Abstract
Oxysterols, oxidized derivatives of cholesterol, are key modulators of central nervous system (CNS) function. Acting as both signaling molecules and cytotoxic mediators, oxysterols can influence glial activity, neuroinflammation and myelination. Glial cells (astrocytes, oligodendrocytes, and microglia) maintain neuronal homeostasis, support synaptic plasticity and mediate CNS repair. Dysregulated glial function contributes to chronic neuroinflammation, a hallmark of neurodegenerative and neuropsychiatric disorders such as Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. In this review article, we discuss the roles of oxysterols in the CNS with a particular focus on their impact on glial cells, neuroinflammation, myelination and neural circuit function. Therapeutically targeting oxysterol metabolism may mitigate oxidative stress, limit apoptosis, enhance glial health and influence myelin repair. Elucidating oxysterol signaling in glia and neurons provides critical insight into neural circuit regulation and identifies promising strategies for treating demyelinating, neurodegenerative and neuropsychiatric disorders.
1 Introduction
Ubiquitously present throughout the body, cholesterol is an essential lipid that has a wide variety of physiological functions. Cholesterol is water insoluble and as such, needs to be bound to complex lipoprotein particles to enter the circulation and travel throughout the body. Low-density lipoproteins (LDL) are the principal carrier of cholesterol from the liver to other tissue via LDL receptors. While high-density lipoproteins (HDL) may be involved in reverse transport from other tissue to the liver (Feingold, 2000). As a major component of the cell membrane, cholesterol helps regulate both integrity and fluidity of the cell. Importantly, cholesterol is also a precursor for a variety of bioactive molecules including vitamin D; the five classes of steroid hormones - glucocorticoids, mineralocorticoids, androgens, estrogens and progesterone; and oxysterols (Wolf, 1999; Gill et al., 2008; Hannich et al., 2011; Miller and Auchus, 2011; Zhang L. et al., 2024). The role that vitamin D and steroid hormones play in facilitating central nervous system (CNS) function has been well documented [see (DeLuca et al., 2013; Lebedev et al., 2024; Sailike et al., 2024) for comprehensive reviews], but the role of oxysterols in neurophysiology remains comparatively underexplored.
Endogenously, oxysterols are formed from the oxidization of cholesterol by either non-enzymatic interactions with reactive oxygen species (ROS); or by enzymatic reactions involving cytochrome P450 enzymes or cholesterol-25-hydroxylase (CH25H). These reactions produce various bioactive oxysterols, including 24S-hydroxycholesterol (24S-OHC), 25-hydroxycholesterol (25-OHC), 26-hydroxycholesterol (26-OHC) and 27-hydroxycholesterol (27-OHC) which are the primary bioactive oxysterols in the brain. Through a wide range of cellular and biochemical mechanistic pathways, these oxysterols have been implicated in cell metabolism, infection, immunity and disease pathology – including neuropsychiatric and neurodegenerative disorders (summarized in Table 1) (Vejux et al., 2007; Töröcsik et al., 2009; Yutuc et al., 2020; Foo et al., 2023). In this review, we explore oxysterol signaling in neurons and glial cells and how these cellular mechanisms may influence brain function. We also explore how altered oxysterol signaling may contribute to the pathophysiology of neurodegenerative disorders.
Table 1
| Disease | Oxysterol | Principal cellular targets | Dominant pathophysiological mechanisms | Functional/clinical consequences | References |
|---|---|---|---|---|---|
| Alzheimer’s disease (AD) | 7β-OHC 7-KC |
Neurons, microglia | Lipid peroxidation product; mitochondrial dysfunction; ROS generation; activation of apoptotic and necroptotic pathways | Neuronal loss, neuroinflammation, disease progression | Anderson et al. (2020), Testa et al. (2016) |
| 24S-OHC | Cortical and hippocampal neurons, astrocytes | Positive allosteric modulation of NMDA receptors; enhanced Ca2+ influx; synaptic hyperexcitability; amplification of Aβ-induced toxicity; altered cholesterol turnover | Early synaptic dysfunction, excitotoxic vulnerability, cognitive decline | Linsenbardt et al. (2014), Sun et al. (2016), Gamba et al. (2011) | |
| 27-OHC | Neurons, astrocytes, cerebrovascular endothelium | BBB influx of peripheral sterols; disruption of cholesterol homeostasis; pro-inflammatory signaling; interference with estrogen receptor signaling | Accelerated neurodegeneration, vascular contributions to cognitive impairment | Björkhem et al. (2009), Heverin et al. (2005) | |
| Multiple sclerosis (MS) | 7β-OHC 7-KC |
Oligodendrocytes, microglia | Oxi-apoptophagy; mitochondrial failure; membrane destabilization; inflammasome activation | Demyelination, axonal degeneration | Nury et al. (2015), Vejux et al. (2021) |
| 25-OHC | Oligodendrocytes, microglia | NLRP3 inflammasome activation; caspase-1–dependent cell death; suppression of OPC differentiation | Failed remyelination, chronic lesion formation | Jang et al., 2016, Romero et al., 2024 | |
| 24S-OHC | Neurons, oligodendrocyte lineage cells | Altered LXR signaling; dysregulated cholesterol efflux; context-dependent effects on myelin repair | Impaired remyelination or compensatory repair depending on disease stage | Meffre et al., 2015, Vejux et al., 2021 | |
| Parkinson’s disease (PD) | 7β-OHC 7-KC |
Dopaminergic neurons (SNpc) | Mitochondrial complex I inhibition; oxidative stress; lipid raft disruption | Nigrostriatal degeneration, motor impairment | Anderson et al. (2020), Gamba et al. (2019) |
| 24S-OHC | Neurons, astrocytes | Altered brain cholesterol turnover, neurodegeneration | Progressive motor symptoms, dopaminergic neuron loss | Leoni et al. (2005), Papassotiropoulos et al. (2002) | |
| 27-OHC | Dopaminergic neurons, astrocytes | Mitochondrial stress; pro-inflammatory glial activation; disruption of dopamine signaling | Progressive motor and non-motor symptoms | Björkhem et al. (2009), Testa et al. (2016) | |
| Psychiatric disorders (e.g., schizophrenia, depression) | 7β-OHC 7-KC |
Neurons, glia | Oxidative stress, lipid peroxidation, membrane dysfunction | Cognitive and mood disturbances, altered synaptic function | Pfrieger and Ungerer (2011) |
| 24S-OHC | Cortical pyramidal neurons, interneurons | Modulation of NMDA receptor function; altered excitation–inhibition balance; impaired synaptic plasticity | Cognitive dysfunction, altered network oscillations | Paul et al. (2013), Sun et al. (2016) | |
| 27-OHC | Limbic neurons, astrocytes | Neuroinflammatory priming; disruption of monoaminergic signaling; oxidative stress | Mood dysregulation, affective symptoms | Segatto et al. (2019); Romero et al. (2024) |
Oxysterols in disease pathophysiology.
Conceptual takeaway: Disease pathophysiology reflects both oxysterol-specific signaling properties and selective cellular vulnerability. Enzymatically generated oxysterols (e.g., 24S-OHC) exert context-dependent neuromodulatory effects, whereas oxidation-derived species (e.g., 7β-OHC and 7-KC) consistently drive mitochondrial dysfunction, inflammation, and cell death across disorders.
2 Oxysterol synthesis in the CNS
Brain-derived 24S-OHC is generated by both neurons and glial cells via cholesterol hydroxylation by cytochrome P450 family 46A1 (CYP46A1; also known as cholesterol 24-hydroxylase) enzyme (Figure 1), and facilitates cholesterol efflux across the blood–brain barrier while also functioning as a key signaling molecule influencing gene expression and synaptic plasticity (Lund et al., 1999; Brachet et al., 2015; Chen et al., 2018; Korinek et al., 2020). The concentration of 24S-OHC within cerebrospinal fluid is associated with neuronal integrity across a range of neurodegenerative diseases (Table 1), with concentrations above 2–10 ng / mL typically indicating increased flux out of degenerating neurons (Leoni et al., 2005). In contrast, 25-OHC, produced via cholesterol hydroxylation by cholesterol 25-hydroxylase (CH25H) enzyme in response to interferons and inflammatory cues (Figure 1), links innate immune activation to cholesterol metabolism, exerting potent immunomodulatory and antiviral effects that may become cytotoxic under chronic neuroinflammatory conditions (Dietschy and Turley, 2004; Bauman et al., 2009; Cyster et al., 2014; Son et al., 2023). Peripherally derived 26- and 27-OHC are generated via cholesterol hydroxylation by cytochrome P450 family 27A1 (CYP27A1; also known as sterol 27-hydroxylase) enzyme (Figure 1).
Figure 1
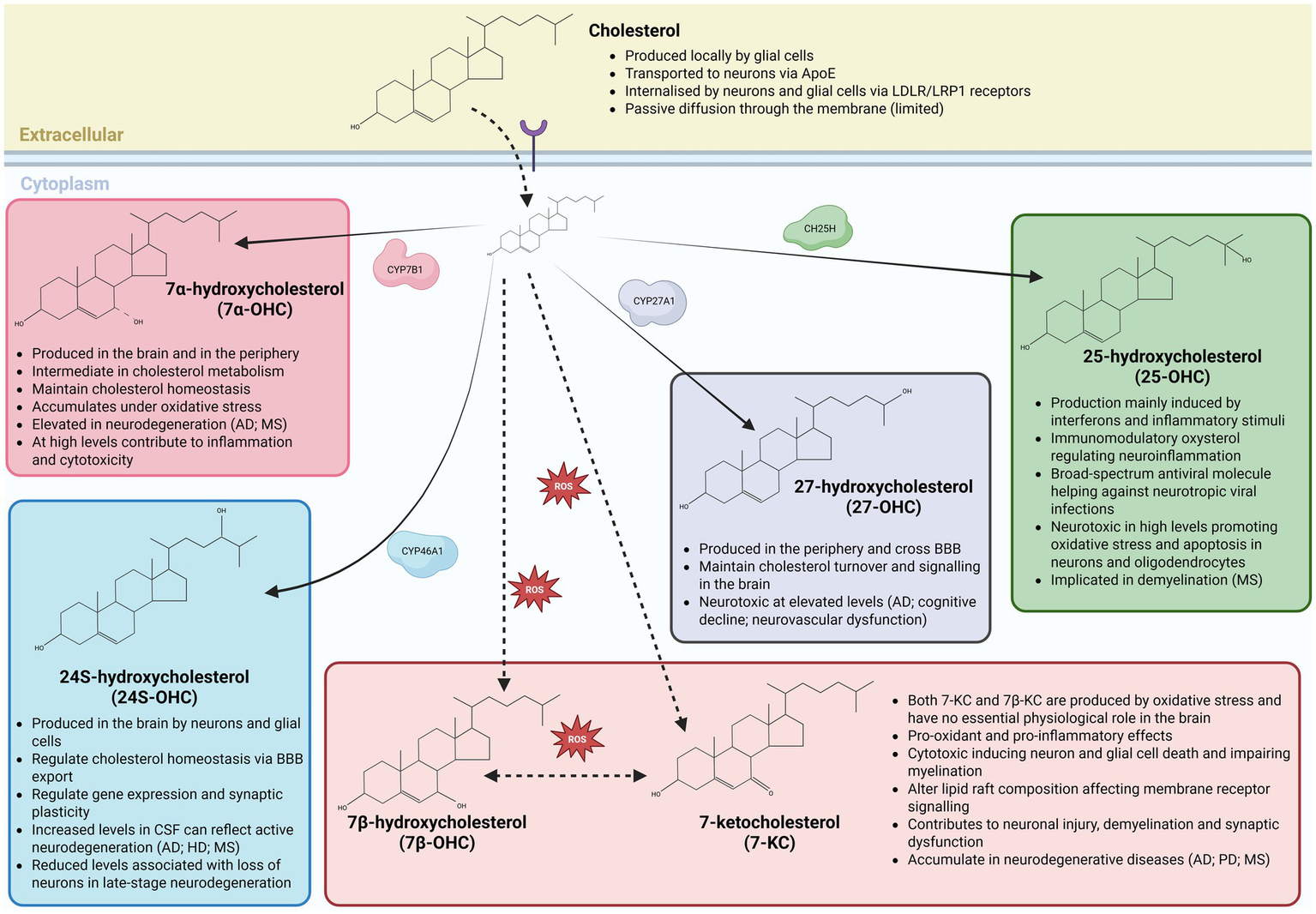
Brain oxysterols: synthesis, transport, and relevance to neuroinflammation and neurodegeneration. Schematic overview of cholesterol metabolism in the central nervous system and the synthesis of major brain-relevant oxysterols through enzymatic and non-enzymatic pathways. Cholesterol is synthesized locally by glial cells and delivered to neurons predominantly via apolipoprotein E (ApoE)–containing lipoproteins, followed by uptake through LDL receptor (LDLR) and LRP1–mediated endocytosis, with limited passive diffusion across membranes. Within neural cells, cholesterol is enzymatically converted into distinct oxysterols with specialized functions. CYP7B1 generates 7α-hydroxycholesterol (7α-OHC), an intermediate in cholesterol metabolism that contributes to cholesterol homeostasis but accumulates under oxidative stress and can promote inflammation and cytotoxicity at elevated levels. Neuron- and glia-expressed CYP46A1 produces 24S-hydroxycholesterol (24S-OHC), the principal brain-derived oxysterol responsible for cholesterol elimination across the blood–brain barrier (BBB) and for modulating gene expression and synaptic plasticity; altered cerebrospinal fluid levels reflect neurodegenerative activity. CYP27A1 generates 27-hydroxycholesterol (27-OHC) primarily in the periphery, which can cross the BBB to influence brain cholesterol turnover and signaling, but becomes neurotoxic at high concentrations (Heverin et al., 2005). Inflammatory cues induce cholesterol-25-hydroxylase (CH25H) to produce 25-hydroxycholesterol (25-OHC), an immunomodulatory oxysterol with antiviral properties that can exacerbate oxidative stress, apoptosis, and demyelination when dysregulated. In parallel, reactive oxygen species (ROS) drive non-enzymatic oxidation of cholesterol to form 7β-hydroxycholesterol (7β-OHC) and 7-ketocholesterol (7-KC), which lack essential physiological roles in the brain and exert pro-oxidant, pro-inflammatory and cytotoxic effects on neurons and glial cells, disrupt lipid raft organization and impair myelination. Accumulation or imbalance of these oxysterols is linked to neurodegenerative and neuroinflammatory disorders, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and multiple sclerosis, underscoring the tight coupling between cholesterol metabolism, oxidative stress, and brain pathology.
Capable of crossing the blood–brain barrier, both 26- and 27-OHC provide a conduit through which systemic lipid metabolism impacts the brain. More specifically, the accumulation of 26- and 27-OHC within the brain can lead to oxidative stress, neurovascular dysfunction and cognitive decline (Diestel et al., 2003; Ma et al., 2010; Ong et al., 2010; Jeitner et al., 2011). Additional oxysterols, including enzymatically generated 7α-hydroxycholesterol (7α-OHC) and oxidative stress–derived 7β-hydroxycholesterol (7β-OHC) and 7-ketocholesterol (7-KC) (Figure 1), further exemplify the dual nature of oxysterol signaling by acting as intermediates of cholesterol turnover under physiological conditions but amplifying pro-inflammatory, pro-oxidant and cytotoxic pathways during disease (Wnętrzak et al., 2022; Véjux and Lizard, 2009; Mahalakshmi et al., 2021; Radhakrishnan et al., 2025). Together, these oxysterols engage shared receptor systems, including LXRs, G protein-coupled receptor 17 (Gpr17) and G protein-coupled receptor 183 (Gpr183; also known as Epstein–Barr virus-induced receptor 2—Ebi2) across neurons and glial cells, positioning oxysterol signaling as a central integrator of metabolic, immune, and degenerative processes in the healthy and diseased brain (Table 2).
Table 2
| Oxysterol | Primary source/production | Enzymes | Key receptors | Protective/homeostatic roles | Toxic/pathological effects in the CNS | References |
|---|---|---|---|---|---|---|
| Cholesterol |
|
Oxysterol precursor | LDLR, LRP1 |
|
|
Dietschy and Turley (2004), Pfrieger and Ungerer (2011) |
| 24S-hydroxycholesterol (24S-OHC) |
|
CYP46A1 | LXRα/β; NMDA receptors |
|
|
Lund et al. (1999), Björkhem et al. (2009), Testa et al. (2016) |
| 7α-hydroxycholesterol (7α-OHC) |
|
CYP7B1 | LXRα/β |
|
|
Russell (2003), Björkhem and Meaney (2004) |
| 27-hydroxycholesterol (27-OHC) |
|
CYP27A1 | LXRα/β; ERα |
|
|
Heverin et al. (2005), Björkhem et al. (2009), Hughes et al. (2013) |
| 25-hydroxycholesterol (25-OHC) |
|
CH25H | LXRα/β |
|
|
Bauman et al. (2009), Cyster et al. (2014) |
| 7β-hydroxycholesterol (7β-OHC) |
|
Non-enzymatic | Interacts with a variety of cellular receptors and pathways to exert its effects |
|
|
Poli et al. (2013), Testa et al. (2016) |
| 7-ketocholesterol (7-KC) |
|
Non-enzymatic | Interacts with several established nuclear receptors and other protein targets, particularly microglia |
|
|
Véjux and Lizard (2009), Anderson et al. (2020), Testa et al. (2016) |
Overview of oxysterol sources and role in the CNS.
Conceptual takeaway: Oxysterols act as context-dependent lipid signals in the CNS, integrating cholesterol metabolism with receptor-mediated transcriptional, synaptic, and immune pathways. Enzymatically produced oxysterols primarily support cholesterol homeostasis and neuronal function, whereas oxysterols generated during inflammation or oxidative stress accumulate to drive neurotoxicity, neuroinflammation, and myelin dysfunction, thereby linking metabolic imbalance to neurodegenerative disease.
3 Oxysterols as a mediator of neuronal function
Oxysterols affect neuron and interneuron function by modulating neurotransmitter release, ion channel activity and nuclear receptor signaling while also influencing oxidative stress, mitochondrial health and glia–neuron interactions (Figure 1). Beneficial oxysterols such as 24S-OHC can enhance plasticity, whereas toxic oxysterols such as 7-KC drive dysfunction and neurodegeneration. Oxysterols modulate neurotransmission both acutely through direct allosteric modulation of ionotropic receptors and membrane effects and chronically through transcriptional regulation via LXRs and oxidative stress pathways. Their actions alter the excitatory/inhibitory balance, which is beneficial for plasticity in normal physiology but pathological when dysregulated. Oxysterols can influence both neurotransmission and neuromodulation by binding to specific receptors such as nuclear receptors or G-protein-coupled receptors, thereby modulating ion channel activity, synaptic plasticity, and neuronal excitability. Through these mechanisms, oxysterols act as key modulators of neuronal communication, linking cholesterol metabolism to dynamic regulation of CNS signaling. In addition to their roles in cholesterol homeostasis, oxysterols participate in neurodevelopment, neuroprotection and the regulation of neuroinflammatory responses, positioning them as important mediators of neuronal health and CNS function. Oxysterol signaling can directly regulate neurotransmission. For instance, oxysterols can alter glutamatergic signaling via direct interference in the function of ionotropic glutamate receptors (NMDA, AMPA, and Kainate), altering gamma-aminobutyric acid (GABA) inhibition and via alterations in neurotransmitters systems like acetylcholine, serotonin and dopamine. Glutamatergic signaling can be influenced by oxysterols via direct regulation of NMDA, AMPA and kainate receptor (KAR) function. As such excess oxysterols (e.g., 7-KC, 27-OHC) can cause glutamate excitotoxicity and neuronal injury (Ma et al., 2010). In neurodegenerative disorders such as MS and AD, dysregulated oxysterol signaling may contribute to glutamate excitotoxicity and demyelination (Diestel et al., 2003; Ong et al., 2010; Jeitner et al., 2011).
3.1 Oxysterol regulation of ion channels
Oxysterols can modulate the function of ion channels (Bezine et al., 2018b), contributing to membrane fluidity and lipid raft composition, the activity of voltage-gated sodium and potassium channels and by acting as a positive allosteric modulators for ionotropic glutamate receptors (Olsen et al., 2012; Filomenko et al., 2015). By embedding into the cell membrane, oxysterols impact cholesterol–sphingolipid-rich domains (lipid raft organization) which can lead to alterations in the clustering and function of ion channels and receptors (Filomenko et al., 2015). Side-chain oxysterols can alter membrane thickness, leading to lateral expansion of the bilayer and increasing permeability. Such effects can influence protein behavior, potentially affecting receptor or channel localization (Olsen et al., 2012). For instance, 7β-OHC and 7-KC have shown to destabilize lipid rafts, whereas 25-OHC tends to stabilize them (Wnętrzak et al., 2022). Many membrane channels are localized to lipid rafts, including ion channels. These rafts influence channel activity through direct lipid-protein interactions, alterations in bilayer properties and recruitment of scaffold proteins such as caveolin, impacting protein gating, trafficking and localization (Dart, 2010). In neuronal membranes, such rafts help maintain the clustering and function of voltage-gated channels and synaptic receptors (NMDA, AMPA, and KAR). When oxysterols disrupt raft integrity, channels and receptors may become miss-localized causing altered ion gating or synaptic signaling. This miss-localization could result in compromised excitability and synaptic plasticity, thereby increasing neuronal vulnerability.
Oxysterols can modulate calcium and potassium channel activity via direct channel interaction or subunit composition in addition to alterations in lipid rafts (Bezine et al., 2018a; Bezine et al., 2018b). Direct modulation of ion channel activity impacts excitability of pyramidal neurons and interneurons increasing calcium influx resulting in hyperexcitability or excitotoxicity (Verma et al., 2022). Oxysterols can influence cytoplasmic Ca2+ levels by binding directly to the channel-forming subunits of calcium channels, thereby modulating their gating or electrophysiological properties (Mackrill, 2011). Disruption of their function can lead to altered firing patterns and excitability in neurons. In cortical pyramidal neurons, the activity of large conductance calcium channels at the soma and dendrites regulates action potential repolarization and dendritic spike repolarization, respectively (Bock and Stuart, 2016). Voltage-gated potassium (Kv) channels, such as the Kv3 subfamily can also be influenced by oxysterols. Kv channels are essential for fast-frequency firing in inhibitory interneurons. Alterations to the structure or function of these channels can lead to an imbalance of inhibitory and excitatory neurotransmission, affecting neuronal excitability (Bezine et al., 2018a). Inhibitory interneurons rely on Kv3 channels for fast firing and are particularly sensitive to disruptions in ion channel function. Oxysterol-induced alterations in these channels can impair inhibitory signaling, leading to excitation and inhibition imbalances resulting in potential hyperexcitability (Faulkner et al., 2024).
3.2 Oxysterol regulation of ionotropic receptors
Recent work has identified oxysterols as important modulators of ionotropic glutamate receptors (iGluRs), positioning these cholesterol-derived metabolites as regulators of excitatory neurotransmission and synaptic plasticity. Acting as endogenous modulators, oxysterols influence receptor function and neuronal excitability, thereby linking brain cholesterol metabolism to glutamatergic signaling in both physiological and pathological contexts (Linsenbardt et al., 2014; Wei et al., 2019; Tang et al., 2020). Accumulating evidence suggests that these lipid mediators can fine-tune synaptic function through direct and indirect interactions with iGluRs, with potential consequences for cognition and neuropsychiatric disease. Though there is limited evidence for direct modulation of GABAergic receptors, the indirect influence of oxysterols on GABAergic signaling is more compelling. These findings highlight the significance of oxysterols in modulating ionotropic receptors, evidencing their potential as therapeutic targets for neurological disorders.
3.2.1 Oxysterol modulation of NMDA receptors
24S-OHC acts as a positive allosteric modulator of NMDARs, enhancing receptor currents and excitatory drive to ultimately influence excitatory neurotransmission. More specifically, 24S-OHC enhances receptor activity by increasing channel opening probability and current amplitude (Paul et al., 2013; Sun et al., 2016) and reducing endogenous 24S-OHC dampens NMDAR function without impacting homeostatic excitability of hippocampal neurons (Sun et al., 2016). The direct oxysterol-receptor interactions appear to be subunit-dependent with receptors containing the NMDA receptor subunit 2B (NR2B) showing greater sensitivity to potentiation by 24S-OHC (Paul et al., 2013; Wei et al., 2019). As NR2B containing NMDARs are thought to have slower activation/deactivation kinetics, leading to an increase in total Ca2+ influx into the cell, it is plausible that potentiation of these receptors by 24S-OHC may promote synaptic plasticity (see Shipton and Paulsen, 2014 a review).
Though increased Ca2+ influx can promote synaptic plasticity, chronic elevation may drive excitotoxicity. In epilepsy, enhanced NMDAR activation by oxysterols may promote seizure susceptibility (Hanin et al., 2021; Nishi et al., 2022). Evidence suggests that 25-OHC and 27-OHC also potentiate NMDARs, though less potently than 24S-OHC. NMDA activation by these oxysterols can contribute to excitotoxic cascades when present at high concentrations contributing to cellular dysfunction. 7-KC can also alter NMDA function, however, instead of potentiation it disrupts receptor function indirectly through oxidative stress and lipid raft destabilization (Wheless and Rho, 2025). In AD, elevated 24S-OHC and 27-OHC are linked to early synaptic dysfunction (Papassotiropoulos et al., 2002; Russell, 2003; Merino-Serrais et al., 2018; Wang et al., 2019; Wang et al., 2022). Excessive oxysterol accumulation in AD and MS contribute to overactivation of NMDARs sustaining Ca2+ influx for longer activating proteases, ROS generation and mitochondrial dysfunction therefore contributing to neurodegeneration (Gamba et al., 2021).
3.2.2 Oxysterol modulation of AMPA receptors
There is growing evidence that oxysterols also modulate AMPA receptor mediated fast excitatory neurotransmission (Wheless and Rho, 2025). Unlike NMDA receptors, AMPARs are less directly sensitive to oxysterols but 24S-OHC, 25-OHC, and 27-OHC can modulate AMPA receptor kinetics, trafficking and synaptic incorporation (Brachet et al., 2015; Korinek et al., 2020). Thus, enhancing excitatory neurotransmission and plasticity in physiology but promoting excitotoxicity in disease context. Oxysterol-induced AMPAR potentiation may lower seizure threshold and worsen excitotoxic damage in people with epilepsy or following ischaemic injury (Wheless and Rho, 2025). In AD, elevated 24S-OHC and 27-OHC reported to alter AMPAR trafficking and impair memory circuits (Merino-Serrais et al., 2018; Staurenghi et al., 2021; Wang et al., 2022). 24S-OHC may weakly potentiate AMPAR-mediated currents in neurons. 24S-OHC acts indirectly by altering membrane cholesterol composition affecting receptor function and mobility (Martin et al., 2014; Korinek et al., 2020). 25-OHC and 27-OHC are reported to alter AMPAR desensitization kinetics and potentially enhance current amplitude in hippocampal neurons (Martin et al., 2014; Korinek et al., 2020). Meanwhile, 7-KC is associated with reduced AMPAR function, possibly by disrupting receptor trafficking and membrane organization. Synaptic plasticity is affected by AMPA receptors being disrupted by oxysterols, such as 24S-OHC which supports early-phase long-term potentiation (LTP), strengthening excitatory transmission (Martin et al., 2014). Conversely, 7-KC accumulation may impair LTP and favor LTD, contributing to cognitive decline in certain neurological disorders (Nury et al., 2020). By increasing AMPAR-mediated excitatory post-synaptic currents (EPSCs), oxysterols shift the excitation/inhibition balance toward excitation, especially when this occurs alongside NMDAR potentiation. This effect can amplify glutamatergic signaling substantially, and sustained AMPAR potentiation, specifically those that lack the 2A subunit, can lead to excitotoxicity due to Ca2+ overload and when sustained for long enough can ultimately lead to neuronal death.
3.2.3 Oxysterol modulation of KA receptors
To date, there is little evidence indicating that oxysterols act as direct allosteric modulators of KARs. However, oxysterols influence KARs indirectly through the membrane lipid environment (Korinek et al., 2020; Wnętrzak et al., 2022; Wnętrzak et al., 2024). KARs are highly sensitive to cholesterol and lipid raft composition. Oxysterols such as 24S-OHC, 25-OHC, 27-OHC and 7-KC intercalate into membranes and alter receptor conformation and trafficking. In neurodegenerative diseases such as AD, amyotrophic lateral sclerosis (ALS) and MS elevated oxysterols disrupt lipid rafts, impairing KAR stability at synapses and contribute to synaptic failure (Grassi et al., 2020; Vallés and Barrantes, 2022; Wnętrzak et al., 2022). KARs can also be disrupted via crosstalk with NMDA and AMPA receptors. For example, KARs share synaptic signaling domains and oxysterol-driven enhancement of glutamatergic tone via NMDA/AMPA receptors indirectly changes KAR activation (Vallés and Barrantes, 2022). Oxysterols could also alter KARs presynaptic modulation by regulating glutamate release probability at excitatory terminals (Lauri et al., 2005). By changing membrane cholesterol and calcium dynamics, oxysterols may increase presynaptic glutamate release via KAR activation amplifying excitation. In epilepsy, dysregulated KAR signaling - already implicated in seizure susceptibility - may be worsened by oxysterol-induced increases in presynaptic glutamate release (Rodríguez-Moreno and Sihra, 2013; Fritsch et al., 2014). KARs contribute to slower EPSCs and metabotropic-like signaling (Lauri et al., 2005). Oxysterols that destabilize cholesterol-rich domains such as 7-KC, can impair KAR clustering at postsynaptic densities, weakening KAR-mediated excitatory currents (Farooqui, 2009; Wnętrzak et al., 2022).
KARs are expressed on both excitatory and inhibitory neurons and as such play a role in maintaining the excitation/inhibition balance within the brain. Oxysterol-driven disruption of KAR function could weaken inhibitory interneuron regulation, tipping networks toward hyperexcitability. KARs contribute to forms of LTP/long term depression (LTD) ratio distinct from NMDARs. Oxysterol-induced shifts in receptor trafficking may bias synapses toward excitotoxic plasticity. KARs – especially those containing Glutamate receptor ionotropic kainate 4 and 5 (GluK4/GluK5), mediate oligodendrocyte and neuronal vulnerability (Lerma and Marques, 2013). Oxysterol accumulation in demyelinated lesions, notably 7-KC and 24S-OHC may exacerbate KAR-mediated excitotoxicity in oligodendrocytes (Matejuk et al., 2025). Oxysterols modulation of KARs indirectly by altering membrane cholesterol dynamics, receptor trafficking and glutamate release shapes excitatory/inhibitory neurotransmission balance potentially contributing to excitotoxicity in epilepsy and neurodegeneration. In summary, iGlu (NMDA, AMPA and KARs) potentiation by oxysterols facilitates LTP strengthening excitatory synapses linking cholesterol metabolism to learning and memory. By boosting glutamatergic drive, oxysterols shift the excitation/inhibition balance toward excitation, contributing to excitotoxicity.
3.2.4 Oxysterol modulation of GABA receptors
Excitation can also be exacerbated by reduced GABAergic signaling (Shore et al., 2020; Jiménez-Balado and Eich, 2021). Disturbances in oxysterol signaling may shift the excitation/inhibition balance, a feature implicated in many neurological disorders including epilepsy and schizophrenia (Shore et al., 2020; Santoro et al., 2024; Crotchett et al., 2025). Oxysterol effects on GABAergic interneurons involve redox balance, cholesterol metabolism and mitochondrial signaling (Poli et al., 2013; Calvo and González, 2016; Beltrán González et al., 2020). Oxysterols like 7-KC promote oxidative stress and ROS generation. ROS can modify GABAA_AA receptor subunits or presynaptic GABA release machinery, impairing synaptic inhibition (Beltrán González et al., 2020). Gamma-Amino-Butyric Acid-A (GABAA) receptor function is highly dependent on membrane cholesterol (Sooksawate and Simmonds, 1998, 2001; Meza et al., 2020; Yuan et al., 2025). Oxysterols can alter GABAergic inhibition via cholesterol depletion and substitution effects (Sooksawate and Simmonds, 1998; Meza et al., 2020). Pathological oxysterols can displace or promote an increase in cholesterol oxidization, leading to reduced GABAA receptor surface stability and faster receptor internalization reducing inhibition (Sooksawate and Simmonds, 1998; Meza et al., 2020; Jiménez-Balado and Eich, 2021). Inhibition can be classified in tonic and basic (Farrant and Nusser, 2005). Tonic inhibition is a persistent, low-level suppression of neuronal excitability mediated by extra synaptic GABAA receptors that respond to ambient levels of the neurotransmitter GABA in the brain. It provides a stable, baseline inhibitory tone that sets the overall excitability of neurons mediated by extra-synaptic GABAA. It sets neuronal excitability thresholds and contributes to network stability. Certain oxysterols may reduce inhibitory tone by destabilizing GABAA receptor function (Calvo and González, 2016; Mele et al., 2019; Michałowski et al., 2025). Tonic GABA currents depend on the stable surface expression of extra-synaptic receptors in cholesterol-rich microdomains (Carver and Reddy, 2013). Pathological oxysterols like 7-KC or 25-OHC can disrupt lipid raft integrity and reduce surface stability of extra-synaptic GABAA receptors (Carver and Reddy, 2013). Disruption of lipid raft integrity can weaken tonic inhibition and increase susceptibility to hyperexcitability. Tonic currents are also sensitive to redox signaling (Calvo and González, 2016). Tonic GABAA receptors are especially redox-sensitive therefore ROS from oxysterols, e.g., 7-KC may reduce their efficacy (Calvo and González, 2016; Anderson et al., 2020; Beltrán González et al., 2020). GABAA receptors are speculated to also be directly modulated by oxysterols. However, to this date there is no direct evidence indicating direct modulation. Altered tonic network excitability can enhance the amplitude and/or prolong the decay of phasic inhibitory postsynaptic currents (IPSCs) (Ataka and Gu, 2006; Labarrera et al., 2013). Oxysterols can also indirectly influence astrocytic cholesterol and GABA metabolism (Labarrera et al., 2013; Mahalakshmi et al., 2021; Liu et al., 2022; Radhakrishnan et al., 2025; Yuan et al., 2025). Elevated oxysterols, especially 7-KC, can impair astrocyte function and glutamate/GABA balance reducing extracellular GABA and weakening tonic inhibition (Mahalakshmi et al., 2021; Radhakrishnan et al., 2025). Conversely, 24S-OHC is produced largely by neurons and may help maintain inhibitory tone under physiological conditions (Leoni and Caccia, 2013; Vitali et al., 2014; Noguchi et al., 2015). Other oxysterols, e.g., 7-KC and 25-OHC can destabilize membrane lipid rafts, altering GABAA receptor trafficking or clustering at synapses (Radhakrishnan et al., 2007; Mahalakshmi et al., 2021; Radhakrishnan et al., 2025). Receptor trafficking and clustering may reduce phasic inhibition efficiency. Phasic inhibition is the rapid, short-lived inhibition of neuronal activity mediated by GABAergic neurotransmitters released from presynaptic terminals that activate GABAA receptors on the postsynaptic membrane (Farrant and Nusser, 2005). In contrast to tonic inhibition, which is a persistent inhibition caused by extracellular GABA activating extra synaptic receptors, phasic inhibition is triggered by discrete synaptic events (Farrant and Nusser, 2005). In other words, it is a fast synaptic IPSCs via clustered GABAA receptors. Since oxysterols strongly enhance glutamatergic signaling, relative dampening or enhancing of GABAergic phasic inhibition directly shifts the excitation/inhibition balance. For example, 24S-OHC might strengthen inhibition exerting a protective effect and 7-KC might weaken inhibition and be pro-excitotoxic. This imbalance (increased glutamatergic drive, reduced inhibitory control) contributes to hyperexcitability and excitotoxicity.
Both ionotropic glutamate and GABA receptors can be indirectly modulated by synaptic vesicle dynamics and altered release probability (Fattorini et al., 2019; Wallace and Sabatini, 2023). Oxysterols can incorporate into membranes, altering lipid raft structure and thereby modulating SNARE complex function and vesicle release probability, affecting glutamate and GABA release (Chamberlain et al., 2001; Salaün et al., 2005; Grassi et al., 2020). AMPA receptors alternate between synaptic and extra-synaptic membranes as part of synaptic plasticity (LTP/LTD). Oxysterols modify lipid raft structure and membrane fluidity, altering AMPAR dynamics and clustering at postsynaptic densities (Filomenko et al., 2015; Wnętrzak et al., 2022). It also modulates receptor endocytosis/exocytosis during activity-dependent plasticity (Kasimov et al., 2017; Petrov, 2024a). As a result, oxysterols can bias plasticity toward potentiation or depression depending on which oxysterol predominates. 7-KC has been shown to impair vesicular cycling and contribute to synaptic dysfunction in neurodegeneration (Mahalakshmi et al., 2021; Shin et al., 2024). Physiological oxysterols, e.g., 24S-OHC enhance tonic and stabilise inhibition by positively modulating δ-subunit–containing GABAA receptors (Leoni and Caccia, 2013; Vitali et al., 2014; Noguchi et al., 2015). Pathological oxysterols, e.g., 7-KC, 25-OHC reduce tonic inhibition via membrane disruption, receptor destabilization, oxidative stress and impaired astrocytic support (Mahalakshmi et al., 2021; Radhakrishnan et al., 2025). Receptor balance is crucial because tonic inhibition is a major controller of excitability therefore oxysterol shift can drive neural networks toward seizure susceptibility or neuroprotection depending on the context. Oxysterols influence phasic inhibition mainly by directly modulating GABAA receptor activity, altering receptor stability in cholesterol-rich membranes and indirectly through oxidative stress effects. Oxysterols like 24S-OHC tend to enhance phasic inhibition, whereas oxysterols such as 7-ketocholesterol (increased in pathological context) reduces it, contributing to network hyperexcitability and possibly seizures or neurodegeneration (Gamba et al., 2021).
3.3 Oxysterol modulation of cholinergic, serotonergic and dopaminergic systems
Neuromodulators are chemical signals that regulate neuronal activity and synaptic transmission over longer timescales and broader spatial domains than classical neurotransmitters. Neuromodulators such as dopamine, serotonin and certain neuropeptides can fine-tune network excitability, synaptic plasticity and overall information processing in the CNS. Oxysterols can influence the cholinergic, serotonergic and dopaminergic systems (Ma et al., 2009; Ullrich et al., 2010; Paul et al., 2017; Kosowski et al., 2021), leading to indirect influence on mood, motor control, cognition and consciousness. Cholesterol metabolism is essential for synaptic vesicle dynamics and oxysterols alter membrane lipid composition disrupting vesicle fusion and neurotransmitter release (Petrov, 2024b). As their name suggests, the primary neurotransmitter for cholinergic neurons is acetylcholine. Cholinergic receptors are divided in two mains types, nicotinic receptors (ligand-gated ion channels) and muscarinic receptors (G-protein-coupled receptors) 0.7-KC and 25-OHC impair choline uptake and ACh release by altering presynaptic terminal integrity and mitochondrial function (ATP supply for vesicle cycling) (Ullrich et al., 2010). Oxysterols also modulate cholinergic receptors (Ullrich et al., 2010; Baenziger et al., 2017; Petrov, 2024a). Membrane cholesterol and oxysterols can regulate nicotinic acetylcholine receptors (nAChRs) stability and gating (Baenziger et al., 2017). Oxysterols may reduce receptor function by disturbing lipid rafts where nAChRs cluster (Baenziger et al., 2017). Oxysterols, especially 27-OHC can also interact with estrogen receptors and G protein-coupled receptors (GPCR) signaling indirectly modulating muscarinic acetylcholine receptors (mAChRs) activity and downstream calcium responses (Wang et al., 2021). Critically relevant to diseases such as AD where both cholinergic decline and altered sterol metabolism occur (Auld et al., 2002; Reiss and Voloshyna, 2012; Comaposada-Baró et al., 2023). Cholinergic neuron loss is a hallmark of AD (Auld et al., 2002) and elevated oxysterols such as 24S-OHC, 27-OHC, 7-KC are detected in AD brains and cerebrospinal fluid (Dias et al., 2022). Such oxysterols may exacerbate cholinergic dysfunction via oxidative stress, mitochondrial damage and impaired receptor signaling. In PD, altered oxysterol metabolism may contribute to degeneration in the cholinergic basal forebrain and striatal interneurons worsening motor and cognitive symptoms (Müller and Bohnen, 2013; Gan et al., 2023; Brahmi et al., 2025). Oxysterols can also impair the cholinergic system by disrupting ACh release, destabilizing nicotinic receptor function, interfering with muscarinic signaling pathways, and aggravating neuroinflammation that normally would be modulated by cholinergic anti-inflammatory signaling receptors (Ullrich et al., 2010; Baenziger et al., 2017; Petrov, 2024a). These mechanisms link oxysterols to cholinergic deficits seen in neurodegenerative conditions like AD.
Dopamine synthesis and release can also be altered by oxysterols (Sacchetti et al., 2009; Hennegan et al., 2024). Dopamine neurons are highly sensitive to oxidative stress (Guo et al., 2018; Bej et al., 2024). Oxysterols like 7-KC and 27-OHC cause mitochondrial dysfunction and ROS production, impairing ATP supply and consequently vesicular dopamine release (Dasari et al., 2010; Wang et al., 2016; Anderson et al., 2020; McComb et al., 2021; Vejux et al., 2025). 24S-OHC has been shown to alter synaptic vesicle dynamics indirectly via effects on lipid rafts and calcium influx. Dopamine receptors’ function is also altered by oxysterols. Membrane cholesterol is crucial for GPCR stability, including D1/D2 receptors (Paila and Chattopadhyay, 2009; Kumar and Chattopadhyay, 2021; Christofidi et al., 2025). Excess oxysterols may destabilize receptor conformation disrupting dopamine receptor signaling. 27-OHC has been reported to reduce dopamine signaling (Marwarha et al., 2011) and contribute to learning and memory deficits in rats (Zhang et al., 2015). In PD, dopaminergic neurons in the substantia nigra accumulate oxysterols under oxidative stress (Hwang, 2013; Doria et al., 2016). Oxysterol 7-KC is cytotoxic to dopaminergic neurons and drives degeneration (Wang et al., 2016; Anderson et al., 2020). Dopaminergic disruption by oxysterols is also observed in psychiatric disorders such as schizophrenia (Sun et al., 2021) and depression (Sun et al., 2023). Dysregulated oxysterol metabolism may indirectly modulate mesolimbic dopamine circuits, affecting motivation and reward.
Oxysterols also affect serotonin synthesis and neuron viability (Dodge et al., 2021). Serotonergic neurons in the raphe nuclei are vulnerable to oxidative stress (Zhang C. et al., 2024). 7-KC and 27-OHC increase ER stress and apoptosis in serotonergic neurons potentially decreasing 5-HT availability. Oxysterols also indirectly disrupt serotonin transporter and receptors. Serotonin transporter function depends on lipid raft integrity and oxysterols disruption of lipid rafts alter serotonin reuptake (Daray et al., 2018). Oxysterols can also indirectly modulate serotonin receptors 5-HT1A and 5-HT2A GPCR signaling by disturbing membrane cholesterol balance (Daray et al., 2018). As such, elevated plasma 27-OHC has been associated with major depressive disorder as a result of impaired serotonin signaling, hypothalamic–pituitary–adrenal (HPA) axis dysregulation and hippocampal neurotoxicity (Daray et al., 2018; Sun et al., 2023; Wang et al., 2023). In AD, serotonergic deficits coexist with high oxysterol levels, compounding cognitive and mood symptoms (Testa et al., 2016).
Oxysterols impair cholinergic, dopaminergic and serotonergic function via oxidative stress, vesicle release impairment, receptor destabilization and transporter dysfunction. 7-KC and 27-OHC are largely neurotoxic, while 24S-OHC has dual roles, sometimes protective through LXRs, sometimes harmful at high levels. These mechanisms are implicated in several neurological disorders. Those neurotransmitter systems can also be modulated by oxysterol-driven LXR signaling (Mouzat et al., 2019). LXRs regulate genes for lipid metabolism but also influence dopamine/serotonin turnover and neuroinflammation (Töröcsik et al., 2009; Mouzat et al., 2019). 27-OHC crosses the blood–brain barrier and acts as an endocrine oxysterol, disrupting striatal dopamine and cortical serotonin simultaneously (Zhang et al., 2015; Kim et al., 2022). This may explain links between hypercholesterolemia and risk of mood disorders and PD (Kim et al., 2022; Sun et al., 2023). Cholinergic system function can also be indirectly modulated via LXR activation. Oxysterols like 24S-OHC and 27-OHC are natural LXR ligands. In neurons, LXRs regulate lipid homeostasis, synapse formation, and plasticity. In interneurons, LXR signaling can affect interneuron survival and development (particularly during cortical wiring). LXR regulates genes linked to cholesterol transport (ABCA1, ApoE), neuroinflammation, and synaptic proteins (Cao et al., 2007; Zhang R. et al., 2024). This influences long-term plasticity and homeostasis of neurotransmitter systems.
4 Oxidative stress, mitochondrial dysfunction, and lipid peroxidation
Oxysterols can act as pro-oxidant molecules and once formed can amplify oxidative stress. This effect is observed in the form of mitochondrial damage, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation and depletion of antioxidants. Some oxysterols - especially 7-KC—disrupt mitochondrial membrane potential, impair electron transport chain activity, and increase ROS leakage (Anderson et al., 2020). Increased oxysterol metabolism can drive a feed-forward cycle of ROS and oxysterol production, further amplifying oxysterol production, causing myelin and neuron damage (Bezine et al., 2018b). Research suggests that certain oxysterols, particularly those produced from non-enzymatic cholesterol oxidation, may be associated with neuroaxonal injury in people with MS. For example, higher levels of 7-KC and 7β-hydroxycholesterol (7β-OHC) are associated with increased levels of serum neurofilament light chain (sNfL, a protein found in the nervous system that is released into the blood and CSF when neurons are damaged commonly used as a marker of neuroaxonal damage) in people with MS. Some studies suggest that oxysterols may be markers of specific disease processes in MS, though more research is needed to fully understand these relationships. Oxysterols can be considered as biomarkers of choice but also as major actors in the pathophysiology of MS (Vejux et al., 2021). 7-KC and 24S-OHC enhance ROS production, leading to oxidative damage in neurons and glial cells. 7-KC and 24S-OHC can activate NADPH oxidases, boosting superoxide production. In vitro studies using neurons treated with 24-OHC in the presence of apocynin—a known inhibitor of NADPH oxidase—evidenced NADPH oxidase–mediated ROS generation under 24S-OHC treatment (Testa et al., 2018). 7-KC has shown to reduce mitochondrial membrane potential in oligodendrocytes (Nury et al., 2018), resulting in mitochondrial damage and reduced ATP production (Nury et al., 2018; Anderson et al., 2020). Similarly, 24S-OHC contributes to oxidative stress via increased ROS production, mitochondrial dysfunction and disruption of antioxidant systems. 24S-OHC can negatively affect the activity of mitochondrial complexes, which are a major site of ROS production. The increased oxidative stress and damage caused by high 24S-OHC levels contribute to neuronal cell death and dysfunction (Cigliano et al., 2019). Increased levels of 24S-OHC are a known factor in the development of AD, where it amplifies the neurotoxic action of amyloid-beta (Aβ). The presence of 24-OHC in the close vicinity of amyloid plaques enhance the adhesion of large amounts of Aβ to the plasma membrane of neurons amplifying the neurotoxic action of Aβ locally increasing ROS steady-state levels (Gamba et al., 2011; Gamba et al., 2021). Both 7-KC and 27-OHC have been linked to mitochondrial dysfunction in AD (Gamba et al., 2019). 27-OHC is elevated in AD patients and is associated with cognitive decline (Leoni and Caccia, 2011). Oxysterols can impair mitochondrial function by disrupting the electron transport chain, reducing ATP production, and increasing apoptosis. Neurons, especially fast-spiking interneurons, are highly energy-demanding and mitochondrial stress makes them vulnerable to dysfunction and loss disrupting network synchrony and function leading to neurodegeneration (Hughes et al., 2013). The combined effects of oxidative stress and mitochondrial dysfunction induced by 7-KC and 27-OHC ultimately contribute to myelin damage and neuronal cell death, key aspects of neurodegenerative diseases (Guo et al., 2013; Singh et al., 2019; Wen et al., 2025).
Oxysterols trigger stress kinases (p38, c-Jun N-terminal kinase—JNK) linking oxidative stress to apoptosis (Lordan et al., 2009). Several oxysterols including 24S-OHC, 25-OHC, 27-OHC, 7-KC can activate Mitogen-Activated Protein Kinase (MAPK) signaling pathways (Extracellular Signal-Regulated Kinase - ERK1/2, JNK, and p38) through the generation of ROS, which engage redox-sensitive kinases upstream of MAPK. Oxysterols generate ROS by damaging mitochondria, activating nitric oxide enzymes, inducing endoplasmic reticulum stress and amplifying inflammatory cascades. Additionally, oxysterols can also interact with other receptors such as LXR which converge on MAPK cascades. Oxysterol activation of JNK and p38 through ROS-dependent signaling, endoplasmic reticulum stress–mediated pathways and inflammatory receptor activation is generally associated with the promotion of apoptosis, while ERK activity inhibits apoptosis (Cross et al., 2000). Oxysterol-driven JNK and p38 activation promotes cell death in neurons and glial cells (Harper and LoGrasso, 2001; Choi et al., 2004). Numerous in vitro studies have demonstrated modifications in ERK-1/2 activity following exposure to oxysterols (Ares et al., 2000; Yoon et al., 2004; Leonarduzzi et al., 2005) therefore altering endogenous apoptosis inhibition. In vitro studies using C6 glioma cells 7β-OH application induced a decrease in ERK-1/2 activity followed by toxicity (Adamczyk et al., 1998). Moreover, administration of 7-KC in Tohoku Hospital Pediatrics-1 (THP-1) cells - a human monocytic leukemia cell line derived from a patient with acute monocytic leukemia—induced ERK-1/2 phosphorylation increasing apoptosis significantly (Berthier et al., 2005). Together, this evidence underscores how oxysterol-driven MAPK signaling can tip the balance toward neurodegeneration, with JNK and p38 activation overriding ERK’s protective effects to promote apoptosis in neurons and glia.
Neurons are inherently susceptible to oxidative stress, partly due to their relatively low levels of antioxidant enzymes such as Glutathione Peroxidase (GPx), Superoxide Dismutase (SOD) and catalase (Dringen, 2000). Oxysterols can consume glutathione (GSH) and inhibit antioxidant enzymes such as glutathione peroxidase and superoxide dismutase. Under oxidative-stress-related conditions, depletion of GSH and impairment of these antioxidant enzymes correlate with neurodegeneration (Testa et al., 2016). 7-KC was linked to increased ROS formation and GSH depletion in neurons, indicating oxidative stress as a key driver of cell damage (Lee et al., 2007). PC12 neuronal-like cells in vitro exposed to 7-KC show a decrease in GSH levels, mitochondrial dysfunction, elevated ROS and activation of caspase-3 apoptotic pathways (Han et al., 2007). The data suggest that mature neurons express low levels of Nuclear Factor E2-related Factor 2 (Nrf2) compared to glial cells (Levings et al., 2023). This inherently limited Nrf2 expression may constrain antioxidant responses, making neurons more susceptible to oxidative stress. Therefore, Nrf2 suppression in neurons potentially contributes to neurodegeneration. All those events contribute to oxidative stress exacerbating mitochondrial dysfunction, further impairing energy metabolism and neuron and glia survival.
Oxysterols also contribute to lipid peroxidation resulting in neuron degeneration (Sultana et al., 2013). Oxysterols initiate chain reactions of lipid peroxidation by producing lipid radicals and reactive aldehydes from polyunsaturated fatty acids in membranes, e.g., 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) (Gęgotek and Skrzydlewska, 2019). A large case–control study found elevated serum MDA levels in people with relapsing–remitting MS (RRMS), particularly during relapse phases. These levels correlated with disability scores (EDSS), with higher MDA detected in people with MS not on disease-modifying therapy (IFN-β) (Ghonimi et al., 2021). A meta-analysis reviewing oxidative stress markers across 31 studies found that MDA levels (and lipid hydroperoxides) in blood and CSF were consistently higher in people with MS compared to healthy controls (Zhang et al., 2020). In neurons, 4-HNE and MDA induce the formation of protein and DNA adducts impairing neuronal function. In AD, elevated 4-HNE adducts accelerate aggregation of tau and amyloid-β followed by synaptic loss (Dalleau et al., 2013; Sultana et al., 2013). In people with MS, lipid aldehyde adducts are found in CSF samples and demyelinated axons and are responsible for exacerbating neurodegeneration (Gonzalo et al., 2012; Zhang et al., 2020). Protein adducts are formed due to binding of 4-HNE and MDA amino acid residues Cys, His and Lys in neuronal proteins such as synaptic and cytoskeleton proteins, ion channels and receptors and mitochondrial enzymes. This leads to synaptic dysfunction, impaired plasticity and vulnerability to excitotoxic cell death of neurons (Neely et al., 1999). DNA adducts are formed when 4-HNE and MDA react with DNA bases resulting in replication error and mutation, activation of DNA damage response and mitochondrial damage. It causes neurons to accumulate DNA lesions and undergo progressive dysfunction (Voulgaridou et al., 2011). Furthermore, lipid peroxidation associated with GSH depletion leads to oxysterol-induced death of neurons via ferroptosis (Ren et al., 2020; Li et al., 2022). Lipid peroxidation also alters membrane fluidity, affecting ion channel function and receptor signaling (Ghosh et al., 1993; Chen and Yu, 1994). Oxidation of lipid rafts and synaptic vesicle membranes alters voltage-gated channels, N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA) and kainate receptor (KAR) localization consequently impairing neuronal function and making neurons vulnerable to excitotoxicity leading to neurodegeneration (Mattson, 1998; Hering et al., 2003; Korinek et al., 2020). Once initiated, lipid peroxidation can further oxidize cholesterol to produce more oxysterols in a feedback loop. These lipid peroxidation products also activate microglia, perpetuating oxidative stress and contributing to chronic neuroinflammation (Farooqui and Farooqui, 2011; Cumaoğlu et al., 2019). Oxysterols both result from and fuel oxidative stress. They damage mitochondria, activate ROS-producing enzymes, and initiate lipid peroxidation, leading to a cascade of events that worsens inflammation and cell death of neurons significantly contributing to neurodegeneration.
5 Roles of oxysterols in glial function and neuroinflammation
Glia are the non-neuronal cells of the CNS that provide essential support and regulation for neuronal function. The three major glial cells are astrocytes, oligodendrocytes and microglia. Astrocytes maintain extracellular ion and neurotransmitter homeostasis, regulate blood–brain barrier integrity and provide metabolic support to neurons. Oligodendrocytes generate and maintain myelin sheaths around axons, enabling rapid saltatory conduction and contributing to axonal health. Microglia are the resident immune cells of the CNS, responsible for surveillance, phagocytosis of debris and modulation of inflammatory responses. Together, these glial populations are crucial not only for maintaining CNS homeostasis but also for facilitating development, plasticity and repair. Dysregulation of these glial cell functions can contribute to chronic neuroinflammation, a hallmark of many neurodegenerative and neuropsychiatric disorders. Chronic neuroinflammation is a central feature of several neurodegenerative diseases like AD, PD and MS. Glial cell activation is a key component of neuroinflammation, accompanied by the activation of other cell types such as peripheral immune cells and neurovascular endothelial cells. Given the pro-inflammatory functions of oxysterols in the peripheral organs, it is plausible that oxysterols could be a key driver of neuroinflammation and neuronal damage in the brain. In AD patients, several oxysterols including 27-OHC, 25-OHC, 7α, OHC, 7-KC, 7β-OHC, 5α,6α-epoxycholesterol, 5β,6β-epoxycholesterol, 4α-OHC and 4β-OHC were increased in frontal and occipital cortex, coincided with an increased expression of pro-inflammatory markers such as IL-1β, IL-6, CCL2, MMP-9, and IL-8 (Testa et al., 2016). In addition, in an LPS-mediated neuroinflammation model, the concentration of 25-OHC in the cortex positively correlated with levels TNF, IL-6, and IL-1β (Romero et al., 2024).
5.1 The influence of oxysterols on oligodendrocyte lineage cells and myelin
5.1.1 Oxysterol regulation of inflammation in oligodendrocyte lineage cells
In murine oligodendrocytes (158 N), 7KC, 7β-OHC, and 24S-OHC have been identified as potent inducers of oxi-apoptophagy, a form of cell death involving the simultaneous activation of oxidative stress, apoptosis and autophagy pathways (Nury et al., 2015; Leoni et al., 2017). Treatment with these oxysterols resulted in reduced cell proliferation and adhesion, mitochondrial activity and nuclear fragmentation. Induction of apoptosis was characterized by caspase-3 activation, PARP degradation and the downregulation of Bcl-2. Oxidative stress was confirmed by the increased ROS production measured through the elevated concentrations of superoxide anion and hydrogen peroxide, while autophagy was indicated by an increased LC3-II/LC3-I ratio. Additionally, 7-KC has been shown to induce peroxisomal dysfunction, evidenced by the reduced expression of ABCD3 transporter and the activation of pexophagy, as observed through electron microscopy. This dysfunction was associated with an impaired peroxisomal β-oxidation, marked by increased levels of very long-chain fatty acids and the reduced expression of key genes involved in the β-oxidation pathway such as ABCD1, ACOX1 and MFP2 (Nury et al., 2018). In mice, stereotactic administration of 25-OHC into the brain has been shown to drive oligodendrocyte apoptosis in an NLRP3-dependent manner (Jang et al., 2016). Brain regions treated with 25-OHC revealed an increased co-localization of caspase-3 expression with MBP, compared to vehicle-treated controls. Furthermore, the 25-OHC-induced loss of oligodendrocytes was prevented in Nlrp3−/− out treated with 25-OHC, highlighting the critical role of the NLRP3 inflammasome in mediating 25-OHC-driven oligodendrocyte death. The pro-apoptotic role of 25-OHC has also been demonstrated in 158 N oligodendrocytes, where it induced apoptosis via increased caspase-3 cleavage activity. Mechanistically, 25-OHC activates the secretory phospholipase A2 type IIA (sPLA2-IIA) promotor and its subsequent expression through the activation of LXRβ and PXR. The activation appears to be dose-dependent, with low dose 25-OHC activating LXRβ signaling, while high doses activate PXR (Trousson et al., 2009). Apart from driving inflammation and cell death, oxysterols have also demonstrated to drive migration. In human MO3.13 oligodendrocytes, administration of 7α,25-OHC led to increased cell migration, an effect that was antagonized by NIBR189 (Velasco-Estevez et al., 2021).
5.1.2 Oxysterols as signaling molecules in the oligodendrocyte lineage
Oxysterol receptors are also expressed by cells glial cells (Figure 2), including cells of the oligodendrocyte lineage (Marinelli et al., 2016; Montani, 2021; Miralles et al., 2023) suggesting a role for oxysterol signaling in regulating these cells. Gpr17 is closely related to the Gpr183 which is known to be activated by 7α-25-DHC (Liu et al., 2011). Recent evidence suggests that 24S-OHC may act as an agonist for Gpr17 (Harrington et al., 2023). In the mouse brain, Gpr17 was found to be expressed by a subpopulation of OPCs (Miralles et al., 2023). This subpopulation of Gpr17+ OPCs displayed a transcriptomic signature distinct from Gpr17-neg OPCs, presenting enrichment towards synapse maturation and regulation of lipid metabolism (GO-term). Gpr17+ OPCs also behave differently under physiological conditions, presenting a reduced rate of differentiation compared to the overall OPC population (Miralles et al., 2023). Gpr17 receptor activation in OPCs triggers Giα interaction blocking adenylate cyclase enzyme and cyclic AMP production (cAMP). Inhibition of cAMP signaling cascade increases the expression of proteins involved in oligodendrocyte differentiation and myelination. When Gpr17 is conditionally knocked out of OPCs in the adult mouse brain, OPC proliferation is reduced while oligodendrogenesis is increased in the motor cortex (Miralles et al., 2023). In addition, a study using siRNA to silence Gpr17 reported an increase in the expression of mature OL markers and altered expression of genes involved in glucose metabolism and lipid biosynthesis (Marangon et al., 2022). Meanwhile, after a CNS insult, Gpr17+ OPCs react with proliferation and differentiation at a higher rate, supporting an important role in repair processes (Miralles et al., 2023). In contrast, Gpr17 activation in oligodendrocyte lineage cells impairs myelination. Receptor activation using Gpr17 agonist MDL29,951 (2-carboxy-4,6-dichloro-1H-indole-3-propionic acid) results in reduced myelin basic protein (MBP) expression. Gpr17 receptor activation in oligodendrocyte lineage cells triggered the Gαi/o signaling pathway, leading to a reduction in the adenylyl cyclase (cAMP) signaling cascade. Blocking cAMP signaling cascade inhibits protein kinase A (PKA) and cAMP response element-binding protein (CREB) reducing MBP expression and oligodendrocyte differentiation. In addition, Gpr17 activation also diminishes MBP expression by lessening stimulation of the exchange protein directly activated by cAMP (EPAC). The data evidence PKA and EPAC role as key downstream effectors of Gpr17 that inhibit oligodendrocyte maturation (Simon et al., 2016).
Figure 2
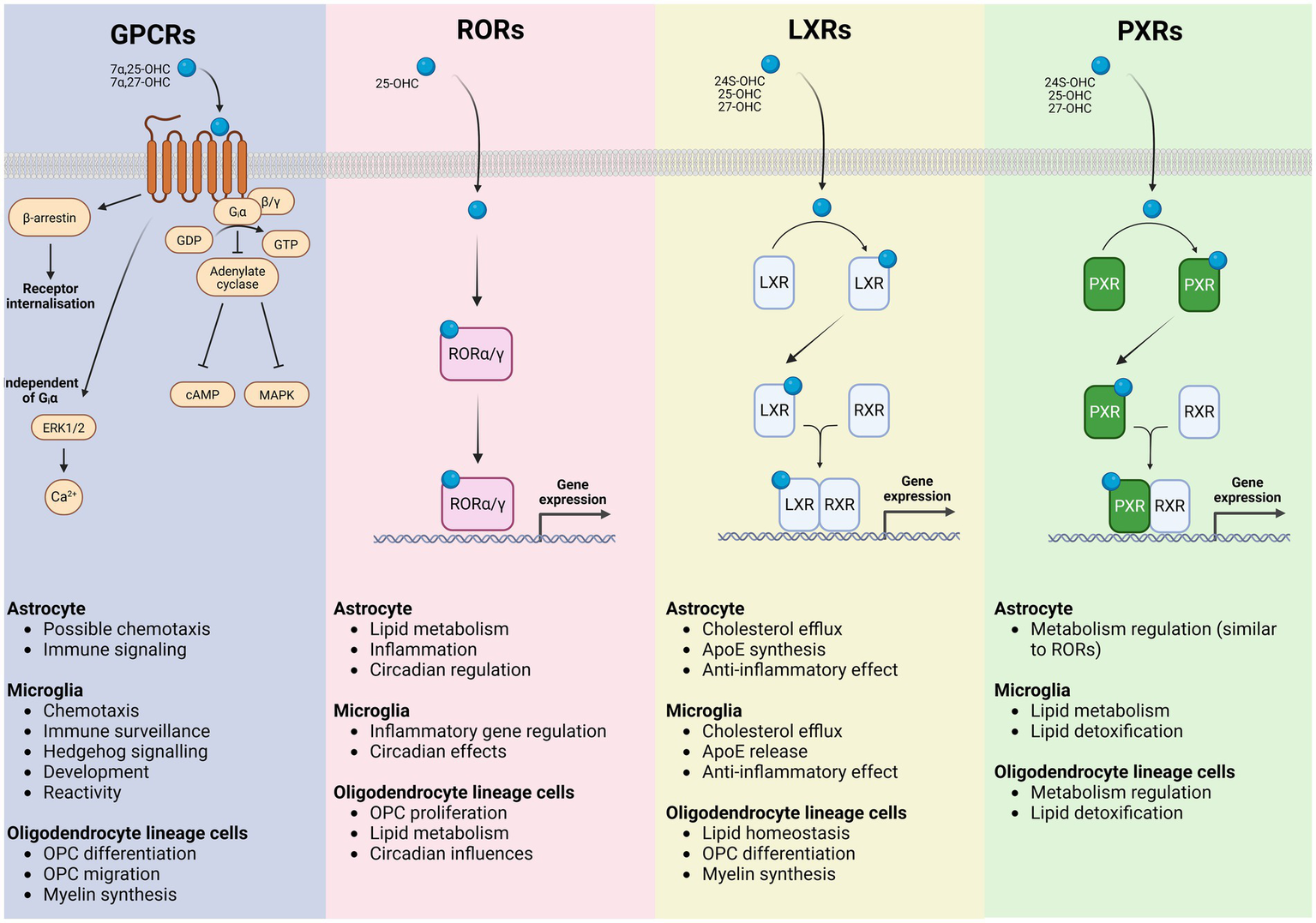
Oxysterol signaling receptors in glial cells and their downstream functional programs. Schematic representation of the major classes of oxysterol-responsive receptors expressed by astrocytes, microglia, and oligodendrocyte lineage cells, illustrating receptor-specific signaling mechanisms and the principal cellular processes they regulate. G protein–coupled receptors (GPCRs) respond to oxysterols including 7α,25-OHC and 7α,27-OHC, activating heterotrimeric G proteins and β-arrestin–dependent pathways that engage adenylate cyclase–cAMP, MAPK, ERK1/2, and Ca2+ signaling cascades, as well as receptor internalization. In glial cells, GPCR signaling contributes to astrocytic immune signaling and chemotactic responses, microglial chemotaxis, immune surveillance, developmental and hedgehog-related signaling, and regulation of microglial reactivity, while influencing OPC migration, differentiation, and myelin synthesis. Retinoic acid receptor–related orphan receptors (RORα/γ) are activated by oxysterols such as 25-OHC and function as ligand-dependent transcription factors that translocate to the nucleus to directly modulate gene expression programs controlling lipid metabolism, inflammatory tone and circadian regulation in astrocytes, inflammatory gene expression and circadian effects in microglia, and OPC proliferation, lipid metabolic pathways, and circadian influences within the oligodendrocyte lineage. Liver X receptors (LXRα/β) are activated by 24S-OHC, 25-OHC, and 27-OHC, heterodimerize with retinoid X receptors (RXRs), and bind to LXR response elements to drive transcription of genes involved in cholesterol efflux and lipid homeostasis. In astrocytes and microglia, LXR signaling promotes cholesterol export, ApoE synthesis and release and exerts anti-inflammatory effects, whereas in oligodendrocyte lineage cells it supports lipid homeostasis, OPC differentiation, and myelin synthesis. Pregnane X receptors (PXRs), activated by several oxysterols including 24S-OHC, 25-OHC, and 27-OHC also heterodimerize with RXRs to regulate transcriptional programs associated with metabolic control and xenobiotic and lipid detoxification. PXR signaling in microglia and oligodendrocyte lineage cells is linked to lipid metabolism and detoxification pathways, with astrocytic roles overlapping those mediated by RORs. Collectively, these receptor systems integrate oxysterol cues to coordinate glial lipid metabolism, inflammatory responses, and myelination, providing a mechanistic framework linking cholesterol-derived metabolites to glial functions in health and disease.
Oxysterols also interact with the nuclear LXRs, critical for lipid homeostasis in oligodendrocytes (Shackleford et al., 2017). Activation of LXRs mediated by oxysterols like 24(S),25-epoxycholesterol (24,25-EC) and 25-OHC has been shown to regulate genes involved in lipid metabolism and myelination (Shackleford et al., 2017). Oxysterols in the cell cytoplasm bind to LXR receptor resulting in dimerization with RXR subunit. LXR-RXR dimer translocate into the cell nucleus where it acts as a transcription factor regulating genes involved in OPC differentiation and myelination. Platelet-derived growth factor receptor alpha (Pdgfrα) and chondroitin sulfate proteoglycan (NG2) are markers exclusively expressed by OPC in the brain and known to be LXR-regulated. Pdgfrα gene and its regulation by LXRs may be one mechanism through which oxysterols signaling helps to control oligodendrocyte numbers in the CNS (Xu et al., 2014; Song et al., 2022). LXRα/β and their ligands in OPCs and oligodendrocytes and have been shown to promote myelination and remyelination in the cerebellum. Using a lysolecithin-induced demyelination of organotypic cerebellar slice cultures, Meffre et al. (2015) could show LXR activation enhanced oligodendrocyte maturation and remyelination. In addition, 25-OHC or synthetic TO901317 activation of LXRs induced myelin gene expression at the promoter, mRNA, and protein levels evidencing a direct transcriptional control of myelin gene expression (Meffre et al., 2015). Altogether, the findings evidenced the transcriptional control of myelin gene expression and the role of LXRs as positive modulators in central myelination and remyelination.
Oligodendrocyte lineage cells respond to oxysterol signaling via Gpr183. In mouse models, signaling via Gpr183 (or Ebi2) improved myelination and remyelination both in vivo (Konieczna-Wolska et al., 2024) and ex vivo (Rutkowska et al., 2017). Similar to Gpr17, Gpr183 activation in OPCs can potentially trigger Giα interaction inhibiting cAMP signaling cascade and increase the expression of proteins involved in oligodendrocyte differentiation and myelination (Rutkowska et al., 2017; Konieczna-Wolska et al., 2024). However, it can also act independently of Giα activating ERK1/2 pathway resulting in increased intracellular calcium signaling enhancing OPC proliferation, differentiation and myelination. Gpr183 activation can also trigger β-arresting activation and receptor internalization, reducing the signaling via Giα pathway enhancing signaling via ERK1/2 pathway. Interestingly, ERK signaling pathway is a known regulator of cell migration, especially in OPCs. As Gpr183 mediates immune cell migration (e.g., T-cell, B-cell, macrophages, eosinophils) towards oxysterol gradients (e.g., 7α,25-OHC), this could suggest a Gpr183 role in OPC chemotactic migration toward oxysterol gradients yet to be explored. It is still unclear whether Gpr183 receptor expression is restricted to a sub-population of OPCs or any specific brain regions, similar to observations from Gpr17 receptor studies in oligodendrocyte lineage cells. Pharmacological blocking of Gpr183 in cerebellar organotypic slice culture transiently delayed MBP expression staggering myelination (Rutkowska et al., 2017). Moreover, application of Gpr183 agonist 7α,25-OHC protected organotypic cerebellar slice cultures from lysolecithin (LPC) induced demyelination and reduced the levels of pro inflammatory cytokines IL1β and IL6 (Rutkowska et al., 2017). In vivo activation of Gpr183 using 7α,25-OHC did not enhance remyelination beyond the levels observed in spontaneously remyelinating tissue in the cuprizone demyelination model (Konieczna-Wolska et al., 2024). As 7α,25-OHC has a very short half-life when administered to mice via oral gavage (~30 min), fluorinating the endogenous 7α,25-OHC molecule to obtain the analogue CF3-7α,25-OHC rendered it more stable and increased its half-life to almost 10 h, allowing longer bioavailability for mice treatment. Interestingly, application of CF3-7α,25-OHC in the cuprizone model showed that extending the bioavailability of 7α,25-OHC is sufficient to accelerate remyelination in vivo. In contrast to the endogenous ligand, the analogue upregulated brain expression of GPR183 receptor and the lipid synthesis in the mouse corpus callosum. Furthermore, the CD4 + transcripts in the cerebellum and CD4 + cell number in the corpus callosum were reduced compared to vehicle-treated mice. These findings suggest a mechanism by which GPR183 / 7α,25-OHC signaling accelerates remyelination and modulates the immune response in the in vivo cuprizone model (Konieczna-Wolska et al., 2024). When Gpr183 is knocked out from the mouse brain of mice that received cuprizone treatment myelin recovery is less efficient (Luxol fast blue and MBP staining) and inflammatory signaling via Abl1 and NFkB1 is altered (Klejbor et al., 2021). Moreover, Gpr183 expression and total cholesterol levels are upregulated during recovery period after cuprizone administration, potentially enhancing remyelination. Gpr183−/− mice that received cuprizone also presented attenuated loss of callosal CC1 + oligodendrocytes, suggesting a possible protective role for Gpr183 (Klejbor et al., 2021). Although there is evidence supporting the importance of oxysterols and their receptors to oligodendrocyte lineage cell function in health and disease, numerous gaps in the knowledge are found and more studies are required to fully understand how oxysterols regulate OPC and oligodendrocyte function and potentially promote myelin repair.
5.1.3 Influence on myelin structure and function
Cholesterol and its derivatives are crucial for the synthesis of membranes, including myelin. They require large amounts of cholesterol for myelin membrane synthesis and structure maintenance. Oligodendrocytes are also the main players in cholesterol production in the brain, accounting for most of its biosynthesis (Dietschy, 2009; Berghoff et al., 2022; Qian et al., 2022). While oligodendrocytes are the primary source of cholesterol biosynthesis in the brain, other cells such as astrocytes and neurons also contribute to cholesterol synthesis to a lesser extent (Zhang and Liu, 2015; Qian et al., 2022). Some oxysterols can act as precursors for cholesterol biosynthesis, while others regulate cholesterol efflux or uptake. For instance, the oxysterol 24-OHC is produced within the neuron by the enzyme CYP46A1 allowing efflux from both neurons and glial cells maintaining brain cholesterol homeostasis (Björkhem, 2006; Segatto et al., 2019). 24-OHC can act as a transporter for cholesterol across the blood brain barrier. 24-OHC activates LXRs acting as transcription factor and regulating cholesterol biosynthesis (Radhakrishnan et al., 2007). Oxysterol activation of LXRs upregulate genes necessary for cholesterol synthesis and myelin formation (Shackleford et al., 2017) regulating myelin gene expression via Wnt/β-Catenin signaling pathway (Makoukji et al., 2011). Genetic ablation of LXR in the cerebellum reduced the expression of myelin basic protein (MBP), proteolipid protein (PLP) and ATP binding cassette subfamily A member 1 (ABCA1), all crucial for myelin formation and maintenance (Meffre et al., 2015; Song et al., 2022). Moreover, LXRs activation using 25-OHC greatly increased the mRNA expression of MBP, PLP and ABCA1 in primary mixed glial cell culture highlighting oxysterol vital role for myelin synthesis and maintenance (Meffre et al., 2015).
Oxysterols influence neural circuit establishment even before myelination takes place. They act during cortical development, influencing neural stem cell/progenitor behaviors and disruption of certain oxysterol metabolic pathways can lead to neurogenic defects and neurodevelopmental disorders (Tomita et al., 2022; De La Fuente et al., 2024). In mice, prior to embryonic stage E18, when the brain is not yet myelinated, CYP46A1, the enzyme that produces 24-OHC is downregulated (Wang et al., 2021). During that period, oxysterols act in oligodendrocyte lineage cell function independent of myelination - a process yet to be understood as the first wave of OPCs arise in the brain at E12. After birth CYP46A1 enzyme expression is upregulated and 24-OHC production in the brain starts - the major oxysterol in the brain after birth - coinciding with myelin genesis. Oxysterol balance keeps maintaining myelin structure throughout adulthood, playing an important role in altering lipid rafts in oligodendrocyte membranes, impacting myelin composition, fluidity and compaction. Oxysterols alter myelin directly either by increasing its fluidity causing raft destabilization, e.g., 7β-OHC or exerting stiffening promoting activity, e.g., 25-OHC (Wnętrzak et al., 2022). Therefore, oxysterols are extremely important for the establishment and maintenance of myelin, directly affecting its function in neural circuits.
Oxysterols can directly and indirectly alter action potential generation and propagation (Bezine et al., 2018b). Ion channel modulation by oxysterols leads to changes in resting membrane potential, the depolarization threshold, rate of depolarization and action potential duration. Oxysterols can also alter action potential propagation and synchronicity via modulation of myelin (Bezine et al., 2018b). Distinct localization of sterols and oxysterols in the mouse brain revealed regional cholesterol metabolism evidencing regional differences coinciding with distinct levels of myelination and consequently distinct neural circuit function (Yutuc et al., 2020). Oxysterols potentially regulate distinct regional myelination levels via nuclear receptors such as LXR (see above), regulating cholesterol turnover influencing membrane fluidity and signaling pathways. It results in regional differences in propagation and synchronicity of action potentials ultimately helping shape neural circuit formation and function.
Oxysterols can also become cytotoxic causing oxidative stress, dysfunction and death of neurons and glial cells altering neural circuits and contributing to demyelinating disorders (Björkhem et al., 2009; Bezine et al., 2018b). For instance, in multiple sclerosis (MS) and other demyelinating disorders, oxysterols may contribute to oligodendrocyte damage by promoting apoptosis and neuroinflammation. Cytotoxic oxysterols can induce caspase-independent myelin figure formation - lipid rich multilamellar cytoplasmic structures - and caspase-dependent polar lipid accumulation (Vejux et al., 2007). Myelin figure formation in the cytoplasm is a caspase-independent event closely linked with the cytotoxicity of oxysterols associated with oligodendrocyte dysfunction and neurodegeneration in demyelinating disorders. Similarly, caspase-dependent polar lipid accumulation resulting from cytotoxic oxysterols activity leads to cellular dysfunction and neurodegeneration. Local oxysterols and cholesterol metabolism also orchestrates remyelination by mediating the activity of LXR nuclear receptors, upregulating expression of genes that promote myelin genesis (Berghoff et al., 2022). The presence of elevated levels of pro-inflammatory oxysterols in the CNS correlate with increased microglial, macrophage and T-cell activation, indirectly impairing OPC differentiation and remyelination (de Freitas et al., 2022). Therefore, oxysterol metabolism dysfunction can lead to neurodegeneration associated with demyelinating disorders, altering the function of neural circuits. Moreover, it evidences the potential of oxysterol signaling pathway pharmacological targeting to prevent demyelination and consequent neurodegeneration and/or promote myelin repair.
5.2 Oxysterols in neuroinflammation
5.2.1 Oxysterols activate neurons and astrocytes to mediate their cytotoxic and pro-inflammatory effect
While several oxysterols have been described to activate pro-inflammatory signaling and cell death pathways in neurons and astrocytes, several studies have proposed that 27-OHC and 24S-OHC are the primary mediators of these effects. Oxysterols exhibit distinct yet overlapping pro-inflammatory and cytotoxic effects in neurons, mediated through cell-specific signaling pathways. For instance, in SH-SY5Y neurons, 27-OHC activates the TGF-β/NF-κB signaling pathway, inducing TNFa production and iNOS expression, while suppressing IL-10 production (Ma et al., 2019). Additionally, another study found that 24-OHC, 27-OHC and 7β-OHC treatment in SH-SY5Y neurons leads to TLR4 activation, which in turn activates COX-2 and mPGES-1 resulting in increased expression of IL-8, MCP-1, β1-integrin, CD46 and MMP-9 (Testa et al., 2014). Apart from triggering the pro-inflammatory signaling cascade, 24S-OHC has also been shown to promote cell death signaling pathways in neurons. In SH-SY5Y neurons, 24S-OHC activates ACAT-1 (Chesnokov et al., 2021), leading to the esterification of 24S-OHC into lipid droplets. This process triggers the downstream SH- signaling of RIPK1 and the activation of necroptosis, independent of caspase-8 activation (Yamanaka et al., 2011). Apart from SH-SY5Y neurons, 27-OHC, 7β-OHC and 24S-OHC have demonstrated to upregulate CD36 and β1-integrin expressions in SK-N-BE cells, whereas only 24S-OHC induces CD36 and β1-integrin expression in NT-2 human neurons, enhancing adhesion of neuronal cells to Aβ1–42. Notably, only 24S-OHC potentiates the neurogenic effects of Aβ1–42 by increasing ROS (Gamba et al., 2011) production. This effect of 24S-OHC augmenting the neurogenic effect of Ab1–42 is also observed in another differentiated human neuroblastoma cell line, MSN (Ferrera et al., 2008). In astrocytes, 27-OHC triggers the TGF-β/TLR4 signaling pathway in C6 astrocytes, inducing iNOS production while suppressing TNFα, IL-1b and IL-10 production (Pfrieger and Ungerer, 2011; Ma et al., 2019). In addition, 27-OHC have also been demonstrated to induce cellular cytotoxicity by increasing ROS production and downregulating antioxidant enzymes (rGSH, GSH-Px and tSOD). This oxidative stress response is exacerbated by the suppression of Nrf2/HO-1 signaling pathway, which is required for the antioxidant response (Ma et al., 2015). These findings highlight the context-dependent effect of 27-OHC in astrocytes, where it may dampen specific pro-inflammatory cytokines while exacerbating oxidative stress response to cause cellular cytotoxicity. Beyond its role in mediating astrocyte migration, the Gpr183/oxysterol has also been implicated in astrocyte migration. In primary mouse astrocyte cultures, antagonism of Gpr183 and CYP7B1 activity using NIBR189 and clotrimazole respectively, reduced LPS-mediated migration. Although 7α,25-OHC was not directly evaluated in the study, these findings further provide evidence supporting the involvement of the Gpr183/oxysterol axis in inflammation-driven astrocyte migration (Caratis et al., 2025a). The pro-inflammatory effects of oxysterols on neurons have also been investigated in vivo. In C57BL/6 J mice, subcutaneous injection of 27-OHC led to increased Aβ1-40 and Aβ1-42 accumulation in the plasma and brain, accompanied by an increased TNFα, IL-6, IL-1b, and IL-17 concentrations in the brain, contributing to cognitive defects (Wang et al., 2020; Jing et al., 2025). In CYP27A1-overexpressing mice, 27-OHC was found to upregulate the expression of S100A8 and RAGE, both associated with pro-inflammatory signaling (Loera-Valencia et al., 2021). These findings were further validated ex vivo, where stimulation of 27-OHC in primary rat astrocytic cultures and primary cortico-hippocampal neurons increased S100A8 and RAGE expression. Mechanistically, the authors demonstrated that 27-OHC activates the RXRγ receptor to increase the accumulation of S100A8 and the subsequent expression of RAGE, contributing to the pro-inflammatory response. Collectively, these studies demonstrated that oxysterols, in particular 24S-OHC and 27-OHC contribute to neuroinflammation by promoting oxidative stress, pro-inflammatory cytokine production and cell adhesion responses.
5.2.2 Oxysterols in microglia activation and migration
Oxysterols can regulate microglial activation. A board-based oxysterol screen in response to neuroinflammation revealed distinct patterns oxysterol production depending on the polarization of microglia (Mutemberezi et al., 2018). In LPS induced, M1-like BV2 microglia cells, sterol-ring oxidized oxysterols such as 4β-OHC, 5α,6β-di-OHC, 7-OHC, 7-KC were increased while side-chain oxysterols such as 25-OHC, 7α,25-OHC, 27-OHC, and 7α,27-OHC were decreased. In contrast, IL-4 induced, M2-polarized BV2 microglia cells showed minimal changes in most oxysterols, with increases observed in 7-OHCone, 7-KC, 7α,25-OHC, and 7α,27-OHC. Further supporting the role of oxysterols in microglial activation, 7-KC has been shown to activate resting BV-2 microglial cells by entering the nucleus and inducing NF-kB and Poly(ADP-ribose)-polymerase-1 (PARP-1) activation (Diestel et al., 2003). This subsequently triggers the downstream activation of iNOS, CD11a and ICAM-1. The 7-KC mediated upregulation of inflammatory and chemotactic phenotypes appears to be cell-state dependent, as 7-KC had a modest effect in amplifying the inflammatory and chemotactic phenotypes in already activated microglia cells (Diestel et al., 2003). In HMC3 microglia cells, both 25-OHC and 27-OHC have shown to activate the PI3K, ERK, and Src Signaling cascades, leading to increased IL-1β production and MHC-II activation (Son et al., 2023). These oxysterols have also shown to induce HSP60 (Kim et al., 2024), which could further contribute to IL-1β production through the activation of the TLR4/p38 MAPK signaling axis (Swaroop et al., 2016). While these studies have provided insights into oxysterol-mediated regulation of microglial activation and polarization, it does not reflect the interplay between the different cell types found within the brain microenvironment. To better reflect the cellular complexity that exists in the brain microenvironment, a study explored the pattern of oxysterol regulation in an LPS-stimulated, primary microglia-astrocytes co-culture system (Mutemberezi et al., 2018). They found that sterol-ring oxysterols like 5α,6β-di-OHC, 7-OHC, 7-OHCone were reduced while side-chain oxysterols such as 25-OHC, 24S-OHC, 7α,25-OHC, and 7α,27-OHC were increased, with the exception of 27-OHC which was found to be reduced. The authors further investigated whether addition of oxysterols to the microglial-astrocyte co-culture would regulate the pro-inflammatory response. Pre-treatment of 4β-OHC, 24(S),25-epoxychol, 25-OHC, and 27-OHC prior to LPS stimulation led to reduced mRNA expression of Il6, Il1b, Mip1a, and Tnf while pre-treatment of 22(R)-OHC and 24S-OHC selectively reduced Il1b and Mip1a expression and 7-OHCone reduced Il1b expression. The co-culture findings contrasted findings from monoculture microglial system where an opposite pattern of oxysterol production was observed highlighting the importance of cellular interactions within the brain microenvironment in regulating oxysterol metabolism. In mouse models, several studies have investigated the Gpr183/oxysterol axis in microglial activation (Romero et al., 2024; Toral-Rios et al., 2024). One study found that Ch25h−/− mice had reduced CD68 expression in the hippocampus in response to peripheral LPS stimulation. This was further accompanied by a reduced expression of gene associated with neuroinflammation (Trem2, Clec7a, Axl). RNA-seq analysis of the hippocampus revealed a sex-specific difference in inflammatory regulation, where female Ch25h−/− mice had reduced expression of genes involved in pro-inflammatory signaling and leukocyte migration. Consistently, Ch25h−/− mice had reduced TNF and IL-6 production, accompanied by impaired NK cell and neutrophil infiltration into the hippocampus. The role of CH25H/25-OHC axis in microglial activation was similarly observed in a PS19 model of tauopathy (Toral-Rios et al., 2024) where CH25H confers protection against tau-mediated neurodegeneration. The authors found that Ch25h−/− mice had reduced microglial activation markers in the hippocampus and entorhinal/piriform cortex, characterized by the reduced IBA1, CD68 and Clec7a expression. Bulk RNA-seq analyses and single nuclei RNA-seq of hippocampal tissue further revealed a downregulation of the pro-inflammatory pathways in the Ch25h−/− mice. They further identified transcription factors (Rela, Nfkb1, Trp53, Sp1, Jun, and Stat3) that were differentially expressed in the Ch25h−/− mice. These findings were corroborated by immunofluorescence analysis, which showed that microglia from Ch25h−/− mice had reduced activation of phosphorylated p65 NF-κB and phosphorylated STAT-3. Together, these findings reinforce the critical role of the CH25H/25-OHC axis as a positive regulator of inflammatory signaling pathways in animal models of neuroinflammation.
Further demonstrating the Gpr183/oxysterol axis in regulating microglial activation, Song et al. (2024) demonstrated that CYP7B1 is critical for microglia activation in the brain. Cyp7b1−/− mice had reduced microglial activation in the lumbar spinal cords characterized by reduced MHC-II, IBA1, Lamp2, and CD68 expression. The protective effect of CYP7B1 deficiency was found to be dependent on the myeloid cells, as EAE progression was delayed in WT mice lacking the myeloid compartment, but not in the Cyp7b1−/− mice. Functional assays performed on primary mouse microglia and microglial-like cells from PBMCs of patients with CYP7B1 mutation further elucidated that phagocytotic activity and pro-inflammatory activation (IL-1β, IFNγ, TNFα, IL6, GM-CSF) was dependent on CYP7B1 expression (Song et al., 2024). Apart from mediating pro-inflammatory signaling, the role of Gpr183/oxysterol axis in regulating microglial migration has been demonstrated in vivo (Jang et al., 2016). Stereotactic administration of 25-OHC into the corpus callosum facilitated the recruitment of IBA1+ microglial, which was accompanied by the activation of caspase-1 in an NLRP3-dependent manner, leading to the increased IL-1β production. Taken together, these studies have highlighted the critical role of 7-KC and the Gpr183/oxysterol axis as a critical mediator of microglial activation and migration.
5.2.3 Oxysterols regulate T cell migration and adhesion
In addition to mediating microglial migration into the brain during neuroinflammation, several studies have highlighted the Gpr183/oxysterol axis in regulating encephalitogenic T cell function within the brain. One study investigated oxysterol kinetics in the brain in response to different models of neuroinflammation (Mutemberezi et al., 2018). In a single dose LPS model, a modest and transient increase in 24(S)-OHC and 27-OHC was observed in the brain, peaking at 4 h post LPS stimulation and returning to baseline by 8 h. In contrast, 5α,6α-epoxychol, 5β,6β-epoxychol, 7-OHC, and 7-KC were found to decrease at 8 h post-LPS stimulation. Interestingly, 7α,25-OHC was the only oxysterol to have sustained production over the course of inflammation. This change in oxysterol levels was found to be only localized in the brain as no difference was observed in the spinal cord. The authors further went on to characterize the oxysterol kinetics during EAE. They found that 5α,6β-di-OHC, 7-KC, and 24(S),25-epoxychol were reduced, whereas 7a,25-OHC was increased in the brainstem over the course of EAE. These findings highlight the dynamic and context-dependent regulation of oxysterols in response to different inflammatory stimuli. Interestingly, across the LPS and EAE models (Mutemberezi et al., 2018), 7α,25-OHC was the only oxysterol observed to be increased in the brain in response to inflammation. Given its well-established role in mediating chemotaxis through Gpr183, sustained production of this oxysterol during inflammation contributes to the recruitment of immune cells during inflammation. Several studies have highlighted the role of the Gpr183/oxysterol axis in mediating immune cell recruitment during neuroinflammation. One study demonstrated that this axis plays a role in the pathogenesis of EAE by mediating the migration of encephalitogenic T cells into the CNS. The authors demonstrated that Ch25h−/− mice had delayed disease onset accompanied by a milder disease severity. They further elucidated that both Ch25h−/− and Gpr183−/− mice had impaired infiltration of IL-17 producing CD44+CD4+ T cells (Th17 cells) into the CNS. Mechanistically, 7α,25-OHC mediates Th17 cells migration in a Gpr183-dependant manner (Chalmin et al., 2015). Another mechanism by which the proliferation of encephalitogenic T cells can be suppressed was demonstrated using an endothelial-specific Ch25h−/− mice. The authors demonstrated that endothelial-specific Ch25h−/− mice have reduced disease severity to EAE. They further elucidated that the loss of CH25H in primary mouse brain microvascular endothelial cells triggered a shift in lipid metabolism, favoring arachidonic acid metabolism and eicosanoid production. This altered lipid profile favors the expansion and recruitment of polymorphonuclear myeloid-derived suppressor cell into the brain, suppressing encephalitogenic T cell proliferation (Ruiz et al., 2023). Deletion of Cyp71b in mice (Cyp7b1−/−) similarly reduces disease onset in the EAE model, which is accompanied by milder disease severity and an impaired infiltration of Th1 and Th17 CD4+ T cells (Song et al., 2024).
The effect of the Gpr183/oxysterol axis in mediating T cell migration into the brain is not disease specific as in a PS19 tauopathy model, Ch25h−/− mice had reduced T cell infiltration in the hippocampus (Toral-Rios et al., 2024). In addition to promoting T cell migration, the Gpr183/oxysterol axis enhances T cell adhesion to endothelial BBB (Caratis et al., 2025b). Using CSF from acute MS patients to mimic the neuroinflammatory environment, Gpr183 is upregulated in human brain vascular pericytes (HBPCs) while HSD3B7 expression is downregulated in the HBPCs and human brain astrocytes (HASTRs). Using a BBB spheroid model consisting of HBPCs, HASTRs and human brain microvascular ECs (HBMECs), they further elucidated that Gpr183 activation with 7α,25-OHC led to reduced VE-cadherin expression, a key component of endothelial junction integrity. This reduction in expression was reversed upon treatment with the Gpr183 antagonist, NIBR189. Similar effects were observed when BBB spheroids were incubated with CSF from MS patients, where VE-cadherin downregulation was reversed following NIBR189 treatment. Furthermore, Gpr183 antagonism reduced CD4+ T cell adhesion to BBB spheroids exposed to CSF of MS patients (Caratis et al., 2025b).
Taken together, these studies highlighted the role of oxysterols in regulating the diverse immune signaling pathways during inflammation – ranging from the induction of pro-inflammatory response and oxidative stress to the modulation of cell migration, demonstrating their potential as therapeutic targets in neuroinflammation.
6 Oxysterols and neurodegeneration
Oxysterols have emerged as critical molecular links between cholesterol metabolism, oxidative stress, and the progressive failure of neural circuits that characterize neurodegenerative disease. Under physiological conditions, brain-enriched oxysterols such as 24S-OHC support neuronal viability by regulating cholesterol turnover, synaptic plasticity and nuclear receptor signaling (Björkhem and Meaney, 2004). However, in aging and disease, the balance between enzymatically generated signaling oxysterols and non-enzymatic, oxidation-derived species becomes profoundly disrupted. Accumulation of cytotoxic oxysterols - including 7-KC, 7β-OHC and peripherally derived 27-OHC - amplifies oxidative stress, perturbs membrane architecture and initiates feed-forward cycles of lipid peroxidation and mitochondrial dysfunction. These processes converge on synaptic failure, impaired axonal integrity and ultimately neuronal loss, positioning oxysterol dysregulation as both a marker and a driver of neurodegeneration. At the synaptic level, oxysterols exert potent effects on neuronal excitability and excitotoxic vulnerability by modulating ionotropic receptor function and ion channel dynamics. Physiological concentrations of 24S-OHC act as positive allosteric modulators of NMDA receptors, facilitating synaptic plasticity and cognitive processes. In contrast, pathological elevations of 24S-OHC, 25-OHC, and 27-OHC sustain excessive calcium influx and destabilize excitatory-inhibitory balance, particularly when combined with oxysterol-induced weakening of GABAergic inhibition. These effects are compounded by oxysterol-driven disruption of cholesterol-rich membrane microdomains, leading to altered trafficking and clustering of glutamate and GABA receptors. Such synaptic dysregulation provides a mechanistic bridge between altered sterol metabolism and the early network hyperexcitability, synaptic loss and cognitive decline observed in AD, epilepsy-associated neurodegeneration and MS. Oxysterols also act as powerful modulators of glial biology, thereby shaping the inflammatory and metabolic landscape of the degenerating brain. In microglia and astrocytes, oxysterols such as 25-OHC and 27-OHC promote pro-inflammatory transcriptional programs through TLR4, NF-κB, and inflammasome signaling, while simultaneously suppressing antioxidant defenses (Pfrieger and Ungerer, 2011; Testa et al., 2014; Ma et al., 2019). Chronic exposure to these sterols biases glia toward neurotoxic phenotypes, sustaining cytokine production, reactive oxygen species generation and impaired metabolic support to neurons. In oligodendrocyte lineage cells, cytotoxic oxysterols directly induce mitochondrial damage, oxidative stress-induced autophagy/apoptosis and inflammasome-dependent cell death, compromising myelin integrity and axonal protection. Conversely, selective activation of oxysterol-responsive receptors such as LXRs and Gpr183 can promote oligodendrocyte differentiation and remyelination, underscoring the context-dependent duality of oxysterol signaling in neurodegeneration. Together, these findings position oxysterols as integrative regulators of neurodegeneration that operate across molecular, cellular and circuit scales. Rather than acting solely as by-products of oxidative damage, oxysterols actively shape disease trajectories by coordinating lipid metabolism, inflammatory signaling, synaptic function and myelin dynamics. This dual nature presents both challenges and opportunities. Indiscriminate suppression of oxysterol pathways may disrupt essential homeostatic functions, whereas pathway-specific modulation of oxysterol synthesis, metabolism or receptor engagement offers a promising strategy to decouple their physiological roles from their neurotoxic consequences. Targeting oxysterol signaling therefore represents a compelling therapeutic avenue to slow neurodegeneration, preserve circuit function and enhance endogenous repair mechanisms across a spectrum of neurological disorders.
7 Protective and therapeutic potential of oxysterols
Oxysterols play a complex role in the central nervous system, contributing to both cellular injury and neuroprotection depending on their type and context. Understanding and targeting oxysterol metabolism has therefore emerged as a promising strategy to mitigate toxicity, support glial health and enhance myelin repair in neurological and psychiatric disorders. Oxysterols metabolism can be modulated with the use of antioxidants, cholesterol-lowering drugs and neuroprotective agents.
Oxidative stress is a major mediator of oxysterol-induced cellular injury, making it a key target for neuroprotective interventions. Targeting oxidative stress with compounds like vitamin E, coenzyme Q10, or polyphenols may help mitigate oxysterol-induced damage. Oxysterol toxicity is closely tied to oxidative stress, making antioxidant strategies a promising therapeutic avenue (Poli et al., 2013). Compounds such as vitamin E, coenzyme Q10 and polyphenols can neutralize reactive oxygen species, thereby reducing oxysterol-driven activation of stress kinases and apoptosis (Niki, 2014; Gutierrez-Mariscal et al., 2020; Didier et al., 2023). By counteracting reactive oxygen species and preserving cellular redox balance, antioxidant therapies offer potential to safeguard neurons and glial cells from oxysterol toxicity and support overall brain health. Antioxidants may protect neurons and glia from oxysterol-induced injury and hold potential as adjunct therapies in neurodegenerative disease.
Modulating cholesterol metabolism has emerged as a promising strategy to control oxysterol levels and protect neural cells from toxicity (Wang et al., 2025). Statins and other cholesterol-modifying therapies may help regulate oxysterol levels. Cholesterol-lowering therapies offer another avenue to limit oxysterol toxicity by reducing their precursor availability. Statins and other cholesterol-modifying agents can decrease cholesterol turnover and dampen the excessive production of oxysterols. By stabilizing cholesterol homeostasis, these drugs may help reduce oxidative stress and apoptosis triggered by oxysterol accumulation, providing therapeutic benefit in conditions such as atherosclerosis and neurodegenerative disease. Importantly, because cholesterol is essential for oligodendrocyte differentiation and myelin synthesis, modulating cholesterol and oxysterol balance could protect glial health. By limiting oxysterol-driven toxicity while preserving adequate cholesterol supply, such interventions may support both neuronal survival and proper myelination. Importantly, cholesterol balance is critical for myelination, as oligodendrocyte differentiation and myelin synthesis require adequate cholesterol supply (Saher et al., 2005). Experimental studies have shown that while excessive statin treatment can impair oligodendrocyte maturation (Paintlia et al., 2005), modulation of cholesterol metabolism may reduce oxysterol toxicity while preserving glial function. Regulation of cholesterol and oxysterol balance such as through judicious use of statins can potentially reduce oxidative stress and apoptosis while supporting oligodendrocyte maturation, myelination and overall glial health.
Drugs targeting mitochondrial function and inflammation may also offer protective benefits. Neuroprotective agents that target mitochondrial dysfunction and inflammation represent another therapeutic strategy against oxysterol-induced damage. Agents such as mitochondrial stabilizers, anti-inflammatory drugs or compounds that enhance energy metabolism can help counteract oxysterol-driven oxidative stress and apoptotic signaling. For example, the mitochondria-targeted antioxidant Mito-Q has been shown to reduce oxidative damage and improve neuronal survival in models of neurodegeneration (Smith et al., 1999), while the anti-inflammatory agent minocycline protects neurons and glia by attenuating microglial activation and caspase-dependent cell death (Yrjänheikki et al., 1999; Zhu et al., 2002). By mitigating mitochondrial stress and dampening neuroinflammation, these approaches may reduce oxysterol toxicity and support long-term neuronal survival. 24S-OHC has also been proposed to exert neuroprotective effects. 24S-HC can activate liver X receptors (LXRs), nuclear receptors that regulate genes involved in cholesterol homeostasis, lipid metabolism and inflammation. Through LXR signaling, 24S-OHC can limit inflammatory responses in the brain and support neuronal and glial health. These findings evidence that certain oxysterols play a beneficial role in maintaining neural function, highlighting their potential as therapeutic modulators in neurodegenerative conditions.
Targeting oxysterol metabolism may offer new therapeutic avenues to support remyelination in conditions where myelin integrity is compromised, e.g., MS, AD, and PD. Modulating the production or signaling of specific oxysterols can potentially reduce oxidative stress, limit apoptosis and promote oligodendrocyte differentiation. Such interventions could benefit people with neurodegenerative or psychiatric disorders where enhancing glial health and myelin repair is critical for restoring neural circuit function.
8 Discussion, conclusion, and future directions
Oxysterols are central yet underappreciated regulators of CNS homeostasis, operating at the intersection of cholesterol metabolism, neural circuit function and neuroinflammation. Collectively, the evidence positions oxysterols not merely as by-products of cholesterol oxidation but as signaling molecules whose actions span neurons, oligodendrocyte lineage cells, astrocytes and microglia. Through convergent effects on ionotropic receptor function, membrane biophysics, nuclear receptor signaling and inflammatory cascades, oxysterols emerge as powerful modulators of excitability, myelination and vulnerability to neurodegeneration. A major conceptual advance is the dualistic nature of oxysterol signaling. Physiological oxysterols, particularly neuron-derived 24S-OHC, support synaptic plasticity, cholesterol homeostasis and myelin maintenance through tightly regulated engagement of NMDA receptors, LXRs and glial metabolic pathways. In contrast, pathological accumulation of oxysterols such as 7-KC, 25-OHC, and 27-OHC drives oxidative stress, mitochondrial dysfunction and chronic neuroinflammation. This balance between adaptive and maladaptive oxysterol signaling appears to be a critical determinant of neuronal and glial resilience across the lifespan. At the level of neural circuits, oxysterols exert profound effects by reshaping the excitation-inhibition balance. Positive allosteric modulation of NMDA receptors by 24S-OHC links cholesterol metabolism directly to synaptic plasticity and cognitive function, yet excessive or prolonged receptor potentiation - particularly in the context of elevated 27-OHC or 7-KC - may predispose circuits to excitotoxicity. In parallel, oxysterol-driven destabilization of lipid rafts alters AMPA, kainate and GABAA receptor trafficking, impairing both phasic and tonic inhibition. Such coordinated enhancement of glutamatergic drive coupled with weakened inhibitory control provides a unifying mechanistic framework for oxysterol contributions to epilepsy, neurodegeneration and neuropsychiatric disorders characterized by network hyperexcitability.
Oxysterols also integrate metabolic and inflammatory signaling through mitochondria- and redox-dependent pathways. Non-enzymatically generated oxysterols amplify reactive oxygen species production, activate stress kinases, and initiate lipid peroxidation, creating a feed-forward cycle that perpetuates neuronal and glial injury. Importantly, neurons are intrinsically vulnerable to these insults due to limited antioxidant capacity, while oligodendrocytes- highly dependent on lipid metabolism - are particularly sensitive to oxysterol-induced mitochondrial and peroxisomal dysfunction. These mechanisms align with clinical observations linking oxysterol signatures to biomarkers of neuroaxonal damage and disease severity in disorders such as MS and AD. Within the glial population, oxysterols emerge as potent regulators of neuroinflammation and myelin dynamics. Microglial and astrocytic responses to oxysterols promote cytokine production, ROS generation and altered migratory behavior, thereby shaping inflammatory microenvironments that influence neuronal survival and OPC differentiation. Notably, oxysterol-sensitive GPCRs such as Gpr17 and Gpr183 define functionally distinct OPC subpopulations and regulate key transitions between proliferation, migration and myelination. In parallel, LXR signaling links oxysterol availability to transcriptional programs controlling cholesterol transport, lipid biosynthesis and myelin gene expression. These findings collectively suggest that oxysterols serve as molecular cues coordinating immune activity, metabolic state and regenerative capacity within the CNS. Importantly, the context-dependent actions of oxysterols provide a plausible explanation for their seemingly paradoxical roles in disease. While excessive oxysterol accumulation exacerbates neuroinflammation and degeneration, appropriately timed and localized oxysterol signaling - particularly via LXR and Gpr183 pathways - can promote remyelination and circuit stabilization. This dichotomy underscores the need to move beyond viewing oxysterols solely as toxic metabolites and instead consider them as dynamic regulators whose effects depend on concentration, cellular source, receptor engagement and disease stage. From a translational perspective, targeting oxysterol metabolism and signaling presents both opportunities and challenges. Modulating enzymatic pathways that govern oxysterol synthesis, selectively engaging protective receptor pathways, or restoring physiological sterol balance may offer novel strategies to limit neurodegeneration while enhancing repair. However, given the breadth of oxysterol actions across cell types and circuits, therapeutic approaches will require precise spatiotemporal control to avoid disrupting essential homeostatic functions.
In conclusion, oxysterols constitute a pivotal molecular axis linking cholesterol metabolism to neural circuit function, glial biology and neurodegenerative disease. Acting as key mediators of oxidative stress, inflammation, mitochondrial dysfunction and protein aggregation, distinct oxysterol species orchestrate adaptive versus pathological responses across neurons and glia. Characterizing specific oxysterol profiles in normal and pathological myelination may reveal metabolic signatures that influence oligodendrocyte function and myelin integrity, providing potential biomarkers and therapeutic targets for demyelinating, neurodegenerative and neuropsychiatric disorders. However, current limitations in oxysterol quantification, including low abundance, structural diversity, and analytical sensitivity, pose challenges to accurately map their spatiotemporal dynamics in the CNS. Advances in high-resolution, cell-type–specific and regionally targeted lipidomic approaches will be critical to overcome these hurdles and fully elucidate oxysterol function. Pharmacologically modulating oxysterol signaling represents a promising strategy to enhance remyelination, support oligodendrocyte differentiation and promote myelin repair, while understanding how oxysterols intersect with other lipid signaling pathways - including sphingolipids and endocannabinoids - could further illuminate mechanisms regulating glial biology. Future studies integrating cell-type specific, regional and temporal analyses of oxysterol signaling will be essential to harness this pathway for therapeutic benefit and to advance our understanding of CNS vulnerability and resilience.
Statements
Author contributions
RR: Writing – review & editing, Project administration, Writing – original draft, Conceptualization, Investigation. CF: Investigation, Writing – review & editing, Writing – original draft. KR: Conceptualization, Supervision, Writing – review & editing, Writing – original draft. CC: Writing – original draft, Funding acquisition, Writing – review & editing, Conceptualization, Resources, Supervision.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by grants from the National Health and Medical Research Council (GNT2012140 and GNT2019167), and Mater Foundation (Equity Trustees and the Trusts of LG McCallum Est).
Acknowledgments
We thank our colleagues at Mater Research Institute – University of Queensland for their advice and support.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author KR declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Adamczyk M. Scherrer E. Kupferberg A. Malviya A. N. Mersel M. (1998). Inhibition of p42/p44 mitogen-activated protein kinase by oxysterols in rat astrocyte primary cultures and C6 glioma cell lines. J. Neurosci. Res.53, 38–50. doi: 10.1002/(SICI)1097-4547(19980701)53:1<38::AID-JNR5>3.0.CO;2-K,
2
Anderson A. Campo A. Fulton E. Corwin A. Jerome W. G. O'Connor M. S. (2020). 7-Ketocholesterol in disease and aging. Redox Biol.29:101380. doi: 10.1016/j.redox.2019.101380
3
Ares M. P. Pörn-Ares M. I. Moses S. Thyberg J. Juntti-Berggren L. Berggren P.-O. et al . (2000). 7β-hydroxycholesterol induces Ca2+ oscillations, MAP kinase activation and apoptosis in human aortic smooth muscle cells. Atherosclerosis153, 23–35. doi: 10.1016/s0021-9150(00)00380-4,
4
Ataka T. Gu J. G. (2006). Relationship between tonic inhibitory currents and phasic inhibitory activity in the spinal cord lamina II region of adult mice. Mol. Pain2:36. doi: 10.1186/1744-8069-2-36,
5
Auld D. S. Kornecook T. J. Bastianetto S. Quirion R. (2002). Alzheimer's disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog. Neurobiol.68, 209–245. doi: 10.1016/s0301-0082(02)00079-5
6
Baenziger J. E. Domville J. A. Therien J. P. D. (2017). The role of cholesterol in the activation of nicotinic acetylcholine receptors. Curr. Top. Membr.80, 95–137. doi: 10.1016/bs.ctm.2017.05.002
7
Bauman D. R. Bitmansour A. D. McDonald J. G. Thompson B. M. Liang G. Russell D. W. (2009). 25-hydroxycholesterol secreted by macrophages in response to toll-like receptor activation suppresses immunoglobulin a production. Proc. Natl. Acad. Sci.106, 16764–16769. doi: 10.1073/pnas.0909142106,
8
Bej E. Cesare P. Volpe A. R. d'Angelo M. Castelli V. (2024). Oxidative stress and neurodegeneration: insights and therapeutic strategies for Parkinson's disease. Neurol. Int.16, 502–517. doi: 10.3390/neurolint16030037
9
Beltrán González A. N. López Pazos M. I. Calvo D. J. (2020). Reactive oxygen species in the regulation of the GABA mediated inhibitory neurotransmission. Neuroscience439, 137–145. doi: 10.1016/j.neuroscience.2019.05.064,
10
Berghoff S. A. Spieth L. Saher G. (2022). Local cholesterol metabolism orchestrates remyelination. Trends Neurosci.45, 272–283. doi: 10.1016/j.tins.2022.01.001
11
Berthier A. Lemaire-Ewing S. Prunet C. Montange T. Vejux A. Pais de Barros J. P. et al . (2005). 7-Ketocholesterol-induced apoptosis: involvement of several pro-apoptotic but also anti-apoptotic calcium-dependent transduction pathways. FEBS J.272, 3093–3104. doi: 10.1111/j.1742-4658.2005.04723.x
12
Bezine M. Maatoug S. Ben Khalifa R. Debbabi M. Zarrouk A. Wang Y. et al . (2018a). Modulation of Kv3.1b potassium channel level and intracellular potassium concentration in 158N murine oligodendrocytes and BV-2 murine microglial cells treated with 7-ketocholesterol, 24S-hydroxycholesterol or tetracosanoic acid (C24:0). Biochimie153, 56–69. doi: 10.1016/j.biochi.2018.02.008,
13
Bezine M. Namsi A. Sghaier R. Ben Khalifa R. Hamdouni H. Brahmi F. et al . (2018b). The effect of oxysterols on nerve impulses. Biochimie153, 46–51. doi: 10.1016/j.biochi.2018.04.013,
14
Björkhem I. (2006). Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J. Intern. Med.260, 493–508. doi: 10.1111/j.1365-2796.2006.01725.x
15
Björkhem I. Cedazo-Minguez A. Leoni V. Meaney S. (2009). Oxysterols and neurodegenerative diseases. Mol. Asp. Med.30, 171–179. doi: 10.1016/j.mam.2009.02.001
16
Björkhem I. Meaney S. (2004). Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol.24, 806–815. doi: 10.1161/01.ATV.0000120374.59826.1b,
17
Bock T. Stuart G. J. (2016). The impact of BK channels on cellular excitability depends on their subcellular location. Front. Cell. Neurosci.10:2016. doi: 10.3389/fncel.2016.00206
18
Brachet A. Norwood S. Brouwers J. F. Palomer E. Helms J. B. Dotti C. G. et al . (2015). LTP-triggered cholesterol redistribution activates Cdc42 and drives AMPA receptor synaptic delivery. J. Cell Biol.208, 791–806. doi: 10.1083/jcb.201407122
19
Brahmi F. Mackrill J. J. Ghzaiel I. Rezig L. Benkhalifa R. Zarrouk A. et al . (2025). Oxysterol-induced inflammation in human diseases: strategies for treatment with natural compounds and synthetic molecules. Molecules30:2883. doi: 10.3390/molecules30132883
20
Calvo D. J. González A. N. B. (2016). Dynamic regulation of the GABAA receptor function by redox mechanisms. Mol. Pharmacol.90, 326–333. doi: 10.1124/mol.116.105205,
21
Cao G. Bales K. R. DeMattos R. B. Paul S. M. (2007). Liver X receptor-mediated gene regulation and cholesterol homeostasis in brain: relevance to Alzheimer's disease therapeutics. Curr. Alzheimer Res.4, 179–184. doi: 10.2174/156720507780362173
22
Caratis F. Karaszewski B. Klejbor I. Furihata T. Rutkowska A. (2025a). Differential expression and modulation of EBI2 and 7α,25-OHC synthesizing (CH25H, CYP7B1) and degrading (HSD3B7) enzymes in mouse and human brain vascular cells. PLoS One20:e0318822. doi: 10.1371/journal.pone.0318822
23
Caratis F. Mruk I. Konieczna-Wolska K. Rojek B. Hałas M. Czaplewska P. et al . (2025b). EBI2-oxysterol signalling regulates VE-cadherin expression and multiple sclerosis CD4(+) T cell attachment to a human tri-cell spheroid blood-brain barrier model. Brain Behav. Immun. Health47:101045. doi: 10.1016/j.bbih.2025.101045,
24
Carver C. M. Reddy D. S. (2013). Neurosteroid interactions with synaptic and extrasynaptic GABA(a) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology230, 151–188. doi: 10.1007/s00213-013-3276-5,
25
Chalmin F. Rochemont V. Lippens C. Clottu A. Sailer A. W. Merkler D. et al . (2015). Oxysterols regulate encephalitogenic CD4(+) T cell trafficking during central nervous system autoimmunity. J. Autoimmun.56, 45–55. doi: 10.1016/j.jaut.2014.10.001
26
Chamberlain L. H. Burgoyne R. D. Gould G. W. (2001). SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc. Natl. Acad. Sci. USA98, 5619–5624. doi: 10.1073/pnas.091502398,
27
Chen J. J. Yu B. P. (1994). Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radic. Biol. Med.17, 411–418. doi: 10.1016/0891-5849(94)90167-8,
28
Chen D. M. Ziolkowski L. Benz A. Qian M. Zorumski C. F. Covey D. F. et al . (2018). A clickable oxysterol Photolabel retains NMDA receptor activity and accumulates in neurons. Front. Neurosci.12:2018. doi: 10.3389/fnins.2018.00923,
29
Chesnokov M. Khan I. Chefetz I. (2021). Induction and detection of Necroptotic cell death in mammalian cell culture. Methods Mol. Biol.2255, 119–134. doi: 10.1007/978-1-0716-1162-3_11
30
Choi W.-S. Eom D.-S. Han B. S. Kim W. K. Han B. H. Choi E.-J. et al . (2004). Phosphorylation of p38 MAPK induced by oxidative stress is linked to activation of both Caspase-8- and −9-mediated apoptotic pathways in dopaminergic neurons*. J. Biol. Chem.279, 20451–20460. doi: 10.1074/jbc.M311164200,
31
Christofidi M. Tzortzini E. Mavromoustakos T. Kolocouris A. (2025). Effects of membrane cholesterol on the structure and function of selected class A GPCRs─challenges and future perspectives. Biochemistry64, 4011–4049. doi: 10.1021/acs.biochem.5c00145,
32
Cigliano L. Spagnuolo M. S. Napolitano G. Iannotta L. Fasciolo G. Barone D. et al . (2019). 24S-hydroxycholesterol affects redox homeostasis in human glial U-87 MG cells. Mol. Cell. Endocrinol.486, 25–33. doi: 10.1016/j.mce.2019.02.013,
33
Comaposada-Baró R. Benito-Martínez A. Escribano-Saiz J. J. Franco M. L. Ceccarelli L. Calatayud-Baselga I. et al . (2023). Cholinergic neurodegeneration and cholesterol metabolism dysregulation by constitutive p75NTR signaling in the p75exonIII-KO mice. Front. Mol. Neurosci.16:1237458. doi: 10.3389/fnmol.2023.1237458,
34
Cross T. G. Scheel-Toellner D. Henriquez N. V. Deacon E. Salmon M. Lord J. M. (2000). Serine/threonine protein kinases and apoptosis. Exp. Cell Res.256, 34–41. doi: 10.1006/excr.2000.4836,
35
Crotchett B. L. M. Reighard S. D. Nasrallah H. A. (2025). GABA dysfunction in schizophrenia: the GABAA receptor as a potential therapeutic target. Biomark. Neuropsychiatry12:100130. doi: 10.1016/j.bionps.2025.100130
36
Cumaoğlu A. Ağkaya A. Özkul Z. (2019). Effect of the lipid peroxidation product 4-Hydroxynonenal on Neuroinflammation in microglial cells: protective role of quercetin and Monochloropivaloylquercetin. Turk. J. Pharm. Sci.16, 54–61. doi: 10.4274/tjps.58966,
37
Cyster J. G. Dang E. V. Reboldi A. Yi T. (2014). 25-hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol.14, 731–743. doi: 10.1038/nri3755,
38
Dalleau S. Baradat M. Guéraud F. Huc L. (2013). Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death Different.20, 1615–1630. doi: 10.1038/cdd.2013.138,
39
Daray F. M. Mann J. J. Sublette M. E. (2018). How lipids may affect risk for suicidal behavior. J. Psychiatr. Res.104, 16–23. doi: 10.1016/j.jpsychires.2018.06.007,
40
Dart C. (2010). Lipid microdomains and the regulation of ion channel function. J. Physiol.588, 3169–3178. doi: 10.1113/jphysiol.2010.191585,
41
Dasari B. Prasanthi J. R. Marwarha G. Singh B. B. Ghribi O. (2010). The oxysterol 27-hydroxycholesterol increases β-amyloid and oxidative stress in retinal pigment epithelial cells. BMC Ophthalmol.10:22. doi: 10.1186/1471-2415-10-22
42
de Freitas F. A. Levy D. Reichert C. O. Cunha-Neto E. Kalil J. Bydlowski S. P. (2022). Effects of oxysterols on immune cells and related diseases. Cells11:251. doi: 10.3390/cells11081251,
43
De La Fuente D. C. Tamburini C. Stonelake E. Andrews R. Hall J. Owen M. J. et al . (2024). Impaired oxysterol-liver X receptor signaling underlies aberrant cortical neurogenesis in a stem cell model of neurodevelopmental disorder. Cell Rep.43:946. doi: 10.1016/j.celrep.2024.113946
44
DeLuca G. C. Kimball S. M. Kolasinski J. Ramagopalan S. V. Ebers G. C. (2013). Review: the role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol.39, 458–484. doi: 10.1111/nan.12020
45
Dias I. H. K. Shokr H. Shephard F. Chakrabarti L. (2022). Oxysterols and oxysterol sulfates in Alzheimer’s disease brain and cerebrospinal fluid. J. Alzheimers Dis.87, 1527–1536. doi: 10.3233/jad-220083,
46
Didier A. J. Stiene J. Fang L. Watkins D. Dworkin L. D. Creeden J. F. (2023). Antioxidant and anti-tumor effects of dietary vitamins A, C, and E. Antioxidants12:632. doi: 10.3390/antiox12030632
47
Diestel A. Aktas O. Hackel D. Hake I. Meier S. Raine C. S. et al . (2003). Activation of microglial poly(ADP-ribose)-polymerase-1 by cholesterol breakdown products during neuroinflammation: a link between demyelination and neuronal damage. J. Exp. Med.198, 1729–1740. doi: 10.1084/jem.20030975,
48
Dietschy J. M. (2009). Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol. Chem.390, 287–293. doi: 10.1515/BC.2009.035,
49
Dietschy J. M. Turley S. D. (2004). Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res.45, 1375–1397. doi: 10.1194/jlr.R400004-JLR200,
50
Dodge J. C. Yu J. Sardi S. P. Shihabuddin L. S. (2021). Sterol auto-oxidation adversely affects human motor neuron viability and is a neuropathological feature of amyotrophic lateral sclerosis. Sci. Rep.11:803. doi: 10.1038/s41598-020-80378-y,
51
Doria M. Maugest L. Moreau T. Lizard G. Vejux A. (2016). Contribution of cholesterol and oxysterols to the pathophysiology of Parkinson's disease. Free Radic. Biol. Med.101, 393–400. doi: 10.1016/j.freeradbiomed.2016.10.008,
52
Dringen R. (2000). Metabolism and functions of glutathione in brain. Prog. Neurobiol.62, 649–671. doi: 10.1016/S0301-0082(99)00060-X
53
Farooqui A. (2009). “Recent developments on Kainate-mediated neurotoxicity and their association with generation of lipid mediators” in Hot topics in neural membrane Lipidology. ed. FarooquiA. A. (New York, NY: Springer US), 215–241.
54
Farooqui T. Farooqui A. A. (2011). Lipid-mediated oxidative stress and inflammation in the pathogenesis of Parkinson's disease. Parkinsons Dis2011:247467. doi: 10.4061/2011/247467,
55
Farrant M. Nusser Z. (2005). Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci.6, 215–229. doi: 10.1038/nrn1625,
56
Fattorini G. Ripoli C. Cocco S. Spinelli M. Mattera A. Grassi C. et al . (2019). Glutamate/GABA co-release selectively influences postsynaptic glutamate receptors in mouse cortical neurons. Neuropharmacology161:107737. doi: 10.1016/j.neuropharm.2019.107737,
57
Faulkner I. E. Pajak R. Z. Harte M. K. Glazier J. D. Hager R. (2024). Voltage-gated potassium channels as a potential therapeutic target for the treatment of neurological and psychiatric disorders. Front. Cell. Neurosci.18:1449151. doi: 10.3389/fncel.2024.1449151,
58
Feingold K. R. (2000). “Introduction to lipids and lipoproteins” in Endotext. eds. FeingoldK. R.AhmedS. F.AnawaltB.BlackmanM. R.BoyceA.ChrousosG.et al. MDText.com, Inc ed (South Dartmouth, MA).
59
Ferrera P. Mercado-Gómez O. Silva-Aguilar M. Valverde M. Arias C. (2008). Cholesterol potentiates beta-amyloid-induced toxicity in human neuroblastoma cells: involvement of oxidative stress. Neurochem. Res.33, 1509–1517. doi: 10.1007/s11064-008-9623-y,
60
Filomenko R. Fourgeux C. Bretillon L. Gambert-Nicot S. (2015). Oxysterols: influence on plasma membrane rafts microdomains and development of ocular diseases. Steroids99, 259–265. doi: 10.1016/j.steroids.2015.02.004
61
Foo C.X. Bartlett S. Chew K.Y. Ngo M.D. Bielefeldt-Ohmann H. Arachchige B.J. et al . (2023). GPR183 antagonism reduces macrophage infiltration in influenza and SARS-CoV-2 infection. Eur. Respir. J.61:2201306. doi: 10.1183/13993003.01306-2022.
62
Fritsch B. Reis J. Gasior M. Kaminski R. M. Rogawski M. A. (2014). Role of GluK1 Kainate receptors in seizures, epileptic discharges, and Epileptogenesis. J. Neurosci.34, 5765–5775. doi: 10.1523/jneurosci.5307-13.2014,
63
Gamba P. Giannelli S. Staurenghi E. Testa G. Sottero B. Biasi F. et al . (2021). The controversial role of 24-S-hydroxycholesterol in Alzheimer's disease. Antioxidants10:740. doi: 10.3390/antiox10050740,
64
Gamba P. Leonarduzzi G. Tamagno E. Guglielmotto M. Testa G. Sottero B. et al . (2011). Interaction between 24-hydroxycholesterol, oxidative stress, and amyloid-β in amplifying neuronal damage in Alzheimer's disease: three partners in crime. Aging Cell10, 403–417. doi: 10.1111/j.1474-9726.2011.00681.x,
65
Gamba P. Staurenghi E. Testa G. Giannelli S. Sottero B. Leonarduzzi G. (2019). A crosstalk between brain cholesterol oxidation and glucose metabolism in Alzheimer’s disease. Front. Neurosci.13:556. doi: 10.3389/fnins.2019.00556,
66
Gan C. Cao X. Wang L. Sun H. Ji M. Zhang H. et al . (2023). Cholinergic basal forebrain atrophy in Parkinson's disease with freezing of gait. Ann. Clin. Transl. Neurol.10, 814–824. doi: 10.1002/acn3.51769,
67
Gęgotek A. Skrzydlewska E. (2019). Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lipids221, 46–52. doi: 10.1016/j.chemphyslip.2019.03.011
68
Ghonimi N. A. M. Elsharkawi K. A. Khyal D. S. M. Abdelghani A. A. (2021). Serum malondialdehyde as a lipid peroxidation marker in multiple sclerosis patients and its relation to disease characteristics. Mult. Scler. Relat. Disord.51:102941. doi: 10.1016/j.msard.2021.102941,
69
Ghosh C. Dick R. M. Ali S. F. (1993). Iron/ascorbate-induced lipid peroxidation changes membrane fluidity and muscarinic cholinergic receptor binding in rat frontal cortex. Neurochem. Int.23, 479–484. doi: 10.1016/0197-0186(93)90133-p,
70
Gill S. Chow R. Brown A. J. (2008). Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog. Lipid Res.47, 391–404. doi: 10.1016/j.plipres.2008.04.002,
71
Gonzalo H. Brieva L. Tatzber F. Jové M. Cacabelos D. Cassanyé A. et al . (2012). Lipidome analysis in multiple sclerosis reveals protein lipoxidative damage as a potential pathogenic mechanism. J. Neurochem.123, 622–634. doi: 10.1111/j.1471-4159.2012.07934.x,
72
Grassi S. Giussani P. Mauri L. Prioni S. Sonnino S. Prinetti A. (2020). Lipid rafts and neurodegeneration: structural and functional roles in physiologic aging and neurodegenerative diseases. J. Lipid Res.61, 636–654. doi: 10.1194/jlr.TR119000427,
73
Guo C. Sun L. Chen X. Zhang D. (2013). Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res.8, 2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009,
74
Guo J. D. Zhao X. Li Y. Li G. R. Liu X. L. (2018). Damage to dopaminergic neurons by oxidative stress in Parkinson's disease (review). Int. J. Mol. Med.41, 1817–1825. doi: 10.3892/ijmm.2018.3406
75
Gutierrez-Mariscal F. M. Arenas-de Larriva A. P. Limia-Perez L. Romero-Cabrera J. L. Yubero-Serrano E. M. López-Miranda J. (2020). Coenzyme Q10 supplementation for the reduction of oxidative stress: clinical implications in the treatment of chronic diseases. Int. J. Mol. Sci.21:7870. doi: 10.3390/ijms21217870
76
Han J. H. Kim Y. J. Han E. S. Lee C. S. (2007). Prevention of 7-ketocholesterol-induced mitochondrial damage and cell death by calmodulin inhibition. Brain Res.1137, 11–19. doi: 10.1016/j.brainres.2006.12.041,
77
Hanin A. Baudin P. Demeret S. Roussel D. Lecas S. Teyssou E. et al . (2021). Disturbances of brain cholesterol metabolism: a new excitotoxic process associated with status epilepticus. Neurobiol. Dis.154:105346. doi: 10.1016/j.nbd.2021.105346,
78
Hannich J. T. Umebayashi K. Riezman H. (2011). Distribution and functions of sterols and sphingolipids. Cold Spring Harb. Perspect. Biol.3:762. doi: 10.1101/cshperspect.a004762,
79
Harper S. J. LoGrasso P. (2001). Signalling for survival and death in neurones: the role of stress-activated kinases, JNK and p38. Cell. Signal.13, 299–310. doi: 10.1016/S0898-6568(01)00148-6,
80
Harrington A. W. Liu C. Phillips N. Nepomuceno D. Kuei C. Chang J. et al . (2023). Identification and characterization of select oxysterols as ligands for GPR17. Br J Pharmacol.180, 4, 401–421 doi: 10.1111/bph.15969
81
Hennegan J. Bryant A. H. Griffiths L. Trigano M. Bartley O. J. M. Bartlett J. J. et al . (2024). Inhibition of 7α,26-dihydroxycholesterol biosynthesis promotes midbrain dopaminergic neuron development. iScience27:108670. doi: 10.1016/j.isci.2023.108670,
82
Hering H. Lin C. C. Sheng M. (2003). Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J. Neurosci.23, 3262–3271. doi: 10.1523/jneurosci.23-08-03262.2003,
83
Heverin M. Meaney S. Lütjohann D. Diczfalusy U. Wahren J. Björkhem I. (2005). Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J. Lipid Res.46, 1047–1052. doi: 10.1194/jlr.M500024-JLR200,
84
Hughes T. M. Rosano C. Evans R. W. Kuller L. H. (2013). Brain cholesterol metabolism, oxysterols, and dementia. J. Alzheimers Dis.33, 891–911. doi: 10.3233/jad-2012-121585
85
Hwang O. (2013). Role of oxidative stress in Parkinson's disease. Exp. Neurobiol.22, 11–17. doi: 10.5607/en.2013.22.1.11,
86
Jang J. Park S. Jin Hur H. Cho H. J. Hwang I. Pyo Kang Y. et al . (2016). 25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome. Nat. Commun.7:13129. doi: 10.1038/ncomms13129
87
Jeitner T. M. Voloshyna I. Reiss A. B. (2011). Oxysterol derivatives of cholesterol in neurodegenerative disorders. Curr. Med. Chem.18, 1515–1525. doi: 10.2174/092986711795328445
88
Jiménez-Balado J. Eich T. S. (2021). GABAergic dysfunction, neural network hyperactivity and memory impairments in human aging and Alzheimer's disease. Semin. Cell Dev. Biol.116, 146–159. doi: 10.1016/j.semcdb.2021.01.005,
89
Jing R. Mu L. Wang C. Liu L. Wang Y. Wang Y. et al . (2025). KaiXinSan improves learning and memory impairment by regulating cholesterol homeostasis in mice overloaded with 27-OHC. J. Steroid Biochem. Mol. Biol.245:106622. doi: 10.1016/j.jsbmb.2024.106622,
90
Kasimov M. R. Fatkhrakhmanova M. R. Mukhutdinova K. A. Petrov A. M. (2017). 24S-hydroxycholesterol enhances synaptic vesicle cycling in the mouse neuromuscular junction: implication of glutamate NMDA receptors and nitric oxide. Neuropharmacology117, 61–73. doi: 10.1016/j.neuropharm.2017.01.030,
91
Kim K. Cho H. R. Kim B. Y. Kim J. Park D. Kwon R. J. et al . (2024). Oxysterol induces expression of 60 kDa chaperone protein on cell surface of microglia. Int. J. Mol. Sci.25:73. doi: 10.3390/ijms25169073,
92
Kim D. Lee K. M. Lee C. Jo Y. S. Muradillaevna M. S. Kim J. H. et al . (2022). Pathophysiological role of 27-hydroxycholesterol in human diseases. Adv. Biol. Regulat.83:100837. doi: 10.1016/j.jbior.2021.100837
93
Klejbor I. Shimshek D. R. Klimaszewska-Łata J. Velasco-Estevez M. Moryś J. Karaszewski B. et al . (2021). EBI2 is expressed in glial cells in multiple sclerosis lesions, and its knock-out modulates remyelination in the cuprizone model. Eur. J. Neurosci.54, 5173–5188. doi: 10.1111/ejn.15359,
94
Konieczna-Wolska K. Caratis F. Opiełka M. Biernacki K. Urbanowicz K. Klimaszewska J. et al . (2024). Accelerated remyelination and immune modulation by the EBI2 agonist 7α,25-dihydroxycholesterol analogue in the cuprizone model. Biomed. Pharmacother.181:117653. doi: 10.1016/j.biopha.2024.117653,
95
Korinek M. Gonzalez-Gonzalez I. M. Smejkalova T. Hajdukovic D. Skrenkova K. Krusek J. et al . (2020). Cholesterol modulates presynaptic and postsynaptic properties of excitatory synaptic transmission. Sci. Rep.10:12651. doi: 10.1038/s41598-020-69454-5
96
Kosowski M. Smolarczyk-Kosowska J. Hachuła M. Maligłówka M. Basiak M. Machnik G. et al . (2021). The effects of statins on neurotransmission and their neuroprotective role in neurological and psychiatric disorders. Molecules26:838. doi: 10.3390/molecules26102838,
97
Kumar G. A. Chattopadhyay A. (2021). Cholesterol-dependent endocytosis of GPCRs: implications in pathophysiology and therapeutics. Biophys. Rev.13, 1007–1017. doi: 10.1007/s12551-021-00878-7,
98
Labarrera C. London M. Angelo K. (2013). Tonic inhibition sets the state of excitability in olfactory bulb granule cells. J. Physiol.591, 1841–1850. doi: 10.1113/jphysiol.2012.241851,
99
Lauri S. E. Segerstråle M. Vesikansa A. Maingret F. Mulle C. Collingridge G. L. et al . (2005). Endogenous activation of kainate receptors regulates glutamate release and network activity in the developing hippocampus. J. Neurosci.25, 4473–4484. doi: 10.1523/jneurosci.4050-04.2005
100
Lebedev A. S. Shevlyakov A. D. Ilyin N. P. Galstyan D. S. Golushko N. I. Kalueff A. V. (2024). The Neurosteroid hormone vitamin D: modern prospects. J. Evol. Biochem. Physiol.60, 2152–2171. doi: 10.1134/S0022093024060024
101
Lee C. S. Park W. J. Han E. S. Bang H. (2007). Differential modulation of 7-Ketocholesterol toxicity against PC12 cells by Calmodulin antagonists and Ca2+ channel blockers. Neurochem. Res.32, 87–98. doi: 10.1007/s11064-006-9230-8,
102
Leonarduzzi G. Gamba P. Sottero B. Kadl A. Robbesyn F. Calogero R. A. et al . (2005). Oxysterol-induced up-regulation of MCP-1 expression and synthesis in macrophage cells. Free Radic. Biol. Med.39, 1152–1161. doi: 10.1016/j.freeradbiomed.2005.06.024,
103
Leoni V. Caccia C. (2011). Oxysterols as biomarkers in neurodegenerative diseases. Chem. Phys. Lipids164, 515–524. doi: 10.1016/j.chemphyslip.2011.04.002
104
Leoni V. Caccia C. (2013). 24S-hydroxycholesterol in plasma: a marker of cholesterol turnover in neurodegenerative diseases. Biochimie95, 595–612. doi: 10.1016/j.biochi.2012.09.025,
105
Leoni V. Lütjohann D. Masterman T. (2005). Levels of 7-oxocholesterol in cerebrospinal fluid are more than one thousand times lower than reported in multiple sclerosis. J. Lipid Res.46, 191–195. doi: 10.1194/jlr.C400005-JLR200,
106
Leoni V. Nury T. Vejux A. Zarrouk A. Caccia C. Debbabi M. et al . (2017). Mitochondrial dysfunctions in 7-ketocholesterol-treated 158N oligodendrocytes without or with α-tocopherol: impacts on the cellular profil of tricarboxylic cycle-associated organic acids, long chain saturated and unsaturated fatty acids, oxysterols, cholesterol and cholesterol precursors. J. Steroid Biochem. Mol. Biol.169, 96–110. doi: 10.1016/j.jsbmb.2016.03.029,
107
Lerma J. Marques J. M. (2013). Kainate receptors in health and disease. Neuron80, 292–311. doi: 10.1016/j.neuron.2013.09.045,
108
Levings D. C. Pathak S. S. Yang Y.-M. Slattery M. (2023). Limited expression of Nrf2 in neurons across the central nervous system. Redox Biol.65:102830. doi: 10.1016/j.redox.2023.102830
109
Li Y. Xiao D. Wang X. (2022). The emerging roles of ferroptosis in cells of the central nervous system. Front. Neurosci.16:1032140. doi: 10.3389/fnins.2022.1032140,
110
Linsenbardt A. J. Taylor A. Emnett C. M. Doherty J. J. Krishnan K. Covey D. F. et al . (2014). Different oxysterols have opposing actions at N-methyl-D-aspartate receptors, Neuropharmacology.85, 232–42 doi: 10.1016/j.neuropharm.2014.05.027
111
Liu J. Feng X. Wang Y. Xia X. Zheng J. C. (2022). Astrocytes: GABAceptive and GABAergic cells in the brain. Front. Cell. Neurosci.16:892497. doi: 10.3389/fncel.2022.892497,
112
Liu C. Yang X. V. Wu J. Kuei C. Mani N. S. Zhang L. et al (2011). Oxysterols direct B-cell migration through EBI2. Nature.475, 7357, 519–23. doi: 10.1038/nature10226
113
Loera-Valencia R. Ismail M. A. Goikolea J. Lodeiro M. Mateos L. Björkhem I. et al . (2021). Hypercholesterolemia and 27-hydroxycholesterol increase S100A8 and RAGE expression in the brain: a link between cholesterol, Alarmins, and neurodegeneration. Mol. Neurobiol.58, 6063–6076. doi: 10.1007/s12035-021-02521-8,
114
Lordan S. Mackrill J. J. O'Brien N. M. (2009). Oxysterols and mechanisms of apoptotic signaling: implications in the pathology of degenerative diseases. J. Nutr. Biochem.20, 321–336. doi: 10.1016/j.jnutbio.2009.01.001
115
Lund E. G. Guileyardo J. M. Russell D. W. (1999). cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. USA96, 7238–7243. doi: 10.1073/pnas.96.13.7238,
116
Ma W. W. Li C. Q. Yu H. L. Zhang D. D. Xi Y. D. Han J. et al . (2015). The oxysterol 27-hydroxycholesterol increases oxidative stress and regulate Nrf2 signaling pathway in astrocyte cells. Neurochem. Res.40, 758–766. doi: 10.1007/s11064-015-1524-2,
117
Ma W. W. Li C. Q. Zhao L. Wang Y. S. Xiao R. (2019). NF-κB-mediated inflammatory damage is differentially affected in SH-SY5Y and C6 cells treated with 27-hydroxycholesterol. Food Sci. Nutr.7, 1685–1694. doi: 10.1002/fsn3.1005,
118
Ma D. K. Ming G. Song H. (2009). Oxysterols drive dopaminergic neurogenesis from stem cells. Cell Stem Cell5, 343–344. doi: 10.1016/j.stem.2009.09.001,
119
Ma M.-T. Zhang J. Farooqui A. A. Chen P. Ong W.-Y. (2010). Effects of cholesterol oxidation products on exocytosis. Neurosci. Lett.476, 36–41. doi: 10.1016/j.neulet.2010.03.078
120
Mackrill J. J. (2011). Oxysterols and calcium signal transduction. Chem. Phys. Lipids164, 488–495. doi: 10.1016/j.chemphyslip.2011.04.001
121
Mahalakshmi K. Parimalanandhini D. Sangeetha R. Livya Catherene M. Beulaja M. Thiagarajan R. et al . (2021). Influential role of 7-Ketocholesterol in the progression of Alzheimer’s disease. Prostaglandins Other Lipid Mediat.156:106582. doi: 10.1016/j.prostaglandins.2021.106582
122
Makoukji J. Shackleford G. Meffre D. Grenier J. Liere P. Lobaccaro J. M. et al . (2011). Interplay between LXR and Wnt/β-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. J. Neurosci.31, 9620–9629. doi: 10.1523/jneurosci.0761-11.2011,
123
Marangon D. Audano M. Pedretti S. Fumagalli M. Mitro N. Lecca D. et al . (2022). Rewiring of glucose and lipid metabolism induced by G protein-coupled receptor 17 silencing enables the transition of oligodendrocyte progenitors to Myelinating cells. Cells11:2369. doi: 10.3390/cells11152369
124
Marinelli C. Bertalot T. Zusso M. Skaper S. D. Giusti P. (2016). Systematic review of pharmacological properties of the oligodendrocyte lineage. Front. Cell. Neurosci.10:27. doi: 10.3389/fncel.2016.00027,
125
Martin M. G. Ahmed T. Korovaichuk A. Venero C. Menchón S. A. Salas I. et al . (2014). Constitutive hippocampal cholesterol loss underlies poor cognition in old rodents. EMBO Mol. Med.6, 902–917. doi: 10.15252/emmm.201303711,
126
Marwarha G. Rhen T. Schommer T. Ghribi O. (2011). The oxysterol 27-hydroxycholesterol regulates α-synuclein and tyrosine hydroxylase expression levels in human neuroblastoma cells through modulation of liver X receptors and estrogen receptors--relevance to Parkinson's disease. J. Neurochem.119, 1119–1136. doi: 10.1111/j.1471-4159.2011.07497.x,
127
Matejuk A. Matejuk S. Offner H. Vandenbark A. A. (2025). Altered lipid metabolism in CNS demyelination and Remyelination are key elements driving progressive MS. Int. J. Mol. Sci.26:8314. doi: 10.3390/ijms26178314
128
Mattson M. P. (1998). Modification of ion homeostasis by lipid peroxidation: roles in neuronal degeneration and adaptive plasticity. Trends Neurosci.21, 53–57. doi: 10.1016/S0166-2236(97)01188-0
129
McComb M. Browne R. W. Bhattacharya S. Bodziak M. L. Jakimovski D. Weinstock-Guttman B. et al . (2021). The cholesterol autoxidation products, 7-ketocholesterol and 7β-hydroxycholesterol are associated with serum neurofilaments in multiple sclerosis. Mult. Scler. Relat. Disord.50:102864. doi: 10.1016/j.msard.2021.102864,
130
Meffre D. Shackleford G. Hichor M. Gorgievski V. Tzavara E. T. Trousson A. et al . (2015). Liver X receptors alpha and beta promote myelination and remyelination in the cerebellum. Proc. Natl. Acad. Sci. USA112, 7587–7592. doi: 10.1073/pnas.1424951112,
131
Mele M. Costa R. O. Duarte C. B. (2019). Alterations in GABAA-receptor trafficking and synaptic dysfunction in brain disorders. Front. Cell. Neurosci.13:77. doi: 10.3389/fncel.2019.00077,
132
Merino-Serrais P. Loera-Valencia R. Rodriguez-Rodriguez P. Parrado-Fernandez C. Ismail M. A. Maioli S. et al . (2018). 27-hydroxycholesterol induces aberrant morphology and synaptic dysfunction in hippocampal neurons. Cereb. Cortex29, 429–446. doi: 10.1093/cercor/bhy274,
133
Meza U. Delgado-Ramírez M. Romero-Méndez C. Sánchez-Armass S. Rodríguez-Menchaca A. A. (2020). Functional marriage in plasma membrane: critical cholesterol level-optimal protein activity. Br. J. Pharmacol.177, 2456–2465. doi: 10.1111/bph.15027,
134
Michałowski M. A. Kłopotowski K. Wiera G. Czyżewska M. M. Mozrzymas J. W. (2025). Molecular mechanisms of the GABA type a receptor function. Q. Rev. Biophys.58:e3. doi: 10.1017/S0033583524000179
135
Miller W. L. Auchus R. J. (2011). The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev.32, 81–151. doi: 10.1210/er.2010-0013,
136
Miralles A. J. Unger N. Kannaiyan N. Rossner M. J. Dimou L. (2023). Analysis of the GPR17 receptor in NG2-glia under physiological conditions unravels a new subset of oligodendrocyte progenitor cells with distinct functions. Glia71, 1536–1552. doi: 10.1002/glia.24356,
137
Montani L. (2021). Lipids in regulating oligodendrocyte structure and function. Semin. Cell Dev. Biol.112, 114–122. doi: 10.1016/j.semcdb.2020.07.016
138
Mouzat K. Chudinova A. Polge A. Kantar J. Camu W. Raoul C. et al . (2019). Regulation of brain cholesterol: what role do liver X receptors play in neurodegenerative diseases?Int. J. Mol. Sci.20:858. doi: 10.3390/ijms20163858,
139
Müller M. L. Bohnen N. I. (2013). Cholinergic dysfunction in Parkinson's disease. Curr. Neurol. Neurosci. Rep.13:377. doi: 10.1007/s11910-013-0377-9,
140
Mutemberezi V. Buisseret B. Masquelier J. Guillemot-Legris O. Alhouayek M. Muccioli G. G. (2018). Oxysterol levels and metabolism in the course of neuroinflammation: insights from in vitro and in vivo models. J. Neuroinflammation15:74. doi: 10.1186/s12974-018-1114-8,
141
Neely M. D. Sidell K. R. Graham D. G. Montine T. J. (1999). The lipid peroxidation product 4-hydroxynonenal inhibits neurite outgrowth, disrupts neuronal microtubules, and modifies cellular tubulin. J. Neurochem.72, 2323–2333. doi: 10.1046/j.1471-4159.1999.0722323.x,
142
Niki E. (2014). Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic. Biol. Med.66, 3–12. doi: 10.1016/j.freeradbiomed.2013.03.022
143
Nishi T. Metcalf C. S. Fujimoto S. Hasegawa S. Miyamoto M. Sunahara E. et al . (2022). Anticonvulsive properties of soticlestat, a novel cholesterol 24-hydroxylase inhibitor. Epilepsia63, 1580–1590. doi: 10.1111/epi.17232,
144
Noguchi N. Urano Y. Takabe W. Saito Y. (2015). New aspects of 24(S)-hydroxycholesterol in modulating neuronal cell death. Free Radic. Biol. Med.87, 366–372. doi: 10.1016/j.freeradbiomed.2015.06.036,
145
Nury T. Sghaier R. Zarrouk A. Ménétrier F. Uzun T. Leoni V. et al . (2018). Induction of peroxisomal changes in oligodendrocytes treated with 7-ketocholesterol: attenuation by α-tocopherol. Biochimie153, 181–202. doi: 10.1016/j.biochi.2018.07.009,
146
Nury T. Yammine A. Menetrier F. Zarrouk A. Vejux A. Lizard G. (2020). “7-Ketocholesterol- and 7β-hydroxycholesterol-induced Peroxisomal disorders in glial, microglial and neuronal cells: potential role in neurodegeneration” in Peroxisome biology: Experimental models, Peroxisomal disorders and neurological diseases. ed. LizardG. (Cham: Springer International Publishing), 31–41.
147
Nury T. Zarrouk A. Mackrill J. J. Samadi M. Durand P. Riedinger J. M. et al . (2015). Induction of oxiapoptophagy on 158N murine oligodendrocytes treated by 7-ketocholesterol-, 7β-hydroxycholesterol-, or 24(S)-hydroxycholesterol: protective effects of α-tocopherol and docosahexaenoic acid (DHA; C22:6 n-3). Steroids99, 194–203. doi: 10.1016/j.steroids.2015.02.003,
148
Olsen B. N. Schlesinger P. H. Ory D. S. Baker N. A. (2012). Side-chain oxysterols: from cells to membranes to molecules. Biochim. Biophys. Acta1818, 330–336. doi: 10.1016/j.bbamem.2011.06.014,
149
Ong W. Y. Kim J. H. He X. Chen P. Farooqui A. A. Jenner A. M. (2010). Changes in brain cholesterol metabolome after excitotoxicity. Mol. Neurobiol.41, 299–313. doi: 10.1007/s12035-010-8099-3,
150
Paila Y. D. Chattopadhyay A. (2009). The function of G-protein coupled receptors and membrane cholesterol: specific or general interaction?Glycoconj. J.26, 711–720. doi: 10.1007/s10719-008-9218-5
151
Paintlia A. S. Paintlia M. K. Khan M. Vollmer T. Singh A. K. Singh I. (2005). HMG-CoA reductase inhibitor augments survival and differentiation of oligodendrocyte progenitors in animal model of multiple sclerosis. FASEB J.19, 1407–1421. doi: 10.1096/fj.05-3861com,
152
Papassotiropoulos A. Lütjohann D. Bagli M. Locatelli S. Jessen F. Buschfort R. et al . (2002). 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J. Psychiatr. Res.36, 27–32. doi: 10.1016/s0022-3956(01)00050-4,
153
Paul R. Choudhury A. Chandra Boruah D. Devi R. Bhattacharya P. Choudhury M. D. et al . (2017). Hypercholesterolemia causes psychomotor abnormalities in mice and alterations in cortico-striatal biogenic amine neurotransmitters: relevance to Parkinson's disease. Neurochem. Int.108, 15–26. doi: 10.1016/j.neuint.2017.01.021,
154
Paul S. M. Doherty J. J. Robichaud A. J. Belfort G. M. Chow B. Y. Hammond R. S. et al . (2013). The major brain cholesterol metabolite 24(S)-hydroxycholesterol is a potent allosteric modulator of N-methyl-d-aspartate receptors. J. Neurosci.33, 17290–17300. doi: 10.1523/jneurosci.2619-13.2013
155
Petrov A. M. (2024a). “Oxysterols in central and peripheral synaptic communication” in Implication of oxysterols and Phytosterols in aging and human diseases. ed. LizardG. (Cham: Springer International Publishing), 91–123.
156
Petrov A. M. (2024b). Oxysterols in central and peripheral synaptic communication. Adv. Exp. Med. Biol.1440, 91–123. doi: 10.1007/978-3-031-43883-7_6,
157
Pfrieger F. W. Ungerer N. (2011). Cholesterol metabolism in neurons and astrocytes. Prog. Lipid Res.50, 357–371. doi: 10.1016/j.plipres.2011.06.002
158
Poli G. Biasi F. Leonarduzzi G. (2013). Oxysterols in the pathogenesis of major chronic diseases. Redox Biol.1, 125–130. doi: 10.1016/j.redox.2012.12.001,
159
Qian L. Chai A. B. Gelissen I. C. Brown A. J. (2022). Balancing cholesterol in the brain: from synthesis to disposal. Expl. Neuroprot. Ther.2, 1–27. doi: 10.37349/ent.2022.00015
160
Radhakrishnan A. Ikeda Y. Kwon H. J. Brown M. S. Goldstein J. L. (2007). Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci.104, 6511–6518. doi: 10.1073/pnas.0700899104,
161
Radhakrishnan K. Zhang Y. Mustapha O. Weigel T.K. Upchurch C.M. Tian X. et al . (2025). 7-ketocholesterol contributes to microglia-driven increases in astrocyte reactive oxygen species in Alzheimer’s disease. bioRxiv [Preprint]. doi: 10.1101/2025.01.19.633810, .
162
Reiss A. B. Voloshyna I. (2012). Regulation of cerebral cholesterol metabolism in Alzheimer disease. J. Investig. Med.60, 576–582. doi: 10.2310/JIM.0b013e318246d973,
163
Ren J.-X. Sun X. Yan X.-L. Guo Z.-N. Yang Y. (2020). Ferroptosis in neurological diseases. Front. Cell. Neurosci.14:218. doi: 10.3389/fncel.2020.00218,
164
Rodríguez-Moreno A. Sihra T. S. (2013). Presynaptic kainate receptor-mediated facilitation of glutamate release involves Ca2+−calmodulin and PKA in cerebrocortical synaptosomes. FEBS Lett.587, 788–792. doi: 10.1016/j.febslet.2013.01.071,
165
Romero J. Toral-Rios D. Yu J. Paul S. M. Cashikar A. G. (2024). 25-hydroxycholesterol promotes brain cytokine production and leukocyte infiltration in a mouse model of lipopolysaccharide-induced neuroinflammation. J. Neuroinflammation21:251. doi: 10.1186/s12974-024-03233-1
166
Ruiz F. Peter B. Rebeaud J. Vigne S. Bressoud V. Roumain M. et al . (2023). Endothelial cell-derived oxysterol ablation attenuates experimental autoimmune encephalomyelitis. EMBO Rep.24:e55328. doi: 10.15252/embr.202255328,
167
Russell D. W. (2003). The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem.72(2003, 137–174. doi: 10.1146/annurev.biochem.72.121801.161712
168
Rutkowska A. Sailer A. W. Dev K. K. (2017). EBI2 receptor regulates myelin development and inhibits LPC-induced demyelination. J. Neuroinflammation14:250. doi: 10.1186/s12974-017-1025-0
169
Sacchetti P. Sousa K. M. Hall A. C. Liste I. Steffensen K. R. Theofilopoulos S. et al . (2009). Liver X receptors and oxysterols promote ventral midbrain neurogenesis in vivo and in human embryonic stem cells. Cell Stem Cell5, 409–419. doi: 10.1016/j.stem.2009.08.019
170
Saher G. Brügger B. Lappe-Siefke C. Möbius W. Tozawa R. Wehr M. C. et al . (2005). High cholesterol level is essential for myelin membrane growth. Nat. Neurosci.8, 468–475. doi: 10.1038/nn1426
171
Sailike B. Onzhanova Z. Akbay B. Tokay T. Molnár F. (2024). Vitamin D in central nervous system: implications for neurological disorders. Int. J. Mol. Sci.25:7809. doi: 10.3390/ijms25147809
172
Salaün C. Gould G. W. Chamberlain L. H. (2005). Lipid raft association of SNARE proteins regulates exocytosis in PC12 cells. J. Biol. Chem.280, 19449–19453. doi: 10.1074/jbc.M501923200
173
Santoro V. Hou M. D. Premoli I. Belardinelli P. Biondi A. Carobin A. et al . (2024). Investigating cortical excitability and inhibition in patients with schizophrenia: a TMS-EEG study. Brain Res. Bull.212:110972. doi: 10.1016/j.brainresbull.2024.110972
174
Segatto M. Tonini C. Pfrieger F. W. Trezza V. Pallottini V. (2019). Loss of mevalonate/cholesterol homeostasis in the brain: a focus on autism Spectrum disorder and Rett syndrome. Int. J. Mol. Sci.20:3317. doi: 10.3390/ijms20133317
175
Shackleford G. G. Grenier J. Abi Habib W. Massaad C. Meffre D. (2017). Liver X receptors differentially modulate central myelin gene mRNA levels in a region-, age- and isoform-specific manner. J. Steroid Biochem. Mol. Biol.169, 61–68. doi: 10.1016/j.jsbmb.2016.02.032,
176
Shin K. C. Ali Moussa H. Y. Park Y. (2024). Cholesterol imbalance and neurotransmission defects in neurodegeneration. Exp. Mol. Med.56, 1685–1690. doi: 10.1038/s12276-024-01273-4,
177
Shipton O. A. Paulsen O. (2014). GluN2A and GluN2B subunit-containing NMDA receptors in hippocampal plasticity. Philos. Transact. R. Soc. B Biol. Sci.369:20130163. doi: 10.1098/rstb.2013.0163,
178
Shore A. N. Colombo S. Tobin W. F. Petri S. Cullen E. R. Dominguez S. et al . (2020). Reduced GABAergic neuron excitability, altered synaptic connectivity, and seizures in a KCNT1 gain-of-function mouse model of childhood epilepsy. Cell Rep.33:108303. doi: 10.1016/j.celrep.2020.108303
179
Simon K. Hennen S. Merten N. Blättermann S. Gillard M. Kostenis E. et al . (2016). The orphan G protein-coupled receptor GPR17 negatively regulates oligodendrocyte differentiation via Gαi/o and its downstream effector molecules*. J. Biol. Chem.291, 705–718. doi: 10.1074/jbc.M115.683953,
180
Singh A. Kukreti R. Saso L. Kukreti S. (2019). Oxidative stress: a key modulator in neurodegenerative diseases. Molecules24:583. doi: 10.3390/molecules24081583,
181
Smith R. A. Porteous C. M. Coulter C. V. Murphy M. P. (1999). Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem.263, 709–716. doi: 10.1046/j.1432-1327.1999.00543.x
182
Son Y. Yeo I. J. Hong J. T. Eo S. K. Lee D. Kim K. (2023). Side-chain immune oxysterols induce Neuroinflammation by activating microglia. Int. J. Mol. Sci.24:288. doi: 10.3390/ijms242015288,
183
Song H. Lv A. Zhu Z. Li R. Zhao Q. Yu X. et al . (2024). CYP7B1 deficiency impairs myeloid cell activation in autoimmune disease of the central nervous system. PNAS Nexus3:pgae334. doi: 10.1093/pnasnexus/pgae334,
184
Song X. Wu W. Warner M. Gustafsson J.-Å. (2022). Liver X receptor regulation of glial cell functions in the CNS. Biomedicine10:2165. doi: 10.3390/biomedicines10092165
185
Sooksawate T. Simmonds M. A. (1998). Increased membrane cholesterol reduces the potentiation of GABAA currents by neurosteroids in dissociated hippocampal neurones. Neuropharmacology37, 1103–1110. doi: 10.1016/S0028-3908(98)00113-0,
186
Sooksawate T. Simmonds M. A. (2001). Influence of membrane cholesterol on modulation of the GABA(a) receptor by neuroactive steroids and other potentiators. Br. J. Pharmacol.134, 1303–1311. doi: 10.1038/sj.bjp.0704360
187
Staurenghi E. Cerrato V. Gamba P. Testa G. Giannelli S. Leoni V. et al . (2021). Oxysterols present in Alzheimer's disease brain induce synaptotoxicity by activating astrocytes: a major role for lipocalin-2. Redox Biol.39:101837. doi: 10.1016/j.redox.2020.101837
188
Sultana R. Perluigi M. Butterfield D. A. (2013). Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med.62, 157–169. doi: 10.1016/j.freeradbiomed.2012.09.027,
189
Sun M. Y. Izumi Y. Benz A. Zorumski C. F. Mennerick S. (2016). Endogenous 24S-hydroxycholesterol modulates NMDAR-mediated function in hippocampal slices. J. Neurophysiol.115, 1263–1272. doi: 10.1152/jn.00890.2015,
190
Sun Z. Yang J. Zhou J. Zhou J. Feng L. Feng Y. et al . (2023). Tissue-specific oxysterols as predictors of antidepressant (Escitalopram) treatment response in patients with major depressive disorder. Biol. Psychiatry Glob. Open Sci.3, 663–672. doi: 10.1016/j.bpsgos.2023.01.004
191
Sun Z. Zhao L. Bo Q. Mao Z. He Y. Jiang T. et al . (2021). Brain-specific oxysterols and risk of schizophrenia in clinical high-risk subjects and patients with schizophrenia. Front. Psych.12:711734. doi: 10.3389/fpsyt.2021.711734,
192
Swaroop S. Sengupta N. Suryawanshi A. R. Adlakha Y. K. Basu A. (2016). HSP60 plays a regulatory role in IL-1β-induced microglial inflammation via TLR4-p38 MAPK axis. J. Neuroinflammation13:27. doi: 10.1186/s12974-016-0486-x,
193
Tang W. Liu D. Traynelis S. F. Yuan H. (2020). Positive allosteric modulators that target NMDA receptors rectify loss-of-function GRIN variants associated with neurological and neuropsychiatric disorders. Neuropharmacology.177:108247 doi: 10.1016/j.neuropharm.2020.108247
194
Testa G. Gamba P. Badilli U. Gargiulo S. Maina M. Guina T. et al . (2014). Loading into nanoparticles improves quercetin's efficacy in preventing neuroinflammation induced by oxysterols. PLoS One9:e96795. doi: 10.1371/journal.pone.0096795,
195
Testa G. Staurenghi E. Giannelli S. Gargiulo S. Guglielmotto M. Tabaton M. et al . (2018). A silver lining for 24-hydroxycholesterol in Alzheimer's disease: the involvement of the neuroprotective enzyme sirtuin 1. Redox Biol.17, 423–431. doi: 10.1016/j.redox.2018.05.009,
196
Testa G. Staurenghi E. Zerbinati C. Gargiulo S. Iuliano L. Giaccone G. et al . (2016). Changes in brain oxysterols at different stages of Alzheimer's disease: their involvement in neuroinflammation. Redox Biol.10, 24–33. doi: 10.1016/j.redox.2016.09.001,
197
Tomita H. Hines K. M. Herron J. M. Li A. Baggett D. W. Xu L. (2022). 7-Dehydrocholesterol-derived oxysterols cause neurogenic defects in Smith-Lemli-Opitz syndrome. eLife11:e067141. doi: 10.7554/eLife.67141,
198
Toral-Rios D. Long J. M. Ulrich J. D. Yu J. Strickland M. R. Han X. et al . (2024). Cholesterol 25-hydroxylase mediates neuroinflammation and neurodegeneration in a mouse model of tauopathy. J. Exp. Med.221:e20232000. doi: 10.1084/jem.20232000,
199
Töröcsik D. Szanto A. Nagy L. (2009). Oxysterol signaling links cholesterol metabolism and inflammation via the liver X receptor in macrophages. Mol. Asp. Med.30, 134–152. doi: 10.1016/j.mam.2009.02.002,
200
Trousson A. Bernard S. Petit P. X. Liere P. Pianos A. El Hadri K. et al . (2009). 25-hydroxycholesterol provokes oligodendrocyte cell line apoptosis and stimulates the secreted phospholipase A2 type IIA via LXR beta and PXR. J. Neurochem.109, 945–958. doi: 10.1111/j.1471-4159.2009.06009.x
201
Ullrich C. Pirchl M. Humpel C. (2010). Effects of cholesterol and its 24S-OH and 25-OH oxysterols on choline acetyltransferase-positive neurons in brain slices. Pharmacology86, 15–21. doi: 10.1159/000314333,
202
Vallés A. S. Barrantes F. J. (2022). The synaptic lipidome in health and disease. BBA Biomembranes1864:184033. doi: 10.1016/j.bbamem.2022.184033
203
Vejux A. Ghzaiel I. Mackrill J. J. Dias I. H. K. Rezig L. Ksila M. et al . (2025). Oxysterols, age-related-diseases and nutritherapy: focus on 7-ketocholesterol and 7β-hydroxycholesterol. Prostaglandins Other Lipid Mediat.178:106993. doi: 10.1016/j.prostaglandins.2025.106993
204
Vejux A. Ghzaiel I. Nury T. Schneider V. Charrière K. Sghaier R. et al . (2021). Oxysterols and multiple sclerosis: physiopathology, evolutive biomarkers and therapeutic strategy. J. Steroid Biochem. Mol. Biol.210:105870. doi: 10.1016/j.jsbmb.2021.105870
205
Vejux A. Kahn E. Menetrier F. Montange T. Lherminier J. Riedinger J. M. et al . (2007). Cytotoxic oxysterols induce caspase-independent myelin figure formation and caspase-dependent polar lipid accumulation. Histochem. Cell Biol.127, 609–624. doi: 10.1007/s00418-006-0268-0,
206
Véjux A. Lizard G. (2009). Cytotoxic effects of oxysterols associated with human diseases: induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Asp. Med.30, 153–170. doi: 10.1016/j.mam.2009.02.006,
207
Velasco-Estevez M. Koch N. Klejbor I. Laurent S. Dev K. K. Szutowicz A. et al . (2021). EBI2 is temporarily upregulated in MO3.13 oligodendrocytes during maturation and regulates Remyelination in the Organotypic cerebellar slice model. Int. J. Mol. Sci.22:342. doi: 10.3390/ijms22094342,
208
Verma M. Lizama B. N. Chu C. T. (2022). Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration. Transl. Neurodegener.11:3. doi: 10.1186/s40035-021-00278-7,
209
Vitali C. Wellington C. L. Calabresi L. (2014). HDL and cholesterol handling in the brain. Cardiovasc. Res.103, 405–413. doi: 10.1093/cvr/cvu148,
210
Voulgaridou G.-P. Anestopoulos I. Franco R. Panayiotidis M. I. Pappa A. (2011). DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat. Res. Fundament. Mol. Mech. Mutag.711, 13–27. doi: 10.1016/j.mrfmmm.2011.03.006,
211
Wallace M. L. Sabatini B. L. (2023). Synaptic and circuit functions of multitransmitter neurons in the mammalian brain. Neuron111, 2969–2983. doi: 10.1016/j.neuron.2023.06.003
212
Wang Y. An Y. Ma W. Yu H. Lu Y. Zhang X. et al . (2020). 27-hydroxycholesterol contributes to cognitive deficits in APP/PS1 transgenic mice through microbiota dysbiosis and intestinal barrier dysfunction. J. Neuroinflammation17:199. doi: 10.1186/s12974-020-01873-7,
213
Wang Y. An Y. Zhang D. Yu H. Zhang X. Wang Y. et al . (2019). 27-hydroxycholesterol alters synaptic structural and functional plasticity in hippocampal neuronal cultures. J. Neuropathol. Exp. Neurol.78, 238–247. doi: 10.1093/jnen/nlz002
214
Wang T. Feng W. Ju M. Yu H. Guo Z. Sun X. et al . (2023). 27-hydroxycholesterol causes cognitive deficits by disturbing Th17/Treg balance and the related immune responses in mild cognitive impairment patients and C57BL/6J mice. J. Neuroinflammation20:305. doi: 10.1186/s12974-023-02986-5,
215
Wang Y. Hao L. Wang T. Liu W. Wang L. Ju M. et al . (2022). 27-hydroxycholesterol-induced dysregulation of cholesterol metabolism impairs learning and memory ability in ApoE ε4 transgenic mice. Int. J. Mol. Sci.23:639. doi: 10.3390/ijms231911639
216
Wang N. Q. Sun P. X. Shen Q. Q. Deng M. Y. (2025). Cholesterol metabolism in CNS diseases: the potential of SREBP2 and LXR as therapeutic targets. Mol. Neurobiol.62, 6283–6307. doi: 10.1007/s12035-024-04672-w
217
Wang H. Yuan L. Ma W. Han J. Lu Y. Feng L. et al . (2016). The cytotoxicity of 27-hydroxycholesterol in co-cultured SH-SY5Y cells and C6 cells. Neurosci. Lett.632, 209–217. doi: 10.1016/j.neulet.2016.08.056,
218
Wang Y. Yutuc E. Griffiths W. J. (2021). Neuro-oxysterols and neuro-sterols as ligands to nuclear receptors, GPCRs, ligand-gated ion channels and other protein receptors. Br. J. Pharmacol.178, 3176–3193. doi: 10.1111/bph.15191
219
Wei X. Nishi T. Kondou S. Kimura H. Mody I. (2019). Preferential enhancement of GluN2B-containing native NMDA receptors by the endogenous modulator 24S-hydroxycholesterol in hippocampal neurons. Neuropharmacology148, 11–20. doi: 10.1016/j.neuropharm.2018.12.028
220
Wen P. Sun Z. Gou F. Wang J. Fan Q. Zhao D. et al . (2025). Oxidative stress and mitochondrial impairment: key drivers in neurodegenerative disorders. Ageing Res. Rev.104:102667. doi: 10.1016/j.arr.2025.102667,
221
Wheless J. W. Rho J. M. (2025). Role of cholesterol in modulating brain hyperexcitability. Epilepsia66, 33–46. doi: 10.1111/epi.18174,
222
Wnętrzak A. Chachaj-Brekiesz A. Kobierski J. Dynarowicz-Latka P. (2024). “The structure of oxysterols determines their behavior at phase boundaries: implications for model membranes and structure–activity relationships” in Implication of oxysterols and Phytosterols in aging and human diseases. ed. LizardG. (Cham: Springer International Publishing), 3–29.
223
Wnętrzak A. Chachaj-Brekiesz A. Stępniak A. Kobierski J. Dynarowicz-Latka P. (2022). Different effects of oxysterols on a model lipid raft – Langmuir monolayer study complemented with theoretical calculations. Chem. Phys. Lipids244:105182. doi: 10.1016/j.chemphyslip.2022.105182,
224
Wolf G. (1999). The role of oxysterols in cholesterol homeostasis. Nutr. Rev.57, 196–198. doi: 10.1111/j.1753-4887.1999.tb06943.x
225
Xu P. Xu H. Tang X. Xu L. Wang Y. Guo L. et al . (2014). Liver X receptor β is essential for the differentiation of radial glial cells to oligodendrocytes in the dorsal cortex. Mol. Psychiatry19, 947–957. doi: 10.1038/mp.2014.60,
226
Yamanaka K. Saito Y. Yamamori T. Urano Y. Noguchi N. (2011). 24(S)-hydroxycholesterol induces neuronal cell death through necroptosis, a form of programmed necrosis*. J. Biol. Chem.286, 24666–24673. doi: 10.1074/jbc.M111.236273,
227
Yoon J. H. Canbay A. E. Werneburg N. W. Lee S. P. Gores G. J. (2004). Oxysterols induce cyclooxygenase-2 expression in cholangiocytes: implications for biliary tract carcinogenesis. Hepatology39, 732–738. doi: 10.1002/hep.20125
228
Yrjänheikki J. Tikka T. Keinänen R. Goldsteins G. Chan P. H. Koistinaho J. (1999). A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. USA96, 13496–13500. doi: 10.1073/pnas.96.23.13496,
229
Yuan Z. Pavel M. A. Hansen S. B. (2025). GABA and astrocytic cholesterol determine the lipid environment of GABAAR in cultured cortical neurons. Commun. Biol.8:647. doi: 10.1038/s42003-025-08026-7,
230
Yutuc E. Angelini R. Baumert M. Mast N. Pikuleva I. Newton J. et al . (2020). Localization of sterols and oxysterols in mouse brain reveals distinct spatial cholesterol metabolism. Proc. Natl. Acad. Sci. USA117, 5749–5760. doi: 10.1073/pnas.1917421117,
231
Zhang C. Bo R. Zhou T. Chen N. Yuan Y. (2024). The raphe nuclei are the early lesion site of gastric α-synuclein propagation to the substantia nigra. Acta Pharm. Sin. B14, 2057–2076. doi: 10.1016/j.apsb.2024.01.015,
232
Zhang S. Y. Gui L. N. Liu Y. Y. Shi S. Cheng Y. (2020). Oxidative stress marker aberrations in multiple sclerosis: a meta-analysis study. Front. Neurosci.14:823. doi: 10.3389/fnins.2020.00823,
233
Zhang J. Liu Q. (2015). Cholesterol metabolism and homeostasis in the brain. Protein Cell6, 254–264. doi: 10.1007/s13238-014-0131-3,
234
Zhang L. Shi Y. Liang B. Li X. (2024). An overview of the cholesterol metabolism and its proinflammatory role in the development of MASLD. Hepatol. Commun.8:34. doi: 10.1097/hc9.0000000000000434,
235
Zhang R. Wuerch E. Yong V. W. Xue M. (2024). LXR agonism for CNS diseases: promises and challenges. J. Neuroinflammation21:97. doi: 10.1186/s12974-024-03056-0,
236
Zhang D. D. Yu H. L. Ma W. W. Liu Q. R. Han J. Wang H. et al . (2015). 27-hydroxycholesterol contributes to disruptive effects on learning and memory by modulating cholesterol metabolism in the rat brain. Neuroscience300, 163–173. doi: 10.1016/j.neuroscience.2015.05.022,
237
Zhu S. Stavrovskaya I. G. Drozda M. Kim B. Y. Ona V. Li M. et al . (2002). Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature417, 74–78. doi: 10.1038/417074a
Summary
Keywords
central nervous system, glia, neurodegeneration, neuroinflammation, oxysterols
Citation
Ricci RP, Foo CX, Ronacher K and Cullen CL (2026) Oxysterol signaling in the central nervous system: cellular mechanisms and implications for neurodegeneration. Front. Mol. Neurosci. 19:1709065. doi: 10.3389/fnmol.2026.1709065
Received
19 September 2025
Revised
23 January 2026
Accepted
26 January 2026
Published
12 February 2026
Volume
19 - 2026
Edited by
Sarah C. Hellewell, Curtin University, Australia
Reviewed by
Xiangnan Zhang, Zhejiang University, China
Sampada Tamhankar, Auburn University, United States
Updates
Copyright
© 2026 Ricci, Foo, Ronacher and Cullen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlie L. Cullen, carlie.cullen@mater.uq.edu.au
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.