- 1Department of Biotechnology, College of Natural and Computational Sciences, Dambi Dollo University, Dambi Dollo, Ethiopia
- 2Department of Applied Biology, College of Applied Natural Sciences, Adama Science and Technology University, Adama, Ethiopia
- 3Department of Applied Physics, College of Applied Natural Sciences, Adama Science and Technology University, Adama, Ethiopia
- 4Department of Applied Chemistry, College of Applied Natural Sciences, Adama Science and Technology University, Adama, Ethiopia
- 5Department of Chemistry, Chemical Engineering and Applied Chemistry, Chungnam National University, Daejeon, Republic of Korea
Propolis, a natural resinous substance produced by honeybees (Apis mellifera L.), is a complex mixture of over 300 bioactive compounds with significant pharmaceutical potential. In light of the escalating global antimicrobial resistance crisis, there is an urgent need for novel antimicrobial agents. This study aimed to synthesize and characterize silver nanoparticles (AgNPs) using Ethiopian propolis and evaluate their antimicrobial and antioxidant properties. The synthesis of propolis-mediated silver nanoparticles (Pro-AgNPs) was optimized and characterized using UV-visible spectrophotometry, scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR), and X-ray diffraction (XRD). The UV-Vis spectra revealed a maximum absorbance at 424 nm, confirming the successful synthesis of AgNPs. FT-IR analysis identified functional groups involved in nanoparticle stabilization, while XRD confirmed the crystalline nature of the nanoparticles. SEM images revealed spherical-shaped nanoparticles with uniform size distribution. The antimicrobial activity of Pro-AgNPs was evaluated against Gram-negative Pseudomonas aeruginosa (ATCC 27853) and Gram-positive Enterococcus faecalis (ATCC 29212) bacteria, demonstrating significant growth inhibition zones of 15.67 ± 0.57 mm and 17.33 ± 1.15 mm, respectively, at a concentration of 50 μg/mL. The antioxidant activity of Pro-AgNPs and propolis extract was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, revealing concentration-dependent radical scavenging activity. Pro-AgNPs exhibited potent antioxidant activity, with an IC50 value of 45.54 ± 0.57 μg/mL. These findings underscore the potential of Pro-AgNPs as natural antimicrobials and antioxidants, with promising applications in pharmaceuticals, cosmetics, and the food industry.
1 Introduction
Propolis is a resinous substance collected from plants by honeybees (Apis mellifera L.). It is a complex mixture containing over 300 compounds, including flavonoids, alkaloids, phenols, terpenoids, and steroids (Rushdi et al., 2014). Its composition and properties vary depending on geographical location and climate (Sangboonruang et al., 2022; Wieczorek et al., 2022). Despite its potential medicinal applications, propolis is limited due to poor water solubility and formulation challenges (Kustiawan et al., 2024). Bees utilize propolis to protect the hive by combating pathogens, sealing gaps, and sanitizing comb cells. Propolis is widely used in pharmaceuticals and is a cornerstone of apitherapy (Afata et al., 2022). The medicinal value of propolis stems from its rich chemical profile, shaped by plant sources, bee metabolism, and additional materials incorporated during production.
Nanotechnology offers a promising avenue to enhance the therapeutic potential of propolis by improving its solubility and bioavailability (Patra et al., 2018). In light of the escalating global antimicrobial resistance crisis, there is an urgent need for novel antimicrobial agents (Tagliabue and Rappuoli, 2018). Utilizing Ethiopian propolis to synthesize silver nanoparticles (AgNPs) could provide a novel approach to addressing this challenge. Reactive oxygen and nitrogen species can harm biological systems, leading to various chronic diseases in humans. Silver nanoparticles are particularly promising due to their potent antibacterial properties and potential as antioxidants. The efficiency of these nanoparticles is influenced by their size, shape, and surface characteristics (Canaparo et al., 2020; Horie and Tabei, 2020; Yuan et al., 2021).
Metals such as silver and gold have been used as antimicrobial agents for millennia. For example, copper salts were documented as astringents as early as 1,500 BC (Gold et al., 2018). Among the noble metals, silver and gold stand out due to their low toxicity and widespread use in nanotechnology (Barsola and Kumari, 2022). Silver nanoparticles, in particular, have gained considerable attention and are more widely utilized than other nanoparticles because of their distinctive characteristics (Zhang et al., 2016). Silver nanoparticles demonstrate broad-spectrum antimicrobial effectiveness, targeting various bacteria, fungi, and viruses. Their ability to disrupt microbial cell membranes and inhibit vital cellular processes, such as DNA replication and protein synthesis, further enhances their antimicrobial potential (Dakal et al., 2016). This versatility makes them particularly valuable in the medical field, where they are used in applications such as wound dressings, antimicrobial coatings, and medical devices. Additionally, their biocompatibility and ability to be functionalized with other compounds allow for tailored applications in drug delivery and diagnostic tools, expanding their utility in modern medicine (Jangid et al., 2024). These nanoparticles can be synthesized using various techniques, including biological, chemical, and physical methods. Biological methods, however, are favored for their environmental friendliness and potential for enhanced morphological control (Ahmed et al., 2021).
Honeybee products like propolis are rich in bioactive compounds and have been extensively investigated for AgNP synthesis. Propolis extracts facilitate the reduction of Ag+ ions to Ag0 nanoparticles, with their properties influenced by factors such as temperature, pH, reaction kinetics, and capping agents (Patil and Chougale, 2021). Ethiopia, the world’s fourth-largest propolis producer with over six million beehives (Teferi, 2018), boasts a unique propolis composition due to its diverse flora and varied environmental conditions (Rushdi et al., 2014). While research on AgNP synthesis from propolis has primarily focused on Latin America, Europe, and North Africa (Dărăban, 2019; Raheem et al., 2020; Khalil et al., 2021; Kustiawan et al., 2024; Barsola and Kumari, 2022), the potential of Ethiopian propolis remains underexplored (Jobir and Shume, 2020). Ethiopian propolis is renowned for its wound-healing, antioxidant, and antibacterial properties, offering potential medicinal applications (Afata et al., 2022). However, its use is hindered by poor water solubility and formulation challenges. Given Ethiopia’s rich biodiversity, investigating the prospect of its propolis for AgNP synthesis and related antimicrobial and antioxidant properties represents a promising research avenue. Propolis has been successfully used to synthesize AgNPs, comprehensive studies that optimize the production process and evaluate both antimicrobial and antioxidant activities are surprisingly rare. To date, there are no published studies that detail the optimized synthesis of AgNPs from Ethiopian propolis extract and subsequently evaluate their antimicrobial and antioxidant efficacy.
Therefore, this study aimed to synthesize, optimize, and characterize AgNPs using Ethiopian propolis. Beyond biosynthesis and characterization, the present study evaluates the antimicrobial efficacy of the AgNPs against selected gram-negative and gram-positive bacteria and assesses their antioxidant effects. By leveraging the unique attributes of Ethiopian propolis, this research seeks to address global challenges posed by antimicrobial resistance and oxidative stress-related diseases. Furthermore, it highlights the potential of synergizing natural products with nanotechnology to develop effective antimicrobial and antioxidant therapies, paving the way for innovative solutions in healthcare, cosmetics, and the food industry.
2 Materials and methods
All chemicals and reagents used in this investigation were of analytical grade, and double distilled water was employed throughout the experimental procedures. Silver nitrate (AgNO3, purity: 99.8%), sodium hydroxide (NaOH, purity: 98%), and ascorbic acid (C6H8O6, purity: 99.9%) were purchased from LobaChemie Pvt. Ltd. (Mumbai, India), Muller-Hinton Agar (MHA), DPPH (C18H12N5O6, purity: 99%), methanol (CH3OH), and ethanol (CH3CH2OH) were from Sigma-Aldrich Pvt Ltd., Germany.
2.1 Description of study site and sample collection
Honeybee propolis samples were collected from the Dambi Dollo University Integrated agricultural research center apiary site in Ethiopia 645 km from Addis Ababa. The collection area, located between 8°32′N latitudes and 34°48′E longitude at altitudes of 1701–1827 m above sea level. The area is dominated by plants such as Coffee arabica, Croton macrostachyus, Vernonia amygdalina, Ficus vasta, Olea africana, Cordia africana, Acacia species, and crops like Zea mays. Figure 1 illustrates the geographical scope of the study.
2.2 Preparation of propolis extract
Propolis was collected using a stainless-steel spatula, following a method adapted from (Rezk et al., 2022). After collection, it was carefully cleaned by hand to remove debris and insect remnants, then frozen at −20°C for 24 h to preserve its integrity. After freezing, the propolis was ground into a fine powder. Precisely 20 g of the powdered propolis were measured and mixed with 200 mL of a 70% ethanol solution. This mixture was heated for 2 hours at 60°C, followed by an additional 2-h incubation at a higher temperature of 80°C. After the extraction, the solution was centrifuged at 5,000 revolutions per minute for 20 min to remove excess alcohol. The resulting liquid, known as the supernatant, was then filtered through Whatman No. 1 filter paper and stored in a refrigerator at 4°C for future use.
2.3 Optimization of synthesis parameters
Key synthesis parameters were meticulously examined, including reaction times of 30, 60, 90, 120, and 150 min; varying volume ratios of silver nitrate (AgNO3) to propolis extract at ratios of 1:1, 1:5, and 1:10; reaction temperatures set at 25°C, 50°C, 70°C, 80°C, and 90°C; and pH levels adjusted to 5, 7, 9, 11, and 13. The formation and growth of AgNPs were monitored throughout the optimization process using UV-visible spectroscopy, which enabled real-time tracking of surface plasmon resonance (SPR) peaks characteristic of silver nanoparticles.
2.4 Synthesis of propolis AgNPs
Propolis-derived AgNPs were synthesized using a 1:1 volume ratio of AgNO3 to propolis extract, which served as both a capping and reducing agent, while AgNO3 acted as the precursor. In the synthesis procedure, 50 mL of propolis extract was gradually added to a solution containing 50 mL of 5 mM AgNO3, with the pH adjusted to 11 using 1 M NaOH. The mixture was stirred at 80°C for 2 hours and covered with aluminum foil to protect it from light exposure. Continuous stirring with a magnetic stirrer ensured thorough mixing. A color change from dark yellow to dark brown signaled the completion of the reaction and the successful formation of AgNPs. The synthesized nanoparticles were then separated through high-speed centrifugation, followed by sequential washing with distilled water and ethanol, and were subsequently dried in an oven. Finally, these AgNPs were characterized and evaluated for their biological activity.
2.5 Characterization of propolis AgNPs
2.5.1 UV-spectrophotometer of Pro-AgNPs
The UV-Vis absorption spectra of the synthesized Pro-AgNPs were recorded using a UV-visible spectrophotometer to confirm nanoparticle biosynthesis and assess the activation of surface plasmon resonance. The absorbance spectra were collected by scanning across the wavelength range of 200–800 nm. A characteristic absorption peak observed in the range of 400–450 nm serves as an indication of the presence of AgNPs (Ratan et al., 2021).
2.5.2 X-ray diffraction pattern analysis
The crystalline properties, particle sizes, and structural characteristics of the synthesized AgNPs were analyzed using an X-ray diffractometer (XRD-7000, Shimadzu Corporation, Japan). X-ray diffraction measurements were conducted over a 2θ range of 20–90° to evaluate the crystallinity and phase of the nanoparticles. The average crystallite size was estimated using the Debye-Scherrer (Equation 1), as detailed by (Gevorgyan et al., 2022). The XRD spectra were recorded using a CuKα filter (λ = 0.15418 nm) in the 2θ/θ scanning mode, spanning from 10° to 90°.
Where, (D) represents the average crystallite size,
2.5.3 Fourier transform infrared spectroscopy (FTIR) analysis
Fourier transform infrared spectroscopy (FTIR) was used to identify the functional groups in the AgNPs, providing insights into their formation and stabilization. This analysis utilized a Nicolet iS10 spectrometer from Thermo Fisher, United States, covering a spectral range of 400–4,000 cm−1. Before the analysis, the Pro-AgNPs were purified, while the ethanolic propolis extract was examined directly in its liquid form using the Attenuated Total Reflectance (ATR) method. The FTIR spectra revealed key functional groups that contribute to the reduction and stabilization of AgNPs, confirming their successful synthesis (Narayanan et al., 2021).
2.5.4 Scanning electron microscopy (SEM)
The shape, structure, and distribution of the synthesized Pro-AgNPs were investigated using Scanning Electron Microscopy (SEM). Pro-AgNPs were prepared for imaging by deposition onto conductive carbon tape on an aluminum stub, followed by a 3-min gold sputter coating. The resulting SEM images provided valuable insights into the physical characteristics of the nanoparticles (Balamurugun et al., 2024).
2.6 Antibacterial activity of silver nanoparticles
Antibacterial activity, evaluated at the Institute of Pharmaceutical Science, Adama Science, and Technology University, demonstrated the effectiveness of both Propolis Extract (Pro-E) and silver nanoparticles synthesized from propolis (Pro-AgNPs) against several bacterial pathogens. These included Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212), Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC 27853). Bacterial suspensions, standardized to 0.5 McFarland, were spread on Mueller-Hinton agar plates for disc diffusion testing. Filter paper discs, impregnated with Pro-E and Pro-AgNPs at concentrations of 12.5, 25, 50, and 100 μg/mL, were placed on the agar. Dimethyl sulfoxide (DMSO) was the negative control, and ciprofloxacin was the positive control. After 24 h of incubation, the inhibition zones were measured to evaluate antimicrobial activity, emphasizing the effectiveness of both Pro-E and Pro-AgNPs as promising antibacterial agents (Gevorgyan et al., 2022).
2.7 In-vitro antioxidant activity of propolis AgNPs
The antioxidant activity of propolis and Pro-AgNPs was analyzed using the DPPH assay. A 0.1 mM DPPH solution prepared in methanol was combined with different concentrations of propolis extract, Pro-AgNPs, and ascorbic acid (used as the standard), along with a negative control. After incubating the mixture for 30 min, the absorbance was recorded at 517 nm. The percentage of inhibition was calculated to determine antioxidant activity, following the procedure described by (Essghaier et al., 2022). The percentage of inhibition was calculated using Equation 2:
Where A control is the absorbance of the control reaction and A sample is the absorbance of the tested sample.
3 Results and discussion
3.1 UV-visible spectroscopy analysis
In the synthesis of Pro-AgNPs, the propolis extract serves as both a reducing and capping agent, which is essential for the synthesis and stabilization of AgNPs. The formation of a dark brown color in the solution signals that the reduction process has occurred, during which Ag+ ions are converted into elemental silver (Melkamu and Bitew, 2021). The formation of AgNPs is further confirmed by the appearance of an SPR peak at 424 nm (Figure 2a), as observed in the UV-Vis spectrophotometric analysis. This SPR peak is a characteristic feature of AgNPs, resulting from the collective oscillation of conduction electrons on the NP surface when exposed to light. The observed SPR peak at 424 nm aligns with previous studies on AgNPs synthesized from various propolis extracts (Barbosa et al., 2019; Al-Saggaf, 2021; Tiri et al., 2021). A peak in the 320–330 nm range in propolis extract suggests the presence of flavonoids, particularly certain types like flavones and flavonols. This finding is consistent with other research on the chemical makeup of propolis extracts (Alanazi and Alenzi, 2024; Ferreira et al., 2024). The consistency across these studies suggests that the SPR peak at 424 nm is a reliable indicator of successful AgNP synthesis using propolis. This reinforces the effectiveness of the synthesis method and underscores propolis’ potential as a sustainable and natural source for producing AgNPs. The observed results are comparable to those obtained using established protocols, further validating the efficacy of the proposed approach.
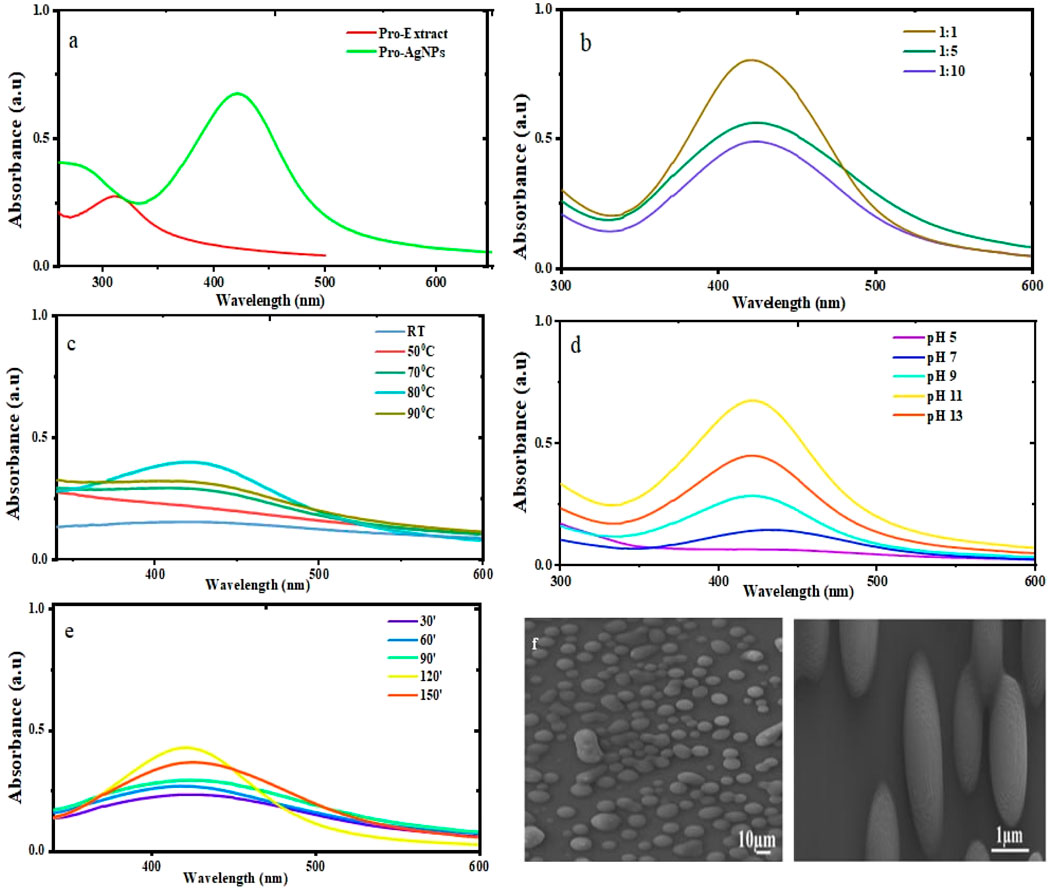
Figure 2. UV-Vis absorption spectra of Pro-AgNPs synthesized under various conditions: (a) Pro-AgNPs synthesized under optimal conditions alongside their corresponding propolis extract. (b) Effect of varying AgNO3 to propolis volume ratios on Pro-AgNP synthesis, with a fixed AgNO3 concentration. (c) Influence of temperature on the synthesis of Pro-AgNPs. (d) pH optimization in the bio-synthesis of Pro-AgNPs. (e) Optimization of reaction time for Pro-AgNP synthesis. (f) SEM images of Propolis mediated AgNPs.
3.1.1 The effects of precursor concentration ratio on AgNPs
The successful synthesis of nanoparticles is highly dependent on the precise control of the relative proportions of the extract and the precursor metal (Soni et al., 2021). In this study, the synthesis of Pro-AgNPs was explored using three different volume ratios of AgNO3 to propolis extract: 1:1, 1:5, and 1:10. While all three ratios resulted in the formation of nanoparticles, as evidenced by the presence of SPR absorption peaks (Figure 2b), the 1:1 ratio demonstrated the most intense absorption peak, indicating the highest nanoparticle yield. This enhanced intensity at the 1:1 ratio can be attributed to the higher concentration of bioactive molecules in the propolis extract, which promotes the efficient reduction of Ag+ ions and stabilizes the resulting nanoparticles (Liu et al., 2023).
A sharper and more defined SPR peak was observed at the 1:5 ratio compared to the 1:10 ratio, suggesting improved nanoparticle formation. However, the peak intensity at the 1:5 ratio was lower than that of the 1:1 ratio, likely due to a reduced concentration of reducing agents in the propolis extract. When the precursor concentration is excessively high relative to the reducing agents, self-quenching can occur, leading to a decrease in SPR peak intensity, as observed in both the 1:1 and 1:5 ratios (Zulfajri et al., 2024). Consequently, the 1:1 ratio of silver nitrate to propolis extract was identified as the optimal condition for Pro-AgNP synthesis, offering a robust foundation for further optimization and ensuring efficient nanoparticle production. This finding underscores the importance of balancing precursor and reducing agent concentrations to achieve high-quality nanoparticles with desirable properties.
3.1.2 The effects of reaction temperature on the synthesis of AgNPs
Temperature critically influences nanoparticle size, morphology, and stability during synthesis. Pro-AgNP synthesis was optimized over a 2-h period at temperatures from room temperature to 90°C. UV-Vis spectroscopy identified 80°C as optimal, yielding the highest nanoparticle stability and concentration (Figure 2c). While temperatures above 80°C offered minimal improvement, precise temperature control remains crucial. Higher temperatures accelerate reaction kinetics, influencing AgNP size and distribution (Rashidi et al., 2024), while lower temperatures produce larger nanoparticles (Sonawane et al., 2023). Maintaining 80°C ensures stable, well-dispersed Pro-AgNPs with minimal aggregation, suitable for various applications. Higher temperatures during synthesis reduce AgNP size due to accelerated nucleation and kinetic energy, enhancing antibacterial efficacy by increasing surface-area-to-volume ratio (Smiechowicz et al., 2021). Similarly, temperature variations can impact the antioxidant capacity of AgNPs by altering their surface reactivity and ability to scavenge free radicals (Yiyit Doğan et al., 2024). Therefore, optimizing temperature conditions is essential to maximize both the antibacterial and antioxidant performance of AgNPs.
3.1.3 The effects of reaction pH on the synthesis of Pro-AgNPs
The properties of synthesized Pro-AgNPs are significantly influenced by the pH of the reaction environment, which plays a pivotal role in determining their size, shape, stability, and other critical characteristics. A thorough understanding of pH effects is essential for controlling the properties of AgNPs during synthesis. In this study, the pH was meticulously adjusted between 5 and 13 using 1 M NaOH to optimize the synthesis process and achieve nanoparticles with desired properties. The absorbance of the solutions was measured using UV-Vis spectrophotometry across a wavelength range of 200–800 nm.
Notably, at pH 11, distinct absorption spectra were observed, confirming the successful formation and stability of Pro-AgNPs under alkaline conditions, as shown in Figure 2d. The intensity of the surface plasmon resonance (SPR) peak is highly pH-dependent, with higher pH values generally leading to increased intensity, while lower (acidic) pH values result in decreased intensity (Ali et al., 2023; Li et al., 2023). This trend indicates that alkaline conditions enhance nanoparticle stability and uniformity, producing stronger SPR signals. In contrast, acidic conditions may reduce intensity due to nanoparticle aggregation or incomplete reduction of silver ions.
Furthermore, nanoparticles synthesized at higher pH levels displayed more uniform shapes and sizes, contributing to improved suspension stability. The pH of the reaction environment plays a critical role in the synthesis of AgNPs, significantly influencing their size, shape, and overall functionality. Variations in pH can alter these physical properties, which directly affect the nanoparticles’ antimicrobial efficacy by influencing their ability to penetrate microbial cell walls (Miranda et al., 2022). For instance, AgNPs synthesized under alkaline conditions exhibit superior antibacterial activity due to their smaller particle size and reduced aggregation, which enhances their ability to infiltrate bacterial cell walls (Chitra and Annadurai, 2014). Additionally, alkaline conditions stabilize capping agents, improving their capacity to donate electrons and neutralize free radicals. Smaller nanoparticles produced at pH 9 demonstrate higher surface reactivity, resulting in enhanced radical scavenging efficiency (Shahzadi et al., 2022). Therefore, precise control of pH is essential to optimize the size, morphology, and functional performance of AgNPs, ensuring their maximum antimicrobial and antioxidant potential. These findings highlight the critical role of pH control in fine-tuning the properties of AgNPs for specific applications, emphasizing its importance in the synthesis process. Alkaline conditions facilitate the efficient reduction of silver ions and stabilize the nanoparticles through interactions with bioactive compounds in propolis, such as flavonoids and phenolic acids (Kurek-Górecka et al., 2022).
3.1.4 The influences of reaction time on Pro-AgNP synthesis
The optimal reaction time for Pro-AgNP synthesis was determined by monitoring nanoparticle formation using UV-Vis spectroscopy every 30 min over a period of 30–150 min (Figure 2e). A reaction time of 120 min yielded the highest nanoparticle production, as evidenced by peak absorbance. Shorter reaction times (30, 60, and 90 min) resulted in lower yields and reduced stability due to incomplete nanoparticle formation. Longer reaction times initially increased nanoparticle production but excessively long durations led to aggregation and less uniform structures. Conversely, shorter times yielded unevenly shaped nanoparticles.
An optimal reaction time is crucial for producing well-defined, uniform nanoparticles (Waktole et al., 2025). Therefore, 120 min was identified as the ideal reaction time for Pro-AgNP synthesis. This duration ensures complete reduction of silver ions and stabilization of nanoparticles, preventing issues such as aggregation or irregular morphology. The results align with previous studies indicating that prolonged reaction times can lead to excessive growth or clustering of nanoparticles, while insufficient time limits their formation (Sonawane et al., 2023). By optimizing the reaction time, this study ensures the production of high-quality Pro-AgNPs with desirable properties for various applications, including antimicrobial and antioxidant therapies. The consistency in size and shape achieved at 120 min underscores the importance of precise temporal control in the synthesis process.
3.2 Scanning electron microscopy analysis
Figure 2f presents the SEM micrograph of Pro-AgNPs, showcasing their surface characteristics. SEM, adept at detailing particle sizes and morphologies, revealed that the Pro-AgNPs primarily possess a spherical shape, mirroring findings from studies on V. amygdalina (Aisida et al., 2020) and Tagetes erecta (Katta and Dubey, 2020). The nanoparticles displayed uniformity in size, confined to the nanoscale, and a narrow size distribution, indicative of high dispersity. This uniformity is beneficial for various applications due to predictable properties and behavior. The biosynthesis method used for Pro-AgNPs effectively minimizes aggregation, as evidenced by the lack of significant nanoparticle clusters. The SEM images confirm that Pro-AgNPs are spherical, uniformly sized on the nanoscale, monodisperse, and largely non-aggregated.
3.3 FTIR analysis of Pro-E and Pro-AgNPs
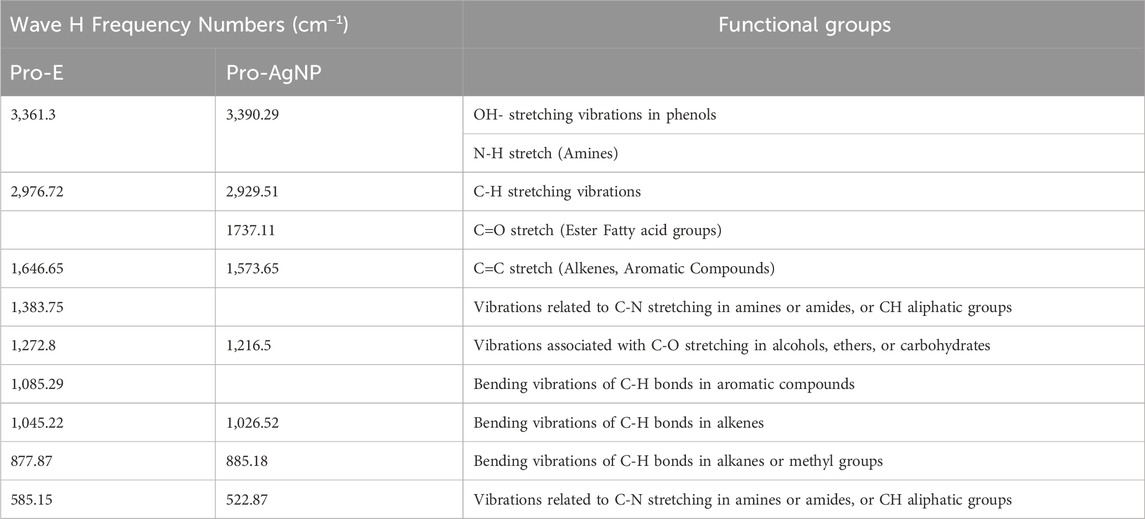
Figure 3b presents the FTIR spectral profiles of the propolis extract and the synthesized Pro-AgNPs. FTIR analysis was employed to identify the functional groups involved in the reduction, stabilization, and capping of AgNPs. The propolis extract spectrum displayed peaks indicative of biomolecules such as O-H stretching (3,361 cm−1), C-H stretching (2,976 cm−1), and C=O stretching (1,646.65 cm−1) (Badiazaman et al., 2023; Ferreira et al., 2023; Peng et al., 2023). In Pro-AgNPs, slight shifts in these peaks were observed, particularly in the C=O stretching region (1737.11 cm−1), suggesting changes in the molecular environment during nanoparticle formation (Figure 3b black). Additionally, peaks at 1,367 cm−1 in Pro-AgNPs indicated the presence of phenol biomolecules, essential for nanoparticle synthesis processes. Peaks around 1,085, 1,045, and 877 cm−1 suggested the presence of carbohydrates (Wang et al., 2021). The synthesis and stability of Pro-AgNPs are influenced by lipids, flavonoids, phenols, and carbohydrates, as supported by previous research (Kulkarni et al., 2023; Asefian and Ghavam, 2024). Table 1 provides a detailed interpretation of the FTIR data, outlining the specific wave number frequencies and corresponding functional groups identified in both the propolis extract and the synthesized Pro-AgNPs.
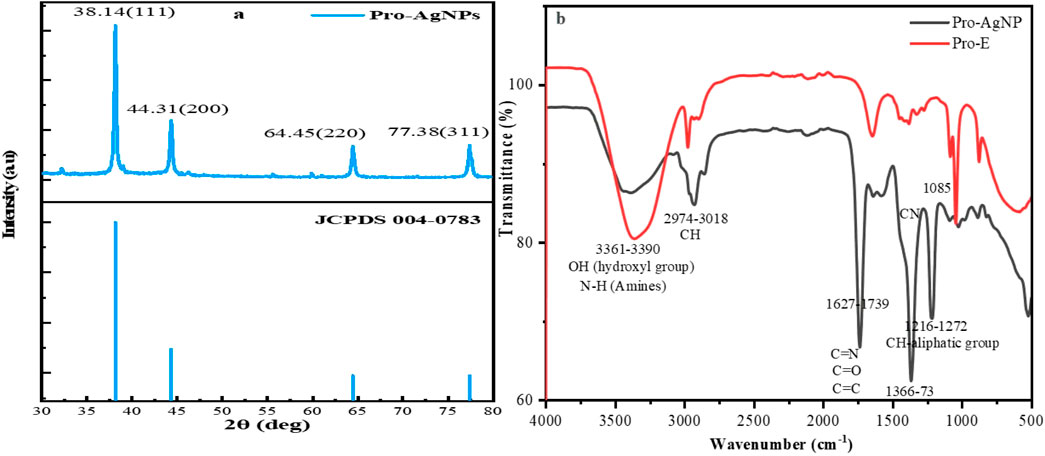
Figure 3. (a) XRD analysis of Propolis mediated AgNPs. (b) FTIR spectra of Propolis extract and Propolis-mediated AgNPs.
3.4 X-ray diffraction analysis
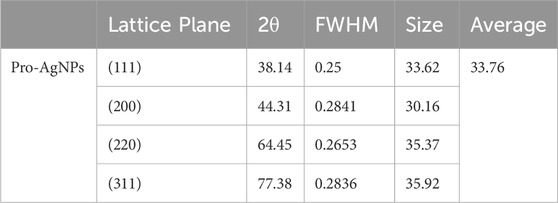
The XRD pattern (Figure 3a) confirmed the crystalline nature of the synthesized Pro-AgNPs, exhibiting characteristic peaks at 2θ values of 38.1°, 44.3°, 64.5°, and 77.4°, corresponding to the (111), (200), (220), and (311) planes of a face-centered cubic (FCC) silver structure (JCPDS reference No. 04-0783). The absence of extraneous peaks indicated the high purity of the synthesized nanoparticles. The observed Miller indices (hkl) values further confirmed the presence of an FCC silver structure, aligning with previous findings (Ali et al., 2023). The sharp, well-defined peaks in the XRD pattern suggested a highly crystalline nature of the Pro-AgNPs, indicating a uniform distribution of crystallite sizes. The absence of peak broadening or peak shifting reveals that the nanoparticles have minimal lattice strain or defects. This uniformity is crucial as it directly influences the nanoparticles’ physical and chemical properties, particularly their high surface area-to-volume ratio, which enhances reactivity.
The consistent crystal size observed in Pro-AgNPs is attributed to the bioactive compounds in propolis extract, which facilitate the reduction of metal ions and the formation of nanoparticles with a narrow size distribution (Huo et al., 2022). These bioactive compounds, including flavonoids, phenolic acids, and terpenes, stabilize the nanoparticles, preventing excessive growth and ensuring a uniform size distribution. The size uniformity of Pro-AgNPs contributes to their stability and performance in various applications, such as antimicrobial treatments where consistent interaction with microbial cells is essential for optimal efficacy (Acharya et al., 2021). The distinctive properties and biological effects of Pro-AgNPs make them highly valuable in a wide range of fields. The size of the Pro-AgNP crystals, as measured by XRD analysis, is a critical factor in their effectiveness in different applications. The specific average crystal size of the Pro-AgNPs, as calculated from these XRD spectra, is detailed in Table 2, underscoring its critical role in determining their effectiveness in different applications.
3.5 Antibacterial activity of propolis extract and propolis AgNPs
Figure 4 illustrates the antimicrobial efficacy of propolis extract and silver nanoparticles against selected bacterial pathogens, determined through the disc diffusion method. Pro-AgNPs at higher concentrations (100 μg/mL) exhibited significant antibacterial activity, with a maximum zone of inhibition (ZOI) of 17.33 ± 1.15 mm against E. faecalis, propolis extract showed a ZOI of 13.67 ± 1.15 mm against P. aeruginosa ATCC 27852. Among the tested bacterial pathogens, E. faecalis exhibited the highest susceptibility to Pro-AgNPs, demonstrating a zone of inhibition of 17.33 ± 1.15 mm at 100 μg/mL. This significant antibacterial effect suggests that E. faecalis is particularly sensitive to Pro-AgNP-induced membrane disruption and oxidative stress, highlighting the potential of Pro-AgNPs as an effective antimicrobial agent, especially against Gram-positive bacteria (Halkai et al., 2018). These findings align with previous studies on green-synthesized AgNPs from Elaeocarpus serratus fruit extract (Balamurugan et al., 2024).
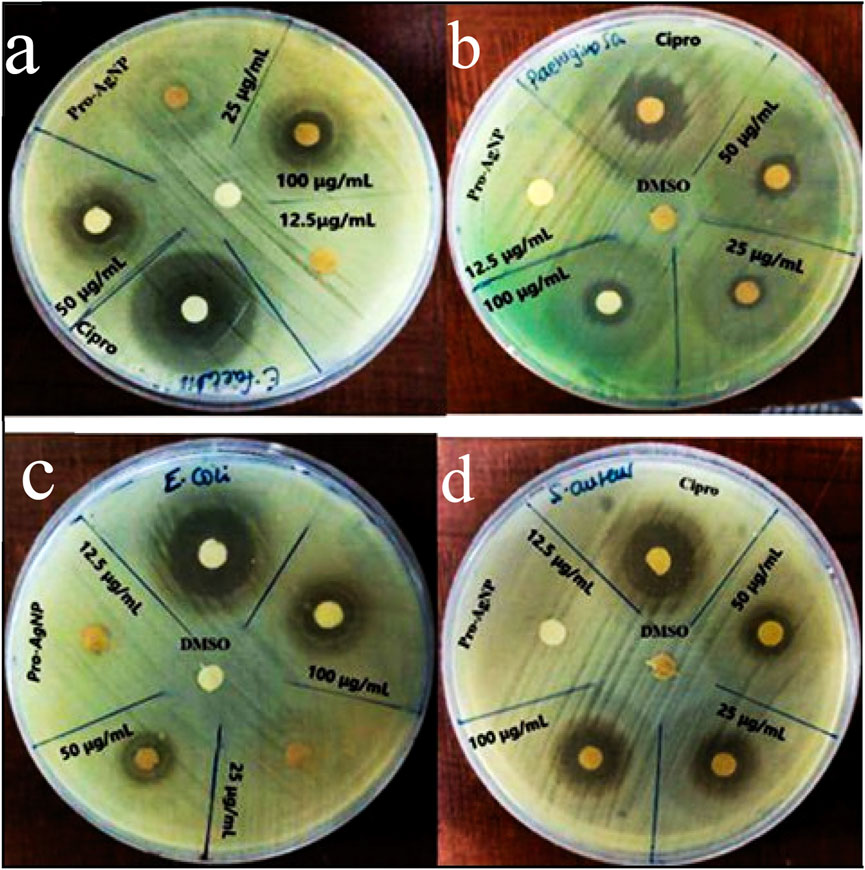
Figure 4. Antibacterial effects of Pro-AgNPs against E. faecalis (a), Pseudomonas aeruginosa (b), E. coli (c), and S. aureus (d). The Pro-AgNPs treatment concentrations were 100, 50, 25, and 12.5 μg/mL. Key: DMSO, dimethyl sulfoxide, Pro-AgNP = Propolis silver nanoparticles.
At 50 μg/mL, Pro-AgNPs effectively inhibited all tested bacteria (Figure 4), indicating broad-spectrum antibacterial properties. AgNPs exert antimicrobial effects by disrupting bacterial cell membranes, inhibiting enzyme activity, and inducing oxidative stress (Adeyemi et al., 2020; Khina and Krutyakov, 2021; Xu et al., 2021; Zhao et al., 2022). Silver ions (Ag+) bind to essential bacterial components, disrupting energy production and DNA replication (Kurek-Górecka et al., 2022). The study underscores the potential of propolis extract and Pro-AgNPs as antimicrobial agents at higher doses, suggesting further investigation to optimize their clinical efficacy. Figure 5 illustrates the comparative antibacterial activity of Pro-E, Pro-AgNPs, and ciprofloxacin against the tested bacterial strains.
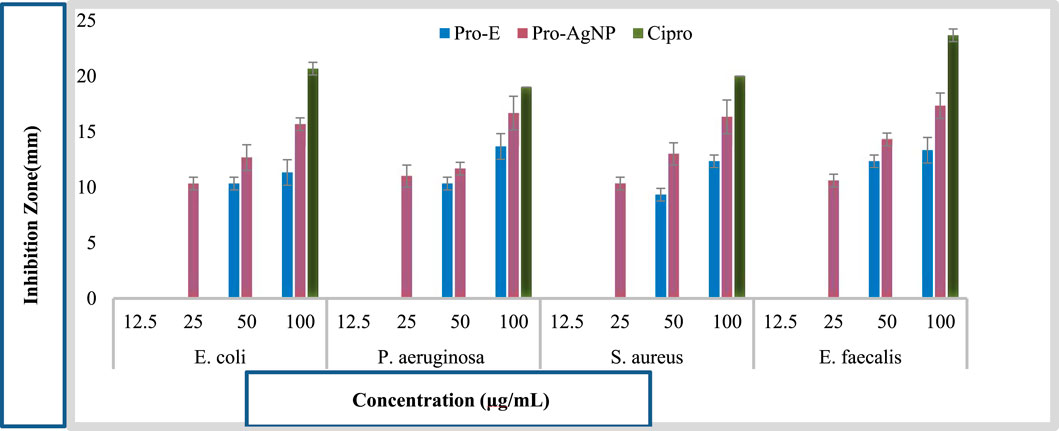
Figure 5. Comparative Antibacterial Activity of Pro-E, Pro-AgNP, and Ciprofloxacin against tested Bacterial Strains.
3.6 Antioxidant activity of silver NPs
3.6.1 2, 2-diphenyl-1-Picrylhydrazyl assay
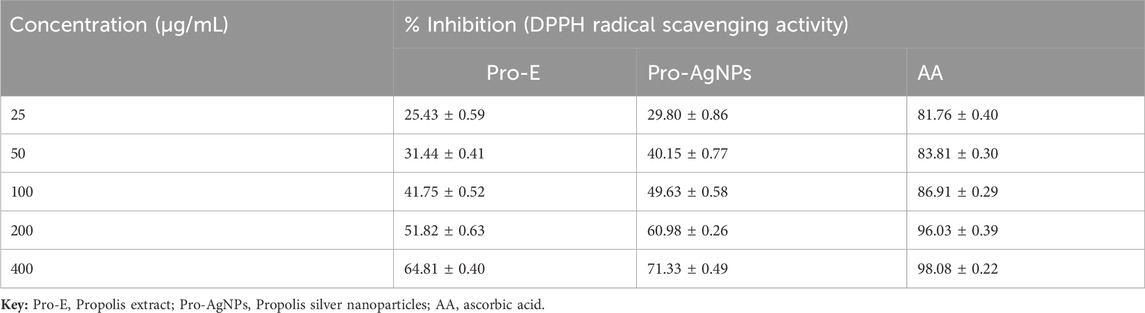
Table 3 shows the DPPH radical scavenging activity, indicating a positive correlation between concentration and antioxidant efficacy for Pro-E, Pro-AgNPs, and ascorbic acid (AA). Initially, Pro-E and Pro-AgNPs had lower scavenging activity than AA at 25 and 50 μg/mL concentrations. Interestingly, the antioxidant capacity of propolis extract and Pro-AgNPs enhanced with increasing concentration, eventually approaching that of AA. Pro-AgNPs exhibited notably good antioxidant activity across all tested concentrations, especially within the 100–400 μg/mL range, underscoring their potent antioxidant potential in agreement with the results (Kurek-Górecka et al., 2022). Our results suggest propolis extracts and their derived AgNPs can serve as valuable natural antioxidants with diverse industrial applications for mitigating oxidative stress, aligning with previous studies (Al-Yousef et al., 2020; Javed et al., 2022).
The study examined the antioxidant capacity of Pro-E, Pro-AgNPs, and ascorbic acid using IC50 values to measure their DPPH radical scavenging ability. Propolis extract showed moderate to high antioxidant activity with an IC50 of 61.56 ± 0.47 μg/mL. Pro-AgNPs had enhanced antioxidant potency (IC50: 45.54 ± 0.57 μg/mL) compared to Pro-E, while AA was the most potent antioxidant with an IC50 of 5.49 ± 0.31 μg/mL. Both Pro-E and Pro-AgNPs exhibited antioxidant properties with increasing efficiency at higher concentrations. Compounds like flavonoids and phenolic acids likely contribute to these properties (Oluwole et al., 2022). These findings suggest the potential of natural extracts and synthesized nanoparticles as antioxidants, warranting further exploration of their practical applications (Chandraker et al., 2021).
4 Conclusion
The biogenic synthesis of Pro-AgNPs using Ethiopian propolis extract confirms its role as a stabilizer and a reducer. The characterization of AgNPs synthesized from propolis extract was performed and confirmed through different analytical methods, incorporating spectroscopic and microscopic techniques. Our study also demonstrated the antimicrobial effectiveness of Pro-AgNPs against gram-negative and gram-positive bacteria. Furthermore, we compared the free radical scavenging efficiency of Pro-AgNPs with propolis extract and ascorbic acid, highlighting their potential as natural antioxidants. These findings represent a promising nanobiotechnological compound obtained from a natural source for various antimicrobial applications, such as protecting medical devices and wound dressings. By integrating the unique properties of Ethiopian propolis with nanotechnology, our work addresses pressing global health challenges and advocates for further research to develop innovative antimicrobial and antioxidant agents and explore diverse applications of natural products and synthesized nanoparticles. However, to ensure the safe and effective translation of Pro-AgNPs, future investigations should prioritize comprehensive cytotoxicity assessments using both in vitro and in vivo models, optimize dosage regimens, and evaluate long-term stability.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
GW: Conceptualization, Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft, Investigation, Writing – review and editing. BC: Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review and editing. AB: Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review and editing. LT: Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acharya, D., Pandey, P., and Mohanta, B. (2021). A comparative study on the antibacterial activity of different shaped silver nanoparticles. Chem. Pap. 75 (9), 4907–4915. doi:10.1007/s11696-021-01722-8
Adeyemi, O. S., Shittu, E. O., Akpor, O. B., Rotimi, D., and Batiha, G. E.-S. (2020). Silver nanoparticles restrict microbial growth by promoting oxidative stress and DNA damage. PubMed 19, 492–500. doi:10.17179/excli2020-1244
Afata, T. N., Nemo, R., Ishete, N., Tucho, G. T., and Dekebo, A. (2022). Phytochemical investigation, physicochemical characterization, and antimicrobial activities of Ethiopian propolis. Arabian J. Chem. 15 (7), 103931. doi:10.1016/j.arabjc.2022.103931
Ahmed, S. F., Mofijur, M., Rafa, N., Chowdhury, A. T., Chowdhury, S., Nahrin, M., et al. (2021). Green approaches in synthesising nanomaterials for environmental nanobioremediation: technological advancements, applications, benefits and challenges. Environ. Res. 204, 111967. doi:10.1016/j.envres.2021.111967
Aisida, S. O., Ugwu, K., Akpa, P. A., Nwanya, A. C., Ejikeme, P. M., Botha, S., et al. (2020). Morphological, optical and antibacterial study of green synthesized silver nanoparticles via Vernonia amygdalina. Mater. Today Proc. 36, 199–203. doi:10.1016/j.matpr.2020.02.931
Alanazi, S., and Alenzi, N. D. (2024). Phytochemical profiling and characterization of flavonoid derivatives from propolis sample and investigation of cytotoxic and antiprotozoal activities. Sci. Rep. 14 (1), 21295. doi:10.1038/s41598-024-72379-y
Ali, M. H., Azad, M. A. K., Khan, K. A., Rahman, M. O., Chakma, U., and Kumer, A. (2023). Analysis of crystallographic structures and properties of silver nanoparticles synthesized using PKL extract and nanoscale characterization techniques. ACS Omega 8 (31), 28133–28142. doi:10.1021/acsomega.3c01261
Al-Saggaf, M. S. (2021). Formulation of insect chitosan stabilized silver nanoparticles with propolis extract as potent antimicrobial and wound healing composites. Int. J. Polym. Sci. 2021, 1–9. doi:10.1155/2021/5578032
Al-Yousef, H. M., Amina, M., Alqahtani, A. S., Alqahtani, M. S., Malik, A., Hatshan, M. R., et al. (2020). Pollen bee aqueous Extract-Based synthesis of silver nanoparticles and evaluation of their Anti-Cancer and Anti-Bacterial activities. Processes 8 (5), 524. doi:10.3390/pr8050524
Asefian, S., and Ghavam, M. (2024). Green and environmentally friendly synthesis of silver nanoparticles with antibacterial properties from some medicinal plants. BMC Biotechnol. 24 (1), 5. doi:10.1186/s12896-023-00828-z
Badiazaman, A. A. M., Mohd, K. S., Zin, N. B. M., Mohamad, H., and Aziz, A. N. (2023). HPTLC Profiling and FTIR Fingerprinting Coupled with Chemometric analysis of Malaysian stingless bee propolis. J. Teknol. 85 (2), 121–131. doi:10.11113/jurnalteknologi.v85.19050
Balamurugan, V., Ragavendran, C., and Arulbalachandran, D. (2024). Eco-friendly green synthesis of AgNPs from Elaeocarpus serratus fruit extract: potential to antibacterial, antioxidant, cytotoxic effects of colon cancerous cells (HT-29) and its toxicity assessments of marine microcrustacean Artemia nauplii. Mol. Biol. Rep. 51 (1), 418. doi:10.1007/s11033-024-09335-6
Barbosa, V. T., Souza, J. K. C., Alvino, V., Meneghetti, M. R., Florez-Rodriguez, P. P., Moreira, R. E., et al. (2019). Biogenic synthesis of silver nanoparticles using Brazilian propolis. Biotechnol. Prog. 35 (6), e2888. doi:10.1002/btpr.2888
Barsola, B., and Kumari, P. (2022). Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: a review. Green Process. Synthesis 11 (1), 659–673. doi:10.1515/gps-2022-0059
Canaparo, R., Foglietta, F., Limongi, T., and Serpe, L. (2020). Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 14 (1), 53. doi:10.3390/ma14010053
Chandraker, S. K., Lal, M., Kumar, A., and Shukla, R. (2021). Justicia adhatoda L. mediated green synthesis of silver nanoparticles and assessment of their antioxidant, hydrogen peroxide sensing, and optical properties. Mater. Technol. 37 (10), 1355–1365. doi:10.1080/10667857.2021.1949525
Chitra, K., and Annadurai, G. (2014). Antibacterial activity of pH-dependent biosynthesized silver nanoparticles against clinical pathogen. BioMed Res. Int. 2014 (1), 1–6. doi:10.1155/2014/725165
Dakal, T. C., Kumar, A., Majumdar, R. S., and Yadav, V. (2016). Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 7, 1831. doi:10.3389/fmicb.2016.01831
Dărăban, A. (2019). The evaluation of antioxidant capacity of propolis originating from western Romania. Farmacia 67 (1), 111–116. doi:10.31925/farmacia.2019.1.15
Essghaier, B., Dridi, R., Mottola, F., Rocco, L., Zid, M. F., and Hannachi, H. (2022). Biosynthesis and characterization of silver nanoparticles from the extremophile plant aeonium haworthii and their antioxidant, antimicrobial and anti-diabetic capacities. Nanomaterials 13 (1), 100. doi:10.3390/nano13010100
Ferreira, A. R., Machado, MTDC, and Chagas, AGCM (2023). Propolis oil extract as an alternative means to the alcoholic extract. Obs. la Econ. Latinoam. 21 (10), 15657–15676. doi:10.55905/oelv21n10-063
Ferreira, LMDCMC, Souza, PDQD, Pereira, R. R., da Silva, E. O., Barbosa, W. L. R., Silva-Júnior, J. O. C., et al. (2024). Preliminary study on the chemical and biological properties of propolis extract from stingless bees from the Northern region of Brazil. Processes 12 (4), 700. doi:10.3390/pr12040700
Gevorgyan, S., Schubert, R., Falke, S., Lorenzen, K., Trchounian, K., and Betzel, C. (2022). Structural characterization and antibacterial activity of silver nanoparticles synthesized using a low-molecular-weight Royal Jelly extract. Sci. Rep. 12 (1), 14077. doi:10.1038/s41598-022-17929-y
Gold, K., Slay, B., Knackstedt, M., and Gaharwar, A. K. (2018). Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. 1 (3), 1800037. doi:10.1002/adtp.201700033
Halkai, K. R., Mudda, J. A., Shivanna, V., Rathod, V., and Halkai, R. (2018). Antibacterial efficacy of biosynthesized silver nanoparticles against Enterococcus faecalis biofilm: an: in vitro: study. Contemp. Clin. Dent. 9 (2), 237–241. doi:10.4103/ccd.ccd_828_17
Horie, M., and Tabei, Y. (2020). Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 55 (4), 331–342. doi:10.1080/10715762.2020.1859108
Huo, C., Hao, Z., Yuan, C., Chen, Y., and Liu, J. (2022). Probing the phytosynthesis mechanism of gold and silver nanoparticles by sequential separation of plant extract and molecular characterization with ultra-high-resolution mass spectrometry. ACS Sustain. Chem. and Eng. 10 (12), 3829–3838. doi:10.1021/acssuschemeng.1c07021
Jangid, H., Singh, S., Kashyap, P., Singh, A., and Kumar, G. (2024). Advancing biomedical applications: an in-depth analysis of silver nanoparticles in antimicrobial, anticancer, and wound healing roles. Front. Pharmacol. 15, 1438227. doi:10.3389/fphar.2024.1438227
Javed, S., Mangla, B., and Ahsan, W. (2022). From propolis to nanopropolis: an exemplary journey and a paradigm shift of a resinous substance produced by bees. Phytotherapy Res. 36 (5), 2016–2041. doi:10.1002/ptr.7435
Jobir, M., and Shume, A. (2020). Comparative study of different Ethiopian propolis: in vivo wound healing, antioxidant, antibacterial, physicochemical - properties and mineral profiles. J. Apitherapy 7 (2), 31. doi:10.5455/ja.20200225125850
Katta, V. K. M., and Dubey, R. S. (2020). Green synthesis of silver nanoparticles using Tagetes erecta plant and investigation of their structural, optical, chemical and morphological properties. Mater. Today Proc. 45, 794–798. doi:10.1016/j.matpr.2020.02.809
Khalil, M., Mohamed, S., and El-Naggar, K. (2021). Green synthesis of silver nanoparticles using Egyptian propolis extract and its antimicrobial activity. Egypt. J. Chem. 0 (0), 0. doi:10.21608/ejchem.2021.104296.4838
Khina, A. G., and Krutyakov, Y. A. (2021). Similarities and differences in the mechanism of antibacterial action of silver ions and nanoparticles. Appl. Biochem. Microbiol. 57 (6), 683–693. doi:10.1134/s0003683821060053
Kulkarni, D., Sherkar, R., Shirsathe, C., Sonwane, R., Varpe, N., Shelke, S., et al. (2023). Biofabrication of nanoparticles: sources, synthesis, and biomedical applications. Front. Bioeng. Biotechnol. 11, 1159193. doi:10.3389/fbioe.2023.1159193
Kurek-Górecka, A., Keskin, Ş., Bobis, O., Felitti, R., Górecki, M., Otręba, M., et al. (2022). Comparison of the antioxidant activity of propolis samples from different geographical regions. Plants 11 (9), 1203. doi:10.3390/plants11091203
Kustiawan, P. M., Syaifie, P. H., Siregar, KAAK, Ibadillah, D., and Mardliyati, E. (2024). New insights of propolis nanoformulation and its therapeutic potential in human diseases. ADMET and DMPK. 12 (1), 1–26.
Li, Y., Liao, Q., Hou, W., and Qin, L. (2023). Silver-based surface plasmon sensors: fabrication and applications. Int. J. Mol. Sci. 24 (4), 4142. doi:10.3390/ijms24044142
Liu, L., Yu, C., Ahmad, S., Ri, C., and Tang, J. (2023). Preferential role of distinct phytochemicals in biosynthesis and antibacterial activity of silver nanoparticles. J. Environ. Manag. 344, 118546. doi:10.1016/j.jenvman.2023.118546
Melkamu, W. W., and Bitew, L. T. (2021). Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) J.F. Gmel plant leaf extract and their antibacterial and antioxidant activities. Heliyon 7 (11), e08459. doi:10.1016/j.heliyon.2021.e08459
Miranda, A., Akpobolokemi, T., Chung, E., Ren, G., and Raimi-Abraham, B. T. (2022). pH alteration in plant-mediated green synthesis and its resultant impact on antimicrobial properties of silver nanoparticles (AgNPs). Antibiotics 11 (11), 1592. doi:10.3390/antibiotics11111592
Narayanan, M., Divya, S., Natarajan, D., Senthil-Nathan, S., Kandasamy, S., Chinnathambi, A., et al. (2021). Green synthesis of silver nanoparticles from aqueous extract of Ctenolepis garcini L. and assess their possible biological applications. Process Biochem. 107, 91–99. doi:10.1016/j.procbio.2021.05.008
Oluwole, O., Fernando, W. B., Lumanlan, J., Ademuyiwa, O., and Jayasena, V. (2022). Role of phenolic acid, tannins, stilbenes, lignans and flavonoids in human health – a review. Int. J. Food Sci. and Technol. 57 (10), 6326–6335. doi:10.1111/ijfs.15936
Patil, R. B., and Chougale, A. D. (2021). Analytical methods for the identification and characterization of silver nanoparticles: a brief review. Mater. Today Proc. 47, 5520–5532. doi:10.1016/j.matpr.2021.03.384
Patra, J. K., Das, G., Fraceto, L. F., Campos, E. V. R., Rodriguez-Torres, M. d. P., Acosta-Torres, L. S., et al. (2018). Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnology 16 (1), 71. doi:10.1186/s12951-018-0392-8
Peng, S., Zhu, M., Li, S., Ma, X., and Hu, F. (2023). Ultrasound-assisted extraction of polyphenols from Chinese propolis. Front. Sustain. Food Syst. 7. doi:10.3389/fsufs.2023.1131959
Raheem, I. A. A., Razek, A. A., Elgendy, A. A., Labah, D. A., and Saleh, N. M. (2020). Egyptian propolis-loaded nanoparticles as a root canal nanosealer: sealing ability and in vivo biocompatibility. Int. J. Nanomedicine 15, 5265–5277. doi:10.2147/ijn.s258888
Rashidi, M. A., Falahi, S., Farhang Dehghan, S., Ebrahimzadeh, H., Ghaneialvar, H., and Zendehdel, R. (2024). Green synthesis of silver nanoparticles by Smyrnium cordifolium plant and its application for colorimetric detection of ammonia. Sci. Rep. 14 (1), 24161. doi:10.1038/s41598-024-73010-w
Ratan, Z. A., Mashrur, F. R., Chhoan, A. P., Shahriar, S. M., Haidere, M. F., Runa, N. J., et al. (2021). Silver nanoparticles as potential antiviral agents. Pharmaceutics 13 (12), 2034. doi:10.3390/pharmaceutics13122034
Rezk, N., Abdelsattar, A. S., Makky, S., Hussein, A. H., Kamel, A. G., and El-Shibiny, A. (2022). New formula of the green-synthesized Au@Ag core@shell nanoparticles using propolis extract presenting high antibacterial and anticancer activity. Amb. Express 12, 1–14. doi:10.1186/s13568-022-01423-7
Rushdi, A. I., El Gamal, A. A., and Mohamed, A. H. (2014). Characteristics and chemical compositions of propolis from Ethiopia. SpringerPlus 3, 1–8.
Sangboonruang, S., Suksomboon, A., Thongboonyou, A., Kantapan, J., Ngo-Giang-Huong, N., Khamduang, W., et al. (2022). Activity of propolis nanoparticles against HSV-2: promising approach to inhibiting infection and replication. Molecules 27 (8), 2560. doi:10.3390/molecules27082560
Shahzadi, I., Aziz Shah, S. M., Shah, M. M., Ismail, T., Fatima, N., Siddique, M., et al. (2022). Antioxidant, cytotoxic, and antimicrobial potential of silver nanoparticles synthesized using Tradescantia pallida extract. Front. Bioeng. Biotechnol. 10, 907551. doi:10.3389/fbioe.2022.907551
Smiechowicz, E., Niekraszewicz, B., and Kulpinski, P. (2021). Optimisation of AgNP synthesis in the production and modification of antibacterial cellulose fibres. Materials 14 (15), 4126. doi:10.3390/ma14154126
Sonawane, J. R., Patil, S., and Kulkarni, A. A. (2023). Understanding the nucleation and growth kinetics of the microwave-assisted synthesis of silver nanowires. Industrial and Eng. Chem. Res. 62 (36), 14199–14211. doi:10.1021/acs.iecr.3c00322
Soni, V., Raizada, P., Singh, P., Cuong, H. N., Rangabhashiyam, S., Saini, A., et al. (2021). Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: a review. Environ. Res. 202, 111622. doi:10.1016/j.envres.2021.111622
Tagliabue, A., and Rappuoli, R. (2018). Changing priorities in vaccinology: antibiotic resistance moving to the top. Front. Immunol. 9, 1068. doi:10.3389/fimmu.2018.01068
Teferi, K. (2018). Status of beekeeping in Ethiopia - a review. J. Dairy and Veterinary Sci. 8 (4). doi:10.19080/jdvs.2018.08.555743
Tiri, R. N. E., Gulbagca, F., Aygun, A., Cherif, A., and Sen, F. (2021). Biosynthesis of Ag-Pt bimetallic nanoparticles using propolis extract: antibacterial effects and catalytic activity on NaBH4 hydrolysis. Environ. Res. 206, 112622. doi:10.1016/j.envres.2021.112622
Waktole, G., Chala, B., Belay, A., and Teshome, L. (2025). Antimicrobial and antioxidant activities of silver nanoparticles synthesized from honeybee-collected pollen. BioNanoScience 15 (1), 144. doi:10.1007/s12668-024-01766-6
Wang, S., Hu, X. Z., Liu, Y. Y., Tao, N. P., Lu, Y., Wang, X. C., et al. (2021). Direct authentication and composition quantitation of red wines based on Tri-step infrared spectroscopy and multivariate data fusion. Food Chem. 372, 131259. doi:10.1016/j.foodchem.2021.131259
Wieczorek, P. P., Hudz, N., Yezerska, O., Horčinová-Sedláčková, V., Shanaida, M., Korytniuk, O., et al. (2022). Chemical variability and pharmacological potential of propolis as a source for the development of new pharmaceutical products. Molecules 27 (5), 1600. doi:10.3390/molecules27051600
Xu, Z., Zhang, C., Wang, X., and Liu, D. (2021). Release strategies of silver ions from materials for bacterial killing. ACS Appl. Bio Mater. 4 (5), 3985–3999. doi:10.1021/acsabm.0c01485
Yiyit Doğan, S. E. M. A., Kaya, S. E. Ç. İ. L., and Kondolot Solak, E. B. R. U. (2024). Green synthesis of silver nanoparticles using origanum onites extract: eff ect of temperature and time on antioxidant and antimicrobial activity. J. Biomed. Res. and Environ. Sci. 5 (9), 1214–1228. doi:10.37871/jbres2009
Yuan, J., Wen, Y., Dionysiou, D. D., Sharma, V. K., and Ma, X. (2021). Biochar as a novel carbon-negative electron source and mediator: electron exchange capacity (EEC) and environmentally persistent free radicals (EPFRs): a review. Chem. Eng. J. 429, 132313. doi:10.1016/j.cej.2021.132313
Zhang, X. F., Liu, Z. G., Shen, W., and Gurunathan, S. (2016). Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 17 (9), 1534. doi:10.3390/ijms17091534
Zhao, H., Wang, M., Cui, Y., and Zhang, C. (2022). Can we arrest the evolution of antibiotic resistance? The differences between the effects of silver nanoparticles and silver ions. Environ. Sci. and Technol. 56 (8), 5090–5101. doi:10.1021/acs.est.2c00116
Keywords: propolis, silver nanoparticles, antimicrobial activity, antioxidant activity, green synthesis
Citation: Waktole G, Chala B, Belay A and Teshome L (2025) Ethiopian honeybee propolis: a novel natural source for the green synthesis of silver nanoparticles with enhanced biological activities. Front. Nanotechnol. 7:1572699. doi: 10.3389/fnano.2025.1572699
Received: 11 February 2025; Accepted: 24 March 2025;
Published: 24 April 2025.
Edited by:
Kevin M. Koo, The University of Queensland, AustraliaReviewed by:
Hongwu Sun, Third Military Medical University, ChinaSandipan Mukherjee, University of Washington, United States
Copyright © 2025 Waktole, Chala, Belay and Teshome. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bayissa Chala, YmF5Y2hhbDA3QGdtYWlsLmNvbQ==
 Gemechis Waktole
Gemechis Waktole Bayissa Chala2*
Bayissa Chala2*