Abstract
Objective:
This study looks into the relationship between serum uric acid levels and the ratio of serum uric acid to high-density lipoprotein cholesterol (UHR) with the likelihood of breast cancer in women. It also evaluates its value as a biomarker related to metabolism for breast cancer.
Methods:
This retrospective study included 500 women (279 breast cancer patients and 221 with benign breast nodules). We calculated the uric acid to high-density lipoprotein cholesterol ratio (UHR) using preoperative lab data. Multivariable logistic regression analysis analyzed its relationship with the likelihood of getting breast cancer. Meanwhile, ROC curves and restricted cubic spline analyses assessed its diagnostic effectiveness and dose–response relationship.
Results:
In the breast cancer group, UHR was significantly higher than that of the benign group (0.10 ± 0.04 vs. 0.09 ± 0.03, p < 0.001). Multivariate analysis showed that for every 100-unit increase in UHR, the odds of developing breast cancer increased by 9 to 13%, and subjects with UHR ≥ 0.091 had a 63% increased odds of developing breast cancer (OR = 1.63, 95% CI: 1.03–2.59). However, UHR showed moderate diagnostic effectiveness (AUC = 0.62), with a sensitivity rate of 80%. Additionally, analysis using restricted cubic splines confirmed a positive linear correlation in a dose–response manner, indicating there was no threshold effect.
Conclusion:
Research has found that UHR (ultra-high risk) is linked to a higher chance of developing breast cancer. This suggests that UHR could help predict breast cancer risk. In the future, we need to confirm our findings with a bigger group of people and keep digging into the reasons behind this.
Introduction
Globally, breast cancer still tops the list of malignancies in women, and its onset stems from a tangled interplay of inherited odds, shifting hormones and the thousand tiny choices that make up daily life (1, 2). In recent years, the association between metabolic disorders and tumor development has become a hot topic in medical research. Among them, the roles of lipid metabolism abnormalities and uric acid metabolism imbalance in the pathological process of breast cancer have gradually received attention (3, 4), however, research on their combined effects remains scarce.
Lipid metabolic reprogramming is one of the core characteristics of tumor cells. High-density lipoprotein cholesterol (HDL-C), often referred to as the “protective cholesterol,” is closely associated with the odds of various cancers when its levels are abnormal (5, 6). A study indicated that regardless of population heterogeneity or how obesity and metabolic status are defined, patients with metabolically healthy obesity (MHO) have a lower cancer odds than those with metabolically unhealthy obesity (MUO) (7). In patients with breast cancer, the level of high-density lipoprotein cholesterol (HDL-C) decreases significantly after chemotherapy. This change is associated with metabolism-related factors such as the patient’s age and body mass index (BMI), which further reflects the close connection between HDL-C metabolism and the disease course of breast cancer (8, 9).
Uric acid is the end product of purine metabolism, and the association between its metabolic abnormalities and chronic inflammation, oxidative stress, as well as the regulation of cell proliferation has been widely confirmed (10, 11). Clinical studies have demonstrated that hyperuricemia may reduce patients’ survival rates by promoting the proliferation and metastasis of breast cancer cells. Meanwhile, uric acid levels exhibit a unique J-shaped association with breast cancer odds, suggesting the dual regulatory potential of disturbed uric acid homeostasis in the pathogenesis of breast cancer (12). Notably, uric acid metabolism and lipid metabolism are not independent processes. Previous studies have observed a significant non-linear association between lipid ratios and hyperuricemia in cancer patients, confirming the close crosstalk between the two in the metabolic network.
Although the individual effects of uric acid and HDL-C have been initially explored, the association between the uric acid to high-density lipoprotein ratio (UA/HDL)—a key indicator integrating their metabolic status—and breast cancer odds remains unclear. Most existing studies have focused on single indicators or other lipid ratios, with a lack of systematic analysis on the UA/HDL ratio. Whether this ratio can more sensitively reflect the association between metabolic imbalance and breast cancer, and whether there are differences in gender or disease stages, remains to be further investigated. Therefore, we hypothesize that the UHR can serve as an integrated biomarker reflecting metabolic disorders and breast cancer odds. The purpose of this study is to explore the correlation between UHR and breast cancer odds, so as to fill the gap in existing research and provide new theoretical basis and practical directions for the early detection, odds stratification and metabolic intervention of breast cancer.
Materials and methods
Study population and data collection
We retrospectively collected the clinical and pathological data of 500 female patients who visited the Affiliated Hospital of Jiangnan University from October 2021 to October 2025, including 279 patients with breast malignant tumors and 221 patients with benign breast nodules (Figure 1). The inclusion criteria were as follows: (1) All cancer patients were pathologically diagnosed with primary breast cancer; (2) Complete pretreatment laboratory data were available; (3) Complete clinicopathological and laboratory data were available. Exclusion criteria: (1) Male patients; (2) Incomplete information and duplicate data; (3) Patients with a history of malignant tumors or complicated with other primary tumors; (4) Patients who have used uric acid-lowering and lipid-lowering drugs. This study was granted ethical approval by the Medical Ethics Committee of the Affiliated Hospital of Jiangnan University (Approval No. JNMS01250043). The data of this retrospective study were anonymized, so the requirement for informed consent was waived.
Figure 1

Flow chart of study subjects.
Data collection
We extracted demographic, clinical and pathological information for 500 individuals from the Affiliated Hospital of Jiangnan University’s database. All patients underwent serum biochemical examinations within one week before surgery, with complete data. The hospital used the Sysmex hematology analyzer for blood analysis and the Roche Cobas C702 biochemical analyzer to detect liver function and collect laboratory data including glucose, albumin, uric acid, cholesterol, triglycerides, high-density lipoprotein and low-density lipoprotein. Patients’ age, presence or absence of hypertension, diabetes mellitus, coronary heart disease, and smoking and drinking histories were collected from their medical records and pathological reports.
The calculation formula for laboratory data is as follows:
Uric Acid to High-Density Lipoprotein Cholesterol Ratio (UHR) = Serum Uric Acid (mg/dL) / High-Density Lipoprotein Cholesterol (mg/dL) * 100 (13).
Statistical analysis
SPSS 26.0 statistical software was used for data processing and analysis in the statistical analysis. In this study, normally distributed continuous data are reported as Mean ± SD, and comparisons between two groups are made using the independent samples t-test. Skewed continuous data are reported as M (Q₁, Q₃) and compared using the Mann–Whitney U test. Categorical data are reported as n (%) and analyzed using the chi-square test or Fisher’s exact test; a p value of less than 0.05 is considered statistically significant for comparisons between groups. From the ROC curve, we derived the Youden index and the area under the curve (AUC) to quantify UHR’s diagnostic performance. Logistic regression was used to analyze the association between the UHR index and breast cancer, and three models were established for evaluation in the data analysis. Model 1 employed univariate logistic regression, whereas Model 2 incorporated multivariate logistic regression, accounting for covariates such as age, blood glucose, albumin, LDL, triglycerides, and total cholesterol. Model 3 extended Model 2 by further adjusting for hypertension, diabetes and coronary heart disease in a multivariate logistic framework. Statistical significance was set at a two-tailed p < 0.05.
Results
Baseline characteristics
Table 1 presents the baseline characteristics of 500 patients in the Affiliated Hospital of Jiangnan University, where the demographic and clinicopathological characteristics of the patients as well as their preoperative laboratory test results are summarized. Figure 2 shows the distribution of the UHR index between the breast nodules and breast cancer groups.
Table 1
| Variables | Total (n = 500) | Non-breast cancer (n = 221) | Breast cancer (n = 279) | P |
|---|---|---|---|---|
| Age, M (Q₁, Q₃) | 48.00 (36.00, 57.00) | 37.00 (29.00, 48.00) | 55.00 (46.50, 61.00) | <0.001 |
| Glucose mmol/L, M (Q₁, Q₃) | 4.94 (4.57, 5.37) | 4.86 (4.57, 5.28) | 5.00 (4.60, 5.46) | 0.028 |
| UA mg/dl, M (Q₁, Q₃) | 4.72 (3.97, 5.58) | 4.53 (3.85, 5.42) | 4.79 (4.00, 5.68) | 0.060 |
| HDL-c mg/dl, M (Q₁, Q₃) | 52.20 (44.47, 59.55) | 54.91 (46.40, 61.10) | 49.50 (42.54, 57.04) | <0.001 |
| UHR, M (Q₁, Q₃) | 9.09 (7.20, 11.33) | 8.44 (6.78, 10.48) | 9.91 (7.53, 12.17) | <0.001 |
| Albumin g/dl, M (Q₁, Q₃) | 4.12 (3.91, 4.45) | 4.18 (3.94, 4.55) | 4.08 (3.88, 4.34) | 0.001 |
| Tg mg/dl, M (Q₁, Q₃) | 102.30 (69.97, 145.48) | 82.37 (60.23, 116.03) | 120.46 (85.91, 165.63) | <0.001 |
| Tc mg/dl, M (Q₁, Q₃) | 184.82 (164.23, 211.50) | 183.66 (160.85, 206.47) | 187.53 (166.45, 214.40) | 0.172 |
| LDL-c mg/dl, M (Q₁, Q₃) | 115.80 (99.76, 135.33) | 114.06 (98.21, 130.69) | 117.15 (100.92, 139.97) | 0.086 |
| Hypertension, n (%) | 0.001 | |||
| No | 431 (86.20) | 203 (91.86) | 228 (81.72) | |
| Yes | 69 (13.80) | 18 (8.14) | 51 (18.28) | |
| Diabetes, n (%) | 0.129 | |||
| No | 476 (95.20) | 214 (96.83) | 262 (93.91) | |
| Yes | 24 (4.80) | 7 (3.17) | 17 (6.09) |
Clinical characteristics of the 500 patients.
Figure 2
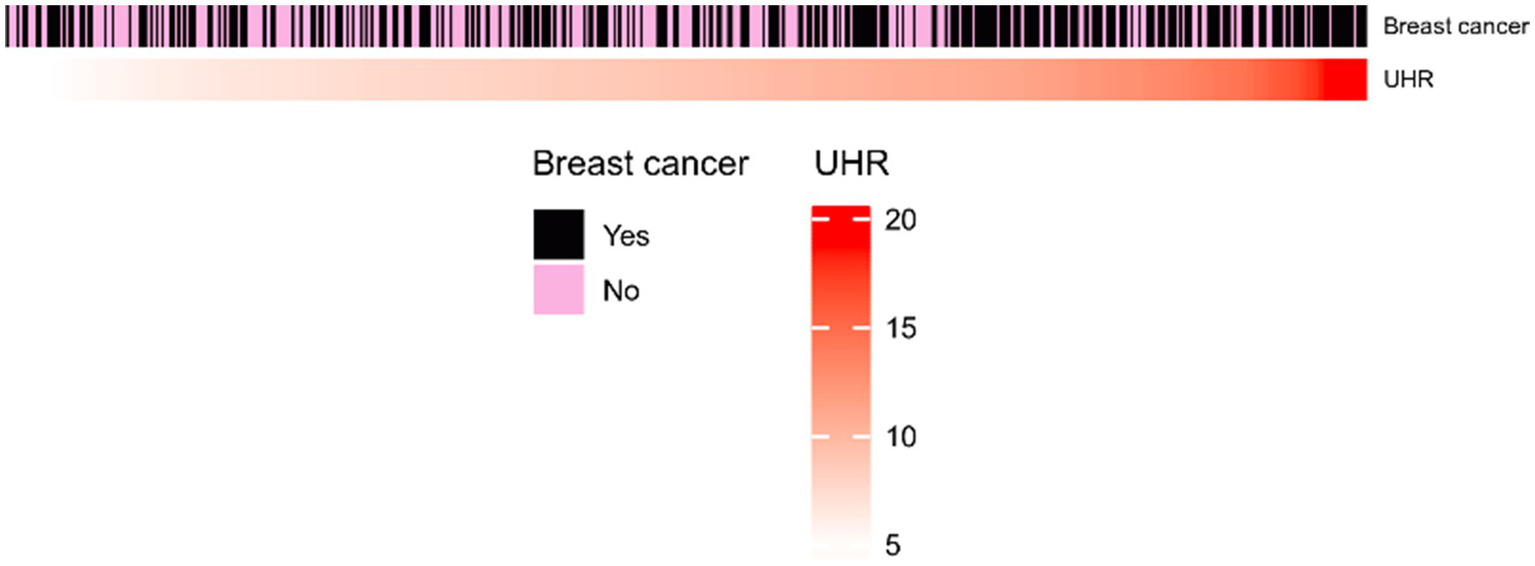
Heatmap of UHR index between breast nodules and breast cancer groups.
In this study, there were a total of 500 participants, of which 221 had breast nodules, making up 44.20%, and 279 had breast cancer, making up 55.80%. There were statistically significant differences between the two groups in age, glucose (mmol/L), HDL-c (mg/dl), UHR, albumin (g/dl), Tg (mg/dl), and hypertension (p < 0.05), and there were no statistically significant differences in UA (mg/dl), Tc (mg/dl), LDL-c (mg/dl), and diabetes (p > 0.05). The tumor group had a significantly older age, with an extremely significant difference, indicating that malignant tumor patients were mainly middle-aged and elderly, which was consistent with previous epidemiological studies (14). The levels of serum albumin and HDL-C were lower in the tumor group, indicating that hypoalbuminemia is an important marker of tumor-related malnutrition and chronic inflammation, and it is also consistent with the phenomenon of “tumor-related lipid metabolism reprogramming” (15). Figure 2 shows that most high UHR values belong to the breast cancer group.
Association between UHR and breast cancer odds
Table 2 illustrates the association between the UHR index, dichotomized at the median, and the odds of breast cancer. In the three models, for each 100-unit increase in UHR, the odds of breast cancer increased by 9–13%, indicating that UHR is an independent odds factor with a robust association. Compared with the Q1 group (UHR < 0.091), the odds of breast cancer in the Q2 group (UHR ≥ 0.091) was significantly increased, with an OR of 1.63 (95% CI: 1.03–2.59, p = 0.038) in Model 3. That is, after comprehensive adjustment for confounding factors, the odds of breast cancer in patients with UHR in the Q2 group was 63% higher than that in the Q1 group. This suggests that UHR is independently and positively correlated with breast cancer odds in a dose-dependent manner, whether as a continuous or categorical variable, and has potential predictive value.
Table 2
| UHR | Model 1 (OR95%CI) | P | Model 2 (OR95%CI) | P | Model 3 (OR95%CI) | P |
|---|---|---|---|---|---|---|
| Continues <0.091 ≥0.091 |
1.13(1.07–1.19) 1 (Reference) 2.09 (1.46–3.00) |
<0.001 —— <0.001 |
1.09(1.02–1.17) 1 (Reference) 1.60 (1.01–2.53) |
0.017 —— 0.046 |
1.10(1.02–1.18) 1 (Reference) 1.63 (1.03–2.59) |
0.011 —— 0.038 |
UHR-breast cancer correlation.
Given that simultaneously testing three models might inflate Type I errors, Hochberg’s correction method was used to adjust the p-values in multiple comparisons. After correcting at the α = 0.05 level, the p-values for the three models (0.001, 0.038, 0.046) were all considered significant, which means there were no false positives.
Diagnostic performance of UHR
The ROC curve analysis (Figure 3) shows UHR’s accuracy in diagnosing breast cancer is 0.62 (95% CI 0.57–0.66). This means its overall diagnostic performance is just okay. So, future research should aim to boost its diagnostic performance through big cohort studies.
Figure 3
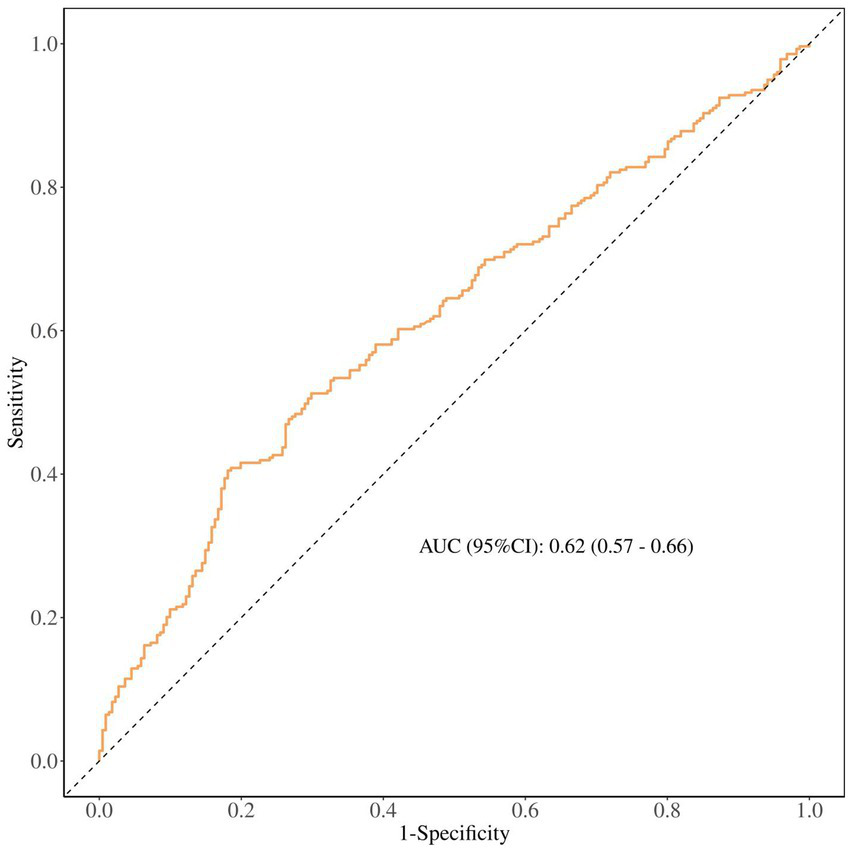
ROC curve analysis.
Table 3 shows how UHR performs in the diagnosis of breast cancer. Its sensitivity is 0.80 (0.75–0.85) and the negative predictive rate is 0.72 (0.65–0.79), showing it can fairly accurately identify people who actually have the disease, pointing to its potential value for initial screening in high-risk groups. However, with a specificity of just 41%, the false positive rate is pretty high, which means that this biomarker increases the number of misdiagnosed individuals. Furthermore, it still needs to improve in other areas.
Table 3
| AUC (95%CI) | Accuracy (95%CI) | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | Cut off |
|---|---|---|---|---|---|---|
| 0.62 (0.57–0.66) | 0.58 (0.54–0.63) | 0.80 (0.75–0.85) | 0.41 (0.35–0.47) | 0.52 (0.46–0.57) | 0.72 (0.65–0.79) | 0.107 |
Performance metrics of the confusion matrix.
PPV, positive predictive value; NPV, negative predictive value.
Analysis of non-linear relationship
Restricted cubic spline (RCS) curves were used to explore the non-linear relationship between UHR and breast cancer odds. Restricted cubic spline regression analysis (Figure 4) showed a positive correlation between UHR and breast cancer odds (P for overall < 0.001, P for nonlinear = 0.338), indicating that the odds increases with the continuous elevation of UHR without a significant threshold effect.
Figure 4
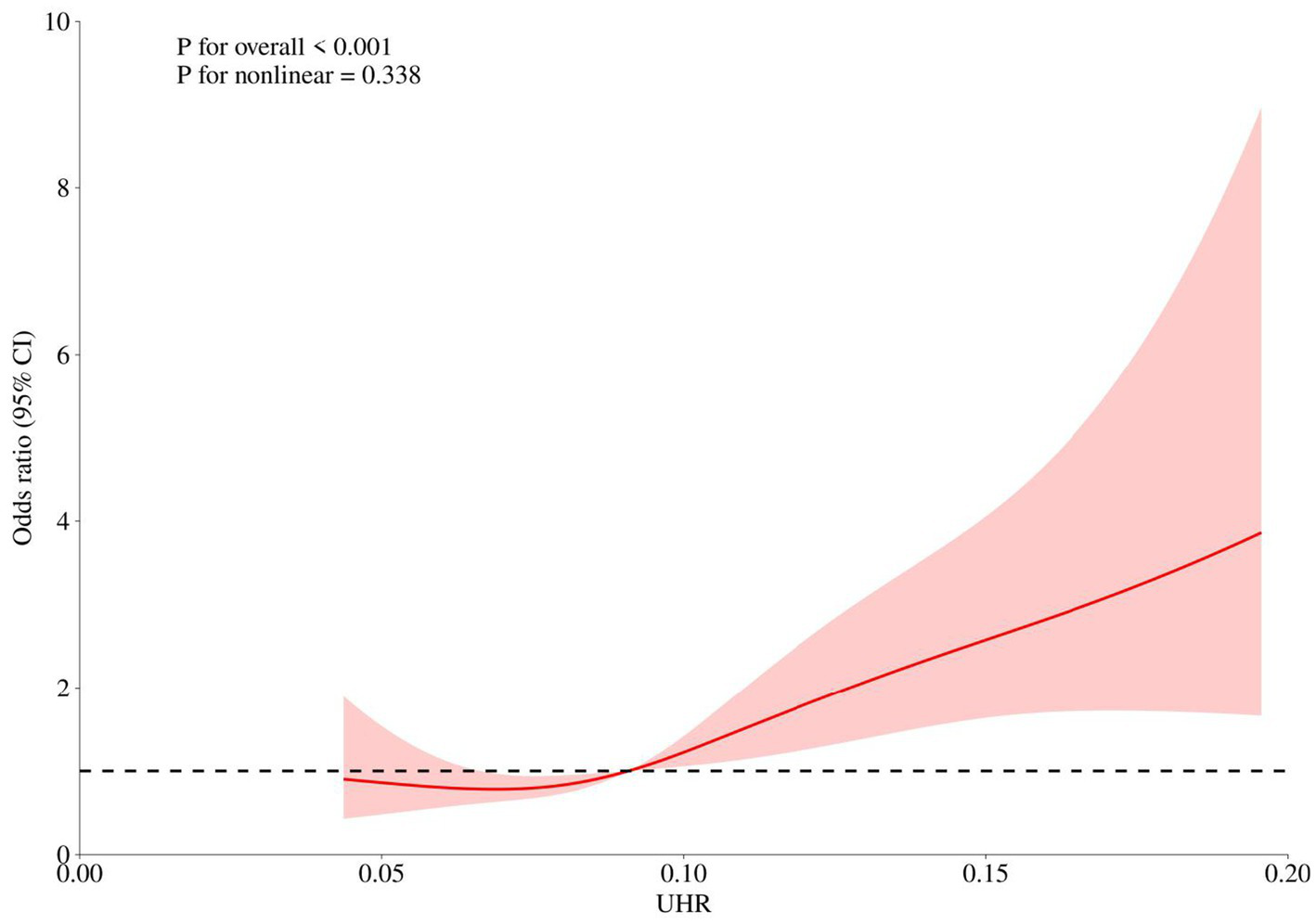
Restricted cubic spline analysis.
Discussion
Through a retrospective analysis, this study systematically explored the association between the serum uric acid to high-density lipoprotein ratio (UHR), a novel composite metabolic index, and the odds of breast cancer in Chinese women. Our main findings confirmed the initial hypothesis: the level of UHR was significantly elevated in breast cancer patients, and it was independently and positively correlated with breast cancer odds in a dose-dependent manner. This association remained robust after full adjustment for various confounding factors such as age, basic metabolic indicators and comorbidities. In addition, receiver operating characteristic (ROC) curve analysis indicated that UHR had moderate diagnostic efficacy, especially showing high sensitivity, which provided preliminary evidence for its use as an auxiliary screening tool.
The core value of this study lies in integrating two independent metabolic pathways, namely uric acid (UA) and high-density lipoprotein cholesterol (HDL-C), into a single indicator (15). On the other hand, HDL-C is known for its strong reverse cholesterol transport capacity, anti-inflammatory and antioxidant properties, and is regarded as “protective cholesterol.” A decrease in its level indicates an impairment of the body’s anti-tumor defense capacity (16, 17). Therefore, the elevation of UHR (i.e., relative increase in UA and/or relative decrease in HDL-C) accurately captures the dual imbalance state in the body where “tumor-promoting forces” are enhanced and “tumor-suppressive protection” is weakened. This may better reflect the overall metabolic disorder background related to tumor occurrence and development than any single indicator. Our findings are highly consistent with the “tumor metabolic reprogramming” theory, which states that cancer cells reshape their own and systemic metabolism to meet the energy and material needs of their rapid proliferation (18).
Our research results are both consistent with existing literature and represent an important advancement. Previous studies have separately reported that hyperuricemia is associated with an increased odds of various cancers (19, 20) as well as the association between decreased HDL-C levels after chemotherapy and poor prognosis in breast cancer patients (21, 22). However, these investigations primarily focused on linear or J-shaped associations of individual biomarkers. By introducing UHR, this study elevates the perspective from “isolated factors” to “network interaction.” We found a linear positive correlation between UHR and breast cancer odds, with no significant nonlinear relationship observed (P for nonlinear = 0.338), which provides a simpler basis for odds assessment in clinical practice. More importantly, in the multivariate model, UHR maintained independent predictive value, while the intergroup differences of certain single indicators (such as TC and LDL-C) were not significant. This suggests that as a composite indicator, UHR may have a stronger ability to identify signals among noise and can more sensitively reveal the metabolic oddss hidden in the population.
Conclusion
In summary, this study is the first to reveal that the uric acid to high-density lipoprotein ratio (UHR) is an independent positive factor associated with the incidence of breast cancer. This research offers a new perspective on understanding the metabolic etiology of breast cancer. Future prospective studies are needed to confirm its clinical usefulness, dig deeper into the biological mechanisms, and ultimately offer new insights into metabolic prevention, as well as accurate diagnosis and treatment strategies for breast cancer.
Limitations
Our limitations include the following: 1. The retrospective design cannot establish the causal sequence; 2. The single-center sample may have regional bias; 3. The lack of an external validation set means the model’s generalization ability remains to be confirmed.
Future research directions
In the future, we can conduct prospective, multi-center cohort studies to verify the dose–response relationship between UHR and breast cancer incidence; carry out stratified studies by gender, menopausal status, and molecular subtypes to clarify the cut-off points and weights of UHR in different subgroups. We can also combine metabolomics and proteomics to clarify the core pathways through which UHR affects tumor proliferation and metastasis. Meanwhile, by designing lifestyle or drug intervention trials, we can evaluate whether reducing UHR can reduce the incidence of breast cancer or improve prognosis.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the Affiliated Hospital of Jiangnan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because all data used in this study were rigorously anonymized and cannot be traced back to individual identities. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because all data used in this study were rigorously anonymized and cannot be traced back to individual identities.
Author contributions
QR: Software, Investigation, Writing – review & editing, Writing – original draft, Project administration, Formal analysis, Methodology. GL: Validation, Data curation, Writing – review & editing, Formal analysis, Methodology, Writing – original draft, Resources, Software, Visualization. EL: Conceptualization, Writing – review & editing, Methodology, Software, Writing – original draft, Formal analysis, Validation. JZ: Formal analysis, Methodology, Writing – original draft, Visualization, Project administration, Investigation, Writing – review & editing. LC: Validation, Writing – review & editing, Writing – original draft, Supervision, Software. XG: Supervision, Writing – original draft, Writing – review & editing, Visualization, Data curation, Project administration, Validation, Resources, Conceptualization.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by General Project of Jiangsu Health Commission (5922598M), Wu Jieping Medical Foundation (320.6750.2024–6-100), General Project of Wuxi Health Commission (M202408 and M202418). Yangtze River Delta Social Assistance Public Service Platform (2025C011).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Xiong X Zheng LW Ding Y Chen Y-F Cai Y-W Wang L-P et al . Breast cancer: pathogenesis and treatments. Signal Transduct Target Ther. (2025) 10:49. doi: 10.1038/s41392-024-02108-4
2.
Kashyap D Pal D Sharma R Garg VK Goel N Koundal D et al . Global increase in breast Cancer incidence: risk factors and preventive measures. Biomed Res Int. (2022) 2022:9605439. doi: 10.1155/2022/9605439
3.
Wan M Pan S Shan B Diao H Jin H Wang Z et al . Lipid metabolic reprograming: the unsung hero in breast cancer progression and tumor microenvironment. Mol Cancer. (2025) 24:61. doi: 10.1186/s12943-025-02258-1
4.
Songyeon A Jun Hyoung P Sandra LG Danthasinghe Waduge Badrajee P Tagari S Vasanta P et al . Metabolomic rewiring promotes endocrine therapy resistance in breast cancer. Cancer Res. (2023) 84:184. doi: 10.1158/0008-5472.CAN-23-0184
5.
Sun M Fritz J Häggström C Bjørge T Nagel G Manjer J et al . Metabolically (un)healthy obesity and risk of obesity-related cancers: a pooled study. J Natl Cancer Inst. (2023) 115:456–467. doi: 10.1093/jnci/djad008
6.
Sarah Tsz Yui Y Eman L Martin Chi Sang W Chi Tim H Ka Chun C Albert L et al . Metabolic dysfunction-associated profiles and subsequent site-specific odds of obesity-related cancers among Chinese patients with diabetes: a retrospective cohort study. BMJ Open. (2024) 14:2414. doi: 10.1136/bmjopen-2023-082414
7.
Xiaonan Z Ruilin P Hang X Tianhai L Shi Q Qiang W et al . The association between metabolic status and odds of cancer among patients with obesity: metabolically healthy obesity vs. metabolically unhealthy obesity. Front Nutr. (2022) 9:3660. doi: 10.3389/fnut.2022.783660
8.
Xu L Dong Q Long Y Tang X Zhang N Lu K et al . Dynamic changes of blood lipids in breast Cancer patients after (neo)adjuvant chemotherapy: a retrospective observational study. Int J Gen Med. (2020) 13:817–23. doi: 10.2147/IJGM.S273056
9.
Yang P He Y Yu X Liu B Wang X Li X et al . Impact of body mass index, weight gain, and metabolic disorders on survival and prognosis in patients with breast cancer who underwent chemotherapy. Chin Med J. (2022) 135:1555–1562. doi: 10.1097/CM9.0000000000001988
10.
Gasse P Riteau N Charron S Girre S Fick L Pétrilli V et al . Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. (2009) 179:903–13. doi: 10.1164/rccm.200808-1274OC
11.
Itahana Y Han R Barbier S Lei Z Rozen S Itahana K et al . The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense. Oncogene. (2015) 34:1799–810. doi: 10.1038/onc.2014.119
12.
Fan K Sun T Yin F . J-shaped association between uric acid and breast cancer risk: a prospective case-control study. J Cancer Res Clin Oncol. (2023) 149:7629–36. doi: 10.1007/s00432-023-04725-y
13.
Sun S Xu H Liu L Luan Z Liu C Zhi F et al . Association of serum uric acid-to-high-density lipoprotein cholesterol ratio (UHR) with risk of myocardial infarction among individuals with diabetes: a cross-sectional analysis using data from NHANES 2005-2020. Eur J Med Res. (2025) 30:554. doi: 10.1186/s40001-025-02845-4
14.
Díaz-Gay M Dos Santos W Moody S Kazachkova M Abbasi A Steele CD et al . Geographic and age variations in mutational processes in colorectal cancer. Nature. (2025) 643:230–40. doi: 10.1038/s41586-025-09025-8
15.
Feng XL Chen HM Yin LJ Chen J Chen LL . Developing a nomogram for risk prediction of the low T3 syndrome. Sci Rep. (2025) 15:4863. doi: 10.1038/s41598-025-89484-1
16.
Alessandro A Giuseppe M Giuseppe M Sebastiano G . Protective effects of high-density lipoprotein on Cancer odds: focus on multiple myeloma. Biomedicine. (2024) 12:514. doi: 10.3390/biomedicines12030514
17.
Nam SY Jo J Cho CM . A population-based cohort study of longitudinal change of high-density lipoprotein cholesterol impact on gastrointestinal cancer risk. Nat Commun. (2024) 15:2923. doi: 10.1038/s41467-024-47193-9
18.
Nakahara R Maeda K Aki S Osawa T . Metabolic adaptations of cancer in extreme tumor microenvironments. Cancer Sci. (2023) 114:1200–7. doi: 10.1111/cas.15722
19.
Liao W Wang Y Zhang W . Serum uric acid and the risk of colorectal cancer: a meta-analysis. Eur J Cancer Prev. (2024) 33:19–28. doi: 10.1097/CEJ.0000000000000834
20.
Mi N Huang J Huang C Lin Y He Q Wang H et al . High serum uric acid may associate with the increased risk of colorectal cancer in females: a prospective cohort study. Int J Cancer. (2022) 150:263–72. doi: 10.1002/ijc.33807
21.
Ying F Xiaoyan D Jiayu W Fei M Peng Y Qing L et al . Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast cancer but not non-TNBCs. Int J Biol Markers. (2015) 30:143. doi: 10.5301/jbm.5000143
22.
Zhang Z Zhang D . Circulating lipids, lipid-lowering drug targets, and breast cancer risk: comprehensive evidence from Mendelian randomization and summary data-based Mendelian randomization. Cancer Causes Control. (2024) 35:983–94. doi: 10.1007/s10552-024-01857-5
Summary
Keywords
breast cancer, high-density lipoprotein cholesterol, metabolic biomarker, odds prediction, UHR, uric acid
Citation
Ren Q, Li G, Liu E, Zhou J, Chen L and Gao X (2026) Association of the uric acid to high-density lipoprotein cholesterol ratio with breast cancer odds: a retrospective study. Front. Med. 13:1741976. doi: 10.3389/fmed.2026.1741976
Received
08 November 2025
Revised
01 January 2026
Accepted
14 January 2026
Published
29 January 2026
Volume
13 - 2026
Edited by
Ming Liu, University of Electronic Science and Technology of China, China
Reviewed by
Shinsuke Hidese, Teikyo University, Japan
Jiayu Zhang, Binzhou Medical University, China
Updates
Copyright
© 2026 Ren, Li, Liu, Zhou, Chen and Gao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Chen, rainbow_lyn@163.com; Xiang Gao, 9862016084@jiangnan.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.