Abstract
Cognitive impairment (COI) is a prevalent complication across a spectrum of brain disorders, underpinned by intricate mechanisms yet to be fully elucidated. Neurons, the principal cell population of the nervous system, orchestrate cognitive processes and govern cognitive balance. Extensive inquiry has spotlighted the involvement of Foxo3a in COI. The regulatory cascade of Foxo3a transactivation implicates multiple downstream signaling pathways encompassing mitochondrial function, oxidative stress, autophagy, and apoptosis, collectively affecting neuronal activity. Notably, the expression and activity profile of neuronal Foxo3a are subject to modulation via various modalities, including methylation of promoter, phosphorylation and acetylation of protein. Furthermore, upstream pathways such as PI3K/AKT, the SIRT family, and diverse micro-RNAs intricately interface with Foxo3a, engendering alterations in neuronal function. Through several downstream routes, Foxo3a regulates neuronal dynamics, thereby modulating the onset or amelioration of COI in Alzheimer’s disease, stroke, ischemic brain injury, Parkinson’s disease, and traumatic brain injury. Foxo3a is a potential therapeutic cognitive target, and clinical drugs or multiple small molecules have been preliminarily shown to have cognitive-enhancing effects that indirectly affect Foxo3a. Particularly noteworthy are multiple randomized, controlled, placebo clinical trials illustrating the significant cognitive enhancement achievable through autophagy modulation. Here, we discussed the role of Foxo3a in neuron-mediated COI and common cognitively impaired diseases.
1 Introduction
Cognitive impairment (COI) is a brain disorder involved in impaired memory, language, reasoning, calculation, executive ability, and attention. Conditions such as Alzheimer’s disease (AD), stroke, ischemic brain injury (IBI), Parkinson (PA), and traumatic brain injury (TBI) are representative examples of COI disorders. Persistent COI seriously affects the recovery of patients’ ability to live autonomously. The development of COI is closely associated with damage in specific brain regions such as the hippocampus (Lisman et al., 2017), dorsolateral prefrontal cortex (Joyce et al., 2024), lateral amygdala (Qin et al., 2024), and pyriform cortex (Qin et al., 2024). In the field of brain rehabilitation therapy in recent years, the restoration of cognitive function has garnered significant recognition as a fundamental aspect of recovering brain functionality. Nevertheless, cognition encompasses a vast array of disorders with intricate etiology, and the precise underlying causes of COI remain incompletely explicated at present.
The core cause of COI pathogenesis at the cellular level involves massive neuronal death and dysfunction (Balakrishnan et al., 2024; Li et al., 2024). Neurons, the predominant cellular entities within the nervous system, establish synaptic connections with one another to form intricate neural circuits, critical for regulating behavioral development and consciousness (Cohen and Greenberg, 2008; Dow-Edwards et al., 2019; Südhof, 2021). Synapses are specialized adhesions for intercellular communication, either chemical or electrical (Miller et al., 2015; Nagy et al., 2018). Chemical synapses rely on various neurotransmitters, including dopamine and epinephrine, etc., to convey signals between neurons (Bito, 2010). Electrical synapses facilitate interneuronal transmission via direct ionic and metabolic coupling facilitated by gap junctions (Shimizu and Stopfer, 2013). Furthermore, interneuronal connections exhibit plasticity, which exert influence over the strength and durability of interneuronal communication, thereby modulating various facets of cognition (Amtul, 2015). Notably, neuronal survival has emerged as a pivotal endogenous factor implicated in the onset of numerous brain disorders, such as AD, stroke, PA, TBI, etc, contributing to the generation of COI. Thus, elucidating the modulation of neuronal function represents a crucial breakthrough in the amelioration of COI.
Forkhead box O3 (Foxo3a), also known as Foxo3, is a member of the Foxo family of transcription factors, which includes Foxo1, Foxo2, Foxo3, and Foxo4. Among these, Foxo3a has been the most extensively studied and recognized. Recent research has delved deeply into the role of Foxo3a in brain diseases, particularly its connection to COI. Foxo3a is known to be expressed in key brain regions like the hippocampus, frontal lobe, and occipital lobe, which play crucial roles in cognitive function (Braidy et al., 2015; Yuan et al., 2016; Cheng et al., 2018). In response to stress, intra-neuronal Foxo3a can activate or inhibit transcriptional regulation by shuttling between the nucleus and cytoplasm (Bahia et al., 2012). Furthermore, as a transcription factor, Foxo3a can also impact neuronal mitochondria, modulate mitochondrial DNA expression, boost mitochondrial adenosine triphosphate (ATP) production and COX activity, and improve mitochondrial function, all of which influence cognitive processes in the brain (Caballero-Caballero et al., 2013; Shi et al., 2016b). By coordinating various downstream signals such as autophagy, inflammation, mitochondrial function, oxidative stress, and apoptosis, Foxo3a has the ability to regulate neuronal behavior and ultimately impact cognition (Wong et al., 2013; Wang et al., 2021; Fu et al., 2023).
Foxo3a has an important role in neuronal response to external stimuli (Maiese et al., 2007; Polter et al., 2009). Individual behavioral changes significantly stimulate cortical Foxo3a activation (Zhou et al., 2012). In the acute immobilization stress (AIS) model, the expressions of peroxidase (Prx) III and Mn-SOD in neurons are upregulated by enhancing Foxo3a expression, forming an antioxidant defense (Jeong et al., 2011). In AD-like brain tissues, nuclear retention of Foxo3a is inversely correlated with DNA damage while positively associated with glutamine synthetase levels and cognitive repair efficacy (Fluteau et al., 2015). As a vital neuronal mediator (Orellana et al., 2023), Foxo3a is essential for the maintenance of neuronal survival. Knockdown of Foxo3a results in substantial neuronal apoptosis in embryonic zebrafish development (Peng et al., 2010). The level of Foxo3a expression varies as age in response to age-related brain damage (Sahin et al., 2013; Rollo et al., 2021). However, excessive activation of Foxo3a can lead to neuronal damage and COI. The regulatory role of Foxo3a on neurons varies significantly across different brain disease contexts. This review mainly discussed intricate relationship between Foxo3a and cognitive disorders, focusing on its effect on neuron.
2 The role of neuron in COI
Neuronal damage is the pathogenetic basis of COI in brain diseases (Figure 1). Elevated levels of neuronal death, inflammation, and oxidative stress have been observed in the hippocampal region of the brain in neurodegenerative conditions such as AD and PA (Ansari et al., 2024; Ramírez-Mendoza et al., 2024). The hippocampus, which is responsible for the storage and orientation of short-term memory, is one of the main anatomical structures involved in the formation of cognitive abilities. The hippocampal tissue mitochondria from AD individuals showed higher levels of reactive oxygen species (ROS), mitochondrial depolarization, reduced ATP, and calcium processing deficits (Olesen et al., 2024). Aging is a key trigger of cognitive deficits. Brain aging develops with age and is accompanied by increased levels of tissue inflammation, with neurons becoming progressively inflamed (Siddiqui et al., 2024). In an inflammatory milieu, activation of nuclear factor kappa B (NF-κB) and NLR family pyrin domain containing 3 (NLRP3) inflammasome leads to upregulation of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β), exacerbating neuronal apoptosis and worsening cognitive impairment (Jin et al., 2019; Liu et al., 2019). Furthermore, neuroinflammation results in detrimental outcomes including mitochondrial fragmentation (Harland et al., 2020) and endoplasmic reticulum stress (Huang et al., 2022). Various neurotoxins, such as perfluorooctane sulfonate (PFOS), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and domoic acid, can trigger neuronal damage (Gratuze et al., 2019; Wu et al., 2019; Petroff et al., 2022; Figure 1). In particular, aluminum, is known to induce severe cognitive deficits through persistent oxidative stress, increased N6-methyladenosine modification of brain-derived neurotrophic factor mRNA, and promotion of neuronal apoptosis (Song et al., 2024; Figure 1). Additionally, exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) has been linked to cerebral and cognitive impairments, with TCDD activating caspase-3 and leading to extensive neuronal apoptosis (Xu et al., 2014; Figure 1). Recent research has shown that chronic exposure to environmental contaminants tris (1-chloro-2-propyl) phosphate (TCPP) can initiate abnormal neuronal oxidative stress and mitochondrial dysfunction, causing significant memory and cognitive deficits in zebrafish (Xia et al., 2021; Figure 1). Notably, depletion of dopamine (DA) neurons disrupts spatial learning, spatial memory, and object memory capacities (Morgan et al., 2015; Figure 1). These findings indicated that neuronal damage plays a pivotal role in the development of COI.
FIGURE 1

Effects of common COI-inducing agents on neurons and cognition.
3 The feature of Foxo3a
The Foxo3a gene is located on the human chromosome 6q21 and spans a total of 124,950 bases. The Foxo3a protein is comprised of 673 amino acids, with a molecular weight of 71,227 Da, displaying a distinct structural regularity (Figure 2). The core of the Foxo3a protein structure is featured by a highly conserved N-terminal Forkhead-associated domain (FAD) and a C-terminal Transactivation domain (TAD). The FAD is responsible for DNA interactions and binding, while the TAD serves as the fundamental structural domain for the transactivation of target genes by Foxo3a. Bidirectional movement between the nucleus and the cytoplasm is necessary for Foxo3a to exert transcriptional activation or repression activity. To fulfill this localization requirement, Foxo3a proteins typically contain two nuclear localization signal domains (NLSs) and one nuclear export signal domain (NESs).
FIGURE 2

Schematic representation of the active structural domain of Foxo3a protein.
Foxo3a binds to gene sequences containing 5′-TAAAA-3′ to exert its transcriptional effects, playing a key role in regulating various cellular processes such as differentiation and apoptosis (Li et al., 2006; Ruankham et al., 2023). For instance, Foxo3a interacts with specific sequences in the promoter region of the pro-apoptotic protein Bim, impacting cell survival (Carbajo-Pescador et al., 2013). Notably, Foxo3a directly regulates transcription of mitochondrial DNA (mtDNA) to mediate mitochondrial respiration (Peserico et al., 2013). The transcriptional regulatory activity of Foxo3a requires the formation of polymeric complexes with multiple proteins (Figure 3). For example, the STAT3/Foxo3a signaling pathway has been found to mitigate excessive autophagy in neuronal PC12 cells (Xu et al., 2023; Figure 3). It has been reported that STAT3 cooperates with 14-3-3 to engage with Foxo3a, thereby sequestering Foxo3a within the cytosol and repressing its activity (Oh et al., 2012). Active aggregates centered on Foxo3a play a critical role in the transcriptional regulation of autophagy genes and the dynamics of autophagy (Xu et al., 2023). Inhibition of Foxo3a is a crucial step for ApoE to inhibit autophagy in the brain (Sohn et al., 2021). Additionally, Foxo3a phosphorylates or acetylates itself by binding to other proteins to regulate its intracellular localization (Figure 3). In cerebellar granule neurons, Foxo3a forms a complex with histone deacetylase 2 (HDAC2), which modulates P21 transcriptional expression and affects apoptosis induced by oxidative stress (Peng et al., 2015). Intriguingly, the interaction between Foxo3a and HDAC2 regulates the acetylation level of histone H4K16 in the p21 promoter region (Peng et al., 2015; Figure 3). SIRT3 serves as a classic acetylation repressor of Foxo3a. It has been suggested that SIRT3 may be involved in deacetylating Foxo3a to regulate Pink1/Parkin-associated mitochondrial autophagy in the brain (Wei et al., 2023). Thus, the interaction of Foxo3a with various factors is a critical step in modulating intracellular location. Besides, Foxo3a can also bind other proteins to alter their activity. It has been demonstrated that Foxo3a binds NF-κB to inhibit its nuclear translocation and reduces the exacerbation of inflammation caused by cerebral ischemia (Tan et al., 2021; Figure 3).
FIGURE 3

The effect of interaction of neuronal Foxo3a with other proteins on Foxo3a and neurons.
4 Regulation of neuronal Foxo3a
4.1 Methylation of promoter regions
The level of methylation in the promoter region of Foxo3a directly correlates with its expression. In cases of ischemia-induced neuronal apoptosis, there is a notable increase in methylation of the Foxo3a gene, leading to a significant suppression of its expression (Meng et al., 2022). Methyl CpG-binding protein 2 (MeCP2) is recruited to the promoter region of neuronal Foxo3a to enhance methylation levels (Meng et al., 2022). MeCP2 is recognized for its robust affinity for methylated DNA and its role in neurons has been extensively investigated. Mutant MeCP2 has been shown to disrupt functional connectivity among cortical regions in adult mice (Rahn et al., 2023). In neuronal cells, MeCP2 requires binding to TCF20 for proper function (Zhou et al., 2022). Dysregulation of TCF20 has also been linked to neurogenesis defects in the mouse cortex (Feng et al., 2020).
4.2 Phosphorylation
The transcriptional activity and subcellular localization of the Foxo3a protein are contingent upon various forms of modification, generally including phosphorylation, acetylation, methylation, and ubiquitination (Wang et al., 2017). Phosphorylation, a predominant inhibitory mechanism for Foxo3a and typically occurs at sites such as Thr-32 (Liu H. et al., 2014), Ser-7 (Ho et al., 2012), and Ser-253 (Li et al., 2006). Upon phosphorylation by the upstream kinase Akt, Foxo3a interacts with 14-3-3 proteins (Sanphui and Biswas, 2013), forming a complex that sequesters it in the cytosol, maintaining an inactive state. In neural-like PC12 cells, attenuation of FOXO3 phosphorylation and reduced Foxo3a recruitment to the promoter region of the pro-apoptotic protein bim were observed following Akt activation and ubiquitination (Xu et al., 2017). Treatment with thapsigargin, an endoplasmic reticulum (ERS) inducer (Figure 3), led to a marked decrease in Thr-32 phosphorylation of Foxo3a and an elevated nuclear-to-cytoplasmic Foxo3a ratio in neuroblastoma cells (Zhu et al., 2004). The diverse activities of the same protein resulting from phosphorylation at distinct sites by various phosphatases may yield contrasting outcomes. Nevertheless, Foxo3a phosphorylation does not invariably signify inactivation and is intricately linked to the interacting protein. For instance, Foxo3a can be phosphorylated and translocated to the nucleus to mediate neuronal death induced by oligomeric β-amyloid (Aβ) through mammalian sterile 20-like kinase 1 (MST1) (Sanphui and Biswas, 2013; Figure 3). The pro-transcriptional function of Foxo3a phosphorylated by Cdk5 has also been documented in neurons (Shi et al., 2016a; Figure 3). Conversely, Akt-mediated phosphorylation of Foxo3a represents a prevailing inhibitory mechanism in neuronal contexts (Wu et al., 2009; Zeng et al., 2017; Dong et al., 2018; Figure 4).
FIGURE 4

A mechanism of neuronal regulation through Foxo3a.
4.3 Acetylation
Acetylation constitutes a vital mechanism for the transcriptional activation of Foxo3a proteins. The Sirtuin (Sirt) family, renowned deacetylases, play a pivotal role in regulating the acetylation of neuronal Foxo3a (Figure 3). In neuroblastoma cells treated with ATRA, high expression of Sirt1 was accompanied by increased Foxo3a deacetylation (Kim et al., 2009). It was further found that in manganese-treated PC12 cells, activation of SIRT1 significantly inhibited the activity of Foxo3a (Zhao et al., 2019). However, acetylation also exerts an inhibitory effect on Foxo3a activation. Downregulation of Sirt1 expression was reported to significantly reduce Foxo3a activation in lidocaine-stimulated PC12 cells (Zheng et al., 2020). The concomitant high expression of Foxo3a and Sirt1 was detected in Parkinson’s-induced neuronal cells (Ubaid et al., 2022). Synergistic interaction of SIRT3 with Foxo3a is critical for the inhibition of hydrogen peroxide-induced oxidative stress in neurons (Liu et al., 2020; Ruankham et al., 2021; Yang et al., 2022). Sirt3-mediated deacetylation of Foxo3a regulates neuronal survival by the modulation of acetylation process (Mishra et al., 2018). Consequently, the functional and active state of SIRT-regulated Foxo3a acetylation is contingent upon the contextual conditions of treatment.
4.4 Upstream pathway of regulating neuronal Foxo3a
Numerous upstream pathways intricately intertwine with Foxo3a activity. AKT emerges as a ubiquitous regulator, exerting influence over Foxo3a through phosphorylation, thereby modulating its functionality. The PI3K/AKT axis has garnered attention for its role in orchestrating neuronal Foxo3a-mediated transcriptional regulation (Figure 4). Within the intricate network of the IGF1 signaling cascade, IGF1, IGF1R, and PI3K emerge as pivotal members. Notably, attenuation of IGF1R expression markedly diminishes AKT and Foxo3a phosphorylation within the murine brain (Liang et al., 2011). Intriguingly, the response of AKT/Foxo3a interplay to endoplasmic reticulum stress (ERS) is perturbed upon ablation of the ERS protein CHOP (Ghosh et al., 2012). Additionally, the upstream factor Triggering receptor expressed on myeloid cells 2 (TREM2) has been shown to potently activate Foxo3a within the AD brain tissues via the transduction of PI3K/AKT signaling (Wang Y. et al., 2020). In glioma stem cells, BMP4 stimulation elicits a decrement in p-AKT/p-Foxo3a signaling alongside an increase in the neuronal marker β-Tubulin III expression (Ciechomska et al., 2020). Moreover, the AMPK/Foxo3a axis exerts a pivotal role in governing autophagy within PC12 cells (He et al., 2022), with the AKT inhibitor SC66 demonstrating efficacy in impeding AMPK activation through modulation of the interaction between EGFR and autophagy protein P62 (Hou et al., 2022). Furthermore, heightened expression of FUN14 Domain Containing 2 (FUNDC2) promotes mitochondrial transport of phosphatidylinositol-3,4,5-trisphosphate (PIP3), which modulates neuronal AkT/Foxo3a signaling in a model of cerebral ischemia/reperfusion, consequently increasing the expression of bim (Shi et al., 2021). SGK1, as an AKT analog, has also been noted to synergize with AKT to mediate neuronal activation of Foxo3a (Sahin et al., 2013). It can be concluded that AKT, as a convergence point of the upstream pathway, has a profound impact on the neuronal cellular localization and functionality of Foxo3a (Sahin et al., 2013).
SIRT-centered signaling represents another pivotal upstream pathway influencing Foxo3a activity. Activation of SIRT3 leads to the deacetylation of Mn-superoxide dismutase (MnSOD), consequently elevating Foxo3a levels in ammonia-neurotoxic animal models (Anamika et al., 2023). Intriguingly, evidence suggests that SIRT1 activation may be dependent on PI3K/AKT signaling (Duan et al., 2019; Figure 4). Conversely, SIRT2 inhibitors AK1 and AGK2 have demonstrated the capacity to attenuate the p-AKT/Foxo3a signaling axis in ischemic stroke models (Duan et al., 2019). Simultaneous overexpression of SIRT1 and Foxo3a markedly mitigates oxidative stress induced by ischemia-reperfusion (Wu et al., 2020). However, during hypoxia-induced neuronal apoptosis, PARP1-dependent inhibition of NAD(+)/SIRT1 signaling promotes the acetylation and nuclear translocation of Foxo3a, and enhances the mRNA expression of Bcl-2/adenovirus E1B 19 kDa-interacting protein (Lu et al., 2014). Predictive E2F binding sites within the FOXO gene promoter region are noted. Moreover, the overexpression of E2F-1 increases the level of Foxo3a to modulate neuronal apoptosis (Nowak et al., 2007). In hepatocellular carcinoma cells, SIRT6 suppresses the transcriptional activity of E2F-1 (Ran et al., 2016), while the effect of this interaction on neuronal survival mediated by Foxo3a remains unexplored. Although SIRT-mediated deacetylation of neuronal Foxo3a has been extensively investigated, its effects necessitate contextual consideration within disease backgrounds and stimulus conditions.
MicroRNAs (miRNAs) are a class of non-coding single-stranded RNA molecules encoded by endogenous genes that regulate the post-transcriptional activity of genes. In recent years, miRNAs have also been extensively studied for their direct or indirect involvement in Foxo3a regulation of neurons. Such as miR-132/212, miRNA-7/211 have been observed to directly or indirectly regulate key molecules of the AKT signaling pathway, PTEN, Foxo3a as well as P300, to regulate the overall state of PI3K/AKT signaling and to affect the neuronal cellular activity (Wong et al., 2013; Salama et al., 2020). Phosphatase and tensin homolog (PTEN) inhibits the phosphorylation activation of neuronal Foxo3a by counteracting PI3K/AKT signaling (Zhao Y. et al., 2018; Zhao et al., 2021). Especially, the inhibition of PI3K/AKT/Foxo3a by miRNA-132 was shown to be an important aspect of sevoflurane-induced neuronal apoptosis (Dong et al., 2018). Additionally, miRNA-132 also affects the activity of Foxo3a by regulating PTEN (Zhao Y. et al., 2018). Several miRNAs such as miR-27a, miR-153-3p and miR-132-3p also directly bind to Foxo3a 3’UTR to manipulate its transcription in neurons (Sun et al., 2017; Wang H. et al., 2020; Fu et al., 2022).
5 Role of Foxo3a in cognitive disorders
Progressive COI associated with aging have emerged as a widely acknowledged concern. The full length Foxo3a isoform expression declined with age (Frankum et al., 2022). For example, Foxo3a expression is significantly down-regulated in the representative age-related disease intervertebral disc (IVD) degeneration (Alvarez-Garcia et al., 2017). The inhibition of PI3K/Akt signaling is accompanied by an increase of age-related renal Foxo3a level (Choi et al., 2012). The expression level of unphosphorylated Foxo3a was negatively correlated with increased age-related mortality (Rollo et al., 2021). SIRT1 is an acetylase of Foxo3a, and downregulation of its level causes significant acceleration of renal aging (Chuang et al., 2017). More importantly, Genetic inquiries have firmly linked mutated Foxo3a with aging and longevity (Brooks-Wilson, 2013). Especially, the rs2802292, rs2764264 and rs13217795 variants of FOXO3 have been associated with extreme longevity (Frankum et al., 2022). Investigations into Foxo3a’s involvement in aging-associated COI have also been undertaken (Baek et al., 2023). AD is a geriatric neurodegenerative disease characterized by representative COI. Clinical trials have shown that serum Foxo3a levels are significantly lower in AD patients compared to those with mild COI, suggesting its potential diagnostic utility in assessing AD risk (Pradhan et al., 2020). Cox proportional hazards modeling predicted that FOXO3 rs2802292 has the potential to inhibit the risk of the advance in AD in hypertensive patients (Chen R. et al., 2023). Possible mechanisms involve its reduction of blood-borne debris accumulation, increased oxidative stress, and inflammatory factor level in the brain due to hypertension-induced disruption of the blood-brain barrier (Chen R. et al., 2023). Further evidence suggests that inhibition of Foxo3a via PI3K/AKT exacerbates the onset of COI in AD mice (Wang Y. et al., 2020), mechanistically which may be related to the modulation of Foxo3a on mitochondrial autophagy for cognitive preservation (Zhou et al., 2024).
Foxo3a also exerts noticeable effects on COI that are not solely attributed to aging. In rats with severe cerebral ischemia/reperfusion-induced COI, a significant decrease in Foxo3a expression was observed, which was subsequently reversed after rehabilitation training (Jin et al., 2021). However, excessive expression of Foxo3a may also negatively impact cognition. TCDD, a common neurotoxin known to impede brain development and promote cognitive impairment, led to marked upregulation of Foxo3a expression in PC12 cells, accompanied by increased apoptosis (Xu et al., 2014). Elevated MST1 expression was detected in mice exhibiting impaired spatial memory, and inhibition of MST1 not only deactivated Foxo3a but also mitigated the memory deficits (Shang et al., 2020). PFOS, an environmental pollutant with widespread occurrence, has been linked to cognitive disorders. Suppression of Foxo3a prevented PFOS-induced apoptosis in PC12 cells (Wu et al., 2019). Excessive autophagy-induced damage to the hippocampus contributes to learning and memory deficits and represents an intrinsic factor underlying cognitive dysregulation in stroke (Gao et al., 2019). Abnormally elevated expression of Foxo3a has been observed in hippocampal neural stem cells affected by zinc deficiency (Han et al., 2015). Remarkably, in the aging brain, hippocampal neurons in the CA1 region exhibited heightened levels of active Foxo3a due to reduced Akt activity (Jackson et al., 2009). Antagonizing the PI3K/AKT/p-Foxo3a signaling cascade through interference of upstream PTEN results in enhanced pro-autophagic activation by Foxo3a, thereby promoting COI in stroke (Zhao et al., 2021).
Foxo3a is intricately connected with numerous upstream and downstream pathways. Various stimuli can induce diverse states and functions of Foxo3a, leading to activation of different downstream targets and thereby exerting distinct effects on neuronal states. Consequently, Foxo3a exhibits diverse effects on different types of COI. Transcriptionally, Foxo3a regulates the expression of several pro-apoptotic factors, including Puma and Bim, both of which are BH3-only pro-apoptotic proteins that synergistically induce neuronal apoptosis. Notably, treatment with Aβ has been shown to directly induce transcriptional regulation of Puma and Bim expression by Foxo3a (Akhter et al., 2014). Moreover, extrasynaptic NMDA receptors, known inducers of nuclear translocation of Foxo3a (Dick and Bading, 2010), have been implicated in neuronal apoptotic processes (Bahia et al., 2012). Foxo3a has also been implicated in mediating corticosterone-induced cell death in PC12 cells, which is closely associated with its activation of pro-apoptotic factor expression (Chang et al., 2023). Conversely, neuronal SIRT3/Foxo3a signaling is markedly upregulated in the presence of protective antioxidant enzymes such as Manganese porphyrins (MnPs) (Cheng et al., 2015). Notably, activation of Foxo3a enhances the expression of the antioxidant enzyme glutamine synthetase in the brain (Fluteau et al., 2015). In summary, Foxo3a modulates the effects of neuron-mediated cognitive alterations, with assessment necessitating integration of disease context and downstream pathways (Table 1).
TABLE 1
| Diseases | Model in vivo or in vitro | Downstream pathway | Biological functions | Potential effect on COI | References |
| AD | Apoe4 mice | Foxo3a upregulates the expressions of autophagy and mitophagy | Foxo3a ameliorates neuroinflammation | ↑ | Sohn et al., 2021; Zhou et al., 2024 |
| Aβ-stimulated neurons | Foxo3a upregulates expressions of bim, puma and caspases-3 | Foxo3a induces apoptosis of neurons | ↓ | Sanphui and Biswas, 2013; Akhter et al., 2014; Saha and Biswas, 2015 | |
| Stroke | MCAO/R rats | Foxo3a alleviates mitochondrial oxidative damage through increasing MnSOD expression | Foxo3a inhibits neurological damage | ↑ | Xie et al., 2022 |
| MCAO/R rats | Foxo3a suppressed the expressions of BRCC3 and NLRP3 | Foxo3a alleviates oxidative stress and neuroinflammatory responses | ↑ | Tan et al., 2024 | |
| tMCAO rats | Foxo3a promotes the activity of CREB factor | Foxo3a attenuates neuroinflammatory responses | ↑ | Chen B. et al., 2023 | |
| IBI | Ovariectomized female rats | High expression of Foxo3a is accompanied by high level of caspase-3 | Foxo3a induces apoptosis of neurons | ↓ | Jover-Mengual et al., 2010 |
| Hypoxia-ischemia rats | Foxo3a upregulates the expressions of bim and caspase-3 | Foxo3a triggers apoptosis of neurons | ↓ | Li et al., 2009, 2013, 2017 | |
| Cerebral I/R mice | Foxo3a promotes the ROS production | Foxo3a reduces apoptosis and excessive ERS | ↑ | Shi et al., 2019 | |
| Cerebral I/R rats | Foxo3a suppressed the activation of nrf2/glutathione signaling pathway | Foxo3a potentially reduces neuronal survival | ↓ | Zaki et al., 2022 | |
| Cerebral I/R rats | Foxo3a inhibits the activation of NF-κB signaling pathway | Foxo3a alleviates neuroinflammatory responses | ↑ | Tan et al., 2021 | |
| OGD-stimulated neurons | Foxo3a upregulate the expression of bax, cleaved caspase 3, but reduces the level of bcl-2 | Foxo3a protect against neuronal cell injury | ↑ | Meng et al., 2022 | |
| PA | Dopaminergic neuronal PC12 cells | Foxo3a activates the autophagy | No significant effect of Foxo3a on neuronal viability | Unknown | He et al., 2022 |
| MPTP mice | Increased level of Foxo3a is accompanied by increased level of MnSOD/NQO1/HO-1, but decreased NF-κB pathway | Foxo3a potentially rescues the loss of dopaminergic neurons | ↑ | Leem et al., 2024 | |
| MPTP mice | Foxo3a upregulate the expression of bim | Foxo3a inhibits the apoptosis of neuron | ↑ | Liu L. et al., 2014 | |
| 6-hydroxy dopamine-treated cells and rats | Foxo3a promotes the expression of puma and FasL | Foxo3a triggers neuron death | ↓ | Sanphui et al., 2020; Bhattacharyya et al., 2023 | |
| TBI | Mice treated with weight drop | Acetylation of Foxo3a increased the level of bim | Acetylation of Foxo3a triggers apoptosis of neuron | ↓ | Xu et al., 2024 |
| Mice struck by convex tip | Foxo3a transcriptionally upregulates AQP4 | Foxo3a leads to cytotoxic edema | ↓ | Kapoor et al., 2013 | |
| Mice treated with weight drop | Foxo3a activates autophagy | Foxo3a initiates neuronal damage in the hippocampus | ↓ | Sun et al., 2018 |
The role of Foxo3a in major models of COI was summarized.
MCAO/R, middle cerebral artery occlusion/reperfusion, tMCAO, transient middle cerebral artery occlusion, IBI, ischemic brain injury, OGD, oxygen-glucose deprivation, MPTP, methyl-4-phenyl-1,2,3,6-tetrahydropyridine, AQP4, Aquaporin 4. “↑” represents promotion of COI, “↓” represents inhibition of COI.
5.1 The downstream pathway of regulating AD by Foxo3a
AD is a prevalent neurodegenerative condition affecting the elderly, characterized by COI. While the aggregation of Aβ and Tau proteins is commonly implicated in AD pathogenesis, the precise etiology of the disease remains elusive due to numerous contributing factors. Foxo3a, a protein extensively studied in the context of AD neurobiology, exhibits a dual modulatory role in neuronal regulation (Figure 5). The involvement of Foxo3a in AD pathophysiology is intricately linked to its interaction with specific disease triggers. Notably, alterations in these triggers can perturb Foxo3a-mediated regulation of AD neurological function.
FIGURE 5
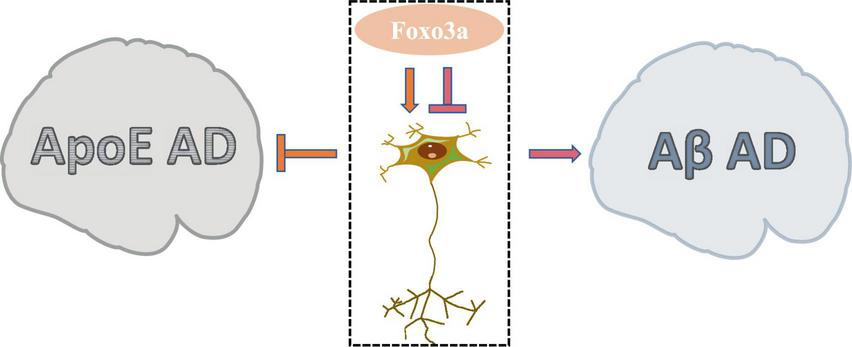
The dual role of Foxo3a in Alzheimer’s disease.
Apolipoprotein E (ApoE) has been widely studied in AD due to its role in lipid and glucose metabolism, as well as its close interplay with neural signaling pathways. Dysregulated ApoE is recognized as a major risk factor for AD (Zhao N. et al., 2018), with ApoE4 being particularly implicated as the most potent pro-AD factor (Lanfranco et al., 2020). In AD brain tissues exhibiting elevated expression of ApoE4, there is a notable increase in the phosphorylation level of Ser253 on Foxo3a, accompanied by its suppressed activity (Sohn et al., 2021). The protection of Foxo3a against APOE-type AD potentially strongly is associated with autophagy. The disruption of autophagy exacerbate the progression of AD (Zhang et al., 2021), wherein the activation of Foxo3a exerts a pivotal role in promoting autophagic processes (Zhou et al., 2024). Moreover, emerging evidence demonstrated that Foxo3a mediates neurodegenerative pathways through the activation of the E3 ubiquitin ligase FBXO32/atrogin-1, thereby modulating autophagosome formation inhibition in AD.
Aβ, the primary constituent of cortical senile plaques, accrues with advancing age and represents a pivotal factor implicated in AD pathology. Aβ dimers have been shown to disrupt synaptic architecture and function, exerting neurotoxic effects. Notably, it has been demonstrated that treatment with Aβ upregulates neuronal Foxo3a expression, thereby directly facilitating the activation of downstream pro-apoptotic mediators including Bim, caspase-3, and PUMA (Sanphui and Biswas, 2013; Akhter et al., 2014; Saha and Biswas, 2015). Gonadotropin-releasing hormone (GnRH) exhibits notable anti-aging properties and exerts modulatory effects on neurodegeneration (Wang et al., 2010). GnRH has been documented to counteract Aβ-induced cytotoxicity (Marbouti et al., 2020). The modulation of hypothalamic on aging processes has been widely investigated (Kim and Choe, 2019). It has been elucidated that Aβ impedes GnRH expression in hypothalamic GnRH neurons by activating Foxo3a through NF-κB signaling pathways (Shi et al., 2020). Furthermore, Aβ-mediated dephosphorylation of Foxo3a in hippocampal neurons leads to its translocation to the mitochondrial nucleus and subsequent modulation of mtDNA expression, culminating in the suppression of cytochrome c oxidase subunit 1 (COX1) and ATP release, thereby precipitating mitochondrial dysfunction (Shi et al., 2016b). Attenuation of Foxo3a activation emerges as a promising therapeutic strategy for mitigating Aβ-associated AD pathology (Qin et al., 2008).
5.2 The downstream pathway of regulating stroke by Foxo3a
Stroke, characterized by the abrupt rupture of cerebral blood vessels or vascular obstruction leading to inadequate blood supply to the brain, results in the impairment of brain tissue. COI is a frequent complication after a stroke that can lead to disability, yet comprehensive and standardized rehabilitative strategies remain elusive (Cumming et al., 2013). The emergence of COI subsequent to stroke is attributed to structural damage within key brain regions including the temporal lobe, hippocampus, and brainstem. Neuronal damage within these regions is posited as a fundamental cellular mechanism underlying COI (Lipton, 1996). An augmentation of neuronal survival to ameliorate post-stroke COI represents a primary focus of contemporary research endeavors.
Ischemic and hemorrhagic strokes represent distinct pathophysiological entities. The overexpression of Foxo3a has been shown to impede the neurorestorative effects of artesunate following middle cerebral artery occlusion (MCAO) (Zhang et al., 2022), underscoring its role in exacerbating MCAO-induced neuronal injury, partly through dysregulation of autophagy (Xie et al., 2022). Conversely, activation of SIRT1 confers neuroprotection in MCAO/R rats, implicating the SIRT1/Foxo3a axis as a promising therapeutic target for MCAO (Tan et al., 2024). Furthermore, Foxo3a exerts influence in the context of hemorrhagic stroke. Activation of p-Foxo3a/CREB signaling contributes to the neuroprotective effects of Swell1 against cerebral ischemic stroke-induced neurological deficits (Chen B. et al., 2023), while phosphorylated Foxo3a impedes CREB nuclear translocation (Chen B. et al., 2023). Notably, CREB serves as a pivotal regulator of MeCP2 and DNMT3B methylases, with CREB-induced DNA hypermethylation identified as a contributing factor to susceptibility of ischemic stroke (Fan et al., 2023).
5.3 The downstream pathway of regulating IBI by Foxo3a
Ischemia induces neuronal apoptosis and disrupts cognitive performance. There is a significant positive correlation between ischemia-activated Foxo3a and neuronal apoptosis (Kuroki et al., 2009; Jover-Mengual et al., 2010; Li et al., 2013). In the early stage of ischemia and hypoxia, the levels of Foxo3a nuclear translocation, Bim and cleaved caspase 3 are significantly increased in brain tissue (Li et al., 2009, 2017). Enhanced oxidative stress activates the expression of mitochondria-associated pro-apoptotic proteins such as caspase 3 and Bax. Blockage of Foxo3a activation downregulates ischemia-induced increase of ROS (Shi et al., 2019). More importantly, it was found that inhibition of Foxo3a expression promotes the activation of the antioxidant pathway Nrf2/glutathione (Zaki et al., 2022). Further mechanistic studies revealed that increasing the methylation level of the Foxo3a promoter and inhibiting Foxo3a expression induces inhibition of the downstream SPRY2-ZEB1 axis and alleviates ischemic neuronal apoptosis (Meng et al., 2022). Additionally, Foxo3a could inhibit ischemia-induced neuroinflammation through direct interaction with NF-κB (Tan et al., 2021). Interestingly, activation of NF-κB was reported to potentially upregulate SPRY2 transcription (Day et al., 2020). Thus, ischemia is a crucial causative factor for COI.
5.4 The downstream pathway of regulating PA by Foxo3a
Parkinson’s disease is a neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons in the midbrain, often accompanied by COI. Recent investigations in dopaminergic PC12 cells have illuminated a significant induction of autophagy following stimulation with the dopamine receptor (D1R) agonist SKF38393 (He et al., 2022). Further mechanistic exploration uncovered the regulatory role of upstream AMPK/ Foxo3a signaling in mediating D1R-dependent autophagy activation (He et al., 2022). In an MPTP-induced Parkinson’s model, the anti-inflammatory and antioxidative properties of creatine were found to be mediated by the activation of SIRT3/ Foxo3a signaling pathways (Leem et al., 2024). Moreover, additional studies unveiled the contribution of SIRT2 to MPTP-induced neuronal apoptosis through Foxo3a deacetylation and subsequent upregulation of Bim expression (Liu L. et al., 2014). The pro-apoptotic function of Foxo3a within Parkinson’s brain tissues, along with its downstream modulation of proteins such as Bim, PUMA, and FasL, underscores its role as a pivotal factor in Parkinson’s pathology (Sanphui et al., 2020; Wu et al., 2021; Bhattacharyya et al., 2023). Hence, Foxo3a emerges as a potential pro-Parkinsonian determinant.
5.5 The downstream pathway of regulating TBI by Foxo3a
TBI resulting from a direct impact or penetrating injury to the head damages brain tissue and affects brain function. Among the myriad complications ensuing TBI, COI stand prominent. Severe instances of TBI manifest a spectrum of cognitive discordances, encompassing visual and linguistic deficits, memory impairment, depressive tendencies, attentional lapses, and impediments in learning (Shuanglong et al., 2024). Notably, profound alterations in hippocampal morphology manifest within the traumatized cerebral milieu, concomitant with a marked decrement in neuronal density (Jahromi et al., 2024). Augmenting neuronal resilience constitutes the principal avenue toward ameliorating COI. Neuronal vitality and functionality are governed by an array of stressors, including oxidative insults and inflammatory cascades (Lu et al., 2024), with alterations in such responses contingent upon the orchestrated interplay of diverse signaling pathways.
Clinical investigations have unveiled a notable elevation in Foxo3a expression within traumatized human brain tissue compared to control group (Maiese et al., 2023). In animal models of traumatic brain TBI, the augmentation in Foxo-positive neuronal populations displays a temporal dependency (Liu et al., 2021). Mechanistically, acetylated Foxo3a was identified as a mediator driving Bim upregulation within traumatized cerebral tissue (Xu et al., 2024). Foxo3a was further delineated to directly stimulate Aquaporin 4 (AQP4) transcription, thereby promoting cytotoxic edema post-TBI and consequent memory deficits (Kapoor et al., 2013). Crucially, attenuation of Aquaporin 4 accumulation mitigates apoptosis and inflammation in TBI-afflicted brain cells (Xing et al., 2023). Additionally, Foxo3a exacerbated TBI-induced COI via activation of LC-3/p62-mediated autophagy (Sun et al., 2018; Liu et al., 2021). Notably, dysregulated autophagy emerges as a significant predisposing factor for compromised TBI recovery (Bao et al., 2016). Collectively, these findings underscore Foxo3a as a pivotal cytokine implicated in TBI pathogenesis.
6 Clinical prospective
Currently, rehabilitation evaluation stands as the principal approach for diagnosing COI. Concurrently, the advancement of technology has elevated imaging to a pivotal role as a cognitive assessment adjunct. Nonetheless, a notable deficiency persists in effective methodologies for evaluating COI from circulatory and pathological perspectives. The identification of a biomarker represents a pivotal advancement in the formulation of diagnostic modalities for cognitive disorders. Recent years have witnessed a significant increase in clinical trials and preclinical investigations, underscoring Foxo3a’s potential as a biomarker for diagnosing cognitive dysregulation.
Aging emerges as a focal risk factor for COI and notably serves as a core causative element in AD. A meta-analysis has underscored a significant association between Foxo3a rs2802292 and exceptional longevity (Revelas et al., 2018). Notably, serum assays conducted on AD patients unveiled markedly diminished serum Foxo3a concentrations compared to those observed in individuals with mild COI (Pradhan et al., 2020). Examination of human cortical tissue further revealed diminished Foxo3a levels in AD patients compared to non-AD counterparts (Sahin et al., 2013). Conversely, a heightened positivity rate for Foxo3a was observed in traumatized human brain tissue compared to non-traumatized specimens (Maiese et al., 2023). While these small-sample clinical studies hint at a potentially robust connection between in vivo Foxo3a alterations and human cognition, larger-scale cohort investigations remain imperative to bolster evidential support. Additionally, further validation is warranted to confirm the diagnostic precision of Foxo3a as a standalone marker for cognitive deficits. A comprehensive approach incorporating additional cognitive impairment biomarkers such as neurofilament light chain, various tau proteins, APOE, Aβ, and the autophagy marker molecule LC3 is essential when utilizing Foxo3a as a diagnostic tool. This integrative strategy is instrumental in enhancing diagnostic accuracy.
Currently, cognitive rehabilitation techniques serve as main treatment options of facilitating cognitive recovery, yet there remains a gap in pharmacological interventions. Foxo3a has emerged as a promising target for mitigating COI. Several studies have demonstrated the potential of clinical drugs that target the Foxo3a signaling pathway to effectively counter COI. Utilized in clinical settings, Chinese herbal medicines or compound formulas such as E. bonariensis, Chaigui granules, and Banxia Xiexin Decoction have exhibited neuroprotective effects by modulating Foxo3a expression in brain tissues, thereby enhancing cognitive function (Shi et al., 2023; Tian et al., 2023; Ibrahim et al., 2024). Additionally, the anti-malarial drug 8-aminoquinoline has demonstrated robust biological activity in improving neuronal mitochondrial function through the SIRT1/3-Foxo3a pathway (Ruankham et al., 2023). Perampanel, a widely prescribed antiepileptic medication, has shown efficacy in inhibiting neuronal damage following subarachnoid hemorrhage by targeting the SIRT3/Foxo3a signaling cascade (Yang et al., 2023). Furthermore, several candidate small molecules targeting neuronal Foxo3a signaling, including 3,14,19-triacetylandrographolide (Zhou et al., 2024), creatine (Leem et al., 2024), carboxy-terminal modulator protein (Miyawaki et al., 2009), estradiol (Jover-Mengual et al., 2010), and Ferulic acid (Picone et al., 2013), have surfaced. Nevertheless, their direct interaction with Foxo3a and specificity necessitate further comprehensive investigation to validate their selective targeting of Foxo3a.
Autophagy stands as a prominent downstream pathway in the regulation of Foxo3a within neurons, exerting a profound impact on cognition. The protective role of autophagy activated by Foxo3a in neurons is demonstrated by the upregulating expressions of ATG5, ATG7, ATG12 and LC3 (Wang et al., 2024). The activation of LC3 II and P62 are required for AMPK/Foxo3a-mediated autophagy machinery (Wan et al., 2020; Liang et al., 2021). Notably, Autophagy is a key protective response in the context of TBI. Inhibition of SKP2/CARM1 signaling by p-AMPK/p-Foxo3a activates autophagic flux in ischemic stroke mice (Zhao et al., 2023). Extensive research has elucidated the pivotal role of autophagy in neuronal function, particularly in the realm of cognitive repair (Li et al., 2023). When Foxo3a acted as an anti-COI factor, it significantly activates PINK1 and Parkin to enhance mitophagy, which in turn restores impaired mitochondria (Zhao et al., 2023). More importantly, upregulation of the autophagy signal BDNF/TrkB significantly attenuates the inflammatory response in hippocampal neurons (Gao et al., 2022). Overexpression of Foxo3a also autophagy flux. It has been highlighted that MAP1LC3B/LC3-associated phagocytosis is closely linked to amyloid β clearance (Lee et al., 2019). Consistently, autophagy enhancement promotes the degradation of Tau protein, thereby alleviating Tauopathy-related neuroinflammation and synapse loss to restore cognition (Zhu et al., 2023). Importantly, mitophagy maintains a balanced mitochondrial homeostasis via removal of the accumulated ROS and toxic fragments.
Spermidine, characterized by its indirect modulation of autophagy and potent antioxidant properties, exhibits the capacity to attenuate brain aging by bolstering neuronal autophagic processes (Xu et al., 2020; Yang et al., 2024). Encouraging findings from a single-center, randomized, double-blind, placebo-controlled Phase IIb trial underscore the efficacy of Spermidine in enhancing various neurocognitive faculties, including behavior and memory (Wirth et al., 2019). Moreover, Spermidine-enriched botanical extracts have demonstrated significant promise in mitigating cognitive decline among older individuals, with favorable safety profiles (Schwarz et al., 2018; Wirth et al., 2018). Importantly, 1.2 mg/day dose supplementation of Spermidine for 3 months had a favorable safety and tolerability profile. Furthermore, lithium has emerged as a facilitator of neuroplasticity through its modulation of autophagy pathways, as supported by clinical observations indicating enhanced hippocampal function following prolonged treatment (Forlenza et al., 2014). A 150 mg/day dose of lithium for 3 months was observed to have lower side effects, but the safety of long-term clinical use of lithium remains to be evaluated in specialized clinical trials (Forlenza et al., 2014). Notably, docosahexaenoic acid (DHA), an essential polyunsaturated fatty acid renowned for its diverse biological activities encompassing anti-aging effects, lipid modulation, and facilitation of brain development, etc. Administration of a 2 g/day dose of DHA over a 24-month period has been shown to elevate brain DHA levels, thereby correlating with enhanced memory, improved learning capabilities, and a potential preventive effect against AD (Pontifex et al., 2018). Several rigorous randomized, controlled clinical trials have corroborated the cognitive benefits of heightened DHA intake among the elderly, mechanistically implicating Aβ-mediated autophagic processes (Yurko-Mauro et al., 2010; Zhang et al., 2017, 2018). Despite the potential of Spermidine and DHA to combat cognitive impairment, there is still a need to consider the limitations of this dietary supplementation, pending more recent pharmacokinetic studies to comprehensively assess the effectiveness of these interventions.
7 Conclusion
Given the yet unclear elucidation of the mechanism underlying COI, there remains a notable absence of a reliable biomarker for its clinical diagnosis. Neurons, serving as the principal cellular constituency of the nervous system, intricately interconnect via synapses to construct elaborate neural networks forming neural circuits. These networks, in turn, orchestrate diverse cognitive functions encompassing memory, learning, and motion, etc. Notably, neuronal apoptosis and dysfunction emerge as pivotal endogenous factors precipitating COI observed in conditions such as AD, stroke, PA, IBI, and TBI. Thus, enhancing neuronal viability and functionality represents a critical frontier in the endeavor to remediate COI.
Foxo3a, functioning as a transcription factor, exerts direct or indirect influence over the activation and transmission of multiple pathways. Foxo3a demonstrated favorable improvement of neuronal activity and function in Apoe4 mice, MCAO/R rats, tMCAO rats, Cerebral I/R mice, Cerebral I/R rats, and MPTP mice as a potential protective factor. Conversely, its activity in Aβ-stimulated neurons, ovariectomized female rats, hypoxia-ischemia rats, and mice struck by convex tip demonstrated significant promotion of neuronal injury. Within the domain of neuron-mediated cognitive dysregulation, mitochondria-associated apoptosis protein including bim, puma and caspases-3, antioxidant pathway Nrf2 signaling and autophagy-related molecules including LC3, p62 and Beclin1, etc, stand out as principal downstream pathways regulated by Foxo3a. The modulation of Foxo3a itself is subject to various modifications, including promoter methylation, protein phosphorylation, and acetylation. Additionally, upstream pathways or molecules impacting Foxo3a encompass PI3K/AKT, SIRT, and micro-RNA. Notably, Foxo3a assumes diverse roles contingent upon the specific disease context within neuron-mediated COI.
Clinical observations suggested the potential of Foxo3a as a diagnostic marker for COI, particularly in AD. In particular, clinical evidence suggests that AD patients have lower levels of Foxo3a in serum and cortical tissues than non-AD or mild AD patients. However, immunohistochemical staining suggests that brain tissue from TBI patients expresses higher levels of Foxo3a compared to non-TBI populations, and these preliminary clinical trials demonstrate the potential of Foxo3a as a biomarker for diagnosing cognitive impairment. Several candidate small molecules have exhibited promise in clinical trials by targeting Foxo3a to facilitate cognitive repair. Notably, compounds such as spermidine, lithium, and DHA, acting upon the downstream autophagic pathway of Foxo3a, have demonstrated effectiveness in ameliorating cognitive disorders among afflicted patients. Additionally, multi-targeted therapy combining the neurofilament light chain, various tau proteins, APOE, Aβ, and the autophagy molecule may be able to improve the effectiveness of these drugs, which needs to be supported by more experiments in the future. More importantly, in-depth investigation and assessment are warranted in future studies to examine the dosage, frequency, side effects evaluation, pharmacokinetics, and precise efficacy of these medications. Large-scale clinical trials are imperative for determining the long-term effectiveness of these drugs. Consequently, elucidating the role of Foxo3a in the process of neuron-mediated cognitive repair warrants diligent investigation.
Statements
Author contributions
Q-QL: Visualization, Writing – original draft, Writing – review & editing. G-HW: Writing – original draft. X-CW: Writing – original draft. X-WX: Writing – review & editing. R-W: Writing – review & editing. B-LY: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Akhter R. Sanphui P. Biswas S. (2014). The essential role of p53-up-regulated modulator of apoptosis (Puma) and its regulation by FoxO3a transcription factor in β-amyloid-induced neuron death.J. Biol. Chem.28910812–10822. 10.1074/jbc.M113.519355
2
Alvarez-Garcia O. Matsuzaki T. Olmer M. Masuda K. Lotz M. (2017). Age-related reduction in the expression of FOXO transcription factors and correlations with intervertebral disc degeneration.J. Orthop. Res.352682–2691. 10.1002/jor.23583
3
Amtul Z. (2015). Neural plasticity and memory: Molecular mechanism.Rev. Neurosci.26253–268. 10.1515/revneuro-2014-0075
4
Anamika Roy A. Trigun S. K. (2023). Hippocampus mitochondrial MnSOD activation by a SIRT3 activator, honokiol, correlates with its deacetylation and upregulation of FoxO3a and PGC1α in a rat model of ammonia neurotoxicity.J. Cell Biochem.124606–618. 10.1002/jcb.30393
5
Ansari M. Al-Jarallah A. Rao M. Babiker A. Bensalamah K. (2024). Upregulation of NADPH-oxidase, inducible nitric oxide synthase and apoptosis in the hippocampus following impaired insulin signaling in the rats: Development of sporadic Alzheimer’s disease.Brain Res.1834:148890. 10.1016/j.brainres.2024.148890
6
Baek J. Shin Y. Ma X. Park H. Hwang Y. Kim D. (2023). Bifidobacterium bifidum and Lactobacillus paracasei alleviate sarcopenia and cognitive impairment in aged mice by regulating gut microbiota-mediated AKT, NF-κB, and FOXO3a signaling pathways.Immun. Ageing20:56. 10.1186/s12979-023-00381-5
7
Bahia P. Pugh V. Hoyland K. Hensley V. Rattray M. Williams R. (2012). Neuroprotective effects of phenolic antioxidant tBHQ associate with inhibition of FoxO3a nuclear translocation and activity.J Neurochem.123182–191. 10.1111/j.1471-4159.2012.07877.x
8
Balakrishnan R. Jannat K. Choi D. (2024). Development of dietary small molecules as multi-targeting treatment strategies for Alzheimer’s disease.Redox Biol.71:103105. 10.1016/j.redox.2024.103105
9
Bao H. Qiu H. Kuai J. Song C. Wang S. Wang C. et al (2016). Apelin-13 as a novel target for intervention in secondary injury after traumatic brain injury.Neural Regen. Res.111128–1133. 10.4103/1673-5374.187049
10
Bhattacharyya P. Biswas A. Biswas S. (2023). Brain-enriched miR-128: Reduced in exosomes from Parkinson’s patient plasma, improves synaptic integrity, and prevents 6-OHDA mediated neuronal apoptosis.Front. Cell. Neurosci.16:1037903. 10.3389/fncel.2022.1037903
11
Bito H. (2010). The chemical biology of synapses and neuronal circuits.Nat. Chem. Biol.6560–563. 10.1038/nchembio.408
12
Braidy N. Poljak A. Grant R. Jayasena T. Mansour H. Chan-Ling T. et al (2015). Differential expression of sirtuins in the aging rat brain.Front. Cell. Neurosci.9:167. 10.3389/fncel.2015.00167
13
Brooks-Wilson A. (2013). Genetics of healthy aging and longevity.Hum. Genet.1321323–1338. 10.1007/s00439-013-1342-z
14
Caballero-Caballero A. Engel T. Martinez-Villarreal J. Sanz-Rodriguez A. Chang P. Dunleavy M. et al (2013). Mitochondrial localization of the forkhead box class O transcription factor FOXO3a in brain.J. Neurochem.124749–756. 10.1111/jnc.12133
15
Carbajo-Pescador S. Steinmetz C. Kashyap A. Lorenz S. Mauriz J. Heise M. et al (2013). Melatonin induces transcriptional regulation of Bim by FoxO3a in HepG2 cells.Br. J. Cancer108442–449. 10.1038/bjc.2012.563
16
Chang P. Liou J. Chen P. Gao W. Wu C. Wu M. et al (2023). The neuroprotective effects of flavonoid fisetin against corticosterone-induced cell death through modulation of ERK, p38, and PI3K/Akt/FOXO3a-dependent pathways in PC12 Cells.Pharmaceutics15:2376. 10.3390/pharmaceutics15102376
17
Chen R. Morris B. Donlon T. Ross G. Kallianpur K. Allsopp R. et al (2023). Incidence of Alzheimer’s disease in men with late-life hypertension is ameliorated by FOXO3 longevity genotype.J. Alzheimers Dis.9579–91. 10.3233/JAD-230350
18
Chen B. Xie C. Shi T. Yue S. Li W. Huang G. et al (2023). Activation of Swell1 in microglia suppresses neuroinflammation and reduces brain damage in ischemic stroke.Neurobiol. Dis.176:105936. 10.1016/j.nbd.2022.105936
19
Cheng H. Xia B. Su C. Chen K. Chen X. Chen P. et al (2018). PI3K/Akt signaling pathway and Hsp70 activate in hippocampus of rats with chronic manganese sulfate exposure.J. Trace Elem. Med. Biol.50332–338. 10.1016/j.jtemb.2018.07.019
20
Cheng K. Guo F. Lu J. Cao Y. Wang T. Yang Q. et al (2015). MnTM-4-PyP modulates endogenous antioxidant responses and protects primary cortical neurons against oxidative stress.CNS Neurosci. Ther.21435–445. 10.1111/cns.12373
21
Choi Y. Kim D. Lee E. Kim J. Ha Y. Kim N. et al (2012). Attenuation of age-related changes in FOXO3a activity and the PI3K/Akt pathway by short-term feeding of ferulate.Age34317–327. 10.1007/s11357-011-9235-3
22
Chuang P. Cai W. Li X. Fang L. Xu J. Yacoub R. et al (2017). Reduction in podocyte SIRT1 accelerates kidney injury in aging mice.Am. J. Physiol. Renal Physiol.313F621–F628. 10.1152/ajprenal.00255.2017
23
Ciechomska I. Gielniewski B. Wojtas B. Kaminska B. Mieczkowski J. (2020). EGFR/FOXO3a/BIM signaling pathway determines chemosensitivity of BMP4-differentiated glioma stem cells to temozolomide.Exp. Mol. Med.521326–1340. 10.1038/s12276-020-0479-9
24
Cohen S. Greenberg M. (2008). Communication between the synapse and the nucleus in neuronal development, plasticity, and disease.Annu. Rev. Cell Dev. Biol.24183–189. 10.1146/annurev.cellbio.24.110707.175235
25
Cumming T. Marshall R. Lazar R. (2013). Stroke, cognitive deficits, and rehabilitation: Still an incomplete picture.Int. J. Stroke838–45. 10.1111/j.1747-4949.2012.00972.x
26
Day E. Sosale N. Xiao A. Zhong Q. Purow B. Lazzara M. (2020). Glioblastoma CELL RESISTANCE to EGFR and MET inhibition can be overcome via blockade of FGFR-SPRY2 bypass signaling.Cell Rep.30:3383-3396.e7. 10.1016/j.celrep.2020.02.014
27
Dick O. Bading H. (2010). Synaptic activity and nuclear calcium signaling protect hippocampal neurons from death signal-associated nuclear translocation of FoxO3a induced by extrasynaptic N-methyl-D-aspartate receptors.J. Biol. Chem.28519354–19361. 10.1074/jbc.M110.127654
28
Dong P. Zhang X. Zhao J. Li D. Li L. Yang B. (2018). Anti-microRNA-132 causes sevoflurane-induced neuronal apoptosis via the PI3K/AKT/FOXO3a pathway.Int. J. Mol. Med.423238–3246. 10.3892/ijmm.2018.3895
29
Dow-Edwards D. MacMaster F. Peterson B. Niesink R. Andersen S. Braams B. (2019). Experience during adolescence shapes brain development: From synapses and networks to normal and pathological behavior.Neurotoxicol. Teratol.76:106834. 10.1016/j.ntt.2019.106834
30
Duan J. Cui J. Zheng H. Xi M. Guo C. Weng Y. et al (2019). Aralia taibaiensis protects against I/R-induced brain cell injury through the Akt/SIRT1/FOXO3a Pathway.Oxid. Med. Cell Longev.2019:7609765. 10.1155/2019/7609765
31
Fan X. Shi G. Zhao Y. Yang J. Feng J. (2023). Neuroprotective effects of oxytocin against ischemic stroke in rats by blocking glutamate release and CREB-mediated DNA hypermethylation.Biomed. Pharmacother.167:115520. 10.1016/j.biopha.2023.115520
32
Feng C. Zhao J. Ji F. Su L. Chen Y. Jiao J. (2020). TCF20 dysfunction leads to cortical neurogenesis defects and autistic-like behaviors in mice.EMBO Rep.21:e49239. 10.15252/embr.201949239
33
Fluteau A. Ince P. Minett T. Matthews F. Brayne C. Garwood C. et al (2015). The nuclear retention of transcription factor FOXO3a correlates with a DNA damage response and increased glutamine synthetase expression by astrocytes suggesting a neuroprotective role in the ageing brain.Neurosci. Lett.60911–17. 10.1016/j.neulet.2015.10.001
34
Forlenza O. V. Coutinho A. N. Aprahamian I. Prando S. Mendes L. L. Diniz B. S. et al (2014). Long-term lithium treatment reduces glucose metabolism in the cerebellum and hippocampus of nondemented older adults: An [18F]FDG-PET study.ACS Chem. Neurosci.5484–489. 10.1021/cn5000315
35
Frankum R. Jameson T. Knight B. Stephens F. Wall B. Donlon T. et al (2022). Extreme longevity variants at the FOXO3 locus may moderate FOXO3 isoform levels.Geroscience441129–1140. 10.1007/s11357-021-00431-0
36
Fu M. Liang X. Zhang X. Yang M. Ye Q. Qi Y. et al (2023). Astaxanthin delays brain aging in senescence-accelerated mouse prone 10: Inducing autophagy as a potential mechanism.Nutr. Neurosci.26445–455. 10.1080/1028415X.2022.2055376
37
Fu X. Liu J. Xie J. Chen G. Zhang H. Meng F. et al (2022). Identification of potential therapeutic and diagnostic characteristics of Alzheimer disease by targeting the miR-132-3p/FOXO3a-PPM1F axis in APP/PS1 mice.Brain Res.1790:147983. 10.1016/j.brainres.2022.147983
38
Gao L. L. Wang Z. H. Mu Y. H. Liu Z. L. Pang L. (2022). Emodin promotes autophagy and prevents apoptosis in sepsis-associated encephalopathy through activating BDNF/TrkB signaling.Pathobiology89135–145. 10.1159/000520281
39
Gao X. Yang J. Li Y. Yu M. Liu S. Han Y. et al (2019). Lanthanum chloride induces autophagy in rat hippocampus through ROS-mediated JNK and AKT/mTOR signaling pathways.Metallomics11439–453. 10.1039/c8mt00295a
40
Ghosh A. Klocke B. Ballestas M. Roth K. A. (2012). CHOP potentially co-operates with FOXO3a in neuronal cells to regulate PUMA and BIM expression in response to ER stress.PLoS One7:e39586. 10.1371/journal.pone.0039586
41
Gratuze M. Josset N. Petry F. Pflieger M. Eyoum Jong L. Truchetti G. et al (2019). The toxin MPTP generates similar cognitive and locomotor deficits in hTau and tau knock-out mice.Brain Res.1711106–114. 10.1016/j.brainres.2019.01.016
42
Han J. Zhao J. Jiang J. Ma X. Liu X. Wang C. et al (2015). Zinc deficiency impairs the renewal of hippocampal neural stem cells in adult rats: Involvement of FoxO3a activation and downstream p27(kip1) expression.J. Neurochem.134879–891. 10.1111/jnc.13199
43
Harland M. Torres S. Liu J. Wang X. (2020). Neuronal mitochondria modulation of lps-induced neuroinflammation.J. Neurosci.401756–1765. 10.1523/JNEUROSCI.2324-19.2020
44
He T. Han C. Liu C. Chen J. Yang H. Zheng L. et al (2022). Dopamine D1 receptors mediate methamphetamine-induced dopaminergic damage: Involvement of autophagy regulation via the AMPK/FOXO3A pathway.Psychopharmacology239951–964. 10.1007/s00213-022-06097-6
45
Ho K. McGuire V. Koo C. Muir K. de Olano N. Maifoshie E. et al (2012). Phosphorylation of FOXO3a on Ser-7 by p38 promotes its nuclear localization in response to doxorubicin.J. Biol. Chem.2871545–1555. 10.1074/jbc.M111.284224
46
Hou B. Li E. Liang J. Liu S. Yang H. Liu L. et al (2022). The unique Akt inhibitor SC66 suppressed AMPK activity and abolished autophagy through the EGFR-p62 pathway.Cell Biol. Int.46311–322. 10.1002/cbin.11732
47
Huang G. Zang J. He L. Zhu H. Huang J. Yuan Z. et al (2022). Bioactive nanoenzyme reverses oxidative damage and endoplasmic reticulum stress in neurons under ischemic stroke.ACS Nano16431–452. 10.1021/acsnano.1c07205
48
Ibrahim W. Sayed R. Abdelhameed M. Omara E. Nassar M. Abdelkader N. et al (2024). Neuroprotective potential of Erigeron bonariensis ethanolic extract against ovariectomized/D-galactose-induced memory impairments in female rats in relation to its metabolite fingerprint as revealed using UPLC/MS.Inflammopharmacology321091–1112. 10.1007/s10787-023-01418-3
49
Jackson T. Rani A. Kumar A. Foster T. (2009). Regional hippocampal differences in AKT survival signaling across the lifespan: Implications for CA1 vulnerability with aging.Cell Death Differ.16439–448. 10.1038/cdd.2008.171
50
Jahromi H. Rafati A. Karbalay-Doust S. Keshavarz S. Naseh M. (2024). The combination treatment of hypothermia and intranasal insulin ameliorates the structural and functional changes in a rat model of traumatic brain injury.Brain Struct. Funct.229947–957. 10.1007/s00429-024-02769-5
51
Jeong H. Jeong H. Song S. Kang J. Seo J. Lee Y. et al (2011). Upregulation of peroxiredeoxin III in the hippocampus of acute immobilization stress model rats and the Foxo3a-dependent expression in PC12 cells.Cell Mol. Neurobiol.311041–1046. 10.1007/s10571-011-9703-4
52
Jin X. Liu M. Zhang D. Zhong X. Du K. Qian P. et al (2019). Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway.CNS Neurosci. Ther.25575–590. 10.1111/cns.13086
53
Jin Z. Zhang Z. Ke J. Wang Y. Wu H. (2021). Exercise-linked irisin prevents mortality and enhances cognition in a mice model of cerebral ischemia by regulating klotho expression.Oxid. Med. Cell Longev.2021:1697070. 10.1155/2021/1697070
54
Jover-Mengual T. Miyawaki T. Latuszek A. Alborch E. Zukin R. Etgen A. (2010). Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway.Brain Res.13211–12. 10.1016/j.brainres.2010.01.046
55
Joyce M. Yang S. Morin K. Duque A. Arellano J. Datta D. et al (2024). β1-adrenoceptor expression on GABAergic interneurons in primate dorsolateral prefrontal cortex: Potential role in stress-induced cognitive dysfunction.Neurobiol. Stress30:100628. 10.1016/j.ynstr.2024.100628
56
Kapoor S. Kim S. Farook J. Mir S. Saha R. Sen N. (2013). Foxo3a transcriptionally upregulates AQP4 and induces cerebral edema following traumatic brain injury.J. Neurosci.3317398–17403. 10.1523/JNEUROSCI.2756-13.2013
57
Kim K. Choe H. (2019). Role of hypothalamus in aging and its underlying cellular mechanisms.Mech. Ageing Dev.17774–79. 10.1016/j.mad.2018.04.008
58
Kim M. Ahn K. Park S. Kang H. Jang B. Oh S. et al (2009). SIRT1 regulates tyrosine hydroxylase expression and differentiation of neuroblastoma cells via FOXO3a.FEBS Lett.5831183–1188. 10.1016/j.febslet.2009.03.007
59
Kuroki K. Virard I. Concannon C. Engel T. Woods I. Taki W. et al (2009). Effects of transient focal cerebral ischemia in mice deficient in puma.Neurosci. Lett.451237–240. 10.1016/j.neulet.2009.01.019
60
Lanfranco M. Ng C. Rebeck G. (2020). ApoE Lipidation as a therapeutic target in Alzheimer’s disease.Int. J. Mol. Sci.21:6336. 10.3390/ijms21176336
61
Lee J. W. Nam H. Kim L. E. Jeon Y. Min H. Ha S. et al (2019). TLR4 (toll-like receptor 4) activation suppresses autophagy through inhibition of FOXO3 and impairs phagocytic capacity of microglia.Autophagy15753–770. 10.1080/15548627.2018.1556946
62
Leem Y. Park J. Park J. Kim D. Kim H. (2024). Creatine supplementation with exercise reduces α-synuclein oligomerization and necroptosis in Parkinson’s disease mouse model.J. Nutr. Biochem.126:109586. 10.1016/j.jnutbio.2024.109586
63
Li B. Deng S. Jiang H. Zhu W. Zhuo B. Du Y. et al (2024). The mechanistic effects of acupuncture in rodent neurodegenerative disease models: A literature review.Front. Neurosci.18:1323555. 10.3389/fnins.2024.1323555
64
Li D. Y. Qu Y. Li J.-H. Zhang L. Xiong T. Mu D.-Z. (2013). [Role of the FOXO3a transcription factor in neuronal apoptosis in neonatal rats with hypoxic-ischemic brain damage].Zhongguo Dang Dai Er Ke Za Zhi151023–1027.
65
Li D. Luo L. Xu M. Wu J. Chen L. Li J. et al (2017). AMPK activates FOXO3a and promotes neuronal apoptosis in the developing rat brain during the early phase after hypoxia-ischemia.Brain Res. Bull.1321–9. 10.1016/j.brainresbull.2017.05.001
66
Li D. Qu Y. Mao M. Zhang X. Li J. Ferriero D. et al (2009). Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia.J. Cereb. Blood Flow Metab.291903–1913. 10.1038/jcbfm.2009.102
67
Li M. Chiu J. Mossman B. Fukagawa N. (2006). Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats.J. Biol. Chem.28140429–40439. 10.1074/jbc.M606596200
68
Li Q. Zhao Y. Guo H. Li Q. Yan C. Li Y. et al (2023). Impaired lipophagy induced-microglial lipid droplets accumulation contributes to the buildup of TREM1 in diabetes-associated cognitive impairment.Autophagy192639–2656. 10.1080/15548627.2023.2213984
69
Liang R. Khanna A. Muthusamy S. Li N. Sarojini H. Kopchick J. et al (2011). Post-transcriptional regulation of IGF1R by key microRNAs in long-lived mutant mice.Aging Cell101080–1088. 10.1111/j.1474-9726.2011.00751.x
70
Liang Z. Zhang T. Zhan T. Cheng G. Zhang W. Jia H. et al (2021). Metformin alleviates cisplatin-induced ototoxicity by autophagy induction possibly via the AMPK/FOXO3a pathway.J. Neurophysiol.1251202–1212. 10.1152/jn.00417.2020
71
Lipton S. (1996). Similarity of neuronal cell injury and death in AIDS dementia and focal cerebral ischemia: Potential treatment with NMDA open-channel blockers and nitric oxide-related species.Brain Pathol.6507–517. 10.1111/j.1750-3639.1996.tb00879.x
72
Lisman J. Buzsáki G. Eichenbaum H. Nadel L. Ranganath C. Redish A. (2017). Viewpoints: How the hippocampus contributes to memory, navigation and cognition.Nat. Neurosci.201434–1447. 10.1038/nn.4661
73
Liu H. Wu X. Luo J. Wang X. Guo H. Feng D. et al (2019). Pterostilbene attenuates astrocytic inflammation and neuronal oxidative injury after ischemia-reperfusion by inhibiting NF-κB phosphorylation.Front. Immunol.10:2408. 10.3389/fimmu.2019.02408
74
Liu H. Yin J. Wang C. Gu Y. Deng M. He Z. (2014). FOXO3a mediates the cytotoxic effects of cisplatin in lung cancer cells.Anticancer Drugs25898–907. 10.1097/CAD.0000000000000117
75
Liu L. Arun A. Ellis L. Peritore C. Donmez G. (2014). SIRT2 enhances 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced nigrostriatal damage via apoptotic pathway.Front. Aging Neurosci.6:184. 10.3389/fnagi.2014.00184
76
Liu J. Wang Y. Hao Y. Wang Z. Yang Z. Wang Z. et al (2020). 5-Heptadecylresorcinol attenuates oxidative damage and mitochondria-mediated apoptosis through activation of the SIRT3/FOXO3a signaling pathway in neurocytes.Food Funct.112535–2542. 10.1039/c9fo03028j
77
Liu X. Gao C. Qi M. Han Y. Zhou M. Zheng L. (2021). Expression of FOXO transcription factors in the brain following traumatic brain injury.Neurosci. Lett.753:135882. 10.1016/j.neulet.2021.135882
78
Lu P. Kamboj A. Gibson S. Anderson C. (2014). Poly(ADP-ribose) polymerase-1 causes mitochondrial damage and neuron death mediated by Bnip3.J. Neurosci.3415975–15987. 10.1523/JNEUROSCI.2499-14.2014
79
Lu S. Ge Q. Yang M. Zhuang Y. Xu X. Niu F. et al (2024). Decoupling the mutual promotion of inflammation and oxidative stress mitigates cognitive decline and depression-like behavior in rmTBI mice by promoting myelin renewal and neuronal survival.Biomed. Pharmacother.173:116419. 10.1016/j.biopha.2024.116419
80
Maiese A. Spina F. Visi G. Del Duca F. De Matteis A. La Russa R. et al (2023). The expression of FOXO3a as a forensic diagnostic tool in cases of traumatic brain injury: An immunohistochemical study.Int. J. Mol. Sci.24:2584. 10.3390/ijms24032584
81
Maiese K. Chong Z. Shang Y. (2007). “Sly as a FOXO”: New paths with Forkhead signaling in the brain.Curr. Neurovasc. Res.4295–302. 10.2174/156720207782446306
82
Marbouti L. Zahmatkesh M. Riahi E. Shafiee Sabet M. (2020). GnRH protective effects against amyloid β-induced cognitive decline: A potential role of the 17β-estradiol.Mol. Cell Endocrinol.518:110985. 10.1016/j.mce.2020.110985
83
Meng L. Feng B. Luan L. Fang Z. Zhao G. (2022). MeCP2 inhibits ischemic neuronal injury by enhancing methylation of the FOXO3a promoter to repress the SPRY2-ZEB1 axis.Exp. Mol. Med.541076–1085. 10.1038/s12276-022-00790-4
84
Miller A. Voelker L. Shah A. Moens C. (2015). Neurobeachin is required postsynaptically for electrical and chemical synapse formation.Curr. Biol.2516–28. 10.1016/j.cub.2014.10.071
85
Mishra N. Lata S. Deshmukh P. Kamat K. Surolia A. Banerjee T. (2018). Insulin signaling pathway protects neuronal cell lines by Sirt3 mediated IRS2 activation.Biofactors44224–236. 10.1002/biof.1413
86
Miyawaki T. Ofengeim D. Noh K. Latuszek-Barrantes A. Hemmings B. Follenzi A. et al (2009). The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death.Nat. Neurosci.12618–626. 10.1038/nn.2299
87
Morgan R. Gibbs J. Melief E. Postupna N. Sherfield E. Wilson A. et al (2015). Relative contributions of severe dopaminergic neuron ablation and dopamine depletion to cognitive impairment.Exp. Neurol.271205–214. 10.1016/j.expneurol.2015.06.013
88
Nagy J. Pereda A. Rash J. (2018). Electrical synapses in mammalian CNS: Past eras, present focus and future directions.Biochim. Biophys. Acta Biomembr.1860102–123. 10.1016/j.bbamem.2017.05.019
89
Nowak K. Killmer K. Gessner C. Lutz W. (2007). E2F-1 regulates expression of FOXO1 and FOXO3a.Biochim. Biophys. Acta1769244–252. 10.1016/j.bbaexp.2007.04.001
90
Oh H. Yu C. Dambuza I. Marrero B. Egwuagu C. (2012). STAT3 protein interacts with Class O Forkhead transcription factors in the cytoplasm and regulates nuclear/cytoplasmic localization of FoxO1 and FoxO3a proteins in CD4(+) T cells.J. Biol. Chem.28730436–30443. 10.1074/jbc.M112.359661
91
Olesen M. Pradenas E. Villavicencio-Tejo F. Porter G. Johnson G. Quintanilla R. (2024). Mitochondria-tau association promotes cognitive decline and hippocampal bioenergetic deficits during the aging.Free Radic. Biol. Med.217141–156. 10.1016/j.freeradbiomed.2024.03.017
92
Orellana A. Mazucanti C. Dos Anjos L. de Sá Lima L. Kawamoto E. Scavone C. (2023). Klotho increases antioxidant defenses in astrocytes and ubiquitin-proteasome activity in neurons.Sci. Rep.13:15080. 10.1038/s41598-023-41166-6
93
Peng K. Li Y. Long L. Li D. Jia Q. Wang Y. et al (2010). Knockdown of FoxO3a induces increased neuronal apoptosis during embryonic development in zebrafish.Neurosci. Lett.48498–103. 10.1016/j.neulet.2010.07.068
94
Peng S. Zhao S. Yan F. Cheng J. Huang L. Chen H. et al (2015). HDAC2 selectively regulates FOXO3a-mediated gene transcription during oxidative stress-induced neuronal cell death.J. Neurosci.351250–1259. 10.1523/JNEUROSCI.2444-14.2015
95
Peserico A. Chiacchiera F. Grossi V. Matrone A. Latorre D. Simonatto M. et al (2013). A novel AMPK-dependent FoxO3A-SIRT3 intramitochondrial complex sensing glucose levels.Cell Mol. Life Sci.702015–2029. 10.1007/s00018-012-1244-6
96
Petroff R. Williams C. Li J. MacDonald J. Bammler T. Richards T. et al (2022). Prolonged, low-level exposure to the marine toxin, domoic acid, and measures of neurotoxicity in nonhuman primates.Environ. Health Perspect.130:97003. 10.1289/EHP10923
97
Picone P. Nuzzo D. Di Carlo M. (2013). Ferulic acid: A natural antioxidant against oxidative stress induced by oligomeric A-beta on sea urchin embryo.Biol. Bull.22418–28. 10.1086/BBLv224n1p18
98
Polter A. Yang S. Zmijewska A. van Groen T. Paik J. Depinho R. et al (2009). Forkhead box, class O transcription factors in brain: Regulation and behavioral manifestation.Biol. Psychiatry65150–159. 10.1016/j.biopsych.2008.08.005
99
Pontifex M. Vauzour D. Minihane A. M. (2018). The effect of APOE genotype on Alzheimer’s disease risk is influenced by sex and docosahexaenoic acid status.Neurobiol. Aging69209–220. 10.1016/j.neurobiolaging.2018.05.017
100
Pradhan R. Yadav S. Prem N. Bhagel V. Pathak M. Shekhar S. et al (2020). Serum FOXO3A: A ray of hope for early diagnosis of Alzheimer’s disease.Mech. Ageing Dev.190:111290. 10.1016/j.mad.2020.111290
101
Qin T. Xia Y. Nazari N. Sepahvand T. Yuan Q. (2024). Correlational patterns of neuronal activation and epigenetic marks in the basolateral amygdala and piriform cortex following olfactory threat conditioning and extinction in rats.Front. Mol. Neurosci.17:1355140. 10.3389/fnmol.2024.1355140
102
Qin W. Zhao W. Ho L. Wang J. Walsh K. Gandy S. et al (2008). Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer’s disease-type amyloid neuropathology and spatial memory deterioration.Ann. N. Y. Acad. Sci.1147335–347. 10.1196/annals.1427.024
103
Rahn R. Yen A. Chen S. Gaines S. Bice A. Brier L. et al (2023). Mecp2 deletion results in profound alterations of developmental and adult functional connectivity.Cereb. Cortex337436–7453. 10.1093/cercor/bhad050
104
Ramírez-Mendoza A. Mendoza-Magaña M. Ramírez-Herrera M. Hernández-Nazara Z. Domínguez-Rosales J. (2024). Nitrooxidative stress and neuroinflammation caused by air pollutants are associated with the biological markers of neurodegenerative diseases.Antioxidants13:326. 10.3390/antiox13030326
105
Ran L. Chen Y. Zhang Z. Tao N. Ren J. Zhou L. et al (2016). SIRT6 overexpression potentiates apoptosis evasion in hepatocellular carcinoma via BCL2-associated x protein-dependent apoptotic pathway.Clin. Cancer Res.223372–3382. 10.1158/1078-0432.CCR-15-1638
106
Revelas M. Thalamuthu A. Oldmeadow C. Evans T. Armstrong N. Kwok J. et al (2018). Review and meta-analysis of genetic polymorphisms associated with exceptional human longevity.Mech. Ageing Dev.17524–34. 10.1016/j.mad.2018.06.002
107
Rollo C. Marsh-Rollo S. Boreham D. Lemon J. (2021). Age-related expression of prominent regulatory elements in mouse brain: Catastrophic decline of FOXO3a.Geroscience431935–1946. 10.1007/s11357-021-00364-8
108
Ruankham W. Songtawee N. Prachayasittikul V. Worachartcheewan A. Suwanjang W. Pingaew R. et al (2023). Promising 8-aminoquinoline-based metal complexes in the modulation of SIRT1/3-FOXO3a axis against oxidative damage-induced preclinical neurons.ACS Omega846977–46988. 10.1021/acsomega.3c06764
109
Ruankham W. Suwanjang W. Wongchitrat P. Prachayasittikul V. Prachayasittikul S. Phopin K. (2021). Sesamin and sesamol attenuate H2O2-induced oxidative stress on human neuronal cells via the SIRT1-SIRT3-FOXO3a signaling pathway.Nutr. Neurosci.2490–101. 10.1080/1028415X.2019.1596613
110
Saha P. Biswas S. (2015). Amyloid-β induced astrocytosis and astrocyte death: Implication of FoxO3a-Bim-caspase3 death signaling.Mol. Cell Neurosci.68203–211. 10.1016/j.mcn.2015.08.002
111
Sahin P. McCaig C. Jeevahan J. Murray J. Hainsworth A. (2013). The cell survival kinase SGK1 and its targets FOXO3a and NDRG1 in aged human brain.Neuropathol. Appl. Neurobiol.39623–633. 10.1111/nan.12023
112
Salama R. Abdel-Latif G. Abbas S. El Magdoub H. Schaalan M. (2020). Neuroprotective effect of crocin against rotenone-induced Parkinson’s disease in rats: Interplay between PI3K/Akt/mTOR signaling pathway and enhanced expression of miRNA-7 and miRNA-221.Neuropharmacology164:107900. 10.1016/j.neuropharm.2019.107900
113
Sanphui P. Biswas S. (2013). FoxO3a is activated and executes neuron death via Bim in response to β-amyloid.Cell Death Dis.4:e625. 10.1038/cddis.2013.148
114
Sanphui P. Kumar Das A. Biswas S. (2020). Forkhead Box O3a requires BAF57, a subunit of chromatin remodeler SWI/SNF complex for induction of p53 up-regulated modulator of apoptosis (Puma) in a model of Parkinson’s disease.J. Neurochem.154547–561. 10.1111/jnc.14969
115
Schwarz C. Stekovic S. Wirth M. Benson G. Royer P. Sigrist S. J. et al (2018). Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline.Aging (Albany NY)1019–33. 10.18632/aging.101354
116
Shang Y. Yan Y. Chen B. Zhang J. Zhang T. (2020). Over-expressed MST1 impaired spatial memory via disturbing neural oscillation patterns in mice.Genes Brain Behav.19:e12678. 10.1111/gbb.12678
117
Shi C. Guo H. Liu X. (2021). Platelet mitochondria transplantation rescues hypoxia/reoxygenation-induced mitochondrial dysfunction and neuronal cell death involving the FUNDC2/PIP3/Akt/FOXO3a axis.Cell Transplant.30:9636897211024210. 10.1177/09636897211024210
118
Shi C. Shi R. Guo H. Shi Y. Liu X. (2020). β-amyloid-induced gonadotropin-releasing hormone decline involving Forkhead transcription factor FOXO3a and nuclear factor-κB.Neuroreport31923–927. 10.1097/WNR.0000000000001488
119
Shi C. Zhu J. Leng S. Long D. Luo X. (2016b). Mitochondrial FOXO3a is involved in amyloid β peptide-induced mitochondrial dysfunction.J. Bioenerg. Biomembr.48189–196. 10.1007/s10863-016-9645-0
120
Shi C. Viccaro K. Lee H. Shah K. (2016a). Cdk5-Foxo3 axis: Initially neuroprotective, eventually neurodegenerative in Alzheimer’s disease models.J. Cell Sci.1291815–1830. 10.1242/jcs.185009
121
Shi W. Tian Y. Li J. (2019). GCN2 suppression attenuates cerebral ischemia in mice by reducing apoptosis and endoplasmic reticulum (ER) stress through the blockage of FoxO3a-regulated ROS production.Biochem. Biophys. Res. Commun.516285–292. 10.1016/j.bbrc.2019.05.181
122
Shi Y. Sheng P. Guo M. Chen K. Zhao Y. Wang X. et al (2023). Banxia Xiexin decoction prevents HT22 cells from high glucose-induced neurotoxicity via JNK/SIRT1/Foxo3a signaling pathway.Curr. Comput. Aided Drug Des.20, 911–927. 10.2174/1573409920666230822110258
123
Shimizu K. Stopfer M. (2013). Gap junctions.Curr. Biol.23R1026–R1031. 10.1016/j.cub.2013.10.067
124
Shuanglong Z. Jiangyuan Y. Meng N. Zheng W. Yunshui Z. Wei S. et al (2024). A meta-analysis of cognitive and functional outcomes in severe brain trauma cases.Front. Behav. Neurosci.18:1349672. 10.3389/fnbeh.2024.1349672
125
Siddiqui N. Sharma A. Kesharwani A. Anurag Parihar V. K. (2024). Exploring role of natural compounds in molecular alterations associated with brain ageing: A perspective towards nutrition for ageing brain.Ageing Res. Rev.97:102282. 10.1016/j.arr.2024.102282
126
Sohn H. Kim S. Park J. Park S. Koh Y. Kim J. et al (2021). ApoE4 attenuates autophagy via FoxO3a repression in the brain.Sci. Rep.11:17604. 10.1038/s41598-021-97117-6
127
Song J. Hao J. Lu Y. Ding X. Li M. Xin Y. (2024). Increased m6A modification of BDNF mRNA via FTO promotes neuronal apoptosis following aluminum-induced oxidative stress.Environ. Pollut.349:123848. 10.1016/j.envpol.2024.123848
128
Südhof T. (2021). The cell biology of synapse formation.J. Cell Biol.220:e202103052. 10.1083/jcb.202103052
129
Sun L. Zhao M. Liu M. Su P. Zhang J. Li Y. et al (2018). Suppression of FoxO3a attenuates neurobehavioral deficits after traumatic brain injury through inhibiting neuronal autophagy.Behav. Brain Res.337271–279. 10.1016/j.bbr.2017.08.042
130
Sun L. Zhao M. Wang Y. Liu A. Lv M. Li Y. et al (2017). Neuroprotective effects of miR-27a against traumatic brain injury via suppressing FoxO3a-mediated neuronal autophagy.Biochem. Biophys. Res. Commun.4821141–1147. 10.1016/j.bbrc.2016.12.001
131
Tan J. Luo J. Meng C. Jiang N. Cao J. Zhao J. (2021). Syringin exerts neuroprotective effects in a rat model of cerebral ischemia through the FOXO3a/NF-κB pathway.Int. Immunopharmacol.90:107268. 10.1016/j.intimp.2020.107268
132
Tan Z. Dong F. Wu L. Xu G. Zhang F. (2024). Transcutaneous electrical acupoint stimulation attenuated neuroinflammation and oxidative stress by activating SIRT1-induced signaling pathway in MCAO/R rat models.Exp. Neurol.373:114658. 10.1016/j.expneurol.2023.114658
133
Tian J. Wu Z. Wu D. Yang C. Gao Y. Yan D. et al (2023). Combining network pharmacology and experimental verification to reveal the mechanism of Chaigui granules in the treatment of depression through PI3K/Akt/mTOR signaling pathways.Metab. Brain Dis.382849–2864. 10.1007/s11011-023-01312-5
134
Ubaid S. Pandey S. Akhtar M. Rumman M. Singh B. Mahdi A. (2022). SIRT1 mediates neuroprotective and neurorescue effects of camel α-lactalbumin and oleic acid complex on rotenone-induced Parkinson’s disease.ACS Chem. Neurosci.131263–1272. 10.1021/acschemneuro.1c00876
135
Wan J. Wang R. Zhou Z. Deng L. Zhang C. Liu C. et al (2020). Saponins of Panax japonicus confer neuroprotection against brain aging through mitochondrial related oxidative stress and autophagy in rats.Curr. Pharm. Biotechnol.21667–680. 10.2174/1389201021666191216114815
136
Wang D. Cao L. Pan S. Wang G. Wang L. Cao N. et al (2021). Sirt3-mediated mitochondrial dysfunction is involved in fluoride-induced cognitive deficits.Food Chem. Toxicol.158:112665. 10.1016/j.fct.2021.112665
137
Wang Y. Lin Y. Wang L. Zhan H. Luo X. Zeng Y. et al (2020). TREM2 ameliorates neuroinflammatory response and cognitive impairment via PI3K/AKT/FoxO3a signaling pathway in Alzheimer’s disease mice.Aging1220862–20879. 10.18632/aging.104104
138
Wang H. Tang X. Huang G. Li Y. Pan R. Zhan J. et al (2020). Long non-coding KCNQ1OT1 promotes oxygen-glucose-deprivation/reoxygenation-induced neurons injury through regulating MIR-153-3p/FOXO3 axis.J. Stroke Cerebrovasc. Dis.29:105126. 10.1016/j.jstrokecerebrovasdis.2020.105126
139
Wang L. Chadwick W. Park S. Zhou Y. Silver N. Martin B. et al (2010). Gonadotropin-releasing hormone receptor system: Modulatory role in aging and neurodegeneration.CNS Neurol. Disord. Drug Targets9651–660. 10.2174/187152710793361559
140
Wang R. Liang Z. Xue X. Mei H. Ji L. Wang B. et al (2024). Microglial FoxO3a deficiency ameliorates ferroptosis-induced brain injury of intracerebral haemorrhage via regulating autophagy and heme oxygenase-1.J. Cell Mol. Med.28e18007. 10.1111/jcmm.18007
141
Wang X. Hu S. Liu L. (2017). Phosphorylation and acetylation modifications of FOXO3a: Independently or synergistically?Oncol. Lett.132867–2872. 10.3892/ol.2017.5851
142
Wei J. Xie J. He J. Li D. Wei D. Li Y. et al (2023). Active fraction of Polyrhachis vicina (Roger) alleviated cerebral ischemia/reperfusion injury by targeting SIRT3-mediated mitophagy and angiogenesis.Phytomedicine121:155104. 10.1016/j.phymed.2023.155104
143
Wirth M. Benson G. Schwarz C. Köbe T. Grittner U. Schmitz D. et al (2018). The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial.Cortex109181–188. 10.1016/j.cortex.2018.09.014
144
Wirth M. Schwarz C. Benson G. Horn N. Buchert R. Lange C. et al (2019). Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline (SmartAge)-study protocol for a randomized controlled trial.Alzheimers Res. Ther.11:36. 10.1186/s13195-019-0484-1
145
Wong H. Veremeyko T. Patel N. Lemere C. Walsh D. Esau C. et al (2013). De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer’s disease.Hum. Mol. Genet.223077–3092. 10.1093/hmg/ddt164
146
Wu H. Meng Q. Zhang Y. Li H. Liu Y. Dong S. et al (2021). Upregulated Nmnat2 causes neuronal death and increases seizure susceptibility in temporal lobe epilepsy.Brain Res. Bull.1671–10. 10.1016/j.brainresbull.2020.11.019
147
Wu P. Ding C. Yan M. Qian B. Wang W. Sun P. et al (2019). Perfluorooctane sulfonate induces apoptosis via activation of FoxO3a and upregulation of proapoptotic Bcl-2 proteins in PC12 cells.J. Toxicol. Sci.44657–666. 10.2131/jts.44.657
148
Wu Y. Huang Y. Cai J. Zhang D. Liu S. Pang B. (2020). LncRNA SNHG12 Improves cerebral ischemic-reperfusion injury by activating SIRT1/FOXO3a pathway through inhibition of autophagy and oxidative stress.Curr. Neurovasc. Res.17394–401. 10.2174/1567202617666200727142019
149
Wu Y. Peng H. Cui M. Whitney N. Huang Y. Zheng J. (2009). CXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathway.J. Neurochem.1091157–1167. 10.1111/j.1471-4159.2009.06043.x
150
Xia M. Wang X. Xu J. Qian Q. Gao M. Wang H. (2021). Tris (1-chloro-2-propyl) phosphate exposure to zebrafish causes neurodevelopmental toxicity and abnormal locomotor behavior.Sci. Total Environ.758:143694. 10.1016/j.scitotenv.2020.143694
151
Xie W. Zhu T. Zhang S. Sun X. (2022). Protective effects of Gypenoside XVII against cerebral ischemia/reperfusion injury via SIRT1-FOXO3A- and Hif1a-BNIP3-mediated mitochondrial autophagy.J. Transl. Med.20:622. 10.1186/s12967-022-03830-9
152
Xing R. Cheng J. Yu J. Li S. Ma H. Zhao Y. (2023). Trifluoperazine reduces apoptosis and inflammatory responses in traumatic brain injury by preventing the accumulation of Aquaporin4 on the surface of brain cells.Int. J. Med. Sci.20797–809. 10.7150/ijms.82677
153
Xu G. Liu J. Yoshimoto K. Chen G. Iwata T. Mizusawa N. et al (2014). 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces expression of p27(kip1) and FoxO3a in female rat cerebral cortex and PC12 cells.Toxicol. Lett.226294–302. 10.1016/j.toxlet.2014.02.019
154
Xu L. Jia Y. Yang X. Han F. Zheng Y. Ni Y. et al (2017). MicroRNA-130b transcriptionally regulated by histone H3 deacetylation renders Akt ubiquitination and apoptosis resistance to 6-OHDA.Biochim. Biophys. Acta Mol. Basis Dis.18631678–1689. 10.1016/j.bbadis.2017.04.012
155
Xu M. Zhao Y. Gong M. He Z. Wang W. Li Y. et al (2024). Dehydroevodiamine ameliorates neurological dysfunction after traumatic brain injury in mice via regulating the SIRT1/FOXO3a/Bim pathway.Phytomedicine125:155321. 10.1016/j.phymed.2023.155321
156
Xu S. Huang P. Yang J. Du H. Wan H. He Y. (2023). Calycosin alleviates cerebral ischemia/reperfusion injury by repressing autophagy via STAT3/FOXO3a signaling pathway.Phytomedicine115:154845. 10.1016/j.phymed.2023.154845
157
Xu T. T. Li H. Dai Z. Lau G. K. Li B. Y. Zhu W. L. et al (2020). Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice.Aging (Albany NY)126401–6414. 10.18632/aging.103035
158
Yang H. Ding C. Cheng M. Sheng Z. Chen L. Chen J. et al (2023). Perampanel attenuates oxidative stress and pyroptosis following subarachnoid hemorrhage via the SIRT3/FOXO3α pathway.Sci. Rep.13:21320. 10.1038/s41598-023-48802-1
159
Yang J. Yu Z. Jiang Y. Zhang Z. Tian Y. Cai J. et al (2024). SIRT3 alleviates painful diabetic neuropathy by mediating the FoxO3a-PINK1-Parkin signaling pathway to activate mitophagy.CNS Neurosci. Ther.30:e14703. 10.1111/cns.14703
160
Yang W. Wang Y. Hao Y. Wang Z. Liu J. Wang J. (2022). Piceatannol alleviate ROS-mediated PC-12 cells damage and mitochondrial dysfunction through SIRT3/FOXO3a signaling pathway.J. Food Biochem.46:e13820. 10.1111/jfbc.13820
161
Yuan S. Liu J. Zhou J. Lu W. Zhou H. Long L. et al (2016). AMPK mediates glucocorticoids stress-induced downregulation of the glucocorticoid receptor in cultured rat prefrontal cortical astrocytes.PLoS One11:e0159513. 10.1371/journal.pone.0159513
162
Yurko-Mauro K. McCarthy D. Rom D. Nelson E. B. Ryan A. S. Blackwell A. et al (2010). Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline.Alzheimers Dement.6456–464. 10.1016/j.jalz.2010.01.013
163
Zaki O. Nassar N. Abdallah D. Safar M. Mohammed R. (2022). Cilostazol alleviates NLRP3 inflammasome-induced allodynia/hyperalgesia in murine cerebral cortex following transient ischemia: Focus on TRPA1/glutamate and Akt/Dopamine/BDNF/Nrf2 Trajectories.Mol. Neurobiol.597194–7211. 10.1007/s12035-022-03024-w
164
Zeng Z. Wang X. Bhardwaj S. Zhou X. Little P. Quirion R. et al (2017). The atypical antipsychotic agent, clozapine, protects against corticosterone-induced death of PC12 cells by regulating the Akt/FoxO3a signaling pathway.Mol. Neurobiol.543395–3406. 10.1007/s12035-016-9904-4
165
Zhang K. Yang Y. Ge H. Wang J. Lei X. Chen X. et al (2022). Neurogenesis and proliferation of neural stem/progenitor cells conferred by artesunate via FOXO3a/p27Kip1 axis in mouse stroke model.Mol. Neurobiol.594718–4729. 10.1007/s12035-021-02710-5
166
Zhang Y. P. Lou Y. Hu J. Miao R. Ma F. (2018). DHA Supplementation improves cognitive function via enhancing Aβ-mediated autophagy in Chinese elderly with mild cognitive impairment: A randomised placebo-controlled trial.J. Neurol. Neurosurg. Psychiatry89382–388. 10.1136/jnnp-2017-316176
167
Zhang Y. P. Miao R. Li Q. Wu T. Ma F. (2017). Effects of DHA supplementation on hippocampal volume and cognitive function in older adults with mild cognitive impairment: A 12-month randomized double-blind, placebo-controlled trial.J. Alzheimers Dis.55497–507. 10.3233/jad-160439
168
Zhang Z. Yang X. Song Y. Tu J. (2021). Autophagy in Alzheimer’s disease pathogenesis: Therapeutic potential and future perspectives.Ageing Res. Rev.72:101464. 10.1016/j.arr.2021.101464
169
Zhao M. Gao J. Cui C. Zhang Y. Jiang X. Cui J. (2021). Inhibition of PTEN ameliorates secondary hippocampal injury and cognitive deficits after intracerebral hemorrhage: Involvement of AKT/FoxO3a/ATG-mediated autophagy.Oxid. Med. Cell Longev.2021:5472605. 10.1155/2021/5472605
170
Zhao Y. Zhao R. Wu J. Wang Q. Pang K. Shi Q. et al (2018). Melatonin protects against Aβ-induced neurotoxicity in primary neurons via miR-132/PTEN/AKT/FOXO3a pathway.Biofactors44609–618. 10.1002/biof.1411
171
Zhao N. Liu C. Qiao W. Bu G. (2018). Apolipoprotein E, receptors, and modulation of Alzheimer’s disease.Biol. Psychiatry83347–357. 10.1016/j.biopsych.2017.03.003
172
Zhao X. Liu Y. Zhu G. Liang Y. Liu B. Wu Y. et al (2019). SIRT1 downregulation mediated Manganese-induced neuronal apoptosis through activation of FOXO3a-Bim/PUMA axis.Sci. Total Environ.6461047–1055. 10.1016/j.scitotenv.2018.07.363
173
Zhao Y. Hong Z. Lin Y. Shen W. Yang Y. Zuo Z. et al (2023). Exercise pretreatment alleviates neuroinflammation and oxidative stress by TFEB-mediated autophagic flux in mice with ischemic stroke.Exp. Neurol.364:114380. 10.1016/j.expneurol.2023.114380
174
Zheng L. Guo F. Li Z. Wang Z. Ma J. Wang T. et al (2020). Dexmedetomidine protects against lidocaine-induced neurotoxicity through SIRT1 downregulation-mediated activation of FOXO3a.Hum. Exp. Toxicol.391213–1223. 10.1177/0960327120914971
175
Zhou J. Hamdan H. Yalamanchili H. Pang K. Pohodich A. Lopez J. et al (2022). Disruption of MeCP2-TCF20 complex underlies distinct neurodevelopmental disorders.Proc. Natl. Acad. Sci. U.S.A.119:e2119078119. 10.1073/pnas.2119078119
176
Zhou W. Chen L. Yang S. Li F. Li X. (2012). Behavioral stress-induced activation of FoxO3a in the cerebral cortex of mice.Biol. Psychiatry71583–592. 10.1016/j.biopsych.2011.08.012
177
Zhou Y. Zhao Q. Zhang Y. Di L. Xue F. Xu W. et al (2024). A new andrographolide derivative ADA targeting SIRT3-FOXO3a signaling mitigates cognitive impairment by activating mitophagy and inhibiting neuroinflammation in Apoe4 mice.Phytomedicine124:155298. 10.1016/j.phymed.2023.155298
178
Zhu J. Jiang X. Chang Y. Wu Y. Sun S. Wang C. et al (2023). Clemastine fumarate attenuates tauopathy and meliorates cognition in Tau mice via autophagy enhancement.Int. Immunopharmacol.123:110649. 10.1016/j.intimp.2023.110649
179
Zhu W. Bijur G. Styles N. Li X. (2004). Regulation of FOXO3a by brain-derived neurotrophic factor in differentiated human SH-SY5Y neuroblastoma cells.Brain Res. Mol. Brain Res.12645–56. 10.1016/j.molbrainres.2004.03.019
Summary
Keywords
cognitive impairment, neurons, Foxo3a, cognitively impaired diseases, brain
Citation
Liu Q-Q, Wu G-H, Wang X-C, Xiong X-W, Rui-Wang and Yao B-L (2024) The role of Foxo3a in neuron-mediated cognitive impairment. Front. Mol. Neurosci. 17:1424561. doi: 10.3389/fnmol.2024.1424561
Received
28 April 2024
Accepted
06 June 2024
Published
19 June 2024
Volume
17 - 2024
Edited by
Koji Fukui, Shibaura Institute of Technology, Japan
Reviewed by
Priya Madhavan, Taylor’s University, Malaysia
Shuichi Shibuya, National Center for Geriatrics and Gerontology (NCGG), Japan
Yuhei Mizunoe, Tokyo University of Science, Japan
Updates
Copyright
© 2024 Liu, Wu, Wang, Xiong, Rui-Wang and Yao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bao-Le Yao, yaobao@mail.gzsrmyy.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.