- 1Department of Pharmacy of Traditional Chinese Medicine, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, China
- 2State Key Laboratory Breeding Base of Systematic Research, Development and Utilization of Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Background: Photodynamic therapy (PDT) displays several beneficial characteristics, but it is still limited by the poor water solubility of photosensitizers, the insufficient targetability of the system, and the hypoxic microenvironment of tumors. It has been demonstrated that Atractylenolide Ⅰ(ATL-1) not only alleviates the tumor hypoxic microenvironment but also has considerable potential in antitumor therapy. Hence the combination of the above two is anticipated to achieve more efficacious antitumor effects.
Methods: Nanosystems of pH-sensitive materials coated with Atractylenolide I and Ce6 were prepared by thin-film hydration method for targeted delivery to the acidic microenvironment of colorectal cancer. The morphology and stability of the nanosystems were evaluated by particle size, zeta potential, and phenology. The in vitro anti-tumor activity and mechanism of the nanosystems were evaluated using CCK-8 assay, live/dead cell double staining assay, ROS level, WB, and flow-through. Establishment of mouse ectopic colorectal cancer model. The safety, in vivo anti-tumor activity, and mechanism of the nanosystems were evaluated by mouse tumor volume change curves, biochemical indexes, and H&E staining.
Results: The results demonstrated that the prepared ATL-1/Ce6-pH Lip had desirable physicochemical properties, drug release behaviors, cellular uptake and cytotoxicity, satisfactory biocompatibility and in vivo antitumor effect.
Conclusion: This paper described the process of co-loading ATL-1 and Ce6 into pH-sensitive liposomes (ATL-1/Ce6-pH Lip), which utilized the tumor-specific acidic microenvironment to facilitate the release of drugs for the synergistic treatment of CRC. Our findings demonstrated that ATL-1 enhanced the oxygen content of the microenvironment, providing the essential raw material for the generation of ROS. At the same time, PDT was observed to obstruct blood vessels in tumor tissues, thereby damaging the blood supply to the tumor tissues. Additionally, PDT was demonstrated to destroy blood vessels in tumor tissues, leading to more exposure of the tumor cells to the drug environment. When it comes to the ATL-1/Ce6-pH Lip, which successfully combine the above two therapeutic approaches, chemotherapy and PDT treatment,a synergistic effect can be expected. In conclusion, the ATL-1/Ce6-pH Lip has considerable potential in the treatment of CRC.
1 Introduction
Cancer is a significant cause of mortality that poses a significant threat to human health. Colorectal cancer (CRC) is the third most common malignant tumor in the world. In 2022, CRC became the second most deadly cancer (Sung et al., 2021). Treatments for CRC patients are currently conventional modalities, including surgery, chemotherapy, radiotherapy, and immunotherapy, etc. However, these approaches often induce severe side effects, invasiveness, and increased resistance to clinical chemotherapeutic agents, as well as non-specific toxicity (Vodenkova et al., 2020). As a result, the treatment effect against CRC can hardly meet the expectation, and a novel therapeutic approach is urgently needed (Shinji et al., 2022).
Among the innovative therapies developed in recent years, photodynamic therapy (PDT) shows several advantages in the treatment of CRC, including minimal invasiveness, reduced systemic toxicity and restrained drug resistance. PDT primarily damages tumor cells by activating photosensitizers (PSs) to generate reactive oxygen species (ROS). However, ROS have a short half-life of less than 40 nanoseconds and are only effective for a limited distance of less than 20 nm after they are generated (van Straten et al., 2017). Therefore, it is preferable to deliver PSs to the key subcellular organelles that are more sensitive to ROS rather than just to tumor cells. It has been shown that Chlorin e6 (Ce6) is primarily localized on the endoplasmic reticulum and is activated by light to generate hydroxyl radicals that affect the synthesis of endoplasmic reticulum proteins and other proteins, resulting in over 90% mortality of tumor cells. In addition, Ce6 has low toxicity and no drug resistance (Hak et al., 2023; Gilson et al., 2019).
Despite the numerous advantages of Ce6-mediated PDT for the treatment of CRC, Ce6 exhibits poor water solubility, low targeting, and difficulty in treating deep tumors due to the low luminescent tissue penetration (Gunaydin et al., 2021; Dinakaran and Wilson, 2023). However, there is a significant limitation on the efficacy of PDT since the cytotoxic mechanism of PDT is the conversion of nontoxic oxygen into toxic ROS, which demonds substantial oxygen that is lack in the tumor microenvironment (Li et al., 2021). Consequently, there is an increasing interest in combining PDT with other therapeutic modalities, such as chemotherapy, with the aim to improve the tumor hypoxic microenvironment and enhancing the targeting behavior in order to achieve synergistic effects, thereby ameliorating the therapeutic efficacy of PDT (Choi et al., 2022).
Recently, researches on traditional Chinese medicines (TCM), particularly with regard to their potential anticancer properties, have yielded encouraging results (Zhou et al., 2023; Ge et al., 2022). Atractylenolide Ⅰ (ATL-1), the primary active ingredient of the traditional Chinese medicine Rhizoma Atractylodis Macrocephalae, has shown promising results against cancer (Bailly, 2021). Studies have demonstrated that ATL-1 inhibits mitochondrial fission and induces mitochondrial dysfunction and apoptosis, which to a certain extent increases the oxygen content for ROS generation and improves the efficiency of PDT (Wang et al., 2009; Sun et al., 2021; Jia et al., 2023; Fukai and Ushio-Fukai, 2020). In addition to this, ATL-1 inhibits tumor cell proliferation through multiple pathways and is effective in inhibiting CRC (Ren et al., 2021; Wang et al., 2020; Qin et al., 2021; Tang et al., 2020). Concurrently, PDT can destroy blood vessels in tumor tissues (by damaging the blood supply to tumor tissues) and expose more tumor cells to the drug environment, thereby enhancing the efficacy of the treatment. However, the therapeutic efficacy of these agents is often limited by their poor water solubility and targeting ability. The rapid development of oncology and nanomaterials calls for the design of a variety of responsive nanocarrier drug delivery systems (NDDS) to provide new strategies for oncology therapy. These systems can efficiently carry and specifically deliver anticancer drugs to the tumor site and increase drug bioavailability (Da Silva et al., 2020).
The tumor microenvironment is distinguished from the normal physiological environment on account of a relatively low oxygen content and pH value (Wu and Dai, 2017). Of the above two characteristics, the acidic micro-environment is prevalent in various tumors and plays a pivotal role in the generation and development of tumors, and also contributes a lot in drug resistance restraining. Therefore, tumor microenvironment-responsive nanodrug carriers have demonstrated remarkable efficacy in controlling drug release at tumor sites, removal of carrier protective shells, and tumor targeting (Xu et al., 2023b; Tian et al., 2022). Based on the acidic micro-environment, the pH-responsive drug delivery system is designed. That is, the system is stable under normal physiological conditions, while after the carrier reaches the tumor site, the acidic micro-environment causes the carrier to undergo a series of variations, such as dissolution, surface charge flipping, and chemical bond rupture, which increases the accumulation of the carrier in the tumor, promotes the cellular uptake, resulting in the specific release of the drug, and eventually enhance the therapeutic effect of the chemotherapeutic drug and reduce the toxic side-effects (Gyanani and Goswami, 2023; Leng et al., 2023; Kansız and Elçin, 2023). According to this, we took advantage of the acidic nature of the tumor microenvironment to construct pH-sensitive drug-delivery liposomes for a better utilization and targeting of Ce6 and ATL-1 (Plana et al., 2022).
This paper described the construction of ATL-1 and Ce6 co-coated pH-sensitive liposomes (ATL-1/Ce6-pH Lip) for the inhibition of CRC. The ATL-1/Ce6-pH Lip can precisely release Ce6 and ATL-1 in an acidic tumor environment. Upon irradiation with a low-dose laser (808 nm), the PDT was found to damage the blood vessels in the tumor tissues, which disrupted the blood supply to the tumor tissue, exposing more tumor cells to the drug environment. Meanwhile, ATL-1 provides raw materials for ROS generation to interfere the function of mitochondria. This allows for the safe and efficient treatment of combining antitumor drugs with PDT, inhibiting the growth and development of CRC, which has a broad prospect of application in the precision treatment of tumors.
2 Materials and methods
2.1 Materials
Atractylenolide Ⅰ (ATL-1, MW 230.30). Soybean Lecithin were purchased from Beijing Xinshengke Technology Co. LTD. (CAS). Cholesterol (CHOL, MW 386.65) was from J&K Scientific (Beijing, China). PhospHatidyl ethanolamine (PE, MW 744.03), and Cholesteryl hemisuccinate (CHEMS, MW 486.73) were purchased from Sigma-Aldrich. Soybean Lecithin (MW 758.06) was purchased from Qingdao Rishui Bio-technologies Co., Ltd. Chlorin e6 (Ce6, MW 596.67) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. 0.01 M PBS (powder, pH 7.2–7.4) were purchased from Shanghai Acmec Biochemical Co., Ltd. Fetal bovine serum (FBS), and Dulbecco’s modified Eagle medium (DMEM) were purchased from GIBCO (Thermo Fisher Scientific, Carlsbad, California, United States).
The Cell Counting Kit-8 (CCK-8) was obtained from APExBIO Technology (Houston, TX, United States). Antibodies were obtained from Abcam (Cambridge, United Kingdom) against Bcl-2 (ab196495). Penicillin-streptomycin Solution (Biosharp). Methylene chloride and methanol were purchased from Shanghai Macklin Biochemical Co., Ltd.
2.2 Cells and animals
The HCT116 cell line was obtained from the Pharmacology Laboratory at the School of Pharmacy, Southwest Medical University in China. Male Balb/c nude mice, aged 4–6 weeks and weighing 20 ± 2 g, were purchased from Gempharmatech Co., Ltd. Certificate of Conformity No. SYXK (Chuan) 2023–034. The animals were reared and tested in accordance with the Ethical Guidelines for the Welfare of Experimental Animals of Southwest Medical University and approved by the Institutional Animal Care and Ethics Committee of Southwest Medical University (License No. 20220624–009). To establish a xenograft colorectal cancer mouse model, 15 × 105 HCT116 cells were injected into the right axillary subcutis of nude mice.
2.3 Preparation and characterization of ATL-1/Ce6-pH Lip
To prepare ATL-1/Ce6-pH Lip, we mixed the lipid material, Ce6, and ATL-1 in dichloromethane (5 mL) at a mass ratio of 7:1:3:1. The mixed solution was evaporated on a rotary evaporator at 45°C to form a lipid film. The lipid film was then rehydrated in PBS (10 mL) for 10 min and sonicated for 2 min with a probe of 65 W , All preparations were filtered through a 220 nm membrane prior to the experiment to remove unencapsulated drug.
Particle size and zeta potential were measured using dynamic light scattering (Nano ZS 90, Malvern, Malvern city, United Kingdom). The samples were diluted to 2 mg/mL with PBS, dispersed onto a copper grid, stained with 10 μL of 1% (v/v) phosphotungstic acid in PBS, and then dried in air before being evaluated for surface topography, size, and morphology using a transmission electron microscope (JEM-200CX, JEOL, Tokyo, Japan).
In order to obtain the irradiation conditions for the administration of ATL-1/Ce6-pH Lip, we placed different concentrations of ATL-1/Ce6-pH Lip under an 808 nm laser with a power of 1.0 W/cm3 and determined the optimal experimental conditions by comparing the heating of the preparation after irradiation for different times.
2.4 Drug loading efficiency (DLE)
The drug encapsulation efficiency (DEE) is the percentage of drug encapsulated in the lipid bilayer of liposomes, relative to the total drug delivery. This rate is used to evaluate the quality of liposomes and to screen for the optimal formulations and processes. It is a key indicator of the effectiveness of drug encapsulation in liposomes. On the other hand, drug loading efficiency (DLE) is used to assess the quality of liposomes, which also facilitates the formulation and the processing optimization. The DLE and DEE of ATL-1/Ce6-pH Lip were determined using the centrifugation method (1 × 104 r/min, 20 min). The 1 mL of the supernatant was precisely extracted and adjusted to 10 mL. The peak area was obtained by injecting and measuring the samples, and the concentration of free ATL-1 was calculated as c. The volume of ATL-1/Ce6-pH Lip was recorded as V. Then, the mass of free ATL-1 was calculated as W = c × V × dilution, and the mass of ATL-1 was known to be M. The concentration of ATL-1 was determined using high-performance liquid chromatography with a mobile phase of acetonitrile and 0.2% phosphate buffer (80:20, v/v) and a measurement wavelength of 274 nm. DLE and DEE were calculated as follows:
2.5 In vitro drug release investigation
The dialysis method was used to assay the release of ATL-1 from ATL-1/Ce6-pH Lip in vitro. ATL-1/Ce6-pH Lip (2 mL, 0.5 mg/mL of ATL-1) in PBS was placed in a dialysis bag with a molecular weight cut-off of 10 kDa (Shanghai Titan Technology), which was immersed in 20 mL of PBS (pH adjusted to 7.4, 6.5, 5.0) containing 0.5% Tween 80 with stirring (100 rpm). At the indicated time points, an aliquot (100 µL) was withdrawn and replaced with the same volume of fresh PBS. The aliquot was assayed for ATL-1 using high-performance liquid chromatography, and the cumulative amount of drug released was calculated.
2.6 Stability evaluation of liposome
The stability of freshly prepared ATL-1/Ce6-pH Lip was investigated by dynamic light scattering respectively in PBS, distilled water, FBS and after storage at 4 °C for 1 week in the untreated state.
2.7 Cellular uptake study
Confocal Laser Scanning Microscope (CLSM, TCS SP2, Leica) and flow cytometer (FCM, Becton Dickinson, Franklin Lakes, NJ, United States) were used to observe the uptake of ATL-1/Ce6-pH Lip by HCT116 cells. HCT116 cells were first cultured overnight in 24-well plates (5× 104 cells per well) placed with coverslips. Subsequently, cells were exposed to ATL-1/Ce6-pH Lip (808 nm), free Ce6 (808 nm) and incubated for 2 h. In all case, the Ce6 concentration was 0.1 mg/mL. After being washed 2–3 times with PBS, the cells were fixed with 4% paraformaldehyde for 30 min and stained with DAPI (blue) for 5 min. The intracellular localization and cell internalization were visualized using CLSM. For flow cytometry, the cells were collected and resuspended after being washed with PBS, and the uptake of cells was measured by FCM.
2.8 In vitro cytotoxicity efficacy
The cytotoxicity of ATL-1 and PDT was investigated with CCK-8 assay on HCT116 cells, which were cultured in 96-well plates (8,000 cells per well) and incubated in different concentrations of ATL-1, ATL-1 Lip, Ce6 (light/no light), Ce6 Lip (light/no light), or ATL-1/Ce6-pH Lip (light/no light). Next, cells were irradiated with laser at 808 nm at 1.0 W/cm3 for 5 min. The IC50 value was obtained to evaluate the synergistic effect of the ATL-1/Ce6-pH Lip treatment.
2.9 Assessment of the ROS generation ability
HCT116 cells were seeded in 12-well plates (10 × 104 cells per well) and placed in an incubator overnight to acquire complete adhesion of the cells to the wall. Respectively, different groups were set up, that is the ATL-1 group, ATL-1 Lip group, Ce6 (light/no light) group, Ce6 Lip (light/no light) group and ATL-1/Ce6-pH Lip (light/no light) group. The light group was exposed to light (808 nm, 5 min, 1.0 W/cm3) after 4 h of incubation. Subsequently, the cells were incubated in a constant temperature incubator in the dark for 12 h. The 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) working solution (2 µM) was prepared before use. The 12-well plates were removed, 1 mL of working solution was added to each well after cleaning and incubated in a constant temperature cell incubator at 37°C in the dark for 30 min. The working solution was removed by suction, the cells were washed 2–3 times with PBS, and the intracellular fluorescence was observed under an inverted microscope.
2.10 Live/dead cell staining assay, and apoptosis evaluation by flow cytometry
To observe the anti-tumor effect of ATL-1/Ce6-pH Lip, HCT116 cells were cultured respectively in 12-well plates (10 × 104 cells per well) with ATL-1, ATL-1 Lip, Ce6 (light/no light), Ce6 Lip (light/no light), ATL-1/Ce6-pH Lip (light/no light), respectively. After illumination and culture according to the method under “2.9”, 1 mL of working solution containing Calcein-AM (1 µM) and propidium iodide (PI, 5 µM) was added respectively. After incubation, the cell survival rates in each group were compared by fluorescence microscopy. The in vitro properties of ATL-1/Ce6-pH Lip were further investigated.
2.11 Western blotting analysis
HCT116 cells were seeded into 6-well plates (10 × 104 cells per well), incubated for 24 h, then treated for 24 h with various drug formulations. As a control, HCT116 cells were cultured alone and treated the same way. Cells were harvested, lysed with radio-immunoprecipitation assay (RIPA) buffer, microcentrifuged to pellet debris, and the supernatant was fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were incubated 12 h with primary rabbit antibodies against Bcl-2 or caspase 3, followed by incubation for 1 h at 25°C with goat anti-rabbit secondary antibody conjugated to horseradish peroxidase. Antibody binding was detected using enhanced chemiluminescence (Image Quant LAS 4000 Mini; Fuji, Tokyo, Japan) and quantified using Gel Image System 4.00 (Tanon, Shanghai, China).
2.12 In vivo dynamic biodistribution
HCT116 cells were subcutaneously injected into the right armpit of nude mice, and the tumor volumes (length × width2/2) were measured by caliper. When the tumor volume reached about 50 cm3, free Ce6 and ATL-1/Ce6-pH Lip were injected into the tail vein of the mice. In vivo imaging (IVIS Spectrum, Perkin Elmer, United States) was used to observe the biodistribution and tumor targeting of the latter two in nude mice at different time points.
2.13 In vivo antitumor effect
Given the excellent chemical activity of ATL-1/Ce6-pH Lip in vitro and its good pharmacokinetics and biodistribution in vivo, we further tested its anti-tumor activity in an ectopic tumor bearing mouse model. After subcutaneous injection of HCT116 cells into the right armpit of nude mice, the tumor volumes (length × width 2/2) were measured using calipers. When the tumor volume reached approximately 50 cm3, the mice were randomly divided into eight groups (blank group, ATL-1 group, ATL-1 Lip group, Ce6 (808 nm) group, Ce6 Lip (808 nm) group, ATL-1/Ce6-pH Lip (light/no light) group) and injected into the tail vein every other day. Six consecutive doses were administered. At the end of the 12th h, the intra-tumor ATL-1/Ce6-pH Lip accumulation peaked, so the ATL-1/Ce6-pH Lip illumination group was laser irradiated at the subcutaneous tumor site 12 h after intravenous injection. After the last administration, the mice were executed, and the tumor tissues were removed, weighed and photographed. Five tissues were collected, and H&E staining was used to examine the histopathological characteristics of the tissues and tumor sections. The cell apoptosis and tumor proliferation were observed by TUNEL and Ki67 immunochemical staining.
2.14 Statistical analysis
The data were recorded as mean ± standard deviation (SD) and analyzed using GraphPadPrism 8.0.1. The pairwise differences were assessed using the two-tailed Student’s t-test, while the differences among the three or more groups were assessed using one-way analysis of variance.
3 Results
3.1 Preparation and characterization of ATL-1/Ce6-pH Lip
Prolonging blood circulation is an important way to make the intravenous nanomedicine more effective (Li et al., 2020). Researches have indicated that nanoparticles (NPs) with a diameter over 200 nm are susceptible to clearance by monocytes and the reticuloendothelial system. Conversely, NPs with smaller particle sizes (<200 nm) exhibit enhanced drug accumulation in tumor cells through augmented permeability and prolonged retention (Xu et al., 2023a; Blanco et al., 2015). DLS showed that the diameter of ATL-1/Ce6-pH Lip was 159.53 ± 19.55 nm (n = 3) (Figure 1A). The zeta potential of ATL-1/Ce6-pH Lip was −40.79 ± 3.06 mV (n = 3), and PDI was 0.213 ± 0.013 (n = 3). This suggests that ATL-1/Ce6-pH Lip is monodispersed and has a suitable diameter for tissue extravasation. As confirmed by TEM images, the morphology of ATL-1/Ce6-pH Lip was homogeneous and spherical, with diameters ranging from 50 to 100 nm, and showed aggregation or slight enlargement due to drug encapsulation compared to naked liposomes. This was in good agreement with the DLS results (Figures 1B,C), indicating that ATL-1/Ce6-pH Lip has a good morphological size. The DEE of ATL-1 in ATL-1/Ce6-pH Lip was 94.37% ± 0.58% (n = 3) as determined by centrifugation, and the DLE was 23.2% ± 0.1%.
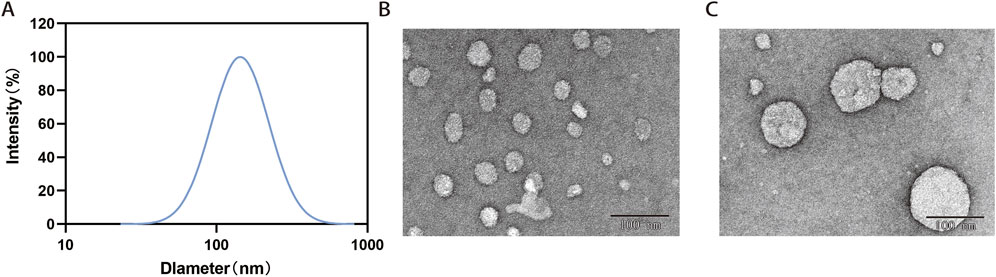
Figure 1. Characterization of ATL-1/Ce6-pH Lip. (A) Particle size distribution. (B) Bare liposomes. (C) Transmission electron micrographs. Scale bar, 100 μm.
It has been shown that a spherical supramolecular structure with a uniform size distribution contributes to the stability of ATL-1/Ce6-pH Lip. The changes in size and Zeta potential of ATL-1/Ce6-pH Lip were assessed through dynamic light scattering studies after 1 week under various solvent conditions, as depicted in Figure (Figures 2A,B). It was observed that there were no significant alterations in the size and Zeta potential of ATL-1/Ce6-pH Lip, indicating its excellent stability. The release of ATL-1 in vitro by ATL-1/Ce6-pH Lip (light/no light) in the pH releasing medium that mimicked the normal physiological environment, tumor environment, and lysosomal environment is shown (Figure 2C). By comparing the amount of drug released at the same time point, it can be seen that the preparation released steadily under the condition of pH = 5.0, which prove that the nano-preparation has sensitive pH responsiveness. Under the light condition, ATL-1/Ce6-pH Lip had a larger initial rate and a faster release rate. After 12 h, the release of ATL-1 tended to be stable, and at the last 72 h, the maximum release of ATL-1/Ce6-pH Lip was 95.17% ± 4.40% (n = 3) at pH 5.0 after light exposure.
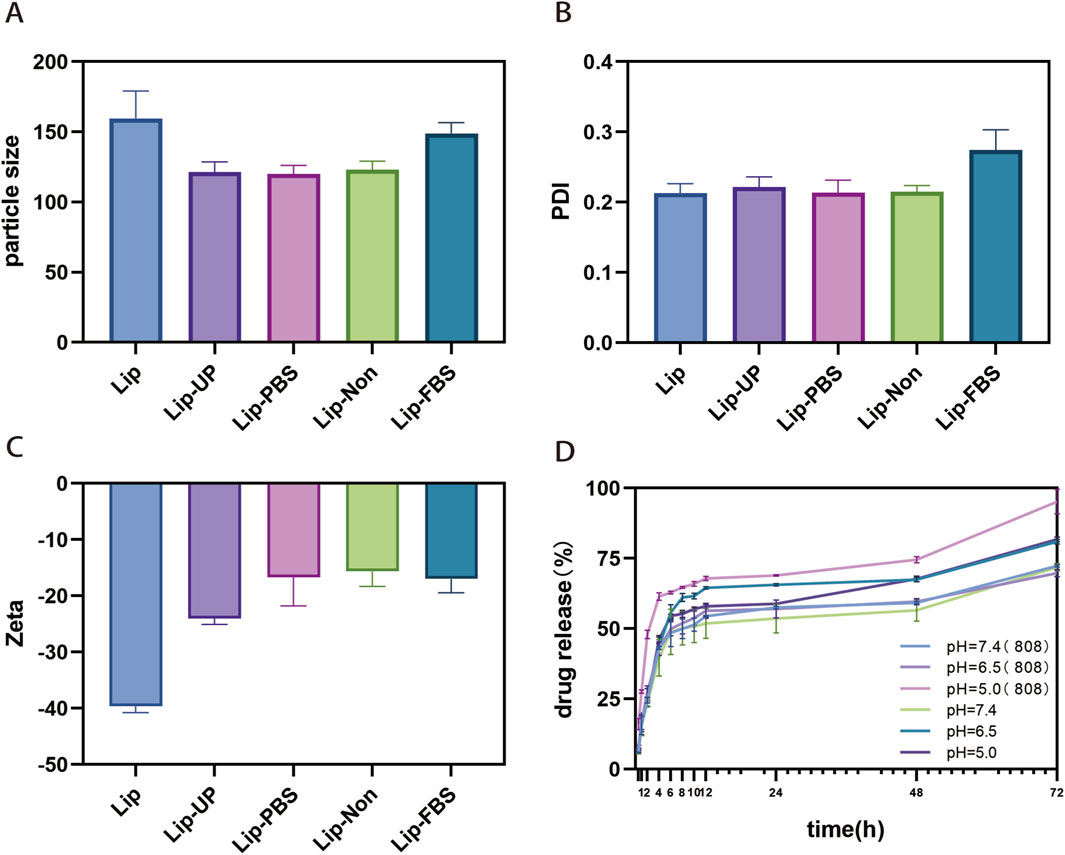
Figure 2. Characterization of ATL-1/Ce6-pH Lip. (A) Stability of ATL-1/Ce6-pH Lip at 4°C in PBS, distilled water or FBS, based on average particle diameter (“particle size”); (n = 3). (B) Stability of ATL-1/Ce6-pH Lip at 4°C in PBS, distilled water or FBS, based on polydispersity index (PDI). (n = 3). (C) Stability of ATL-1/Ce6-pH Lip at 4°C in PBS, distilled water or FBS, based on Zeta potential (Zeta). (n = 3). (D) The release of ATL-1 at pH 5.0, 6.5, 7.4 (light/no light). (n = 3).
After irradiating the drug formulations using 808 nm laser, the warming of the different groups is shown in Figure 3, where the PBS group only warmed up to 10.1°C, whereas ATL-1/Ce6-pH Lip at a concentration of 20 μg/mL Ce6 reached a temperature of 49.2°C after irradiation for 5 min. Considering the acclimatization of the mice, the final light condition was determined to be 1.0 W/cm3 for 5 min (Figures 3A,B).
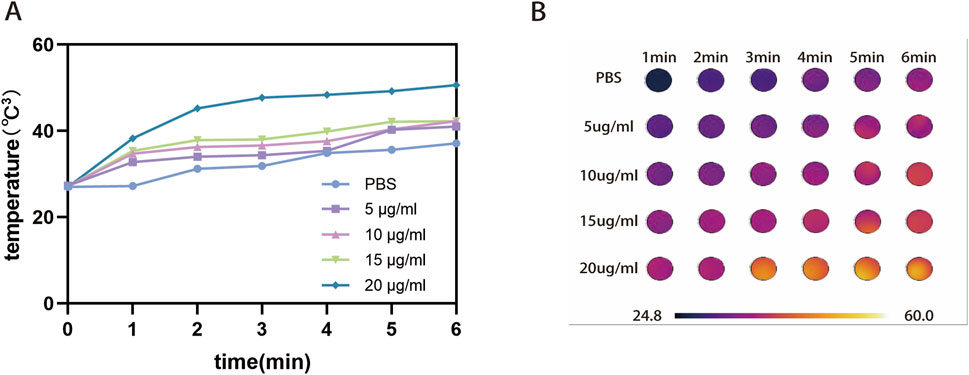
Figure 3. Heating of pharmaceutical preparations by light irradiation. (A) Line graph of warming trend for PBS and different concentrations of ATL-1/Ce6-pH Lip (5, 10, 15, 20 μg/mL). (B) Temperature graph of warming trend for PBS and different concentrations of ATL-1/Ce6-pH Lip (5, 10, 15, 20 μg/mL).
3.2 Cellular uptake
Since Ce6 itself is fluorescent, its fluorescence was used to follow the internalization of nanoparticles by CLSM and FCM in HCT116 cells (λex = 633 nm, λem = 650–710 nm) (Gilson et al., 2019; Tan et al., 2016). First, we examined the cellular uptake capacity of ATL-1/Ce6-pH Lip using CLSM, as shown in Figure 4A, where a clear red fluorescence was detected in the cytoplasm after 2 h of incubation. FCM results showed that ATL-1/Ce6-pH Lip was efficiently taken up by HCT116 cells, and the uptake of ATL-1/Ce6-pH Lip was significantly higher than that of the free Ce6 (Figure 4B).
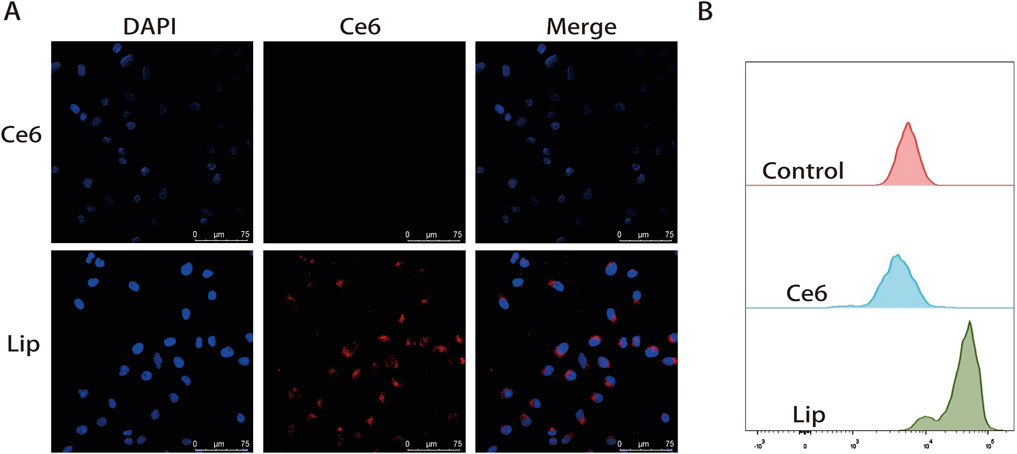
Figure 4. Uptake of drug formulations by HCT116 tumor cells. (A) Confocal micrographs showing uptake of drug formulations by HCT116 cells cultured alone. Scale bar, 50 μm. (B) FCM image of Ce6 fluorescence intensity.
3.3 Effect of NPs combined with PDT on HCT116
The chemo-PDT cytotoxicity of ATL-1/Ce6-pH Lip was determined by CCK-8 assay. The IC50 of blank group, ATL-1 group, ATL-1 Lip group, Ce6 (808 nm) group, Ce6 Lip (808 nm) group, ATL-1/Ce6-pH Lip (light/no light) group at 24 h were 51.05, 27.91, 36.84, 32.41, 17.46, 15.84 (µg/mL), respectively (Figures 5A,B). It can be seen from the figure that after 24 h of incubation, the anticancer activity of ATL-1/Ce6-pH Lip+808 nm laser irradiation group was the strongest followed by the ATL-1/Ce6-pH Lip (no light) group, ATL-1 Lip group, Ce6 Lip (808 nm) group, Ce6 (808 nm) group and ATL-1 group. In addition, a concentration-dependent manner was observed in the preparation groups, indicating that ATL-1/Ce6-pH Lip exhibited excellent anticancer activity.
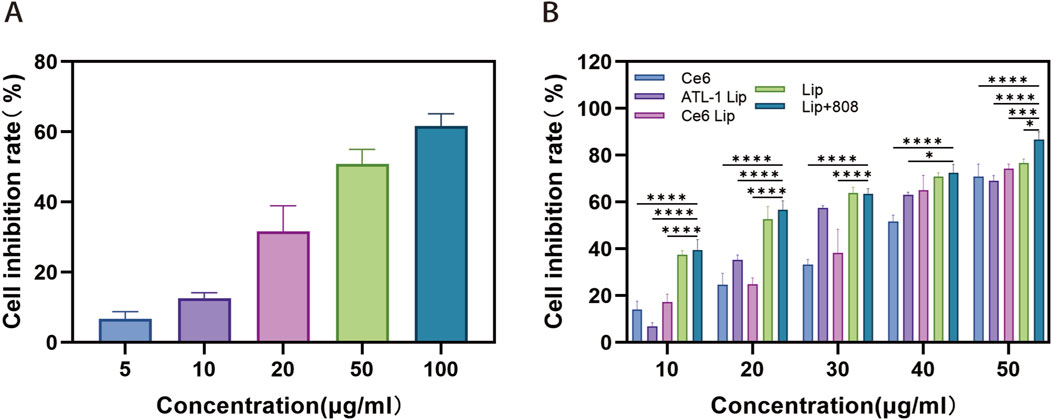
Figure 5. Cytotoxicity of different drug formulations against HCT116 tumor cells. (A,B) Cytotoxicity was assessed using the Cell Counting Kit-8 assay with HCT116 tumor cells cultured with (A) ATL–1, (B) ATL–1 Lip, Ce6 (808 nm), Ce6 Lip (808 nm), Lip or Lip (808 nm). Data are mean ± SD (n = 5). ****p < 0.0001, ***p < 0.001, *p < 0.05.
3.4 Assessment of the ROS generation ability
The extent of ROS generated after 808 nm laser irradiation induced by ATL-1/Ce6-pH Lip was assessed using the DCFH-DA in PBS solution. The results demonstrated that ATL-1 Lip and ATL-1/ Ce6-pH Lip under 808 nm laser irradiation produced strong green fluorescence, suggesting a greater ROS production. However, the control group, ATL-1 group, Ce6 (808 m) group, Ce6 Lip (808 nm) group, and ATL-1/Ce6-pH Lip (no light) group failed to exhibit a significant green fluorescence. The results indicated that ATL-1 was capable of inducing the generation of ROS, and under 808 nm laser irradiation, ATL-1/Ce6-pH Lip was able to produce a higher amount of ROS, thus exerting an inhibitory effect (Figure 6A). Figure 6B shows the results of semi-quantitative analysis of the mean fluorescence intensity of the different drug formulation groups with the following formula:
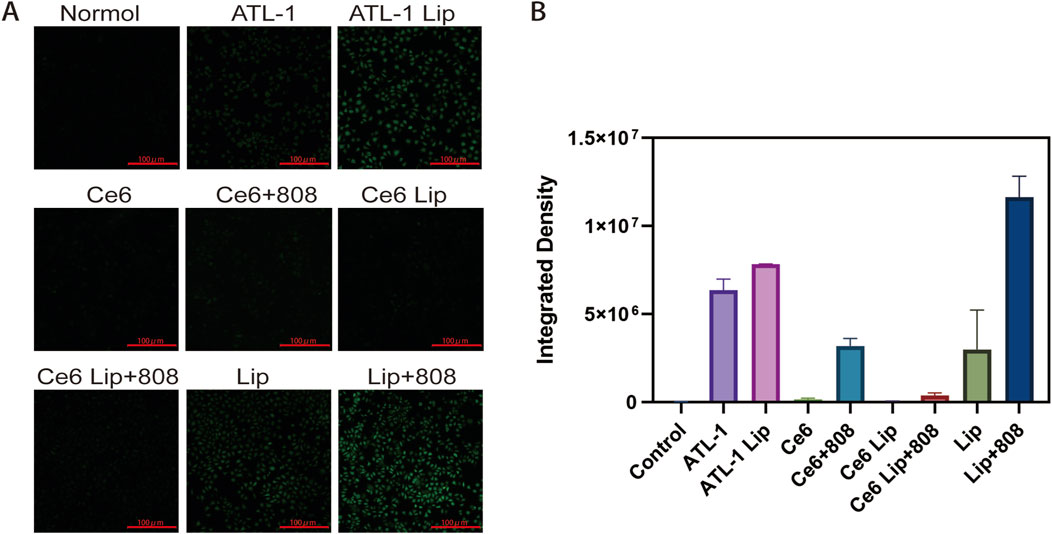
Figure 6. Effects of drug formulations on ROS generation ability. Cultures were exposed to the formulations described in Figure 5. “Control” cultures were not exposed to any formulation. (A) HCT116 cells were cultured alone in the presence of 2′,7′- dichlorodihydrofluorescein diacetate, which is converted by reactive oxygen species into a green fluorescent product. Representative micrographs. Scale bar, 100 μm. (B) Mean fluorescence intensity (Mean), n = 3.
3.5 Live/dead cell staining assay
As shown in Figure 7, the ATL-1/Ce6-pH Lip +808 nm group had more red fluorescence (that is, dead cells) and less green fluorescence (that is, surviving cells). The intensity of red fluorescence in each treatment group was in the order of: ATL-1/Ce6-pH Lip (808) group > ATL-1/Ce6-pH Lip group > Ce6 Lip (808 nm) group > ATL-1 Lip group > Ce6 (808 nm) group > Ce6 Lip group > Ce6 group > ATL-1 group > blank group. In the control group, more obvious green fluorescence was observed, without red fluorescence. The results showed that ATL-1/Ce6-pH Lip had a significant anticancer effect when irradiated with 808 nm laser. The anti-tumor activity of the groups treated with ATL-1 and Ce6 alone was remarkably less than that of ATL-1/Ce6-pH Lip after illumination, indicating that the drug of this preparation has a strong synergistic effect with PDT.
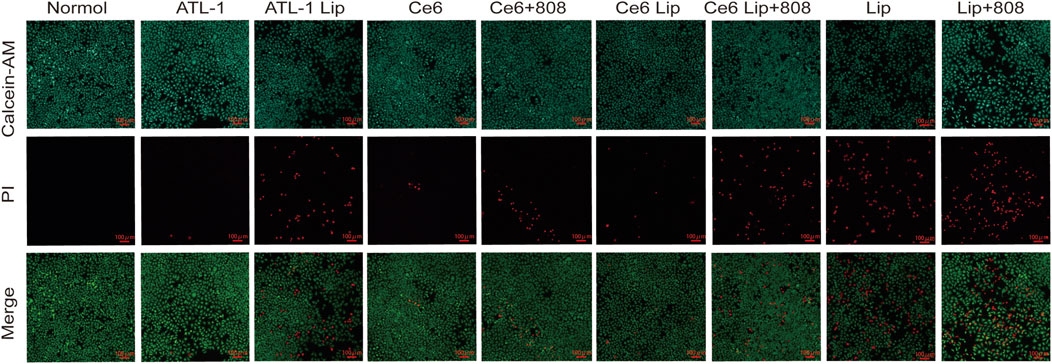
Figure 7. Cytotoxicity in tumor cells in vitro. Cytotoxicity against HCT116 monocultures was assessed through staining with calcein-AM and propidium iodide. Live cells appear green; dead cells, red. Scale bar, 100 μm, n = 3.
3.6 Western blotting analysis
Bcl-2 is one of the key proteins in the regulation of cell apoptosis, whose high expression inhibits apoptosis and accelerates cell growth, which in turn leads to the development of malignant tumors, and thus is closely related to the generation, development and treatment of tumors. In contrast, caspase 3, a apoptosis indicator of cellular function, belongs to the critical execution proteins downstream of apoptosis. Both the cellular Bcl-2 and Caspase 3 protein expression levels were detected by Western blot method after treatment of HCT116 cells with different preparations (Figure 8A). Semi-quantitative results showed that ATL-1/Ce6-pH Lip group could effectively inhibit Bcl-2 protein expression and upregulate Caspase 3 protein expression, thus displaying anti-tumor effects, further verifying the cytotoxicity results of CCK-8 (Figures 8B,C). The results demonstrated that ATL-1/Ce6-pH Lip was able to reduce the expression of anti-apoptotic protein Bcl-2 and increase the expression of pro-apoptotic protein Caspase 3.
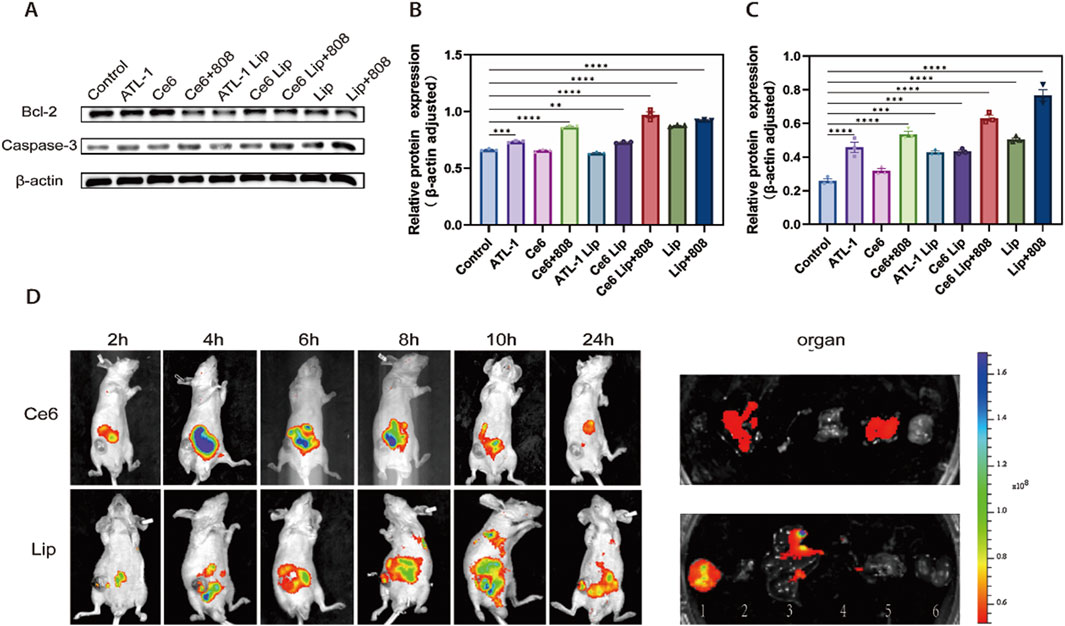
Figure 8. The results of Western blotting and dynamic biodistribution in vivo. (A) Western blotting against Bcl-2 and caspase-3 in total soluble protein fractions. Cultures were exposed to the formulations described in Figure 5. (B) Quantitative analysis of Bcl-2. (C) Quantitative analysis of caspase-3. (D) Inject HCT116 cells into the right armpit of nude mice; after tumors attained adequate size, the animals were systemically injected with indicated Ce6 or ATL-1/Ce6-pH Lip. Representative fluorescence images in 24 h, and the fluorescence of major organs (1: tumor; 2: heart; 3: liver; 4: spleen; 5: lungs; 6: kidney.). ****p < 0.0001, ***p < 0.001, **p < 0.01, n = 3.
3.7 In vivo dynamic biodistribution
The accumulation of nanoparticles in the tumor will affect the final therapeutic effect. The biodistribution of ATL-1/Ce6-pH Lip and free Ce6 in Babl/c nude mice carrying ectopic colorectal cancer can be directly determined by the in vivo imaging technology (shown in Figure 8D) ATL-1/Ce6-pH Lip peaked at 12 h in mice, and ATL-1/Ce6-pH Lip sticked in tumors after 48 h. Meanwhile, ex vivo imaging and semi-quantitative analysis of major organs also showed that ATL-1/Ce6-pH Lip fluorescence was significantly stronger than that of the Ce6, and these results indicated that ATL-1/Ce6-pH Lip had a significant tumor accumulation and retention effect with a wide therapeutic window.
3.8 In vivo anti-tumor effect
The results above demonstrated that ATL-1/Ce6-pH Lip showed good accumulation and permeability in tumor, and therefore, we performed experiments to evaluate the combined treatment effect of ATL-1/Ce6-pH Lip in HCT116 mice. The result of anti-tumor experiments in vivo showed that compared with the blank group, the other groups all had anti-tumor effects, with the ATL-1/Ce6-pH Lip+808 group displaying the strongest anti-tumor effect (Figure 9A). After drug administration and light irradiation, the tumor volume was inhibited to 174.63 mm3 (n = 6), and the treatment effect of ATL-1@pH Lip and Ce6@pH Lip groups was better than that of the free ATL-1 and the free Ce6 groups. This finding suggested that the formulation had significant tumor targeting ability. In addition, the tumor volume of the ATL-1/Ce6-pH Lip+808 group was 0.18 times that of the blank group, showing a notable tumor inhibition effect (Figure 9B). During the administration period, no significant weight loss was observed in each experimental group compared with the blank group, indicating that ATL-1/Ce6-pH Lip had better biological safety (Figure 9C). These data suggested that the combination of chemotherapy and phototherapy of ATL-1/Ce6-pH Lip had prominent anti-tumor ability and presented a greater therapeutic advantage than free drugs with individual treatment approaches.
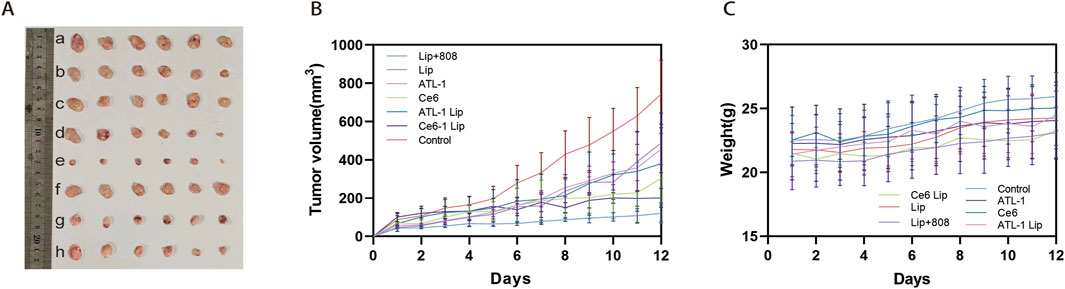
Figure 9. Antitumor efficacy of drug formulations in vivo. HCT116 cells were injected into the right axilla of nude mice; after the tumors had reached the appropriate size, the animals were given systemic injections of the drug formulations described in Figure 5. “Control” animals were injected with PBS. (A) Photographs of tumors excised on day 12 after injection. (B) Tumor growth curve. (C) Mouse body weight curve. n = 5.
Biological safety was evaluated by blood biochemical analysis of Balb/c nude mice and H&E examination of normal organs of mice in different experimental groups. The results showed that there was no necrosis, apoptosis or inflammation of the cells in the major organs after treatment with the nanopreparations, and the nuclei were largely fusiform or spherical without apparent changes, exibiting favorable biocompatibility (Figure 10). Additionally, the damage in the tumor showed that the ATL-1/Ce6-pH Lip+808 group was the most serious. Secondly, the expression of Ki-67 was in the following order: control, ATL-1/Ce6-pH Lip (808 nm), ATL-1/Ce6-pH Lip, ATL-1 Lip, Ce6 Lip, ATL-1, and Ce6, which were consistent with the anti-tumor effects described above in vivo (Figure 10A). TUNEL results further confirmed the anti-tumor effect of ATL-1/Ce6-pH Lip (808 nm), which showed the highest degree of apoptosis (Figure 11B).
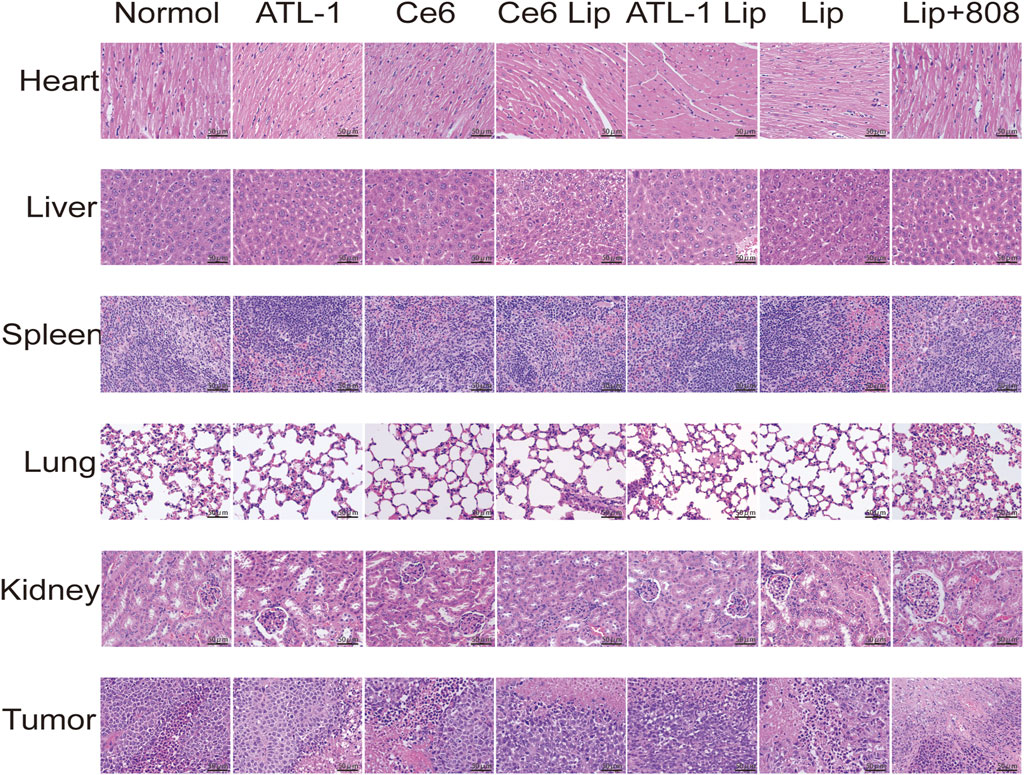
Figure 10. Safety evaluation of drug formulations in vivo. H&E staining of organ and tumor tissue sections of mice on day 12 after injection. Scale bar, 50 μm.
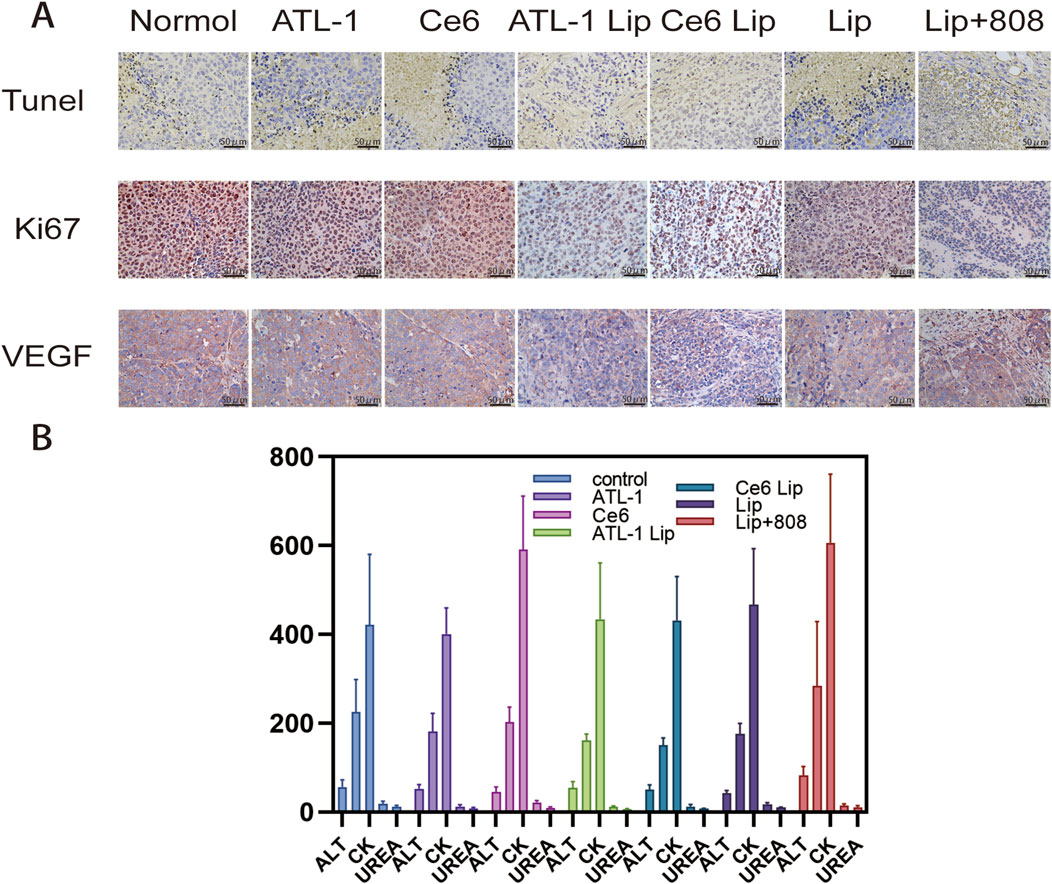
Figure 11. Mechanism of action studies and serum indices of drug preparations in vivo. (A) (1) TUNEL staining of tumor tissue sections on day 12 after injection. Scale bar, 400 μm. (2) Expression of Ki67 and VEGF in tumor tissue sections on the 12th day after injection. Scale bar, 40 μm. (B) Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CREA), uridylyltransferase (UREA), and creatine kinase (CK) on day 12 after injection.
Finally, the results of blood physiological and biochemical parameters showed that alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CREA), UREA and creatine kinase (CK) of the mice treated with ATL-1/Ce6-pH Lip+808 were not significantly different from those of the PBS-treated mice (Figure 1B).
4 Discussion
CRC is one of the most common human malignant tumors in the digestive tract with high mortality. Current therapies for cancer, such as transplantation, minimally invasive, local excision, and molecularly targeted therapies, are unable to completely inhibit the growth and metastasis of tumor cells, so there is an urgent need for research, development, and innovation of new agents to counteract it (Dey et al., 2025).
In recent years, it has been found that natural products of traditional Chinese medicines are highly effective and low toxicity, thus have been included in the development of novel antitumor drugs. ATL -1 is one of the compound variants extracted from the traditional Chinese medicine Atractylodes macrocephala. In previous studies, ATL-1 was found to have various anti-tumor activities, such as downregulation of CTGF expression in fibroblasts, activation of the MAPK-JNK/c-Jun signaling pathway, and inhibition of the EPAS1 pathway (Ren et al., 2021; Wang et al., 2021; Li et al., 2024). In addition, it was also found that ATL-1 can disrupt tumor cell glucose metabolism and produce excess oxygen through the regulation of AKT/mTOR pathway, which shows that ATL-1 has the potential to be used for molecularly targeted cancer therapy (Wang et al., 2020; Xie et al., 2024). At the same time, this reminds us of PDT, a photodynamic therapy that has received much attention in recent years (Modi et al., 2025). PDT has been approved for the treatment of certain cancers and precancerous lesions due to its high spatiotemporal selectivity, low invasiveness and low systemic toxicity (Huis In 't Veld et al., 2023). However, the therapeutic efficacy of PDT itself is limited by the fact that it exacerbates hypoxia in the tumor microenvironment by consuming oxygen to generate highly reactive singlet oxygen and thereby producing cytotoxicity (Shabnum et al., 2024). Therefore, we hypothesized that co-administration of ATL-1 and PDT might improve the therapeutic effect of PDT on the basis of the original tumor growth inhibition of ATL-1. At the same time, PDT is able to destroy tumor tissue, exposing more of the drug to the tumor environment and helping to address challenges such as deep-seated tumors, metastasis or recurrence.
In addition, we also considered that both ATL-1 and PS have poor water solubility, low permeability, and low bioavailability for direct drug delivery, so we chose the commonly used nanoformulations as drug carriers. It is well known that the acidic microenvironment induced by massive anaerobic glycolysis is one of the important features of malignant tumors, and with the in-depth study of acidic TEM, it becomes a new target for tumor-targeted drug delivery nano-formulations, which is of great significance for the design of pH-responsive nano-formulations (Yang et al., 2021).
In summary, the ATL-1/Ce6-pH Lip formulation exhibited encouraging antitumor activity, thereby establishing a novel, viable approach for the treatment of additional tumors. Nevertheless, the study is not without its limitations. First of all, in the initial phase of large-scale liposome production, there is a necessity to optimize the parameters to ensure stability between batches. This optimization should address the issues of particle size growth and drug precipitation during storage (Yu et al., 2021). Furthermore, there is a need to investigate the potential chronic toxicity of long-term repeated use of the drug. Following these investigations, there is ample opportunity for research in subsequent clinical translation. Furthermore, the antitumor activity of ATL-1/Ce6-pH Lip was investigated in this study through a mouse model of ectopic colon cancer, but its efficacy in situ colon cancer needs to be further investigated due to the problems of light source penetration depth limitation, immunosuppressive microenvironment, high pressure in colon cancer mesenchyme, and dense extracellular matrix (ECM) that significantly limits drug penetration. In conclusion, we found that the combination of ATL-1 and PDT has good anti-tumor activity, but this is only the result of the comprehensive comparison, in the subsequent more in-depth study, we will use network pharmacology to analyze the interaction between the two, to further clarify the key targets and core pathways, and ultimately to verify the synergistic mechanism through experiments.
5 Conclusion
In summary, we have successfully developed an efficient and multifunctional nano-carrier that integrates drug therapy with photodynamic therapy. The ATL-1/Ce6-pH Lip nanosystem had optimal particle size, zeta potential, PDI, encapsulation efficiency, and anti-tumor activity. Specifically released in an acidic environment, ATL-1/Ce6-pH Lip demonstrated excellent cytotoxicity in vitro. Significant cellular uptake and ROS generation were also observed by direct in vitro assays, which were able to significantly enhance the inhibitory effect of PDT (Figure 12).
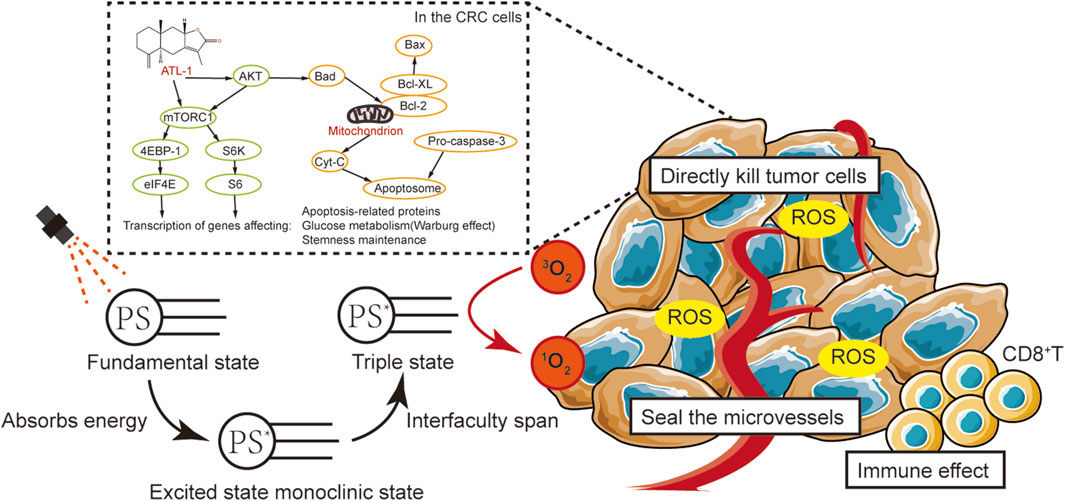
Figure 12. Summary of results of this study: The aim of this study was to preliminarily explore the effects of ATL-1/Ce6-pH Lip on the behavior of CRC and its related mechanisms. We preliminarily hypothesized that ATL-1 is able to affect tumor cells by inhibiting the activation of the AKT/mTOR signaling pathway, by promoting apoptosis, and by altering glucose metabolism (inhibiting the Warburg effect). This enables ATL-1/Ce6-pH Lip to improve the therapeutic efficacy of PDT by increasing the oxygen content at the tumor site.
In addition, the synergistic therapeutic effect and tumor suppression of ATL-1/Ce6-pH Lip was also evident despite the more complex biodistribution or clearance and tumor hypoxic microenvironment in the in vivo studies. These properties rendered the ATL-1/Ce6-pH Lip a promising carrier for combined drug therapy and photodynamic therapy of cancer cells, offering novel insights into the treatment modality of colorectal cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Experimental Animal Ethics Committee of Southwest Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YZ: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. QY: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review and editing. SF: Data curation, Writing – original draft. XF: Investigation, Visualization, Writing – review and editing. SC: Resources, Supervision, Writing – review and editing. HL: Software, Writing – review and editing. JN: Supervision, Validation, Writing – original draft. YL: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review and editing. DZ: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the State Key Laboratory of Southwestern Chinese Medicine Resources under [Grant number SKLTCM202309], the key research and development project fund of social development of luzhou Science and technology Bureau under [Grant number 2022-SYF-56] and The Student’s Platform for innovation and entrepreneurship training Program under [Grant number 202310632046].
Acknowledgments
We gratefully acknowledge technical support by the Public Platform of Advanced Detecting Instruments, Public Center of Experimental Technology, Southwest Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bailly, C. (2021). Atractylenolides, essential components of Atractylodes-based traditional herbal medicines: antioxidant, anti-inflammatory and anticancer properties. Eur. J. Pharmacol. 891, 173735. doi:10.1016/j.ejphar.2020.173735
Blanco, E., Shen, H., and Ferrari, M. (2015). Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951. doi:10.1038/nbt.3330
Choi, J., Sun, I. C., Sook Hwang, H., Yeol Yoon, H., and Kim, K. (2022). Light-triggered photodynamic nanomedicines for overcoming localized therapeutic efficacy in cancer treatment. Adv. Drug Deliv. Rev. 186, 114344. doi:10.1016/j.addr.2022.114344
Da Silva, F. L. O., Marques, M. B. F., Kato, K. C., and Carneiro, G. (2020). Nanonization techniques to overcome poor water-solubility with drugs. Expert Opin. Drug Discov. 15, 853–864. doi:10.1080/17460441.2020.1750591
Dey, S., Ghosh, M., and Dev, A. (2025). Signalling and molecular pathways, overexpressed receptors of colorectal cancer and effective therapeutic targeting using biogenic silver nanoparticles. Gene 936, 149099. doi:10.1016/j.gene.2024.149099
Dinakaran, D., and Wilson, B. C. (2023). The use of nanomaterials in advancing photodynamic therapy (PDT) for deep-seated tumors and synergy with radiotherapy. Front. Bioeng. Biotechnol. 11, 1250804. doi:10.3389/fbioe.2023.1250804
Fukai, T., and Ushio-Fukai, M. (2020). Cross-talk between NADPH oxidase and mitochondria: role in ROS signaling and angiogenesis. Cells 9, 1849. doi:10.3390/cells9081849
Ge, H., Xu, C., Chen, H., Liu, L., Zhang, L., Wu, C., et al. (2022). Traditional Chinese medicines as effective reversals of epithelial-mesenchymal transition induced-metastasis of colorectal cancer: molecular targets and mechanisms. Front. Pharmacol. 13, 842295. doi:10.3389/fphar.2022.842295
Gilson, R. C., Tang, R., Gautam, K. S., Grabowska, D., and Achilefu, S. (2019). Trafficking of a single photosensitizing molecule to different intracellular organelles demonstrates effective hydroxyl radical-mediated photodynamic therapy in the endoplasmic reticulum. Bioconjug Chem. 30, 1451–1458. doi:10.1021/acs.bioconjchem.9b00192
Gunaydin, G., Gedik, M. E., and Ayan, S. (2021). Photodynamic therapy-current limitations and novel approaches. Front. Chem. 9, 691697. doi:10.3389/fchem.2021.691697
Gyanani, V., and Goswami, R. (2023). Key design features of lipid nanoparticles and electrostatic charge-based lipid nanoparticle targeting. Pharmaceutics 15, 1184. doi:10.3390/pharmaceutics15041184
Hak, A., Ali, M. S., Sankaranarayanan, S. A., Shinde, V. R., and Rengan, A. K. (2023). Chlorin e6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 6, 349–364. doi:10.1021/acsabm.2c00891
Huis In 'T Veld, R. V., Heuts, J., Ma, S., Cruz, L. J., Ossendorp, F. A., and Jager, M. J. (2023). Current challenges and opportunities of photodynamic therapy against cancer. Pharmaceutics 15, 330. doi:10.3390/pharmaceutics15020330
Jia, W., Jin, B., Xu, W., Liu, S., Mao, X., Peng, H., et al. (2023). pH-responsive and actively targeted metal-organic framework structures for multimodal antitumor therapy and inhibition of tumor invasion and metastasis. ACS Appl. Mater. Interfaces 15, 50069–50082. doi:10.1021/acsami.3c11909
Kansız, S., and Elçin, Y. M. (2023). Advanced liposome and polymersome-based drug delivery systems: considerations for physicochemical properties, targeting strategies and stimuli-sensitive approaches. Adv. Colloid Interface Sci. 317, 102930. doi:10.1016/j.cis.2023.102930
Leng, Q., Imtiyaz, Z., Woodle, M. C., and Mixson, A. J. (2023). Delivery of chemotherapy agents and nucleic acids with pH-dependent nanoparticles. Pharmaceutics 15, 1482. doi:10.3390/pharmaceutics15051482
Li, Q., Zeng, K., Chen, Q., Han, C., Wang, X., Li, B., et al. (2024). Atractylenolide I inhibits angiogenesis and reverses sunitinib resistance in clear cell renal cell carcinoma through ATP6V0D2-mediated autophagic degradation of EPAS1/HIF2α. Autophagy 21, 619–638. doi:10.1080/15548627.2024.2421699
Li, Y., Zhao, L., and Li, X. F. (2021). Hypoxia and the tumor microenvironment. Technol. Cancer Res. Treat. 20, 15330338211036304. doi:10.1177/15330338211036304
Li, Z., Xiao, C., Yong, T., Li, Z., Gan, L., and Yang, X. (2020). Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem. Soc. Rev. 49, 2273–2290. doi:10.1039/c9cs00575g
Modi, S. K., Mohapatra, P., Bhatt, P., Singh, A., Parmar, A. S., Roy, A., et al. (2025). Targeting tumor microenvironment with photodynamic nanomedicine. Med. Res. Rev. 45, 66–96. doi:10.1002/med.22072
Plana, D., Palmer, A. C., and Sorger, P. K. (2022). Independent drug action in combination therapy: implications for precision oncology. Cancer Discov. 12, 606–624. doi:10.1158/2159-8290.cd-21-0212
Qin, Y., Yu, Y., Yang, C., Wang, Z., Yang, Y., Wang, C., et al. (2021). Atractylenolide I inhibits NLRP3 inflammasome activation in colitis-associated colorectal cancer via suppressing drp1-mediated mitochondrial fission. Front. Pharmacol. 12, 674340. doi:10.3389/fphar.2021.674340
Ren, Y., Lv, C., Zhang, J., Zhang, B., Yue, B., Luo, X., et al. (2021). Alantolactone exhibits antiproliferative and apoptosis-promoting properties in colon cancer model via activation of the MAPK-JNK/c-Jun signaling pathway. Mol. Cell Biochem. 476, 4387–4403. doi:10.1007/s11010-021-04247-6
Shabnum, S. S., Siranjeevi, R., Raj, C. K., Saravanan, A., Vickram, A. S., Chopra, H., et al. (2024). Advancements in nanotechnology-driven photodynamic and photothermal therapies: mechanistic insights and synergistic approaches for cancer treatment. RSC Adv. 14, 38952–38995. doi:10.1039/d4ra07114j
Shinji, S., Yamada, T., Matsuda, A., Sonoda, H., Ohta, R., Iwai, T., et al. (2022). Recent advances in the treatment of colorectal cancer: a review. J. Nippon. Med. Sch. 89, 246–254. doi:10.1272/jnms.jnms.2022_89-310
Sun, C., Zhang, X., Yu, F., Liu, C., Hu, F., Liu, L., et al. (2021). Atractylenolide I alleviates ischemia/reperfusion injury by preserving mitochondrial function and inhibiting caspase-3 activity. J. Int. Med. Res. 49, 300060521993315. doi:10.1177/0300060521993315
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Tang, D., Xu, X., Ying, J., Xie, T., and Cao, G. (2020). Transfer of metastatic traits via miR-200c in extracellular vesicles derived from colorectal cancer stem cells is inhibited by atractylenolide I. Clin. Transl. Med. 10, e139. doi:10.1002/ctm2.139
Tan, X., Pang, X., Lei, M., Ma, M., Guo, F., Wang, J., et al. (2016). An efficient dual-loaded multifunctional nanocarrier for combined photothermal and photodynamic therapy based on copper sulfide and chlorin e6. Int. J. Pharm. 503, 220–228. doi:10.1016/j.ijpharm.2016.03.019
Tian, H., Zhang, T., Qin, S., Huang, Z., Zhou, L., Shi, J., et al. (2022). Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 15, 132. doi:10.1186/s13045-022-01320-5
Van Straten, D., Mashayekhi, V., De Bruijn, H. S., Oliveira, S., and Robinson, D. J. (2017). Oncologic photodynamic therapy: basic principles, current clinical status and future directions. Cancers (Basel) 9, 19. doi:10.3390/cancers9020019
Vodenkova, S., Buchler, T., Cervena, K., Veskrnova, V., Vodicka, P., and Vymetalkova, V. (2020). 5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol. Ther. 206, 107447. doi:10.1016/j.pharmthera.2019.107447
Wang, C., Duan, H., and He, L. (2009). Inhibitory effect of atractylenolide I on angiogenesis in chronic inflammation in vivo and in vitro. Eur. J. Pharmacol. 612, 143–152. doi:10.1016/j.ejphar.2009.04.001
Wang, K., Huang, W., Sang, X., Wu, X., Shan, Q., Tang, D., et al. (2020). Atractylenolide I inhibits colorectal cancer cell proliferation by affecting metabolism and stemness via AKT/mTOR signaling. Phytomedicine 68, 153191. doi:10.1016/j.phymed.2020.153191
Wang, M., Li, X. Z., Zhang, M. X., Ye, Q. Y., Chen, Y. X., and Chang, X. (2021). Atractylenolide-I sensitizes triple-negative breast cancer cells to paclitaxel by blocking CTGF expression and fibroblast activation. Front. Oncol. 11, 738534. doi:10.3389/fonc.2021.738534
Wu, T., and Dai, Y. (2017). Tumor microenvironment and therapeutic response. Cancer Lett. 387, 61–68. doi:10.1016/j.canlet.2016.01.043
Xie, Y., Wang, Y., Chen, L., Long, J., Zhu, R., and Zheng, H. (2024). Atractylenolide I regulates colorectal cancer cells' biological processes and glycolysis via KRT7. Pak J. Pharm. Sci. 37, 1213–1222.
Xu, M., Qi, Y., Liu, G., Song, Y., Jiang, X., and Du, B. (2023a). Size-dependent in vivo transport of nanoparticles: implications for delivery, targeting, and clearance. ACS Nano 17, 20825–20849. doi:10.1021/acsnano.3c05853
Xu, Q., Lan, X., Lin, H., XI, Q., Wang, M., Quan, X., et al. (2023b). Tumor microenvironment-regulating nanomedicine design to fight multi-drug resistant tumors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 15, e1842. doi:10.1002/wnan.1842
Yang, M., Li, J., Gu, P., and Fan, X. (2021). The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact. Mater. 6, 1973–1987. doi:10.1016/j.bioactmat.2020.12.010
Yu, J. Y., Chuesiang, P., Shin, G. H., and Park, H. J. (2021). Post-processing techniques for the improvement of liposome stability. Pharmaceutics 13, 1023. doi:10.3390/pharmaceutics13071023
Keywords: CRC, ATL-1, Ce6, pH sensitive liposome, PDT
Citation: Zhu Y, Ye Q, Feng S, Fu X, Chen S, Liu H, Ning J, Li Y and Zhang D (2025) Efficient pH-sensitive nanosystem containing Atractylenolide Ⅰ for improving tumor hypoxic microenvironment and photothermal conversion capability for synergistic treatment of colorectal cancer. Front. Nanotechnol. 7:1587439. doi: 10.3389/fnano.2025.1587439
Received: 04 March 2025; Accepted: 20 June 2025;
Published: 14 July 2025.
Edited by:
Prem Shankar Gupta, Maharishi Markandeshwar University, Mullana, IndiaReviewed by:
Shivani R. Pandya, Narnarayan Shastri Institute of Technology, IndiaSandipan Mukherjee, University of Washington, United States
Soniya Rani, Gandhi Institute of Technology and Management (GITAM), India
Copyright © 2025 Zhu, Ye, Feng, Fu, Chen, Liu, Ning, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaling Li, bHlsYXBvdGhlY2FyeUBzd211LmVkdS5jbg==; Dan Zhang, emhhbmdkYW5Ac3dtdS5lZHUuY24=
†These authors have contributed equally to this work
 Yuqing Zhu1†
Yuqing Zhu1† Yaling Li
Yaling Li Dan Zhang
Dan Zhang