Abstract
Acupuncture is a common complementary and alternative therapy around the world, but its mechanism remains still unclear. In the past decade, some studies indicated that transient receptor potential vanilloid (TRPV) channels play a great role in the response of acupuncture stimulation. In this article, we discussed the relationship between acupuncture and TRPV channels. Different from inhibitors and agonists, the regulation of acupuncture on TRPV channels is multi-targeted and biphasic control. Acupuncture stimulation shows significant modulation on TRPV1 and TRPV4 at the autonomic nervous system (ANS) including central and peripheral nervous systems. On the contrary, the abundant expression and functional participation of TRPV1 and TRPV4 were specific to acupuncture stimulation at acupoints. The enhancement or inhibition of TRPV channels at different anatomical levels will affect the therapeutic effect of acupuncture. In conclusion, TRPV channels help to understand the principle of acupuncture stimulation, and acupuncture also provides a potential approach to TRPV-related trials.
Introduction
Acupuncture originated in China over 3,000 years ago. In acupuncture theory, stimulation at certain areas, also known as acupoints, with needles could dynamically harmonize Yin-Yang and Qi to cure diseases (Kaptchuk, 2002). Nowadays, acupuncture is confined as an important complementary and alternative therapy all over the world (Rubin, 2019; Slomski, 2019). Some systematic reviews and meta-analysis have confirmed that acupuncture has encouraging effects on pain (He et al., 2020) and obesity (Kim et al., 2018).
Neurobiological mechanisms underlying acupuncture’s effectiveness have been widely discussed in modern research. Acupuncture is effective on the endocannabinoid system (Hu et al., 2017), purinergic signaling (Tang et al., 2016), and neuro-immune microenvironment (Gong et al., 2020). Current studies have shown that acupuncture plays a great role in the autonomic nervous system (ANS) (Shu et al., 2016). Liu et al. (2021) performed studies on acupuncture relieving inflammation, which showed that both vagal-adrenal axis and NPY-expressing sympathetic pathway (Liu et al., 2020) could be regulated by acupuncture stimulation at certain acupoints through specific autonomic pathways. It is worth mentioning that either anti-inflammatory or pro-inflammatory effects of acupuncture depend on the state of diseases. Although several biological correlates in ANS may explain the principle of acupuncture (Lim et al., 2016), the biological basis of stimulation at acupoints affecting physiology and pathology of internal organs remains unknown.
Since transient receptor potential vanilloid (TRPV) 1, which is sensitive to both heat and capsaicin, was first found in neurons in 1997 (Caterina et al., 1997), other five TRPV family members including TRPV2-6 have been identified in the next few years (Smith et al., 2002; Patapoutian et al., 2003; van de Graaf et al., 2003). Recent studies showed that TRPV channels are widely expressed in various excitable and non-excitable cell types in the human body. TRPV channels not only respond to thermosensation (Paricio-Montesinos et al., 2020) but also play a great role in the physiological or pathological processes of pain, inflammation, immunity, diabetes, and obesity (Jordt and Ehrlich, 2007; Samanta et al., 2018).
Physical stimulation such as pressure, vibration, pain, and temperature can be felt by sensory receptors at the peripheral nervous system, and the signal will be further passed to the central nervous system (CNS) through dorsal root ganglia (DRG). The operation of acupuncture produces pain, pressure, and vibration at the acupoints for neurobiological regulations. Hence, it is reasonable to assume that there is a specific relationship between acupuncture and TRPV channels. To the best of our knowledge, some studies have shown that acupuncture is closely associated with TRPV1 and TRPV4, which is elaborated in this review.
Transient receptor potential vanilloid channels
As sensory receptors, TRPV channels are sensitive to various tissue-damaging signals and have a strong link with signaling pathways such as phosphorylation of protein kinase A (PKA) and protein kinase C (PKC) (Por et al., 2013). Hence, TRPV channels are also considered as nociceptors (Satheesh et al., 2016). TRPV channels in the CNS play a great role not only in pain but also in neuropsychiatric disorders, such as depression, stress, and anxiety (Singh et al., 2019). It was found that all kinds of tissues and organs with TRPV channels expression contribute to a plethora of physiological or pathophysiological effects (Seebohm and Schreiber, 2021).
TRPV1 has been the most widely studied TRPV channel in the past decade. TRPV1 expresses in both neuronal cells, such as peripheral sensory neurons (C- and Aδ-fibers), DRG, trigeminal ganglia, and vagal ganglia (Cao et al., 2013), and non-neuronal cells, such as skin and muscles (Cavanaugh et al., 2011). After TRPV1 was knocked out, mice exhibited no vanilloid-evoked pain behavior and impaired nociception shown in inflammation pain mice models (Caterina et al., 2000). But it should be noted that TRPV1 agonists are also effective on neuropathic pain. Agonists could over-activate TRPV1 channels and lead to internalization and subsequent desensitization of afferent nerve endings or degeneration of neurons by Ca2+-induced neurotoxicity (Fischer et al., 2020).
Capsaicin, an important component in spiciness with a spicy sensation, could selectively block pain signals at primary afferent neurons targeting TRPV1 (Szolcsanyi, 2008). Besides capsaicin, TRPV1 could be activated by many physical and chemical stimuli, and activation pathways exist for specific stimuli (Yang and Zheng, 2017). The extracellular pore domain and transmembrane domains of TRPV1 could respond to different stimulation from physical and chemical inputs (Zheng, 2013). Many small molecules as potential analgesics aimed to inhibit TRPV1, but most of them failed in clinical trials due to severe side effects (Carnevale and Rohacs, 2016). Until now, only 8% capsaicin patch was approved by the European Union and the US Food and Drug Administration (FDA) for the treatment of postherpetic neuralgia, namely, peripheral neuropathic pain (Basith et al., 2016).
Similar to TRPV1, TRPV4 channels are broadly expressed in organs and tissues to participate in many physiological and pathophysiological processes. The therapeutic effect of TRPV4 antagonism on pain, gastrointestinal disorders, and respiratory diseases has been suggested through animal studies (Grace et al., 2017). GSK2798745, a potent and selective TRPV4 inhibitor, has been investigated in early phase clinical trials for heart failure (Brooks et al., 2019). To date, TRPV4 agonists could lead to unpredictable toxicity during systemic activation of TRPV4 and only suggested local delivery (Lawhorn et al., 2020).
Acupuncture stimulation
Acupuncture is a treatment that involves inserting needles at specific acupoints in the body. In traditional Chinese medicine theory, the key to the curative effect of acupuncture treatment lies in the human body’s response to acupuncture stimulation, including heaviness, numbness, soreness, and distension, which is also called “De Qi” (Mao et al., 2007). It is believed that acupuncture stimulation could activate mechanically sensitive pain fibers and various types of lesser-known deep tissue receptors (Zhao, 2008).
The analgesic effect of acupuncture has been studied for a long time. Brain imaging shows that acupuncture could alter activation patterns in brain areas against pain processing (Huang et al., 2012). Endorphins (Han, 2004), serotonin (Zhang Y. et al., 2012), and various neuromodulators and neurotransmitters in CNS and/or peripheral nervous system (PNS) can be regulated by acupuncture stimulation. A systematic review based on quantitative sensory testing (QST) has found that acupuncture significantly changed the sensory threshold and activated neuromodulation (Baeumler et al., 2014). A large number of preclinical trials have shown that the analgesic effect of electroacupuncture is optimistic, and the peripheral, spinal, and supraspinal mechanisms related to the activation of a variety of bioactive chemicals show more difference in health than that in pain conditions (Zhang et al., 2014).
Acupuncture affects transient receptor potential vanilloid channels
A total of 24 animal studies were included in this article, and acupuncture/electroacupuncture showed significantly beneficial effects. The regulation of acupuncture/electroacupuncture to TRPV channels differs in models (Table 1). To help readers understand the implementation methods of acupuncture, we listed the acupoints of the animal related to this article in Figure 1 (Jin et al., 2018).
TABLE 1
| Models | Acupuncture delivery | Acupoints | Target organs | Main results | References |
| Mice, Inflammatory pain models | MA | ST36 | Muscle at ST36 | TRPV1 and TRPV4 channels were abundantly expressed | Wu et al., 2014 |
| epimysium at ST36 | |||||
| Subcutaneous loose connective tissue at ST36 | |||||
| Neural tissue at ST36 | |||||
| Mice, Inflammatory pain models | EA, 2 Hz 1 mA | ST36 | DRG | TRPV1 channel overexpression was decreased | Liao et al., 2017 |
| SCDH | |||||
| Mice, Inflammatory pain models | EA, 2 Hz 1 mA | ST36 | DRG | TRPV1 channel overexpression was decreased | Yang et al., 2017 |
| Spinal cord | |||||
| Mice, Inflammatory pain models | EA, 2 Hz 1 mA | LI4 | Prefrontal cortex | TRPV1 channel overexpression was decreased | Yen et al., 2019 |
| Hypothalamus | |||||
| Periaqueductal gray | TRPV1 channel suppression was reversed | ||||
| Mice, Inflammatory pain models | EA, 2 Hz 1 mA | ST36 | Cerebellum lobules V, VIa and VII | TRPV1 channel overexpression was decreased | Inprasit and Lin, 2020 |
| Mice, Chronic pain and depression models | EA, 2 Hz 1 mA | ST36 | Cerebellum lobules VI, VII, VIII | TRPV1 channel inhibition was revised | Lottering and Lin, 2021 |
| Rats, Inflammatory pain models | EA, 2 Hz, 100 Hz, 2/100 Hz 0.5-1.0-1.5 mA | ST36 | L4-6 DRG neurons | TRPV1 channel overexpression was decreased high-frequency EA was more effective | Fang et al., 2018 |
| Mice, Fibromyalgia models | EA, 2 Hz 1 mA | ST36 | DRG neurons | TRPV1 channel overexpression was decreased | Lin et al., 2015 |
| Spinal cord | |||||
| TRPV4 channel overexpression was decreased | |||||
| Mice, Fibromyalgia models | EA, 2 Hz 1 mA | ST35 | Thalamus | TRPV1 channel overexpression was decreased | Hsu et al., 2020 |
| Amygdala | |||||
| Somatosensory cortex | |||||
| Rats, carcinoma cell inoculation to cancer pain models | EA, 2 Hz 1 mA | ST36 | DRG neurons | TRPV1 channel overexpression was decreased | Zhang Z. et al., 2012 |
| Mice, cold stress-induced nociception and depression models | EA, 2 Hz 1 mA | ST36 | Medial prefrontal cortex | TRPV1 channel suppression was reversed | Lin et al., 2020 |
| Hippocampus | TRPV1 channel overexpression was decreased | ||||
| Periaqueductal gray | |||||
| Amygdala | |||||
| Rats, high fat diet-induced obese models | EA, 10 Hz 1 mA | ST36 | Medulla regions | TRPV1 channel suppression was reversed | Ji et al., 2013 |
| Skin at ST36 | |||||
| Rats, paclitaxel-induced peripheral neuropathy models | EA, 2 Hz 0.5–1.5 mA | ST36 and BL60 | L4-6 DRG neurons | TRPV1 channel overexpression was decreased | Li et al., 2019 |
| Rats, MCAo models | EA, 2 Hz 2 mA | GV20 | Hippocampal CA1 areas | TRPV1 channel overexpression was decreased | Lin and Hsieh, 2010 |
| Rats, MCAo models | EA, 2/100 Hz 2 mA | GV20, BL23 and, SP6 | Hippocampal | TRPV1 channel overexpression was decreased | Long et al., 2019 |
| Mice | EA, 2 Hz 1 mA | ST36 | DRG neurons | TRPV1 channel was upregulated | Choowanthanapakorn et al., 2015 |
| Spinal cord | |||||
| Mice, motion sickness models | EA, 2 Hz 1 mA | PC6 | Thalamus | TRPV1 channel overexpression was decreased | Inprasit et al., 2018 |
| Hypothalamic | |||||
| Brain stem | |||||
| Rats | EA, 1 mA | BL40 | Subepidermal nerve fibers at BL40 | TRPV1 channel was upregulated | Abraham et al., 2011 |
| Mice | MA | ST36 | Peripheral DRG neurons | Components of the TRPV1-related signaling pathway was upregulated | Chen et al., 2018 |
| EA 2, 15, 50 Hz and 1 mA | Somatosensory cortex | ||||
| Rats | EA 2, 15 Hz and 1 mA | ST36 | Splenic CD4 + T cells | TRPV1 channel was upregulated | Chen et al., 2017 |
| Rats, gastric distension to cardiovascular reflexes models | MA | P5 and P6 | C7-8 DRG neurons | TRPV1 channel was upregulated | Guo et al., 2018 |
| EA, 2 Hz 0.3–0.5 mA |
Acupuncture affects TRPV channels.
MA, manual acupuncture; EA, electroacupuncture; MCAo, middle cerebral artery occlusion; DRG, dorsal root ganglion; SCDH, spinal cord dorsal horn.
FIGURE 1

Acupoints in murine. Acupuncture delivery is through the insertion of needles into the muscle at acupoints with 1–3 mm depth and triggering of a local reaction by means of manual manipulation or electrical stimulation. Sham acupuncture is usually performed in the tissue adjacent to the targeted acupoint without manual operation or electrode connection.
The relationship between acupuncture and TRPV channels involves the whole PNS, including sensory receptors and afferent nerves. Acupuncture could significantly increase the subepidermal nerve fibers with high expression of TRPV1 (Abraham et al., 2011). DRG neurons are believed to act as the bridge for acupuncture stimulation projecting to CNS. However, the effect of acupuncture on TRPV channels at DRG may be completely opposite in different models. In studies focused on diseases related to pain, the overexpression of TRPV1 channel in DRG neurons by drugs or surgery, which causes pain, would be reversed by acupuncture (Zhang Z. et al., 2012; Lin et al., 2015; Liao et al., 2017; Yang et al., 2017). But in other studies based on normal animals or obese models, acupuncture enhanced the expression of TRPV1 channels in DRG neurons (Ji et al., 2013; Choowanthanapakorn et al., 2015; Chen et al., 2018). Acupuncture at different acupoints would lead to TRPV channel changes in different DRG neurons. Stimulation at ST36 and BL40 is likely passed to L4-6 DRG neurons (Li et al., 2019), while acupuncture at P5 and P6 targets at C7-8 DRG neurons (Guo et al., 2018). Meanwhile, the effect of electroacupuncture on regulating TRPV channels is positively correlated with frequency (Fang et al., 2018).
It is noticed that acupuncture stimulation could modulate TRPV channels in the brain and spinal cord (Liao et al., 2017; Yang et al., 2017; Inprasit et al., 2018). Similar to PNS, the regulation of acupuncture on CNS is also bidirectional. Acupuncture significantly inhibits the trend of TRPV channels’ overexpression in pain-related models but promotes TRPV channels’ expression in obesity (Ji et al., 2013) or normal condition (Choowanthanapakorn et al., 2015). Meanwhile, stimulation at acupoints could lead to a multi-targeted effect on the central nervous system. For example, after stimulation at ST36, the changes of TRPV channels in the spinal cord (Yang et al., 2017); cerebellum lobules V, VIa, VI, VII, and VIII (Inprasit and Lin, 2020; Lottering and Lin, 2021); hippocampus; periaqueductal gray; and medial prefrontal cortex (Lin et al., 2020) could be observed.
In addition to nerves, acupuncture stimulation could also directly regulate the expression of TRPV1 and TRPV4 channels in different anatomical layers of skin at acupoints including muscles, epimysium, and subcutaneous loose connective tissue (Ji et al., 2013; Wu et al., 2014). It is noticed that electroacupuncture at ST36 enhances immune cytokines by promoting the TRPV1 channels in splenic CD4+ T cells (Chen et al., 2017). Mast cells could also be activated by acupuncture through TRPV2 channels to release histamine (Huang et al., 2018).
Transient receptor potential vanilloid channels influence the effect of acupuncture
The use of TRPV gene knockout, agonist, and antagonism provides us with an opportunity to understand the relationship between acupuncture and TRPV from another perspective (Table 2). When acupuncture enhances TRPV expression in wild models, the effect of acupuncture could be significantly inhibited after TRPV gene knockout (Yu et al., 2016; Huang et al., 2018). If TRPV over-expression is related to the progress of disease such as hyperpathia, the TRPV gene knockout mimics the analgesic effect of acupuncture (Liao et al., 2017; Yang et al., 2017). Delivery of TRPV agonist or antagonist on different areas of PNS could lead to different effects of acupuncture. The injection of TRPV1 antagonist into ST36 could mimic the acupuncture-like analgesic effect, but it was not replicated through the injection of TRPV4 agonist (Wu et al., 2014). The injection of TRPV1 antagonist into P5 and P6 could inhibit the modulation of sympathoexcitatory responses in manual acupuncture but not in electroacupuncture (Guo et al., 2018). A study performed by Fang et al. (2018) found that the injection of capsaicin into the dorsum of the foot could exhibit an analgesic effect similar to acupuncture. But Li et al. (2019) found that the injection of capsaicin into the dorsum of the foot reversed the effect of acupuncture and TRPV1 antagonist showed a contrary result.
TABLE 2
| Models | Intervention on TRPV | Methods | Main results | References |
| Mice inflammatory pain models | TRPV1 agonist | Capsaicin injected into ST36 | Replicated the acupuncture-like analgesic effect | Wu et al., 2014 |
| TRPV4 agonist | GSK1016790A injected into ST36 | Did not induce an analgesic effect | ||
| Mice inflammatory pain models | TRPV1 antagonist | TRPV1 gene knockout | Replicated the acupuncture-like analgesic effect | Liao et al., 2017 |
| Mice inflammatory pain models | TRPV1 antagonist | TRPV1 gene knockout | Replicated the acupuncture-like analgesic effect | Yang et al., 2017 |
| Mice inflammatory pain models | TRPV1 antagonist | TRPV1 gene knockout | Replicated the acupuncture-like analgesic effect | Yen et al., 2019 |
| Mice, chronic pain and depression models | TRPV1 antagonist | TRPV1 gene knockout | There is no significant difference with the model group | Lottering and Lin, 2021 |
| Mice inflammatory pain models | TRPV1 agonist | Capsaicin injected into the dorsum of the foot | Replicated the acupuncture-like analgesic effect | Fang et al., 2018 |
| Mice, fibromyalgia models | TRPV1 antagonist | TRPV1 gene knockout | Replicated the acupuncture-like analgesic effect | Lin et al., 2015 |
| Mice, cold stress-induced nociception and depression models | TRPV1 antagonist | TRPV1 gene knockout | Replicated the acupuncture-like analgesic effect | Lin et al., 2020 |
| Rats, paclitaxel-induced peripheral neuropathy models | TRPV1 agonist | Capsaicin injected into dorsal part of the ipsilateral hind paw | Inhibited the analgesic effect of acupuncture | Li et al., 2019 |
| TRPV1 antagonist | AMG9810 injected into dorsal part of the ipsilateral hind paw | Replicated the analgesic effect of acupuncture | ||
| Rats, MCAo models | TRPV1 agonist | Capsaicin, subcutaneous injection | Inhibited the analgesic effect of acupuncture | Long et al., 2019 |
| TRPV1 antagonist | AMG-517, intraperitoneal injection | Replicated the analgesic effect of acupuncture | ||
| Mice | TRPV1 antagonist | TRPV1 gene knockout | Inhibited the weight-loss effect of acupuncture | Choowanthanapakorn et al., 2015 |
| Mice, motion sickness models | TRPV1 antagonist | TRPV1 gene knockout | Replicated the acupuncture-like relieving motion sickness symptoms effect | Inprasit et al., 2018 |
| Mice | TRPV1 antagonist | TRPV1 gene knockout | Inhibited the phosphorylated effect of acupuncture | Chen et al., 2018 |
| Rats | TRPV1 antagonist | TRPV1 gene knockout | Inhibited the CD4 + T cells active effect of acupuncture | Chen et al., 2017 |
| Rats, gastric distension to cardiovascular reflexes models | TRPV1 antagonist | SiRNA, injected into C7-8 DRG neurons | Inhibited the inhibition of reflex increases in blood pressure by MA but not in EA | Guo et al., 2018 |
| Iodoresiniferatoxin, injected into P5 and P6 | Inhibited the modulation of sympathoexcitatory responses by MA but not in EA | |||
| Mice | TRPV1 antagonist | TRPV1 gene knockout | Inhibited the analgesic effect of EA and significant in higher intensity | Xin et al., 2016 |
| Rats, acute adjuvant arthritis models | TRPV2 antagonist | TRPV2 gene knockout | Inhibited the acupuncture activation effect of mast cells and analgesic effect | Huang et al., 2018 |
| Rats | TRPV1 antagonist | TRPV1 gene knockout | Inhibited the effect of acupuncture in suppressing the motor activity of the jejunum in an intensity-dependent manner | Yu et al., 2016 |
TRPV channels could influence the effect of acupuncture.
Acupuncture and transient receptor potential vanilloid channels participate in complex molecular networks
No matter how acupuncture regulates TRPV channels or how TRPV channels influence the effect of acupuncture as described above, TRPV channels mediate the communication between acupuncture and body tissues (Table 3). Through TRPV channels, acupuncture could regulate complex molecular networks, including adenosine triphosphate (ATP), extracellular signal-regulated kinase (ERK), toll-like receptor 4 (TLR4), and others. Phosphorylation of downstream molecules was widely found after acupuncture-regulated TRPV channels (Inprasit et al., 2018). Inprasit and Lin (2020) and Lottering and Lin (2021) performed a series of studies to clarify the role of acupuncture and TRPV in the cerebellum lobules. Both complete Freund’s adjuvant (CFA) and acid saline (AS) could lead to chronic pain, but the expression of TRPV1 in the cerebellum lobules is completely opposite. Although it is observed that the analgesic effect of acupuncture at ST36 is obvious in both studies, the regulation of TRPV1 and phosphorylation of molecules in MAPK pathways by acupuncture are quite different. Behavioral results indicate that the mechanism of TRPV1 and related inflammatory factors in pain is not single and decisive in different models. The AS model restores the general concept of hyperalgesia caused by the pathological overexpression of TRPV and inflammation, and the influence of TRPV1 and inflammatory factors on pain sensation was not significant in the CFA model. In addition, the low expression of TRPV1 and inflammatory factors also leads to the formation of depression in mice after the injection of CFA.
TABLE 3
| Models | Acupuncture delivery | Acupoints | TRPV | Targets | Related molecules or pathways | References |
| Mice inflammatory pain models | MA | ST36 | TRPV1 and TRPV4 | Cell membrane at muscle, epimysium, and neuron | Promote ATP signaling | Wu et al., 2014 |
| Mice inflammatory pain models | EA, 2 Hz 1 mA | ST36 | TRPV1 | DRG and SCDH | Inhibit PI3K, AKT, CREB, NF-κB, Nav1.7, and Nav1.8 | Liao et al., 2017 |
| Mice inflammatory pain models | EA, 2 Hz 1 mA | ST36 | TRPV1 | DRG and Spinal cord | Inhibit pPKA, pPI3K, pPKC, pERK, pp38, pJNK, pCREB, pNF-κB, Nav1.7, Nav1.8, GFAP, S100B, and RAGE | Yang et al., 2017 |
| Mice inflammatory pain models | EA, 2 Hz 1 mA | LI4 | TRPV1 | Brain | Inhibit pPKA, pPI3K, pPKC, pERK, pp38, pJNK, pCREB, pNF-κB, Nav1.7, Nav1.8 | Yen et al., 2019 |
| Mice inflammatory pain models | EA, 2 Hz 1 mA | ST36 | TRPV1 | Cerebellum lobules V, VIa and VII | Inhibit pPI3K, pmTOR, pAkT, pERK, pPKCε, pPKAIIα, pNFkB, pCREB, and S100B | Inprasit and Lin, 2020 |
| Mice, chronic pain and depression models | EA, 2 Hz 1 mA | ST36 | TRPV1 | Cerebellum lobules VI, VII, VIII | Promote pmTOR, pPI3K, NMDAR1, pPKCε, pAkt, TrkB, pNFκB, GABAAα1, pPKAIIα, pCREB, and Perk | Lottering and Lin, 2021 |
| Mice fibromyalgia models | EA, 2 Hz 1 mA | ST36 | TRPV1 and TRPV4 | L5 DRG neurons | Inhibit pERK signaling | Lin et al., 2015 |
| Mice fibromyalgia models | EA, 2 Hz 1 mA | ST35 | TRPV1 | Brain | inhibit pERK signaling | Hsu et al., 2020 |
| Mice, cold stress-induced nociception and depression models | EA, 2 Hz 1 mA | ST36 | TRPV1 | Medial prefrontal cortex, hippocampus and periaqueductal gray | Promote pPKA, pPI3K, pPKC, pAKT, pmTOR, pERK, pp38, pJNK, pCREB, and pNFκB | Lin et al., 2020 |
| Rats, high fat diet-induced obese models | EA, 10 Hz 1 mA | ST36 | TRPV1 | Nucleus tractus solitarius/gracile nucleus regions | Promote nNOS | Ji et al., 2013 |
| Skin at ST36 | ||||||
| Rats, paclitaxel-induced peripheral neuropathy models | EA, 2 Hz 0.5 to 1.5 mA | ST36 and BL60 | TRPV1 | L4-6 DRG neurons | Inhibit TLR4 and MyD88 signaling | Li et al., 2019 |
| Rats, MCAo models | EA, 2/100 Hz 2 mA | GV20, BL23 and, SP6 | TRPV1 | Hippocampal | Inhibit pp38 | Long et al., 2019 |
| Mice | EA, 2 Hz 1 mA | ST36 | TRPV1 | DRG and spinal cord | Promote pPKA, pPKC, and pERK signaling | Choowanthanapakorn et al., 2015 |
| Mice, motion sickness models | EA, 2 Hz 1 mA | PC6 | TRPV1 | Thalamus | Inhibit pPI3K, pAKT, pmTOR, pERK, pp38, npJNK, pCREB, and pNFκB | Inprasit et al., 2018 |
| Rats | EA, 1 mA | BL40 | TRPV1 | Subepidermal nerve fibers, C-fibers and A-δ fibers | Promote nNOS | Abraham et al., 2011 |
| Mice | MA and EA 2, 15, 50 Hz and 1 mA | ST36 | TRPV1 | DRG and somatosensory cortex | Promote ppPKA, pPI3K, pPKC-pERK, pAKT and pNR1-pCaMKII pathway, | Chen et al., 2018 |
| Rats | EA 2, 15 Hz and 1 mA | ST36 | TRPV1 | Splenic CD4 + T cells | Promote Ca2 + signaling | Chen et al., 2017 |
| Rats, gastric distension to cardiovascular reflexes models | MA | P5 and P6 | TRPV1 | C7-8 DRG neurons | Promote pERK signaling | Guo et al., 2018 |
| EA, 2 Hz 0.3-0.5 mA | Group III and IV bimodal sensory afferent nerves | |||||
| Rats acute adjuvant arthritis models | MA | ST36 | TRPV2 | Mast cells | Active histamine H1 and adenosine A1 receptor | Huang et al., 2018 |
| Rats | EA, 2 to 15 Hz | ST25 | TRPV1 | - | Promote sympathetic pathway | Yu et al., 2016 |
Acupuncture and TRPV channels play a great role in related molecules and pathways.
ATP, adenosine triphosphate; DRG, dorsal root ganglion; pERK, phosphoactivation of extracellular signal-regulated kinase; pPKA, phosphorylated protein kinase A; pCREB, cAMP-response-element-binding protein; NTS, nucleus tractus solitarius; nNOS, neuronal nitric oxide synthase; TLR4, toll-like receptor 4; MyD88, myeloid differentiation primary response 88.
Conclusion and future directions
Current studies have identified that there is a special relationship between acupuncture and TRPV channels including TRPV1 and TRPV4. First, stimulation at local acupoints can lead to systemic changes in TRPV channels, and the regulation of acupuncture to TRPV varies in different diseases or noxious stimuli. Second, the abundant expression and functional participation of TRPV1 and TRPV4 were specific to acupoints, and the enhancement or inhibition of TRPV channels at different anatomical levels will affect the therapeutic effect of acupuncture. Third, acupuncture and TRPV channels participate in complex molecular networks and that may explain the mechanism of acupuncture. All of the concepts are presented in Figure 2.
FIGURE 2
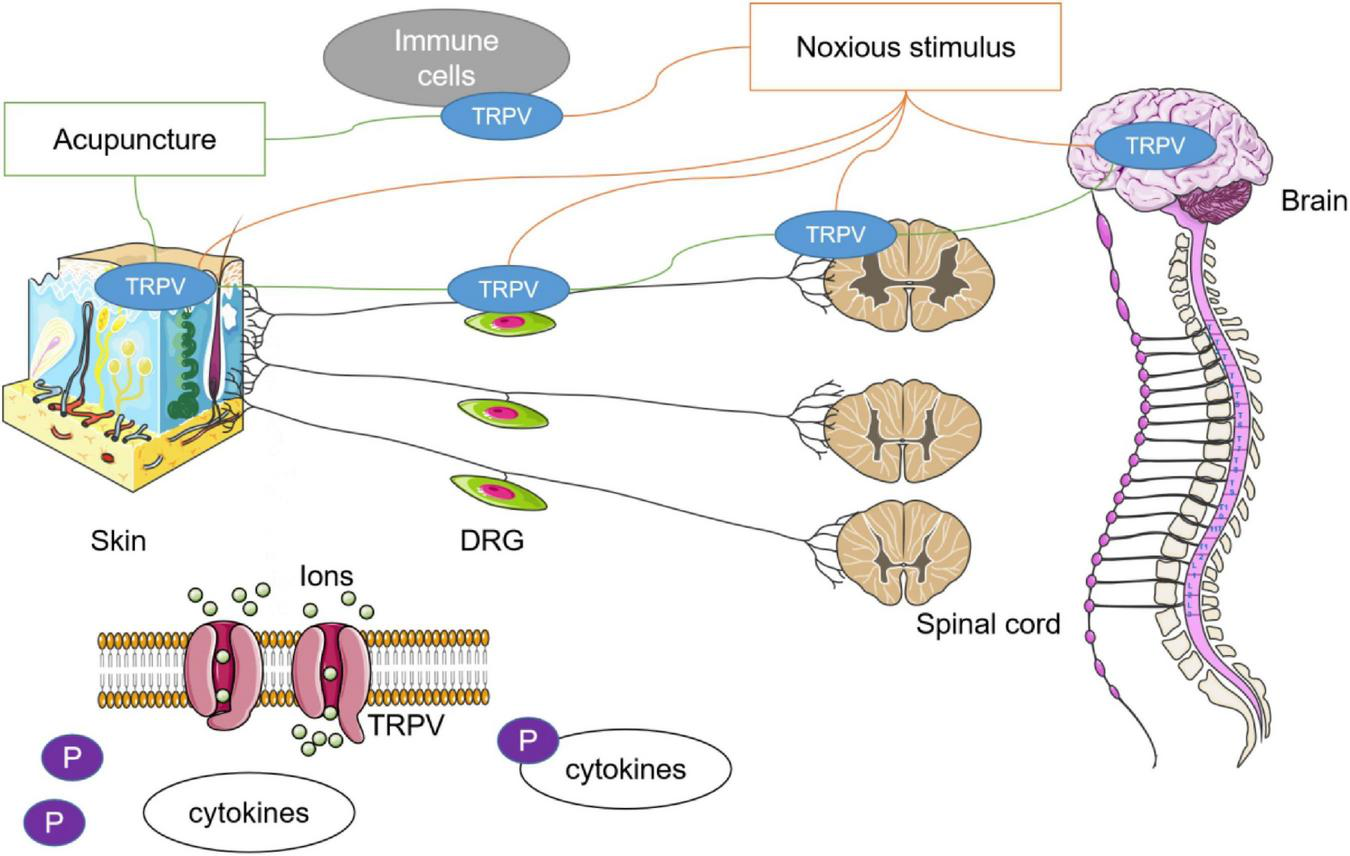
Relationship between acupuncture and TRPV channels. Noxious stimulus could lead to the imbalance of TRPV channel in multiple tissues. In many studies, it has been found that the therapeutic effect of acupuncture may be related to the agitation or antagonism of TRPV1 and TRPV4. The role of acupuncture in achieving systemic conditions through stimulation of specific acupoints may be closely dependent on the autonomic nervous system. Phosphorylation is an important mechanism in which acupuncture regulates downstream cytokines through TRPV channels.
The regulation of acupuncture to TRPV channels is significantly different from that of TRPV agonist or antagonist. Similar to the balance of Yin and Yang pursued in acupuncture theory, acupuncture exhibits a bidirectional regulation to TRPV channels in multiple targets and contributes to an overall improvement of clinical symptoms and physiological functions in different diseases or stages of illness. In the past decades, TRPV1-targeted drugs have been long studied for human pain conditions (Iftinca et al., 2021). Many drugs attempting to block TRPV1 may lead to mixed results (Basso and Altier, 2017). Only the efficacy of topical use of TRPV agonists such as capsaicin patches has been recognized, but the systemic administration was not suggested because of the adverse effects on blood pressure, breathing, and other reflex pathways (Lu et al., 2020). The regulation of acupuncture to the p38 signaling pathway via TRPV in several nervous system diseases has been discussed by Wei and Hsieh (2020). Similar to this article, the role of acupuncture to p38 signal pathways is bidirectional, but it can improve the symptoms of a disease.
TRPV channels may also help us understand the principle of acupuncture. TRPV channels tend to be highly expressed at acupoints after acupuncture stimulation. Compared with sham acupuncture, only acupuncture stimulation at acupoints could cause TRPV response. The expression of TRPV and the effect of using TRPV agonist or antagonist were also different from various acupuncture methods. For example, TRPV is sensitive to the intensity and duration of electroacupuncture, which suggests that there might be a more precise adjustment between acupuncture and TRPV channels.
There are still some limitations. All studies in this review were based on animal models. Therefore, the results in humans are inconclusive. In a single study, the regulatory relationship between acupuncture and TRPV was clear, but these effects became complex after consideration of similar studies. This prevents us from simply defining acupuncture as an agonist or inhibitor, as capsaicin does. At the same time, only a few articles compared the changes of acupuncture efficacy after local use of TRPV-related drugs, making it difficult to evaluate acupuncture and existing TRPV agonists or antagonist. As described earlier, activation of TRPV is structurally specific and selective. However, there are no studies on the changes of TRPV structure after acupuncture intervention. With the cryo-EM resolution revolution, the structural insights into the gating mechanisms of TRPV channels developed rapidly (Pumroy et al., 2020), and the role of TRPV channels in the endoplasmic reticulum has also been noticed (Haustrate et al., 2020). Compared with the current TRPV-related drugs, the effectiveness and safety of acupuncture in pain have been widely discussed (Cherkin et al., 2003; Manheimer et al., 2005). In conclusion, acupuncture has a strong relationship with TPPV1 and TRPV4. Acupuncture may provide a viable intervention target to TRPV channels. TRPV channels also help us understand how acupuncture works, especially for pain-related diseases. But the mechanism is unclear between acupuncture and TRPV channels, and further study is still needed.
Statements
Author contributions
DL: conceptualization. DL, LL, H-mZ, Y-dZ, M-fZ, J-xL, and Z-mY: data curation. DL, LL, and H-mZ: writing—original draft preparation. F-xL and RC: writing—review and editing. All authors read and agreed to the published version of the manuscript and contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81774420, 81774401, 82105009, and 82274634), the Traditional Chinese Medicine Scientific Research Project of Hubei Provincial Health Commission (No. ZY2021Q031), and the Wuhan Medical Research Project (Nos. WX19Y18 and WZ22Q32).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
- TRPV
transient receptor potential vanilloid
- ANS
autonomic nervous system
- CNS
central nervous system
- PNS
peripheral nervous system
- MA
manual acupuncture
- EA
electroacupuncture
- MCAO
middle cerebral artery occlusion
- DRG
dorsal root ganglion
- SCDH
spinal cord dorsal horn
- ATP
adenosine triphosphate
- DRG
dorsal root ganglion
- Perk
phosphoactivation of extracellular signal-regulated kinase
- pPKA
phosphorylated protein kinase A
- pCREB
cAMP-response-element-binding protein
- NTS
nucleus tractus solitarius
- nNOS
neuronal nitric oxide synthase
- TLR4
toll-like receptor 4
- MyD88
myeloid differentiation primary response 88.
References
1
Abraham T. S. Chen M. L. Ma S. X. (2011). TRPV1 expression in acupuncture points: Response to electroacupuncture stimulation.J. Chem. Neuroanat.41129–136. 10.1016/j.jchemneu.2011.01.001
2
Baeumler P. I. Fleckenstein J. Takayama S. Simang M. Seki T. Irnich D. et al (2014). Effects of acupuncture on sensory perception: A systematic review and meta-analysis.PLoS One9:e113731. 10.1371/journal.pone.0113731
3
Basith S. Cui M. Hong S. Choi S. (2016). Harnessing the therapeutic potential of capsaicin and its analogues in pain and other diseases.Molecules21:966. 10.3390/molecules21080966
4
Basso L. Altier C. (2017). Transient Receptor Potential Channels in neuropathic pain.Curr. Opin. Pharmacol.329–15. 10.1016/j.coph.2016.10.002
5
Brooks C. A. Barton L. S. Behm D. J. Eidam H. S. Fox R. M. Hammond M. et al (2019). Discovery of GSK2798745: A clinical candidate for inhibition of transient receptor potential vanilloid 4 (TRPV4).ACS Med. Chem. Lett.101228–1233. 10.1021/acsmedchemlett.9b00274
6
Cao E. Cordero-Morales J. F. Liu B. Qin F. Julius D. (2013). TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids.Neuron77667–679. 10.1016/j.neuron.2012.12.016
7
Carnevale V. Rohacs T. (2016). TRPV1: A target for rational drug design.Pharmaceuticals99:52. 10.3390/ph9030052
8
Caterina M. J. Leffler A. Malmberg A. B. Martin W. J. Trafton J. Petersen-Zeitz K. R. et al (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor.Science288306–313. 10.1126/science.288.5464.306
9
Caterina M. J. Schumacher M. A. Tominaga M. Rosen T. A. Levine J. D. Julius D. et al (1997). The capsaicin receptor: A heat-activated ion channel in the pain pathway.Nature389816–824. 10.1038/39807
10
Cavanaugh D. J. Chesler A. T. Jackson A. C. Sigal Y. M. Yamanaka H. Grant R. et al (2011). Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells.J. Neurosci.315067–5077. 10.1523/JNEUROSCI.6451-10.2011
11
Chen H. C. Chen M. Y. Hsieh C. L. Wu S. Y. Hsu H. C. Lin Y. W. et al (2018). TRPV1 is a responding channel for acupuncture manipulation in mice peripheral and central nerve system.Cell Physiol. Biochem.491813–1824. 10.1159/000493627
12
Chen L. Xu A. Yin N. Zhao M. Wang Z. Chen T. et al (2017). Enhancement of immune cytokines and splenic CD4+ T cells by electroacupuncture at ST36 acupoint of SD rats.PLoS One12:e175568. 10.1371/journal.pone.0175568
13
Cherkin D. C. Sherman K. J. Deyo R. A. Shekelle P. G. (2003). A review of the evidence for the effectiveness, safety, and cost of acupuncture, massage therapy, and spinal manipulation for back pain.Ann. Intern. Med.138898–906. 10.7326/0003-4819-138-11-200306030-00011
14
Choowanthanapakorn M. Lu K. W. Yang J. Hsieh C. L. Lin Y. W. (2015). Targeting TRPV1 for body weight control using TRPV1(-/-) mice and electroacupuncture.Sci. Rep.5:17366. 10.1038/srep17366
15
Fang J. Q. Du J. Y. Fang J. F. Xiao T. Le X. Q. Pan N. F. et al (2018). Parameter-specific analgesic effects of electroacupuncture mediated by degree of regulation TRPV1 and P2X3 in inflammatory pain in rats.Life Sci.20069–80. 10.1016/j.lfs.2018.03.028
16
Fischer M. Ciotu C. I. Szallasi A. (2020). The mysteries of Capsaicin-Sensitive afferents.Front. Physiol.11:554195. 10.3389/fphys.2020.554195
17
Gong Y. Li N. Lv Z. Zhang K. Zhang Y. Yang T. et al (2020). The neuro-immune microenvironment of acupoints-initiation of acupuncture effectiveness.J. Leukoc. Biol.108189–198. 10.1002/JLB.3AB0420-361RR
18
Grace M. S. Bonvini S. J. Belvisi M. G. McIntyre P. (2017). Modulation of the TRPV4 ion channel as a therapeutic target for disease.Pharmacol. Ther.1779–22. 10.1016/j.pharmthera.2017.02.019
19
Guo Z. L. Fu L. W. Su H. F. Tjen-A-Looi S. C. Longhurst J. C. (2018). Role of TRPV1 in acupuncture modulation of reflex excitatory cardiovascular responses.Am J. Physiol. Regul. Integr. Comp. Physiol.314R655–R666. 10.1152/ajpregu.00405.2017
20
Han J. S. (2004). Acupuncture and endorphins.Neurosci. Lett.361258–261. 10.1016/j.neulet.2003.12.019
21
Haustrate A. Prevarskaya N. Lehen’Kyi V. (2020). Role of the TRPV channels in the endoplasmic reticulum calcium homeostasis. Cells9:317. 10.3390/cells9020317
22
He Y. Guo X. May B. H. Zhang A. L. Liu Y. Lu C. et al (2020). Clinical evidence for association of acupuncture and acupressure with improved cancer pain: A systematic review and Meta-Analysis.JAMA Oncol.6271–278. 10.1001/jamaoncol.2019.5233
23
Hsu H. C. Hsieh C. L. Lee K. T. Lin Y. W. (2020). Electroacupuncture reduces fibromyalgia pain by downregulating the TRPV1-pERK signalling pathway in the mouse brain.Acupunct. Med.38101–108. 10.1136/acupmed-2017-011395
24
Hu B. Bai F. Xiong L. Wang Q. (2017). The endocannabinoid system, a novel and key participant in acupuncture’s multiple beneficial effects.Neurosci. Biobehav. Rev.77340–357. 10.1016/j.neubiorev.2017.04.006
25
Huang M. Wang X. Xing B. Yang H. Sa Z. Zhang D. et al (2018). Critical roles of TRPV2 channels, histamine H1 and adenosine A1 receptors in the initiation of acupoint signals for acupuncture analgesia.Sci. Rep.8:6523. 10.1038/s41598-018-24654-y
26
Huang W. Pach D. Napadow V. Park K. Long X. Neumann J. et al (2012). Characterizing acupuncture stimuli using brain imaging with FMRI–a systematic review and meta-analysis of the literature.PLoS One7:e32960. 10.1371/journal.pone.0032960
27
Iftinca M. Defaye M. Altier C. (2021). TRPV1-Targeted drugs in development for human pain conditions.Drugs817–27. 10.1007/s40265-020-01429-2
28
Inprasit C. Lin Y. W. (2020). TRPV1 responses in the cerebellum lobules v, VIa and VII using electroacupuncture treatment for inflammatory hyperalgesia in murine model.Int. J. Mol. Sci.21:3312. 10.3390/ijms21093312
29
Inprasit C. Lin Y. W. Huang C. P. Wu S. Y. Hsieh C. L. (2018). Targeting TRPV1 to relieve motion sickness symptoms in mice by electroacupuncture and gene deletion.Sci. Rep.8:10365. 10.1038/s41598-018-23793-6
30
Ji B. Hu J. Ma S. (2013). Effects of electroacupuncture Zusanli (ST36) on food intake and expression of POMC and TRPV1 through afferents-medulla pathway in obese prone rats.Peptides40188–194. 10.1016/j.peptides.2012.10.009
31
Jin C. Lu Y. Lu M. Cai H. Zhang J. (2018). [Discussion on the cognition and development process of experimental animal acupoints]. Zhongguo Zhen Jiu38, 963–966. 10.13703/j.0255-2930.2018.09.015
32
Jordt S. E. Ehrlich B. E. (2007). TRP channels in disease.Subcell. Biochem.45253–271. 10.1007/978-1-4020-6191-2_9
33
Kaptchuk T. J. (2002). Acupuncture: Theory, efficacy, and practice.Ann. Intern. Med.136374–383. 10.7326/0003-4819-136-5-200203050-00010
34
Kim S. Y. Shin I. S. Park Y. J. (2018). Effect of acupuncture and intervention types on weight loss: A systematic review and meta-analysis.Obes. Rev.191585–1596. 10.1111/obr.12747
35
Lawhorn B. G. Brnardic E. J. Behm D. J. (2020). Recent advances in TRPV4 agonists and antagonists.Bioorg. Med. Chem. Lett.30:127022. 10.1016/j.bmcl.2020.127022
36
Li Y. Yin C. Li X. Liu B. Wang J. Zheng X. et al (2019). Electroacupuncture alleviates Paclitaxel-Induced peripheral neuropathic pain in rats via suppressing TLR4 signaling and TRPV1 upregulation in sensory neurons.Int. J. Mol. Sci.20:5917. 10.3390/ijms20235917
37
Liao H. Y. Hsieh C. L. Huang C. P. Lin Y. W. (2017). Electroacupuncture Attenuates CFA-induced Inflammatory Pain by suppressing Nav1.8 through S100B, TRPV1, Opioid, and Adenosine Pathways in Mice.Sci. Rep.7:42531. 10.1038/srep42531
38
Lim H. D. Kim M. H. Lee C. Y. Namgung U. (2016). Anti-Inflammatory Effects of Acupuncture Stimulation via the Vagus Nerve.PLoS One11:e151882. 10.1371/journal.pone.0151882
39
Lin J. G. Hsieh C. L. Lin Y. W. (2015). Analgesic effect of electroacupuncture in a mouse fibromyalgia model: Roles of TRPV1. TRPV4, and pERK.PLoS One10:e128037. 10.1371/journal.pone.0128037
40
Lin Y. W. Chou A. Su H. Su K. P. (2020). Transient receptor potential V1 (TRPV1) modulates the therapeutic effects for comorbidity of pain and depression: The common molecular implication for electroacupuncture and omega-3 polyunsaturated fatty acids.Brain Behav. Immun.89604–614. 10.1016/j.bbi.2020.06.033
41
Lin Y. W. Hsieh C. L. (2010). Electroacupuncture at Baihui acupoint (GV20) reverses behavior deficit and long-term potentiation through N-methyl-d-aspartate and transient receptor potential vanilloid subtype 1 receptors in middle cerebral artery occlusion rats.J. Integr. Neurosci.9269–282. 10.1142/s0219635210002433
42
Liu S. Wang Z. Su Y. Qi L. Yang W. Fu M. et al (2021). A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis.Nature598641–645. 10.1038/s41586-021-04001-4
43
Liu S. Wang Z. F. Su Y. S. Ray R. S. Jing X. H. Wang Y. Q. et al (2020). Somatotopic organization and intensity dependence in driving distinct NPY-Expressing sympathetic pathways by electroacupuncture.Neuron108436–450. 10.1016/j.neuron.2020.07.015
44
Long M. Wang Z. Zheng D. Chen J. Tao W. Wang L. et al (2019). Electroacupuncture pretreatment elicits neuroprotection against cerebral Ischemia-Reperfusion injury in rats associated with transient receptor potential vanilloid 1-Mediated Anti-Oxidant stress and Anti-Inflammation.Inflammation421777–1787. 10.1007/s10753-019-01040-y
45
Lottering B. Lin Y. W. (2021). TRPV1 responses in the cerebellum lobules VI, VII, VIII using electroacupuncture treatment for chronic pain and depression comorbidity in a murine model.Int. J. Mol. Sci.22:5028. 10.3390/ijms22095028
46
Lu M. Chen C. Lan Y. Xiao J. Li R. Huang J. et al (2020). Capsaicin-the major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems.Food Funct.112848–2860. 10.1039/d0fo00351d
47
Manheimer E. White A. Berman B. Forys K. Ernst E. (2005). Meta-analysis: Acupuncture for low back pain.Ann. Intern. Med.142651–663. 10.7326/0003-4819-142-8-200504190-00014
48
Mao J. J. Farrar J. T. Armstrong K. Donahue A. Ngo J. Bowman M. A. et al (2007). De qi: Chinese acupuncture patients’ experiences and beliefs regarding acupuncture needling sensation–an exploratory survey.Acupunct. Med.25158–165. 10.1136/aim.25.4.158
49
Paricio-Montesinos R. Schwaller F. Udhayachandran A. Rau F. Walcher J. Evangelista R. A. et al (2020). The sensory coding of warm perception.Neuron106830–841. 10.1016/j.neuron.2020.02.035
50
Patapoutian A. Peier A. M. Story G. M. Viswanath V. (2003). ThermoTRP channels and beyond: Mechanisms of temperature sensation.Nat. Rev. Neurosci.4529–539. 10.1038/nrn1141
51
Por E. D. Gomez R. Akopian A. N. Jeske N. A. (2013). Phosphorylation regulates TRPV1 association with beta-arrestin-2. Biochem. J.451, 101–109. 10.1042/BJ20121637
52
Pumroy R. A. Fluck E. R. Ahmed T. Moiseenkova-Bell V. Y. (2020). Structural insights into the gating mechanisms of TRPV channels. Cell Calcium87:102168. 10.1016/j.ceca.2020.102168
53
Rubin R. (2019). Medicare proposes coverage of acupuncture for lower back pain.JAMA322:716. 10.1001/jama.2019.11573
54
Samanta A. Hughes T. Moiseenkova-Bell V. Y. (2018). Transient receptor potential (TRP) channels.Subcell. Biochem.87141–165. 10.1007/978-981-10-7757-9_6
55
Satheesh N. J. Uehara Y. Fedotova J. Pohanka M. Busselberg D. Kruzliak P. et al (2016). TRPV currents and their role in the nociception and neuroplasticity.Neuropeptides571–8. 10.1016/j.npep.2016.01.003
56
Seebohm G. Schreiber J. A. (2021). Beyond hot and spicy: TRPV channels and their pharmacological modulation.Cell Physiol. Biochem.55108–130. 10.33594/000000358
57
Shu Q. Wang H. Litscher D. Wu S. Chen L. Gaischek I. et al (2016). Acupuncture and Moxibustion have Different Effects on Fatigue by Regulating the Autonomic Nervous System: A Pilot Controlled Clinical Trial.Sci. Rep.6:37846. 10.1038/srep37846
58
Singh R. Bansal Y. Parhar I. Kuhad A. Soga T. (2019). Neuropsychiatric implications of transient receptor potential vanilloid (TRPV) channels in the reward system. Neurochem. Int.131:104545. 10.1016/j.neuint.2019.104545
59
Slomski A. (2019). Acupuncture may reduce menopausal symptoms.JAMA321:1558. 10.1001/jama.2019.4593
60
Smith G. D. Gunthorpe M. J. Kelsell R. E. Hayes P. D. Reilly P. Facer P. et al (2002). TRPV3 is a temperature-sensitive vanilloid receptor-like protein.Nature418186–190. 10.1038/nature00894
61
Szolcsanyi J. (2008). Hot target on nociceptors: Perspectives, caveats and unique features.Br. J. Pharmacol.1551142–1144. 10.1038/bjp.2008.374
62
Tang Y. Yin H. Y. Rubini P. Illes P. (2016). Acupuncture-Induced analgesia: A neurobiological basis in purinergic signaling.Neuroscientist22563–578. 10.1177/1073858416654453
63
van de Graaf S. F. Hoenderop J. G. Gkika D. Lamers D. Prenen J. Rescher U. et al (2003). Functional expression of the epithelial Ca(2+) channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex.Embo. J.221478–1487. 10.1093/emboj/cdg162
64
Wei T. H. Hsieh C. L. (2020). Effect of Acupuncture on the p38 Signaling Pathway in Several Nervous System Diseases: A Systematic Review.Int. J. Mol. Sci.21:4693. 10.3390/ijms21134693
65
Wu S. Y. Chen W. H. Hsieh C. L. Lin Y. W. (2014). Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: Mechanosensitive TRPV1 as an “acupuncture-responding channel”.BMC Complement. Altern. Med.14:96. 10.1186/1472-6882-14-96
66
Xin J. Su Y. Yang Z. He W. Shi H. Wang X. et al (2016). Distinct roles of ASIC3 and TRPV1 receptors in electroacupuncture-induced segmental and systemic analgesia.Front. Med.10465–472. 10.1007/s11684-016-0482-7
67
Yang F. Zheng J. (2017). Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin.Protein Cell8169–177. 10.1007/s13238-016-0353-7
68
Yang J. Hsieh C. L. Lin Y. W. (2017). Role of Transient Receptor Potential Vanilloid 1 in Electroacupuncture Analgesia on Chronic Inflammatory Pain in Mice.Biomed. Res. Int.2017:5068347. 10.1155/2017/5068347
69
Yen C. M. Wu T. C. Hsieh C. L. Huang Y. W. Lin Y. W. (2019). Distal electroacupuncture at the LI4 acupoint reduces CFA-Induced inflammatory pain via the brain TRPV1 signaling pathway.Int. J. Mol. Sci.20:4471. 10.3390/ijms20184471
70
Yu Z. Zhang N. Lu C. X. Pang T. T. Wang K. Y. Jiang J. F. et al (2016). Electroacupuncture at ST25 inhibits jejunal motility: Role of sympathetic pathways and TRPV1.World J. Gastroenterol.221834–1843. 10.3748/wjg.v22.i5.1834
71
Zhang R. Lao L. Ren K. Berman B. M. (2014). Mechanisms of acupuncture-electroacupuncture on persistent pain.Anesthesiology120482–503. 10.1097/ALN.0000000000000101
72
Zhang Y. Zhang R. X. Zhang M. Shen X. Y. Li A. Xin J. et al (2012). Electroacupuncture inhibition of hyperalgesia in an inflammatory pain rat model: Involvement of distinct spinal serotonin and norepinephrine receptor subtypes.Br. J. Anaesth.109245–252. 10.1093/bja/aes136
73
Zhang Z. Wang C. Gu G. Li H. Zhao H. Wang K. et al (2012). The effects of electroacupuncture at the ST36 (Zusanli) acupoint on cancer pain and transient receptor potential vanilloid subfamily 1 expression in Walker 256 tumor-bearing rats.Anesth. Analg.114879–885. 10.1213/ANE.0b013e318246536d
74
Zhao Z. Q. (2008). Neural mechanism underlying acupuncture analgesia.Prog. Neurobiol.85355–375. 10.1016/j.pneurobio.2008.05.004
75
Zheng J. (2013). Molecular mechanism of TRP channels.Compr. Physiol.3221–242. 10.1002/cphy.c120001
Summary
Keywords
acupuncture, TRPV channels, autonomic nervous system, central nervous system, peripheral sensory nervous
Citation
Luo D, Liu L, Zhang H-m, Zhou Y-d, Zhou M-f, Li J-x, Yu Z-m, Chen R and Liang F-x (2022) Relationship between acupuncture and transient receptor potential vanilloid: Current and future directions. Front. Mol. Neurosci. 15:817738. doi: 10.3389/fnmol.2022.817738
Received
18 November 2021
Accepted
25 January 2022
Published
03 November 2022
Volume
15 - 2022
Edited by
Siyi Yu, Chengdu University of Traditional Chinese Medicine, China
Reviewed by
Sang Hoon Lee, University of Cincinnati, United States; Cyril Rivat, Université de Montpellier, France
Updates
Copyright
© 2022 Luo, Liu, Zhang, Zhou, Zhou, Li, Yu, Chen and Liang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Chen, unioncr@163.comFeng-xia Liang, fxliang5@hotmail.com
†These authors have contributed equally to this work
This article was submitted to Neuroplasticity and Development, a section of the journal Frontiers in Molecular Neuroscience
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.