- 1Department of Ophthalmology, Tongji Medical College, Union Hospital, Huazhong University of Science and Technology, Wuhan, China
- 2Aier Eye Hospital of Wuhan University, Wuhan, China
Objective: Thyroid-associated ophthalmopathy (TAO) is an autoimmune orbital disorder characterized by pathological alterations including extraocular muscle fibrosis and orbital inflammation. Cerium oxide nanoparticles (CeO2-NPs, CNPs) are gaining popularity in ophthalmology due to their potential antifibrotic and anti-inflammatory properties. This study aims to investigate the inhibitory effects of CNPs on fibrosis and inflammation in TAO orbital fibroblasts (OFs) derived from TAO patients.
Methods: OFs obtained by primary culture of orbital adipose tissue from 8 TAO patients. Probing the safe action concentration of CNPs and Anisomycin using CCK8 and detecting intracellular reactive oxygen species (ROS) generation using ROS Assay kit. Wound-Healing Assay was used to examine the degree of fibrosis of OFs. The expression of Fibronectin, COL1A1, α-Smooth muscle action, Hyaluronan Synthase 2, c-Jun N-terminal Kinase (JNK) and pJNK were detected by the RT-PCR and WB, and Hyaluronic Acid secretion was detected by ELISA. Inflammatory factors Interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNFα) expression were detected by RT-PCR and ELISA.
Results: CNPs below 100 μg/mL and Anisomycin below 5 μM did not affect OFs proliferation. CNPs inhibit intracellular ROS generation. CNPs inhibit OFs fibrosis and suppress the expression of fibrosis indicators. These antifibrotic effects were mediated by inhibition of JNK phosphorylation, and were reversible by a JNK agonist. Furthermore, CNPs reduce both mRNA levels and secretion of inflammatory factors, IL-6 and TNF-α.
Conclusion: CNPs demonstrated the ability to inhibit fibrosis in TAO OFs by reducing JNK phosphorylation, as well as dose-dependently suppressed ROS generation and inflammatory response in TAO OFs.
1 Introduction
Thyroid-associated ophthalmopathy (TAO), also referred to as Graves’ orbitopathy (GO), manifests as an organ-specific autoimmune condition within the context of Graves’ disease (GD) (Zhu et al., 2023). As the predominant extrathyroidal manifestation of Graves’ disease (GD), thyroid-associated ophthalmopathy (TAO) develops in ∼25% of GD patients clinically (Comi et al., 2025). The annual incidence of TAO is 0.54–0.9 (males) versus 2.67–3.3 (females) per 100,000. Mild disease predominates (94%–95% of cases), with moderate/severe forms comprising 5%–6% (Bartalena et al., 2020). The primary ocular manifestations of TAO include exophthalmos, eyelid retraction, and restricted extraocular myopathy (Bartalena et al., 2021). In severe instances, TAO can lead to incomplete eyelid closure, corneal ulcers, optic neuropathy, and potential blindness. Advanced pathological alterations in TAO encompass orbital adipose tissue expansion, fibrosis of the extraocular muscles, and infiltration of inflammatory factors (Diao et al., 2022).
Currently, the etiology of TAO is not fully understood, however, it is believed that the activation of autoimmune T cells and thyroid-stimulating hormone receptor (TSHR) autoantibodies may be significant factors (Shen et al., 2023). Activated immune T and B cells migrate into the periorbital connective tissue, releasing cytokines including IL-1β, IL-6, TNF-α, TGF-β1, and autoantibodies such as IgG, ultimately resulting in connective tissue inflammation, fibrosis, and remodeling (Xu et al., 2022). Oxidative stress also plays a pivotal role in the pathogenesis of TAO. Elevated reactive oxygen species (ROS) levels or diminished antioxidant capacity result in oxidative damage to cellular membranes, lipid peroxidation, and DNA oxidation (Ma et al., 2024).
Treatment varies at different stages of TAO, including orbital decompression surgery, orbital ratiotherapy and medication (Oculoplastic and Orbital Disease Group of Chinese Ophthalmological Society of Chinese Medical Association, 2022). The pathophysiological complexity and interpatient heterogeneity in TAO necessitate personalized management. First-line pharmacotherapy, Mycophenolate mofetil (MMF) plus glucocorticoids (GC) (Längericht et al., 2020) pulse therapy and intravenous teprotumumab (anti-IGF-1R monoclonal antibody) (Markham, 2020), demonstrates robust efficacy when initiated early, modifying disease progression and minimizing complications. Emerging agents [e.g., novel immunosuppressants, somatostatin analogues, and biologic mAbs such as Rituximab (anti-CD20 monoclonal antibody) (Kang et al., 2022), Belimumab (anti-targeting B cell stimulating factor (BAFF) antibody) (Salvi et al., 2019), Tocilizumab (anti-IL-6 antibody) (Boutzios et al., 2023)] show promising efficacy in ongoing trials. However, their cost-effectiveness and safety profiles, particularly long-term adverse events, require rigorous evaluation before clinical translation. Therefore, the research on therapeutic drugs for TAO is still a hot topic at this stage.
Inorganic nanoparticles (NPs) have emerged as versatile platforms for novel therapeutic applications, enabling targeted disease intervention at early pathological stages with efficacy surpassing current treatments (Pelaz et al., 2017). These NPs demonstrate exceptional utility as drug delivery vehicles and dynamic scaffolds that modulate conjugated biomolecule activity (DuRoss et al., 2019). Chen et al. demonstrated that nanoparticles (NPs) synthesized from PLGA-encapsulated TSHR-A subunit and rapamycin (TSHR-A + Rapa NPs) can target dendritic cells (DCs) to modulate immune tolerance, representing a novel potential therapeutic strategy for Graves’ disease (Chen et al., 2025). Cerium oxide nanoparticles (CeO2-NPs, CNPs), nanocrystals derived from Cerium, is well known for its antioxidant properties (Casals et al., 2020). Since CNPs can converte between Ce3+ and Ce4+, it is considered as an antioxidant with auto-regenerative free radical scavenging activity. At present, CNPs have been found to be remarkably effective in eliminate ROS (Li X. et al., 2022), anti-inflammatory (Wei et al., 2021) and anti-fibrosis (Boey et al., 2021), and its neuroprotective effects have numerous applications in ophthalmology, including age-related macular degeneration, retinitis pigmentosa (Maccarone et al., 2020) and acute damage induced by high intensity light exposure (Tisi et al., 2019). The role of CNPs in treating TAO remains unexplored in current literature. Since the pathology of TAO includes oxidative stress, fibrosis of orbital fibroblasts (OFs) and infiltration of inflammatory factors, which is consistent with the role of CNPs, we wished to explore the therapeutic role of CNPs in TAO, and investigate the mechanism.
In this study, we selected OFs derived from orbital tissue of TAO patients, to validate the effect and mechanism of CNPs in TAO extraocular muscle fibrosis and inflammatory infiltration.
2 Methods and materials
2.1 Materials
Dulbecco’s modified Eagle medium (DMEM), penicillin-streptomycin mixture and 0.25% trypsin-ethylene diamine tetraacetic acid (EDTA) (1х) were purchased from Servicebio (China). Fetal bovine serum (FBS) was purchased from Procell (China). CNPs (544841-5G) was purchased from Sigma-Aldrich (United States). TGF-β (HY-P78168), IL-1β (HY-P7028), Anisomycin (HY-18982) were purchased from MedChemExpress (China).
2.2 Samples
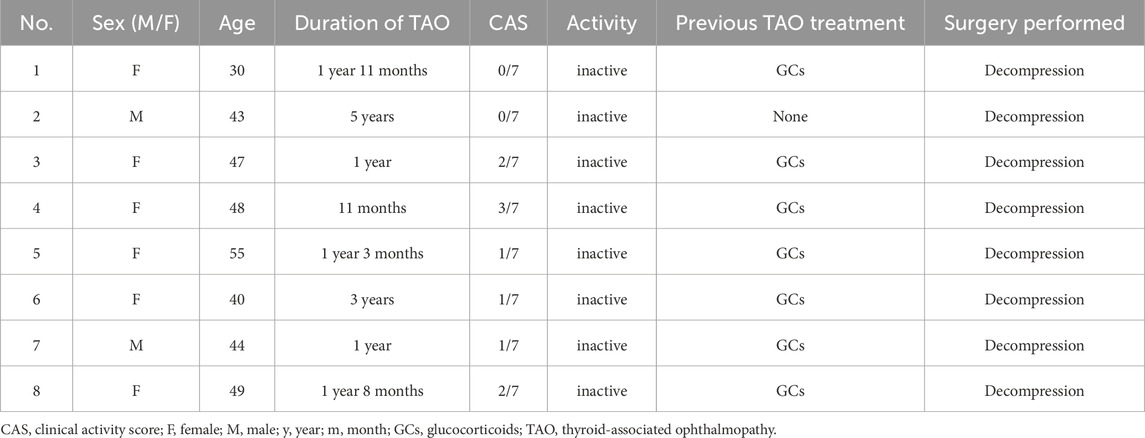
The study design and protocol were approved by the Ethics Committee of Huazhong University of Science and Technology Union hospital attached to Tongji University Medical School (UHCT230552), and informed consent was obtained from the patients for the collection of specimens. Orbital adipose tissues were taken from 8 patients, aged between 18 and 65 years old, having no other ocular diseases and major systemic diseases. The clinical and patient information is shown in Table 1. The 7-item clinical activity score (CAS) scheme was used to assess GO activity. When the sum is ≤3/7, TAO is defined as inactive. The severity and clinical activity of GO were graded according to the NOSPECS classification.
2.3 Primary culture of OFs
Orbital adipose tissue samples were collected and washed three times with phosphate-buffered saline (PBS). Then samples were cut into small pieces of 1–2 mm3 and distributed evenly on the bottom of a culture flask. After adding 0.5–1 mL DMEM, the tissue was put into a 37°C cell incubator with 5% CO2. When about 80% of the bottom of the flask was occupied, the OFs were digested with 0.25% trypsin for passage. Cells from passages 4–8 were used for subsequent experiments.
2.4 Detection of cell proliferation activity
Different concentrations of CNPs and Anisomycin were added to 96-well plates for 2 days. Cell counting kit (CCK)-8 was used to detect the absorbance at 450 nm by using a microplate reader to obtain the action concentration of each drug.
2.5 Detection of antioxidative activity
Intracellular ROS generation was assessed using ROS Assay Kit (S0033S, Beyotime, China). DCFH-DA was diluted to 10 μmol/L in serum-free DMEM, added to differently-treated OFs, and avoid the light in 37°C for 20 min. Cells were washed for 3 times before imaging with a laser confocal scanning microscopy (IX51, OLYMPUS, Japan).
2.6 Wound-healing assay
Cell migration was detected using an in vitro scratch-wound healing assay. 1*106 OFs were seeded on 6-well dishes. When cells reached 90%–100% confluence, different treatments were performed after 12 h FBS-free culture. Linear scratch wounds were made with a 200-μL pipette tip. Images were captured at 0h, 24 h and 48 h under a microscope, and the area of each scratch wound was analysed by ImageJ software.
2.7 Enzyme linked immunosorbent assay (ELISA)
Hyaluronic Acid (HA), Interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNFα) secreted by OFs were detected with corresponding ELISA kits (Elabscience, China). The supernatant was collected after cell processing and assayed according to the manufacturer’s protocols. All experiments were performed in duplicate.
2.8 Reverse transcription-polymerase chain reaction (RT-PCR)
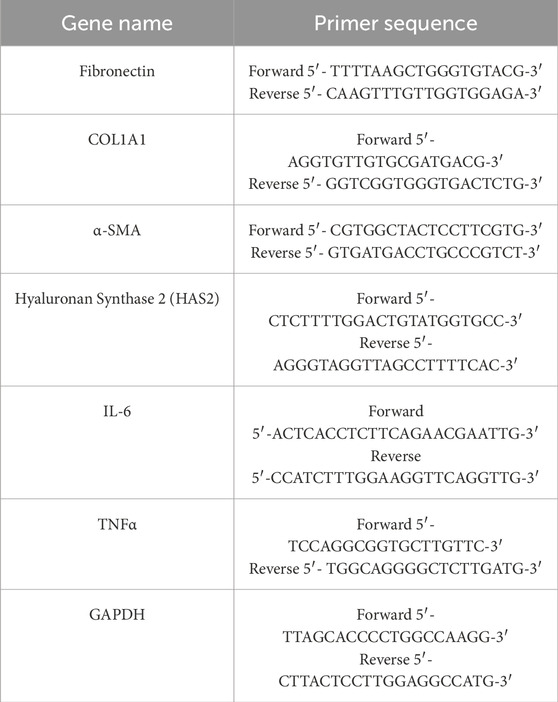
Total RNA was extracted from OFs through using the FastPure Cell/Tissue Total RNA Isolation kit (RC101, Vazyme, China) according to the manufacturer’s instructions, and the RNA was reversely transcribed to a complementary DNA (cDNA) by using the HiScript All-in-one RT SuperMix kit (R333-01, Vazyme, China). RT-PCR was performed by using the ChamQ Universal SYBR qPCR Master Mix kit (Q711-02, Vazyme, China). Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as an internal control. Primer sequences were shown in Table 2.
2.9 Western blotting (WB)
After adding Radioimmunoprecipitation assay lysis buffer, the supernatant was collected, and the protein concentration was detected by using a Bicinchoninic Acid (BCA) Protein Assay kit (BL521A, Biosharp, China). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, blocked with 5% Skim milk powder for 1 h at room temperature, and incubated with primary antibodies overnight at 4°C. The primary antibodies used were Fibronectin (1:500, Abmart, China), α-SMA (1:500, Abmart, China), COL1A1 (1:500, Abmart, China), c-Jun N-terminal Kinase (JNK) (1:500, Abmart, China), pJNK (1:500, Abmart, China) and GAPDH (1:10,000, Abclonal, China). After incubated with horseradish peroxidase-labeled goat anti-rabbit secondary antibody (1:2,000, Servicebio, China) for 1 h at 37°C, the protein intensity was detected through using electrochemiluminescence chemiluminescence reagent (ATVK07071, Abbkine, China) and analyzed by using ImageJ software.
2.10 Statistical analysis
Graphpad Prism 9.0 and SPSS27.0 software were used for data analysis, and each experimental group was compared with the control group respectively. T-test was applied to analyze the differences between the two groups of data. Shapiro-Wilk Test was used to determine whether the distribution was normal, and Levene’s Test was used to conduct a homogeneity analysis of variance. If the distribution is normal and the variance is homogeneous, an independent sample T-test can be conducted. Mann-Whitney rank sum test was used for non-compliance. The data differences among multiple groups were compared. If the data of each group were normally distributed and the variance was homogeneous, one-way ANOVA was used. As for those which do not meet the requirements, Kruskal–Wallis H test was selected. The above experiments were conducted for more than 3 times, and the results were expressed as mean ± standard deviation. When the P-value was <0.05, the difference was considered statistically significant.
3 Results
3.1 Action concentration of CNPs and anisomycin on OFs
CCK-8 was used to detect the action concentration of CNPs and Anisomycin on OFs. As shown in Figures 1A,B, CNPs ≤100 μg/mL, Anisomycin ≤5 μM did not affect cell activity. Therefore, 10, 50, 100 μg/mL CNPs and 5 μM Anisomycin were respectively chosen for subsequent experiments.
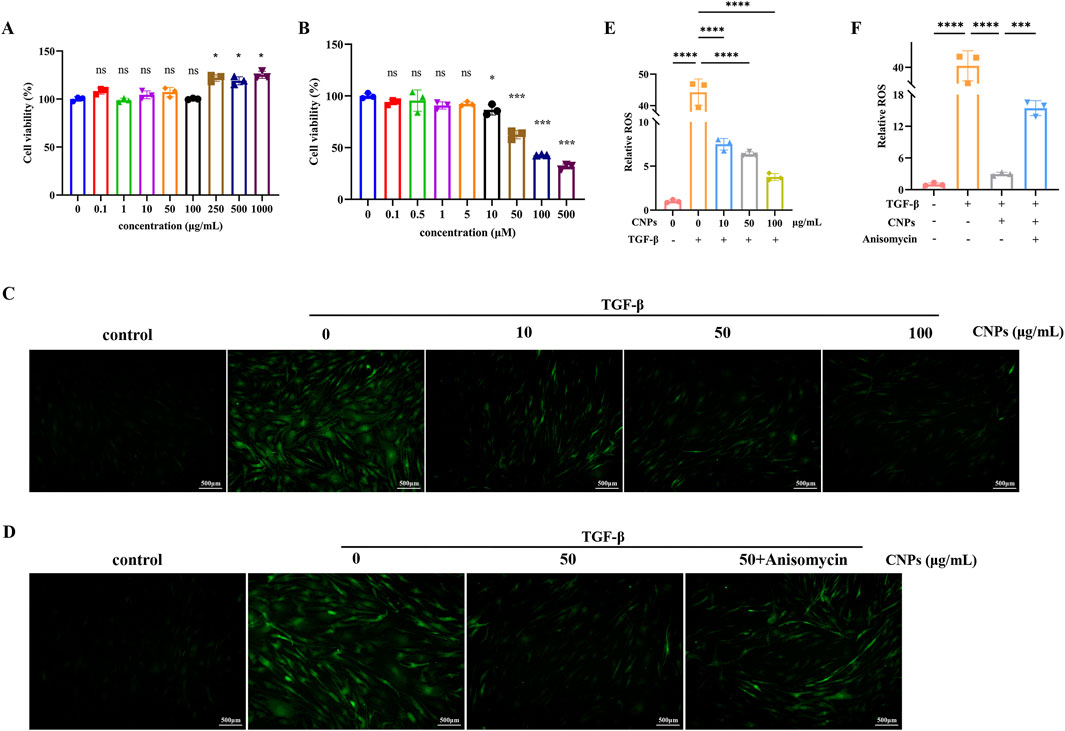
Figure 1. Action concentration of drugs and ROS generation in OFs. (A,B) Cell viability measurement using the CCK-8 assay after treatment with CNPs or Anisomycin for 2 days. (C) ROS generation of OFs after treatment with 0, 10, 50 and 100 μg/mL CNPs and 5 ng/mL TGF-β. (D) ROS generation of OFs in control, TGF-β, CNPs + TGF-β and CNPs + Anisomycin + TGF-β groups. (E,F) Fluorescence intensity analysis in Figures (C,D). Data are presented as mean ± SEM (n ≥ 3). ns:P > 0.05, *P < 0.05, ***P < 0.001, ****P < 0.0001.
3.2 Effect of CNPS on the inhibition of fibrosis and HA secretion in TAO OFs
Different concentrations (10, 50, 100 μg/mL) CNPs and 5 ng/mL TGF-β were simultaneously administered to OFs, fibrosis indexes and HA secretion were detected after 2 days. Scratch assays in Figures 2A,B showed significantly higher migration rate of OFs after addition of TGF-β (P < 0.05). Meanwhile, addition of CNPs to OFs resulted in a decrease in OFs migration rate (P < 0.05), and the gradient of migration rate decreased with increasing CNPs concentration. The mRNA level of fibrosis indicators Fibronectin, COL1A1 and α-SMA in 10, 50 and 100 μg/mL of CNPs groups presented significant reduction, which were 0.45, 0.18 and 0.14-fold; 0.73, 0.53 and 0.36-fold; 0.28, 0.23 and 0.14-fold lower than TGF-β group, respectively (Figure 2D). The WB results exhibited the same trend (Figure 2E). Figure 2C demonstrated the secretion of HA by cells after the addition of different concentrations of CNPs. Similarly, HA secretion was inversely correlated with CNPs concentrations. The mRNA level of HAS2 were 0.46, 0.18 and 0.22-fold, compared with TGF-β group. In summary, CNPs inhibits OFs fibrosis and HA secretion in a dose-dependent trend. Based on this, we selected intermediate concentrations of CNPs (50 μg/mL) for subsequent mechanistic explorations.
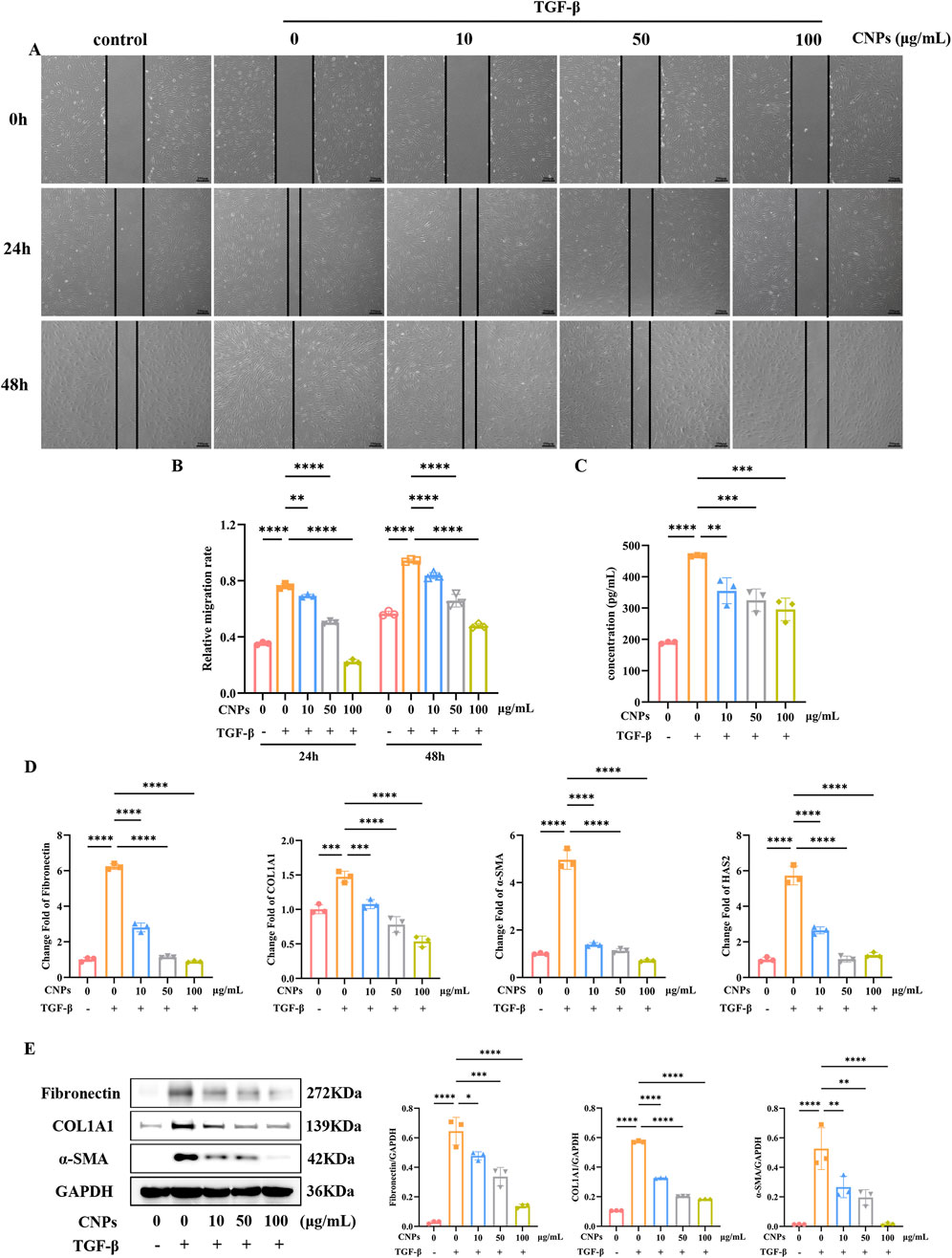
Figure 2. CNPs exert an inhibitory effect on OFs fibrosis. (A,B) Wound-Healing assay and cell migration analysis after treatment with 0, 10, 50 and 100 μg/mL CNPs and 5 ng/mL TGF-β, at 24 h and 48 h. (C) Secretion of HA after treatment with different concentrations of CNPs. (D) mRNA levels of Fibronectin, COL1A1, α-SMA and HAS2 after treatment with CNPs. (E) The WB results for Fibronectin, COL1A1 and α-SMA in each group, and the protein levels were quantified and normalized to the level of GAPDH for each sample. Data are presented as mean ± SEM (n ≥ 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.3 JNK pathway mediates CNPs inhibits the process of fibrosis of OFs
Addition of CPNs inhibits intracellular c-Jun N-terminal Kinase (JNK) phosphorylation of OFs. After adding CNPs and inducing fibrosis for 2 days, intracellular fibrosis indicators and pJNK/JNK were examined, which showed a significant reduction in intracellular pJNK/JNK level.
Anisomycin was chosen to verify the inhibition of JNK phosphorylation in CNPs-mediated inhibition of fibrosis in OFs. Wound-Healing Assay showed the migration rate of OFs. Compared to CNPs group, CNPs + Anisomycin group exhibited significant increase, either 24 h or 48 h after fibrosis induction (P < 0.05) (Figure 3A). The mRNA expression of Fibronectin, COL1A1, α-SMA and HAS2 in CNPs + Anisomycin group were significantly higher than CNPs group, which were 1.50, 1.47, 1.66 and 1.47-fold, respectively (Figure 3B). Protein level of Fibronectin, COL1A1 and α-SMA in OFs followed the same trend as mRNA. CNPS induced lower JNK phosphorylation than that in TGF-β group and CNPs + Anisomycin, which were 1.29 and 1.51-fold (Figure 3C). These suggest that CNPs inhibits fibrosis of OFs via reducing the phosphorylation of JNK.
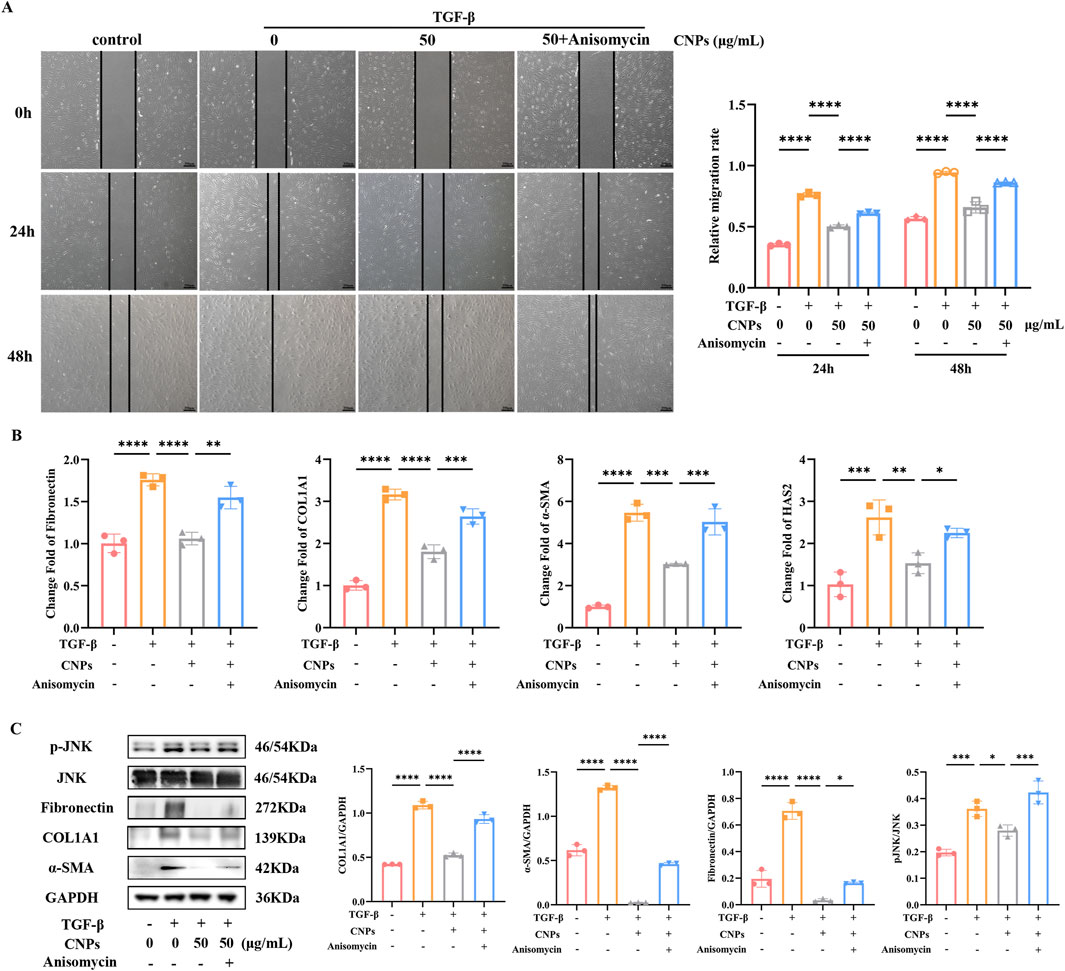
Figure 3. CNPs regulates JNK phosphorylation during OFs fibrosis inhibition. (A) Wound-Healing assay and cell migration analysis in control, TGF-β, CNPs + TGF-β and CNPs + Anisomycin + TGF-β groups. (B) mRNA levels of Fibronectin, COL1A1, α-SMA and HAS2 in each group. (C) The WB results for COL1A1, α-SMA and pJNK/JNK in each group, and the protein levels were quantified and normalized to the level of GAPDH for each sample. Data are presented as mean ± SEM (n ≥ 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.4 Effect of CNPs on the inhibition of ROS generation in TAO OFs
Different concentrations (10, 50, 100 μg/mL) CNPs and 5 ng/mL TGF-β were simultaneously administered to OFs, occurring with 50 μg/mL CNPs + 5 μM Anisomycin group, and intracellular ROS level were observed by microscopy. As shown in Figures 1C,E, compared with induction group, intracellular ROS generation were significant decreased in 10, 50, 100 μg/mL CNPs group in a dose-dependent manner. Furthermore, intracellular ROS level of CNPs + Anisomycin group showed somewhat elevated compared to CNPs group (Figures 1D,F).
3.5 Effect of CNPs on the inhibition of secretion of inflammatory factors in TAO OFs
Different concentrations (10, 50, 100 μg/mL) CNPs and 5 ng/mL IL-1β were simultaneously administered to OFs, the mRNA level and secretion of inflammatory factors, IL-6 and TNFα, were detected after 24 h. The expression of IL-6 and TNFα were significantly downregulated, which were 0.62, 0.49 and 0.28-fold; 0.65, 2.21 and 0.31-fold, respectively, as determined using RT-PCR (Figure 4A). Trends in IL-6 and TNFα secreted by OFs are identical, which detected by ELISA, and presented 0.80, 0.72 and 0.67-fold; 0.78, 0.71 and 0.54-fold, lower than induction group (Figure 4B). Both mRNA level and secretion of IL-6 and TNFα decrease with increasing CNPs concentration.
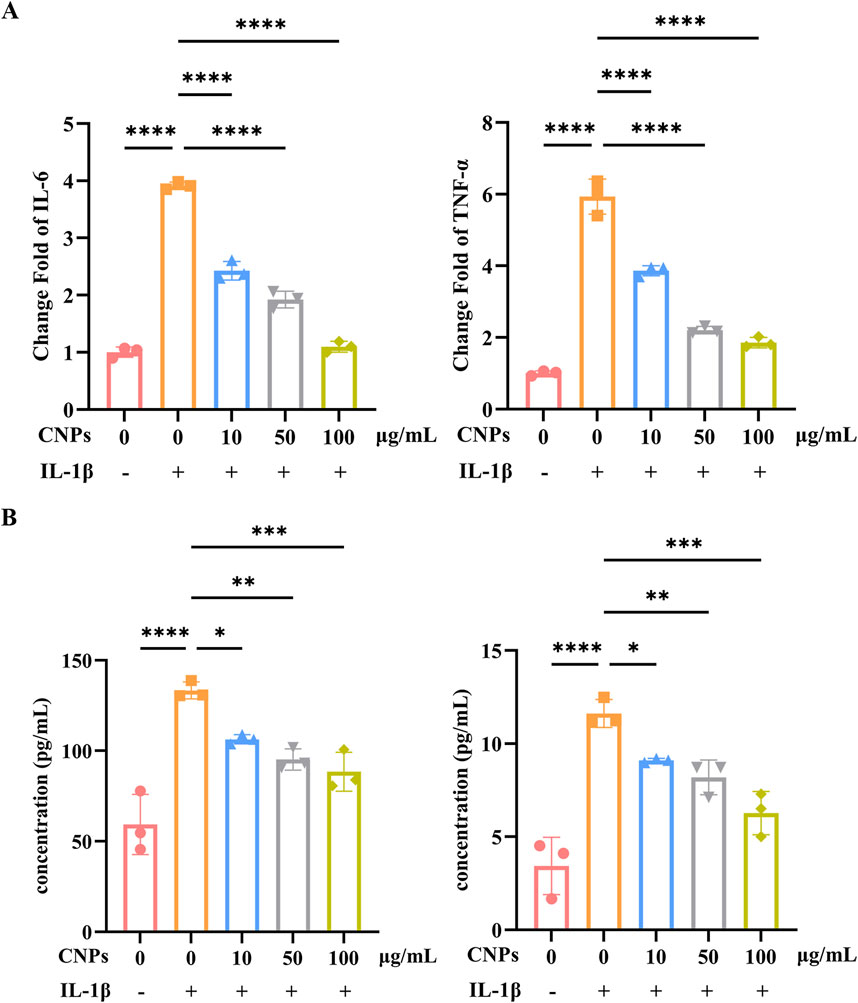
Figure 4. CNPs exert an inhibitory effect on OFs inflammation. (A) mRNA levels of IL-6 and TNF-α after treatment with 0, 10, 50 and 100 μg/mL CNPs and 5 ng/mL IL-1β. (B) Secretion of IL-6 and TNF-α after treatment with different concentrations of CNPs. Data are presented as mean ± SEM (n ≥ 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
4 Discussion
Thyroid-associated ophthalmopathy (TAO) is a refractory orbital disease characterized by specific immune reactivity and pathological remodeling of periocular tissue (Li Z. et al., 2022). Oxidative stress is one of the most important mechanisms underlying the pathogenesis of TAO, leading to elevated ROS, which could result in fibrosis and inflammation. Extraocular muscle fibrosis and inflammatory reaction are mean pathologic changes in TAO. Acute TAO is driven by Th1-mediated inflammation (Antonelli et al., 2014), chronic inactive TAO is characterized by progressive fibrosis. Inflammatory infiltration and accumulation of glycosaminoglycans (GAG) lead to edematous-infiltrative changes in periocular tissues (Łacheta et al., 2019) while TGF-β stimulated differentiation of OFs into myofibroblasts (Hao et al., 2020), which in turn leads to ocular dyskinesia, restrictive extraocular myopathy, and other clinical symptoms in patients. Currently, conventional treatments for TAO include glucocorticoids, immunosuppressants, orbital radiotherapy (Zhang X. et al., 2023), and strabismus correction, but the available treatments have many side effects, such as cushingoid features, elevated blood pressure, weight gain, muscle pain and osteoporosis (Zang et al., 2011). In the active phase, patients feel red and painful in eyes because of vasodilatation and inflammatory cell infiltration caused by active inflammation, and respond well to immunosuppressive therapy. In the inactive phase, with fibrosis taking place, patients show painless motility deficit in eyes, and immunosuppressive therapy is useless (Lin et al., 2021). Surgical interventions provide only symptomatic relief, with many patients experiencing poor prognoses. In recent years, targeted therapies for TAO have developed rapidly. Monoclonal antibody drugs, for example, Teprotumumab (Markham, 2020) and Rituximab (Kang et al., 2022), have emerged as novel therapies for TAO. Teprotumumab, a monoclonal antibody that inhibits IGF-1R, is the first disease-modifying therapy approved in the United States for the treatment of TAO in the United States (Nie and Lamb, 2022). Rituximab, a monoclonal antibody specifically binding CD20, was the first biologic therapies applied to the treatment of active TAO (Genere and Stan, 2019). Although these medications are more effective than traditional treatments, their prohibitive cost limits accessibility. Consequently, the search for a safe, cost-effective, and highly effective TAO therapeutic agent is the current focus of scientific research.
CNPs are cerium oxide particles with a diameter of less than 100 nm, which exhibit strong antioxidant capacity attributed to the unique electronic orbital structure of cerium atoms, which enables the interconversion between Ce4+ and Ce3+ states (Singh et al., 2020). In recent years, CNPs have garnered significant research interest within the medical field, particularly for their neuroprotective and antioxidant properties mediated through the reduction of ROS generation. They also have numerous applications in ophthalmology. The protective role of CNPs in retinal disease in rats (Wong et al., 2013) and mice (Cai et al., 2016) has been demonstrated. Chen et al. found that intravitreal injection of CNPs prior to light exposure effectively protects photoreceptor health and retinal function in albino rats (Chen et al., 2006). CNPs also prevented light-induced degradation of RPE and accumulation of lipofuscin (Tisi et al., 2019). In addition, the investigators tested the lens epithelial cell toxicity of CNPs in vitro (Pierscionek et al., 2012) to confirm that CNPs are also a potential cataract treatment strategy. At the same time, the anti-fibrotic and anti-inflammatory studies of CNPs are accumulating. CNPs exert an inhibitory effect on hepatic fibrosis in mice by reducing ROS production (Godugu et al., 2023) and significantly attenuate the inflammatory response in rats with CCL4-induced liver injury (Oró et al., 2016). Therefore, we assessed the suppression effect of CNPs on TAO OFs fibrosis and inflammation, and explored the mechanism.
In our study, we firstly verified the safe concentration of CNPs on OFs. We demonstrated that CNPs inhibited the fibrotic process and inflammatory response of TAO OFs in a dose-dependent manner. Meanwhile, we found a gradient decrease in intracellular ROS generation with increasing CNPs concentration.
In one study, CNPs were found to downregulate the p38/JNK signalling pathway after intravitreal injection and to inhibit neovascularisation in vldlr−/− mice (Cai et al., 2014). Li et al. further reported that CNPs downregulate JNK phosphorylation in human skin fibroblasts after UVA irradiation to against UVA radiation-induced photoaging (Li et al., 2019). These suggested that CNPs inhibit JNK phosphorylation. JNK, a primary member of the Mitogen-Activated Protein Kinase (MAPK) family, regulates diverse biological processes including cell proliferation, differentiation, cytoskeleton construction, and apoptosis (de Los Reyes Corrales et al., 2021). Evidence implicates resistin promotes cardiac fibroblast to myofibroblast transformation by activating JNK pathway (Singh et al., 2021). Zhang et al. reported that targeting GPR65 alleviates hepatic inflammation and fibrosis by suppressing the JNK pathways (Zhang K. et al., 2023). Otherwise, Astragalus polysaccharide alleviated alcoholic-induced hepatic fibrosis by inhibiting TLR4/JNK/NF-κB/MyD88 pathway (Sun et al., 2023). These suggested that attenuating JNK phosphorylation inhibits fibrosis.
Accordingly, we assessed the degree of cellular JNK phosphorylation upon the addition of 50 μg/mL CNPs when fibrosis was induced and found that the level of CNPs significantly reduced phosphorylated JNK compared to untreated controls. Co-treatment with the JNK agonist Anisomycin revealed an increase in the cellular JNK phosphorylation level and a significant rebound in intracellular ROS generation and fibrosis compared to the addition of CNPs alone. Oxidative stress is associated with the JNK pathway (Gehi et al., 2022). Study showed that blocking JNK pathway alleviates alcoholic liver fibrosis by inhibiting oxidative stress (Liu et al., 2022). In vitro JNK inhibitor reduces oxidative stress in SH-SY5Y cells (Rehfeldt et al., 2021). Collectively the above experimental results suggest that CNPs are greatly likely to alleviate oxidative stress by attenuating JNK phosphorylation, thereby inhibiting fibrosis of OFs. Simultaneously, suppress OFs inflammatory response in a dose-dependent manner.
Nevertheless, our study has certain shortcomings. First, there is no more recognized and stable animal model of TAO for validation in in vivo experiments. Second, our investigation provided a preliminary exploration of the mechanisms by which CNPs inhibit fibrosis in OFs. Third, the sample size of eight patients was relatively small. Given CNPs’ demonstrated efficacy in treating other ophthalmic diseases, we believe that the results of this study are valuable for the use of CNPs in the treatment of TAO.
5 Conclusion
The present study confirmed that CNPs inhibited TAO OFs fibrosis by attenuating JNK phosphorylation, while dose-dependently suppressing TAO OFs inflammatory response and oxidative stress. These findings establish a theoretical foundation for utilizing CNPs, a novel, cost-effective, and safer therapeutic agent, in the treatment of TAO extraocular muscle fibrosis and inflammation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Huazhong University of Science and Technology Union hospital attached to Tongji University Medical School (UHCT230552). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
B-WW: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. RZ: Data curation, Funding acquisition, Methodology, Supervision, Writing – review and editing. YJ: Data curation, Writing – original draft. XO: Resources, Writing – original draft. F-GJ: Conceptualization, Project administration, Supervision, Writing – review and editing. X-HW: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of Hunan Province (Grant number 2024JJ9014), The Funding for Scientific Research Projects from Wuhan Municipal Health (Grant number WX23Q06) and Natural Science Foundation of Xiangjiang Philanthropy Foundation (SKY25055).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Antonelli, A., Ferrari, S. M., Corrado, A., Franceschini, S. S., Gelmini, S., Ferrannini, E., et al. (2014). Extra-ocular muscle cells from patients with Graves’ ophthalmopathy secrete α(CXCL10) and β (CCL2) chemokines under the influence of cytokines that are modulated by PPARγ. Autoimmun. Rev. 13 (11), 1160–1166. doi:10.1016/j.autrev.2014.08.025
Bartalena, L., Kahaly, G. J., Baldeschi, L., Dayan, C. M., Eckstein, A., Marcocci, C., et al. (2021). The 2021 european group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 185 (4), G43–G67. doi:10.1530/EJE-21-0479
Bartalena, L., Piantanida, E., Gallo, D., Lai, A., and Tanda, M. L. (2020). Epidemiology, natural history, risk factors, and prevention of graves' orbitopathy. Front. Endocrinol. (Lausanne) 11, 615993. doi:10.3389/fendo.2020.615993
Boey, A., Leong, S. Q., Bhave, S., and Ho, H. K. (2021). Cerium oxide nanoparticles alleviate hepatic fibrosis phenotypes in vitro. Int. J. Mol. Sci. 22 (21), 11777. doi:10.3390/ijms222111777
Boutzios, G., Chatzi, S., Goules, A. V., Mina, A., Charonis, G. C., Vlachoyiannopoulos, P. G., et al. (2023). Tocilizumab improves clinical outcome in patients with active corticosteroid-resistant moderate-to-severe Graves' orbitopathy: an observational study. Front. Endocrinol. (Lausanne) 14, 1186105. doi:10.3389/fendo.2023.1186105
Cai, X., Seal, S., and McGinnis, J. F. (2014). Sustained inhibition of neovascularization in vldlr-/- mice following intravitreal injection of cerium oxide nanoparticles and the role of the ASK1-P38/JNK-NF-κB pathway. Biomaterials 35 (1), 249–258. doi:10.1016/j.biomaterials.2013.10.022
Cai, X., Seal, S., and McGinnis, J. F. (2016). Non-toxic retention of nanoceria in murine eyes. Mol. Vis. 22, 1176–1187.
Casals, E., Zeng, M., Parra-Robert, M., Fernández-Varo, G., Morales-Ruiz, M., Jiménez, W., et al. (2020). Cerium oxide nanoparticles: advances in biodistribution, toxicity, and preclinical exploration. Small 16 (20), e1907322. doi:10.1002/smll.201907322
Chen, J., Patil, S., Seal, S., and McGinnis, J. F. (2006). Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 1 (2), 142–150. doi:10.1038/nnano.2006.91
Chen, K., Yang, Y., Wu, Y., Cao, W., Zhao, Y., Wang, S., et al. (2025). PLGA nanoparticles encapsulating TSHR-A and rapamycin enhance the induction of dendritic cell-specific immune tolerance in mice with Graves’ disease. Biomed. Mater 20 (2), 025045. doi:10.1088/1748-605X/adbaa3
Comi, S., Cosentino, G., Lanzolla, G., Menconi, F., Maglionico, M. N., Posarelli, C., et al. (2025). Long-term outcome of Graves’ orbitopathy following treatment with sirolimus. J. Endocrinol. Invest. 48 (3), 607–618. doi:10.1007/s40618-024-02470-8
de Los Reyes Corrales, T., Losada-Pérez, M., and Casas-Tintó, S. (2021). JNK pathway in CNS pathologies. Int. J. Mol. Sci. 22 (8), 3883. doi:10.3390/ijms22083883
Diao, J., Chen, X., Mou, P., Ma, X., and Wei, R. (2022). Potential therapeutic activity of berberine in thyroid-associated ophthalmopathy: inhibitory effects on tissue remodeling in orbital fibroblasts. Invest. Ophthalmol. Vis. Sci. 63 (10), 6. doi:10.1167/iovs.63.10.6
DuRoss, A. N., Neufeld, M. J., Rana, S., Thomas, C. R., and Sun, C. (2019). Integrating nanomedicine into clinical radiotherapy regimens. Adv. Drug Deliv. Rev. 144, 35–56. doi:10.1016/j.addr.2019.07.002
Gehi, B. R., Gadhave, K., Uversky, V. N., and Giri, R. (2022). Intrinsic disorder in proteins associated with oxidative stress-induced JNK signaling. Cell Mol. Life Sci. 79 (4), 202. doi:10.1007/s00018-022-04230-4
Genere, N., and Stan, M. N. (2019). Current and emerging treatment strategies for graves’ orbitopathy. Drugs 79 (2), 109–124. doi:10.1007/s40265-018-1045-9
Godugu, C., Khurana, A., and Saifi, M. A. (2023). Rare earth cerium oxide nanoparticles attenuated liver fibrosis in bile duct ligation mice model. J. Trace Elem. Med. Biol. 75, 127102. doi:10.1016/j.jtemb.2022.127102
Hao, M., Sun, J., Zhang, Y., Zhang, D., Han, J., Zhang, J., et al. (2020). Exploring the role of SRC in extraocular muscle fibrosis of the Graves’ ophthalmopathy. Front. Bioeng. Biotechnol. 8, 392. doi:10.3389/fbioe.2020.00392
Kang, S., Hamed Azzam, S., Minakaran, N., and Ezra, D. G. (2022). Rituximab for thyroid-associated ophthalmopathy. Cochrane Database Syst. Rev. 6 (6), CD009226. doi:10.1002/14651858.CD009226.pub3
Łacheta, D., Miśkiewicz, P., Głuszko, A., Nowicka, G., Struga, M., Kantor, I., et al. (2019). Immunological aspects of graves’ ophthalmopathy. Biomed. Res. Int. 2019, 7453260. doi:10.1155/2019/7453260
Längericht, J., Krämer, I., and Kahaly, G. J. (2020). Glucocorticoids in Graves’ orbitopathy: mechanisms of action and clinical application. Ther. Adv. Endocrinol. Metabolism 11, 2042018820958335. doi:10.1177/2042018820958335
Li, X., Han, Z., Wang, T., Ma, C., Li, H., Lei, H., et al. (2022a). Cerium oxide nanoparticles with antioxidative neurorestoration for ischemic stroke. Biomaterials 291, 121904. doi:10.1016/j.biomaterials.2022.121904
Li, Y., Hou, X., Yang, C., Pang, Y., Li, X., Jiang, G., et al. (2019). Photoprotection of cerium oxide nanoparticles against UVA radiation-induced senescence of human skin fibroblasts due to their antioxidant properties. Sci. Rep. 9 (1), 2595. doi:10.1038/s41598-019-39486-7
Li, Z., Wang, M., Tan, J., Zhu, L., Zeng, P., Chen, X., et al. (2022b). Single-cell RNA sequencing depicts the local cell landscape in thyroid-associated ophthalmopathy. Cell Rep. Med. 3 (8), 100699. doi:10.1016/j.xcrm.2022.100699
Lin, C., Song, X., Li, L., Li, Y., Jiang, M., Sun, R., et al. (2021). Detection of active and inactive phases of thyroid-associated ophthalmopathy using deep convolutional neural network. BMC Ophthalmol. 21 (1), 39. doi:10.1186/s12886-020-01783-5
Liu, Y., Kuang, Q., Dai, X., Zhan, M., Zhou, L., Zhu, L., et al. (2022). Deficiency in inactive rhomboid Protein2 (iRhom2) alleviates alcoholic liver fibrosis by suppressing inflammation and oxidative stress. Int. J. Mol. Sci. 23 (14), 7701. doi:10.3390/ijms23147701
Ma, C., Li, H., Lu, S., and Li, X. (2024). Thyroid-associated ophthalmopathy and ferroptosis: a review of pathological mechanisms and therapeutic strategies. Front. Immunol. 15, 1475923. doi:10.3389/fimmu.2024.1475923
Maccarone, R., Tisi, A., Passacantando, M., and Ciancaglini, M. (2020). Ophthalmic applications of cerium oxide nanoparticles. J. Ocul. Pharmacol. Ther. 36 (6), 376–383. doi:10.1089/jop.2019.0105
Markham, A. (2020). Teprotumumab: first approval. Drugs 80 (5), 509–512. doi:10.1007/s40265-020-01287-y
Nie, T., and Lamb, Y. N. (2022). Teprotumumab: a review in thyroid eye disease. Drugs 82 (17), 1663–1670. doi:10.1007/s40265-022-01804-1
Oculoplastic and Orbital Disease Group of Chinese Ophthalmological Society of Chinese Medical Association (2022). Chinese guideline on the diagnosis and treatment of thyroid-associated ophthalmopathy (2022). Zhonghua Yan Ke Za Zhi. 58 (9), 646–668. doi:10.3760/cma.j.cn112142-20220421-00201
Oró, D., Yudina, T., Fernández-Varo, G., Casals, E., Reichenbach, V., Casals, G., et al. (2016). Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J. Hepatol. 64 (3), 691–698. doi:10.1016/j.jhep.2015.10.020
Pelaz, B., Alexiou, C., Alvarez-Puebla, R. A., Alves, F., Andrews, A. M., Ashraf, S., et al. (2017). Diverse applications of nanomedicine. ACS Nano 11 (3), 2313–2381. doi:10.1021/acsnano.6b06040
Pierscionek, B. K., Li, Y., Schachar, R. A., and Chen, W. (2012). The effect of high concentration and exposure duration of nanoceria on human lens epithelial cells. Nanomedicine 8 (3), 383–390. doi:10.1016/j.nano.2011.06.016
Rehfeldt, S. C. H., Laufer, S., and Goettert, M. I. (2021). A highly selective in vitro JNK3 inhibitor, FMU200, restores mitochondrial membrane potential and reduces oxidative stress and apoptosis in SH-SY5Y cells. Int. J. Mol. Sci. 22 (7), 3701. doi:10.3390/ijms22073701
Salvi, M., and Covelli, D. (2019). B cells in Graves' orbitopathy: more than just a source of antibodies? Eye (Lond). 33 (2), 230–234. doi:10.1038/s41433-018-0285-y
Shen, F., Liu, J., Fang, L., Fang, Y., and Zhou, H. (2023). Development and application of animal models to study thyroid-associated ophthalmopathy. Exp. Eye Res. 230, 109436. doi:10.1016/j.exer.2023.109436
Singh, K. R., Nayak, V., Sarkar, T., and Singh, R. P. (2020). Cerium oxide nanoparticles: properties, biosynthesis and biomedical application. RSC Adv. 10 (45), 27194–27214. doi:10.1039/d0ra04736h
Singh, R., Kaundal, R. K., Zhao, B., Bouchareb, R., and Lebeche, D. (2021). Resistin induces cardiac fibroblast-myofibroblast differentiation through JAK/STAT3 and JNK/c-Jun signaling. Pharmacol. Res. 167, 105414. doi:10.1016/j.phrs.2020.105414
Sun, X., Zheng, Y., Tian, Y., Xu, Q., Liu, S., Li, H., et al. (2023). Astragalus polysaccharide alleviates alcoholic-induced hepatic fibrosis by inhibiting polymerase I and transcript release factor and the TLR4/JNK/NF-κB/MyD88 pathway. J. Ethnopharmacol. 314, 116662. doi:10.1016/j.jep.2023.116662
Tisi, A., Passacantando, M., Lozzi, L., Riccitelli, S., Bisti, S., and Maccarone, R. (2019). Retinal long term neuroprotection by cerium oxide nanoparticles after an acute damage induced by high intensity light exposure. Exp. Eye Res. 182, 30–38. doi:10.1016/j.exer.2019.03.003
Wei, F., Neal, C. J., Sakthivel, T. S., Kean, T., Seal, S., and Coathup, M. J. (2021). Multi-functional cerium oxide nanoparticles regulate inflammation and enhance osteogenesis. Mater Sci. Eng. C Mater Biol. Appl. 124, 112041. doi:10.1016/j.msec.2021.112041
Wong, L. L., Hirst, S. M., Pye, Q. N., Reilly, C. M., Seal, S., and McGinnis, J. F. (2013). Catalytic nanoceria are preferentially retained in the rat retina and are not cytotoxic after intravitreal injection. PLoS One 8 (3), e58431. doi:10.1371/journal.pone.0058431
Xu, Z., Ye, H., Xiao, W., Sun, A., Yang, S., Zhang, T., et al. (2022). Metformin attenuates inflammation and fibrosis in thyroid-associated ophthalmopathy. Int. J. Mol. Sci. 23 (24), 15508. doi:10.3390/ijms232415508
Zang, S., Ponto, K. A., and Kahaly, G. J. (2011). Clinical review: intravenous glucocorticoids for Graves' orbitopathy: efficacy and morbidity. J. Clin. Endocrinol. Metab. 96 (2), 320–332. doi:10.1210/jc.2010-1962
Zhang, K., Zhang, M. X., Meng, X. X., Zhu, J., Wang, J. J., He, Y. F., et al. (2023b). Targeting GPR65 alleviates hepatic inflammation and fibrosis by suppressing the JNK and NF-κB pathways. Mil. Med. Res. 10 (1), 56. doi:10.1186/s40779-023-00494-4
Zhang, X., Zhao, Q., and Li, B. (2023a). Current and promising therapies based on the pathogenesis of Graves' ophthalmopathy. Front. Pharmacol. 14, 1217253. doi:10.3389/fphar.2023.1217253
Keywords: thyroid-associated ophthalmopathy, cerium oxide nanoparticles, orbital fibroblasts, JNK phosphorylation, fibrosis, inflammation
Citation: Wang B-W, Zhu R, Jin Y, Ouyang X, Jiang F-G and Wang X-H (2025) Cerium oxide nanoparticles attenuates fibrosis and inflammation in thyroid-associated ophthalmopathy via JNK pathway. Front. Mol. Biosci. 12:1580062. doi: 10.3389/fmolb.2025.1580062
Received: 20 February 2025; Accepted: 10 July 2025;
Published: 23 July 2025.
Edited by:
Paolo Tortora, University of Milano-Bicocca, ItalyReviewed by:
Poupak Fallahi, University of Pisa, ItalyMuling Zeng, Spanish National Research Council (CSIC), Spain
Copyright © 2025 Wang, Zhu, Jin, Ouyang, Jiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fa-Gang Jiang, ZmdqaWFuZ0BodXN0LmVkdS5jbg==; Xing-Hua Wang, eGluZ2h1YV93YW5nQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Bo-Wen Wang
Bo-Wen Wang Ru Zhu
Ru Zhu Ying Jin1
Ying Jin1 Xing-Hua Wang
Xing-Hua Wang