- 1Laboratory of Synthetic Glycovaccines, N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, Russia
- 2Laboratory of Glycoconjugate Chemistry, N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, Russia
- 3N. N. Blokhin National Medical Research Center of Oncology, Ministry of Health of the Russian Federation, Moscow, Russia
Mannans are polysaccharide antigens expressed on the cell wall of different fungal species including Saccharomyces cerevisiae and Candida spp. These fungi are components of the normal intestinal microflora, and the presence of antibodies to fungal antigens is known to reflect the features of the patient’s immune system. Thus, titers of IgG and IgA antibodies against Saccharomyces cerevisiae mannan (ASCA) are markers for clinical diagnostics of inflammatory bowel diseases. The complex organization and heterogeneity of cell-wall mannans may reduce the quality and reproducibility of ELISA results due to interference by different antigenic epitopes. In this research, we analyzed the levels of IgG antibodies in the sera of healthy donors and patients with colorectal cancer using an array of synthetic oligosaccharides related to distinct fragments of fungal mannan. This study aimed to establish the influence of oligosaccharide structure on their antigenicity. Variations in the structure of the previously established ASCA epitope (changing type of linkage, chain length, and the presence of branches) significantly modified the ability of ligands to bind to circulating antibodies in blood sera. The study showed that surface presentation density of the ligand critically affects the results of enzyme immunoassay. The transition from natural coating antigens to their corresponding synthetic mimetics with a defined structure opens new opportunities for improving existing ELISA test systems, as well as developing diagnostic kits with new properties.
1 Introduction
Fungal cell-wall polysaccharides are dominant surface antigens stimulating immune reactions in humans (Erwig and Gow, 2016). The heterogeneity and high variability of substructures in polysaccharides lead to a multiplicity of so-called “antigenic factors” (Suzuki, 1997) and the generation of a repertoire of antibodies with varying specificity (Solovev et al., 2023). Moreover, the ability of different carbohydrate structures to elicit an antibody response varies depending on their structure. Thus, the use of oligosaccharide ligands as model antigens with distinct structures related to the fragments of polysaccharide components of the fungal cell wall opens the possibility of a comparative study of their immunological properties (Krylov and Nifantiev, 2020). This was demonstrated by several examples. Particularly, our previous works showed that β-oligomannoside fragments of Candida albicans mannan (Figure 1A) generated a higher antibody response than antigenic factors with solely α-linked chains (Solovev et al., 2023). The specificity of the monoclonal antibody EBCA-1 used in sandwich immune assay to detect Candida mannan was recently reinvestigated, and it was shown that EBCA-1 recognizes the trisaccharide β-Man-(1→2)-α-Man-(1→2)-α-Man and not homo-α-(1→2)-linked pentamannoside, as reported previously (Jacquinot et al., 1998; Krylov et al., 2021). The study of the immunogenic properties of Aspergillus fumigatus galactomannan using a library of synthetic oligosaccharide antigens showed that oligogalactofuranoside fragments but not oligomannoside chains are responsible for immune reaction and elicitation of anti-galactomannan antibodies (Wong et al., 2020).
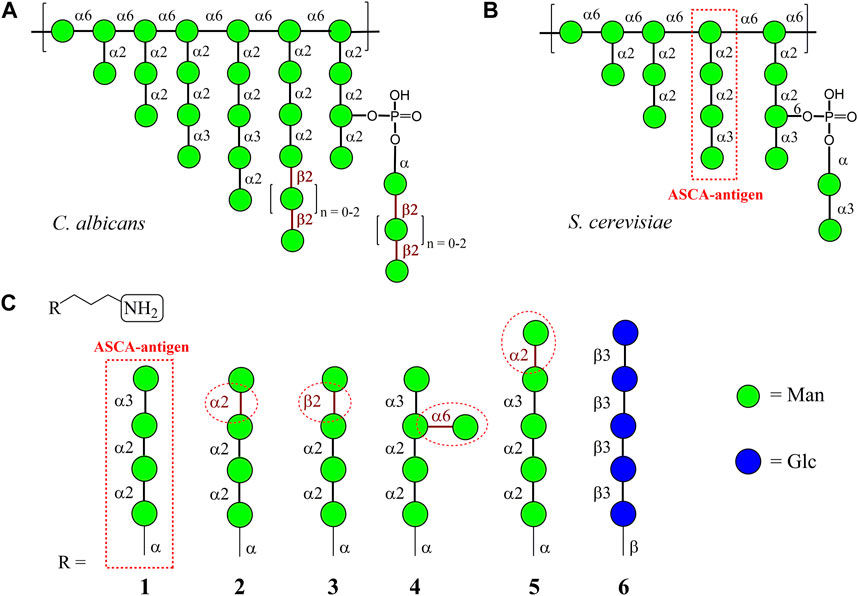
FIGURE 1. Tentative structures of C. albicans (Suzuki, 1997) (A) and S. cerevisiae (Vinogradov et al., 1998) (B) mannans; (C) carbohydrate sequences in the oligomannosides R-(CH2)3NH2 1–5 used for glycoarray creation in this work. Penta-β-(1→3)-Glucoside 6, the fragment of β-D-glucan of the fungal cell wall, was used as the control sample.
Saccharomyces cerevisiae is the common yeast utilized in biotechnology in many food fermentations and other industrial processes (Parapouli et al., 2020). This yeast is an important part of the normal fungal microbiota and is generally regarded as safe; however, recent studies have provided evidence of its involvement in a range of superficial and systemic diseases (Enache-Angoulvant and Hennequin, 2005).
The first cellular component interacting with the host immune system is the yeast cell wall (Erwig and Gow, 2016). Orlean (2012) describedthe architecture and biosynthesis of the S. cerevisiae cell wall in detail. Its main carbohydrate component is a branched mannan (Figure 1B), which amounts to approximately half of the total mass of the cell wall and plays an important role in immune response and its regulation (Orlean, 2012).
The detection of antibodies against S. cerevisiae mannan, known as ASCA, is used for the differential diagnosis of inflammatory bowel diseases (Szilagyi et al., 2014) such as Crohn’s disease and ulcerative colitis (Main et al., 1988). A comparative study of different ASCA-detecting assays revealed their moderate sensitivity (41%–76%) and good specificity (86%–98%) (Vermeire et al., 2001). The role of antibodies to S. cerevisiae mannan in inflammatory bowel diseases is not clearly determined. However, a growing number of studies have detected high levels of ASCA in patients affected with autoimmune diseases, including antiphospholipid syndrome, systemic lupus erythematosus, type 1 diabetes mellitus, rheumatoid arthritis, spondyloarthritis, and hidradenitis suppurativa (Rinaldi et al., 2013; Maillet et al., 2016; Assan et al., 2020). Probably, an imbalance of immune tolerance to commensal microbiota (e.g., S. cerevisiae) can trigger systemic autoimmune disorders (Temme et al., 2021). Thus, anti-S. cerevisiae mannan antibodies have high potential as clinical diagnostic markers and require a more detailed study of their immunological properties.
In this paper, we describe the results of screening ASCA-related antibodies in the sera of patients with colorectal cancer and healthy controls using glycoconjugates containing distinct oligosaccharide ligands present in the chains of S. cerevisiae mannan. To the best of our knowledge, such comparative studies have never been performed. The used glycoconjugates were selected instead of natural polysaccharides because of substantial variations in ASCA titers between assays from different manufacturers (Vermeire et al., 2001) in studies where polysaccharide antigens were employed.
2 Materials and methods
2.1 Conjugates of synthetic oligosaccharides with biotin and polyacrylamide
Biotinylated conjugates of ligand 1 (Figure 1C) were chemically synthesized by coupling of parent aminospacered oligosaccharides (Karelin et al., 2007) with an activated biotin derivative containing a hydrophilic hexaethylene glycol linker (Tsvetkov et al., 2011), as previously described (Krylov et al., 2018).
For preparation of polyacrylamide (PAA) conjugates, solutions of parent oligosaccharides (Karelin et al., 2007; Argunov et al., 2011; Karelin et al., 2016; Yashunsky et al., 2016; Krylov and Nifantiev, 2020) (1 eq) and p-nitrophenyl polyacrylate (190 mmol of p-nitrophenol groups per 1 mg; 5, 10, 20, or 40 eq) in dry DMF were incubated for 2 h at 25°C. Subsequently, n-butylamine (2.5 eq) was added, and the mixture was incubated for another 2 h at 25°C. Ethanolamine (20 µL) was added, and the reaction mixture was incubated overnight at room temperature. The reaction mixture was concentrated under reduced pressure on a rotovap, and the dry solid was purified with size-exclusive chromatography on Sephadex LH-20 in MeCN:H2O (1:1). PAA conjugate fractions were concentrated under reduced pressure on a rotovap and then lyophilized to yield beige solid. 1H NMR spectra of obtained conjugates were recorded in D2O on a Bruker AV600 (600 MHz) spectrometer.
2.2 Human donor sera
The collection of biological material was carried out in the Laboratory of Clinical Biochemistry at the N. N. Blokhin National Medical Research Center of Oncology of the Ministry of Health of Russia. Prior to use, blood serum samples were stored at −152°C. The study included 30 patients with colorectal cancer (15 women aged 56.5 ± 13.1 and 15 men aged 58.6 ± 8.9 years). In all patients, colorectal cancer was detected for the first time and confirmed by histological examination of the tumor. Straight intestine cancer was diagnosed in five patients, blind intestine cancer in four patients, sigmoid colon cancer in 11 patients, ascending part of the colon in five patients, and descending part of the colon in five patients. Three patients with CRC had stage 1 of the disease, nine had stage II, 12 had stage III, and six had stage IV. According to the histological structure, all tumors are characterized as adenocarcinoma. The control group consisted of 18 healthy donors. The information about patients and healthy donors is summarized in Supplementary Tables S1, S2. The healthy donors included nine women aged 50.1 ± 17.7 years and nine men aged 43 ± 17.1 years. This study was approved by the Ethics Committee of the N. N. Blokhin National Medical Research Center of Oncology of the Ministry of Health of the Russian Federation on 11 May 2022 (Protocol No. 5 as of 05/11/2022). Before screening, human blood sera was diluted 400 times in PBS containing 0.05% Tween-20 and 0.1% BSA.
2.3 Glycoarray
Polyacrylamide conjugates of oligosaccharides 1–6 were absorbed on the wells of polystyrene plates (Xema, Russia) (100 µL of a 5 μg/mL solution in PBS) for 24 h at 4°С. Subsequently, the wells were washed with PBS one time and blocked with casein (Sigma-Aldrich, Germany) for 1 h at 37°С. After shaking out and drying, the plates were ready to use. The wells of 96-well avidin-coated plates (Xema, Russia) were coated with biotin-tagged oligosaccharide 1 (Figure 1) (100 µL of 200 pmol/mL solution in PBS containing 0.05% Tween-20 and 0.1% BSA) and then incubated for 2 h at 37°C. After washing three times, the plates were ready to use. The ready-to-use plate provided in the Anti-Saccharomyces cerevisiae Kit EV2841-9601G (EUROIMMUN AG, Germany) was used as a natural antigen reference.
The plates were incubated with calibration sera provided in the Anti-Saccharomyces cerevisiae Kit EV2841-9601G (EUROIMMUN AG, Germany) or diluted human sera for 45 min at 37°C. After washing three times, the wells were treated with conjugates of anti-human IgG Ab with peroxidase (d5000; IMTEK, Russia) and incubated for 30 min at 37°C. The plates were washed five times, and color was developed using 100 µL of TMB monocomponent substrate (Xema, Russia) for 15 min. The reaction was stopped with 50 µL of 1 M sulfuric acid. Absorbance was measured at 450 nm using a Multiskan GO plate reader (Thermo Fisher Scientific, United States). All measurements were repeated independently and performed twice in triplicate.
2.4 Statistical analysis
One-way analysis of variance (ANOVA) was used to compare the differences between six carbohydrate ligands. The antibody levels between the two groups were compared using the Mann–Whitney test. The bars represent the mean values with standard deviation (SD).
3 Results
Mannans of S. cerevisiae (Vinogradov et al., 1998), C. albicans (Suzuki, 1997), and other fungi are highly branched polysaccharide antigens that have common and specific structural fragments (Figures 1A, B). For example, both polysaccharides include fragments related to a similar group of antigenic factors composed of α-(1→2)/α-(1→3)-linked mannoside residues. On the other hand, side chains with β-(1→2)-linked mannose residues, which have the highest impact on antibody response (Sendid et al., 2021), were found only in Candida spp. (Singh et al., 2023). The oligomannoside sequence within S. cerevisiae mannan corresponding to antibodies associated with Crohn’s disease was assigned to be the following mannotetraoside: Man(1→3)Man(1→2)Man(1→2)Man (Sendid et al., 1996; Chevalier et al., 2005), which is illustrated in Figure 1B. Therefore, the corresponding oligosaccharide 1 (Karelin et al., 2007) was selected in this study as a basis for the creation of structurally related glycoarray (Figure 1C). Ligands 2 (Karelin et al., 2007) and 3 (Karelin et al., 2016) stem from 1 after formally replacing the terminal α-(1→3)-mannoside fragment with α-(1→2)- and β-(1→2)-mannoside units, respectively. Additional glycosylation of ligand 1 leads to the formation of ligands 4 and 5. Branched oligosaccharide 4 (Argunov et al., 2011) contains the branch at O(6) of the central mannose residue, and pentasaccharide 5 is glycosylated with α-(1→2)-mannose at the terminal end of the original antigen 1. The β-(1→3)-glucan fragment 6 (Yashunsky et al., 2016) was taken as a representative of another class of fungal cell-wall polysaccharides and used as a negative control ligand.
Two types of glycoconjugates as coating antigens for ELISA studies were used in this work. They included biotinylated conjugates (Figure 2A), which were prepared by coupling of compounds 1–6 with activated pentafluorophenyl ester of hexaethylene glycol-tagged biotin (Tsvetkov et al., 2011). Obtained biotinylated oligosaccharides were immobilized on avidin-coated plates and used for sera screening. It appeared that biotinylated α-linked mannosides 1, 2, 4, and 5 did not show any significant signals for healthy donor sera (Figure 3) or for patients with colorectal cancer (Solovev et al., 2023). We hypothesized that loading of oligosaccharide ligands onto microtiter well surfaces with higher density may improve the sensitivity of ELISA and permit better detection of low-affinity antibodies.
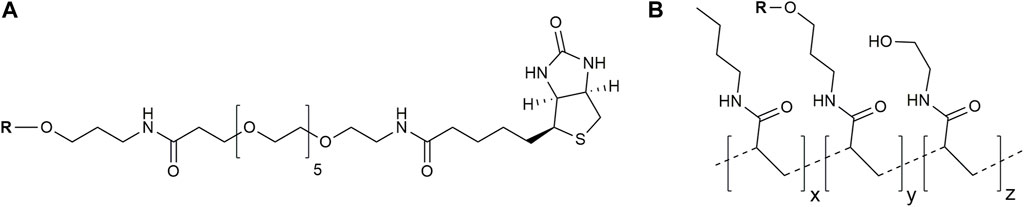
FIGURE 2. Structures of used glycoconjugates: biotinylated conjugates (A) and PAA-based conjugates (B) (the tentative structure is shown). R, oligosaccharide ligand.
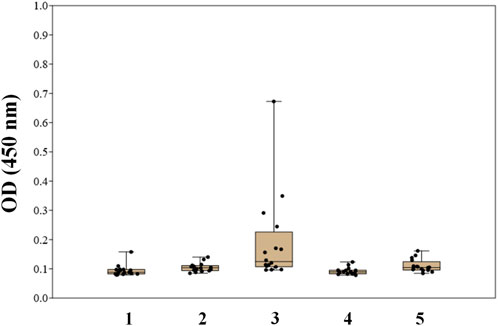
FIGURE 3. Screening results of IgG antibodies to oligosaccharides 1–5 in the blood sera of healthy donors using biotinylated conjugates of oligosaccharides 1–5 (Solovev et al., 2023).
To prove this concept, polyacrylamide-based conjugates (see tentative structure in Figure 2B) with different percentages of oligomannoside 1 related to Crohn’s disease marker were obtained (see Section 2.1). The desired content of loaded carbohydrate ligands in synthesized PAA conjugates of 20, 10, 5, and 2.5 mol% was confirmed using 1H NMR spectroscopy. Intensities of carbohydrate signals (Figure 4) decreased with a reduction in carbohydrate incorporation, while polymeric matrix signals had a constant profile. Integration of the anomeric signals in 1H NMR spectra of conjugates confirmed the content of attached oligosaccharide ligands.
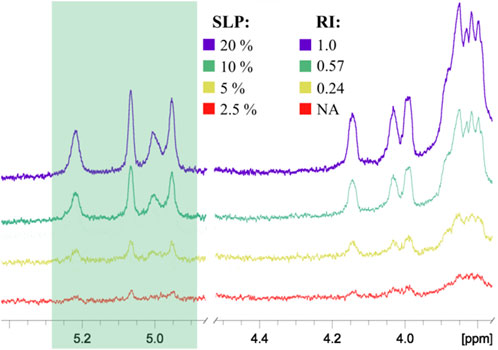
FIGURE 4. Comparison of 1H NMR spectra of PAA conjugates with different percentages of oligosaccharides: SLP, stoichiometric ligand percentage; RI, relative integral intensity of the anomeric signals shown in the green box. All spectra were normalized by the intensity of the PAA matrix signals. The RI of the conjugate with the highest ligand content was taken as 1.00. NA, integration data are not available due to low signal intensity.
Commercially available diagnostic plates with loaded ASCA antigen and the plates coated with PAA and biotinylated conjugates of ligand 1 were examined via ELISA using the calibrant samples (2, 20, and 200 U/mol) and control sera (positive and negative) provided in the ASCA diagnostic kit (Figure 5). The optical density for ASCA-positive samples decreased with a decrease in the content of ligand 1 in PAA conjugates, while biotinylated ligand 1 did not differentiate between positive and negative serum samples. This observation may be due to the low amount of ligand 1 on the surface of the well, resulting in the inability to form detectable signals. On the contrary, the results obtained using synthetic PAA-based conjugates permitted the detection of much better signals, which depended on the degree of conjugation of carbohydrate ligand 1. The conjugate with the highest loading of 20% demonstrated a slightly better result compared with commercially available plates coated with natural ASCA antigen.
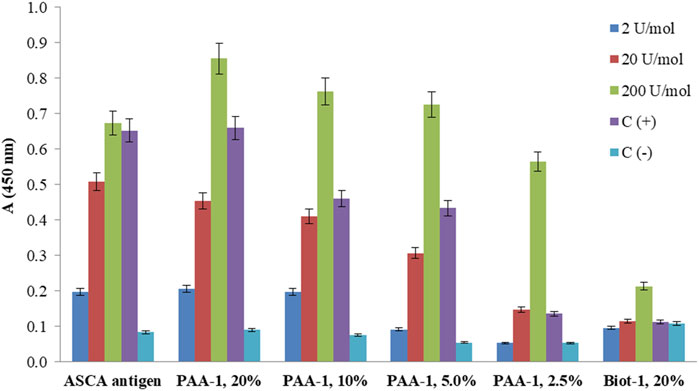
FIGURE 5. ELISA of calibrant samples (ASCA: 2, 20, and 200 U/mol), ASCA-positive, and ASCA-negative control sera [C(+) and C(−)] using a commercially available diagnostic kit coated with natural ASCA antigen and synthetically prepared coating antigens: PAA-1 with different contents of attached ligand 1. The screening of biotin-1 conjugates loaded onto avidin-coated wells showed low signals. The bars represent the mean values with standard deviation (SD).
Taking into account the obtained results, we also prepared PAA conjugates of synthetic oligosaccharides 2–6 with 20% molar content of incorporated ligands and applied them to screen antibodies in the sera of healthy donors (N = 18) and patients with colorectal cancer (N = 30). The information about patients and healthy donors is summarized in Supplementary Tables S1, S2. The raw screening data are provided in Supplementary Table S3. The spread of the obtained results is presented as а box plot demonstrating median optical density and quartiles (Figure 6).
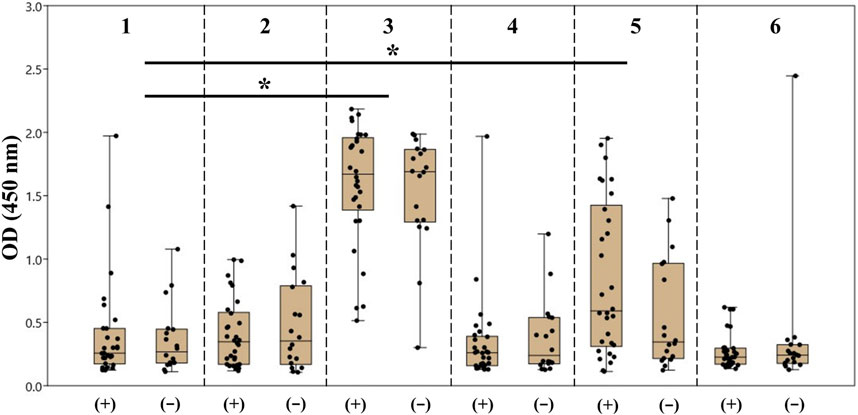
FIGURE 6. Screening results of the antibodies of the IgG class specific to oligosaccharides 1–6 in the blood sera of patients with colorectal cancer (+) and healthy donors (−). The data are presented as the box plot: the median value, upper and bottom quartiles, and upper and bottom boundaries are given for each antigen. The dots on the plot correspond to the average absorbance at 450 nm (OD450) in the ELISA for each serum sample. The sets of values were compared by the Mann–Whitney method (*p < 0.05, ns p > 0.05).
It should be noted that the formal replacement of the terminal α-(1→3)-mannoside residue with the β-(1→2)-mannoside one (1 → 3) or addition of the α-(1→2)-mannose residue to the non-reducing end of the original tetramannoside (1 → 5) significantly increased the dispersion and median value of observed optical density for both groups of samples. The result of comparison between carbohydrate ligands 1–6 with one-way ANOVA is provided in Supplementary Table S4. Of note, the median value and dispersion of optical density measured using ASCA tetramannoside 1 were the same for the serum samples of colorectal cancer patients and healthy donors. Alternatively, ligand 5 showed statistical relevance to an elevated median and wider dispersion for sera from patients with colorectal cancer (Table 1). It is noticeable that the observed level of anti-5 IgG was higher in the sera of colorectal patients. The reason for the observed phenomenon should be further studied in the larger cohorts.
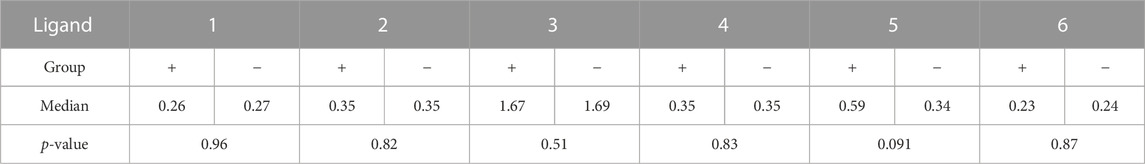
TABLE 1. Median values of antibody levels (OD) to synthetic oligosaccharides 1–6 for the two groups and the result of their comparison using the Mann–Whitney test.
4 Discussion
The type of antigen coating and its immobilization protocol have a dramatic impact on ELISA results. Immobilization via biotin–avidin or biotin–streptavidin pairs is widely used and has certain advantages (Komarova et al., 2018; Matveev et al., 2018; Matveev et al., 2019; Kazakova et al., 2020; Krylov and Nifantiev, 2020; Wong et al., 2020). The streptavidin molecule is composed of four biotin-binding subunits, with an average distance of approximately 2.5 nm between biotin molecules. For a standard commercial microtiter plate with a biotin-binding capacity of 5 pmol per well, the calculated average distance between biotin-binding streptavidin subunits is 6.1 nm, assuming their uniform distribution (Krylov and Nifantiev, 2020). These distances exceed the size of the immobilized oligosaccharide molecules and exclude the interaction of neighboring ligands. Nevertheless, carbohydrate chains tightly attached to the polyacrylamide matrix are intertwined with each other, which may be attributed to their new antigenic properties. We found that PAA conjugates with 20% carbohydrate loading are more suitable for detecting ASCA-related antibodies.
We hypothesize that the high density of oligosaccharide ligands on the surface promotes the formation of multicenter interactions with several binding sites in immunoglobulins G and M. High-density carbohydrate screens have the advantage of the fixation of low-affinity antibodies. Moreover, low-affinity IgM antibodies with 10 binding centers are more easily detectable than IgG antibodies with two binding centers. Nevertheless, carbohydrate displays with low ligand density are more suitable for fine differentiation of antigenic properties of different ligands (Solovev et al., 2023).
The PAA conjugates of such type were used to form an array of oligosaccharides related to ASCA tetramannoside. Following the pioneering works by B. Sendid, D. Poulain, J. M. Mallet, and other studies based on the use of synthetic oligosaccharides, we investigated how the structural modifications in α-mannan chains affect their antigenicity. Particularly, we showed, for the first time, that the elongation of the ASCA epitope (α-Man-(1→3)-Man-α-(1→2)-Man-α-(1→2)-Man-α-) with an additional α-(1→2)-linked mannose residue significantly increases its recognition by human serum antibodies that can be associated with its higher immunogenicity. The high level of antibodies against oligomannoside 3 with β-(1→2)-linkage confirms the high immunogenicity of the corresponding epitope that is present in the polysaccharides of the natural fungal microbiota of the human intestine, which correlates with the previously reported results (Krylov et al., 2018; Sendid et al., 2021; Singh et al., 2023). The elevated median level of optical density for patients with colorectal cancer versus healthy donors was observed only for oligosaccharide 5 (Table 1). Of note, this ligand was recently introduced as a new high-affinity ligand for DC-SIGN, a basic receptor of the innate immune system (Krylov et al., 2023). Due to the low sensitivity and specificity of existing screening methods, much effort is focused on finding new potential biomarkers for colon cancer (Jimenez-Luna et al., 2021). Tumor and normal tissues are characterized by different glycosylation profiles (Zhao et al., 2018); in particular, high-mannose N-glycans are regarded as markers of malignant progression in the early stages of colorectal cancer (Boyaval et al., 2022). Considering these factors, deeper investigation of the immunological properties of mannosides is required, which may open new opportunities for improving existing ELISA test systems, as well as developing fungal diagnostic kits with new properties.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the N. N. Blokhin National Medical Research Center of Oncology of the Ministry of Health of the Russian Federation on 11 May 2022 (Protocol No. 5 as of 05/11/2022). All of the experimental procedures involving human blood sera were conducted in accordance with the ethical standards of the National Committee on Research Ethics (Russia) and the Declaration of Helsinki of 1964 and its subsequent changes or comparable ethical principles. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
VK: conceptualization, supervision, validation, writing–original draft, and writing–review and editing. AK: investigation and writing–review and editing. AP: investigation and writing–review and editing. PT: investigation, resources, and writing–review and editing. DY: investigation and writing–review and editing. NK: conceptualization, resources, supervision, and writing–review and editing. NN: conceptualization, funding acquisition, resources, supervision, and writing–review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Ministry of Science and Higher Education of the Russian Federation (Theme No. FFZZ-2022-0010).
Acknowledgments
The authors thank Dr. A. G. Gerbst and Ms. A. I. Tokatly for reading this manuscript and for their critical discussion. The authors also thank Drs. D. A. Argunov and A. S. Dmitrenok for recording NMR spectra at the Department of Structural Studies at the Zelinsky Institute of Organic Chemistry, Moscow, Russia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2023.1296828/full#supplementary-material
References
Argunov, D. A., Karelin, A. A., Grachev, A. A., Tsvetkov, Y. E., and Nifantiev, N. E. (2011). A new synthesis of the 3, 6-branched hexasaccharide fragment of the cell wall mannan in Candida albicans, corresponding to the antigenic factor 4. Russ. Chem. Bull. 60, 1004–1011. doi:10.1007/s11172-011-0157-0
Assan, F., Gottlieb, J., Tubach, F., Lebbah, S., Guigue, N., Hickman, G., et al. (2020). Anti-Saccharomyces cerevisiae IgG and IgA antibodies are associated with systemic inflammation and advanced disease in hidradenitis suppurativa. J. Allergy Clin. Immunol. 146, 452–455. doi:10.1016/j.jaci.2020.01.045
Boyaval, F., Dalebout, H., Van Zeijl, R., Wang, W., Fariña-Sarasqueta, A., Lageveen-Kammeijer, G. S. M., et al. (2022). High-mannose N-glycans as malignant progression markers in early-stage colorectal cancer. Cancers 14, 1552. doi:10.3390/cancers14061552
Chevalier, R., Esnault, J., Vandewalle, P., Sendid, B., Colombel, J.-F., Poulain, D., et al. (2005). Synthetic yeast oligomannosides as biological probes: α-d-Manp (1→3) α-d-Manp (1→2) α-d-Manp and α-d-Manp (1→3) α-d-Manp (1→2) α-d-Manp (1→2) α-d-Manp as Crohn's disease markers. Tetrahedron 61, 7669–7677. doi:10.1016/j.tet.2005.05.098
Enache-Angoulvant, A., and Hennequin, C. (2005). Invasive Saccharomyces infection: a comprehensive review. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 41, 1559–1568. doi:10.1086/497832
Erwig, L. P., and Gow, N. A. R. (2016). Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 14, 163–176. doi:10.1038/nrmicro.2015.21
Jacquinot, P. M., Plancke, Y., Sendid, B., Strecker, G., and Poulain, D. (1998). Nature of Candida albicans-derived carbohydrate antigen recognized by a monoclonal antibody in patient sera and distribution over Candida species. FEMS Microbiol. Lett. 169, 131–138. doi:10.1111/j.1574-6968.1998.tb13309.x
Jimenez-Luna, C., González-Flores, E., Ortiz, R., Martínez-González, L. J., Antúnez-Rodríguez, A., Expósito-Ruiz, M., et al. (2021). Circulating PTGS2, JAG1, GUCY2C and PGF mRNA in peripheral blood and serum as potential biomarkers for patients with metastatic colon cancer. J. Clin. Med. 10, 2248. doi:10.3390/jcm10112248
Karelin, A. A., Tsvetkov, Y. E., Kogan, G., Bystricky, S., and Nifantiev, N. E. (2007). Synthesis of oligosaccharide fragments of mannan from Candida albicans cell wall and their BSA conjugates. Russ. J. Bioorg. Chem. 33, 119–130. doi:10.1134/s106816200701013x
Karelin, A. A., Tsvetkov, Y. E., Paulovičová, E., Paulovičová, L., and Nifantiev, N. E. (2016). A blockwise approach to the synthesis of (1→2)-linked Oligosaccharides corresponding to fragments of the acid-stable β-mannan from the Candida albicans cell wall. Eur. J. Org. Chem. 2016, 1173–1181. doi:10.1002/ejoc.201501464
Kazakova, E. D., Yashunsky, D. V., Krylov, V. B., Bouchara, J.-P., Cornet, M., Valsecchi, I., et al. (2020). Biotinylated oligo-α-(1 → 4)-d-galactosamines and their N-acetylated derivatives: α-stereoselective synthesis and immunology application. J. Am. Chem. Soc. 142, 1175–1179. doi:10.1021/jacs.9b11703
Komarova, B. S., Wong, S. S. W., Orekhova, M. V., Tsvetkov, Y. E., Krylov, V. B., Beauvais, A., et al. (2018). Chemical synthesis and application of biotinylated oligo-α-(1→3)-D-glucosides to study the antibody and cytokine response against the cell wall α-(1→3)-D-glucan of Aspergillus fumigatus. J. Org. Chem. 83, 12965–12976. doi:10.1021/acs.joc.8b01142
Krylov, V. B., Gómez-Redondo, M., Solovev, A. S., Yashunsky, D. V., Brown, A. J. P., Stappers, M. H. T., et al. (2023). Identification of a new DC-SIGN binding pentamannoside epitope within the complex structure of Candida albicans mannan. Cell Surf. 10, 100109. doi:10.1016/j.tcsw.2023.100109
Krylov, V. B., and Nifantiev, N. E. (2020). Synthetic oligosaccharides mimicking fungal cell wall polysaccharides. Curr. Top. Microbiol. Immunol. 425, 1–16. doi:10.1007/82_2019_187
Krylov, V. B., Petruk, M. I., Grigoryev, I. V., Lebedin, Y. S., Glushko, N. I., Khaldeeva, E. V., et al. (2018). Study of the carbohydrate specificity of antibodies against Aspergillus fumigatus using the library of synthetic mycoantigens. Russ. J. Bioorg. Chem. 44, 80–89. doi:10.1134/s1068162017060073
Krylov, V. B., Solovev, A. S., Puchkin, I. A., Yashunsky, D. V., Antonets, A. V., Kutsevalova, O. Y., et al. (2021). Reinvestigation of carbohydrate specificity of EBCA-1 monoclonal antibody used for the detection of Candida mannan. J. Fungi 7, 504. doi:10.3390/jof7070504
Maillet, J., Ottaviani, S., Tubach, F., Roy, C., Nicaise-Rolland, P., Palazzo, E., et al. (2016). Anti-Saccharomyces cerevisiae antibodies (ASCA) in spondyloarthritis: prevalence and associated phenotype. Jt. Bone Spine 83, 665–668. doi:10.1016/j.jbspin.2015.10.011
Main, J., McKenzie, H., Yeaman, G. R., Kerr, M. A., Robson, D., Pennington, C. R., et al. (1988). Antibody to Saccharomyces cerevisiae (bakers’ yeast) in Crohn’s disease. BMJ 297, 1105–1106. doi:10.1136/bmj.297.6656.1105
Matveev, A. L., Krylov, V. B., Emelyanova, L. A., Solovev, A. S., Khlusevich, Y. A., Baykov, I. K., et al. (2018). Novel mouse monoclonal antibodies specifically recognize Aspergillus fumigatus galactomannan. PloS One 13, e0193938. doi:10.1371/journal.pone.0193938
Matveev, A. L., Krylov, V. B., Khlusevich, Y. A., Baykov, I. K., Yashunsky, D. V., Emelyanova, L. A., et al. (2019). Novel mouse monoclonal antibodies specifically recognizing β-(1→3)-D-glucan antigen. PloS One 14, e0215535. doi:10.1371/journal.pone.0215535
Orlean, P. (2012). Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 192, 775–818. doi:10.1534/genetics.112.144485
Parapouli, M., Vasileiadis, A., Afendra, A.-S., and Hatziloukas, E. (2020). Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 6, 1–31. doi:10.3934/microbiol.2020001
Rinaldi, M., Perricone, R., Blank, M., Perricone, C., and Shoenfeld, Y. (2013). Anti-Saccharomyces cerevisiae autoantibodies in autoimmune diseases: from bread baking to autoimmunity. Clin. Rev. Allergy Immunol. 45, 152–161. doi:10.1007/s12016-012-8344-9
Sendid, B., Colombel, J. F., Jacquinot, P. M., Faille, C., Fruit, J., Cortot, A., et al. (1996). Specific antibody response to oligomannosidic epitopes in Crohn’s disease. Clin. Diagn. Lab. Immunol. 3, 219–226. doi:10.1128/cdli.3.2.219-226.1996
Sendid, B., Lecointe, K., Collot, M., Danzé, P.-M., Damiens, S., Drucbert, A.-S., et al. (2021). Dissection of the anti-Candida albicans mannan immune response using synthetic oligomannosides reveals unique properties of β-1,2 mannotriose protective epitopes. Sci. Rep. 11, 10825. doi:10.1038/s41598-021-90402-4
Singh, R. K., Reuber, E. E., Bruno, M., Netea, M. G., and Seeberger, P. H. (2023). Synthesis of oligosaccharides to identify an immunologically active epitope against Candida auris infection. Chem. Sci. 14, 7559–7563. doi:10.1039/d3sc01242e
Solovev, A. S., Tsarapaev, P. V., Krylov, V. B., Yashunsky, D. V., Kushlinskii, N. E., and Nifantiev, N. E. (2023). A repertoire of anti-mannan Candida albicans antibodies in the blood sera of healthy donors. Russ. Chem. Bull. 72, 263–268. doi:10.1007/s11172-023-3731-3
Suzuki, S. (1997). Immunochemical study on mannans of genus Candida. I. Structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34. Curr. Top. Med. Mycol. 8, 57–70.
Szilagyi, A., Leighton, H., Burstein, B., and Xue, X. (2014). Latitude, sunshine, and human lactase phenotype distributions may contribute to geographic patterns of modern disease: the inflammatory bowel disease model. Clin. Epidemiol. 6, 183–198. doi:10.2147/CLEP.S59838
Temme, J. S., Butler, D. L., and Gildersleeve, J. C. (2021). Anti-glycan antibodies: roles in human disease. Biochem. J. 478, 1485–1509. doi:10.1042/BCJ20200610
Tsvetkov, Y. E., Burg-Roderfeld, M., Loers, G., Ardá, A., Sukhova, E. V., Khatuntseva, E. A., et al. (2011). Synthesis and molecular recognition studies of the HNK-1 trisaccharide and related oligosaccharides. The specificity of monoclonal anti-HNK-1 antibodies as assessed by surface plasmon resonance and STD NMR. J. Am. Chem. Soc. 134, 426–435. doi:10.1021/ja2083015
Vermeire, S., Joossens, S., Peeters, M., Monsuur, F., Marien, G., Bossuyt, X., et al. (2001). Comparative study of ASCA (Anti-Saccharomyces cerevisiae antibody) assays in inflammatory bowel disease. Gastroenterology 120, 827–833. doi:10.1053/gast.2001.22546
Vinogradov, E., Petersen, B., and Bock, K. (1998). Structural analysis of the intact polysaccharide mannan from Saccharomyces cerevisiae yeast using 1H and 13C NMR spectroscopy at 750 MHz. Carbohydr. Res. 307, 177–183. doi:10.1016/s0008-6215(98)00042-1
Wong, S. S. W., Krylov, V. B., Argunov, D. A., Karelin, A. A., Bouchara, J.-P., Fontaine, T., et al. (2020). Potential of chemically synthesized oligosaccharides to define the carbohydrate moieties of the fungal cell wall responsible for the human immune response, using Aspergillus fumigatus galactomannan as a model. mSphere 5, 00688–e719. doi:10.1128/mSphere.00688-19
Yashunsky, D. V., Tsvetkov, Y. E., Grachev, A. A., Chizhov, A. O., and Nifantiev, N. E. (2016). Synthesis of 3-aminopropyl glycosides of linear β-(1→3)-D-glucooligosaccharides. Carbohydr. Res. 419, 8–17. doi:10.1016/j.carres.2015.10.012
Keywords: mannan, Saccharomyces cerevisiae, Candida albicans, fungi, antibodies, IgG, human sera, colorectal cancer
Citation: Krylov VB, Kuznetsov AN, Polyanskaya AV, Tsarapaev PV, Yashunsky DV, Kushlinskii NE and Nifantiev NE (2023) ASCA-related antibodies in the blood sera of healthy donors and patients with colorectal cancer: characterization with oligosaccharides related to Saccharomyces cerevisiae mannan. Front. Mol. Biosci. 10:1296828. doi: 10.3389/fmolb.2023.1296828
Received: 19 September 2023; Accepted: 20 November 2023;
Published: 07 December 2023.
Edited by:
Giuseppe Stefanetti, University of Urbino Carlo Bo, ItalyReviewed by:
Hans-Christian Siebert, RI-B-NT, GermanyArkady Sinitsyn, Lomonosov Moscow State University, Russia
Copyright © 2023 Krylov, Kuznetsov, Polyanskaya, Tsarapaev, Yashunsky, Kushlinskii and Nifantiev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vadim B. Krylov, dl9rcnlsb3ZAaW9jLmFjLnJ1; Nikolay E. Nifantiev, bmVuQGlvYy5hYy5ydQ==
 Vadim B. Krylov
Vadim B. Krylov Anton N. Kuznetsov
Anton N. Kuznetsov Alina V. Polyanskaya
Alina V. Polyanskaya Pavel V. Tsarapaev1,3
Pavel V. Tsarapaev1,3 Nikolay E. Nifantiev
Nikolay E. Nifantiev