Abstract
Introduction:
Thirty-day mortality remains a critical indicator of perioperative safety and quality of care in patients undergoing non-cardiac surgery. Identifying predictive factors for 30-day mortality is critical for crisis mitigation and improving patient outcomes. This study aimed to identify key predictors of 30-day mortality in non-cardiac surgery patients and develop a predictive model to aid clinical decision-making.
Methods:
This retrospective observational study analyzed data from patients aged ≥18 years who underwent non-cardiac surgery at a tertiary care hospital between January and August 2022. Data on demographics, comorbidities, intraoperative variables, and postoperative complications were collected. Multivariate logistic regression identified independent predictors of 30-day mortality, and a nomogram was constructed to facilitate individualized risk assessment.
Results:
Among 7,528 patients, 76 (1.0%) died within 30 days. Independent predictors of mortality included preoperative ventilatory support (odds ratio [OR] 8.76; 95% confidence interval [CI]: 3.58–21.42; p < 0.001), postoperative sepsis (OR 5.86; 95% CI: 2.23–15.37; p < 0.001), coma (OR 18.56; 95% CI: 6.81–50.55; p < 0.001), myocardial infarction (OR 26.35; 95% CI: 4.46–155.63; p < 0.001), and cardiac arrest (OR 57.9; 95% CI: 18.22–184.05; p < 0.001). The nomogram demonstrated good calibration at lower risk levels, with slight overestimation at higher probabilities. The area under the receiver operating characteristic curve indicated excellent discrimination.
Discussion:
The developed nomogram serves as a useful tool for perioperative risk stratification in non-cardiac surgery. Incorporating both preoperative and postoperative factors, aids in early identification of high-risk patients and supports targeted clinical interventions. External validation and refinement of the model are recommended for broader applicability.
1 Introduction
Thirty-day mortality remains a key indicator of perioperative safety and quality of care in patients undergoing non-cardiac surgery. Despite advances in surgical techniques, anesthesia, and perioperative management, postoperative mortality continues to pose a significant clinical burden, particularly among older patients and those with multiple comorbidities. Understanding perioperative factors associated with 30-day mortality is therefore essential to improve risk stratification, guide clinical decision-making, and optimize perioperative management strategies.
Among the contributors to perioperative mortality, cardiac arrest represents one of the most catastrophic events. Although relatively uncommon occurring in approximately 7–30 per 10,000 non-cardiac surgical cases (1, 2). Intraoperative cardiac arrest is associated with extremely poor outcomes. Previous studies report 30-day mortality rates of 60–80% following perioperative cardiac arrest, particularly in elderly patients and those with significant comorbidities (1, 3). Complications following cardiac arrest, including septicemia, ventilator dependence, renal impairment, and major bleeding, further contribute to low survival rates (4, 5).
In addition to cardiac arrest, multiple perioperative factors have been shown to influence 30-day mortality. These include advanced age, higher American Society of Anesthesiologists (ASA) physical status classification, reduced functional capacity, clinical sepsis, emergency surgery, and end-stage renal disease (5–8). Many of these factors are also associated with an increased risk of intraoperative and postoperative cardiac arrest, highlighting the overlapping and cumulative nature of perioperative risk. Recognition of these predictors may allow anesthesiologists and surgeons to identify high-risk patients, implement targeted interventions, and potentially reduce postoperative mortality.
While prior studies have extensively examined risk factors for intraoperative cardiac arrest and its associated outcomes, there remains limited evidence on comprehensive predictive models specifically designed to estimate 30-day mortality in patients undergoing non-cardiac surgery. Accordingly, this study aimed to identify key perioperative predictors of 30-day mortality and to develop a predictive scoring system to support perioperative risk stratification in this patient population.
2 Materials and methods
2.1 Institutional review board approval
This study (REC.65-131-8-1) was approved by the Institutional Ethics Committee of the Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand on April 22, 2022. The requirement for written informed consent was waived because this study utilized only retrospective clinical data collected during routine practice.
2.2 Study design and setting
This retrospective observational study was conducted at Songklanagarind Hospital, a tertiary care facility in Southern Thailand. The study included all patients at the center from January 2022 to August 2022 who met the following criteria: aged ≥18 years and underwent non-cardiac surgery. Patients <18 years who underwent non-cardiac surgery were excluded to maintain a focus on identifying associations between predictive factors and 30-day mortality in the target population (Figure 1). The data for the study were collected between September 1, 2022, and September 30, 2023.
Figure 1

Flow chart for the study.
2.3 Data collection
Data on demographics (age, sex, body mass index), ASA physical status classification, type of surgery, patient comorbidities, and anesthesia techniques were collected from electronic medical and anesthesia records. The primary outcome was 30-day mortality, defined as all-cause death occurring at any time from the start of surgery up to 30 days postoperatively. Intraoperative mortality was not analyzed as a separate outcome but was included as a subset of the 30-day mortality endpoint, ensuring that all deaths occurring during surgery and the postoperative period were captured within a single, clinically meaningful outcome measure.
Sepsis was defined according to clinical documentation consistent with Sepsis-3 criteria, including suspected or confirmed infection with associated organ dysfunction occurring within the peri- or post-operative period. Myocardial infarction was defined as new ischemic symptoms, electrocardiographic changes, or elevated cardiac biomarkers documented by the treating physician within 30 days after surgery. Renal failure was defined as acute kidney injury requiring renal replacement therapy or a documented diagnosis of acute renal failure during hospitalization. Altered consciousness (coma) was defined as a documented Glasgow Coma Scale score ≤8.
2.4 Statistical analysis
The data were analyzed using R studio version 4.3.1 to evaluate the differences between survivors and non-survivors within 30 days of surgery. Continuous variables are summarized as means with standard deviations for normally distributed data or medians with interquartile ranges (IQRs) for non-normally distributed data. Student’s t-test was employed to compare normally distributed continuous variables between groups, whereas the Wilcoxon rank-sum test was applied for non-normal distributed variables.
Categorical variables are expressed as frequencies and percentages. The chi-squared test was used to compare proportions for variables with large sample sizes and sufficient expected frequencies. For categorical variables with small sample sizes or when expected cell frequencies were <5, Fisher’s exact test was applied for validity.
Univariate analyses were conducted to identify factors associated with 30-day mortality. Variables with a p-value <0.20 in univariate analysis were included in a multivariate logistic regression model to identify independent predictors of mortality. Given that there were 76 deaths, we ensured that only 7–8 variables were included in the final model to maintain statistical validity and avoid overfitting. The selected variables were determined based on clinical relevance and statistical significance. Logistic regression analysis results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was defined as a p-value < 0.05.
The predictive performance of the model was evaluated using a calibration plot to compare the predicted probabilities of mortality with observed outcomes. Bootstrapping with 1,000 resamples was conducted to correct for optimism and overfitting in the model. The model’s discrimination ability was evaluated using receiver operating characteristic (ROC), which quantified its capacity to distinguish between survivors and non-survivors.
This comprehensive statistical approach ensured robust analysis, accounting for data characteristics and providing reliable insights into the factors influencing 30-day mortality in patients undergoing non-cardiac surgery.
3 Results
3.1 Baseline patient characteristics and mortality rates
A total of 7,528 patients were included in the study, of whom 76 (1.0%) experienced 30-day mortality. Patients who died were significantly older, with a median age of 66 years (IQR 49–73.2), compared to 53 years (IQR 36–66) for survivors (p < 0.001). A higher proportion of patients in the mortality group had an ASA classification of 3, 4, 5 accounted for 57.9, 23.7, and 10.5% of deaths, respectively, (p < 0.001). Emergency surgeries were more common in the mortality group (59.2%) than in survivors (23.0%, p < 0.001). Key preoperative factors significantly associated with higher mortality are summarized in Table 1.
Table 1
| Characteristics | Alive (N = 7,452) | Death (N = 76) | p-value |
|---|---|---|---|
| Male, n (%) | 2,933 (39.4) | 43 (56.6) | 0.003 |
| Age, median (IQR) | 53 (36, 66) | 66 (49, 73.2) | <0.001 |
| Weight, median (IQR) | 62.3 (54.2, 72) | 59.5 (50, 70) | 0.005 |
| Height, median (IQR) | 160 (155, 165) | 160.5 (155, 170) | 0.198 |
| ASA classification, n (%) | <0.001 | ||
| 1 | 254 (3.4) | 0 (0) | |
| 2 | 4,727 (63.5) | 6 (7.9) | |
| 3 | 2,369 (31.8) | 44 (57.9) | |
| 4 | 90 (1.2) | 18 (23.7) | |
| 5 | 9 (0.1) | 8 (10.5) | |
| Case type, n (%) | <0.001 | ||
| Elective | 5,605 (75.3) | 31 (40.8) | |
| Emergency | 1,715 (23) | 45 (59.2) | |
| OPD | 124 (1.7) | 0 (0) | |
| History of ischemic heart disease, n (%) | 305 (4.1) | 8 (10.5) | 0.012 |
| History of congestive heart failure, n (%) | 32 (0.4) | 1 (1.3) | 0.771 |
| History of peripheral arterial disease, n (%) | 113 (1.5) | 3 (3.9) | 0.214 |
| History of arrhythmia, n (%) | 275 (3.7) | 16 (21.1) | <0.001 |
| Smoking, n (%) | 1,126 (15.1) | 24 (32) | <0.001 |
| Diabetic mellitus, n (%) | 1,265 (17) | 20 (26.7) | 0.039 |
| History of cerebrovascular disease, n (%) | <0.001 | ||
| Fully recovered | 235 (3.2) | 3 (4) | |
| Partially recovered | 196 (2.6) | 10 (13.3) | |
| History of cancer, n (%) | 1,662 (22.3) | 27 (36) | 0.007 |
| On ventilator, n (%) | 165 (2.2) | 43 (56.6) | <0.001 |
| Preoperative sepsis, n (%) | 111 (1.5) | 23 (30.3) | <0.001 |
| Altered consciousness, n (%) | 113 (1.5) | 26 (34.2) | <0.001 |
| Preoperative hyperthermia, n (%) | 470 (6.4) | 37 (51.4) | <0.001 |
| Preoperative hypertensive emergency, n (%) | 81 (1.1) | 5 (6.7) | <0.001 |
| Preoperative use of inotropes, n (%) | 49 (0.7) | 20 (26.7) | <0.001 |
| Preoperative red blood cell transfusion, n (%) | 157 (2.1) | 26 (34.7) | <0.001 |
| Preoperative hyperglycemia, n (%) | 227 (8.1) | 15 (28.8) | <0.001 |
| Preoperative use of acetaminophen, n (%) | 2,370 (31.8) | 14 (18.4) | 0.018 |
| Preoperative hematocrit, median (IQR) | 37.5 (33.8, 40.8) | 30.4 (26.2, 37.3) | <0.001 |
| Preoperative platelet count, median (IQR) | 254,000 (201,000, 311,000) | 233,000 (148,000, 305,250) | 0.148 |
| Creatinine, median (IQR) | 0.7 (0.5, 0.9) | 0.8 (0.6, 1.4) | <0.001 |
| Albumin, median (IQR) | 9 (4, 9) | 3.3 (2.6, 5.6) | <0.001 |
Baseline characteristics of patients undergoing non-cardiac surgery categorized by 30-day mortality.
IQR, interquartile range; ASA, American Association of Anesthesiologists; FiO2, fraction of inspired oxygen; INR, international normalized ratio; PTT, partial thromboplastin time; OPD, outpatient department.
3.2 Intraoperative factors
Mortality was associated with cases performed outside the OR (17.1% vs. 5.7%, p < 0.001), intraoperative tachycardia (34.2% vs. 16.5%, p < 0.001), and intraoperative cardiac arrest (5.3% vs. 0.1%, p < 0.001). Additionally, intraoperative hypotension (69.7% vs. 48.5%, p < 0.001) and the use of inotropic agents (60.5% vs. 16.3%, p < 0.001) were significantly more prevalent in the mortality group (Table 2).
Table 2
| Intraoperative techniques and complications | Alive (N = 7,452) | Death (N = 76) | p value |
|---|---|---|---|
| Intraoperative period | |||
| Area, n (%) | <0.001 | ||
| Operating room | 7,029 (94.3) | 63 (82.9) | |
| Remote area | 423 (5.7) | 13 (17.1) | |
| Duration of anesthetic time, median (IQR) | 130 (75, 210) | 115 (78.8, 205) | 0.514 |
| Invasive monitor, n (%) | 1,284 (17.2) | 43 (56.6) | <0.001 |
| Arterial line, n (%) | 1,275 (17.1) | 43 (56.6) | <0.001 |
| Central line, n (%) | 213 (2.9) | 20 (26.3) | <0.001 |
| Endotracheal tube, n (%) | 4,493 (60.3) | 70 (92.1) | <0.001 |
| Laryngeal mask airway, n (%) | 74 (1) | 0 (0) | 0.773 |
| Tracheostomy tube, n (%) | 137 (1.8) | 4 (5.3) | 0.077 |
| Intraoperative inotrope, n (%) | 1,214 (16.3) | 46 (60.5) | <0.001 |
| Adrenaline, n (%) | 26 (0.3) | 12 (15.8) | <0.001 |
| Dopamine, n (%) | 15 (0.2) | 7 (9.2) | <0.001 |
| Levophed, n (%) | 1,203 (16.1) | 45 (59.2) | <0.001 |
| Intraoperative hypotension, n (%) | 3,613 (48.5) | 53 (69.7) | <0.001 |
| Intraoperative tachycardia, n (%) | 1,227 (16.5) | 26 (34.2) | <0.001 |
| Intraoperative hyperglycemia, DTX > 180 mg/dL | 169 (2.3) | 9 (11.8) | <0.001 |
| Duration of surgery, median (IQR) | 90 (50, 165) | 82.5 (50, 180) | 0.883 |
| Estimated blood loss, median (IQR) | 50 (5, 300) | 50 (10, 300) | 0.404 |
| Intraoperative cardiac arrest, n (%) | 7 (0.1) | 4 (5.3) | <0.001 |
| Phase of arrest, n (%) | <0.001 | ||
| Induction | 3 (0) | 2 (2.6) | |
| Anesthetic course | 4 (0.1) | 0 (0) | |
| Transport to recovery room | 0 (0) | 1 (1.3) | |
| Postoperative period | |||
| Postoperative 24 h | 0 (0) | 1 (1.3) | |
| EKG in arrest, n (%) | <0.001 | ||
| Asystole | 2 (0) | 2 (2.6) | |
| PEA | 3 (0) | 1 (1.3) | |
| VF/VT | 2 (0) | 1 (1.3) | |
| Cause of arrest, n (%) | <0.001 | ||
| Bleeding | 4 (0.1) | 2 (2.6) | |
| Cardiac | 2 (0) | 1 (1.3) | |
| Hypoxia | 0 (0) | 1 (1.3) | |
| Other | 1 (0) | 0 (0) | |
| Intraoperative death, n (%) | 4 (0.1) | 1 (1.3) | 0.044 |
Intraoperative techniques and complications stratified by 30-day mortality.
IQR, interquartile range; N2O, nitrous oxide; DTX, dextrostrix; EKG, electrocardiogram; PEA, pulseless electrical activity; VF, ventricular fibrillation; VT, ventricular tachycardia.
3.3 Postoperative complications
Patients in the mortality group exhibited significantly higher rates of complications within 30 days. Sepsis (68.4%), pneumonia (55.3%), and renal failure (43.4%) were the most common complications among patients who died, all markedly elevated compared to the occurrence in survivors (p < 0.001; Table 3).
Table 3
| Postoperative complications | Alive (N = 7,452) | Dead (N = 76) | p-value |
|---|---|---|---|
| Pneumonia | 153 (2.1) | 42 (55.3) | <0.001 |
| Prolonged intubation | 70 (0.9) | 26 (34.2) | <0.001 |
| Reintubation | 38 (0.5) | 6 (7.9) | <0.001 |
| Coma | 30 (0.4) | 56 (73.7) | <0.001 |
| Sepsis | 141 (1.9) | 52 (68.4) | <0.001 |
| DVT/PE | 32 (0.4) | 10 (13.2) | <0.001 |
| Renal failure | 75 (1) | 33 (43.4) | <0.001 |
| Stroke | 14 (0.2) | 6 (7.9) | <0.001 |
| Myocardial infarction | 10 (0.1) | 7 (9.2) | <0.001 |
| Cardiac arrhythmia | 43 (0.6) | 21 (27.6) | <0.001 |
| Cardiac arrest | 8 (0.1) | 50 (65.8) | <0.001 |
| Reoperation | 80 (1.1) | 6 (7.9) | <0.001 |
| Multiple organ failure | 6 (0.1) | 16 (21.1) | <0.001 |
Postoperative complications within 30 days stratified by mortality status.
Data are presented as numbers (%) unless otherwise indicated. DVT, deep vein thrombosis; PE, pulmonary embolism.
3.4 Multivariate analysis
Key predictors of 30-day mortality were identified through multivariate analysis, with the following variables showing strong associations: preoperative ventilatory support (OR 8.76; 95% CI: 3.58–21.42; p < 0.001), postoperative sepsis (OR 5.86; 95% CI: 2.23–15.37; p < 0.001), coma (OR 18.56; 95% CI: 6.81–50.55, p < 0.001), deep vein thrombosis or pulmonary embolism (OR 7.91; 95% CI: 1.8–34.7; p < 0.001), and myocardial infarction (OR 26.35; 95% CI: 4.46–155.63; p < 0.001) (Table 4).
Table 4
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| Odds ratio | p-value | Odds ratio | p-value | |
| On ventilator | 8.1 (2.86,22.95) | <0.001 | 8.76 (3.58, 21.42) | <0.001* |
| Preoperative sepsis | 2.67 (0.73,9.77) | 0.147 | ||
| Preoperative RBC transfusion | 1.37 (0.74,2.55) | 0.427 | ||
| Adrenaline | 37.86 (7.32,195.92) | <0.001 | 27.32 (6.74, 110.67) | <0.001* |
| Intraoperative tachycardia | 2.58 (1.05,6.35) | 0.044 | ||
| Coma | 18.52 (6.27,54.75) | <0.001 | 18.56 (6.81, 50.55) | <0.001* |
| Postoperative sepsis | 6 (2.19, 16.39) | 0.001 | 5.86 (2.23, 15.37) | <0.001* |
| DVT/PE | 8.08 (1.89, 34.44) | 0.008 | 7.91 (1.8, 34.7) | <0.001* |
| Myocardial infarction | 38.22 (7.06, 207.04) | <0.001 | 26.35 (4.46, 155.63) | <0.001* |
| Cardiac arrest | 64.47 (18.06, 230.12) | <0.001 | 57.9 (18.22, 184.05) | <0.001* |
Univariate and multivariate analyses of factors associated with 30-day mortality.
*Value is statistically significant. RBC, red blood cells; DVT, deep vein thrombosis; PE, pulmonary embolism.
Cardiac arrest emerged as the strongest independent predictor of 30-day mortality (OR 57.9; 95% CI: 18.22–184.05; p < 0.001). Additionally, the use of adrenaline during surgery markedly increased the risk (OR 27.32; 95% CI: 6.74–110.67; p < 0.001) (Table 4).
3.5 Nomogram and predictive model
A predictive nomogram was developed to incorporate key clinical variables, including preoperative ventilatory support, postoperative sepsis, myocardial infarction, and cardiac arrest. The tool allowed individualized risk assessment, with higher total scores corresponding to an increased probability of 30-day mortality (Figure 2).
Figure 2
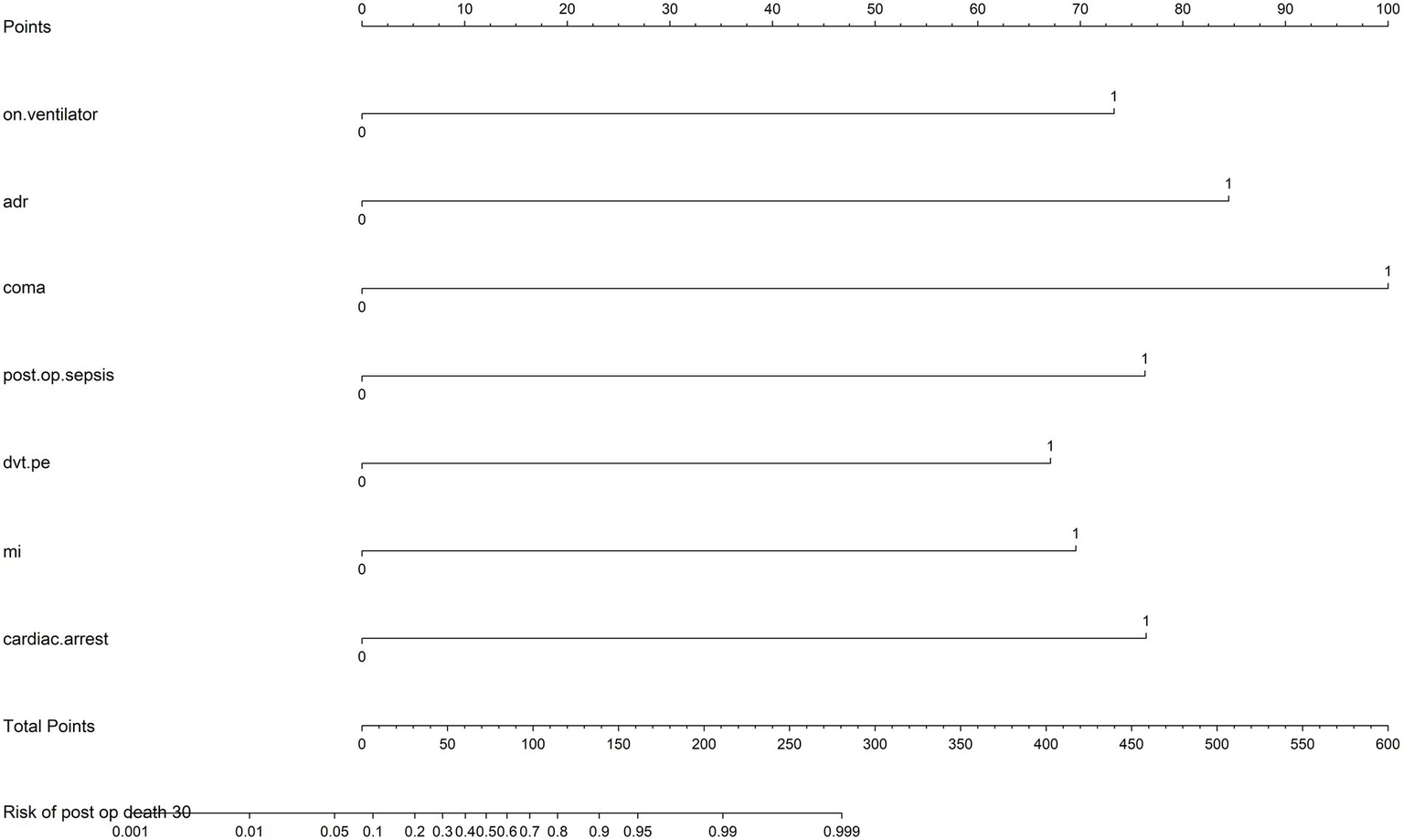
Nomogram for individualized risk assessment of 30-day mortality rates. The nomogram incorporates key predictors, including preoperative on ventilator support (on.ventilator), intraoperative use adrenaline (ADR), postoperative coma, postoperative sepsis (post.op.sepsis), deep vein thrombosis or pulmonary embolism (dvt.pe), myocardial infarction (MI), and cardiac arrest (cardiac.arrest). Each predictor is assigned a score based on its impact on risk, which can be summed to calculate the total points. The total points correspond to the predicted risk of 30-day mortality rates, displayed on the bottom scale.
The nomogram’s performance was evaluated using calibration analysis, which demonstrated good accuracy at lower risk levels. However, a slight overestimation of mortality risk was observed for higher-risk probabilities (Figure 3). The model’s discriminatory ability was assessed using the area under the receiver operating characteristic (AUROC) curve as 0.96.
Figure 3

Calibration plot of the predictive nomogram for 30-day mortality risk. The calibration plot compares predicted probabilities from the nomogram with observed 30-day mortality rates. The dashed diagonal line represents the ideal calibration, while the solid line indicates the model’s performance. Overestimation is noted at higher probabilities. Mean absolute error = 0.013; mean squared error = 0.00061; quantile of absolute error = 0.021.
4 Discussion
This study evaluated factors associated with 30-day mortality in patients undergoing non-cardiac surgery and developed a predictive nomogram to support risk stratification. The findings highlight the importance of patient characteristics, intraoperative events, and postoperative complications contributing to mortality. These insights emphasize the critical impact of comprehensive perioperative management in improving surgical outcomes and optimizing risk stratification.
4.1 Key predictors of 30-day mortality
The multivariate analysis identified several key predictors of 30-day mortality, including preoperative ventilatory support, postoperative sepsis, coma, deep vein thrombosis, myocardial infarction, and cardiac arrest. Cardiac arrest demonstrated the highest OR, underscoring its critical impact on patient outcomes.
4.2 Preoperative factors
The association between preoperative factors, such as ventilatory support and preoperative sepsis, and increased mortality highlights the vulnerability of patients with compromised physiological reserves. Of these factors, preoperative ventilatory support emerged as the strongest independent predictor of mortality.
These findings are consistent with those of previous studies that identified preoperative physiological derangements as critical determinants of adverse outcomes. For instance, Kazaure et al. (4) reported that renal impairment, disseminated cancer, and preoperative sepsis are independent predictors of poor survival. Similarly, Kaiser et al. (5) reported that ASA classification, advanced age, functional status, SIRS/sepsis, and disseminated cancer were the most substantial determinants of 30-day mortality. In contrast, our study found that preoperative sepsis, while significant in univariate analysis, did not remain significant in multivariate models. This distinction indicates the nuanced interplay between preoperative factors and their relative contributions to mortality risk. Additionally, Nuttall et al. (9) identified sepsis as the leading cause of death within 30 days postoperatively, followed by malignancy, cardiovascular disease, neurological disease, and hemorrhage. Magor et al. (10) also observed that patients requiring unplanned postoperative ventilatory support had a significantly higher mortality rate, with an adjusted OR of 5.8 (95% CI: 3.8–8.8, p < 0.001), a finding consistent with our result. Collectively, these findings emphasize the relevance of early identification and targeted optimization of modifiable factors. For example, the prompt treatment of infections and the management of physiological derangements can significantly mitigate risks and improve patient survival.
4.3 Intraoperative factors
The intraoperative period presents several critical challenges, particularly factors like tachycardia and the use of inotropes, such as adrenaline, which are strongly associated with increased mortality. However, the role of tachycardia in perioperative outcomes remains complex and incompletely understood. Ruetzler et al. (11) found no significant relationship between various measures of tachycardia and composite outcomes of myocardial injury and death, a finding that aligns with our results. In contrast, an earlier retrospective analysis of 41,140 patients found that a heart rate of at least 90 beats per minute was independently associated with an increased risk of myocardial injury (adjusted OR 1.22; 95% CI: 1.06–1.39; p = 0.005) (12). Similarly, Jindawatthana et al. (13) demonstrated that preoperative vasopressor use was an independent risk factor significantly associated with 30-day mortality (adjusted relative risk 1.90; 95% CI: 1.08–3.32; p = 0.025), consistent with our findings.
Maintaining hemodynamic stability is paramount, as even transient disturbances can trigger adverse outcomes in high-risk patients. The strong association between cardiac arrest and mortality underscores the importance of the immediate recognition and management of perioperative crises, using standardized protocols and advanced monitoring technologies. These measures are essential to minimize intraoperative risks and improve surgical outcomes, particularly in vulnerable populations.
4.4 Postoperative complications
This study confirmed that complications such as sepsis, myocardial infarction, deep vein thrombosis, and altered consciousness are significant predictors of mortality. These findings align with those from previous studies demonstrate the critical role of postoperative complications in surgical mortality. For instance, Park et al. (14) reported five post-operative complications, myocardial injury after non-cardiac surgery [MINS], major bleeding, sepsis, stroke, and acute kidney injury requiring dialysis, as independent predictors of death. Similarly, Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators et al. (15) identified three complications with the largest attributable fractions (AF) for mortality: major bleeding (15.6%; adjusted hazard ratio [HR] 2.6; 95% CI 2.2–3.1; AF 17.0%); MINS (13.0%; adjusted HR 2.2; 95% CI 1.9–2.6; AF 15.9%); and sepsis (4.5%; adjusted HR 5.6; 95% CI 4.6–6.8; AF 12.0%). Additionally, postoperative cardiac arrest is independently associated with worse survival, further emphasizing the significant impact of severe postoperative events on mortality outcomes (4).
Early recognition and aggressive management of postoperative complications are critical for improving patient outcomes. Timely interventions, such as the administration of antibiotics for sepsis, early mobilization to reduce the risk of thromboembolic events, and vigilant cardiac monitoring, play a pivotal role in mitigating these risks. The strong association between these complications and mortality underscores the importance of structured postoperative care protocols.
Overall, the identification of high-risk patients based on these predictors allows clinicians to implement targeted and timely interventions, ultimately improving survival outcomes and reducing the burden of postoperative complications.
4.5 Nomogram as a predictive tool
The nomogram is, therefore, best interpreted as a perioperative risk aggregation tool, reflecting the cumulative impact of preoperative vulnerability, intraoperative instability, and early postoperative complications, rather than a purely causal or preoperative prediction model. We acknowledge that several variables included in the model, such as intraoperative cardiac arrest, use of inotropic agents, and severe hypotension, may lie directly on the causal pathway to death. Their inclusion may enhance statistical discrimination while limiting the usefulness of the model for early risk prediction.
The nomogram developed in this study provides a practical framework for estimating individual 30-day mortality risk (Figure 2) once major perioperative events have occurred. By integrating key predictors, including ventilatory support, sepsis, myocardial infarction, and cardiac arrest, the model facilitates stratification of patients according to evolving perioperative risk. Although calibration was good at lower predicted risk levels, the model slightly overestimated mortality at higher probabilities (Figure 3), consistent with the inclusion of catastrophic perioperative events. Consequently, the nomogram should be applied cautiously and be primarily used to support perioperative clinical decision-making and resource allocation, rather than as a tool for early or preoperative prognostication.
4.6 Clinical implications
These findings highlight the need for a multidisciplinary approach to perioperative care. Addressing modifiable preoperative factors, maintaining intraoperative hemodynamic stability, and implementing rigorous postoperative monitoring are crucial strategies to reduce surgical mortality. Incorporating the nomogram into clinical workflows could further enhance the clinician’s ability to identify high-risk patients and effectively prioritize interventions.
4.7 Future directions
Future studies should focus on validating the nomogram in external cohorts to assess its generalizability. Additionally, advanced predictive modeling techniques such as machine learning could improve the model’s accuracy and applicability. Evaluating the impact of targeted interventions based on risk stratification would provide critical insights into improving perioperative care for high-risk patients.
4.8 Limitations
This study has certain limitations that should be considered when interpreting the results. First, the retrospective design may introduce selection and information biases, as the data were collected from past records without real-time verification. Second, the small number of intraoperative cardiac arrest cases limit the ability to construct a reliable predictive model for this specific outcome. As a result, the primary focus shifted to identifying predictors of 30-day mortality. Third, the findings are based on data from a single tertiary care hospital, which may affect the generalizability of the results to other settings. Lastly, although the model demonstrated strong discrimination, several of the included variables, such as intraoperative cardiac arrest, use of inotropic agents, and severe hypotension, lie on the causal pathway to death. Their inclusion likely inflated model performance and limits the usefulness of the nomogram as an early or purely preoperative prediction tool. As a result, the nomogram should be interpreted as a perioperative risk aggregation model rather than a causal predictor of mortality. Future research should aim for external validation of the nomogram in diverse populations and consider using advanced machine-learning techniques to improve the model’s predictive accuracy.
5 Conclusion
This study identifies critical predictors of 30-day mortality in non-cardiac surgery and provides a nomogram for risk stratification. While postoperative factors play an important role, the inclusion of preoperative predictors offers some early prognostic value. Further refinement and validation of the model will enhance its clinical applicability. Therefore, the nomogram is best interpreted as a dynamic perioperative risk aggregation tool rather than a purely preoperative prediction model.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee of the Faculty of Medicine, Prince of Songkla University, Songkhla. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study was retrospective study.
Author contributions
MS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TK: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SL: Writing – review & editing, Methodology, Validation. KSu: Data curation, Resources, Visualization, Writing – review & editing. KSa: Data curation, Resources, Visualization, Writing – review & editing. DY: Data curation, Resources, Visualization, Writing – review & editing. OM: Data curation, Resources, Visualization, Writing – review & editing. MB: Data curation, Resources, Visualization, Writing – review & editing. SK: Data curation, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by the Faculty of Medicine, Prince of Songkla University.
Acknowledgments
We thank Editage for English language editing and Sasira Choocham for statistical consultation.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2026.1749782/full#supplementary-material
Abbreviations
ASA, American Society of Anesthesiologist; SIRS, Systemic inflammatory response syndrome; IQR, Interquartile range; OR, Odds ratio; CI, Confidence interval; MINS, Myocardial injury after non-cardiac surgery; VISION, Vascular events in non-cardiac surgery patients cohort evaluation; AF, Attributable fractions; HR, Hazard ratio; ROC, Receiver operating characteristic; AUROC, Area under the ROC.
References
1.
Goswami S Brady JE Jordan DA Li G . Intraoperative cardiac arrests in adults undergoing noncardiac surgery: incidence, risk factors, and survival outcome. Anesthesiology. (2012) 117:1018–26. doi: 10.1097/ALN.0b013e31827005e9,
2.
Charuluxananan S Punjasawadwong Y Suraseranivongse S Srisawasdi S Kyokong O Chinachoti T et al . The Thai anesthesia incidents study (Thai study) of anesthetic outcomes: II. Anesthetic Profiles and Adverse Events. J Med Assoc Thail. (2005) 88:S14–29.
3.
Yongyukantorn K Oofuvong M . Risk factors of intraoperative and 24-hour postoperative cardiac arrest in geriatric patients in non-cardiac surgery. J Gerontol Geriatr. (2020) 68:159–67. doi: 10.36150/2499-6564-381
4.
Kazaure HS Roman SA Rosenthal RA Sosa JA . Cardiac arrest among surgical patients: an analysis of incidence, patient characteristics, and outcomes in ACS-NSQIP. JAMA Surg. (2013) 148:14–21. doi: 10.1001/jamasurg.2013.671,
5.
Kaiser HA Saied NN Kokoefer AS Saffour L Zoller JK Helwani MA . Incidence and prediction of intraoperative and postoperative cardiac arrest requiring cardiopulmonary resuscitation and 30-day mortality in non-cardiac surgical patients. PLoS One. (2020) 15:e0225939. doi: 10.1371/journal.pone.0225939,
6.
Sprung J Warner ME Contreras MG Schroeder DR Beighley CM Wilson GA et al . Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: a study of 518,294 patients at a tertiary referral center. Anesthesiology. (2003) 99:259–69. doi: 10.1097/00000542-200308000-00006,
7.
Nunnally ME O’Connor MF Kordylewski H Westlake B Dutton RP . The incidence and risk factors for perioperative cardiac arrest observed in the national anesthesia clinical outcomes registry. Anesth Analg. (2015) 120:364–70. doi: 10.1213/ANE.0000000000000527,
8.
Siriphuwanun V Punjasawadwong Y Lapisatepun W Charuluxananan S Uerpairojkit K . Incidence of and factors associated with perioperative cardiac arrest within 24 hours of anesthesia for emergency surgery. Risk Manag Healthc Policy. (2014) 7:155–62. doi: 10.2147/RMHP.S67935,
9.
Nuttall GA Merren MP Naranjo J Portner ER Ambrose AR Rihal CS . Perioperative mortality: a retrospective cohort study of 75,446 noncardiac surgery patients. Mayo Clin Proc Innov Qual Outcomes. (2024) 8:435–42. doi: 10.1016/j.mayocpiqo.2024.07.002,
10.
Magor R Dabush-Elisha I Aviram D Karol D Syn-Hershko A Schvartz R et al . In-hospital mortality of patients requiring unplanned postoperative ventilatory support: a multicenter observational study. Perioper Med (Lond). (2022) 11:44. doi: 10.1186/s13741-022-00276-x,
11.
Ruetzler K Yilmaz HO Turan A Zimmerman NM Mao G Hung MH et al . Intra-operative tachycardia is not associated with a composite of myocardial injury and mortality after noncardiac surgery: a retrospective cohort analysis. Eur J Anaesthesiol. (2019) 36:105–13. doi: 10.1097/EJA.0000000000000925,
12.
Ladha KS Beattie WS Tait G Wijeysundera DN . Association between preoperative ambulatory heart rate and postoperative myocardial injury: a retrospective cohort study. Br J Anaesth. (2018) 121:722–9. doi: 10.1016/j.bja.2018.06.016,
13.
Jindawatthana I Pathomkajonkul N Wongsripuemtet P . Factors associated with 30-day mortality after perioperative cardiac arrest in adults undergoing non-cardiac surgery: a seven-year observational study from Siriraj Hospital. Ann Transl Med. (2023) 11:342. doi: 10.21037/atm-23-762,
14.
Park LJ Borges FK Ofori S Nenshi R Jacka M Heels-Ansdell D et al . Association between complications and death within 30 days after general surgery: a vascular event in noncardiac surgery patients cohort evaluation (VISION) substudy. Ann Surg. (2024) 282:1007–13. doi: 10.1097/SLA.0000000000006372,
15.
Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators Spence J LeManach Y Chan MTV Wang CY Sigamani A et al . Association between complications and death within 30 days after noncardiac surgery. CMAJ. (2019) 191:E830–7. doi: 10.1503/cmaj.190221
Summary
Keywords
30-day mortality, nomogram, non-cardiac surgery, perioperative complications, risk predictors
Citation
Saetang M, Kunapaisal T, Limvatanalert S, Sukitpaneenit K, Saelim K, Yongsata D, Muangsong O, Bunkerd M and Kampeng S (2026) Key predictors of 30-day mortality in non-cardiac surgery: development, key risk factors, and calibration of a risk model. Front. Med. 13:1749782. doi: 10.3389/fmed.2026.1749782
Received
19 November 2025
Revised
20 January 2026
Accepted
20 January 2026
Published
30 January 2026
Volume
13 - 2026
Edited by
Mohammad H. Abukhalil, Al-Hussein Bin Talal University, Jordan
Reviewed by
Wenzheng Bao, Xuzhou University of Technology, China
Lana Alzayadneh, School of Medicine, Jordan
Updates
Copyright
© 2026 Saetang, Kunapaisal, Limvatanalert, Sukitpaneenit, Saelim, Yongsata, Muangsong, Bunkerd and Kampeng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thitikan Kunapaisal, thitikan070@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.