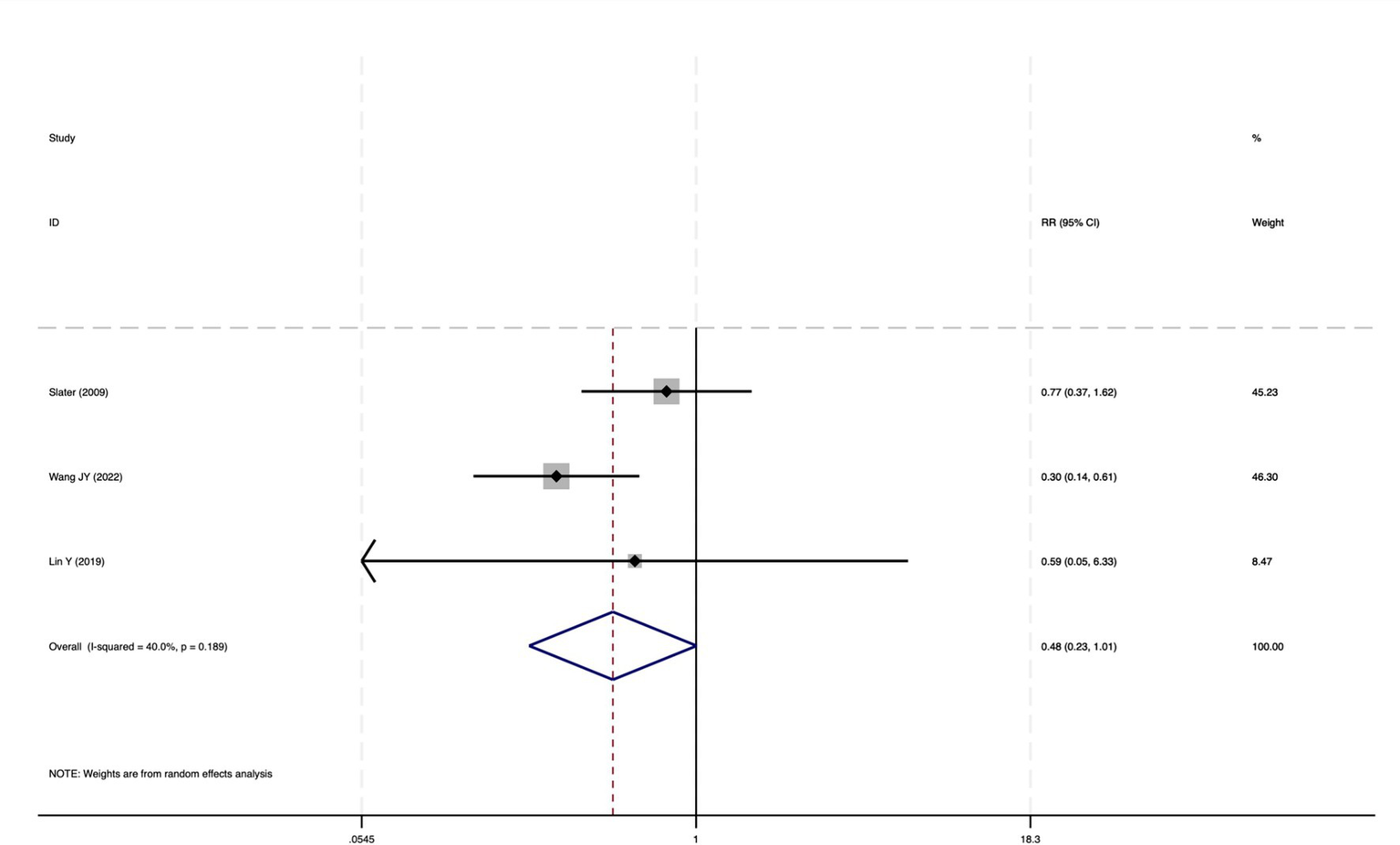
The forest plot of the impact of cerebral oxygen saturation on POD after excluding Chinese-language studies.
Figure 17
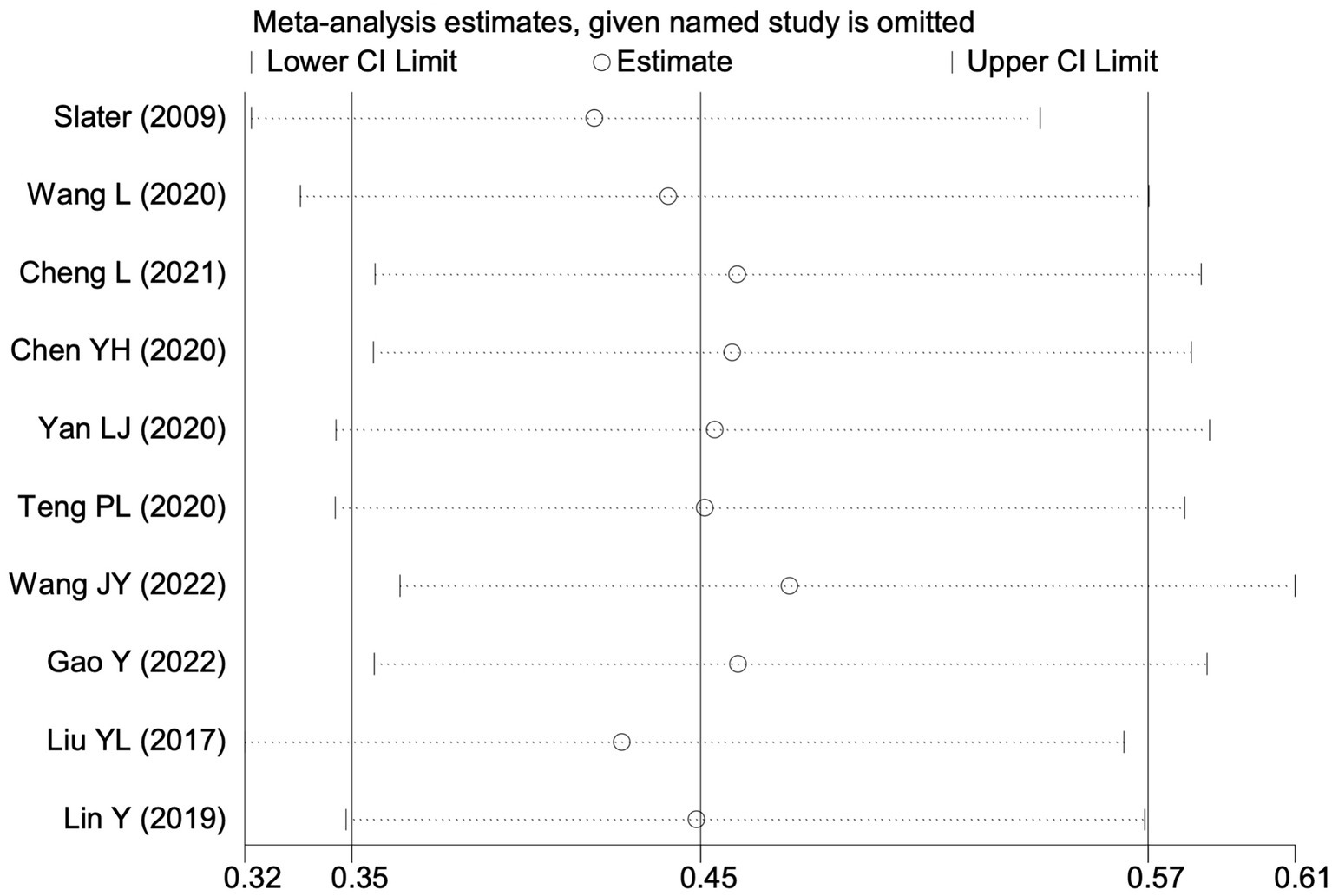
Sensitivity analysis of the effect of intraoperative cerebral oximetry monitoring on the incidence of POD using the leave-one-out method.
Figure 18
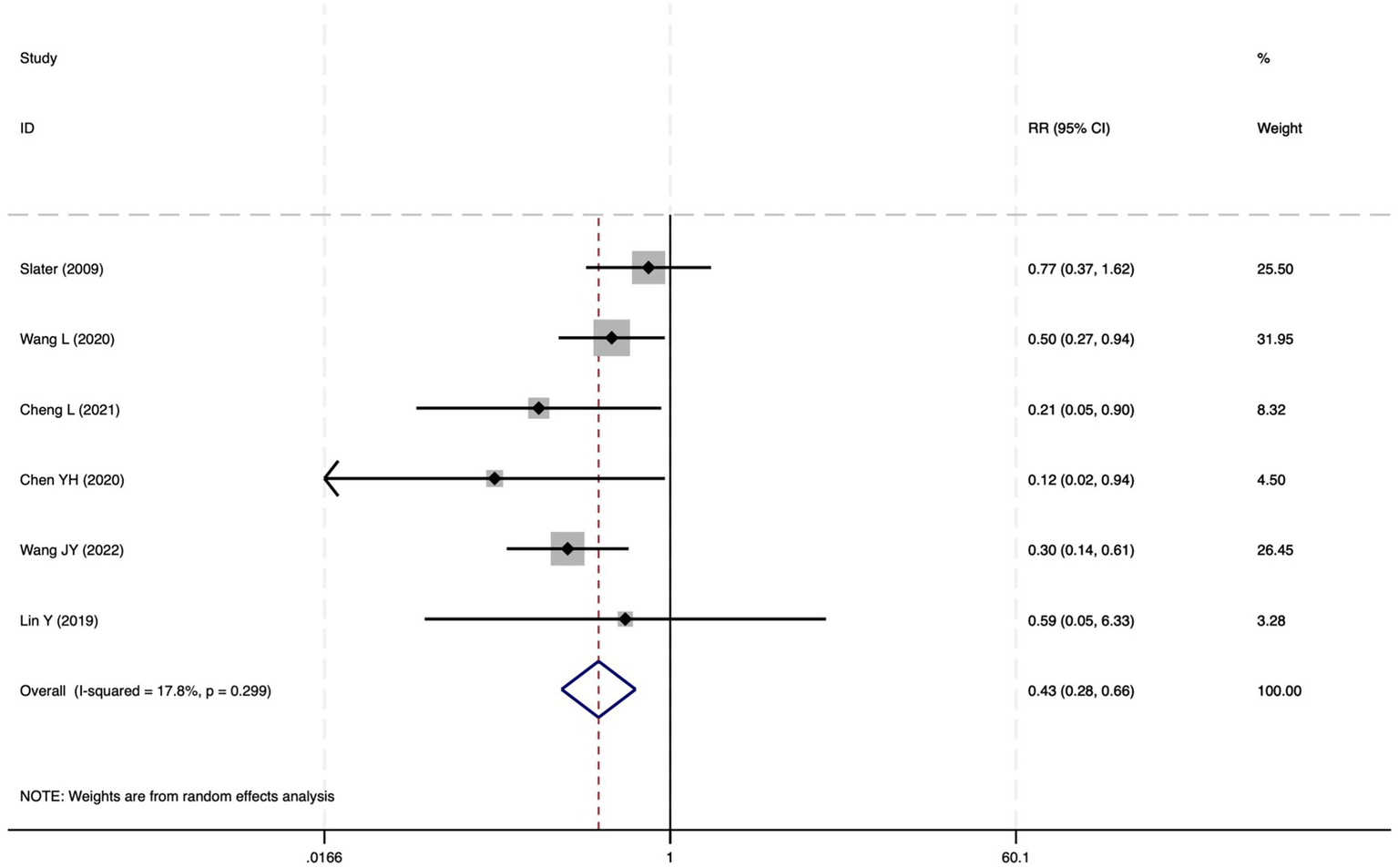
The forest plot of the impact of cerebral oxygen saturation on POD after excluding low-quality studies.
Figure 19
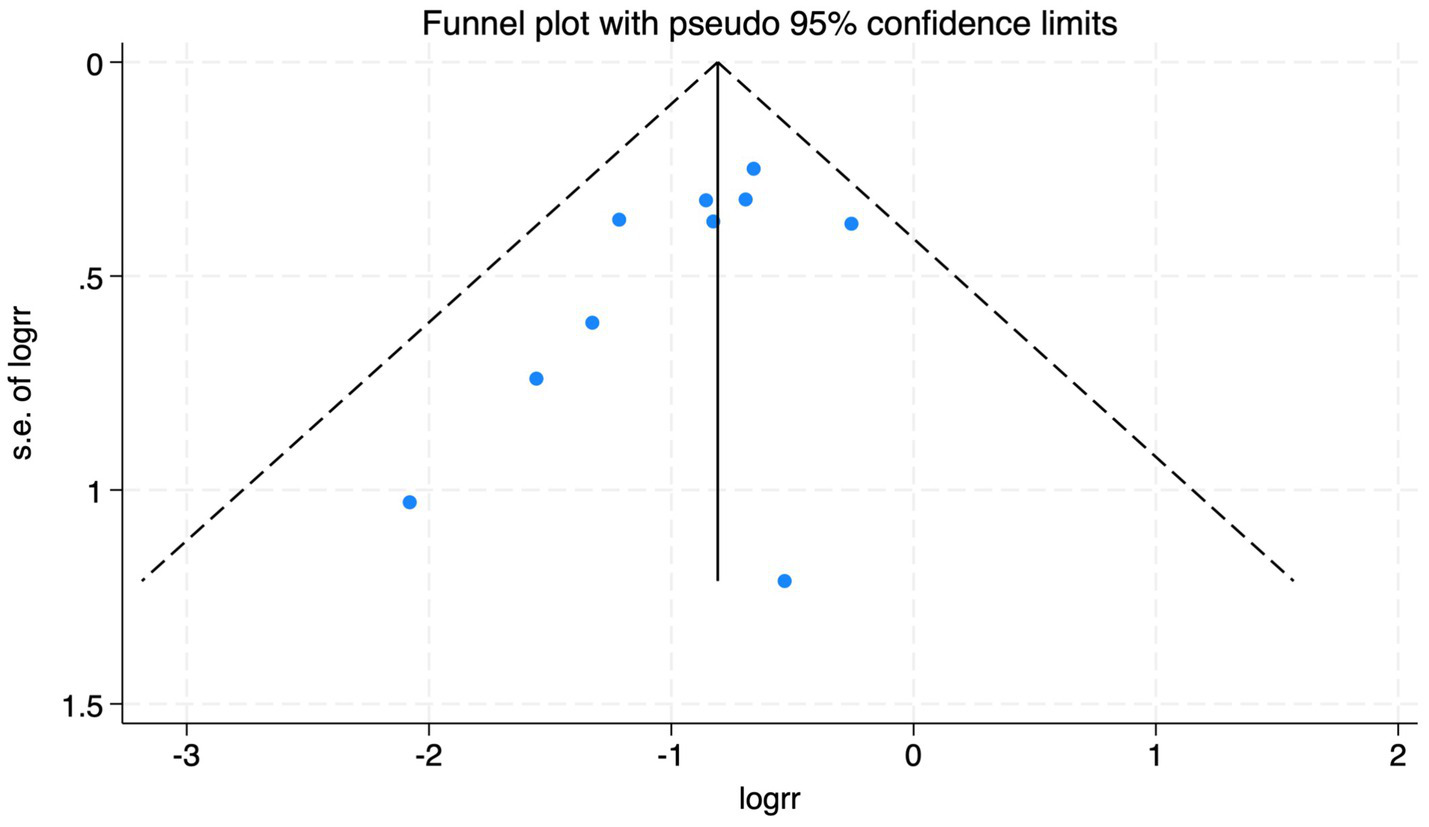
The funnel plot of the impact of cerebral oxygen saturation on POD.
Figure 20
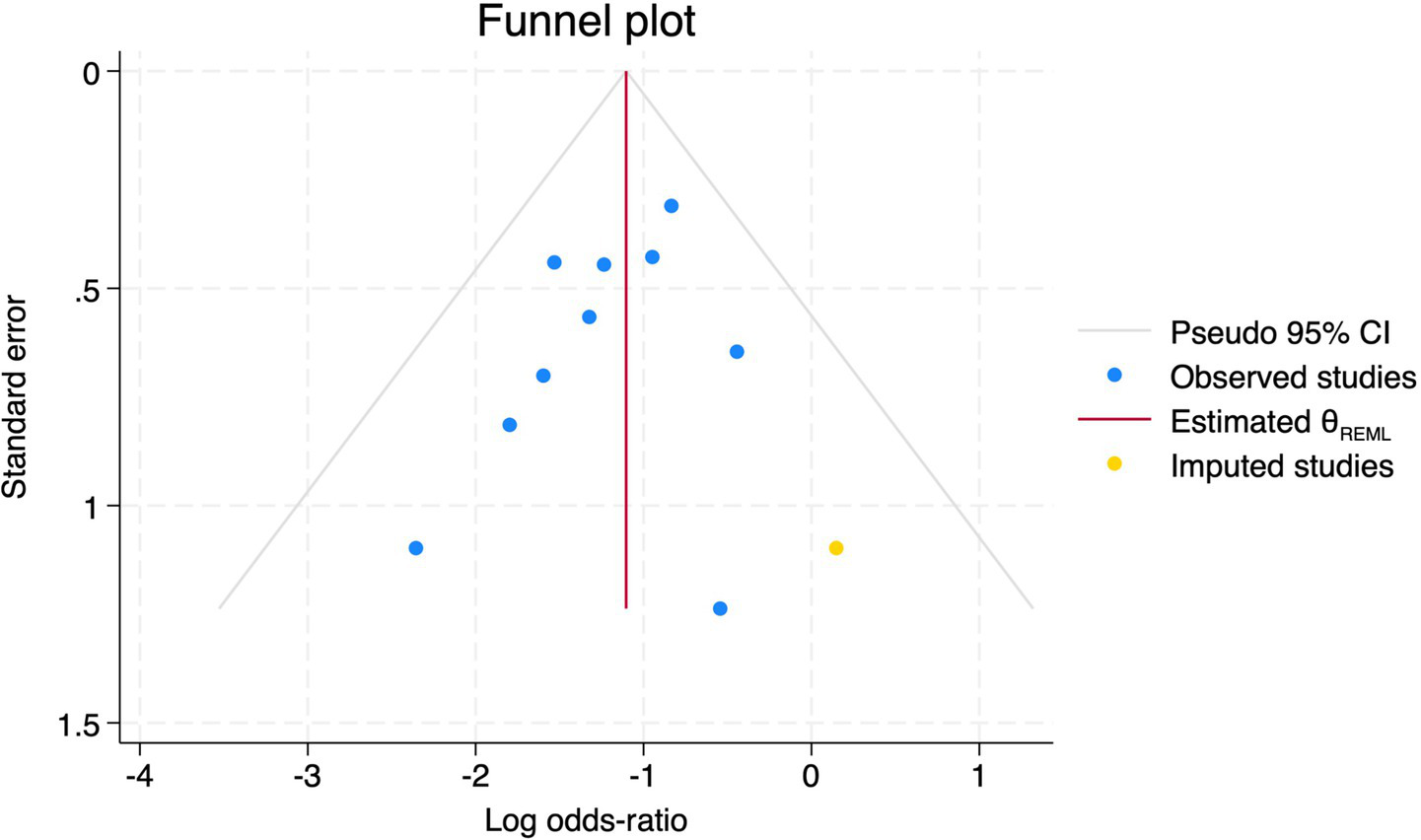
Trim and fill method for POD.
Economic evaluation of cerebral oximetry in reducing postoperative neurological complications
The calculated effect indicators (Table 2) of ARR, NNT, and CBR collectively demonstrated that cerebral oximetry was associated with a substantial reduction in the risk of PND, POCD, and POD. The NNT to prevent one case of PND was approximately 5.0 (95% CI: 4.2, 6.0), which suggested that monitoring five patients could prevent one additional case. Similarly, the NNTs for POCD and POD were approximately 4.5 (95% CI: 3.7, 5.6) and 5.7 (95% CI: 4.4, 8.1), respectively. Such low NNT values indicated that the intervention is clinically meaningful because a substantial number of adverse outcomes can be prevented by offering rScO2 monitoring to a relatively small number of patients.
Table 2
| Outcome | Subgroup | Experimental group | Control group | RR (95% CI) | NNT (95% CI) | ARR (%) (95% CI) | CBR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n1) | Events (a1) | Risk (p1) | Total (n2) | Events (a2) | Risk (p2%) | ||||||
| PND | Overall | 1,295 | 209 | 16.1% | 1,329 | 483 | 36.3% | 0.47 (0.41 ~ 0.54) | 5.0 (4.2 ~ 6.0) | 20.2 (16.7 ~ 23.7) | 0.058 (0.047 ~ 0.070) |
| Cardiac | 416 | 61 | 14.7% | 442 | 118 | 26.7% | 0.57 (0.44 ~ 0.73) | 8.3 (5.6 ~ 15.9) | 12.0 (6.3 ~ 17.7) | 0.096 (0.066 ~ 0.188) | |
| Orthopedic | 344 | 46 | 13.4% | 354 | 128 | 36.2% | 0.39 (0.29 ~ 0.52) | 4.4 (3.5 ~ 6.0) | 22.8 (16.7 ~ 28.9) | 0.051 (0.041 ~ 0.063) | |
| Abdominal | 264 | 49 | 18.6% | 257 | 87 | 33.9% | 0.56 (0.38 ~ 0.82) | 6.5 (4.2 ~ 14.7) | 15.3 (6.8 ~ 23.8) | 0.075 (0.049 ~ 0.153) | |
| Other | 271 | 53 | 19.6% | 276 | 150 | 54.3% | 0.38 (0.28 ~ 0.51) | 2.9 (2.3 ~ 3.8) | 34.7 (26.3 ~ 43.1) | 0.034 (0.027 ~ 0.045) | |
| POCD | Overall | 750 | 139 | 18.5% | 772 | 315 | 40.8% | 0.47 (0.39 ~ 0.57) | 4.5 (3.7 ~ 5.6) | 22.3 (17.9 ~ 26.7) | 0.052 (0.042 ~ 0.064) |
| Cardiac | 296 | 50 | 16.9% | 310 | 95 | 30.6% | 0.56 (0.42 ~ 0.75) | 7.3 (4.9 ~ 14.5) | 13.7 (6.9 ~ 20.5) | 0.085 (0.058 ~ 0.166) | |
| Orthopedic | 254 | 34 | 13.4% | 264 | 98 | 37.1% | 0.37 (0.26 ~ 0.52) | 4.2 (3.2 ~ 6.1) | 23.7 (16.4 ~ 31.0) | 0.049 (0.037 ~ 0.072) | |
| Abdominal | 90 | 27 | 30% | 87 | 40 | 46% | 0.64 (0.37 ~ 1.12) | 6.3 (2.9 ~ ∞) | 16.0 (−3.0 ~ 35.0) | 0.073 (0.034 ~ ∞) | |
| Other | 110 | 28 | 25.5% | 111 | 82 | 73.9% | 0.33 (0.17 ~ 0.64) | 2.1 (1.5 ~ 3.1) | 48.4 (32.1 ~ 64.7) | 0.024 (0.017 ~ 0.036) | |
| POD | Overall | 545 | 70 | 12.8% | 557 | 168 | 30.2% | 0.45 (0.35 ~ 0.57) | 5.7 (4.4 ~ 8.1) | 17.4 (12.3 ~ 22.5) | 0.066 (0.051 ~ 0.092) |
| Cardiac | 120 | 11 | 9.2% | 132 | 23 | 17.4% | 0.56 (0.28 ~ 1.10) | 12.2 (5.3 ~ ∞) | 8.2 (−2.3 ~ 18.7) | 0.141 (0.062 ~ ∞) | |
| Orthopedic | 90 | 12 | 13.3% | 90 | 30 | 33.3% | 0.35 (0.11 ~ 1.15) | 5.0 (2.7 ~ 30.3) | 20.0 (3.3 ~ 36.7) | 0.058 (0.032 ~ 0.585) | |
| Abdominal | 174 | 22 | 12.6% | 170 | 47 | 27.6% | 0.43 (0.21 ~ 0.87) | 6.7 (3.9 ~ 23.8) | 15.0 (4.2 ~ 25.8) | 0.078 (0.047 ~ 0.231) | |
| Other | 161 | 25 | 15.5% | 165 | 68 | 41.2% | 0.38 (0.26 ~ 0.57) | 3.9 (2.8 ~ 6.2) | 25.7 (16.1 ~ 35.3) | 0.045 (0.033 ~ 0.060) | |
Clinical and economic benefits of cerebral oxygen saturation monitoring for preventing postoperative neurocognitive disorders.
NNT, number needed to treat; ARR, attributable risk; CBR, Cost–Benefit Ratio, calculated as intervention cost per benefit unit; CI, confidence interval; PND, perioperative neurocognitive disorders; POCD, postoperative cognitive dysfunction; POD, postoperative delirium.
From an economic perspective, the CBR analysis was based on estimates reported in the literature, which suggest that cerebral oximetry costs approximately $200 per patient (46), whereas the occurrence of postoperative neurological complications (including PND, POCD, and POD) would result in an additional average cost of approximately $17,275 per case (47). This cost differential was applied uniformly across all neurological outcomes to enable a standardized economic evaluation. The intervention cost of rScO2 monitoring to prevent one case of PND is approximately $1,000 (5 × $200), which is markedly lower than the associated financial burden of postoperative neurological complications. These findings, together with favorable CBR values, suggest that cerebral oximetry is not only clinically beneficial but also cost-effective, supporting its broader adoption in perioperative care.
Overall, the RR values ranged from 0.43 to 0.47, which corresponds to a relative risk reduction of over 50%. The ARR exceeded 17% for all outcomes, with NNTs falling predominantly between 4 and 6, indicating strong clinical impact. Subgroup analyses revealed particularly pronounced effects for orthopedic and miscellaneous surgeries, which showed higher ARRs and lower NNTs, although the benefit was relatively modest for cardiac surgeries. The CBR values further underscore the potential of cerebral oximetry for broader perioperative implementation, especially during procedures involving a higher baseline risk of neurocognitive impairment.
Discussion
We conducted a meta-analysis of 28 RCTs to systematically evaluate the impact of intraoperative rScO2 monitoring on the incidence of PND, POCD, and POD. The results indicated that the use of rScO2 monitoring and corresponding interventions during surgery significantly reduced the incidence of PND, POCD, and POD across various surgery types. Our findings align with previous studies, underscoring the importance of rScO2 monitoring in perioperative management, and provide strong evidence for broader clinical implementation of this monitoring strategy (48) to offer additional brain protection and a solid foundation for improving patients’ long-term quality of life (49).
In addition to the clinical benefits, rScO2 monitoring demonstrates good economic value. Based on the NNT values for PND, POCD, and POD (ranging from 4.5 to 5.7) and an estimated cost of $200 per patient, the total cost of preventing a single neurocognitive event was significantly lower than the average economic burden of such complications, which can reach $17,275 per case. Therefore, monitoring rScO2 is not only effective but also cost-effective, especially in those undergoing orthopedic surgery. Walsh et al. (50) conducted a cost analysis on 100 patients undergoing cardiac surgery and compared those who underwent cerebral oxygen monitoring (via the INVOS system) with those without monitoring. They found that although the INVOS device itself came with a cost, the patient group that used INVOS significantly reduced their postoperative hospital stay (including in both the intensive care unit and the general wards), thereby enabling a considerable cost saving. Overall, approximately £102,000 could be saved for every 100 patients who undergo heart surgery, which indicates that rScO2 monitoring not only significantly reduces the risk of postoperative neurological complications but also offers significant economic benefits by shortening the length of hospital stay.
In non-cardiac surgeries, such as orthopedic and abdominal surgeries, the preventive effect of rScO2 monitoring on POCD and POD was particularly pronounced. The protective effect of rSO2 monitoring on preventing POCD is more obvious in non-cardiac surgeries. This might be because global cerebral ischemia and hypoxia caused by systemic hypotension or anemia in non-cardiac surgeries are more easily reflected through rSO2 monitoring, while the distribution of cerebral ischemia in cardiac surgeries may be uneven, resulting in a less significant effect of rSO2 monitoring compared to non-cardiac surgeries (51). This may be because patients undergoing non-cardiac surgeries experience less physiological fluctuation than those undergoing cardiac surgeries; thus, rScO2 monitoring allows more efficient response to localized cerebral hypoxia. By contrast, during cardiac surgeries, where patients experience more complex and dramatic circulatory changes and greater instability in cerebral blood flow and oxygen supply (52), rScO2 monitoring offers critical protection, which, in turn, significantly reduces the incidence of PND. This demonstrates the clinical value of rScO2 monitoring across different types of surgeries and further validates its wide applicability as an intraoperative monitoring tool. Therefore, we recommend that rScO2 monitoring be implemented broadly in various surgeries to improve patients’ postoperative cognitive outcomes.
Notably, the studies included in this meta-analysis encompassed various types of surgeries, including cardiac procedures (e.g., coronary artery bypass grafting and valve replacement) and non-cardiac surgeries (e.g., orthopedic and abdominal surgeries). The interventions were based on NIRS monitoring of rScO2 and included personalized management strategies, such as adjustments to inspired oxygen concentration, circulatory management, and other measures aimed at optimizing cerebral oxygen supply (53). Such diversity in procedures highlights the robustness of the observed intervention effects and suggests that the benefits of rScO2 monitoring vary depending on the type of surgery and patient population.
Despite these findings, this meta-analysis has several limitations. First, the number of included studies was limited, which may contribute to statistical heterogeneity and affect the robustness of the findings. Second, patient-reported outcomes and functional measures, such as quality of life, were not assessed. Therefore, the comprehensiveness of postoperative recovery evaluation was limited. Third, the lack of standardized thresholds for rScO2 monitoring across studies restricts the reproducibility and clinical applicability of the results. Fourth, only two studies reported outcomes at 3 months, limiting the strength of conclusions regarding long-term effects. Fifth, about 60% of patients were recruited in Chinese centers; although excluding Chinese-language studies did not change the PND result, the number of non-Chinese trials was small for some outcomes (e.g., POD), so broad generalisability should be cautioned and multicentre data from other regions are needed. Finally, we did not stratify PND, POCD, or POD by severity or duration, which may limit the clinical relevance of the findings. Until consensus definitions exist, we recommend that future trials adopt CAM/CAM-ICU for POD and the ISPOCD 1-SD rule for POCD assessed at 1 week and 3 months to facilitate comparison and clinical translation. Supplementary Table 3 shows that most studies used the same three-step bundle (raise FiO2, MAP and Hb), sensitivity analyses found similar RRs after excluding trials that waited until rScO2 < 80% baseline, suggesting the benefit comes from the bundle, not the trigger value. Future research should address these issues via standardized protocols, longer follow-up periods, and comprehensive neurocognitive outcome measures.
Overall, intraoperative rScO2 monitoring is an effective means of preventing PNDs in patients undergoing general anesthesia. Although our findings are encouraging, larger, multicenter RCTs are needed to validate our results further. Given the significant results of rScO2 monitoring and management, further investigation into its specific mechanisms and clinical applications will provide anesthesiologists with more precise guidance on the management of general anesthesia surgeries. The traditional focus of anesthesia management has seen a shift from merely ensuring stable vital signs to also considering the potential impact of anesthesia on the brain. By implementing rScO2 monitoring, not only is patient safety during surgery ensured but also cognitive health is safeguarded.
Conclusion
This study revealed that rScO2 monitoring, as an intraoperative intervention, significantly reduces the occurrence of PND. Moreover, it has wide clinical applicability across all types of surgeries. Its widespread implementation is recommended to improve the postoperative cognitive outcomes of patients undergoing surgery, especially given its cost-effectiveness in reducing postoperative neurocognitive complications.
What is known?
Inadequate cerebral oxygen supply during anesthesia and surgery is a risk factor for perioperative neurocognitive disorders (PNDs).
Although previous studies have explored the impact of intraoperative rScO2 monitoring on cognitive function, results have been inconsistent; moreover, outcomes were not classified by surgery type.
What is new?
This meta-analysis demonstrated that intraoperative rScO2 monitoring significantly reduces the incidence of PND, POCD, and POD.
RScO2 monitoring is beneficial for both cardiac and non-cardiac surgeries, showing its broad applicability across different patient populations.
Our findings support the widespread implementation of rScO2 monitoring as an effective intervention to improve postoperative cognitive outcomes.
Our analysis revealed favorable economic benefits with a low CBR, which indicates that rScO2 monitoring is a cost-effective strategy for preventing postoperative neurocognitive complications.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JQ: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing, Resources. GW: Methodology, Validation, Data curation, Writing – review & editing. DG: Funding acquisition, Investigation, Methodology, Writing – original draft. JL: Data curation, Software, Writing – review & editing. JZ: Investigation, Software, Writing – review & editing, Data curation. MG: Methodology, Software, Writing – original draft. XH: Resources, Writing – original draft, Formal analysis, Project administration, Writing – review & editing, Methodology, Software, Investigation, Data curation. XM: Funding acquisition, Writing – review & editing, Writing – original draft, Investigation, Project administration.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by the Scientific research projects within Changzhi People’s Hospital (YNLX20240001, YNLX20240017) and Health Development Promotion Project – Love from the Heart -Shanxi Anesthesia Research Incubation Program (YXHA-ARP-012).
Acknowledgments
We sincerely thank all the staff members who contributed to this study, particularly for their assistance in data collection and manuscript preparation. We thank Sarina Iwabuchi, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2026.1677218/full#supplementary-material
Abbreviations
PND, perioperative neurocognitive disorders; POCD, postoperative cognitive dysfunction; POD, postoperative delirium; ASA, American Society of Anesthesiology; BMI, Body Mass Index; RCT, randomized controlled trial; rScO2, Regional Cerebral Oxygen Saturation; NNT, Number Needed to Treat; CBR, Cost–Benefit Ratio; ARR, Absolute Risk Reduction; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; RR, Relative Risk; CI, Confidence Interval.
References
1.
Evered LA Goldstein PA . Reducing perioperative neurocognitive disorders (PND) through depth of anesthesia monitoring: a critical review. Int J Gen Med. (2021) 14:153–62. doi: 10.2147/IJGM.S242230,
2.
Varpaei HA Farhadi K Mohammadi M Khafaee Pour Khamseh A Mokhtari T . Postoperative cognitive dysfunction: a concept analysis. Aging Clin Exp Res. (2024) 36:133. doi: 10.1007/s40520-024-02779-7,
3.
Thedim M Vacas S . Postoperative delirium and the older adult: untangling the confusion. J Neurosurg Anesthesiol. (2024) 36:184–9. doi: 10.1097/ANA.0000000000000971,
4.
Li R Zhang Y Zhu Q Wu Y Song W . The role of anesthesia in peri-operative neurocognitive disorders: molecular mechanisms and preventive strategies. Fundam Res. (2023) 4:797–805. doi: 10.1016/j.fmre.2023.02.007,
5.
Glumac S Kardum G Sodic L Bulat C Covic I Carev M et al . Longitudinal assessment of preoperative dexamethasone administration on cognitive function after cardiac surgery: a 4-year follow-up of a randomized controlled trial. BMC Anesthesiol. (2021) 21:129. doi: 10.1186/s12871-021-01348-z,
6.
Qiu L Ma Y Ge L Zhou H Jia W . Efficacy of cerebral oxygen saturation monitoring for perioperative neurocognitive disorder in adult noncardiac surgical patients: a systematic review and Meta-analysis of randomized controlled trials. World Neurosurg. (2025) 194:123570. doi: 10.1016/j.wneu.2024.123570,
7.
Lavinio A . Cerebral circulation II: pathophysiology and monitoring. BJA Educ. (2022) 22:282–8. doi: 10.1016/j.bjae.2022.02.005,
8.
Colak Z Borojevic M Bogovic A Ivancan V Biocina B Majeric-Kogler V . Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: a randomized, prospective study. Eur J Cardiothorac Surg. (2015) 47:447–54. doi: 10.1093/ejcts/ezu193,
9.
Momeni M Meyer S Docquier MA Lemaire G Kahn D Khalifa C et al . Predicting postoperative delirium and postoperative cognitive decline with combined intraoperative electroencephalogram monitoring and cerebral near-infrared spectroscopy in patients undergoing cardiac interventions. J Clin Monit Comput. (2019) 33:999–1009. doi: 10.1007/s10877-019-00253-8,
10.
Moher D Liberati A Tetzlaff J Altman DG PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097,
11.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
12.
DerSimonian R Laird N . Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2,
13.
Begg CB Mazumdar M . Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
14.
Egger M Smith DG Schneider M Minder C . Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629,
15.
Duval S Tweedie R . Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x,
16.
De Vries FEE Wallert ED Solomkin JS Allegranzi B Egger M Dellinger EP et al . A systematic review and meta-analysis including GRADE qualification of the risk of surgical site infections after prophylactic negative pressure wound therapy compared with conventional dressings in clean and contaminated surgery. Medicine (Baltimore). (2016) 95:e4673. doi: 10.1097/MD.0000000000004673,
17.
Christensen PM Kristiansen IS . Number-needed-to-treat (NNT)--needs treatment with care. Basic Clin Pharmacol Toxicol. (2006) 99:12–6. doi: 10.1111/j.1742-7843.2006.pto_412.x,
18.
Masters R Anwar E Collins B Cookson R Capewell S . Return on investment of public health interventions: a systematic review. J Epidemiol Community Health. (2017) 71:827–34. doi: 10.1136/jech-2016-208141,
19.
Wang L Li XZ Yu WG Dong R Wang MS Bi YL et al . The effect on bleeding volume and postoperative recovery of regional cerebral oxygen saturation guides controlled hypotension in elderly patients with hypertension undergoing spinal surgery. Natl Med J China. (2020) 100:3230–4. doi: 10.3760/cma.j.cn112137-20200515-01554
20.
Cheng L Jiang Y Zheng LD . Effect of goal-directed management of cerebral oxygen monitoring on postoperative delirium in elderly patients undergoing thoracoscopic radical esophagectomy. Anhui Med Pharm J. (2021) 25:1190–3. doi: 10.3969/j.issn.1009-6469.2021.06.032
21.
Chen YH Wan HF Zhang YX . Effect of interventional intraoperative cerebral oxygen saturation monitoring on postoperative delirium in elderly patients with spinal tuberculosis. Chin J Gen Pract. (2020) 18:415–8. doi: 10.16766/j.cnki.issn.1674-4152.001261
22.
Yan L Li C Wang G Ma X Dai B Xie W et al . Effects of controlled hypotension on postoperative delirium in elderly patients with hypertension under cerebral oxygen saturation monitoring. J Clin Anesthesiol. (2020) 36:857–60. doi: 10.12089/jca.2020.09.005
23.
Teng PL Xu DR Lv F Liu HH Wang N Feng XX . Effect of regional cerebral oxygen saturation guided lung protective ventilation on postoperative delirium in elderly. J Clin Anesthesiol. (2020) 36:1009–12. doi: 10.12089/jca.2020.10.017
24.
Xu QR Bo HL Li Y Yu YJ . Effect of rSO2-guided low-dose norepinephrine on postoperative cognitive dysfunction in elderly patients undergoing hip replacement under general anesthesia. Chin J Anesthesiol. (2021) 41:662–6. doi: 10.3760/cma.j.cn131073.20210301.00606
25.
Chen ZQ Gao MH Cai Q . Effects of intraoperative cerebral oxygen saturation on early postoperative cognitive dysfunction in elderly patients with intrathecal anesthesia. Chin J Biomed Eng. (2021) 27:391–6. doi: 10.3760/cma.j.cn115668-20200527-00132
26.
Su XZ Zhang Y Wang LP . Effect of goal-oriented technique of cerebral oxygen saturation monitoring on perioperative cerebral protection in elderly patients. China Med Herald. (2021) 18:95–8. doi: 10.20047/j.issn1673-7210.2021.07.024
27.
Wang JY Li M Wang P Fang P . Goal-directed therapy based on rScO2 monitoring in elderly patients with one-lung ventilation: a randomized trial on perioperative inflammation and postoperative delirium. Trials. (2022) 23:687. doi: 10.1186/s13063-022-06654-6,
28.
Ballard C Jones E Gauge N Aarsland D Nilsen OB Saxby BK et al . Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLoS One. (2012) 7:e37410. doi: 10.1371/journal.pone.0037410,
29.
Murkin JM Adams SJ Novick RJ Quantz M Bainbridge D Iglesias I et al . Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. (2007) 104:51–8. doi: 10.1213/01.ane.0000246814.29362.f4,
30.
Slater JP Guarino T Stack J Vinod K Bustami RT Brown JM 3rd et al . Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. (2009) 87:36–44. doi: 10.1016/j.athoracsur.2008.08.070,
31.
Trafidło T Gaszyński T Gaszyński W Nowakowska-Domagała K . Intraoperative monitoring of cerebral NIRS oximetry leads to better postoperative cognitive performance: a pilot study. Int J Surg. (2015) 16:23–30. doi: 10.1016/j.ijsu.2015.02.009,
32.
Murniece S Soehle M Vanags I Mamaja B . Near infrared spectroscopy based clinical algorithm applicability during spinal neurosurgery and postoperative cognitive disturbances. Medicina (Kaunas). (2019) 55:179. doi: 10.3390/medicina55050179,
33.
Uysal S Lin HM Trinh M Park CH Reich DL . Optimizing cerebral oxygenation in cardiac surgery: a randomized controlled trial examining neurocognitive and perioperative outcomes. J Thorac Cardiovasc Surg. (2020) 159:943–953.e3. doi: 10.1016/j.jtcvs.2019.03.036,
34.
Chen YJ Luo DX . Monitoring and management of cerebral oxygen saturation during perianaesthesia for postoperative delirium and recovery in elderly fracture patients effect. Heilongjiang Med J. (2022) 35:1408–10. doi: 10.14035/j.cnki.hljyy.2022.06.062
35.
Gao Y Zhang ZQ Hu J Chen XP He Y Qu HY et al . Application of local cerebral oxygen saturation monitoring in minimally invasive coronary artery bypass grafting. Transl Med J. (2022) 11:159–63. doi: 10.3969/j.issn.2095-3097.2022.03.008
36.
Min XZ Gao GH Guo R Wang NH . Application value of the intervening measure of cerebral oxygen saturation monitoring technique in reducing the incidence rate of postoperative cognitive dysfunction. J Bengbu Med Coll. (2019) 44:1461–4. doi: 10.13898/j.cnki.issn.1000-2200.2019.11.007
37.
Wang X Qi L Wang L . Effect of regional cerebral oxygen saturation monitoring on perioperative circulation and inflammatory factors in elderly hypertensive patients undergoing spinal fixation surgery. Orthopaedics. (2023) 14:358–62. doi: 10.3969/j.issn.1674-8573.2023.04.012
38.
Şahan C Sungur Z Çamcı E Sivrikoz N Sayin Ö Gurvit H et al . Efeitos das alterações no oxigênio cerebral durante cirurgia de revascularização do miocárdio sobre a disfunção cognitiva no pós-operatório em pacientes idosos: estudo piloto [Effects of cerebral oxygen changes during coronary bypass surgery on postoperative cognitive dysfunction in elderly patients: a pilot study]. Braz J Anesthesiol. (2018) 68:142–8. doi: 10.1016/j.bjan.2017.10.005
39.
Yang S Xiao W Wu H Liu Y Feng S Lu J et al . Management based on multimodal brain monitoring May improve functional connectivity and post-operative Neurocognition in elderly patients undergoing spinal surgery. Front Aging Neurosci. (2021) 13:705287. doi: 10.3389/fnagi.2021.705287,
40.
Liang RR Cui XY Wang CY Fan ZL . Application of cerebral oxygen saturation monitoring in perioperative period of pheochromocytoma. Chin J Endourol (Electron Ed). (2020) 14:343–7. doi: 10.3877/cma.j.issn.1674-3253.2020.05.006
41.
Liu Y . A randomized controlled study on reducing postoperative delirium in elderly esophageal cancer patients guided by cerebral oxygen saturation and BIS monitoring. Shang Hai: Second Military Medical University (2017).
42.
Lin Y Chen MF Chen LW Wang JB Zhang H Li RM . Midterm cerebral outcomes of Stanford type a aortic dissection in patients who underwent novel triple-branched stent graft implantation combined with intraoperative monitoring of regional cerebral oxygen saturation. J Card Surg. (2019) 34:774–81. doi: 10.1111/jocs.14130,
43.
Song HJ Hu YY Tong L Wang Y Cheng Z Zhao X et al . Effects of goal-oriented management of cerebral oxygen saturation on early postoperative neurocognitive impairment in elderly spinal surgery patients. J Chin Physician. (2021) 23:1012–6. doi: 10.3760/cma.j.cn431274-20200410-00433
44.
Casati A Fanelli G Pietropaoli P Proietti R Tufano R Danelli G et al . Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg. (2005) 101:740–7. doi: 10.1213/01.ane.0000166974.96219.cd,
45.
Hekimoglu Sahin S Copuroglu E Delen E Tutunculer B Sut N Colak A et al . The effect of cerebral oxygen saturation changes on early postoperative neuropsychological function in patients undergoing cranial surgery. Turk Neurosurg. (2023) 33:618–25. doi: 10.5137/1019-5149.JTN.37194-21.3,
46.
Zhang J Shen H Wang H Xiao F Deng L Chen X et al . Intraoperative application of regional cerebral oxygen saturation monitoring for geriatric patients in China: a survey. Front Med (Lausanne). (2023) 10:1165821. doi: 10.3389/fmed.2023.1165821,
47.
Boone MD Sites B von Recklinghausen FM Mueller A Taenzer AH Shaefi S . Economic burden of postoperative neurocognitive disorders among US Medicare patients. JAMA Netw Open. (2020) 3:e208931. doi: 10.1001/jamanetworkopen.2020.8931,
48.
Wong ZZ Chiong XH Chaw SH Hashim NHBM Abidin MFBZ Yunus SNB et al . The use of cerebral oximetry in surgery: a systematic review and meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. (2022) 36:2002–11. doi: 10.1053/j.jvca.2021.09.046,
49.
Zhang CY Yang YS Pei MQ Chen XL Chen WC He HF . The Association of Cerebral Oxygen Desaturation with postoperative cognitive dysfunction in older patients: a review. Clin Interv Aging. (2024) 19:1067–78. doi: 10.2147/CIA.S462471,
50.
Walsh D Bennett M Bennett S . Cost analysis of patients undergoing cardiac surgery managed with or without cerebral oximetry (INVOS). BMC Proceedings. London: BioMed Central, 2012, 6: O16.
51.
Ding X Zha T Abudurousuli G Zhao C Chen Z Zhang Y et al . Effects of regional cerebral oxygen saturation monitoring on postoperative cognitive dysfunction in older patients: a systematic review and meta-analysis. BMC Geriatr. (2023) 23:123. doi: 10.1186/s12877-023-03804-6,
52.
Vretzakis G Georgopoulou S Stamoulis K Stamatiou G Tsakiridis K Zarogoulidis P et al . Cerebral oximetry in cardiac anesthesia. J Thorac Dis. (2014) 6 Suppl 1:S60–9. doi: 10.3978/j.issn.2072-1439.2013.10.22,
53.
Nielsen HB . Systematic review of near-infrared spectroscopy determined cerebral oxygenation during non-cardiac surgery. Front Physiol. (2014) 5:93. doi: 10.3389/fphys.2014.00093,
54.
Folstein MF Folstein SE McHugh PR . "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6,
55.
Nasreddine ZS Phillips NA Bédirian V Charbonneau S Whitehead V Collin I et al . The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x,
56.
Ely EW Inouye SK Bernard GR Gordon S Francis J May L et al . Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. (2001) 286:2703–10. doi: 10.1001/jama.286.21.2703,
57.
Trzepacz PT . The delirium rating scale. Its use in consultation-liaison research. Psychosomatics. (1999) 40:193–204. doi: 10.1016/S0033-3182(99)71235-1,
Summary
Keywords
cerebral oxygen saturation, cognitive dysfunction, delirium, economic evaluation, meta-analysis, neuropsychological tests, perioperative neurocognitive disorders, postoperative cognitive complications
Citation
Qin J, Wang G, Gu D, Li J, Zhang J, Ge M, He X and Ma X (2026) The impact of cerebral oxygen saturation monitoring on perioperative neurocognitive disorders: a meta-analysis and economic analysis. Front. Med. 13:1677218. doi: 10.3389/fmed.2026.1677218
Received
31 July 2025
Revised
01 January 2026
Accepted
05 January 2026
Published
23 January 2026
Volume
13 - 2026
Edited by
Bülent Taner Karadağ, Marmara University, Türkiye
Reviewed by
Bo Gui, Nanjing Medical University, China
Sungho Ahn, Pusan National University Yangsan Hospital, Republic of Korea
Updates
Copyright
© 2026 Qin, Wang, Gu, Li, Zhang, Ge, He and Ma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng He, 393120823@qq.com; Xiaoyan Ma, 66846396@qq.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.