Abstract
Purpose:
To investigate the tilt and decentration differences and influencing factors of intraocular lenses (IOLs) with plate-haptic and C-loop haptic designs one month after cataract surgery, using the combination of swept-source optical coherence tomography (SS-OCT) and a customized convolutional neural network (CNN) algorithm.
Methods:
Participants were categorized into two groups based on the haptic design of their implanted IOLs. Group A included 37 patients (37 eyes) with the ATTORBI 709 M plate-haptic design, while Group B comprised 42 patients (42 eyes) with the PY-60 AD C-loop design. SS-OCT examinations were performed both before and one month after cataract surgery. A customized CNN algorithm was developed to generate data for IOL tilt and decentration, using the corneal vertex as a reference.
Results:
This study included 79 patients (79 eyes). Before surgery, there were no statistically significant differences in ocular parameters between the two groups (all p > 0.05). Significant difference in IOL decentration was observed (Group A & Group B: 0.16 ± 0.08 mm & 0.42 ± 0.28 mm, p < 0.001). In contrast, the difference in IOL tilt was not significant (Group A & Group B: 4.60 ± 1.27° & 4.63 ± 1.93°, p = 0.948). Both groups displayed a predominant inferotemporal tilt of the crystalline lens and IOL, but decentration did not show a distinct distribution pattern. Multivariate linear regression confirmed a correlation between IOL tilt in both groups and crystalline lens tilt (p < 0.003).
Conclusion:
The plate-haptic design IOL shows less decentration than the C-loop design. Clinicians should consider this potential difference in IOL stability when selecting IOLs with varying haptic designs.
Introduction
The decentration and tilt of intraocular lenses (IOLs) following cataract surgery are critical determinants of patients’ visual quality. Research indicates that when IOL tilt exceeds 7° or decentration surpasses 0.4 mm, there is a notable increase in postoperative wavefront aberrations and a decrease in the capability of cylinder correction, especially for eyes with premium multifocal, Toric, and extended depth of focus (EDOF) IOL implantations in different corneal conditions (1–5). Variations in IOL haptic designs, such as C-loop and plate-haptic configurations, as well as differences in overall size, may influence the IOL’s orientation (6, 7). Clinically, ultrasound biomicroscopy (UBM) can be employed to analyze crystalline lens and IOL positioning, though it is a contact-based procedure that requires the patient to be supine. The supine position and the lack of a stable fixation target may affect measurement reliability (8). Purkinje imaging can assess the position of the crystalline lens or IOL by detecting light reflections from different refractive surfaces. Moreover, its reliance on the IOL’s curvature radii for calculations can introduce errors, particularly with relatively flat IOLs (8, 9). Furthermore, the potential shift in the pupil center with varying pupil sizes suggests that using the pupil axis as a reference can lead to fluctuations in tilt and decentration measurements, thereby reducing their repeatability (10–12).
Pentacam Scheimpflug imaging and swept-source optical coherence tomography (SS-OCT) enable rapid anterior segment imaging, facilitating the measurement of tilt and decentration metrics. However, the Pentacam requires a minimum pupil diameter of 6 mm to detect the crystalline lens posterior surface accurately, and fluctuations in the pupil axis can also affect measurement reliability (10, 11, 13, 14). Some commercial SS-OCT devices automatically provide crystalline lens or IOL tilt and decentration values via integrated software (2, 15, 16). Yet, the lack of a universally accepted analytical algorithm leads to variability in the determination of these metrics across different imaging qualities (9). Our research on IOL tilt and decentration analysis has progressed significantly over the past decade. In 2013, we conducted initial analyses using time-domain OCT, referencing the pupillary plane (17). By 2021, we had advanced to using SS-OCT images and the corneal vertex as a reference axis, achieving repeatable measurements of IOL tilt and decentration (18). While these earlier methods provided reliable results, they were not fully automated. To enhance analysis efficiency, our team published a robust spatial-position analysis algorithm in 2023. This algorithm, designed for both crystalline lenses and IOLs, is based on multitask learning and was developed using a dataset of 1,251 SS-OCT images from 180 patients. It demonstrated excellent inter-observer performance for segmentation and calculation, with Dice coefficients higher than 0.968 (19). In the current study, we apply this same algorithm to evaluate IOL tilt and decentration, continuing to use the corneal vertex as a dependable reference (18).
The aims of various IOL haptic designs and their subsequent modifications are twofold: first, to enhance the IOL’s postoperative stability, and second, to reduce the frequency of posterior capsule opacification. These haptic configurations predominantly encompass the open-loop plate haptic design (such as the Akreos Adapt), the whole plate haptic design (such as Zeiss 509 M), the single-piece C-loop design (such as AcrySof SA60AT and Lenstec SOFTEC HD), the open-loop single-piece C-loop design (such as Rayner 920H), and the three-piece C-loop design (such as AcrySof MA60AC) (11). However, the existing literature indicates a scarcity of direct comparative analyses between the three-piece C-loop design (PY-60 AD) and the whole-plate haptic design (Zeiss 709 M). The PY-60 AD is an aspheric, three-piece, hydrophobic acrylic IOL with modified C-loop haptics, a 5-degree angulation, and a contact length of 12.5 mm. In contrast, the Zeiss 709 M is an aspheric, single-piece, hydrophilic acrylic plate haptic IOL, characterized by its lack of haptic angulation and a 12.53 mm contact length (calculated with Pythagorean theorem).
This study aims to conduct an analytical comparison of the short-term postoperative spatial positioning of the PY-60 AD and 709 M IOLs. Furthermore, the study seeks to elucidate the pertinent factors influencing the postoperative positioning of these IOLs.
Materials and methods
This cross-sectional, retrospective, observational study recruited 79 patients diagnosed with senile cataracts who underwent phacoemulsification and IOL implantation at the Shanxi Eye Hospital from January 2021 to May 2024. Patients were categorized into Group A (implanted with a plate-haptic design, AT TORBI 709 M, Carl Zeiss) and Group B (implanted with a C-loop design, PY-60 AD, HOYA, Japan). The study conformed to the Declaration of Helsinki, receiving approval from the Ethics Committee of Shanxi Eye Hospital Affiliated to Shanxi Medical University (No. 2019LL130), and all participants provided informed consent.
Participants eligible for this study included: senile cataract, 21.0 mm ≤ axial length (AL) ≤ 26.0 mm, and postoperative best-corrected visual acuity (BCVA) ≥ 12/20. Exclusion criteria encompassed coexisting ocular diseases (such as corneal pathologies, lens dislocation, or retinal disorders), systemic ailments that might influence ocular health (such as diabetes, hypertension, or hyperthyroidism), and intraoperative complications (including posterior capsule rupture, incomplete anterior capsulorhexis, or sulcus implantation of IOL), as well as severe preoperative refractive media opacities that hindered data collection.
For briefing the surgical procedure, following topical anesthesia, a 2.2 mm main clear cornea incision was created in the superonasal quadrant for the left eye or the superotemporal quadrant for the right eye. Subsequently, an ophthalmic viscosurgical device (OVD) was injected into the anterior chamber, and a 5.0–5.5 mm diameter continuous curvilinear capsulorhexis was manually created. The nucleus and cortical material were efficiently emulsified and aspirated. IOL was implanted, and the clear corneal incision was hydrated.
All enrolled patients underwent anterior segment SS-OCT imaging system (ANTERION; software version 1.3.4.0; Heidelberg Engineering, Heidelberg, Germany) prior to and one month after the cataract surgery. ANTERION uses a 1,300-nm light source to obtain ocular biometric parameters, including AL, anterior chamber depth (ACD), central corneal thickness (CCT), and lens thickness (LT), as well as anterior segment cross-sectional images.
Statistical analyses were conducted using SPSS software (version 22.0; SPSS, Chicago, IL, USA). The Shapiro–Wilk test was employed to assess the normality of the variables. For data following a normal distribution, an independent samples t-test was used. Otherwise, the Mann–Whitney U test was applied. Univariate and multivariate linear regression analyses were employed to evaluate the correlation of IOL position and preoperative biometric values, including AL, ACD, and LT, as well as the tilt and decentration of the crystalline lens. Model collinearity was assessed using variance inflation factors (VIFs). Statistical significance was determined at p < 0.05.
The sample size was calculated using MedCalc software (version 20.216; MedCalc Software Ltd). With a Type I error rate of 0.05 and a Type II error rate of 0.10, sample size calculations were performed. Based on a previous study, a clinically significant mean tilt difference of 1.32 degrees, with standard deviations of 0.89 and 1.83 degrees for Group 1 and Group 2, respectively, required a minimum of 27 eyes per group with a 1:1 ratio. Additionally, for a mean decentration difference of 0.1 mm and standard deviations of 0.13 mm and 0.06 mm for Group 1 and Group 2, a minimum of 23 eyes per group was needed with a 1:1 ratio (11).
Results
A total of 79 patients (79 eyes) were included in this study. Table 1 shows no statistically significant differences in preoperative ocular biometrics, postoperative BCVA, and astigmatism magnitude between the two groups (all p > 0.05).
Table 1
| Parameters | Group A (37 eyes) | Group B (42eyes) | p |
|---|---|---|---|
| Age (years) | 67.24 ± 11.17 | 69.57 ± 9.55 | 0.321 |
| Male/Female | 16/21 | 19/23 | 0.859 |
| AL (mm) | 23.47 ± 0.87 | 23.10 ± 1.02 | 0.094 |
| CCT (μm) | 528 ± 31 | 527 ± 27 | 0.957 |
| ACD (mm) | 3.26 ± 0.41 | 3.15 ± 0.38 | 0.231 |
| LT (mm) | 4.41 ± 0.45 | 4.44 ± 0.38 | 0.695 |
| WTW (mm) | 11.30 ± 0.46 | 11.27 ± 0.51 | 0.790 |
| K flat (D) | 43.76 ± 1.70 | 44.42 ± 1.55 | 0.076 |
| K steep (D) | 45.72 ± 1.62 | 45.23 ± 1.63 | 0.178 |
| Crystalline lens tilt (°) | 3.50 ± 1.00 | 3.33 ± 1.15 | 0.473 |
| Crystalline lens decentration (mm) | 0.16 ± 0.11 | 0.20 ± 0.14 | 0.152 |
| Postoperative BCVA (decimal) | 0.88 ± 0.30 | 0.85 ± 0.30 | 0.703 |
| Postoperative astigmatism magnitude (D) | −0.21 ± 0.52 | −0.30 ± 0.89 | 0.626 |
Baseline characteristics of the two groups.
AL, axial length; ACD, anterior chamber depth; BCVA, best corrected visual acuity; CCT, central corneal thickness; D, diopter; K, keratometry; IOL, intraocular lens; LT, lens thickness; WTW, white to white.
The postoperative IOL tilt in Group A and Group B was (4.60 ± 1.27)° and (4.63 ± 1.93)°, respectively, with no statistically significant difference (p = 0.948, Cohen’s d = 0.02). However, the difference in postoperative IOL decentration between Group A (0.16 ± 0.08 mm) and Group B (0.42 ± 0.28 mm) was statistically significant (p < 0.001, Cohen’s d = 1.26). As shown in Figure 1, the crystalline lens and the IOL in the two groups tended to incline toward the inferotemporal quadrant, whereas the decentration did not exhibit an obviously distinct distribution pattern (Figure 2). In Group A, 89.19% (33/37) of the crystalline lenses and 94.59% (35/37) of the IOLs inclined toward the inferotemporal quadrant with a tilt degree of less than 7°, and 91.89% (34/37) of the crystalline lenses had a decentration of less than 0.4 mm. All the IOL decentrations were less than 0.4 mm. In Group B, 95.24% (40/42) of the crystalline lenses and 69.05% (29/42) of the IOLs inclined toward the inferotemporal quadrant with a tilt degree of less than 7°, with 92.86% (39/42) of the crystalline lenses and 57.14% (24/42) of the IOLs with decentration of less than 0.4 mm.
Figure 1
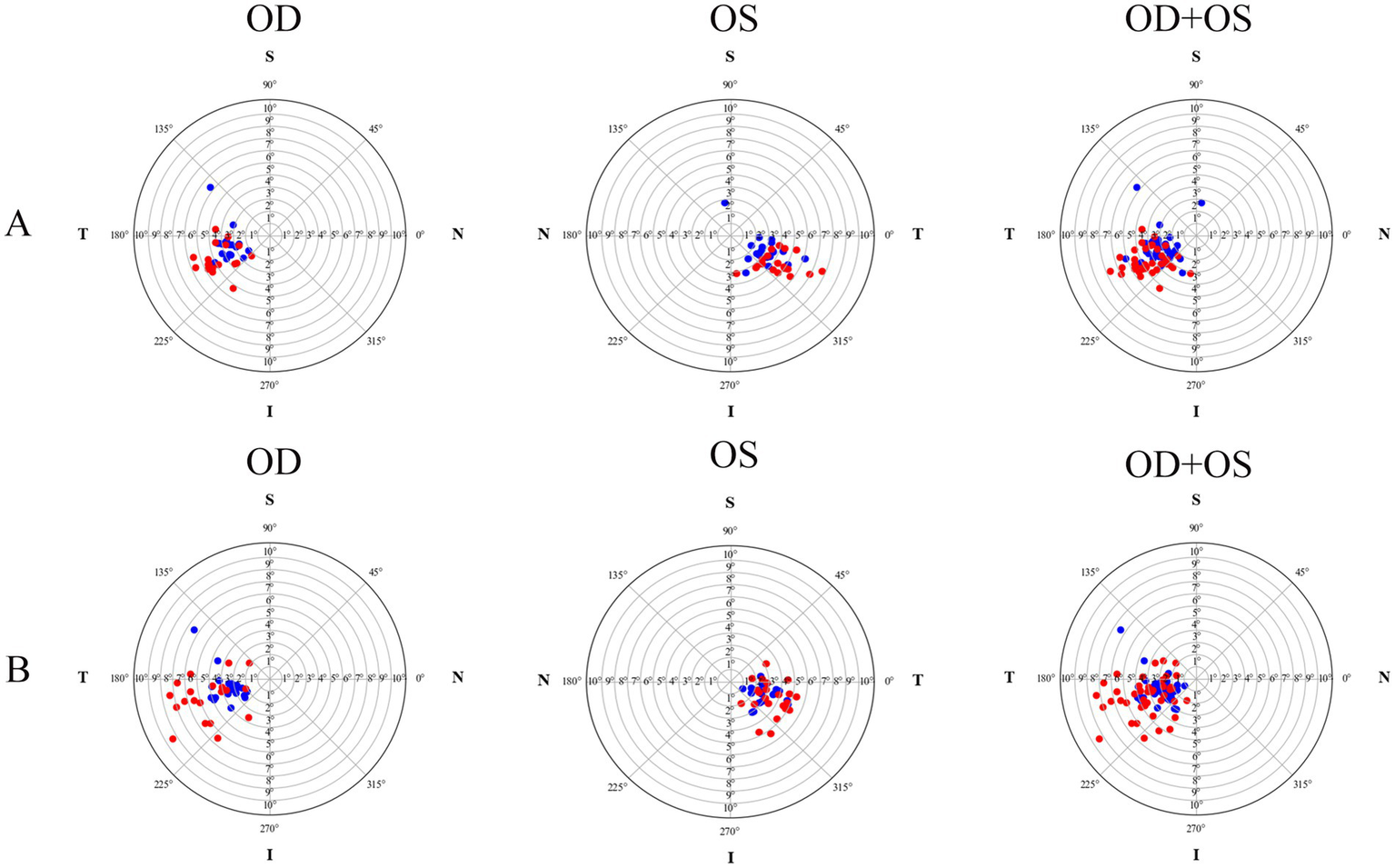
The tilt distribution pattern of crystalline lenses and intraocular lenses for both the plate-haptic group (A) and the C-loop group (B). The blue markers indicate crystalline lenses, while the red markers represent the IOLs. OD refers to the right eye, and OS denotes the left eye.
Figure 2
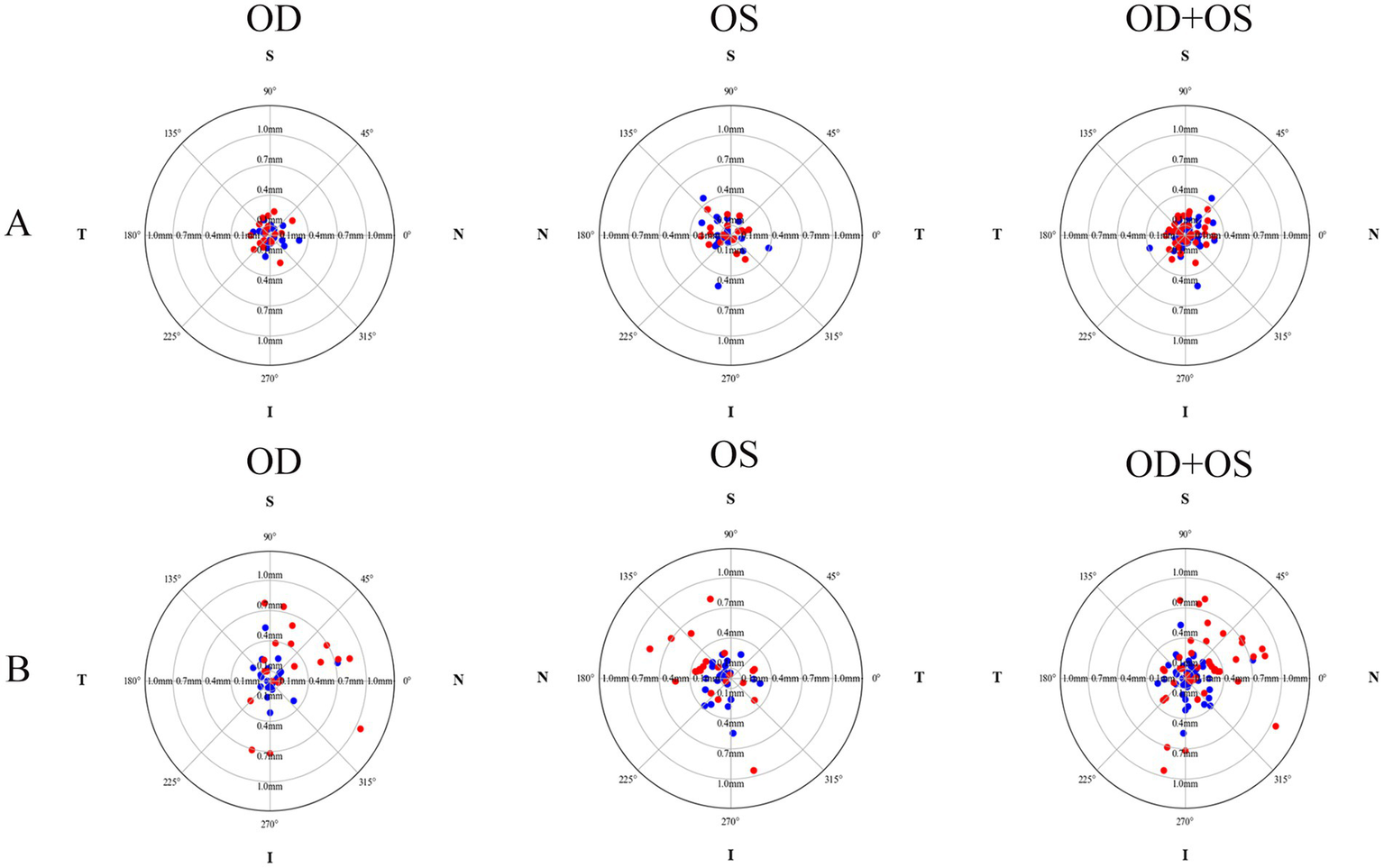
The decentration distribution pattern of crystalline lenses and intraocular lenses for both the plate-haptic group (A) and the C-loop group (B). The blue markers indicate the crystalline lenses, while the red markers represent the IOLs. OD refers to the right eye, and OS denotes the left eye.
In Group A, both univariate (β = 0.767, p < 0.001) and multivariate (β = 0.738, p < 0.001) linear regression analyses demonstrated a significant correlation between IOL tilt and preoperative crystalline lens tilt. Similarly, in Group B, both univariate (β = 0.774, p = 0.002) and multivariate (β = 0.759, p = 0.003) linear regression analyses confirmed that IOL tilt was exclusively correlated with preoperative lens tilt (Table 2).
Table 2
| Parameters | Group A (37 eyes) | Group B (42 eyes) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate linear regression | Multivariate linear regression | Univariate linear regression | Multivariate linear regression | |||||||||
| R2 | β (95%CI) | p | β (95%CI) | p | VIF | R2 | β (95%CI) | p | β (95%CI) | p | VIF | |
| AL (mm) | 0.030 | −0.254 (−0.749, 0.242) | 0.306 | −0.154 (−0.567, 0.259) | 0.453 | 1.099 | 0.006 | −0.148 (−0.749, 0.452) | 0.620 | −0.345 (−1.001, 0.310) | 0.292 | 1.469 |
| ACD (mm) | 0.032 | −0.557 (−1.615, 0.501) | 0.292 | −0.171 (−1.284, 0.943) | 0.757 | 1.751 | 0.006 | 0.384 (−1.232, 2.001) | 0.633 | 1.046 (−1.212, 3.305) | 0.354 | 2.403 |
| LT (mm) | 0.039 | 0.550 (−0.391, 1.492) | 0.243 | 0.267 (−0.695, 1.229) | 0.575 | 1.640 | 0.003 | 0.278 (−1.335, 1.890) | 0.730 | 1.142 (−0.785, 3.068) | 0.237 | 1.763 |
| Lens tilt (°) | 0.367 | 0.767 (0.421, 1.113) | <0.001 | 0.783 (0.441, 1.124) | <0.001 | 1.010 | 0.212 | 0.774 (0.297, 1.251) | 0.002 | 0.759 (0.267, 1.250) | 0.003 | 1.038 |
| Lens decentration (mm) | 0.043 | −2.345 (−6.150, 1.459) | 0.219 | −2.277 (−5.502, 0.949) | 0.160 | 1.123 | 0.027 | 2.218 (−2.024, 6.459) | 0.297 | 2.312 (−1.638, 6.263) | 0.243 | 1.046 |
Univariate and multivariate linear regression analyses of IOL tilt with preoperative parameters in both groups.
AL, axial length; ACD, anterior chamber depth; LT, lens thickness. For Group A, the multiple linear regression yielded an R2 of 0.462 and an Adjusted R2 of 0.376. For Group B, the multiple linear regression resulted in an R2 of 0.279 and an Adjusted R2 of 0.179.
Both univariate and multivariate linear regression analyses indicated that IOL decentration in both groups was not significantly correlated with AL, ACD, LT, preoperative crystalline lens tilt, or decentration (all p > 0.05, Table 3). Moreover, no significant correlations between postoperative BCVA, postoperative astigmatism magnitude, and IOL tilt and decentration (all p > 0.05).
Table 3
| Parameters | Group A (37 eyes) | Group B (42 eyes) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate linear regression | Multivariate linear regression | Univariate linear regression | Multivariate linear regression | |||||||||
| R2 | β (95%CI) | p | β (95%CI) | p | VIF | R2 | β (95%CI) | p | β (95%CI) | p | VIF | |
| AL (mm) | 0.041 | −0.019 (−0.051, 0.013) | 0.231 | −0.021 (−0.054, 0.013) | 0.219 | 1.099 | 0.008 | 0.025 (−0.063, 0.112) | 0.574 | −0.032 (−0.137, 0.072) | 0.533 | 1.469 |
| ACD (mm) | 0.005 | −0.014 (−0.084, 0.055) | 0.677 | 0.022 (−0.069, 0.112) | 0.629 | 1.751 | 0.067 | 0.192 (−0.037, 0.420) | 0.098 | 0.307 (−0.053, 0.667) | 0.092 | 2.403 |
| LT (mm) | 0.040 | 0.037 (−0.025, 0.098) | 0.233 | 0.059 (−0.019, 0.137) | 0.133 | 1.640 | 0.002 | −0.036 (−0.271, 0.199) | 0.760 | 0.167 (−0.140, 0.474) | 0.277 | 1.763 |
| Lens tilt (°) | 0.003 | 0.005 (−0.024, 0.033) | 0.746 | 0.002 (−0.026, 0.030) | 0.894 | 1.010 | 0.023 | 0.037 (−0.041, 0.114) | 0.342 | 0.025 (−0.053, 0.104) | 0.516 | 1.038 |
| Lens decentration (mm) | 0.042 | 0.150 (−0.097, 0.398) | 0.226 | 0.209 (−0.053, 0.471) | 0.114 | 1.123 | 0.045 | 0.418 (−0.195, 1.031) | 0.176 | 0.356 (−0.274, 0.985) | 0.259 | 1.046 |
Univariate and multivariate linear regression analyses of IOL decentration with preoperative parameters in both groups.
AL, axial length; ACD, anterior chamber depth; LT, lens thickness. For Group A, the multiple linear regression yielded an R2 of 0.161 and an Adjusted R2 of 0.025. For Group B, the multiple linear regression resulted in an R2 of 0.140 and an Adjusted R2 of 0.020.
Discussion
Building on our previous research, this study utilized a CNN with SS-OCT to compare the positioning of monofocal IOLs featuring plate-haptic and three-piece C-loop designs. The findings revealed that the three-piece C-loop design showed greater postoperative decentration than the plate-haptic design, although no significant difference in tilt was observed. The crystalline lens and IOL predominantly leaned toward the inferotemporal quadrant, with a notable positive correlation between their tilts.
An IOL usually displays a reasonable degree of tilt and decentration. The factors contributing to IOL tilt and decentration can be attributed primarily to three aspects (8, 20–22): (1) Patient factors: Research findings revealed that highly myopic eyes with AL ≥ 30 mm are prone to IOL decentration after surgery due to zonular laxity (23). Moreover, AL negatively correlates with IOL tilt (2, 24). Additionally, capsular bag contraction creates uneven circumferential traction forces on the IOL, further increasing the risk of IOL tilt and decentration (25). Furthermore, gravitational forces and zonular support change when patients transition from sitting to supine positions, potentially affecting IOL positioning. (2) IOL material and design: Table 4 offers a retrospective summary of previous studies, indicating that IOLs with various three-piece C-loop designs typically display more pronounced tilt and decentration values. In contrast, IOLs with open or full-plate haptic designs exhibit minimal tilt and decentration. On the other hand, hydrophobic IOLs, compared to hydrophilic IOLs, can reduce the risk of capsular bag contraction (21), thereby alleviating IOL tilt and decentration. Additionally, angulated haptic-optic junction position, orientation, and sharp-edge designs (26, 27), which may contribute to IOL decentration, negative dysphotopsia, and the incidence of posterior capsule opacification (PCO), further influence IOL stability. (3) Surgical factors: intraoperative posterior capsular rupture, zonular dehiscence, eccentric or irregular capsulorhexis (21), and vitreous prolapse could also increase the IOL tilt and decentration (28).
Table 4
| Author | Year | IOL types (eyes) | Method | Result | ||
|---|---|---|---|---|---|---|
| Tilt (°) | Decentration (mm) | |||||
| Mutlu et al. (38) | 2005 | Single-piece C-loop: SA30AL (43 eyes) Three-piece C-loop: MA30BA (45 eyes) |
Purkinje | SA30AL (2.70 ± 0.84) MA30BA (2.72 ± 0.55) |
SA30AL (0.34 ± 0.08) MA30BA (0.39 ± 0.13) |
|
| Hayashi et al. (39) | 2005 | Single-piece C-loop: SA30AL (30 eyes) Three-piece C-loop: MA60BM (30 eyes) |
Scheimpflug | SA30AL (1.64 ± 1.00) MA60BM (1.67 ± 0.94) |
SA30AL (0.24 ± 0.18) MA60BM (0.22 ± 0.13) |
|
| Crnej et al. (40) | 2011 | Three-piece C-loop: MA60AC (15 eyes) Single-piece C-loop: SA60AT (15 eyes) Open-loop plate haptic: Akreos Adapt (30 eyes) |
Purkinje | Akreos Adapt (Vertical 1.50 ± 1.10; Horizontal 2.90 ± 0.90) SA60AT (2.20 ± 7.20) MA60AC (5.30 ± 2.40) |
Akreos Adapt (Vertical 0.40 ± 0.20; Horizontal 0.40 ± 0.20) SA60AT (0.40 ± 0.30) MA60AC (0.60 ± 0.80) |
|
| Zhong et al. (41) | 2016 | Single-piece C-loop: ZCB00 (40 eyes) Three-piece C-loop: ZA9003 (40 eyes) |
Scheimpflug | ZA9003 (1.01 ± 0.45) ZCB00 (1.06 ± 0.49) |
ZA9003 (0.20 ± 0.17) ZCB00 (0.22 ± 0.17) |
|
| Meng et al. (42) | 2020 | Single-piece C-loop: ZMB00 (59 eyes) Full plate haptic: 839MP (63 eyes) |
OPD-Scan III | - | 21.0 mm<AL ≤ 24.5 mm | 839MP (0.14 ± 0.08, 30 eyes) |
| ZMB00 (0.19 ± 0.11, 28 eyes) | ||||||
| AL>24.5 mm | 839MP (0.16 ± 0.10, 33 eyes) | |||||
| ZMB00 (0.41 ± 0.15, 31 eyes) | ||||||
| Xiao et al. (11) | 2021 | Open-loop single-piece C-loop: 920H (25 eyes) Single-piece C-loop: Softec HD (24 eyes) full plate haptic: 509 M (19 eyes) |
CASIA2 | 509 M (4.96 ± 0.89) 920H (5.52 ± 1.74) Softec HD (6.28 ± 1.83) |
509 M (0.12 ± 0.06) 920H (0.19 ± 0.12) Softec HD (0.22 ± 0.13) |
|
IOL tilt and decentration with various haptic designs from previous studies.
AL, axial length; −, no data reported; Vertical, IOL was positioned vertically; Horizontal, IOL was positioned horizontally.
Kimura et al. (10) measured crystalline lens tilt and decentration under natural pupil conditions in 41 eyes of cataract patients, reporting a tilt of 5.15 ° toward the inferotemporal direction relative to the corneal topographic axis and a decentration of 0.11 mm. Gu et al. (2) evaluated 56 subjects with SN60WF implantation under similar conditions, finding the lens and IOL tilt to be (4.90 ± 1.81)° and (4.75 ± 1.66)°, respectively, while the lens and IOL decentration both were (0.21 ± 0.02) mm. Preoperative crystalline lenses and postoperative IOLs were tilted toward the inferotemporal direction in the right and left eyes. Additionally, Kim et al. conducted an IOL tilt and decentration analysis 12 months post-surgery in 78 subjects, yielding results of (4.20 ± 1.70)° for tilt and (0.24 ± 0.15) mm for decentration (29). Although all of these studies used SS-OCT as the imaging modality, there are both similarities and discrepancies in the findings. These variations may be attributed to differences in the baseline characteristics of the study populations, sample sizes, IOL types, and the analysis algorithms implemented. In the present study, both groups of crystalline lenses and IOLs generally tilted toward the inferotemporal direction, exhibiting mirror symmetry between the eyes, which aligns with previous research results (24, 30).
Unlike the IOL types examined in previous studies (Table 4), this research includes a single-piece plate-haptic IOL and a three-piece IOL (31, 32). The postoperative decentration measured in Group A (0.16 ± 0.08 mm) was significantly smaller than in Group B (0.42 ± 0.28 mm). The disparity may be attributed to differences in IOL design and size. The 709 M has four distinct points contacting the capsular bag with an approximate total contact length of 12.53 mm. Conversely, the PY-60 AD incorporates a two-point contact configuration, with an estimated total contact length of 12.50 mm. The integrity of IOL positioning can be compromised by factors such as postoperative anterior capsule contraction, capsulotomy size, and centration, all of which contribute to an asymmetrical distribution of forces on the IOL optic. The PY-60 AD’s two-point contact design is hypothesized to be more susceptible to these asymmetrical forces than the 709 M’s four-point design, potentially leading to greater decentration.
In this study, IOL tilt showed a positive correlation with crystalline lens tilt in both groups; however, IOL decentration did not correlate significantly with preoperative ocular parameters. Gu et al. (2) used CASIA2 to measure tilt and decentration in 56 eyes (with AL ranging from 21.38 to 32.27 mm) that received a single-piece C-loop SN60WF IOL during surgery. In a multiple linear regression analysis, they found that postoperative IOL tilt was significantly related to preoperative lens tilt (β = 0.565, p < 0.001), AL (β = −0.173, p = 0.033), and LT (β = −1.009, p = 0.011). Furthermore, IOL decentration correlated with lens decentration (β = 0.620, p < 0.001) and AL (β = 0.041, p < 0.001). The differences observed between their results and those of this study may be partly attributable to variations in measurement equipment, the characteristics of the study populations (with AL ranging from 21.00 to 26.0 mm), the duration of follow-up, and the reference axes (this study utilized the corneal vertex as the reference, while CASIA2 relied on the corneal topography axis (33)). Nevertheless, there is a consistent positive correlation between the crystalline lens tilt and IOL tilt across these studies.
While a certain degree of IOL tilt (up to 2–3°) and decentration (0.2–0.3 mm) are often clinically unnoticed with many IOL designs, it is important to recognize that these values are averages, and individual patient experiences may vary (9, 34). Optical bench analyses further demonstrate that decentration can result in a significant reduction in modulation transfer function values and an increase in wavefront aberration errors (35). Research by Ruiz-Alcocer et al., using an optical bench, has shown that IOL tilt and decentration can diminish through-focus optical quality, particularly with premium IOLs and post-LASIK corneas (3–5). The lack of significant correlation between postoperative astigmatism magnitude, BCVA, and IOL tilt and decentration in this study is primarily attributable to the relatively narrow range of tilt and decentration observed and to the use of a monofocal lens.
This study presents several limitations: Firstly, as a retrospective study, the intraocular higher-order aberrations (HOAs) were not assessed. Secondly, the follow-up period in the current study is relatively short, and the results from the first postoperative month mainly reflect the immediate postoperative force. They may not be directly used to infer long-term stability differences. Also, the manual capsulorhexis quality, especially the capsulorhexis size, circularity, centration, and the IOL material properties, IOL weight, could also affect the IOL stability in the long term (36, 37). Therefore, future prospective investigations with a larger sample size, longer follow-up, and more capsulorhexis metrics, visual quality parameters (including HOAs) will offer deeper insights into the long-term stability and visual impact of IOL positioning. However, we still believe that the current study will provide valuable information on premium IOLs and Toric IOLs with similar haptic designs for clinical reference in the short term (4, 5).
In conclusion, this research underscores that plate-haptic design monofocal IOLs exhibit less postoperative decentration compared to C-loop design IOLs. Additionally, a significant positive correlation was observed between the crystalline lens and the IOL’s spatial position. Therefore, clinicians should consider the preoperative position of the crystalline lens and the potential differences in IOL decentration across different haptic designs during IOL selection.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Shanxi Eye Hospital Affiliated to Shanxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JY: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. KL: Data curation, Formal analysis, Methodology, Software, Writing – review & editing. FC: Data curation, Formal analysis, Writing – review & editing. JW: Data curation, Formal analysis, Methodology, Writing – review & editing. ZW: Data curation, Formal analysis, Methodology, Software, Writing – review & editing. XW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by Fundamental Research Program of Shanxi Province (Grant No. 202403021211120) statement for human subjects. The institutional review board of the Shanxi Eye Hospital Affiliated to Shanxi Medical University approved the study protocol (No. 2019LL130).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Holladay JT Piers PA Koranyi G van der Mooren M Norrby NE . A new intraocular lens design to reduce spherical aberration of pseudophakic eyes. J Refract Surg. (2002) 18:683–91. doi: 10.3928/1081-597x-20021101-04
2.
Gu X Chen X Yang G Wang W Xiao W Jin G et al . Determinants of intraocular lens tilt and decentration after cataract surgery. Ann Transl Med. (2020) 8:921. doi: 10.21037/atm-20-1008,
3.
Ruiz-Alcocer J Martínez-Alberquilla I Rementería-Capelo LA De Gracia P Lorente-Velázquez A . Changes in optical quality induced by tilt and decentration of a trifocal IOL and a novel extended depth of focus IOL in eyes with corneal myopic ablations. J Refract Surg. (2021) 37:532–7. doi: 10.3928/1081597x-20210518-03
4.
Ruiz-Alcocer J Lorente-Velázquez A de Gracia P Madrid-Costa D . Optical tolerance to rotation of trifocal toric intraocular lenses as a function of the cylinder power. Eur J Ophthalmol. (2021) 31:1007–13. doi: 10.1177/1120672120926845,
5.
Ruiz-Alcocer J Lorente-Velázquez A Hernández-Verdejo JL De Gracia P Madrid-Costa D . Optical performance of a trifocal IOL and a novel extended depth of focus IOL combined with different corneal profiles. J Refract Surg. (2020) 36:435–41. doi: 10.3928/1081597x-20200519-02,
6.
Zhu X Meng J He W Rong X Lu Y . Comparison of the rotational stability between plate-haptic toric and C-loop haptic toric IOLs in myopic eyes. J Cataract Refract Surg. (2020) 46:1353–9. doi: 10.1097/j.jcrs.0000000000000259
7.
Gao X Zhou D Jiang Y Liu Z Zhang J Deng G . Comparison of clinical outcomes and capsular bag stability between plate-haptic and C-loop haptic Toric IOLs in cataract eyes. Eur J Ophthalmol. (2025) 35:1992–2000. doi: 10.1177/11206721251349041,
8.
Chen XY Wang YC Zhao TY Wang ZZ Wang W . Tilt and Decentration with various intraocular lenses: a narrative review. World J Clin Cases. (2022) 10:3639–46. doi: 10.12998/wjcc.v10.i12.3639,
9.
Ashena Z Maqsood S Ahmed SN Nanavaty MA . Effect of intraocular lens tilt and decentration on visual acuity, dysphotopsia and wavefront aberrations. Vision. (2020) 4:41. doi: 10.3390/vision4030041,
10.
Kimura S Morizane Y Shiode Y Hirano M Doi S Toshima S et al . Assessment of tilt and decentration of crystalline lens and intraocular lens relative to the corneal topographic axis using anterior segment optical coherence tomography. PLoS One. (2017) 12:e0184066. doi: 10.1371/journal.pone.0184066,
11.
Xiao Z Wang G Zhen M Zhao Z . Stability of intraocular lens with different haptic design: a swept-source optical coherence tomography study. Front Med. (2021) 8:705873. doi: 10.3389/fmed.2021.705873,
12.
Donnenfeld E . The pupil is a moving target: centration, repeatability, and registration. J Refract Surg. (2004) 20:S593–6. doi: 10.3928/1081-597x-20040901-35,
13.
Li L Wang K Yan Y Song X Liu Z . Research on calculation of the IOL tilt and decentration based on surface fitting. Comput Math Methods Med. (2013) 2013:572530. doi: 10.1155/2013/572530,
14.
Nishi Y Hirnschall N Crnej A Gangwani V Tabernero J Artal P et al . Reproducibility of intraocular lens decentration and tilt measurement using a clinical purkinje meter. J Cataract Refract Surg. (2010) 36:1529–35. doi: 10.1016/j.jcrs.2010.03.043,
15.
Montés-Micó R Nilsson M Brautaset R Venkataraman AP Domínguez-Vicent A . Analysis of crystalline lens tilt and decentration using a fully automated swept-source optical coherence tomography biometer. J Refract Surg. (2025) 41:e155–63. doi: 10.3928/1081597x-20241230-03,
16.
Gonzalez-Lopez F Delgado-Tirado S Perez-Izquierdo R Gonzalez-Gonzalez D Medel D Saez-Corrales A et al . Tilt and decentration of the crystalline lens and intraocular lens after femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. (2025). doi: 10.1097/j.jcrs.0000000000001770,
17.
Wang X Dong J Wang X Wu Q . IOL tilt and Decentration estimation from 3 dimensional reconstruction of OCT image. PLoS One. (2013) 8:e59109. doi: 10.1371/journal.pone.0059109,
18.
Dong J Wang XL Deng M Wang XG . Three-dimensional reconstruction and swept-source optical coherence tomography for crystalline lens tilt and decentration relative to the corneal vertex. Transl Vis Sci Technol. (2021) 10:13. doi: 10.1167/tvst.10.9.13,
19.
Li K Yang G Chang S Yao J He C Lu F et al . Comprehensive assessment of the anterior segment in refraction corrected OCT based on multitask learning. Biomed Opt Express. (2023) 14:3968–87. doi: 10.1364/boe.493065,
20.
Chen X Gu X Wang W Xiao W Jin G Wang L et al . Characteristics and factors associated with intraocular lens tilt and decentration after cataract surgery. J Cataract Refract Surg. (2020) 46:1126–31. doi: 10.1097/j.jcrs.0000000000000219,
21.
Findl O Hirnschall N Draschl P Wiesinger J . Effect of manual capsulorhexis size and position on intraocular lens tilt, centration, and axial position. J Cataract Refract Surg. (2017) 43:902–8. doi: 10.1016/j.jcrs.2017.04.037,
22.
Jeon YY Park N Lee H Eah KS Han J Chung HS et al . Analysis of intraocular lens tilt and decentration after cataract surgery in eyes with high myopia using the anterior segment optical coherence tomography. Sci Rep. (2024) 14:27987. doi: 10.1038/s41598-024-78759-8,
23.
Wang L Jin G Zhang J Chen X Tan X Wang W et al . Clinically significant intraocular lens decentration and tilt in highly myopic eyes: a swept-source optical coherence tomography study. Am J Ophthalmol. (2022) 235:46–55. doi: 10.1016/j.ajo.2021.08.017,
24.
Wang L de Guimaraes Souza R Weikert MP Koch DD . Evaluation of crystalline lens and intraocular lens tilt using a swept-source optical coherence tomography biometer. J Cataract Refract Surg. (2019) 45:35–40. doi: 10.1016/j.jcrs.2018.08.025
25.
Choi M Lazo MZ Kang M Lee J Joo CK . Effect of number and position of intraocular lens haptics on anterior capsule contraction: a randomized, prospective trial. BMC Ophthalmol. (2018) 18:78. doi: 10.1186/s12886-018-0742-1,
26.
Buehl W Findl O . Effect of intraocular lens design on posterior capsule opacification. J Cataract Refract Surg. (2008) 34:1976–85. doi: 10.1016/j.jcrs.2008.07.029,
27.
Shetty N Patel Y Ranade R Jain A Narasimhan R Bansod A et al . Evaluation of change in the contact of IOL with the posterior capsule with respect to the orientation of haptics of the iol using intraoperative spectral domain optical coherence tomography. Indian J Ophthalmol. (2025) 73:396–400. doi: 10.4103/ijo.Ijo_348_24,
28.
Tan X Liu Z Chen X Zhu Y Xu J Qiu X et al . Characteristics and risk factors of intraocular lens tilt and decentration of phacoemulsification after pars plana vitrectomy. Transl Vis Sci Technol. (2021) 10:26. doi: 10.1167/tvst.10.3.26,
29.
Kim BZ Lee H Jeon YY Eah KS Park N Chung HS et al . Assessment of intraocular lens tilt and decentration after femtosecond laser-assisted and conventional cataract surgery at 12 months and beyond. BMC Ophthalmol. (2024) 24:459. doi: 10.1186/s12886-024-03720-2,
30.
Li Z Zhu Z Li X Meng Z Qu W Zhao Y . Age-related changes in crystalline lens tilt and decentration: swept-source oct study. J Cataract Refract Surg. (2021) 47:1290–5. doi: 10.1097/j.jcrs.0000000000000632
31.
Bascaran L Mendicute J Macias-Murelaga B Arbelaitz N Martinez-Soroa I . Efficacy and stability of at Torbi 709 M toric IOL. J Refract Surg. (2013) 29:194–9. doi: 10.3928/1081597x-20130129-02
32.
Morgan-Warren PJ Smith JA . Intraocular lens-edge design and material factors contributing to posterior-capsulotomy rates: comparing Hoya Fy60ad, Py60ad, and Acrysof Sn60wf. Clin Ophthalmol. (2013) 7:1661–7. doi: 10.2147/opth.S48824,
33.
Zhang F Zhang J Li W Zhou L Feng D Zhang H et al . Correlative comparison of three ocular axes to tilt and decentration of intraocular lens and their effects on visual acuity. Ophthalmic Res. (2020) 63:165–73. doi: 10.1159/000504716,
34.
Ale JB . Intraocular lens tilt and decentration: a concern for contemporary IOL designs. Nepal J Ophthalmol. (2011) 3:68–77. doi: 10.3126/nepjoph.v3i1.4281
35.
Pérez-Gracia J Ávila FJ Ares J Vallés JA Remón L . Misalignment and tilt effect on aspheric intraocular lens designs after a corneal refractive surgery. PLoS One. (2020) 15:e0243740. doi: 10.1371/journal.pone.0243740,
36.
Kránitz K Takacs A Miháltz K Kovács I Knorz MC Nagy ZZ . Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J Refract Surg. (2011) 27:558–63. doi: 10.3928/1081597x-20110623-03,
37.
Chen Y Meng J Cheng K Lu Q Wei L Lu Y et al . Influence of IOL weight on long-term IOL stability in highly myopic eyes. Front Med. (2022) 9:835475. doi: 10.3389/fmed.2022.835475,
38.
Mutlu FM Erdurman C Sobaci G Bayraktar MZ . Comparison of tilt and decentration of 1-piece and 3-piece hydrophobic acrylic intraocular lenses. J Cataract Refract Surg. (2005) 31:343–7. doi: 10.1016/j.jcrs.2004.06.022,
39.
Hayashi K Hayashi H . Comparison of the stability of 1-piece and 3-piece acrylic intraocular lenses in the lens capsule. J Cataract Refract Surg. (2005) 31:337–42. doi: 10.1016/j.jcrs.2004.06.042,
40.
Crnej A Hirnschall N Nishi Y Gangwani V Tabernero J Artal P et al . Impact of intraocular lens haptic design and orientation on decentration and tilt. J Cataract Refract Surg. (2011) 37:1768–74. doi: 10.1016/j.jcrs.2011.04.028,
41.
Zhong X Long E Chen W Xiang W Liu Z Chen H et al . Comparisons of the in-the-bag stabilities of single-piece and three-piece intraocular lenses for age-related cataract patients: a randomized controlled trial. BMC Ophthalmol. (2016) 16:100. doi: 10.1186/s12886-016-0283-4,
42.
Meng J He W Rong X Miao A Lu Y Zhu X . Decentration and tilt of plate-haptic multifocal intraocular lenses in myopic eyes. Eye Vis. (2020) 7:17. doi: 10.1186/s40662-020-00186-3,
Summary
Keywords
cataract surgery, decentration, haptic, intraocular lens, tilt
Citation
Yao J, Li K, Chai F, Wang J, Wang Z and Wang X (2026) IOL tilt and decentration: a comparison of different haptic designs using CNN and SS-OCT in a short term. Front. Med. 13:1750166. doi: 10.3389/fmed.2026.1750166
Received
19 November 2025
Revised
07 January 2026
Accepted
07 January 2026
Published
22 January 2026
Volume
13 - 2026
Edited by
Weihua Yang, Shenzhen Eye Hospital, China
Reviewed by
Pablo De Gracia, University of Detroit Mercy, United States
Jin Li, Wenzhou Medical University, China
Updates
Copyright
© 2026 Yao, Li, Chai, Wang, Wang and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaogang Wang, movie6521@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.