- 1Department of Pharmacy, Baotou Medical College, Baotou, China
- 2The Second Affiliated Hospital of Baotou Medical College, Baotou, China
- 3The First Affiliated Hospital of Baotou Medical College, Baotou, China
- 4Institute of Bioactive Substance and Function of Mongolian Medicine and Chinese Materia Medica, Baotou Medical College, Baotou, China
Introduction: Hepatic Fibrosis (HF), a pathological remodeling process triggered by persistent liver damage, is marked by the excessive buildup of extracellular matrix (ECM), leading to a gradual deterioration of liver function and an increased likelihood of advancing to cirrhosis and liver failure.
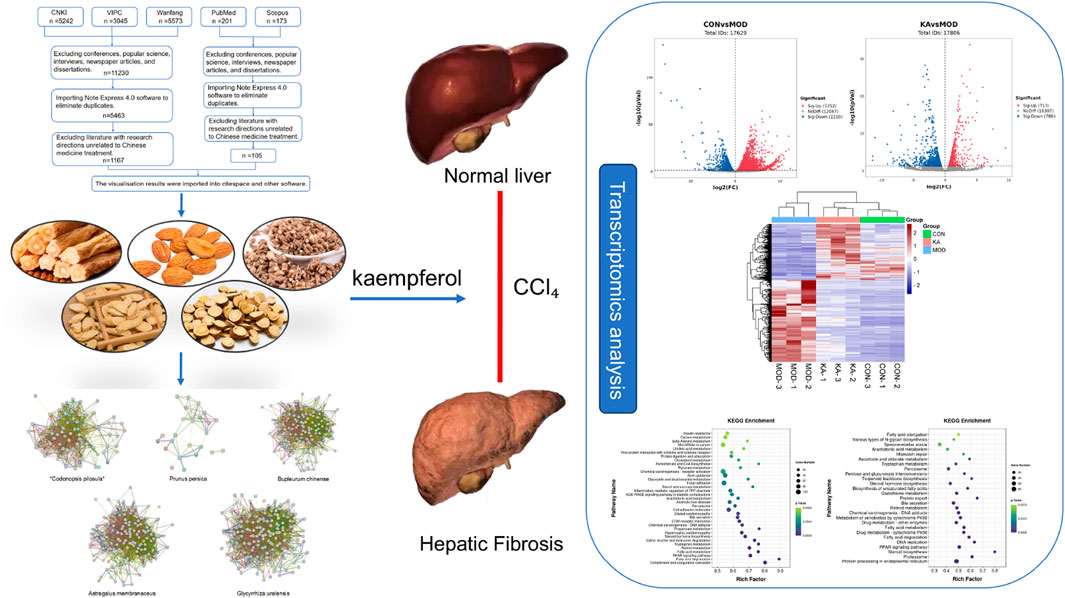
Methods: This study adopts a systematic pharmacology methodology, initially employing bibliometric analysis to identify traditional Chinese medicine (TCM) formulations and individual herbs with potential anti-HF properties. Subsequently, a multi-dimensional network analysis is conducted to pinpoint core active components. Experimental investigations involve the construction of a carbon tetrachloride (CCl4)-induced rat model of liver fibrosis, complemented by transcriptomic technology to systematically elucidate the mechanisms of action of the active components in TCM.
Results: In this study, kaempferol (KA), identified as the principal active compound with anti-fibrotic properties, was selected from traditional Chinese medicine (TCM) and TCM prescriptions through a combination of bibliometric analysis and network pharmacology. Pharmacodynamic evaluations, including pathological section analyses, demonstrated that KA effectively mitigated the fibrotic process and decreased collagen deposition. Further corroborated by ELISA experiments, kaempferol exhibited pronounced anti-fibrotic effects, inhibited inflammatory responses, restored liver function indices, and ameliorated the progression of liver fibrosis. Mechanistic investigations revealed that KA modulated fatty acid metabolism, retinol metabolism, and arachidonic acid metabolism by regulating the expression of key metabolic enzyme genes such as SCD, SCD2, FADS2, and CYP4A8, and significantly influenced the activity of the PPAR signaling pathway. Additionally, it impacted the dysregulation of lipid metabolism and inflammatory response pathways, significantly inhibited hepatic stellate cell (HSC) activation, and reduced ECM accumulation.
Discussion: This finding elucidates the mechanism by which KA attenuates HF through multi-target regulation, and provides a theoretical basis for metabolic reprogramming-based therapeutic strategies with translational valu.
1 Introduction
Hepatic fibrosis (HF) is a pathological process associated with chronic liver disease, characterized by the abnormal deposition of extracellular matrix (ECM) (George et al., 2019). The pathological progression of HF encompasses inflammatory activation (Zhangdi et al., 2019), disruption of oxidative stress balance (Lin et al., 2018), and the persistent activation of pro-fibrotic signaling pathways, eventually advancing to liver failure or hepatocellular carcinoma (HCC). This condition has become a major global public health issue (Yang et al., 2023). In this context, hepatic stellate cells (HSCs) act as key effector cells, driving the fibrotic process through complex interactions with various cells, cytokines, and signaling pathways (Kitano and Bloomston, 2016). Although etiological treatment is regarded as the gold standard for preventing and managing HF (Wang et al., 2022), its effects are often slow, and it is challenging to reverse advanced fibrotic lesions. While silymarin is a widely utilized therapeutic agent for liver fibrosis, it suffers from poor oral bioavailability, rapid hepatic metabolism, and challenges in maintaining effective blood drug concentrations (Vanzan et al., 2024). The development of combination regimens with other antifibrotic agents remains in its infancy. Consequently, traditional Chinese medicine (TCM) has emerged as a significant area of interest in liver fibrosis treatment due to its multi-target regulatory properties (Jia et al., 2022).
TCM formulations facilitate systematic intervention in the pathological processes of HF through the synergistic modulation of disease-associated biological networks by multiple components (Li et al., 2022). This “multi-component, multi-pathway” mechanism of action provides unique benefits in addressing complex diseases, as it allows for a more comprehensive and synergistic therapeutic approach (Liu et al., 2024). Furthermore, TCM has been widely applied in the clinical treatment of HF due to its significant safety and economic benefits. Significant progress has been made in the research on the treatment of HF.
The effective active ingredient for the treatment of HF was identified as kaempferol (KA) through data screening of extensive literature and analysis of web-based pharmacological data. KA is an important natural flavonoid compound that is widely found in a variety of medicinal plants, including the Asteraceae family, as well as in cruciferous vegetables and rutabaga fruits (Chen et al., 2023). This compound demonstrates a wide range of biological activities, addressing critical pathological processes such as free radical scavenging, inflammation inhibition (Alam et al., 2020), and tumor cell cycle regulation (Ma et al., 2023). KA exhibits notable hepatoprotective properties by counteracting chemically induced liver damage, with its mechanism of action intricately linked to the regulation of hepatocyte metabolic homeostasis (Wang et al., 2015). In comparison to silymarin, a well-known treatment for liver fibrosis, kaempferol exhibits a broader multi-target mechanism of action, enabling a more comprehensive regulation of critical pathways involved in liver fibrosis, such as TGF-β/Smad and PI3K/Akt (El-Hawary et al., 2019; Xia et al., 2025), Kaempferol demonstrates greater potential in inhibiting the activation of hepatic stellate cells and promoting collagen degradation. Additionally, kaempferol is characterized by its wide availability and low production cost (Alam et al., 2020), making it a more economical option while maintaining efficacy. This cost-effectiveness can better accommodate the long-term medication needs of patients and enhance treatment adherence.
This study introduces an innovative research paradigm, namely, “from macro screening to micro analysis”. Initially, bibliometric techniques are employed to systematically analyze the omics characteristics of TCM anti-HF research, facilitating the identification of potential active components. Subsequently, network pharmacology is utilized to construct a multi-dimensional interaction network encompassing “component-target-pathway” (Zheng et al., 2024), relationships, thereby elucidating key pharmacodynamic substances and their central targets (Xiang et al., 2024). Concurrently, network pharmacology has demonstrated significant multi-level systematic advantages in the study of traditional Chinese medicine. Firstly, this approach allows for a comprehensive analysis of the synergistic mechanisms among multiple components and facilitates the precise optimization of compound compatibility ratios (Li et al., 2025). Secondly, it enables the efficient identification of key active ingredients within a single herb and elucidates its multi-target synergistic action mode (Su et al., 2025). Furthermore, focusing on a single compound not only broadens its potential therapeutic applications but also provides precise guidance for the optimization of its molecular structure (Zheng et al., 2025). Subsequently, in vivo experiments were conducted to verify the pharmacodynamic effects of KA on HF in SD rats induced by carbon tetrachloride and treated with KA, and the pharmacodynamic effects of KA on HF were analyzed by combining with pathological sections and relevant biochemical indexes, and further histological analyses were carried out to reveal the specific mechanism of KA’s antifibrotic effects. This methodological framework not only clarifies the multi-dimensional mechanisms underlying TCM but also lays a foundational theoretical groundwork for the innovation of new pharmacological treatments aimed at combating HF.
2 Methods
2.1 Bibliometric analysis
2.1.1 Data sources
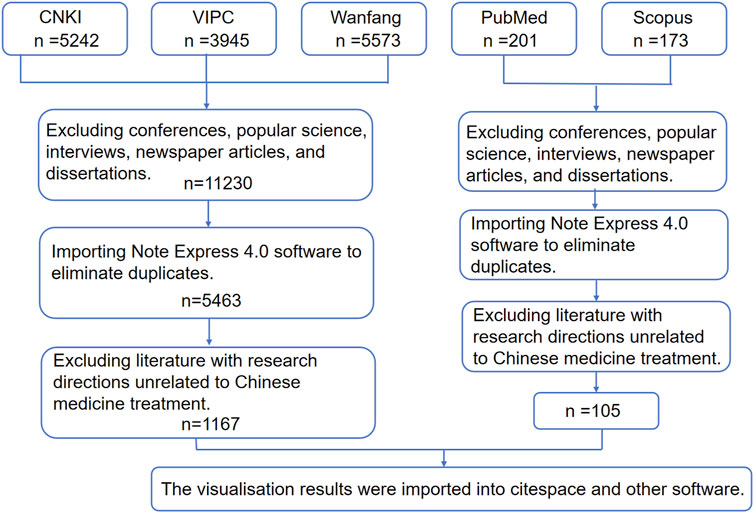
The literature review was conducted using data collected from five databases: CNKI, Wanfang Data, VIP, Scopus, and PubMed. The search strategy employed precise subject terms, including “hepatic fibrosis,” “liver fibrosis,” “Chinese medicine,” and “Chinese medicine prescription,” covering the period from 1 January 2018, to 1 January 2023.
2.1.2 Literature screening criteria
Inclusion Criteria: (1) Clinical trials or experimental studies explicitly using TCM formulations or single herbs for HF intervention. (2) Original research with detailed prescription compositions. Exclusion Criteria: (1) Duplicate publications. (2) Studies lacking detailed prescription compositions (e.g., labeled as “standardized formula” without specific components). (3) Studies involving subjects with concurrent conditions like tumors, autoimmune diseases, or other comorbidities. (4) Non-research literature (e.g., popular science articles, interviews, conference summaries).
2.1.3 Data processing
Using NoteExpress 4.0 for duplicate removal, two researchers independently reviewed titles and abstracts, with a senior researcher resolving disagreements. Documents meeting criteria were exported to Refworks format to build a structured database, and then a keyword co-occurrence network visualization was generated using VOSviewer version 1.6.19, with a minimum word frequency threshold set at five occurrences. Finally, a frequency matrix of Chinese medicine ingredients was constructed in Microsoft Excel 2021 to identify high-frequency candidates based on frequency-efficacy correlation.
2.2 Network pharmacology analysis
2.2.1 Screening of active component and target prediction
Based on in accordance with the standards recordoutlined in the Chinese Pharmacopoeia (2020 edition), the active components of Codonopsis pilosula, Prunus persica, Bupleurum chinense, Glycyrrhiza uralensis, and Astragalus membranaceus were identified using the TCM Systems Pharmacology Database and Analysis Platform (TCMSP; https://tcmsp-e.com/). Following international pharmacokinetic screening criteria (Yan et al., 2018).
2.2.2 Integration of disease-related targets
Disease-related targets were extracted from GeneCards (https://www.genecards.org/) V5.13 and the Online Mendelian Inheritance in Man (OMIM; https://omim.org/) database using the search terms “Hepatic Fibrosis” and “Liver Fibrosis.” The intersection of targets from TCM and disease-related targets was determined using the JVenn (Zhang Y. et al., 2022) interactive tool (https://jvenn.toulouse.inrae.fr/). Significant intersection targets were determined through hypergeometric testing, with a false discovery rate (FDR)-adjusted p -value of less than 0.05.
2.2.3 Construction of multi-dimensional interaction networks
The shared targets were imported into STRING version 11.5 (https://string-db.org/), with a confidence threshold set at ≥0.4 (Martino et al., 2021), to construct a high-density protein-protein interaction (PPI) network for the organism Homo sapiens. Cytoscape version 3.9.1 was employed to calculate node characteristic parameters, including degree centrality, betweenness centrality, and closeness centrality. Utilizing inverse pharmacophore mapping, the top five core targets (Degree ≥2 × Median) and their corresponding binding active components were identified.
2.3 Experimental animals and drug preparation
This study received approval from the Experimental Animal Ethics Committee of Baotou Medical College (Approval No.: 2022-96-1). A cohort of forty healthy male Sprague-Dawley (SD) rats (Rattus norvegicus, strain code: VAF/Plus®), classified as SPF-grade, aged between 6 and 8 weeks and weighing 190-220 g, were procured from the National Institutes for Food and Drug Control [Laboratory Animal Production License No.: SCXK (Jing) 2017-0005]. The rats underwent a 7-day acclimatization period in a controlled environment, maintained at a temperature of 22 °C ± 1 °C and a relative humidity of 50% ± 5%, with unrestricted access to sterilized irradiated feed (AIN-93G standard formula) and drinking water with a pH of 2.5–3.0. KA, (CAS: 520-18-3, with a purity of ≥98%, sourced from Shanghai Yuanye Bio-Technology Co., Ltd.) was utilized in this study. A suspension of KA (KA701216 Shanghai Jieshikai Biotechnology Co.) at a concentration of 5 mg/mL was prepared using 0.5% sodium carboxymethyl cellulose (CMC-Na, Sigma-Aldrich, C5678) as the solvent, followed by ultrasonic oscillation at 40 kHz for 30 min. The suspension was freshly prepared prior to use and stored at 4 °C in a dark environment.
2.4 HF model construction and drug administration
Following a 7-day acclimatization period, the experimental animals were allocated into four groups using a stratified randomization method based on body weight: the blank control group (CON), the model group (MOD), the silymarin positive control group, and the kaempfenol intervention group (KA), with each group comprising 10 animals. The Reynaert chronic heart failure induction method, as modified, was employed (Huang et al., 2018). The MOD, silymarin positive control, and KA groups received subcutaneous injections of a 40% carbon tetrachloride (CCl4) suspension in corn oil (Sigma-Aldrich, 319961). An initial induction dose of 5 mL/kg was administered during the first week, followed by a maintenance dose of 3 mL/kg biweekly for a duration of 7 weeks, ensuring a minimum dosing interval of 72 h. The CON group was administered an equivalent volume of sterile corn oil. Commencing on the first day of model induction, drug interventions were conducted daily between 8:00 and 10:00 a.m. The specific intervention protocols were as follows: (1) The CON and MOD groups received 0.9% normal saline via oral gavage at a dosage of 10 mL/kg. (2) The silymarin positive control group was administered 100 mg/kg of silymarin, dissolved in a 10 mL/kg solution, via oral gavage. (3) In the kaempferol group, a suspension of kaempferol at a dosage of 100 mg/kg was dissolved in a 10 mL/kg solution and administered orally via gavage. The allocation of subjects into groups was conducted by independent researchers utilizing a computer-generated random number system. To ensure rigorous blinding, the study employed a stringent triple-blind design. Specifically, the experimental operators, responsible for drug administration and sample collection, were not involved in the grouping process and remained unaware of the treatment assignments. Histopathological evaluations were independently performed by two senior pathologists using a double-blind approach, with neither pathologist having access to the group allocation information. Data analysts were only exposed to anonymized, encoded data throughout the analysis process until the completion of statistical evaluations. The chosen dose of 100 mg/kg was selected to achieve a local hepatic concentration within the effective range of 2–10 μM, as established by in vitro studies. Additionally, this dosage has been demonstrated to significantly ameliorate liver fibrosis markers in several animal studies, while maintaining a favorable safety profile. This selection of dose was designed to address the requirements for anti-fibrotic therapy while ensuring the safety of medication (Cao et al., 2023) Continuous monitoring was conducted to assess the following parameters: (1) Changes in biological rhythms, including food intake and frequency of voluntary activities. (2) Evaluation of survival status, focusing on coat gloss and mucosal coloration. (3) Dynamic Body Weight Monitoring: Body weight was measured biweekly, on Mondays and Thursdays, with an accuracy of ±0.1 g.
2.5 Observation of liver tissue morphology and sample collection in rats
Following an 8-week regimen of continuous drug administration, rats were anesthetized 24 h after the final dose using an intraperitoneal injection of sodium pentobarbital solution (3%, 30 mg/kg). Post-euthanasia, immediate assessments of the liver’s color, texture, morphology, and volume were conducted. Subsequently, 5 mL of blood was drawn from the abdominal aorta, with serum being separated and preserved at −80 °C. A portion of the liver tissue was preserved in a 4% paraformaldehyde solution, while the remaining tissue was rapidly frozen in liquid nitrogen, transferred to cryotubes, and stored at −80 °C for subsequent analyses.
2.6 Determination of biochemical markers of liver fibrosis in rats
Adequate amounts of rat liver tissue from each group were taken. The levels of key biochemical indices were measured and analyzed using enzyme-linked immunosorbent assay (ELISA) with an ELISA enzyme labeled instrument, operated according to the instructions of the corresponding kit. These indices included inflammatory factors such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α); liver function indices including aspartate aminotransferase (AST) and alanine aminotransferase (ALT); and fibrosis-related indices such as hyaluronic acid (HA), laminin (LN), type III procollagen (PC-III), and type IV collagen (COL-IV) in the liver tissues.
2.6.1 Sample preparation and sequencing
Upon completion of the experiment, three biological replicate samples were collected from each experimental group (CON, MOD, KA) and stored at −80 °C for no longer than 6 weeks. Total RNA was extracted utilizing the TRIzol® method, and any genomic DNA contaminants were removed using DNase I (RNase-free, Thermo Fisher, EN0521). RNA integrity was evaluated via 1% agarose gel electrophoresis (100 V, 30 min), ensuring a 28S/18S ratio of ≥1.8, while RNA purity was assessed using the NanoDrop 2000 spectrophotometer (Thermo Fisher), with acceptable A260/A280 ratios ranging from 1.8 to 2.1 and A260/A230 ratios of ≥2.0. The RNA Integrity Number (RIN) was assessed utilizing the Agilent 2100 Bioanalyzer with the RNA Nano 6000 chip, where RIN values of 7.0 or higher were deemed acceptable. mRNA sequencing libraries were prepared in accordance with the standard protocol of the NEBNext® UltraTM II RNA Library Prep Kit (NEB, E7770S). Sequencing was executed on the Illumina NovaSeq 6000 platform, employing 150 base pair paired-end reads (2 × 150 PE).
2.6.2 Bioinformatics analysis
In this study, we employed FASTP (version 0.19.7) for the initial processing of raw sequencing data, specifically the raw reads. During this phase, sequences of low quality (phred score <20) and contaminating adapter sequences were excised, resulting in a dataset of cleaned reads with a clean data ratio of ≥95%. Subsequently, these cleaned reads were aligned to the reference genome, Rattus norvegicus mRatBN7.2, utilizing HISAT2 (version 2.2.1). The calculation of Fragments Per Kilobase of transcript per Million mapped reads (FPKM) values was conducted using StringTie (version 2.1.7). For datasets comprising biological replicates, differentially expressed genes (DEGs) were identified using EdgeR (version 3.42.4), applying thresholds of a false discovery rate (FDR) < 0.05 and an absolute log2 fold change (|log2FC|) ≥ 1. Functional enrichment analyses of Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were conducted utilizing ClusterProfiler (version 4.6.2), with an adjusted p-value threshold of <0.05. Visualization of the regulatory and clustering characteristics of differentially expressed mRNAs was achieved through the generation of volcano plots and correlation heatmaps using the OmicStudio cloud platform (https://www.omicstudio.cn/tool).
2.7 Statistical analysis
Statistical evaluations were performed utilizing SPSS software, version 26.0. Experimental data were presented as mean ± standard deviation (
3 Results
3.1 Fundamental characteristics of the literature on TCM in the treatment of HF
This study initially identified a total of 14,760 publications related to the application of TCM in the treatment of HF through a systematic search of domestic databases. The literature was subsequently filtered based on predefined inclusion and exclusion criteria. Initially, 3,530 non-research documents, including conference abstracts, popular science articles, news reports, and dissertations, were excluded. Following this, 5,767 duplicate entries were removed using the Note Express 4.0 literature management software. Additionally, 4,296 articles that did not pertain to TCM treatment of HF were excluded. In PubMed, the search formula “(TCM) AND (liver fibrosis) AND (hepatic fibrosis)” yielded 134 articles, whereas the formula “((TCM prescription) AND (liver fibrosis)) AND (liver fibrosis)” retrieved 37 articles. In Scopus, the corresponding search methods resulted in 102 and 71 articles, respectively. After excluding incomplete, irrelevant, and duplicate articles, the total number of relevant articles was reduced to 105, which constitutes only 8% of similar studies found in the Chinese databases. Consequently, our study undertook a comprehensive analysis of the literature available in CNKI, Wanfang, and VIP, while also incorporating relevant international studies from databases such as PubMed and Scopus. Ultimately, 1,167 papers that satisfied the criteria were retained for analysis. Figure 1 illustrates the detailed procedure involved in the literature screening process.
3.2 Keyword analysis
3.2.1 Keyword Co-occurrence analysis
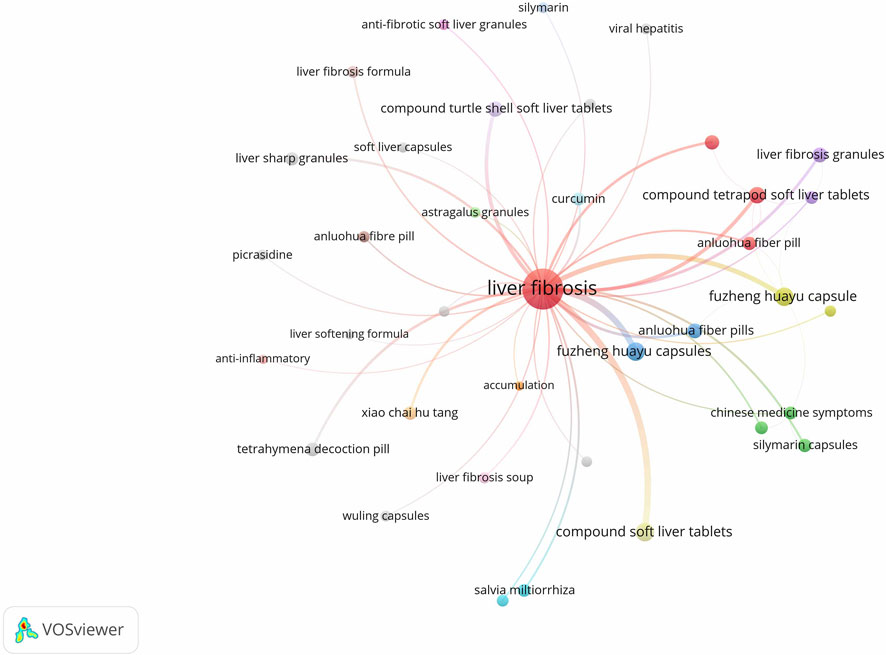
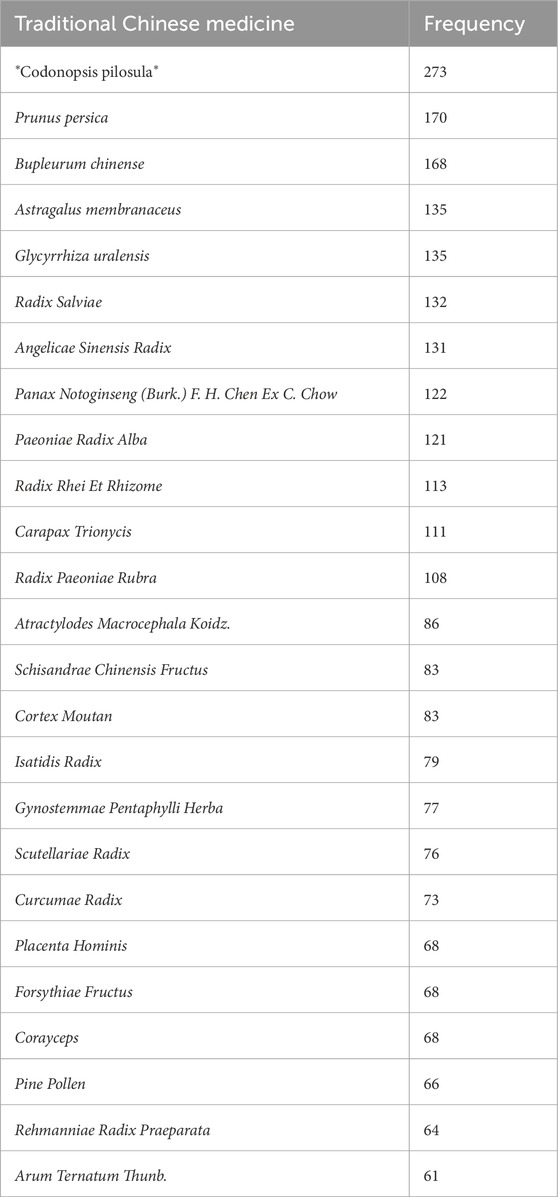
Keywords were analyzed for co-occurrence using VOSviewer 1.6.19 software, with the frequency of keywords serving as an indicator of research interest in the field. The database included a total of 1,816 keywords, of which 153 appeared with a frequency of five or more. A co-occurrence analysis was conducted on keywords associated with herbal prescriptions and monomers, resulting in the generation of a keyword co-occurrence network graph (Figure 2). In this graph, each node represents a keyword, and the size of the node corresponds to the frequency of the keyword. The connecting lines between nodes signify the co-occurrence relationships between the keywords. Notably, keywords such as “Compound Jianjia Soft Liver Tablets”, “Fuzheng Huayu Capsule”, “Tetrapod Decoction Pill”, “Anluohua Fibre Pill”, “Fuzheng Huayu Capsules”, “Baojia Decoction Pills”, “Anluo Huayu Pills”, and “Huangqi” exhibit higher frequencies of occurrence. Statistical analysis of keyword frequencies reveals the top 25 single drugs, as presented in Table 1.
3.3 Acquisition of active ingredients and targets
A total of 92 active ingredients were identified from Glycyrrhiza uralensis. After integrating the active ingredient targets and eliminating duplicate entries, 183 unique targets were obtained. In the study of *Codonopsis pilosula*, 21 active ingredients were identified. Upon integrating the active ingredient targets and eliminating duplicates, 85 unique targets were obtained. Astragalus membranaceus was found to contain 20 active ingredients, resulting in 163 unique targets after target integration and duplicate removal. The investigation of Prunus persica revealed 23 active ingredients, leading to 31 unique targets after processing. Conversely, Bupleurum chinense comprised 17 active ingredients, yielding 150 unique targets following the same procedure.
3.4 Common targets of five TCMs with HF
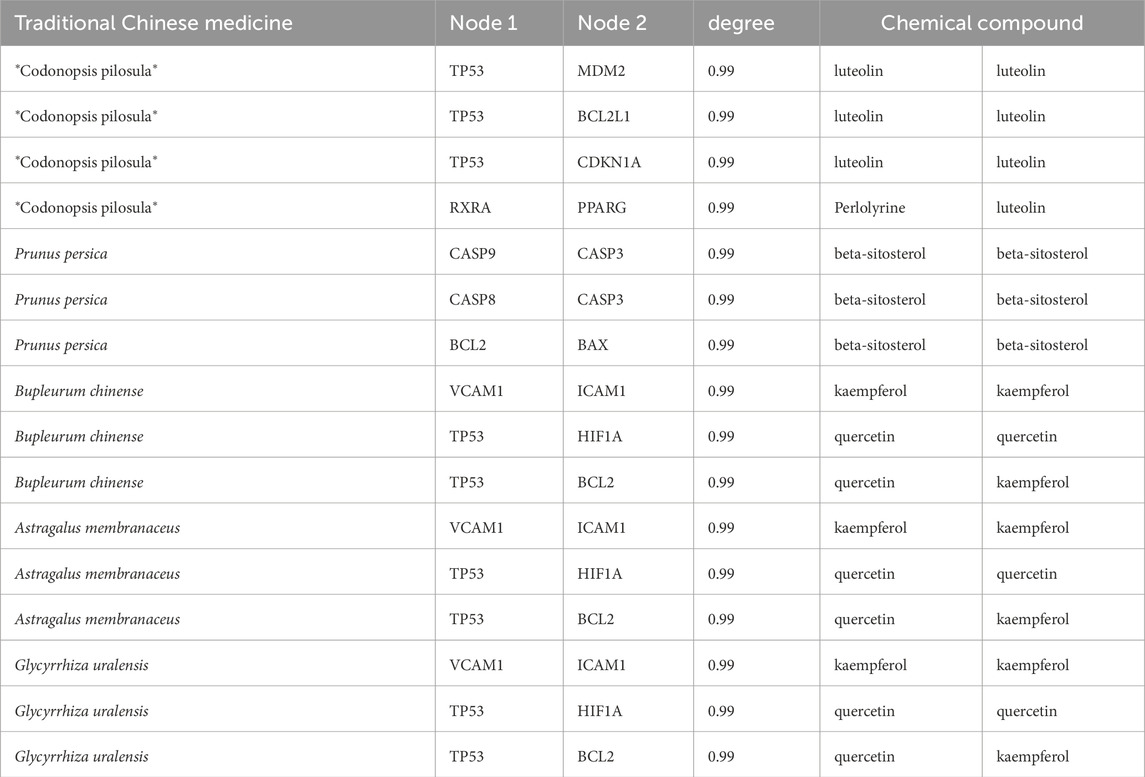
In this study, a total of 9,804 targets associated with HF were identified. Common targets were determined using Venny 2.1 to find intersections with each TCM (Figure 3, Supplementary Material 1).
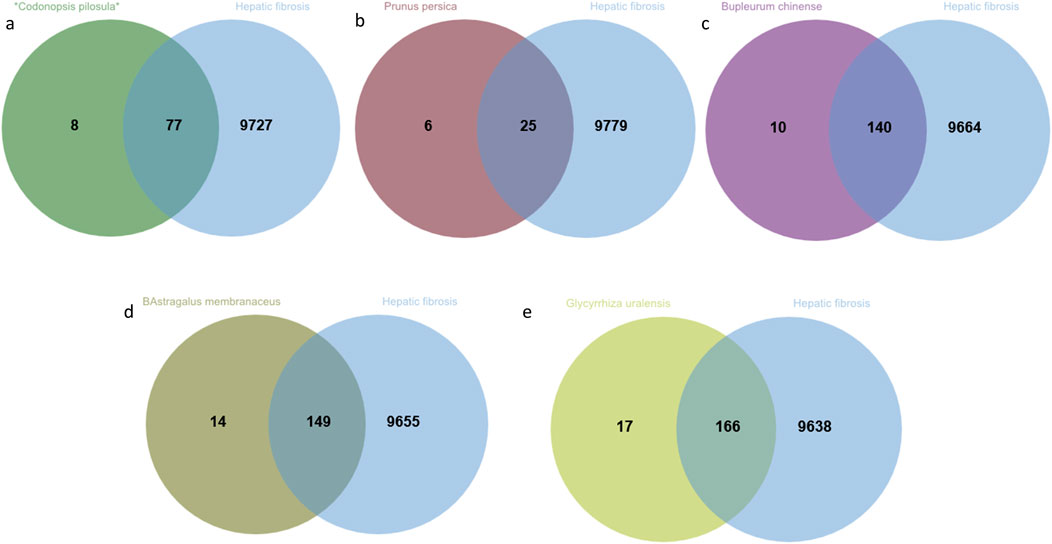
Figure 3. The intersecting targets of *Codonopsis pilosula*、Prunus persica、Bupleurum chinense、Astragalus membranaceus and Glycyrrhiza uralensis with HF. (a) *Codonopsis pilosula* and HF; (b) Prunus persica and HF; (c) Bupleurum chinense and HF; (d) Astragalus membranaceus and HF; (e) Glycyrrhiza uralensis and HF.
3.5 Protein-protein interaction (PPI) network of five Chinese herbal medicines for the treatment of HF
After uploading the intersecting targets identified from the screening to the STRING database (https://string-db.org/), with the species specified as “Homo sapiens” and hidden no-joints, a protein-protein interaction (PPI) network diagram was generated (Figure 4). Through analysis of the interaction scores between the nodes, the top five core targets were identified, including 25 key targets such as GAPDH, TP53, Akt1, IL-6, and TNF. Subsequently, the active ingredients of TCMs associated with these targets were further screened, and their frequency of occurrence was quantified (Table 2). Notably, compounds such as quercetin, KA, and β-sitosterol, found in TCMs like *Codonopsis pilosula*, Prunus persica, Bupleurum chinense, Astragalus membranaceus and Glycyrrhiza uralensis exhibited high target binding frequency and demonstrated significant correlation within the target network.
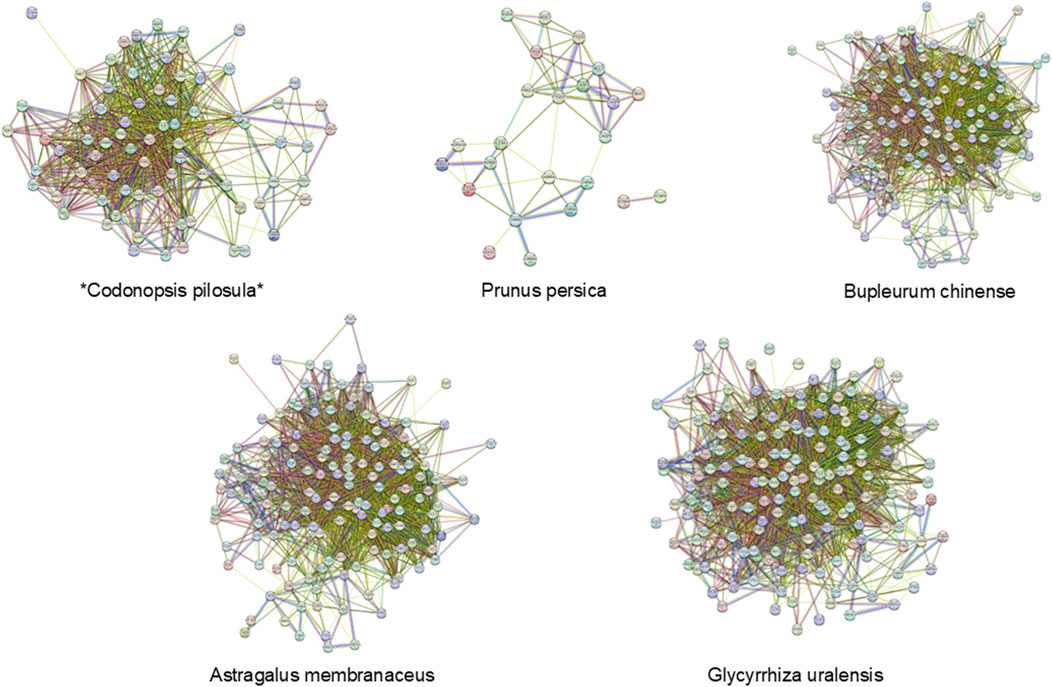
Figure 4. The protein interaction network of *Codonopsis pilosula*, Prunus persica, Bupleurum chinense, Astragalus membranaceus and Glycyrrhiza uralensis in the treatment of HF.
3.6 Morphological alterations in rat liver tissue appearance
In the CON group, the liver tissue of normal rats exhibited a reddish-brown color, uniformity, a smooth surface, and a soft texture, as depicted in Figure 5a. Conversely, the livers of rats in the MOD group presented a brownish-yellow hue and were swollen, with an uneven, fibrotic surface and a hard texture, characterized by blotches and an inconsistent color, as shown in Figure 5b. Notably, there was a significant improvement in liver color and texture in the silymarin group (Figure 5c) as well as in the KA group (Figure 5d).
Figure 5. Histopathological evaluation of rat liver tissue. (a–d) Macroscopic appearance and morphology of liver tissue: (a) Normal group, (b) Model group, (c) Silymarin-treated group, (d) KA-treated group. (e–l) Histopathologic analysis by H&E and Masson trichrome staining: (e,i) The normal group; (f,j) The model group; (g,k) The silymarin group; (h,i) The KA treatment group. (m) Histopathological scores among each group. Comparisons with the normal control group, the statistical significance indicated by ***P < 0.001, **P < 0.01, and *P < 0.05. Comparisons with the model group are indicated by ###P < 0.001, ##P < 0.01, and #P < 0.05.
3.7 Histological changes in rat liver
Histological examination of normal rat liver revealed structurally intact tissue. However, administration of CCl4 for 8 weeks in the model group led to extensive HF, as determined by both qualitative and quantitative histopathological assessments. In comparison to the normal liver morphology (Figures 5e, i), CCl4-induced fibrosis was characterized by disrupted tissue architecture, fiber extension, formation of large fibrous septa, separation into pseudolobules, and collagen accumulation (Figures 5f,j). In the liver slices of rats that were treated with silymarin (Figures 5g,k), KA (Figures 5h,i), and CCl4 respectively for 8 weeks, these changes were significantly reduced. The scores for the KA-treated group demonstrated a significant improvement (P < 0.05) compared to the fibrotic group, as assessed by the Noetherian index of HF. However, statistically significant differences remained between the MOD and CON groups and between the MOD and silymarin groups, while no differences were detected between the scores of the CON and KA groups (Figure 5m).
3.8 Determination of biochemical markers of liver fibrosis in rats
Relative to the CON group, the serum levels of ALT (0.03907 ± 0.00062 ng·mL−1), HA (0.33969 ± 0.00001 ng·mL−1), LN (0.04569 ± 0.00069 ng·mL−1) and COL-IV (0.05395 ± 0.00069 ng·mL−1) were significantly elevated (P < 0.001) in the model group. Additionally, the levels of IL-1β, AST and PC-III (P < 0.01), and TNF-α (P < 0.05) were also significantly increased. Conversely, the serum concentrations of ALT (0.02930 ± 0.00134 ng·mL−1), AST (0.06445 ± 0.00134 ng·mL−1), HA (0.33867 ± 0.00003 ng·mL−1), LN (0.04441 ± 0.00002 ng·mL−1) (P < 0.001), COL-IV, PC-III, IL-1β (P < 0.01), and TNF-α (P < 0.05) were significantly reduced in the KA group compared to the MOD group (Figure 6).
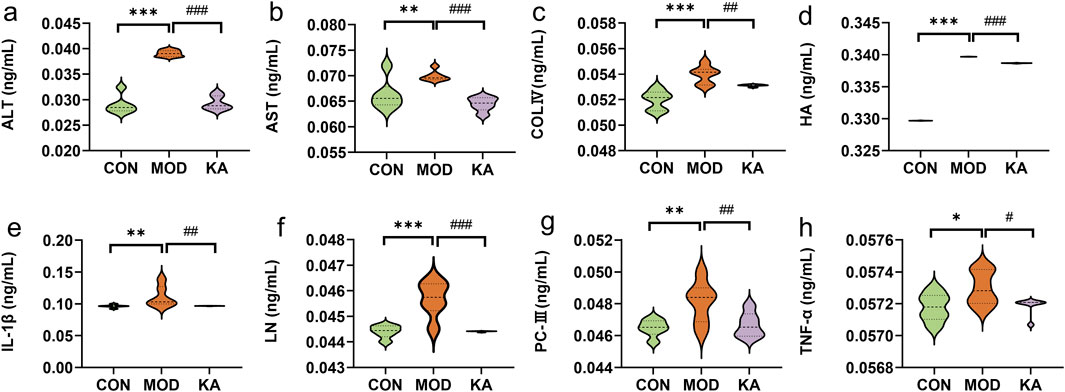
Figure 6. Analysis of biochemical markers. (a) ALT; (b) AST; (c) COL-Ⅳ; (d) HA; (e) IL-1β; (f) LN; (g) PC-Ⅲ; (h) TNF-α. Statistical comparison with the normal control group is denoted as follows: ***P < 0.001; **P < 0.01; *P < 0.05. Comparisons with the model group are indicated as ###P < 0.001; ##P < 0.01; #P < 0.05. CON: the control group, MOD: the model group, and KA: the kaempferol-administration group.
3.9 Transcriptomic analysis of KA-Treated HF rats
The study involved the analysis of the control (CON), model (MOD), and KA-treated groups to identify DEGs. The volcano plot depicted in Figure 7a illustrates that 5,562 DEGs were significantly expressed between the control and model groups, comprising 3,352 upregulated and 2,210 downregulated genes. Similarly, the volcano plot in Figure 7b reveals that 1,499 DEGs were significantly expressed between the model and KA-treated groups, with 713 genes upregulated and 786 downregulated. A total of 895 DEGs were identified across the CON, MOD, and KA groups, as illustrated by the Venn diagram in Figure 7c. Following KA treatment, the expression of 725 DEGs was restored, as shown in Figure 7d, with 295 genes upregulated and 430 downregulated (Figure 7e). These findings suggest that KA treatment may normalize certain gene expression disorders. To further elucidate the potential mechanisms underlying changes in DEGs, all prospective biomarkers underwent KEGG enrichment and functional analyses. This process identified several major enriched pathways at the transcriptomic level in both the CON and MOD groups (Figure 7f), including tryptophan metabolism, complement and coagulation cascades, fatty acid degradation, the PPAR signaling pathway, and fatty acid metabolism, among others. Similarly, the major enriched pathways identified at the transcriptomic level in the KA and MOD groups (Figure 7g) encompassed steroid biosynthesis, protein processing in the endoplasmic reticulum, the PPAR signaling pathway, and protein export, among others. Notably, ten enriched pathways were identified, including arachidonic acid metabolism, tryptophan metabolism, steroid hormone biosynthesis, retinoid metabolism, fatty acid metabolism, fatty acid degradation, and the PPAR signaling pathway. The ten enrichment pathways identified exhibited significant overlap with the primary enrichment pathways observed in the CON and MOD groups. These pathways were subsequently analyzed to elucidate the roles of the CYP4A8, SCD, FADS2, and SCD2 genes in processes such as fatty acid degradation, the PPAR signaling pathway, fatty acid metabolism, retinol metabolism, and arachidonic acid and tryptophan metabolism. Additionally, retinol metabolism, arachidonic acid metabolism, and other enrichment pathways (as depicted in Figure 7h) demonstrated enhanced relevance within the transglutomics mechanism associated with KA treatment for liver fibrosis. Further investigation into the gene functions was conducted using Gene Ontology (GO) analysis on the DEGs from the MOD and KA groups. The GO enrichment analysis (illustrated in Figure 7i) revealed that the enriched biological functions predominantly involved responses to endoplasmic reticulum stress, fatty acid metabolic processes, components of the endoplasmic reticulum, binding to cofactors, oxidoreductase activity acting on paired donors, and interactions with or reduction of molecular oxygen.
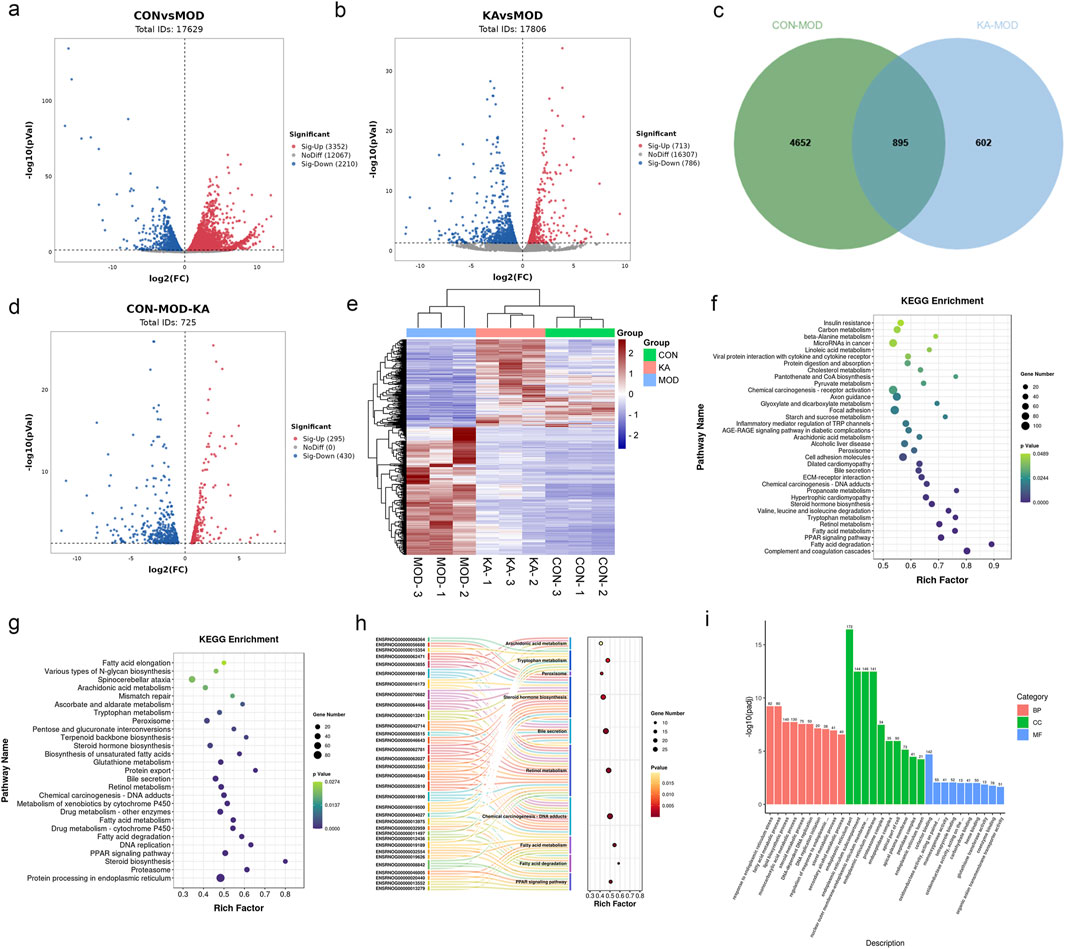
Figure 7. The transcriptomic analysis results of kaempferol - treated HF rats. (a) Volcano plot of DEGs between the CON and MOD groups; (b) Volcano plot of DEGs between the KA and MOD groups; (c) The intersection of DEGs among the CON, MOD, and KA groups; (d) The regulatory changes of DEGs between the CON and the MOD group after KA administration; (e) Clustering heatmap to analyze the regulation of differentially expressed gene content among the CON, MOD, and KA groups, with red indicating high expression and blue indicating low expression; (f) KEGG enrichment analysis of DEGs between the CON and MOD groups; panel; (g) KEGG enrichment analysis of DEGs between the KA and MOD groups; (h) Sankey bubble map of the intersection enrichment pathway among the CON, MOD, and KA groups; and panel; (i) GO functional analysis of DEGs. CON: the control group, MOD: the model group, and KA: the KA-administration group.
3.10 Regulation of gene expression by KA in HF rats
Through comprehensive analyses, this study identified ten key signaling pathways that are closely associated with the CCl4-induced HF rat model. In this study, particular attention was directed towards the DEGs within these pathways, with an emphasis on the signaling pathways significantly implicated in the pathological processes of HF, as identified through KEGG database analysis. Four DEGs were identified as playing critical regulatory roles in the pathways of fatty acid degradation, PPAR signaling, fatty acid metabolism, retinol metabolism, and arachidonic acid metabolism. Notably, Stearoyl-CoA Desaturase 2 (SCD2) exhibited a marked upregulation in expression within the model group, whereas its expression was significantly downregulated following treatment with the intervention drug, KA. Furthermore, Cytochrome P450 Family 4 Subfamily A Member 8 (CYP4A8) and Fatty Acid Desaturase 2 (FADS2) did not demonstrate significant changes in expression within the model group; however, their expression levels were significantly diminished post-kaempferol intervention. Concurrently, the expression of the SCD gene was downregulated in the model group and further decreased following kaempferol treatment (Figure 8).
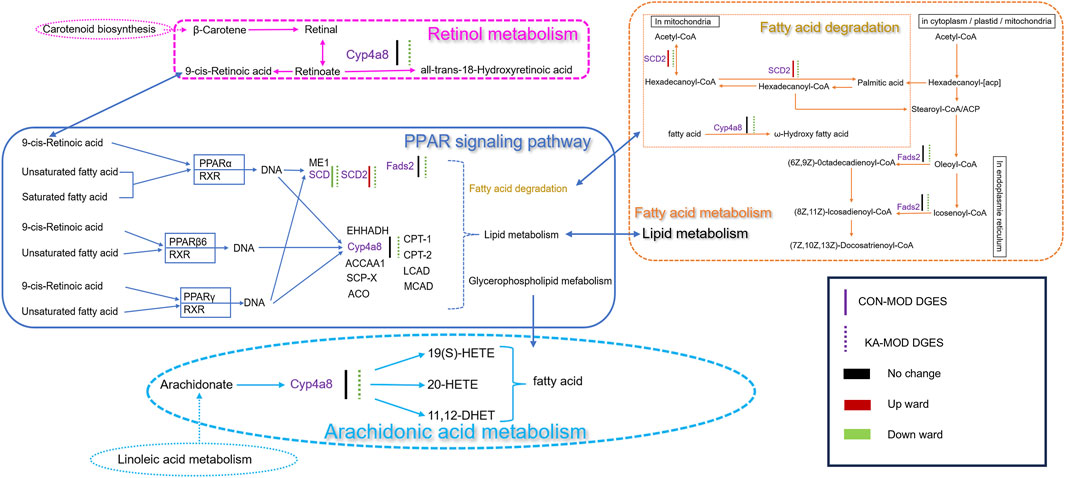
Figure 8. The relevance analysis mechanism diagram. CON: the control group, MOD: the model group, and KA: the kaempferol-administration group.
4 Discussion
This research investigates the pathological mechanisms underlying HF and explores intervention strategies rooted in TCM. HF, a common pathological response to chronic liver injury, involves the excessive accumulation of ECM resulting from metabolic dysregulation. The excessive accumulation of type I collagen (collagen I) serves as the primary molecular foundation for liver tissue remodeling (Kisseleva, 2016). This pathological process is predominantly driven by persistent factors such as viral hepatitis and alcoholic liver injury, and its global prevalence has markedly increased in conjunction with the rising incidence of metabolic syndrome (Altamirano-Barrera et al., 2019). At the cellular and molecular levels, the phenotypic transformation of HSCs plays a central role in the development and progression of fibrosis. When exposed to a chronic inflammatory microenvironment, HSCs transition from vitamin A-rich quiescent cells into myofibroblasts, acquiring proliferative, migratory, and contractile characteristics. This transformation promotes an imbalance in ECM synthesis and degradation through signaling pathways such as TGF-β/Smad (VanHook, 2019). TCM offers distinct theoretical advantages and extensive practical experience in the prevention and treatment of HF. It operates under guiding principles such as “promoting blood circulation to remove stasis” and “soothing the liver to regulate qi.” TCM formulations, including Biejiajian Pills and Fuzheng Huayu Formula, as well as monomeric components like tanshinone IIA and curcumin, have been clinically validated to significantly enhance liver histopathological scores and serum fibrosis markers (Zhang and Schuppan, 2014; Liu et al., 2018). This study utilizes a systems pharmacology approach, integrating molecular docking and network analysis, to elucidate the dynamic network mechanisms through which TCM active components modulate HSC activation and ECM metabolism via multi-target synergistic effects.
Additionally, we utilize integrated bibliometric and network pharmacology approaches to systematically explore the current research landscape and mechanistic foundations of TCM in the management of HF. Initially, a bibliometric analysis was conducted, integrating keyword co-occurrence and timeline mapping of dynamic changes, to utilize visualization techniques for a comprehensive elucidation of research hotspots and developmental trends concerning TCM formulations and individual herbs in the treatment of HF. Subsequently, network pharmacology methods were employed to identify TCM components with potential anti-hepatic fibrosis activity, which were then experimentally validated using a rat model of HF induced by a combination of corn oil and CCl4. Additionally, transcriptomic analysis was utilized to thoroughly investigate the molecular mechanisms and associated gene targets through which these active TCM components modulate HF. The results demonstrate that active components of TCM exhibit anti-hepatic fibrosis effects through multi-target and multi-pathway mechanisms. The experimental findings of this study not only offer scientific validation for understanding the pharmacological mechanisms of TCM active components in treating HF but also lay a critical theoretical groundwork for further in-depth research and clinical applications of TCM in this domain.
To enhance the recognition of TCM in the treatment of HF and to ensure the adequacy and credibility of references, this study performed a comprehensive search across three authoritative databases: CNKI, the Wanfang Data Knowledge Service Platform, and the VIP Network. This comprehensive literature review facilitated the identification of research hotspots related to TCM formulations and their active components through keyword visualization analysis. The study identified five high-frequency TCM components: Codonopsis Radix, Persicae Semen, Radix Bupleuri, Hedysarum Multijugum Maxim., and Glycyrrhiza uralensis Fisch. Utilizing network pharmacology approaches, a protein-protein interaction (PPI) network was developed, allowing for the identification of primary TCM components with potential anti-hepatic fibrosis activity based on relevant scoring systems. In our study, we concentrated on compounds that have been thoroughly documented in the existing literature for their specific therapeutic effects pertinent to our research objectives. Although quercetin and β-sitosterol are indeed noteworthy, our preliminary analyses revealed that the compounds we selected demonstrated a more direct relevance to the mechanisms we intended to investigate. Among these components, KA emerged as the most frequently occurring. KA, a flavonoid secondary metabolite, is extensively distributed across various plant species (Chen et al., 2023). Previous studies have highlighted its notable biological activities, such as anti-inflammatory, antioxidant, and anticancer properties (Periferakis et al., 2023). In recent years, significant attention has been directed towards the pharmacokinetic properties of kaempferol (KA) and its potential therapeutic applications in liver disease treatment. Through a systematic investigation of the oral pharmacokinetics and tissue distribution of KA and its metabolites, Liu Y. et al. (2022) identified a distinctive triple cycle pathway for this compound, which markedly enhances the local bioavailability of flavonoids in target tissues and extends their retention time in the liver and intestine (Liu X. et al., 2022). Complementary findings by Barve et al. (2009) demonstrated that kaempferol achieves peak plasma concentration within 1–2 h post-oral administration, indicative of its rapid absorption characteristics (Barve et al., 2009). Nevertheless, kaempferol undergoes extensive metabolic transformations, such as glucuronidation in the gastrointestinal tract and liver, which result in its relatively low systemic bioavailability. In a 2016 study utilizing a rat model, Zhou, Wang, and colleagues demonstrated that the oral bioavailability of kaempferol (KA) ranged from only 2.1%–3.1%. Despite this low bioavailability, subsequent toxicological assessments confirmed a favorable safety profile for KA, as no significant adverse effects on liver and kidney function were observed (Zhou et al., 2016). More recently, a 2023 study by Kim, Song, and collaborators revealed that kaempferol effectively mitigated hepatocyte necrosis and inhibited inflammatory responses in a carbon tetrachloride (CCl4)-induced liver injury model, thereby exhibiting significant hepatoprotective properties (Kim et al., 2023). Collectively, these findings indicate that kaempferol’s distinctive pharmacokinetic characteristics, coupled with its pronounced liver-protective efficacy, render it a highly promising candidate for the treatment of hepatic fibrosis (HF), notwithstanding its limited systemic bioavailability. To substantiate the anti- Hepatic fibrosis effects of KA, this study utilized a CCl4-induced rat model of HF. Through transcriptomic analysis, the study elucidated the molecular mechanisms by which KA modulates key genes and associated signaling pathways involved in HF. These findings provide critical experimental evidence supporting its potential clinical application.
The experiment revealed the pharmacodynamic changes of KA in anti-liver fibrosis. Clinically, ALT and AST are vital indicators of liver function. ALT is predominantly found in the cytoplasm of hepatocytes, with intracellular concentrations that are 1,000–3,000 times higher than those in serum (George et al., 2022). A mere 1% damage to hepatocytes can result in a doubling of serum enzyme levels. AST is most prevalent in myocardial cells, followed by the liver, skeletal muscle, and kidneys. Within hepatocytes, AST is predominantly located in the mitochondria, with a smaller fraction present in the cytoplasm. In cases of severe hepatocyte damage, the integrity of mitochondrial membranes is compromised, leading to the release of AST into the bloodstream and a consequent elevation in serum AST activity (Lavrynenko et al., 2020). IL-1β and TNF-α are critical inflammatory cytokines (Kaffe et al., 2018), that exhibit a positive correlation with inflammatory responses. Markers of serum fibrosis include HA, LN, PC-III, and COL-IV. PC-III, COL-IV, and LN constitute major structural elements of the basement membrane, playing roles in cell adhesion and signal transduction. Their overexpression during pulmonary fibrosis results in aberrant repair of the basement membrane (Ohta et al., 1995; Florea et al., 2016), thereby exacerbating the progression of fibrosis. Hyaluronic acid (HA), a polysaccharide abundantly found in the ECM, accumulates during inflammation and fibrosis, potentially contributing to tissue repair and fibrotic processes (Yang et al., 2019). The experimental findings indicated that serum biochemical markers were markedly elevated in the MOD group but decreased to levels comparable to the CON group following KA administration. Histological evaluations using H&E and Masson staining revealed substantial damage to the alveolar interstitium and liver tissue architecture in the MOD group, in contrast to normal liver tissue. Post-KA administration, the damage was significantly ameliorated, and liver tissue morphology was restored to a condition resembling that of the CON group. These results collectively confirm the successful establishment of a CCl4-induced rat model of liver fibrosis and demonstrate that KA exerts a notable ameliorative and therapeutic effect on liver fibrosis.
Transcriptome analysis of KA against HF in the present study showed that the regulation of KA affected the relevant metabolic and signaling pathways. The degradation and metabolism of aliphatic acids are critically involved in the initiation and progression of HF. As the primary metabolic organ, the liver possesses the ability to synthesize, decompose, and metabolize aliphatic acids (Heeren and Scheja, 2021). Under pathological conditions such as obesity, hyperlipidemia, and, the liver often exhibits excessive lipid accumulation (Heeren and Scheja, 2021). The expansion of adipocytes, infiltration of leukocytes, and release of cytokines collectively contribute to lipid accumulation in hepatocytes, thereby exacerbating the onset and progression of HF (Shen et al., 2019). Furthermore, disruptions in fatty acid degradation and metabolism can induce oxidative stress and inflammatory responses, further accelerating the development of HF (Li et al., 2024). This study identifies SCD2, FADS2, and the PPAR signaling pathway as pivotal regulators of aliphatic acid synthesis and metabolism. SCD2, a member of the stearoyl-CoA desaturase family serves a vital function as the rate-limiting enzyme in the synthesis of monounsaturated fatty acids (MUFAs) (O’Neill et al., 2020), thereby promoting aliphatic acid synthesis and participating in various metabolic processes within hepatocytes. Its expression level is positively correlated with the severity of HF. FADS2 is a crucial enzyme involved in the desaturation of aliphatic acids and is essential for the biosynthesis of long-chain polyunsaturated fatty acids (PUFAs) (Yamane et al., 2021). The upregulation of FADS2 expression markedly promotes the progression of HF, resulting in the accelerated synthesis of unsaturated fatty acids and a disruption of intracellular lipid metabolism within hepatocytes (Kothapalli et al., 2023). The PPAR signaling pathway constitutes the core regulatory network of fatty acid metabolism, and its activation can exert anti-liver fibrosis effects through a multi-target synergistic mechanism. Research has demonstrated that KA significantly alters hepatic lipid metabolism by down-regulating the expression of the SCD and CYP4A8 genes. The inhibition of the SCD gene leads to a reduction in the production of monounsaturated fatty acids and diminishes the activation of HSC through lipotoxicity (Subedi et al., 2020). KA suppresses the expression of its downstream target, Cyp4a, through the downregulation of SCD2 and the activation of the PPARα signaling pathway. This process results in a reduction of ROS production, alleviation of oxidative stress-induced hepatocyte injury, and a consequent decrease in serum ALT/AST levels (Shearn et al., 2014). Concurrently, the downregulation of CYP4A8 decreases the excessive production of ω-hydroxylated fatty acids (Gato et al., 2012). Collectively, these changes alleviate endoplasmic reticulum stress and oxidative damage caused by abnormal lipid accumulation. This metabolic remodeling further activates the PPARα/γ isoforms: activation of PPARα enhances fatty acid β-oxidation, accelerates lipid clearance, and reduces lipid peroxide accumulation (Zhang Z. et al., 2022). Simultaneously, PPARγ nuclear translocation not only improves insulin sensitivity but also inhibits HSC activation and collagen deposition by directly suppressing the TGF-β/Smad pathway. Furthermore, the synergistic activation of PPARβ/δ can inhibit inflammatory pathways, such as NF-κB, and reduce the release of pro-fibrotic factors, including TNF-α and IL-6. This establishes a cascade reaction network characterized by “metabolic regulation, inflammation suppression, and fibrosis remission.” (Titus et al., 2023). This study investigates the complex regulatory mechanisms that confer anti-liver fibrosis effects by enhancing liver lipid homeostasis, mitigating oxidative stress, and inhibiting HSC activation. KA mitigates ECM deposition through a multi-target regulatory mechanism by inhibiting SCD2, which is crucial for lipid metabolic reprogramming in HSC activation. This inhibition prevents the lipid-dependent transformation of HSCs into myofibroblasts and reduces ECM component secretion like COL-IV and LN. Concurrently, FADS2 and CYP4A8 facilitate HSC proliferation and fibrosis via the TGF-β1 and MAPK/ERK pathways, respectively. KA effectively inhibits both FADS2 and CYP4A8, blocking these signaling pathways, thereby reducing HSC activation and fibrosis markers such as HA and PC-III, demonstrating its anti-liver fibrosis properties (Yan et al., 2022; Li et al., 2020).
Furthermore, retinol metabolism and arachidonic acid metabolism are integral to the potential therapeutic effects of KA on HF. Research has demonstrated that retinol metabolism is integral to liver regeneration and the mechanisms underlying disease development, encompassing processes such as inflammation, fatty liver disease, fibrosis, and cirrhosis (Wang et al., 2023). Exposure of the liver to CCl4 results in hepatocyte damage and the activation of hematopoietic stem cells, concurrently disrupting retinol metabolism. These alterations further exacerbate the progression of HF and cirrhosis (Ping et al., 2023). Enzymes and bioactive products derived from arachidonic acid metabolism are indicative of inflammatory responses (Liu X. et al., 2022). CYP4A8, a cytochrome P450 enzyme from the CYP4A subfamily, is primarily expressed in the liver and plays a role in fatty acid metabolism, particularly in the ω- and (ω-1)-hydroxylation of long-chain fatty acids (Yamaguchi et al., 2002). CYP4A8 may possess anti-inflammatory properties by modulating the metabolism of fatty acids, arachidonic acid, and retinoic acid, thereby playing a pivotal role in mitigating HF (Nakamura et al., 1994; Hoch et al., 2000).
This study utilized transcriptomic sequencing technology in conjunction with experimental validation methods to systematically examine the therapeutic effects of KA on CCl4-induced HF in rats, as well as its underlying pharmacological mechanisms. The findings indicated that the therapeutic efficacy of KA is intricately linked to the regulation of aliphatic acid degradation and metabolism, retinol metabolism, and arachidonic acid metabolism. KA significantly downregulated the expression of key genes such as SCD, SCD2, FADS2, and Cyp4A8, inhibited the release of inflammatory cytokines in hepatic tissue, and reduced the synthesis of aliphatic acids in the liver. These effects collectively helped reduce oxidative stress and inflammatory responses, thereby promoting the recovery of CCl4-induced liver damage. Additionally, KA may further impact lipid metabolism and inflammatory responses through modulation of the PPAR signaling pathway, offering a molecular mechanism-based rationale for its potential application in the treatment of HF. This research elucidates the potential mechanisms by which KA may contribute to the treatment of HF, while also identifying significant molecular targets and providing theoretical foundations for the development of innovative anti-fibrotic therapeutic strategies. CCl4 is a well-established hepatotoxic compound frequently utilized in experimental models to induce liver fibrosis, thereby facilitating the study of chronic liver disease pathogenesis. Nonetheless, it is crucial to recognize that CCl4 can also induce pulmonary fibrosis, a phenomenon that is not yet fully understood. A significant gap exists in comprehending the correlation between the CCl4-induced pulmonary fibrosis model in rats and pulmonary fibrosis in humans, particularly concerning pathogenesis and pathological characteristics. This study aims to elucidate the relevance of the CCl4-induced rat model in replicating the pathogenesis of human pulmonary fibrosis. By systematically comparing and analyzing the pathological changes, molecular regulatory networks, and cellular response characteristics of both models, this research endeavors to provide a robust theoretical foundation and a novel experimental framework for preclinical investigations into pulmonary fibrosis. Moreover, this study not only advances the understanding of the multi-organ toxicity mechanisms associated with CCl4 but also provides a crucial foundation in translational medicine for the development of novel therapeutic strategies targeting pulmonary fibrosis. Future research efforts will focus on elucidating the mechanisms of KA against HF through the integration of various omics approaches. Our study employed a rigorously designed animal model that accurately recapitulated the pathophysiological characteristics of liver fibrosis observed in humans. Through a blinded, randomized experimental protocol with an adequate sample size, we demonstrated a potent antifibrotic effect of kaempferol, thereby minimizing the risk of false-positive results. These findings underscore not only the therapeutic potential of kaempferol but also the translational value of our preclinical data for future clinical applications. This research establishes a robust theoretical foundation for subsequent clinical studies, following a clear translational pathway from mechanistic research to evidence generation, ultimately aiming at patient benefit. Although current investigations remain at the preclinical stage, utilizing both cellular and animal models, the observed strong efficacy and favorable safety profile of kaempferol suggest its significant promise as a potential clinical candidate for liver fibrosis treatment. These findings warrant further exploration through clinical trials to validate their therapeutic potential in human patients.
5 Conclusion
By integrating bibliometric and network pharmacology approaches, the study systematically screened and validated the anti-hepatic fibrosis mechanisms of the active component KA found in TCM. In a CCl4-induced rat model of HF, KA exhibited significant ameliorative effects on pathological conditions. Transcriptomic analysis indicated that KA facilitates the activation of the PPAR signaling pathway, reprogramming of fatty acid metabolism, regulation of retinol homeostasis, and maintenance of the dynamic balance within the arachidonic acid metabolic network by modulating core target genes such as SCD1/SCD2, FADS2, and CYP4A8. The research demonstrated that KA significantly suppresses the activation of HSCs and the accumulation of ECM by concurrently targeting the dysregulation of lipid metabolism and the inflammatory response pathways. This study provides a theoretical basis for the clinical use of KA in the treatment of liver fibrosis.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/bioproject/, PRJNA992181.
Ethics statement
The animal study was approved by the animal experiments were approved by the Ethical Committee of Baotou Medical College (no. 2022-96). All methods are performed in accordance with relevant guidelines and regulations. The study animal protocol was based on the requirements of the ARRIVE Guidelines report. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JL: Conceptualization, Writing – original draft. XWS: Conceptualization, Writing – original draft. XS: Data curation, Writing – original draft. XF: Data curation, Writing – original draft. SN: Formal Analysis, Funding acquisition, Writing – original draft. HC: Methodology, Writing – original draft. SS: Supervision, Writing – original draft. MY: Formal Analysis, Validation, Writing – original draft. RZ: Validation, Writing – original draft. PW: Methodology, Writing – original draft. JQ: Validation, Funding acquisition, Writing – original draft. WB: Funding acquisition, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the National Natural Science Foundation of China (82460834), the Natural Science Foundation of Inner Mongolia Autonomous Region, China (2024MS08025, 2024QN08090, 2025LHMS08049), the Inner Mongolia Health Science and Technology Project in 2022 (202202226), the Baotou Health Science and Technology Program (wskjjk2022065, 2024wsjkkj70), the Baotou Medical College Graduate Research and Innovation Project (BYKYCX202463, BYKYCX202503), the Baotou Medical College 2025 “Bud Plan” Project (HLJH202508), the Science and Technology Program of the Joint Fund of Scientific Research for the Public Hospitals of Inner Mongolia Academy of Medical Sciences (2023GLLH0183, 2024GLLH0529), College Student Innovation and Entrepreneurship Training Program (S202510130003) and Baotou Medical College Pharmacy Quality Improvement and Cultivation Discipline Construction Project (YXTZ202506).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2025.1607103/full#supplementary-material
References
Alam, W., Khan, H., Shah, M. A., Cauli, O., and Saso, L. (2020). Kaempferol as a dietary anti-inflammatory agent: current therapeutic standing. Molecules 25 (18), 4073. doi:10.3390/molecules25184073
Altamirano-Barrera, A., Barranco-Fragoso, B., and Méndez-Sánchez, N. (2019). Management strategies for liver fibrosis. Ann. Hepatology 16 (1), 48–56. doi:10.5604/16652681.1226814
Barve, A., Chen, C., Hebbar, V., Desiderio, J., Saw, C. L. L., and Kong, A. N. (2009). Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. and Drug Dispos. 30, 356–365. doi:10.1002/bdd.677
Cao, R., Cao, C., Hu, X., Du, K., Zhang, J., Li, M., et al. (2023). Kaempferol attenuates carbon tetrachloride (CCl4)-induced hepatic fibrosis by promoting ASIC1a degradation and suppression of the ASIC1a-mediated ERS. Phytomedicine 121, 155125. doi:10.1016/j.phymed.2023.155125
Chen, J., Zhong, H., Huang, Z., Chen, X., You, J., and Zou, T. (2023). A critical review of kaempferol in intestinal health and diseases. Antioxidants 12 (8), 1642. doi:10.3390/antiox12081642
El-Hawary, S. S. E.-D., El Zalabani, S. M., Selim, N. M., Ibrahim, M. A., Wahba, F. A., El Badawy, S. A., et al. (2019). Phenolic constituents of Chrysophyllum oliviforme L. leaf down-regulate TGF-β expression and ameliorate CCl4-Induced liver fibrosis: evidence from in vivo and in silico studies. Antioxidants 8 (12), 646. doi:10.3390/antiox8120646
Florea, F., Koch, M., Hashimoto, T., and Sitaru, C. (2016). Autoimmunity against laminins. Clin. Immunol. 170, 39–52. doi:10.1016/j.clim.2016.07.021
Gato, W. E., Hales, D. B., and Means, J. C. (2012). Hepatic gene expression analysis of 2-aminoanthracene exposed Fisher-344 rats reveal patterns indicative of liver carcinoma and type 2 diabetes. J. Toxicol. Sci. 37 (5), 1001–1016. doi:10.2131/jts.37.1001
George, J., Tsuchishima, M., and Tsutsumi, M. (2019). Molecular mechanisms in the pathogenesis of N-nitrosodimethylamine induced hepatic fibrosis. Cell Death and Dis. 10 (1), 18. doi:10.1038/s41419-018-1272-8
George, J., Tsuchishima, M., and Tsutsumi, M. (2022). Epigallocatechin-3-gallate inhibits osteopontin expression and prevents experimentally induced hepatic fibrosis. Biomed. and Pharmacother. 151, 113111. doi:10.1016/j.biopha.2022.113111
Heeren, J., and Scheja, L. (2021). Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 50, 101238. doi:10.1016/j.molmet.2021.101238
Hoch, U., Zhang, Z., Kroetz, D. L., and Ortiz de Montellano, P. R. (2000). Structural determination of the substrate specificities and regioselectivities of the rat and human fatty acid omega-hydroxylases. Archives Biochem. Biophysics 373 (1), 63–71. doi:10.1006/abbi.1999.1504
Huang, G.-R., Wei, S.-J., Huang, Y.-Q., Xing, W., Wang, L.-Y., and Liang, L.-L. (2018). Mechanism of combined use of vitamin D and puerarin in anti-hepatic fibrosis by regulating the Wnt/β-catenin signalling pathway. World J. Gastroenterology 24 (36), 4178–4185. doi:10.3748/wjg.v24.i36.4178
Jia, W., Wang, K., Zhang, S., Lu, W., Du, A., Li, J., et al. (2022). Integrating network pharmacology and in vivo experimental validation to reveal the alleviation of mailuoning oral liquid on non-alcoholic fatty liver disease. Phytomedicine 104, 154326. doi:10.1016/j.phymed.2022.154326
Kaffe, E., Fiorotto, R., Pellegrino, F., Mariotti, V., Amenduni, M., Cadamuro, M., et al. (2018). β-Catenin and interleukin-1β-dependent chemokine (C-X-C motif) ligand 10 production drives progression of disease in a mouse model of congenital hepatic fibrosis. Hepatology 67 (5), 1903–1919. doi:10.1002/hep.29652
Kim, M.-J., Song, Y.-R., Kim, Y. E., Bae, S.-J., Lee, W.-Y., Bak, S.-B., et al. (2023). Kaempferol stimulation of autophagy regulates the ferroptosis under the oxidative stress as mediated with AMP-Activated protein kinase. Free Radic. Biol. Med. 208, 630–642. doi:10.1016/j.freeradbiomed.2023.09.008
Kisseleva, T. (2016). The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology 65 (3), 1039–1043. doi:10.1002/hep.28948
Kitano, M., and Bloomston, P. M. (2016). Hepatic stellate cells and microRNAs in pathogenesis of liver fibrosis. J. Clin. Med. 5 (3), 38. doi:10.3390/jcm5030038
Kothapalli, K. S. D., Park, H. G., Kothapalli, N. S. L., and Brenna, J. T. (2023). FADS2 function at the major cancer hotspot 11q13 locus alters fatty acid metabolism in cancer. Prog. Lipid Res. 92, 101242. doi:10.1016/j.plipres.2023.101242
Lavrynenko, O., Alatrach, M., Abdul-Ghani, M., Cersosimo, E., Alkhouri, N., Puckett, C. L., et al. (2020). 184-OR: liver fibrosis scores do not predict response to therapy in T2DM. Diabetes 69 (Suppl. 1), 184-OR. doi:10.2337/db20-184-or
Li, M., Xu, C., Ma, Y., Ye, R., Chen, H., Xie, D., et al. (2020). Effects of dietary n-3 highly unsaturated fatty acids levels on growth, lipid metabolism and innate immunity in juvenile golden pompano (Trachinotus ovatus). Fish and Shellfish Immunol. 105, 177–185. doi:10.1016/j.fsi.2020.06.060
Li, Y., Li, Y., Zhang, J., Ji, L., Li, M., Sun, X., et al. (2022). Current perspective of traditional Chinese medicines and active ingredients in the therapy of hepatocellular carcinoma. J. Hepatocell. Carcinoma 9, 41–56. doi:10.2147/jhc.s346047
Li, M., Kong, X., Jian, X., Bo, Y., Miao, X., Chen, H., et al. (2024). Fatty acids metabolism in ozone-induced pulmonary inflammatory injury: evidence, mechanism and prevention. Sci. Total Environ. 933, 173222. doi:10.1016/j.scitotenv.2024.173222
Li, J.-T., Gu, A., Tang, N.-N., Sun, Z.-Y., Zhang, G., and Li, M.-Y. (2025). Exploring anti-tumor potential of food and medicine homology substances: an in-silico evaluation of citri grandis exocarpium against gallbladder cancer. Food and Med. Homol. doi:10.26599/FMH.2026.9420084
Lin, L., Zhou, F., Shen, S., and Zhang, T. (2018). Fighting liver fibrosis with naturally occurring antioxidants. Planta Medica 84 (18), 1318–1333. doi:10.1055/a-0757-0008
Liu, L., Zhou, Y., Dai, D., Xia, H., Zhao, K., and Zhang, J. (2018). Protective effects of Kangxian ruangan capsule against nonalcoholic fatty liver disease fibrosis in rats induced by MCD diet. Biomed. and Pharmacother. 108, 424–434. doi:10.1016/j.biopha.2018.06.134
Liu, X.-Y., Zhang, W., Ma, B.-F., Sun, M.-M., and Shang, Q.-H. (2024). Advances in research on the effectiveness and mechanism of active ingredients from traditional Chinese medicine in regulating hepatic stellate cells autophagy against hepatic fibrosis. Drug Des. Dev. Ther. 18, 2715–2727. doi:10.2147/dddt.s467480
Liu, X., Wang, L., Tan, S., Chen, Z., Wu, B., and Wu, X. (2022). Therapeutic effects of berberine on liver fibrosis are associated with lipid metabolism and intestinal flora. Front. Pharmacol. 13, 814871. doi:10.3389/fphar.2022.814871
Liu, Y., Lu, Y., Li, X., Zhang, Z., Sun, L., Wang, Y., et al. (2022). Kaempferol suppression of acute colitis is regulated by the efflux transporters BCRP and MRP2. Eur. J. Pharm. Sci. 179, 106303. doi:10.1016/j.ejps.2022.106303
Ma, X., Zhang, X., Wang, X., Wang, C., and Ma, Y. (2023). The role of kaempferol in gynaecological malignancies: progress and perspectives. Front. Pharmacol. 14, 1310416. doi:10.3389/fphar.2023.1310416
Martino, E., Chiarugi, S., Margheriti, F., and Garau, G. (2021). Mapping, structure and modulation of PPI. Front. Chem. 9, 718405. doi:10.3389/fchem.2021.718405
Nakamura, M., Imaoka, S., Miura, K., Tanaka, E., Misawa, S., and Funae, Y. (1994). Induction of cytochrome P450 isozymes in rat renal microsomes by cyclosporin A. Biochem. Pharmacol. 48 (9), 1743–1746. doi:10.1016/0006-2952(94)90460-x
Ohta, K., Mortenson, R. L., Clark, R. A., Hirose, N., and King, T. E. (1995). Immunohistochemical identification and characterization of smooth muscle-like cells in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 152 (5 Pt 1), 1659–1665. doi:10.1164/ajrccm.152.5.7582311
O’Neill, L. M., Guo, C.-A., Ding, F., Phang, Y. X., Liu, Z., Shamsuzzaman, S., et al. (2020). Stearoyl-CoA Desaturase-2 in murine development, metabolism, and disease. Int. J. Mol. Sci. 21 (22), 8619. doi:10.3390/ijms21228619
Periferakis, A., Periferakis, A.-T., Troumpata, L., Periferakis, K., Scheau, A.-E., Savulescu-Fiedler, I., et al. (2023). Kaempferol: a review of current evidence of its antiviral potential. Int. J. Mol. Sci. 24 (22), 16299. doi:10.3390/ijms242216299
Ping, D.-B., Sun, X., Peng, Y., and Liu, C.-H. (2023). Cyp4a12-mediated retinol metabolism in stellate cells is the antihepatic fibrosis mechanism of the Chinese medicine Fuzheng Huayu recipe. Chin. Med. 18 (1), 51. doi:10.1186/s13020-023-00754-4
Shearn, C. T., Mercer, K. E., Orlicky, D. J., Hennings, L., Smathers-McCullough, R. L., Stiles, B. L., et al. (2014). Short term feeding of a high fat diet exerts an additive effect on hepatocellular damage and steatosis in liver-specific PTEN knockout mice. PLoS One 9, e96553. doi:10.1371/journal.pone.0096553
Shen, X., Guo, H., Xu, J., and Wang, J. (2019). Inhibition of lncRNA HULC improves hepatic fibrosis and hepatocyte apoptosis by inhibiting the MAPK signaling pathway in rats with nonalcoholic fatty liver disease. J. Cell. Physiology 234 (10), 18169–18179. doi:10.1002/jcp.28450
Su, M., Zhu, J., Bai, L., Cao, Y., and Wang, S. (2025). Exploring manzamine a: a promising anti-lung cancer agent from marine sponge Haliclona sp. Front. Pharmacol. 16, 1525210. doi:10.3389/fphar.2025.1525210
Subedi, A., Liu, Q., Sharon, D., Cathelin, S., Bader, G. D., Xu, C., et al. (2020). Nicotinamide phosphoribosyltransferase inhibitors induce apoptosis of AML stem cells through dysregulation of lipid metabolism. Blood 28 (10), 25–26. doi:10.1182/blood-2020-142404
Titus, C., Hoque, M. T., and Bendayan, R. (2023). PPAR agonists for the treatment of neuroinflammatory diseases. Trends Pharmacol. Sci. 45 (1), 9–23. doi:10.1016/j.tips.2023.11.004
VanHook, A. M. (2019). Protecting against liver fibrosis. Science 583, 19–585. doi:10.1126/science.366.6465.583-s
Vanzan, D. F., Goma, E. P., Locatelli, F. R., Honorio, T. d. S., Furtado, P. d. S., Rodrigues, C. R., et al. (2024). Evaluation of silybin nanoparticles against liver damage in murine schistosomiasis mansoni infection. Pharmaceutics 16 (5), 618. doi:10.3390/pharmaceutics16050618
Wang, M., Sun, J., Jiang, Z., Xie, W., and Zhang, X. (2015). Hepatoprotective effect of kaempferol against alcoholic liver injury in mice. Am. J. Chin. Med. 43 (2), 241–254. doi:10.1142/s0192415x15500160
Wang, F.-D., Zhou, J., and Chen, E.-Q. (2022). Molecular mechanisms and potential new therapeutic drugs for liver fibrosis. Front. Pharmacol. 13, 787748. doi:10.3389/fphar.2022.787748
Wang, L., Li, D., Zhu, Z., Liao, Y., Wu, J., Liu, Y., et al. (2023). Knockout of Sema4D alleviates liver fibrosis by suppressing AOX1 expression. Pharmacol. Res. 195, 106886. doi:10.1016/j.phrs.2023.106886
Xia, C., Zhang, J., Chen, H., Jiang, W., Zhou, S., Zheng, H., et al. (2025). Kaempferol improves mitochondrial homeostasis via mitochondrial dynamics and mitophagy in diabetic kidney disease. Int. Immunopharmacol. 162, 115121. doi:10.1016/j.intimp.2025.115121
Xiang, X., You, S., Zeng, Z., Xu, J., Lin, Y., Liu, Y., et al. (2024). Exploration of the hypoglycemic mechanism of Fuzhuan brick tea based on integrating global metabolomics and network pharmacology analysis. Front. Mol. Biosci. 10, 1266156. doi:10.3389/fmolb.2023.1266156
Yamaguchi, Y., Kirita, S., Hasegawa, H., Aoyama, J., Imaoka, S., Minamiyama, S., et al. (2002). Contribution of CYP4A8 to the formation of 20-Hydroxyeicosatetraenoic acid from arachidonic acid in rat kidney. Drug Metabolism Pharmacokinet. 17 (2), 109–116. doi:10.2133/dmpk.17.109
Yamane, D., Hayashi, Y., Matsumoto, M., Nakanishi, H., Imagawa, H., Kohara, M., et al. (2021). FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chem. Biol. 29 (5), 799–810.e4. doi:10.1016/j.chembiol.2021.07.022
Yan, R., Yang, Y., and Chen, Y. (2018). Pharmacokinetics of Chinese medicines: strategies and perspectives. Chin. Med. 13, 24. doi:10.1186/s13020-018-0183-z
Yan, L., Sundaram, S., Rust, B. M., Palmer, D. G., Johnson, L. K., and Zeng, H. (2022). Consumption of a high-fat diet alters transcriptional rhythmicity in liver from pubertal mice. Front. Nutr. 9, 1068350. doi:10.3389/fnut.2022.1068350
Yang, Y. M., Noureddin, M., Liu, C., Ohashi, K., Kim, S. Y., Ramnath, D., et al. (2019). Hyaluronan synthase 2-mediated hyaluronan production mediates Notch1 activation and liver fibrosis. Sci. Transl. Med. 11 (496), eaat9284. doi:10.1126/scitranslmed.aat9284
Yang, X., Li, Q., Liu, W., Zong, C., Wei, L., Shi, Y., et al. (2023). Mesenchymal stromal cells in hepatic fibrosis/cirrhosis: from pathogenesis to treatment. Cell. and Mol. Immunol. 20 (6), 583–599. doi:10.1038/s41423-023-00983-5
Zhang, L., and Schuppan, D. (2014). Traditional chinese Medicine (TCM) for fibrotic liver disease: hope and hype. J. Hepatology 61 (1), 166–168. doi:10.1016/j.jhep.2014.03.009
Zhang, Y., Liang, J., Cao, N., Gao, J., Song, L., and Tang, X. (2022). Coal dust nanoparticles induced pulmonary fibrosis by promoting inflammation and epithelial-mesenchymal transition via the NF-κB/NLRP3 pathway driven by IGF1/ROS-mediated AKT/GSK3β signals. Cell Death Discov. 8 (1), 500. doi:10.1038/s41420-022-01291-z
Zhang, Z., Diao, P., Zhang, X., Nakajima, T., Kimura, T., and Tanaka, N. (2022). Clinically relevant dose of pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), lowers serum triglyceride levels by targeting hepatic PPARα in mice. Biomedicines 10 (7), 1667. doi:10.3390/biomedicines10071667
Zhangdi, H.-J., Su, S.-B., Wang, F., Liang, Z.-Y., Yan, Y.-D., Qin, S.-Y., et al. (2019). Crosstalk network among multiple inflammatory mediators in liver fibrosis. World J. Gastroenterology 25 (33), 4835–4849. doi:10.3748/wjg.v25.i33.4835
Zheng, W., Lei, M., Yao, Y., Zhan, J., Zhang, Y., and Zhou, Q. (2024). Mechanisms underlying the therapeutic effects of Semen cuscutae in treating recurrent spontaneous abortion based on network pharmacology and molecular docking. Front. Mol. Biosci. 11, 1282100. doi:10.3389/fmolb.2024.1282100
Zheng, M., Liu, H., Zhang, R., Guo, X., Shao, Q., Zhang, J., et al. (2025). Exploring the mechanism of Sinisan in the treatment of ulcerative colitis with depression based on UPLC-Q-Orbitrap-MS combined with network pharmacology, molecular docking, and experimental validation. J. Ethnopharmacol. 347 (347), 119696. doi:10.1016/j.jep.2025.119696
Keywords: bibliometrics, network pharmacology, kaempferol, hepatic fibrosis, transcriptomics
Citation: Liu J, Song X, Song X, Fu X, Niu S, Chang H, Shi S, Yang M, Zhao R, Wang P, Qi J and Bai W (2025) Bibliometrics-guided cyberpharmacology and transcriptomics for multidimensional analysis of the antihepatic fibrosis mechanism of kaempferol. Front. Mol. Biosci. 12:1607103. doi: 10.3389/fmolb.2025.1607103
Received: 07 April 2025; Accepted: 12 August 2025;
Published: 29 August 2025.
Edited by:
Shaohui Wang, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Zheng Xu, Guangzhou University of Chinese Medicine, ChinaZiheng Xu, Guangxi University of Chinese Medicine, China
Copyright © 2025 Liu, Song, Song, Fu, Niu, Chang, Shi, Yang, Zhao, Wang, Qi and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wanfu Bai, MTAyMDEwMTE0QGJ0bWMuZWR1LmNu; Jun Qi, MzM5NzY5OTBAcXEuY29t
†These authors have contributed equally to this work
 Jiali Liu
Jiali Liu Xiaowen Song1†
Xiaowen Song1† Xinni Song
Xinni Song Xinyue Fu
Xinyue Fu Hong Chang
Hong Chang Songli Shi
Songli Shi Ruiqi Zhao
Ruiqi Zhao Peng Wang
Peng Wang Wanfu Bai
Wanfu Bai