- Yan’an Medical College, Yan’an University, Yan’an, China
Ubiquitination and deubiquitination are common forms of protein post-translational modifications that play crucial roles in the regulation of intracellular homeostasis. As a member of deubiquitination enzyme USP family, USP36 maintains the stability of substrate proteins by mediating their deubiquitination, thereby playing a significant role in various pathophysiological processes. Here we focus on discussing how USP36 participates in regulating ribosome biosynthesis and responds to ribotoxic stress response. Furthermore, this review has elucidated the role of USP36 in regulating DNA replication stress, hypoxia adaptation, oxidative stress, and selective autophagy, as well as the related molecular mechanisms. This review is very helpful for understanding the role of USP36 in pathophysiological process and exploring the possibility of USP36 as a target for disease treatment.
1 Introduction
As one of the most critical post-translational modifications, ubiquitination involves the transfer of ubiquitin onto a substrate protein, forming an isopeptide bond, in turn resulting in the alteration of the stability and/or activity of the substrate (Liu F., 2024; Lacoursiere et al., 2022; Dikic and Schulman, 2023). This process is catalyzed by the collaboration of E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin ligases. Among them, E3 ubiquitin ligases are of crucial importance, as they determine the specificity and extent of ubiquitination of substrate proteins (Zhang, 2025). Conversely, deubiquitination modification is to break the isopeptide bond between ubiquitin and the substrate protein, and deubiquitination enzymes (DUBs) playing a pivotal catalytic role in this cascade reaction (Lim et al., 2013).
There are mainly nine classes of DUBs, including ubiquitin-specific proteases (USPs), ovarian tumor domain proteases (OTUs), ubiquitin C-terminal hydrolases (UCHs), Machado-Joseph domain (or Josephin domain)-containing proteins (MJDs), JAMM/MPN domain metalloproteases (JAMMs), Zinc finger with UFM1-specific peptidase domain protein (ZUFSP/ZUP1), MIU-containing novel DUB family (MINDY), monocyte chemotactic protein-induced protein (MCPIP) and permuted papain fold peptidase of dsDNA viruses and eukaryotes (PPPDE) (Ge, 2022; Ren, 2023). Among these DUBs, the USPs family contains the largest number of members and has been the most extensively studied (Cruz et al., 2021). Enzymes of the USP family can recognize their substrate proteins and deubiquitinate them, maintaining substrate stability, thereby counteracting the effects of E3 ligases on specific cellular substrates. This property has a direct impact on diseases, including cancer (Dewson et al., 2023). The intracellular localization of USPs varies, such as the nucleolus, Golgi body, and endoplasmic reticulum, which helping USPs deubiquitination modification of substrate proteins with different subcellular localization (Clague et al., 2019). Numerous evidences suggest that ubiquitin-specific peptidase 36 (USP36) plays a crucial role in regulating various physiological and pathological processes, including ribosome biosynthesis, ribotoxic stress response, DNA replication stress, hypoxia adaptation, oxidative stress, and selective autophagy (Fang, 2023; Ren et al., 2019; Bhattacharya et al., 2020; Meng et al., 2019). Thus the present article comprehensively reviewed the regulatory functions of USP36 in these pathophysiological processes and the underlying molecular mechanisms.
2 USP36 is involved in regulating ribosome biosynthesis and ribosomal stress response
2.1 The role of USP36 in ribosome biosynthesis
(1) The role of USP36 in the transcriptional synthesis of rRNA
The synthesis of ribosomal RNA (rRNA) is the initial and rate-limiting step in ribosome biosynthesis (Hori et al., 2023). 47S pre-rRNA serves as the precursor for the three mature rRNAs in eukaryotes: 28S rRNA, 18S rRNA, and 5.8S rRNA. This precursor rRNA is transcribed and synthesized under the catalysis of RNA polymerase I (RNA Pol I), using ribosomal DNA (rDNA) located in the fibrillar center (FC) of the nucleolar region as the template (Bowman et al., 2020). Research has found that USP36 can maintain the stability of RNA PolⅠby reversing its ubiquitin-proteasome degradation, thereby increasing the synthesis rate of 47S pre-rRNA and ultimately participating in regulating the biosynthesis rate of ribosomes (Richardson et al., 2012; Vanden Broeck and Klinge, 2024). Similarly, USP36 can increase the stability of DEAH-box helicase 33 (DHX33) through deubiquitination modificatio, while DHX33 can promote the synthesis of 47S pre-rRNA by enhancing the binding strength between RNA PolⅠand rDNA (Hori et al., 2023; Fraile et al., 2018). These results suggest that USP36 can enhance the protein stability of DHX33 through its deubiquitinating activity, thus promoting 47S pre-rRNA synthesis. USP36 can also maintain the deubiquitinated state of Fanconi anemia complementation group I (FANCI), stabilizing the interaction between FANCI and the large subunit of RNA Pol I (RPA194) in the FC, in turn facilitating the synthesis of 47S pre-rRNA (Sondalle et al., 2019). Additionally, USP36 interacts with snail family transcriptional repressor 1 (SNAIL1) to remove the polyubiquitin chains at positions K146 and K206 of the SNAIL1, achieving the stabilization of SNAIL1 and consequently upregulating the transcriptional synthesis of 47S pre-rRNA (Qin, 2023). Furthermore, it has been reported that knockdown of USP36 can elevate the ubiquitination level of histone H2A, leading to the inhibition of rRNA transcription (Li et al., 2019; Endo et al., 2009a). In summary, USP36 can participate in regulating the transcriptional synthesis of 47S pre-rRNA through mediating the deubiquitination modification of multiple nucleolar proteins such as RNA Pol I, and thereby promote ribosome biogenesis. The production of higher-level nucleolar proteins and the formation of ribosomes can meet the requirements for the high proliferation ability of tumor cells. Given that USP36 is highly expressed in various tumor cells, it is possible that USP36 may have certain carcinogenic potential by regulating ribosome biosynthesis (Figure 1A).
(2) The role of USP36 in the processing and maturation of precursor rRNA
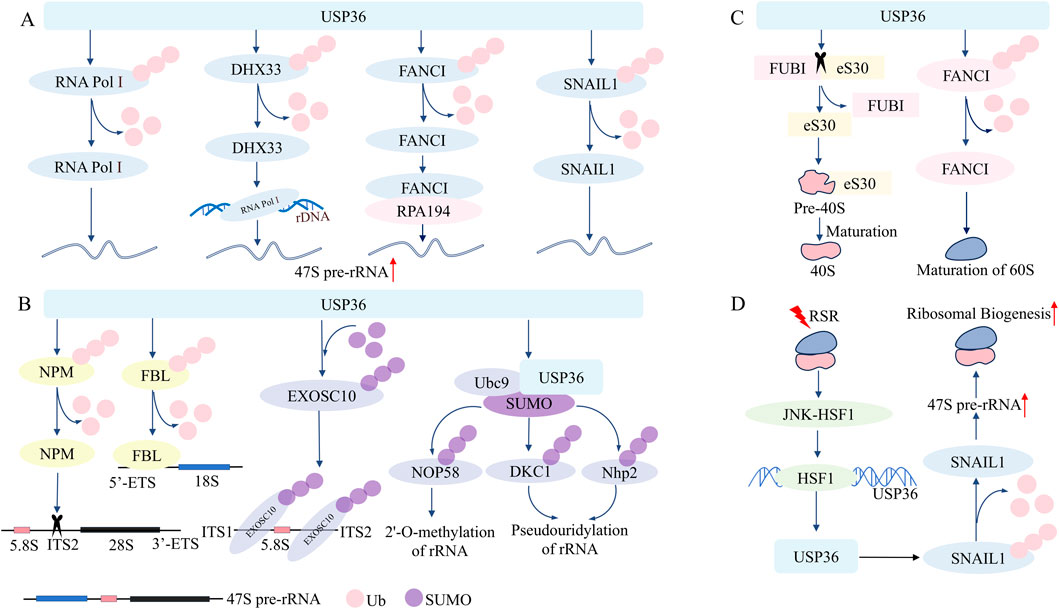
Figure 1. USP36 plays a key role in the regulation of ribosome biosynthesis and ribosomal stress response. (A) USP36 enhances the synthesis of 47S pre-rRNA by deubiquitinating RNA Pol Ⅰ, DHX33, FANCI, and SNAIL1. (B) USP36 promotes the processing of 47S pre-rRNA by deubiquitinating NPM and FBL, and participates in the maturation and processing of 18S rRNA and 5.8s rRNA by sumoylating EXOSC10 and UBC9. (C) USP36 promotes the maturation of the 40S subunit by cleaving FUBI-eS30, and promotes the maturation of the 60S subunit by deubiquitinating FANCI. (D) USP36 combats RSR by promoting ribosome biosynthesis.
USP36 not only participates in the transcriptional synthesis of rRNA but also in the processing and modification of precursor rRNA (Figure 1B). After synthesis, 47S pre-rRNA undergoes a series of fine regulations to remove the external transcribed spacer (ETS) and internal transcribed spacer (ITS), generating three mature rRNAs: 28S rRNA, 18S rRNA, and 5.8S rRNA (Jiao, 2023; Dorner, 2023). USP36 interacts with nucleophosmin (NPM) and stabilizes NPM through deubiquitination modification. Then NPM recognizes and cleaves specific sequences within the 5.8S-ITS2 region of 47S pre-rRNA through its endoribonuclease activity, thereby facilitating the processing of 47S pre-rRNA (Savkur and Olson, 1998; Endo et al., 2009b; Frazier et al., 2021). Similarly, USP36 colocalizes with fibrillarin (FBL) and mediates the deubiquitination modification of the latter to enhance its stability (Endo et al., 2009a; Endo et al., 2009b). FBL can bind to the 5' ETS of the 47S pre-rRNA precursor through its C-terminal methyltransferase domain, enabling the FBL-associated 47S pre-rRNA to transfer from the FC/dense fibrillar component (DFC) boundary to the DFC for further processing (Yao et al., 2019). Consistent with this, downregulation of USP36 leads to depletion of FBL, accompanied by a reduction in 28S rRNA and 18S rRNA due to impaired processing and maturation of 47S pre-rRNA (Tafforeau et al., 2013).
On the other hand, USP36 is able to regulate the processing and maturation of precursor rRNA through mediating the SUMOylation of certain key nucleolar proteins (Figure 1B). USP36 interacts with the C-terminal Lasso domain of exosome component 10 (EXOSC10) via the basic amino acid extension sequence (amino acids 801–1121) in its C-terminal region, functioning as an E3 SUMO ligase for EXOSC10 (Chen, 2023). By mediating the SUMOylation modification of EXOSC10, USP36 enhances the binding affinity of EXOSC10 to the 18S-ITS1 and 5.8S-ITS2 sequences of 47S pre-rRNA, thereby participating in the regulation of 18S rRNA and 5.8S rRNA maturation (Chen, 2023; Ryu, 2021). Notably, the N-terminal region of USP36 containing the USP domain (amino acids 1–420) can also exert E3 SUMO ligase activity (Ryu, 2021). The N-terminus of USP36 binds simultaneously to Ubc9 (a E2 SUMO-conjugating enzyme) and SUMO, thus mediating the SUMO modification of some protein components (such as Nop58, Nhp2, DKC1) in the small nucleolar ribonucleoproteins complex (Ryu, 2021; Akimoto et al., 2022). Among these, Nop58 is involved in 2'-O-methylation of rRNA, while DKC1 and Nhp2 participate in pseudouridylation, all of which are crucial steps in rRNA maturation (Lui and Lowe, 2013; Wu et al., 2020; Watkins and Bohnsack, 2012). USP36 facilitates the processing and maturation of 47S pre-rRNA. Unlike conventional models, USP36 functions not only as a deubiquitinase but also as an E3 SUMO ligase, catalyzing SUMOylation. Notably, USP36 stabilizes NPM and FBL through deubiquitination. Through SUMOylation of EXOSC10, Nop58, Nhp2 and DKC1, USP36 modulates their functions without changing their mRNA or protein levels (Ryu, 2021).
(3) The role of USP36 in the processing and maturation of ribosome
USP36 is crucial for the maturation of both 40S small subunit and 60S large subunit of the ribosome (Sondalle et al., 2019; van den Heuvel, 2021; O'Dea, 2023). USP36 directly catalyzes the cleavage of the ribosome ubiquitin-like fusion protein FUBI-eS30 into FUBI and eS30. Following this, eS30 integrates into the precursor 40S small subunit, thereby promoting the processing and maturation of the latter. Conversely, if FUBI cannot be effectively removed by USP36, the ribosomal 40S small subunit carrying the FUBI fragment will not be functional in protein translation (van den Heuvel, 2021; O'Dea, 2023). On the other hand, USP36 maintains FANCI in a deubiquitinated state in the nucleolus, ensuring its stability. FANCI is essential for the transcription of precursor rRNA and the processing of the ribosomal 60S large subunit precursor rRNA (Sondalle et al., 2019). In summary, USP36 plays a key role in the processing and maturation of ribosome (Figure 1C).
2.2 The role of USP36 in ribotoxic stress response
The ribotoxic stress response (RSR) refers to the response of cells to translation abnormalities, mainly occurring when ribosomes are dysfunctional or overloaded (Snieckute, 2022; Vind et al., 2020). When triple-negative breast cancer cells undergo RSR, the JNK-HSF1 signaling pathway is activated, then USP36 is transcriptionally activated by HSF1. And USP36 stabilizes the SNAIL1 protein through its deubiquitinating activity, thus promoting the precursor rRNA synthesis and ribosome biosynthesis, so as to combat RSR (Qin, 2023) (Figure 1D). These findings suggest that USP36 regulates ribosome biogenesis through multiple mechanisms and tumor initiation and progression by deubiquitinating and SUMOylating various nucleolar proteins. Therefore, targeting USP36 and its regulated mechanisms is expected to be a promising strategy for tumor therapy (Qin, 2023).
3 USP36 is involved in regulating DNA replication stress
DNA replication stress refers to the slowdown or stalling of the progression of replication forks during DNA synthesis, posing a threat to the replication stability of genome (Da Costa, 2023; Berti et al., 2020). DNA replication damage and replication fork stalling can trigger DNA replication stress during DNA replication (Saxena and Zou, 2022). Cells respond to DNA replication stress through various mechanisms, including the reversal of replication forks, the uncoupling of replication forks, and the restart of stalled replication forks (Gaillard et al., 2015). Studies have demonstrated that USP36 is crucial for the effective restart of replication forks (Yan et al., 2020). USP36 is localized in the nucleolus under normal conditions, maintaining the integrity of nucleolar functions. However, when cells experience DNA replication stress, USP36 migrates from the nucleolus to the nucleoplasm and colocalizes with the DNA damage-tolerant polymerase PrimPol at stalled replication forks through its N-terminal USP domain (Yan et al., 2020; Diaz-Talavera, 2022). Yan et al. found that there is a positive correlation between the expression levels of USP36 and PrimPol in ovarian cancer, and USP36 can increase the protein stability of PrimPol through deubiquitination modification, thereby counteracting DNA replication stress (Yan et al., 2020). While knockdown of USP36 significantly increases the sensitivity of ovarian cancer cells to the DNA replication stress inducer hydroxyurea, while restoring PrimPol expression can reverse this phenomenon (Yan et al., 2020). Taken together, these findings suggest that USP36 stabilizes PrimPol through deubiquitination, thus playing a role in counteracting DNA replication stress (Figure 2A) (Yan et al., 2020). High DNA replication stress in cancer cells may lead to genomic instability, and targeting replication stress can help identify new cancer susceptibility loci (Wen, 2025; Da Cos and ta, 2023). Therefore, targeted inhibition of USP36 to block PrimPol-mediated replication fork restart, combined with the use of DNA replication stress pathway inhibitors, may enhance the chemosensitivity of ovarian cancer cells, providing a novel approach for ovarian cancer treatment. Meanwhile, the development of highly selective small-molecule inhibitors targeting USP36 will offer a more specific tool for the precise regulation of DNA replication stress and overcoming tumor chemoresistance.
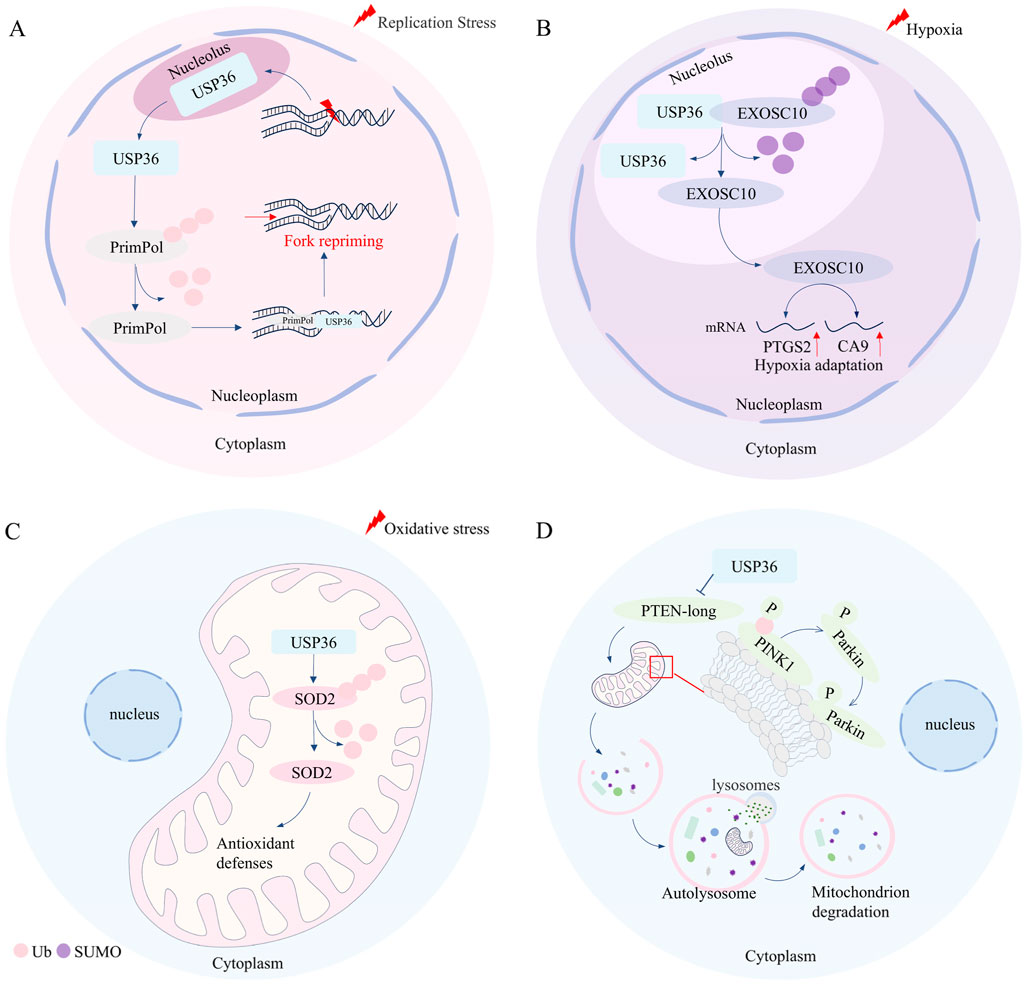
Figure 2. USP36 is involved in the regulation of DNA replication stress, hypoxia adaptation, oxidative stress, and selective autophagy. (A) In the condition of DNA replication stress, USP36 facilitates the restart of stalled replication forks by mediating the deubiquitination of PrimPol. (B) Under hypoxic conditions, USP36 upregulates the expression of hypoxia-related genes such as PTGS2 and CA9 by mediating the de-SUMOylation of EXOSC10, thereby regulating hypoxic adaptation. (C) USP36 can protect cells from mitochondrial oxidative damage through mediating the deubiquitination of SOD2. (D) USP36 inhibits the translation of the long form of PTEN, thereby promoting the formation of pSer65-Ub modification on PINK1 on the outer mitochondrial membrane, and marking the damaged mitochondria for autophagic degradation.
4 USP36 is involved in regulating hypoxia adaptation
The activation of gene transcription mediated by HIF is a crucial mechanism for cells to maintain their survival and function under hypoxic conditions (Yuan, 2024; Batie, 2022). However, USP36 has been reported to be involved in regulating hypoxia adaptation independent of HIF in cervical cancer (Filippopoulou et al., 2020; Chachami et al., 2019; Filippopoulou, 2024). Under normoxic conditions, USP36 and EXOSC10 are co-localized in the nucleolus, where USP36 can function as an E3 SUMO ligase to mediate the SUMOylation of EXOSC10 (Filippopoulou, 2024). However, under hypoxic conditions, EXOSC10 dissociates from USP36 and migrates from the nucleolus to the nucleoplasm along with its deSUMOylation. The deSUMOylation of EXOSC10 upregulates the expression levels of hypoxia-responsive genes (such as PTGS2,CA9, etc.) (Filippopoulou, 2024) (Figure 2B). These findings suggest that USP36 plays a significant regulatory role in hypoxia adaptation.
5 USP36 is involved in regulating oxidative stress
Oxidative stress refers to the imbalance of intracellular redox homeostasis, that is, reactive oxygen species are excessively produced, which cannot be offset by the action of antioxidants (Akiyama and Ivanov, 2024). Studies have suggested that USP36 play a role in regulating oxidative stress (Kim et al., 2011) (Figure 2C). USP36 interacts with superoxide dismutase 2 (SOD2) in mitochondria, in turn mediating the deubiquitination modification of SOD2, thereby enhancing the antioxidant capacity of SOD2 (Kim et al., 2011). Further research has demonstrated that the interaction between USP36 and SOD2 is time-dependent and occurs at COP9 signalosome subunit 3 (COPS3) (Kim et al., 2011; Pariano, 2021). The researcher also fund that Anakinra can protect cells from mitochondrial oxidative stress damage by promoting the interaction between SOD2 and USP36-COPS3 (Pariano, 2021). This suggests that targeted regulation of USP36 can enhance SOD2-mediated antioxidant capacity and improve mitochondrial function, providing a potential direction for therapeutic intervention in various diseases, such as Aspergillus fumigatus infection-related pneumonia, mitochondrial dysfunction-associated cardiovascular aging, and age-related neurodegenerative diseases.
6 USP36 is involved in regulating the selective autophagy
Autophagy is a process where cells degrade proteins and organelles for reuse, which is crucial for maintaining intracellular homeostasis (Liu, 2023). Based on the selectivity of substrates, autophagy can be broadly classified into two types: selective autophagy and non-selective autophagy (Liu J., 2024; Glick et al., 2010). For selective autophagy, selective autophagy receptors transport specific substrates (such as damaged organelles, aggregated protein or invading bacteria) to autophagosomes for degradation trough binding with LC3 (Vargas, 2023). While, non-selective autophagy is typically induced by factors like rapamycin, nutrient deprivation, or energy starvation (Liu J., 2024). Growing eidence indicates that USP36 plays a vital role in selective autophagy (Geisler et al., 2019; Wang, 2018; Pickrell and Youle, 2015; Ye, 2023) (Figure 2D). USP36 promotes the phosphorylation of Ser65 of ubiquitin (pSer65-Ub) of PINK1 on the mitochondrial outer membrane by inhibiting the translation of the long isoform of phosphatase and tensin homologue (PTEN-long) (Geisler et al., 2019). The activation of PINK1 promotes the recruitment of Parkin from the cytoplasm to the mitochondria, and then ubiquitinates mitochondrial outer membrane protein and marks damaged mitochondria for autophagic degradation (Geisler et al., 2019; Wang, 2018; Pickrell and Youle, 2015). Furthermore, USP36 can also upregulate the protein expression levels of ATG14L and Beclin-1, thereby promoting Parkin-dependent mitophagy during mitosis (Geisler et al., 2019; Ye, 2023). However, it has been reported that USP36 inhibits the selective autophagy in Drosophila (Taillebourg et al., 2012). Specifically, USP36 reduces the number of LC3-labeled autophagosomes without affecting the activity of the mTOR pathway (Taillebourg et al., 2012). However, the nuclear localization of USP36 limits the research on its mechanisms of direct action on mitophagy, and further exploration of its downstream targets is required. Additionally, further studies are needed to investigate the applicability of USP36 in regulating selective autophagy across different biological models.
7 Conclusion
This review has comprehensively elaborated the role of USP36 in various pathophysiological processes, including ribosome biosynthesis, ribotoxic stress response, DNA replication stress, hypoxic adaptation, oxidative stress, and autophagy activity. USP36 stabilizes RNA Pol I, DHX33, FANCI, SNAIL1, and H2A through its deubiquitinating activity, thereby promoting the synthesis of precursor rRNA. On the other hand, USP36 plays a key role in the processing and maturation of precursor rRNA through mediating the deubiquitination modification of NPM and FBL or mediating the SUMOylation of EXOSC10, Nop58, Nhp2, and DKC1. USP36 further mediates the cleavage of FUBI-eS30 and the deubiquitination of FANCI, promoting the maturation of the ribosome. Additionally, under conditions of ribosomal toxic stress, USP36 can be transcriptionally activated by HSF1 to counteract this stress. It is noteworthy that USP36 also plays a crucial regulatory role in other cellular processes such as DNA replication stress, hypoxic adaptation, oxidative stress, and selective autophagy (Table 1).
Research has shown that USP36 promotes tumor progression by deubiquitinating a variety of proteins. USP36 promotes the progression of esophageal squamous cell carcinoma (ESCC) through the Hippo/YAP axis; USP36 enhances the invasiveness of glioblastoma by stabilizing SNAIL2, and USP36 accelerates the progression of hepatocellular carcinoma (HCC) through synergistic action with TP53 (Sun, 2022; Li, 2023; Chang, 2023). The proliferation of tumor cells is highly dependent on ribosome biogenesis, and USP36 has been proven to promote ribosome biogenesis through multiple mechanisms. However, how USP36 regulates ribosome biogenesis in tumor cells has not been fully studied. In addition, specific inhibitors have been developed for other deubiquitinases in the USP family (such as USP1 and USP7), but inhibitors targeting USP36 have not been reported yet (Huang, 2023; Guo, 2024). Therefore, in the future, it is still necessary to deeply study the mechanism of action between USP36 and ribosome biogenesis in tumor cells and explore its potential as a new target for tumor therapy. Meanwhile, the development of specific inhibitors for USP36 should be accelerated to provide new strategies for precise tumor diagnosis and treatment.
Author contributions
LS: Writing – review and editing, Conceptualization, Writing – original draft. MG: Writing – original draft. QK: Writing – original draft. YG: Writing – original draft. XL: Writing – original draft. FL: Conceptualization, Writing – review and editing, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Programs from the National Natural Science Foundation of China (82260530), the Scientific Research Project of Shaanxi Provincial Department of Education (24JK0726) and the Start-up Project of Doctoral Research in Yan 'an University (YDBK 2020-04).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akimoto, G., Fernandes, A. P., and Bode, J. W. (2022). Site-specific protein ubiquitylation using an engineered, chimeric E1 activating enzyme and E2 SUMO Conjugating enzyme Ubc9. ACS Cent. Sci. 8 (2), 275–281. doi:10.1021/acscentsci.1c01490
Akiyama, Y., and Ivanov, P. (2024). Oxidative stress, transfer RNA metabolism, and protein synthesis. Antioxid. Redox Signal 40 (10-12), 715–735. doi:10.1089/ars.2022.0206
Batie, M. (2022). Regulation of chromatin accessibility by hypoxia and HIF. Biochem. J. 479 (6), 767–786. doi:10.1042/BCJ20220008
Berti, M., Cortez, D., and Lopes, M. (2020). The plasticity of DNA replication forks in response to clinically relevant genotoxic stress. Nat. Rev. Mol. Cell Biol. 21 (10), 633–651. doi:10.1038/s41580-020-0257-5
Bhattacharya, U., Neizer-Ashun, F., Mukherjee, P., and Bhattacharya, R. (2020). When the chains do not break: the role of USP10 in physiology and pathology. Cell Death Dis. 11 (12), 1033. doi:10.1038/s41419-020-03246-7
Bowman, J. C., Petrov, A. S., Frenkel-Pinter, M., Penev, P. I., and Williams, L. D. (2020). Root of the tree: the significance, evolution, and origins of the ribosome. Chem. Rev. 120 (11), 4848–4878. doi:10.1021/acs.chemrev.9b00742
Chachami, G., Stankovic-Valentin, N., Karagiota, A., Basagianni, A., Plessmann, U., Urlaub, H., et al. (2019). Hypoxia-induced changes in SUMO conjugation affect transcriptional regulation under low oxygen. Mol. Cell Proteomics 18 (6), 1197–1209. doi:10.1074/mcp.RA119.001401
Chang, G. (2023). USP36 promotes tumorigenesis and drug sensitivity of glioblastoma by deubiquitinating and stabilizing ALKBH5. Neuro Oncol. 25 (5), 841–853. doi:10.1093/neuonc/noac238
Chen, Y. (2023). The ubiquitin-specific protease USP36 SUMOylates EXOSC10 and promotes the nucleolar RNA exosome function in rRNA processing. Nucleic Acids Res. 51 (8), 3934–3949. doi:10.1093/nar/gkad140
Clague, M. J., Urbe, S., and Komander, D. (2019). Breaking the chains: deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol. 20 (6), 338–352. doi:10.1038/s41580-019-0099-1
Cruz, L., Soares, P., and Correia, M. (2021). Ubiquitin-Specific proteases: players in cancer cellular processes. Pharm. (Basel) 14 (9). doi:10.3390/ph14090848
Da Costa, A. A. B. A. (2023). Targeting replication stress in cancer therapy. Nat. Rev. Drug Discov. 22 (1), 38–58. doi:10.1038/s41573-022-00558-5
Dewson, G., Eichhorn, P. J. A., and Komander, D. (2023). Deubiquitinases in cancer. Nat. Rev. Cancer 23 (12), 842–862. doi:10.1038/s41568-023-00633-y
Diaz-Talavera, A. (2022). PrimPol: a breakthrough among DNA replication enzymes and a potential new target for cancer therapy. Biomolecules 12 (2). doi:10.3390/biom12020248
Dikic, I., and Schulman, B. A. (2023). An expanded lexicon for the ubiquitin code. Nat. Rev. Mol. Cell Biol. 24 (4), 273–287. doi:10.1038/s41580-022-00543-1
Dorner, K. (2023). Ribosome biogenesis factors-from names to functions. EMBO J. 42 (7), e112699. doi:10.15252/embj.2022112699
Endo, A., Matsumoto, M., Inada, T., Yamamoto, A., Nakayama, K. I., Kitamura, N., et al. (2009a). Nucleolar structure and function are regulated by the deubiquitylating enzyme USP36. J. Cell Sci. 122 (Pt 5), 678–686. doi:10.1242/jcs.044461
Endo, A., Kitamura, N., and Komada, M. (2009b). Nucleophosmin/B23 regulates ubiquitin dynamics in nucleoli by recruiting deubiquitylating enzyme USP36. J. Biol. Chem. 284 (41), 27918–27923. doi:10.1074/jbc.M109.037218
Fang, Y. (2023). Deubiquitination complex platform: a plausible mechanism for regulating the substrate specificity of deubiquitinating enzymes. Acta Pharm. Sin. B 13 (7), 2955–2962. doi:10.1016/j.apsb.2023.02.019
Filippopoulou, C. (2024). Hypoxia-driven deSUMOylation of EXOSC10 promotes adaptive changes in the transcriptome profile. Cell Mol. Life Sci. 81 (1), 58. doi:10.1007/s00018-023-05035-9
Filippopoulou, C., Simos, G., and Chachami, G. (2020). The role of sumoylation in the response to hypoxia: an overview. Cells 9 (11). doi:10.3390/cells9112359
Fraile, J. M., Campos-Iglesias, D., Rodríguez, F., Astudillo, A., Vilarrasa-Blasi, R., Verdaguer-Dot, N., et al. (2018). Loss of the deubiquitinase USP36 destabilizes the RNA helicase DHX33 and causes preimplantation lethality in mice. J. Biol. Chem. 293 (6), 2183–2194. doi:10.1074/jbc.M117.788430
Frazier, M. N., Pillon, M. C., Kocaman, S., Gordon, J., and Stanley, R. E. (2021). Structural overview of macromolecular machines involved in ribosome biogenesis. Curr. Opin. Struct. Biol. 67, 51–60. doi:10.1016/j.sbi.2020.09.003
Gaillard, H., Garcia-Muse, T., and Aguilera, A. (2015). Replication stress and cancer. Nat. Rev. Cancer 15 (5), 276–289. doi:10.1038/nrc3916
Ge, F. (2022). Deubiquitinating enzymes: promising targets for drug resistance. Drug Discov. Today 27 (9), 2603–2613. doi:10.1016/j.drudis.2022.06.009
Geisler, S., Jäger, L., Golombek, S., Nakanishi, E., Hans, F., Casadei, N., et al. (2019). Ubiquitin-specific protease USP36 knockdown impairs parkin-dependent mitophagy via downregulation of Beclin-1-associated autophagy-related ATG14L. Exp. Cell Res. 384 (2), 111641. doi:10.1016/j.yexcr.2019.111641
Glick, D., Barth, S., and Macleod, K. F. (2010). Autophagy: cellular and molecular mechanisms. J. Pathol. 221 (1), 3–12. doi:10.1002/path.2697
Guo, N. (2024). USP7 as an emerging therapeutic target: a key regulator of protein homeostasis. Int. J. Biol. Macromol. 263 (Pt 1), 130309. doi:10.1016/j.ijbiomac.2024.130309
Hori, Y., Engel, C., and Kobayashi, T. (2023). Regulation of ribosomal RNA gene copy number, transcription and nucleolus organization in eukaryotes. Nat. Rev. Mol. Cell Biol. 24 (6), 414–429. doi:10.1038/s41580-022-00573-9
Huang, P. (2023). Ubiquitin-specific peptidase 1: assessing its role in cancer therapy. Clin. Exp. Med. 23 (7), 2953–2966. doi:10.1007/s10238-023-01075-4
Jiao, L. (2023). Ribosome biogenesis in disease: new players and therapeutic targets. Signal Transduct. Target Ther. 8 (1), 15. doi:10.1038/s41392-022-01285-4
Kim, M., Ramakrishna, S., Lim, K. H., Kim, J. H., and Baek, K. H. (2011). Protein stability of mitochondrial superoxide dismutase SOD2 is regulated by USP36. J. Cell Biochem. 112 (2), 498–508. doi:10.1002/jcb.22940
Lacoursiere, R. E., Hadi, D., and Shaw, G. S. (2022). Acetylation, phosphorylation, ubiquitination (oh my!): following post-translational modifications on the ubiquitin road. Biomolecules 12 (3). doi:10.3390/biom12030467
Li, S. (2023). USP32 deubiquitinase: cellular functions, regulatory mechanisms, and potential as a cancer therapy target. Cell Death Discov. 9 (1), 338. doi:10.1038/s41420-023-01629-1
Li, F., Han, H., Sun, Q., Liu, K., Lin, N., Xu, C., et al. (2019). USP28 regulates deubiquitination of histone H2A and cell proliferation. Exp. Cell Res. 379 (1), 11–18. doi:10.1016/j.yexcr.2019.03.026
Lim, K., Ramakrishna, S., and Baek, K. (2013). Molecular mechanisms and functions of cytokine-inducible deubiquitinating enzymes. Cytokine Growth Factor Rev. 24 (5), 427–431. doi:10.1016/j.cytogfr.2013.05.007
Liu, S. (2023). Autophagy: regulator of cell death. Cell Death Dis. 14 (10), 648. doi:10.1038/s41419-023-06154-8
Liu, F. (2024a). Ubiquitination and deubiquitination in cancer: from mechanisms to novel therapeutic approaches. Mol. Cancer 23 (1), 148. doi:10.1186/s12943-024-02046-3
Liu, J. (2024b). Selective autophagy in cancer: mechanisms, therapeutic implications, and future perspectives. Mol. Cancer 23 (1), 22. doi:10.1186/s12943-024-01934-y
Lui, L., and Lowe, T. (2013). Small nucleolar RNAs and RNA-guided post-transcriptional modification. Essays Biochem. 54, 53–77. doi:10.1042/bse0540053
Meng, J., Ai, X., Lei, Y., Zhong, W., Qian, B., Qiao, K., et al. (2019). USP5 promotes epithelial-mesenchymal transition by stabilizing SLUG in hepatocellular carcinoma. Theranostics 9 (2), 573–587. doi:10.7150/thno.27654
O'Dea, R. (2023). Molecular basis for ubiquitin/Fubi cross-reactivity in USP16 and USP36. Nat. Chem. Biol. 19 (11), 1394–1405. doi:10.1038/s41589-023-01388-1
Pariano, M. (2021). Anakinra activates superoxide dismutase 2 to mitigate inflammasome activity. Int. J. Mol. Sci. 22 (12). doi:10.3390/ijms22126531
Pickrell, A. M., and Youle, R. J. (2015). The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 85 (2), 257–273. doi:10.1016/j.neuron.2014.12.007
Qin, K. (2023). USP36 stabilizes nucleolar Snail1 to promote ribosome biogenesis and cancer cell survival upon ribotoxic stress. Nat. Commun. 14 (1), 6473. doi:10.1038/s41467-023-42257-8
Ren, J. (2023). Deubiquitylating enzymes in cancer and immunity. Adv. Sci. (Weinh) 10 (36), e2303807. doi:10.1002/advs.202303807
Ren, G., Zhang, X., Xiao, Y., Zhang, W., Wang, Y., Ma, W., et al. (2019). ABRO1 promotes NLRP3 inflammasome activation through regulation of NLRP3 deubiquitination. EMBO J. 38 (6). doi:10.15252/embj.2018100376
Richardson, L. A., Reed, B. J., Charette, J. M., Freed, E. F., Fredrickson, E. K., Locke, M. N., et al. (2012). A conserved deubiquitinating enzyme controls cell growth by regulating RNA polymerase I stability. Cell Rep. 2 (2), 372–385. doi:10.1016/j.celrep.2012.07.009
Ryu, H. (2021). The deubiquitinase USP36 promotes snoRNP group SUMOylation and is essential for ribosome biogenesis. EMBO Rep. 22 (6), e50684. doi:10.15252/embr.202050684
Savkur, R. S., and Olson, M. O. (1998). Preferential cleavage in pre-ribosomal RNA byprotein B23 endoribonuclease. Nucleic Acids Res. 26 (19), 4508–4515. doi:10.1093/nar/26.19.4508
Saxena, S., and Zou, L. (2022). Hallmarks of DNA replication stress. Mol. Cell 82 (12), 2298–2314. doi:10.1016/j.molcel.2022.05.004
Snieckute, G. (2022). Ribosome stalling is a signal for metabolic regulation by the ribotoxic stress response. Cell Metab. 34 (12), 2036–2046.e8. doi:10.1016/j.cmet.2022.10.011
Sondalle, S. B., Longerich, S., Ogawa, L. M., Sung, P., and Baserga, S. J. (2019). Fanconi anemia protein FANCI functions in ribosome biogenesis. Proc. Natl. Acad. Sci. U. S. A. 116 (7), 2561–2570. doi:10.1073/pnas.1811557116
Sun, W. (2022). Gene signature and prognostic value of ubiquitin-specific proteases members in hepatocellular carcinoma and explored the immunological role of USP36. Front. Biosci. Landmark Ed. 27 (6), 190. doi:10.31083/j.fbl2706190
Tafforeau, L., Zorbas, C., Langhendries, J. L., Mullineux, S. T., Stamatopoulou, V., Mullier, R., et al. (2013). The complexity of human ribosome biogenesis revealed by systematic nucleolar screening of Pre-rRNA processing factors. Mol. Cell 51 (4), 539–551. doi:10.1016/j.molcel.2013.08.011
Taillebourg, E., Gregoire, I., Viargues, P., Jacomin, A. C., Thevenon, D., Faure, M., et al. (2012). The deubiquitinating enzyme USP36 controls selective autophagy activation by ubiquitinated proteins. Autophagy 8 (5), 767–779. doi:10.4161/auto.19381
van den Heuvel, J. (2021). Processing of the ribosomal ubiquitin-like fusion protein FUBI-eS30/FAU is required for 40S maturation and depends on USP36. Elife 10. doi:10.7554/eLife.70560
Vanden Broeck, A., and Klinge, S. (2024). Eukaryotic ribosome assembly. Annu. Rev. Biochem. 93 (1), 189–210. doi:10.1146/annurev-biochem-030222-113611
Vargas, J. N. S. (2023). The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 24 (3), 167–185. doi:10.1038/s41580-022-00542-2
Vind, A. C., Genzor, A. V., and Bekker-Jensen, S. (2020). Ribosomal stress-surveillance: three pathways is a magic number. Nucleic Acids Res. 48 (19), 10648–10661. doi:10.1093/nar/gkaa757
Wang, L. (2018). PTEN-L is a novel protein phosphatase for ubiquitin dephosphorylation to inhibit PINK1-Parkin-mediated mitophagy. Cell Res. 28 (8), 787–802. doi:10.1038/s41422-018-0056-0
Watkins, N. J., and Bohnsack, M. T. (2012). The box C/D and H/ACA snoRNPs: key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip. Rev. RNA 3 (3), 397–414. doi:10.1002/wrna.117
Wen, Z. (2025). STING agonists: a range of eminent mediators in cancer immunotherapy. Cell Signal 134, 111914. doi:10.1016/j.cellsig.2025.111914
Wu, H., Qin, W., Lu, S., Wang, X., Zhang, J., Sun, T., et al. (2020). Long noncoding RNA ZFAS1 promoting small nucleolar RNA-mediated 2'-O-methylation via NOP58 recruitment in colorectal cancer. Mol. Cancer 19 (1), 95. doi:10.1186/s12943-020-01201-w
Yan, Y., Xu, Z., Huang, J., Guo, G., Gao, M., Kim, W., et al. (2020). The deubiquitinase USP36 regulates DNA replication stress and confers therapeutic resistance through PrimPol stabilization. Nucleic Acids Res. 48 (22), 12711–12726. doi:10.1093/nar/gkaa1090
Yao, R., Xu, G., Wang, Y., Shan, L., Luan, P. F., Wang, Y., et al. (2019). Nascent Pre-rRNA sorting via phase separation drives the assembly of dense fibrillar components in the human nucleolus. Mol. Cell 76 (5), 767–783. doi:10.1016/j.molcel.2019.08.014
Ye, J. (2023). Targeting autophagy and beyond: deconvoluting the complexity of Beclin-1 from biological function to cancer therapy. Acta Pharm. Sin. B 13 (12), 4688–4714. doi:10.1016/j.apsb.2023.08.008
Yuan, X. (2024). Targeting hypoxia-inducible factors: therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 23 (3), 175–200. doi:10.1038/s41573-023-00848-6
Keywords: ubiquitin-specific peptidase 36, ribosome biosynthesis, DNA replication stress, hypoxia adaptation, oxidative stress, selective autophagy
Citation: Shao L, Guo M, Kou Q, Guo Y, Li X and Li F (2025) The role of USP36 in ribosome biogenesis and other pathophysiological processes. Front. Mol. Biosci. 12:1650908. doi: 10.3389/fmolb.2025.1650908
Received: 28 June 2025; Accepted: 04 August 2025;
Published: 20 August 2025.
Edited by:
José Rodrigo Pauli, State University of Campinas, BrazilCopyright © 2025 Shao, Guo, Kou, Guo, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Li, MTg3OTI4NzMxOThAMTYzLmNvbQ==
 Linxin Shao
Linxin Shao Mengqi Guo
Mengqi Guo Qianrui Kou
Qianrui Kou Ya Guo
Ya Guo Xin Li
Xin Li Fang Li
Fang Li