Abstract
Objectives:
To compare knee range of motion and muscle strength between individuals with symptomatic knee osteoarthritis and healthy controls, and to assess how Kellgren–Lawrence grade and measurement protocols affect these outcomes.
Methods:
A systematic search of PubMed, Scopus, and Web of Science identified studies comparing knee flexion/extension range of motion or flexor/extensor strength between patients with knee osteoarthritis and controls. Risk of bias was assessed with Joanna Briggs Institute tools. Pooled mean and standardized mean differences with 95% confidence intervals were calculated using random-effects meta-analyses.
Results:
Thirty studies were included. Compared with healthy controls, individuals with knee osteoarthritis showed significantly reduced knee flexion [MD = 16.30°, 95%CI (11.40, 21.21)] and extension [MD = 4.25°, 95%CI (2.30, 6.19)], with greater flexion loss in advanced KL grades. Knee osteoarthritis participants also demonstrated significantly lower strength across all contraction types: isometric [extensors: SMD = 0.86, 95%CI (0.57, 1.14); flexors: SMD = 0.52, 95%CI (0.30, 0.74)], concentric [extensors: SMD = 1.07, 95%CI (0.65, 1.50); flexors: SMD = 0.77, 95%CI (0.43, 1.12)], and eccentric extensor strength. Strength deficits were consistent across Kellgren–Lawrence grades, knee joint angles, and angular velocities during testing.
Conclusions:
Individuals with symptomatic knee osteoarthritis present with marked reductions in knee range of motion and strength. While range of motion impairments worsen with disease severity, strength deficits are stable across Kellgren–Lawrence grades and measurement protocols. Given the very low to low certainty of evidence, results should be interpreted with caution.
1 Introduction
Knee osteoarthritis (KOA) is one of the most prevalent forms of osteoarthritis (OA) (1), affecting 16% of individuals aged ≥15 years and 23% of those over 40 years worldwide (2). Clinical guidelines state that a confident diagnosis of KOA can be made based solely on symptoms and signs (3), although radiographic imaging remains a common tool for confirming the presence of KOA and grade severity using the Kellgren–Lawrence (KL) classification (4). KOA represents a considerable physical and psychological burden through pain, stiffness, mobility restrictions, disability, and psychological distress (5–8). Mobility restrictions may be linked to limited knee range of motion (RoM) (9), given that most daily activities require full knee extension and ~110° of flexion (10). In addition, reduced function in people with KOA can result from strength deficits, particularly weakness of the knee extensors and flexors (11–13).
Comparisons between individuals with KOA and healthy controls may help identify functional limitations that contribute to or result from the disease, while also providing important information for diagnostic purposes. To date, no systematic review has comprehensively compared knee RoM between these groups. Existing reviews on muscle impairment are outdated, lack meta-analyses or focus only on hip joint (14, 15), despite newer studies (16–18). Therefore, the aim of our systematic review is to evaluate differences in knee joint strength and RoM between individuals with symptomatic KOA and healthy controls. Furthermore, we aim to compare different population subgroups and determine whether strength and mobility deficits are conditioned by specific factors (e.g., KL grade or by variations in measurement protocols). We hypothesize that individuals with KOA will demonstrate poorer outcomes across all strength and RoM measures compared to healthy individuals. Based on Liu et al. (19), who reported a negative correlation between KOA severity and balance, we hypothesize that RoM and strength deficits will be greater in higher than in lower KL grades. Because tibiofemoral compressive forces peak at ~90° flexion (20), we hypothesize that isometric deficits will be greater at this angle than at others. We also expect smaller isokinetic differences at higher velocities, as KOA patients spend less time in painful positions during faster movements.
2 Materials and methods
2.1 Search strategy and eligibility criteria
The search was conducted in February 2025 across PubMed, Scopus, and Web of Science using Boolean operators and combinations of terms for knee osteoarthritis (e.g., “knee osteoarthritis,” “knee OA,” “gonarthrosis”) and for healthy status (e.g., “healthy,” “normal,” “asymptomatic,” “without KOA”). Outcome-related keywords (e.g., RoM or muscle strength) were excluded to avoid missing studies that reported these measures only as baseline characteristics. Search strategies for the other databases were adapted accordingly, with no filters applied. No additional restrictions were applied.
The inclusion criteria were defined using the PICOS tool (21):
-
P (population): Individuals with symptomatic KOA, with a clearly defined radiographic severity according to the KL grading scale.
-
I (Intervention): N/A.
-
C (Comparison): Healthy individuals without KOA or any other knee-related conditions.
-
O (Outcome): Studies were eligible if they reported quantitative measures of (a) knee joint range of motion, specifically flexion and/or extension, assessed in degrees using objective instruments (e.g., goniometer); and/or (b) muscle strength of the knee extensors and/or flexors, measured under isometric or isokinetic conditions, with contraction type (concentric or eccentric) clearly defined and assessed using validated devices (e.g., isokinetic dynamometer, hand-held dynamometer, or comparable strength testing apparatus).
-
S (Study design): Observational studies (cross-sectional, case-control, cohort with healthy controls) and baseline data from clinical trials that included a healthy control group. Only articles published in English were included. Abstracts, conference proceedings, and unpublished studies were not included in this review.
Titles and abstracts were screened for relevance, followed by full-text assessment against predefined PICOS criteria. The literature search was conducted by the first author (M. O. Z.), a trained physiotherapist and PhD researcher experienced in systematic reviews and meta-analyses, under the supervision of the senior author (N.Š.), an experienced researcher in musculoskeletal rehabilitation and systematic review methodology. Reference lists of relevant reviews and included studies were also searched to identify additional articles. Eligible records were imported into Microsoft® Excel® LTSC MSO software for further processing. The included studies were directly relevant to the research question, as they compared individuals with symptomatic knee osteoarthritis to healthy controls and reported quantitative measures of knee range of motion and/or muscle strength. These outcomes were essential for assessing functional impairment and differences in musculoskeletal performance associated with knee osteoarthritis, thereby allowing valid evaluation of the study hypothesis.
2.2 Data extraction
Two independent reviewers extracted and coded data. Discrepancies were resolved by consensus to ensure reliability. The extracted data included: (a) Participant characteristics (sex, age, height, weight, BMI); (b) KOA characteristics [symptom presence; compartment involvement (medial or lateral); laterality (unilateral or bilateral); joint location (tibiofemoral or patellofemoral); surgical status (scheduled for knee surgery, e.g., TKA or osteotomy); and KL grade]; (c) Control group characteristics [matching criteria and OA exclusion method (radiographic confirmation or absence of signs and symptoms)]; (d) Measurement procedures for RoM and muscle strength; (e) Outcomes, specifically RoM and muscle strength values for each direction of movement. We sought all results compatible with these outcome domains. In clinical trials that included an intervention, we extracted and analyzed data from measurements taken prior to the intervention (i.e., baseline data). When summary statistics were not reported as mean (M) ± standard deviation (SD), data were converted to this format using established formulae (e.g., from confidence intervals, or median and interquartile range). Data were carefully collected into Microsoft® Excel® LTSC MSO software. If the data were presented in a graphical form, we used WebPlotDigitizer software (version 5.2) to obtain the M and SDs. For missing data, study authors were contacted via e-mail and ResearchGate. A reminder was sent after 14 days, and data were deemed irretrievable if no response followed the second inquiry. Potential confounding factors, such as age, sex, and body mass index, were assessed based on the information provided in the included studies. Most studies reported comparable characteristics between participants with KOA and healthy controls. When differences were present, they were acknowledged in the narrative synthesis.
2.3 Assessment of the quality of the included studies and certainty of evidence
The quality of the included studies was identified using the Joanna Briggs Institute (JBI) checklist for analytical cross-sectional studies which assesses study quality based on eight items (22). As the statistical analysis item was not applicable, a maximum of seven items was used: 6–7 “Yes” responses indicated high, 4–5 moderate, and 0–3 low quality. Disagreements were resolved by discussion. Quality assessment was not blinded to study results. To explore potential sources of heterogeneity, subgroup analyses were performed according to Kellgren–Lawrence grade and measurement protocols. Study quality was also considered when interpreting the pooled results.
The certainty of evidence was assessed using the GRADE framework (23), covering risk of bias, imprecision, inconsistency, indirectness, and publication bias. Ratings ranged from high to very low. As most studies were observational, the approach was adapted: certainty was downgraded if >25% of studies fell below quality thresholds, if CIs were wide or total N < 300, if heterogeneity was substantial (I2 > 50%), if evidence was indirect, or if publication bias was suspected.
2.4 Data analysis
The meta-analysis was carried out in Review Manager (Version 5.4, The Cochrane Collaboration, 2020). Means, standard deviations, and sample sizes for both, the KOA and healthy control groups were entered into the meta-analytical model. If data normalized to participant demographics (e.g., weight, BMI, age) were available, these values were included in the analysis. We conducted meta-analyses of continuous outcomes to compare individuals with KOA and healthy controls. A random-effects model with the inverse variance method was used to calculate pooled between-group differences. For outcomes measured using the same units and scales (e.g., RoM in degrees), MDs with 95% CIs were calculated. For outcomes assessed using different measurement instruments or protocols (e.g., muscle strength in N, Nm, Nm/kg, N/BMI, among others) standardized mean differences (SMDs) with 95% CIs were used. Accordingly, the effect sizes representing between-group differences were expressed as MD (for RoM, as it was consistently measured in degrees using a goniometer) and SMD (for muscle strength, as different units and measurement devices were used). Meta-analyses required ≥3 studies, and subgroup analyses ≥2 per subgroup. Where sufficient studies were available, subgroups were formed based on KL grade and on measurement protocols (e.g., knee angle during isometric testing or angular velocity during isokinetic testing). To avoid double-counting participants, data from studies that reported outcomes separately for each KL grade were combined using established formulae for pooling summary statistics across groups (23). In cases where isometric strength was reported at multiple knee angles, we selected the value most commonly used across studies (60 ° for isometric strength of both knee extensors and flexors). Similarly, when concentric strength was reported at multiple angular velocities, we used the value most frequently reported (60 °/s for concentric strength of knee extensors and flexors, or the value closest to 60 °/s when 60 °/s was not available). For meta-analyses comparing concentric strength of knee extensors and flexors across different velocities, we included the higher velocity values within the subgroup representing higher angular speeds. Several studies contributed data to more than one outcome-specific meta-analysis. Consequently, participant numbers overlap across figures representing different outcomes. In subgroup analyses, the same healthy control group was sometimes used as a comparator for multiple disease severity or protocol-specific subgroups. Subgroup comparisons were based on independent effect size estimates, and no participant data were double-counted within any single meta-analysis; therefore, this overlap does not affect the validity of the statistical analyses or the test for subgroup differences. Across analyses involving subgroup stratification, the total number of participants displayed in the forest plots reflects repeated use of the same samples across subgroups and therefore does not represent the number of unique individuals included in the analyses. The exact total number of unique control participants is explicitly reported in the figure captions and is also stated in the Results section.
Statistical heterogeneity was assessed using the I2 statistic, interpreted according to Cochrane guidelines (0–30% low, 30–60% moderate, 50–90% substantial, 75–100% considerable). Significance was set at P ≤ 0.05. For substantial heterogeneity, sensitivity analyses were performed by excluding studies that differed markedly from the pooled effect.
Study characteristics and risk of bias assessments were tabulated. Results of individual studies and pooled effect sizes were displayed using forest plots generated in Review Manager (RevMan 5.4) where appropriate. Additional syntheses are summarized narratively. Risk of bias due to missing results (reporting bias) was assessed using funnel plots when at least 10 studies were available for a given outcome. Funnel plots were generated in Review Manager (RevMan 5.4) and visually inspected for asymmetry.
3 Results
3.1 General overview of the search results
We found 30 studies that were included in the review, 28 of which were eligible for the meta-analysis. Several studies initially appeared to meet the inclusion criteria but were excluded upon full-text review because they did not provide data for healthy control participants, reporting outcomes only for individuals with KOA (24–29). The detailed summary of the search process is shown in Figure 1 (30).
Figure 1

Flowchart of the study search and selection process.
All included studies involved participants with symptomatic KOA, defined either by self-reported pain or by the American College of Rheumatology criteria (31). Some studies examined unilateral KOA (32–34), others bilateral (35, 36) and several included both (11, 17, 37–44). With respect to compartment involvement, some focused on medial KOA (16, 17, 44, 45), others on predominantly medial degeneration (46, 47) while one on both compartments (38). One study included participants awaiting total knee arthroplasty (48). Controls were typically free of KOA, either radiographically or symptomatically. Studies that did not clearly define control status or specify whether the affected leg was tested were included, but sensitivity analyses were performed with and without them. The details of each study are presented in Table 1.
Table 1
| Author (year) | KOA participants | Healthy participants | Measurement details | Results/conclusions | ||||
|---|---|---|---|---|---|---|---|---|
| Sample size, sex (M/F) |
Age (years) in
mean ±SD |
KL grade | Sample size, sex (M/F) | Similar to the KOA subjects in terms of: | KOA exclusion method | |||
| Aily et al. (40) | G1 (middle-aged): n = 20 (10M/10F) G2 (older): n = 20 (10M/10F) | G1: 45.3 ± 2.7 G2: 74.3 ± 2.8 |
KL 2–3 | G1 (middle-aged): n = 20 (10M/10F) G2 (older): n = 20 (10M/10F) | Sex, age, BMI | No symptoms, KL 0 or 1 |
a) Isometric strength of knee extensors
Machine: Biodex system dynamometer (Biodex Medical systems 3 Pro, Shirley, New York, USA) Starting position: Seated with knees flexed at 90° Repetitions and rest: three maximal contractions held for 3s each, with a 1min rest between contractions b) Concentric isokinetic strength of knee extensors Machine: Biodex System dynamometer (Biodex Medical Systems 3 Pro, Shirley, New York, USA) Starting position: Seated with knees flexed at 90° Repetitions and rest: five maximal contractions, 1min rest in between Speed of movement: 60°/s |
Middle-aged individuals with KOA had significantly lower isometric and concentric torque compared to their healthy participants (P < 0.05). Similarly, older adults with KOA also showed significantly lower isometric and concentric torque than healthy older adults (ś < 0.05) |
| Aily et al. (39) | n = 23 (11M/12F) | 61.9 ± 9.5 | KL 2–3 | n = 23 (11M/12F) | Sex, age, BMI | No knee pain, KL 0 or 1 |
Isometric strength of knee extensors
Machine: Isokinetic dynamometer (Multi-Joint System 3, Biodex Medical System, New York, USA) Starting position: Seated with knees flexed at 60° Repetitions and rest: three maximal contractions held for 3s each, with a 1min rest between contractions |
Isometric torque was significantly lower in the KOA group compared to the healthy group (P < 0.001) when torque was normalized to body mass. In contrast, the difference in non-normalized isometric torque did not reach statistical significance (P = 0.070) |
| Baert et al. (41) | G1 (early KOA): n = 21 (21F) G2 (established KOA): n = 24 (24F) | G1 (early KOA): 65.5 ± 7.6 G2 (established KOA): 64.0 ± 7.5 |
G1 (early KOA): KL 0, 1, or 2− G2 (established KOA): ≥ 2+ | n = 20 (20F) | Sex, age, weight, height, BMI | Asymptomatic, no history of knee pain, KL 0 or 1 |
Isometric strength of knee extensors and flexors
Machine: Biodex System 3 Pro (Biodex Medical Systems, New York) Starting position: Seated position with 60° and 90° of knee flexion Repetitions and rest: Each test was performed three times with maximal contraction held for 5s and 10s rest between trials |
Participants with early and established KOA demonstrated significantly lower isometric knee extensor torque at 60° and 90° of knee flexion compared to healthy controls. For knee flexor torque, participants with established KOA showed significantly lower values than controls at both angles, whereas no significant differences were observed between the early KOA group and the control group at either position |
| Baert et al. (46) | G1 (early KOA): n = 14 (14F) G2 (established KOA): n = 12 (12F) | G1 (early KOA): 65.4 ± 8.9 G2 (established KOA): 68.3 ± 6.8 |
G1 (early KOA): KL 0, 1, or 2− G2 (established KOA): ≥ 2+ | n = 14 (14F) | Sex, age, weight, height, BMI | Asymptomatic, no history of knee pain, KL 0 or 1 |
Isometric strength of knee extensors and flexors
Machine: Biodex System 3 Pro (Biodex Medical Systems, NY, USA) Starting position: Seated position with 60° of knee flexion Repetitions and rest: each test was performed three times with maximal contraction held for 5s and 10s rest between trials |
The established KOA group showed significantly lower isometric strength in both knee extension (P = 0.001) and flexion (P = 0.019) compared to the healthy control group. In contrast, the early KOA group had significantly lower isometric extension strength (P = 0.007), while their isometric flexion strength was lower than that of healthy controls, but the difference was not statistically significant (P = 0.693) |
| Diraçoglu et al. (35) | n = 51 (51F) | 55.6 ± 9.7 | KL 1–2 | n = 43 (43F) | Age, BMI, sex | Without clinical or radiological evidence of KOA |
Concentric isokinetic strength of knee extensors and flexors
Machine: Biodex System 3-Pro (Biodex Medical Systems, Inc, New York, USA) Starting position: Seated, range of motion of the knee was kept at 0°-90° Repetitions and rest: Four repetitions for speed of movement of 60°/s and 180°/s and 20 repetitions for speed of movement of 240°/s Speed of movement: 60°/s, 180°/s, 240°/s |
Patients with KOA exhibited significantly lower isokinetic concentric torque of both the knee flexors and extensors at all tested movement speeds |
| Gapeyeva et al. (48) | n = 10 (10F) | 63.0 ± 7.1 | KL 3–4 | n = 10 (10 F) | Sex, age | Without painful joints or any other criteria listed for the patients group |
a) Knee flexion RoM
Active RoM, measured by Gollehon Extendable Goniometer (Lafayette Instrument, USA) b) Isometric strength of knee extensors Machine: Custom made chair equipped with a chair-fixed standard calibrated strain gauge transducer DST 1778 (Russia) Starting position: Seated with knees and hips at 90° and 110°, respectively Repetitions and rest: three repetitions of maximal contractions that were held for approximately 3s. A rest period of 2 min was allowed between the attempts |
Subjects with KOA had significantly lower RoM compared to healthy controls (P < 0.01) and significantly lower isometric strength of the knee extensors (P < 0.001) |
| Unver Kocak et al. (49) | G1: n = 33 (8M/25F) G2: n = 30 (4M/26F) G3: n = 59 (11M/48F) G4: n = 61 (2M/59F) |
G1: 50.9 ± 11.0 G2: 59.6 ± 9.0 G3: 65.7 ± 9.2 G4: 66.9 ± 7.7 |
G1: KL1 G2: KL2 G3: KL 3 G4: KL 4 |
n = 35 (20M/15F) | / | No history of knee pain or other symptoms |
Knee flexion RoM
Active RoM measured by universal goniometer |
There were no significant differences in knee flexion RoM between healthy subjects and individuals with KOA of KL grades 1 and 2 (P > 0.05). However, healthy subjects had significantly higher values compared to those with KL grades 3 and 4 (P < 0.000) |
| Kumar et al. (45) | n = 16 (8M/8F) | 65.2 ± 9.5 | KL ≥ 2 | n = 12 (6M/6F) | Age, weight, BMI | KL ≤ 1 |
Isometric strength of knee extensors
Machine: Isokinetic dynamometer (Kin Com Isokinetic International. Harrison, TN 37341) Starting position: Knee flexed to 90°Repetitions and rest: Three maximal voluntary isometric contractions |
The KOA group had lower quadriceps strength but it was not statistically significant (P = 0.16) |
| Liikavainio et al. (11); Lyytinen et al. (79) |
G1: n = 12 (12M) G2: n = 15 (15M) G3: n = 19 (19M) G4: n = 8 (8M) |
G1: 57.7 ± 5.8 G2: 58.7 ± 5.8 G3: 59.1 ± 5.1 G4: 61.2 ± 4.1 |
G1: KL 1 G2: KL2 G3: KL3 G4: KL4 |
n = 53 (53M) | Age, sex | No Knee OA according to the clinical criteria of the American College of Rheumatology (31) |
a) Knee flexion and extension RoM
Active knee flexion and passive knee extension RoM measured in a supine position with a standard goniometer b) Isometric strength of knee extensors and flexors Machine: Calibrated dynamometer Starting position: sitting position with knee and hip angles fixed at 70° Repetitions and rest: as many maximal actions until the peak value no longer increased |
The control subjects showed significantly higher knee extension torque values (P < 0.01), knee flexion torque (P = 0.024), as well as greater knee flexion (P < 0.01) and extension (P < 0.01) RoM compared to all subjects with KOA |
| Ling et al. (51) | n = 21 (10M/11F) | 65.49 ± 2.8 | KL 1–4 | n = 18 (12M/6F) | Age, sex | No knee symptoms, KL0 |
Isometric strength of knee extensors
Machine: KIN-Com 125E dynamometer (Chattecx, Chattanooga, TN) Starting position: knee angle of 60° of flexion Repetitions and rest: three trials |
Participants with KOA exhibited lower isometric knee extensor strength than controls (P = 0.016) |
| Rodriguez-Lopez et al. (18) | n = 18 (18F) | 69.6 ± 7.3 | KL 2–4 | n = 26 (26F) | Age, sex, BMI | Asymptomatic and no history of knee pain |
Isometric strength of knee extensors
Machine: biodex Medical System 3 dynamometer (Biodex Medical Systems, Shirley, NY) Starting position: Knee joint angle of 90° Repetitions and rest: four 5s maximal voluntary isometric ramp contractions, separated by 20s rest interval |
KOA subjects showed lower isometric strength compared to control subjects (P = 0.001) |
| Sanchez-Ramirez et al. (52) | G1 (early KOA): n = 14 (14F) G2 (established KOA): n = 19 (19F) |
G1: 70.4 ± 4.6 G2: 68.37 ± 6.7 |
G1: KL 1 G2: KL ≥ 2 | n = 14 (14F) | Age, sex, BMI, weight, height | No history of knee symptoms or characteristics associated with knee OA, KL 0 |
Isometric strength of knee extensors and flexors
Machine: biodex System 3 Pro (Biodex Medical System, Shirley, NY, USA) Starting position: measured in 60° of flexion position Repetitions and rest: three trials of each leg |
Control subjects demonstrated significantly higher isometric knee flexor torque compared to the established KOA group (P = 0.011). However, no significant differences were observed between the control group and the early KOA group for knee flexor strength (P = 0.217). There were also no significant differences in isometric knee extensor torque among the groups (P = 0.362) |
| Rutherford et al. (47) | G1: n = 38 (27M/11F) G2: n = 33 (28M/5F) G3: n = 11 (6M/5F) |
G1: 56 ± 8 G2: 59 ± 8 G3: 59 ± 8 |
G1: KL2 G2: KL3 G3: KL4 |
n = 35 (16M/19F) | Age | Asymptomatic |
Isometric strength of knee extensors and flexors
Machine: Starting position: knee extension strength was measured at 45° of knee flexion in a seated position, and knee flexion strength at 55° of knee flexion in the same position Repetitions and rest: two three second maximal isometric contractions were completed for each muscle group. A minimum 60s rest period separated each contraction |
Asymptomatic individuals had greater quadriceps strength than the KL 2 group (P < 0.05). Other differences between groups were not significant |
| Tan et al. (53) | n = 30 (30F) | 63 ± 6.85 | KL 2–3 | n = 30 (30F) | Sex | / |
Isometric strength of knee extensors and flexors
Machine: cybex-350 isokinetic dynamometer system (Lumex Inc., Ronkokoma, New York) Starting position: at 30° and 60° of knee flexion Repetitions and rest: six maximal tries were made at each flexion degree, with resting interval of 20s between each try |
Isometric maximum peak torque loss of knee flexors and extensors was found in KOA group with respect to controls |
| Vårbakken et al. (37) | n = 31 (15M/16F) | 55.3 ± 8.0 | KL 2–4 | n = 28 (10M/18F) | Sex, BMI, height, weight | Without knee pain or knee complaints |
Concentric isokinetic strength of knee extensors and flexors
Machine: biodex System 4 Dynamometer (Biodex Medical Systems, NY, USA) Starting position: seated, with back rest tilted 70° Repetitions and rest: five consecutive maximum strength tests Speed of movement: 60°/s |
There was a significant difference in knee extensor torque between groups (P = 0.012), while no significant differences were observed in knee flexor torque (P = 0.114) |
| Yagi et al. (17) | n = 22 (22F) | 69.5 ± 5.4 | KL 2–4 | n = 15 (15F) | Age, sex, height | Asymptomatic |
a) Knee flexion and extension RoM
Passive knee RoM was measured in flexion and extension with a goniometer b) Isometric strength of knee extensors Machine: dynamometer Biodex System 4, Biodex Medical Systems, Inc Starting position: seated, with 45° of knee flexion Repetitions and rest: two repetitions of maximum voluntary contraction for 5s |
Healthy controls exhibited significantly greater knee RoM in both flexion (P = 0.003) and extension (P = 0.024). Isometric MVC torque was also significantly greater in the control group (P < 0.001) |
| Yang et al. (32) | n = 18 (14M/4F) | 63.88 ± 6.5 | KL 2–3 | n = 18 (13M/5F) | Age, sex, BMI, weight, height | / |
Concentric isokinetic strength of knee extensors and flexors
Machine: biodex isokinetic dynamometer (System 4, Biodex Inc., Shirley, NY, United States) Starting position: seated repetitions and rest: the concentric/concentric pattern was repeated 3 times, with 5 min break between each set of tests Speed of movement: 60°/s, 120°/s, 180°/s |
Compared to healthy controls, KOA patients had significantly smaller peak torque at all speeds (P < 0.05) |
| Baker et al. (16) | n = 20 (10M/10F) | 62 ± 7 | KL 1–3 | n = 20 (11M/9F) | Age, height | Asymptomatic |
Isometric strength of knee extensors and flexors
Machine: a Human Norm Isokinetic Dynamometer (Computer Sports Medicine Inc., USA) Starting position: at 45° of knee flexion Repetitions and rest: two, 3s MVIC trials were completed, separated by 40s of rest |
No significant between group strength differences were found for knee extensors (P = 0.195) and flexors (P = 0.067) |
| Childs et al. (43) | n = 24 (56%F) | 62 ± 10 | KL ≥ 2 | n = 24 (56%F) | Age, sex, height | No history of knee OA, KL ≤ 1 | Knee flexion and extension RoM / | Subjects with KOA had significantly less flexion and extension knee RoM (P < 0.01) compared to healthy individuals |
| Emrani et al. (50) | n = 20 (M and F) | 44.6 ± 2.3 | KL 1–2 | n = 20 (M and F) | Age, weight, height, activity level | No clinical or radiological sign of KOA |
a) Concentric isokinetic strength of knee extensors and flexors
Machine: biodex System 2 isokinetic dynamometer (Biodex Medical System, Shirley, NY, USA) Starting position: Seated with a backrest at 90° angle. During the test subjects pushed the lever arm of the device through the RoM between 10° and 90° of knee flexion Repetitions and rest: two sets of tests, in order of speed. Each test consisted of a continuous maximal flexion-extension and was repeated five times. A 1 min rest was allowed between each two sets of tests, and a 3 min rest was given after each angular speed. A 20 min rest was allowed between the two legs Speed of movement: 90°/s, 150°/s b) Knee flexion and extension RoM Measured with goniometer while the subject was supine with the hip extended |
There were significant differences between the two groups with regard to isokinetic torque at both angular speeds for knee flexors and extensors (P < 0.00). There were no significant differences in the values of lower extremity RoM (P > 0.05) |
| Hortobágyi et al. (36) | n = 20 (5M/15F) | 57.5 ± 7.3 | KL ≥ 2 | n = 20 (5M/15F) | Sex | No knee OA, no knee pain |
a) Isometric strength of knee extensors
Machine: Dynamometer (Kin-Com, AP125; Chattecx Inc, Chattanooga, TN) Starting position: 1.14 radians of knee flexion Repetitions and rest: subjects performed three maximal isometric effort 5s trials with 1 min of rest between trials b) Concentric and eccentric isokinetic knee extensors strength Machine: Dynamometer (Kin-Com, AP125; Chattecx Inc, Chattanooga, TN) Starting position: Repetitions and rest: subjects performed three maximal effort eccentric and concentric isokinetic quadriceps contractions. Each quadriceps contraction was followed by a hamstring contraction with a 1s pause between the efforts. There was 1min of rest between conditions Speed of movement: 1.57 radians per second |
Overall, KOA patients produced 63% less quadriceps force than control subjects (P < 0.05) |
| Lohnes et al. (34) | n = 16 (6M/10F) | 61 ± 6 | KL 0–3 | n = 16 (6M/10F) | Age, sex | Asymptomatic |
Isometric strength of knee flexors and extensors
Machine: Human Norm Isokinetic Dynamometer (Computer Sports Medicine Inc., USA) Starting position: 45° of knee flexion Repetitions and rest: two, three second maximal isometric contractions with a 40s rest period separated each contraction |
There were no significant differences between KOA and healthy control group for knee flexion (P = 0.187) and knee extension (P = 0.217) strength |
| Noehren et al. (54) | n = 24 (14M/10F) | 60.2 ± 5.5 | KL 2–3 | n = 15 (5M/10F) | Activity, age, BMI | KL ≤ 1 in both knees and no evidence of patellofemoral OA |
Concentric isokinetic knee extensor strength
Machine: HUMAN NORM isokinetic dynamometer (CSMi, Stoughton, MA, USA) Starting position: Seated, knee extensor strength was tested through a joint arc from 90° to 30° of knee flexion Repetitions and rest: five trials spaced by rest period of 30–60s Speed of movement |
KOA subjects had significantly weaker quadriceps than the control subjects (P = 0.003) |
| Rice et al. (75) | n = 15 (7M/8F) | 63.0 ± 9.7 | KL 2–4 | n = 15 (7M/8F) | Age, sex, height, mass | No history of knee injury or pathology |
Isometric strength of knee extensors and flexors
Machine: Starting position: Repetitions and rest: three quadriceps MVCs (6s) followed by three hamstring MVCs (6s) with a 2 min rest period between each maximum-effort contraction |
Quadriceps peak torque was significantly lower in the KOA group compared with the control group (P = 0.005). While hamstrings peak torque was lower in the KOA group compared with the control group, this difference did not reach statistical significance (P = 0.101) |
| Serrao et al. (55) | n = 22 (22M) | 52 ± 8.1 | KL 1–2 | n = 18 (18M) | Sex | No joint disorders, no radiographic alterations, KL = 0, no history of lower limb pain, illness, injury, trauma or fracture |
Concentric and eccentric isokinetic knee extensor strength
Machine: Isokinetic dynamometer (Biodex Multi-Joint System 3, Biodex Medical Inc, Shirley, NY) Starting position: Seated with knee flexed at 90°. Range of motion: from 20° to 90° Repetitions and rest: five maximal voluntary concentric and five maximal voluntary eccentric contractions at an angular speed of 90°/s and 10 maximal concentric and 10 maximal eccentric contractions at an angular speed of 180°/s. The rest between each type of contraction and velocities lasted for 5 min. Speed of movement: 90°/s and 180°/s |
In the analysis of concentric knee extensor strength, no differences were found between the groups at any angular speed (P = 0.73 at 90°/s; P = 0.97 at 180°/s). For eccentric knee extensor strength, differences were found between the groups at 90°/s (P = 0.01) and at 180°/s (P = 0.04), with higher values for the control group |
| Teoli et al. (42) | n = 22 (6M/16F) | 60 ± 7 | KL 1–4 | n = 22 (6M/16F) | Age, sex, BMI | No current lower extremity pain, no diagnosis of lower extremity OA |
Isometric knee extensor strength
Machine: Isokinetic dynamometer (Humac Cybex NORM, Computer Sports Medicine Inc., Massachusetts, US) Starting position: Seated with 45° of knee flexion Repetitions and rest: two 5s MVIC trials with a 30s rest period between trials |
No significant differences were observed in knee extensor strength between groups (P = 0.335) |
| Ucurum et al. (33) | n = 35 (35F) | 59.63 ± 7.81 | KL 2–3 | n = 35 (35F) | Age, sex, weight, height, BMI | No current pain and no OA symptoms |
Isometric knee flexor and extensor strength
Machine: Digital hand-held dynamometer (Lafayette Model-01165, Lafayette Instrument Company, Lafayette IN, USA) Starting position: Seated with a tight-body angle of 90° and knee flexion of 60° Repetitions and rest: three repetitions with a 30s period of rest in between |
Extensor strength in KOA group was lower than extensor strength of dominant and nondominant knees of the controls and the effect sizes were moderate (P = 0.037 and d=0.51 for dominant knee; P = 0.039 and d=0.50 for non dominant knee). No significant difference was found between contralateral and ipsilateral knees and groups for knee flexor strength (P > 0.05) |
| Yagi et al. (17) | n = 18 (18F) | 69.7 ± 5.9 | KL 2–4 | n = 22 (22F) | Age, height, weight, BMI | No knee pain |
Knee flexion and extension RoM
Measured in supine using a two-arm goniometer (Sakai Medical Co., Ltd.) |
Healthy control group had significantly more knee extension and flexion RoM compared to control group (P < 0.001) |
| Zhang et al. (3) | n = 25 (25F) | 64.42 ± 2.95 | KL 1–3 | n = 22 (22F) | / | / |
Concentric and eccentric isokinetic knee extensor strength
Machine: Multi-joint dynamometer (Con-trex multi joint model, CMV AG, Dubendorf, Switzerland) Starting position: Seated Repetitions and rest: three repetitions Speed of movement: 90°/s |
Compared with the control group, the KOA exhibited lower absolute peak knee extension torque, relative peak knee extension and relative flexion torque |
Characteristics of included studies.
n, numerus; KOA, knee osteoarthritis; OA, osteoarthritis; G, group; M, mean or male; SD, standard deviation; F, female; KL, Kellgren-Lawrence Classification System; RoM, range of motion; MVC, maximal voluntary contraction; MVIC, Maximal voluntary isometric contraction; min, minute; s, second.
3.2 Assessment of the quality of the included studies
As shown in Supplementary Table S1, the methodological quality of the included studies was predominantly high, with 27 of 30 studies meeting 6–7 JBI criteria and the remaining two classified as moderate; no study was rated low quality.
3.3 Differences in knee flexion range of motion between individuals with knee osteoarthritis and healthy controls
Six studies (311 KOA; 159 controls) (11, 17, 43, 44, 48, 49) investigated knee flexion RoM differences (Figure 2). Healthy individuals showed 16.30° greater flexion than those with KOA [MD = 16.30, 95% CI (11.40–21.21), P < 0.0001], though heterogeneity was high (I2 = 82%). Excluding studies with unclear measurement or control status (43, 49) increased the difference to 18.19 ° and heterogeneity to I2 = 87% (P < 0.0001). Certainty of evidence was very low, despite all studies being rated high quality.
Figure 2

Meta-analysis of differences in knee flexion range of motion between individuals with kne osteoarthritis and healthy controls. Mean differences (MD) are expressed in degrees (°).
One study (50) also assessed flexion but lacked values for inclusion; it reported no significant differences (P > 0.05) in lower extremity RoM between patients with KOA and healthy controls.
To explore the impact of osteoarthritis severity, we conducted a separate meta-analysis (two studies, 237 KOA; 88 controls) (11, 49) comparing knee flexion RoM between healthy controls and individuals with KOA, stratified by KL grade (Figure 3). The results showed significant subgroup differences (P < 0.00001), indicating that the magnitude of the difference in knee flexion RoM between healthy individuals and those with KOA depends on the KL grade. When examining the overall effect within each subgroup, significant differences between healthy individuals and those with KOA were found only in KL grade 3 (P < 0.00001) and KL grade 4 (P < 0.00001). In contrast, no significant differences were observed between healthy individuals and those with KL grade 1 (P = 0.64) or KL grade 2 (P = 0.40). Moreover, one study (49) reported greater knee flexion in individuals with KL grades 1 and 2 compared to healthy controls. The largest difference in knee flexion RoM was observed between healthy individuals and those with KOA classified as KL grade 4, with a mean difference of 27.02° in favor of the healthy group [MD = 27.02, 95% CI (21.75, 32.29), P < 0.00001].
Figure 3

Meta-analysis of knee flexion range of motion differences according to Kellgren–Lawrence grades of osteoarthritis severity. Mean differences (MD) are expressed in degrees (°). The total number of controls displayed in the forest plot reflects repeated use of the same control samples across Kellgren–Lawrence grade subgroups; the number of unique healthy control participants included in this analysis was 88.
3.4 Differences in knee extension range of motion between individuals with knee osteoarthritis and healthy controls
Four studies included in the meta-analysis (118 KOA; 114 controls) (11, 17, 43, 44) investigated differences in knee extension RoM between individuals with KOA and healthy subjects (Figure 4). Overall, healthy individuals demonstrated 4.25° greater knee extension RoM compared to those with KOA [MD = 4.25, 95% CI (2.30, 6.19), P < 0.0001]. However, study effects were highly heterogeneous (I2 = 81%). After repeating the meta-analysis with the exclusion of studies that did not clearly report whether measurements were performed on the affected leg in KOA participants or did not explicitly state that control participants were free of KOA (43), heterogeneity decreased to I2 = 0%. The between-group difference decreased to 3.17°, remaining statistically significant [MD = 3.17, 95% CI (2.33, 4.01), P < 0.00001]. Certainty of evidence was very low, despite all studies being rated high quality.
Figure 4
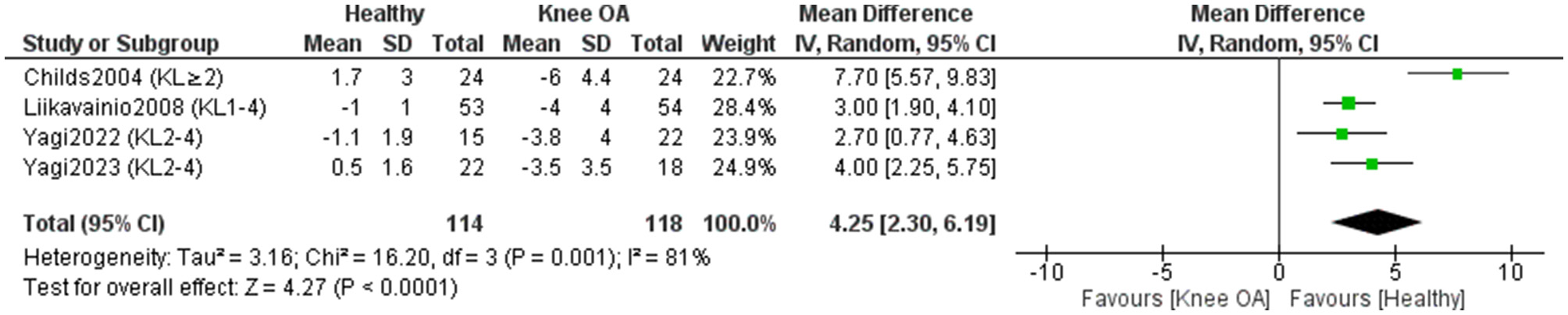
Meta-analysis of differences in knee extension range of motion between individuals with knee osteoarthritis and healthy controls. Mean differences (MD) are expressed in degrees (°).
One study (50) assessed knee extension RoM but lacked measurement values and was excluded from the meta-analysis; it reported no significant differences (P > 0.05) between KOA patients and controls.
3.5 Differences in isometric knee extensor strength between individuals with knee osteoarthritis and healthy controls
Seventeen studies included in the meta-analysis (493 KOA; 383 controls) (11, 16–18, 34, 38–42, 45–48, 51–53) examined differences in isometric knee extensor strength between individuals with KOA and healthy controls (Figure 5). Overall, the results showed a significant (P < 0.00001) and large effect in favor of healthy individuals [SMD = 0.86, 95% CI (0.57, 1.14)]. One study (48) reported an especially large positive effect. When this study was excluded, the effect size remained large [SMD = 0.80, 95%CI (0.58, 1.02)] and statistically significant (P < 0.00001), while heterogeneity decreased from I2 = 72% to I2 = 55%. A sensitivity analysis was conducted by excluding studies that did not clearly report whether measurements in KOA participants were taken from the affected limb or failed to explicitly confirm that control participants were free of KOA (39, 51, 53). In this analysis, heterogeneity increased to I2 = 76%, and the effect size remained large [SMD = 0.84, 95% CI (0.49, 1.18)] and statistically significant (P < 0.00001). Two studies (33, 36) could not be included in the meta-analysis due to the absence of precise numerical data. However, the authors consistently reported reduced isometric knee extensor strength in individuals with KOA compared to healthy controls (33, 36). The certainty of evidence was low. Fifteen studies were rated as high quality and one as moderate quality according to the JBI checklist. Visual inspection of the funnel plot suggests no substantial asymmetry, indicating a low risk of publication bias. Most studies were symmetrically distributed around the pooled effect size, with no evidence of systematically missing small studies.
Figure 5

Meta-analysis of differences in isometric knee extensor strength between individuals with knee osteoarthritis and healthy controls.
To explore the impact of osteoarthritis severity, we conducted a separate meta-analysis comparing isometric knee extensor strength between healthy controls and individuals with KOA, stratified by KL grade (Figure 6). This meta-analysis included four studies (171 KOA; 120 controls) (11, 47, 51, 52). The results showed that subgroup differences were not statistically significant (P = 0.39), indicating that individuals with KOA, regardless of KL grade, exhibit a similar reduction in isometric knee extensor strength compared to healthy controls. When examining the overall effect within each subgroup, significant differences in isometric knee extensor strength between healthy individuals and those with KOA were observed in participants with KL grades 2–4 (P < 0.05), but not in those with KL grade 1 (P = 0.08). The effect size increased with higher KL grades, with the largest effect observed in the KL 4 subgroup (SMD = 1.21).
Figure 6

Meta-analysis of isometric knee extensor strength differences according to Kellgren–Lawrence grades of osteoarthritis severity. The total number of controls displayed in the forest plot reflects repeated use of the same control samples across Kellgren–Lawrence grade subgroups; the number of unique healthy control participants included in this analysis was 120.
To determine whether isometric knee extensor strength differs between individuals with KOA and healthy controls depending on the knee flexion angle during testing, we conducted a separate meta-analysis (Figure 7). The analysis revealed no statistically significant subgroup differences based on the knee flexion angle (P = 0.39). These findings indicate that the magnitude of the strength deficit in individuals with KOA, compared to healthy controls, does not substantially vary across different knee flexion angles during isometric testing. The effect size increased with greater knee flexion angles, with the largest effect observed at 90 ° of knee flexion (SMD = 1.30), indicating that the strength differences between healthy individuals and those with KOA are more pronounced when measurements are taken in a more flexed knee position. Even after removing the study that showed a substantially higher positive effect and was considered an outlier (48), the subgroup with measurements taken at 90° of knee flexion still demonstrated the largest effect size (SMD = 1.01).
Figure 7

Meta-analysis of isometric knee extensor strength differences according to the knee flexion angle during strength assessment.
3.6 Differences in isometric knee flexor strength between individuals with knee osteoarthritis and healthy controls
Nine studies included in the meta-analysis (321 KOA; 217 controls) (11, 16, 34, 38, 41, 46, 47, 52, 53) examined differences in isometric knee flexor strength between individuals with KOA and healthy controls (Figure 8). Overall, the results showed a statistically significant (P < 0.00001) and moderate effect in favor of healthy individuals [SMD = 0.52, 95% CI (0.30, 0.74)]. A sensitivity analysis was performed by excluding studies that did not clearly report whether measurements in KOA participants were taken from the affected limb or did not explicitly confirm that control participants were free of KOA (52, 53). In this analysis, the effect size remained moderate [SMD = 0.42, 95% CI (0.20, 0.63)] and statistically significant (P < 0.0001). One study (33) also assessed isometric knee flexor strength but was not included in the meta-analysis, as the exact measurement values could not be extracted from the study. The authors reported no statistically significant differences in knee flexor strength between patients with KOA and healthy controls (P > 0.05) (33). Certainty of evidence was low, with most studies rated high quality and one moderate (JBI).
Figure 8

Meta-analysis of differences in isometric knee flexor strength between individuals with knee osteoarthritis and healthy controls.
A subgroup meta-analysis (3 studies; 150 KOA, 102 controls) (11, 47, 52) found no significant differences by KL grade (P = 0.56), indicating similar isometric knee flexor strength deficits across KOA severities (Figure 9).
Figure 9

Meta-analysis of isometric knee flexor strength differences according to Kellgren–Lawrence grades of osteoarthritis severity. The total number of controls displayed in the forest plot reflects repeated use of the same control samples across Kellgren–Lawrence grade subgroups; the number of unique healthy control participants included in this analysis was 102.
3.7 Differences in concentric isokinetic knee extensor strength between individuals with knee osteoarthritis and healthy controls
Eight studies included in the meta-analysis (279 KOA; 250 controls) (32, 35, 37, 40, 50, 54–56) examined differences in concentric isokinetic knee extensor strength between individuals with KOA and healthy controls (Figure 10). Overall, the results showed a statistically significant (P < 0.00001) and large effect in favor of healthy individuals [SMD = 1.07, 95% CI (0.65, 1.50)]. However, heterogeneity was high (I2 = 78%). One study (54) stood out due to a markedly positive effect in favor of healthy individuals, while another study (55) showed a positive effect in favor of individuals with KOA. When these two studies were excluded and the meta-analysis was repeated, the overall effect remained large (SMD = 0.89) and statistically significant (P < 0.00001), while the heterogenity decreased significantly (I2=0%). A sensitivity analysis was conducted by excluding studies that did not clearly report whether measurements in KOA participants were taken from the affected limb or did not explicitly confirm that control participants were free of KOA (32, 54, 56). In this analysis, heterogeneity was moderate (I2 = 32%), while the effect size remained large [SMD = 0.80, 95% CI (0.53, 1.06)] and statistically significant (P < 0.00001). The certainty of evidence was low. All studies were rated as high quality and one as moderate quality according to the JBI checklist.
Figure 10
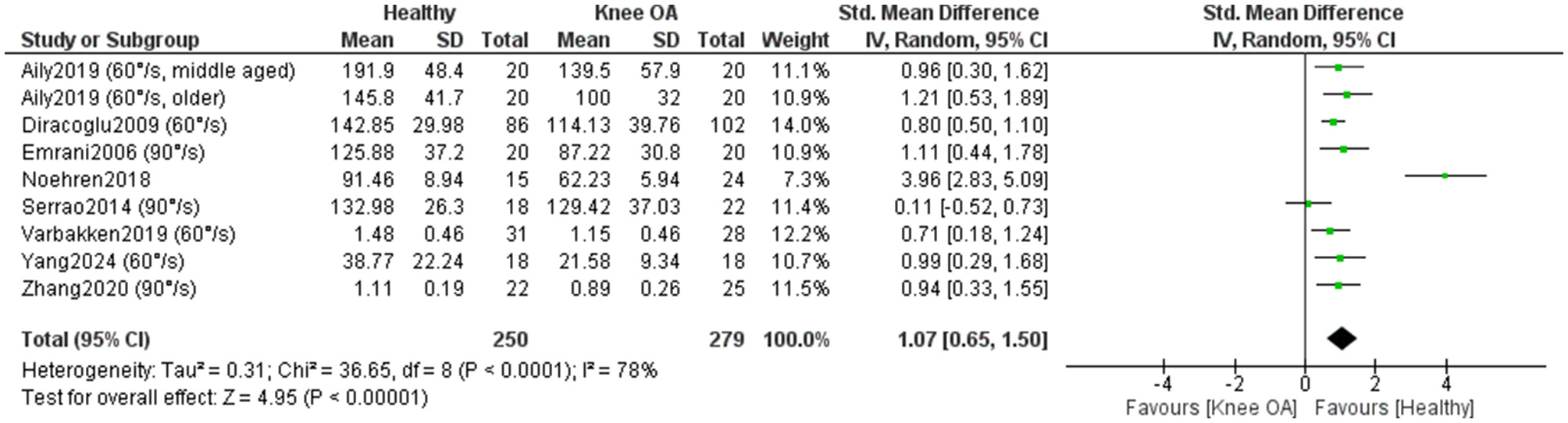
Meta-analysis of concentric isokinetic knee extensor strength differences between individuals with knee osteoarthritis and healthy controls.
One study (36) could not be included in the meta-analysis due to the lack of precise numerical data. Nevertheless, the study also reported reduced concentric knee extensor strength in individuals with KOA, who produced 56% lower torque compared to healthy controls.
A subgroup meta-analysis (7 studies; 375 KOA, 235 controls) (32, 35, 37, 40, 50, 55, 56) found no significant differences by angular velocity (P = 0.61), indicating that concentric knee extensor deficits in KOA are consistent across low and moderate testing speeds (Figure 11).
Figure 11

Meta-analysis of concentric isokinetic knee extensor strength differences according to speed of movement during strength assessment. The total number of controls displayed in the forest plot reflects repeated use of the same control samples across movement-velocity subgroups; the number of unique healthy control participants included in this analysis was 235.
3.8 Differences in concentric isokinetic knee flexor strength between individuals with knee osteoarthritis and healthy controls
Four studies included in the meta-analysis (175 KOA; 159 controls) (35, 37, 50, 56) examined differences in concentric isokinetic knee flexor strength between individuals with KOA and healthy controls (Figure 12). Overall, the results showed a statistically significant (p < 0.0001) and large effect in favor of healthy individuals [SMD = 0.77, 95% CI (0.43, 1.12)] with a small heterogeneity across studies (I2 = 49%). A sensitivity analysis was conducted by excluding studies that did not clearly report whether measurements in KOA participants were taken from the affected limb or did not explicitly confirm that control participants were free of KOA (56). In this analysis, heterogeneity was moderate (I2 = 53%), while the effect size remained large [SMD = 0.84, 95% CI (0.44, 1.24)] and statistically significant (P < 0.0001). The certainty of evidence was low. All studies were rated as high quality and one as moderate quality according to the JBI checklist.
Figure 12

Meta-analysis of concentric isokinetic knee flexor strength differences between individuals with knee osteoarthritis and healthy controls.
A subgroup meta-analysis (4 studies; 175 KOA, 159 controls) (35, 37, 50, 56) found no significant differences by angular velocity (P = 0.64), indicating similar concentric knee flexor strength deficits in KOA across low and moderate movement velocities (Figure 13).
Figure 13

Meta-analysis of concentric isokinetic knee flexor strength differences according to speed of movement during strength assessment.
3.9 Differences in eccentric isokinetic knee extensor strength between individuals with knee osteoarthritis and healthy controls
Two studies reported lower eccentric extensor strength in KOA than in healthy controls (36, 55). Hortobágy et al. (36), reported that OA patients produced 76% lower eccentric forces compared to healthy subjects. Serrão et al. (55) demonstrated significantly reduced eccentric isokinetic knee extensor strength at both 90 °/s (P = 0.01) and 180 °/s (P = 0.04) in individuals with early-stage KOA compared with healthy controls.
4 Discussion
This systematic review examined differences in knee joint RoM and muscle strength between individuals with and without KOA. Thirty studies were included, 28 in the meta-analysis. Results showed reduced knee flexion and extension RoM in KOA, with flexion deficits increasing with higher KL grades. Healthy controls demonstrated greater isometric, concentric, and eccentric strength of the knee extensors and flexors. Isometric strength deficits in KOA were consistent across KL grades and knee flexion angles, while concentric strength differences were similar across testing velocities.
4.1 Differences in knee range of motion between individuals with knee osteoarthritis and healthy controls
Deficits in knee RoM in individuals with KOA compared to healthy individuals may be attributed to a variety of factors. On one hand, KOA patients may be limited by pain or fear of pain, which prevents them from achieving a greater RoM, even if the joint itself would otherwise be capable of moving through a larger range (57, 58). On the other hand, knee RoM may be mechanically restricted by structural changes in bone and cartilage, such as the presence of osteophytes and reduced joint space (59). It can also be limited by soft tissue restrictions, including shortening or tightness of the knee flexor and extensor muscles (60, 61), knee effusion (62–64) or by excess fat, which mechanically restricts inter-segmental movement in the body (65). The latter may often contribute to limited knee RoM, as the prevalence of KOA is reported to be 3.59 times higher among individuals with obesity compared to non-obese individuals (66). It is important to recognize that all these factors restricting RoM contribute to a vicious cycle—disuse and unloading of the knee joint throughout its full RoM can, in itself, lead to articular and periarticular tissue changes, which further limit knee RoM. This is supported by studies conducted on rats, which showed that prolonged immobilization in full knee flexion led to replacement of the articular cartilage with bone tissue on the anterior region of the tibial articular surface (67, 68). It is possible that similar changes may also occur in individuals with KOA who do not load the knee in a neutral position—i.e., 0 ° of extension. This lack of full extension appears to be quite common, as all studies included in the review report mean knee extension values in individuals with KOA that do not reach 0 °.
Our meta-analysis showed that the deficit in knee RoM increases with higher KL grades. Specifically, individuals with KL grade four exhibited a flexion deficit of 27.02 degrees compared to healthy controls, while those with KL grade one had a deficit of only 2.42 degrees. Similar findings were reported by Hilfiker et al. (60), who found that radiographic severity is independently associated with deficits in both knee extension and flexion in patients with KOA. These results support the traditional biomedical model, indicating that the extent of structural pathology (degeneration) affects joint function. Additionally, this highlights the importance of early intervention: in individuals with lower KL grades, strategies should focus on preserving mobility and preventing functional decline, potentially reducing the severity of deficits as the disease progresses.
4.2 Differences in knee muscle strength between individuals with knee osteoarthritis and healthy controls
Our results showed that individuals with KOA have reduced isometric, concentric, and eccentric strength of the knee muscles compared to healthy individuals. Similar to deficits in knee RoM, muscle weakness can also arise from a variety of factors. Studies have shown that individuals with KOA spend approximately two-thirds of their daily time in sedentary behaviour (61). While most of the general population does not meet physical activity guidelines, people with hip or knee osteoarthritis are still approximately 25% less active than those without OA (69). Sedentary behaviour can lead to a loss of muscle mass and a reduction in muscle cross-sectional area (70) which in turn decreases the muscle's ability to generate force (71, 72). According to strength inhibition theory, peak muscle force can be inhibited by pain (73). The presence of muscle pain may reduce muscle strength due to altered reflex pathways, where nociceptive input from group III and IV afferents facilitates inhibitory effects on agonist moto neurons, thereby decreasing maximal voluntary force production (74). Altered reflex pathways can be influenced not only by pain but also by joint swelling, inflammation, joint laxity, and damage to sensory receptors (75). There is evidence that up to 50% of individuals with KOA are unable to fully activate their quadriceps muscle (76). Therefore, to improve peak muscle force in KOA patients, it is important not only to engage in strength training, but also to include interventions aimed at enhancing muscle activation, such as cryotherapy, transcutaneous electrical nerve stimulation, or neuromuscular electrical stimulation (75).
Our meta-analysis found that deficits in isometric strength between individuals with and without KOA were similar across different KL grades of KOA severity. The strength deficits did not appear to increase with higher KL grades. These findings suggest that reduced muscle strength may not be associated with structural joint changes. It is possible that the cause of reduced muscle strength lies elsewhere—perhaps in the presence of symptoms rather than structural joint changes. Previous studies have shown that only 47% of individuals with KL grade 2–4 report experiencing pain, and up to 23.5% of those with KL grade 4 report no pain (77, 78). On the other hand, our findings may indicate that muscle strength does not decline proportionally with the progression of structural joint changes.
We also found that deficits in isometric knee extensor strength in individuals with KOA compared to healthy controls were independent of the knee flexion angle used during testing, and that deficits in concentric strength were independent of movement velocity. These findings may guide future research on muscle strength assessment. Given that similar strength deficits are observed regardless of joint position and movement speed, it would be reasonable to conduct strength assessments under conditions that minimize symptoms and maximize patient comfort. We would expect greater discomfort in the 90 ° of knee flexion position, as this angle generates higher compressive forces on the tibiofemoral joint (20). It is important to keep in mind that strength measurements may be invalid if performed in the presence of pain (73), which further highlights the importance of adapting testing conditions to the patient's symptoms.
5 Strengths and limitations
According to the JBI scale, most of the studies included in the meta-analysis were of high methodological quality, with the majority achieving a score of seven. However, the effect sizes were highly heterogeneous in some of the meta-analyses. In the meta-analyses comparing RoM between individuals with and without KOA, we included both active and passive RoM measurements, which may have influenced the results. Additionally, the individuals with and without KOA were not matched using the same criteria across studies. If differences in age or other demographic characteristics existed between groups, they may have affected the magnitude of the observed differences. Some conclusions in the analyses are based on results observed in individuals with KOA across different KL grades. It is possible that different findings would have emerged if the analyses had been conducted separately for each KL grade. Some studies did not report whether pain was present during measurements, which could compromise the validity of the results. Only English-language publications were included, which may have led to language bias. An important limitation of this review is that balance-related outcomes were not included. Balance impairments and falls are highly prevalent in individuals with KOA and represent a major clinical concern. As balance measures were outside the scope of the present review, the relationship between knee RoM, muscle strength, and balance function could not be addressed. Another limitation is that potential regional and cultural differences in functional demands were not considered. In some regions, particularly in parts of Asia, daily activities such as dining or sitting on the floor require deep knee flexion, making knee RoM particularly important. As most included studies originated from Western countries, the functional relevance of knee flexion deficits may differ across populations, which should be considered when interpreting the findings.
6 Conclusions
This review confirms that individuals with symptomatic KOA have reduced knee RoM and muscle strength across all contraction types. Flexion deficits worsen with disease severity, whereas strength deficits remain stable across KL grades, suggesting links to factors such as pain or muscle inhibition rather than structural changes. Similar deficits across knee joint angles and angular velocities during testing indicate that assessment protocols can be flexibly adapted to patient comfort in clinical practice. Conclusions were drawn within the limitations of the available observational data, and no causal inferences were made. Future studies assessing knee strength and RoM should more precisely report whether participants have symptomatic KOA, whether control subjects are truly free of KOA (either symptomatically or radiographically), whether pain was present during measurements, and which leg was tested. To enable more detailed subgroup analyses, future research should also report measurement outcomes stratified by individual KL grades.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. Existing datasets from previously published studies were analyzed as part of the systematic review and meta-analysis. All data were extracted from peer-reviewed articles available in the public domain.
Author contributions
MO: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. NŠ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This research was financially supported by the Slovenian Research and Innovation Agency (research program KINSPO-Kinesiology for the effectiveness and prevention of musculoskeletal injuries in sports (P5-0443) and grant number 58589 for junior researcher Manca Opara Zupančič).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was used in the creation of this manuscript. The author(s) used AI tools (ChatGPT, version 4o, OpenAI, San Francisco, CA) to assist with translation into English. The tool was not used to analyze the literature or draw insights from papers as part of the review process. After using this tool/service, the author(s) reviewed and edited the content as needed, and they take full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2026.1737973/full#supplementary-material
References
1.
Steinmetz JD Culbreth GT Haile LM Rafferty Q Lo J Fukutaki KG et al . Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the global burden of disease study 2021. Lancet Rheumatol. (2023) 5:e508–22. doi: 10.1016/S2665-9913(23)00163-7
2.
Cui A Li H Wang D Zhong J Chen Y Lu H . Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. E Clinic Med. (2020) 29–30:100587. doi: 10.1016/j.eclinm.2020.100587
3.
Zhang W Doherty M Peat G Bierma-Zeinstra MA Arden NK Bresnihan B et al . EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. (2010) 69:483–9. doi: 10.1136/ard.2009.113100
4.
Kohn MD Sassoon AA Fernando ND . Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. (2016) 474:1886–93. doi: 10.1007/s11999-016-4732-4
5.
Litwic A Edwards MH Dennison EM Cooper C . Epidemiology and burden of osteoarthritis. Br Med Bull. (2013) 105:185–99. doi: 10.1093/bmb/lds038
6.
Wise BL Niu J Zhang Y Wang N Jordan JM Choy E et al . Psychological factors and their relation to osteoarthritis pain. Osteoarthritis Cartilage. (2010) 18:883–7. doi: 10.1016/j.joca.2009.11.016
7.
Merry del Val B Shukla SR Oduoye MO Nsengiyumva M Tesfaye T Glinkowski WM . Prevalence of mental health disorders in knee osteoarthritis patients: a systematic review and meta-analysis. Annals of Medicine Surgery. (2024) 86:4705–13. doi: 10.1097/MS9.0000000000002258
8.
Wallis JA Taylor NF Bunzli S Shields N . Experience of living with knee osteoarthritis: a systematic review of qualitative studies. BMJ Open. (2019) 9:e030060. doi: 10.1136/bmjopen-2019-030060
9.
Epskamp S Dibley H Ray E Bond N White J Wilkinson A et al . Range of motion as an outcome measure for knee osteoarthritis interventions in clinical trials: an integrated review. Phys Ther Rev. (2020) 25:462–81. doi: 10.1080/10833196.2020.1867393
10.
Rowe PJ Myles CM Walker C Nutton R . Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: how much knee motion is sufficient for normal daily life?Gait Posture. (2000) 12:143–55. doi: 10.1016/S0966-6362(00)00060-6
11.
Liikavainio T Lyytinen T Tyrväinen E Sipilä S Arokoski JP . Physical function and properties of quadriceps femoris muscle in men with knee osteoarthritis. Arch Phys Med Rehabil. (2008) 89:2185–94. doi: 10.1016/j.apmr.2008.04.012
12.
Hughes MA Myers BS Schenkman ML . The role of strength in rising from a chair in the functionally impaired elderly. J Biomech. (1996) 29:1509–13. doi: 10.1016/S0021-9290(96)80001-7
13.
Casaña J Calatayud J Silvestre A Sánchez-Frutos J Andersen LL Jakobsen MD et al . Knee extensor muscle strength is more important than postural balance for stair-climbing ability in elderly patients with severe knee osteoarthritis. Int J Environ Res Public Health. (2021) 18:3637. doi: 10.3390/ijerph18073637
14.
Deasy M Leahy E Semciw AI . Hip strength deficits in people with symptomatic knee osteoarthritis: a systematic review with meta-analysis. J Orthop Sports Phys Ther. (2016) 46:629–39. doi: 10.2519/jospt.2016.6618
15.
Alnahdi AH Zeni JA Snyder-Mackler L . Muscle impairments in patients with knee osteoarthritis. Sports Health. (2012) 4:284–92. doi: 10.1177/1941738112445726
16.
Baker M Stanish W Rutherford D . Walking challenges in moderate knee osteoarthritis: a biomechanical and neuromuscular response to medial walkway surface translations. Hum Mov Sci. (2019) 68:102542. doi: 10.1016/j.humov.2019.102542
17.
Yagi M Taniguchi M Tateuchi H Hirono T Yamagata M Umehara J et al . Relationship between individual forces of each quadriceps head during low-load knee extension and cartilage thickness and knee pain in women with knee osteoarthritis. Clin Biomech. (2022) 91:105546. doi: 10.1016/j.clinbiomech.2021.105546
18.
Rodriguez-Lopez C Beckwée D Luyten FP Van Assche D Van Roie E . Reduced knee extensor torque production at low to moderate velocities in postmenopausal women with knee osteoarthritis. Scand J Med Sci Sports. (2021) 31:2144–55. doi: 10.1111/sms.14035
19.
Liu C Wan Q Zhou W Feng X Shang S . Factors associated with balance function in patients with knee osteoarthritis: an integrative review. Int J Nurs Sci. (2017) 4:402–9. doi: 10.1016/j.ijnss.2017.09.002
20.
Wilk KE Escamilla RF Fleisig GS Barrentine SW Andrews JR Boyd ML et al . Comparison of tibiofemoral joint forces and electromyographic activit during open and closed kinetic chain exercises. Am J Sports Med. (1996) 24:518–27. doi: 10.1177/036354659602400418
21.
Methley AM Campbell S Chew-Graham C McNally R Cheraghi-Sohi S PICO . PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. (2014) 14:579. doi: 10.1186/s12913-014-0579-0
22.
Moola S Munn Z Tufanaru C Aromataris E Sears K Sfetcu R et al . “Chapter 7: systematic reviews of etiology and risk.,” In:AromatarisEMunnZ, editors. JBI Manual for Evidence Synthesis. JBI (2020) https://synthesismanual.jbi.global [Accessed June 1, 2025] doi: 10.46658/JBIRM-17-06
23.
Higgins J Thomas J Chandler J Cumpston M Li T Page M . Cochrane Handbook for Systematic Reviews of Interventions. (2008) https://search.library.wisc.edu/catalog/9910060197402121 [Accessed September 18, 2025]
24.
Kuwahara W Nakanishi K Kurumadani H Shimada N Asaeda M Deie M et al . Total knee arthroplasty for patients with medial knee osteoarthritis improves trunk movement during gait. J Back Musculoskelet Rehabil. (2020) 33:727–34. doi: 10.3233/BMR-181383
25.
Skvortsov D Kaurkin S Prizov A Altukhova A Goncharov E Nikitin A . Gait analysis and knee joint kinematics before a and 6 month after of corrective valgus osteotomy at patients with medial knee arthritis. Int Orthop. (2022) 46:1573–82. doi: 10.1007/s00264-022-05370-9
26.
Nagano Y Naito K Saho Y Torii S Ogata T Nakazawa K et al . Association between in vivo knee kinematics during gait and the severity of knee osteoarthritis. Knee. (2012) 19:628–32. doi: 10.1016/j.knee.2011.11.002
27.
Manoy P Yuktanandana P Tanavalee A Tanpowpong T Ittipanichpong T Honsawek S . Telomere shortening is associated with poor physical performance in knee osteoarthritis. Biomed Rep. (2020) 13:27. doi: 10.3892/br.2020.1334
28.
Gu C Mao Y Dong H Cui Y Fu M . Nomogram in knee instability: 3D gait analysis of knee osteoarthritis patients. Indian J Orthop. (2022) 56:1554–64. doi: 10.1007/s43465-022-00644-1
29.
Hiyama Y Takahashi R Tanaka T Misaki S . Quantitative ultrasound of the heel in women with knee osteoarthritis. J Clin Densitom. (2021) 24:557–62. doi: 10.1016/j.jocd.2021.01.001
30.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
31.
Altman R Asch E Bloch D Bole G Borenstein D Brandt K et al . Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. (1986) 29:1039–49. doi: 10.1002/art.1780290816
32.
Yang K Ding Y Chu L Cheng C Yu X Xu H et al . Altered activation patterns of the sensory-motor cortex in patients with knee osteoarthritis during knee isokinetic movement at different speeds. Front Bioeng Biotechnol. (2024) 12: 1444731. doi: 10.3389/fbioe.2024.1444731
33.
Ucurum SG Kirmizi M Umay Altas E Ozer Kaya D . Postural stability and its relation to knee flexor/extensor strength ratio in women with mild to moderate unilateral knee osteoarthritis: a case-control study. Somatosens Mot Res. (2024) 41:69–76. doi: 10.1080/08990220.2023.2175809
34.
Lohnes J Urquhart N Wong I Stanish W Rutherford D . Do individuals with knee osteoarthritis walk with distinct knee biomechanics and muscle activation characteristics? An investigation of knee osteoarthritis, hip osteoarthritis, and asymptomatic groups. Gait Posture. (2023) 99:14–9. doi: 10.1016/j.gaitpost.2022.10.011
35.
Diraçoglu D Baskent A Yagci I Ozçakar L Aydin R . Isokinetic strength measurements in early knee osteoarthritis. Acta Reumatol Port. (2009) 34:72–7.
36.
Hortobágyi T Garry J Holbert D Devita P . Aberrations in the control of quadriceps muscle force in patients with knee osteoarthritis. Arthritis Care Res. (2004) 51:562–9. doi: 10.1002/art.20545
37.
Vårbakken K Lorås H Nilsson KG Engdal M Stensdotter AK . Relative difference in muscle strength between patients with knee osteoarthritis and healthy controls when tested bilaterally and joint-inclusive: an exploratory cross-sectional study. BMC Musculoskelet Disord. (2019) 20:593. doi: 10.1186/s12891-019-2957-6
38.
Rice DA McNair PJ Lewis GN . Mechanisms of quadriceps muscle weakness in knee joint osteoarthritis: the effects of prolonged vibration on torque and muscle activation in osteoarthritic and healthy control subjects. Arthritis Res Ther. (2011) 13:R151. doi: 10.1186/ar3467
39.
Aily JB de Noronha M Ferrari RJ Mattiello SM . Differences in fatty infiltration in thigh muscles and physical function between people with and without knee osteoarthritis and similar body mass index: a cross-sectional study. BMC Musculoskelet Disord. (2025) 26:109. doi: 10.1186/s12891-025-08347-y
40.
Aily JB de Noronha M de Almeida AC Pedroso MG Maciel JG Mattiello-Sverzut AC et al . Evaluation of vastus lateralis architecture and strength of knee extensors in middle-aged and older individuals with knee osteoarthritis. Clin Rheumatol. (2019) 38:2603–11. doi: 10.1007/s10067-019-04539-9
41.
Baert IAC Mahmoudian A Nieuwenhuys A Jonkers I Staes F Luyten FP et al . Proprioceptive accuracy in women with early and established knee osteoarthritis and its relation to functional ability, postural control, and muscle strength. Clin Rheumatol. (2013) 32:1365–74. doi: 10.1007/s10067-013-2285-4
42.
Teoli A Martel-Pelletier J Abram F Pelletier J-P Robbins SM . Vastus medialis intramuscular fat is associated with reduced quadriceps strength, but not knee osteoarthritis severity. Clin Biomech. (2022) 96:105669. doi: 10.1016/j.clinbiomech.2022.105669
43.
Childs JD Sparto PJ Fitzgerald GK Bizzini M Irrgang JJ . Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech. (2004) 19:44–9. doi: 10.1016/j.clinbiomech.2003.08.007
44.
Yagi M Taniguchi M Tateuchi H Yamagata M Hirono T Asayama A et al . Properties of the iliotibial band and their relationships with gait parameters among patients with knee osteoarthritis. J Orthop Res. (2023) 41:1177–85. doi: 10.1002/jor.25466
45.
Kumar D Manal KT Rudolph KS . Knee joint loading during gait in healthy controls and individuals with knee osteoarthritis. Osteoarthritis Cartilage. (2013) 21:298–305. doi: 10.1016/j.joca.2012.11.008
46.
Baert IAC Jonkers I Staes F Luyten FP Truijen S Verschueren SMP . Gait characteristics and lower limb muscle strength in women with early and established knee osteoarthritis. Clin Biomech. (2013) 28:40–7. doi: 10.1016/j.clinbiomech.2012.10.007
47.
Rutherford DJ Hubley-Kozey CL Stanish WD . Changes in knee joint muscle activation patterns during walking associated with increased structural severity in knee osteoarthritis. J Electrom Kinesiol. (2013) 23:704–11. doi: 10.1016/j.jelekin.2013.01.003
48.
Gapeyeva H Buht N Peterson K Ereline J Haviko T Pääsuke M . Quadriceps femoris muscle voluntary isometric force production and relaxation characteristics before and 6 months after unilateral total knee arthroplasty in women. Knee Surg Sports Traumatol Arthrosc. (2007) 15:202–11. doi: 10.1007/s00167-006-0166-y
49.
Unver Kocak F Unver B Karatosun V Bakirhan S . Associations between radiographic changes and function, pain, range of motion, muscle strength and knee function score in patients with osteoarthritis of the knee. J Phys Ther Sci. (2009) 21:93–7. doi: 10.1589/jpts.21.93
50.
Emrani A Bagheri H Hadian MR Jabal-Ameli M Olyaei GR Talebian S . Isokinetic strength and functional status in knee osteoarthritis. J Phys Ther Sci. (2006) 18:107–14. doi: 10.1589/jpts.18.107
51.
Ling SM Conwit RA Talbot L Shermack M Wood JE Dredge EM et al . Electromyographic patterns suggest changes in motor unit physiology associated with early osteoarthritis of the knee. Osteoarthritis Cartilage. (2007) 15:1134–40. doi: 10.1016/j.joca.2007.03.024
52.
Sanchez-Ramirez DC Malfait B Baert I van der Leeden M van Dieën J Lems WF et al . Biomechanical and neuromuscular adaptations during the landing phase of a stepping-down task in patients with early or established knee osteoarthritis. Knee. (2016) 23:367–75. doi: 10.1016/j.knee.2016.02.002
53.
Tan J Balci N Sepici V Gener FA . Isokinetic and isometric strength in osteoarthrosis of the knee. A comparative study with healthy women. Am J Phys Med Rehabil. (1995) 74:364–9. doi: 10.1097/00002060-199509000-00008
54.
Noehren B Kosmac K Walton RG Murach KA Lyles MF Loeser RF et al . Alterations in quadriceps muscle cellular and molecular properties in adults with moderate knee osteoarthritis. Osteoarthritis Cartilage. (2018) 26:1359–68. doi: 10.1016/j.joca.2018.05.011
55.
Serrão PRMS Vasilceac FA Gramani-Say K Lessi GC Oliveira AB Reiff RBM et al . Men with early degrees of knee osteoarthritis present functional and morphological impairments of the quadriceps femoris muscle. Am J Phys Med Rehabil. (2015) 94:70–81. doi: 10.1097/PHM.0000000000000143
56.
Zhang X Pan X Deng L Fu W . Relationship between knee muscle strength and fat/muscle mass in elderly women with knee osteoarthritis based on dual-energy X-Ray absorptiometry. Int J Environ Res Public Health. (2020) 17:573. doi: 10.3390/ijerph17020573
57.
Bennett D Hanratty B Thompson N Beverland DE . The influence of pain on knee motion in patients with osteoarthritis undergoing total knee arthroplasty. Orthopedics. (2009) 32:252–6. doi: 10.3928/01477447-20090401-19
58.
Gür O Başar S Esen E Ataoglu B Turanli S . The relationship of kinesiophobia and pain catastrophizing with pain, range of motion, muscle strength and function in osteoarthritis. Int J Disabil Sports Health Sci. (2021) 4:130–9. doi: 10.33438/ijdshs.980343
59.
Ozdemir F Tukenmez O Kokino S Turan FN . How do marginal osteophytes, joint space narrowing and range of motion affect each other in patients with knee osteoarthritis. Rheumatol Int. (2006) 26:516–22. doi: 10.1007/s00296-005-0016-0
60.
Hilfiker R Jüni P Nüesch E Dieppe PA Reichenbach S . Association of radiographic osteoarthritis, pain on passive movement and knee range of motion: a cross-sectional study. Man Ther. (2015) 20:361–5. doi: 10.1016/j.math.2014.11.014
61.
Reid DA McNair PJ . Effects of an acute hamstring stretch in people with and without osteoarthritis of the knee. Physiotherapy. (2010) 96:14–21. doi: 10.1016/j.physio.2009.06.010
62.
Holm B Kristensen MT Bencke J Husted H Kehlet H Bandholm T . Loss of knee-extension strength is related to knee swelling after total knee arthroplasty. Arch Phys Med Rehabil. (2010) 91:1770–6. doi: 10.1016/j.apmr.2010.07.229
63.
Hill CL Gale DG Chaisson CE Skinner K Kazis L Gale ME et al . Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. (2001) 28:1330–7.
64.
Paschos NK Giotis D Abuhemoud K Georgoulis AD . Effectiveness of aspiration in knee joint effusion management: a prospective randomized controlled study. Knee Surg Sports Traumatol, Arthrosc. (2014) 22:226–32. doi: 10.1007/s00167-013-2379-1
65.
Park W Ramachandran J Weisman P Jung ES . Obesity effect on male active joint range of motion. Ergonomics. (2010) 53:102–8. doi: 10.1080/00140130903311617
66.
Shao W Hou H Han Q Cai K . Prevalence and risk factors of knee osteoarthritis: a cross-sectional survey in Nanjing, China. Front Public Health. (2024) 12:1441408. doi: 10.3389/fpubh.2024.1441408
67.
Watanabe M Campbell TM Reilly K Uhthoff HK Laneuville O Trudel G . Bone replaces unloaded articular cartilage during knee immobilization. A longitudinal study in the rat. Bone. (2021) 142:115694. doi: 10.1016/j.bone.2020.115694
68.
Campbell TM Reilly K Laneuville O Uhthoff H Trudel G . Bone replaces articular cartilage in the rat knee joint after prolonged immobilization. Bone. (2018) 106:42–51. doi: 10.1016/j.bone.2017.09.018
69.
Wallis JA Webster KE Levinger P Taylor NF . What proportion of people with hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta-analysis. Osteoarthritis Cartilage. (2013) 21:1648–59. doi: 10.1016/j.joca.2013.08.003
70.
Mo Y Zhou Y Chan H Evans C Maddocks M . The association between sedentary behaviour and sarcopenia in older adults: a systematic review and meta-analysis. BMC Geriatr. (2023) 23:877. doi: 10.1186/s12877-023-04489-7
71.
Jones EJ Bishop PA Woods AK Green JM . Cross-sectional area and muscular strength. Sports Med. (2008) 38:987–94. doi: 10.2165/00007256-200838120-00003
72.
Petterson SC Barrance P Buchanan T Binder-Macleod S Snyder-Mackler L . Mechanisms underlying quadriceps weakness in knee osteoarthritis. Med Sci Sports Exerc. (2008) 40:422–7. doi: 10.1249/MSS.0b013e31815ef285
73.
Merkle SL Sluka KA Frey-Law LA . The interaction between pain and movement. J Hand Ther. (2020) 33:60–6. doi: 10.1016/j.jht.2018.05.001
74.
Graven-Nielsen T Lund H Arendt-Nielsen L Danneskiold-Samsøe B Bliddal H . Inhibition of maximal voluntary contraction force by experimental muscle pain: a centrally mediated mechanism. Muscle Nerve. (2002) 26:708–12. doi: 10.1002/mus.10225
75.
Rice DA McNair PJ . Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum. (2010) 40:250–66. doi: 10.1016/j.semarthrit.2009.10.001
76.
Lewek MD Rudolph KS Snyder-Mackler L . Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthopaedic Res. (2004) 22:110–5. doi: 10.1016/S0736-0266(03)00154-2
77.
Son KM Hong JI Kim D-H Jang D-G Crema MD Kim HA . Absence of pain in subjects with advanced radiographic knee osteoarthritis. BMC Musculoskelet Disord. (2020) 21:640. doi: 10.1186/s12891-020-03647-x
78.
Hannan MT Felson DT Pincus T . Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. (2000) 27:1513–7.
79.
Lyytinen T Liikavainio T Bragge T Hakkarainen M Karjalainen PA Arokoski JP . Postural control and thigh muscle activity in men with knee osteoarthritis. J Electromyogr Kinesiol. (2010) 20:1066–74. doi: 10.1016/j.jelekin.2010.05.005
Summary
Keywords
arthritis, osteoarthritis, knee, rehabilitation, risk factors
Citation
Opara Zupančič M and Šarabon N (2026) Disease severity affects knee range of motion but not strength deficits in knee osteoarthritis: a systematic review and meta-analysis. Front. Med. 13:1737973. doi: 10.3389/fmed.2026.1737973
Received
02 November 2025
Revised
23 January 2026
Accepted
28 January 2026
Published
13 February 2026
Volume
13 - 2026
Edited by
Xianhua Cai, General Hospital of Central Theater Command, China
Reviewed by
Feza Korkusuz, Hacettepe University, Türkiye
Erik Cattrysse, Vrije University Brussels, Belgium
Updates
Copyright
© 2026 Opara Zupančič and Šarabon.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nejc Šarabon, nejc.sarabon@fvz.upr.si
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.