- 1Division of Plant Pathology, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 2Department of Botany, B.R. Ambedkar Bihar University, Muzaffarpur, Bihar, India
- 3Department of Applied Sciences and Humanities, Faculty of Engineering and Technology, Jamia Millia Islamia, New Delhi, India
- 4Department of Physical Sciences, Sant Baba Bhag Singh University, Jalandhar, Punjab, India
- 5Department of Chemistry, Jamia Millia Islamia, New Delhi, India
- 6Department of Electronics and Communication Engineering, Noida Institute of Engineering and Technology, Noida, Uttar Pradesh, India
- 7Electronics Engineering Section, University Polytechnic, Jamia Millia Islamia, New Delhi, India
- 8Division of Agricultural Chemicals, ICAR-Indian Agricultural Research Institute, New Delhi, India
This is the first study that reports in vivo antifungal efficacy of cucumber-derived green synthesized silver and gold nanoparticles (Ag and Au NPs) against root-rot disease of mungbean caused by Macrophomina phaseolina. Numerous spectral and morphological studies including UV–Vis, FT-IR, TGA, DLS, TEM, SEM, and EDX were performed that determine the successful preparation of Ag/Au NPs and confirmed their particle size and crystalline structure. The biosynthesized NPs were first tested under in vitro condition which showed 55%–74% mycelial inhibition of M. phaseolina. In vivo experiment clearly demonstrated that both the nanoparticles significantly controlled root-rot disease of mungbean. Maximum control of disease (47%) was found with Ag NPs at 100 ppm with significant improvement in the plant growth (38%–45%) and yield (59%). Au NPs (100 ppm) also gave a satisfactory control of root-rot disease (42%) and increased grain yield of infected plants by 49%. As the results of TGA revealed that Ag NPs were thermally more stable than Au NPs, hence, additionally we explored the impact of Ag NPs on thermally-activated delayed fluorescent (TADF) organic LED performance to broaden their application potential. We found that incorporating Ag NPs into hole-injection layer significantly enhances the performance of TADF organic LED. This research not only demonstrates the potential of green-synthesized Ag and Au NPs as effective nanofungicides for mungbean root-rot disease management, but also highlights their promising role in enhancing the performance of advanced electronic devices such as TADF organic LEDs, thus broadening their scope of application in both agriculture and optoelectronics.
1 Introduction
Nanotechnology has emerged recently as very important tool to boost the agricultural output through the use of nanoparticles either by suppressing economically important plant diseases or by serving as plant growth regulators. Nanoparticles (NPs) refers to atoms that has a diameter in the range of 1 nm–100 nm (Auffan et al., 2009; Parveen et al., 2022). NPs of some noble metals, e.g., silver, platinum, gold, copper, zinc, magnesium, and titanium, have gained great attention in recent years (Kanimozhi et al., 2022; Shubhashree et al., 2022; Lal et al., 2021). “Green” synthesis of nanoparticles has gained importance during past few years to attain sustainability in the environment (Spinoso-Castillo et al., 2017). Although, chemical as well as physical methods are available to synthesize NPs, yet at same time they are accompanied with hazardous chemicals posing toxicity.
In this instance, plant-mediated synthesis of the metal NPs is an alternativedue to its low toxicity, environmental friendliness, short time consumption and cost effectiveness (Gengan et al., 2013). Furthermore, plants are easily available and serve as excellent source of secondary metabolites including polyphenols, polysaccharides, flavonoids, proteins, terpenoids, alkaloids, ketones, aldehydes, amines, and tannins, that perform as good reducing, stabilizing and capping agents during formation of metal NPs from the metal ions (Dhayalan et al., 2018; Niluxsshun et al., 2021). Fruit extracts contain strong reducing, stabilizing as well as capping agents (Jain et al., 2009). Some reports are available in the literature showing synthesis of silver NPs by using fruit extract of cucumber, European wine grape, bilimbi, papaya and apple (Jain et al., 2009; Rimal Isaac et al., 2013; Roy et al., 2015) as well as synthesis of gold NPs by using the fruit extract of bilimbi, red raspberry, strawberry, and blackberry (Rimal Isaac et al., 2013; Demirbas et al., 2019).
Plant diseases pose a serious threat to cultivation of several economically important crops (Khan et al., 2010; Shahid et al., 2023a; Shahid et al., 2023b; Shahid et al., 2024a). Hence, its management is very necessary to prevent the losses (Khan et al., 2012; Shahid et al., 2023c; Shahid et al., 2024b). Plant disease management using NPs is progressing increasingly throughout the globe. Metal NPs specifically, have been widely explored and reported to have strong antifungal characteristics, hence since few years they have been utilized for the suppression of plant pathogenic fungi (Ahmed et al., 2016; Kong et al., 2020). Mungbean is an important legume crop and its productivity is greatly affected by root-rot disease caused by fungus, M. phaseolina which is a very destructive disease that causes substantial losses to the crop (Shahid and Khan, 2016a; Tandel et al., 2010). Current studies on management aspects of root-rot of mungbean have shown that various methods have been used for controlling the pathogenic fungus, but its management is very challenging because of the seed and soil borne nature of the pathogen which makes the disease management very difficult. In the recent years, a lot of efforts have been constantly conducted to find the alternatives to the chemical fungicides used to control soil-borne infections because of the adverse effects of chemicals on human and environment (Meena et al., 2020; Khan et al., 2011; Khan et al., 2017; Ahmad et al., 2020). In order to overcome this problem, use of nanoparticles can be served as an alternative method for its management which is eco-friendly and effective. Although, efficacy to control some of the plant diseases has been reported for a variety of nanoparticles including silver NPs, gold NPs, magnesium NPs, copper NPs, titanium NPs etc., (Dizaj et al., 2014; Abou-Salem et al., 2022; Abdallah et al., 2022), but these NPs are still being explored for numerous other important plant diseases against which these have not been tested so far.
Even though some researchers have reported the antifungal effect of only silver NPs (but not gold NPs) against M. phaseolina only under in vitro condition, however, to date, there is no data on testing the effect of biosynthesized silver and gold NPs against root-rot disease of mungbean caused by M. phaseolina under in vivo condition. In particular, very little is known about the influence of gold NPs on the plant diseases. Hence in this paper, we report for the first time the in vivo effects of green synthesized silver and gold nanoparticles against root-rot disease of mungbean caused by M. phaseolina.
In addition, the effect of Ag NPs was also examined on the performance of advanced electronic devices such as thermally-activated delayed fluorescent (TADF) organic LED through incorporation of Ag NPs into hole-injection layer in order to widen their application potential. Hence, this study also aimed to evaluate the influence of Ag NPs on the optical and electrical properties of TADF OLEDs, focusing on parameters such as luminance efficiency, current density, and external quantum efficiency (EQE). By leveraging the plasmonic effects of Ag NPs, enhancements in charge injection, light extraction, and recombination efficiency were anticipated (Liang et al., 2024; Georgiopoulou et al., 2024; Lee et al., 2023). Comparative simulations were conducted with and without Ag NPs to assess their impact, providing insights into the potential of nanoparticle incorporation as a strategy for optimizing next-generation OLED devices.
2 Materials and methods
2.1 Collection of cucumber fruits and chemicals
The freshly harvested fruits of cucumber were collected from the experimental fields of ICAR-Indian Agricultural Research Institute (IARI), New Delhi, India. The extract of cucumber (Cucumis sativus) fruits was used for green synthesis of nanoparticles. For preparing the fruit extract, fresh and clean cucumbers were selected (Figure 1). Silver nitrate (AgNO3) and chloroauric acid (AuHCl4) required for the synthesis of NPs were purchased from Sigma-Aldrich, India Ltd. and were of analytical grade. All the other chemicals that were used during the synthesis of NPs were used as such without further purification. Triple distilled deionized water (H2O) was used throughout the synthesis.
2.2 Isolation of the pathogen
The root-rot fungus, M. phaseolina was isolated from mungbean plants showing symptoms of wilting and rotting from IARI fields. The fungus was identified through cultural and morphological characters (Dhingra and Sinclair, 1978), and the species was confirmed. Thereafter, the fungus was sub-cultured on Petri plates poured with Potato Dextrose Agar (PDA) and pure culture was maintained (Figure 2). Koch’s postulates were followed to confirm the pathogenicity of the root-rot fungus on mungbean plants.
2.3 Green synthesis of Ag NPs and Au NPs
To prepare the cucumber (Cucumis sativus) fruit extract, fresh cucumbers (∼100 g) were cut into small pieces and then crushed by means of a grinder. The crushed material was thereafter filtered and centrifuged for 5 min at 5,000 rpm. After centrifugation, a clear soup of cucumber extract was obtained. 0.01 M of aqueous solution of AgNO3/AuHCl4 was prepared for the synthesis of Ag/Au NPs. To reduce Ag+/Au+ ions present in AgNO3/AuHCl4 solution, cucumber fruit extract (100 mL) was added drop wise to each solution. The reacting mixtures were put under incubation at 25°C–30°C and after few hours, characteristic colour change in the solutions was observed. On completion of the reaction, each mixture was centrifuged for 40 min at 10,000 rpm which lead to separation of the colloidal particles from other components of the reaction mixture. The supernatant of the centrifuge tube was discarded and the precipitate which was accumulated at the bottom was redispersed in 10 mL of triply distilled deionized H2O. The centrifugation of the formed suspension was done again for 20 min at 5,000 rpm to get complete removal of the residue. Finally, the formed pellet inside the centrifuge tube was collected and dried completely to obtain the powdered form of nanoparticles. The biosynthesized silver and gold NPs were then characterized through different instrumental analytical techniques to confirm the formation of nanoparticles.
2.4 Characterization techniques
2.4.1 Ultraviolet–visible spectroscopy (UV–Vis)
The UV–visible spectra of cucumber synthesized Ag NPs and Au NPs was recorded on a UV-visible spectrometer (Lambda 25 PerkinElmer UV Win Lab 6.0.4.0738/L) at room temperature in the range of 200–600 nm in a quartz cuvette using distilled water as a solvent with scan rate of 600 nm/min.
2.4.2 Fourier transforms infrared (FT-IR) spectroscopy
FT-IR spectra of the cucumbersynthesized Ag NPs and Au NPs were recorded on a Perkin Elmer Spectrum Version 10.4.00 spectrometer, Germany using KBr pellet. The measurements were done in between 400 and 4,000 cm−1.
2.4.3 Dynamic light scattering (DLS)
The particle size of Ag NPs and Au NPs synthesized from cucumberand its distribution were confirmed by spectroscatter 201 instrument. The samples of NPs are ultrasonicated for 30 min and the analysis was carried out at constant temperature (20°C) for both the samples of NPs at central instrumentation facility (CIF), Jamia Millia Islamia, New Delhi, India.
2.4.4 Transmission electron microscopy (TEM)
The morphology and microstructure of the particles were studied by high resolution transmission electron microscope. A TEM instrument, JEM1011JEOL, Japan, 2013 electron microscope with photographic systems at IARI, New Delhi was used to ascertain the size and shape of the cucumber synthesized Ag NPs and Au NPs. The powdered nanoparticles were dissolved in sterilized distilled water and sonicated for 30 min in a sonicator. It was deposited on a copper coated grid at room temperature and the grid was allowed for drying before the TEM measurement was done to confirm the formation of Ag NPs and Au NPs, and to study their sizes and shapes.
2.4.5 Scanning electron microscopy (SEM)
The morphological analysis of Ag NPs and Au NPs synthesized fromcucumber was carried out using a JSM-6510, SEM Version 1.0 at an accelerating voltage of 15 kV, to ascertain the morphology of the NPs derived from cucumber.
2.4.6 Energy-dispersive X-ray analysis (EDX)
EDX analyse the types and the quantity of elements at the nanomaterial surface or in the vicinity of surface to provide specimen map. EDX spectra shows the chemical composition of the nanoparticles which confirms the formation of Ag NPs and Au NPs synthesized from cucumber.
2.4.7 Thermal gravimetric analysis (TGA)
The thermal stability of Ag NPs and Au NPs was investigated by thermogravimetric analysis (TGA) using thermal analyzer-V2.2A DuPont 9,900. The samples of the NPs were heated from 300°C–800°C in the nitrogen atmosphere at the flow rate of 200°C /min.
2.5 Antifungal effect of Ag NPs and Au NPs
2.5.1 In vitro efficacy of Ag NPs and Au NPs against the root-rot pathogen, M. phaseolina
In vitro test is necessary before conducting the experiment under in vivo condition to ensure the efficacy of the tested NPs. Antifungal activity of Ag NPs and Au NPs under in vitro condition was investigated using poisoned food technique (Dhingra and Sinclair, 1985). Each of the two nanoparticles was prepared at concentrations of 25, 50, and 100 ppm (µg/mL). The different concentrations of each NPs were separately amended with sterilized PDA medium and then pouring was done in petri plates of 9 cm diameter. Thereafter, the fungus was inoculated in the amended PDA plates. A 5 mm disc was cut from 7 days old culture of M. phaseolina and placed centrally in the Petri plate. A PDA medium without nanoparticles served as a control. Each treatment was replicated three times. The plates were then incubated at 25°C ± 2°C. The mycelial growth of the fungus was measured when the linear growth of the control colony had been completed (after 7 days).After incubation, the diameter of the fungal colony was measured and the percent growth inhibition was then calculatedaccording to Vincent’s formula (Vincent, 1947):
Where, PI = Percent growth inhibition.
C = Diameter of colony of the test pathogen in control (mm).
T = Diameter of colony of the test pathogen in treatment (mm).
2.5.2 In vivo efficacy of Ag NPs and Au NPs on the root-rot disease of mungbean inoculated with the root-rot pathogen, M. phaseolina
The in vivo experiment was conducted at the Division of Plant Pathology, IARI, New Delhi, India. Pots of 12-inch diameter were filled with a mixture of steam sterilized loam soil + farmyard manure in ratio of 3:1. The pots were organized in a completely randomized block (CRD) design.
The pathogen inoculum was mass culturedon sorghum seeds in 500 mL conical flasks by inoculating with mycelial discs of 3 mm diameter cut from 5 days old culture of M. phaseolina (3 discs/flask) and incubated for 7 days at 25°C ± 2°C (Figure 3). The fungus colonized sorghum seeds were then grinded along with distilled water using a mixer grinder. 20 mL of this suspension (having inoculum level of 5 gM. phaseolina colonized seeds/kg soil) was added in the pots filled with soil and mixed thoroughly. The inoculation was completed 2 days prior to seed sowing.
The seeds of mungbean (Vigna radiata) cv. T-44 were sown in each of the pot and at same time, 50 mL of each nanoparticle concentration (25, 50, and 100 ppm) was added to the pots each alone. Before the incorporation into the soil, the suspension of each nanoparticle was sonicated for 30 min with the help of sonicator. Pots without NPs and fungus served as control (uninoculated), while pots without NPs but having fungus served as control (inoculated). There were total 14 treatments and 3 replications/treatment in the experiment.
Plant growth parameters were estimated at harvesting time, i.e., after 3.5 months of sowing. For calculating the grain yield/plant, the pods were summed up from three individual harvests since harvesting of the pods was done three times in a season (2.5, 3.0, and 3.5 months). Disease severity in terms of root-rot index was recorded at harvest on a 0–5 scale (0: No rotting, 1: 1%–20% rotting, 2: 21%–40% rotting, 3: 41%–60% rotting, 4: 61%–80% rotting and 5: 81%–100% rotting).
2.6 Statistical analysis
Data recorded from 3 replicates for each of the treatment were averaged and the mean value was presented in the tables and graphs. R statistical software (R Development Core Team, 2011) was utilized for analysis of the collected data. Least Significant Difference (LSD) was calculated for all the variables for comparing the mean differences of individual treatments at P ≤ 0.05 (Dospekhov, 1984). Single factor ANOVA was performed for collected data on root-rot severity, while two factor ANOVA was done for the data onplant growth and yield. The F-values were also calculated and standard errors has been calculated and marked in the figures.
2.7 OLED simulation methods
The simulation of thermally activated delayed fluorescent (TADF) organic light-emitting diodes (OLEDs) was performed to investigate the impact of silver nanoparticles (Ag NPs) on device performance. Two distinct device configurations were modelled. The first configuration, without Ag NPs, consisted of the following layers: ITO/PEDOT:PSS/TCTA/mTDBA-vDBNA/TmPyPb/LiF/Al. In this structure, ITO served as the transparent anode, PEDOT acted as the hole injection layer (HIL), TCTA was used as the hole transport layer (HTL), mTDBA was the TADF emissive layer, TmPyPb functioned as the electron transport layer (ETL), and LiF/Al formed the electron injection layer (EIL) and cathode. This standard configuration served as the baseline for comparison.
The second configuration incorporated Ag NPs into the PEDOT layer, forming a composite hole injection layer. The resulting structure was ITO/PEDOT:PSS/TCTA/mTDBA-vDBNA/TmPyPb/LiF/Al. The integration of Ag NPs was aimed at leveraging localized surface plasmon resonance (LSPR) effects to enhance charge injection, light extraction, and recombination efficiency. These plasmonic effects were anticipated to improve the overall optical and electrical performance of the TADF OLED.
Both configurations were analysed using advanced simulation tools to model their optical and electrical properties. Optical simulations were conducted to study the influence of Ag NPs on light management, focusing on outcoupling efficiency and the modification of internal optical modes due to LSPR effects. Electrical simulations evaluated charge transport dynamics, examining parameters such as current density, recombination rates, and energy loss mechanisms. The comparative analysis of these simulations provided a detailed understanding of how the incorporation of Ag NPs affects the performance of TADF OLEDs, highlighting their potential to optimize next-generation OLED designs.
3 Results
3.1 Characterization of the synthesized Ag NPs and Au NPs
The silver and gold nanoparticles synthesized from cucumber through green synthesis approach were confirmed through their characterization in terms of their size, shape, and morphology using the following techniques.
3.1.1 UV-vis spectroscopy of Ag NPs and Au NPs
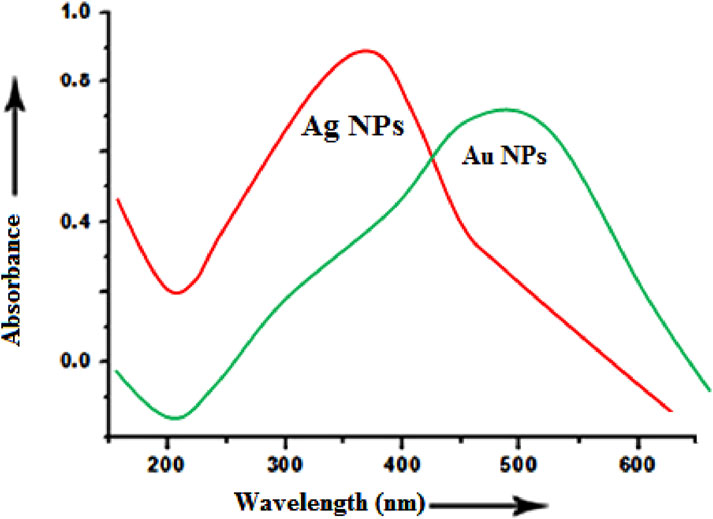
The fruit extract of cucumber contains organic biomolecules which are supposed to be effective reducing and capping agents for synthesis of silver and gold nanoparticles. A couple of hours after the extract addition, the reacting mixture turned into light brown from colourless solution suggesting the formation of colloidal Ag nanoparticles in the mixture. The colour intensified with reaction time and became dark brown after 8 h of incubation. The formation of Ag NPs was monitored gradually by scanning the mixture under UV-Vis spectrometer at regular intervals. The peak absorbance of Ag NPs was observed at ∼ 350–380 nm which may correspond to the surface plasmon resonance (SPR) phenomenon of Ag nanoparticles (Parveen et al., 2022). The appearance of broad peaks with very low intensity was observed in the range of ∼ 460–540 nm which may be assigned to Au NPs of large diameter. UV-Vis spectroscopy showed the spectra of Ag NPs and Au NPs (Figure 4). The possible chemical reactions for the biological synthesis of the Ag NPs and Au NPs respectively are as follows-
3.1.2 Fourier transform infrared spectroscopy (FT-IR) of Ag NPs and Au NPs
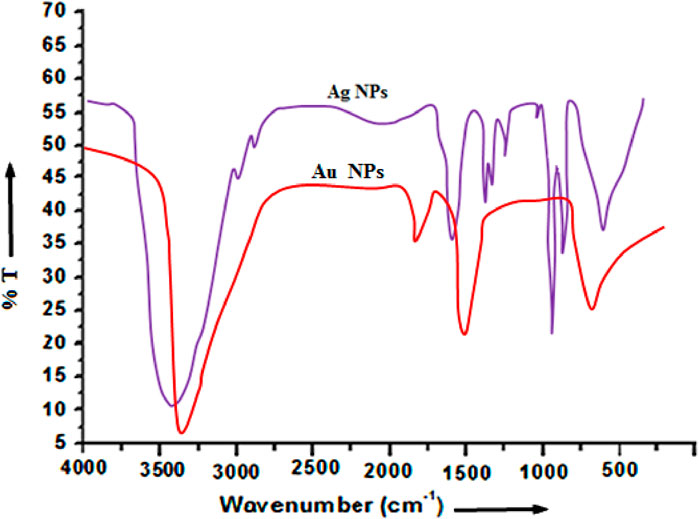
The recorded FTIR spectrum in transmittance mode of the dry Ag and Au nanoparticles obtained from cucumber (Cucumis sativus) is depicted in Figure 5. In order to identify the nature, purity and possible biomolecules responsible for the reduction of the metal precursors of the green synthesized NPs infrared study was carried out. The IR spectrum consists of two main regions: functional group and fingerprint. The organic compounds show absorption band in the functional group region whereas the metal NPs shows the absorption band in the fingerprint region. The spectrum consists of around seven distinct peaks in the entire range of recorded spectrum. From this analysis, it may be concluded that the bioactive functional molecules like phenols, amines, etc. present in the fruit extract of cucumber might reduce the silver ionsand stabilize the colloidal particles during interaction. The FTIR spectra of Ag and Au NPs obtained from Cucumis sativus extract recorded major and minor shifts of the peaks because of reduction, capping, as well as stabilization of the synthesized NPs (Parveen et al., 2022). In case of Ag NPs and Au NPs, strong bands appeared at around ∼ 3,416 at ∼ 3,440 cm−1 respectively may be attributed to hydroxyl group of alcohols and phenols. This prominent decrease in intensity may be due to the reduction of AgNO3 and AuHCl4. This small shift in absorption frequency signifies the participation of the–OH group in the process of reduction. The band appearing at ∼1611 cm−1 in the spectra of Ag NPs is attributed to the possible involvement of the CO-(NH) group in the reduction of Ag nanoparticles. Moreover, bands at ∼1,377 and ∼1065 cm−1correspond to aliphatic –CH stretching, geminal methyl, and ether linkage respectively.
This data indicates that the synthesized Ag NPs and Au NPs using the cucumber aqueous extracts is encompassed by organic materials like proteins and other metabolites such as terpenoids having functional groups of amines, ketones, alcohols, aldehydes, phenolic compound, carboxylic acids and alkaloids which help in the formation of Ag NPs and Au NPs. The –OH groups of alcohols and carbonyl groups from the amino acid residues and peptides of proteins have a strong affinity to bind metals so that they can act as encapsulating agents and thus protect the Ag and Au nanoparticles from agglomeration. Thus the FT-IR spectrum reveals the successful formation of Ag NPs and Au NPs.
3.1.3 DLS measurement of Ag NPs and Au NPs
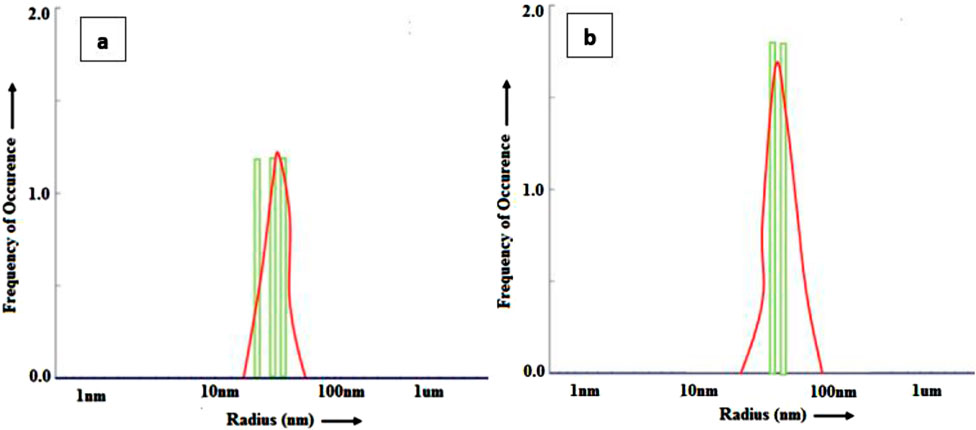
DLS is a reliable technique for determining particle size distribution inaqueous solution. The DLS histogram of Ag NPs and Au NPs using cucumber (Cucumis sativus) extract is shown in Figures 6A,B. Size of NPs obtained from cucumber samples ranged 15–65 nm for Ag NPs while in Au NPs it was around 20–70 nm respectively. The dynamic light scattering (DLS) techniqueis used to determine the particle size and particle size distribution profile of very small particles in the suspension. In this technique a monochromatic light beam, such as a laser, hits the moving particles and changes the wavelength of the incoming light. This change is related to the particle size. Figure 6 shows the average mean particle size of Ag and Au nanoparticles. The average diameterof Ag NPs and Au NPs are of ∼30 nm and ∼35 nm respectively.
3.1.4 Transmission electron microscopy (TEM) of Ag NPs and Au NPs
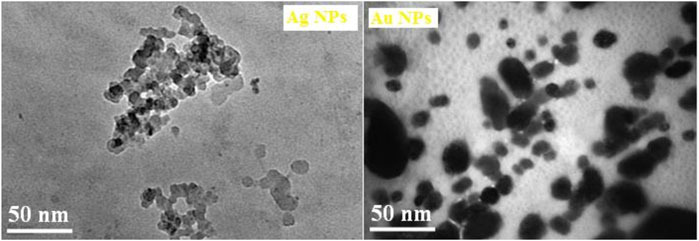
Transmission electron microscopy (TEM) was performed on selected samples of nanoparticles in order to confirm the successful formation of the nanoparticles as well as to study their sizes and shapes. The TEM image as depicted in Figure 7 shows the morphology and microstructure of the NPs and it has been clearly revealed that Ag NPs are polydispersed having typical nanochain morphology connecting spherical nanoparticles with an average diameter of about 30–40 nm, whereas the Au NPs are almost monodispersed which indicates no agglomeration and have spherical shape with an average diameter of about 35–45 nm, respectively. Furthermore, the Ag NPs showed a tendency to join together with the adjacent ones to form the elongated structures. The nature as well as distribution of NPs are significantly different in both the NPs which may be attributed to the difference in the size distribution and change in the reactivity of Ag and Au NPs.
3.1.5 Scanning electron microscopy (SEM) analysis of Ag NPs and Au NPs
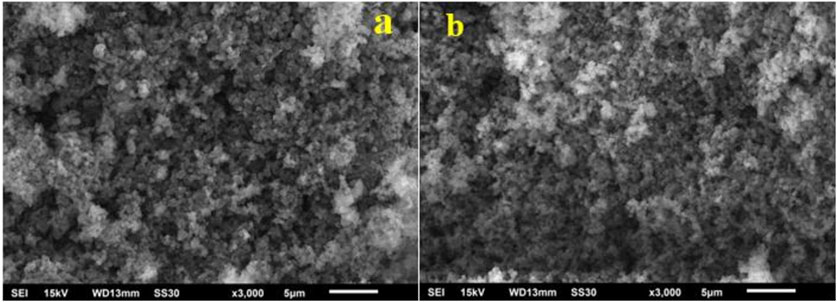
The shape and morphology of the green synthesized Ag NPs and Au NPs were also characterized by scanning electron microscopy (SEM). Figure 8 shows the micrographs of Ag NPs and Au NPs synthesized from the extracted material of cucumber and they were found to be nearly irregular pebbles like shape presumably because of high degree of agglomeration. Further, it has been noticed that the agglomeration occurred due to the intermolecular attraction among the silver and gold nanoparticles is different for both the nanoparticles and an increase in the size of the synthesized AuNPs has also been observed. It has been taken into notice that the capping agent exhibits the stabilization of the Ag NPs and Au NPs as these were not in direct association with the aggregated form. As per the SEM analysis, the larger nanoparticles of gold may be the result of aggregation of the smaller nanoparticles of gold which is also evident from the SEM micrographs.
3.1.6 Energy-dispersive X-ray analysis (EDX) of Ag NPs and Au NPs
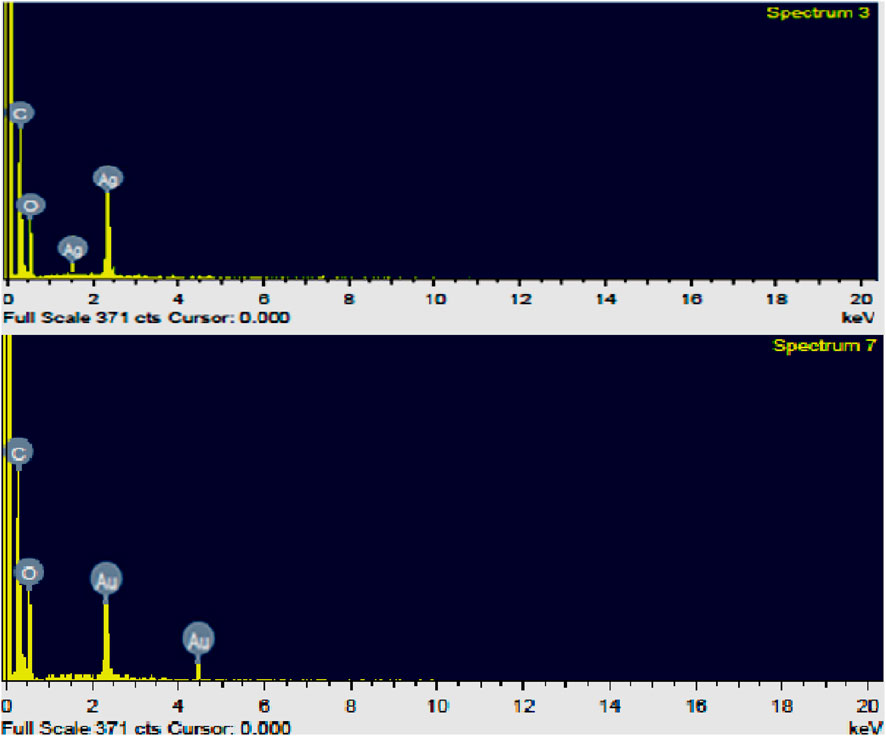
For analysis of the stoichiometric chemical composition of the various elements of the green synthesized Ag NPs and Au NPs, EDX analytical technique was implemented for confirmation of the presence and distribution of dissimilar constituent elements inside the nanoparticles. The qualitative compositions of the synthesized nanoparticles obtained from the position of the strong peak (keV) indicate the highest counts/second thereby confirming the successful formation of Ag NPs and Au NPs, respectively (Figure 9). EDX results confirm the intense peaks relating to the elements such as Ag, Au, C and O respectively without any unwanted signals which verifies the purity as well as formation Ag NPs and AuNPs synthesized from cucumber.
3.1.7 Thermal gravimetric analysis (TGA) of Ag NPs and Au NPs
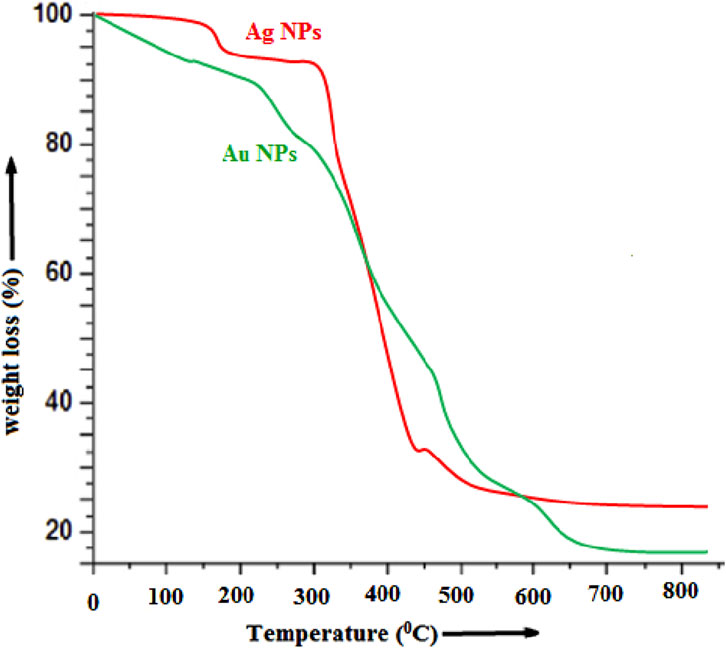
The thermal gravimetric analysis (TGA) curve of the biosynthesized Ag NPs and Au NPs is depicted in Figure 10 which gives the information related to the percent weight loss of Ag upon increasing temperature which has been recorded in the temperature range of 25°C–800°C. For heating purposes, ceramic crucible was utilized and the measurements were carried out at a heating rate of 20°C min−1 in a nitrogen atmosphere. The initial weight loss was observed below 200°C temperature which corresponds to the liberation of water content and adsorbed moisture on the sample surface.
From the TGA graph it is observed that Ag NPs showed a two-step random weight loss profile. This loss occurs in the temperature range of ∼290–540°C that can be attributed to the loss of the plant residue and almost no weight loss below 100°C and above 550°C. The TGA results of Ag NPs markedly showed a significant increase in the decomposition temperature in comparison to Au NPs, which indicate higher thermal stability of the Ag NPs. The initial weight loss of ∼5–7% may be attributed to the loss of water molecules adsorbed on Ag NPs. The overall weight losses of Ag NPs and Au NPs are ∼70.5% and ∼83% as shown in Figure 10. Actually, it is a very important observation that reveals that Ag NPs are more stable than Au NPs.
3.2 In vitro efficacy of Ag NPs and Au NPs against the root-rot pathogen, M. phaseolina
The antifungal effect of Ag NPs and Au NPs on the mycelial growth of M. phaseolina under the in vitro condition was determined by calculating the percentage of inhibition. Both the nanoparticles showed inhibition of mycelial growth of the fungus at all the concentrations. The suppression of mycelial growth in the petri plates with treatments was calculated as a percentage compared to the fungus grown in the petri plates without any treatments. The results from Figure 11 showed that Ag NPs were highly inhibitory on M. phaseolina than Au NPs. Maximum inhibition was observed with 100 ppm AgNPs (74%), followed by 100 ppm AuNPs (68%) and 50 ppm AgNPs (66%) in comparison with the control (Figure 11). The rate of inhibition was directly correlated to the treatment concentration.
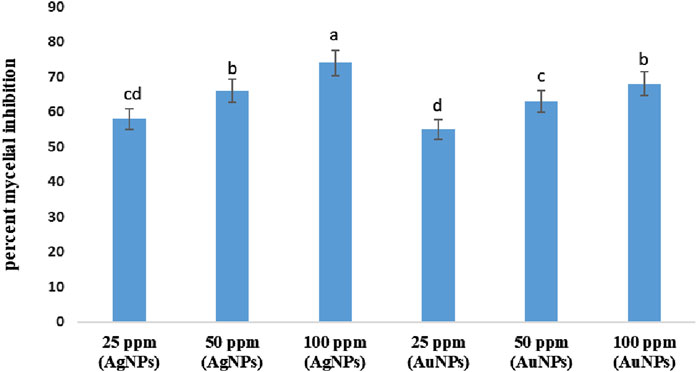
Figure 11. Effect of Ag NPs and Au NPs at different concentrations on the mycelial growth of M. phaseolina. Error bars show standard error.
3.3 In vivo efficacy of Ag NPs and Au NPs on the root-rot disease of mungbean inoculated with the root-rot pathogen, M. phaseolina
3.3.1 Disease severity
Mungbean plants inoculated with the root-rot pathogen, M. phaseolina were stunted in growth (Figure 12B). They showed symptoms of chlorosis at the seedling stage as well as wilting and drying at the flowering stage. The infected plants bear shrunken pods showing necrotic lesions and contained deformed seeds. Major part of the root was rotten and dark brown with almost no lateral roots as well as root hairs.
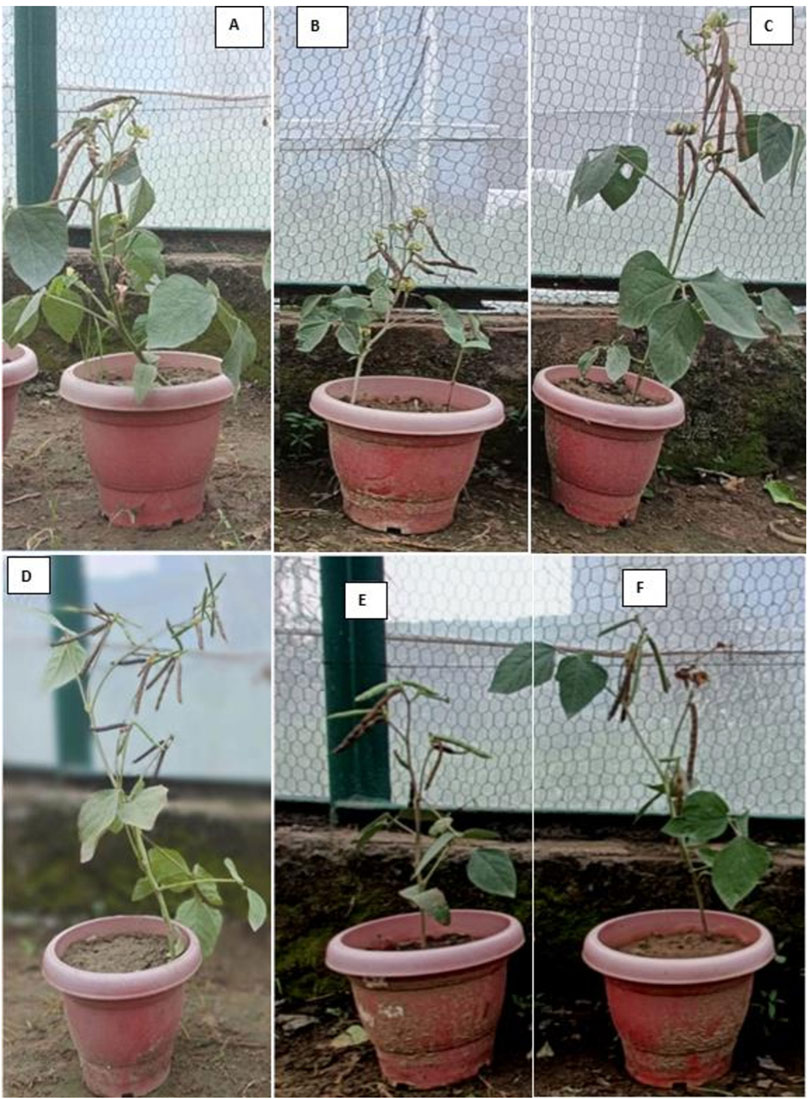
Figure 12. Healthy mungbean plant (A), M. phaseolina infected mungbean plant (B), Mungbean plant on treatment with 100 ppm Au NPs (C), Mungbean plant on treatment with 100 ppm Ag NPs (D), Mungbean plant on treatment with 100 ppm Au NPs + M. phaseolina (E), Mungbean plant on treatment with 100 ppm Ag NPs + M. phaseolina (F).
The present results demonstrated strong antifungal activity of the NPs against the mungbean root-rot pathogen, M. phaseolina under in vivo condition. The results clearly revealed that the root-rot disease severity in mungbean plants decreased due to treatments with both the NPs. The mungbean plants inoculated with M. phaseolina scored 4.0 disease severity, in terms of the root-rot index on a 0–5 scale (Figure 13). The highest efficacy in decreasing the root-rot index was observed with Ag NPs at 100 ppm concentration which showed a marked significant decline of 47% in the disease severity. Au NPs at 100 pm was next in effectiveness in reducing the disease severity (42%) which is at par with 50 ppm Ag NPs (40%). Rest of the treatments decreased the root-rot severity by 27%–30% (Figure 13).
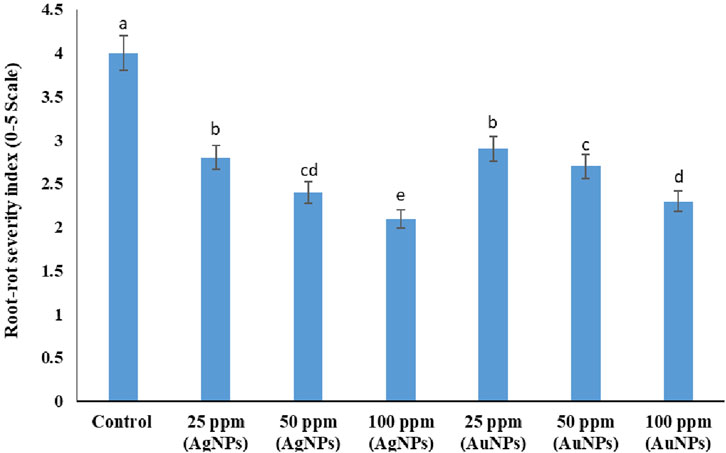
Figure 13. Effect of Ag NPs and Au NPs at different concentrations on the root-rot severity of mungbean inoculated with M. phaseolina. Error bars show standard error.
3.3.2 Plant growth
In the pots not inoculated with M. phaseolina, treatment with both NPs improved the growth parameters up to 18% indicating their ability as plant growth promoters (Figures 12C,D). The 25 ppm concentration of both the NPs caused only a little stimulating effect on growth parameters of uninoculated plants (6%–10%, Table 1). Inoculation with the root-rot pathogen led to 41% and 32% reduction in the lengths of shoot and root of mungbean plants, respectively over the uninoculated control (P ≤ 0.05, Figures 12A,B). Treatment with NPs in the plants inoculated with M. phaseolina significantly improved plant growth (9%–17%) over control. Ag NPs at 100 ppm had the greatest impact on enhancing the shoot and root lengths (38%–45%, Figure 12E), followed by 100 ppm Au NPs (35%–41%, Figure 12F).

Table 1. Effect of Ag NPs and Au NPs at different concentrations on the plant growth of mungbean inoculated or not inoculated with M. phaseolina.
3.3.3 Plant dry weight
Application of NPs significantly improved (8%–24%) the dry weight of mungbean plants grown in non-infested soil over the uninoculated control (P ≤ 0.05). The root-rot pathogen reduced the plant dry weight by 38%–43% as compared to control (Table 1). Ag NPs at 100 ppm concentration caused highest increase in dry weight of shoot (44%) and root (41%) of the infected plants. Plants exposed to Ag NPs at 100 ppm showed significant improvements (P ≤ 0.05) in the shoot dry weight (38%) and root dry weight (36%).
3.3.4 Yield
Treatments with the NPs in the non-infested pots significantly increased the yield parameters of mungbean plants, being maximum (19%–24%) with 100 ppm Ag NPs. Inoculation with the root-rot fungus caused a marked decline in the pod formation (52%), seed formation (29%) and grain yield by 56% (P ≤ 0.05, Table 2). The NPs treatments checked the suppressive effect of the pathogen leading to significant enhancement in the yield of infected plants (P ≤ 0.05). Ag NPs at 100 ppm concentration was found to have highest antifungal effect and it significantly improved the pods/plant by 56% and seeds/pod by 33% resulting in 59% improvement in the grain yield/plant. Treatment with Au NPs (100 ppm)was next in effectiveness and it resulted in 49% recovery of the grain yield with 43% and 27% increment in the pods/plant and seeds/pod respectively over inoculated control. Rest of the NPs treatments also provided antifungal effects on mungbean yield over control, but to a lesser extent (Table 2).
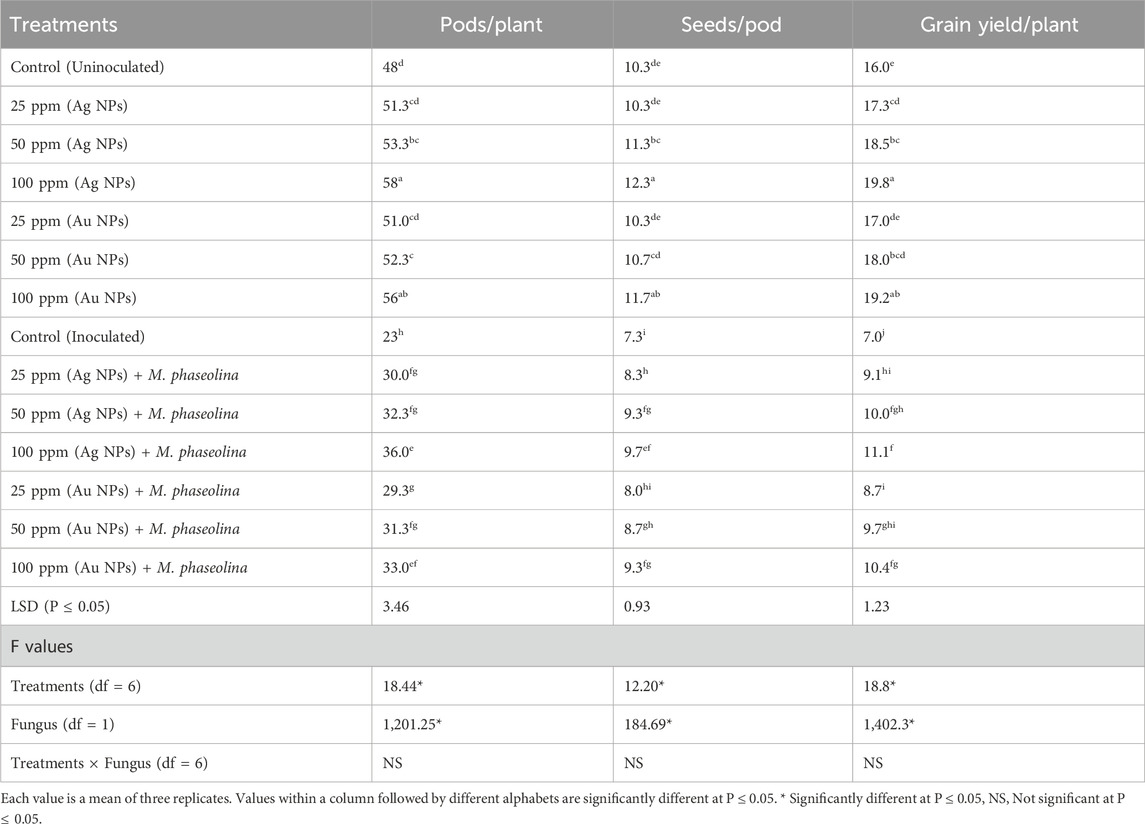
Table 2. Effect of Ag NPs and Au NPs at different concentrations on the yield of mungbean inoculated or not inoculated with M. phaseolina.
3.4 Effects of nanoparticles (Ag NPs) on organic TADF LED
To explore another application of sustainably synthesized nanoparticles, the effect of Ag NPs was also examined on the performance of advanced electronic devices such as thermally-activated delayed fluorescent (TADF) organic LED through incorporation of Ag NPs into hole-injection layer in order to widen their application potential. Since TGA analysis results noticeably showed higher thermal stability of the Ag NPs than Au NPs, therefore Ag NPs was selected for the incorporation into hole-injection layer (HIL) of blue TADF organic LEDs. The effect of Ag NPs on the characteristics of blue TADF organic LED was investigated by designing and simulation of blue TADF organic LED using two different hole-injection layers (HILs) with and without Ag NPs.
Figure 14 displays the energy-level diagram of blue TADF organic LED simulated by using two different HILs doped with and without Ag NPs. Energy-level diagram also demonstrates how the alignment of energy-levels influences the charge injection and transport. Indium tin oxide (ITO) is incorporated as an anode having a work function of 4.9 eV. Initially, PEDOT: PSS, having a HOMO level of 4.9 eV, was used as a HIL in proposed organic LED. TCTA, having a HOMO level of 5.5 eV was incorporated as hole transport layer. As observed, PEDOT: PSS based device has higher hole injection barrier (0.6 eV), which may create charge imbalance in emissive layer. To reduce hole injection barrier and increase the conductivity of PEDOT: PSS, Ag NPs were doped into PEDOT: PSS, which has HOMO level of 5.2 eV. Ag NPs doped HIL displays reduced hole injection barrier of 0.3 eV, which enhances the injection of positive charge carriers into HTL. mTDBA-Si and v-DBNA are newly reported efficient host and emitter for fabricating highly efficient blue TADF organic LEDs. TmPyPb was incorporated as an electron transport layer, having a LUMO of 2.7 eV, which is almost near the work function of cathode (2.9 eV).
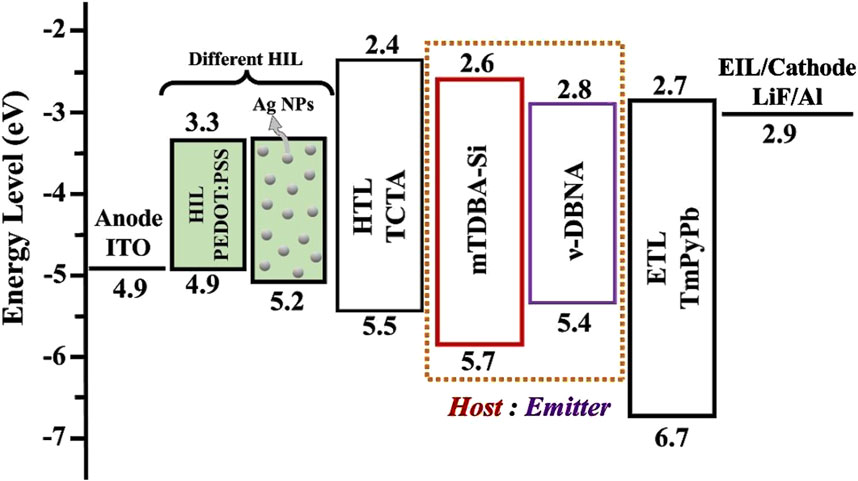
Figure 14. Energy-level diagram of blue TADF organic LEDs designed with different hole injection layer with and without Ag NPs doping.
Figure 15 shows the comparison of charge carrier densities and recombination rate in the blue TADF organic LED with and without Ag NPs doped in hole-injection layer. Figure 15A displays that the incorporation of Ag NPs in HIL significantly impacts the charge carrier dynamics.
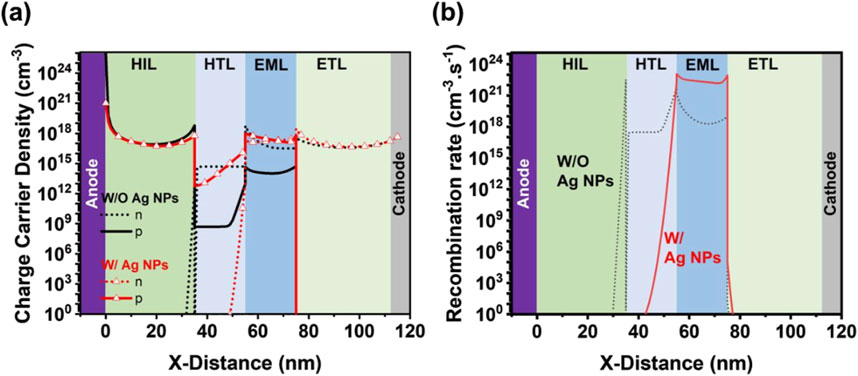
Figure 15. Comparison of (a) charge carrier densities and (b) recombination rate of blue TADF organic LEDs with and without Ag NPs doped hole-injection layer.
In Figure 15A, the charge carrier’s density is higher in the blue organic LED, which indicates enhanced charge injection and transport into emissive layer. This enhancement in the charge carrier’s density may be attributed due to the improved conductivity of HILs and localized surface plasmon effects of the Ag NPs. As observed from Figure 15B, the recombination rate in blue organic LED with Ag NPs is also higher, which suggest that the improved positive charge carrier injection and transport contribute to more efficient electron-hole recombination in the emissive layer. Efficient hole-electron injection leads in higher brightness and better performance as compared to the device without Ag NPs.
Figure 16 compares the stack contributions in blue TADF organic LEDs designed and simulated (a) without Ag NPs and (b) with Ag NPs, which highlights the impact of Ag NPs on LEDs performance.

Figure 16. Comparison of stacks contribution in blue TADF organic LED simulated (a) without Ag NPs and (b) with Ag NPs.
As observed from Figures 16A,B, in the presence of Ag NPs, the organic LEDs exhibits enhanced surface plasmon mode, which improve the interaction between electromagnetic field and excitons in the emissive layer. This interaction leads to increased efficiency and higher brightness. However, Ag NPs introduced the absorption losses, as the metal nanoparticles can absorb the emitted light and generate extra electron-hole pair. In contrast, Figure 16A shows that the organic LED without Ag NPs experienced less surface plasmon and Absorption losses. Additionally, this device exhibits waveguide mode, leading to reduced performance as compared to counterpart having Ag NPs.
4 Discussion
Nanotechnology offers promising as well as eco-friendly strategies for the management of plant diseases (Shahid et al., 2024c; El-Khawaga et al., 2024). Management of plant diseases by the use of nanotechnology is evolving day by day across the world. Metal nanoparticles like silver and gold are considered to be one amongst the most reported as well as effective antifungal agents. Nowadays, special response has been given to plant-based or greensynthesis of metal NPs (Shahid et al., 2024c). In the present study, the same plant-based approach has been followed and fresh cucumbers were used as base for green synthesis of silver as well as gold nanoparticles. Green synthesis of NPs is considered a better alternative over the chemical synthesis because it is more environmental friendly, low in cost and easy to produce (Spinoso-Castillo et al., 2017). In comparison to green synthesis, chemical synthesis results in adsorption of several harmful compounds on surface of synthesized nanoparticles (Rahimi et al., 2019).
In the present study, silver and gold NPs have been successfully synthesized from cucumbers through green synthesis. The synthesized NPs have been confirmed and characterized in terms of their size, shape, and morphology using several techniques i.e., FTIR, UV–Vis, TEM, SEM, TGA, DLS and EDX. Parveen et al. (2022) reported the biogenic synthesis of silver and gold NPs from the leaf extract of Acacia auriculiformis and characterized the NPs through spectral and morphological studies viz., FTIR, UV–Vis, TGA, SEM, TEM and EDX. Jeevitha et al. (2023) also utilized UV-visible spectroscopy, FT-IR and SEM for the characterization of silver nanoparticles synthesized from cucumber and our results are in conformity with it. Green synthesis of silver nanoparticles from leaves of Ipomoea carnea and its confirmation through the above characterization techniques have also done by Islam et al. (2024). In the present study, UV-visible spectroscopy showed the peak absorbance at ∼350–380 nm which may correspond to the surface plasmon resonance (SPR) phenomenon of silver nanoparticles and confirmed the formation of Ag NPs (Raza et al., 2013). The present results also reveal the successful formation of Au NPs which are in consistency with the findings of Annamalai et al. (2013) who have biologically synthesized eco-friendly Au NPs using the leaf extract of Euphorbia hirta L and characterized the synthesized NPs through different instrumental techniques such as FTIR, TEM, and XRD etc.
The present in vivo experiment has shown the devastating effect of the disease and the inoculated plants scored 4.0 disease severity on a scale of 0–5 in terms of root-rot index. The present results on disease severity are in consistency with the findings of Shahid and Khan (2019). Ikram and Dawar (2014) has also reported the destructive effect of M. phaseolina on mungbean plants.
Effective management for root-rot disease is extremely difficult and farmers mostly rely on chemical fungicides. Although fungicides give a good control of the disease (Shahid and Khan, 2016b), but at same time it is very hazardous to the environment. In the current years, substantial efforts have been madefor developing safe methods ofmanagement whichare environmental friendly (Khan et al., 2022; Jabran et al., 2024). Hence to limit the use of synthetic fungicides, the present study focussed on the use of green synthesized nanoparticles for the management of root-rot disease of mungbean which have not been tested earlier. In the current study, different concentrations (25, 50, and 100 ppm) of silver and gold NPs were evaluated against the disease. The present results clearly revealed that silver NPs were more effective in comparison to gold NPs, along with their corresponding concentrations under in vivo as well as in vitro conditions. Ag NPs have comparatively greater effectiveness than other NPs due to their unique characteristics. The present results are in agreement with the findings of Derbalah et al. (2022) who reported that Ag NPs inhibited the mycelial growth of M. phaseolina under in vitro conditions and also reduced the disease severity of charcoal rot on strawberry plants under in vivo conditions. Aleksandrowicz-Trzcinska et al. (2018) also reported the suppressive effect of Ag NPs and Cu NPs against Rhizoctonia solani, Fusarium oxysporum and other pathogens.
Several researchers have proved strong inhibitory effect of Ag NPs against a range of fungal plant pathogens like Fusarium oxysporum, Macrophomina phaseolina, Sclerotinia minor, Alternaria alternata, Colletotrichum gloeosporioides, Bipolaris sorokiniana etc. (Min et al., 2009; Pandey et al., 2018), and they explained the reason behind this that Ag NPs caused extensive damage to the hyphal wall and cell membrane which finally led to hyphal death. On comparing the different concentrations used in the present study, it was noticed that on increasing the concentration to 100 ppm, the antifungal effect of each NPs also increased. Ag NPs treatment at 25, 50, and 100 ppm concentrations inhibited mycelium development by 58%, 66%, and 74%, respectively. The fungal growth inhibition by silver nanoparticles depends upon its concentration used (Min et al., 2009; Aguilar-Mendez et al., 2011). Akpinar et al. (2021) also reported that treatment of Ag NPs at 25, 37.5, and 50 ppm concentrations caused 50%, 75%, and 90% respective inhibitions in the mycelial growth of F. oxysporum f. sp. radicis-lycopersici.
The obtained data clearly determines that treatment with the NPs increased the plant growth as well as yield of mungbean in the plants not inoculated with M. phaseolina which proved that nanoparticles alsoacted as plant growth stimulators. Mirzal et al. (2022) reported that nanoparticles enhanced the growth and yield of mungbean plants which varied at different concentrations. Several researchers have reported the role of Ag NPs as plant growth stimulators in mungbean (Najafi and Jamei, 2014), fenugreek plants (Sadak, 2019), and wheat (Razzaq et al., 2016) which causes improvement in their yield also. The nanoparticles promote the photosynthetic activity through increase in chlorophyll content as well as nitrogen metabolism, that ultimately increases the growth and weight of plants (Farghaly and Nafady, 2015; Latif et al., 2017). This supports the present findings that silver is a good plant growth stimulator.
In the plants infected with M. phaseolina pathogen, the nanoparticles treatments significantly improved the plant growthas well as yield of mungbean plants, being maximum with silver NPs at 100 ppm (38%–45% and 59%, respectively). The improved growth and yield of plants treated with nanoparticles was attributed to reduction in the root-rot disease as well as plant growth promoting activity of nanoparticles which stimulated the root growth increasing the availability of certain nutrients to the plants (Sharon et al., 2010). The present results are consistent with the findings of Mahmoud et al. (2022) who reported the potential antifungal efficacy of silver nanoparticles at a concentration of 100 ppm suppressing charcoal rot of faba bean caused by M. phaseolina and increasing the growth parameters of infected faba bean plants.
The present experiment demonstrated strong antifungal effect by both Ag NPs and Au NPs, however, the former was comparatively more inhibitory than the latter. Nanoparticles have been reported to directly inhibit the growth of phytopathogenic fungi which might be because of high surface to volume ratio of NPs that lead to an increase in ROS (reactive oxygen species) production, including the free radicals. This is in similarity with the reports of Dakal et al. (2016) who noticed that induction of cell membrane damage by free radicals is caused by both Ag NPs and Au NPs, and this cell membrane disruption permits the movement of NPs into the cell cytoplasm, that causes DNA damage leading to death of the microorganism. Karimi and Sadeghi (2019) also observed that as the cell membrane disintegrates, death of cells occurs because of damage in the membrane structure and loss of osmotic balance, thereby collapsing antioxidant machinery and cellular virulence.
The simulation results revealed that the incorporation of Ag NPs into the PEDOT:PSS layer significantly enhanced the performance of TADF OLEDs compared to the configuration without Ag NPs. The plasmonic effects of Ag NPs improved charge injection and recombination efficiency, leading to higher current density and luminance (Deng et al., 2018). Additionally, the light extraction efficiency was enhanced due to localized surface plasmon resonance (LSPR), which reduced optical losses and improved outcoupling (Chen et al., 2024). These improvements resulted in notable increases in external quantum efficiency (EQE) and device stability, demonstrating the potential of Ag NPs integration to optimize the optical and electrical performance of TADF OLEDs for advanced applications.
5 Conclusion
The present investigation has revealed the strong antifungal effect of Ag NPs and Au NPs on the root-rot disease of mungbean under in vivo condition which has not been reported earlier. This demonstrated the importance of Ag and Au NPs in the management of this devastating disease, as there is an urgent and increasing need to minimize the environmental impact of chemical pesticides and to develop an environment friendly alternative for its management. In this study, Ag and Au NPs have been successfully synthesized from fruit extract of cucumber through green synthesis. The successful formation of the biologically synthesized NPs was confirmed through UV–Vis, FT-IR, DLS, TEM, SEM, TGA, and EDX. Both the nanoparticles were shown to have promising antifungal potential. Ag NPs showed a more effective impact than Au NPs. Besides showing antifungal efficacy, the NPs also acted as efficient plant growth promoters. This study concluded that 100 ppm Ag NPs was the most efficient treatment for significantly managing the root-rot disease of mungbean as well as improving its plant growth and yield. Au NPs (100 ppm) also gave satisfactorily control the disease at P ≤ 0.05. In this respect, our findings substantiate the use of silver and gold nanoparticles in the management of root-rot disease, indicating the promising prospects of nanofungicides. However, further trials are needed to be performed in farmer’s fields at different locations before the commercial use of these nanoparticles for root-rot management of mungbean. Moreover, incorporation of Ag NPs in blue TADF organic LEDs enhances surface plasmon modes, leading to improved charge injection and recombination efficiency, though it introduces additional absorption losses.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. ArK: Formal Analysis, Methodology, Project administration, Visualization, Writing – original draft, Writing – review and editing, Investigation. MK: Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review and editing, Data curation. NA: Data curation, Methodology, Resources, Software, Writing – review and editing. AbK: Data curation, Formal Analysis, Resources, Software, Writing – review and editing. MN: Conceptualization, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review and editing. NR: Data curation, Formal Analysis, Software, Writing – review and editing. SR: Conceptualization, Methodology, Project administration, Resources, Writing – original draft, Writing – review and editing. SA: Writing – review and editing, Resources, Methodology. PK: Data curation, Investigation, Methodology, Resources, Validation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to acknowledge the Division of Plant Pathology, ICAR-Indian Agricultural Research Institute, New Delhi-110012, India and Department of Applied Sciences and Humanities, Jamia Millia Islamia, New Delhi-110025, India.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdallah, Y., Hussien, M., Omar, M. O. A., Elashmony, R. M. S., Alkhalifah, D. H. M., and Hozzein, W. N. (2022). Mungbean (vignaradiata) treated with magnesium nanoparticles and its impact on soil borne Fusarium solani and Fusarium oxysporum in clay soil. Plants 11, 1514. doi:10.3390/plants11111514
Abou-Salem, E., Ahmed, A. R., Elbagory, M., and Omara, A. E. D. (2022). Efficacy of biological copper oxide nanoparticles on controlling damping-off disease and growth dynamics of sugar beet (Beta vulgaris L.) Plants. Sustainability 14, 12871. doi:10.3390/su141912871
Aguilar-Mendez, M. A., Martin-Martinez, E. S., Ortega-Arroyo, L., Cobia-Portillo, G., and Sanchez-Espindola, E. (2011). Synthesis and characterization of silver nanoparticles: effect on phytopathogenColletotrichumgloesporioides. J. Nanopart. Res. 13, 2525–2532. doi:10.1007/s11051-010-0145-6
Ahmad, S. I., Ahmad, R., Khan, M. S., Kant, R., Shahid, S., Gautam, L., et al. (2020). Chitin and its derivatives: structural properties and biomedical applications. Int. J. Biol. Macromol. 164, 526–539. doi:10.1016/j.ijbiomac.2020.07.098
Ahmed, A. I., Yadav, D. R., and Lee, Y. S. (2016). Applications of nickel nanoparticles for control of fusarium wilt on lettuce and tomato. Int. J. Innov. Res. Sci. Eng. Technol. 5, 7378–7385. doi:10.15680/ijirset.2016.0505132
Akpinar, I., Unal, M., and Sar, T. (2021). Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum F. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 3, 506. doi:10.1007/s42452-021-04524-5
Aleksandrowicz-Trzcinska, M., Szaniawski, A., Olchowik, J., and Drozdowski, S. (2018). “Effects of copper and silver nanoparticles on growth of selected species of pathogenic and wood-decay fungi in vitro,”, 94. Canada: Canadian Science Publishing, 109–116. doi:10.5558/tfc2018-017
Annamalai, A., Christina, V. L. P., Sudha, D., Kalpana, M., and Lakshmi, P. T. V. (2013). Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L. leaf extract. Colloids Surf. B 108, 60–65. doi:10.1016/j.colsurfb.2013.02.012
Auffan, M., Rose, J., Bottero, J. Y., Lowry, G. V., Jolivet, J. P., and Wiesner, M. R. (2009). Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 4, 634–641. doi:10.1038/nnano.2009.242
Chen, S. H., Fang, J. K., Du, C. H., Shih, M. H., Liang, H. C., and Chiang, H. P. (2024). Enhanced photoluminescence of DCJTB by silver nanohole arrays with ring-shaped silver nanoparticles over hyperbolic metamaterials. Opt. Mater. 150, 115174. doi:10.1016/j.optmat.2024.115174
Dakal, T. C., Kumar, A., Majumdar, R. S., and Yadav, V. (2016). Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 7, 1831. doi:10.3389/fmicb.2016.01831
Demirbas, A., Büyükbezirci, K., Celik, C., Kislakci, E., Karaagac, Z., Gokturk, E., et al. (2019). Synthesis of long-term stable gold nanoparticles benefiting from red raspberry (rubusidaeus), strawberry (fragariaananassa), and blackberry (rubusfruticosus) extracts–gold ion complexation and investigation of reaction conditions. ACS Omega 4, 18637–18644. doi:10.1021/acsomega.9b02469
Deng, L., Zhou, Z., Jia, B., Zhou, H., Peng, L., Shang, W., et al. (2018). Improved electron injection and efficiency in blue organic light-emitting diodes using coupled electric field near cathode. Org. Electron. 53, 346–352. doi:10.1016/j.orgel.2017.12.004
Derbalah, A., Essa, T., Kamel, S. M., Omara, R. I., Abdelfatah, M., Elshaer, A., et al. (2022). Silver oxide nanostructures as a new trend to control strawberry charcoal rot induced by Macrophominaphaseolina. Pest Manag. Sci. 78, 4638–4648. doi:10.1002/ps.7084
Dhayalan, M., Denison, M. I. J., Ayyar, M., Gandhi, N. N., Krishnan, K., and Abdulhadi, B. (2018). Biogenic synthesis, characterization of gold and silver nanoparticles from Coleus forskohlii and their clinical importance. J. Photochem. Photobiol. B 183, 251–257. doi:10.1016/j.jphotobiol.2018.04.042
Dhingra, O. B., and Sinclair, J. B. (1978). Biology and pathology of macrophominaphaseolina. Brazil: Universidade Federal de Viscosa, 166.
Dhingra, O. D., and Sinclair, J. B. (1985). Basic plant pathology methods. Boca Raton, Florida: CRC Press, Inc., 132–163.
Dizaj, S. M., Lotfpour, F., Barzegar-Jalali, M., Zarrintan, M. H., and Adibkia, K. (2014). Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. 44, 278–284. doi:10.1016/j.msec.2014.08.031
Dospekhov, B. A. (1984). Field experimentation: statistical procedures. Moscow: Mir Publishers, 352.
El-Khawaga, A. M., and Mukhtar, S. (2024). “ShumailaShahid, Sustainable Nanomaterials as promising antibacterial agents,” in Sustainable nanomaterials: synthesis and environmental applications. Editors I. Uddin, and I. Ahmad (Singapore), 203–225.
Farghaly, F. A., and Nafady, N. A. (2015). Green synthesis of silver nanoparticles using leaf extract of Rosmarinus officinalis and its effect on tomato and wheat plants. J. Agric. Sci. 7, 277–287. doi:10.5539/jas.v7n11p277
Gengan, R., Anand, K., Phulukdaree, A., and Chuturgoon, A. (2013). A549 lung cell line activity of biosynthesized silver nanoparticles using Albiziaadianthifolia leaf. Colloids Surf. B 105, 87–91. doi:10.1016/j.colsurfb.2012.12.044
Georgiopoulou, Z., Verykios, A., Soultati, A., Chroneos, A., Hiskia, A., Aidinis, K., et al. (2024). Plasmonic enhanced OLED efficiency upon silver-polyoxometalate core-shell nanoparticle integration into the hole injection/transport layer. Sci. Rep. 14, 28888. doi:10.1038/s41598-024-79977-w
Ikram, N., and Dawar, S. (2014). Impact of biocontrol agents in combination with Prosopisjuliflora (Swartz) DC, in controlling the root-infecting fungi of leguminous crops. Arch. Phytopathol. Plant Prot. 47, 930–937. doi:10.1080/03235408.2013.825993
Islam, A. K. M. S., Bhuiyan, R., Nihad, S. A. I., Akter, R., Khan, M. A. I., Akter, S., et al. (2024). Green synthesis and characterization of silver nanoparticles and its efficacy against Rhizoctoniasolani, a fungus causing sheath blight disease in rice. PLoS ONE 19, e0304817. doi:10.1371/journal.pone.0304817
Jabran, M., Ali, M. A., Muzammil, S., Zahoor, A., Ali, F., Hussain, S., et al. (2024). Exploring the potential of nanomaterials (NMs) as diagnostic tools and disease resistance for crop pathogens. Chem. Biol. Technol. Agric. 11, 75. doi:10.1186/s40538-024-00592-y
Jain, D., Daima, H. K., Kachhwaha, S., and Kothari, S. L. (2009). Dig. J. Nanomater. Bios. 4, 723. Available online at: https://www.researchgate.net/publication/215790280_Synthesis_of_Plant-Mediated_Silver_Nanoparticles_using_Papaya_Fruit_Extract_and_Evaluation_of_their_Anti_Microbial_Activities
Jeevitha, M., Singh, M. M., Zehra, S., Prashant, B. R., Kumar, B., Ranjan, R., et al. (2023). Green synthesis of cucumber tree leaf extract's silver nanoparticles. J. Cardiovasc. Dis. Res. 14, 1656–1677. doi:10.48047/jcdr.2023.14.08.174
Kanimozhi, S., Durga, R., Sabithasree, M., Kumar, A. V., Sofiavizhimalar, A., Kadam, A. A., et al. (2022). Biogenic synthesis of silver nanoparticle using cissusquadrangularis extract and itsin vitro study. J. King Saud Univ. Sci. 34, 101930. doi:10.1016/j.jksus.2022.101930
Karimi, E., and Sadeghi, A. (2019). Toxicity efect of silver nanoparticles on two plant growth promoting Streptomyces spp. strains, phytopathogenic fungi Fusarium solani and phytopathogenic oomycetes Pythium aphanidermatum and Pythium ultimum. Modares. J. Biotechnol. 10, 23–27. Available online at: http://biot.modares.ac.ir/article-22-16269-en.html
Khan, F., Atif, M., Khan, M. S., Shahid, S., Nami, S. A. A., et al. (2022). Synthesis, classification and properties of hydrogels: their applications in drug delivery and agriculture. J. Mater. Chem. B 10, 170–203. doi:10.1039/d1tb01345a
Khan, M. R., Anwer, M. A., and Shahid, S. (2011). ShumailaShahid, Management of graymold of chickpea, Botrytis cinereawith bacterial and fungal biopesticides using differentmodes of inoculation and application. BiologicalControl 57, 13–23. doi:10.1016/j.biocontrol.2011.01.004
Khan, M. R., Ashraf, S., Shahid, S., and Anwer, M. A. (2010). Response ofsome chickpea cultivars to foliar, seed and soil inoculations with Botrytis cinerea. PhytopathologiaMeditteranea 49, 275–286. Available online at: https://www.jstor.org/stable/26458653
Khan, M. R., Ashraf, T., and Shahid, S. (2012). Managementofriceroot-knotnematode by soil application of carbofuran at different intervals. Ann. PlantProtectionSciences 20, 444–448. Available online at: https://www.indianjournals.com/ijor.aspx?target=ijor:apps&volume=20&&issue=2&article=046
Khan, M. R., Shahid, S., Mohidin, F. A., and Mustafa, U. (2017). InteractionofFusariumoxysporumf.sp.gladioliandMeloidogyneincognitaongladioluscultivarsanditsmanagementthroughcormtreatmentwith biopesticides and pesticides. Biol. Control 115, 95–104. doi:10.1016/j.biocontrol.2017.09.010
Kong, F., Wang, J., Han, R., Ji, S., Yue, J., Wang, Y., et al. (2020). Antifungal activity of magnesium oxide nanoparticles: effect on the growth and key virulence factors of Candida albicans. Mycopathologia 185, 485–494. doi:10.1007/s11046-020-00446-9
Lal, M., Sharma, P., and Ram, C. (2021). Calcination temperature effect on titanium oxide (TiO2) nanoparticles synthesis. Optik 241, 166934–167014. doi:10.1016/j.ijleo.2021.166934
Latif, H. H., Ghareib, M., and Abu Tahon, M. (2017). Phytosynthesis of silver nanoparticles using leaf extracts from Ocimumbasilicum and Mangifiraindica and their effect on some biochemical attributes of Triticumaestivum. GesundePflanzen 69, 39–46. doi:10.1007/s10343-017-0385-9
Lee, D. W., Cho, E., Jeon, Y., and Kwon, S. J. (2023). Characterization of the material and electrical properties depending on the Mg:Ag ratio as a cathode for TEOLED. Mater. Chem. Phys. 303, 127742. doi:10.1016/j.matchemphys.2023.127742
Liang, N., Xin, X., Yin, M., Chen, R., Yang, H., Tian, R., et al. (2024). Hybrid plasmon mode enhancing the lifetime and forward-directional emission for solution-processed OLEDs. Adv. Funct. Mater. 34, 2400449. doi:10.1002/adfm.202400449
Mahmoud, Y., Mohamed, A., and Elshahawy, I. E. (2022). Antifungal activity of photo-biosynthesized silver nanoparticles (AgNPs) from organic constituents in orange peel extract against phytopathogenicMacrophominaphaseolina. Eur. J. Plant Pathol. 162, 725–738. doi:10.1007/s10658-021-02434-1
Meena, R. S., Kumar, S., Datta, R., Lal, R., Vijayakumar, V., Brtnicky, M., et al. (2020). Impact of agrochemicals on soil microbiota and management: a review. Land 9, 34. doi:10.3390/land9020034
Min, J. S., Kim, K. S., Kim, S. W., Jung, J. H., Lamsal, K., Kim, S. B., et al. (2009). Effects of colloidal silver nanoparticles on sclerotium-forming phytopathogenic fungi. Plant Pathol. J. 25, 376–380. doi:10.5423/ppj.2009.25.4.376
Mirzal, F. S., Aftab, Z., Danish Ali, M., Aftab, A., Anjum, T., Rafiq, H., et al. (2022). Green synthesis and application of GO nanoparticles to augment growth parameters and yield in mungbean (Vignaradiata L.). Front. Plant Sci. 13, 1040037. doi:10.3389/fpls.2022.1040037
Najafi, S., and Jamei, R. (2014). Effect of silver nanoparticles and Pb(NO3)2 on the yield and chemical composition of mungbean (Vignaradiata). J. Stress Physiol. Biochem. 10, 316–325. Available online at: https://www.researchgate.net/publication/371788777_Effect_of_Silver_Nanoparticles_and_PbNO_3_2_on_the_Yield_and_Chemical_Composition_of_Mung_beanVigna_radiata_Effect_of_Silver_Nanoparticles_and_PbNO32_Effect_of_Silver_Nanoparticles_and_PbNO_3_2_on_the
Niluxsshun, M. C. D., Masilamani, K., and Mathiventhan, U. (2021). Green synthesis of silver nanoparticles from the extracts of fruit peel of Citrus tangerina, Citrus sinensis, and Citrus limon for antibacterial activities. Bioinorg. Chem. Appl. 2021, 1–8. doi:10.1155/2021/6695734
Pandey, S., Giri, K., Kumar, R., Mishra, G., and Raja, R. R. (2018). Nanopesticides: opportunities in crop protection and associated environmental risks. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 88, 1287–1308. doi:10.1007/s40011-016-0791-2
Parveen, M., Kumar, A., Khan, M. S., Rehman, R., Furkan, M., Khan, R. H., et al. (2022). Comparative study of biogenically synthesized silver and gold nanoparticles of Acacia auriculiformis leaves and their efficacy against Alzheimer's and Parkinson's disease. Int. J. Biol. Macromol. 203, 292–301. doi:10.1016/j.ijbiomac.2022.01.116
Rahimi, H., and Doostmohammadi, M. (2019). “Nanoparticle synthesis, applications, and toxicity,” in Applications of nanobiotechnology. Editors M. Stoytcheva, and R. Zlatev (London, UK: IntechOpen).
Raza, S., Yan, W., Stenger, N., Wubs, M., and Mortensen, N. A. (2013). Blueshift of the surface plasmon resonance in silver nanoparticles: substrate effects. Opt. Express 21, 27344. doi:10.1364/oe.21.027344
Razzaq, A., Ammara, R., Jhanzab, H. M., Mahmood, T., Hafeez, A., and Hussain, S. (2016). A noval nanomaterial to enhance growthand yield of wheat. J. Nanosci. Technol. 2, 55–58. Available online at: https://www.researchgate.net/publication/303737670_A_Novel_Nanomaterial_to_Enhance_Growth_and_Yield_of_Wheat
Rimal Isaac, R. S., Sakthivel, G., and Murthy, Ch. (2013). Green synthesis of gold and silver nanoparticles using averrhoa bilimbi fruit extract. J. Nanotech. 6, 906592. doi:10.1155/2013/906592
Roy, K., Sarkar, C. K., and Ghosh, C. K. (2015). Single-step novel biosynthesis of silver nanoparticles using CucumisSativus fruit extract and study of its photcatalytic and antibacterial activity. Dig. J. Nanomater. Biostructures 10, 107–115. Available online at: https://chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://chalcogen.ro/107_Roy.pdf
Sadak, M. S. (2019). Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonellafoenumgraecum). Bull. Natl. Res. Cent. 43, 38–46. doi:10.1186/s42269-019-0077-y
Shahid, S. (2023a). “Plant diseases-A major limiting factor in production of pulses (Mung bean and Chickpea),” in Recent research in agriculture and plant Sciences. Editors S. Shahid, P. Singh, and B. Neog (Lucknow, India: MKSES Publication), 1–18. Available online at: https://www.magazines.cornous.com/article/agroscience-today/fusarium-wilt-a-major-problem-in-cucurbits
Shahid, S., Choudhary, H., and Jat, G. S. (2023b). Fusarium wilt- A major problem in cucurbits. Agroscience Today 4, 570–572. https://www.magazines.cornous.com/article/agroscience-today/fusarium-wilt-a-major-problem-in-cucurbits
Shahid, S., Choudhary, H., and Tara, K. K. (2024a). Cross-pathogenecity of FormaeSpeciales of Fusarium oxysporum in cucurbits. Agroscience Today 5, 0825–0829. Available online at: https://www.magazines.cornous.com/article/agroscience-today/cross-pathogenecity-of-formae-speciales-of-ifusarium-oxysporumi-in-cucurbits
Shahid, S., Choudhary, H., and Tara, K. K. (2024b). Biological control of Fusarium wilt of cucurbits. Agroscience Today 5, 820–824. Available online at: https://www.magazines.cornous.com/article/agroscience-today/biological-control-of-fusarium-wilt-of-cucurbits
Shahid, S., and Khan, M. R. (2016a). Management of root-rot of mungbeancausedbyMacrophominaphaseolinathroughseedtreatmentwithfungicides. IndianPhytopathology 69, 128–136.
Shahid, S., and Khan, M. R. (2016b). BiologicalControlofRoot-rotonMungbeanPlantsIncitedbyMacrophominaphaseolinaThroughMicrobialAntagonists. Plant Pathology J. 15, 27–39. doi:10.3923/ppj.2016.27.39
Shahid, S., and Khan, M. R. (2019). Evaluation of biocontrol agents for themanagementofroot-rotofmungbeancausedbyMacrophominaphaseolina. Indian Phytopathol. 72, 89–98. doi:10.1007/s42360-018-0098-8
Shahid, S., Khan, M. S., Kumar, A., Rahman, S., Arshad, M., Kaushik, P., et al. (2024c). “Role of nanomaterials in sustainable agriculture,” in Sustainable nanomaterials: synthesis and environmental applications. Editors I. Uddin, and I. Ahmad (Singapore: Springer Nature), 227–248.
Shahid, S., Sharma, B. B., Khan, A. A., and Ansari, N. H. (2023c). Management of plant diseases by surfactants. Int. J. Trop. Agric. 41, 121–127. Available online at: https://serialsjournals.com/abstract/71992_16_shumaila_shahid.pdf
Sharon, M., Choudhary, A. K., and Kumar, R. (2010). Nanotechnology in agricultural diseases and food safety. J. Phytol. 2, 83–92. Available online at: https://www.researchgate.net/publication/284063192_Nanotechnology_in_agricultural_diseases_and_food_safety
Shubhashree, K. R., Reddy, R., Gangula, A. K., Nagananda, G., Badiya, P. K., Ramamurthy, S. S., et al. (2022). Green synthesis of copper nanoparticles using aqueous extracts from Hyptissuaveolens (L.). Mater. Chem. Phys. 280, 125795–125799. doi:10.1016/j.matchemphys.2022.125795
Spinoso-Castillo, J. L., Chavez-Santoscoy, R. A., Bogdanchikova, N., Perez-Sato, J. A., Morales-Ramos, V., and Bello-Bello, J. J. (2017). Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tissue Organ Cult. 129, 195–207. doi:10.1007/s11240-017-1169-8
Tandel, D. H., Sabalpara, A. N., and Pandya, J. R. (2010). Efficacy of phytoextracts on Macrophominaphaseolina (Tassi) Goid causing leaf blight of green gram. Int. J. Pharma BiolSci 2, 1–5. Available online at: https://www.researchgate.net/publication/291871135_Efficacy_of_phytoextracts_on_Macrophomina_phaseolina_Tassi_goid_causing_leaf_blight_of_green_gram
Keywords: silver nanoparticles, gold nanoparticles, cucumber, mungbean, antifungal activity, TADF organic LED
Citation: Shahid S, Kumar A, Khan MS, Ansari NH, Kareem A, Nagar MR, Rizvi NZ, Rahman S, Ahmad S and Kaushik P (2025) Sustainable synthesis of cucumber-derived gold and silver nanoparticles: impact on mungbean root-rot disease suppression, and organic LED performance. Front. Nanotechnol. 7:1572639. doi: 10.3389/fnano.2025.1572639
Received: 07 February 2025; Accepted: 25 April 2025;
Published: 29 May 2025.
Edited by:
Iseli L. Nantes, Universidade Federal do ABC, BrazilReviewed by:
Patricia Targon Campana, University of São Paulo, BrazilMarcelo Yudi Icimoto, Federal University of São Paulo, Brazil
Copyright © 2025 Shahid, Kumar, Khan, Ansari, Kareem, Nagar, Rizvi, Rahman, Ahmad and Kaushik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shumaila Shahid, c2h1bWFpbGEyNGFtdUBnbWFpbC5jb20=, c2h1bWFpbGFAaWFyaS5yZXMuaW4=
 Shumaila Shahid
Shumaila Shahid Arvind Kumar2
Arvind Kumar2 Mohd Shoeb Khan
Mohd Shoeb Khan Safikur Rahman
Safikur Rahman Parshant Kaushik
Parshant Kaushik