- 1Neural Stem Cell Research Lab, Research Department, National Neuroscience Institute, Singapore, Singapore
- 2Neuroscience and Behavioral Disorders Program, DUKE-NUS Graduate Medical School, Singapore, Singapore
- 3Centre for Molecular Neuropathology, Lee Kong Chian School of Medicine, Nanyang Technology University, Singapore, Singapore
The growing burden of neurodegenerative diseases (NDD) on healthcare systems, driven by global aging population, has increased interest in modelling the blood-brain barrier (BBB). While microfluidic platforms have been widely used to model the BBB, they remain limited by complex fabrication techniques, low-throughput, and restricted control over BBB geometry. Recent advancements in three-dimensional (3D) bioprinting offer promising strategies to overcome these constraints and to enable the generation of physiologically relevant BBB models. This review examines the recent progress in 3D bioprinting approaches to model human in vitro BBB, with a focus on their applications in NDD research. We first summarise current 3D bioprinting techniques and strategies, including the selection of bioinks and geometry design. Subsequently, we address the evaluation methods for in vitro BBB modelling and their relevance to disease modelling. Finally, we identify key challenges and future directions aimed at improving resolution, reproducibility, and functional 3D-printed BBB constructs for use in NDD modelling and drug development.
1 Introduction
Neurodegenerative diseases (NDD), including Alzheimer’s disease (AD) and Parkinson’s disease (PD), affect approximately 15% of the global population and pose a growing challenge to healthcare systems worldwide (Liu et al., 2022). A critical factor in NDD pathogenesis is the dysfunction of the blood-brain barrier (BBB) (Sweeney et al., 2018; Tran et al., 2022), which plays a central role in maintaining central nervous system (CNS) homeostasis by tightly regulating molecular exchange between the bloodstream and the brain (Kadry et al., 2020; Segarra et al., 2021). The BBB protects neural tissue from neurotoxic plasma components, blood cells, and pathogens, while ensuring optimal neuronal function.
Evidence suggests that disruption of BBB, leading to the loss of selective permeability, may precede and contribute to neuronal degeneration by allowing the entry of neurotoxic plasma components, inflammatory mediators, and immune cells into the CNS (Bell et al., 2010; Takata, 2021). This breach can amplify neuroinflammation and impair clearance of pathological proteins such as accumulated amyloid-beta (Aβ) in AD (Mawuenyega et al., 2010) and misfolded alpha-synuclein in PD (Stefanis et al., 2012). Understanding the mechanisms underlying BBB dysfunction and evaluating strategies to restore barrier integrity are therefore essential for advancing NDD research and therapy development.
Modelling the BBB has thus become essential for elucidating NDD pathogenesis and evaluating CNS-targeted therapies. However, due to the restrictive permeability and structural complexity of the human BBB, accurate modelling remains challenging. Traditional modelling approaches include the two-dimensional (2D) Transwell co-culture platform, which typically involves endothelial cells (EC) on the apical surface (representing the blood side) and other supporting cells on the basolateral surface (representing the brain side). The two compartments are separated by a porous membrane which allows molecular exchange and intercellular communication (Stone et al., 2019).
Although the 2D Transwell system has provided valuable insights, it is limited in its ability to recapitulate spatial organization, dynamic flow conditions and microenvironment of the human BBB (Yan et al., 2011). Furthermore, animal models often fail to fully recapitulate human BBB physiology, limiting their translational relevance (Badawi et al., 2024; Helman et al., 2016). These limitations have therefore driven the development of more advanced systems that better mimic native human BBB physiology.
Advanced technologies such as microfluidic organ-on-a-chip platforms and stem cell-derived BBB models have emerged in response. Among these, three-dimensional (3D) bioprinting stands out for its ability to generate spatially organized, customizable, and physiologically relevant in vitro BBB constructs. By integrating vascular geometry, multicellular interactions, extracellular matrix (ECM) composition, and perfusable flow, 3D bioprinting provides a powerful platform for studying BBB dysfunction in NDD and accelerating CNS-targeted drug development.
In this review, we explore the use of 3D bioprinting for modelling human BBB, with a focus on applications in NDD research (Figure 1). We begin by outlining the anatomical and functional features of the BBB, followed by key design considerations for in vitro models. We then compare existing modelling approaches while emphasising the advantages of 3D bioprinting. Subsequently, we discuss bioprinting techniques, bioink optimisation, and geometric design strategies, and conclude with functional assessment methods and the potential application of bioprinted BBB models in NDD research.
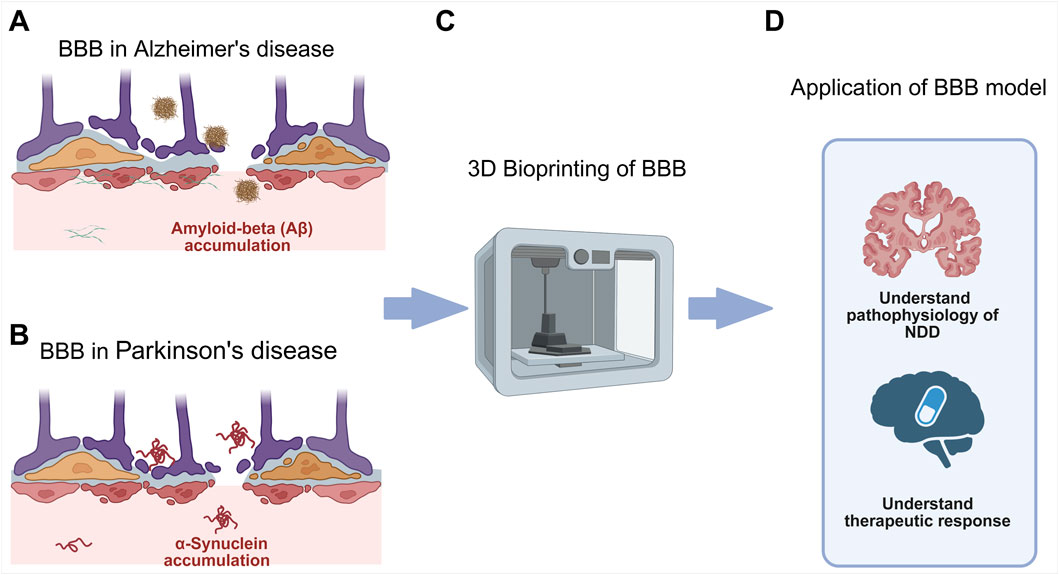
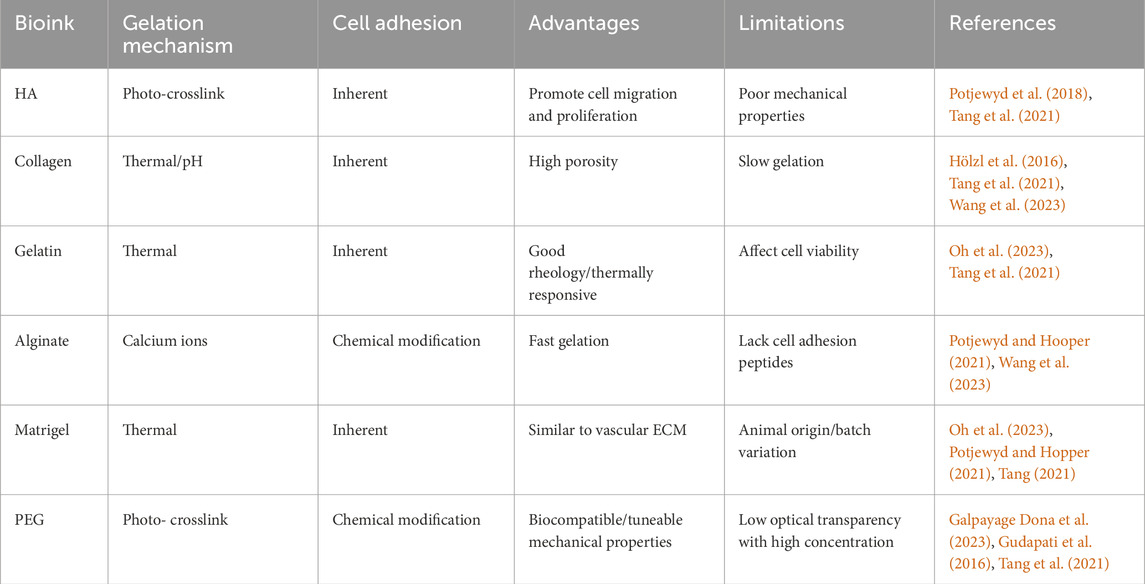
Figure 1. (A) BBB dysfunction in AD which involves accumulation of amyloid plaque. (B) BBB dysfunction in PD which involves accumulation of misfolded alpha-synuclein protein. (C) 3D bioprinting to create in vitro models of the BBB in NDD. (D) Application of BBB model in NDD research, to elucidate NDD pathophysiology for identification of novel therapeutic targets and therapy development via high-throughput drug screening.
1.1 Anatomy of the BBB
Accurate BBB modelling requires replication of both its cellular composition and the structure of the surrounding ECM. The main cellular components of the BBB include brain microvascular endothelial cells (BMEC), pericytes (PC), astrocytes (AC) (Figure 2). BMEC differ markedly from EC in peripheral tissues. They exhibit low rate of transcytosis and are interconnected by complex tight junctions (TJ) that restrict paracellular flux and diffusion (Rubin et al., 1999).
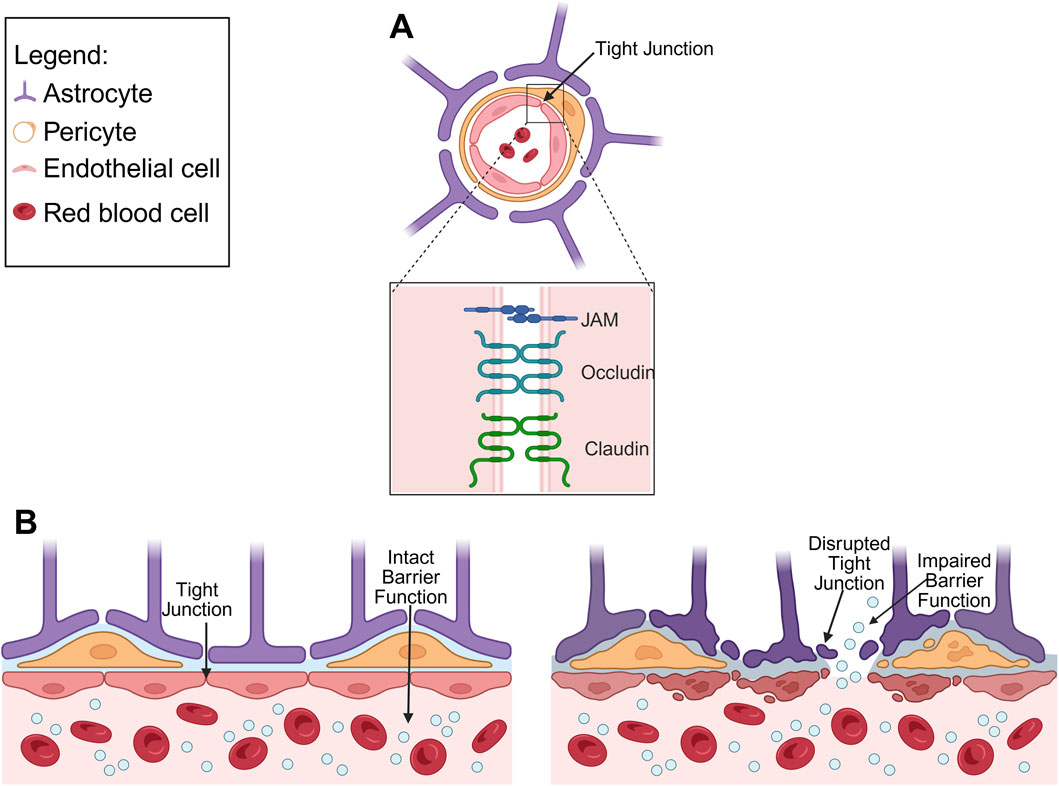
Figure 2. (A) Schematic diagram depicting anatomy of the healthy BBB, highlighting TJ proteins (junctional adhesion molecule (JAM), occludin and claudin). (B) Comparison of healthy BBB and diseased BBB, illustrating disrupted TJ and impaired barrier function.
PC, embedded within the basement membrane, regulate BBB permeability by modulating the expression of TJ and adherent junction proteins in BMEC, thereby influencing barrier tightness and vascular stability (Sweeney et al., 2016; Sweeney et al., 2019). AC contribute to the regulation of BBB permeability through their end-feet, which envelop the blood vessels and form close physical interactions with BMEC. These astrocytic processes also respond to CNS injury and help maintain homeostasis (Pekny et al., 2016). The BBB ECM differs from other tissues, lacking hyaluronic acid (HA) and consisting primarily of collagen IV, laminin, nidogen, perlecan and fibronectin (Reed et al., 2019). These components provide both structural support and biochemical cues that influence BBB cellular behaviour and barrier function.
TJ, which are fundamental to BBB integrity, comprise of membrane proteins including occludin, claudins and JAM (Stamatovic et al., 2016). Occludin forms oligomers that regulate solute diffusion across the TJ that can be disrupted under pathological conditions such as hypoxia-regeneration (Lochhead et al., 2010). Claudins, a family of tetraspan transmembrane proteins, determine the tissue, size, and charge properties of the TJ (Krause et al., 2008). Among these, claudin-3 and claudin-5 are particular important for maintaining BBB integrity, and reduced claudin-5 expression increased barrier permeability (Luissint et al., 2012). JAMs, members of the immunoglobulin superfamily, regulate TJ assembly through interactions with cell polarity related proteins, thereby reducing permeability (Hudson et al., 2021). Notably, JAM-1 is involved in the early stages of TJ formation and is essential for BBB integrity (Jia et al., 2013).
1.2 Key design considerations for in vitro BBB modelling
Physiologically relevant BBB models aim to replicate in vivo function as closely as possible, encompassing appropriate cellular composition and structural integrity. Key design considerations are summarised in Figure 3. One of the most critical aspects is the inclusion of all three key BBB cell types–BMEC, AC and PC (Jamieson et al., 2017). Incorporating these cells enhances TJ formation and barrier tightness, allowing more accurate replication of the anatomical and functional complexity of the BBB (Vetter et al., 2025). Beyond cellular composition, model reproducibility and homogeneity are also crucial considerations when developing NDD specific BBB models to ensure consistent and reliable disease modelling (Winkelman et al., 2021). Variability in cell sourcing, culture conditions, or scaffold composition can significantly impact barrier properties and reduce translational relevance.
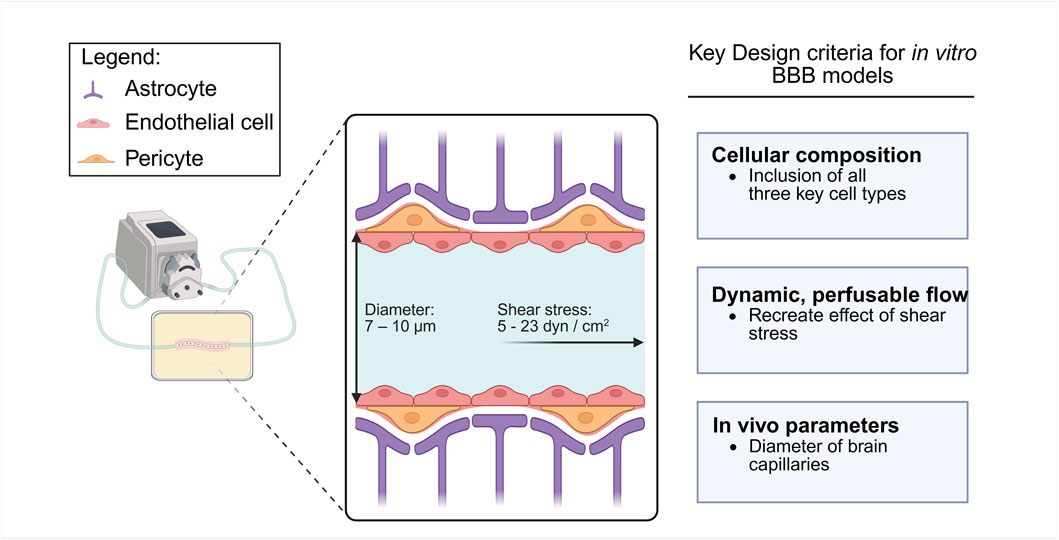
Figure 3. Schematic diagram of key design considerations for in vitro BBB modelling. Key design criteria include: 1) cellular composition consisting of AC, EC and PC, 2) a dynamic and perfusable flow system with physiological shear stress, and 3) physical parameters similar to in vivo brain capillaries.
Another important criterion is the inclusion of dynamic, perfusable flow (Bolden et al., 2023; Potjewyd et al., 2021). Perfusable models that simulate capillary blood flow recreate shear stress experienced by EC in vivo that is reported to be between 5 and 23 dyn/cm2 in human brain capillaries (Wang et al., 2020). This mechanical stimulus influences cell alignment, morphology, and upregulation of TJ proteins which are key elements for maintaining barrier integrity and function (Yue et al., 2020). By integrating controlled flow conditions, in vitro BBB systems can better emulate the native microenvironment, enabling the study of vascular contributions to NDD pathogenesis and for evaluating therapeutic strategies.
Finally, in vitro BBB models should strive to reproduce in vivo physiological parameters. High-resolution fabrication is required to achieve structural features comparable to brain capillaries, which are 7–10 μm in diameter (Pandey et al., 2016; Wong et al., 2013). Another key metric is the trans-epithelial electrical resistance (TEER), which reflects barrier integrity; physiologically relevant models should aim for in vivo TEER values ranging from 1,500 to 8,000 Ωcm2 (Crone et al., 1982; Reichel et al., 2003; Wolff et al., 2015).
1.3 Current in vitro BBB modelling approaches
Various techniques have been developed to construct in vitro BBB models, including microfluidics and 3D bioprinting (Table 1). Among these, microfluidic approaches are currently more prevalent in the literature, in part due to their ability to incorporate dynamic flow and mimic physiological shear-stress conditions (Jagtiani et al., 2022).
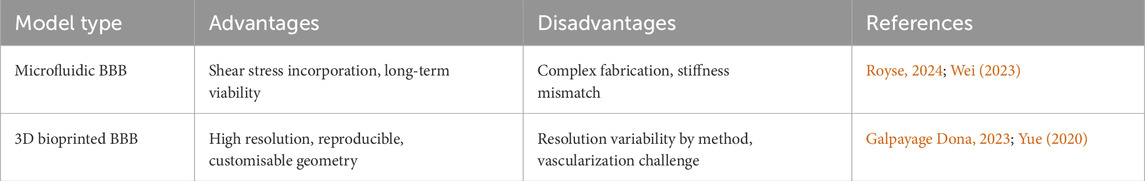
Table 1. Advantages and disadvantages of microfluidic against 3D bioprinting methods for BBB modelling.
Microfluidic models enable perfusion, allowing the development of dynamic BBB models with improved barrier tightness and functionality. EC cultured under flow conditions showed elongated cell morphology and higher localisation of TJ proteins which are features associated with enhanced barrier integrity (Wei et al., 2023).
Additionally, perfusion also supports cell viability by facilitating metabolite and nutrient diffusion, promoting long-term culture maintenance. However, stiff materials used in microfluidic devices can alter mechanotransduction signalling due to stiffness mismatches with native tissue (Potjewyd et al., 2021). Furthermore, complex fabrication procedures and small construct dimensions can limit meaningful multicellular interactions, which are essential for replicating the BBB multicellular nature (Royse et al., 2024).
To address these challenges, 3D bioprinting has recently been integrated with microfluidic devices, offering a promising hybrid approach. 3D bioprinting enables spatially controlled deposition of multiple cell types and ECM components, supporting the creation of high-resolution, reproducible and customisable models (Tang et al., 2021; Yue et al., 2020). Galpayage Dona et al. demonstrated the use of digital light processing (DLP)-based bioprinting to encapsulated human AC within a vascular lumen surrounded by PC and primary human BMEC, successfully generating a perfusable microvascular network that replicated key BBB features (Galpayage Dona et al., 2023). While microfluidics currently dominates the field, 3D bioprinting offers superior architectural control and scalability, making it a promising platform for next-generation BBB models.
2 3D bioprinting strategies for BBB modelling
3D bioprinting utilises computer aided design models to fabricate precise 3D structures. These models can be developed from medical imaging data such as radiological images, allowing for the recreation of anatomically accurate tissue architectures. When combined with chemical crosslinking, 3D bioprinting can generate high-resolution, multicellular structures that closely mimic native tissue environments (Potjewyd et al., 2021). Importantly, this technique enables reproducible and consistent manufacturing of in vitro models (Jagtiani et al., 2022), allowing for better standardization and comparability across studies.
2.1 Bioprinting techniques for BBB fabrication
Three major categories of 3D bioprinting technologies are commonly employed in tissue engineering applications: inkjet-based, extrusion-based and light-assisted printing (LAP) methods (Cho et al., 2019) (Figure 4). Inkjet-based bioprinting involves the deposition of controlled volumes of bioink at predefined locations, either through thermal inkjet bioprinting or piezoelectric inkjet bioprinting, which differ in how they overcome surface tension to eject bioink droplets from the nozzle. Although inkjet bioprinting allows fabrication of complex tissue constructs with different compositions and is both affordable and versatile, its use in BBB modelling is limited by difficulties in generating porous, tissue-like constructs and the requirement for low-viscosity bioinks, which restricts material choices (Gudapati et al., 2016).
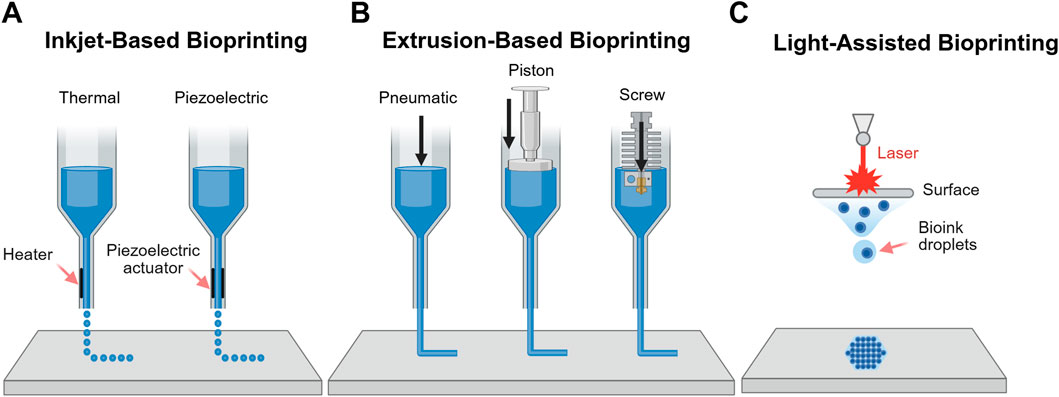
Figure 4. Schematic representation of 3D bioprinting methods. (A) Inkjet-based bioprinting which operates via thermal or piezoelectric mechanisms (B) Extrusion-based bioprinting controlled via pneumatic, piston or screw systems; and (C) LAP.
Extrusion-based bioprinting deposits continuous filaments of biomaterial through a nozzle, controlled pneumatically or mechanically. This approach accommodates a broader range of bioink viscosities and supports very high cell densities. However, it is limited by lower resolution compared to other methods, the risk of nozzle clogging and reduced cell viability due to shear stress during extrusion (Hölzl et al., 2016). Notably, co-axial extrusion enables the fabrication of hollow fibres that mimic capillary geometry, making it particularly promising for modelling BBB (Mohan TS et al., 2022).
LAP methods, such as DLP and two-photon polymerisation techniques, offer precise control over material properties and high-resolution printing (Galpayage Dona et al., 2023). As these methods are nozzle-free, they eliminate shear stress on cells during printing, preserving cell viability (Hölzl et al., 2016). Although there are concerns over cytotoxicity from photo-crosslinking (Mironi-Harpaz et al., 2012; parhi, 2017), multiple studies have shown that these effects are minimal, with no significant impact on cell viability (Galpayage Dona et al., 2023; Haring et al., 2019). Among the available technologies, LAP methods are currently the most widely used for BBB bioprinting due to their superior resolution and precision.
2.2 Bioink development and optimization
Selecting an appropriate bioink is crucial in 3D bioprinting to recapitulate the BBB complex architecture and function. Ideal bioinks must fulfil criteria such as biocompatibility, printability, mechanical integrity, and the ability to support BBB-specific cellular functions. Bioinks are typically categorized as either natural or synthetic.
Natural bioinks, such as HA, collagen, gelatin, alginate, and Matrigel, provide intrinsic biological cues but often lack mechanical robustness. HA supports cell migration and proliferation (Potjewyd et al., 2018) but exhibits poor mechanical strength, requiring combination with other polymers to enhance structural stability and printability (Tang et al., 2021). Collagen, particularly type IV, is a native component of the BBB ECM, offering high bioactivity through Arginine-Glycine-Aspartic (RGD) motifs (Hölzl et al., 2016; Tang et al., 2021). However, its slow gelation and low stiffness restrict independent use, requiring reinforcement with additional agents (Potjewyd et al., 2018). Gelatin, a hydrolysed form of collagen, retains bioactive domains (Asim et al., 2023) but lacks photo-crosslinkable groups. This limitation can be overcome by chemical modification (e.g., methacrylamide or thiol-ene functionalisation), which enhances print fidelity and reproducibility (Dobos et al., 2021). Gelatin also enables modular designs that permit post-printing dissolution and tissue remodelling, making it suitable for soft-tissue BBB constructs (Jagtiani et al., 2022). A notable application is its combination with fibrinogen for the coculture of BMEC, AC, and PC, which improved cell morphology compared with conventional 2D cultures (Tung et al., 2024). Alginate, derived from brown algae, undergoes rapid ionic crosslinking with calcium, allowing physiological gelation while maintaining cell viability. Although rigidity and porosity are calcium concentration dependent, no adverse effects on cell morphology or function were reported (Oh et al., 2023). Blending alginate with low viscosity collagen yields a compliant bioink suitable for mimicking native BBB tissue (Potjewyd et al., 2021). Lastly, Matrigel, a thermosensitive ECM extract rich in laminin and collagen IV, supports differentiation and barrier formation (Oh et al., 2023; Tang et al., 2021). Nonetheless, its murine origin and batch variability compromise reproducibility and limit clinical translation.
Synthetic bioinks such as polyethylene glycol (PEG) provide well-controlled mechanical properties and reproducible performance. When functionalised as PEG-diacrylate (PEGDA) or PEG-norbornene, PEG enables photo-crosslinking, allowing precise spatial patterning and reducing cell death during live-cell printing (Gudapati et al., 2016; Paone et al., 2024). However, PEG lacks inherent bioactivity and therefore requires modification with ECM-derived peptides to promote cell-material interactions. Functional motifs such as RGD and Isoleucine-Lysine-Valine-Alanine-Valine (IKVAV) facilitate cell adhesion and spreading (Matthiesen et al., 2023), while Histidine-Alanine-Valine-Aspartate-Isoleucine (HAVDI) supports endothelial monolayer formation and TJ assembly, as evidenced by increased localization of zonula occludens-1 (ZO-1) even in the absence of flow (Paone et al., 2024). Incorporating these bioactive peptides into PEG-based inks preserves mechanical stability while substantially enhancing biological functionality.
As summarized in Table 2, natural bioinks excel in cell-matrix interactions but often require mechanical reinforcement, whereas synthetic bioinks are structurally tuneable yet need biofunctionalisation for physiological relevance. Current challenges include improving reproducibility, vascularisation, long-term stability, and scalability of the 3D bioinks. To address these issues, hybrid bioinks combining natural and synthetic components, enhanced with cell-instructive peptides, is a promising strategy. Future directions should prioritise advanced crosslinking strategies, peptide-based customization and biofunctionalization, standardized formulations, and validation in perfused, shear-responsive systems to better model BBB physiology and improve translational relevance.
2.3 Geometry design considerations
In addition to the choice of bioinks, the geometrical fidelity is critical for BBB modelling. Ideally, the model should closely replicate the dimensions and structure of microvascular capillaries, which vary in diameter according to the anatomical location of the microvessel (DeStefano et al., 2018). Accurately mimicking these capillary dimensions is essential for reproducing physiological shear stress and cellular organisation. However, achieving such high-resolution features with direct bioprinting methods can be technically challenging. In contrast, high-resolution bioprinting methods, such as the biomimetic model developed by Marino A. et al. Achieved resolutions similar to that of the in vivo dimensions using two photon lithography (TPL) (Marino et al., 2018).
Beyond resolution, the model’s architectural design should accurately reflect the cylindrical geometry of the native microvessel. To achieve this, indirect bioprinting methods which incorporates a removable sacrificial biomaterial have been used in order to create cylindrical channels that can be subsequently seeded with EC, thereby ensuring a physiologically relevant structure (Potjewyd et al., 2018). This method allows for the fabrication of perfusable, physiologically relevant constructs that support cellular alignment and barrier formation under flow conditions.
3 Functional assessment of BBB models
Rigorous functional assessment is essential to validate in vitro BBB models and confirm they capture the physiological and pathological features of the native BBB. As illustrated in Figure 5, the functionality of in vitro BBB models can be evaluated based on integrity, permeability, cellular function and key molecular expression (DeStefano et al., 2018).
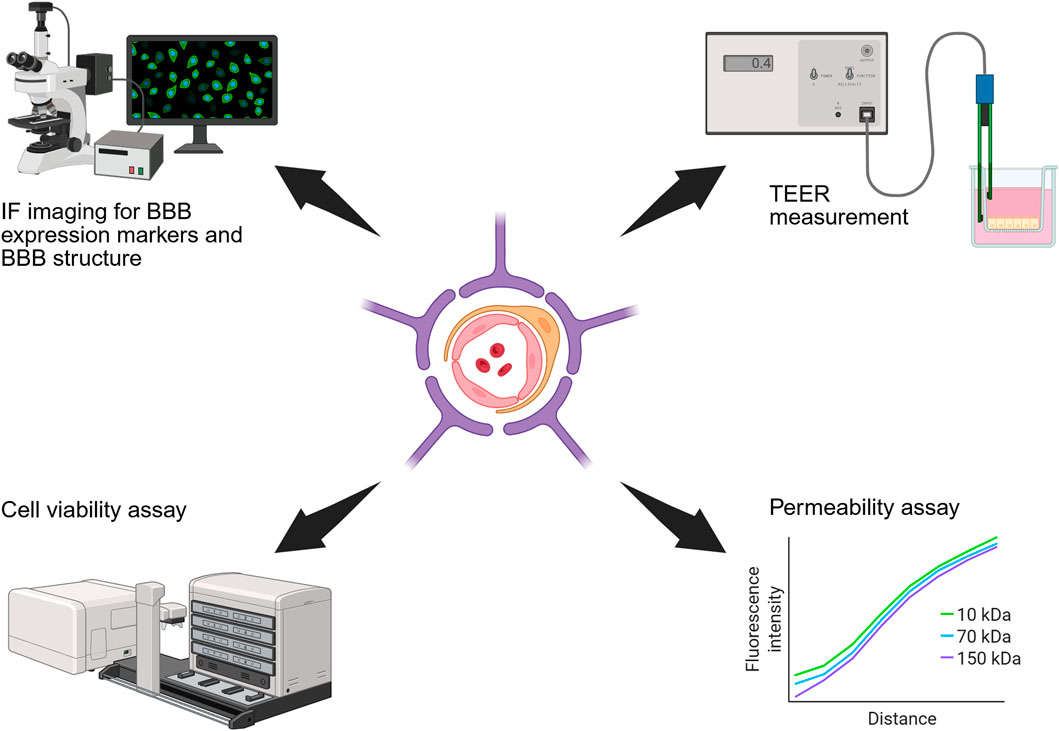
Figure 5. Schematic diagram of methods for functional assessment of BBB models. These include IF staining for BBB specific markers, TEER measurement, cell viability assay, and permeability assays.
BBB integrity is commonly assessed by immunofluorescence (IF) staining of TJ proteins (ZO-1, claudin-5, JAMs) and efflux transporters (e.g., P-glycoprotein) which are indicative of barrier formation (Langen et al., 2019). TEER remains the gold standard for non-destructive, real-time assessment of barrier tightness, although electrode placement and opacity can introduce variability (Srinivasan et al., 2015; Wei et al., 2023). It should be noted that TEER is influenced by the applied voltage and does not provide information regarding the transcellular transport of charged compounds (Hajal et al., 2021). Barrier permeability to solutes and overall barrier tightness is typically evaluated via tracer diffusion assays (e.g., FITC-dextran) (Bednarek et al., 2022), while live/dead imaging confirms cell viability and morphology is consistent with native BBB architecture (Bikmulina et al., 2022). Achieving native-like cellular morphologies is key to developing an accurate BBB model, as it reflects successful recapitulation of the physiological environment.
4 Applications of 3D bioprinting in modelling BBB in NDD research
The aetiologies of NDD involve intricate cross-talk between dysfunctional BMEC, PC, AC and neurons. 3D bioprinting, which allows precise, spatially controlled deposition of bioinks and multiple cell types, facilitates the creation of these complex cellular ecosystems, making it highly valuable in NDD research. Moreover, the incorporation of induced pluripotent stem cells (iPSCs) allows for the generation of humanised and patient-specific BBB models, thereby eliminating interspecies differences (Brown et al., 2019; Ito K.et al., 2011) and enabling personalised investigations into NDD pathophysiology and therapeutic response (Pérez-López et al., 2023).
Techniques such as TPL and DLP have been used to construct microvascular structures that mimic the native BBB microenvironment. Marino and colleagues employed a 3D BBB model by incorporating bEND.3 EC and U87 glioblastoma cells within microtubes of approximately 10 µm in diameter using the TPL technique (Marino et al., 2018). The bEnd.3 cells efficiently covered the tubular structures and the model demonstrated key BBB features, including TJ maturation (confirmed by ZO-1 IF) and barrier integrity (assessed via dextran diffusion), thus providing a powerful platform for high-throughput drug screening across the BBB. However, the absence of AC and PC in this biohybrid system resulted in a TEER of 75 ± 2 Ω cm2, substantially lower than in vivo values, limiting its suitability for studying NDD.
To address this limitation, Galpayage Dona et al. developed a more comprehensive model using DLP bioprinting that incorporated all major BBB cell types, AC, PC, and EC (Galpayage Dona et al., 2023). In this model, vascular structures were continuously perfused to activate mechanotransduction pathways and promote maturation. Treatment with Tumor necrosis factor alpha (TNF-α), known to decrease barrier tightness, significantly increased dextran leakage, confirming the model’s responsiveness to neuroinflammation, a common hallmark of NDD. To better simulate neuroinflammation, pro-inflammatory cytokines (e.g., TNF-α, IL-1β) or lipopolysaccharide have been introduced into the vascular lumen of bioprinted BBB models, enabling real-time assessment of barrier breakdown, upregulation of adhesion molecules (e.g., vascular cell adhesion molecule-1 (VCAM-1)), and immune cell adhesion and transmigration (Knox et al., 2022; Wei et al., 2023).
It is worth to notice that interleukin-6 (IL-6), elevated in AD and linked to BBB disruption (Lyra et al., 2021; Wu et al., 2015), has not yet been employed in 3D bioprinted BBB studies. IL-6, released by activated AC, triggers signal transducer and activator of transcription 3 (STAT3) pathways in AC and EC, inducing matrix metalloproteinase-9 (MMP-9) and vascular endothelial growth factor (VEGF) expression, leading to degradation of TJ proteins such as claudin-5, occludin, and ZO-1, thereby increasing BBB permeability (Gryka-Marton et al., 2025; Hu et al., 2025; Rose-John, 2017). This pathway is strongly associated with vascular dysfunction (Rose-John, 2017; Yang et al., 2022). Hence, targeting IL-6/STAT3 signalling to restore BBB function in diseased AC and EC may offer an effective strategy for developing novel therapeutics against NDD.
Beyond investigating inflammatory effects, 3D bioprinted BBB model also hold promise for evaluating the impact of natural compounds and virus infections in NDD pathogenesis (Abdelsalam et al., 2023; Kumar et al., 2018; Yousif et al., 2021). Similarly, 3D bioprinted models can be exposed to Aβ-induced toxicity to simulate AD (Yue et al., 2020) or subjected to oxygen-glucose deprivation to mimic ischemic stroke, a major risk factor for vascular dementia and other NDD, allowing studies on oxidative stress and reperfusion injury on BBB in a human context (Cho et al., 2015).
To further enhance DLP-printed model fidelity and matrix-cell interactions, Paone et al. created a tuneable, perfusable BBB model using DLP-printed PEG-norbornene hydrogels functionalised with HAVDI/IKVAV peptides, which promoted endothelial adhesion and TJ formation, as confirmed by ZO-1 staining, dextran permeability assays, and live/dead cell assays (Paone et al., 2024). Nonetheless, the current limitation for DLP-printed BBB model is the low resolution of polymerized layers, preventing is achievement of capillary-scale lumens.
Genetic mutations have also been incorporated into 3D bioprinting approaches to establish NDD models that exhibiting disease hallmarks. By transducing amyloid precursor protein (APP) genes with familial AD mutations to neural stem cells (NSC), Zhang and colleagues used 3D bioprinting technology to create a coaxial core-shell structure comprising a high-density cell suspension and Matrigel in the core, surrounded by alginate in the shell (Yi Zhang et al., 2022). This 3D printed AD model displayed superior self-assembly, extended cell survival, more complex metabolic activity, and differentiation rich in Aβ, highlighting 3D bioprinting as a promising tool for studying AD pathology and developing therapeutics. More recently, Royse et al. used stereolithography to bioprint a BBB model incorporating all key cell types expressing exogenous low-density lipoprotein receptor related protein 1 (LRP1) for studies of Aβ clearance, enabling mechanistic and pharmacological investigations modelling AD in NDD contexts (Royse et al., 2024).
Collectively, these studies demonstrate that 3D bioprinting enables the generation of biomimetic, multicellular, and perfusable BBB models that recapitulate key pathological features of NDD (Table 3), making them suited for advancing our understanding of BBB dysfunction, high-throughput drug screening and developing effective novel therapeutics for NDD.
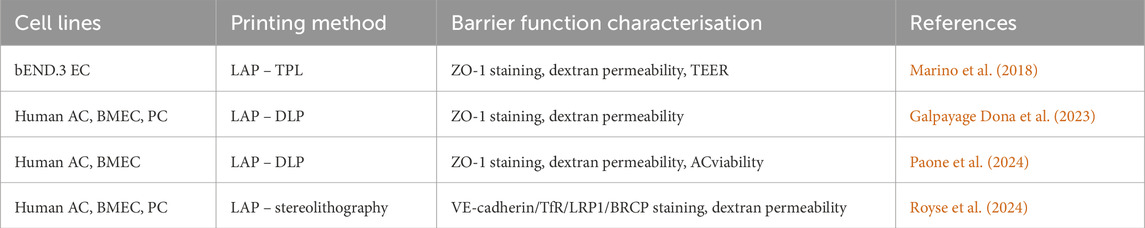
Table 3. Compilation of 3D bioprinted BBB models applied in NDD studies. VE-cadherin: vascular endothelial cadherin; BCRP: breast cancer resistance protein; TfR: transferrin receptor.
5 Conclusion and future perspectives
3D bioprinting enables precise spatial control in BBB models, creating perfusable, physiologically relevant structures through layer-by-layer deposition of biomaterials. By integrating multiple BBB cell types with brain-specific ECM components under digital design, it offers a reproducible platform for in vitro studies. However, challenges remain, including the lack of bioinks that mimic brain ECM, limited sub-capillary resolution, and immature tissue phenotypes.
Future progress will likely stem from combining bioprinted vascular networks with microfluidic systems and incorporating iPSC-derived cells into dynamic platforms for higher-throughput screening. Advances in BBB-specific bioinks and high-resolution printing will be key to producing reproducible, human-relevant BBB constructs for NDD modelling and CNS drug discovery.
Author contributions
ST: Data curation, Formal Analysis, Validation, Writing – original draft, Writing – review and editing. LQ: Conceptualization, Data curation, Project administration, Supervision, Validation, Writing – original draft, Writing – review and editing. JL: Writing – review and editing. LZ: Funding acquisition, Resources, Supervision, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was funded by NMRC-OFIRG24jul-0093.
Acknowledgments
The authors thank the National Neuroscience Institute (NNI) Academic Research Achievement Programme (NARA) for institutional support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelsalam, S. A., Renu, K., Zahra, H. A., Abdallah, B. M., Ali, E. M., Veeraraghavan, V. P., et al. (2023). Polyphenols mediate neuroprotection in cerebral ischemic Stroke-An update. Nutrients 15 (5), 1107. doi:10.3390/nu15051107
Asim, S., Tabish, T. A., Liaqat, U., Ozbolat, I. T., and Rizwan, M. (2023). Advances in gelatin bioinks to optimize bioprinted cell functions. Adv. Healthc. Mater. 12 (17), e2203148. doi:10.1002/adhm.202203148
Badawi, A. H., Mohamad, N. A., Stanslas, J., Kirby, B. P., Neela, V. K., Ramasamy, R., et al. (2024). In vitro blood-brain barrier models for neuroinfectious diseases: a narrative review. Curr. Neuropharmacol. 22 (8), 1344–1373. doi:10.2174/1570159X22666231207114346
Bednarek, R. (2022). In vitro methods for measuring the permeability of cell monolayers. Methods Protoc. 5 (1), 17. doi:10.3390/mps5010017
Bell, R. D., Winkler, E. A., Sagare, A. P., Singh, I., LaRue, B., Deane, R., et al. (2010). Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron (Cambridge, Mass.) 68 (3), 409–427. doi:10.1016/j.neuron.2010.09.043
Bikmulina, P., Kosheleva, N., Efremov, Y., Antoshin, A., Heydari, Z., Kapustina, V., et al. (2022). 3D or not 3D: a guide to assess cell viability in 3D cell systems. Soft Matter 18 (11), 2222–2233. doi:10.1039/D2SM00018K
Bolden, C. T., Skibber, M. A., Olson, S. D., Zamorano Rojas, M., Milewicz, S., Gill, B. S., et al. (2023). Validation and characterization of a novel blood–brain barrier platform for investigating traumatic brain injury. Sci. Rep. 13 (1), 16150. doi:10.1038/s41598-023-43214-7
Brown, T. D., Nowak, M., Bayles, A. V., Prabhakarpandian, B., Karande, P., Lahann, J., et al. (2019). A microfluidic model of human brain (μHuB) for assessment of blood brain barrier. Bioeng. and Transl. Med. 4 (2), e10126. doi:10.1002/btm2.10126
Cho, H., Seo, J. H., Wong, K. H. K., Terasaki, Y., Park, J., Bong, K., et al. (2015). Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology. Sci. Rep. 5 (1), 15222. doi:10.1038/srep15222
Cho, D.-W., Kim, B. S., Jang, J., Gao, G., Han, W., and Singh, N. K. (2019). 3D bioprinting: modeling in vitro tissues and organs using tissue-specific bioinks, 1st 2019. doi:10.1007/978-3-030-32222-9
Crone, C., and Olesen, S. P. (1982). Electrical resistance of brain microvascular endothelium. Brain Res. 241 (1), 49–55. doi:10.1016/0006-8993(82)91227-6
DeStefano, J. G., Jamieson, J. J., Linville, R. M., and Searson, P. C. (2018). Benchmarking in vitro tissue-engineered blood-brain barrier models. Fluids Barriers CNS 15 (1), 32. doi:10.1186/s12987-018-0117-2
Dobos, A., Gantner, F., Markovic, M., Van Hoorick, J., Tytgat, L., Van Vlierberghe, S., et al. (2021). On-chip high-definition bioprinting of microvascular structures. Biofabrication 13 (1), 015016. doi:10.1088/1758-5090/abb063
Galpayage Dona, K. N. U., Ramirez, S. H., and Andrews, A. M. (2023). A next-generation 3D tissue-engineered model of the human brain microvasculature to Study the blood-brain barrier. Bioeng. (Basel) 10 (7), 817. doi:10.3390/bioengineering10070817
Gryka-Marton, M., Grabowska, A. D., and Szukiewicz, D. (2025). Breaking the barrier: the role of proinflammatory cytokines in BBB dysfunction. Int. J. Mol. Sci. 26 (8), 3532. doi:10.3390/ijms26083532
Gudapati, H., Dey, M., and Ozbolat, I. (2016). A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials 102, 20–42. doi:10.1016/j.biomaterials.2016.06.012
Hajal, C., Le Roi, B., Kamm, R. D., and Maoz, B. M. (2021). Biology and models of the blood-brain barrier. Annu. Rev. Biomed. Eng. 23 (1), 359–384. doi:10.1146/annurev-bioeng-082120-042814
Haring, A. P., Thompson, E. G., Tong, Y., Laheri, S., Cesewski, E., Sontheimer, H., et al. (2019). Process- and bio-inspired hydrogels for 3D bioprinting of soft free-standing neural and glial tissues. Biofabrication 11 (2), 025009. doi:10.1088/1758-5090/ab02c9
Helman, A. M., and Murphy, M. P. (2016). Vascular cognitive impairment: modeling a critical neurologic disease in vitro and in vivo. Biochimica Biophysica Acta. Mol. Basis Dis. 1862 (5), 975–982. doi:10.1016/j.bbadis.2015.12.009
Hölzl, K., Lin, S., Tytgat, L., Van Vlierberghe, S., Gu, L., and Ovsianikov, A. (2016). Bioink properties before, during and after 3D bioprinting. Biofabrication 8 (3), 032002. doi:10.1088/1758-5090/8/3/032002
Hu, C., Li, H., Cui, J., Li, Y., Zhang, F., Li, H., et al. (2025). Integrative analysis identifies IL-6/JUN/MMP-9 pathway destroyed blood-brain-barrier in autism mice via machine learning and bioinformatic analysis. Transl. Psychiatry 15 (1), 239. doi:10.1038/s41398-025-03452-x
Hudson, N., and Matthew, C. (2021). Tight junctions of the neurovascular unit. Front. Mol. Neurosci. 14, 752781. doi:10.3389/fnmol.2021.752781
Ito, K. U. Y., Ohtsuki, S., Aizawa, S., Kawakami, H., Katsukura, Y., Kamiie, J., et al. (2011). Quantitative membrane protein expression at the blood-brain barrier of adult and younger cynomolgus monkeys. J. Pharm. Sci. 100 (9), 3939–3950. doi:10.1002/jps.22487
Jagtiani, E., Yeolekar, M., Naik, S., and Patravale, V. (2022). In vitro blood brain barrier models: an overview. J. Control. Release 343, 13–30. doi:10.1016/j.jconrel.2022.01.011
Jamieson, J. J., Searson, P. C., and Gerecht, S. (2017). Engineering the human blood-brain barrier in vitro. J. Biol. Eng. 11 (1), 37. doi:10.1186/s13036-017-0076-1
Jia, W., Martin, T. A., Zhang, G., and Jiang, W. G. (2013). Junctional adhesion molecules in cerebral endothelial tight junction and brain metastasis. Anticancer Res. 33 (6), 2353–2359.
Kadry, H., Noorani, B., and Cucullo, L. (2020). A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS, 17, 69(1). doi:10.1186/s12987-020-00230-3
Knox, E. G., Aburto, M. R., Clarke, G., Cryan, J. F., and O'Driscoll, C. M. (2022). The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 27 (6), 2659–2673. doi:10.1038/s41380-022-01511-z
Krause, G., Winkler, L., Mueller, S. L., Haseloff, R. F., Piontek, J., and Blasig, I. E. (2008). Structure and function of claudins. Biochimica Biophysica Acta. Biomembr. 1778 (3), 631–645. doi:10.1016/j.bbamem.2007.10.018
Kumar, B., Asha, K., Khanna, M., Ronsard, L., Meseko, C. A., and Sanicas, M. (2018). The emerging influenza virus threat: status and new prospects for its therapy and control. Arch. Virol. 163 (4), 831–844. doi:10.1007/s00705-018-3708-y
Langen, U. H., Ayloo, S., and Gu, C. (2019). Development and cell biology of the blood-brain barrier. Annu. Rev. Cell Dev. Biol. 35 (1), 591–613. doi:10.1146/annurev-cellbio-100617-062608
Liu, L.-Y., Lu, Y., Shen, L., Li, C.-B., Yu, J.-T., Yuan, C. R., et al. (2022). Prevalence, risk and protective factors for mild cognitive impairment in a population-based study of Singaporean elderly. J. Psychiatric Res. 145 (1), 111–117. doi:10.1016/j.jpsychires.2021.11.041
Lochhead, J. J., McCaffrey, G., Quigley, C. E., Finch, J., DeMarco, K. M., Nametz, N., et al. (2010). Oxidative stress increases blood–brain barrier permeability and induces alterations in occludin during hypoxia–reoxygenation. J. Cereb. Blood Flow Metabolism 30 (9), 1625–1636. doi:10.1038/jcbfm.2010.29
Luissint, A.-C., Artus, C., Glacial, F., Ganeshamoorthy, K., and Couraud, P.-O. (2012). Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 9 (1), 23. doi:10.1186/2045-8118-9-23
Lyra, E. S. N. M., Goncalves, R. A., Pascoal, T. A., Lima-Filho, R. A. S., Resende, E. P. F., Vieira, E. L. M., et al. (2021). Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer's disease. Transl. Psychiatry 11 (1), 251. doi:10.1038/s41398-021-01349-z
Marino, A., Tricinci, O., Battaglini, M., Filippeschi, C., Mattoli, V., Sinibaldi, E., et al. (2018). A 3D real-scale, biomimetic, and biohybrid model of the blood-brain barrier fabricated through two-photon lithography. Small 14 (6), 1702959. doi:10.1002/smll.201702959
Matthiesen, I., Jury, M., Rasti Boroojeni, F., Ludwig, S. L., Holzreuter, M., Buchmann, S., et al. (2023). Astrocyte 3D culture and bioprinting using peptide functionalized hyaluronan hydrogels. Sci. Technol. Adv. Mater. 24 (1), 2165871. doi:10.1080/14686996.2023.2165871
Mawuenyega, K. G., Sigurdson, W., Ovod, V., Munsell, L., Kasten, T., Morris, J. C., et al. (2010). Decreased clearance of CNS β-Amyloid in alzheimer's disease. Sci. Am. Assoc. Adv. Sci. 330 (6012), 1774. doi:10.1126/science.1197623
Mironi-Harpaz, I., Wang, D. Y., Venkatraman, S., and Seliktar, D. (2012). Photopolymerization of cell-encapsulating hydrogels: crosslinking efficiency versus cytotoxicity. Acta Biomater. 8 (5), 1838–1848. doi:10.1016/j.actbio.2011.12.034
Mohan, T. S. D. P., Nesaei, S., Ozbolat, V., and Ozbolat, I. T. (2022). 3D coaxial bioprinting: process mechanisms, bioinks and applications. Prog. Biomed. Eng. (Bristol) 4 (2), 022003. doi:10.1088/2516-1091/ac631c
Oh, H., Kang, M., Bae, E., Jung, Y., Cho, J., Poirier, J., et al. (2023). Fabrication of hydrogel microchannels using aqueous two-phase printing for 3D blood brain barrier. Biochip J. 17 (3), 369–383. doi:10.1007/s13206-023-00110-6
Pandey, P. K., Sharma, A. K., and Gupta, U. (2016). Blood brain barrier: an overview on strategies in drug delivery, realistic in vitro modeling and in vivo live tracking. Tissue Barriers 4 (1), e1129476. doi:10.1080/21688370.2015.1129476
Paone, L. S., Benmassaoud, M. M., Curran, A., Vega, S. L., and Galie, P. A. (2024). A 3D-printed blood-brain barrier model with tunable topology and cell-matrix interactions. Biofabrication 16 (1), 015005. doi:10.1088/1758-5090/ad0260
Pekny, M., and Pekna, M. (2016). Reactive gliosis in the pathogenesis of CNS diseases. Biochimica Biophysica Acta. Mol. Basis Dis. 1862 (3), 483–491. doi:10.1016/j.bbadis.2015.11.014
Pérez-López, A., Torres-Suárez, A. I., Martín-Sabroso, C., and Aparicio-Blanco, J. (2023). An overview of in vitro 3D models of the blood-brain barrier as a tool to predict the in vivo permeability of nanomedicines. Adv. Drug Deliv. Rev. 196, 114816. doi:10.1016/j.addr.2023.114816
Potjewyd, G. K. K., and Hooper, N. M. (2021). 3D hydrogel models of the neurovascular unit to investigate blood-brain barrier dysfunction. Neuronal Signal 5 (4). doi:10.1042/NS20210027
Potjewyd, G., Moxon, S., Wang, T., Domingos, M., and Hooper, N. M. (2018). Tissue engineering 3D neurovascular units: a biomaterials and bioprinting perspective. Trends Biotechnol. 36 (4), 457–472. doi:10.1016/j.tibtech.2018.01.003
Reed, M. J., Damodarasamy, M., and Banks, W. A. (2019). The extracellular matrix of the blood–brain barrier: structural and functional roles in health, aging, and Alzheimer’s disease. Tissue Barriers 7 (4), 1651157. doi:10.1080/21688370.2019.1651157
Reichel, A., Begley, D. J., and Abbott, N. J. (2003). An overview of in vitro techniques for blood-brain barrier studies. Methods Mol. Med. 89, 307–324. doi:10.1385/1-59259-419-0:307
Rose-John, S. (2017). The soluble interleukin 6 receptor: advanced therapeutic options in inflammation. Clin. Pharmacol. Ther. 102 (4), 591–598. doi:10.1002/cpt.782
Royse, M. K., Fowler, M., Mai, A. K., He, Y., Durante, M. R., Buist, N., et al. (2024). Development of a 3D printed perfusable in vitro blood–brain barrier model for use as a scalable screening tool. Biomaterials Sci. 12 (17), 4363–4375. doi:10.1039/D4BM00663A
Rubin, L. L., and Staddon, J. M. (1999). The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 22 (1), 11–28. doi:10.1146/annurev.neuro.22.1.11
Segarra, M., Aburto, M. R., and Acker-Palmer, A. (2021). Blood–brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. Regul. Ed 44 (5), 393–405. doi:10.1016/j.tins.2020.12.002
Srinivasan, B., Kolli, A. R., Esch, M. B., Abaci, H. E., Shuler, M. L., and Hickman, J. J. (2015). TEER measurement techniques for in vitro barrier model systems. J. Laboratory Automation 20 (2), 107–126. doi:10.1177/2211068214561025
Stamatovic, S. M., Johnson, A. M., Keep, R. F., and Andjelkovic, A. V. (2016). Junctional proteins of the blood-brain barrier: new insights into function and dysfunction. Tissue Barriers 4 (1), e1154641. doi:10.1080/21688370.2016.1154641
Stefanis, L. (2012). α-Synuclein in parkinson's disease. Cold Spring Harb. Perspect. Med. 2 (2), a009399. doi:10.1101/cshperspect.a009399
Stone, N. L., England, T. J., and O’Sullivan, S. E. (2019). A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells [Original Research]. Front. Cell. Neurosci., 13–2019. doi:10.3389/fncel.2019.00230
Sweeney, M. D., Ayyadurai, S., and Zlokovic, B. V. (2016). Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 19 (6), 771–783. doi:10.1038/nn.4288
Sweeney, M. D., Sagare, A. P., and Zlokovic, B. V. (2018). Blood–brain barrier breakdown in alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14 (3), 133–150. doi:10.1038/nrneurol.2017.188
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R., and Zlokovic, B. V. (2019). Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 99 (1), 21–78. doi:10.1152/physrev.00050.2017
Takata, F., Nakagawa, S., Matsumoto, J., and Dohgu, S. (2021). Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front. Cell. Neurosci. 15 (1), 661838. doi:10.3389/fncel.2021.661838
Tang, M., Rich, J. N., and Chen, S. (2021). Biomaterials and 3D bioprinting strategies to model glioblastoma and the blood–brain barrier. Adv. Mater. Weinh. 33 (5), e2004776. doi:10.1002/adma.202004776
Tran, M., Heo, C., Lee, L. P., and Cho, H. (2022). Human mini-blood-brain barrier models for biomedical neuroscience research: a review. Biomaterials Res. 26 (82), 82. doi:10.1186/s40824-022-00332-z
Tung, Y. T., Chen, Y. C., Derr, K., Wilson, K., Song, M. J., and Ferrer, M. (2024). A 3D bioprinted human neurovascular unit model of glioblastoma tumor growth. Adv. Healthc. Mater. 13 (15), e2302831. doi:10.1002/adhm.202302831
Vetter, J., Palagi, I., Waisman, A., and Blaeser, A. (2025). Recent advances in blood-brain barrier-on-a-chip models. Acta Biomater. 197, 1–28. doi:10.1016/j.actbio.2025.03.041
Wang, X., Xu, B., Xiang, M., Yang, X., Liu, Y., Liu, X., et al. (2020). Advances on fluid shear stress regulating blood-brain barrier. Microvasc. Res. 128, 103930. doi:10.1016/j.mvr.2019.103930
Wang, S., Bai, L., Hu, X., Yao, S., Hao, Z., Zhou, J. J., et al. (2023). 3D bioprinting of neurovascular tissue modeling with collagen-based low-viscosity composites. Adv. Healthc. Mater. 12 (25), e2300004. doi:10.1002/adhm.202300004
Wei, W., Cardes, F., Hierlemann, A., and Modena, M. M. (2023). 3D in vitro blood-brain-barrier model for investigating barrier insults. Adv. Sci. 10 (11), e2205752. doi:10.1002/advs.202205752
Winkelman, M. A., Kim, D. Y., Kakarla, S., Grath, A., Silvia, N., and Dai, G. (2021). Interstitial flow enhances the formation, connectivity, and function of 3D brain microvascular networks generated within a microfluidic device. Lab a Chip 22 (1), 170–192. doi:10.1039/D1LC00605C
Wolff, A., Antfolk, M., Brodin, B., Tenje, M., of Medicine, F., fakulteten, M., et al. (2015). In vitro blood–brain barrier models—an overview of established models and new microfluidic approaches. J. Pharm. Sci. 104 (9), 2727–2746. doi:10.1002/jps.24329
Wong, A. D., Ye, M., Levy, A. F., Rothstein, J. D., Bergles, D. E., and Searson, P. C. (2013). The blood-brain barrier: an engineering perspective. Front. Neuroengineering 6 (7), 7. doi:10.3389/fneng.2013.00007
Wu, Y. Y., Hsu, J. L., Wang, H. C., Wu, S. J., Hong, C. J., and Cheng, I. H. (2015). Alterations of the neuroinflammatory markers IL-6 and TRAIL in alzheimer's disease. Dement. Geriatr. Cogn. Dis. Extra 5 (3), 424–434. doi:10.1159/000439214
Yan, L., Moriarty, R. A., and Stroka, K. M. (2011). Recent progress and new challenges in modeling of human pluripotent stem cell-derived blood-brain barrier. Theranostics 11 (20), 10148–10170. doi:10.7150/thno.63195
Yang, J., Ran, M., Li, H., Lin, Y., Ma, K., Yang, Y., et al. (2022). New insight into neurological degeneration: inflammatory cytokines and blood-brain barrier. Front. Mol. Neurosci. 15, 1013933. doi:10.3389/fnmol.2022.1013933
Yi Zhang, H. C., Long, X., and Xu, T. (2022). Three-dimensional-engineered bioprinted in vitro human neural stem cell self-assembling culture model constructs of Alzheimer’s disease. Bioact. Mater. 11, 192–205. doi:10.1016/j.bioactmat.2021.09.023
Yousif, A. S., Ronsard, L., Shah, P., Omatsu, T., Sangesland, M., Bracamonte Moreno, T., et al. (2021). The persistence of interleukin-6 is regulated by a blood buffer system derived from dendritic cells. Immunity 54 (2), 235–246.e5. doi:10.1016/j.immuni.2020.12.001
Keywords: blood-brain barrier, 3D bioprinting, neurodegenerative diseases, bioink, disease modeling
Citation: Tan S, Qiu L, Lee JWL and Zeng L (2025) Advances in 3D bioprinting for modeling the blood-brain barrier in neurodegenerative diseases. Front. Mol. Biosci. 12:1703403. doi: 10.3389/fmolb.2025.1703403
Received: 18 September 2025; Accepted: 31 October 2025;
Published: 13 November 2025.
Edited by:
Stefania Scalise, Università di Catanzaro, ItalyReviewed by:
Larance Ronsard, Ragon Institute, United StatesNitin Kamble, University of Cincinnati Medical Center, United States
Copyright © 2025 Tan, Qiu, Lee and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zeng, bGlfemVuZ0BubmkuY29tLnNn
†These authors have contributed equally to this work
 Shirleen Tan
Shirleen Tan Lifeng Qiu
Lifeng Qiu Jolene Wei Ling Lee
Jolene Wei Ling Lee Li Zeng
Li Zeng