Abstract
Background:
Anticoagulation is essential during continuous renal replacement therapy (CRRT) for acute kidney injury to maintain circuit patency and balance bleeding risks.
Objective:
Systematically compare the anticoagulant efficacy and safety of nafamostat mesylate (NM) versus heparin in CRRT.
Methods:
We searched China National Knowledge Infrastructure, Wanfang Database, China Biology Medicine, PubMed, Embase, Cochrane Library, and Web of Science up to June 30, 2025 for randomized or non-randomized controlled trials. A meta-analysis was performed using RevMan version 5.4 software.
Results:
Seven retrospective cohort studies comprising were included. No significant differences between the two groups regarding filter lifespan [MD = -1.05; 95% CI (-5.92, 3.83); P = 0.67] and anticoagulation efficacy [OR = 2.64; 95% CI (0.41, 17.11); P = 0.31] were shown. No difference in the risk of bleeding events was shown [OR = 0.57; 95% CI (90.27, 1.21); P = 0.14]. The length of hospital stay in the NM group was significantly shortened [MD = –3.43; 95% CI (–5.53 to –1.33); P = 0.001]. NM showed significantly greater reduction in thrombin time (TT) compared to heparin [MD = –3.44; 95% CI (–5.33, –1.56); P = 0.0003), while no significant differences were observed in activated partial thromboplastin time (APTT) [MD = –5.35; 95% CI (–16.41, 5.72); P = 0.34) or international normalized ratio (INR) [MD = –0.46; 95% CI (–1.12, 0.20); P = 0.17].
Conclusion:
NM demonstrates similar filter lifespan and bleeding safety to heparin in CRRT. NM may shorten hospital stay and differentially affects coagulation indicators, supporting its use in individualized anticoagulation. But because of poor evidence, the conclusions must be interpreted with caution.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/, identifier CRD420251077749.
1 Background
In the intensive care unit, up to 50% of patients develop acute kidney injury, which has a poor clinical prognosis (1). However, early detection and treatment can help restore kidney function. Continuous renal replacement therapy (CRRT) has been widely used for critically ill patients with acute kidney injury (2). Through a slow and isotonic blood purification process—during which electrolyte imbalances are corrected and volume overload prevented—CRRT can effectively eliminate inflammatory mediators (3). In so doing, this therapy plays a unique role in maintaining homeostasis of the internal environment. Premature filter failure is precipitated when the contact coagulation cascade is activated via blood–material interactions in extracorporeal circuits, resulting in rates of therapy discontinuation that exceed 34% (4). Frequent clotting events shorten the duration of treatment, increase healthcare costs and staff workload, and lead to elevated blood loss and transfusion requirements in patients (5).
Unfractionated heparin (UFH) is the most widely used anticoagulant. By enhancing antithrombin III activity, its use potently inhibits coagulation factors IIa and Xa, enabling rapid and reversible anticoagulation to be achieved (6). Beyond inhibiting the coagulation cascade, unfractionated heparin exhibits anti-inflammatory, immunomodulatory, and endothelium-protective functions (7). However, therapeutic dosing of heparin elevates bleeding risk, with an incidence of hemorrhagic complications of up to 40% being reported in the literature (8). This concern is particularly pronounced in patients with a high risk of bleeding at baseline (9).
Nafamostat mesylate (NM) exhibits an ultrashort half-life of 8 min and has specific inhibitory properties against coagulation factors IIa, VIIa, and Xa. In Japan, NM is utilized in 85% of cases of anticoagulation during CRRT (10). Its anti-inflammatory effect, achieved through inhibiting protease-activated receptor-1, provides additional benefits for patients with sepsis (11). The conclusions yielded by current studies regarding the safety and efficacy of NM are inconsistent: Some studies have demonstrated that NM significantly prolongs filter lifespan and reduces clotting rates (12). However, studies suggest that its benefits may be partially offset by potential adverse effects such as hyperkalemia and allergic reactions (13, 14).
Treatment guidelines recommend both NM and heparin regimens for anticoagulation during CRRT in patients who are critically ill (9). Consequently, this study aimed, through meta-analysis, to systematically evaluate the anticoagulant efficacy, safety, and adverse complications of administering NM versus heparin to patients who underwent CRRT.
2 Methods
The review and analysis were conducted in accordance with the PRISMA Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (15). Our research plan was registered in PROSPERO (registration number CRD420251077749).
2.1 Inclusion and exclusion criteria
The inclusion criteria were formulated according to the patient population, intervention, comparison, and outcome (PICO) framework. The inclusion criteria were: (1) a study design compliant with those of randomized controlled trials, prospective cohort studies, or retrospective cohort studies; (2) interventions involving a direct comparison of NM versus unfractionated heparin for anticoagulation in CRRT; (3) study participants who were adult patients (aged ≥ 18 years) who met the Kidney Disease: Improving Global Outcomes (KDIGO)2012 criteria for initiating CRRT; and (4) outcome measures wherein at least one predefined outcome (including filter lifespan, bleeding events, coagulation parameters, anticoagulation efficacy, or length of hospital stay) was reported. Studies in which anticoagulation regimens that employed no anticoagulation, citrate anticoagulation, or argatroban anticoagulation, or those with non-extractable outcomes data or loss to follow-up of > 20% were excluded. Anticoagulation efficacy was defined as the percentage of patients exhibiting dialyzer coagulation graded as 0 or I. Coagulation severity was assessed according to the following criteria (16):
-
Grade 0: No coagulation or only a few clotted fibers.
-
Grade I: Less than 10% clotted fibers or the presence of small fiber bundles with clotting.
-
Grade II: Less than 50% clotted fibers or significant coagulation.
-
Grade III: More than 50% clotted fibers, a substantial rise in venous pressure, or the necessity for dialyzer replacement.
At the end of hemodialysis, the coagulation status within the dialyzer was examined. The anticoagulation efficacy rate was then calculated as the percentage of patients with coagulation graded as 0 or I, using the following formula: Anticoagulation efficacy rate = (Number of patients with Grade 0 or I coagulation/Total number of patients assessed) × 100%. Bleeding events were defined as instances of overt bleeding, a requirement for transfusion, or a reduction in hemoglobin exceeding 2 g/Dl (17).
2.2 Search strategy
A structured search strategy was employed to retrieve relevant literature from the following databases: China National Knowledge Infrastructure, Wanfang Database, China Biology Medicine, PubMed, Embase, Cochrane Library, and Web of Science. The timeframe of the search extended from the inception of each database through June 30, 2025. No language restrictions were applied to ensure broad coverage of both English and Chinese-language publications. Keywords included “nafamostat mesylate,” “heparin,” and “continuous renal replacement therapy.” Combining professional terminology with synonym expansion, the following search term combinations were constructed:
-
(“continuous renal replacement therapy”[tiab] OR “CRRT”[tiab] OR “continuous venovenous hemofiltration”[tiab] OR “CVVH”[tiab] OR “continuous hemodiafiltration”[tiab] OR “CHDF”[tiab]) OR (“renal replacement therapy”[Mesh] OR “hemofiltration”[Mesh])
-
(“nafamostat”[tiab] OR “nafamostat mesilate”[tiab])
-
(“heparin”[tiab] OR “unfractionated heparin”[tiab] OR “UFH”[tiab]) OR (“heparin”[Mesh])
-
Combinations 1 AND 2 AND 3
2.3 Literature screening and data extraction
The retrieved documents were imported into NoteExpress V4.0 (AegeanSoftware, Beijing, China). After removing duplicates, two reviewers trained in evidence-based methodology independently screened all the records by reviewing titles and abstracts as preliminary assessment for inclusion. After the initial screening, they read the full text of the articles that remained. The reviewers extracted data from the included studies, encompassing the first author’s name, year of publication, country, study design, sample size, patient characteristics, CRRT modality, anticoagulation regimen, and outcome measures.
2.4 Quality evaluation
Two reviewers independently assessed methodological quality using the Cochrane Risk of Bias tool (RoB 1.0) from the Cochrane Handbook for Systematic Reviews (Version 5.1.0) (18) as well as the Newcastle–Ottawa Scale (19). The Cochrane Handbook for Systematic Reviews focuses on seven critical domains: randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. A three-tier classification system of low risk of bias–high risk of bias–unclear for item-by-item assessment was adopted. Appraisal of the methodological quality of the included cohort studies was performed using the ?Newcastle–Ottawa Scale, a validated tool used to evaluate eight domains across three core dimensions. Quality was assessed across three domains: selection of subjects, comparability of cohorts, and outcome assessment. Studies were classified as high (score, 6–8 points), moderate (4–5 points), or low (≤ 3 points) quality.
2.5 Data extraction
The quality of the literature was independently assessed by two reviewers trained in systematic analysis. Standardized operating procedures were implemented in the evaluation process, utilizing a two-way independent review system with methods for cross-verification and adjudication of discrepancies to minimize subjective bias; thereby, the reliability and reproducibility of the evaluation outcomes were ensured. When two reviewers disagreed on the assessment of a specific item in a study, they first resolved discrepancies through reexamining the original literature for discussion. If consensus remained unattainable, a third senior reviewer with certification in conducting Cochrane systematic reviews was engaged for a three-way review, with the final consensus serving as the definitive assessment of risk of bias for the relevant study item. All evaluation results were documented in predesigned standardized forms and subsequently incorporated into evidence synthesis after the accuracy was confirmed through two-way independent verification.
2.6 Statistical methods
The meta-analysis was performed using Review Manager 5.4 software and STATA statistical software (version 18.0) on data extracted from the literature, with the odds ratio (OR) and 95% confidence interval (CI) as effect measures. When heterogeneity testing indicated an I2 value of ≤ 50% and P-value of ≥ 0.1, a fixed-effect model was employed to calculate the pooled OR and 95% CI. An I2 value of > 50% and P-value of < 0.1 indicated significant heterogeneity. In such instances, sources were investigated via sensitivity or subgroup analyses. If homogeneity remained unattainable, the random effects model was applied for statistical synthesis. P < 0.05 indicated that the difference was statistically significant. We assessed the potential publication bias of the meta-analysis by using Egger’s tests.
3 Results
3.1 Literature search
The initial database retrieval identified 291 relevant studies. After removing duplicates, 185 studies were retained for initial screening. Through title and abstract screening, 58 studies were selected for full-text retrieval. After comprehensive assessment of the full text, seven studies were ultimately included in the meta-analysis (16, 17, 20–24). The literature screening process is detailed in Supplementary material 1.
3.2 Basic characteristics and quality of the included literature
The characteristics of the included literature are shown in Table 1. A total of seven studies were included in this research for a meta-analysis, all of which were cohort studies (16, 17, 20–24). The studies originated from East Asia: four from China (16, 21, 23, 24), two from Japan (20, 22), and one from South Korea (17). The patient population primarily consisted of adult patients who were critically ill, in intensive care units, and who required CRRT. The dosing regimens in the NM group comprised both fixed (10–50 mg/h) and weight-adjusted (0.1–0.5 mg/kg/h) strategies. In the heparin group, heparin was mainly administered, with the dosage ranging from 1 to 20 U/kg/h. All the included studies reported filter clotting and or longevity-related outcomes. Four studies (17, 22–24) reported hemorrhagic complications that were attributable to the therapy. Control for confounding and completeness of follow-up represent pervasive methodological limitations in cohort studies. The results of the quality appraisal of all the studies included are presented in Table 2.
TABLE 1
| Research | Research type | Nationality | Sample size (NM vs. heparin) | Patient characteristic | CRRT mode | Anticoagulation method | Primary outcome indicator |
|---|---|---|---|---|---|---|---|
| Kameda et al. | Cohort study | Japan | 129 vs. 157 | Adult patients requiring CRRT in ICU | CRRT | NM 10 mg/h vs. heparin 400 U/h | Filter life, hospital stay, mortality rate, mechanical ventilation time, inflammatory biomarkers |
| Liu and Li | Cohort study | China | 33 vs. 32 | End-stage renal failure patients on hemodialysis | CVVH | NM 20-50 mg/h vs. heparin5-10 mg/h | Clinical efficacy, anticoagulation effective rate (filter coagulation classification), coagulation indicators |
| Hwang et al. | Cohort study | Korea | 25 vs. 56 | Adult patients requiring CRRT in ICU | CVVH | NM 10-30 mg/h vs. heparin 1-20 U/kg/h | Filter life, coagulation indicators, bleeding events, survival rate |
| Makino et al. | Cohort study | Japan | 76 vs. 25 | Adult patients requiring CRRT in ICU | CRRT | NM 20 mg/h vs. heparin 400 IU/h | Filter lifespan, bleeding events |
| Miao and Chen | Cohort study | China | 50 vs. 48 | Patients with AKI due to sepsis | CVVH | NM 0.4 mg/kg/h vs. heparin 5-15 U/kg/h | Clinical efficacy, length of stay in the ICU, APACHE II score, immune indicators, renal function indicators, bleeding events, hyperkalemia |
| Zhan et al. | Cohort study | China | 40 vs. 40 | Patients with acute kidney injury (AKI) | CRRT | NM 0.4 mg/kg/h vs. heparin 3-15 U/kg/h | Filter lifespan, coagulation indicators, renal function indicators, bleeding events, hyperkalemia, hospital stay |
| Li et al. | Cohort study | China | 44 vs. 42 | Patients with CRRT (mainly with low platelet count) | CRRT | NM 0.1-0.5 mg/(kg/h) vs. heparin 5-15 U/(kg/h) | Anticoagulation effective rate (filter coagulation classification), coagulation indicators, and blood biochemical indicators |
General characteristics of included literature.
NM, Nafamostat mesylate; CRRT, Continuous renal replacement therapy; AKI, acute kidney injury; ICU, intensive care unit; RCT; randomized controlled trials; CVVH, continuous veno-venous hemofiltration.
TABLE 2
| Study ID | Selection | Comparability | Outcome | Total (9*) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort (*) | Selection of non-exposed cohort (*) | Ascertainment of exposure (*) | Demonstration that outcome of interest was not present at start of study (*) | Comparability of cohorts (**) | Assessment of outcome (*) | Length of follow up(*) | Adequacy of follow up (*) | ||
| Miao and Chen | * | * | * | ** | * | * | 7 | ||
| Hwang et al. | * | * | * | * | * | * | 6 | ||
| Kameda et al. | * | * | * | ** | * | * | 7 | ||
| Liu and Li | * | * | * | * | * | 5 | |||
| Makino et al. | * | * | * | * | ** | * | * | 8 | |
| Li et al. | * | * | * | * | * | * | 6 | ||
| Zhan et al. | * | * | * | * | * | * | 6 | ||
Newcastle-Ottawa quality assessment scale results of cohort studies.
*, ** means getting one point.
3.3 Outcomes analysis
3.3.1 Filter lifespan
Four studies (17, 20, 22, 24) reported filter lifespan as an outcome measure. Significant heterogeneity was observed among them (P < 0.00001; I2 = 91%), and a random effects model was applied for analysis. The results showed that no statistically significant difference in the lifespan of the anticoagulant filter between the NM and heparin groups was present [mean difference (MD) = –1.05; 95% CI (–5.92, 3.83); P = 0.67) (Figure 1).
FIGURE 1

Forest plot comparing the filter lifespan between the nafamostat mesylate and heparin groups. CI, confidence interval; IV, intravenous; SD, standard deviation.
3.3.2 Effectiveness in achieving anticoagulation
Two studies (16, 21) reported effectiveness in achieving anticoagulation as an outcome measure. Moderate heterogeneity was observed among them (P = 0.08; I2 = 66%), and a random effects model was used for analysis: The results showed that no statistically significant difference in the effective rate of anticoagulation between the NM and the heparin groups was present (OR = 2.64; 95% CI [0.41, 17.11]; P = 0.31) (Figure 2).
FIGURE 2

Forest plot comparing the effective rate of anticoagulation between the nafamostat mesylate and heparin groups. CI, confidence interval; M-H, Mantel-Haenszel.
3.3.3 Bleeding events
Four studies (17, 22–24) reported bleeding events as an outcome measure. No significant heterogeneity was observed among the included studies (P = 0.17; I2 = 40%), and a fixed effects model was applied for analysis. The results showed that no statistically significant difference in bleeding events between the NM and heparin groups was present (OR = 0.57; 95% CI [0.27, 1.21]; P = 0.14) (Figure 3).
FIGURE 3

Forest plot comparing bleeding events between the nafamostat mesylate and heparin groups.
3.3.4 Length of hospital stay
Two studies (23, 24) reported length of hospital stay as an outcome measure. Significant heterogeneity was observed between the two (P = 0.01; I2 = 85%), and a random effects model was used for analysis. The results showed a significant difference in the length of hospital stay between the NM and heparin groups (MD = -3.43; 95% CI [-5.53, -1.33]; P = 0.001) (Figure 4).
FIGURE 4

Forest plot comparing length of hospital stay between the nafamostat mesylate and heparin groups. CI, confidence interval; IV, intravenous; SD, standard deviation.
3.3.5 Coagulation indicators
Four studies (16, 17, 21, 24) reported the APTT as an outcome measure. Significant heterogeneity was observed among the included studies (P < 0.00001; I2 = 99%), and a random effects model was used for analysis. The results showed no statistically significant difference in the coagulation index, APTT, between the NM and heparin groups (MD = -5.35; 95% CI [-16.41, 5.72]; P = 0.34) (Figure 5A).
FIGURE 5
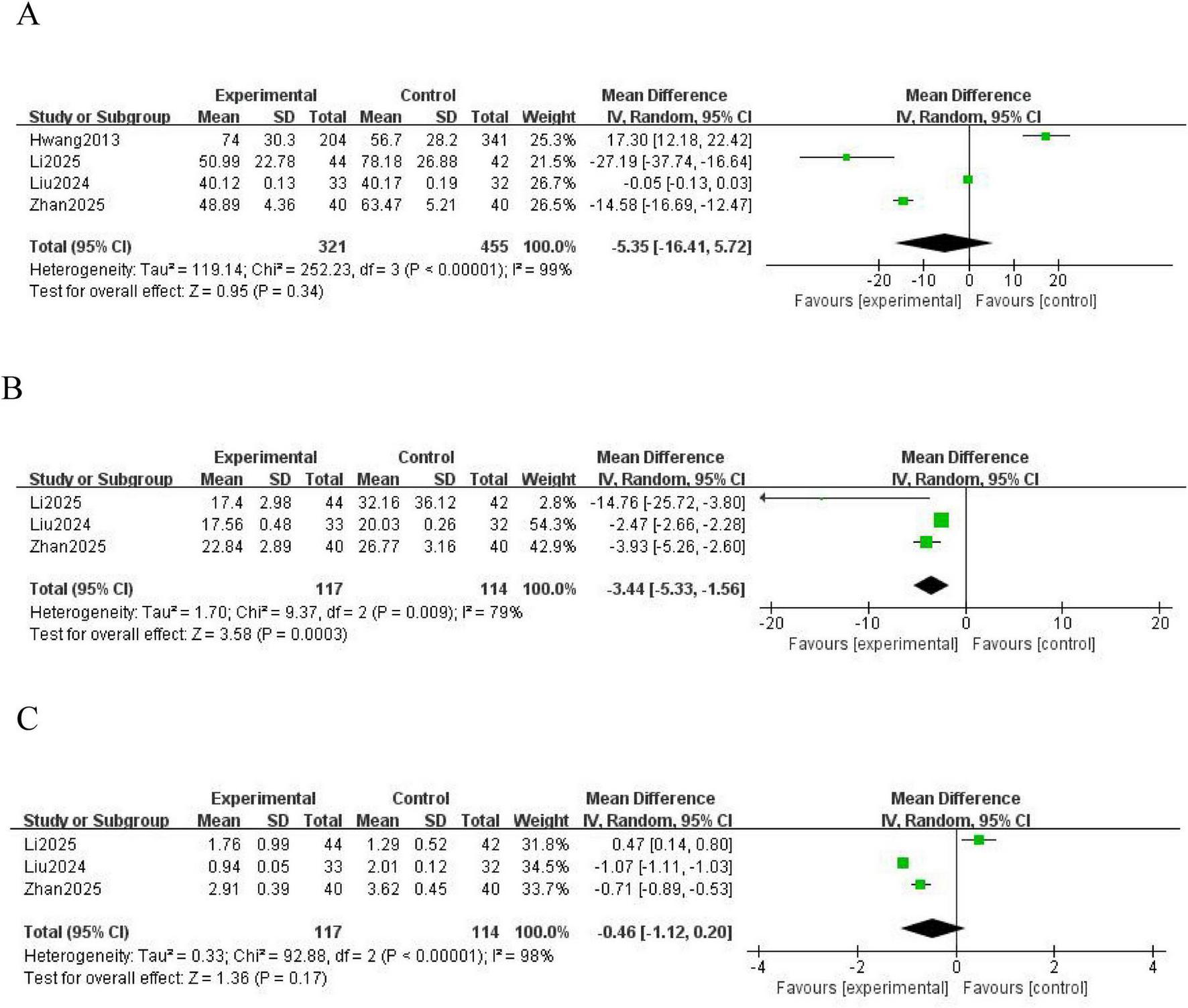
Forest plot comparing coagulation indicators between the nafamostat mesylate and heparin groups. (A) Forest plot of APTT. (B) Forest plot of TT. (C) Forest plot of INR. APTT, activated partial thromboplastin time; TT, thrombin time; INR, international normalized ratio; CI, confidence interval; IV, intravenous; SD, standard deviation.
Three studies (16, 21, 24) reported the TT as an outcome measure. Significant heterogeneity was observed among the included studies (P = 0.009; I2 = 79%), and a random effects model was used for analysis. The results showed a statistically significant difference in the coagulation index, TT, between the NM and heparin groups (MD = -3.44; 95% CI [-5.33, -1.56]; P = 0.0003) (Figure 5B).
Three studies (16, 21, 24) reported the INR as an outcome measure. Significant heterogeneity was observed among these studies (P < 0.00001; I2 = 98%), and a random effects model was used for analysis. The results showed no statistically significant difference in the coagulation index, INR, between the NM and heparin groups (MD = -0.46; 95% CI [-1.12, 0.20]; P = 0.17) (Figure 5C).
3.3.6 Subgroup analyses
To explore the substantial heterogeneity observed in several outcomes, pre-specified subgroup analyses were conducted based on anticoagulant dosing strategy. The subgroup analysis of filter lifespan revealed a statistically significant difference based on dosing strategy (P = 0.002). In studies employing a fixed-dose regimen, filter lifespan was significantly shorter in the NM group compared to the heparin group (MD = -7.62; 95% CI [-14.78, -0.47]; P = 0.04; Supplementary material 2). In contrast, no significant difference was found between groups in studies using a weight-adjusted dose (MD = 3.65; 95% CI [-2.14, 9.44]; P = 0.22; Supplementary material 2). The effect of NM on APTT was significantly modified by the dosing strategy (P = 0.008). The overall pooled result (MD = -5.35; 95% CI [-16.41, 5.72]; P = 0.34; Supplementary material 3a) masked divergent effects between the weight-adjusted and fixed-dose subgroups. An additional analysis by CRRT modality also indicated significant effect modification (P = 0.008; Supplementary material 3b). For INR (P = 0.11; Supplementary material 3c) and TT (P = 0.30; Supplementary material 3d), the subgroup analyses did not show statistically significant differences between weight-adjusted and fixed-dose subgroups, indicating that dosing strategy was not a major source of heterogeneity for these parameters.
3.3.7 Sensitivity analysis and publication bias
Sensitivity analysis was performed using the one-study-removal approach to assess the stability of the effect size of all the studies combined. The results indicated that no single trial influenced the outcome measures, confirming the robustness of the findings. Egger’s regression analysis showed that no significant publication bias was found in the main outcome of filter lifespan (P = 0.780).
4 Discussion
In this meta-analysis, we pooled data from seven studies, providing the first systematic comparison of the safety and efficacy of using NM and heparin as the anticoagulant during CRRT. In the comparison of efficacy, no statistically significant difference was found in filter lifespan between the NM and heparin groups. Similarly, regarding safety, no statistically significant difference in the incidence of bleeding events between the two groups was shown (P > 0.05). For clinical outcomes, a significantly shorter length of hospital stay was shown in the NM group (P < 0.05). No statistically significant differences were shown for the coagulation monitoring indicators APTT and INR (P > 0.05), whereas a statistically significant difference was shown for the TT (P < 0.05).
The results of the present study showed no significant difference in filter lifespan between the NM or heparin groups (MD = -1.05; 95% CI [-5.92, 3.83]; P = 0.67). This contrasts with the conclusions reached in some studies (12) in which the filter lifespan was significantly prolonged when NM was used. Through in-depth analysis of sources of heterogeneity in the included studies, we identified diversity in dosing regimens as a key contributing factor. The dosage of NM covered a fixed dose of 10–50 mg/h and a weight-adjusted dose of 0.1–0.5 mg/kg/h, whereas the dosage of heparin ranged from 1 to 20 U/kg/h. NM selectively inhibits coagulation factors XIIa and XIa, and kallikreins, thereby blocking the intrinsic coagulation pathway. Heparin relies on Antithrombin III to inhibit coagulation factors XIIa and XIa (25, 26). Although their anticoagulation targets differ, both NM and heparin effectively delay the deposition of fibrin within the filter circuit. The cohort study conducted by Kameda et al. (20) involving 286 patients in the intensive care unit demonstrated a median filter lifespan of 38.2 h in the NM group versus 36.5 h in the heparin group (P = 0.78).
No statistically significant difference in the incidence of bleeding events was shown between the two groups (OR = 0.57; 95% CI [0.27, 1.21]; P = 0.14), providing critical evidence for selecting an anticoagulant for patients at high risk of bleeding. However, due to the limited number of studies reporting this result, it must be regarded as a preliminary conclusion. Also, the potential influence of publication bias on this non-differential outcome should be taken into consideration. The risk of bleeding associated with heparin correlates with its comprehensive suppression of the coagulation cascade, whereas the targeted inhibition exhibited by NM minimizes interference with physiological hemostasis (27). Additionally, the risk of heparin Induced thrombocytopenia (HIT) must be considered (28). In the cohort studied by Makino (22), two cases of suspected HIT were noted in the heparin group, which may partially explain the discrepancy in length of hospital stay. However, in this study, the administration of NM did not significantly reduce the number of bleeding events. This finding may be related to the following factors: Firstly, the proportion of patients at high risk of bleeding included in the studies was insufficient; Secondly, the dose adjustment of NM was not fully matched to individual patient variations. Notably, the utilization rate of NM for anticoagulation in CRRT in the Japanese guidelines reaches 85% (29); because NM has an ultra-short half-life, it is particularly suitable for use in clinical scenarios in which frequent interruption of anticoagulation therapy is required. Clinically, when administering NM, particular attention must be paid to the continuity of the administration route to avoid fluctuations in the anticoagulant effect due to interruption of infusion. Similarly, during administration of heparin therapy, vigilance against concurrent risks of anticoagulation failure and bleeding caused by ATIII depletion is required (30).
The length of hospital stay in the NM group was significantly shorter than that in the heparin group (MD = -3.43; 95% CI [-5.53, -1.33]; P = 0.001). However, this favorable result is based on only two studies, and its robustness should be interpreted with caution until confirmed by larger, prospective investigations. The mechanism underlying this finding may involve the multifaceted pharmacological effects of NM. Besides its anticoagulant action, NM inhibits protease-activated receptor-1, thereby reducing the release of inflammatory mediators such as interleukin 6 and tumor necrosis factor-alpha (31). The study conducted by Miao and Chen (23) confirmed that patients with sepsis-associated acute kidney injury who were treated with NM demonstrated a greater reduction in Sequential Organ Failure Assessment scores compared to those treated with heparin. This reduction accelerated the recovery of organ function and consequently shortened the length of hospital stay. However, vigilance is required against adverse reactions, such as hyperkalemia, that the administration of NM may induce (23). Zhan et al. (24) reported an incidence of hyperkalemia in the NM group of 12.5%. Clinical monitoring should include an increased frequency of electrolyte testing, especially in patients with renal insufficiency, while emphasizing control of the infusion rate and management of the electrolyte balance.
The APTT primarily reflects the functionality of the intrinsic coagulation pathway. Heparin significantly prolongs the APTT by potently inhibiting coagulation factors Xa and IIa through enhanced ATIII activity (32). NM inhibits intrinsic coagulation factors such as XIIa and Xa. However, its ultra-short half-life and regionally specific anticoagulation properties result in high local concentrations within the extracorporeal circuit, while rapid systemic clearance minimizes its impact on the systemic APTT (33). NM is rapidly metabolized by hepatic carboxylesterases, with approximately 80% of it being cleared by the liver, while dialysis devices can partially remove it (34). Therefore, the risk of systemic accumulation is low. Heparin is primarily cleared by the kidneys. In cases of renal insufficiency, the half-life of heparin is prolonged, resulting in greater fluctuations in the APTT. TT directly reflects the activity of coagulation factor IIa. NM potently inhibits thrombin, thereby significantly shortening the TT. Although heparin inhibits factor IIa, its action is dependent on ATIII and subject to interference from heparinase, PF4, thus limiting its impact on the TT (35). This property makes heparin particularly suitable for use in patients at high risk of bleeding. Li et al. (16) demonstrated that, in patients with platelet counts of < 50 × 109/L, the rate of bleeding reached 14.3% in the heparin group, whereas it only reached 6.8% in the NM group. This indicates that, for clinical monitoring, priority should be given to the TT values when administering NM, but that the APTT should serve as the primary indicator when administering heparin therapy. Clinically, the frequency of monitoring coagulation and interpretation of these parameters must be adjusted based on the type of anticoagulant administered. The INR primarily reflects the extrinsic coagulation pathway. No significant difference in the INR was shown between the two groups, because neither NM nor heparin primarily targets this pathway. This indicates that the effect of NM on vitamin K is similar to that of heparin. However, the rapid inhibitory effect of NM on thrombin may interfere with global coagulation assessments such as thromboelastogram. Clinical staff must integrate the mechanism of action of the anticoagulant to comprehensively interpret coagulation reports.
In the subgroup analysis, we found that the anticoagulant dosing strategy served as a major effect modifier, particularly for filter lifespan and APTT. The inferior performance of NM under fixed-dose regimens suggests that a fixed, non-individualized dosing strategy may lead to under-anticoagulation, compromising filter longevity. This underscores the paramount importance of utilizing weight-adjusted dosing to optimize the efficacy of NM in CRRT, ensuring adequate anticoagulation while potentially minimizing adverse effects. Furthermore, the influence of CRRT modality on APTT highlights the complexity of comparing anticoagulation across different technical settings. These findings shift the focus from a simple drug-versus-drug comparison to a more nuanced understanding that includes protocol-specific factors, advocating for personalized anticoagulation management in critically ill patients undergoing CRRT.
4.1 Limitations
This study had the following limitations: First, significant heterogeneity was observed for several outcomes, which although explored through subgroup analyses, remains a consideration. Second, the generalizability of the findings may be limited as all included studies were conducted in East Asia; the applicability of the results to other populations with different genetic backgrounds, clinical practices, or risk profiles requires further investigation. Third, we explicitly acknowledge that all included studies were non-randomized in design.
While the results are consistent and provide valuable insights from real-world clinical practice, the conclusions must be interpreted with caution. Finally, the number of studies reporting key outcomes like hospital stay and bleeding events was relatively small, which may limit the robustness of those specific conclusions.
5 Conclusion
The findings of this meta-analysis demonstrated that NM and heparin provide comparable filter longevity and hemorrhagic safety profiles when used as anticoagulants during CRRT. NM exhibited the distinct advantages of significantly shortening length of hospital stay and optimizing regulation of coagulation parameters. In clinical practice, formulating tailored monitoring protocols necessitates the integration of individual patient characteristics with the pharmacological properties of the anticoagulant. A pressing need for more high-quality RCT research exists to establish a more robust evidence base for developing anticoagulation strategies in CRRT. Future large-scale studies, specifically powered to detect differences in patient-centered outcomes like hospital stay and bleeding events, and crucially, conducted in diverse geographic settings beyond East Asia, are essential to confirm the generalizability of our results and to establish NM’s role in a global context.
Statements
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YW: Data curation, Formal analysis, Funding acquisition, Writing – original draft. QH: Methodology, Resources, Software, Writing – original draft. DW: Data curation, Methodology, Writing – review & editing. RX: Data curation, Formal analysis, Writing – original draft. XY: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. LZ: Formal analysis, Funding acquisition, Resources, Software, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This project was supported by the Patient Safety Research Center of Zigong Academy of Medical Sciences (Grant no. HZAQ-2024-02) and Medical Quality (Evidence-Based) Management research project of Hospital Management Research Institute, National Health Commission (Grant no. ylzlxz24g093).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2026.1713412/full#supplementary-material
References
1.
Susantitaphong P Cruz D Cerda J Abulfaraj M Alqahtani F Koulouridis I et al World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. (2013) 8:1482–93. 10.2215/CJN.00710113
2.
Duan R Li Y Zhang R Hu X Wang Y Zeng J et al Reversing acute kidney injury through coordinated interplay of anti-inflammation and iron supplementation. Adv Mater. (2023) 35:e2301283. 10.1002/adma.202301283
3.
Horkan C Purtle S Mendu M Moromizato T Gibbons F Christopher K . The association of acute kidney injury in the critically ill and postdischarge outcomes: a cohort study*.Crit Care Med. (2015) 43:354–64. 10.1097/CCM.0000000000000706
4.
Oudemans-van Straaten H Wester J de Pont A Schetz M . Anticoagulation strategies in continuous renal replacement therapy: can the choice be evidence based?Intensive Care Med. (2006) 32:188–202. 10.1007/s00134-005-0044-y
5.
Tolwani A Wille K . Regional citrate anticoagulation for continuous renal replacement therapy: the better alternative?Am J Kidney Dis. (2012) 59:745–7. 10.1053/j.ajkd.2012.03.003
6.
Matsuura R Komaru Y Miyamoto Y Yoshida T Yoshimoto K Yamashita T et al HMGB1 Is a prognostic factor for mortality in acute kidney injury requiring renal replacement therapy. Blood Purif. (2023) 52:660–7. 10.1159/000530774
7.
Bouchard J Weidemann C Mehta RL . Renal replacement therapy in acute kidney injury: intermittent versus continuous? How much is enough?. Adv Chronic Kidney Dis. (2008) 15:235–247. 10.1053/j.ackd.2008.04.004
8.
Uchino S Bellomo R Morimatsu H Morgera S Schetz M Tan I et al Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. (2007) 33:1563–70. 10.1007/s00134-007-0754-4
9.
Baek N Jang H Huh W Kim Y Kim D Oh H et al The role of nafamostat mesylate in continuous renal replacement therapy among patients at high risk of bleeding. Ren Fail. (2012) 34:279–85. 10.3109/0886022X.2011.647293
10.
Lang Y Zheng Y Qi B Zheng W Wei J Zhao C et al Anticoagulation with nafamostat mesilate during extracorporeal life support. Int J Cardiol. (2022) 366:71–9. 10.1016/j.ijcard.2022.07.022
11.
Shinoda T . Anticoagulation in acute blood purification for acute renal failure in critical care.Contrib Nephrol. (2010) 166:119–25. 10.1159/000314861
12.
Lin Y Shao Y Liu Y Yang R Liao S Yang S et al Efficacy and safety of nafamostat mesilate anticoagulation in blood purification treatment of critically ill patients: a systematic review and meta-analysis. Ren Fail. (2022) 44:1263–79. 10.1080/0886022X.2022.2105233
13.
Kim J Park J Jang S Kim J Song Y Lee H et al Fatal anaphylaxis due to nafamostat mesylate during hemodialysis. Allergy Asthma Immunol Res. (2021) 13:517–9. 10.4168/aair.2021.13.3.517
14.
Kim H Lee K Oh J Jung C Choi D Kim Y et al Cardiac arrest caused by nafamostat mesilate. Kidney Res Clin Pract. (2016) 35:187–9. 10.1016/j.krcp.2015.10.003
15.
Page M McKenzie J Bossuyt P Boutron I Hoffmann T Mulrow C et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71
16.
Li T Wang X Yang J . Analysis of the efficacy and safety of nemonistat mesylate in continuous blood purification anticoagulation for critically ill patients.Chinese J Clin Doctors. (2025) 53:181–5. 10.3969/j.issn.2095-8552.2025.02.012
17.
Hwang S Hyun Y Moon S Lee S Yoon S . Nafamostat mesilate for anticoagulation in continuous renal replacement therapy.Int J Artif Organs. (2013) 36:208–16. 10.5301/IJAO.5000191
18.
Higgins J Altman D Gøtzsche P Jüni P Moher D Oxman A et al The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. 10.1136/bmj.d5928
19.
Zhang Y Huang L Wang D Ren P Hong Q Kang D . The ROBINS-I and the NOS had similar reliability but differed in applicability: a random sampling observational studies of systematic reviews/meta-analysis.J Evid Based Med. (2021) 14:112–22. 10.1111/jebm.12427
20.
Kameda S Fujii T Ikeda J Kageyama A Takagi T Miyayama N et al Unfractionated heparin versus nafamostat mesylate for anticoagulation during continuous kidney replacement therapy: an observational study. BMC Nephrol. (2023) 24:12. 10.1186/s12882-023-03060-1
21.
Liu K Li Z . Efficacy and safety of Nafamostat mesylate in patients with end-stage renal failure.World J Clin Cases. (2024) 12:68–75. 10.12998/wjcc.v12.i1.68
22.
Makino S Egi M Kita H Miyatake Y Kubota K Mizobuchi S . Comparison of nafamostat mesilate and unfractionated heparin as anticoagulants during continuous renal replacement therapy.Int J Artif Organs. (2016) 39:16–21. 10.5301/ijao.5000465
23.
Miao M Chen Z . Impact of nafamostat mesylate combined with continuous renal replacement therapy on clinical outcomes, immune function, and oxidative stress markers in patients with sepsis-associated acute kidney injury.Br J Hosp Med. (2025) 86:1–13. 10.12968/hmed.2024.0615
24.
Zhan X Qu X Wu D Wang G Bai S Ji T . Effects of nafamostat mesilate and systemic unfractionated heparin anticoagulation on coagulation, renal function, and 28-day survival status in critically ill aki crrt patients.Iran J Kidney Dis. (2025) 19:41–9. 10.52547/5j9v7385
25.
Wu M Sapin-Minet A Gaucher C . Heparin, an active excipient to carry biosignal molecules: Applications in tissue engineering - a review.Int J Biol Macromol. (2025) 312:143959. 10.1016/j.ijbiomac.2025.143959
26.
Nadarajah L Fan S Forbes S Ashman N . Major bleeding in hemodialysis patients using unfractionated or low molecular weight heparin: a single-center study.Clin Nephrol. (2015) 84:274–9. 10.5414/CN108624
27.
Hogwood J Mulloy B Lever R Gray E Page C . Pharmacology of heparin and related drugs: an update.Pharmacol Rev. (2023) 75:328–79. 10.1124/pharmrev.122.000684
28.
Koster A Nagler M Erdoes G Levy J . Heparin-induced thrombocytopenia: perioperative diagnosis and management.Anesthesiology. (2022) 136:336–44. 10.1097/ALN.0000000000004090
29.
Hirayama T Nosaka N Okawa Y Ushio S Kitamura Y Sendo T et al AN69ST membranes adsorb nafamostat mesylate and affect the management of anticoagulant therapy: a retrospective study. J Intensive Care. (2017) 5:46. 10.1186/s40560-017-0244-x
30.
Lee YK Lee HW Choi KH Kim BS . The ability of nafamostat mesilate to prolong filter patency during continuous renal replacement therapy in patients at high risk of bleeding: a randomized controlled study.PLoS One. (2014) 9:e108737. 10.1371/journal.pone.0108737
31.
He Q Wei Y Qian Y Zhong M . Pathophysiological dynamics in the contact, coagulation, and complement systems during sepsis: potential targets for nafamostat mesilate.J Intensive Med. (2024) 4:453–67. 10.1016/j.jointm.2024.02.003
32.
Beurskens D Huckriede J Schrijver R Hemker H Reutelingsperger C Nicolaes G . The anticoagulant and nonanticoagulant properties of heparin.Thromb Haemost. (2020) 120:1371–83. 10.1055/s-0040-1715460
33.
Han S Kim H Kim K Whang S Hong K Lee W et al Use of nafamostat mesilate as an anticoagulant during extracorporeal membrane oxygenation. J Korean Med Sci. (2011) 26:945–50. 10.3346/jkms.2011.26.7.945
34.
Park J Her C Min H Kim D Park S Jang H . Nafamostat mesilate as a regional anticoagulant in patients with bleeding complications during extracorporeal membrane oxygenation.Int J Artif Organs. (2015) 38:595–9. 10.5301/ijao.5000451
35.
Chung S Lee M Bae S Park J Jeon O Lee H et al Potentiation of anti-angiogenic activity of heparin by blocking the ATIII-interacting pentasaccharide unit and increasing net anionic charge. Biomaterials. (2012) 33:9070–9. 10.1016/j.biomaterials.2012.09.002
Summary
Keywords
anticoagulation, continuous renal replacement therapy, heparin, meta-analysis, nafamostat mesylate
Citation
Wang Y, He Q, Wen D, Xu R, Yu X and Zhao L (2026) Efficacy and safety of nafamostat mesylate versus heparin anticoagulation in adult kidney disease patients using continuous renal replacement therapy: a systematic review and meta-analysis. Front. Med. 13:1713412. doi: 10.3389/fmed.2026.1713412
Received
26 September 2025
Revised
21 January 2026
Accepted
28 January 2026
Published
17 February 2026
Volume
13 - 2026
Edited by
Rongli Yang, Central Hospital of Dalian University of Technology, China
Reviewed by
Shaobin He, The Second Affiliated Hospital of Fujian Medical University, China
Jian-Ming Zheng, Fudan University, China
Updates
Copyright
© 2026 Wang, He, Wen, Xu, Yu and Zhao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingning Zhao, smallwen123@126.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.